Abstract
We have recently shown that in polymorphonuclear leukocytes, 11-keto boswellic acids (KBAs) induce Ca2+ mobilisation and activation of mitogen-activated protein kinases (MAPK). Here we addressed the effects of BAs on central signalling pathways in human platelets and on various platelet functions.
We found that β-BA (10 μM), the 11-methylene analogue of KBA, caused a pronounced mobilisation of Ca2+ from internal stores and induced the phosphorylation of p38 MAPK, extracellular signal-regulated kinase (ERK)2, and Akt. These effects of β-BA were concentration dependent, and the magnitude of the responses was comparable to those obtained after platelet stimulation with thrombin or collagen.
Based on inhibitor studies, β-BA triggers Ca2+ mobilisation via the phospholipase (PL)C/inositol-1,4,5-trisphosphate pathway, and involves Src family kinase signalling.
Investigation of platelet functions revealed that β-BA (⩾10 μM) strongly stimulates the platelet-induced generation of thrombin in an ex-vivo in-vitro model, the liberation of arachidonic acid (AA), and induces platelet aggregation in a Ca2+-dependent manner.
In contrast to β-BA, the 11-keto-BAs (KBA or AKBA) evoke only moderate Ca2+ mobilisation and activate p38 MAPK, but fail to induce phosphorylation of ERK2 or Akt, and do not cause aggregation or significant generation of thrombin.
In summary, β-BA potently induces Ca2+ mobilisation as well as the activation of pivotal protein kinases, and elicits functional platelet responses such as thrombin generation, liberation of AA, and aggregation.
Keywords: Boswellic acids, platelets, calcium, mitogen-activated protein kinases, Akt, arachidonic acid, thrombin
Introduction
Platelets play critical roles in vascular thrombosis and inflammation. Activation of platelets may lead to secretion of granular contents and release of arachidonic acid (AA), shape change, adhesion, and aggregation (Holmsen, 1994). Agonists of platelets can be subdivided into strong activators, such as thrombin or collagen, and weak agonists including platelet-activating factor (PAF), adenosine diphosphate (ADP), serotonin, or thromboxane (TX)A2 that require autocrine stimulation for the entire platelet response (Holmsen, 1994). Furthermore, platelets are partially activated when brought in close contact with surfaces, for example by adhesion to leukocytes or by aggregation.
Soluble platelet agonists, such as thrombin, ADP, PAF, or TXA2, typically bind to specific G protein-coupled receptors (GPCRs), leading to the activation of phospholipase (PL)C (Ruggeri, 2002). PLC isoenzymes in turn, produce diacylglycerols (DAGs) and inositol-1,4,5-trisphosphate (IP3), the latter releases Ca2+ via IP3 receptors from the endoplasmatic reticulum (ER) (Rhee, 2001). GPCR stimulation may also lead to activation of phosphatidylinositol 3-kinase (PI 3-K) isoforms, resulting in Akt phosphorylation. Moreover, mitogen-activated protein kinase (MAPK) cascades, signalling pathways distal of G proteins, are activated upon platelet stimulation (Papkoff et al., 1994; Kramer et al., 1995). Altogether, Ca2+, MAPKs, and PI 3-K/Akt regulate important platelet functions, for example, activation of cytosolic phospholipase (cPL)A2 that liberates AA from phospholipids. In fact, agonist-stimulated platelets generate abundant AA (Kroll & Schafer, 1989), that is mainly metabolised to biologically active prostanoids, including TXA2, and to 12(S)-hydro(pero)xyeicosatetraenoic acid (Yoshimoto & Takahashi, 2002).
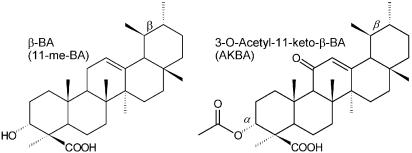
The pentacyclic triterpenes boswellic acids (BAs, Figure 1) are thought to be responsible for the pharmacological actions of Boswellia serrata (BS) extracts, observed in several models of inflammation (Safayhi & Sailer, 1997). 5-lipoxygenase (5-LO) (Safayhi et al., 1992), leukocyte elastase (Safayhi et al., 1997), IκB kinases (Syrovets et al., 2005), and topoisomerases (Syrovets et al., 2000) are molecular targets of BAs. The anti-inflammatory properties of BAs have been attributed to inhibition of 5-LO (Safayhi & Sailer, 1997) but also to suppressed lipopolysaccharide-mediated TNF-α induction in monocytes (Syrovets et al., 2005). Moreover, BAs induce apoptosis of tumor cells (Glaser et al., 1999; Liu et al., 2002), accompanied by decreased ERK phosphorylation (Park et al., 2002) and enhanced caspase activity (Liu et al., 2002).
Figure 1.
Chemical structures of β-BA and AKBA. AKBA lacking the 11-acetyl group yields KBA; 3-O-acetylation of β-BA yields Aβ-BA.
The functional properties and the potencies of the BAs depend on their structure, in particular on the absence or presence of the 11-keto group (Safayhi et al., 1992; Altmann et al., 2002; Liu et al., 2002). Thus, 11-keto-BA (KBA) and 3-O-acetyl-11-keto-BA (AKBA, Figure 1), but not the 11-methylene (11-me) analogues β-BA and Aβ-BA, potently inhibit 5-LO (Safayhi et al., 1992), induce caspase activation (Liu et al., 2002), Ca2+ mobilisation, and MAPK activation in polymorphonuclear leukocytes (PMNL) (Altmann et al., 2002), whereas 11-me-BAs were more efficient in inhibition of topoisomerases (Syrovets et al., 2000) and induction of apoptosis (Glaser et al., 1999).
Recently, we demonstrated that 11-keto-BAs can activate PMNL by mobilisation of Ca2+ and stimulation of MAPKs (Altmann et al., 2002), coupled to functional PMNL responses (Altmann et al., 2004). In the present study we identified β-BA as an agonist for platelets inducing essential signal transduction pathways as well as functional platelet responses, for example release of endogenous AA, thrombin generation, and Ca2+-dependent aggregation.
Methods
Materials
BAs were kindly provided by Dr J. Jauch (Saarbruecken, Germany). Argatroban was a gift from Mitsubishi Pharma (Tokio, Japan) and WEB 2086 was a gift from Boehringer Ingelheim (Ingelheim, Germany). Collagen was from Nykomed Pharma (Unterschleißheim, Germany). U-73122, Calbiochem (Bad Soden, Germany); BAPTA/AM, Fura-2/AM, and forskolin, Alexis (Grünberg, Germany); NF-449 and wortmannin, Biotrend (Köln, Germany); thrombin receptor-activating peptide (TRAP), Bachem (Weil am Rhein, Germany); MRS-2179 and all other chemicals were obtained from Sigma (Deisenhofen, Germany).
Cells
Platelets were freshly isolated from human venous blood of healthy adult donors (St Markus Hospital, Frankfurt, Germany) as described (Albert et al., 2002). Washed platelets were finally resuspended in PBS pH 7.4 and 1 mg ml−1 glucose (PG buffer) or in PBS pH 7.4 and 1 mg ml−1 glucose plus 1 mM CaCl2 (PGC buffer). For incubations with solubilised compounds, ethanol or DMSO was used as vehicle, never exceeding 1% (vol by vol). For functional platelet test (AA release, thrombin generation, aggregation, flow-cytometry of platelet activation markers) platelet-rich plasma (PRP) was prepared from freshly drawn blood (in 3.13% citrate, designated ‘citrate-chelated PRP') from healthy adult donors by centrifugation for 7 min at 750 × g. Depending on the experimental setup, PRP was recalcified to obtain a final [Ca2+] of 1.5 mM, or diluted (14%, vol by vol) in a buffer containing 20 mM HEPES, 140 mM NaCl, 10 mM glucose, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2. Alternatively, platelets were isolated from PRP to yield washed platelets. Some flow-cytometry experiments were also carried out using whole blood (in 3.13% citrate).
Viability assessment
Washed platelets (∼3 × 108 ml−1 PG buffer) were prewarmed for 15 min at 37°C. Then, CaCl2 (1 mM) and any agent (DMSO, β-BA, AKBA, thrombin, or Ca2+ ionophore A23187) were added, and samples were incubated for another 15 min at 37°C. The particle distribution pattern in each sample was then determined using a Sysmex Cell Counter (Norderstedt, Germany) and compared to the DMSO sample (negative control, viable cells) and the A23187 sample (cell fragmentation and lysis due to ionophore action).
Measurement of intracellular Ca2+ levels
Platelets (6 × 108 ml−1 PG buffer) were incubated with 2 μM Fura-2/AM for 30 min at 37°C. After washing, 108 cells ml−1 PG buffer were incubated in a thermally controlled (37°C) fluorimeter cuvette in a spectrofluorometer (Aminco-Bowman series 2, Thermo Spectronic, Rochester, NY, U.S.A.) with continuous stirring. At 2 min prior stimulation, 1 mM CaCl2 or 1 mM EDTA was added. The fluorescence was measured and [Ca2+]i was calculated according to Grynkiewicz et al. (1985).
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blotting
Platelets (109 ml−1 PG buffer containing 1 mM CaCl2 or 1 mM EDTA plus 30 μM BAPTA/AM, respectively) were incubated with the indicated stimuli at 37°C. The reaction was stopped by addition of the same volume of ice-cold 2 × SDS–PAGE sample loading buffer (SDS-b). Samples for SDS–PAGE (aliquots corresponding to 106 cells in 20 μl volume) were prepared, and proteins were separated as described (Werz et al., 2002). Correct loading of the gel and transfer of proteins to the nitrocellulose membrane was confirmed by Ponceau staining. Western blotting using phospho-specific antibodies (New England Biolabs (Beverly, MA, U.S.A.), 1 : 1000 dilution, each) against pERK1/2 (Thr202/Tyr204), pp38 MAPK (Thr180/Tyr182), or pAkt (Ser473), was performed as described (Werz et al., 2002).
Determination of release of [3H]-labelled AA from intact platelets
PRP was labelled with 19.2 nM. [3H]AA (1 μCi ml−1, specific activity 200 Ci mmol−1) for 2 h at 37°C in the presence of 100 μM aspirin. Then, cells were washed twice with PBS pH 5.9 plus 1 mM MgCl2, 11.5 mM NaHCO3, 1 g l−1 glucose, and 1 mg ml−1 fatty acid-free BSA, and finally resuspended in PG buffer (108 ml−1). Preparation of cells at pH 5.9 is thought to minimise temperature-induced activation (Maurer-Spurej et al., 2001). After 15 min at RT, 1 mM CaCl2 was added 2.5 min prior stimulation with the indicated agents at 37°C. After 5 min, incubations were put on ice for 10 min, followed by centrifugation (5000 × g, 15 min). Aliquots (300 μl) of the supernatants were measured (Micro Beta Trilux, Perkin Elmer) to detect the amounts of [3H]-labelled AA released into the medium.
Measurement of thrombin generation
Thrombin generation was assessed by using a fluorometric assay, based on the cleavage of a thrombin-specific fluorogenic substrate resulting from stimulation of recalcified or citrate-chelated PRP, yielding the so-called endogenous thrombin potential (ETP) (Hemker et al., 2000). In all, 80 μl of PRP and 20 μl of buffer containing the thrombin generation trigger were added to each well of a 96-well microtitre plate. The Fluoroskan Ascent Type 374 plate fluorometer (Labsystems; Finland) was used with excitation wavelength 390 nm, emission wavelength 460 nm, and a measurement integration time per well of 20 ms. The first derivative of the fluorescence–time curve reflects the course of thrombin activity in the sample. The parameter of interest in the samples using recalcified PRP was the maximal generation rate which is the peak of the first derivative (ETP peak velocity, relative fluorescence units (RFU) min−1) of the thrombin generation curve, or, due to low peak values in Ca2+-free samples, the ETP–area under the curve (AUC).
Measurement of platelet activation markers CD62 and PAC-1 by flow cytometry
Whole blood samples (containing 3.13% sodium citrate), recalcified PRP, or washed platelets resuspended in PGC were incubated with β-BA, AKBA, TRAP, or vehicle (DMSO, control) for 2 or 15 min at RT. To measure CD62 and PAC-1, samples were diluted 1 : 1 in 20 mM HEPES, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 5.6 mM glucose, 1 g l−1 BSA, pH 7.4, and aliquots of 5 μl were incubated with saturating concentrations of CD41-PC7, CD62-PE, and PAC1-FITC at RT for 15 min in the dark. Samples were fixed with formaldehyde 1% (in PBS), washed twice (CellWash, Becton-Dickinson, Heidelberg, Germany), and resuspended in 300 μl PBS. Isotype-matched IgG and IgM antibodies were used to correct for the nonspecific binding of the specific antibodies. P-selectin (represented by CD62) and PAC-1 antigen expression were quantified using Cellquest software (Becton-Dickinson). Three-color flow cytometric analysis was used with logarithmic modes set for all channels. A gate was set around the platelet population (CD41), and 5000 events were acquired from each probe. The percentage of CD62-positive cells (%) as well as their mean channel fluorescence intensity (MFI) was determined at a level which yields a value of 1% for mouse IgG1-PE labelled sample. A histogram of PAC1-FITC against cell events was generated and MFI of the total platelet population was recorded.
Acquisition of data was carried out using a FACSCalibur flow cytometer with CELLQuest™ (Becton-Dickinson). The instrument calibration and compensation was assured daily with calibration beads (CaliBRITE™ Beads, Becton-Dickinson) and FACSComp™. Fluorescence-conjugated antibodies CD41-PC7, CD62-PE, and PE-labelled isotype IgG1 control were obtained from Beckman Coulter (Krefeld, Germany), PAC1-FITC and FITC-isotype IgM were from Becton-Dickinson.
Ex vivo platelet aggregation (turbidimetric)
Aggregation of platelets in pure or diluted PRP was determined using a turbidimetric light-transmittance device. For aggregation, the response to 30 μM β-BA, 30 μM AKBA, or (as positive controls) 2 U ml−1 thrombin, or 1 μg ml−1 collagen is given as per cent of the maximal light transmission Amax. In Ca2+-containing samples, CaCl2 was added right before the start of the measurement. Aggregation was recorded for 15 min.
Statistics
Statistical evaluation of the data was performed by one-way ANOVAs for independent or correlated samples followed by Tukey HSD post hoc tests. Where appropriate, Student's t-test for paired observations was applied. A P-value of <0.05 (*) or <0.01 (**) was considered significant.
Results
BAs evoke Ca2+ mobilisation in washed human platelets
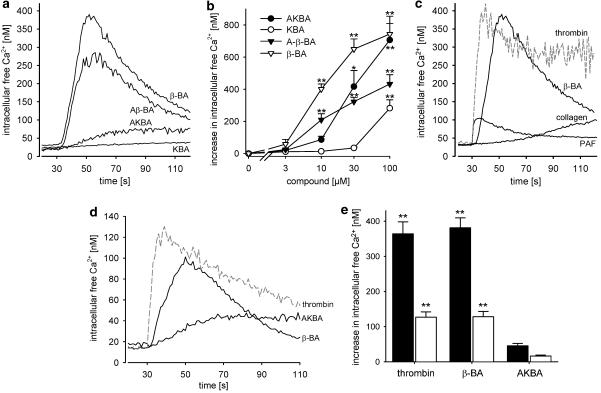
In the presence of extracellular Ca2+ (1 mM), BAs lacking the 11-keto moiety (Aβ-BA and β-BA, 10 μM each, chemical structure see Figure 1) induced a transient but robust elevation of [Ca2+]i in washed platelets that peaked 18–30 s following exposure, whereas KBA was ineffective and AKBA caused only a weak and rather slow Ca2+ mobilisation (Figure 2a and b). β-BA was effective already at 3 μM, though not yet significant (Figure 2b). At 10 μM, the maximum increase in [Ca2+]i (381±28 nM) elicited was comparable to that obtained by thrombin (0.5 U ml−1; 364±34 nM), and exceeded the signal obtained by PAF (100 nM; 62±5 nM, Figure 2c). However, thrombin- and PAF-induced Ca2+ mobilisation was more rapid, peaking 5–10 s after exposure and (for thrombin) was more sustained. Collagen (8 μg ml−1) caused a slow and only moderate elevation of [Ca2+]i (78±7 nM, after 90 s). 11-Keto BAs caused significant Ca2+ mobilisation at higher concentrations (⩾20–30 μM) (Figure 2b), which again was rather slow. Thus, the potencies and the kinetics differ between 11-keto-BAs and their 11-me analogues.
Figure 2.
BAs induce intracellular Ca2+ mobilisation. To Fura-2-loaded platelets (108 ml−1 PG buffer), 1 mM CaCl2 (a–c and e) or 1 mM EDTA (d and e) was added 2 min prior stimulation, and [Ca2+]i was determined. (a) Ca2+ mobilisation in the presence of extracellular Ca2+. BAs (10 μM, each) were added 30 s after the measurement was started. (b) Concentration–response curves of BAs in the presence of extracellular Ca2+. The maximal increase in [Ca2+]i obtained within 100 s of measurement is given. (c) Ca2+ mobilisation induced by various agonists. The following agonists were used: β-BA (10 μM), thrombin (0.5 U ml−1), collagen (8 μg ml−1), and PAF (100 nM). (d) Ca2+ mobilisation in the absence of extracellular Ca2+. BAs (10 μM, each) or thrombin (0.5 U ml−1) were added 30 s after the measurement was started. (e) Comparison of Ca2+ mobilisation in the presence (black bars) or absence (white bars) of 1 mM of extracellular Ca2+. The maximal increase in [Ca2+]i after stimulation with thrombin (0.5 U ml−1) or BAs (10 μM, each) was determined within 100 s of measurement. Values are given as mean+s.e., n=5; curves are representative for at least five experiments. One-way ANOVAs followed by Tukey HSD tests were applied to data related to unstimulated controls in (b) and (e) *P<0.05 or **P<0.01.
[Ca2+]i was also measured in the absence of extracellular Ca2+. β-BA, Aβ-BA (not shown), and AKBA as well as thrombin evoked an internal Ca2+ release with similar kinetics observed for the total Ca2+ response in the presence of extracellular Ca2+, respectively (Figure 2D, compare Figure 2a). Nevertheless, in the absence of extracellular Ca2+, elevation of [Ca2+]i was reduced to about 37±14% for thrombin and 28±17% for β-BA, as compared to the total Ca2+ response (Figure 2e).
β-BA-, but not AKBA-induced Ca2+ mobilisation is PLC dependent
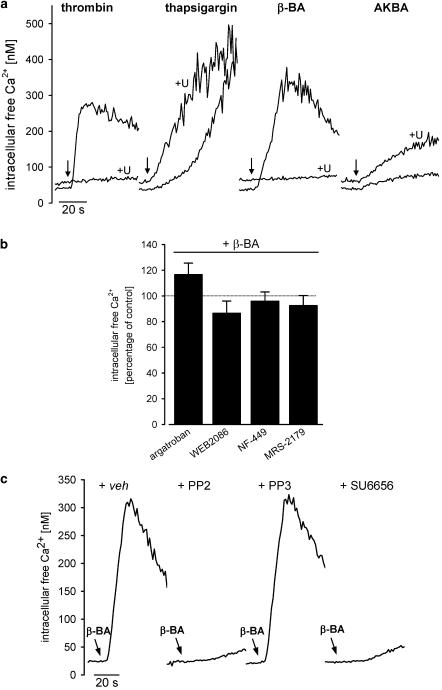
U-73122 (3 μM) and 2-aminoethoxydiphenylborate (2-APB, 50 μM) were used to examine the participation of PLC in BA-induced Ca2+ mobilisation. Cells were stimulated with β-BA or AKBA (10 μM each), with thrombin (positive control), or thapsigargin (TG, negative control). Thrombin evokes Ca2+ elevations via a GPCR/PLC-dependent pathway (Coughlin, 2000), whereas TG induces Ca2+ mobilisation by inhibition of the ER Ca2+-ATPase, thus circumventing PLC and GPCR signalling (Gouy et al., 1990). Thrombin- and β-BA-induced Ca2+ elevation was strongly suppressed by U-73122 (Figure 3a), both, in the presence and in the absence of extracellular Ca2+ (Table 1). In contrast, Ca2+ mobilisation induced by TG was not suppressed by U-73122, and the response to AKBA was even potentiated (Figure 3a, Table 1). Generally, U-73122 preincubation already caused a slight elevation of the resting Ca2+ levels (Figure 3a). U-73343, the inactive analogue of U-73122, had no effect (not shown). 2-APB (50 μM) (Maruyama et al., 1997), an inhibitor of IP3-mediated elevations in cytosolic [Ca2+], also suppressed Ca2+ mobilisation induced by β-BA in the presence (73±7% inhibition, n=3) and in the absence of Ca2+ (54±11% inhibition, n=3). An equal reduction was found for the thrombin response (not shown). Collectively, our data indicate that Ca2+ mobilisation induced by β-BA is mediated by the PLC/IP3 signalling pathway.
Figure 3.
Modulation of β-BA-induced Ca2+ mobilisation by pharmacological inhibitors. (a) Effects of U-73122. Fura-2-loaded platelets (108 ml−1 PG buffer) were preincubated with U-73122 (3 μM, trace labelled ‘+U') or vehicle (DMSO) for 15 min. CaCl2 (1 mM) was added 2 min prior stimulation with thrombin (0.5 U ml−1), thapsigargin (0.1 μM), or BAs (10 μM, each), and [Ca2+]i was determined. Curves are representative for at least five experiments. (b) Effects of argatroban (100 ng ml−1), WEB 2086 (30 μM), NF 449 (1 μM), or MRS-2179 (10 μM). Fura-2-loaded platelets (108 ml−1 PG buffer) were preincubated with the indicated compounds or vehicle (DMSO) for 15 min. CaCl2 (1 mM) was added 2 min prior stimulation with 10 μM β-BA. The maximal increase in [Ca2+]i determined within 100 s of measurement is expressed as percentage of control (10 μM β-BA). Values are given as mean+s.e., n=5. (c) Effects of Src family kinase inhibitors. Fura-2-loaded platelets (108 ml−1 PG buffer) were preincubated with PP2 (3 μM), PP3 (3 μM), SU6656 (10 μM), or vehicle (DMSO) for 15 min. CaCl2 (1 mM) and β-BA (10 μM) were added, and [Ca2+]i was determined. Curves are representative for at least four experiments.
Table 1.
Effects of U-73122 on Ca2+ mobilisation in the absence and presence of Ca2+
| +Ca2+ residual signal (percentage of control) | −Ca2+ (+EDTA) residual signal (percentage of control) | |
|---|---|---|
| Thrombin | 10±4 (n=5)** | 12±3 (n=5)** |
| TG | 90±12 (n=5) | 116±19 (n=4) |
| β-BA | 5±2 (n=6)** | 23±8 (n=6)** |
| AKBA | 170±68 (n=5) | 202±41 (n=5) |
Fura-2-loaded platelets (108 ml−1 PG buffer) were preincubated with U-73122 (3 μM) for 15 min. CaCl2 (1 mM) or EDTA (1 mM) were added, and after 2 min, platelets were stimulated with thrombin (0.5 U ml−1), TG (0.1 μM), or BAs (10 μM, each). Maximum amplitudes were compared to control measurements in the absence of U-73122. Data are expressed as mean+s.e., n=4–6 (see table). Statistical analysis (t-tests for correlated samples, inhibitor versus control samples for each stimulus) was performed prior to data normalisation
P<0.01.
Since β-BA could first induce the generation of an endogenous platelet agonist that in turn causes PLC/IP3-coupled elevation of [Ca2+]i, antagonists of typical platelet stimuli were utilised to unravel such an autocrine mode of action. The thrombin antagonist argatroban (100 ng ml−1) failed to significantly suppress the effects of β-BA (Figure 3b), whereas it completely blocked thrombin-induced Ca2+ mobilisation (not shown). Similarly, the PAF receptor antagonist WEB 2086 (30 μM) as well as the purinergic receptor antagonists NF-449 (1 μM, targeting P2X1) and MRS-2179 (10 μM, targeting P2Y1, and P2Y12) did not markedly affect β-BA-induced elevation of [Ca2+]i (Figure 3b), although these compounds abolished the responses induced by their respective agonists (not shown).
Src family kinases are involved in β-BA-induced Ca2+ mobilisation
The role of Src family kinases in β-BA-induced Ca2+ mobilisation was assessed using the selective Src family kinase inhibitors PP2 (and its inactive analogue PP3) (Hanke et al., 1996) and SU6656 (Blake et al., 2000). PP2 (3 μM) blunted the Ca2+ response initiated by β-BA (92±2% inhibition, n=7, see Figure 3c) whereas the inactive analogue PP3 (3 μM) was hardly effective (89±8% residual activity, n=4, see Figure 3c). Also, the structurally unrelated Src kinase inhibitor SU6656 (10 μM) likewise abolished the β-BA signal (93±1% inhibition, n=4, Figure 3c). In sharp contrast, no such inhibitory effects of PP2 on Ca2+ signals induced by thrombin, PAF, or AKBA were apparent (not shown).
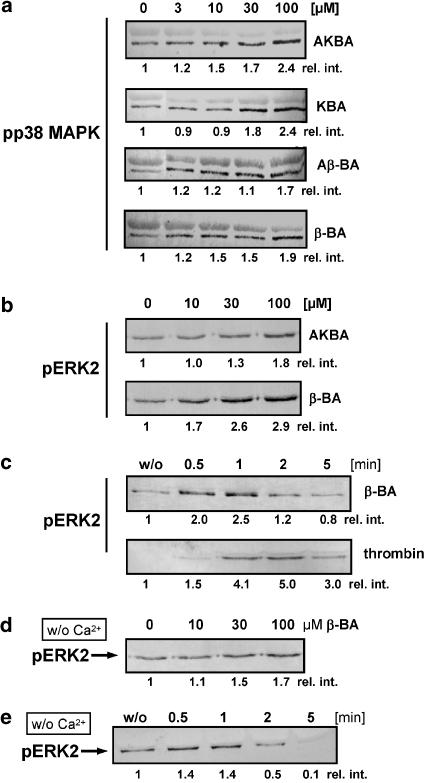
β-BA induces MAPK activation and Akt/PKB phosphorylation
All BAs tested led to a concentration-dependent activation of p38 MAPK in platelets (Figure 4a). Moreover, β-BA (and Aβ-BA, not shown) also concentration-dependently (10–100 μM) activated ERK2 (Figure 4b), which was maximal 30–60 s upon stimulation, slightly preceding thrombin-induced ERK2 activation (maximum after 2 min) (Figure 4c). The EC50 for thrombin to activate p38 MAPK and ERK2 was determined at ≈1 U ml−1 (not shown). AKBA (and KBA, not shown) were virtually ineffective to activate ERK2. U-73122 (not shown) as well as the Ca2+ chelators EDTA and BAPTA/AM, moderately reduced β-BA-induced ERK2 activation (Figure 4d and e), indicating that PLC and Ca2+ may contribute, but are not absolutely required.
Figure 4.
BAs induce the activation of MAPKs. Activation of p38 MAPK (a) and ERK2 (b). Platelets (109 ml−1 PGC buffer) were stimulated with the indicated concentrations of the BAs at 37°C. Reactions were terminated after 1 min to assess ERK2 phosphorylation, and after 1.5 min to assess p38 MAPK phosphorylation. Samples were subjected to SDS–PAGE and Western blotting using phospho-specific antibodies against the dually phosphorylated form of the MAPKs. (c) Time course of ERK2 activation in the presence of Ca2+. Platelets (109 ml−1 PGC buffer) were stimulated with β-BA (30 μM) or thrombin (1 U ml−1) at 37°C for the indicated times and phosphorylation of ERK2 was determined. (d) ERK2 activation by β-BA in the absence of Ca2+. Platelets (109 ml−1 PG buffer containing 1 mM EDTA and 30 μM BAPTA/AM) were preincubated for 15 min at RT, stimulated with the indicated concentrations of β-BA for 1 min and phosphorylation of ERK2 was determined. (e) Time course of ERK2 activation by β-BA in the absence of Ca2+. Platelets (109 ml−1 PG buffer containing 1 mM EDTA and 30 μM BAPTA/AM) were preincubated for 15 min at RT, stimulated with 30 μM β-BA, and phosphorylation of ERK2 was determined after the times indicated. The relative intensities (rel. int.) of blot bands were determined by densitometry using the BioRad Quantitate One software. The results shown are representative of at least three independent experiments.
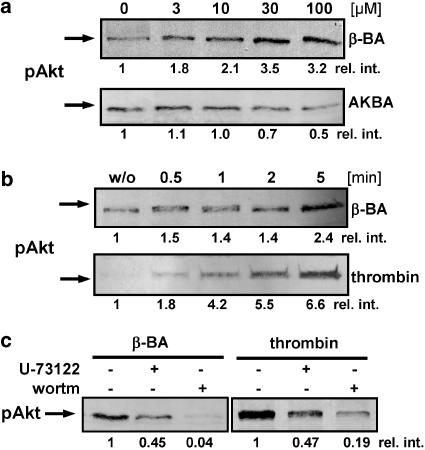
β-BA concentration-dependently increased the phosphorylation of Akt at Ser473, whereas AKBA had no significant effect (Figure 5a). Again, the effects of β-BA were comparable to thrombin. Thus, Akt phosphorylation induced by β-BA was most pronounced after 2–5 min (Figure 5b), and was completely blocked by the PI 3-K inhibitor wortmannin (wortm., 200 nM) and strongly blunted by U-73122 (Figure 5c). Moreover, removal of total Ca2+ with EDTA and BAPTA/AM abolished the effect of β-BA (not shown). Similar inhibitory effects on Akt phosphorylation by wortmannin and U-73122 (Figure 5c) as well as by Ca2+ removal (not shown) were seen when thrombin was used as stimulus.
Figure 5.
β-BA induces phosphorylation of Akt. (a) Concentration–response experiments. Platelets (109 ml−1 PGC buffer) were stimulated with the indicated concentrations of BAs for 4 min at 37°C and Akt phosphorylated at Ser473 was assessed. (b) Time course of Akt phosphorylation. Platelets (109 ml−1 PGC buffer) were incubated with β-BA (30 μM) or thrombin (1 U ml−1) at 37°C for the indicated times and Akt phosphorylation was assessed. (c) Effects of U-73122 and wortmannin (wortm) on Akt phosphorylation. Platelets (109 ml−1 PGC buffer) were preincubated with 3 μM U-73122 or 200 nM wortmannin as indicated and then stimulated with β-BA (30 μM) or thrombin (1 U ml−1), respectively, for 4 min at 37°C and Akt phosphorylation was assessed. The relative intensities (rel. int.) of blot bands were determined by densitometry using the BioRad Quantitate One software. Results are representative of at least three independent experiments.
Induction of cell viability assessment and AA release
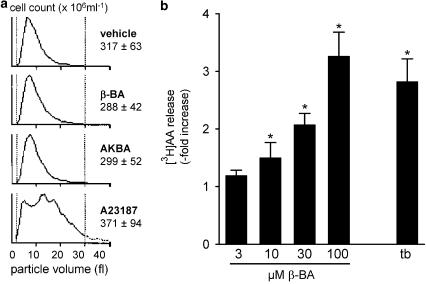
The effects of BAs on platelet viability were determined by analysis of the particle distribution pattern of washed platelet samples treated with β-BA, AKBA (30 μM, each), DMSO, or Ca2+ ionophore A23187 (5 μM) for 15 min at 37°C. Particle size spreading (given by the particle volume, unit: fl, horizontal axis) and numbers were similar for β-BA, AKBA, and DMSO, whereas ionophore-induced platelet lysis caused a heterogeneous dispersion (Figure 6a). Thus, exposition of platelets to β-BA for 15 min does not seem to affect cell viability.
Figure 6.
β-BA induces the liberation of AA; effects on cell viability. (a) Analysis of cell viability. Washed platelets were resuspended in PGC buffer and exposed to the indicated stimuli (vehicle (DMSO), 30 μM β-BA, 30 μM AKBA, 5 μM A23187, from top) for 15 min at 37°C. The particle size distribution pattern and particle number in each sample was determined. DMSO (negative control, viable cells) and A23187 samples (positive control, lysed cells) were used as reference. Curves are representative for at least three independent determinations. Cell count values are given as mean+s.e., n=3–4. (b) β-BA induces the release of AA. Platelets were labelled with [3H]AA for 2 h. CaCl2 (1 mM) was added to the cells (108 in 1 ml PG buffer), and after 2.5 min, cells were stimulated with the indicated concentrations of β-BA or 2 U ml−1 thrombin (tb). [3H]AA released into the medium was measured after 5 min at 37°C. Data are expressed as increase over unstimulated cells, values are given as mean+s.e., n=5. Statistical analysis (directed t-tests for correlated samples) was applied to original data prior to normalisation, *P<0.05.
An elevation of [Ca2+]i and/or activation of members of the MAPK family are considered important for the liberation of AA by the cPLA2 (Gijon & Leslie, 1999). Incubation of [3H]AA-labelled platelets with β-BA caused a concentration-dependent increase in the amounts of [3H]AA released into the medium. At 30 μM, β-BA was equipotent to 2 U ml−1 thrombin (Figure 6b).
Effects of β-BA on aggregation
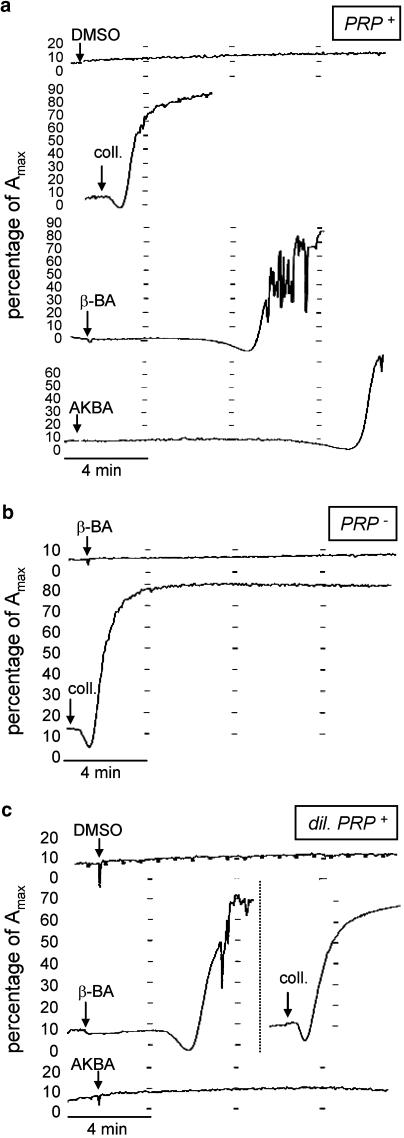
In recalcified (1.5 mM CaCl2, free Ca2+ was calculated as approx. 1 mM) PRP, β-BA (10 or 30 μM) stimulated aggregation 6–8 min after addition (Figure 7a, middle left trace). In contrast, aggregation induced by collagen (1 μg ml−1) was much more rapid (Figure 7a, middle right trace). Spontaneous aggregation due to unspecific platelet activation (e.g. stirring) occurred after >12–15 min. Similarly, in samples that received AKBA (30 μM), aggregation was first evident after approx. 12 min (Figure 7a, lower trace). In contrast, in citrate-chelated PRP (no Ca2+), β-BA (30 μM) caused no aggregation, whereas collagen remained a full agonist (Figure 7b). Also, no unspecific aggregation was observed in the absence of extracellular Ca2+. The Ca2+ dependency of the β-BA effect was confirmed using PRP diluted in a Ca2+-containing HEPES buffer (14% PRP, final concentration). Under these conditions, aggregation induced by β-BA (30 μM) was more rapid (after 4–6 min) than in pure PRP (Figure 7c). DMSO and AKBA (30 μM) were inactive, and collagen again acted as an immediate and full agonist (Figure 7c). It should be noted that in diluted PRP without Ca2+, none of the stimuli induced aggregation (not shown). Together, β-BA-stimulated aggregation strictly depended on the presence of extracellular Ca2+.
Figure 7.
Effects of β-BA on platelet aggregation. (a) Aggregation in recalcified PRP (PRP+). Samples were stimulated with vehicle (DMSO, 0.3%), β-BA (30 μM), collagen (1 μg ml−1), or AKBA (30 μM) as indicated and aggregation curves were recorded (maximum 15 min). The aggregation response is given as per cent of the maximal light transmission Amax. Curves are representative for at least five independent determinations. (b) Aggregation in citrate-chelated PRP. The experimental conditions were the same as above, n=4. (c) Aggregation in PRP diluted in Ca2+-containing HEPES buffer, n=5.
Thrombin generation and expression of activation markers
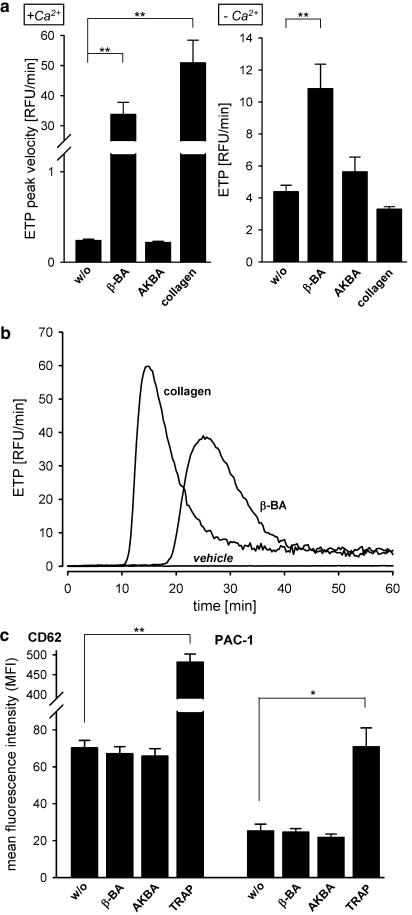
β-BA was tested for its ability to generate thrombin from PRP, expressed as the ETP. β-BA (10 μM) potently stimulated thrombin generation, whereas AKBA (10 μM) was inactive (Figure 8a, left panel). Although collagen was only moderately superior to β-BA in the peak thrombin generation velocity, there was again a delayed onset of the β-BA effect, visualised by the kinetic progression of the ETP (Figure 8b). In the absence of Ca2+, neither stimulus induced a marked increase in the ETP over time although analysis of the ETP-AUCs revealed a slight stimulatory effect of β-BA (10 μM) as compared to DMSO and collagen that both were inactive (Figure 8a, right panel).
Figure 8.
Thrombin generation and activation marker expression. (a) Thrombin generation was assessed in recalcified PRP (given as ETP peak velocity, left bar chart), or citrate-chelated PRP (given as ETP-AUC, right bar chart). PRP and buffer containing the indicated stimuli were added to each well of a 96-well microtitre plate. β-BA (10 μM), AKBA (10 μM), collagen (2 μg ml−1, final concentrations each), and vehicle (DMSO) were tested for their ability to induce thrombin generation. Data are expressed as mean+s.e., n=4 (β-BA, AKBA, collagen) or n=8 (vehicle). One-way ANOVA and Tukey HSD tests were performed, **P<0.01. (b) Representative original traces of the ETP kinetic progression. Cells in recalcified PRP were stimulated as described above. (c) Expression of the platelet activation markers CD62 and PAC-1. Flow cytometry in recalcified PRP was performed as described in the Methods section. Expression of CD62 (left bar chart) and PAC-1 (right bar chart) after stimulation with vehicle (DMSO), β-BA (30 μM), AKBA (30 μM), or TRAP (10 μM) is given. The percentage of CD62-positive cells (%) as well as their mean channel fluorescence intensity (MFI) was determined (left diagram). Right, a histogram of PAC1-FITC against cell events was generated and MFI of total platelet population was recorded., n=4. One-way ANOVA and Tukey HSD tests were performed, *P<0.05 or **P<0.01.
Finally, the expression of the activation markers PAC-1 (the activated GPIIb/IIIa-receptor for fibrinogen) and CD62, which indicates the release of platelet alpha-granules, were assessed. Incubations were carried out in (I) whole blood (containing 3.13% citrate), (II) recalcified PRP, and (III) washed platelets in Ca2+-containing PGC buffer, for 2 or 15 min. Neither β-BA (30 or 100 μM) nor AKBA (30 μM) led to a significant expression of CD62 and PAC-1 under all experimental settings (I–III), whereas TRAP (used as positive control) was a strong activator (Figure 8c).
Discussion
We identified 11-me-BAs (i.e. β-BA) as naturally occurring compounds that induce central signalling pathways and that elicit select functions in human platelets. Depending on the structure of the BAs, the effectiveness and the routes, utilised to activate downstream signalling pathways and functional responses, are highly distinct. For β-BA, Src family kinases and the PLC/IP3 pathway seem to be involved in Ca2+ mobilisation, and β-BA causes activation of ERK2 and the PI 3-K/Akt route. Moreover, β-BA induces the release of AA, a pronounced generation of thrombin, and Ca2+-dependent platelet aggregation. In contrast, AKBA-induced Ca2+ mobilisation is not connected to Src family kinases and PLC/IP3 signalling, and AKBA failed to induce phosphorylation of Akt and ERK2, as well as functional platelet responses.
Among the BAs tested for Ca2+ mobilisation, β-BA is the most potent analogue. At 10 μM, the effectiveness of β-BA exceeded that of PAF or collagen, and was comparable with that of the potent platelet agonist thrombin. Such β-BA concentrations are in the range of β-BA levels in human plasma (10.1 μM), determined after oral application of 4 × 786 mg BS extract/day within 10 days (Buchele & Simmet, 2003). The 3-O-acetyl group slightly hampers (receptor-)activation and the 11-keto moiety significantly decreases the potency and also alters the signalling routes in platelets. In sharp contrast to platelets, only 11-keto BAs, but not 11-me-BAs, caused stimulation of PMNL (Altmann et al., 2002; 2004). Possibly, PMNL and platelets selectively express closely related but not identical receptors specific for AKBA or 11-me-BAs, respectively. Important receptors for soluble agonists known to regulate [Ca2+]i in platelets are the purinergic P2X1 and P2Y1/12 receptors, the TXA2 receptor, the PAF receptor, the 5-HT2A receptor, and the PAR-1 and -4 (Jackson et al., 2003). Whether β-BA acts at one (or more) of these receptors is unknown. However, antagonists of thrombin (argatroban), PAF (WEB 2086), and ADP (NF449 and MRS2179) did not affect β-BA-induced Ca2+ mobilisation.
Thrombin is the most potent platelet agonist acting via PAR-1 and -4. PARs are coupled to trimeric Gq/Gi/G12/13 proteins enabling the Gα and Gβγ subunits to stimulate PLC-β subtypes (Lee et al., 1996; Coughlin, 2000), resulting in IP3-dependent Ca2+ mobilisation from intracellular storage sites with concomitant store-operated Ca2+ entry (Rosado & Sage, 2001). In analogy to thrombin, β-BA caused Ca2+ mobilisation from internal stores, which was sensitive to U-73122 and to 2-APB, confirming the involvement of PLC/IP3. However, it should be noted that PLC/IP3-independent effects of U-73122 (Broad et al., 1999) and 2-APB on cellular Ca2+ influx systems have been reported (Dobrydneva & Blackmore, 2001). Of interest, the proximal routes mediating PLC/IP3-dependent Ca2+ mobilisation appear to be different for β-BA and thrombin (or PAF). Thus, Src family kinase inhibitors abolished the β-BA-induced response, but not the responses elicited by thrombin or PAF. PLC-γ is the most abundant PLC isoform in platelets (Lee et al., 1996) and is an operative element in Ca2+ mobilisation mediated by adhesion receptors (Rhee, 2001). Whereas, soluble ligands such as thrombin, ADP, PAF, or TXA2 act via GPCRs to stimulate PLC-β isoenzymes, the PLC-γ isoforms are regulated through phosphorylation by Src family kinases (Rhee, 2001). In analogy to agonists that act via adhesion receptors but unlike thrombin, β-BA may utilise the Src family kinases/PLC-γ pathway to induce Ca2+ mobilisation. Another difference between β-BA- and thrombin-mediated Ca2+ mobilisation is the significant delay of the response to β-BA as compared to the rapid effect of thrombin. Possibly, aside of acting as a direct ligand at a certain (adhesion) receptor, β-BA may first induce the generation of an endogenous agonist that in turn causes PLC-γ/IP3-coupled Ca2+ mobilisation via (adhesion) receptors. Attempts to unravel a putative autocrine mode of action are in progress in our laboratory.
Typical platelet agonists such as thrombin, collagen, or TXA2 activate PI 3-K and its downstream effector Akt, important mediators of agonist-induced platelet activation (Kim et al., 2004), as well as p38 MAPK and ERKs (Papkoff et al., 1994; Kramer et al., 1995; Saklatvala et al., 1996). The MAPK are a point of convergence of complex signalling networks, regulating cell proliferation and differentiation (Papkoff et al., 1994). In platelets, the functions of MAPK are mainly uncharacterised and the signal transduction steps are poorly understood. All BAs tested activated p38 MAPK with similar efficacy, but only β-BA (and Aβ-BA) rapidly and significantly activated ERK2. Also, β-BA, but not AKBA, evoked Akt phosphorylation, and in analogy to thrombin, the PI 3-K and/or the PLC/Ca2+ pathway is involved. Therefore, the receptor for BAs mediating p38 MAPK activation might be different from that transmitting signals to activate ERK2 and Akt. The latter (11-me-BA specific) receptor may also mediate increases in [Ca2+]i, generation of thrombin, release of AA and aggregation, since AKBA and KBA failed to elicit these events.
Investigation of the platelet functions elicited by β-BA provided controversial results. As a rule, the distinct responses of activated platelets depend on the strength (potency) of the agonist, and these responses can be ordered in an activation sequence: (1) aggregation, (2) granule secretion, (3) AA liberation, and (4) acid hydrolase secretion (Steen & Holmsen, 1987). For the induction of these responses, the magnitude of Ca2+ mobilisation is an important parameter. In fact, β-BA (10–30 μM) substantially elevated [Ca2+]i and potently induced thrombin generation, being equipotent in this respect with collagen at 2 μg ml−1 in a model utilising native platelets. Also, β-BA potently evoked the liberation of free AA from washed platelets, although at concentrations slightly higher than those required for Ca2+ mobilisation, probably due to the presence of fatty acid-free albumin that may bind BAs. In general, liberation of free AA is a response distal of aggregation and degranulation, and its induction normally requires a potent agonist-activating platelets with high strength. Surprisingly, however, the efficacy of β-BA was much reduced for the induction of aggregation. In contrast to collagen, the response of β-BA was strictly dependent on the presence of extracellular Ca2+ and was characterised by a prolonged lag phase (4–8 min), a rather slow initial decrease in light transmission, and a submaximal slope of the aggregation curve. This response in some way resembles the ‘unspecific' aggregation induced by shear stress (stirring), normally occurring after 12–15 min, in contrast to the rapid (<1 min) signal evoked by a strong agonist (i.e. collagen). Therefore, β-BA may rather facilitate aggregation by other factors than being a full agonist. Moreover, β-BA failed to induce degranulation and fibrinogen receptor activation (CD62, PAC-1 expression). Together, despite the pronounced elevation of [Ca2+]i, only select functional platelet responses were observed after stimulation with β-BA. Along these lines it was found that platelets in polycythaemia vera exhibit decreased aggregation after stimulation with PAF, although an equal increase in [Ca2+]i was seen as compared to platelets from healthy donors (Le Blanc et al., 2000). Also, a patient was described with defective platelet aggregation in response to ionophore A23187, despite normal increases in [Ca2+]i (Fuse et al., 1999). Hence, elevation of [Ca2+]i in platelets is one important signalling step for eliciting various platelet responses, but must not necessarily lead to the induction of all Ca2+-dependent platelet functions. It is conceivable that β-BA on one hand is a platelet agonist that potently induces central signalling pathways (Ca2+ mobilisation, MAPK/Akt phosphorylation) and select responses such as thrombin generation and AA release, but on the other hand lacks the stimulation of certain signalling components or executing molecules particularly important for a rapid aggregation, degranulation, and fibrinogen receptor activation.
At present, our findings cannot be readily related to the anti-inflammatory properties of BS extracts, observed in animal models or in studies with human subjects (Safayhi & Sailer, 1997). Nevertheless, due to its high effectiveness and the importance of the signalling molecules and the select platelet functions induced, the receptor(s) mediating the actions of β-BA in platelets warrant further elucidation. Since the effective concentrations of β-BA (10 μM) are in range of β-BA levels in human plasma (see above), one should be aware of its pharmacological actions on platelets when administering BS extracts to patients.
Acknowledgments
We thank Sven George for expert technical assistance and Dr Günter Lambrecht for critical reading of the manuscript and helpful discussion. This study was supported by grants from the Fonds der Chemischen Industrie, from the Deutsche Forschungsgemeinschaft (GRK 757), the Deutsche Pharmazeutische Gesellschaft, and from the European Union (LEUCHRON, QLRT-2000-01521).
Abbreviations
- AA
arachidonic acid
- Aβ-BA
3-O-acetyl-boswellic acid
- ADP
adenosine diphosphate
- AKBA
3-O-acetyl-11-keto-boswellic acid
- 2-APB
2-aminoethoxydiphenylborate
- AUC
area under the curve
- BA
boswellic acid
- cPLA2
cytosolic phospholipase A2
- DAG
diacylglycerol
- ER
endoplasmatic reticulum
- ERK
extracellular signal-regulated kinase
- ETP
endogenous thrombin potential
- GPCR
G protein-coupled receptor
- IP3
inositol-1,4,5-trisphosphate
- KBA
11-keto-boswellic acid
- LO
lipoxygenase
- MAPK
mitogen-activated protein kinase
- MFI
mean fluorescence intensity
- PAF
platelet-activating factor
- PG buffer
PBS plus 1 mg ml−1 glucose
- PGC buffer
PBS containing 1 mg ml−1 glucose and 1 mM CaCl2
- PI 3-K
phosphatidylinositol 3-kinase
- PLC
phospholipase C
- PMNL
polymorphonuclear leukocytes
- PRP
platelet rich plasma
- RFU
relative fluorescence units
- SDS-b
2 × sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample loading buffer
- TG
thapsigargin
- TRAP
thrombin receptor-activating peptide
- TXA2
thromboxane A2
References
- ALBERT D., ZÜNDORF I., DINGERMANN T., MÜLLER W.E., STEINHILBER D., WERZ O. Hyperforin is a dual inhibitor of cyclooxygenase-1 and 5-lipoxygenase. Biochem. Pharmacol. 2002;64:1767–1775. doi: 10.1016/s0006-2952(02)01387-4. [DOI] [PubMed] [Google Scholar]
- ALTMANN A., FISCHER L., SCHUBERT-ZSILAVECZ M., STEINHILBER D., WERZ O. Boswellic acids activate p42(MAPK) and p38 MAPK and stimulate Ca(2+) mobilization. Biochem. Biophys. Res. Commun. 2002;290:185–190. doi: 10.1006/bbrc.2001.6153. [DOI] [PubMed] [Google Scholar]
- ALTMANN A., PÖCKEL D., FISCHER L., SCHUBERT-ZSILAVECS M., STEINHILBER D., AND WERZ O. Coupling of boswellic acid-induced Ca2+-mobilization and MAPK activation to lipid metabolism and formation of reactive oxygen species in human leukocytes. Br. J. Pharmacol. 2004;141:223–232. doi: 10.1038/sj.bjp.0705604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAKE R.A., BROOME M.A., LIU X., WU J., GISHIZKY M., SUN L., COURTNEIDGE S.A. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol. Cell. Biol. 2000;20:9018–9027. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROAD L.M., CANNON T.R., SHORT A.D., TAYLOR C.W. Receptors linked to polyphosphoinositide hydrolysis stimulate Ca2+ extrusion by a phospholipase C-independent mechanism. Biochem. J. 1999;342:199–206. [PMC free article] [PubMed] [Google Scholar]
- BUCHELE B., SIMMET T. Analysis of 12 different pentacyclic triterpenic acids from frankincense in human plasma by high-performance liquid chromatography and photodiode array detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;795:355–362. doi: 10.1016/s1570-0232(03)00555-5. [DOI] [PubMed] [Google Scholar]
- COUGHLIN S.R. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- DOBRYDNEVA Y., BLACKMORE P. 2-Aminoethoxydiphenyl borate directly inhibits store-operated calcium entry channels in human platelets. Mol. Pharmacol. 2001;60:541–552. [PubMed] [Google Scholar]
- FUSE I., HIGUCHI W., UESUGI Y., HATTORI A., AIZAWA Y. Relationship between intracellular calcium-dependent process and protein-tyrosine phosphorylation in human platelets: studies of platelets from a patient with defective A23187-induced platelet aggregation. Clin. Lab. Haematol. 1999;21:29–32. doi: 10.1046/j.1365-2257.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- GIJON M.A., LESLIE C.C. Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J. Leukoc. Biol. 1999;65:330–336. doi: 10.1002/jlb.65.3.330. [DOI] [PubMed] [Google Scholar]
- GLASER T., WINTER S., GROSCURTH P., SAFAYHI H., SAILER E.R., AMMON H.P., SCHABET M., WELLER M. Boswellic acids and malignant glioma: induction of apoptosis but no modulation of drug sensitivity. Br. J. Cancer. 1999;80:756–765. doi: 10.1038/sj.bjc.6690419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOUY H., CEFAI D., CHRISTENSEN S.B., DEBRE P., BISMUTH G. Ca2+ influx in human T lymphocytes is induced independently of inositol phosphate production by mobilization of intracellular Ca2+ stores. A study with the Ca2+ endoplasmic reticulum-ATPase inhibitor thapsigargin. Eur. J. Immunol. 1990;20:2269–2275. doi: 10.1002/eji.1830201016. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HANKE J.H., GARDNER J.P., DOW R.L., CHANGELIAN P.S., BRISSETTE W.H., WERINGER E.J., POLLOK B.A., CONNELLY P.A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- HEMKER H.C., GIESEN P.L., RAMJEE M., WAGENVOORD R., BEGUIN S. The thrombogram: monitoring thrombin generation in platelet-rich plasma. Thromb. Haemost. 2000;83:589–591. [PubMed] [Google Scholar]
- HOLMSEN H. Significance of testing platelet functions in vitro. Eur. J. Clin. Invest. 1994;24:3–8. doi: 10.1111/j.1365-2362.1994.tb02418.x. [DOI] [PubMed] [Google Scholar]
- JACKSON S.P., NESBITT W.S., KULKARNI S. Signaling events underlying thrombus formation. J. Thromb. Haemost. 2003;1:1602–1612. doi: 10.1046/j.1538-7836.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- KIM S., JIN J., KUNAPULI S.P. Akt activation in platelets depends on Gi signaling pathways. J. Biol. Chem. 2004;279:4186–4195. doi: 10.1074/jbc.M306162200. [DOI] [PubMed] [Google Scholar]
- KRAMER R.M., ROBERTS E.F., STRIFLER B.A., JOHNSTONE E.M. Thrombin induces activation of p38 MAP kinase in human platelets. J. Biol. Chem. 1995;270:27395–27398. doi: 10.1074/jbc.270.46.27395. [DOI] [PubMed] [Google Scholar]
- KROLL M.H., SCHAFER A.I. Biochemical mechanisms of platelet activation. Blood. 1989;74:1181–1195. [PubMed] [Google Scholar]
- LE BLANC K., BERG A., PALMBLAD J., SAMUELSSON J. Defective platelet aggregation in polycythaemia vera is not caused by impaired calcium signaling, phospholipase D activation or decreased amounts of focal adhesion proteins. Eur. J. Haematol. 2000;65:322–330. doi: 10.1034/j.1600-0609.2000.065005322.x. [DOI] [PubMed] [Google Scholar]
- LEE S.B., RAO A.K., LEE K.H., YANG X., BAE Y.S., RHEE S.G. Decreased expression of phospholipase C-beta 2 isozyme in human platelets with impaired function. Blood. 1996;88:1684–1691. [PubMed] [Google Scholar]
- LIU J.J., NILSSON A., OREDSSON S., BADMAEV V., ZHAO W.Z., DUAN R.D. Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells. Carcinogenesis. 2002;23:2087–2093. doi: 10.1093/carcin/23.12.2087. [DOI] [PubMed] [Google Scholar]
- MARUYAMA T., KANAJI T., NAKADE S., KANNO T., MIKOSHIBA K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1, 4, 5)P3-induced Ca2+ release. J. Biochem. (Tokyo) 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- MAURER-SPUREJ E., PFEILER G., MAURER N., LINDNER H., GLATTER O., DEVINE D.V. Room temperature activates human blood platelets. Lab. Invest. 2001;81:581–592. doi: 10.1038/labinvest.3780267. [DOI] [PubMed] [Google Scholar]
- PAPKOFF J., CHEN R.H., BLENIS J., FORSMAN J. p42 mitogen-activated protein kinase and p90 ribosomal S6 kinase are selectively phosphorylated and activated during thrombin-induced platelet activation and aggregation. Mol. Cell. Biol. 1994;14:463–472. doi: 10.1128/mcb.14.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK Y.S., LEE J.H., BONDAR J., HARWALKAR J.A., SAFAYHI H., GOLUBIC M. Cytotoxic action of acetyl-11-keto-beta-boswellic acid (AKBA) on meningioma cells. Planta. Med. 2002;68:397–401. doi: 10.1055/s-2002-32090. [DOI] [PubMed] [Google Scholar]
- RHEE S.G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSADO J.A., SAGE S.O. Role of the ERK pathway in the activation of store-mediated calcium entry in human platelets. J. Biol. Chem. 2001;276:15659–15665. doi: 10.1074/jbc.M009218200. [DOI] [PubMed] [Google Scholar]
- RUGGERI Z.M. Platelets in atherothrombosis. Nat. Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- SAFAYHI H., SAILER E.R. Anti-inflammatory actions of pentacyclic triterpenes. Planta. Med. 1997;63:487–493. doi: 10.1055/s-2006-957748. [DOI] [PubMed] [Google Scholar]
- SAFAYHI H., MACK T., SABIERAJ J., ANAZODO M.I., SUBRAMANIAN L.R., AMMON H.P. Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. J. Pharmacol. Exp. Ther. 1992;261:1143–1146. [PubMed] [Google Scholar]
- SAFAYHI H., RALL B., SAILER E.R., AMMON H.P. Inhibition by boswellic acids of human leukocyte elastase. J. Pharmacol. Exp. Ther. 1997;281:460–463. [PubMed] [Google Scholar]
- SAKLATVALA J., RAWLINSON L., WALLER R.J., SARSFIELD S., LEE J.C., MORTON L.F., BARNES M.J., FARNDALE R.W. Role for p38 mitogen-activated protein kinase in platelet aggregation caused by collagen or a thromboxane analogue. J. Biol. Chem. 1996;271:6586–6589. doi: 10.1074/jbc.271.12.6586. [DOI] [PubMed] [Google Scholar]
- STEEN V.M., HOLMSEN H. Current aspects on human platelet activation and responses. Eur. J. Haematol. 1987;38:383–399. doi: 10.1111/j.1600-0609.1987.tb01434.x. [DOI] [PubMed] [Google Scholar]
- SYROVETS T., BUCHELE B., GEDIG E., SLUPSKY J.R., SIMMET T. Acetyl-boswellic acids are novel catalytic inhibitors of human topoisomerases I and IIalpha. Mol. Pharmacol. 2000;58:71–81. doi: 10.1124/mol.58.1.71. [DOI] [PubMed] [Google Scholar]
- SYROVETS T., BUCHELE B., KRAUSS C., LAUMONNIER Y., SIMMET T. Acetyl-boswellic acids inhibit lipopolysaccharide-mediated TNF-alpha induction in monocytes by direct interaction with IkappaB kinases. J. Immunol. 2005;174:498–506. doi: 10.4049/jimmunol.174.1.498. [DOI] [PubMed] [Google Scholar]
- WERZ O., BURKERT E., FISCHER L., SZELLAS D., DISHART D., SAMUELSSON B., RADMARK O., STEINHILBER D. Extracellular signal-regulated kinases phosphorylate 5-lipoxygenase and stimulate 5-lipoxygenase product formation in leukocytes. FASEB J. 2002;16:1441–1443. doi: 10.1096/fj.01-0909fje. [DOI] [PubMed] [Google Scholar]
- YOSHIMOTO T., TAKAHASHI Y. Arachidonate 12-lipoxygenases. Prostaglandins Other Lipid Mediat. 2002;68-69:245–262. doi: 10.1016/s0090-6980(02)00034-5. [DOI] [PubMed] [Google Scholar]