Abstract
The utility of halothane-anaesthetized guinea-pigs as an in vivo model for predicting the clinical potential of a drug to induce QT interval prolongation was assessed using the electrocardiogram and monophasic action potential (MAP) recordings with electrical ventricular pacing.
Intravenous administration of D-sotalol (0.3 mg kg−1) and terfenadine (0.3 mg kg−1), blockers of a rapid component of delayed rectifier potassium currents, prolonged the QT interval by 32±7 and 23±6 ms, respectively, whereas chromanol 293B (1 mg kg−1), a blocker of a slow component of delayed rectifier potassium currents, lengthened it by 33±8 ms. The extent of the QT interval prolongation by these drugs was greater than those in previous reports using pentobarbital-anaesthetized guinea-pigs.
The MAP duration at the control was shortened by decreasing the pacing cycle length from 400 to 200 ms, but the MAP duration at each cycle length was prolonged by D-sotalol.
The formulas of Van de Water, Matsunaga, Fridericia and Bazett showed good correlation of the repolarization period when compared with the MAP duration at a pacing cycle length of 400 ms.
The halothane-anaesthetized guinea-pig model may possess enough sensitivity to detect drug-induced QT interval prolongation, indicating that halothane anaesthesia can reduce the repolarization reserve of the heart in vivo.
Keywords: QT prolongation, D-sotalol, terfenadine, chromanol 293B, guinea-pigs, halothane, monophasic action potential, corrected QT interval
Introduction
Drug-induced QT interval prolongation is often associated with the onset of torsades de pointes (TdP) resulting in life-threatening ventricular arrhythmia, which is mainly caused by suppression of a rapid component of delayed rectifier potassium currents (IKr) (Belardinelli et al., 2003; Redfern et al., 2003). To avoid the occurrence of such arrhythmias, a number of new drug candidates have been carefully evaluated according to the guideline ICH S7B for safety pharmacology studies (The ICH Steering Committee, 2005), which recommends a combination of in vitro and in vivo methods. The acquired long QT syndrome is often induced in patients with several risk factors, leading to a reduced repolarization reserve (Lengyel et al., 2004). Since animal hearts usually have a more inherent repolarization reserve than those of human because of a faster heart rate (Biliczki et al., 2002; Lengyel et al., 2004), no model can predict quantitatively the dose of a drug developing TdP. There are, however, a number of in vivo and in vitro models that predict potential liability on a yes/no basis (Hey et al., 1996a, 1996b; Minematsu et al., 1999; Hamlin et al., 2004; Testai et al., 2004).
Recently, an inhaled anaesthetic, halothane, has been demonstrated to decrease the repolarization reserve in an in vivo canine model, which can unmask the selective IKr blocker sematilide-induced QT interval prolongation (Takahara et al., 2005). Furthermore, the sensitivity of the halothane-anaesthetized canine model for detecting the drug-induced QT interval prolongation is suggested to be similar to that of the phase I clinical studies (Satoh et al., 2005). Therefore, the halothane-anaesthetized canine model is considered to be useful for predicting the drug-induced QT interval prolongation in the human.
In the present study, we assessed the utility of halothane-anaesthetized guinea-pigs as an in vivo model for predicting the QT interval prolongation by new drugs, since guinea-pigs are less expensive than dogs and are preferable when only a limited amount of drug is available. Guinea-pigs have been widely utilized for assessing the electropharmacological effects of cardiac ion channel modulators (Takahara et al., 1999; Noda & Hashimoto, 2004). In order to precisely analyse the effects of a drug on the repolarization process of the heart, we first recorded the monophasic action potentials (MAPs) before and after administration of a representative IKr blocker D-sotalol (Campbell, 1987; McComb et al., 1987), under electrical ventricular pacing of various cycle lengths (Sugiyama & Hashimoto, 2002; Sugiyama et al., 2003). Next, we assessed the effects of a histamine H1 blocker, terfenadine (a noncardiac drug that induces the QT interval prolongation), a specific blocker of a slow component of delayed rectifier potassium currents (IKs) chromanol 293B and a histamine H2 blocker famotidine (a negative control drug) (Bosch et al., 1998; Sugiyama et al., 2003; Tarantino et al., 2005) to validate the halothane-anaesthetized guinea-pig model.
Methods
Experiments were carried out using 18 male Hartley guinea-pigs weighing 393±36 g (SLC, Hamamatsu, Japan). All experiments were performed in accordance with the rules and regulations of the Committee for Research of the University of Yamanashi.
Measurement of haemodynamic and electrophysiological parameters
The guinea-pigs were initially anaesthetized with 4% halothane. After a tracheal cannula was inserted, 1% halothane vaporized with 100% oxygen was inhaled with a rodent ventilator (SN-480-7, Shinano, Tokyo, Japan). The tidal volume and respiratory rate were set at 10 ml kg−1 and 60 strokes min−1, respectively. Body temperature was maintained at 37°C with a heating pad. The right carotid artery and jugular vein were cannulated for continuous monitoring of the systemic blood pressure and drug administration, respectively. The surface lead II electrocardiogram (ECG) was obtained from the limb electrodes. The haemodynamic and electrophysiological parameters were continuously monitored using a polygraph system (RM-6000, Nihon Kohden), and analysed using a real-time full automatic data analysis system (MP/VAS 3 for Macintosh ver 1.0, Physio-Tech, Tokyo, Japan).
Experiment 1: Effects of D-sotalol on the ventricular repolarization process of the halothane-anaesthetized guinea-pig heart
After midline thoracotomy and pericardial incision, the MAP electrode (BS4 73-0409, Harvard Apparatus, Hollistone, MA, U.S.A.) was attached onto the epicardium of the left ventricle using the mounting bar (BS4 73-0566, Harvard Apparatus) with spring holder (BS4 73-3015, Harvard Apparatus). The signals were amplified with a DC preamplifier (Model 300, EP Technologies Inc., Sunnyvale, CA, U.S.A.). The duration of the MAP signals was measured as an interval, along a line horizontal to the diastolic baseline, from the MAP upstroke to the desired repolarization level, and the interval (ms) at 90% repolarization level was defined as MAP90, which correlates well with the QT interval (Stroobandt et al., 1985). The heart was electrically driven using a cardiac stimulator (SEC-3102, Nihon Kohden, Tokyo, Japan) with silver electrodes placed on the epicardium of the right ventricle. The stimulation pulses were rectangular in shape, 1–2 V (about twice the threshold voltage) and of 1 ms duration. The MAP90 was measured during the sinus rhythm and electrical ventricular stimulation at a pacing cycle length of 200, 250, 300 or 400 ms. After the basal assessment, D-sotalol in a low dose of 0.3 mg kg−1 was intravenously administered, and each parameter was assessed 5, 10, 20 and 30 min after the drug administration. Next, D-sotalol in a high dose of 3.0 mg kg−1 was additionally administered and each parameter was assessed in the same manner.
Experiment 2: Effects of terfenadine, chromanol 293B and famotidine on ECG parameters
This experiment was performed without thoracotomy under the monitoring of ECG. After the basal assessment, terfenadine in a low dose of 0.3 mg kg−1 was intravenously administered, and each parameter was assessed 5, 10, 20 and 30 min after the drug administration. Next, terfenadine in a high dose of 3.0 mg kg−1 was additionally administered and each parameter was assessed in the same manner. Similarly, effects of chromanol 293B in doses of 0.1 and 1.0 mg kg−1 and famotidine in a dose of 0.3 mg kg−1 on ECG parameters were assessed.
Drugs
D-Sotalol was provided from the Chemical Division of Yamanashi Research Center of Clinical Pharmacology (Yamanashi, Japan). Terfenadine (Sigma, St Louis, MO, U.S.A.), chromanol 293B (Tocris, Ellisville, MO, U.S.A.), famotidine (Astellas Pharma Inc., Tokyo, Japan), halothane (Takeda Chemical Industries, Tokyo, Japan) and heparin calcium (Mitsui Pharmaceuticals, Tokyo, Japan) were purchased. D-Sotalol and famotidine were dissolved in saline, and terfenadine was dissolved in 1% lactate. Chromanol 293B was initially dissolved in dimethylsulphoxide and diluted with saline before use as described previously (Yang et al., 2004).
Data analysis
Each measurement of the ECG and MAP was the mean of three consecutive recordings. Corrected QT intervals were calculated using the formulas of Bazett (QTc(B)), Fridericia (QTc(F)), Van de Water (QTc(V)) and Matsunaga (QTc(M)) (Bazett, 1920; Fridericia, 1920; Van de Water et al., 1989; Matsunaga et al., 1997) as follows: Bazett's formula: QTc=QT/(RR/1000)1/2, Fridericia's formula: QTc=QT/(RR/1000)1/3, Van de Water's formula: QTc=QT−0.087 × (RR−1000), and Matsunaga's formula: QTc=log 600 × QT/log RR, where a unit of the RR interval is given in ms. To assess the reliability of the corrected QT interval for this model, we compared the MAP90 values corrected by these rate correction formulas for the QT interval with those at a pacing cycle length of 400 ms (Takahara et al., 2004). The data are expressed as the mean±s.e.m. Statistical significances within a parameter were evaluated by one-way, repeated-measures analysis of variance (ANOVA), followed by Contrast for mean values comparison. A P-value <0.05 was considered to be statistically significant.
Results
Experiment 1: Effects of D-sotalol on the ventricular repolarization process of the halothane-anaesthetized guinea-pig heart
Effects on the heart rate
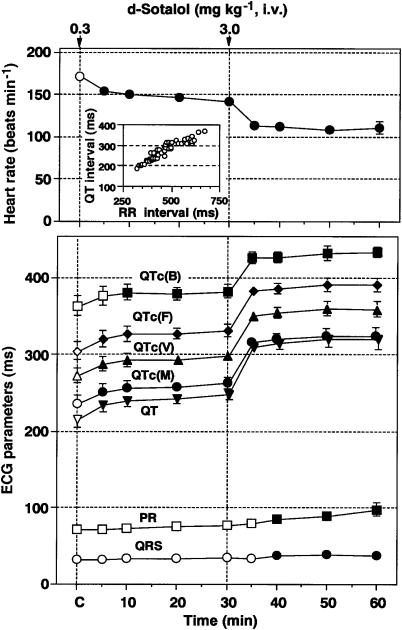
Haemodynamic parameters were constant throughout the whole experimental period. The time course of changes in the heart rate is summarized in Figure 1 (upper panel), of which the predrug control value was 171±6 beats min−1 (n=6). After the i.v. injection of the low dose (0.3 mg kg−1) of D-sotalol, the heart rate gradually decreased for 5–30 min. After the i.v. injection of the high dose (3 mg kg−1) of D-sotalol, the heart rate further decreased for 5–30 min.
Figure 1.
Time courses of the effects of D-sotalol on the heart rate, PR interval, QRS width and QT interval, QTc(B) (corrected by Bazett's formula), QTc(F) (corrected by Fridericia's formula), QTc(V) (corrected by Van de Water's formula) and QTc(M) (corrected by Matsunaga's formula). Intravenous administration of D-sotalol in doses of 0.3 and 3.0 mg kg−1 prolonged the QT interval by 32±7 and 104±16 ms, respectively. The inset figure shows QT–RR relationship. Data are presented as mean±s.e.m. (n=6). The closed symbols represent the significant differences from each predrug control (C) value by P<0.05.
Effects on the ECG
The time courses of changes in the ECG parameters are summarized in Figure 1 (lower panel, n=6), and typical tracings of the effects of D-sotalol on the ECG are depicted in Figure 2a. The predrug control values of the PR interval, QRS width, QT interval, QTc(B), QTc(F), QTc(V) and QTc(M) were 72±2, 32±3, 216±11, 363±13, 305±12, 273±10 and 236±11 ms, respectively. After the low-dose injection, the QT interval, QTc(B), QTc(F), QTc(V) and QTc(M) were prolonged and significant changes were detected in the QT interval, QTc(F), QTc(V) and QTc(M) for 5–30 min, and in the QTc(B) for 10–30 min. Meanwhile, no significant change was detected in the PR interval or QRS width. After the high-dose injection, the PR interval, QRS width, QT interval, QTc(B), QTc(F), QTc(V) and QTc(M) were prolonged. Significant changes were detected in the PR interval and QRS width for 10–30 min, whereas these were observed in the QT interval, QTc(B), QTc(F), QTc(V) and QTc(M) for 5–30 min. No ventricular premature beat was observed during the whole experimental period.
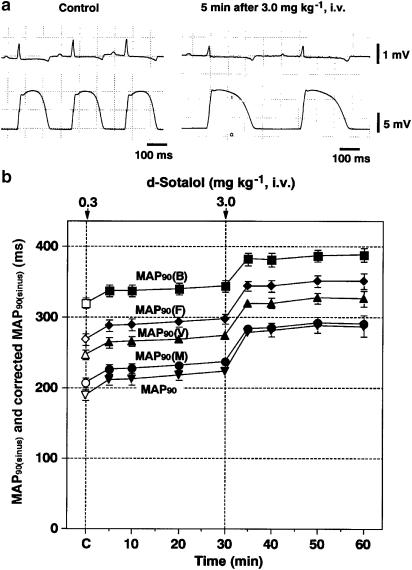
Figure 2.
Effects of D-sotalol on the MAPs recorded from the left ventricular muscle during the sinus rhythm. (a) Typical tracings of the surface lead II ECG and MAP signals during the sinus rhythm before (Control) and 5 min after 3.0 mg kg−1 of D-sotalol injection. (b) Time courses of the effects of D-sotalol on the MAP duration at 90% repolarization level (MAP90). The values of MAP90(B), MAP90(F), MAP90(V) or MAP90(M) were calculated using formula of Bazett, Fridericia, Van de Water or Matsunaga, respectively. Data are presented as mean±s.e.m. (n=6). The closed symbols represent the significant differences from each predrug control (C) value by P<0.05.
Effects on the MAP signals
Typical tracings of the effects of D-sotalol on the MAP signals during the sinus rhythm are depicted in Figure 2a, and the time courses of changes in the MAP90 during the sinus rhythm are summarized in Figure 2b (n=6). The predrug control value of the MAP90 and those corrected by formulas of Bazett (MAP90(B)), Fridericia (MAP90(F)), Van de Water (MAP90(V)) and Matsunaga (MAP90(M)) were 190±8, 320±8, 269±8, 247±7 and 207±7 ms, respectively. After the low-dose injection, the MAP90, MAP90(B), MAP90(F), MAP90(V) and MAP90(M) were prolonged for 5–30 min. After the high-dose injection, MAP90, MAP90(B), MAP90(F), MAP90(V) and MAP90(M) were prolonged for 5–30 min.
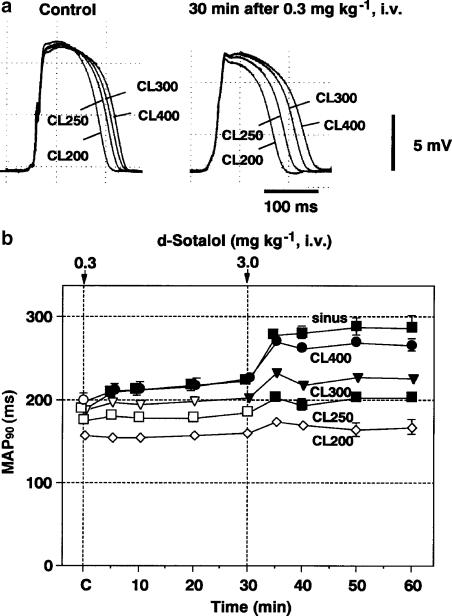
Typical tracings of the effects of D-sotalol on the MAP signals during the electrical ventricular pacing at 200, 250, 300 and 400 ms are shown in Figure 3a, and the time courses of changes in the MAP90 during the electrical ventricular pacing are summarized in Figure 3b (n=6). The predrug control values of the MAP90 during the electrical ventricular pacings at 400, 300, 250 and 200 ms (MAP90(CL400), MAP90(CL300), MAP90(CL250) and MAP90(CL200), respectively) were 199±8, 188±6, 176±5 and 158±6 ms, respectively. After the low-dose injection, the MAP90(CL400) and MAP90(CL300) were prolonged and significant changes were detected in the MAP90(CL400) for 5–30 min and the MAP90(CL300) at 30 min. Meanwhile, no significant change was detected in the MAP90(CL250) or MAP90(CL200). After the high-dose injection, the MAP90(CL400), MAP90(CL300) and MAP90(CL250) were prolonged for 5–30 min, whereas no significant change was detected in the MAP90(CL200).
Figure 3.
Effects of D-sotalol on the MAPs recorded from the left ventricular muscle during the ventricular pacing. (a) Typical tracings of the MAP signals electrically driven at pacing cycle lengths of 200, 250, 300 and 400 ms (CL200, CL250, CL300 and CL400, respectively) before (Control) and 30 min after 0.3 mg kg−1 of D-sotalol injection. (b) Time courses of the effects of D-sotalol on the MAP duration at 90% repolarization level (MAP90). Data are presented as mean±s.e.m. (n=6). The closed symbols represent the significant differences from each predrug control (C) value by P<0.05.
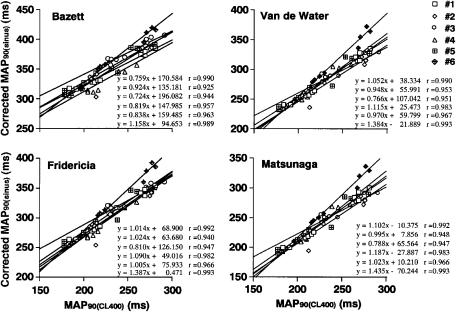
Correlation between the MAP90(CL400) and corrected MAP90(sinus) by the Bazett, Fridericia, Van de Water and Matsunaga formulas
The relationships between the MAP90(CL400) and corrected MAP90(sinus) are summarized in Figure 4 for each formula. The averages of the correlation coefficients obtained from six animals between the MAP90(CL400) and corrected MAP90(sinus) by the Bazett, Fridericia, Van de Water and Matsunaga formulas were 0.961±0.010, 0.970±0.009, 0.973±0.008 and 0.972±0.009, respectively, which were comparable to each other.
Figure 4.
Linear regression analysis between the duration of the monophasic action potentials at 90% repolarization level (MAP90) during the sinus rhythm corrected by the formula of Bazett, Fridericia, Van de Water or Matsunaga (corrected MAP90(sinus)) versus the MAP90 during the electrical ventricular pacing at a pacing cycle length of 400 ms (MAP90(CL400)).
Experiment 2: Effects of terfenadine, chromanol 293B and famotidine on ECG parameters
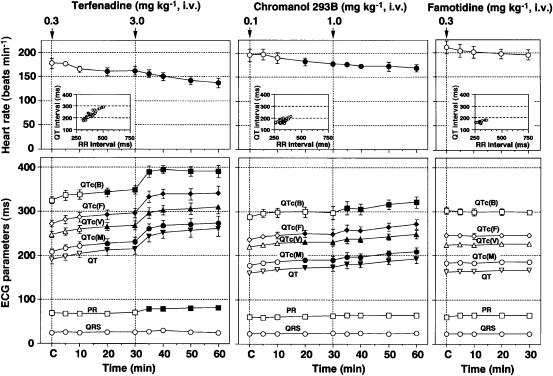
The time courses of changes in the heart rate and ECG parameters are summarized in Figure 5. The low dose of 0.3 mg kg−1 of terfenadine decreased the heart rate and prolonged the QT interval, QTc(B), QTc(F), QTc(V) and QTc(M) for 20–30 min. The high dose of 3.0 mg kg−1 of terfenadine decreased the heart rate and prolonged the PR interval, QT interval, QTc(B), QTc(F), QTc(V) and QTc(M) for 5–30 min (left panel). The low dose of 0.1 mg kg−1 of chromanol 293B decreased the heart rate and prolonged the QT interval, QTc(F), QTc(V) and QTc(M) for 20–30 min. The high dose of 1.0 mg kg−1 of chromanol 293B decreased the heart rate and prolonged the QT interval, QTc(B), QTc(F), QTc(V) and QTc(M) for 5–30 min (middle panel). After the administration of 0.3 mg kg−1 of famotidine, no significant change was detected in each of the heart rate or ECG parameters (right panel). No ventricular premature beat was observed in each experimental group.
Figure 5.
Time courses of the effects of terfenadine, chromanol 293B and famotidine on the heart rate, PR interval, QRS width and QT interval, QTc(B) (corrected by Bazett's formula), QTc(F) (corrected by Fridericia's formula), QTc(V) (corrected by Van de Water's formula) and QTc(M) (corrected by Matsunaga's formula). The inset figures show QT–RR relationship. In the terfenadine group, predrug control values of the heart rate, PR interval, QRS width, QT interval, QTc(B), QTc(F), QTc(V), and QTc(M) were 177±10 beats min−1, 69±5, 24±5, 190±9, 325±7, 272±8, 247±7 and 208±7 ms, respectively (n=4), and terfenadine in doses of 0.3 and 3.0 mg kg−1 prolonged the QT interval by 23±6 and 89±19 ms, respectively. In the chromanol 293B group, the predrug control values were 196±13 beats min−1, 60±2, 22±2, 160±4, 287±6, 236±4, 220±3 and 178±3 ms, respectively (n=4), and chromanol 293B in doses of 0.1 and 1.0 mg kg−1 prolonged the QT interval by 14±7 and 33±8 ms, respectively. In the famotidine group, the predrug control values were 224±4 beats min−1, 61±3, 23±4, 162±4, 302±8, 245±5, 224±4 and 183±4 ms, respectively (n=4), and no significant change was detected after administration of famotidine. Data are presented as mean±s.e.m. The closed symbols represent the significant differences from each predrug control (C) value by P<0.05.
Discussion
Recently, the pentobarbital-anaesthetized guinea-pig has been proposed as an experimental model for preliminary screening of the torsadogenic properties of drugs (Carlsson et al., 1997; Testai et al., 2004; Yang et al., 2004). In the present study, we assessed the utility of halothane-anaesthetized guinea-pigs as an in vivo model for predicting drug-induced QT interval prolongation. The dose-dependent QT interval prolongation by D-sotalol was confirmed in this model. More importantly, the extent of the QT interval prolongation by the low dose of 0.3 mg kg−1 (+33 ms, +15%) in this study corresponded to the clinically reported effects of 1.5 mg kg−1, i.v. (+60 ms, +16%) (McComb et al., 1987), indicating that our model can effectively predict the drug-induced QT interval prolongation in clinical patients. On the other hand, the QT interval prolongation by D-sotalol in this study was >1.5 times greater than that in a previous study with the pentobarbital-anaesthetized guinea-pigs, in which <+10% QT prolongation was shown by 0.3 mg kg−1, i.v. of D-sotalol (Carlsson et al., 1997). Moreover, in the present halothane-anaesthetized guinea-pig model, the extents of the QT interval prolongation by terfenadine (0.3 mg kg−1, i.v.) and chromanol 293B (1.0 mg kg−1, i.v.) were +23 and +33 ms, respectively, each of which was greater than that in previous reports using pentobarbital-anaesthetized guinea-pigs (Testai et al., 2004; Yang et al., 2004). Similar effects of anaesthetics were observed in our recent study using the halothane- and pentobarbital-anaesthetized dogs (Takahara et al., 2005). It should be noted that the extent of the QT interval prolongation by a recommended dose for IKs blockade of chromanol 293B (1.0 mg kg−1; Yang et al., 2004) was about three times less than those by IKr blockers D-sotalol and terfenadine in this model.
Generally, it is known that excessive and potentially dangerous QT prolongation is rarely observed by pharmacological blockade of ‘one type' of outward K+ current, including the transient outward (Ito), inward rectifier (IK1) potassium currents, IKs and IKr, because the other intact potassium currents may sufficiently compensate the repolarizing ability, that is, repolarization reserve (Biliczki et al., 2002). Previous electrophysiological studies have demonstrated that the clinical anaesthetic concentration of halothane inhibits the IKr, IKs, IK1 and Ito in the isolated cardiomyocytes (Davies et al., 2000; Stadnicka et al., 2000; Li & Correa, 2002; Shibata et al., 2004). This electrophysiological profile of halothane together with the high sensitivity of this model for the drug-induced QT prolongation indicate that the repolarization reserve may have decreased in the halothane-anaesthetized guinea-pig heart.
As clearly shown in Figure 3b, the MAP duration of the in vivo guinea-pig heart was shortened by decreasing the pacing cycle length, but the duration of each cycle length was prolonged by D-sotalol. These electrophysiological profiles of the drug are in accordance with previous reports describing the effects of IKr blockers on the isolated ventricular muscle (Nakaya et al., 1993; Baskin & Lynch, 1994). Thus, the current in vivo heart model can be useful for precisely analysing the electrophysiological effects of a drug on the ventricular repolarization process.
To better apply the mathematical methods for rate correction of repolarization period to the halothane-anaesthetized guinea-pig model, we calculated the correlation between the MAP90 at a fixed pacing rate (MAP90(CL400)) and that during the sinus rhythm corrected by four types of formulas (corrected MAP90(sinus)). As shown in Figure 4, the high correlation coefficients (r) were observed in all algorithms, which were essentially comparable to each other. These results suggest that utilization of any of the four formulas may be reliable for detecting the drug-induced QT interval prolongation.
In conclusion, the halothane-anaesthetized guinea-pig model is sensitive enough to predict the drug-induced QT prolongation, indicating that halothane anaesthesia can reduce the repolarization reserve of the in vivo heart. The formulas of Van de Water, Matsunaga, Fridericia and Bazett will be useful for rate correction of the QT interval with high reliability in this model.
Acknowledgments
The work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (#17590216) and the Yamanashi Research Center of Clinical Pharmacology. We thank Mr Y. Nakamura for his technical assistance.
Abbreviations
- ECG
electrocardiogram
- IK1
inward rectifier potassium currents
- IKr
rapid component of delayed rectifier potassium currents
- IKs
slow component of delayed rectifier potassium currents
- Ito
transient outward potassium currents
- MAP
monophasic action potentials
- MAP90
duration of MAP at 90% repolarization level
- QTc
corrected QT interval
- QTc(B)
QTc calculated with the formula of Bazett
- QTc(F)
QTc calculated with the formula of Fridericia
- QTc(M)
QTc calculated with the formula of Matsunaga
- QTc(V)
QTc calculated with the formula of Van de Water
- TdP
torsades de pointes
References
- BASKIN E.P., LYNCH J.J., JR Comparative effects of increased extracellular potassium and pacing frequency on the class III activities of methanesulfonanilide IKr blockers dofetilide, D-sotalol, E-4031, and MK-499. J. Cardiovasc. Pharmacol. 1994;24:199–208. [PubMed] [Google Scholar]
- BAZETT H.C. An analysis of the time relations of electrogram. Heart. 1920;7:353–370. [Google Scholar]
- BELARDINELLI L., ANTZELEVITCH C., VOS M.A. Assessing predictors of drug-induced torsade de pointes. Trends Pharmacol. Sci. 2003;24:619–625. doi: 10.1016/j.tips.2003.10.002. [DOI] [PubMed] [Google Scholar]
- BILICZKI P., VIRÁG L., IOST N., PAPP J.G., VARRÓ A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br. J. Pharmacol. 2002;137:361–368. doi: 10.1038/sj.bjp.0704881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSCH R.F., GASPO R., BUSCH A.E., LANG H.J., LI G.R., NATTEL S. Effects of the chromanol 293B, a selective blocker of the slow, component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovasc. Res. 1998;38:441–450. doi: 10.1016/s0008-6363(98)00021-2. [DOI] [PubMed] [Google Scholar]
- CAMPBELL T.J. Cellular electrophysiological effects of D- and DL-sotalol in guinea-pig sinoatrial node, atrium and ventricle and human atrium: differential tissue sensitivity. Br. J. Pharmacol. 1987;90:593–599. doi: 10.1111/j.1476-5381.1987.tb11210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSSON L., AMOS G.J., ANDERSSON B., DREWS L., DUKER G., WADSTEDT G. Electrophysiological characterization of the prokinetic agents cisapride and mosapride in vivo and in vitro: implications for proarrhythmic potential. J. Pharmacol. Exp. Ther. 1997;282:220–227. [PubMed] [Google Scholar]
- DAVIES L.A., HOPKINS P.M., BOYETT M.R., HARRISON S.M. Effects of halothane on the transient outward K+ current in rat ventricular myocytes. Br. J. Pharmacol. 2000;131:223–230. doi: 10.1038/sj.bjp.0703565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIDERICIA L.S. Die systolendauer in elektrokardiogramm bei normalen menschen und bei herzkranken. Acta Med. Scand. 1920;53:469–486. [Google Scholar]
- HAMLIN R.L., CRUZE C.A., MITTELSTADT S.W., KIJTAWORNRAT A., KEENE B.W., ROCHE B.M., NAKAYAMA T., NAKAYAMA H., HAMLIN D.M., ARNOLD T. Sensitivity and specificity of isolated perfused guinea pig heart to test for drug-induced lengthening of QTc. J. Pharmacol. Toxicol. Methods. 2004;49:15–23. doi: 10.1016/j.vascn.2003.08.003. [DOI] [PubMed] [Google Scholar]
- HEY J.A., DEL PRADO M., KREUTNER W., EGAN R.W. Cardiotoxic and drug interaction profile of the second generation antihistamines ebastine and terfenadine in an experimental animal model of torsade de pointes. Arzneimittelforschung. 1996a;46:159–163. [PubMed] [Google Scholar]
- HEY J.A., DEL PRADO M., SHERWOOD J., KREUTNER W., EGAN R.W. The guinea pig model for assessing cardiotoxic proclivities of second generation antihistamines. Arzneimittelforschung. 1996b;46:834–837. [PubMed] [Google Scholar]
- LENGYEL C., DÉZSI L., BILICZKI P., HORVÁTH C., VIRÁG L., IOST N., NÉMETH M., TÁLOSI L., PAPP J.G., VARRÓ A. Effect of a neuroprotective drug, eliprodil on cardiac repolarisation: importance of the decreased repolarisation reserve in the development of proarrhythmic risk. Br. J. Pharmacol. 2004;143:152–158. doi: 10.1038/sj.bjp.0705901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI J., CORREA A.M. Kinetic modulation of HERG potassium channels by the volatile anesthetic halothane. Anesthesiology. 2002;97:921–930. doi: 10.1097/00000542-200210000-00026. [DOI] [PubMed] [Google Scholar]
- MATSUNAGA T., MITSUI T., HARADA T., INOKUMA M., MURANO H., SHIBUTANI Y. QT corrected for heart rate and relation between QT and RR intervals in beagle dogs. J. Pharmacol. Toxicol. Methods. 1997;38:201–209. doi: 10.1016/s1056-8719(97)00098-1. [DOI] [PubMed] [Google Scholar]
- McCOMB J.M., McGOVERN B., McGOWAN J.B., RUSKIN J.N., GARAN H. Electrophysiologic effects of D-sotalol in humans. J. Am. Coll. Cardiol. 1987;10:211–217. doi: 10.1016/s0735-1097(87)80182-1. [DOI] [PubMed] [Google Scholar]
- MINEMATSU T., OHTANI H., SATO H., IGA T. Pharmacokinetic/pharmacodynamic analysis of tacrolimus-induced QT prolongation in guinea pigs. Biol. Pharm. Bull. 1999;22:1341–1346. doi: 10.1248/bpb.22.1341. [DOI] [PubMed] [Google Scholar]
- NAKAYA H., TOHSE N., TAKEDA Y., KANNO M. Effects of MS-551, a new class III antiarrhythmic drug, on action potential and membrane currents in rabbit ventricular myocytes. Br. J. Pharmacol. 1993;109:157–163. doi: 10.1111/j.1476-5381.1993.tb13546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NODA Y., HASHIMOTO K. Development of a halothane–adrenaline arrhythmia model using in vivo guinea pigs. J. Pharmacol. Sci. 2004;95:234–239. doi: 10.1254/jphs.fp0030423. [DOI] [PubMed] [Google Scholar]
- REDFERN W.S., CARLSSON L., DAVIS A.S., LYNCH W.G., MACKENZIE I., PALETHORPE S., SIEGL P.K., STRANG I., SULLIVAN A.T., WALLIS R., CAMM A.J., HAMMOND T.G. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- SATOH Y., SUGIYAMA A., TAKAHARA A., ANDO K., WANG K., HONSHO S., HASHIMOTO K. The antipsychotic and antiemetic drug prochlorperazine delays the ventricular repolarization of the in situ canine heart. J. Pharmacol. Sci. 2005;97:101–106. doi: 10.1254/jphs.fpj04038x. [DOI] [PubMed] [Google Scholar]
- SHIBATA S., ONO K., IIJIMA T. Sevoflurane inhibition of the slowly activating delayed rectifier K+ current in guinea pig ventricular cells. J. Pharmacol. Sci. 2004;95:363–373. doi: 10.1254/jphs.fp0040024. [DOI] [PubMed] [Google Scholar]
- STADNICKA A., BOSNJAK Z.J., KAMPINE J.P., KWOK W.M. Modulation of cardiac inward rectifier K+ current by halothane and isoflurane. Anesth. Analg. 2000;90:824–833. doi: 10.1097/00000539-200004000-00010. [DOI] [PubMed] [Google Scholar]
- STROOBANDT R., BRACHMANN J., BOURGEOIS I., WIELDERS P., KUBLER W., SENGES J. Simultaneous recording of atrial and ventricular monophasic action potentials: monophasic action potential duration during atrial pacing, ventricular pacing, and ventricular fibrillation. Pacing Clin. Electrophysiol. 1985;8:502–511. doi: 10.1111/j.1540-8159.1985.tb05852.x. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA A., HASHIMOTO K. Effects of a typical IKr channel blocker sematilide on the relationship between ventricular repolarization, refractoriness and onset of torsades de pointes. Jpn. J. Pharmacol. 2002;88:414–421. doi: 10.1254/jjp.88.414. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA A., SATOH Y., TAKAHARA A., NAKAMURA Y., SHIMIZU-SASAMATA M., SATO S., MIYATA K., HASHIMOTO K. Famotidine does not induce long QT syndrome: experimental evidence from in vitro and in vivo test systems. Eur. J. Pharmacol. 2003;466:137–146. doi: 10.1016/s0014-2999(03)01559-0. [DOI] [PubMed] [Google Scholar]
- TAKAHARA A., SUGIYAMA A., SATOH Y., HASHIMOTO K. QT-prolonging effects of a histamine H1 receptor antagonist terfenadine assessed in the in vivo canine heart model: comparison of the rate-correcting methods for the ventricular repolarization periods. Yamanashi Med. J. 2004;19:89–99. [Google Scholar]
- TAKAHARA A., SUGIYAMA A., SATOH Y., WANG K., HONSHO S., HASHIMOTO K. Halothane sensitizes the canine heart to pharmacological IKr blockade. Eur. J. Pharmacol. 2005;507:169–177. doi: 10.1016/j.ejphar.2004.11.045. [DOI] [PubMed] [Google Scholar]
- TAKAHARA A., UNEYAMA H., SASAKI N., UEDA H., DOHMOTO H., SHOJI M., HARA Y., NAKAYA H., YOSHIMOTO R. Effects of AH-1058, a new antiarrhythmic drug, on experimental arrhythmias and cardiac membrane currents. J. Cardiovasc. Pharmacol. 1999;33:625–632. doi: 10.1097/00005344-199904000-00016. [DOI] [PubMed] [Google Scholar]
- TARANTINO P., APPLETON N., LANSDELL K. Effect of trazodone on hERG channel current and QT-interval. Eur. J. Pharmacol. 2005;510:75–85. doi: 10.1016/j.ejphar.2005.01.009. [DOI] [PubMed] [Google Scholar]
- TESTAI L., CALDERONE V., SALVADORI A., BRESCHI M.C., NIERI P., MARTINOTTI E. QT prolongation in anaesthetized guinea-pigs: an experimental approach for preliminary screening of torsadogenicity of drugs and drug candidates. J. Appl. Toxicol. 2004;24:217–222. doi: 10.1002/jat.975. [DOI] [PubMed] [Google Scholar]
- THE ICH STEERING COMMITTEE. The nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals 2005. The international conference on harmonization of technical requirements for registration of pharmaceuticals for human use (ICH). The Guideline was recommended for adoption at Step 4 of the ICH process in May 2005 ()
- VAN DE WATER A., VERHEYEN J., XHONNEUX R., RENEMAN R.S. An improved method to correct the QT interval of the electrocardiogram for change in heart rate. J. Pharmacol. Methods. 1989;22:207–217. doi: 10.1016/0160-5402(89)90015-6. [DOI] [PubMed] [Google Scholar]
- YANG Z., SHI G., LI C., WANG H., LIU K., LIU Y. Electrophysiologic effects of nicorandil on the guinea pig long QT1 syndrome model. J. Cardiovasc. Electrophysiol. 2004;15:815–820. doi: 10.1046/j.1540-8167.2004.03632.x. [DOI] [PubMed] [Google Scholar]