Abstract
Nitric oxide (NO) is an important mediator of gastric mucosal defense. Sildenafil (SILD), a cyclic GMP-specific phosphodiesterase inhibitor, promotes an increase in cGMP concentrations in the gastrointestinal tract. cGMP mediates many of the biological actions of NO.
We tested the hypothesis that SILD could increase mucosal defense against indomethacin-induced gastropathy in rats.
SILD (1, 4 or 10 mg kg−1, p.o.) pretreatment significantly reduced (P<0.01) the gastric damage and the increase in gastric myeloperoxidase (MPO) activity elicited by indomethacin (20 mg kg−1 p.o.), with the maximal effect at the dose of 10 mg kg−1.
L-NAME (3, 10 or 20 mg kg−1, i.p.) dose dependently reversed the protective effects of SILD, an effect not seen when L-arginine (L-ARG) (200 mg kg−1, i.p.) was co-administered with L-NAME.
Indomethacin-induced leukocyte adhesion, assessed by intravital microscopy, was decreased (P<0.01) by SILD, and this effect was reversed by L-NAME cotreatment.
Indomethacin elicited a decrease in gastric blood flow and in gastric PGE2 levels. SILD was able to prevent the decrease in gastric blood flow (P<0.01), without diminishing the inhibitory effect of indomethacin on prostaglandin synthesis.
These results indicate that SILD, acting via NO-dependent mechanisms, prevents indomethacin-induced gastropathy, possibly through a reduction of leukocyte adhesion and maintenance of gastric blood flow.
Keywords: Sildenafil, gastric damage, neutrophil, gastric blood flow, nitric oxide
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are one of the most widely used classes of drugs in the world. NSAID-induced gastric damage is the major side effect of this kind of drug (Wolfe et al., 1999). It is generally believed that the mechanism of NSAID-induced gastric damage is related to the ability of these agents to inhibit gastric prostaglandin generation (Vane, 1971). However, evidence has been recently produced that leukocyte adherence to the vascular endothelium (Asako et al., 1992a, 1992b), microcirculatory disturbances (Ashley et al, 1985; Gana et al., 1987), superoxide radicals and protease liberation may be relevant pathogenic mechanisms in NSAID gastropathy (Wallace, 1997).
Nitric oxide (NO) is involved in the relaxation of the arterial smooth muscle through stimulation of the enzyme guanylate cyclase, with subsequent formation of cGMP (Reffelmann & Kloner, 2003). In the gastrointestinal tract, NO is a crucial mediator of gastrointestinal mucosal defense but, paradoxically, it may also contribute to mucosal damage in some circumstances (Muscara & Wallace, 1999). NO plays a critical role in modulating several components of mucosal defence, including gastric blood flow and mucus secretion (Wallace & Miller, 2000). However, recently, using knockout mice, we showed that iNOS-generated NO is involved in gastric damage induced by indomethacin (Souza et al., 2004).
Sildenafil (SILD) is a selective and potent inhibitor of cGMP-specific phosphodiesterase (PDE5), which catalyzes hydrolysis of cGMP and has a relaxant effect on the smooth muscle cells of the arterioles supplying the human corpus cavernosum (Chuang et al., 1998; Gibson, 2001). SILD modifies gastroduodenal motility in both humans (Bortolotti et al., 2001) and animals (Rosalmeida et al., 2003). However, effects of SILD on gastric resistance to injury have not been reported. In the present study, we evaluated the protective effect of SILD on gastric damage induced by indomethacin, a potent NSAID. We also studied the role of NO, leukocyte adherence and gastric blood flow in mediating these effects of SILD.
Methods
Animals
Male Wistar rats (weight 200–250 g) were fasted 18–24 h before the experiments. The rats were housed in cages in temperature-controlled rooms and received water and food ad libitum. All treatments and surgical procedures were performed in accordance with the Guide for Care and Use of Laboratory Animals, National Institutes of Health (Bethesda, MD, U.S.A.).
Drugs
L-NAME and L-arginine (L-ARG) were purchased from Sigma Chemicals (St Louis, MO, U.S.A.) and indomethacin was purchased from Prodome Química e Farmacêutica (São Paulo, SP, Brazil). SILD citrate was kindly provided by Pfizer (Sandwich, Kent, U.K.). Vehicle solutions consisted of Tris (Merck, São Paulo, SP, Brazil) buffer or saline (SAL).
Effects of SILD on indomethacin-induced gastric damage
The rats were treated with saline or SILD (1, 4 or 10 mg kg−1 by gavage). After 30 min, indomethacin (20 mg kg−1) was administered by gavage. The control (CONT) group received only the vehicle (Tris buffer). After 3 h, the rats were killed and their stomachs rapidly removed, opened by an incision along the greater curvature and pinned out on a wax platform. The hemorrhagic or ulcerative lesions were counted and their lengths measured with a digital caliper. A gastric damage score was then calculated as the sum of the lengths of all linear erosions (Santucci et al., 1994), which was measured by an observer who was unaware of the treatment given to the rats (CLS). Full-thickness pieces of the gastric corpus were then weighed, frozen and stored at −70°C until the assay for myeloperoxidase (MPO) activity (Bradley et al., 1982). MPO is an enzyme found primarily in the azurophilic granules of the neutrophils and therefore has been used extensively as a biochemical marker of the granulocyte infiltration into various tissues, including the gastrointestinal tract.
Role of NO in the protective effect of SILD
In order to study the role of NO in the SILD protective effect, the rats were treated with L-NAME (3, 10 or 20 mg kg−1, i.p.) or with L-arginine (200 mg kg−1, i.p.) + L-NAME (20 mg kg−1, i.p.). After 30 min, the rats received SILD (10 mg kg−1, by gavage). After 30 min, gastric damage was induced by intragastric instillation of indomethacin (20 mg kg−1). The control group received only vehicle. After 3 h, gastric damage was determined as described above. Finally, full-thickness pieces of the gastric corpus were weighed, frozen and stored at −70°C until the assay for MPO activity.
Gastric MPO activity
The extent of granulocyte accumulation in the gastric mucosa was measured by MPO activity assay as previously described (Bradley et al., 1982). Briefly, 50–100 mg of gastric tissue was homogenized in 1 ml of hexadecyltrimethyl-ammonium bromide (HTAB) buffer for each 50 mg of tissue. The homogenate was then centrifuged at 2000 × g. for 7 min at 4°C. MPO activity in the resuspended pellet was assayed by measuring the change in absorbance at 450 nm using o-dianisidine and H2O2. The results were reported as the MPO units/mg of tissue. A unit of MPO activity was defined as that converting 1 μmol of hydrogen peroxide to water in 1 min at 22°C.
Intravital microscopy
It was described that NSAID-induced neutrophil adherence could contribute to the pathogenesis of gastric mucosal injury (Wallace, 1997). In order to verify that SILD treatment could influence indomethacin-induced leukocyte adherence, intravital microscopy was performed on postcapillary mesentery venules in rats deprived of food for 18–20 h, as described previously (Baez, 1969; Fortes et al., 1991). The rats were treated with vehicle (saline) or SILD (10 mg kg−1). After 30 min, indomethacin (20 mg kg−1) or vehicle (Tris buffer) was administrated intragastrically. After 1 h, rats were anesthetized with chloral hydrate (400 mg kg−1) and mesenteric tissue was exposed for microscopic examination in situ. Vessels selected for study were third-order venules. The endothelial–leukocyte interaction was studied in a segment of the vessel. A leukocyte was considered to be adherent if it remained stationary for >30 s (Granger et al., 1989). Adherent cells were expressed as the number per 100-μm length of venule. Cells were counted using five different fields for each rat to avoid variability due to sampling. Data were then averaged for each rat.
To determine if NO was involved in the effects of SILD, another group of rats was pretreated with vehicle (saline), L-NAME (50 mg kg−1, s.c.) or L-arginine (500 mg kg−1, s.c.) + L-NAME (50 mg kg−1, subcutaneously (s.c.)). After 30 min, the rats received SILD (10 mg kg−1) or vehicle (saline) by gavage. At the end of 30 min, indomethacin (20 mg kg−1) was administrated and intravital microscopy was performed 1 h later as described above.
Gastric blood flow
Conventional NSAIDs have been shown to cause a decrease in gastric blood flow (Ashley et al., 1985; Gana et al., 1987; Kitahora & Guth, 1987), and this has been suggested to contribute significantly to the pathogenesis of injury associated with these agents (Wallace, 1997). To determine if SILD could prevent the decrease in gastric blood flow induced by indomethacin, experiments were carried out in which gastric blood flow was measured by Laser-Doppler flowmetry, as described in detail previously (Ferraz et al., 1995). An ex vivo gastric chamber preparation was used. The exposed stomach was bathed with 100 mmol l−1 hydrochloric acid throughout the experiment. A Laser-Doppler probe was placed on the surface of the dorsal, corpus region of the stomach for continuous recording of blood flow (Wallace et al., 2000). After a 15-min basal period, SILD (10 mg kg−1), indomethacin (20 mg kg−1), the combination of SILD (10 mg kg−1) and indomethacin (20 mg kg−1), or vehicle (saline) were injected i.p. (n=4–6 rats per group). Blood flow over the hour that followed was expressed as a percentage of the flow rate in the basal period.
Prostaglandin E2 assay
To evaluate the effects of SILD on the indomethacin-induced inhibition of gastric prostaglandin E2 (PGE2) synthesis, groups of rats were treated orally with saline or SILD (10 mg kg−1). After 30 min, indomethacin (20 mg kg−1) was administered orally. The control group received only the vehicles (saline and Tris buffer, respectively). After 3 h, the rats were sacrificed and a sample of the corpus region of the stomach was excised, weighed and added to a tube containing 1 ml of sodium phosphate buffer (10 mmol l−1; pH 7.4). The tissue was minced with scissors for 30 s, then placed in a shaking water bath (37°C) for 20 min. The samples were centrifuged (9000 × g) for 1 min, and the concentration of PGE2 in the supernatant was determined by ELISA by using a commercially available kit (Wallace et al., 2000).
Statistical analysis
Statistical analysis was performed using one way analysis of variance (ANOVA) followed by the Newman–Keuls test. Statistical significance was set at P<0.05.
Results
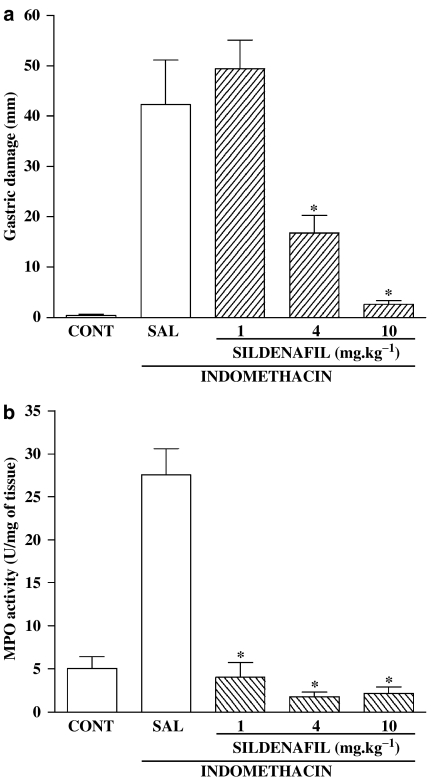
Indomethacin administration resulted in extensive hemorrhagic damage in the corpus region of the stomach, as well as a marked increase in granulocyte infiltration (MPO activity) (Figure 1). Pretreatment with SILD resulted in a dose-dependent reduction in the extent of gastric damage, with near-complete protection observed with a dose of 10 mg kg−1. SILD also prevented the increase in gastric MPO activity caused by indomethacin.
Figure 1.
Effect of SILD on gastric damage and gastric MPO activity increase induced by indomethacin. The rats were treated with SAL or SILD. After 30 min, indomethacin (20 mg kg−1) was administered. The control group (CONT) was treated with vehicles (Tris buffer and saline). Gastric lesions (a) and gastric MPO activity (b) were determined after 3 h. The results are expressed as the mean±s.e.m. of at least five rats per group. (*) P<0.05, when compared with the indomethacin group. ANOVA and Newman–Keuls test.
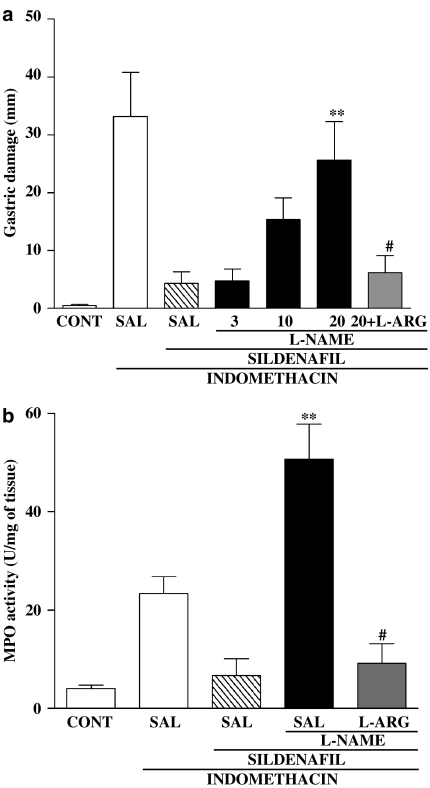
The protective effect of SILD against indomethacin-induced gastric damage, was prevented, in a dose-dependent manner, by L-NAME (Figure 2). L-NAME also reversed the effect of SILD on the indomethacin-induced increase in gastric MPO activity. Indeed, L-NAME increased gastric MPO substantially above that seen with indomethacin alone. These effects of L-NAME were abolished by coadministration of L-arginine, suggesting that the effects of L-NAME were related to its ability to inhibit NO synthesis.
Figure 2.
Involvement of NO in the protective effect of SILD in the indomethacin-induced gastrophaty. The rats were treated with saline (SAL), L-NAME (3, 10 and 20 mg kg−1) or L-NAME (20 mg kg−1) + L-Arginine (L-ARG, 200 mg kg−1). After 30 min, SILD or SAL was administe by gavage. After 30 min, the rats received indomethacin (20 mg kg−1). The control group (CONT) received only the vehicles (Tris buffer+saline). After 3 h, gastric damage and gastric MPO activity were determined. The results are expressed as the mean±s.e.m. of at least five rats per group. (**) P<0.05 when compared with the SILD group (panels a and b) and (#) P<0.05 when compared with the L-NAME group (panels a and b). ANOVA and Newman–Keuls test.
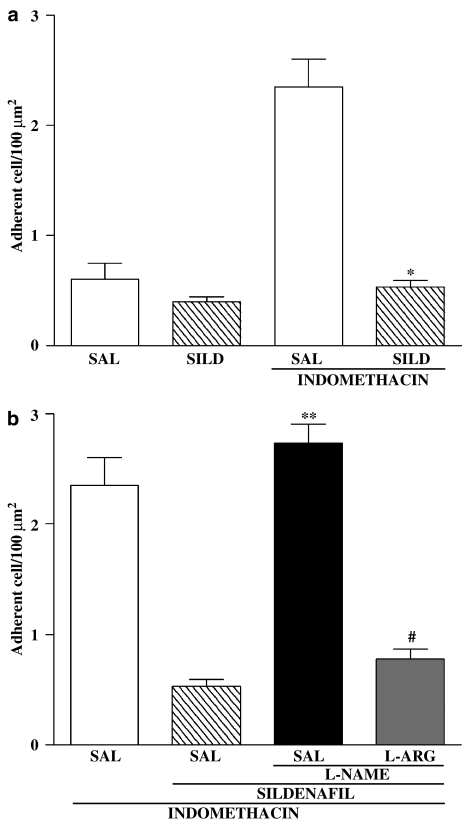
Indomethacin administration resulted in a marked increase in leukocyte adherence to postcapillary venular endothelium (Figure 3). Prior administration of SILD completely prevents this effect of indomethacin. Treatment with L-NAME alone, but not with L-NAME plus L- arginine, reversed this inhibitory effect of SILD.
Figure 3.
Role of nitric oxide in the SILD decreased leukocyte adherence induced by indomethacin. Rats were treated with saline (SAL) or SILD. After 30 min, indomethacin or saline was administe. After 1 h, intravital microscopy was performed (panel a). Another group of rats were pretreated by L-NAME (50 mg kg−1, s.c.)+SAL or with L-arginine (L-ARG, 500 mg kg−1, s.c.) + L-NAME (50 mg kg−1, s.c.). After 30 min, the rats received SILD (10 mg kg−1) or vehicle (saline) by gavage. At the end of 30 min, indomethacin (20 mg kg−1) was administrated and intravital microscopy was performed 1 h later. The results are expressed as the mean±s.e.m. of at least five rats per group. (*) P<0.05 when compared with the indomethacin group (a), (**) P<0.05 when compared with the SILD group (b) and (#) P<0.05 when compared with the L-NAME group (b). ANOVA and Newman–Keuls test.
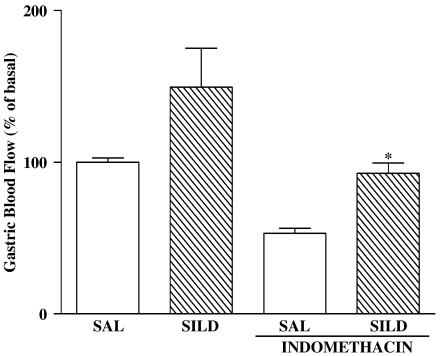
Administration of SILD to otherwise-untreated rats resulted in a significant increase in gastric blood flow (Figure 4). Indomethacin administration caused a significant decrease in gastric blood flow. Pretreatment with SILD prevented the indomethacin-induced decrease in gastric blood flow.
Figure 4.
Effect of SILD tretament on the gastric blood flow decrease induced by indomethacin. After a 15-min basal period, SILD (SILD, 10 mg kg−1), indomethacin (20 mg kg−1), the combination of SILD and indomethacin, or SAL was injected i.p. (n=4–6 rats per group), and gastric blood flow was continuously measured by laser-Doppler flowmetry for 60 min. Blood flow over the hour that followed was expressed as a percentage of the flow rate in the basal period. The results are expressed as the mean±s.e.m. of at least five rats per group. (*) P<0.05 when compared with the indomethacin group ANOVA and Newman–Keuls test.
The results described above suggest that SILD was able to prevent several effects of indomethacin that could contribute to gastric damage. It was possible that SILD interfered with the ability of indomethacin to suppress gastric prostaglandin synthesis, which would explain the observations described above. However, this was not the case. As shown in Table 1, indomethacin significantly reduced PGE2 by the stomach (by ∼70%). Pretreatment with SILD did not interfere with the ability of indomethacin to suppress gastric PG synthesis.
Table 1.
Effects of indomethacin and sildenafil on gastric PGE2 synthesis
| Treament | PGE2 (pg/mg) |
|---|---|
| Vehicle+vehicle | 244±23 |
| Sildenafil+Tris buffer | 267±31 |
| Saline+indomethacin | 78±14* |
| Sildenafil+indomethacin | 85±21* |
Data are presented as the mean±s.e.m. of five rats per group.
P<0.01 compared to the vehicle+vehicle group (ANOVA and Newman–Keuls test).
Discussion
SILD is a drug commonly used in the treatment of erectile dysfunction. It inhibits the metabolism of cGMP, resulting in increased relaxation of the smooth muscle surrounding arterioles supplying the human corpus cavernosum (Rosalmeida et al., 2003). This effect of SILD is attributable to inhibition of phosphodiesterase-type 5 (Moreland et al., 1998). Levels of cGMP in vascular smooth muscle are increased in response to activation of soluble guanylate cyclase by NO. NO plays a critical role in modulating several gastrointestinal functions, including gastrointestinal motility (Ueki et al., 1988), and several elements of mucosal defence, including blood flow (Whittle et al., 1981), neutrophil adhesion (Kubes et al., 1991; May et al., 1991) and mucus secretion (Allen et al., 1993; Wallace & Miller, 2000). Recently, it was demonstrated that SILD could reduce gastrointestinal motility in man (Bortolotti et al., 2001) and in animals (Rosalmeida et al., 2003). However, we are unaware of any previous studies examining the effects of SILD on gastric mucosal defense. Thus, the results of the present study demonstrate that SILD, acting via NO-dependent mechanisms, can protect the stomach against indomethacin-induced damage. Our results are consistent with the hypothesis that this protective effect of SILD is mediated via inhibition of indomethacin-induced leukocyte adhesion to vascular endothelium and maintenance of gastric blood flow.
The evidence that the protective effects of SILD were NO dependent includes the following: treatment with L-NAME (a competitive, nonselective NOS inhibitor) abrogated the protective effect of SILD, while the protective effect could be restored by coadministration of L-arginine (a substrate of NOS). These observations are consistent with previous observations that NO-releasing agents protected against experimental NSAID-induced damage (Wallace et al., 1994a, 1994b; Calatayud et al., 1999), while NO donors accelerated gastric ulcer healing in rats (Elliott et al., 1995).
There is substantial evidence that the damage produced in the stomach of the animals after the administration of NSAIDs is mediated by neutrophils. NSAIDs trigger the adherence of neutrophils to the vascular endothelium within the gastric and mesenteric microcirculation (Asako et al., 1992b; Wallace et al., 1991; 1993). We observed that SILD treatment decreased indomethacin-induced leukocyte adherence in postcapillary mesentery venules, as well as reducing granulocyte infiltration into gastric tissue (measured by MPO assay). Recently, in guinea-pig models of inflammatory airway disease, Toward et al. (2004) showed that SILD decreased leukocyte efflux in bronchoalveolar lavage fluid. This effect of SILD on leukocyte migration is at least in part dependent on the presence of NO, because treatment with L-NAME prevented the SILD-induced decrease in leukocyte adherence and infiltration. Consistent with our results, there is evidence that NO inhibits the expression of adhesion molecules on endothelial cells, which is an important step in neutrophil migration (Kubes et al., 1991; Fox-Robichaud et al., 1999; Benjamim et al., 2002). Thus, it is possible that the protective effect of SILD against indomethacin-induced gastric damage is mediated by inhibition of leukocyte adherence, and this is a NO-dependent process.
One of the most important components of mucosal defense is the vascular response to irritants. When challenged with an irritant, gastric blood flow increases. This response is likely aimed at diluting the irritant and at buffering any back-diffusing acid (Wallace, 1997). Administration of NSAIDs has been shown to reduce mucosal blood flow (Ashley et al., 1985; Gana et al., 1987; Kitahora & Guth, 1987; Wallace et al., 1994a, 1994b), and in doing so results in an increased susceptibility to damage. NO is a crucial mediator of mucosal blood flow (Whittle, 1993). In the present study, we showed that SILD increased basal gastric blood flow and prevented indomethacin-induced decreases in gastric blood flow. Others have observed similar effects of SILD in other tissues, such as the human corpus cavernosum (Ballard et al., 1998) and brachial artery (Desouza et al., 2002). The effects of SILD on gastric blood flow could be an important component of the mechanism of action in terms of protecting the stomach from damage induced by indomethacin.
Inhibition of gastric prostaglandin synthesis is central to the ability of NSAIDs to cause gastric damage (Vane, 1971). Agents that interfere with the ability of NSAIDs to suppress gastric prostaglandin synthesis will reduce the ability of those agents to cause damage. The ability of SILD to protect the stomach against indomethacin-induced damage is not, however, related to interference with the ability of this agent to suppress gastric prostaglandin synthesis. We observed that the inhibitory effect of indomethacin on gastric PG synthesis was not altered by prior administration of SILD.
In summary, our results indicate that SILD, by amplifying the effects of endogenous NO, prevents indomethacin-induced gastric damage. While there are many mechanisms through which this effect could be produced, our data support the hypothesis that inhibition of indomethacin-induced leukocyte adhesion and maintenance of gastric blood flow are of primary importance. SILD may have utility as a protective agent against NSAID-induced gastropathy in a clinical setting.
Acknowledgments
We gratefully acknowledge the technical assistance of Maria Silvandira Freire França. Grants from Pfizer Pharmaceuticals Group (Viagra Research Grant # 998) and CNPq (Brazil) supported this work. Dr Wallace is supported by grants from the Canadian Institutes of Health Research, and he holds an Alberta Heritage Foundation for Medical Research Senior Scientist Award and a Canada Research Chair in Inflammation Research.
Abbreviations
- ANOVA
analysis of variance
- CONT
control
- HTAB
hexadecyltrimethyl-ammonium bromide
- L-ARG
L- arginine
- MPO
myeloperoxidase
- NO
nitric oxide
- NSAIDs
nonsteroidal antiinflammatory drugs
- PDE5
phosphodiesterase- type 5
- PGE2
prostaglandin E2
- SAL
saline
- SILD
sildenafil
References
- ALLEN A., FLEMSTROM G., GARNER A., KIVILAAKSO E. Gastroduodenal mucosal protection. Physiol. Rev. 1993;73:823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- ASAKO H., KUBES P., WALLACE J.L., GAGINELLA T., WOLF R.E., GRANGER D.N. Indomethacin-induced leukocyte adhesion in mesenteric venules: role of lipoxygenase products. Am. J. Physiol. 1992a;262:G903–G908. doi: 10.1152/ajpgi.1992.262.5.G903. [DOI] [PubMed] [Google Scholar]
- ASAKO H., KUBES P., WALLACE J.L., WOLF R.E., GRANGER D.N. Modulation of leukocyte adhesion in mesenteric venules by aspirin and salicylate. Gastroenterology. 1992b;103:146–152. doi: 10.1016/0016-5085(92)91107-f. [DOI] [PubMed] [Google Scholar]
- ASHLEY S.W., SONNENSCHEIN L.A., CHEUNG L.Y. Focal gastric mucosal blood flow at the site of aspirin-induced ulceration. Am. J. Surg. 1985;149:53–59. doi: 10.1016/s0002-9610(85)80009-x. [DOI] [PubMed] [Google Scholar]
- BAEZ S. Simultaneous measurements of radii and wall thickness of microvessels in the anesthetized rat. Cric. Res. 1969;25:315–329. doi: 10.1161/01.res.25.3.315. [DOI] [PubMed] [Google Scholar]
- BALLARD S.A., GINGELL C.J., TANG K., TURNER L.A., PRICE M.E., NAYLOR A.M. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J. Urol. 1998;159:2164–2171. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- BENJAMIM C.F., SILVA J.S., FORTES Z.B., OLIVEIRA M.A., FERREIRA S.H., CUNHA F.Q. Inhibition of leukocyte rolling by nitric oxide during sepsis leads to reduced migration of active microbicidal neutrophils. Infect. Immunol. 2002;70:3602–3610. doi: 10.1128/IAI.70.7.3602-3610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORTOLOTTI M., MARI C., LOPILATO C., LA ROVERE L., MIGLIOTI M. Sildenafil inhibits gastroduodenal motility. Aliment. Pharmacol. Ther. 2001;15:157–161. doi: 10.1046/j.1365-2036.2001.00917.x. [DOI] [PubMed] [Google Scholar]
- BRADLEY P.P., CHRISTENSEN R.D., ROTHSTEIN G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982;60:618–622. [PubMed] [Google Scholar]
- CALATAYUD S., SANZ M.J., CANET A BELLO R., DE ROJAS F.D., ESPLUGUES J.V. Mechanisms of gastroprotection by transdermal nitroglycerin in the rat. Br. J. Pharmacol. 1999;127:1111–1118. doi: 10.1038/sj.bjp.0702649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUANG A.T., STRAUSS J.D., MURPHY R.A. Sildenafil, atype cGMP phosphodiesterase inhibitor, specifically amplifies endogenous cGMP-dependent relaxation in rabbit corpus cavernosum smooth in vitro. J. Urol. 1998;160:257–261. [PubMed] [Google Scholar]
- DESOUZA C., PARULKAR A., LUMPKIN D., AKERS D., FONSECA V.A. Acute and prolonged effects of sildenafil on brachial artery flow-mediated dilatation in type 2 diabetes. Diabetes Care. 2002;25:1336–1339. doi: 10.2337/diacare.25.8.1336. [DOI] [PubMed] [Google Scholar]
- ELLIOTT S.N., MCKNIGHT W., CIRINO G., WALLACE J.L. A nitric oxide-releasing nonsteroidal anti-inflammatory drug accelerates gastric ulcer healing in rats. Gastroenterology. 1995;109:524–530. doi: 10.1016/0016-5085(95)90341-0. [DOI] [PubMed] [Google Scholar]
- FERRAZ J.G., MCKNIGHT W., SHARKEY K.A., WALLACE J.L. Impaired vasodilatory responses in the gastric microcirculation of cirrhotic rats. Gastroenterology. 1995;108:1183–1191. doi: 10.1016/0016-5085(95)90218-x. [DOI] [PubMed] [Google Scholar]
- FORTES Z.B., FARSKY S.P., OLIVEIRA M.A., GARCIA-LEME J. Direct vital microscopic study of defective leokocyte-endothelial interactions in diabetes melitus. Diabetes. 1991;40:1267–1273. doi: 10.2337/diab.40.10.1267. [DOI] [PubMed] [Google Scholar]
- FOX-ROBICHAUD A., PAYNE D., KUBES P. Inhaled NO reaches distal vasculatures to inhibit endothelium- but not leukocyte-dependent cell adhesion. Am. J. Physiol. 1999;277:L1224–L1231. doi: 10.1152/ajplung.1999.277.6.L1224. [DOI] [PubMed] [Google Scholar]
- GANA T.J., HUHLEWYCH R., KOO J. Focal gastric mucosal blood flow in aspirin-induced ulceration. Ann. Surg. 1987;205:399–403. doi: 10.1097/00000658-198704000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A. Phosphodiesterase 5 inhibitors and nitregic transmission- from Zaprinast to Sildenafil. Eur. J. Pharmacol. 2001;411:1–10. doi: 10.1016/s0014-2999(00)00824-4. [DOI] [PubMed] [Google Scholar]
- GRANGER D.N., BENOIT J.N., SUZUKI M., GRISHAM M.B. Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am. J. Physiol. 1989;257:G683–G688. doi: 10.1152/ajpgi.1989.257.5.G683. [DOI] [PubMed] [Google Scholar]
- KITAHORA T., GUTH P.H. Effect of aspirin plus hydrochloric acid on the gastric mucosal microcirculation. Gastroenterology. 1987;93:810–817. doi: 10.1016/0016-5085(87)90444-6. [DOI] [PubMed] [Google Scholar]
- KUBES P., SUZUKI M., GRANGER D.N. Nitric oxide: an endogenous modulator of eukocyte adhesion. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY G.R., CROOK P., MOORE P.K., PAGE C.P. The role of nitric oxide as an endogenous regulator of platelet and neutrophil activation within the pulmonary circulation of the rabbit. Br. J. Pharmacol. 1991;102:759–763. doi: 10.1111/j.1476-5381.1991.tb12246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORELAND R.B., GOLDSTEIN I., TRAISH A. Sildenafil, a novel inhibitor of phopshodiesterase type 5 in human corpus cavernosum muscle cells. Life Sci. 1998;62:309–318. doi: 10.1016/s0024-3205(98)00158-1. [DOI] [PubMed] [Google Scholar]
- MUSCARA M.N., WALLACE J.L. Nitric oxide. V. Therapeutic potential of nitric oxide donors and inhibitors. Am. J. Physiol. 1999;276:G1313–G1316. doi: 10.1152/ajpgi.1999.276.6.G1313. [DOI] [PubMed] [Google Scholar]
- REFFELMANN T., KLONER R.A. Therapeutic potential of phosphodiesterase 5 inhibition for cardiovascular disease. Circulation. 2003;108:239–244. doi: 10.1161/01.CIR.0000081166.87607.E2. [DOI] [PubMed] [Google Scholar]
- ROSALMEIDA M.C., SARAIVA L.D., DA GRAÇA J.R., BARRETO B.I., DA NÓBREGA M.V., GONDIM F.A., ROLA F.H., SANTOS A.A. Sildenafil, a phosphodiesterase-5 inhibitor, delays gastric emptying and gastrointestinal transit of liquid in awake rats. Dig. Dis. Sci. 2003;48:2064–2068. doi: 10.1023/a:1026151227729. [DOI] [PubMed] [Google Scholar]
- SANTUCCI L., FIORUCCI S., GIANSANTI M. Pentoxifylline prevents indomethacin-induced acute gastric mucosal damage in rats - role of tumor-necrosis-factor-alpha. Gut. 1994;35:909–915. doi: 10.1136/gut.35.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA M.H., LEMOS H.P., OLIVEIRA R.B., CUNHA F.Q. Gastric damage and granulocyte infiltration induced by indomethacin in tumour necrosis factor receptor 1 (TNF-R1) or inducible nitric oxide synthase (iNOS) deficient mice. Gut. 2004;53:791–796. doi: 10.1136/gut.2002.012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOWARD T.J., SMITH N., BROADLEY K.J. Effect of phosphodiesterase-5 inhibitor, sildenafil (Viagra), in animal models of airways disease. Am. J. Respir. Crit. Care. Med. 2004;169:227–234. doi: 10.1164/rccm.200211-1372OC. [DOI] [PubMed] [Google Scholar]
- UEKI S., TAKEUCHI K., OKABE S. Gastric motility is an important factor in the pathogenesis of indomethacin-induced gastric mucosal lesions in rats. Dig. Dis. Sci. 1988;33:209–216. doi: 10.1007/BF01535735. [DOI] [PubMed] [Google Scholar]
- VANE J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MILLER M.J. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–520. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., ARFORS K.E., MACKNIGHT G.W. A monoclonal antibody against the CD18 leukocyte adhesion molecule prevents indomethacin-induced gastric damage in the rabbit. Gastroenterology. 1991;100:878–883. doi: 10.1016/0016-5085(91)90259-n. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MCKNIGHT W., MIYASAKA M., TAMATANI T., PAULSON J., ANDERSON D.C., GRANGER D.N., KUBES P. Role of endothelial adhesion molecules in NSAID-induced gastric mucosal injury. Am. J. Physiol. 1993;265:G993–G998. doi: 10.1152/ajpgi.1993.265.5.G993. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MCKNIGHT W., REUTER B.K., VERGNOLLE N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119:706–714. doi: 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., REUTER B., CICALA C., MCKNIGHT W., GRISHAM M., CIRINO G. A diclofenac derivative without ulcerogenic properties. Eur. J. Pharmacol. 1994a;257:249–255. doi: 10.1016/0014-2999(94)90136-8. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., REUTER B.K., CIRINO G. Nitric oxide-releasing non-steroidal anti-inflammatory drugs: a novel approach for reducing gastrointestinal toxicity. J. Gastroenterol. Hepatol. 1994b;9:S40–S44. doi: 10.1111/j.1440-1746.1994.tb01300.x. [DOI] [PubMed] [Google Scholar]
- WHITTLE B.J. Neuronal and endothelium-derived mediators in the modulation of the gastric microcirculation: integrity in the balance. Br. J. Pharmacol. 1993;110:3–17. doi: 10.1111/j.1476-5381.1993.tb13763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTLE B.J., KAUFFMAN G.L., MONCADA S. Vasoconstriction with thromboxane A2 induces ulceration of the gastric mucosa. Nature. 1981;292:472–474. doi: 10.1038/292472a0. [DOI] [PubMed] [Google Scholar]
- WOLFE M.M., LICHTENSTEIN D.R., SINGH G. Gastrointestinal toxicity of nonsteroidal anti-inflammatory drugs. N. Engl. J. Med. 1999;340:1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]