Abstract
Bisphosphonates are inhibitors of tumor cell growth as well as of bone resorption by inducing cell apoptosis. However, little is known regarding the mechanisms by which the drug induces cell apoptosis. The aim of the present study was to determine the effect of alendronate, one of the nitrogen-containing bisphosphonates on the phoshoinositide 3-kinase (PI3K)–Akt–NFκB pathway, the major cell survival pathway.
The PI3K–Akt–NFκB pathway was activated in the osteosarcoma cell line MG-63 treated with tumor necrosis factor-α or insulin. Saos-2 was also used in some experiments. This was assessed by the production of phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3), increased PI3K activity, phosphorylation of Akt at serine 473 and threonine 308, increase in activity of the inhibitor of nuclear factor κB (IκB) kinase (IKK) and finally phosphorylation of IκB and its subsequent degradation.
Pretreatment with alendronate at 100 μM for 24 h prior to the stimulation with tumor necrosis factor-α or insulin partially inhibited the IκB phosphorylation and degradation. These events were more clearly observed in the presence of inhibitors of proteasomes, which are responsible for the degradation of IκB. The drug also partially inhibited the activity of IKK, but almost fully inhibited the phosphorylation of Akt and the production of PtdIns(3,4,5)P3.
The inhibitory effect of alendronate on IκB phosphorylation and degradation was not attenuated by the exogenous addition of geranylgeraniol to replenish the cytosolic isoprenyl lipid substrate.
The present findings demonstrate that alendronate inhibited the PI3K–Akt–NFκB cell survival pathway at the point of PI3K activation, thus indicating the presence of new targets of alendronate.
Keywords: Akt, apoptosis, bisphosphonate, cell survival pathway, NFκB, osteosarcoma, phosphoinositides
Introduction
Bisphosphonates (BP) are inhibitors of bone resorption. They have been used for more than 30 years clinically, and are now most widely used in the treatment of patients who have bone diseases such as osteoporosis, Paget's disease, hypercalcemia and metastatic cancer in the bones (Russell & Rogers, 1999). The possibility of their use in the treatment of rheumatoid arthritis, periodontal disease (Binderman et al., 2000; Kaynak et al., 2003) and reconstruction of bone in orthopedics (van der Poest Clement et al., 2002) is also being investigated. BP are analogues of pyrophosphates, and can be separated into two classes based on their structure, non-nitrogen-containing BP and nitrogen-containing BP (or amino-BP). Non-nitrogen-containing BP can be metabolically incorporated into nonhydrolysable analogues of ATP for osteoclast apoptosis (Firth et al., 1997). It has been suggested that amino-BP affect osteoclast function by inhibiting protein prenylation of the small GTP-binding regulatory protein (G protein) in the mevalonate pathway (Luckman et al., 1998). Briefly, amino-BP inhibit the synthesis of farnesyldiphosphate and geranylgeranyldiphosphate which are absolutely required for the prenylation of small G proteins, since this modification is essential to anchor the proteins in plasma membranes allowing participation in protein–protein interactions. Amino-BP also affect osteoclast function, including causing loss of the ruffled border, disruption of the actin cytoskeleton and induction of osteoclast apoptosis (Sato & Grasser, 1990; Fisher et al., 1999). It has also been reported that BP inhibit the differentiation of osteoclast precursors (Schmidt et al., 1996). Although Halasy-Nagy et al. (2001) reported that the use of amino-BP, alendronate and risedronate, to inhibit bone resorption did not cause cell apoptosis, it is generally accepted that the inhibition of bone resorption by this drug can be attributed to the induction of osteoclast apoptosis (Hughes et al., 1995; Rodan & Fleisch, 1996; Ebetino et al., 1998).
Recent reports have also suggested that amino-BP can act directly on the growth of tumor cells by inducing the apoptosis of tumor cells such as myeloma cells (Shipman et al., 1998), breast cancer cells (Jagdev et al., 2001) and osteosarcoma cells (Cheng et al., 2004). However, the mechanism of the effect on cancer cells in bone has not been investigated.
The Akt activation pathway is known to be a major cell survival pathway (Li et al., 1999; Ozes et al., 1999), which subsequently activates the nuclear transcription factor, nuclear factor κB (NFκB). This regulates the gene expression involved in immunity, stress responses, inflammation and the inhibition of apoptosis, thus providing appropriate conditions for cell survival (Foo & Nolan, 1999; Ozes et al., 1999). Akt is one of the targets of phoshoinositide 3-kinase (PI3K) (Toker & Cantley, 1997), and contains the pleckstrin homology domain which directly binds phosphatidylinositol 3,4,5-trisphosphate (PtdIns(3,4,5)P3), a product of PI3K activation.
In the present study, we investigated the mechanisms by which amino-BP exert antitumor actions. We determined the effect of alendronate, one of the amino-BP currently widely used clinically, on each step of the cellular signaling involved in the cell survival pathways as described above, using the osteosarcoma cell line MG-63. In some experiments, another cell line, Saos-2, was also used. We mainly used tumor necrosis factor-α (TNFα) to stimulate the pathway, and insulin in some experiments, because we have experience with experiments using TNFα (Sandra et al., 2002). Alendronate was found to inhibit PI3K activation, an initial step of the cell survival pathway examined. The subsequent processes, including the activation of Akt and NFκB, were also inhibited, thus indicating a new target for alendronate.
Methods
Cell culture and treatment with alendronate
MG-63 and Saos-2, supplied by Teijin Pharma (Tokyo, Japan), were cultured in Dulbecco's minimum essential medium (DMEM) and 10% fetal bovine serum in a humidified atmosphere of 5% CO2 at 37°C. The medium was changed every 3 days. The cells were passaged twice a week. Cells were cultured in a six-well dish overnight in medium containing serum, and then cultured for 12 h in a serum-free medium, followed by treatment with alendronate at 100 μM for 24 h prior to the stimulation with TNFα or insulin. In assays using a proteasomal inhibitor, lactacystin (10 μM) or N-acetyl-leucine-leucine-norleucine-CHO (ALLN, 50 μM), the chemical was added 2 h before the stimulation.
Experiments on cell death
MG-63 cells (30 × 104 cells) were cultured as described above, followed by starvation for 12 h, with or without TNFα at 5 ng ml−1. Alendronate (100 μM) and geranylgeraniol (GGOH, 20 μM) were then added and cultivated with the cells for 24 h. Cells attached to dishes were collected and viability was assessed using the trypan blue exclusion test.
Immunoblotting
Following the stimulation with 5 ng ml−1 TNFα for the period indicated or 10 ng ml−1 insulin for 10 min, cells were incubated with lysis buffer (20 mM Hepes buffer at pH 7.2, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 50 mM sodium fluoride, 40 mM sodium β-glycerophosphate, 2 mM sodium orthovanadate, 30 mM sodium pyrophosphate and a cocktail of protease inhibitors (see below)) on ice for 10 min and scraped. Extracts were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to a polyvinylidene difluoride sheet. After blocking with 5% skimmed milk in a Tris-buffered saline (150 mM NaCl and 20 mM Tris-HCl at pH 7.2), the sheet was incubated with the first antibody of interest. The secondary antibody was horseradish peroxidase-conjugated donkey anti-rabbit IgG antibody, followed by detection using the ECL system (Amersham). The cocktail of protease inhibitors contained 10 μM aprotinin, 10 μM pepstatin A, 10 μM leupeptin and 1 mM p-amidinophenylmethanesulfonyl fluoride. Concentration of TNFα used (5 ng ml−1) was determined based on the results described in Results section.
Inhibitor of NFκB (IκB) kinase (IKK) activity assay
Following the stimulation with 5 ng ml−1 TNFα for the period indicated, cells were scraped and lysed in an ice-cold buffer containing 20 mM Tris-HCl at pH 8.0, 500 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10 mM β-glycerophosphate, 10 mM sodium fluoride, 10 mM p-nitrophenyl phosphate, 300 μM sodium orthovanadate, 1 mM benzamidine, 1 mM dithiothreitol, 0.25% Nonidet P-40 and the protease inhibitor mixture described above. The supernatant, obtained by centrifugation at 12,000 × g for 10 min at 4°C was immunoprecipitated with anti-IKKβ antibody. Half of the immunoprecipitated samples were analyzed for IKK activity using a method similar to that described by Subha & Kundu (2003). Briefly, the immunoprecipitates were incubated with recombinant IκB-α (C-15, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) in a kinase assay buffer (20 mM Tris-HCl at pH 7.7, 2 mM MgCl2, 10 μM ATP containing 3 μCi of [γ-32P]ATP, 10 mM β-glycerophosphate, 10 mM sodium fluoride, 10 mM p-nitrophenyl phosphate, 300 μM sodium orthovanadate, 1 mM benzamidine, 1 mM dithiothreitol and a protease inhibitor mixture) at 30°C for 10 min. The reaction was stopped by the addition of SDS-sample buffer. The sample was resolved by SDS–PAGE, followed by autoradiography. The remaining half of the immunoprecipitates was analyzed for quantification of IKKβ by immunoblotting using the specific antibody.
PI3K activity assay
MG-63 cells treated with or without 100 μM alendronate for 24 h, or together with 25 μM LY294002 for 2 h were stimulated with 5 ng ml−1 TNFα for 10 min. The cells were scraped and lysed in 500 μl of lysis buffer (20 mM Tris-HCl buffer at pH 8.0, 137 mM NaCl, 1 mM MgCl2, 10 % Nonidet P-40, 1 mM dithiothreitol, 0.4 mM sodium orthovanadate and 1 mM p-amidinophenylmethanesulfonyl fluoride). The cell lysate collected by centrifugation was incubated with 25 μl of protein G-Sepharose, which had previously been conjugated with polyclonal anti-PI3K (p85α) antibody (Santa Cruz Biotechnology) overnight at 4°C. The beads were washed with each of the following buffers: buffer A (PBS containing 1% Nonidet P-40 and 1 mM dithiothreitol), buffer B (0.1 M Tris-HCl buffer at pH 7.6, 0.5 M LiCl and 1 mM dithiothreitol) and buffer C (10 mM Tris-HCl buffer at pH 7.6, 0.1 M NaCl and 1 mM dithiothreitol), followed by incubation with 0.5 mg ml−1 phosphoinositide fraction in a reaction mixture (50 mM MgCl2, 100 mM Hepes buffer at pH 7.6, 250 μM ATP containing 5 μCi of [γ-32P]ATP at 30°C for 10 min). The reaction was stopped by adding 15 μl of 4 N HCl and 130 μl of CHCl3-methanol (1 : 1 by volume). After vortexing for 30 s, 30 μl from the phospholipid-containing chloroform phase was spotted onto a potassium oxalate-impregnated silica-gel 60 plate and developed in a thin layer chromatography tank with a solvent containing CHCl3-methanol-NH4OH-H2O (60 : 27 : 20 : 11.3 by volume). The plate was activated by 15 min at 110°C before spotting. After the solvent front reached the top, the plate was dried and labeled phospholipids were detected by autoradiography on X-Omat film (Kodak).
PtdIns(3,4,5)P3 production assay
MG-63 cells treated with 100 μM alendronate for 24 h or 25 μM LY294002 for 2 h were washed three times with saline containing 30 mM Hepes buffer at pH 7.4, 110 mM NaCl, 10 mM KCl, 1 mM MgCl2 and 10 mM glucose, followed by incubation with [32P]orthophosphate (125 μCi ml−1) for 3 h at 37°C. After extensive washing, cells were stimulated with 5 ng ml−1 TNFα or 10 ng ml−1 insulin for 10 min, and then any activity was halted by adding 1 ml of 10% trichlorocetic acid. After 15 min on ice for quenching, the dishes were scraped and washed once with 0.5 ml of 10% trichloroacetic acid. The cell lysates were centrifuged for 5 min at 13,000 × g, and the resulting pellet was washed with 5% trichloroacetic acid and 1 mM EDTA. Lipids were extracted for 20 min in 0.75 ml of CHCl3-methanol-HCl (40 : 80 : 1 by volume) containing the antioxidant BHT (0.63 mg ml−1) and phosphoinositides as cold carrier. The phases were then split by the addition of 0.25 ml of CHCl3 and 0.45 ml of 0.1 M HCl. The lower phase, obtained after 1 min centrifugation at 13,000 × g, was re-extracted once with 0.45 ml of the synthetic lower phase. The lower phases were pooled and dried down by N2 bubbling. Then, phospholipids were dissolved into 50 μl of methanol-CHCl3 (1 : 5 by volume) and spotted onto a potassium oxalate-impregnated silica-gel 60 plate and developed in a thin layer chromatography tank with a solvent containing CHCl3-methanol-acetone-CH3COOH-H2O (7 : 5 : 2 : 2 : 2 by volume). After the solvent front reached the top, the plate was dried and labeled phospholipids were detected by autoradiography on X-Omat film (Kodak).
Radioligand binding assay
Cells were cultured in CulturPlate-96 (Perkin-Elmer Life Sciences, Norwalk, CT, U.S.A.) overnight, starved for 12 h and then incubated with 100 μM alendronate. After washing with ice-cold phosphate-buffered saline containing 0.1% bovine serum albumin, the cells were incubated with [125I]TNFα (Perkin-Elmer Life Sciences) at various concentrations for 2 h at 4°C. The cells were washed three times with phosphate-buffered saline, and then the radioactivity obtained after mixing with 200 μl of Microscint™ 40 (Packard) was counted using Top Count NXT™ (Perkin-Elmer Life Sciences). Nonspecific binding was also assayed in the presence of 1000-fold excess of unlabeled TNFα, and was subtracted from the total binding to yield the specific binding.
Reagents
Alendronate (4-amino-1-hydroxybutylidene-1, 1-bisphosphonate) was a gift from Teijin Pharma (Tokyo, Japan). A stock solution of alendronate (10 mM) was prepared in Dulbecco's phosphate-buffered saline (Sigma, St Louis, MO, U.S.A.), and sterilized by filtration. Human recombinant TNFα, lactacystin and ALLN were obtained from Calbiochem (San Diego, CA, U.S.A.). LY294002 and geranylgeraniol (GGOH) were from Sigma.
Statistical analysis
Values are expressed as mean±s.e. Statistical evaluation was performed using Student's t-test for paired data. P<0.05 was considered statistically significant.
Results
Alendronate inhibited the phosphorylation of IκB
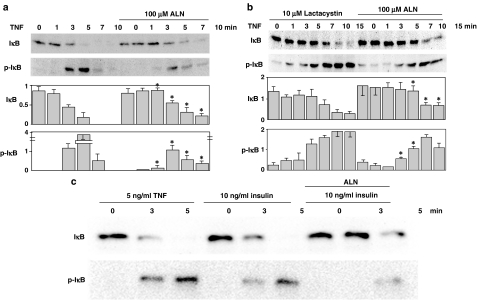
We first analyzed the phosphorylation and degradation of IκB, which is a prerequisite for the entry of NFκB into the nucleus and its subsequent activation, in the osteosarcoma cell line MG-63 in response to stimulation with TNFα at 5 ng ml−1. As shown in Figure 1a, the amount of IκB in MG-63 cells decreased as the duration of stimulation by TNFα increased, as assessed by imunoblotting with a specific antibody against IκB. Conversely, the phosphorylation at serine 32 (Ser32) increased, as assessed by a phospho-specific antibody, although little phosphorylation at Ser32 was seen at 7 and 10 min after stimulation. This was probably because IκB was almost completely degraded, as little IκB was seen at these time points. These events were concentration-dependent; TNFα at 10 ng ml−1 induced almost complete phosphorylation and degradation of IκB within 2 min after stimulation, which was too fast to detect, while the drug at 1 ng ml−1 induced the phosphorylation and degradation at 7 min after the stimulation (results not shown). Treatment with alendronate at 100 μM for 24 h prior to the stimulation with TNFα partially inhibited the phosphorylation at Ser32 and retarded the decrease in the amount of IκB. Similar results were obtained with another osteosarcoma cell line, Saos-2 cells (data not shown). Alendronate at 10 μM induced little effect, but at 500 μM it exhibited toxic effects to cause cell detachment from the culture dishes. Alendronate at 100 μM added to MG-63 cells minimally and maximally required the incubation for 9 and 12–24 h, respectively, for the inhibition, as examined for 3, 6, 9, 12 and 24 h. Therefore, TNFα at 5 ng ml−1, and alendronate at 100 μM for 24 h were employed in following experiments.
Figure 1.
Phosphorylation and degradation of IκB. (a) MG-63 cells (30 × 104) were starved for 12 h and then treated with or without 100 μM alendronate for 24 h, followed by the stimulation with 5 ng ml−1 TNFα for 1, 3, 5, 7 and 10 min. Cell lysates were analyzed by immunoblotting using polyclonal anti-phospho-IκB-α (Ser32) antibody (Cell Signaling Technology, Inc.) and polyclonal anti-specific-IκB-α (Cell Signaling Technology, Inc.). (b) MG-63 cells (30 × 104) were starved for 12 h and incubated with 10 μM lactacystin for 2 h, followed by treatment with 100 μM alendronate for 24 h and then 5 ng ml−1 TNFα for 1, 3, 5, 7, 10 and 15 min. Cell lysates were analyzed by immunoblotting as described above. Upper panels and lower graphs indicate typical immunoblots and a summary of five separate experiments shown as the mean±s.e., respectively. *Indicates a significant difference when compared with that seen in the control cells at the same time point. (c) MG-63 cells were treated as described in (a), followed by stimulation with 5 ng ml−1 TNFα or 10 ng ml−1 insulin for the period indicated. Cell lysates were immunoblotted as described above. The blot shown is typical of three experiments.
Phosphorylation of IκB is known to initiate ubiquitination followed by proteasomal degradation (Karin & Ben-Neriah, 2000). In the assay employed, the phosphorylation of IκB represented not only the levels of phosphorylation but also the remaining amount of IκB. Therefore, it could have been difficult to discriminate the target site inhibited by alendronate, the inhibition of the phosphorylation or the degradation. However, at 3 min after stimulation, the phosphorylation was inhibited even though the levels of IκB remained similar to those before stimulation, indicating that alendronate inhibits the phosphorylation process, but not the proteasomal degradation process. For better discrimination, same experiments were performed using MG-63 cells, which had been pretreated with 10 μM lactacystin, an inhibitor of the proteasome, for 2 h (Figure 1b). The amount of IκB in lactacystin-treated cells was greater than that in control cells (compared with that shown in Figure 1a, in which same amounts of cells were analyzed using the same anti-IκB antibody), indicating that constitutive degradation of IκB occurs during the cultivation of cells. The degradation and the phosphorylation of IκB appeared to be prolonged in response to the stimulation with TNFα. Alendronate inhibited the phosphorylation of IκB and further prolonged the degradation of IκB. Similar results were observed with another proteasome inhibitor, ALLN at 50 μM, which was added in place of lactacystin (results not shown).
Insulin (10 ng ml−1), a ligand involved in the Akt activation pathway also caused the phosphorylation and degradation of IκB in MG-63 cells and the effect was partially inhibited by pretreatment with alendronate, in a similar manner to when TNFα was used (Figure 1c).
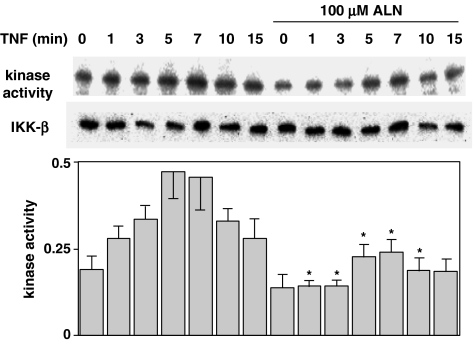
Alendronate inhibited the activation of IKK
IκB is phosphorylated by IKK, comprising IKKα, IKKβ and IKKγ (Rothwarf et al., 1998). We examined IKK activity using immunoprecipitates obtained by incubating anti-IKKβ antibody with cells treated with TNFα and alendronate. As shown in Figure 2, IKK activity, as determined by in vitro phosphorylation of IκB increased with TNFα stimulation to a peak at 5 and 7 min, and then decreased with further incubation. Alendronate at 100 μM inhibited this process. Treatment with alendronate for 24 h prior to the stimulation with TNFα (at time 0) caused decreased IKK activity, indicating that activation was constitutive.
Figure 2.
Activity of IKK. MG-63 cells (1 × 106) were starved for 12 h and treated with or without 100 μM alendronate for 24 h, followed by stimulation with 5 ng ml−1 TNFα for the period indicated. Lysates were immunoprecipitated by polyclonal anti-IKKβ antibody (Santa Cruz Biotechnology, Inc.). The procedures used are described in ‘Methods'. Upper panels indicate typical immunoblots, and the lower graph indicates the results expressed as the density of anti-phospho-IκB relative to that of anti-IKKβ in the mean±s.e. of five independent experiments. *Indicates a significant difference when compared with that seen in the control cells at the same time point.
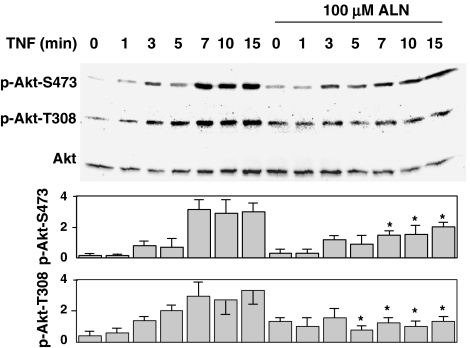
Alendronate inhibited the phosphorylation of Akt
One of the upstream pathways of IKK activation is Akt activation (Ozes et al., 1999). Akt activity was assessed by analyzing the phosphorylation at serine 473 (S473) and threonine 308 (T308) (Alessi et al., 1996; Sandra et al., 2002). The phosphorylation at both residues, as assessed by phospho-specific antibodies, was stimulated by TNFα in a time-dependent manner, and this was inhibited by pretreatment with alendronate (Figure 3).
Figure 3.
Phosphorylation of Akt. MG-63 cells (30 × 104) were starved for 12 h and treated with or without 100 μM alendronate for 24 h, followed by stimulation with 5 ng ml−1 TNFα for the period indicated. Cell lysates were analyzed by immunoblotting using polyclonal anti-phospho-Akt (Ser473) (New England BioLabs Inc.) or polyclonal anti-phospho-Akt (Thr308) (New England BioLabs Inc.) for the phosphorylation assay, and polyclonal anti-Akt (New England BioLabs Inc.) for the quantification. The upper panel and lower graph show typical immunoblots and the results are expressed as the density of anti-phospho-Akt relative to that of anti-Akt and shown as the mean±s.e. of five independent experiments, respectively. *Indicates a significant difference when compared with that seen in the control cells at the same time point.
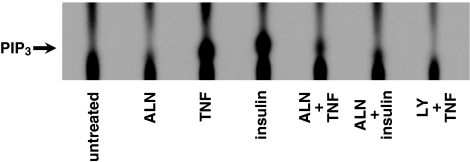
Alendronate inhibited the activation of PI3K
Activation of Akt caused by phosphorylation at residues S473 and T308 is catalyzed by activation of phosphoinositide-dependent kinase (PDK). Consequently, we examined the production of PtdIns(3,4,5)P3 in cells and PI3K activity using cell extracts (Figure 4). MG-63 cells, metabolically labeled with 32P-orthophosphate, were stimulated with TNFα at 5 ng ml−1 for 10 min, and the lipid extract from cell membranes was analyzed for the production of PtdIns(3,4,5)P3. As shown in Figure 4, production of PtdIns(3,4,5)P3 was detected within 10 min of stimulation with TNFα. The position of PtdIns(3,4,5)P3 on the plate for thin layer chromatography was confirmed by analyzing an authentic PtdIns(3,4,5)P3 followed by iodine vapor detection and the phospholipid extracts from [3H]inositol-labeled cells. The production of PtdIns(3,4,5)P3 was almost completely inhibited when cells were pretreated with 25 μM of LY294002. These results suggest that stimulation of TNFα resulted in PI3K activation in MG-63 cells and this action is LY294002-sensitive, as observed in other cell types (Ozes et al., 1999; Sandra et al., 2002). Alendronate had no effect alone, but partially inhibited the production of PtdIns(3,4,5)P3, although not as much as LY294002. Similar inhibition by alendronate was also observed in cells stimulated with insulin (10 ng ml−1).
Figure 4.
Activity of PI3K. MG-63 cells (1 × 106) were starved for 12 h and treated with or without 100 μM alendronate for 24 h or 25 μM LY294002 for 2 h, followed by incubation with [32P]orthophosphate (125 μCi ml−1) for 3 h at 37°C. After extensive washing, cells were stimulated with 5 ng ml−1 TNFα or 10 ng ml−1 insulin for 10 min. PtdIns(3,4,5)P3 production assay was performed as described in ‘Methods'. Similar results were seen in four other experiments.
Cellular extracts prepared from MG-63 cells treated with TNFα or insulin with/without alendronate or LY294002, as described in the Methods section, were analyzed for in vitro PI3K activity. TNFα stimulated PI3K, which was almost completely inhibited by alendronate, similarly to LY294002 (data not shown).
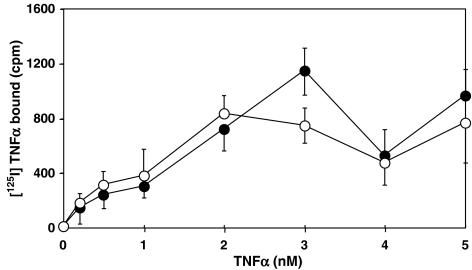
Alendronate had no effect on TNFα ligand receptor binding
We next examined the receptor binding of TNFα to MG-63 cell surfaces treated with alendronate by ligand binding assay. As shown in Figure 5, there was little difference in the specific binding of [125I]TNFα to cell surface receptors of alendronate-treated and control cells.
Figure 5.
Radio ligand binding assay. MG-63 cells (1 × 104 cells in a well) were starved for 12 h in 96-well plates (CulturPlate-96) and treated with or without 100 μM alendronate for 24 h. After washing with a phosphate-buffered saline three times, cells were incubated with 0.2, 0.5, 1.0, 2.0, 3.0, 4.0 or 5.0 nM [125I]TNFα (Amersham Bioscience) for 2 h at 4°C. After washing with PBS three times, Microscint™ 40 (Packard) was added to each well, followed by scintillation counting using Top Count NXT™ (Perkin-Elmer). Open and closed symbols represent the specific binding obtained with control and alendronate-treated cells, respectively. Nonspecific binding assayed in the presence of 1000-fold excess amount of unlabeled TNFα was within 150–300 c.p.m. Each point indicates the mean±s.e. of six independent measurements.
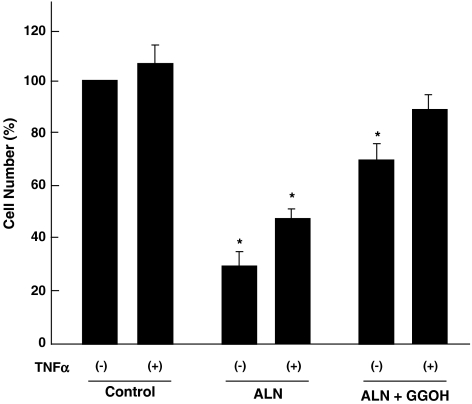
Cell death induced by alendronate
The results obtained indicate that alendronate caused cell death by inhibiting a cell survival pathway mediated by PI3K/Akt/NFκB. This was corroborated by counting viable cells after treatment with alendronate. As shown in Figure 6, alendronate caused a decrease in viable cells to about 30%, while TNFα at 5 ng ml−1 slightly, but not significantly, increased the number of viable cells. Alendronate was also effective in the presence of TNFα, but less so. A higher concentration of TNFα (1 μg ml−1) caused considerable cell death (data not shown), as suggested by its name and as seen in other cell types (Sandra et al., 2002; Bezzi et al., 2003).
Figure 6.
Experiments for cell death. MG-63 cells (30 × 104 cells) were cultured as described above, followed by starvation for 12 h, with or without TNFα at 5 ng ml−1. Alendronate (100 μM), with or without GGOH (20 μM), was then added for 24 h, either in the presence or absence of TNFα. Cells attached to dishes were collected and then viability was assessed using the Trypan blue exclusion test. *Indicates a significant difference from the control cells.
The effect of GGOH on alendronate activity with respect to cell survival and the inhibition of IκB phosphorylation
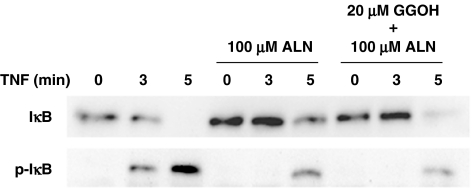
As described in the ‘Introduction', it has been suggested that amino-BP effects are exerted by inhibiting prenylation of the small G protein (Luckman et al., 1998). These effects are prevented by the addition of exogenous isoprenoid lipids, such as farnesol and GGOH which replenish the cytosolic isoprenoid substrate (Shipman et al., 1998; Benford et al., 1999; Fisher et al., 1999). Therefore, we examined the effects of exogenous addition of GGOH on cell viability, and the phosphorylation and degradation of IκB. As shown in Figure 6, GGOH at 20 μM partly rescued cells from the death induced by alendronate. Increasing the concentration of GGOH to 50 μM did not increase the extent of cell rescue. Since Benford et al. (1999) reported that the effect of GGOH on cell apoptosis required 48 h using a macrophage cell line, we also analyzed cell viability up to 48 h, but cell viability was not further improved. This indicated that the cell death induced by alendronate was partially, but not completely, caused by the inhibition of small G protein function probably through the inhibition of synthesis of farnesyldiphosphate or geranylgeranyldiphosphate. Figure 7 shows the effect of GGOH on the phosphorylation and degradation of IκB. TNFα induced the phosphorylation and degradation of IκB in a time-dependent manner, which was inhibited by alendronate as seen in Figure 1. GGOH did not modify the effect of alendronate, indicating that the effect of alendronate on the IκB pathway is mediated by a mechanism(s) other than the inhibition of synthesis of farnesyldiphosphate or geranylgeranyldiphosphate.
Figure 7.
The effect of geranylgeraniol on phosphorylation and degradation of IκB. MG-63 cells starved for 12 h were treated with alendronate with or without GGOH (20 μM) for 24 h, followed by stimulation with 5 ng ml−1 TNFα for 3 or 5 min. Cell lysates were immunoblotted as described above. The blot shown is typical of three experiments.
Discussion
BP are widely used for the treatment of patients who have bone diseases, such as osteoporosis, Paget's disease, hypercalcemia and metastatic cancer in bone (Russell & Rogers, 1999). One of the rationales for this usage is to inhibit bone resorption by inducing osteoclast apoptosis (Rodan & Fleisch, 1996; Coxon et al., 1998). Amino-BP such as alendronate inhibit the prenylation of small G proteins (Luckman et al., 1998), thus causing the inhibition of small G protein function. They also cause disruption of the cytoskeleton, and induction of osteoclast apoptosis. In addition to this application, there are several reports showing that alendronate is effective against cancer cells by inducing apoptosis in in vivo and in vitro experimental systems (Suri et al., 2001; Cheng et al., 2004), although the mechanisms for the anticancer action of alendronate are still unclear. Therefore, the present study was undertaken to clarify the mechanisms underlying the antitumor action of amino-BP, with special reference to the effect on the PI3K–Akt–NFκB cell survival pathway.
NFκB is composed of DNA-binding subunits (p50 and p52) and subunits with transcriptional activity (p65(RelA), RelB or c-Rel), which dimerize in various combinations. The primary form of NFκB is a heterodimer of the p50 and RelA subunits and is localized mainly in the cytoplasm in an inactive form bound to an inhibitory protein termed IκB (Siebenlist et al., 1994; Shao et al., 1999; Simeonidis et al., 1999). NFκB activation occurs via phosphorylation of IκB at Ser32 and Ser36, and Ser32 phosphorylation is essential for the release of active NFκB. NFκB is activated by stimulation of the IKK complex, which phosphorylates IκB. The IKK complex is composed of three subunits, IKKα, IKKβ and IKKγ (Ghosh & Karin, 2002). IKK phosphorylation by Akt is essential for NFκB activation (Li et al., 1999; Ozes et al., 1999). In the present study, we examined the effect of alendronate on this Akt–IκB–NFκB pathway, known to be one of the major cell survival pathways (Datta et al., 1999). We found that alendronate inhibited the production of PtdIns(3,4,5)P3, probably caused by the inhibition of PI3K activity and, therefore, the following processes including Akt/IκB/NFκB were also inhibited. In spite of almost the full inhibition of the production of PtdIns(3,4,5)P3, 100 μM alendronate only partially inhibited IKK activity and the following phosphorylation of IκB. IKK can be activated by phosphorylation by several kinases, that is, Akt might be just one of the enzymes involved in the phosphorylation of IKK (Datta et al., 1999). The IKK complex can be activated by various kinases such as NIK, MEKK1, Cot, NAK, MEKK3, PKCβ PKCδ and PKD (Lee et al., 1997; 1998; Malinin et al., 1997; Woronicz et al., 1997; Lin et al., 1998; 1999; 2000; Lallena et al., 1999; Storz & Toker, 2003). There are several reports that MEKK1 (one of MAPKKK) was phosphorylated by TNFα to activate IKK (Nemoto et al., 1998; Zhou et al., 2003). Therefore, we examined whether alendronate inhibited the MAPK pathway by analyzing the phosphorylation of MAPK in response to TNFα stimulation. Alendronate had no effect on the phosphorylation of p44/p42 MAPK, as assessed by a phospho-specific antibody to p44/p42 MAPK using the same cell extract as for a phospho-IκB antibody. Thus, mechanisms other than Akt activation, which are not affected by alendronate, are also involved in the activation of IKK. However, inhibition of PI3K–Akt could not be fully compensated by other pathways to activate IKK, and, therefore, the involvement of Akt in the activation of IKK–IκB–NFκB is physiologically relevant, indicating that this pathway is a pharmacologically relevant target.
As described above, alendronate is known to affect the functions of small G proteins, including Ras, by inhibiting isoprenylation, and thus their localization at the plasma membrane (Luckman et al., 1998). Ras is involved in the activation of PI3K (Rodriguez-Viciana et al., 1994; Mansell et al., 2001), suggesting the mechanism of action of alendronate. However, PI3K could be activated by a variety of mechanisms, including the recruitment of the PI3K holoenzyme through binding to tyrosine phosphorylated receptors and/or adaptor proteins to the SH2 domain in the p85–p55 regulatory subunit, an event which is not related to the function of Ras (Toker & Cantley, 1997; Wu et al., 2001; Sandra et al., 2002). Therefore, alendronate appears to influence additional mechanisms involved in the activation of PI3K, in which Ras does not play a part.
There are many reports that the exogenous addition of GGOH or farnesol protects a variety of cell types from apoptosis induced by amino-BP: myeloma cells (Shipman et al., 1998), Caco-2 cells (Suri et al., 2001), J774 cells (Benford et al., 1999) and osteoclasts (Fisher et al., 1999; Halasy-Nagy et al., 2001). Therefore, we examined the effect of GGOH on cell death, and the phosphorylation and degradation of IκB. GGOH partially prevented cell death, but showed little effect on the profile of IκB. These results clearly indicate that alendronate has a target other than that which inhibits the synthesis of farnesyldiphosphate or geranylgeranyldiphosphate, which would probably be PI3K, and the subsequent activation of Akt, IKK and NFκB.
Alendronate, at concentrations lower than 100 μM and in periods of treatment shorter than 12 h, had no effect on the phosphorylation of IκB. It has been reported that alendronate, at concentrations from 10−5 to 10−12 M, resulted in a significant increase in osteosarcoma cell proliferation, but at a concentration of 10−4 M inhibited cell proliferation (Plotkin et al., 1999; Im et al., 2004). It was shown that apoptosis of J774 cells occurred after 16 h of continuous treatment with 100 μM alendronate (Coxon et al., 1998). Thus, the conditions employed in this study with respect to the concentration and the duration of treatment appear to be ordinal, indicating that the events observed here are pharmacologically relevant.
Plotkin et al. (1999) reported that amino-BPs, etidronate, pamidronate, olpadronate and alendronate protect osteocytes and osteoblasts from apoptosis, probably through the early activation of extracellular signal-regulated kinases. However, the concentrations required were approximately three orders of magnitude lower than those required for the promotion of osteoclast apoptosis in vitro (Hughes et al., 1995) as well as that in the present study. Therefore, the antiapoptotic effect is most likely to be mediated by stimulation of receptors involved in cell growth pathways, which are shared by amino-BP.
Bezzi et al. (2003) reported that zoledronate, an amino-bisphosphonate also inhibited the TNFα-induced phosphorylation of Akt (referred to as PKB in the paper) in human umbilical vein endothelial cells, thus causing the cell death, consistent with the results obtained here. However, the inhibitory effect was seen in a sustained activation process at 6 h, but not in a short period up to 30 min.
In conclusion, alendronate inhibits the cell survival pathway stimulated by the PI3K/Akt/NFκB pathway by inhibiting the initial step, the activation of PI3K, thus causing apoptosis of osteosarcoma cells.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to N.M. and M.H.).
Abbreviations
- ALLN
N-acetyl-leucine-leucine-norleucine-CHO
- BP
bisphosphonate
- GGOH
geranylgeraniol
- IKK
IκB kinase
- IκB
inhibitor of NFκB
- NFκB
nuclear factor κB
- PI3K
phosphoinositide 3-kinase
- PtdIns(3,4,5)P3
phosphatidylinositol 3,4,5-trisphosphate
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- TNFα
tumor necrosis factor-α
References
- ALESSI D.R., ANDJELKOVIC M., CAUDWELL B., CRON P., MORRICE N., COHEN P., HEMMINGS B.A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- BENFORD H.L., FRITH J.C., AURIOLA S., MÖNKKÖNEN J., ROGERS M.J. Farnesol and geranylgeraniol prevent activation of caspases by aminobisphosphonates: biochemical evidence for two distinct pharmacological classes of bisphosphonate drugs. Mol. Pharmacol. 1999;56:131–140. doi: 10.1124/mol.56.1.131. [DOI] [PubMed] [Google Scholar]
- BEZZI M., HASMIM M., BIELER G., DORMOND O., RUEGG C. Zoledronate sensitizes endothelial cells to tumor necrosis factor-induced programmed cell death. J. Biol. Chem. 2003;278:43603–43614. doi: 10.1074/jbc.M308114200. [DOI] [PubMed] [Google Scholar]
- BINDERMAN I., ADULT M., YAFFE A. Effectiveness of local delivery of alendronate in reducing alveolar bone loss following periodontal surgery in rats. Periodontology. 2000;71:1236–1240. doi: 10.1902/jop.2000.71.8.1236. [DOI] [PubMed] [Google Scholar]
- CHENG Y.Y., HUANG L., LEE K.M., XU J.K., ZHENG M.H., KUMTA S.M. Bisphosphonates induce apoptosis of stromal tumor cells in giant cell tumor of bone. Calcif. Tissue Int. 2004;75:71–77. doi: 10.1007/s00223-004-0120-2. [DOI] [PubMed] [Google Scholar]
- COXON F.P., BENFORD H.L., RUSSELL R.G., ROGERS M. Protein synthesis is required for caspase activation and induction of apoptosis by bisphosphonate drugs. Mol. Pharmacol. 1998;54:631–638. [PubMed] [Google Scholar]
- DATTA S.R., BRUNET A., GREENBERG M.E. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- EBETINO F.H., FRANCIS M.D., ROGERS M.J., RUSSELL R.G.G. Mechamisms of action of etidoronate and other bisphosphonates. Rev. Contemp. Pharmacother. 1998;9:233–243. [Google Scholar]
- FIRTH J.C., MÖNKKÖNEN J., BLACKBURN G.M., RUSSELL R.G., ROGERS M.J. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5′-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. J. Bone Miner. Res. 1997;12:1358–1367. doi: 10.1359/jbmr.1997.12.9.1358. [DOI] [PubMed] [Google Scholar]
- FISHER J.E., ROGERS M.J., HALASY J.M., LUCKMAN S.P., HUGHES D.E., MASARACHIA P.J., WESOLOWSKI G., RUSSELL R.G., RODAN G.A., RESZKA A.A. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc. Natl. Acad. Sci. U.S.A. 1999;96:133–138. doi: 10.1073/pnas.96.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOO S.Y., NOLAN G.P. NF-κB to the rescue: RELs, apoptosis and cellular transformation. Trends Genet. 1999;15:229–235. doi: 10.1016/s0168-9525(99)01719-9. [DOI] [PubMed] [Google Scholar]
- GHOSH S., KARIN M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- HALASY-NAGY J.M., RODAN G.A., REZUKA A.A. Inhibition of bone resorption by alendronate and risedronate dose not require osteoclast apoptosis. Bone. 2001;29:553–559. doi: 10.1016/s8756-3282(01)00615-9. [DOI] [PubMed] [Google Scholar]
- HUGHES D.E., WRIGHT K.R., UY H.L., SASAKI A., YONEDA T., ROODMAN G.D., MUNDY G.R., BOYCE B.F. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Miner. Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- IM G., QURESHI S.A., KENNY J., RUBASH H.E., SHANBHAG A.S. Osteoblast proliferation and maturation by bisphosphonate. Biomaterials. 2004;25:4105–4115. doi: 10.1016/j.biomaterials.2003.11.024. [DOI] [PubMed] [Google Scholar]
- JAGDEV S.P., COLEMAN R.E., SHIPMAN C.M., ROSTAMI-H A., CROUCHER P.I. The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: evidence for synergy with paclitaxel. Br. J. Cancer. 2001;84:1126–1134. doi: 10.1054/bjoc.2001.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARIN M., BEN-NERIAH Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- KAYNAK D., MEFFERT R., BOSTANCI H., GUNHAN O., OZKAYA O.G. A histopathological investigation on the effect of systemic administration of the bisphosphonate alendronate on resorptive phase following mucoperiosteal flap surgery in the rat mandible. J. Periodontol. 2003;74:1348–1354. doi: 10.1902/jop.2003.74.9.1348. [DOI] [PubMed] [Google Scholar]
- LALLENA M.J., DIAZ-MECO M.T., BREN G., PAYA C.V., MOSCAT J. Activation of IkappaB kinase beta by protein kinase C isoforms. Mol. Cell. Biol. 1999;19:2180–2188. doi: 10.1128/mcb.19.3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE F.S., HAGLER J., CHEN Z.J., MANIATIS T. Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- LEE F.S., PETERS R.T., DANG L.C., MANIATIS T. MEKK1 activates both IkappaB kinase alpha and IkappaB kinase beta. Proc. Natl. Acad. Sci. U.S.A. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Z.W., CHU W., HU Y., DELHASE M., DEERINCK T., ELLISMAN M., JOHNSON R., KARIN M. The IKK subunit of IκB kinase (IKK) is essential for nuclear factor B activation and prevention of apoptosis. J. Exp. Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN X., CUNNINGHAM E.T., JR, MU Y., GELEZIUNAS R., GREENE W.C. The proto-oncogene Cot kinase participates in CD3/CD28 induction of NF-kappaB acting through the NF-kappaB-inducing kinase and IkappaB kinases. Immunity. 1999;10:271–280. doi: 10.1016/s1074-7613(00)80027-8. [DOI] [PubMed] [Google Scholar]
- LIN X., MU Y., CUNNINGHAM JR E.T., MARCU K.B., GELEZIUNAS R., GREENE W.C. Molecular determinants of NF-kappaB-inducing kinase action. Mol. Cell. Biol. 1998;18:5899–5907. doi: 10.1128/mcb.18.10.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN X., O'MAHONY A., MU Y., GELEZIUNAS R., GREENE W.C. Protein kinase C-theta participates in NF-kappaB activation induced by CD3-CD28 costimulation through selective activation of IkappaB kinase beta. Mol. Cell. Biol. 2000;20:2933–2940. doi: 10.1128/mcb.20.8.2933-2940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCKMAN S.P., HUGHES D.E., COXON F.P., GRAHAM R., RUSSELL G., ROGERS M.J. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J. Bone Miner. Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- MALININ N.L., BOLDIN M.P., KOVALENKO A.V., WALLACH D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- MANSELL A., KHELEF N., COSSART P., O'NEILL L.A.J. Internalin B activates nuclear factor-κB via Ras, phosphoinositide 3-kinase, and Akt. J. Biol. Chem. 2001;276:43597–43603. doi: 10.1074/jbc.M105202200. [DOI] [PubMed] [Google Scholar]
- NEMOTO S., DIDONATO J.A., LIN A. Coordinate regulation of IkappaB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-kappaB-inducing kinase. Mol. Cell. Biol. 1998;18:7336–7343. doi: 10.1128/mcb.18.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZES O.N., MAYO L.D., GUSTIN J.A., PFEFFER S.R., PFEFFER L.M., DONNER D.B. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- PLOTKIN L.I., WEINSTEIN R.S., PARFITT A.M., ROBERSON P.K., MAMOLAGAS S.C., BELLIDO T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J. Clin. Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODAN G.A., FLEISCH H.A. Bisphosphonates: mechanisms of action. J. Clin. Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ-VICIANA P., WARNE P.H., DHAND R., VANHAESEBROECK B., GOUT I., FRY M.J., WATERFIELD M.D., DOWNWARD J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- ROTHWARF D.M., ZANDI E., NATOLI G., KARIN M. IKK-γ is an essential regulatory subunit of the IkB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- RUSSELL R.G.G., ROGERS M.J. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25:97–106. doi: 10.1016/s8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- SANDRA F., MATSUKI N.A., TAKEUCHI H., IKEBE T., KANEMATSU T., OHISHI M., HIRATA M. TNF inhibited the apoptosis by activation of Akt serine/threonine kinase in the human head and neck squamous cell carcinoma. Cell Signal. 2002;14:771–778. doi: 10.1016/s0898-6568(02)00025-6. [DOI] [PubMed] [Google Scholar]
- SATO M., GRASSER W. Effects of bisphosphonates on isolated rat osteoclasts as examined by reflected light microscopy. J. Bone Miner. Res. 1990;5:31–40. doi: 10.1002/jbmr.5650050107. [DOI] [PubMed] [Google Scholar]
- SCHMIDT A., RUTLEDGE S.J., ENDO N., OPAS E.E., TANAKA H., WESOLOWSKI G., LEU Z., HUANG C.T., RAMACHANDARAN C., RODAN S.B., RODAN G.A. Protein-tyrosine phosphatase activity regulates osteoclast formation and function: inhibition by alendronate. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3068–3073. doi: 10.1073/pnas.93.7.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAO R., HU M.C., ZHOU B.P., LIN S.Y., CHIAO P.J., VON LINDERN R.H., SPOHN B., HUNG M.C. E1A sensitizes cells to tumor necrosis factor-induced apoptosis through inhibition of IkappaB kinases and nuclear factor kappaB activities. J. Biol. Chem. 1999;274:21495–21498. doi: 10.1074/jbc.274.31.21495. [DOI] [PubMed] [Google Scholar]
- SHIPMAN C.M., CROUCHER P.I., RUSSELL R.G., HELFRICH M.H., ROGERS M.J. The bisphosphonate incadronate (YM175) causes apoptosis of human myeloma cells in vitro by inhibiting the mevalonate pathway. Cancer Res. 1998;58:5294–5297. [PubMed] [Google Scholar]
- SIEBENLIST U., FRANZOSO G., BROWN R. Structure, regulation and function of NF-kappa B. Annu. Rev. Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- SIMEONIDIS S., STAUBER D., CHEN G., HENDRICSON W.A., THANOS D. Mechanisms by which IkappaB protein control NF-kappaB activity. Proc. Natl. Acad. Sci. U.S.A. 1999;96:49–54. doi: 10.1073/pnas.96.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STORZ P., TOKER A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J. 2003;22:109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUBHA P., KUNDU G.C. Osteopontin induces nuclear factor kappa B-madiated promatrix metalloproteinase-2 activation through I kappa B alpha/IKK signaling pathways, and curcumim (diferulolylmethane) down-regulates these pathways. J. Biol. Chem. 2003;278:14487–14497. doi: 10.1074/jbc.M207309200. [DOI] [PubMed] [Google Scholar]
- SURI S., MÖNKKÖNEN J., TASKINEN M., PESONEN J., BLANK M.A., PHIPPS R.J., ROGERS M.J. Nitrogen-containing bisphosphonates induce apoptosis of Caco-2 cells in vitro by inhibiting the mevalonate pathway: a model of bisphosphonate-induced gastrointestinal toxicity. Bone. 2001;29:336–343. doi: 10.1016/s8756-3282(01)00589-0. [DOI] [PubMed] [Google Scholar]
- TOKER A., CANTLEY L.C. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- VAN DER POEST CLEMENT E., VAN ENGELAND M., ADER H., ROOS J.C., PATKA P., LIPS P. Alendronate in the prevention of bone loss after a fracture of the lower leg. J. Bone Miner. Res. 2002;17:2247–2255. doi: 10.1359/jbmr.2002.17.12.2247. [DOI] [PubMed] [Google Scholar]
- WORONICZ J.D., GAO X., CAO Z., ROTHE M., GOEDDEL D.V. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- WU C.J., O'ROURKE D.M., FENG G.S., JOHNSON G.R., WANG Q., GREENE M.I. The tyrosine phosphatase SHP-2 is required for mediating phosphatidylinositol 3-kinase/Akt activation by growth factors. Oncogene. 2001;20:6018–6025. doi: 10.1038/sj.onc.1204699. [DOI] [PubMed] [Google Scholar]
- ZHOU L., TAN A., IASVOVSKAIA S., LI J., LIN A., HERSHENSON M.B. Ras and mitogen-activated protein kinase kinase kinase-1 coregulate activator protein-1- and nuclear factor-kappaB-mediated gene expression in airway epithelial cells. Am. J. Resp. Cell Mol. Biol. 2003;28:762–769. doi: 10.1165/rcmb.2002-0261OC. [DOI] [PubMed] [Google Scholar]