Abstract
We evaluated a potential role for proteinase-activated receptor 4 (PAR4) in a rodent paw inflammation model, with a focus on two main features of inflammation: (1) oedema and (2) granulocyte recruitment.
A PAR4 antagonist (Pepducin P4pal-10; palmitoyl-SGRRYGHALR-NH2) reduced both the oedema and granulocyte recruitment induced by a localized administration of carrageenan in the rat hind paw, pointing to a key role for PAR4 in this inflammation model.
Further, intraplantar injection in the mouse hind paw of a PAR4 agonist (AYPGKF-NH2), but not its standard PAR4-inactive peptide control (YAPGKF-NH2), caused an inflammatory reaction characterized by oedema (increased paw thickness) and granulocyte recruitment (increased paw myeloperoxidase activity). The PAR4 agonist-induced effects were inhibited in mice pretreated with pepducin P4pal10.
These PAR4 agonist-mediated effects were not affected by pretreatment with inhibitors of either NO production or prostaglandin release (L-NAME and indomethacin, respectively).
However, selective immuno-depletion of neutrophils significantly reduced PAR4 agonist-induced oedema formation.
Moreover, AYPGKF-NH2-induced oedema was also reduced by pretreatment with either a kinin B2 receptor antagonist (icatibant) or a tissue or plasma kallikrein inhibitor (FE999024 and FE999026, respectively), but not with a kinin B1 receptor antagonist (SSR240612).
We conclude: (1) that PAR4 plays an important role in the inflammatory response as it mediates some of the hallmarks of inflammation and (2) that PAR4-mediated oedema is dependent on the recruitment of neutrophils and components of the kallikrein–kinin system.
Keywords: Proteinase-activated receptors, inflammation, oedema, neutrophils, kallikrein, kinin receptors, mouse
Introduction
Proteinase-activated receptors (PARs) are members of the G protein-coupled receptor superfamily. They are characterized by their unique mechanism of activation involving serine proteinases such as thrombin and trypsin. A specific proteolytic cleavage of the amino terminal sequence unmasks a new amino terminal sequence consisting of a tethered ligand that binds to and activates the receptor (Hollenberg & Compton, 2002). To date, four PARs have been cloned. They are numbered 1–4 according to their order of discovery, with PAR4 being the most recent member added to the PAR family (Vu et al., 1991; Nystedt et al., 1995; Ishihara et al., 1997; Kahn et al., 1998; Xu et al., 1998). PARs 1, 3 and 4 were first recognized as targets for thrombin whereas PAR2 was seen as a trypsin receptor. However, this view has changed in the past years with the demonstration that PARs can be activated by other serine proteinases and that the same proteinase can activate more than one PAR (Ossovskaya & Bunnett, 2004). For example, PAR4 is now known to be activated by thrombin, trypsin, the activated factor X of the coagulation cascade, cathepsin G and trypsin IV (Kahn et al., 1998; Xu et al., 1998; Camerer et al., 2000; Sambrano et al., 2000; Cottrell et al., 2004). Interestingly, with the exception of PAR3, PARs can also be activated by short synthetic peptides of 5 or 6 amino acids that mimic the tethered ligand (Hollenberg & Compton, 2002). Receptor-selective PAR-activating peptides (PAR-APs) have proved of considerable value to uncover the physiological and pathophysiological roles played by these novel receptors. The PAR4-AP, AYPGKF-NH2 (AYP), which was designed based on the mouse PAR4-tethered ligand sequence, is a good example of such experimentally useful synthetic peptides (Faruqi et al., 2000; Hollenberg et al., 2004). This selective PAR4 agonist does not affect either PAR1 or PAR2 (Hollenberg & Compton, 2002).
Besides its role in thrombin-induced platelet aggregation, the potential physiological or pathophysiological roles for PAR4 are still essentially unknown. PAR4 was first described as an important thrombin receptor for the aggregation of both human and mouse platelets (Kahn et al., 1998). Mice lacking the PAR4 gene show an increased bleeding time, emphasizing the importance of PAR4 signalling for coagulation events in vivo (Sambrano et al., 2001). Studies carried out in vitro suggest a role for PAR4 in gut motor function or as a signal for the release of inflammatory mediators such as cytokines or prostaglandins (Asokananthan et al., 2002; Mule et al., 2004). It has been proposed that PAR4 is the likely candidate to mediate thrombin-induced leukocyte rolling and adherence in rat mesenteric venules, as monitored by intravital microscopy (Vergnolle et al., 2002). In that work, the selective PAR4-AP, AYPGKF-NH2, but not the selective PAR1-AP, reproduced the proinflammatory effects of thrombin (Vergnolle et al., 2002). The mechanism(s) by which PAR4 recruits leukocytes to the endothelium is unknown, but PAR4 has been detected by immunohistochemistry at the surface of rat neutrophils as well as in the endothelial and smooth muscle cells of the aorta (Vergnolle et al., 2002). Further evidence for the presence of functional PAR4 on endothelial cells of different origins has also been reported (Kataoka et al., 2003). Moreover, recently published preliminary results show that the PAR4-AP, AYPGKF-NH2, can mediate the formation of oedema in a rat paw, independent of neuropeptide release from sensory neurons and of mast cell degranulation (Hollenberg et al., 2004).
Taken together, these observations strongly support a possible involvement of PAR4 in inflammation. Therefore, we sought to investigate the role and mechanisms of action of PAR4 in the inflammatory response by using a rodent paw inflammation model. This model, which has been extensively used to characterize the inflammatory response mediated by the activation of either PAR1 or PAR2 (Vergnolle et al., 1999a, 1999b; Steinhoff et al., 2000; de Garavilla et al., 2001), allowed us to focus on two hallmarks of inflammation at the same time: oedema and granulocyte recruitment. Here, we investigated (1) the potential role for PAR4 activation in the generation of carrageenan-induced inflammation, and (2) the involvement of nitric oxide, prostaglandins, neutrophils and the kallikrein–kinin system in PAR4-mediated inflammation.
Methods
Animals
Male C57bl/6 mice (4–6 weeks old; 20–25 g) and male Wistar rats (200–250 g) were obtained from Charles River Laboratories (Montréal, Québec, Canada). The rodents had free access to food and water and were housed under constant temperature (22°C) and photoperiod (12 h light–dark cycle). All experimental procedures were approved by the Animal Care Committee of the University of Calgary and were performed in accordance with the guidelines established by the Canadian Council on Animal Care.
Paw oedema assay
All paw oedema assays reported in this paper were performed by following the general guidelines described below. Prior to the administration of the PAR4-AP, a basal measurement of the paw thickness of each mouse was recorded using an electronic calliper (Fisher Scientific, Hampton, NH, U.S.A.). For the carrageenan-induced inflammation, the rat paw volume, rather than its thickness, was measured by using a hydroplethismometer (Ugo Basile, Milan, Italy). The compounds to be tested or the vehicle were then administered by intraplantar (i.pl.) injection (n=5–9 rodent per group for all experiments). These injections were performed under light halothane anaesthesia for the rats. Each compound injected into the paw, in a final volume of 10 μl per mouse paw or 100 μl per rat paw, was diluted in sterile saline (0.9% NaCl). As an index of oedema formation, paw thickness or volume was then measured every hour for 6 h after the injection.
Experiments were designed to determine the involvement and potential mechanisms of action of PAR4 in inflammation. In the first experimental series, rats were pretreated or not with the PAR4 antagonist, pepducin palmitoyl-SGRRYGHALR-NH2 (P4pal10; (Covic et al., 2002); 0.5 mg kg−1, i.p.), 1 h prior to the i.pl. injection of carrageenan (2% in saline). Carrageenan was used to induce a general inflammatory reaction. In the second experimental series, mice received i.pl. administration of the PAR4-AP (AYPGKF-NH2; 50 μg), the standard PAR4-inactive control peptide (YAPGKF-NH2; 50 μg) or the vehicle (saline) to determine the inflammatory effects that could be mediated by a direct activation of PAR4. In some groups, mice were pretreated with pepducin P4pal10 (same as above), Nω-nitro-L-arginine methyl ester (L-NAME; 25 mg kg−1 diluted in saline, i.p. injection 1 h before i.pl. injections), indomethacin (5 mg kg−1 diluted in 2% carboxymethylcellulose, given orally 1 h before i.pl. injections), rat anti-mouse Ly-6G antibody clone RB6-8C5 or rat IgG2bκ control antibody (Hestdal et al., 1991; 125 μg diluted in saline, i.p. injection 18 h before i.pl. injections), FE999024 or FE999026 (((2S,2R)-2-(2′-amino-3′-(4″chlorophenyl)propanoyl-amino-N-(3-guanidinopropyl)-3-(1-naphthyl)propanoamide or ((2′S,2″R)-4-(2′(2″-(carboxymethylamino)-3″-cyclohexyl-propanoylamino)-3′-phenyl-propanoylamino)piperidine-1-carboxyamidine), respectively; (Evans et al., 1996a, 1996b); 60 μmol kg−1 diluted in saline, i.p. injection 1 h before i.pl. injections), [(2R)-2-[((3R)-3-(1,3-benzodioxol-5-yl)-3-{[(6-methoxy-2-naphthyl)sulfonyl]amino}propanoyl)amino]-3-(4-{[(2R,6S)-2,6-dimethylpiperidinyl]methyl}phenyl)-N-isopropyl-N-methylpropanamide hydrochloride] (SSR240612); (Gougat et al., 2004); 10 mg kg−1 diluted in saline, i.p. injection 1 h before i.pl. injections) or icatibant (Hock et al., 1991; 50 μg kg−1 diluted in saline, coinjection in the paw at t=0 h). Except for icatibant, which was injected in the mouse paws in a final volume of 10 μl at the same time as AYPGKF-NH2, all other inhibitors or antagonists were administered in a final volume of 200 μl. The control groups always received a similar pretreatment with the corresponding vehicle.
Myeloperoxidase activity assay
Tissue myeloperoxidase activity assay was performed as an index of granulocyte recruitment at the end of each experiment, as previously described (Steinhoff et al., 2000; Asfaha et al., 2002). Briefly, the injected paws were cut and weighed prior to their homogenization in a 0.5% hexadecyltrimethylammonium bromide phosphate buffered (pH 6.0) solution using a polytron PT10-35 homogenizer (Kinematica, Lucerne, Switzerland). The homogenates were then centrifuged at 13,000 × g for 3 min at 4°C in a microcentrifuge. Five aliquots of each supernatant were then transferred into 96-well plates before the addition of a solution containing 3,3′-dimethoxybenzidine and 1% hydrogen peroxide. In parallel, a number of standard dilutions of pure myeloperoxidase were also tested for their activity to construct a standard curve (OD as a function of units of enzyme activity). Optical density readings at 450 nm were taken at 1 min (which corresponds to the linear portion of the enzymatic reaction) using a Spectra Max Plus plate reader linked to the SOFTmax Pro 3.0 software (Molecular Devices Corp., Sunnyvale, CA, U.S.A.). The myeloperoxidase activity found in the paws was expressed as units of enzyme per milligrams of tissue.
Calcium-signalling assay
Calcium signalling was measured as described previously (Compton et al., 2001). Kirsten virus sarcoma-transformed rat kidney cells (KNRK cells) were grown to near confluence in 75 cm2 culture flasks in Dulbecco's modified Eagle medium (DMEM) containing 10% FBS and 1% antibiotics. Cells were harvested using a nonenzymatic dissociation buffer. Harvested KNRK cells were then incubated in 1 ml of DMEM containing 10% FBS, 0.25 mM sulphinpyrazone and 22 μM Fluo-3 acetoxymethyl ester (Molecular Probes Inc., Eugene, OR, U.S.A.) for 25 min at room temperature with gentle shaking. Cells were then washed and resuspended in calcium assay buffer (mM: NaCl 150, KCl 3, CaCl2 1.5, glucose 10, HEPES 20, sulphinpyrazone 0.25, pH 7.4). Fluorescence measurements were performed on a Perkin-Elmer fluorescence spectrometer 650-10S, with an excitation wavelength of 480 nm and emission recorded at 530 nm. Cell suspensions (2 ml) in 4 ml cuvettes were stirred with a magnetic flea bar and maintained at 24°C. The signal produced (E530) by the addition of a test agonist was measured as a percentage of the fluorescence peak height yielded by the addition of 2 μM calcium ionophore (A23187).
Chemicals
All peptides (AYPGKF-NH2; YAPGKF-NH2; P4pal10, pepducin P4pal10, (Covic et al., 2002)) were obtained from the Peptide Synthesis Facility of the University of Calgary (Calgary, Alberta, Canada; peplab@ucalgary.ca, Dr Dennis McMaster, Director). The antibody used to deplete the mice of their granulocytes (anti-mouse Ly-6G antibody clone RB6-8C5; (Hestdal et al., 1991)) and the control antibody (rat IgG2bκ antibody) were purchased from eBioscience (San Diego, CA, U.S.A.). The tissue and plasma kallikrein inhibitors (FE999024 and FE999026, respectively; also known as CH-2856 and CH-4215, respectively; Evans et al., 1996a, 1996b) were a kind gift from Dr D. Michael Evans (Ferring Research Ltd, Southampton, U.K.) and the nonpeptidic kinin B1 receptor antagonist, SSR240612 (Gougat et al., 2004), was a kind gift from Dr Denis Riochet (Sanofi-Synthelabo Recherche, France). The myeloperoxidase, isolated from human neutrophils and used as a standard, was obtained from EMD Biosciences Inc. (San Diego, CA, U.S.A.). All other drugs and reagents were purchased from Sigma-Aldrich (St Louis, MO, U.S.A.), most notably L-NAME (the nonselective nitric oxide synthase inhibitor), indomethacin (the nonselective cyclooxygenase inhibitor), icatibant (the specific B2 kinin receptor antagonist; (Hock et al., 1991)), carrageenan and calcium ionophore (A23187).
Statistical analysis
All results are reported as mean±s.e.m. They were analyzed using the Instat 3.0 statistics software (GraphPad Software, San Diego, CA, U.S.A.). Comparisons among groups were performed using the paired ANOVA test for repeated measures followed by the parametric Dunnett's test. For all statistical analysis, an associated probability (P-value) of less than 5% was considered significant.
Results
Role for PAR4 activation in inflammatory processes
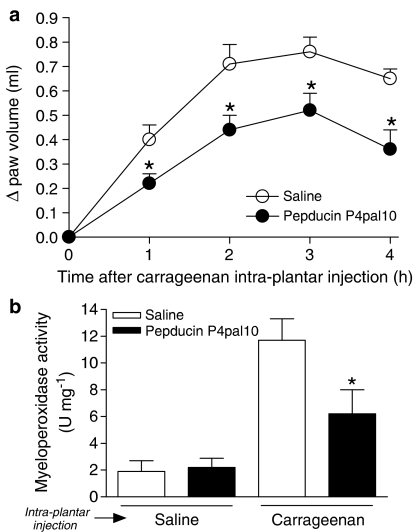
In order to determine if PAR4 participates in the inflammatory response, we studied the effects of the PAR4 antagonist, pepducin P4pal10 (0.5 mg kg−1), in a rat model of paw inflammation (i.pl. injection of 2% carrageenan in saline) classically used to test anti-inflammatory drugs. It has previously been demonstrated that this dose of pepducin P4pal10 can prevent the thrombin-mediated aggregation of mouse platelets in vivo (Covic et al., 2002). As expected (Levy, 1969), carrageenan provoked the formation of a substantial oedema over a 4 h period as well as the recruitment of granulocytes (Figure 1). Interestingly, the pepducin P4pal10 treatment significantly reduced both the oedema (Figure 1a) and the granulocyte infiltration (Figure 1b) induced by carrageenan. These results pointed strongly to the involvement of PAR4 in the carrageenan-induced inflammatory response.
Figure 1.
Effect of a PAR4 antagonist on a general inflammatory reaction induced by carrageenan in the rat paw. Pepducin P4pal10 (0.5 mg kg−1)-dependent reduction of the carrageenan (200 μg)-mediated oedema formation (a) and granulocyte recruitment (b). Saline was used as a control for both the pepducin P4pal10 and carrageenan injections. Values are mean±s.e.m. of n=8 per group. *Significantly different from the vehicle+carrageenan group, P<0.05.
Inflammation induced by PAR4 activation
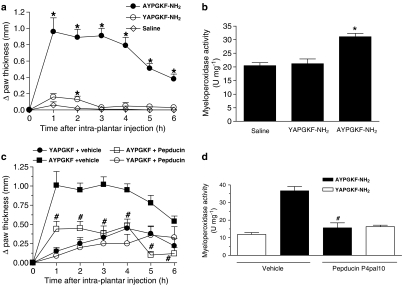
Next, we evaluated the ability of a direct activation of PAR4 to induce an inflammatory response. In this experiment, we used AYPGKF-NH2 as the PAR4 agonist and YAPGKF-NH2 as a standard PAR4-inactive control peptide. This choice of peptides was based on a structure–activity relationship study showing that this combination of receptor probes is optimal to study the functions of PAR4 in vivo (Hollenberg et al., 2004). Here, injection of AYPGKF-NH2 (50 μg) into the mouse paw caused a rapid oedema (statistically significant at less than 1 h) that lasted for 6 h (Figure 2a and c) and a substantial granulocyte recruitment (Figure 2b and d) when compared to the control peptide YAPGKF-NH2 (50 μg) or the saline vehicle. These two inflammatory events were markedly attenuated in mice pretreated with the PAR4 antagonist, pepducin P4pal10, supporting the hypothesis that the peptide AYPGKF-NH2 acts through PAR4. For both parameters (oedema and granulocyte recruitment), the standard PAR4-inactive control peptide had little or no effect when compared to the saline vehicle (Figure 2a and b). On occasion, the control peptide YAPGKF-NH2 induced a slightly greater response than saline, a response which appeared to be independent of PAR4 in view of the lack of effect of pepducin P4pal10 pretreatment (Figure 2c and d). These observations showed that the PAR4-AP induces two important hallmarks of inflammation: oedema and granulocyte recruitment.
Figure 2.
Direct inflammatory effects of PAR4 activation in the mouse paw. The oedema formation (a) and granulocyte recruitment (b) were evaluated following an intraplantar injection of the PAR4-AP, AYPGKF-NH2 (50 μg), the control inactive peptide, YAPGKF-NH2 (50 μg), or the saline vehicle. The effect of a pepducin P4pal10 (0.25 mg kg−1) pretreatment was also evaluated against the oedema formation (c) and granulocyte recruitment (d) induced by AYPGKF-NH2 (50 μg) and YAPGKF-NH2 (50 μg). Values are mean±s.e.m. of n=5–8 per group. *Significantly different from the saline vehicle or the control peptide group (a and b), P<0.05; #significantly different from the vehicle+AYPGKF-NH2 group (c and d), P<0.05.
Nitric oxide and prostanoids are not involved in PAR4-induced inflammation
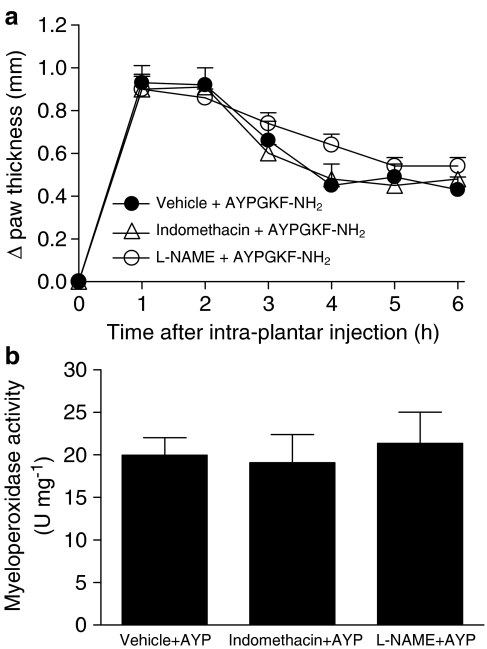
Since NO and prostaglandins are two major inflammatory mediators (Ushikubi et al., 2000; Guzik et al., 2003), we evaluated their respective contribution to the PAR4-mediated proinflammatory effects. Mice were pretreated with either L-NAME (a nonselective inhibitor of nitric oxide synthases; 25 mg kg−1; Vergnolle et al., 1999a, 1999b) or indomethacin (a nonspecific inhibitor of cyclooxygenases; 5 mg kg−1; Vergnolle et al., 1999a, 1999b) prior to the i.pl. administration of AYPGKF-NH2. Neither of these two inhibitors affected oedema formation (Figure 3a) or granulocyte recruitment (Figure 3b) induced by PAR4 activation.
Figure 3.
Noninvolvement of NO and prostanoids in PAR4-mediated oedema formation and granulocyte recruitment in the mouse paw. Effect of indomethacin (cyclooxygenases inhibitor, 5 mg kg−1) or L-NAME (nitric oxide synthases inhibitor, 25 mg kg−1) on oedema formation (a) and on granulocyte recruitment (b) observed following PAR4 activation with AYPGKF-NH2 (50 μg). Values are mean±s.e.m. of n=8–9 per group. No statistically significant difference was observed between groups.
Contribution of neutrophils to PAR4-induced oedema
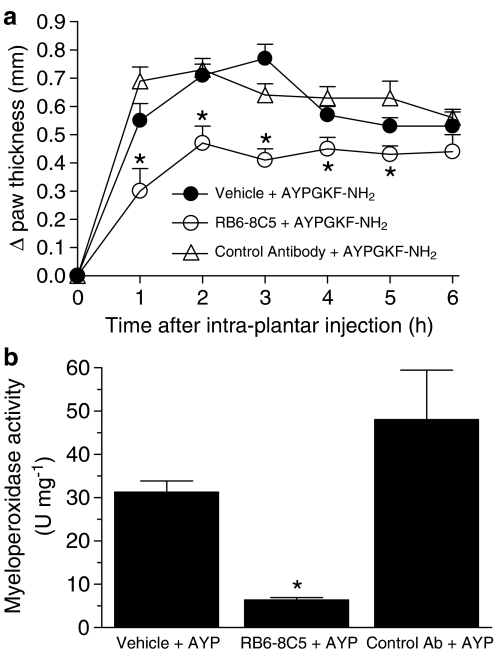
Neutrophils are considered a major component of the innate immune system due to their scavenging roles and because of the many proinflammatory mediators they can release (Scapini et al., 2000). In order to determine if neutrophil recruitment following the activation of PAR4 might participate in oedema formation, we pretreated groups of mice with a granulocyte-depleting antibody (rat anti-mouse Ly-6G antibody clone RB6-8C5, 125 μg; a dose that has previously shown to deplete mice from their circulating neutrophils by 95%; (Bonder et al., 2004)), a control antibody (rat IgG2bκ control antibody; 125 μg) or the vehicle before the i.pl. administration of AYPGKF-NH2. The marked decrease in myeloperoxidase activity reflected a dramatic reduction in the migration of granulocytes into the paw (Figure 4b). Further, neutrophil-depleted mice showed a significant reduction of the PAR4-induced oedema throughout the first 5 h compared to control antibody-injected mice (Figure 4a). These results suggest that PAR4-recruited neutrophils play an important role in the formation of oedema, possibly by releasing or generating inflammatory factors.
Figure 4.
Role for neutrophils in the PAR4-mediated oedema formation in the mouse paw. A granulocyte-depleting antibody (anti-mouse Ly-6G antibody clone RB6-8C5, 125 μg) causes a marked reduction of myeloperoxidase, indicative of neutropenia (b) as well as reducing the oedema (a) mediated by AYPGKF-NH2 (50 μg) whereas a control antibody (rat IgG2bκ antibody, 125 μg) does not. Values are mean±s.e.m. of n=8 per group. *Significantly different from the vehicle+AYPGKF-NH2 group, P<0.05.
Contribution of the kallikrein–kinin system in PAR4-induced oedema
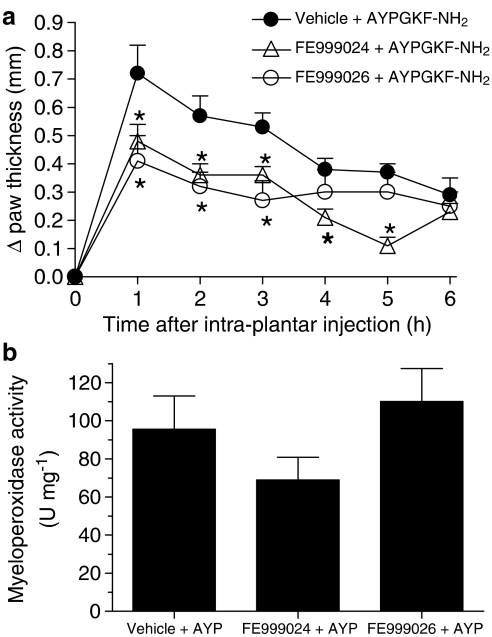
To study further the mechanisms of PAR4-induced inflammation, we hypothesized that PAR4 activation can trigger kallikrein activity, which in turn might participate in the formation of oedema by producing active kinins. To test this hypothesis, we pretreated groups of mice with inhibitors of either tissue or plasma kallikreins (FE999024 and FE999026, respectively; 60 μmol kg−1) before the i.pl. administration of AYPGKF-NH2. The doses administered of these inhibitors have been shown to be highly selective for their respective kallikrein target in vitro as well as in vivo in a rat model of acute pancreatitis (Griesbacher et al., 2002). The tissue kallikrein inhibitor, FE999024, significantly reduced the PAR4-AP-induced oedema throughout the first 5 h, whereas the plasma kallikrein inhibitor, FE999026, was effective in reducing oedema for only the first 3 h (Figure 5a). The data suggest that both kallikreins can play distinct roles in PAR4-induced oedema.
Figure 5.
Role for the tissue and plasma kallikreins in PAR4-mediated oedema formation in the mouse paw. Effect of FE999024 (tissue kallikrein inhibitor, 60 μmol kg−1) or FE999026 (plasma kallikrein inhibitor, 60 μmol kg−1) on the oedema formation (a) and granulocyte recruitment (b) observed following PAR4 activation with AYPGKF-NH2 (50 μg). Values are mean±s.e.m. of n=8 per group. *Significantly different from the vehicle+AYPGKF-NH2 group, P<0.05.
Since kallikreins are responsible for the release of active kinins, we next investigated a possible role for activation of the two known kinin receptors (the inducible B1 and the constitutive B2; (Marceau et al., 1998) in PAR4-induced oedema. We observed that pretreatment of mice with the kinin B1 receptor antagonist (SSR240612; 10 mg kg−1; a concentration known to inhibit the kinin B1 receptor-mediated oedema in the mouse paw; (Gougat et al., 2004) had no effect on PAR4-induced oedema (Figure 6a). In contrast, mice pretreated with the B2 receptor antagonist (icatibant; 50 μg kg−1; (Decarie et al., 1996) showed a reduced oedema in response to the PAR4 agonist at all of the observed time points (Figure 6b). These results demonstrated the involvement of the kinin B2 (but not the B1) receptor in PAR4-induced oedema.
Figure 6.
Role for the kinin B2 receptor, but not the B1, in PAR4-mediated oedema formation in the mouse paw. Effect of SSR240612 (kinin B1 receptor antagonist, 10 mg kg−1) or of icatibant (kinin B2 receptor antagonist, 50 μg kg−1) on the oedema formation (a and b, respectively) and granulocyte recruitment (c) observed following PAR4 activation with AYPGKF-NH2 (50 μg). Values are mean±s.e.m. of n=8 per group. *Significantly different from the vehicle+AYPGKF-NH2 group, P<0.05; #significantly different from either the vehicle+saline or the icatibant+saline groups, P<0.05.
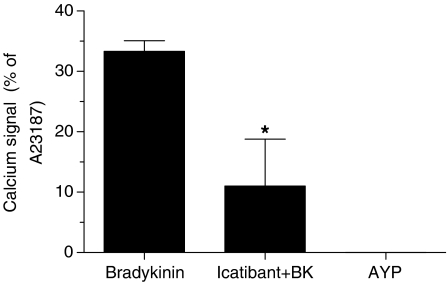
We have also evaluated the possibility that the PAR4-AP, AYPGKF-NH2, could activate directly the B2 receptor. To test this hypothesis, we have performed a calcium-signalling assay using a KNRK cell line that possesses functional B2 receptors but not PAR4. Bradykinin, at a concentration of 10 nM, induced a rapid calcium response (Figure 7). This response was clearly mediated by the B2 receptor as the bradykinin-induced calcium signal was abrogated by 30 nM of the specific antagonist icatibant. The PAR4-AP, AYPGKF-NH2, at a concentration of 200 μM, failed to induce a calcium response in these kinin B2 receptor responsive cells (Figure 7). Thus, it is unlikely that AYPGKF-NH2 interacts directly with the B2 receptor to activate some signalling pathways.
Figure 7.
The PAR4-AP, AYPGKF-NH2, does not activate directly the kinin B2 receptor. Calcium signal emission was recorded at 530 nm in KNRK cells following stimulation with bradykinin (10 nM) or AYPGKF-NH2 (200 μM). Some cells were pretreated with icatibant (kinin B2 receptor antagonist; 30 nM). Values are mean±s.e.m. of n=3 experiments and are expressed as the percentage of the fluorescence peak height yielded by the addition of 2 μM calcium ionophore (A23187). *Significantly different from the bradykinin group, P<0.05.
Although affecting the oedema response, none of the inhibitors or antagonists of the kallikrein–kinin system mentioned above had a statistically significant effect on granulocyte recruitment mediated by PAR4 activation (Figures 5b and 6c).
Discussion
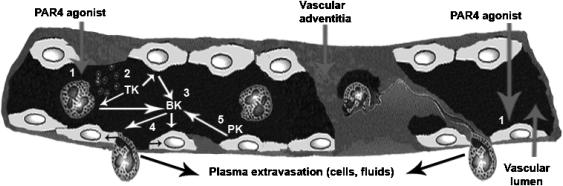
A main conclusion that can be drawn from our study is that activation of PAR4 can play a key role in generating two hallmarks of the inflammatory response: oedema and granulocyte infiltration. In this context, neutrophils and the kallikrein–kinin system are important contributors. Thus, PAR4 can take its place alongside PAR1 and PAR2 as an important receptor for the regulation of the inflammatory response (Ossovskaya & Bunnett, 2004). The overall scheme we propose for this inflammatory role of PAR4 is summarized in Figure 8.
Figure 8.
Proposed model for the mechanisms of action of PAR4-mediated oedema formation. In the blood vessel depicted schematically, we propose that PAR4 activation could occur both on endothelial cells and neutrophils to affect vascular permeability (1). Activated neutrophils would release tissue kallikrein activity (2). Tissue kallikrein would then cleave kininogens found at the surface of neutrophils and endothelial cells to produce active kinins (3). These kinins would in turn bind to and activate endothelial B2 receptors to increase vascular permeability, resulting in oedema and the migration of neutrophils into the tissue (4). With the breach of endothelial integrity, plasma kallikrein could then add to the response following activation of the contact system (5). TK, tissue kallikrein; PK, plasma kallikrein; BK, bradykinin.
Owing to the relatively recent discovery of PAR4 (Kahn et al., 1998; Xu et al., 1998), its functions are still largely unknown. There is strong evidence that this receptor is involved in platelet-mediated haemostasis in humans and rodents. However, the role that PAR4 activation plays in other events related to hæmostasis and inflammation has yet to be clarified. The data we present here substantially extend our preliminary work in which we showed, without exploring the underlying mechanisms, that a selective PAR4-AP can cause oedema in the rat (Hollenberg et al., 2004). Added to this oedema, our new data also demonstrate that AYPGKF-NH2 increases the recruitment of granulocytes to the site of inflammation in the mouse paw. Moreover, the blockade of PAR4 with a specific antagonist, pepducin P4pal10 (Covic et al., 2002), reduced both the oedema and granulocyte recruitment responses caused by the inflammatory stimulant, carrageenan, when injected in the rat paw. Previous work had singled out PAR4 as the likely candidate to mediate thrombin-induced leukocyte rolling and adherence in rat mesenteric venules (Vergnolle et al., 2002). Our present observations demonstrate further the involvement of PAR4 activation in inflammatory processes, particularly in the generation of oedema and inflammatory cell recruitment. Although oedema and granulocyte infiltration were significantly reduced by the PAR4 antagonist in the carrageenan inflammation model, these parameters were not completely abolished. This result suggests that although PAR4 plays a prominent role in carrageenan-induced inflammation, other mediators are undoubtedly also involved.
In a rat paw inflammation model, we found that oedema caused by the activation of PAR1 or PAR2 (Vergnolle et al., 1999a, 1999b) results from the release of neuropeptides from sensory neurons (Steinhoff et al., 2000; de Garavilla et al., 2001). In contrast, the PAR4-mediated oedema response differs, in that a neurogenic mechanism does not appear to be involved nor does mast cell degranulation play a role (Hollenberg et al., 2004). In common with the PAR1-triggered inflammatory response (Vergnolle et al., 1999b), we found that NO production and eicosanoid synthesis do not play a major role in the inflammatory events mediated by PAR4. Taken together, our observations suggest that PAR4 is unique with regards to its inflammatory mechanisms of action when compared to the other PARs. Further, the PAR4-mediated inflammatory mechanisms appear to differ from the classical inflammatory pathways (NO and prostaglandin generation) often triggered by other G protein-coupled receptor systems, such as PAR2 (Vergnolle et al., 1999a).
Since PAR4 agonists, including thrombin, can induce leukocyte rolling and adhesion in the vasculature (Vergnolle et al., 2002) and since PAR4-induced oedema is not mediated by sensory neurons and mast cells (Hollenberg et al., 2004), a potential role for PAR4 activation of leukocytes, particularly neutrophils, was hypothesized. Here, our data show (1) that the recruitment of granulocytes goes hand-in-hand with oedema formation and (2) that the presence of neutrophils per se is a major contributor to the development of PAR4-induced oedema, particularly within the first hour of the oedema response. Whether or not the PAR4-triggered activation of platelets might also play some role in the neutrophil activation process represents an important topic for our work in the future. The neutrophils rapidly recruited to the site of inflammation undoubtedly release a number of inflammatory mediators that contribute to oedema (see our proposed model in Figure 8). In this regard, we identified components of the kallikrein–kinin system as the potential mediators linking neutrophil recruitment to oedema formation (Figure 8). Indeed, inhibitors of both plasma and tissue kallikreins reduced the formation of oedema to the same extent as did the depletion of neutrophils. Neutrophils are known to possess all of the components of the kallikrein–kinin system: (1) tissue and plasma kallikreins, (2) high and low molecular weight kininogens and (3) the kinin B1 and B2 receptors (Figueroa et al., 1989; Gustafson et al., 1989; Henderson et al., 1994; Rajasekariah et al., 1997). Since thrombin can increase the release of kallikrein activity by neutrophils (Cohen et al., 1991) and considering that kallikreins are involved in the oedema triggered by PAR4, our results support the hypothesis that PAR4 could be the target responsible for thrombin-induced kallikrein release at the site of inflammation.
Given that our work links kallikrein activity to PAR4-induced oedema, we suggest that active kinins are produced locally from the cleavage of kininogens and could thereby activate local kinin receptors. In keeping with this hypothesis, blockade of the kinin B2 receptor led to a reduction in oedema comparable to that caused by either neutrophil depletion or the kallikrein inhibitors. This result strongly suggests that endothelial cell kinin B2 receptor activation, caused by locally produced kinins, is responsible for a large proportion of PAR4-mediated oedema (Figure 8). It is now recognized that activated neutrophils are able to produce biologically active kinins from kininogens (Stuardo et al., 2004). Moreover, supporting this indirect activation of the B2 receptor by newly produced kinins following PAR4 stimulation is the observation that the PAR4-AP AYPGKF-NH2 failed to induce a calcium response through the B2 receptor. Interestingly, the lack of effect of the B1 receptor antagonist showed that the kinin B1 receptor, which expression is induced by inflammatory mediators (Marceau et al., 1998), plays no role in the PAR4-mediated oedema response. Added to the importance of the kallikrein–kinin system, the contact system (in which plasma kallikrein and high molecular weight kininogen are major components) present at the surface of endothelial cells would also be activated in the course of PAR4-induced inflammation (Figure 8). This activation would occur as soon as there is a breach in the endothelium to allow plasma extravasation (Colman & Schmaier, 1997).
Since a residual oedema response is still observed after either neutrophil depletion or kallikrein–kinin system inhibition, we also propose that PAR4 activation on endothelial cells could mediate signalling events increasing vascular permeability (Figure 8). PAR4 was detected at the surface of leukocytes, endothelial cells and smooth muscle cells (Vergnolle et al., 2002), suggesting that PAR4 could be activated both on leukocytes and endothelial cells to induce inflammatory signs. Further, multiple signalling pathways are triggered in endothelial cells of different origins following PAR4 agonist exposure (Kataoka et al., 2003). The combined activation of PAR4 and the kinin B2 receptor on endothelial cells could explain the magnitude of the response observed as well as its sustained duration. Indeed, the kinetics of desensitization for both receptors could explain the time course of oedema formation. The B2 receptor is a receptor known to be desensitized rapidly by G protein-coupled receptor kinases (Blaukat et al., 2001). PAR4 appears to be slowly desensitized by a mechanism that is still unclear (Shapiro et al., 2000). The effects of the inhibitors of the kallikrein–kinin system are more evident within the first hour, underscoring the importance in early oedema events of the B2 receptor, before its desensitization. The residual sustained response is more likely the result of a direct activation of PAR4 on endothelial cells which could signal for hours.
In conclusion, our data illustrate that PAR4 participates in the inflammatory response by mediating at least two of the hallmarks of inflammation: oedema and granulocyte recruitment. Both neutrophils and the kallikrein–kinin system appear to be key mediators in the oedema response mediated by PAR4.
Acknowledgments
We are grateful to Dr Dennis McMaster, director of the University of Calgary Peptide Synthesis Facility (peplab@ucalgary.ca), for provision of the synthetic peptides. We thank Kevin Chapman, Laurie Cellars, Zhenguo Yu and Bernard Renaux for their expert technical assistance and Linda Nadeau for her help with the scheme describing our inflammation model (Figure 7). We also thank Dr D. Michael Evans (Ferring Research Ltd) and Dr Denis Riochet (Sanofi-Synthelabo Recherche) for their gifts of inhibitors of the kallikrein–kinin system. This work was supported by Canadian Institutes of Health Research (CIHR) Proteinases and Inflammation Group Grant, by CIHR operating grant funds (M.D.H. and N.V.) and by a Servier International Alliance Stratégique grant (to M.D.H. and N.V.). S.H. is the recipient of a postdoctoral fellowship from the Alberta Heritage Foundation for Medical Research and Neuroscience Canada. N.V. is an Alberta Heritage Foundation for Medical Research Scholar and a Canadian Institutes for Health Research Investigator.
Abbreviations
- AYP
AYPGKF-NH2
- FE999024
((2S, 2R)-2-(2′-amino-3′-(4″chlorophenyl)propanoyl-amino-N-(3-guanidinopropyl)-3-(1-naphthyl)propanoamide
- FE999026
((2′S, 2″R)-4-(2′(2″-(carboxymethylamino)-3″-cyclohexyl-propanoylamino)-3′-phenyl-propanoylamino)piperidine-1-carboxyamidine)
- i.pl.
intraplantar
- KNRK
Kirsten virus sarcoma-transformed rat kidney epithelial cell
- L-NAME
Nω-nitro-L-arginine methyl ester
- PAR
proteinase-activated receptor
- PAR-AP
proteinase-activated receptor-activating peptide
- pepducin P4pal10
palmitoyl-SGRRYGHALR-NH2
- SSR240612
[(2R)-2-[((3R)-3-(1,3-benzodioxol-5-yl)-3-{[(6-methoxy-2-naphthyl)sulfonyl]amino}propanoyl)amino]-3-(4-{[(2R,6S)-2,6-dimethylpiperidinyl]methyl}phenyl)-N-isopropyl-N-methylpropanamide hydrochloride]
References
- ASFAHA S., BRUSSEE V., CHAPMAN K., ZOCHODNE D.W., VERGNOLLE N. Proteinase-activated receptor-1 agonists attenuate nociception in response to noxious stimuli. Br. J. Pharmacol. 2002;135:1101–1106. doi: 10.1038/sj.bjp.0704568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASOKANANTHAN N., GRAHAM P.T., FINK J., KNIGHT D.A., BAKKER A.J., MCWILLIAM A.S., THOMPSON P.J., STEWART G.A. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J. Immunol. 2002;168:3577–3585. doi: 10.4049/jimmunol.168.7.3577. [DOI] [PubMed] [Google Scholar]
- BLAUKAT A., PIZARD A., BREIT A., WERNSTEDT C., HENC-GELAS F., MULLER-ESTERL W., DIKIC I. Determination of bradykinin B2 receptor in vivo phosphorylation sites and their role in receptor function. J. Biol. Chem. 2001;276:40431–40440. doi: 10.1074/jbc.M107024200. [DOI] [PubMed] [Google Scholar]
- BONDER C.S., AJUEBOR M.N., ZBYTNUIK L.D., KUBES P., SWAIN M.G. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J. Immunol. 2004;172:45–53. doi: 10.4049/jimmunol.172.1.45. [DOI] [PubMed] [Google Scholar]
- CAMERER E., HUANG W., COUGHLIN S.R. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN W.M., WU H.F., FEATHERSTONE G.L., JENZANO J.W., LUNDBLAD R.L. Linkage between blood coagulation and inflammation: stimulation of neutrophil tissue kallikrein by thrombin. Biochem. Biophys. Res. Commun. 1991;176:315–320. doi: 10.1016/0006-291x(91)90926-x. [DOI] [PubMed] [Google Scholar]
- COLMAN R.W., SCHMAIER A.H. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood. 1997;90:3819–3843. [PubMed] [Google Scholar]
- COMPTON S.J., RENAUX B., WIJESURIYA S.J., HOLLENBERG M.D. Glycosylation and the activation of proteinase-activated receptor 2 (PAR(2)) by human mast cell tryptase. Br. J. Pharmacol. 2001;134:705–718. doi: 10.1038/sj.bjp.0704303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTTRELL G.S., AMADESI S., GRADY E.F., BUNNETT N.W. Trypsin IV: a novel agonist of protease-activated receptors 2 and 4. J. Biol. Chem. 2004;279:13532–13539. doi: 10.1074/jbc.M312090200. [DOI] [PubMed] [Google Scholar]
- COVIC L., MISRA M., BADAR J., SINGH C., KULIOPULOS A. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat. Med. 2002;8:1161–1165. doi: 10.1038/nm760. [DOI] [PubMed] [Google Scholar]
- DE GARAVILLA L., VERGNOLLE N., YOUNG S.H., ENNES H., STEINHOFF M., OSSOVSKAYA V.S., D'ANDREA M.R., MAYER E.A., WALLACE J.L., HOLLENBERG M.D., ANDRADE-GORDON P., BUNNETT N.W. Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism. Br. J. Pharmacol. 2001;133:975–987. doi: 10.1038/sj.bjp.0704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DECARIE A., ADAM A., COUTURE R. Effects of captopril and Icatibant on bradykinin (BK) and des [Arg9] BK in carrageenan-induced edema. Peptides. 1996;17:1009–1015. doi: 10.1016/0196-9781(96)00145-3. [DOI] [PubMed] [Google Scholar]
- EVANS D.M., JONES D.M., PITT G.R., ASHWORTH D., DE C.F., VERHEYEN F., SZELKE M. Synthetic inhibitors of human tissue kallikrein. Immunopharmacology. 1996a;32:117–118. doi: 10.1016/0162-3109(95)00069-0. [DOI] [PubMed] [Google Scholar]
- EVANS D.M., JONES D.M., PITT G.R., SUEIRAS-DIAZ J., HORTON J., ASHWORTH D., OLSSON H., SZELKE M. Selective inhibitors of plasma kallikrein. Immunopharmacology. 1996b;32:115–116. [PubMed] [Google Scholar]
- FARUQI T.R., WEISS E.J., SHAPIRO M.J., HUANG W., COUGHLIN S.R. Structure function analysis of protease activated receptor 4 tethered ligand peptides: determinants of specificity and utility in assays of receptor function. J. Biol. Chem. 2000;275:19728–19734. doi: 10.1074/jbc.M909960199. [DOI] [PubMed] [Google Scholar]
- FIGUEROA C.D., MACIVER A.G., BHOOLA K.D. Identification of a tissue kallikrein in human polymorphonuclear leucocytes. Br. J. Haematol. 1989;72:321–328. doi: 10.1111/j.1365-2141.1989.tb07711.x. [DOI] [PubMed] [Google Scholar]
- GOUGAT J., FERRARI B., SARRAN L., PLANCHENAULT C., PONCELET M., MARUANI J., ALONSO R., CUDENNEC A., CROCI T., GUAGNINI F., URBAN-SZABO K., MARTINOLLE J.P., SOUBRIE P., FINANCE O., LE F.G. SSR240612 [(2R)-2-[((3R)-3-(1,3-benzodioxol-5-yl)-3-[[(6-methoxy-2-naphthyl)sulfonyl]amino]propanoyl)amino]-3-(4-[[2R,6S)-2,6-dimethylpiperidinyl]methyl]phenyl)-N-isopropyl-N-methylpropanamide hydrochloride], a new nonpeptide antagonist of the bradykinin B1 receptor: biochemical and pharmacological characterization. J. Pharmacol. Exp. Ther. 2004;309:661–669. doi: 10.1124/jpet.103.059527. [DOI] [PubMed] [Google Scholar]
- GRIESBACHER T., RAINER I., TIRAN B., EVANS D.M. Involvement of tissue kallikrein but not plasma kallikrein in the development of symptoms mediated by endogenous kinins in acute pancreatitis in rats. Br. J. Pharmacol. 2002;137:692–700. doi: 10.1038/sj.bjp.0704910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAFSON E.J., SCHMAIER A.H., WACHTFOGEL Y.T., KAUFMAN N., KUCICH U., COLMAN R.W. Human neutrophils contain and bind high molecular weight kininogen. J. Clin. Invest. 1989;84:28–35. doi: 10.1172/JCI114151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUZIK T.J., KORBUT R., MEK-GUZIK T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- HENDERSON L.M., FIGUEROA C.D., MULLER-ESTERL W., BHOOLA K.D. Assembly of contact-phase factors on the surface of the human neutrophil membrane. Blood. 1994;84:474–482. [PubMed] [Google Scholar]
- HESTDAL K., RUSCETTI F.W., IHLE J.N., JACOBSEN S.E., DUBOIS C.M., KOPP W.C., LONGO D.L., KELLER J.R. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J. Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- HOCK F.J., WIRTH K., ALBUS U., LINZ W., GERHARDS H.J., WIEMER G., HENKE S., BREIPOHL G., KONIG W., KNOLLE J. Hoe 140 a new potent and long acting bradykinin-antagonist: in vitro studies. Br. J. Pharmacol. 1991;102:769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLENBERG M.D., COMPTON S.J. International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol. Rev. 2002;54:203–217. doi: 10.1124/pr.54.2.203. [DOI] [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., SANDHU S., HOULE S., VERGNOLLE N. Proteinase-activated receptor-4: evaluation of tethered ligand-derived peptides as probes for receptor function and as inflammatory agonists in vivo. Br. J. Pharmacol. 2004;143:443–454. doi: 10.1038/sj.bjp.0705946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIHARA H., CONNOLLY A., ZENG D., KAHN M., ZHENG Y., TIMMONS C., TRAM T., COUGHLIN S. Protease-activated receptor-3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- KAHN M., ZHENG Y., HUANG C., BIGORNIA V., ZENG D., MOFF S., FARESE R., TAM C., COUGHLIN S. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- KATAOKA H., HAMILTON J.R., MCKEMY D.D., CAMERER E., ZHENG Y.W., CHENG A., GRIFFIN C., COUGHLIN S.R. Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood. 2003;102:3224–3231. doi: 10.1182/blood-2003-04-1130. [DOI] [PubMed] [Google Scholar]
- LEVY L. Carrageenan paw edema in the mouse. Life Sci. 1969;8:601–606. doi: 10.1016/0024-3205(69)90021-6. [DOI] [PubMed] [Google Scholar]
- MARCEAU F., HESS J.F., BACHVAROV D.R. The B1 receptors for kinins. Pharmacol. Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- MULE F., PIZZUTI R., CAPPARELLI A., VERGNOLLE N. Evidence for the presence of functional protease activated receptor 4 (PAR(4)) in the rat colon. Gut. 2004;53:229–234. doi: 10.1136/gut.2003.021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., LARSSON A.K., STROMBECK B., SUNDELIN J. Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur. J. Biochem. 1995;232:84–89. doi: 10.1111/j.1432-1033.1995.tb20784.x. [DOI] [PubMed] [Google Scholar]
- OSSOVSKAYA V.S., BUNNETT N.W. Protease-activated receptors: contribution to physiology and disease. Physiol. Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- RAJASEKARIAH P., WARLOW R.S., WALLS R.S. High affinity bradykinin binding to human inflammatory cells. Biochem. Mol. Biol. Int. 1997;43:279–290. doi: 10.1080/15216549700204061. [DOI] [PubMed] [Google Scholar]
- SAMBRANO G.R., HUANG W., FARUQI T., MAHRUS S., CRAIK C., COUGHLIN S.R. Cathepsin G activates protease-activated receptor-4 in human platelets. J. Biol. Chem. 2000;275:6819–6823. doi: 10.1074/jbc.275.10.6819. [DOI] [PubMed] [Google Scholar]
- SAMBRANO G.R., WEISS E.J., ZHENG Y.W., HUANG W., COUGHLIN S.R. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature. 2001;413:74–78. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- SCAPINI P., LAPINET-VERA J.A., GASPERINI S., CALZETTI F., BAZZONI F., CASSATELLA M.A. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- SHAPIRO M.J., WEISS E.J., FARUQI T.R., COUGHLIN S.R. Protease-activated receptors 1 and 4 are shutoff with distinct kinetics after activation by thrombin. J. Biol. Chem. 2000;275:25216–25221. doi: 10.1074/jbc.M004589200. [DOI] [PubMed] [Google Scholar]
- STEINHOFF M., VERGNOLLE N., YOUNG S., TOGNETTO M., AMADESI S., ENNES H., TREVISANI M., HOLLENBERG M.D., WALLACE J.L., CAUGHEY G., MITCHELL S., WILLIAMS L., GEPPETTI P., MAYER E., BUNNETT N. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- STUARDO M., GONZALEZ C.B., NUALART F., BORIC M., CORTHORN J., BHOOLA K.D., FIGUEROA C.D. Stimulated human neutrophils form biologically active kinin peptides from high and low molecular weight kininogens. J. Leukoc. Biol. 2004;75:631–640. doi: 10.1189/jlb.1103546. [DOI] [PubMed] [Google Scholar]
- USHIKUBI F., SUGIMOTO Y., ICHIKAWA A., NARUMIYA S. Roles of prostanoids revealed from studies using mice lacking specific prostanoid receptors. Jpn. J. Pharmacol. 2000;83:279–285. doi: 10.1254/jjp.83.279. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N., DERIAN C.K., D'ANDREA M.R., STEINHOFF M., ANDRADE-GORDON P. Characterization of thrombin-induced leukocyte rolling and adherence: a potential pro-inflammatory role for Proteinase-Activated Receptor-4 (PAR-4) J. Immunol. 2002;169:1467–1473. doi: 10.4049/jimmunol.169.3.1467. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N., HOLLENBERG M.D., WALLACE J.L. Pro- and anti-inflammatory actions of thrombin: a distinct role for proteinase-activated receptor-1 (PAR1) Br. J. Pharmacol. 1999a;126:1262–1268. doi: 10.1038/sj.bjp.0702408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., HOLLENBERG M.D., SHARKEY K., WALLACE J.L. Characterization of the inflammatory response to proteinase-activated receptor-2 (PAR-2)-activating peptides in the rat paw. Br. J. Pharmacol. 1999b;127:1083–1090. doi: 10.1038/sj.bjp.0702634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VU T., HUNG D., WHEATON V., COUGHLIN S. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- XU W., ANDERSEN H., WHITMORE T., PRESNELL S., YEE D., CHING A., GILBERT T., DAVIE E., FOSTER D. Cloning and characterization of human protease-activated receptor-4. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]