Abstract
We previously reported that four lignans isolated from the bark of Machilus thunbergii Sieb. et Zucc. (Lauraceae) protected primary cultures of rat cortical neurons from neurotoxicity induced by glutamate.
Among the lignans, meso-dihydroguaiarectic acid (MDGA) and licarin A significantly attenuated glutamate-induced neurotoxicity when added prior to or right after the excitotoxic glutamate challenge.
The neuroprotective activities of two lignans appeared to be more effective in protecting neurons against neurotoxicity induced by NMDA than that induced by kainic acid.
MDGA and licarin A diminished the calcium influx that routinely accompanies with the glutamate-induced neurotoxicity, and inhibited the subsequent overproduction of cellular nitric oxide and peroxide to the level of control cells. They also preserved cellular activities of antioxidative enzymes such as superoxide dismutase, glutathione peroxidase and glutathione reductase reduced in the glutamate-injured neuronal cells.
Thus, our results suggest that MDGA and licarin A significantly protect primary cultured neuronal cells against glutamate-induced oxidative stress, via antioxidative activities.
Keywords: Lignans, antioxidative activity, meso-dihydroguaiaretic acid, glutamate, licarin A, neuroprotective activity
Introduction
Glutamate, an excitatory amino acid, activates different types of ion channel-forming receptors and G-protein-coupled receptors and plays its essential roles – neuronal survival, synaptogenesis, neuronal plasticity, memory, learning and behavior – in the central nervous system (CNS) (Sucher et al., 1996; Michaelis, 1998). However, high concentration of glutamate cause neuronal cell death within the CNS, and may be involved in neuropsychiatric and neuropathological disorders such as Parkinson's disease, Alzheimer's disease, epilepsy, seizures, ischemic stroke and spinal cord trauma (Lipton & Rosenberg, 1994; Cacabelos et al., 1996; Lee et al., 1999). Thus, neuroprotection against glutamate-induced neurotoxicity has been a therapeutic strategy to treat neurodegenerative diseases (Rajendra et al., 2004).
We previously employed primary cultures of rat cortical cells injured by glutamate as an in vitro assay system to isolate neuroprotective compounds from natural products, which protect neurons against glutamate-induced neurotoxicity (Kim et al., 1998). In our previous report, we found that lignans isolated from the bark of Machilus thunbergii had significant neuroprotective activities in our in vitro screening system (Ma et al., 2004).
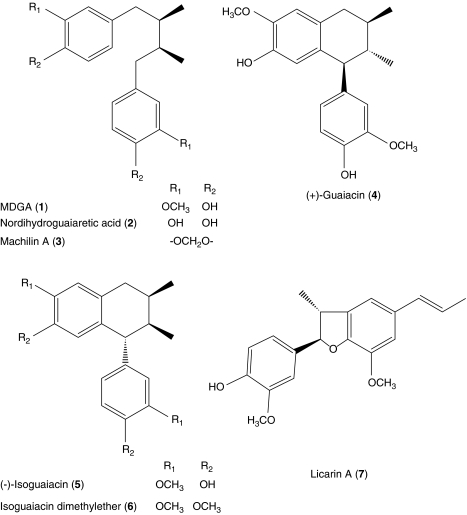
In the present study, we have investigated the structure–activity relationship of these isolated compounds (1–6 shown in Figure 1). Furthermore, we attempted to elucidate the neuroprotective mechanisms of MDGA and licarin A, which showed the most potent protection against glutamate-induced toxicity equally among the isolated compounds, although their structures are quite different.
Figure 1.
Structures of lignans isolated from M. thunbergii.
Methods
Materials
All chemicals for rat cortical cell cultures and biochemical assays were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO, U.S.A.), unless stated otherwise. Fetal bovine serum was obtained from Hyclone Co. (Logan, UT, U.S.A.). DL-2-Amino-5-phosphonovaleric acid (APV), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and MK-801 (dizocilpine maleate) used as positive controls were purchased from Research Biochemicals International (Natick, MA, U.S.A.). Urethane and Triton X-100 were purchased from Junsei Chemical Co. (Tokyo, Japan) and Yakuri Chemical Co. (Osaka, Japan), respectively. All lignans were isolated from the bark of M. thunbergii and their purity were higher than 95.0%, respectively (Ma et al., 2004).
Cell culture
Primary cultures of mixed cortical cells containing both neuronal and glial cells were prepared from 17- to 19-day-old fetal Sprague–Dawley rats as described previously (Kim et al., 1998). In brief, the trypsin-dissociated cortical cells were plated on multiwell culture plates (Corning, NY, U.S.A.) coated with collagen at a density of 1 × 106 cells well−1 and poly-L-lysine at a density of 2 × 105 cells well−1, respectively. The cortical cells were grown in DMEM containing 10% heat-inactivated fetal bovine serum with penicillin (100 IU ml−1) and streptomycin (100 μg ml−1) at 37°C in a humidified atmosphere of 95% air–5% CO2. Cultures were allowed to mature for 17 days before being used for experiments. Our mixed cortical cultures consisted of approximately 70–75% cells immunopositive for neuron-specific enolase and 25–30% cells immunopositive for glial fibrillary acidic protein as determined by immunocytochemical staining methods (Kim et al., 2004). All experiments were performed with Ethical Approval of Seoul National University.
Neurotoxicity and cell viability
Test compounds were dissolved in DMSO (final culture concentration, 0.1%); preliminary studies indicated that the solvent had no effect on cell viability of control and glutamate-treated cells at the concentration used (Kim et al., 1998). Cortical cell cultures (17 days old) were washed with DMEM and incubated with compounds for 1 h. The cultured cells were then exposed to 100 μM L-glutamate for 30 min. After 24 h incubation in the presence of test compounds, the cultures were assessed for the extent of neuronal damage by measuring lactate dehydrogenase (LDH) in the media. In some experiments, the cultures were treated with the appropriate lignans, MDGA and licarin A, either 1 h before exposure or after exposure to 100 μM L-glutamate for 1 h. After an additional 24 h incubation in the absence (pretreatment) or presence (post-treatment) of lignans, the cultures were assessed by measuring LDH in the media. In some experiments, cultures were pretreated with lignans for 1 h before exposure to 50 μM NMDA in HEPES-buffered salt solution containing 15 mM glucose and 10 μM glycine (pH 7.4) for 30 min or to 50 μM KA and 10 μM MK-801 (used to prevent NMDA receptor activation after the release of endogenous glutamate) for 3 h. After exposure to NMDA or KA, the cultures were then washed and further maintained in DMEM for 24 h in the absence of lignans. Neuronal viability was measured by the LDH assay, which reflects cellular integrity (Koh & Choi, 1987). Data are expressed as the percentage protection relative to vehicle-treated control cultures. Values shown are the mean±s.d. of three experiments (three to four cultures per experiment).
Measurement of intracellular calcium and nitric oxide (NO) contents
The intracellular calcium was determined by ratio fluorometry using Ca2+-specific dye, Fura 2-AM (Kim et al., 1998). In brief, 1 h before exposure to 100 μM glutamate, cultures grown on 48-well plates were treated with lignans and 5 μM Fura-2 AM in phosphate-buffered saline (pH 7.2) at 37°C in a humidified atmosphere of 95% air–5% CO2. The change of [Ca2+]i was measured 10 min after exposure to glutamate. Fura-2 fluorescence was measured with a spectroflurometer by exciting cells at 340 and 380 nm and measuring light emission at 520 nm (Grynkiewicz et al., 1985). The level of NO formed was determined by measuring the content of nitrite released into the medium using the method of Dawson et al. (1994). The culture medium was reacted with Griess reagent and the absorbance was then read at 550 nm. The concentration was determined against a nitrite standard curve. Values shown are the mean±s.d. of three experiments (three to four cultures per experiment).
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay
A measure of 100 μl of a 300 μM solution of DPPH in ethanol was added to 100 μl of a solution containing MDGA and licarin A in DMSO. The reaction mixtures were mixed and transferred into 96-well plates. After incubation for 30 min, the absorbance was determined using a microplate reader at 515 nm. The EC50 value, defined as the amount of antioxidant necessary to decrease the initial DPPH concentration by 50%, was calculated from the results (Kim et al., 2003). Values shown are the mean±s.d. of three experiments (three to four cultures per experiment).
Measurement of cellular peroxide
The relative level of free radicals, that is peroxide, in cultured cells was measured with the oxidation-sensitive compound, 2,7-DCF-DA by the method of Goodman & Mattson (1994). Cells were loaded with DCF-DA (50 μM, 50 min incubation) followed by three washes in HBSS. DCF fluorescence was then determined after 3 h incubation by measuring light emitted at 530 nm of exciting cells with light at 485 nm. Values shown are the mean±s.d. of three experiments (three to four cultures per experiment).
Assay for the activity of antioxidant enzymes
Cells from three culture plates were pooled in 2 ml of 0.1 M phosphate buffer (pH 7.4) and homogenized. The homogenate was centrifuged for 30 min at 3000 × g at 4°C and the supernatant (cytosolic and mitochondrial fractions) collected (Gibson & Skelf, 1988) for the measurements of antioxidative enzyme activity and glutathione (GSH) content. The activity of superoxide dismutase was determined according to the method of McCord & Fridovich (1969) by xanthine–xanthine oxidase reaction. GSSG reductase activity was measured according to the method of Carlberg & Mannervik (1975) based on the reduction of GSSG by GSSG reductase and NADPH. GSH peroxidase activity was determined by quantifying the rate of oxidation of GSH to GSSG by cumene hydroperoxide, a reaction catalyzed by GSH peroxide (Flohe & Gunzler, 1984). Values shown are the mean±s.d. of three experiments (three to four cultures per experiment).
Total GSH content measurement
Total GSH in the supernatant was determined spectrophotometrically using the enzymatic cycling method (Tietz, 1969). Values shown are the mean±s.d. of three experiments (three to four cultures per experiment).
Protein assay
Protein content was measured by the method of Lowry et al. (1951) with bovine serum albumin as a standard.
Statistical analysis
Statistical significance was determined by one-way ANOVA and, if significant, group means were compared by post hoc analysis using Tukey multiple comparison of means. Values shown are the mean±s.d. of three experiments (three to four cultures per experiment).
Results
We previously reported isolation of lignans from the bark of M. thunbergii Sieb. et Zucc. (Lauraceae) and the neuroprotective activities of these compounds in vitro (Ma et al., 2004). Thus, we have investigated the structure–activity relationship of lignans isolated from M. thunbergii using a test system consisting of primary cultures of rat cortical neurons injured with glutamate (Table 1; an MTT assay showed the same trend as the LDH assay; data not shown). In our culture system, MK-801 and CNQX, well-known positive controls against glutamate-induced neurotoxicity, showed effective neuroprotective activities at a concentration of 10 μM (Table 1). The potency of neuroprotective activity throughout the treatment paradigm was in the order: MDGA⩾licarin A⩾nordihydroguaiaretic acid >(+)-guaiacin=(−)-isoguaiacin. The neuroprotective potency for compounds other than those described above is not given because their abilities to protect neurons in our assay system were lower than 30%.
Table 1.
Neuroprotective activities of lignans isolated from M. thunbergii on primary cultures of rat cortical cells injured by glutamate
| Compounds |
EC50 (× 10−6 M) |
Emax (%)a |
|---|---|---|
| MDGA (1) | 0.24±0.04 | 58.5±1.2*** at 1 μM |
| Nordihydroguaiaretic acid (2) | 2.10±0.38 | 43.2±3.4** at 1 μM |
| Machilin A (3) | ND | 23.6±2.1* at 10 μM |
| (+)-Guaiacin (4) | 38.4±0.76 | 36.1±1.3** at 1 μM |
| (−)-Isoguaiacin (5) | 56.2±0.82 | 37.9±2.6* at 10 μM |
| Isoguaiacin dimethylether (6) | ND | 25.1±2.8* at 1 μM |
| Licarin A (7) | 0.32±0.02 | 54.2±1.9*** at 1 μM |
| MK-801 | 0.38±0.01 | 83.4±5.1*** at 10 μM |
| APV | 32.1±0.51 | 63.8±3.3*** at 50 μM |
| CNQX | 1.2±0.07 | 60.8±2.4*** at 10 μM |
ND=not determined.
Cortical cell cultures were washed with DMEM and incubated with test compounds for 1 h. The cultures were then exposed to 100 μM glutamate for 1 h. After 24 h incubation, cultures were assessed for the extent of neuronal damage (throughout the experiment). Values shown are the mean±s.d. of three experiments (three to four cultures per experiment).
LDH released from control and glutamate-treated cultures were 117±3 and 287±9 mU ml−1, respectively. Protection (%) was calculated as 100 × (LDH released from glutamate+test compound-treated cultures−LDH released from glutamate-injured cultures)/(LDH released from control cultures−LDH released from glutamate-injured cultures). Cell viabilities of control and glutamate-treated cells were representative as 100 and 0%, respectively. Glutamate-injured cells differ significantly from the control at a level of P<0.001. MK-801, dizocilpine maleate, a noncompetitive antagonist of the NMDA receptor; APV, DL-2-amino-5-phosphonovaleric acid, a competitive antagonist of the NMDA receptor; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione, non-NMDA receptor antagonist.
*P<0.05, **P<0.01, ***P<0.001 vs glutamate-injured cells (ANOVA and Tukey).
We tried to investigate the action mechanisms of MDGA and licarin A, the most potent neuroprotective lignans, using glutamate-injured primary cultures of rat cortical cells (see Table 1). Therefore, the neuroprotective activities of MDGA and licarin A against glutamate-induced neurotoxicity were initially investigated by a timed exposure to two lignans before or after glutamate treatment. As shown in Table 2, both MDGA and licarin A significantly attenuated neurotoxicity induced by glutamate in the pre- or post-treatment paradigm at the concentration ranging from 0.1 to 10 μM, but a strict-concentration dependence was not observed. The neuroprotective activity of MDGA reached a plateau at the concentration ranging from 1 to 5 μM. The activities of MK-801 and CNQX also reached a plateau at the high concentration (100 μM) in our primary culture systems.
Table 2.
Neuroprotective activity of MDGA or licarin A on glutamate-induced neurotoxicity in primary cultures of rat cortical cells
|
Concentration (μM) |
Protection (%) | ||
|---|---|---|---|
| Pretreatmenta | Post-treatmentb | ||
| Controlc | 100.0±2.1 | ||
| Glutamate injured | 0.0±0.5d | ||
| MDGA+glutamate | 0.1 | 30.6±3.7* | 29.0±11.1 |
| 1.0 | 46.7±0.7*** | 46.3±2.7** | |
| 10.0 | 42.1±4.3** | 43.1±3.8** | |
| Licarin A+glutamate | 0.1 | 34.5±2.8* | 30.8±2.1* |
| 1.0 | 41.2±3.9** | 40.5±4.1** | |
| 10.0 | 38.1±1.8** | 36.7±2.7** | |
Pretreatment: MDGA or licarin A was pretreated for 1 h prior to the glutamate insult.
Post-treatment: MDGA or licarin A was treated after the glutamate insult.
The value of LDH released from control and glutamate-injured cultures was 117±3 and 328±7 mU ml−1, respectively. Protection (%) was calculated as 100 × (LDH released from glutamate+test compound-treated cultures−LDH released from glutamate-injured cultures)/(LDH released from control cultures−LDH released from glutamate-injured cultures). The values shown are the mean±s.d. of three experiments (three to four cultures per experiment).
Glutamate-injured cells differ significantly from the control at a level of P<0.001.
*P<0.05, **P<0.01, ***P<0.001 vs glutamate-injured cells (ANOVA and Tukey).
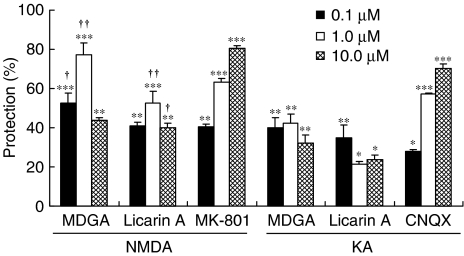
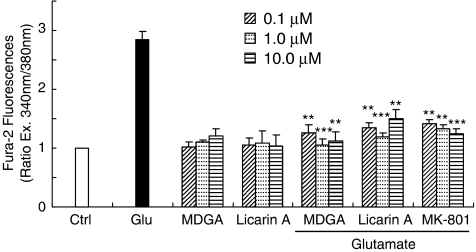
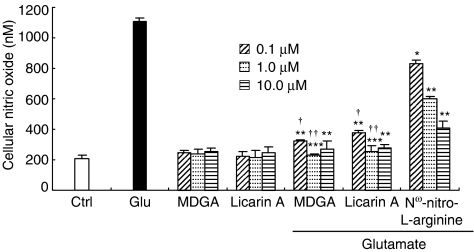
In order to reveal how MDGA and licarin A protected against glutamate-induced neurotoxicity, two excitotoxins, NMDA and KA, were used to induce selective receptor-mediated neurotoxicity in primary cultures of rat cortical cells. Both MDGA and licarin A showed neuroprotective activities on cortical cultures regardless of whether NMDA or KA was used as a neurotoxicant. However, they protected cultures slightly more selectively against NMDA-induced neurotoxicity of primary cultured rat cortical cells than from KA-induced neurotoxicity (Figure 2). Furthermore, this was confirmed by the results of MDGA and licarin A on the cellular calcium level in the early stages of glutamate-induced neurotoxicity. The [Ca2+]i provoked by glutamate treatment was significantly and effectively inhibited by the treatment of MDGA and licarin A, respectively (Figure 3). Then, we estimated whether MDGA and licarin A could effectively reduce NO content in cultured cortical cells treated with glutamate since NO synthase could be overactivated by excess Ca2+ in cultured cells. Both MDGA and licarin A significantly reduced overproduction of NO in cortical cells exposed to glutamate (Figure 4).
Figure 2.
Neuroprotective activity of MDGA or licarin A on NMDA- or KA-injured rat cortical cells. The value of LDH releases from control, NMDA- or KA-injured cultures was 117±3, 210±8 and 230±6 mU ml−1, respectively. Values shown are mean±s.d. of three experiments (three to four cultures per experiment). Protection (%) was calculated as 100 × (LDH released from NMDA/KA+test compound-treated cultures−LDH released from NMDA/KA-injured cultures)/(LDH released from control cultures−LDH released from NMDA/KA-injured cultures). NMDA- or KA-injured cultures differed significantly from control (P<0.001). *P<0.05, **P<0.01, ***P<0.001 vs excitotoxin-injured cells; †P<0.01, ††P<0.001 vs KA+each lignan (ANOVA and Tukey).
Figure 3.
Effect of MDGA or licarin A on intracellular [Ca2+] in glutamate-injured rat cortical cells. Cultures were treated with lignans and 5 μM Fura-2 AM in phosphate-buffered saline (pH 7.2) 1 h before exposure to 100 μM glutamate. The change of [Ca2+]i was measured 10 min after the exposure to glutamate. The values shown are mean±s.d. of three experiments (three to four cultures per experiment). *P<0.05, **P<0.01, ***P<0.001 vs glutamate-injured cells (ANOVA and Tukey).
Figure 4.
Effect of MDGA or licarin A on intracellular NO in glutamate-injured rat cortical cells. Cortical cultures were pretreated with MDGA or licarin A 1 h before glutamate-induced neurotoxicity. The values shown are mean±s.d. of three experiments (three to four cultures per experiment). Nω-nitro-L-arginine is a well-known NOS inhibitor. Glutamate-injured value differs significantly from the control at a level of P<0.001. *P<0.05, **P<0.01, ***P<0.001 vs glutamate-injured cells; †P<0.001 vs each value of Nω-nitro-L-arginine-treated cells (ANOVA and Tukey).
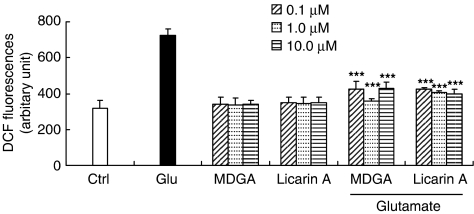
Glutamate-induced toxicity is known to involve such free radicals as hydroxyl radicals and superoxide anions (Dykens et al., 1987; Lafon-Cazal et al., 1993). We measured the effects of MDGA and licarin A on the content of cellular peroxides using the specific fluorescent dye, 2,7-DCF-DA (Figure 5). Indeed, MDGA and licarin A effectively reduced cellular peroxides in cultured rat cortical cells exposed to glutamate. When cultured cortical cells were insulted with glutamate, the cellular peroxide content was increased up to 3 h after the insult (Kim et al., 2002a). However, the cortical cells were pretreated with MDGA or licarin A; the content of cellular peroxides at 24 h after the glutamate insult was significantly reduced. Furthermore, we determined direct free radical scavenging activities of MDGA and licarin A using DPPH radical. We found that MDGA directly scavenged DPPH free radical in our studies (IC50: 15.0 μM); however, licarin A showed weak direct scavenging activity of DPPH free radicals (IC50: 75.0 μM).
Figure 5.
Effect of MDGA or licarin A on cellular oxidation in primary cultures of rat cortical cells. Cultures were pretreated with lignans 1 h before the glutamate insult. The relative content of intracellular peroxide was determined using the fluorescent dye 2,7-DCF-DA. The values shown are mean±s.d. of three experiments (three to four cultures per experiment). Glutamate-injured value differs significantly from the control at a level of P<0.001. *P<0.05, **P<0.01, ***P<0.001 vs glutamate-injured cells (ANOVA and Tukey).
Glutamate-induced oxidative stress is also known to deplete intracellular GSH (Almeida et al., 1998). As such, we further investigated the effect of MDGA and licarin A on GSH level using such GSH depletors as BSO and DEM. Treatment with BSO or DEM caused the GSH depletion in cells regardless of whether the cells were insulted with glutamate or not (Table 3). The pretreatment of the cultured cortical cells with DEM or BSO also rendered these cells more susceptible to glutamate insult. MDGA or licarin A itself did not influence the GSH level in the normal control cultures. They did not restore the reduced level of GSH induced by the treatment of BSO or DEM. However, these two lignans significantly prevented the GSH depletion in glutamate-induced toxicity (Table 3).
Table 3.
Effect of MDGA or licarin A on glutathione content in glutamate-injured rat cortical cell cultures
| Glutathione (μmol mg−1 protein) | |||
|---|---|---|---|
| w/o Lignan | MDGA | Licarin A | |
| Control | 2.42±0.21 | 2.38±0.24 | 2.26±0.14 |
| BSO treated | 1.15±0.10 | 1.17±0.19 | 1.32±0.20 |
| DEM treated | 1.14±0.24 | 1.18±0.15 | 1.21±0.25 |
| Glu treated | 0.86±0.12 | 2.05±0.11** | 1.91±0.21** |
| BSO+Glu treated | 0.75±0.19† | 1.52±0.17** | 1.44±0.17** |
| DEM+Glu treated | 0.73±0.17† | 1.55±0.21** | 1.46±0.23** |
At 1 h before the treatment of 1 μM MDGA or licarin A, cortical cells were pretreated with 50 μM BSO or DEM for 1 h. The cultures were then exposed to 100 μM glutamate and maintained for additional 24 h. The values shown are mean±s.d. of three experiments (three to four cultures per experiment).
*P<0.01; **P<0.001 vs each value in the absence of lignans; †P<0.001 vs each value of BSO or DEM only (ANOVA and Tukey).
Furthermore, treatment with MDGA or licarin A significantly preserved the activities of superoxide dismutase (SOD), GSH peroxidase (GSH-px) and GSH reductase (GSH-R) to the control level in primary cultures of rat cortical cells injured with glutamate (Table 4).
Table 4.
Effect of MDGA or licarin A on activities of antioxidative enzymes in glutamate-injured rat cortical cell cultures
|
Concentration (μM) |
SOD (U mg−1 protein) |
GSH-R (mU mg−1 protein) |
GSH-px (mU mg−1 protein) |
|
|---|---|---|---|---|
| Control | 34.7±2.7 | 39.7±0.4 | 37.7±0.5 | |
| Glutamate injured | 15.8±2.1 | 19.8±2.1 | 21.8±2.4 | |
| MDGA | 0.1 | 34.0±2.2 | 38.9±2.7 | 38.1±1.8 |
| 1.0 | 33.9±1.7 | 39.1±2.9 | 37.5±1.5 | |
| 10.0 | 34.5±1.9 | 39.5±3.4 | 37.9±1.4 | |
| Licarin A | 0.1 | 33.7±3.1 | 38.7±4.7 | 38.0±1.5 |
| 1.0 | 34.1±2.7 | 39.1±2.9 | 37.9±2.4 | |
| 10.0 | 35.0±4.7 | 40.4±3.7 | 38.3±0.9 | |
| MDGA+glutamate | 0.1 | 27.4±4.1** | 31.5±2.4** | 32.5±2.1** |
| 1.0 | 30.8±2.5** | 33.4±3.8** | 36.3±3.4*** | |
| 10.0 | 28.7±2.0** | 27.1±1.8** | 28.1±1.8** | |
| Licarin A+glutamate | 0.1 | 25.7±2.8* | 22.7±2.8* | 24.2±1.6* |
| 1.0 | 29.4±3.5** | 27.6±2.4** | 28.9±2.1** | |
| 10.0 | 26.7±2.4* | 24.9±2.5* | 24.6±2.4* |
Cortical cell cultures were pretreated with MDGA or licarin A for 1 h before exposure to 100 μM glutamate and then maintained for 24 h. The values shown are mean±s.d. of three experiments (three to four cultures per experiment). Glutamate-injured value differs significantly from the control at a level of P<0.001.
*P<0.05, **P<0.01, ***P<0.001 vs glutamate-injured cells (ANOVA and Tukey).
Discussion
We previously reported that MDGA and licarin A were found to have neuroprotective activities against glutamate-induced neurotoxicity in cultured rat cortical cells by our activity-guided isolation system (Ma et al., 2004). MDGA significantly attenuated glutamate-induced neurotoxicity when added prior to or after an excitotoxic glutamate challenge in our culture system. At high concentration (over 10 μM), however, MDGA did not show the improvement in the cell survival rate due to inherent cytotoxicity. For example, when only MDGA was administered to normal control cultures at concentrations ranging from 10.0 to 50.0 μM, the percentage cell viability significantly dropped (10.0 μM: 84.3±2.4%, P<0.5; and 50.0 μM: 73.9±1.6%, P<0.01 of untreated normal control, respectively). However, we found that the decrease of neuroprotection by the treatment of MK-801 or CNQX also happens in our culture system even though they did not show any significant cytotoxicity at higher concentrations (over 100 μM).
On the basis of the results in Table 1, we suggest that the substitution on the phenyl nucleus of a lignan might contribute to exert neuroprotective activity. When the neuroprotective activities of structural derivatives (1–6) of MDGA, dibenzylbutane and arylnaphthalene lignans, were compared, the activity of machilin A (3), which has two methylenedioxy moieties, was lower than that of MDGA (1) or nordihydroguaiaretic acid (2) by 35%. Moreover, (−)-isoguaiacin (5) showed stronger activity than isoguaiacin dimethylether (6), which has four methoxy groups without hydroxy group.
Glutamate is the major excitatory neurotransmitter in the mammalian CNS and can activate three ionotropic receptors, NMDA, AMPA and KA. Treatment of primary cultured rat cortical cells with NMDA induces NMDA receptor-mediated neurotoxicity characterized by acute influx of calcium (Choi et al., 1988). On the other hand, KA-induced neurotoxicity is attributed to an initial rapid influx of Na+, leading to passive influx of Cl− and H2O and excessive generation of ROS (Farooqui et al., 2001). Both MDGA and licarin A significantly attenuated glutamate-induced neurotoxicity during the pre- and post-treatment paradigms in our culture system. Therefore, we postulate that MDGA and licarin A might act on both of the early stage and the consequent receptor-mediated responses in our culture system.
MDGA and licarin A protected neuronal cells more selectively against toxicity induced by NMDA compared to KA-induced toxicity (Figure 2). Overactivation at NMDA receptors triggers an excessive entry of Ca2+, initiating a series of cytoplasmic and nuclear processes that promote neuronal cell death. Indeed, Ca2+-dependent enzymes like calpains and endonucleases can degrade essential proteins and DNA, respectively, which can induce cell death through necrosis. Ca2+ also activates NO synthase, increasing the presence of NO in the neuron and in surrounding areas. NO reacts with superoxide anion (O2−) to form the strong oxidizing compound, peroxynitrite, which causes nitration in proteins and oxidation of lipids, proteins and DNA, leading to cell death. In our culture system, MDGA and licarin A could effectively inhibit the increase of Ca2+ influx in the early stages of glutamate-induced neurotoxicity and subsequent NO overproduction (Figure 4) (McDonald & Johnston, 1990). In addition, we could suppose that peroxynitrite radical was also suppressed by the treatment with MDGA and licarin A. Superoxide anion has a higher affinity for NO than for SOD under certain conditions (Kohno et al., 1995). The decrease in O2− inactivation via a reduction in SOD activity promoted the overproduction of peroxynitrite (Greene & Greenamyre, 1996). Our results in Table 4 showed that MDGA and licarin A significantly preserved SOD activity in glutamate-injured cells. This might lead to the scavenging of potent free radicals and keeping the level of •O2− low. Thus, we could suggest that the retention of SOD activity by the treatment with MDGA and licarin A promotes O2− inactivation and, in turn, inhibits overproduction of NO and peroxynitrile radical.
Even though MDGA and licarin A almost completely inhibited Ca2+ influx and NO overproduction probably induced by overactivation of NMDA receptor, their neuroprotective activities against the glutamate-injured neurons in culture was lower than expected. This observation could be explained by the fact that our cultured cells intoxicated with glutamate were more effectively protected from excitotoxicity when NMDA- and non-NMDA receptor-mediated cellular responses were blocked at the same time (Kim et al., 2002b). In our cultures, cotreatment with both 10 μM MK-801 and 10 μM CNQX was more effective in protecting neurons from glutamate-induced toxicity (over 95% neurons survived) than treatment with either 10 μM MK-801 (80% neurons survived) or 10 μM CNQX alone (60% neurons survived). Indeed, MDGA and licarin A showed significant protection against NMDA-induced neurotoxicity in our culture system; however, they showed significant but weaker protection against KA-induced neurotoxicity in cultured cells (Figure 2).
All living cells have developed mechanisms for protection against oxidative stress. In general, GSH plays a major role in the elimination of a large number of nucleophilic toxicants such as oxidative radicals. In normal cells, GSH levels are decreased by oxidative radicals but are promptly restored to normal levels. The depletion of GSH alone did not result in a severe leakage of LDH from primary cultured cells (Casey et al., 1995); however, glutamate insult to cells rapidly and continuously decreased cellular GSH levels and inactivated many related antioxidant enzymes including superoxide dismutase, GSH-px and GSH-R (Yasuda et al., 1980). Thus, toxic free radicals such as •O2−, H2O2 were kept high in response (Yu et al., 2000). Such defects in GSH metabolism might cause oxidative stress, which has been implicated in several neurologic and neurodegenerative diseases (Bains & Shaw, 1997; Schulz et al., 2000). MDGA and licarin A significantly preserved the level of GSH in glutamate-injured rat cortical cultures (Table 3). However, these lignans might not accelerate GSH synthesis since they did not affect GSH depletion when the cultured cells were exposed to BSO and DEM. BSO is known to deplete GSH by inhibition of glutamylcysteine synthetase and DEM to deplete GSH via a reaction catalyzed by glutathione-S-transferase (Kim et al., 2002a). In our culture system, MDGA and licarin A maintained the levels of not only SOD but also GSH-px and GSH-R. Therefore, MDGA and licarin A could be supposed to facilitate GSH redox system in glutamate-injured cortical cells through preserving GSSG-R and GSH-px activities.
We also found that MDGA directly scavenged DPPH free radical, but licarin A has low DPPH free radical scavenging activity (IC50; 15 and 75 μM, respectively). These results suggest that the difference in free radical scavenging activity resulted from the structural differences. MDGA is a dibenzylbutane lignan, but licarin A is a dihydrobenzofuran neolignan. However, free radical scavenging by MDGA was not supposed as a pivotal mechanism since licarin A having weak DPPH scavenging activity showed almost equal degree of neuroprotection in our culture system.
At present, the cellular and molecular mechanisms that underlie the action of MDGA and licarin A are not fully understood. However, our results show that MDGA and licarin A significantly protect primary cultured neuronal cells against glutamate-induced oxidative stress via antioxidative activities. Therefore, we conclude that MDGA and licarin A might offer useful therapeutic choices in the treatment of neurodegenerative disorders caused by excitotoxicity.
Acknowledgments
This research was supported by a grant (M103KV010019-04K2201-01940) from Brain Research Center of the 21st Century Frontier Research Program funded by the ministry of Science and Technology, the Republic of Korea.
Abbreviations
- AMPA
2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl) propionic acid
- APV
DL-2-amino-5-phosphonovaleric acid
- BSO
buthionine sulfoximine
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DEM
diethylmaleate
- DPPH
1,1-diphenyl-2-picrylhydrazyl
- GSH
reduced glutathione
- GSH-px
glutathione peroxidase
- GSSG-R
glutathione reductase
- KA
kainic acid
- LDH
lactate dehydrogenase
- MDGA
meso-dihydroguaiaretic acid
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NMDA
N-methyl-D-aspartic acid
- NO
nitric oxide
- SOD
superoxide dismutase
References
- ALMEIDA A., HEALES S.J., BOLANOS J.P., MEDINA J.M. Glutamate neurotoxicity is associated with nitric oxide-mediated mitochondrial dysfunction and glutathione depletion. Brain Res. 1998;709:209–216. doi: 10.1016/s0006-8993(98)00064-x. [DOI] [PubMed] [Google Scholar]
- BAINS J.S., SHAW C.A. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res. Brain Res. Rev. 1997;25:335–358. doi: 10.1016/s0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- CACABELOS R., RODRIGUEZ B., CARRERA C., CAAMANO J., BEYER K., LAO J.I., SELLERS M.A. APOE-related frequency of cognitive and noncognitive symptoms in dementia. Meth. Find Exp. Clin. Pharmacol. 1996;18:693–706. [PubMed] [Google Scholar]
- CARLBERG I., MANNERVIK B. Purification and characterization of the flavoenzyme glutathione redcutase from rat liver. J. Biol. Chem. 1975;250:5475–5480. [PubMed] [Google Scholar]
- CASEY S.A., BREWSTER D., VIAU C., ACOSTA D. Effect of glutathione depletin and oxidative stress on the in vitro cytotoxicity of velnacrine maleate. Toxicol. Lett. 1995;76:257–265. doi: 10.1016/0378-4274(95)80011-2. [DOI] [PubMed] [Google Scholar]
- CHOI D.W., KOH J., PETERS S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J. Neurosci. 1988;8:185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON V.L., BRAHMBHATT H.P., MONG J.A., DAWSON T.M. Expression of inducible nitric oxide synthase causes delayed neurotoxicity in primary mixed neuronal–glial cortical cultures. Neuropharmacology. 1994;33:1425–1430. doi: 10.1016/0028-3908(94)90045-0. [DOI] [PubMed] [Google Scholar]
- DYKENS J.A., STERN A., TREKNER E. Mechanism of kainate toxicity to cerebellar neurons in vitro is analogous to reperfusion tissue injury. J. Neurochem. 1987;49:1222–1228. doi: 10.1111/j.1471-4159.1987.tb10014.x. [DOI] [PubMed] [Google Scholar]
- FAROOQUI A.A., YI O.W., LU X.R., HALLIWELL B., HORROCKS L.A. Neurochemical consequences of kainate-induced toxicity in brain: involvement of arachidonic acid release and prevention of toxicity by phospholipase A2 inhibitors. Brain Res. Brain Res. Rev. 2001;38:61–78. doi: 10.1016/s0169-328x(01)00214-5. [DOI] [PubMed] [Google Scholar]
- FLOHE L., GUNZLER W.A. Assays of glutathione peroxidase. Method Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- GIBSON G.G., SKELF P.Techniques and experiments illustrating drug metabolism Introduction to Drug Metabolism 1988New York: Chapman & Hall; 239–271.ed. Gibson, G.G. & Skelf, P. pp [Google Scholar]
- GOODMAN Y., MATTSON M.P. Selected forms of β-amyloid precursor protein protect hippocampal neurons against amyloid β-peptide induced oxidative injury. Exp. Neurol. 1994;128:1–12. doi: 10.1006/exnr.1994.1107. [DOI] [PubMed] [Google Scholar]
- GREENE J.G., GREENAMYRE J.T. Manipulation of membrane potential modulates malonate-induced striatal excitotoxicity in vivo. J. Neurochem. 1996;66:637–643. doi: 10.1046/j.1471-4159.1996.66020637.x. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of calcium indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- KIM S.R., KOO K.A., SUNG S.H., MA C.J., YOON J.S., KIM Y.C. Iridoids from Scrophularia buergeriana attenuate glutamate-induced neurotoxicity in rat cortical cultures. J. Neurosci. Res. 2003;74:948–995. doi: 10.1002/jnr.10828. [DOI] [PubMed] [Google Scholar]
- KIM S.R., PARK M.J., LEE M.K., SUNG S.H., PARK E.J., KIM J., KIM S.Y., OH T.H., MARKELONIS G.J., KIM Y.C. Flavonoids of Inula britannica protect cultured cortical cells from necrotic cell death induced by glutamate. Free Rad. Biol. Med. 2002a;32:596–604. doi: 10.1016/s0891-5849(02)00751-7. [DOI] [PubMed] [Google Scholar]
- KIM S.R., SUNG S.H., JANG Y.P., MARKELONIS G.J., OH T.H., KIM Y.C. E-p-methoxycinnamic acid protects cultured cells against neurotoxicity induced by glutamate. Br. J. Pharmacol. 2002b;135:1281–1291. doi: 10.1038/sj.bjp.0704576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM S.R., SUNG S.H., KANG S.Y., KOO K.A., KIM S.H., MA C.J., LEE H.S., PARK M.J., KIM Y.C. Aristolactam BII of Saururus chinensis attenuates glutamate-induced neurotoxicity in rat cortical cultures probably by inhibiting nitric oxide production. Planta Med. 2004;70:391–396. doi: 10.1055/s-2004-818964. [DOI] [PubMed] [Google Scholar]
- KIM Y.C., KIM S.R., MARKELONIS G.J., OH T.H. Ginsenoside Rb1 and Rg3 protect cultured rat cortical cells from glutamate-induced neurodegeneration. J. Neurosci. Res. 1998;53:426–432. doi: 10.1002/(SICI)1097-4547(19980815)53:4<426::AID-JNR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- KOH J.Y., CHOI D.W. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase. J. Neurosci. Meth. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- KOHNO K., OHTA S., FURUTA S., KOHNO K., KUMON Y., SASAKI S. Intraventricular administration of nitric oxide synthase inhibitors prevents delayed neuronal death in gerbil hippocampal CA1 neurons. Neurosci. Lett. 1995;199:65–68. doi: 10.1016/0304-3940(95)12018-y. [DOI] [PubMed] [Google Scholar]
- LAFON-CAZAL M., PIETRI S., CULCASI M., BOCKAERT J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- LEE J.M., ZIPFEL G.J., CHOI D.W. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399 (Suppl 6738):7–14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- LIPTON S.A., ROSENBERG P.A. Excitatory amino acids as a final common pathway for neurologic disorders. N. Eng. J. Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- LOWRY O., ROSEBROUGH H., FARR A., RANDALL R. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MA C.J., SUNG S.H., KIM Y.C. Neuroprotective lignans from the bark of Machilus thunbergii. Planta Med. 2004;70:79–80. doi: 10.1055/s-2004-815463. [DOI] [PubMed] [Google Scholar]
- MCCORD J.M., FRIDOVICH I. Superoxide dismutase. J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- MCDONALD J.W., JOHNSTON M.V. Physiological and pathophysiological roles of excitatory amino acids during CNS development. Brain Res. Brain Res. Rev. 1990;15:41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- MICHAELIS E.K. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog. Neurobiol. 1998;54:369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- RAJENDRA W., ARMUGAM A., JEYASEELAN K. Neuroprotection and peptide toxins. Brain Res. Brain Res. Rev. 2004;45:125–141. doi: 10.1016/j.brainresrev.2004.04.001. [DOI] [PubMed] [Google Scholar]
- SCHULZ J.B., LINDENAU J., SEYFRIED J., DICHGANS J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- SUCHER N.J., AWOBULUYI M., CHOI Y.B., LIPTON S.A. NMDA receptors: from genes to channels. Trends Pharmacol. Sci. 1996;17:348–355. [PubMed] [Google Scholar]
- TIETZ F. Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione. Anal. Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- YASUDA H., IZUMI N., SHIMADA O., KOBAYAKAWA T., NAKANISHI T. The protective effect of tinoridine against carbon tetrachloride hepatotoxicity. Toxicol. Appl. Pharmacol. 1980;52:407–413. doi: 10.1016/0041-008x(80)90335-x. [DOI] [PubMed] [Google Scholar]
- YU Y.U., KANG S.Y., PARK H.K., SUNG S.H., LEE E.J., KIM S.Y., KIM Y.C. Antioxidant lignans from Machilus thunbergii protect CCl4-injured primary cultures of rat hepatocytes. J. Pharm. Pharmcol. 2000;52:1163–1169. doi: 10.1211/0022357001774949. [DOI] [PubMed] [Google Scholar]