Abstract
A possible role of arginine vasopressin (AVP) V1b receptor subtype in stress-related disorders has been recently highlighted by the discovery of the agonist [1-deamino-4-cyclohexylalanine] AVP (d[Cha4]AVP) and the antagonist SSR149415. Both compounds have been proposed to target specifically V1b receptors, since the reported affinities for the related V1a, V2 and oxytocin receptors are in the micromolar or submicromolar range. In the present study, we further investigated the binding affinities of d[Cha4]AVP and SSR149415 at recombinant human vasopressin V1b (hV1b) and oxytocin (hOT) receptors expressed in Chinese hamster ovary (CHO) cells and functional properties of both compounds at hV1b, hV1a, hV2 and hOT receptors.
d[Cha4]AVP bound to hV1b receptors and hOT receptors with pKi values of 9.68±0.06 and 7.68±0.09, respectively. SSR149415 showed pKi values of 9.34±0.06 at hV1b and 8.82±0.16 at hOT receptors.
d[Cha4]AVP stimulated [Ca2+]i increase in hV1b-CHO cells with a pEC50 value of 10.05±0.15. It showed pEC50 values of 6.53±0.17 and 5.92±0.02 at hV1a and hV2 receptors, respectively, and behaved as a weak antagonist at hOT receptors (pKB=6.31±0.12). SSR149415 inhibited the agonist-induced [Ca2+]i increase with pKB values of 9.19±0.07 in hV1b-CHO and 8.72±0.15 in hOT-CHO cells. A functional pKi value of 7.23±0.10 was found for SSR1494151 at hV1a receptors, whereas it did not inhibit 20 nM AVP response at hV2 receptors up to 3 μM.
Data obtained confirmed the high potency and selectivity of d[Cha4]AVP at hV1b receptors, but revealed that SSR149415, in addition to the high potency at hV1b receptors, displays a significant antagonism at hOT receptors.
Keywords: Human, vasopressin, V1b receptors, oxytocin receptors, V1a receptors, V2 receptors, d[Cha4]AVP, SSR149415
Introduction
Arginine vasopressin (AVP) is a cyclic hypothalamic nonapeptide that exhibits many physiological effects, such as facilitation of water reabsorption by the kidney, contraction of the smooth muscle in arterioles (Jard et al., 1987), stimulation of hepatic glycolysis, gluconeogenesis, esterification and oxidation of free fatty acids (Hems & Whitton, 1973), platelet aggregation (Haslam & Rosson, 1972) and adrenocorticotropin hormone (ACTH) secretion (Antoni, 1993).
AVP effects are mediated by at least three different 7-transmembrane G-protein-coupled receptor (GPCR) subtypes that have been defined on the basis of their tissue distribution and pharmacology. The V1a receptor subtype is mainly distributed in the vascular wall, central nervous system (CNS), liver, kidney and platelets (Hirasawa et al., 1994). V1b receptors are predominantly found in the anterior pituitary but they are also significantly expressed in CNS areas such as hippocampus, frontal, piriform and cingulate cortex, caudate putamen, medial habenula, central amygdala and hypothalamus (Lolait et al., 1995; Hernando et al., 2001; Griebel et al., 2002; Stemmelin et al., 2005).
The V2 receptor subtype is distributed almost exclusively in the kidney (Jard, 1998). Activation of V1a and V1b receptors stimulates phosphatidylinositol (inositol phosphate (IP)) hydrolysis and mobilises intracellular calcium, whereas V2 receptors are positively coupled to adenylyl cyclase (Thibonnier et al., 1998a). The neurohypophyseal hormone oxytocin (OT), which differs from AVP by only two amino acids, induces uterine contraction and milk ejection through a GPCR linked to IP hydrolysis and calcium mobilisation. OT and AVP receptors are highly homologous and AVP binds to the OT receptor with appreciable affinity, causing agonist-like effects (Thibonnier et al., 1998b).
The wide range of physiological effects mediated by AVP and OT makes the potential use of selective pharmacological agents extremely valuable for the treatment of human diseases. These receptors have been proposed as potential targets for the treatment of debilitating disorders such as congestive heart failure, arterial hypertension, dysmenorrhoea (V1a receptors), hyponatremia, water-retaining diseases, ocular hypertension (V2 receptors) and preterm labour (OT receptors) (Paranjape & Thibonnier, 2001; Thornton et al., 2001). Intense efforts in this field have resulted in the discovery of selective V1a receptor antagonists such as OPC-21268 and SR49059 (Yamamura et al., 1991; Serradeil-Le Gal et al., 1993), selective V2 receptor antagonists such as SR121463 and OPC-31260 (Yamamura et al., 1992; Serradeil-Le Gal, 2001) and selective OT receptor antagonists such as L-366,509 and L-371,257 (Evans et al., 1992; Williams et al., 1995).
More recently, further research in this area led to the identification of d[Cha4]AVP (Derick et al., 2002) and SSR149415 (Serradeil-Le Gal et al., 2002) as high-affinity and selective agents at V1b receptors with respect to other AVP/OT receptor subtypes. d[Cha4]AVP is a peptido mimetic V1b agonist in vitro and its administration in rats stimulates ACTH and corticosterone release without showing vasopressor or antidiuretic responses (Derick et al., 2002). SSR149415 has been shown to be a potent V1b antagonist in vitro and has been demonstrated to inhibit AVP-induced ACTH release in vivo (Serradeil-Le Gal et al., 2002). Interestingly, SSR149415 also showed anxiolytic and antidepressant-like properties in preclinical models after systemic administration (Griebel et al., 2002).
The selectivity of both SSR149415 and d[Cha4]AVP at V1b receptors was established on the basis of binding affinity at recombinant and native AVP and OT receptors, whereas functional studies were carried out extensively only at human vasopressin V1b (hV1b) receptors (Serradeil-Le Gal et al., 2002).
In this study, a functional characterisation of d[Cha4]AVP and SSR149415 at human recombinant V1b, V1a, V2 and OT receptors was carried out. Our results confirmed high potency and selectivity of d[Cha4]AVP at hV1b receptors and revealed that the compound exhibits weak antagonist properties at hOT receptors. SSR149415 was confirmed to be a potent antagonist at hV1b receptors, but, in contrast with published data (Serradeil-Le Gal et al., 2002), the compound showed a significant antagonism at hOT receptors. Functional data were supported by radioligand binding studies, in which SSR149415 showed nanomolar affinity at hOT receptors.
Methods
Materials
SSR149415, d[Cha4]AVP, SR49059 and L-371,257 were synthesised at Medicinal Chemistry department, GlaxoSmithKline (Verona, Italy). [3H]AVP (2.22 TBq mmol−1) and [3H]OT (1.2 TBq mmol−1) were purchased from Perkin-Elmer Life Sciences (Monza, Italy). AVP, OT, Bacitracin, Leupeptin, Pefabloc, Pepstatin A, Probenecid, bovine serum albumin (BSA) and 3-amino-1,2,4-triazole (AT) were purchased from Sigma (Milan, Italy). (d(CH2)5 1Tyr(Me)2,Thr4,Orn8,Tyr-NH29)-vasotocin was purchased from Bachem (Wehil am Rhein, Germany). Fluo-4 and Fluorescein-d-B-glucopyranoside (FDGlu) were purchased from Molecular Probes (Paisley, U.K.). All drugs to be tested were diluted primarily in dimethyl sulphoxide (DMSO) and further diluted in the assay buffer to give a final DMSO concentration not exceeding 1%.
Cell culture
Chinese hamster ovary (CHO) cells were stably transfected with hV1b, hV1a or hOT receptors using pCIN1 vectors (Rees et al., 1996). Transfected cells were selected by antiobiotic (G418/geneticin) resistance. Individual clones have been functionally validated by FLIPR. Cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% decomplemented fetal calf serum and, 5 mM glutamine, in an atmosphere of 95% air and 5% CO2 at 37°C.
Membrane preparation
When confluent, cells were harvested in phosphate-buffered saline (PBS) containing 5 mM EDTA and centrifuged at 913 × g for 8 min at 4°C. Cells were then resuspended in 10 volumes of HEPES (50 mM) buffer (pH 7.4), containing Leupeptin (0.1 mM), Bacitracin (40 μg ml−1), EDTA (1 mM), Pefabloc (1 mM) and Pepstatin A (2 μM) and homogenised using a Polytron. The suspension was centrifuged at 48,000 × g for 20 min at 4°C. The final pellet was resuspended in 10 volumes of the same buffer and rehomogenised. Suspensions of membrane were then frozen at −80°C until required. Protein concentration was determined by the Bio-Rad Protein assay using BSA as internal standard.
Binding experiments
Binding assays were carried out in 96 deep-well plates (Whatman). In saturation experiments, increasing concentrations of [3H]AVP (2 pM–5 nM) and [3H]OT (4 pM–10 nM) were incubated with 14 μg well−1 of hV1b and 16 μg well−1 of hOT receptor preparations, respectively, for 60 min at room temperature in a final volume of 400 μl of 50 mM Tris-HCl, pH 7.4, 10 mM MgCl2 and 0.1% BSA (binding buffer). Nonspecific binding was determined by the presence of 1 μM AVP (hV1b receptors) or 1 μM OT (hOT receptors). In competition experiments, increasing concentrations of displacing compounds were incubated as above in the presence of 1 nM [3H]AVP (hV1b receptors) or 1 nM [3H]OT (hOT receptors).
Reactions were stopped by rapid filtration through GF/C filterplates (Packard) presoaked in ice-cold binding buffer using a cell harvester. Filters were washed three times with 1.5 ml of ice-cold 0.9% w v−1 NaCl, and radioactivity was counted in a microplate scintillation counter (Top Count, Packard). In each independent experiment, every concentration of displacer was tested in duplicate.
Intracellular Ca2+ measurements in hV1b, hOT and hV1a-CHO cells
hV1b-CHO and hOT-CHO cells were seeded into black-walled, clear bottom 96-well plates at a density of 50,000 cells per well and cultured overnight. Cells were then incubated for the labelling in the culture medium containing the fluorescent calcium indicator Fluo-4 AM (2 μM), the organic anion transport blocker Probenecid (5 mM) and HEPES (20 mM) for 30 min in a humidified atmosphere of 5% CO2. After washing with Hanks' balanced salts solution (HBSS) containing 20 mM HEPES and 2.5 mM Probenecid (wash buffer), cells were incubated for 15 min at 37°C in wash buffer containing 0.02% BSA (assay buffer) either in the absence (control) or presence of antagonists. The plates were then placed into an FLIPR (Molecular Devices, Sunnyvale, CA, U.S.A.) to monitor cell fluorescence (λex=488 nm, λem=510–570 nm) before and after the addition of different concentrations of agonists in assay buffer.
FLIPR experiments were carried out by using a laser setting of 1.0 W and a 0.4 s CCD (charge-coupled device) camera shutter speed.
FLIPR assays in hV1a-CHO cells were carried out as above in 384-well format by plating 10,000 cells per well. The ability of test compounds to increase [Ca2+]i or to inhibit 1 nM AVP-induced response was evaluated.
hV2 receptor yeast reporter assay
hV2 receptors and yeast chimeric G-α subunits containing the 5′ C-terminal amino acids of human G-αs were transfected in a modified Saccharomyces cerevisiae strain following the method described by Brown et al. (2000).
Receptor activation was determined by measuring the induction of exogluconase activity under the control of the HIS3 inducible reporter gene. Agonist stimulation of the pheromone mating pathway leads to expression of HIS3 and production of exogluconase, an enzyme that cleaves fluorescein-di-β-glucopyranoside (FDGlu) to yield fluoroscein (which is directly proportional to cell number).
Yeast cells were grown overnight to exponential phase in synthetic complete growth media (21 mg l−1 L-arginine, 102 mg l−1 L-aspartic acid, 102 mg l−1 L-glutamic acid, 31 mg l−1 L-lysine HCL, 21 mg l−1 L-methionine, 51 mg l−1 L-phenyalanine, 384 mg l−1 L-serine, 205 mg l−1 L-threonine, 31 mg l−1 L-tyrosine, 153 mg l−1 L-valine, pH 5.5, 6.7 g l−1 yeast nitrogen base without amino acids, 20 g l−1 glucose and 0.024 g l−1 histidine,) at 30°C with constant shaking at 200 r.p.m. Cells were diluted to 0.02 OD units at 600 nm in synthetic complete assay media (synthetic complete growth media pH 7.0, minus histidine, supplemented with 3.6 g l−1 NaH2PO4·2H2O, 3.7 g l−1 NaH2PO4 anhydrous, 10 μM FDGlu, 5 mM 3-AT). Assays were completed in 384-well black-walled, microtitre plates (NUNC, U.K.) containing compound and yeast cells in 50 μl synthetic complete assay media. The plates were incubated at 30°C for 24 h and fluorescence read at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. For antagonist studies, 20 nM AVP was added to plates containing antagonist and yeast cells in synthetic complete assay media.
Data analysis
Binding assays
Radioligand binding data were analysed by nonlinear regression analysis using GraphPad Prism 4.0 (GraphPad Software, CA, U.S.A.). Determination of KD and receptor density (Bmax) of [3H]AVP and [3H]OT at respective receptors was made by elaborating saturation experiments using one-site binding (hyperbola) equation. Curve fitting from competition binding experiments was determined by using one site competition equation after checking with F-test (P<0.05) that Hill slopes in the four parameter logistic equation were not statistically different from 1.0. Half-maximal inhibitory concentration (IC50) values were converted to Ki using the Cheng–Prusoff equation (Cheng & Prusoff, 1973). Results are expressed as mean pKi±s.e.m.
Functional assays
In FLIPR experiments, functional responses were measured as fluorescence intensity (FI) produced after receptor stimulation minus basal FI. Agonist EC50 values were determined by fitting the concentration–response curves with a four-parameter logistic equation in GraphPad Prism. The potency of antagonists at hV1b and hOT receptors was determined by using Schild analysis (Arunlakshana & Schild, 1959). Alternatively, Gaddum equation (pKB=log(DR−1)−log[antagonist], where DR is the ratio between the EC50 value of the agonist in the presence of the antagonist and the EC50 of agonist alone), was used to estimate an apparent pKB value of antagonists.
The antagonism at hV1a receptors was expressed as functional (f) pKi by using the adapted Cheng–Prusoff equation Ki=IC50/(1+[L])/EC50, where IC50 is the concentration of antagonist required for 50% inhibition of the maximum response, [L] is the concentration of AVP and EC50 is the AVP concentration giving the 50% of maximal response. Results are expressed as mean±s.e.m. All experiments were independently repeated at least three times in duplicate.
Results
Saturation binding experiments
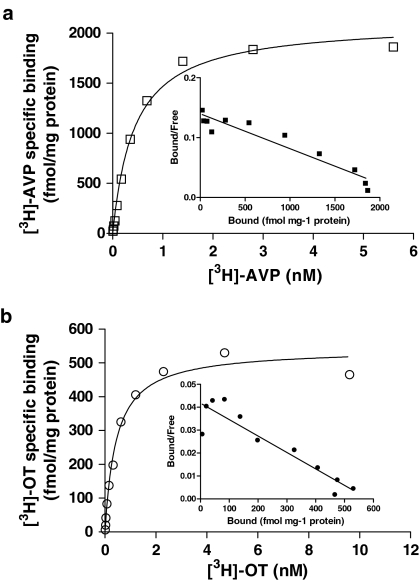
Saturation experiments on membranes prepared from hV1b-CHO and hOT-CHO cells showed that [3H]AVP and [3H]OT binding at respective receptors is saturable (Figure 1a and b). Scatchard analysis gave linear plots consistent with the presence of a single class of high-affinity binding sites. The negative log of the apparent dissociation constant (pKd) was 9.33±0.06 (n=5) for [3H]AVP at hV1b receptors and 9.40±0.03 (n=3) for [3H]OT at hOT receptors. The calculated maximum binding capacity (Bmax) was 2177±147 and 506±25 fmol mg−1 protein for hV1b and hOT receptors, respectively.
Figure 1.
Saturation experiments on [3H]AVP (a) and [3H]OT (b) binding to receptors on plasma membranes prepared from CHO cells transfected with human V1b and OT receptors, respectively. Inset: Scatchard linear transformation of the data. Data shown are representative of specific binding curves from five (hV1b) and three (hOT) independent experiments performed in duplicate.
Competition binding experiments
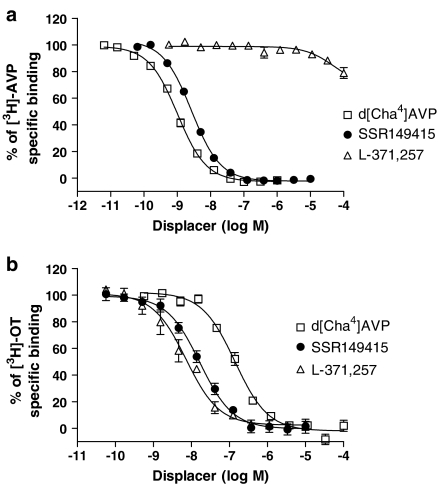
Compounds tested in competition binding experiments both at hV1b and hOT receptors are listed in Table 1. The V1b agonist d[Cha4]AVP showed pKi values of 9.68±0.06 and 7.68±0.09 at hV1b and hOT receptors, respectively (Figure 2a and b). SSR149415 showed pKi values of 9.34±0.06 at hV1b (Figure 2a) and 8.82±0.16 at hOT receptors (Figure 2b).
Table 1.
Binding pKi values of different AVP and OT receptor agonists and antagonists at hV1b and hOT receptors
| hV1b | hOT | V1b/OT Selectivity ratio | |
|---|---|---|---|
| AVP | 9.38±0.03 (n=4) | 9.26±0.13 (n=3) | 1.3 |
| OT | 6.97±0.13 (n=3) | 9.61±0.03 (n=3) | 0.002 |
| SR49059 | 6.34±0.22 (n=3) | 7.00±0.06 (n=3) | 0.2 |
| (d(CH2)5 1Tyr(Me)2,Thr4,Orn8,Tyr-NH29)-vasotocin | <6 (n=3) | 9.99±0.08 (n=3) | <0.001 |
| L-371,257 | <6 (n=3) | 8.83±0.04 (n=3) | <0.001 |
| d[Cha4]AVP | 9.68±0.06 (n=5) | 7.68±0.09 (n=4) | 100 |
| SSR149415 | 9.34±0.06 (n=8) | 8.82±0.16 (n=5) | 3.2 |
Data are expressed as mean±s.e.m. of n independent experiments performed in duplicate.
Figure 2.
Inhibition of [3H]AVP binding at hV1b receptors (a) and [3H]OT binding at hOT receptors (b) by d[Cha4]AVP, SSR149415 and L-371,257. Results are expressed as % of specific radioligand binding against the log concentration of each compound and values are mean±s.e.m. The data were taken from three independent experiments in which d[Cha4]AVP, SSR149415 and L-371,257 were tested in the same assay.
Intracellular Ca2+ increase
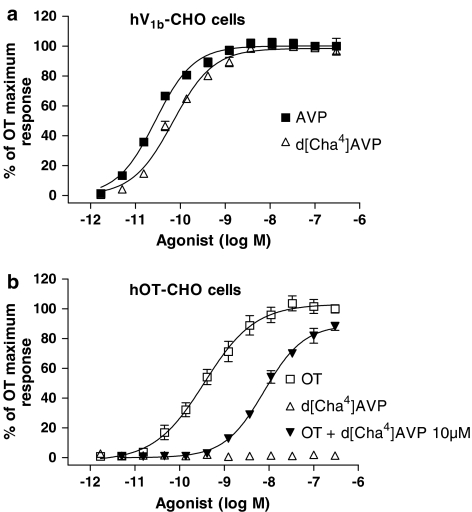
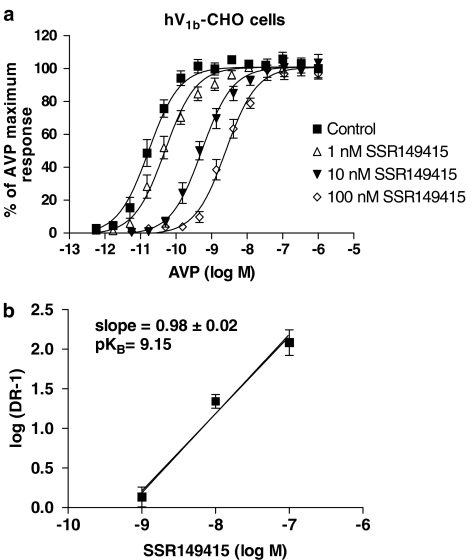
Addition of AVP and OT at hV1b-CHO and hOT-CHO cells, respectively, resulted in an increase in [Ca2+]i in a concentration-dependent manner (AVP pEC50=10.50±0.07 (n=19), OT pEC50=8.93±0.09 (n=16); Figure 3a and b). The V1b agonist d[Cha4]AVP stimulated the [Ca2+]i increase in hV1b-CHO cells with a pEC50 value of 10.05±0.15 (Figure 3a, Table 2). When added to hOT-CHO cells, d[Cha4]AVP did not show any agonist-like response up to 10 μM concentration, but showed a weak antagonism, since it inhibited the OT response with an apparent pKB value of 6.31±0.12 (Figure 3b). SSR149415 (1–100 nM) showed a competitive antagonism at hV1b receptors (Schild's slope=0.98±0.02) with a pKB value of 9.15±0.10, calculated according to Schild's analysis (Figure 4). The OT antagonist L-371,257 showed nanomolar potency at hOT receptors, with an apparent pKB of 8.19±0.13 (n=3) when tested at 10 nM concentration. At higher concentrations (100 nM, 1 μM), L-371,257 produced an unsurmountable antagonism, since it caused depression of the maximal OT responses along with rightward shifts of the agonist concentration–response curves, thus precluding Schild's analysis (Figure 5a). L-371,257 did not produce any inhibition of AVP response at hV1b receptors up to 1 μM concentration (n=3, data not shown). When tested at hOT receptors, SSR149415 (10 nM–1 μM) did not induce [Ca2+]i response, but inhibited the OT response in an apparent unsurmountable way with an apparent pKB value of 8.72±0.15, as calculated at 10 nM concentration (Figure 5b). At hV1a receptors, AVP showed a pEC50 of 9.87±0.06 (n=8), whereas d[Cha4]AVP displayed weak agonist properties (pEC50=6.53±0.17; Table 2). SSR149415 potency in inhibiting AVP response was in the submicromolar range (fpKi=7.23±0.14; Table 2). In the same assay, the V1a antagonist SR49059 showed an fpKi of 9.95±0.06 (n=4, data not shown).
Figure 3.
Increase in [Ca2+]i concentrations after agonist stimulation as determined in FLIPR experiments. (a) Concentration–response curves of AVP and d[Cha4]AVP in hV1b-CHO cells. (b) Concentration–response curves of OT in the absence or presence of 10 μM d[Cha4]AVP cells. d[Cha4]AVP alone did not stimulate any [Ca2+]i increase up to 10 μM concentration in hOT-CHO cells. Curves depicted are the mean fit of three independent experiments performed in duplicate.
Table 2.
Functional activities of d[Cha4]AVP and SSR149415 at human recombinant vasopressin and oxytocin receptors
| [Ca2+]i measurement | Yeast growth assay | |||
|---|---|---|---|---|
| hV1b | hV1a | hOT | hV2 | |
| d[Cha4]AVP pEC50 (pKB*) | 10.05±0.15 (n=3) | 6.53±0.17 (n=4) | 6.31±0.12* (n=3) | 5.92±0.02 (n=3) |
| SSR149415 pKB/fpKi | 9.19±0.07 (n=6) | 7.23±0.10 (n=7) | 8.72±0.15 (n=8) | Inactive up to 3 μM (n=3) |
*=pKB. Data are expressed as mean±s.e.m. of n independent experiments performed in duplicate.
Figure 4.
Inhibition of AVP-induced [Ca2+]i increase in hV1b-CHO cells by SSR149415. (a) Concentration–response curves for AVP in the absence or presence of 1, 10 and 100 nM SSR149415. Curves depicted are the mean fit of three independent experiments performed in triplicate. (b) Schild analysis of the antagonism produced by SSR1494151. Data are expressed as mean±s.e.m.
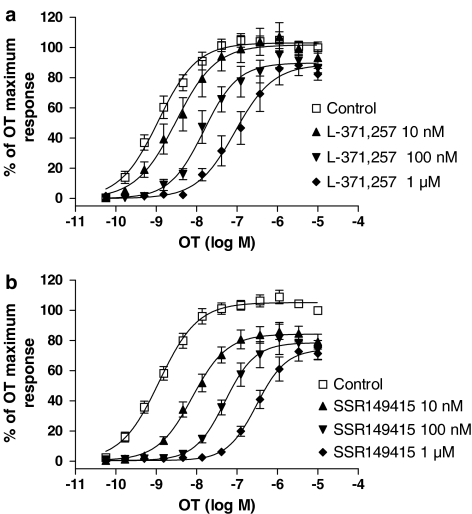
Figure 5.
Inhibition of OT-induced [Ca2+]i increase in hOT-CHO cells by L-371,257A and SSR149415. Concentration–response curves for OT in the absence or presence of 10 nM, 100 nM and 1 μM L-371,257 (a), or 10 nM, 100 nM and 1 μM SSR149415 (b). Curves depicted are the mean fit of three independent experiments performed in duplicate.
hV2 receptor assay in yeast
At hV2 receptors, AVP showed a pEC50 of 8.53±0.06 (n=6), whereas d[Cha4]AVP displayed weak agonist properties (pEC50=5.92±0.02, n=3). SSR149415 did not inhibit 20 nM AVP response up to 3 μM concentration (n=3).
Discussion
The absence of selective pharmacological agents at V1b receptors had severely limited the investigation of the physiological role of these receptors, until the recent discovery of d[Cha4]AVP as the first selective peptido mimetic agonist (Derick et al., 2002), and SSR149415 as the first selective nonpeptide antagonist (Serradeil-Le Gal et al., 2002) at V1b receptors.
Since only binding data at V1a, V2 and OT receptors have been produced from the above studies for SSR149415 and d[Cha4]AVP, in this work we carried out an extensive functional characterisation of the two compounds at human recombinant AVP and OT receptors to complete their in vitro pharmacological profile. Binding data at hV1b and hOT receptors were also provided to establish a correlation with functional experiments.
In agreement with data reported in the literature (Derick et al., 2002; Cheng et al., 2004), our study confirmed that d[Cha4]AVP is a potent agonist at hV1b receptors with more than 100-fold selectivity with respect to the other AVP/OT receptor subtypes. Interestingly, in FLIPR functional experiments, d[Cha4]AVP did not stimulate [Ca2+]i increase but displayed weak antagonist properties at hOT receptors. The potency calculated for d[Cha4]AVP in functional experiments is about 10-fold lower compared to the affinity calculated here in [3H]OT binding experiments. The discrepancy between binding and functional data can be due to the particular chemico–physical properties of this peptido-mimetic compound. As measured by liquid chromatography/mass spectrometry (LC/MS), d[Cha4]AVP content in the buffer used in functional assays was about three-fold lower compared to that measured in binding assay buffer (data not shown), suggesting a poor solubility or a nonspecific binding of the compound to plastic surfaces in the functional assay conditions. Hence, it can be hypothesised that data obtained in the functional assay may underestimate the actual potency of d[Cha4]AVP. However, both binding and functional data show that d[Cha4]AVP is at least 100-fold selective at hV1b versus hOT receptors, in agreement with data published by Derick et al. (2002).
In functional experiments, SSR149415 inhibited in a competitive manner the AVP response at hV1b receptors with a nanomolar potency. Consistent results were obtained in [3H]AVP binding experiments, where SSR149415 showed an affinity value in agreement with functional potency, and with data previously reported by Serradeil-Le Gal et al. (2002). SSR149415 was confirmed to be a weak antagonist at human V1a receptor with potency in the submicromolar range, whereas it did not significantly inhibit AVP response at V2 receptors up to 3 μM concentration. However, in functional assays at hOT receptors, SSR149415 inhibited the [Ca2+]i increase induced by OT with high potency, comparable to that of L-371,257, a high-affinity ligand and potent antagonist at both human and rat OT receptors (Williams et al., 1995, 1999).
SSR149415 and L-371,257 displayed an unsurmountable antagonism versus OT at hOT receptors. In FLIPR assay, receptors are preincubated with the antagonist before their challenge with the agonist. Under these conditions, agonists can only produce full receptor stimulation if the antagonist in question is both competitive and sufficiently rapidly dissociating to make accessible the entire receptor population at the time at which the maximal response is measured. Hence, the apparent noncompetitive antagonism displayed by SSR1494151 and L-371,257 may be attributable to a lack of equilibrium between OT and antagonists with hOT receptors within the short duration of rapid transient effects of OT on intracellular calcium. A similar observation was reported by Miller et al. (1999) about the apparent noncompetitive antagonism at H(1)-histamine receptors found in FLIPR experiments by known competitive antagonists. This hypothesis is supported by the finding that L-371,257 has been shown to be a competitive OT receptor antagonist in the isolated uterus of rats (Pettibone et al., 1995).
In agreement with functional experiments, in [3H]OT binding experiments, SSR149415 and L-371,257 showed similar pKi values at hOT receptors. Serradeil-Le Gal et al. (2002) reported that in binding assays performed on human recombinant receptors, SSR149415 shows 100-fold higher affinity at V1b with respect to OT receptors. We report herein a low selectivity profile for SSR149415. Even though SSR149415 showed about three-fold higher affinity and potency at hV1b compared to hOT receptors, the activity at the latter receptors is in the nanomolar range.
One possible explanation for the difference in SSR149415 affinity observed in the two studies could be attributed to the radioligand chosen in the OT binding assays. In the work of Serradeil-Le Gal et al. (2002), the OT antagonist [d(CH2)5Tyr(Me)2, Thr4, Orn8 [125I]Tyr9-NH2]vasotocin was utilised, whereas in the present study, we used the endogenous agonist [3H]OT. It has been demonstrated that the OT antagonist (d(CH2)5 1Tyr(Me)2,Thr4,Orn8,Tyr-NH29)-vasotocin binds to transmembrane regions different from those participating in OT binding at the porcine OT receptor (Postina et al., 1996), even though the observation that this antagonist exhibits a subnanomolar affinity versus [3H]OT indicates a mutually exclusive binding. It could be hypothesised that SSR149415 shows different affinities versus [3H]OT and the radiolabelled antagonist, resulting in an apparent lower affinity of SSR149415 at OT receptors. However, an unequivocal interpretation of the functional data obtained with SSR149415 at hOT receptors is hampered by the lack of similar data in the literature.
A possible role of AVP and V1b receptors in mood disorders is supported by an increasing body of evidence. In particular, it has been proposed that the HPA axis hyperactivity observed in prolonged stressful states and in patients affected by major depression could be maintained by increased secretion of AVP (Aguilera & Rabadan-Diehl, 2000; Scott & Dinan, 2002). The anxiolytic and antidepressant profile shown by SSR149415 in preclinical models after systemic administration (Griebel et al., 2002, 2003) suggests that the behavioural effects are not only related to the antagonism at V1b receptors located in the pituitary, but also to the blockade of central V1b receptors (Griebel et al., 2002; Stemmelin et al., 2005). Furthermore, a reduction in aggressive behaviour after systemic administration of SSR149415 in hamsters has been reported (Blanchard et al., 2005) confirming the findings in V1b knockout mice (Wersinger et al., 2002). The evidence that SSR149415 is not highly selective with respect to OT receptors could question whether the anxiolytic- and antidepressant-like effects observed in vivo after SSR149415 administration (Griebel et al., 2002) are mediated only by antagonism at V1b receptors. The effect of OT receptors on anxiety-related behaviour is controversial. A large body of evidence supports the idea that stimulation of OT receptors inhibits HPA axis activation (Neumann et al., 2000) and that OT mediates anxiolytic-like effects after central infusion in rats (Bale et al., 2001). Furthermore, an increase in anxiety-related behaviour has been observed in female OT-deficient mice (Mantella et al., 2003; Amico et al., 2004). Studies on the behavioural effects after systemic administration of OT receptor antagonists are missing, even though a decrease of immobility time in the forced swimming test has been recently reported, after administration into amygdala of an OT receptor antagonist in rat (Ebner et al., 2005), a possible indication of antidepressant-like effects. Alltogether, these studies do not elucidate if the effects of SSR149415 are exclusively mediated by antagonism at V1b receptors. The observation of possible behavioural effects induced by central administration of the selective V1b receptor agonist d[Cha4]AVP could help in understanding the role of V1b receptors located in the CNS. Further studies are warranted to clarify the implications of our results obtained on human recombinant receptors. Even though a significant improvement in the comprehension of the functional role of V1b receptors has been made possible by the discovery of d[Cha4]AVP and SSR149415, there is still the need of additional pharmacological tools at these receptors to provide an unequivocal demonstration of the therapeutic potential of these agents.
Acknowledgments
We acknowledge Steven Ratcliffe and Elisabetta Piga for the synthesis of d[Cha4]AVP and SSR149415.
Abbreviations
- ACTH
adrenocorticotropin hormone
- AT
3-amino-1,2,4-triazole
- AVP
[arginine8] vasopressin
- Bmax
receptor density
- BSA
bovine serum albumin
- [Ca2+]i
intracellular calcium
- CHO
Chinese hamster ovary
- CNS
central nervous system
- d[Cha4]AVP
[1-deamino-4-cyclohexylalanine] arginine vasopressin
- DMSO
dimethyl sulphoxide
- FDGlu
fluorescein-d-B-glucopyranoside
- FLIPR
fluorescence imaging plate reader
- GPCR
G-protein-coupled receptors
- HEPES
4-2-(hydroxyethyl)piperazine-1-ethanesulphonic acid
- HPA
hypothalamic-pituitary-adrenal
- IC50
half-maximal inhibitory concentration
- IP
inositol phosphate
- L-371,257
(1-{1-[4-[(N-acetyl-4-piperidinyl)oxy]-2-methoxybenzoyl]piperidin-4-yl}-4H-3,1-benzoxazin-2(1H)-one)
- OT
oxytocin
- PBS
phosphate-buffered saline
- SR49059
((2S)1-{(2R, 3S)-5-chloro-3-(2-chloro-phenyl)-1-(3,4-dimethoxybenzene-sulphonyl)-3-hydroxy-2,3-dihydro-1H-indole-2-carbonyl}pyrrolidine-2-carboxamide)
- SSR149415
((2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulphonyl]-3-(2-methoxy-phenyl)-2-oxo-2,3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide)
References
- AGUILERA G., RABADAN-DIEHL C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regul. Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- AMICO J.A., MANTELLA R.C., VOLLMER R.R., Li X. Anxiety and stress responses in female oxytocin deficient mice. J. Neuroendocrinol. 2004;16:319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- ANTONI F.A. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front. Neuroendocrinol. 1993;14:76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALE T.L., DAVIS A.M., AUGER A.P., DORSA D.M., McCARTHY M.M. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J. Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLANCHARD R.J., GRIEBEL G., FARROKHI C., MARKHAM C., YANG M., BLANCHARD D.C. AVP V(1b) selective antagonist SSR149415 blocks aggressive behaviors in hamsters. Pharmacol. Biochem. Behav. 2005;80:189–194. doi: 10.1016/j.pbb.2004.10.024. [DOI] [PubMed] [Google Scholar]
- BROWN A.J., DYOS S.L., WHITEWAY M.S., WHITE J.H., WATSON M.A., MARZIOCH M., CLARE J.J., COUSENS D.J., PADDON C., PLUMPTON C., ROMANOS M.A., DOWELL S.J. Functional coupling of mammalian receptors to the yeast mating pathway using novel yeast/mammalian G protein alpha-subunit chimeras. Yeast. 2000;16:11–22. doi: 10.1002/(SICI)1097-0061(20000115)16:1<11::AID-YEA502>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- CHENG L.L., STOEV S., MANNING M., DERICK S., PENA A., MIMOUN M.B., GUILLON G. Design of potent and selective agonists for the human vasopressin V1b receptor based on modifications of [deamino-cys1]arginine vasopressin at position 4. J. Med. Chem. 2004;47:2375–2388. doi: 10.1021/jm030611c. [DOI] [PubMed] [Google Scholar]
- CHENG Y., PRUSOFF W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- DERICK S., CHENG L.L., VOIROL M.J., STOEV S., GIACOMINI M., WO N.C., SZETO H.H., BEN MIMOUN M., ANDRES M., GAILLARD R.C., GUILLON G., MANNING M. [1-deamino-4-cyclohexylalanine] arginine vasopressin: a potent and specific agonist for vasopressin V1b receptors. Endocrinology. 2002;143:4655–4664. doi: 10.1210/en.2002-220363. [DOI] [PubMed] [Google Scholar]
- EBNER K., BOSCH O.J., KROMER S.A., SINGEWALD N., NEUMANN I.D. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology. 2005;30:223–230. doi: 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- EVANS B.E., LEIGHTON J.L., RITTLE K.E., GILBERT K.F., LUNDELL G.F., GOULD N.P., HOBBS D.W., DIPARDO R.M., VEBER D.F., PETTIBONE D.J., CLINESCHMIDT B.V., ANDERSON P.S., FREIDINGER R.M. Orally active, nonpeptide oxytocin antagonists. J. Med. Chem. 1992;35:3919–3927. doi: 10.1021/jm00099a020. [DOI] [PubMed] [Google Scholar]
- GRIEBEL G., SIMIAND J., SERRADEIL-LE GAL C., WAGNON J., PASCAL M., SCATTON B., MAFFRAND J.P., SOUBRIE P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc. Natl. Acad. Sci. U.S.A. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIEBEL G., SIMIAND J., STEMMELIN J., GAL C.S., STEINBERG R. The vasopressin V1b receptor as a therapeutic target in stress-related disorders. Curr. Drug Targets CNS Neurol. Disord. 2003;2:191–200. doi: 10.2174/1568007033482850. [DOI] [PubMed] [Google Scholar]
- HASLAM R.J., ROSSON G.M. Aggregation of human blood platelets by vasopressin. Am. J. Physiol. 1972;223:958–967. doi: 10.1152/ajplegacy.1972.223.4.958. [DOI] [PubMed] [Google Scholar]
- HEMS D.A., WHITTON P.D. Stimulation by vasopressin of glycogen breakdown and gluconeogenesis in the perfused rat liver. Biochem. J. 1973;136:705–709. doi: 10.1042/bj1360705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNANDO F., SCHOOTS O., LOLAIT S.J., BURBACH J.P. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology. 2001;142:1659–1668. doi: 10.1210/endo.142.4.8067. [DOI] [PubMed] [Google Scholar]
- HIRASAWA A., SHIBATA K., KOTOSAI K., TSUJIMOTO G. Cloning, functional expression and tissue distribution of human cDNA for the vascular-type vasopressin receptor. Biochem. Biophys. Res. Commun. 1994;203:72–79. doi: 10.1006/bbrc.1994.2150. [DOI] [PubMed] [Google Scholar]
- JARD S. Vasopressin receptors. A historical survey. Adv. Exp. Med. Biol. 1998;449:1–13. [PubMed] [Google Scholar]
- JARD S., BARBERIS C., AUDIGIER S., TRIBOLLET E. Neurohypophyseal hormone receptor systems in brain and periphery. Prog. Brain Res. 1987;72:173–187. doi: 10.1016/s0079-6123(08)60206-x. [DOI] [PubMed] [Google Scholar]
- LOLAIT S.J., O'CARROLL A.M., MAHAN L.C., FELDER C.C., BUTTON D.C., YOUNG W.S., III, MEZEY E., BROWNSTEIN M.J. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6783–6787. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANTELLA R.C., VOLLMER R.R., LI X., AMICO J.A. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology. 2003;144:2291–2296. doi: 10.1210/en.2002-0197. [DOI] [PubMed] [Google Scholar]
- MILLER T.R., WITTE D.G., IRELAND L.M., KANG C.H., ROCH J.M., MASTERS J.N., ESBENSHADE T.A., HANCOCK A.A. Analysis of apparent noncompetitive responses to competitive H(1)-histamine receptor antagonists in fluorescent imaging plate reader-based calcium assays. J. Biomol. Screen. 1999;4:249–258. doi: 10.1177/108705719900400506. [DOI] [PubMed] [Google Scholar]
- NEUMANN I.D., WIGGER A., TORNER L., HOLSBOER F., LANDGRAF R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J. Neuroendocrinol. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- PARANJAPE S.B., THIBONNIER M. Development and therapeutic indications of orally-active non-peptide vasopressin receptor antagonists. Expert Opin. Invest. Drugs. 2001;10:825–834. doi: 10.1517/13543784.10.5.825. [DOI] [PubMed] [Google Scholar]
- PETTIBONE D.J., GUIDOTTI M., HARRELL C.M., JASPER J.R., LIS E.V., O'BRIEN J.A., REISS D.R., WOYDEN C.J., BOCK M.G., EVANS B.E., FREIDINGER R.M., WILLIAMS P.D., MURPHY M.G. Progress in the development of oxytocin antagonists for use in preterm labor. Adv. Exp. Med. Biol. 1995;395:601–612. [PubMed] [Google Scholar]
- POSTINA R., KOJRO E., FAHRENHOLZ F. Separate agonist and peptide antagonist binding sites of the oxytocin receptor defined by their transfer into the V2 vasopressin receptor. J. Biol. Chem. 1996;271:31593–31601. doi: 10.1074/jbc.271.49.31593. [DOI] [PubMed] [Google Scholar]
- REES S., COOTE J., STABLES J, GOODSON S., HARRIS S., LEE M.G. Bicistronic vector for the creation of stable mammalian cell lines that predisposes all antibiotic-resistant cells to express recombinant protein. Biotechniques. 1996;20:102–104. doi: 10.2144/96201st05. [DOI] [PubMed] [Google Scholar]
- SCOTT L.V., DINAN T.G. Vasopressin as a target for antidepressant development: an assessment of the available evidence. J. Affect. Disord. 2002;72:113–124. doi: 10.1016/s0165-0327(02)00026-5. [DOI] [PubMed] [Google Scholar]
- SERRADEIL-LE GAL C. An overview of SR121463, a selective non-peptide vasopressin V(2) receptor antagonist. Cardiovasc. Drug Rev. 2001;19:201–214. doi: 10.1111/j.1527-3466.2001.tb00065.x. [DOI] [PubMed] [Google Scholar]
- SERRADEIL-LE GAL C., WAGNON J., GARCIA C., LACOUR C., GUIRAUDOU P., CHRISTOPHE B., VILLANOVA G., NISATO D., MAFFRAND J.P., LE FUR G., GUILLON G., CANTAU B., BARBERIS C., TRUEBA M., ALA Y., JARD S. Biochemical and pharmacological properties of SR 49059, a new, potent, nonpeptide antagonist of rat and human vasopressin V1a receptors. J. Clin. Invest. 1993;92:224–231. doi: 10.1172/JCI116554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERRADEIL-LE GAL C., WAGNON J., SIMIAND J., GRIEBEL G., LACOUR C., GUILLON G., BARBERIS C., BROSSARD G., SOUBRIE P., NISATO D., PASCAL M., PRUSS R., SCATTON B., MAFFRAND J.P., LE FUR G. Characterization of (2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxy-phenyl)-2-oxo-2,3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide (SSR149415), a selective and orally active vasopressin V1b receptor antagonist. J. Pharmacol. Exp. Ther. 2002;300:1122–1130. doi: 10.1124/jpet.300.3.1122. [DOI] [PubMed] [Google Scholar]
- STEMMELIN J., LUKOVIC L., SALOME N., GRIEBEL G. Evidence that the lateral septum is involved in the antidepressant-like effects of the vasopressin V1b receptor antagonist, SSR149415. Neuropsychopharmacology. 2005;30:35–42. doi: 10.1038/sj.npp.1300562. [DOI] [PubMed] [Google Scholar]
- THIBONNIER M., BERTI-MATTERA L.N., DULIN N., CONARTY D.M., MATTERA R. Signal transduction pathways of the human V1-vascular, V2-renal, V3-pituitary vasopressin and oxytocin receptors. Prog. Brain Res. 1998a;119:147–161. doi: 10.1016/s0079-6123(08)61568-x. [DOI] [PubMed] [Google Scholar]
- THIBONNIER M., CONARTY D.M., PRESTON J.A., WILKINS P.L., BERTI-MATTERA L.N., MATTERA R. Molecular pharmacology of human vasopressin receptors. Adv. Exp. Med. Biol. 1998b;449:251–276. doi: 10.1007/978-1-4615-4871-3_34. [DOI] [PubMed] [Google Scholar]
- THORNTON S., VATISH M., SLATER D. Oxytocin antagonists: clinical and scientific considerations. Exp. Physiol. 2001;86:297–302. doi: 10.1113/eph8602186. [DOI] [PubMed] [Google Scholar]
- WERSINGER S.R., GINNS E.I., O'CARROLL A.M., LOLAIT S.J., YOUNG W.S., III Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol. Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- WILLIAMS P.D., BOCK M.G., EVANS B.E., FREIDINGER R.M., GALLICCHIO S.N., GUIDOTTI M.T., JACOBSON M.A., KUO M.S., LEVY M.R., LIS E.V., MICHELSON S.R., PAWLUCZYK J.M., PERLOW D.S., PETTIBONE D.J., QUIGLEY A.G., REISS D.R., SALVATORE C., STAUFFER K.J., WOYDEN C.J. Nonpeptide oxytocin antagonists: analogs of L-371,257 with improved potency. Bioorg. Med. Chem. Lett. 1999;9:1311–1316. doi: 10.1016/s0960-894x(99)00181-x. [DOI] [PubMed] [Google Scholar]
- WILLIAMS P.D., CLINESCHMIDT B.V., ERB J.M., FREIDINGER R.M., GUIDOTTI M.T., LIS E.V., PAWLUCZYK J.M., PETTIBONE D.J., REISS D.R., VEBER D.F., WOYDEN C.J. 1-(1-[4-[(N-acetyl-4-piperidinyl)oxy]-2-methoxybenzoyl]piperidin-4-yl)-4H-3,1-benzoxazin-2(1H)-one (L-371,257): a new, orally bioavailable, non-peptide oxytocin antagonist. J. Med. Chem. 1995;38:4634–4636. doi: 10.1021/jm00023a002. [DOI] [PubMed] [Google Scholar]
- YAMAMURA Y., OGAWA H., CHIHARA T., KONDO K., ONOGAWA T., NAKAMURA S., MORI T., TOMINAGA M., YABUUCHI Y. OPC-21268, an orally effective, nonpeptide vasopressin V1 receptor antagonist. Science. 1991;252:572–574. doi: 10.1126/science.1850553. [DOI] [PubMed] [Google Scholar]
- YAMAMURA Y., OGAWA H., YAMASHITA H., CHIHARA T., MIYAMOTO H., NAKAMURA S., ONOGAWA T., YAMASHITA T., HOSOKAWA T., MORI T., TOMINAGA M., YABUUCHI Y. Characterization of a novel aquaretic agent, OPC-31260, as an orally effective, nonpeptide vasopressin V2 receptor antagonist. Br. J. Pharmacol. 1992;105:787–791. doi: 10.1111/j.1476-5381.1992.tb09058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]