Abstract
Neuropeptide Y (NPY) is a prominent enteric neuropeptide with prolonged antisecretory effects in mammalian intestine. Veratridine depolarises neurons consequently causing epithelial anion secretion across mouse colon mucosa. Our aim was to characterise functionally, veratridine-stimulated mucosal responses and to determine the roles for NPY, Y1, and Y2 receptors in modulating these neurogenic effects.
Colon mucosae (with intact submucous innervation) from wild-type mice (+/+) and knockouts lacking either NPY (NPY−/−), Y1−/− or Y2−/− were placed in Ussing chambers and voltage clamped at 0 mV. Veratridine-stimulated short-circuit current (Isc) responses in +/+, Y1 or Y2 antagonist pretreated +/+ colon, Y1−/− and NPY−/− colon were insensitive to cholinergic blockade by atropine (At; 1 μM) and hexamethonium (Hex; 10 μM). Tetrodotoxin (TTX, 100 nM) abolished veratridine responses, but had no effect upon carbachol (CCh) or vasoactive intestinal polypeptide (VIP)-induced secretory responses.
To establish the functional roles for Y1 and Y2 receptors, +/+ tissues were pretreated with either the Y1 or Y2 receptor antagonist (BIBO3304 (300 nM) or BIIE0246 (1 μM), respectively) and veratridine responses were compared with those from Y1−/− or Y2−/− colon. Neither BIBO3304 nor Y1−/− altered veratridine-induced secretion, but Y1 agonist responses were abolished in both preparations. In contrast, the Y2 antagonist BIIE0246 significantly amplified veratridine responses in +/+ mucosa. Unexpectedly, NPY−/− colon exhibited significantly attenuated veratridine responses (between 1 and 5 min).
We demonstrate that electrogenic veratridine responses in mouse colon are noncholinergic and that NPY can act directly upon epithelia, a Y1 receptor effect. The enhanced veratridine response observed in +/+ tissue following BIIE0246, indicates that Y2 receptors are located on submucosal neurons and that their activation by NPY will inhibit enteric noncholinergic secretory neurotransmission.
We also demonstrate Y1 and Y2 receptor-mediated antisecretory tone in +/+ colon and show selective loss of each in Y1 and Y2 null colon respectively. In NPY−/− tissue, only Y1-mediated tone was present, this presumably being mediated by endogenous endocrine peptide YY. Y2 tone was absent from NPY−/− (and Y2−/−) colon and we conclude that NPY activation of neuronal Y2 receptors attenuates secretory neurotransmission thereby providing an absorptive electrolyte tone in isolated colon.
Keywords: Neuropeptide Y receptors, mouse colon, veratridine, mucosal ion transport, submucosal neurons
Introduction
Neuropeptide Y (NPY) is a prominent neuropeptide located within myenteric and submucosal plexus neurons throughout the mouse colon, the latter providing a dense network of immunoreactive fibres within the lamina propria (Sang & Young, 1996). NPY and peptide YY (PYY), an intestinal hormone released from endocrine cells that are abundant in the descending colon (Ekblad & Sundler, 2002), preferentially stimulate Y1 and Y2 receptors. PYY(3–36), hydrolysed from PYY (Medeiros & Turner, 1994) and a Y2 receptor preferring fragment (Michel et al., 1998) is the predominant form of postprandial circulating PYY (Grandt et al., 1994), and has been associated with decreased food intake in mice and humans (Batterham et al., 2002).
By virtue of their primary Gi-protein coupling in intestinal epithelia, NPY and PYY are both antisecretory peptides and cause an inhibition of ongoing adenylate cyclase activity (Servin et al., 1989). Studies of mouse isolated colon mucosa demonstrate an antisecretory role for NPY, PYY and the Y1 receptor preferring analogue of PYY, Pro34PYY, in reducing vasoactive intestinal polypeptide (VIP)-stimulated short circuit current (Isc; Holliday et al., 2000). As expected Pro34PYY responses were sensitive to pretreatment with the Y1 receptor antagonist ((R)-N-[[4-(aminocarbonylaminomethyl)phenyl) methyl]-N2-(diphenylacetyl)-argininamide-trifluoroacetate (BIBO3304) (Wieland et al., 1998), confirming an antisecretory role for this type of Y receptor in the mouse colon (Holliday et al., 2000). However, PYY responses were only partially attenuated by the Y1 antagonist, demonstrating the ability of PYY to activate Y1 and Y2 receptors in mouse colon mucosa (Holliday et al., 2000). A role for the Y2 receptor was subsequently confirmed by Cox et al. (2001) using the selective Y2 receptor antagonist, BIIE0246 (Doods et al., 1999) and the combination of BIBO3304 and BIIE0246 was shown to abolish the antisecretory effects of PYY (Cox et al., 2001 and NPY (Cox et al., unpublished).
Pancreatic polypeptide (PP) is also a member of the NPY peptide family and causes a decrease in Isc in murine and human colon mucosae (though not rat small intestine or colon mucosae) by preferentially stimulating Y4 receptors (Tough & Cox, 1996; Cox et al., 2001; Cox & Tough, 2002). However, the antisecretory effects of PP in mouse colon are significantly smaller than those of either Pro34PYY or PYY (Cox et al., 2001) suggesting that the former peptide and its receptor have a less significant role in regulating ion secretion, than the Y1 receptor. The Y5 receptor agonist Ala31, Aib32hNPY was only active at μM concentrations in mouse colon mucosa (Cox et al., 2001) while y6 receptors do not appear to be present in adult mouse (Gregor et al., 1996). Taken together these data suggest that Y1 and Y2 receptors are the predominant Y receptor types mediating the antisecretory actions of NPY and PYY in mouse colon, a finding analogous to the human colon (Cox & Tough, 2002).
Immunohistochemical evidence for both epithelial and neuronal Y1 receptors has been described in rat (Jackerott & Larsson, 1997) and human (Peaire et al., 1997; Mannon et al., 1999) intestine. In rat small intestine, Y1 receptor immunoreactivity was colocalised with NPY on submucosal cell bodies and on both submucosal and myenteric nerve terminals (Jackerott & Larsson, 1997). Colocalisation was also demonstrated between a fraction of Y1-positive nerve cell bodies and VIP and a separate Y1-expressing population plus nitric oxide synthase (NOS) in submucosal cell bodies. A similar pattern of colocalisation between Y1 and NOS was reported in human myenteric neurons, while NPY and Y1 receptor colocalisation was observed in Henle's plexus neurons (Peaire et al., 1997). Y1 receptor immunoreactivity exhibited a basolateral distribution in both crypt and surface epithelia of human colon (Mannon et al., 1999) and this matched the sidedness of Y1 receptor-mediated responses observed in isolated human colon mucosa (Cox & Tough, 2002).
The lack of a commercially available Y2 receptor antibody has precluded a detailed localisation of this receptor type. Nevertheless Y2 receptor mRNA was found to be highly expressed in rat proximal and distal colon (Feletou et al., 1998), in the muscle of the ileum and ascending colon, as well as in the mucosal layers of the human ileum and descending colon (Ferrier et al., 2002). Taken together these studies suggest that Y2 and Y1 receptor activation can result in direct actions upon epithelial function as well as indirect neuronal modulatory roles in particular species and tissues. In the present study, we used the plant-derived alkaloid, veratridine, to depolarise intrinsic neurons (as a result of increased voltage-sensitive Na+ permeability, Catterall, 1980), in order to elucidate functionally the pre- versus postjunctional modulatory roles afforded by Y1 and Y2 receptors in mouse descending colon.
Veratridine-stimulated responses had previously been characterised in mouse jejunum mucosal preparations and produced an increase in Isc, associated with an increase in net Cl− secretion (Sheldon et al., 1990). Both veratridine and veratrine were shown to stimulate the release of enteric neurotransmitters, for example, substance P (SP), VIP (Belai & Burnstock, 1988) and acetylcholine (ACh; Yau et al., 1986) from guinea pig myenteric neurons, and also VIP release from myenteric synaptosomes (Allescher et al., 1996). However, a role for NPY in contributing to, or modulating, veratridine-stimulated ion secretion has yet to be characterised. Using selective Y1 and Y2 receptor antagonists (BIBO3304 and BIIE0246, respectively) and mice lacking either the peptide (NPY−/−) or one receptor type (Y1−/− and Y2−/−) we now describe discrete functional roles for NPY and these two Y receptors in the mouse colon.
Methods
Tissue preparation
Descending colon was collected from both adult (>10 weeks old) Y1+/+, Y2+/+, NPY+/+, Y1−/−, Y2−/− and NPY−/− mice (all on the same mixed background, 129Sv/C57Bl6) which were asphyxiated with CO2, followed by cervical dislocation. Tissues were immediately placed in fresh Krebs–Henseleit (KH) solution with the following composition (in mM): NaCl 118, KCl 4.7, NaHCO3 25, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5, glucose 11.1 (pH 7.4) and routinely provided six mucosal preparations (with intact submucosal plexus innervation) termed ‘mucosal preparations' throughout. These preparations were obtained by removing both overlying smooth muscle layers with the accompanying myenteric plexi, by dissection under a microscope. Mucosae were then placed in modified Ussing chambers (exposed area of 0.14 cm2) bathing both sides with 5 ml oxygenated (95% O2/5% CO2) KH, maintained at 37°C.
Mucosae were voltage clamped at 0 mV using an automatic voltage clamp (DVC 1000, World Precision Instruments, Stevenage, U.K.) as previously described (Cox et al., 2001). All agents were added subsequently to the basolateral reservoir and changes in Isc were recorded continuously. At least three animals contributed to each different data group. Tissues were not paired, and experiments with wild-type (+/+) and knockout (−/−) mucosae were usually carried out on different days and not in parallel. Male and female mice were used in this study and data were pooled, as no significant differences between genders were found.
Investigation of neurogenic Isc responses in mouse colon: TTX-sensitivity and antagonism of cholinoceptor responses
To investigate the tetrodotoxin (TTX, 100 nM) sensitivity of carbachol (CCh,10 μM) and VIP (30 nM) responses, TTX was added to tissues 15 min prior to either agonist, and forskolin (10 μM) was an internal control added at the end of each experiment. To investigate antagonism of muscarinic (atropine (At), 10 μM) and nicotinic (hexamethonium (Hex), 1 μM) receptors, these antagonists were added to untreated tissues 15 min prior to either CCh (10 μM) or nicotine (10 μM).
Chemical stimulation of enteric neurons with veratridine and modulation by BIBO3304 and BIIE0246
To stimulate all intrinsic neurons expressing voltage-sensitive Na+ channels, veratridine (30 μM) was added to colonic preparations. Veratridine time-course experiments were recorded for 60 min, before TTX was added. To determine the contribution of ACh to veratridine-induced responses in wild-type and knockout tissues, a combination of At (10 μM) and Hex (1 μM) was added to untreated tissues 15 min before veratridine. To examine the modulation of veratridine-induced responses, the Y1 antagonist, BIBO3304 (300 nM) or Y2 antagonist, BIIE0246 (1 μM) were added 15 min prior to veratridine. Previous studies in our laboratory have demonstrated maximum inhibition of Y1 or Y2 agonist responses, respectively, by 300 nM BIBO3304 or 1 μM BIIE0246 (Cox et al., 2001; Cox et al., unpublished). In fact, these competitive antagonists both increased Isc per se, revealing different degrees of Y1 and Y2 receptor-mediated inhibitory tone. The inactive enantiomer of Y1 antagonist BIBP3226, 1 μM BIBP3435, was also used as an internal control, previous studies with +/+ mouse colon mucosa having shown it to have no effect upon Y1 agonist responses, in contrast with BIBP3226 (1 μM) which abolished them (Cox et al., unpublished). Each antagonist effect was analysed separately from their subsequent modulatory effect upon veratridine responses. The latter were recorded for 15 min, within which time the peak Isc had been achieved and was beginning to wane. Respective agonists were then added to confirm specific antagonism, followed finally by TTX (100 nM).
Statistical analysis
Electrogenic Isc responses were converted to μA cm−2 and are quoted throughout as the mean±1 s.e.m. Student's unpaired t-test was used to compare either individual time points for veratridine responses, or agonist responses±antagonist and a P-value of less than 0.05 was considered statistically significant. The responses of male and female Y1+/+, Y2+/+ and NPY+/+ animals (collectively abbreviated, +/+) to veratridine, were pooled as their genetic backgrounds were a similar mixture of 129Sv and C57Bl6 and importantly, that no significant differences were observed between these groups of animals (data not shown).
Materials
Pro34PYY, PYY(3–36) and VIP were purchased from Bachem Ltd (Merseyside, U.K.) and aliquots were frozen and stored at −20°C, undergoing a single freeze-thaw cycle only. BIBO3304 (N2-(diphenylacetyl)-N-[(4-hydroxy-phenyl)methyl]-D-arginine amide), BIBP3226 (N2-(diphenylacetyl)-N-[(4-hydroxy-phenyl)methyl]-D-arginine amide), BIBP3435 (S)-N2-diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]-argininamide, and BIIE0246 ((S)-N2-[[1-[2-[4-(R,S)-5,11-Dihydro-6(6H)-oxidobenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N-[2-[2-[1,2-dihydro-3,5(4H)-dioxo-1,2-diphenyl-3H-1,2,4-triazol-4-yl]ethyl]-argininamide) were obtained from Boehringer Ingelheim Pharma KG (Biberach an der Riss, Germany). The proposed VPAC1 antagonist [Acetyl-His1, D-Phe2, Lys15, Arg16, Leu17]VIP(3–7)/GRF(8–27) (PG97–269) (referred to as PG 97–269) was a gift from Patrick Robberecht (Universite Libre de Bruxelles, Belgium). Veratridine, CCh, nicotine, At, Hex and TTX were all purchased from Sigma (Poole, U.K.).
Results
Characterisation of veratridine-stimulated mucosal responses
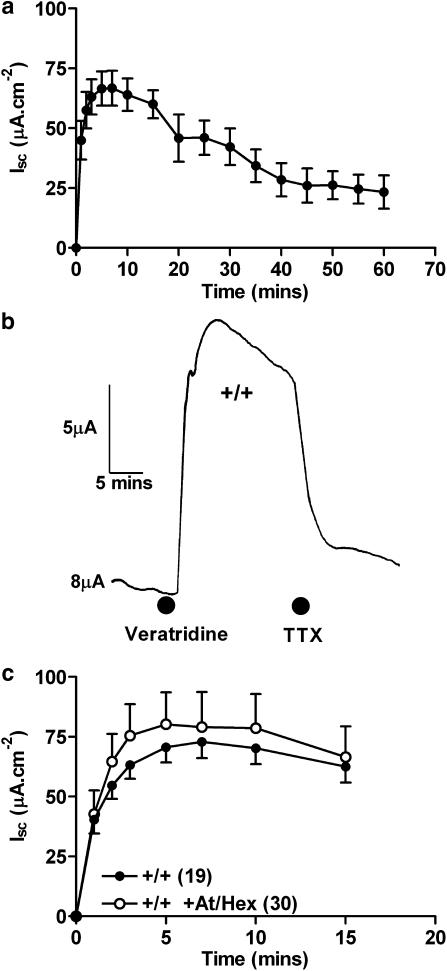
Veratridine was added at time 0 min to the basolateral reservoir and resulted in an immediate increase in Isc, which peaked 5–7 min later and then gradually returned towards basal Isc levels over a period of 60 min (Figure 1a). Addition of TTX 15 min after veratridine reduced the intrinsic neuronal-elevated Isc by >90% (Figure 1b). Pretreatment of tissues with a combination of both muscarinic and nicotinic antagonists (At and Hex) completely blocked subsequent CCh and nicotine Isc responses, respectively (data not shown), but did not significantly alter veratridine-induced changes in Isc in wild-type colon mucosa (Figure 1c). These data indicate that submucosal plexi cholinergic neurotransmission is not a significant component of the colonic mucosal response to veratridine.
Figure 1.
The pooled veratridine (30 μM) Isc responses over a period of 60 min (a) n=5–19, and the sensitivity of a representative veratridine response to TTX (100 nM at 15 min, in b). In (c), a comparison of veratridine responses±cholinergic blockade with atropine (At, 1 μM) and hexamethonium (Hex, 10 μM) is shown. Numbers of observations are otherwise given in parenthesis and data (in a and c) is the mean±1 s.e.m. A Student's unpaired t-test was used to compare veratridine responses±At/Hex and P>0.05 for all time points shown.
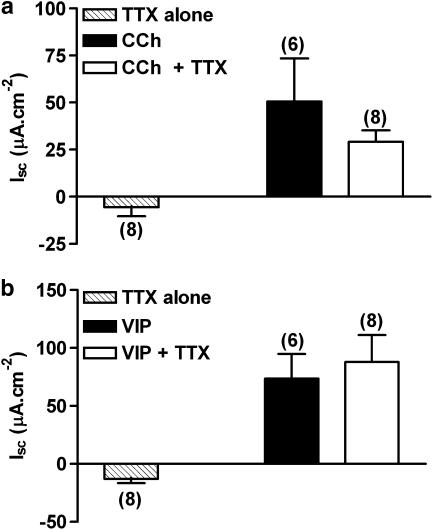
Sensitivity of CCh and VIP responses to TTX pretreatment
TTX was added to mucosal sheets once a stable basal Isc was achieved and it caused a small sustained decrease in Isc per se (Figure 2a and b). Previously we reported that the Y1 receptor preferred agonist, Pro34PYY stimulated prolonged reductions in Isc that were insensitive to TTX pretreatment while in contrast, Y2-preferred agonist PYY(3–36) responses were significantly reduced (>80%) by the neurotoxin (Cox et al., 2003; Hyland et al., 2003). Neither CCh (Figure 2a) nor VIP-induced peak Isc responses (Figure 2b) were altered by TTX. These data suggests the responses to CCh and VIP are predominantly, if not exclusively, epithelial in origin in mouse descending colon mucosa. We also tested the VPAC1 antagonist, PG97–269 in the hope that it would block VIP-induced anion secretion, however, this analogue had no significant effect (up to a concentration of 3 μM) upon subsequent VIP (30 nM) responses (data not shown).
Figure 2.
The effects of TTX pretreatment alone (100 nM, hatched bars) and upon subsequent CCh (10 μM, a) and VIP (30 nM, b) responses. Each bar is the mean±1 s.e.m. with n numbers shown in parenthesis. Student's unpaired t-test was used to compare control and TTX-treated data groups for either agonist and no significant differences were observed.
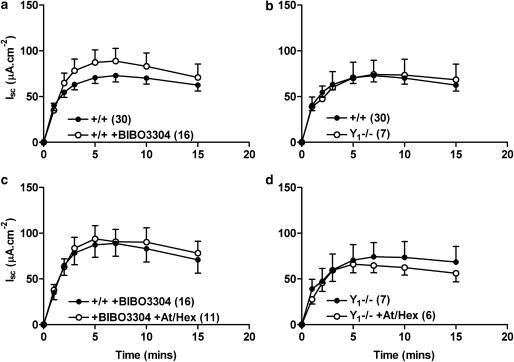
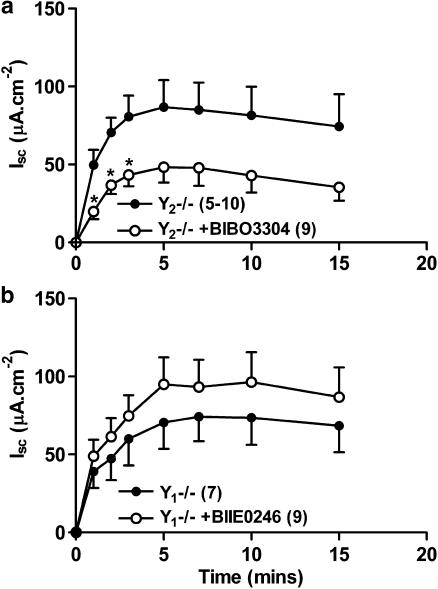
Lack of regulation of veratridine-induced increases in Isc by the Y1 receptor
Neither pretreatment of +/+ tissue with the Y1 receptor antagonist, BIBO3304 (300 nM, Figure 3a) nor knockout of the Y1 receptor (Figure 3b) significantly altered the time-course of veratridine-induced ion transport. Cholinergic blockade did not alter veratridine-stimulated Isc in either Y1 antagonist pretreated or Y1−/− colon mucosae (Figure 3c and d) but did abolish subsequent responses to CCh and nicotine (data not shown).
Figure 3.
The effects of either Y1 receptor antagonism (BIBO3304, 300 nM, in a) or Y1 receptor knockout (Y1−/−, in b) upon veratridine-induced (30 μM, added at time 0 min) Isc responses. Cholinergic blockade with atropine (At, 1 μM) and hexamethonium (Hex, 10 μM, c and d) did not significantly alter veratridine responses in either BIBO3304-pretreated tissue (c) or in Y1−/− tissue (d). Each pooled mucosal veratridine response was compared wild-type (+/+) responses. Data are the mean±1 s.e.m. and n numbers are given in parenthesis.
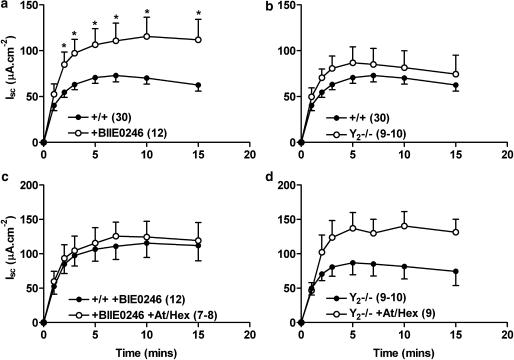
Regulation of veratridine-induced increases in Isc by the Y2 receptor
In contrast, pretreatment of +/+ tissue with the Y2 antagonist BIIE0246 significantly increased veratridine responses (between 2 and 15 min, Figure 4a). A small but insignificant increase in veratridine-induced secretion was observed in Y2−/− tissue compared with +/+ colon (Figure 4b). As seen with Y1−/− colon, and +/+ tissues pretreated with a Y1 antagonist, there was no effect of cholinergic blockade upon +/+ neurogenic responses in the presence of Y2 anatgonist, BIIE0246 (Figure 4c). However, pretreatment of Y2−/− tissue with At and Hex markedly increased veratridine-induced responses indicating a potential role for ACh in modulating submucosal neurotransmission in Y2−/− colon. CCh responses were significantly decreased in Y2−/− colon; 56.8±18.8 μA cm−2 (+/+, n=4) compared with 18.8±5.8 μA cm−2 (Y2−/−, n=9, P<0.05) in Y2−/− colon epithelia.
Figure 4.
The effects of either Y2 receptor antagonism (BIIE0246, 1 μM, a), or Y2 receptor knockout (Y2−/−, b) upon veratridine-induced Isc responses compared with wild-type (+/+) controls. The effects of cholinergic blockade (atropine, At, 1 μM and hexamethonium, Hex, 10 μM) upon BIIE0246-pretreated +/+ tissue (in c), or Y2−/− tissue (in d) are shown for comparison. Veratridine (30 μM) was added at time 0 min. Each point is the mean±1 s.e.m and n numbers are given in parenthesis. A Student's unpaired t-test was used to compare individual time points and significant differences are shown; *P<0.05.
Antagonism of Y1 and Y2 receptors in Y2−/− and Y1−/− colon, respectively
Addition of BIBO3304 alone to Y2−/− tissue reduced veratridine-induced responses (Figure 5a) significantly so, 1–3 min after veratridine addition. This is in contrast to +/+ tissue where the Y1 antagonist had no significant effect upon veratridine-stimulated Isc (Figure 3a). Pretreatment of Y1−/− colon mucosa with Y2 antagonist, BIIE0246 increased neurogenic changes in Isc (Figure 5b), but to a lesser extent than observed with +/+ colon (Figure 4a).
Figure 5.
The effect of either Y1 receptor antagonist pretreatment (BIBO3304, 300 nM) of Y2−/− colon (in a) or Y2 antagonism (BIIE0246, 1 μM) of Y1−/− mucosa (in b). Veratridine (30 μM) was added at time 0 min and data are the mean±1 s.e.m., with n numbers shown in parenthesis. A Student's unpaired t-test was used for comparison between individual pairs of time points in a; *P<0.05.
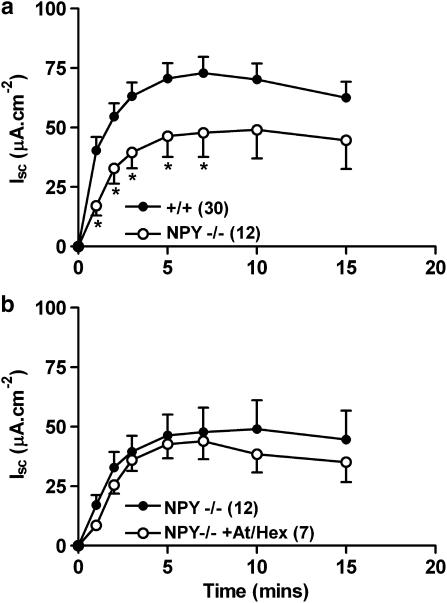
The effects of NPY knockout on veratridine-stimulated secretion in mouse descending colon
Somewhat surprisingly, veratridine responses were significantly blunted (between 1 and 7 min, Figure 6a) in NPY−/− tissue compared with +/+ controls. When NPY−/− mucosae were pretreated with At and Hex (Figure 6b) there was no significant alteration in the profile of the neurogenic responses, as seen previously with both +/+ (Figure 1c) and Y1−/− (Figure 3d) tissues. The sensitivity of NPY−/− colon to exogenous Y1 agonist, Pro34PYY (EC50 16.3 nM (14.1–20.0) in +/+ colon, compared with 16.8 nM (10.3–25.8) in NPY−/−) or the Y2 receptor-preferred fragment, PYY(3–36) (EC50 12.4 nM (5.4–28.7) in +/+ tissues, compared with 29.4 nM (17.9–48.4) in NPY−/− colon) was not significantly different, either in terms of potency or efficacy.
Figure 6.
A comparison of NPY−/− and wild-type (+/+) colon mucosal veratridine responses in (a); and the effects of cholinergic blockade upon the same in NPY−/− colon in (b). Veratridine (30 μM) was added at time 0 min, and each data point is the mean±1 s.e.m. with n numbers in parenthesis. A Student's unpaired t-test was used to compare individual pairs of time points and *P<0.05.
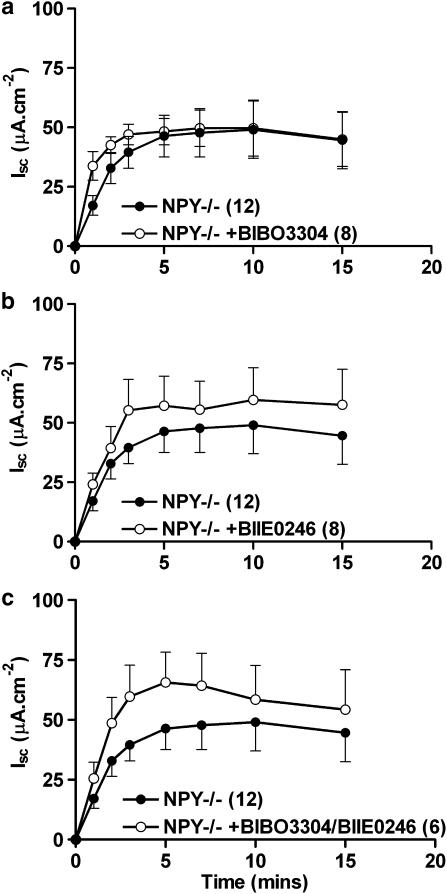
The effects of individual and combined Y1 and Y2 receptor antagonism upon NPY−/− colon
In order to investigate a role for enteroendocrine cell-derived PYY and PYY(3–36) upon submucosal neuromodulation, we measured veratridine-stimulated changes in Isc in the presence of BIBO3304 and/or BIIE0246 in NPY−/− colon. BIBO3304 pretreatment of NPY−/− tissue caused a more rapid rate of Isc increase after veratridine (1–3 min) but this was not significant (Figure 7a). BIIE0246 pretreatment of NPY−/− colon mucosae resulted in a nonsignificant amplification of the neurogenic response (the maximum difference at 3 min was, 39.5±6.7 μA cm−2, n=12; and following BIIE0246 pretreatment, 55.3±13.0 μA cm−2, n=8; Figure 7b). The combination of BIBO3304 and BIIE0246 (Figure 7c) appeared additive but again, this change in the veratridine response was not statistically significant.
Figure 7.
The effects of either single, Y1 receptor antagonism (in a, BIBO3304, 300 nM) or in (b); Y2 receptor blockade (BIIE0246, 1 μM) or in (c); combined Y1 and Y2 antagonism in NPY−/− colon throughout. Veratridine (30 μM) was added at time 0 min. Data are the mean±1 s.e.m. with n numbers given in parenthesis. Student's unpaired t-test comparisons revealed there were no significant differences between any time points.
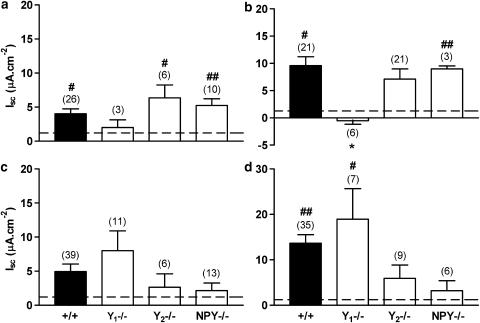
The effects of either BIBO3304 or BIIE0246 alone upon naive and VIP-stimulated mucosae
The competitive Y1 receptor antagonist, BIBO3304 when added to untreated +/+ colon (Figure 8a) or to VIP-stimulated (30 nM for 15 min) tissue (Figure 8b) resulted in a significant elevation of Isc per se, compared to vehicle controls (DMSO) and both responses were significantly larger than controls (P<0.01). Like BIBO3304, the Y1 antagonist, BIBP3226 (1 μM) also raised basal Isc (9.6±2.0 μA cm−2, n=6) while its inactive enantiomer, BIBP3435 (1 μM) had no significant effect (0.6±0.4 μA cm−2, n=6). Y1-mediated Pro34NPY responses (30 nM) were abolished by BIBP3226 (controls; −47.3±7.8 μA cm−2, n=6, and plus BIBP3226; −0.6±0.6 μA cm−2, n=6) while BIBP3435 had no significant effect upon agonist responses (−39.6±13.1 μA cm−2, n=6).
Figure 8.
Blockade of endogenous Y1-inhibitory tone (by BIBO3304, 300 nM) in (a): untreated controls, or (b): following VIP (30 nM) pretreatment of different genotype mouse colon mucosae. Inhibition of Y2-inhibitory tone (with BIIE0246, 1 μM) in naive (c) or VIP-pretreated (d) wild-type (+/+), Y1−/−, Y2−/− or NPY−/− colon mucosae. The dashed line in all histograms represents the vehicle control (DMSO, 0.01%). Data are the mean±1 s.e.m. with n numbers shown in parenthesis. A one-way ANOVA using Dunnett's post-test was used to compare +/+ responses with those in −/− tissues, and *P⩽0.05 was considered significant. A Student's unpaired t-test was used to compare BIBO3304 and BIIE0246 effects with vehicle controls and these significant differences are denoted by hatches (#P⩽0.05, ##P⩽0.01).
The effect of Y1 antagonist, BIBO3304 was predictably absent from Y1−/− colon both in naive tissues (Figure 8a) and following VIP pretreatment (Figure 8b). In untreated +/+ colon mucosa, BIBO3304's effects were insensitive to At and Hex (in controls 6.9±1.4 μA cm−2 (n=24) compared with 6.3±1.3 μA cm−2 (n=12) in experimentals) which suggests that submucous plexus-derived ACh does not contribute to Y1 tone. Interestingly, Y1 inhibitory tone was significant in basal and VIP-pretreated Y2−/− and NPY−/− tissue (Figure 8a and b) and comparable with that observed in +/+ tissues.
The Y2 receptor antagonist, BIIE0246 when added to untreated +/+ tissue did not significantly elevate basal Isc above vehicle control (DMSO; Figure 8c), however, following VIP, it did significantly increase Isc in both +/+ and Y1−/− tissue (Figure 8d). Furthermore, the effects of the competitive Y2 antagonist were predictably absent from Y2−/− tissue and also notably from NPY−/− colonic tissues±VIP (Figure 8c and d). The inhibitory Y2 tone revealed by BIIE0246 following VIP pretreatment of +/+ and Y1−/− tissues was therefore absent from both Y2−/− and NPY−/− mucosae.
Discussion
Characterisation of veratridine-stimulated mucosal responses
Different types of enteric neuron have been classified in guinea pig colon; these include cholinergic secretomotor and noncholinergic secretomotor neurons, interneurons and sensory neurons (Furness, 2000; Furness et al., 2004). In this study, we have used the veratrum alkaloid, veratridine to nonselectively stimulate submucosal neurons. The mixture of neurotransmitters released from stripped mouse jejunum (Sheldon et al., 1990) and rabbit colon (Plass et al., 1994) results ultimately in a sustained mucosal response, recorded as a prolonged increase in Isc. Following stimulation of intrinsic submucosal neurons in mouse descending colon, the balance of neurotransmitter release also appears to be prosecretory and resulted in a prolonged increase in Isc (Figure 1a and b).
Although approximately 20% of submucosal neurons in the mouse colon are cholinergic (Sang & Young, 1998), veratridine-induced responses were not significantly altered by the combination of muscarinic and nicotinic antagonists, At and Hex (Figure 1c). This was also the case in stripped mouse jejunum (Sheldon et al., 1990). These data would suggest that veratridine-stimulated responses in mouse colon and jejunum are predominantly noncholinergic, and implicate a major role for noncholinergic submucosal neurotransmission in contributing to the observed increase in Isc.
NOS and VIP have been colocalised in submucosal cell bodies and fibres in mouse colon (Sang & Young, 1996) however, our preliminary data suggested we would be unable to investigate a role for either neurotransmitter in regulating veratridine-stimulated secretion in this study. In our hands the proposed VPAC1 receptor antagonist, PG 97–269 (Gourlet et al., 1997), did not significantly alter VIP-induced secretory responses in mouse colon. This suggests either that the analogue has low affinity for murine VPAC1 receptors, or the involvement of other VIP receptor types, possibly VPAC2 in mediating the effects of the neuropeptide in this tissue. In addition, the VIP fragment VIP(10–28), which displayed noncompetitive antagonism of VIP responses in rat intestine (Cox & Cuthbert, 1989) did not significantly alter VIP responses in mouse colon (data not shown). Thus, the lack of a selective VPAC receptor antagonist precluded further investigation of this most likely of secretomotor transmitters and its contribution to veratridine-induced mucosal responses.
We also encountered problems when attempting to investigate a role for NO in veratridine-stimulated Isc responses in mouse colon. At similar concentrations used by Rao et al. (1994) in mouse ileum, we found that the NOS inhibitor, Nω-nitro-L-arginine did not alter Isc in colon mucosa. At a low concentration (137 μM) and at higher concentrations (1 and 2 mM) the NO substrate, L-Arg did not alter Isc, however at 3 mM a sustained increase in Isc was observed. It is unlikely that the latter was a specific effect however, as this response was mimicked by D-Arg (3 mM, data not shown).
Y1 receptor-mediated mechanisms
Neither Y1 receptor blockade nor Y1 receptor knockout had significant effects upon veratridine-induced changes in Isc in +/+ mouse colon (Figure 3a). Nevertheless the small increase in veratridine-induced secretion in the presence of BIBO3304 may indicate a minor prejunctional neuronal role for Y1 receptors in wild-type colon. Y1 receptors were colocalised with NOS in human submucosal colonic neurons (Peaire et al., 1997), and in rat small intestinal submucosal neurons (Jackerott & Larsson, 1997). In the hypothalamus NPY actions mediated via Y1 (and Y2) receptors can directly stimulate NOS-containing neurons (Fetissov et al., 2003). Our study has not demonstrated whether NO results in a secretory or antisecretory response in mouse colon. However, if NO resulted in the former, then blockade of prejunctional Y1 receptors could potentially cause release of NO and subsequently enhance secretory responses following veratridine stimulation. This mechanism may also explain the significant attenuation of veratridine responses observed in Y2−/− colon (Figure 5a) following BIBO3304 pretreatment.
Y1 receptors have also been colocalised with VIP in nerve cell bodies throughout the rat intestine in submucosal neurons (Jackerott & Larsson, 1997). If a similar pattern of colocalisation is present in mouse colon submucous innervation, then blockade of Y1 receptors with BIBO3304 could potentially increase VIP release, thereby enhancing veratridine-stimulated secretion. This mechanism might offer an explanation for the slight enhancement observed with BIBO3304 upon veratridine-stimulated ion secretion in +/+ mouse colon (Figure 3a). However, we know that Y1 receptors are located predominantly on epithelia in mouse colon and we suggest that their activation by endogenous PYY provides the majority of the inhibitory Y1 tone we observe in +/+ and NPY−/− (Figure 7a and c) mouse colon mucosa.
Y2 receptor-mediated mechanisms
In contrast to Y1 receptor blockade, Y2 receptor antagonism significantly increased veratridine-induced changes in Isc, although the increase observed in Y2−/− colon was not significant (Figure 4a and b). This suggests that in +/+ colon, Y2 receptors have a significant role in the modulation of enteric submucosal neurotransmission. Use of BIIE0246 has confirmed a prejunctional role for Y2 receptor modulation of neurotransmitter release in a wide range of central and peripheral tissues such as the kidney and spleen (Malmström et al., 2002a, 2002b), hippocampus (Weiser et al., 2000), hypothalamus (King et al., 2000), rat heart, and guinea pig trachea and vas deferens (Smith-White et al., 2001). The Y2 receptor has an autoinhibitory role in the regulation of NPY release. Addition of BIIE0246 to rat hypothalamic slices after neuronal stimulation (King et al., 2000), and following electrical field stimulation of spleen and kidney sympathetic nerves (Malmström et al., 2002a) results in a significant increase in NPY release. Previous studies have demonstrated a role for Y2 receptor-mediated blockade of inhibitory postsynaptic potentials in guinea pig submucosal neurons (Cunningham et al., 1994). Therefore, increased NPY release in the presence of BIIE0246 could subsequently inhibit the release of an antisecretory neurotransmitter (e.g. NPY) in mouse colon submucosal neurons, observed as an increase in veratridine-induced secretion in this study (Figure 4a). Consistent with a prejunctional location for Y2 receptors, previous studies have shown that BIIE0246 effects per se were TTX sensitive (Cox et al., 2003) and here we found Y2-inhibitory tone was absent from NPY−/− colon (Figure 8c and d), suggesting that it is NPY dependent in mouse descending colon.
Y2 receptor knockout increased veratridine-induced secretion but not significantly so and the unusual sensitivity to cholinergic blockade (Figure 4d), accompanied by changes in sensitivity to CCh (data not shown), might suggest a reorganisation of cholinergic neural circuits in the colonic submucous plexi of this null mouse. Further characterisation of veratridine effects in the presence of At and Hex is required in Y2−/− tissue, in order to determine which neurotransmitters are contributing to the enhanced cholinergic component observed in the colon from these knockout mice. Another unexpected finding with Y2−/− colon mucosa was that the Y1 antagonist BIBO3304, significantly attenuated veratridine responses (between 1 and 3 min; Figure 5a) in contrast to a lack of effect in +/+ colon. Thus, absence of Y2 receptors may either have uncovered a functional role for prejunctional Y1 receptors or a compensatory ectopic expression of neuronal Y1 receptors in this null mouse. The latter is unlikely because we have demonstrated that there is no change in the TTX insensitivity of Y1-mediated, Pro34PYY (30 nM) responses in Y2−/− tissues (Cox et al., unpublished). The mechanism by which Y1 receptor blockade decreases veratridine-induced responses in Y2−/− colon could involve nitr-ergic or VIP-ergic pathways, as both NOS and VIP are colocalised with different populations of Y1-positive submucous neuron cell bodies in rat intestine and human colon (Jackerott & Larsson 1997; Peaire et al., 1997). Blockade of Y1 receptors that would usually attenuate antisecretory, rather than secretagogue neurotransmission (e.g. VIP) would result in the blunting of neurogenic responses, as we observed in Y2−/− colon mucosa (Figure 5a).
Absence of NPY reveals PYY-mediated inhibitory mechanisms in mouse colon
NPY−/− mice provided a model that allowed conclusions to be drawn about the mechanism by which endocrine PYY and its product PYY(3–36) modulate submucosal neurotransmission. The decrease in veratridine-stimulated secretion that we observed was unexpected, as NPY added to rat (Cox & Cuthbert, 1988) and mouse (Holliday et al., 2000; Cox et al., 2001) colon mucosae is antisecretory and causes prolonged, concentration-dependent decreases in Isc. In the guinea pig ENS however, Y2 receptors have been implicated in the attenuation of inhibitory postsynaptic potentials in caecal submucosal neurons (Cunningham et al., 1994). This could offer a potential explanation for the significant decrease in veratridine-induced secretion in NPY−/− mouse colon. NPY, depending upon the neurochemical coding of the target neuron, could potentially inhibit secretory, or disinhibit antisecretory neurotransmitter release in the vicinity of the epithelial lining, thereby decreasing veratridine-induced neurogenic increases in Isc (Figure 9). In addition to which, knockout of the NPY gene identified a potential prejunctional neuromodulatory role for PYY and its product PYY(3–36) in mouse colon, as the Y2 antagonist, BIIE0246 alone and in combination with the Y1 antagonist, increased veratridine responses (Figure 7a–c) in the absence of NPY.
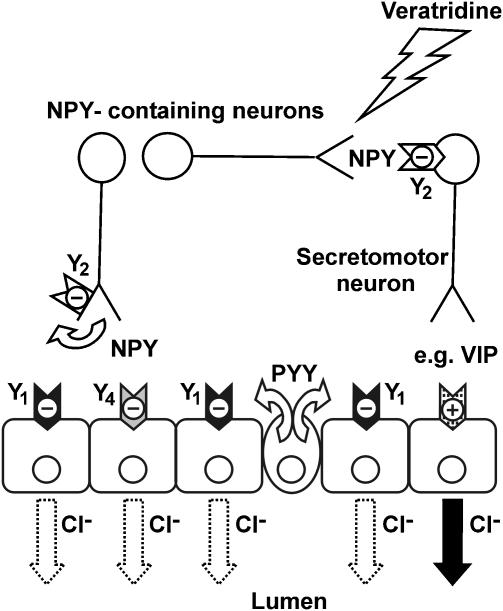
Figure 9.
Schematic diagram depicting potential sites of action for neuronal NPY and endocrine PYY upon different targets in wild-type mouse descending colon mucosa. Veratridine nonselectively stimulates all intrinsic submucous neurons. Endocrine PYY may coactivate neuronal Y2 receptors as well as epithelial Y1 receptors, the latter predominating and resulting in a sustained inhibition of epithelial Cl− secretion. NPY released from submucosal secretomotor neurons can feedback to inhibit further NPY release (a Y2 receptor-mediated effect) and also, when released from interneurons, can inhibit (again via Y2 receptors) other NANC secretomotor neurons. The NANC neurotransmitter in this final secretomotor neuron cannot as yet be positively identified, but is most likely to be VIP. Stimulation of epithelial VIP receptors results in activation of epithelial Cl− secretion, while epithelial Y1 (or Y4) receptor activation will inhibit mucosal anion secretion.
Therefore, in mouse descending colon the antisecretory effect of NPY and PYY is modulated by an indirect mechanism involving Y2 receptors located on noncholinergic neurons (Figure 9). Further studies characterising the neurochemical coding of these noncholinergic submucosal Y2 neurons, that may also express NPY, VIP and/or NOS (Sang & Young, 1996), would greatly enhance our understanding of the nature of neurotransmitter release regulated by this Y receptor type.
Y1 and Y2 receptor-mediated antisecretory tone in the colon
The use of competitive antagonists, selective for either the Y1 or the Y2 receptor has allowed us to establish different ongoing antisecretory tone, the latter being sensitive to TTX and therefore submucous neuron mediated (Cox et al., 2001; Hyland et al., 2003). Using different null mice we observed a predictable loss of Y1 tone from Y1−/− colon (Figure 8a and b) and Y2 tone from Y2−/− colon mucosa (Figure 8c and d). In NPY−/− colon, where the endogenous agonist for both receptor types is PYY released from endocrine cells, Y2 tone is not apparent (Figure 8d), while Y1 tone is present and to the same degree as observed in +/+ colon (Figure 8a and b). We conclude therefore that endogenous PYY predominantly provides the epithelial Y1 receptor-mediated antisecretory tone, while neuronal NPY activates Y2 receptors on, as yet unidentified submucosal nerves to provide Y2 receptor antisecretory tone in mouse colon.
This study has demonstrated the complexity of NPY and Y receptor regulation of epithelial Cl− secretion in mouse colon. The functional end point, a change in Isc is a result of a coordinated effect of neuronal and epithelial Y receptor stimulation. We have demonstrated differential roles for neuronal Y2 receptors and epithelial Y1 receptors. Therefore NPY (or PYY and its product, PYY(3–36)) activating prejunctional Y2 receptors on NANC neurons, depending on the nature of the neuron (secretory or antisecretory), will indirectly attenuate epithelial transport mechanisms, measured as reductions in Isc. Our data would suggest that these intrinsic neurons are secretory in nature. From a therapeutic perspective in vivo human studies have demonstrated a profound antisecretory role for PYY in the small intestine of ileostomy patients (Playford et al., 1990), and i.v. infusion of NPY-inhibited PGE2-stimulated fluid secretion in healthy human volunteers (Holzer-Petsche et al., 1991). Our data describes several pathways that may regulate the antisecretory effect of NPY and PYY in mouse colon. This is particularly relevant as studies from our laboratory using mouse (Holliday et al., 2000; Cox et al., 2001; Hyland et al., 2003) and human (Cox & Tough, 2002; Hyland et al., 2003) isolated colon have demonstrated significant similarities in Y receptor function. Specifically, Y1 receptors are predominantly epithelial, while Y2 receptor mechanisms are neuronal (Cox & Tough, 2002; Hyland et al., 2003) and significant Y1 and Y2 receptor-mediated antisecretory tone exists in both the mouse and the human-isolated colon.
Acknowledgments
This work was funded by the Biotechnology and Biological Sciences Research Council, Genomics and Animal Function Initiative. Dr Herbert Herzog (Garvan Institute for Medical Research, Sydney, Australia) kindly provided wild type, Y1 and Y2 receptor knockout mice, while the NPY+/+ and NPY−/− mice were obtained from Prof David Wynick (Depeartment of Medicine, University of Bristol, U.K.). We thank Boehringer Ingelheim Pharma KG for the Y1 and Y2 receptor antagonists, and Professor Patrick Robberecht (Universite Libre de Bruxelles, Belgium) for PG 97–269. We also thank Professor Keith Sharkey (Department of Physiology and Biophysics, University of Calgary) for helpful discussions in the preparation of this manuscript and Iain Tough for providing the BIBP3226 and BIBP3435 data included in this study.
Abbreviations
- At
atropine
- BIBO3304
((R)-N-[[4-(aminocarbonylaminomethyl)phenyl)methyl]-N2-(diphenylacetyl)-argininamide-trifluoroacetate
- BIBP3226
N2-(diphenylacetyl)-N-[(4-hydroxy-phenyl)methyl]-D-arginine amide
- BIBP3435
(S)-N2-diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]-argininamide (acetate salt)
- BIIE0246
((S)-N2-[[1-[2-[4-[(R,S)-5,11-Dihydro-6(6H)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl] cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5(4H)-dioxo-1,2-diphenyl-3H-1,2,4-triazol-4-yl]ethyl]-argininamide)
- CCh
carbachol
- DMSO
dimethylsulphoxide
- Hex
Hexamethonium
- KH
Krebs–Henseleit
- NOS
nitric oxide synthase
- NPY
neuropeptide Y
- NPY−/−
NPY knockout mice
- PG97–269
[Acetyl-His1, D-Phe2, Lys15, Arg16, Leu17]VIP(3–7)/GRF(8–27)
- PP
pancreatic polypeptide
- Pro34PYY
human [Leu31, Pro34]PYY
- PYY
peptide YY
- PYY(3–36)
peptide YY(3–36)
- TTX
tetrodotoxin
- VIP
vasoactive intestinal polypeptide
- Y1−/−
Y1 receptor knockout
- Y2−/−
Y2 receptor knockout
- +/+
all wild type including NPY+/+, Y1+/+ and Y2+/+
References
- ALLESCHER H.D., KURJAK M., HUBER A., TRUDRUNG P., SCHUSDZIARRA V. Regulation of VIP release from rat enteric nerve terminals: evidence for a stimulatory effect of NO. Am. J. Physiol. 1996;271:G568–G574. doi: 10.1152/ajpgi.1996.271.4.G568. [DOI] [PubMed] [Google Scholar]
- BATTERHAM R.L., COWLEY M.A., SMALL C.J., HERZOG H., COHEN M.A., DAKIN C.L., WREN A.M., BRYNES A.E., LOW M.J., GHATEI M.A., CONE R.D., BLOOM S.R. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- BELAI A., BURNSTOCK G. Release of calcitonin gene-related peptide from rat enteric nerves is Ca2+-dependent but is not induced by K+ depolarization. Regul. Pept. 1988;23:227–235. doi: 10.1016/0167-0115(88)90030-4. [DOI] [PubMed] [Google Scholar]
- CATTERALL W.A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu. Rev. Pharmacol. Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- COX H.M., CUTHBERT A.W. Neuropeptide Y antagonises secretagogue evoked chloride transport in rat jejunal epithelium. Pflugers Arch. 1988;413:38–42. doi: 10.1007/BF00581226. [DOI] [PubMed] [Google Scholar]
- COX H.M., CUTHBERT A.W. Secretory actions of vasoactive intestinal polypeptide, peptide histidine isoleucine and helodermin in rat small intestine: the effects of putative VIP antagonists upon VIP-induced ion secretion. Regul. Pept. 1989;26:127–135. doi: 10.1016/0167-0115(89)90004-9. [DOI] [PubMed] [Google Scholar]
- COX H.M., POLLOCK E.L., TOUGH I.R., HERZOG H. Multiple Y receptors mediate pancreatic polypeptide responses in mouse colon mucosa. Peptides. 2001;22:445–452. doi: 10.1016/s0196-9781(01)00355-2. [DOI] [PubMed] [Google Scholar]
- COX H.M., TOUGH I.R. Neuropeptide Y, Y1, Y2 and Y4 receptors mediate Y agonist responses in isolated human colon mucosa. Br. J. Pharmacol. 2002;135:1505–1512. doi: 10.1038/sj.bjp.0704604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX H.M., TOUGH I.R., HYLAND N.P. Neuropeptide Y Y1 and Y2 receptors mediate inhibitory tone in murine and human colon mucosae. Neurogastroenterol. Motil. 2003;15:195–237. [Google Scholar]
- CUNNINGHAM S.M., MIHARA S., LEES G.M. Y2-receptor-mediated selective inhibition of slow, inhibitory postsynaptic potential in submucous neurons of guinea-pig caecum. Br. J. Pharmacol. 1994;113:883–888. doi: 10.1111/j.1476-5381.1994.tb17075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOODS H., GAIDA W., WIELAND H.A., DOLLOINGER H., SCHNORRENBERG G., ESSER F., ENGEL W., EBERLEIN W., RUDOLF K. BIIE0246: A selective and high affinity neuropeptide Y Y2 receptor antagonist. Eur. J. Pharmacol. 1999;384:R3–R5. doi: 10.1016/s0014-2999(99)00650-0. [DOI] [PubMed] [Google Scholar]
- EKBLAD E., SUNDLER F. Distribution of pancreatic polypeptide and peptide YY. Peptides. 2002;23:251–261. doi: 10.1016/s0196-9781(01)00601-5. [DOI] [PubMed] [Google Scholar]
- FELETOU M., RODRIGUEZ M., BEAUVERGER P., GERMAIN M., IMBERT J., DROMAINT S., MACIA C., BOURRIENNE A., HENLIN J.M., NICOLAS J.P., BOUTIN J.A., GALIZZI J.P., FAUCHERE J.L., CANET E., DUHAULT J. NPY receptor subtypes involved in the contraction of the proximal colon of the rat. Regul. Pept. 1998;75–76:221–229. doi: 10.1016/s0167-0115(98)00072-x. [DOI] [PubMed] [Google Scholar]
- FERRIER L., SEGAIN J., BONNET C., CHERBUT C., LEHUR P., JARRY A., GALMICHE J., BLOTTIERE H. Functional mapping of NPY/PYY receptors in rat and human gastrointestinal tract. Peptides. 2002;23:1765. doi: 10.1016/s0196-9781(02)00133-x. [DOI] [PubMed] [Google Scholar]
- FETISSOV S.O., XU Z.Q., BYRNE L.C., HASSANI H., ERNFORS P., HÖKFELT T. Neuropeptide y targets in the hypothalamus: nitric oxide synthesizing neurons express Y1 receptor. J. Neuroendocrinol. 2003;15:754–760. doi: 10.1046/j.1365-2826.2003.01051.x. [DOI] [PubMed] [Google Scholar]
- FURNESS J.B. Types of neurons in the enteric nervous system. J.Auton. Nerv. Syst. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- FURNESS J.B., JONES C., NURGALI K., CLERC N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog. Neurobiol. 2004;72:143–164. doi: 10.1016/j.pneurobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- GOURLET P., DE NEEF P., CNUDDE J., WAELBROECK M., ROBBERECHT P. In vitro properties of a high affinity selective antagonist of the VIP1 receptor. Peptides. 1997;18:1555–1560. doi: 10.1016/s0196-9781(97)00230-1. [DOI] [PubMed] [Google Scholar]
- GRANDT D., SCHIMICZEK M., BEGLINGER C., LAYER P., GOEBELL H., EYSSELEIN V.E., REEVE J.R. Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1–36 and PYY 3–36. Regul. Pept. 1994;51:151–159. doi: 10.1016/0167-0115(94)90204-6. [DOI] [PubMed] [Google Scholar]
- GREGOR P., FENG Y., DECARR L.B., CORNFIELD L.J., MCCALEB M.L. Molecular characterization of a second mouse pancreatic polypeptide receptor and its inactivated human homologue. J. Biol. Chem. 1996;271:27776–27781. doi: 10.1074/jbc.271.44.27776. [DOI] [PubMed] [Google Scholar]
- HOLLIDAY N.D., POLLOCK E.L., TOUGH I.R., COX H.M. PYY preference is a common characteristic of neuropeptide Y receptors expressed in human, rat, and mouse gastrointestinal epithelia. Can. J. Physiol. Pharmacol. 2000;78:126–133. [PubMed] [Google Scholar]
- HOLZER-PETSCHE U., PETRITSCH W., HINTERLEITNER T., EHERER A., SPERK G., KREJS G.J. Effect of neuropeptide Y on jejunal water and ion transport in humans. Gastroenterology. 1991;101:325–330. doi: 10.1016/0016-5085(91)90007-8. [DOI] [PubMed] [Google Scholar]
- HYLAND N.P., SJÖBERG F., TOUGH I.R., HERZOG H., COX H.M. Functional consequences of neuropeptide Y Y2 receptor knockout and Y2 antagonism in mouse and human colonic tissues. Br. J. Pharmacol. 2003;139:863–871. doi: 10.1038/sj.bjp.0705298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKEROTT M., LARSSON L.I. Immunocytochemical localization of the NPY/PYY Y1 receptor in enteric neurons, endothelial cells, and endocrine-like cells of the rat intestinal tract. J. Histochem. Cytochem. 1997;45:1643–1650. doi: 10.1177/002215549704501207. [DOI] [PubMed] [Google Scholar]
- KING P.J., WILLIAMS G., DOODS H., WIDDOWSON P.S. Effect of a selective neuropeptide Y Y2 receptor antagonist, BIIE0246 on neuropeptide Y release. Eur. J. Pharmacol. 2000;396:R1–R3. doi: 10.1016/s0014-2999(00)00230-2. [DOI] [PubMed] [Google Scholar]
- MALMSTRÖM R.E., LUNDBERG J.N., WEITZBERG E. Effects of the neuropeptide Y Y2 receptor antagonist BIIE0246 on sympathetic transmitter release in the pig in vivo. Naunyn Schmiedebergs Arch. Pharmacol. 2002a;365:106–111. doi: 10.1007/s00210-001-0516-8. [DOI] [PubMed] [Google Scholar]
- MALMSTRÖM R.E., LUNDBERG J.O., WEITZBERG E. Auto-inhibitory function of the sympathetic prejunctional neuropeptide Y Y2 receptor evidenced by BIIE0246. Eur. J. Pharmacol. 2002b;439:113–119. doi: 10.1016/s0014-2999(02)01371-7. [DOI] [PubMed] [Google Scholar]
- MANNON P.J., KANUNGO A., MANNON R.B., LUDWIG K.A. Peptide YY/neuropeptide Y Y1 receptor expression in the epithelium and mucosal nerves of the human colon. Regul. Pept. 1999;83:11–19. doi: 10.1016/s0167-0115(99)00035-x. [DOI] [PubMed] [Google Scholar]
- MEDEIROS M.D., TURNER A.J. Processing and metabolism of peptide-YY: pivotal roles of dipeptidylpeptidase-IV, aminopeptidase-P, and endopeptidase-24.11. Endocrinology. 1994;134:2088–2094. doi: 10.1210/endo.134.5.7908871. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., BECK-SICKINGER A., COX H., DOODS H.N., HERZOG H., LARHAMMAR D., QUIRION R., SCHWARTZ T., WESTFALL T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- PEAIRE A.E., KRANTIS A., STAINES W.A. Distribution of the NPY receptor subtype Y1 within human colon: evidence for NPY targeting a subpopulation of nitrergic neurons. J. Auton. Nerv. Syst. 1997;67:168–175. doi: 10.1016/s0165-1838(97)00101-x. [DOI] [PubMed] [Google Scholar]
- PLASS H., WACHTER C., TURNHEIM K. Neurogenic chloride secretion induced by scorpion venom and veratrine in rabbit colon. Naunyn. Schmiedebergs. Arch. Pharmacol. 1994;350:403–409. doi: 10.1007/BF00178959. [DOI] [PubMed] [Google Scholar]
- PLAYFORD R.J., DOMIN J., BEACHAM J., PARMAR K.B., TATEMOTO K., BLOOM S.R., CALAM J. Preliminary report: role of peptide YY in defence against diarrhoea. Lancet. 1990;335:1555–1557. doi: 10.1016/0140-6736(90)91378-n. [DOI] [PubMed] [Google Scholar]
- RAO R.K., RIVIERE P.J., PASCAUD X., JUNIEN J.L., PORRECA F. Tonic regulation of mouse ileal ion transport by nitric oxide. J. Pharmacol. Exp. Ther. 1994;269:626–631. [PubMed] [Google Scholar]
- SANG Q., YOUNG H.M. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res. 1996;284:39–53. doi: 10.1007/s004410050565. [DOI] [PubMed] [Google Scholar]
- SANG Q., YOUNG H.M. The identification and chemical coding of cholinergic neurons in the small and large intestine of the mouse. Anat. Rec. 1998;251:185–199. doi: 10.1002/(SICI)1097-0185(199806)251:2<185::AID-AR6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- SERVIN A.L., ROUYER-FESSARD C., BALASUBRAMANIAM A., SAINT P.S., LABURTHE M. Peptide-YY and neuropeptide-Y inhibit vasoactive intestinal peptide- stimulated adenosine 3′,5′-monophosphate production in rat small intestine: structural requirements of peptides for interacting with peptide-YY-preferring receptors. Endocrinology. 1989;124:692–700. doi: 10.1210/endo-124-2-692. [DOI] [PubMed] [Google Scholar]
- SHELDON R.J., MALARCHIK M.E., BURKS T.F., PORRECA F. Effects of nerve stimulation on ion transport in mouse jejunum: responses to Veratrum alkaloids. J. Pharmacol. Exp. Ther. 1990;252:636–642. [PubMed] [Google Scholar]
- SMITH-WHITE M.A., HARDY T.A., BROCK J.A., POTTER E.K. Effects of a selective neuropeptide Y Y2 receptor antagonist, BIIE0246, on Y2 receptors at peripheral neuroeffector junctions. Br. J. Pharmacol. 2001;132:861–868. doi: 10.1038/sj.bjp.0703879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOUGH I.R., COX H.M. Selective inhibition of neuropeptide Y Y1 receptors by BIBP3226 in rat and human epithelial preparations. Eur. J. Pharmacol. 1996;310:55–60. doi: 10.1016/0014-2999(96)00372-x. [DOI] [PubMed] [Google Scholar]
- WEISER T., WIELAND H.A., DOODS H.N. Effects of the neuropeptide Y Y2 receptor antagonist BIIE0246 on presynaptic inhibition by neuropeptide Y in rat hippocampal slices. Eur. J. Pharmacol. 2000;404:133–136. doi: 10.1016/s0014-2999(00)00478-7. [DOI] [PubMed] [Google Scholar]
- WIELAND H.A., ENGEL W., EBERLEIN W., RUDOLF K., DOODS H.N. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br. J. Pharmacol. 1998;125:549–555. doi: 10.1038/sj.bjp.0702084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAU W.M., DORSETT J.A., YOUTHER M.L. Calcium-dependent stimulation of acetylcholine release by substance P and vasoactive intestinal polypeptide. Eur. J. Pharmacol. 1986;120:241–243. doi: 10.1016/0014-2999(86)90547-9. [DOI] [PubMed] [Google Scholar]