Abstract
A disturbance in body water homeostasis is a common feature in advanced cirrhosis. This disturbance is always associated with the existence of ascites and is characterized by an inability to adjust the amount of water excreted in the urine to the amount of water ingested. Vasopressin (AVP) is of major importance in the pathogenesis of water retention and hyponatremia in cirrhosis.
The current study assessed the renal, hormonal and hemodynamic effects induced by 10-day chronic oral administration of RWJ-351647 (0.5 mg kg−1 daily), a new nonpeptide V2-AVP antagonist, in rats with CCl4-induced cirrhosis, ascites and severe water retention. Urine volume (UV), urine osmolality and sodium and potassium excretion were measured daily. At the end of the study, systemic hemodynamic parameters were also assessed.
Long-term administration of RWJ-351647 has an aquaretic effect in rats with cirrhosis, ascites, water retention and hypo-osmolality. It increases UV (ANOVA: F=7.32, P<0.0001) and reduces urine osmolality (ANOVA: F=12.69, P<0.0001) throughout the entire period of treatment, thereby leading to a greater renal ability to excrete a water load at the end of the 10-day treatment period (the percentage of water load excreted improved from 30±8 to 92±21%, P<0.025).
The nonpeptide AVP V2-receptor antagonist RWJ-351647 also increased sodium excretion without affecting creatinine clearance and blood pressure.
These data suggest that RWJ-351647 could be therapeutically useful in the treatment of water retention in human cirrhosis.
Keywords: Cirrhosis, hyponatremia, water retention, aquaretics, AVP-receptor antagonist
Introduction
Vasopressin (AVP) is a polypeptide hormone that is released from the posterior pituitary gland in response to changes in blood pressure and plasma osmolality. The biological effects of AVP are mediated by three receptor subtypes: V1a, V2 and V1b. V1 receptors mediate phospholipase C activation and intracellular calcium mobilization. The V1a receptor is located in vascular smooth muscle cells and cardiomyocytes, and modulates vessel vasoconstriction and myocardial function, whereas activation of V1b receptors mainly induces adenocorticotrophin release from the pituitary gland. V2 receptors are distributed on renal collecting duct principle cells and mediate the well-known antidiuretic effects of AVP by activating a cAMP-dependent pathway. AVP is of major importance in the pathogenesis of the water retention and dilutional hyponatremia in cirrhosis with ascites (Arroyo et al., 1994). Two families of agents, which are able to inhibit AVP activity and possess aquaretic effects, have emerged. The first type consists in the κ-opioid receptor agonists, which increase urine volume (UV) and decrease urinary osmolality in humans and experimental animals mainly by inhibiting AVP release (Slizgi & Ludens, 1982; Hamon & Jouquey, 1990). However, κ-opioid agonists are of limited value because they may display some adverse effects, which are probably related to their central mechanism of action (Bellisant et al., 1996). The second type includes several AVP nonpeptide receptor antagonists (Sawyer et al., 1981; Yamamura et al., 1992).
Dual V1a/V2-AVP antagonists have also been described. Investigations performed in our laboratory recently showed that the receptor AVP antagonist, Conivaptan, has an aquaretic effect in rats with cirrhosis, ascites, water retention and hypo-osmolality. However, the renal effects of this agent only occur during the first 5 days of treatment, and after a 10-day treatment period marked water retention is still observed (Fernández-Varo et al., 2003).
A new nonpeptide V2-AVP receptor antagonist, called RWJ-351647, has recently been introduced (Matthews et al., 2004). This compound has been found to be a potent aquaretic in both rats and primates and, therefore, could be of therapeutic value for water retention and dilutional hyponatremia in cirrhosis. At present, however, there is no study on the effect of this new V2-AVP receptor antagonist in cirrhosis with ascites.
Therefore, the current study was aimed to assess the renal, hormonal and hemodynamic effects induced by the chronic oral administration of RWJ-351647 in rats with CCl4-induced cirrhosis, ascites and severe water retention.
Methods
Cirrhosis induction protocol and assessment of water retention
The study was performed in 32 conscious adult male Wistar rats with cirrhosis, ascites and impaired free water excretion. Cirrhosis was induced by repetitive CCl4 inhalation (Clària & Jiménez, 1999). The rats were fed ‘ad libitum' with standard chow and distilled water containing phenobarbital (0.3 g l−1) as drinking fluid. After 1 week of phenobarbital treatment, inhalation of CCl4 was begun. Rats were placed in a gas chamber (70 × 25 × 30 cm3) and air was bubbled (1 l min−1) through a flask containing CCl4. Animals were exposed to the CCl4 vapor atmosphere twice a week (Monday and Friday) starting with 0.5 min per exposure. Afterwards, the duration of exposure was increased to 1 min after three sessions, to 2 min after three more sessions, to 3 min after three more sessions, to 4 min after three more sessions, and then 5 min until the animals developed ascites and water retention. Cirrhotic rats were obtained from a group of 150 animals submitted to the cirrhosis induction protocol. In all, 118 of these animals could not be included in the study for the following reasons: 101 rats died before the development of impairment of water excretion and 17 animals died before completing the experimental protocol. At 2 weeks after cirrhotic rats developed ascites, all animals were placed in metabolic cages and the renal ability to excrete free water was determined once weekly (Tuesday) in each rat submitted to the cirrhosis induction program as follows: 2 h after removing food and water from the metabolic cage, a water load (50 ml kg−1 bw) was administered via a gastric tube inserted following a short inhalation of isoflurane anesthesia. Immediately afterwards, the animals were reintroduced into their metabolic cages where each volume of spontaneously voided urine was collected separately. After 3 h, and following abdominal massage, a final urine sample was obtained. The osmolality of each urine sample was measured. Total volume was measured gravimetrically. The renal ability to excrete free water was estimated through the minimum urinary osmolality (mUOsm) of spontaneously voided samples obtained after the water load and by calculating the percentage (%) of the water load excreted during the 3-h urine collection period. When a significant impairment in the renal ability to excrete free water was detected (% of water load excreted <60% and mUOsm>160 mOsm kg−1), animals were included in the protocol. All studies in cirrhotic rats were performed 24 h after exposing the animals to a short inhalation session of CCl4 (2 min) to avoid spontaneous improvement in free water excretion.
Titration experiments of RWJ-351647 in cirrhotic rats
In all, 20 cirrhotic rats with ascites and impaired renal water excretion were included in the study and randomly assigned to one of the following groups: (1) Single intragastric administration of RWJ-351647 (0.5 mg kg−1, dissolved in 0.5% methyl cellulose), (2) single intragastric administration of RWJ-351647 (1 mg kg−1, dissolved in 0.5% methyl cellulose), (3) single intragastric administration of RWJ-351647 (2.5 mg kg−1, dissolved in 0.5% methyl cellulose and (4) intragastric administration of 0.5% methyl cellulose (4.2 ml kg−1).
Measurements of water intake, UV, urine osmolality (UOsm), urinary excretion of Na+/K+ (UNaV, UKV) and creatinine (UCreatV) were made 1 day prior to the water overload and over the 24 h following treatment. Then, animals were submitted to a second water overload, as previously described, and the mUOsm, % of water excreted and urine creatinine concentration were determined in the 3-h urine collection. Thereafter, the rats were killed and blood samples were obtained to measure serum Na+, K+, creatinine and osmolality.
Chronic effect of RWJ-351647 in cirrhotic rats
In all, 12 cirrhotic rats with ascites and impaired renal water excretion were included in the study and randomly assigned to one of the following groups: (1) intragastric administration of RWJ-351647 (0.5 mg kg−1, dissolved in 0.5% methyl cellulose) administered daily for 10 days (six cirrhotic rats) and (2) intragastric administration of 0.5% methyl cellulose (4.2 ml kg−1) administered daily for 10 days (six cirrhotic rats).
Measurements of 24-h UV and osmolality (UOsm) and UNaV were made 1 day prior to the water overload and for 9 consecutive days after inclusion in the protocol. An aliquot of each 24-h urine collection was frozen at −30°C until analyzed to determine urinary excretion of urea (UureaV), creatinine (UcreatV), vasopressin (UAVPV) and aldosterone (UALDV).
On the 10th day, animals were submitted to a second water overload, as previously described, and the mUOsm and % of water excreted were determined in the 3-h urine collection. Thereafter, animals were included in the following protocol.
At the end of the renal excretory function studies, all cirrhotic rats were anesthetized with Inactine (0.5 ml kg−1 bw) and prepared with PE-50 polyvinyl catheters in the left femoral artery to assess systemic hemodynamic parameters. A blood sample (0.5 ml) was obtained to measure standard parameters of hepatic and renal function. Ringer solution (0.5 ml) replaced the volume of blood sample collected. A midline abdominal incision (2 cm) was made, and the portal vein cannulated through an ileocolic vein with a PE-50 catheter to measure portal pressure (PP, mmHg). After verifying the achievement of free blood reflux, the catheter was fixed to the mesentery with cyanoacrylate glue and the abdomen closed with silk sutures. The right jugular vein was also isolated and a PE-50 catheter placed in the right atrium. A thermocouple (Columbus Instruments, Columbus, OH, U.S.A.) was advanced to the aortic arch through a left carotid approach to monitor the intra-arterial temperature during cardiac output (CO, ml min−1) measurement. Arterial and ileocolic vein catheters were connected to highly sensitive transducers (Hewlett Packard, Avondale, PA, U.S.A.), which were calibrated before each study. Mean arterial pressure (MAP, mmHg), stroke volume (SV, ml min−1) and heart rate (HR, beats min−1) were determined in a microcomputer system (Cardiomax IIR, Columbus Instruments, Columbus, OH, U.S.A.). MAP, HR and PP were continuously recorded in a multichannel system (MX4P and MT4, Lectromed Ltd, Jersey, Channel Islands, U.K.). CO was measured by thermodilution following the administration of a bolus of 200 μl of Ringer solution (20–23°C) into the right atrium. A spring-loaded syringe was used (Hamilton Syringe, model CR-700-200) to insure a constant injection rate and volume. Cardiac index (CI, ml min−1 100 g bw) was calculated by dividing CO by animal weight. Total peripheral resistance (TPR, mmHg min−1 ml−1) was obtained using the formula TPR=MAP/CO. Hemodynamic parameters were allowed to equilibrate for 1 h and values of MAP, PP, HR, CO, SV and TPR recorded. Then, animals were killed and the liver specimens were collected from the middle liver lobe of each animal, fixed in 10% buffered formalin and stained with hematoxylin and eosin, reticulin and Masson's trichrome for histological examination. The study was performed according to the criteria of the Investigation and Ethics Committee of the Hospital Clínic Universitari.
Measurements
Serum and urinary osmolality were determined from osmometric depression of the freezing point (Advanced Instruments Osmometer 3300, Needham, HTs, MA, U.S.A.) and sodium concentration by flame photometry (IL 943, Instrumentation Laboratory, Lexington, MA, U.S.A.). Urinary AVP was determined by radioimmunoassay (Bühlman Laboratories AG, Basel, Switzerland) of unextracted samples as described previously (Camps et al., 1987). The urinary concentration of aldosterone was measured with the use of a commercial kit (Coat-A-Count Aldosterone, Diagnostic and Products Corporation, Los Angeles, CA, U.S.A.), in urine samples (0.5 ml) adjusted to pH 1.0 with 1 ml of 0.2 N HCl and kept during 20 h at 30°C. Using this procedure, most aldosterone-18-glucuronide is transformed into aldosterone (Jiménez et al., 1985). Creatinine and urea were measured by the alkaline picrate technique and enzymatic urease GLDH, respectively, with ADVIA 1650 Instrument (Bayer Diagnostic Europe Ltd, Pittsburgh, PA, U.S.A.).
Serum RWJ-351647 concentrations were determined using validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) methodologies. The assay supporting the pharmacokinetic study in rats had a lower limit of quantification (LLOQ) of 0.1 ng ml−1 (0.19 nM).
Statistical analysis
Statistical analysis of the results was performed by one-way or two-way analysis of variance (ANOVA), the Newman–Keuls and Bonferroni tests, and the paired and unpaired Student's t-tests when appropriate. Results are given as mean±s.e.m. and considered significant at a P-level of 0.05 or less.
Results
Histological examination of the liver specimens obtained from CCl4-treated rats showed cirrhosis in all animals, with no significant differences between rats receiving RWJ-351647 or vehicle.
Table 1 shows that cirrhotic rats included in the dose–response protocol were investigated after they had developed marked sodium retention (Clària et al., 1991; Jiménez et al., 2000; Fernández-Varo et al., 2003) and severely impaired renal ability to excrete free water. The impairment of water excretion occurred within the range of 13–30 weeks after starting the cirrhosis induction program. Ascites preceded the impairment in free water excretion by at least 2 weeks. No differences were found in the baseline renal response to water overload and UNaV in cirrhotic rats receiving the administration of a single oral dose of the V2-AVP receptor antagonist or vehicle.
Table 1.
Body weight, renal response to the water load and urinary excretion of sodium in baseline conditions in cirrhotic rats prior to receiving different doses of RWJ-351647 or vehicle
| Vehicle (n=5) | RWJ-351647 (0.5 mg kg−1) (n=5) | RWJ-351647 (1 mg kg−1) (n=5) | RWJ-351647 (2.5 mg kg−1) (n=5) | |
|---|---|---|---|---|
| Body wt (g) | 508±13 | 510±30 | 457±22 | 493±18 |
| % Water load excreted | 28±5 | 20±6 | 18±5 | 25±6 |
| mUOsm (mOsm kg−1) | 347±100 | 470±91 | 538±293 | 435±269 |
| UNaV (μEq min−1) | 0.40±0.1 | 0.2±0.08 | 0.18±0.06 | 0.2±0.09 |
At 24 h after the oral administration (Table 2), no significant differences were observed in the renal response to the water load, mUOsm, and serum sodium and serum osmolality between cirrhotic rats receiving a single oral dose of RWJ-351647 or vehicle. Neither were differences observed in creatinine release. The absence of modifications in renal water handling 24 h after the administration of RWJ-351647 to cirrhotic rats is likely due to the fact that the diuretic effect of RWJ-351647 peaks 4–8 h after its administration and does not extend over periods of time longer than 10 h. The results obtained with the highest dose of antagonist (2.5 mg kg−1, dissolved in 0.5% methyl cellulose) are not shown because this dose induced a massive diuretic effect in cirrhotic rats with ascites that eventually led to animal death due to dehydration.
Table 2.
Body weight, renal response to the water load, CCreat and urinary excretion of sodium, serum sodium and serum osmolality 24 h after the administration of a single oral dose of the V2-AVP receptor antagonist or vehicle to cirrhotic rats included in the protocol
| Vehicle (n=5) | RWJ-351647 (0.5 mg kg−1) (n=5) | RWJ-351647 (1 mg kg−1) (n=5) | |
|---|---|---|---|
| Body wt (g) | 504±10 | 480±24 | 420±23 |
| % Water load excreted | 38±8 | 30±10 | 33±14 |
| mUOsm (mOsm kg−1) | 211±91 | 394±212 | 525±379 |
| CCreat (ml min−1) | 1.26±0.4 | 3.8±2.4 | 6.2±2.4 |
| Serum sodium (mEq l−1) | 136±0.6 | 140±2 | 139±3 |
| Serum osmolality (mOsm kg−1) | 285±1 | 291±4 | 294±5 |
Table 3 shows urine flow, urine osmolality, and sodium, potassium and creatinine excretion in baseline conditions and during the initial 24 h after the administration of the corresponding dose of RWJ-351647 or vehicle. At doses of 0.5 and 1 mg kg−1 of RWJ-351647, a significant increase in UV and a marked decrease in UOsm were observed. No differences in UNaV, UKV and UCreatV were found between these two groups of treated cirrhotic rats. The serum levels of RWJ-351647 measured 24 h after dosing were 0.55±0.16 and 1.58±0.81 nM for animals given 0.5 and 1.0 mg kg−1, respectively. In two animals given 2.5 mg kg−1, the average serum levels after 24 h were 19.7 nM.
Table 3.
Values of UV, UOsm, UNaV, and UCreatV in baseline conditions and during the initial 24 h after the administration of a single oral dose of the V2-AVP receptor antagonist or vehicle to cirrhotic rats included in the protocol
| Vehicle (n=5) | RWJ-351647 (0.5 mg kg−1) (n=5) | RWJ-351647 (1 mg kg−1) (n=5) | ||||
|---|---|---|---|---|---|---|
| BASAL | 24 h | BASAL | 24 h | BASAL | 24 h | |
| UV (ml day−1) | 10.3±2 | 10.9±2 | 9.7±1 | 86±26*,a | 9±3 | 81±17**,b |
| UOsm (mOsm kg−1) | 1919±176 | 1916±198 | 1928±137 | 434±119**,c | 1653±474 | 326±106***,c |
| UNaV (mEq day−1) | 0.37±0.2 | 0.36±0.17 | 0.28±0.1 | 2.5±1 | 0.26±0.09 | 1.1±0.5 |
| UKV (mEq day−1) | 2.5±0.4 | 2.9±0.7 | 2.5±0.2 | 2.7±0.5 | 1.4±0.5 | 1.56±0.3 |
| UCreatV (mg h−1) | 0.54±0.09 | 0.57±0.1 | 0.47±0.03 | 0.34±0.07 | 0.33±0.08 | 0.37±0.04 |
aP<0.025, bP<0.01 and cP<0.001 vs vehicle (unpaired Student's t-test).
*P<0.05, **P<0.025 and ***P<0.01 vs basal at the same doses (paired Student's t-test).
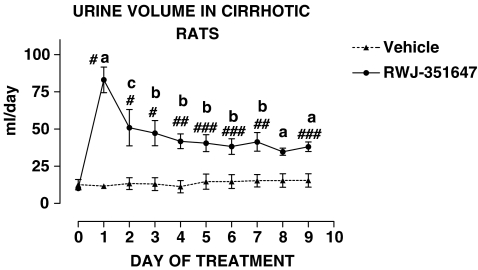
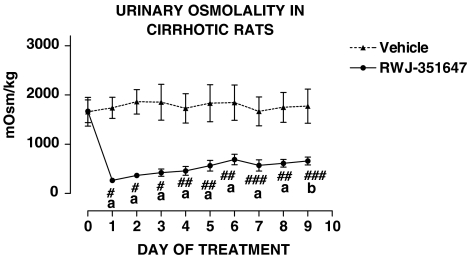
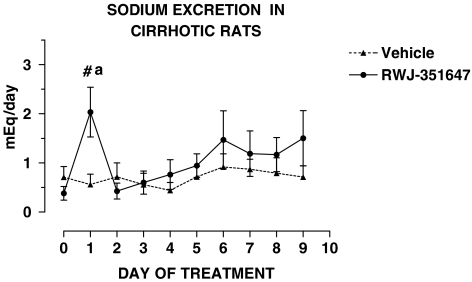
Table 4 shows that cirrhotic rats included in the chronic treatment protocol were investigated after they had developed marked sodium retention and severely impaired renal ability to excrete free water. The impairment of water excretion occurred within the range of 12–35 weeks after starting the cirrhosis induction program. Ascites preceded the impairment in free water excretion by at least 2 weeks. No differences were found in the baseline renal response to water overload and UNaV in cirrhotic rats receiving the V2-AVP receptor antagonist or vehicle. The effect of RWJ-351647 or vehicle on urine flow and urinary osmolality is shown in Figures 1 and 2, respectively. No significant changes were observed in any of these parameters throughout the study in cirrhotic rats receiving vehicle. In contrast, the V2-AVP receptor antagonist significantly increased urine flow (ANOVA: F=7.32, P<0.0001) and decreased UOsm (ANOVA: F=12.69, P<0.0001) in cirrhotic rats throughout the entire period of treatment. The aquaretic effect of RWJ-351647 was, however, more intense during the first 2 days of treatment and remained rather constant thereafter. As shown in Figure 3, RWJ-351647 also induced a significant natriuretic effect (ANOVA: F=2.13, P<0.05) in cirrhotic rats during the first 24 h and disappeared thereafter.
Table 4.
Body weight, renal response to the water load and urinary excretion of sodium in baseline conditions in cirrhotic rats prior to chronic treatment with RWJ-351647 or vehicle
| RWJ-351647 (n=6) | Vehicle (n=6) | |
|---|---|---|
| Body wt (g) | 510±23 | 540±17 |
| % Water load excreted | 38±8 | 32±6 |
| mUOsm (mOsm kg−1) | 254±70 | 233±27 |
| UNaV (μEq min−1) | 0.26±0.14 | 0.49±0.1 |
Figure 1.
UV in basal conditions and during the entire period of treatment in cirrhotic rats with ascites and water retention receiving RWJ-351647 (0.5 mg kg−1 bw) or vehicle. (a) P<0.001, (b) P<0.01 and (c) P<0.05 vs basal values (one-way ANOVA and Newman–Keuls tests). #P<0.001, ##P<0.01 and ###P<0.025 vs vehicle on the same day of treatment (two-way ANOVA and Bonferroni test).
Figure 2.
Urinary osmolality in basal conditions and during the entire period of treatment in cirrhotic rats with ascites and water retention receiving RWJ-351647 (0.5 mg kg−1 bw) or vehicle. (a) P<0.01 and (b) P<0.25 vs basal values (one-way ANOVA and Newman–Keuls tests). #P<0.001, ##P<0.01 and ###P<0.05 vs vehicle on the same day of treatment (two-way ANOVA and Bonferroni test).
Figure 3.
Urinary sodium excretion in basal conditions and during the entire period of treatment in cirrhotic rats with ascites and water retention receiving RWJ-351647 (0.5 mg kg−1 bw) or vehicle. (a) P<0.05 vs basal values (one-way ANOVA and Newman–Keuls tests). #P<0.05 vs vehicle on the same day of treatment (two-way ANOVA and Bonferroni test).
After completing the study (Table 5), no significant differences were observed in serum sodium and serum osmolality between cirrhotic rats treated or not treated with RWJ-351647. Treatment with RWJ-351647, however, was associated with a significant amelioration in the renal response to the water load, with the figures of % of water load excreted being within normal values.
Table 5.
Body weight, renal response to the water load, serum sodium and serum osmolality in the cirrhotic rats after completing chronic treatment with RWJ-351647 or vehicle
| RWJ-351647 (n=6) | Vehicle (n=6) | |
|---|---|---|
| Body wt (g) | 517±37 | 533±29 |
| % Water load excreted | 92±21 | 33±13a |
| mUOsm (mOsm kg−1) | 156±72 | 314±122 |
| Serum sodium (mEq l−1) | 145±1 | 143±1 |
| Serum osmolality (mOsm kg−1) | 298±3 | 297±4 |
P<0.05 with respect to treated rats (unpaired Student's t-test).
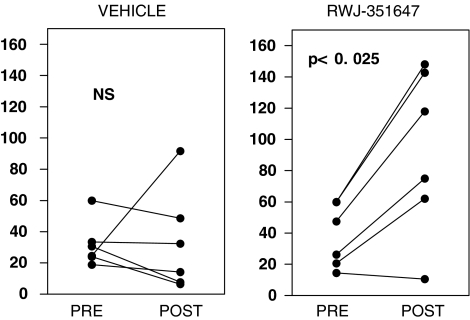
Figure 4 shows the individual values of the % of water load excreted obtained in baseline conditions and after completing the treatment in cirrhotic rats. The improvement in the renal water handling in cirrhotic rats was so remarkable that, at the end of the treatment, only one cirrhotic animal still showed a % of water load excreted lower than 60% (Figure 4).
Figure 4.
Individual values of the % of the water load excreted observed in cirrhotic rats with ascites and impaired water excretion before and after completing the daily chronic oral administration of vehicle or RWJ-351647 (0.5 mg kg−1 bw). NS: not significant (paired Student's t-test).
Table 6 shows the average values of UNaV, UKV, UUreaV, UCreatV, UALDV and UAVPV during the entire period of treatment and creatinine clearance (CCreat) at the end of the study in cirrhotic rats receiving vehicle or RWJ-351647. Chronic administration of RWJ-351647 to cirrhotic rats was associated with increased sodium and AVP excretion, a diminution in UALDV and no changes in CCreat in comparison to cirrhotic rats receiving vehicle.
Table 6.
Average values of UNaV, UUreaV, UCreatV, UALDV, and UAVPV during the entire period of treatment and creatinine clearance (CCreat) at the end of the study in cirrhotic rats included in the study
| RWJ-351647 (n=6) | Vehicle (n=6) | |
|---|---|---|
| UNaV (mEq day−1) | 1.12±0.1a | 0.7±0.07 |
| UKV (mEq day−1) | 2.2±0.1 | 2.3±0.1 |
| UUreaV (mg h−1) | 22.9±1.0 | 23.0±0.7 |
| UCreatV (mg h−1) | 0.44±0.01 | 0.45±0.01 |
| UALDV (ng day−1) | 44.6±3b | 134.3±14 |
| UAVPV (ng day−1) | 12.5±0.74b | 6.0±0.8 |
| CCreat (ml min−1) | 1.4±0.18 | 1.3±0.12 |
aP<0.05 and bP<0.0001 vs vehicle (unpaired Student's t-test).
Table 7 shows MAP, CO, SV, HR and PP obtained after completing the 10-day treatment period in cirrhotic rats included in the systemic hemodynamic study. Two cirrhotic rats (one treated with the V2-AVP receptor antagonist and one receiving vehicle) died within 5 h after the administration of the second water overload; thus, the table shows values of five and five cirrhotic rats receiving vehicle or RWJ-351647, respectively. Treatment with RWJ-351647 did not result in any significant difference as compared to cirrhotic rats receiving vehicle.
Table 7.
MAP, PP, HR, SV, CO, CI, and TPR in cirrhotic rats receiving RWJ-351647 or vehicle
| RWJ-351647 (n=5) | Vehicle (n=5) | |
|---|---|---|
| MAP (mmHg) | 91±3 | 92±4 |
| PP (mmHg) | 11.4±0.24 | 12.6±0.6 |
| HR (beats min−1) | 387±14 | 382±12 |
| SV (ml min−1) | 1002±91 | 994±157 |
| CO (ml min−1) | 390±29 | 377±37 |
| CI (ml min−1 100 g bw) | 3.9±0.3 | 3.7±0.4 |
| TPR (mmHg min ml−1) | 0.24±0.02 | 0.27±0.04 |
At the end of the hemodynamic study, serum levels of RWJ-351647 in treated cirrhotic rats were of 2.70±0.62 nM. In the reference group of control rats studied under identical conditions, this figure was of 0.27±0.05 nM. RWJ-351647 did not induce any significant effect on UV, UOsm, and UNaV, in these animals.
Discussion
The development of selective inhibitors of AVP activity represents a promising treatment to antagonize the effects of elevated plasma AVP concentrations. Increased AVP levels play a major role in the pathogenesis of several oedematous disorders with dilutional hyponatremia such as advanced cardiac failure, nephrotic syndrome and syndrome of inappropriate secretion of antidiuretic hormone and cirrhosis (Arroyo & Jiménez, 2003). AVP-mediated water reabsorption in the collecting tubule evolves from several sequential steps initiated by the interaction of the hormone with the V2-AVP receptor, which induces an increase in intracellular cAMP that ultimately results in the insertion of the water channel aquaporin-2 (AQP2) into the luminal membrane of the cell (Nielsen et al., 2002). This facilitates active water transport from the tubular lumen to the intracellular space. Therefore, from a theoretical point of view, water reabsorption can be modified by reducing the circulating levels of AVP, by specifically blocking AVP receptors, by modifying cAMP formation or by inhibiting the activity of the AVP-regulated water channel (Jard, 1988).
The first possibility can be accomplished by administering κ-opioid receptor agonists (Slizgi & Ludens, 1982). However, although these compounds have proven to be effective in inducing aquaresis in humans and experimental animals (Bosch-Marcé et al., 1995), their potential central side effects have precluded their clinical use, so far. On the other hand, the last two possibilities cannot be considered since renal cAMP inhibition with demeclocycline may deteriorate renal function under certain pathological conditions (Pérez-Ayuso et al., 1984), and no inhibitor of AQP2 is currently available. Consequently, it is not surprising that AVP-receptor blockade has been the therapeutic strategy most extensively investigated.
The feasibility of interfering with AVP binding as a potentially useful strategy was first demonstrated by several V2-AVP receptor antagonists. However, their peptide nature hampered their clinical use because of poor oral bioavailability, partial agonistic action or species-specific properties (Allison et al., 1988). Manning et al. (1987) demonstrated that the ring structure of AVP is not necessary for the interaction of the hormone with the AVP receptor, thereby allowing development of nonpeptide selective V2-AVP receptor antagonists. A number of compounds have been evaluated. Among the most extensively characterized are those of the OPC family (31260 and 41061) and VPA-985 (Albright et al., 1998) and SR121463 (Serradeil-Le Gal, 1998). Single oral administration of OPC-31260 has been shown to induce a potent aquaretic effect in humans, dogs and rats under normal or pathological conditions. This compound, however, failed to show continuous aquaresis in cirrhotic rats in a 10-day treatment regime and displayed antagonist V1 activity (V1/V2 antagonism: 1/10) in human renal tissue (Jiménez et al., 2000). More recently, OPC-41061 has shown improved selectivity for V2 receptors (V1/V2 antagonism: 1/29) and produces aquaresis after multiple dosing in normal rats (Wong & Verbalis, 2001). VPA-985 is a highly selective V2 antagonist that increases water excretion, serum sodium and osmolality. Its aquaretic efficacy has been tested in cirrhotic patients who experienced a dose-dependent augmentation in urinary flow and osmolality at single doses up to 300 mg. In these patients, orthostatic pulse rate elevation or hypotension, secondary to fluid loss, were recorded (Guyader et al., 2002; Wong et al., 2003). In addition, VPA-985 is able to correct hyponatremia in patients with cirrhosis and ascites (Gerbes et al., 2003). Finally, the pharmacological and aquaretic properties of SR121463 have been characterized in several in vivo and in vitro models. This agent is devoid of any agonistic effect and shows a highly competitive affinity for V2 receptors in rat and human kidney. Previous investigations have demonstrated that SR121463 has a powerful aquaretic effect in cirrhotic rats with ascites, water retention and hypo-osmolality (Jiménez et al., 2000). The effect of a dual V1/V2-AVP receptor antagonist, namely Conivaptan, has also been investigated. This agent has shown to display aquaretic efficacy in several experimental models characterized by oedema formation and hyponatremia (Tahara et al., 1997). Furthermore, Conivaptan improves water metabolism in patients with acute or congestive heart failure (Udelson et al., 2001; Lee et al., 2003). Recently, Conivaptan also displayed an aquaretic effect in cirrhotic rats with ascites, although the effect only lasted for the first 5 days of treatment and at the end of the study animals still showed water retention when challenged with a water load (Fernández-Varo et al., 2003).
As expected, and in comparison to previous studies performed in our laboratory under identical experimental conditions (Bosch-Marce et al., 1999; Jiménez et al., 2000; Fernández-Varo et al., 2003), cirrhotic rats receiving vehicle showed marked sodium retention (UNaV in normal rats is ≈1.2 mEq min−1) which occurred in the setting of pronounced hyperaldosteronism (UALDV in normal rats is ≈21.1 ng day−1) and a marked increase in UAVPV (UAVPV in normal rats is ≈2.3 ng day−1), without significant changes in creatinine, urea or K+ excretion. This was associated with marked hyperkinetic circulation characterized by arterial hypotension (MAP in normal rats is ≈123 mmHg), portal hypertension (PP in normal rats is ≈6 mmHg), high CO (CO in normal rats is ≈215 ml min−1) and decreased peripheral resistance (TPR in normal rats is ≈0.58 mmHg min ml−1).
In the current study, the long-term oral administration of the V2-receptor antagonist RWJ-351647 to cirrhotic rats with ascites and water retention resulted in an acute increase in the urinary flow rate and a reduction in UOsm. However, contrary to what occurs with most V2-AVP receptor antagonists (Jiménez et al., 2000), RWJ-351647 displayed aquaretic efficacy throughout the entire period of treatment and, at the end of the investigation, all but one cirrhotic rat receiving the V2-AVP receptor antagonist exhibited renal water handling within the normal range. The sustained effect of RWJ-351647 was related to its chemical structure based on oxazinobenzodiazepine templates that result in excellent V2 affinity (Ki=0.9 nM) and favorable oral bioavailability (Matthews et al., 2004).
An unexpected result of the current investigation was the remarkable reduction in aldosterone excretion induced by RWJ-351647 in cirrhotic rats, which was associated with a significant improvement in sodium excretion during the first day of treatment. During the entire period of treatment, the average UALDV in cirrhotic animals chronically treated with the V2-AVP receptor antagonist was around three-fold lower than in cirrhotic rats treated with vehicle. Conversely, the average UNaV was approximately 60% higher in cirrhotic-treated rats than in cirrhotic animals receiving vehicle. A possible effect of RWJ-351647 on the renin–angiotensin system is unlikely since no differences in MAP were observed between treated or nontreated cirrhotic rats. An alternative explanation could be a direct inhibitory effect of RWJ-351647 on aldosterone synthesis.
AVP hypersecretion in cirrhosis is generally considered to be a compensatory mechanism to the arterial vasodilation occurring in advanced liver disease (Arroyo et al., 1994). Blockade of V1-AVP receptors in cirrhotic rats further deteriorates MAP in these animals (Clària et al., 1991). Conversely, it is theoretically possible that any increase in the circulating levels of AVP in experimental cirrhosis may have hemodynamic consequences. Chronic administration of RWJ-351647 actually increased AVP levels since the urinary excretion of this hormone was approximately twice that in rats treated with this agent than in those receiving vehicle. However, the increase in AVP levels did not result in any effect on MAP and/or TPR since long-term administration of RWJ-351647 did not modify any parameter in either cirrhotic rats treated or not treated with the antagonist.
In summary, the results of the current investigation indicate that long-term administration of RWJ-351647 has a potent and sustained aquaretic effect in rats with cirrhosis, ascites, water retention and hypo-osmolality. In fact, this agent increased UV and reduced UOsm throughout the entire period of treatment, resulting in a greater renal ability to excrete a water load. The nonpeptide V2-AVP receptor antagonist also increased UNaV without affecting CCreat and MAP. These data suggest that RWJ-351647 may be therapeutically useful in the treatment of water retention in human cirrhosis.
Acknowledgments
This work was supported by grants from Dirección General de Investigación Científica y Técnica (SAF 03/02597), Fondo de Investigación Sanitaria (FIS 02/0588) and Johnson & Johnson Pharmaceutical Research & Development. J.M.-L. had a grant from Dirección General de Investigación Científica y Técnica (SAF 03/02597).
Abbreviations
- AVP
vasopressin
- CCreat
creatinine clearance
- CI
cardiac index
- CO
cardiac output
- HR
heart rate
- MAP
mean arterial pressure
- mUOsm
minimum urinary osmolality
- PP
portal pressure
- SV
stroke volume
- TPR
total peripheral resistance
- UALDV
urinary excretion of aldosterone
- UAVPV
urinary excretion of vasopressin
- UCreatV
urinary excretion of creatinine
- UKV
urine potassium
- UNaV
urine sodium
- UOsm
urine osmolality
- UureaV
urinary excretion of urea
- UV
urine volume
- %
percentage
References
- ALBRIGHT J.D., REICH M.F., DELOS SANTOS E.G., DUSZA J.P., SUM F.W., VENKATESAN A.M., COUPET J., CHAN P.S., RU X., MAZANDARANI H., BAILEY T. 5-Fluoro-2-methyl-N-[4-(5H-pyrrolo[2,1-c]-[1,4]benzodiazepin-10(11H)-ylcarbonyl)-3-chlorophenyl]benzamide (VPA-985): an orally active arginine vasopressin antagonist with selectivity for V2 receptors. J. Med. Chem. 1998;41:2442–2444. doi: 10.1021/jm980179c. [DOI] [PubMed] [Google Scholar]
- ALLISON N.L., ALBRIGHTSON-WINSLOW C.R., BROOKS D.P., STASSEN F.L., HUFFMAN W.F., STOTE R.M., KINTER L.B.Species heterogeneity and antidiuretic hormone antagonists: what are the predictors Vasopressin: Cellular and Integrative Functions 1988New York, NY: Raven Press Ltd; 207–214.ed. Cowley, A.W., Liard, J.F. & Ausiello, D.A. pp [Google Scholar]
- ARROYO V., CLÀRIA J., SALÓ J., JIMÉNEZ W. Antidiuretic hormone and pathogenesis of water retention in cirrhosis with ascites. Semin. Liver Dis. 1994;14:44–58. doi: 10.1055/s-2007-1007297. [DOI] [PubMed] [Google Scholar]
- ARROYO V., JIMÉNEZ W. Clinical need for antidiuretic hormone antagonists in cirrhosis. Hepatology. 2003;37:13–15. doi: 10.1053/jhep.2003.50025. [DOI] [PubMed] [Google Scholar]
- BELLISANT E., DENOLLE T., SINNASSAMY P., BICHET D.G., GIUDICELLI J.F., LECOZ F., GANDON J.M., ALLAIN H. Systemic and regional hemodynamic and biological effects of a new κ-opioid agonist, niravoline, in healthy volunteers. J. Pharmacol. Exp. Ther. 1996;278:232–242. [PubMed] [Google Scholar]
- BOSCH-MARCÉ M., JIMÉNEZ W., ANGELI P., LEIVAS A., CLÀRIA J., GRAZIOTTO A., ARROYO V., RIVERA F., RODÉS J. Aquaretic effect of the κ-opioid agonist RU 51599 in cirrhotic rats with ascites and water retention. Gastroenterology. 1995;109:217–223. doi: 10.1016/0016-5085(95)90287-2. [DOI] [PubMed] [Google Scholar]
- BOSCH-MARCE M., POO J.L., JIMÉNEZ W., BORDAS N., LEIVAS A., MORALES-RUIZ M., MUÑOZ R.M., PÉREZ M., ARROYO V., RIVERA F., RODÉS J. Comparison between two aquaretic drugs (niravoline vs OPC-31260) in cirrhotic rats with ascites and water retention. J. Pharmacol. Exp. Ther. 1999;289:194–201. [PubMed] [Google Scholar]
- CAMPS J., SOLÁ J., ARROYO V., PÉREZ-AYUSO R.M., GAYA J., RIVERA F., RODÉS J. Temporal relationship between the impairment of free water excretion and antidiuretic hormone hypersecretion in rats with experimental cirrhosis. Gastroenterology. 1987;93:498–505. doi: 10.1016/0016-5085(87)90911-5. [DOI] [PubMed] [Google Scholar]
- CLÀRIA J., JIMÉNEZ W.Renal dysfunction and ascites in carbon-tetrachloride-induced cirrhosis in rats Ascites and Renal Dysfunction in Liver Disease. Pathogenesis Diagnosis and Treatment 1999Blackwell: Science Inc; 379–396.ed. Arroyo, V., Ginès, P., Rodés, J., Schrier, R.W., pp [Google Scholar]
- CLÀRIA J., JIMÉNEZ W., ARROYO V., LA VILLA G., LÓPEZ C., ASBERT M., CASTRO A., GAYA J., RIVERA F., RODÉS J. Effect of V1-vasopressin receptor blockade on arterial pressure in conscious rats with cirrhosis and ascites. Gastroenterology. 1991;100:494–501. doi: 10.1016/0016-5085(91)90222-7. [DOI] [PubMed] [Google Scholar]
- FERNÁNDEZ-VARO G., ROS J., CEJUDO-MARTÍN P., CANO C., ARROYO V., RIVERA F., RODÉS J., JIMÉNEZ W. Effect of the V1a/V2-AVP receptor antagonist, Conivaptan, on renal water metabolism and systemic hemodynamics in rats with cirrhosis and ascites. J. Hepatol. 2003;38:755–761. doi: 10.1016/s0168-8278(03)00116-8. [DOI] [PubMed] [Google Scholar]
- GERBES A.L., GÜLBERG V., GINÈS P., DECAUX G., GROSS P., GANDJINI H., DJIAN J., The VPA Study Group Therapy of hyponatremia in cirrhosis with a vasopressin receptor antagonist: a randomized double-blind multicenter trial. Gastroenterology. 2003;124:933–939. doi: 10.1053/gast.2003.50143. [DOI] [PubMed] [Google Scholar]
- GUYADER D., PATAT A., ELLIS-GROSSE E.J., ORCZYK G.P. Pharmacodynamic effect of a nonpeptide antidiuretic hormone V2 antagonist in cirrhotic patients with ascites. Hepatology. 2002;36:1197–1205. doi: 10.1053/jhep.2002.36375. [DOI] [PubMed] [Google Scholar]
- HAMON G., JOUQUEY S. Kappa agonists and vasopressin secretion. Horm. Res. 1990;34:129–132. doi: 10.1159/000181811. [DOI] [PubMed] [Google Scholar]
- JARD S. Mechanisms of action of vasopressin and vasopressin antagonists. Kidney Int. 1988;34:S38–S42. [PubMed] [Google Scholar]
- JIMÉNEZ W., MARTINEZ-PARDO A., ARROYO V., BRUIX J., RIMOLA A., GAYA J., RIVERA F., RODÉS J. Temporal relationship between hyperaldosteronism, sodium retention and ascites formation in rats with experimental cirrhosis. Hepatology. 1985;5:245–250. doi: 10.1002/hep.1840050215. [DOI] [PubMed] [Google Scholar]
- JIMÉNEZ W., SERRADEIL-LE GAL C., ROS J., CANO C., CEJUDO P., MORALES-RUIZ M., ARROYO V., PASCAL M., RIVERA F., MAFFRAND J.P., RODÉS J. Long-term aquaretic efficacy of a selective nonpeptide V2-vasopressin receptor antagonist, SR121463, in cirrhotic rats. J. Pharmacol. Exp. Ther. 2000;295:83–90. [PubMed] [Google Scholar]
- LEE C.R., WATKINS M.L., PETTERSON J.H., GATTIS W., O'CONNOR C.M., GHEORGHIADE M., ADAMS K.F. Vasopressin: a new target for the treatment of heart failure. Am. Heart J. 2003;146:9–18. doi: 10.1016/S0002-8703(02)94708-3. [DOI] [PubMed] [Google Scholar]
- MANNING M., PRZYBYLSKI J.P., OLMA A., KLIS W.A., KRUSZYNSKI M., WO N.C., PELTON G.H., SAWYER W.H. No requirement of cyclic conformation of antagonists in binding to vasopressin receptors. Nature. 1987;329:839–840. doi: 10.1038/329839a0. [DOI] [PubMed] [Google Scholar]
- MATTHEWS J.M., HOEKSTRA W.J., DYATKIN A.B., HECKER L.R., HLASTA D.J., POULTER B.L., ANDRADE-GORDON P., DE GARAVILLA L., DEMAREST K.T., ERICSON E., GUNNET J.W., HAGEMAN W., LOOK R., MOORE J.B., REYNOLDS C.H., MARYANOFF B.E. Potent nonpeptide vasopressin receptor antagonists based on oxazino- and thiazinobenzodiazepine templates. Bioorg. Med. Chem. Let. 2004;14:2747–2752. doi: 10.1016/j.bmcl.2004.03.083. [DOI] [PubMed] [Google Scholar]
- NIELSEN S., FROKIAER J., MARPLES D., KWON T.H., AGRE P., KNEPPER M.A. Aquaporins in the kidney: from molecules to medicine. Physiol. Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- PÉREZ-AYUSO R.M., ARROYO V., CAMPS J., JIMÉNEZ W., RODAMILANS M., RIMOLA A., GAYA J., RIVERA F., RODÉS J. Effect of demeclocycline on renal function and urinary prostaglandin E2 and kallikrein in hyponatremic cirrhosis. Nephron. 1984;36:30–37. doi: 10.1159/000183112. [DOI] [PubMed] [Google Scholar]
- SAWYER W.H., PANG P.K.T., SETO J., MCENROE M., LAMMEK B., MANNING M. Vasopressin analogues that antagonize antidiuretic responses by rats to the antidiuretic hormone. Science (Washington, DC) 1981;312:49–51. doi: 10.1126/science.7209515. [DOI] [PubMed] [Google Scholar]
- SERRADEIL-LE GAL C. Nonpeptide antagonist for vasopressin receptors-pharmacology of SR 121463A, a new potent and highly selective V2 receptor antagonist. Vasopressin Oxytocin. 1998;449:427–438. [PubMed] [Google Scholar]
- SLIZGI G.R., LUDENS J.H. Studies on the nature and mechanism of the diuretic activity of the opioid analgesic ethylketocyclazocine. J. Pharmacol. Exp. Ther. 1982;220:585–591. [PubMed] [Google Scholar]
- TAHARA A., TOMURA Y., WADA K., KUSAYAMA T., TSUKADA J., TAKANASHI M., YATSU T., UCHIDA W., TANAKA A. Pharmacological profile of YM087, a novel potent nonpeptide vasopressin V1A and V2 receptor antagonist, in vitro and in vivo. J. Pharmacol. Exp. Ther. 1997;282:301–308. [PubMed] [Google Scholar]
- UDELSON J.E., SMITH W.B., HENDRIX G.H., PAICHAUD C.A., GHAZZI M., THOMAS I., GHALI J.K., SELARU P., CHANOINE F., PRESSLER M.L., KONSTAM M.A. Acute hemodynamic effects of Conivaptan, a dual V1A vasopressin receptor antagonist, in patients with advanced heart failure. Circulation. 2001;104:2417–2423. doi: 10.1161/hc4501.099313. [DOI] [PubMed] [Google Scholar]
- WONG F., BLEI A.T., BLENDIS L.M., THULUVATH P.J., for The North American VPA-985 Study Group A vasopressin receptor antagonist (VPA-985) improves serum sodium concentration in patients with hyponatremia: a multicenter randomized placebo-controlled trial. Hepatology. 2003;37:182–191. doi: 10.1053/jhep.2003.50021. [DOI] [PubMed] [Google Scholar]
- WONG LL., VERBALIS J.G. Vasopressin V2 receptor antagonists. Cardiovasc. Res. 2001;51:391–402. doi: 10.1016/s0008-6363(01)00315-7. [DOI] [PubMed] [Google Scholar]
- YAMAMURA Y., OGAWA H., YAMASHITA H., CHIHARA T., MIYAMOTO H., NAKAMURA S., ONOGAWA T., YAMASHITA T., HOSOKAWAM T., MORI T., TOMINAGA M., YABUUCHI Y. Characterization of a novel aquaretic agent, OPC-31260, as an orally effective, nonpeptide vasopressin V2 receptor antagonist. Br. J. Pharmacol. 1992;105:787–791. doi: 10.1111/j.1476-5381.1992.tb09058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]