Abstract
Recent evidence supports additional subtypes of vasodilator β-adrenoceptor (β-AR) besides the ‘classical' β2. The aim of this study was to investigate the distribution of β-ARs in the wall of rat mesenteric resistance artery (MRA), to establish the relative roles of β-ARs in smooth muscle and other cell types in mediating vasodilatation and to analyse this in relation to the functional pharmacology.
We first examined the vasodilator β-AR subtype using ‘subtype-selective' agonists against the, commonly employed, phenylephrine-induced tone. Concentration-related relaxation was produced by isoprenaline (pEC50: 7.70±0.1) (β1 and β2). Salbutamol (β2), BRL 37344 (β3) and CGP 12177 (atypical β) caused relaxation but were 144, 100 and 263 times less potent than isoprenaline; the ‘β3-adrenoceptor agonist' CL 316243 was ineffective.
In arteries precontracted with 5-HT or U 46619, isoprenaline produced concentration-related relaxation but salbutamol, BRL 37344, CGP 12177 and CL 316243 did not. SR 59230A, CGP 12177 and BRL 37344 caused a parallel rightward shift in the concentration–response curve to phenylephrine indicating competitive α1-AR antagonism, explaining the false-positive ‘vasodilator' action against phenylephrine-induced tone. Endothelial denudation but not L-NAME slightly attenuated isoprenaline-mediated vasodilatation in phenylephrine and U 46619 precontracted MRA.
The β-AR fluorescent ligand BODIPY TMR-CGP 12177 behaved as an irreversible β1-AR antagonist in MRA and bound to the surface and inside vascular smooth muscle cells in intact vascular wall. β-ARs in smooth muscle cells were observed in a perinuclear location, consistent with the location of Golgi and endoplasmic reticulum.
Binding of BODIPY TMR-CGP 12177 was inhibited by BAAM (1 μM) in all three vascular tunics, confirming the presence of β-ARs in adventitia, media and intima. Binding in adventitia was observed in both neuronal and non-neuronal cell types. Lack of co-localisation with a fluorescent ligand for α-ARs confirms the selectivity of BODIPY TMR-CGP 12177 for β-ARs over α-ARs.
Our results support the presence of functional vasodilator β1-ARs and show that they are mainly located in smooth muscle cells. Furthermore, we have demonstrated, for the first time, the usefulness of BODIPY TMR-CGP 12177 for identifying β-AR distribution in the ‘living' vascular wall.
Keywords: Adrenoceptors; β-adrenoceptors; fluorescent ligands; imaging; vascular smooth muscle, mesenteric resistance artery
Introduction
In their groundbreaking study that divided β-adrenoceptors (ARs) into two subtypes, Lands et al. (1967) classified vasodilator β-ARs as β2. Since then, several studies have shown that β1-ARs can also participate in the relaxation of blood vessels (O'Donnell & Wanstall, 1984; Graves & Poston, 1993). The involvement of one or other β-AR subtype has been postulated to vary not only with the vascular bed but also with species (Guimaraes & Moura, 2001). Recently, Chruscinski et al. (2001) showed, by a combination of receptor knockouts and classical pharmacology, that vasodilator β1-ARs were common and widespread amongst mouse blood vessels whereas β2-ARs were confined to a narrower range of vessels. The participation of the β3-AR subtype, originally described in adipose tissue and the gastrointestinal tract (Arch & Kaumann, 1993; Arch, 2002), has been implicated in rat and dog blood vessels (Shen et al., 1996; MacDonald et al., 1999; Tagaya et al., 1999). A fourth atypical β-AR subtype that shares some properties with the β3-AR subtype has been reported in cardiac and adipose tissue (Kaumann & Molenaar, 1996; Galitzky et al., 1997; Malinowska & Schlicker, 1997) and also in several rat blood vessels (Oriowo, 1994; 1995; Dumas et al., 1998; Brawley et al., 2000a; Kozlowska et al., 2003). Brahmadevara et al. (2003); (2004) provided evidence in rat thoracic aorta against the presence of β3-ARs, suggesting that the relaxation induced by the ‘atypical β-AR agonists' could be explained by an α-AR-blocker action against the agonist (phenylephrine) commonly used to contract the vessels rather than to an interaction with β3-ARs.
It is generally assumed that vasodilatation caused via β-ARs involves vascular smooth muscle cells rather than any other cell type in the vascular wall, although the role of other cell types cannot be discounted. Nevertheless, using autoradiography, β-ARs have been located in the media, adventitia and/or endothelium in canine (Molenaar et al., 1988a) and human (Nakai et al., 1986; Molenaar et al., 1988b; Amenta et al., 1991). Radioligand binding studies using cultured bovine endothelial cells have also reported the presence of β-ARs (Steinberg et al., 1984). Thus, although no evidence exists for the function of adventitial β-AR, a role for endothelial β-ARs in vasodilatation mediated by β-AR agonists remains controversial (Guimaraes & Moura, 2001; Vanhoutte, 2001). Some studies in blood vessels of rat (Moncada et al., 1991; Oriowo, 1994) and human (Molenaar et al., 1988b) support the concept that β-AR agonist-induced vasodilatation is endothelium independent. In contrast, other studies suggest that the relaxation mediated by β-ARs in rat (Gray & Marshall, 1992; Trochu et al., 1999; Brawley et al., 2000b; Georgescu et al., 2005) and mouse (Akimoto et al., 2002) blood vessels relies on the presence of the endothelium. The studies indicating that the β2-AR should no longer be thought of as the principal vascular subtype also prompts reanalysis of the function and distribution of the vascular β-AR subtypes.
We have undertaken visualisation and functional analysis of β-ARs in a standard resistance artery model to compare receptor location and vasodilator function.
BODIPY-TMR-CGP 12177 is a fluorescent ligand for β-ARs that has been described as a long-lasting β2-AR partial agonist in cells expressing the recombinant human β2-AR (Baker et al., 2003). Its affinity for β1-ARs is not known, although there is no prima facie case to assume that it would be selective between β-AR subtypes. In addition, its pharmacology has not been reported in the rat resistance vasculature so it was necessary to first validate the receptor subtype present in the small mesenteric arteries and then to test it as an antagonist of these receptors.
The main objective of this study was to employ the fluo-ligand, with confocal microscopy, to show the cellular distribution of β-ARs in the wall of a ‘live' resistance artery and to resolve whether smooth muscle, adventitia and/or endothelial β-ARs exist and to relate this to vasodilation. To attain this objective, it was necessary to (i) characterise the β-AR subtype that mediates vasodilatation in the preconstricted mesenteric resistance artery (MRA), (ii) consider the participation of endothelium in β-AR mediated vasodilatation. (iii) evaluate the β-AR agonist and/or antagonist capacity of BODIPY-TMR-CGP 12177 since the class of β-blocker from which it is derived can have diverse actions and (iv) examine the binding of the fluorescent ligand in MRA.
Methods
Tissue preparation
Male Wistar Kyoto rats, 6-month old, (Autonomous University of Madrid, Spain) were decapitated and the mesenteric arcade was removed and placed in physiological salt solution (PSS) of the following composition (in mM): NaCl 112.0, KCl 4.7, CaCl2 2.5, KH2PO4 1.1, MgSO4 1.2, NaHCO3 25.0 and glucose 11.1, maintained at 4°C and continuously gassed with 95% O2 and 5% CO2. Segments (1.8–2 mm) of third-order branches of the mesenteric arteries were dissected free of fat and connective tissue. In some vessels, the endothelial layer was removed by passing a human hair through the lumen. Vessels were mounted on wires on an isometric myograph (mod 410 A, J.P. Trading, Denmark) filled with PSS kept at 37°C. The vessels were then allowed to equilibrate for 30 min and set up with a ‘normalised' internal circumference 0.9 L100 estimated to be 0.9 times the passive circumference at 100 mmHg transmural pressure. This was calculated for each individual vessel based on the passive length-tension characteristics of the artery and the Laplace relationship (Mulvany & Halpern, 1977). This procedure optimised active force generation by these vessels and the internal diameters referred to were derived from this calculation. After the normalization procedure, the vessels were allowed to equilibrate for a further 30 min. The tissue was then contracted three times with KCl 100 mM every 5 min to ensure that the size of the contractile response was constant. After a 30 min period, each ring was contracted with phenylephrine (2–3 μM) and relaxed with acetylcholine (ACh, 1–10 μM) to verify the functionality of the endothelium. Only preparations that relaxed more than 80% were classified as endothelium intact and those that failed to relax (0%) were classified as endothelium removed. After washing, the tissues were left to equilibrate for a further 30 min before starting the experiments.
Functional experiments
In the first series of experiments, MRA with or without endothelium were incubated (30 min) with β-AR antagonists, or vehicle, before being exposed to a concentration of either phenylephrine (α1-AR agonist), [9,11,-dideoxy-9α-11α-epoxymenthane prostaglandin-F2α] (U 46619, thromboxane A2 mimetic) or serotonin (5-HT) that produces a contractile response between 70 and 80% of 100 mM KCl-induced contraction. The contractile response was allowed to stabilize and a concentration–response curve (CRC) was obtained to test for vasodilator β-AR agonism of each compound. When the vessels were precontracted with either U 46619 or 5-HT, phentolamine (10 μM) was also present.
In a second series of experiments, CRCs to phenylephrine were performed in the presence and absence of the test drugs (putative β3-AR agonists and antagonists) to establish their α-AR antagonist capacity.
In addition, the fluo-ligand BODIPY-TMR-CGP 12177 was assessed for potential β-AR agonism (versus phenylephrine-induced tone) and antagonism (versus isoprenaline) following protocols similar to those in the first series. In addition, to determine if this compound binds irreversibly to β-ARs, in a separate series of experiments, tissues were incubated (30 min) with BODIPY-TMR-CGP 12177 (1 μM) or DMSO and then washed successively every 5 min for 30 min after which the vessels were contracted with phenylephrine and a CRC to isoprenaline was constructed.
β-AR localization by confocal microscopy
MRA segments were incubated (1 h, 37°C) with the irreversible β-AR antagonist bromoacetylalprenolol menthane (BAAM; 1 μM) or vehicle (0.0001% ethanol) and washed every 10 min over 30 min followed by incubation (15 min) with BODIPY-TMR-CGP 12177 (1 μM). Some vessels were coincubated (1 h) at room temperature with 1 μM quinazolinyl piperazine borate-dipyrromethene (QAPB, Miquel et al., 2005), an α-AR fluorescent ligand (Shafaroudi et al., 2005) and BODIPY-TMR-CGP (1 μM). Vessels were then mounted in PSS on slides with a small well (400 μM depth) made of silicon spacers to avoid vessel compression. The vessels were visualised with either a Leica TCS SP2 or BIORAD Radiance 2100 confocal system fitted to an inverted microscope comprising argon and helium–neon laser sources. Serial optical sections (stacks of images) from the adventitia to the lumen (z step=0.5 μm) were captured with either × 63 or × 40 oil objectives (NA=1.3) using 488 or 543 nm excitation of the helium–neon laser as appropriate. A minimum of two stacks of different regions was captured in each arterial segment. For comparative studies, images were taken under identical conditions of laser intensity, brightness and contrast.
In a separate set of experiments (to visualise endothelial cells), MRA were pressure-fixed (70 mmHg) with 4% phosphate-buffered paraformaldehyde (pH=7.6) for 1 h and washed in three changes of phosphate-buffered saline solution (pH=7.4). Transverse sections (100 μm) were cut on to gelatine-coated slides and incubated with BODIPY-TMR-CGP 12177 (1 μM, 3 h). Some sections were preincubated with BAAM (1 μM, 3 h, 37°C) or vehicle (0.0001% ethanol) prior to incubation with BODIPY-TMR-CGP 12177.
Imaging methods
Serial confocal sections were stored as a series of TIFF images for offline processing and analysis. Metamorph (Universal Imaging) software was used for image processing and final presentation. Orthogonal (cross and transverse) sectioning was performed by loading a full TIFF series in IMARIS (Bitplane) software and selecting an xz and yz section line to produce sectional views of the data.
Drugs
Acetylcholine HCl, CGP 12177, CL 316243, isoprenaline, NG-nitro-L-arginine methylester, papaverine, phenylephrine HCl, propranolol HCl, salbutamol, 5-HT and SR 59230A were purchased from Sigma Chemical Co. (Poole, Dorset, U.K.); BODIPY-TMR-CGP 12177 and QAPB (quinazolinyl piperazine-borate-dipyrromethene, marketed under the name of BODIPY FL-prazosin) from Molecular Probes (Eugene, OR, U.S.A.) (an Invitrogen company); BAAM (bromoacetylalprenolol menthane) from Research BiochemicaI Incorporated (RBI, Poole, Dorset, U.K.); BRL 37344 from Tocris (Northpoint, Fourth Way, Avonmouth, U.K.); U 46619 from Calbiochem (Merck Biosciences Ltd, Beeston, Nottingham, U.K.) BAAM (10 mM) and U 46619 were dissolved in absolute ethanol and diluted with physiological salt solution. BODIPY-TMR-CGP 12177 (10 mM) was diluted in DMSO, aliquoted and stored at −20°C; further dilutions were made with physiological salt solution on the day of the experiment. All other chemicals used were of analytical grade.
Data and statistical analysis
Location parameters were estimated for each experiment as pEC50 (the negative logarithm of the concentration required to cause 50% of the maximum response). Agonist concentration ratios (CR) were calculated from the EC50 values and, where appropriate, estimates of antagonist affinities expressed as pKB values were obtained from the equation pKB=log (agonist CR−1)−log [antagonist]. Data were compared using either unpaired Student's t-test (pEC50) or two-way analysis of variance (ANOVA, CRC) as appropriate. Differences were considered statistically significant when P<0.05. ‘n' is indicated in brackets in the figures and represents the number of animals used. Only one ring/animal/agonist was used.
Results
β-AR-mediated vasodilatation
The nonselective β-AR agonist isoprenaline, the β2-AR agonist salbutamol, the atypical partial β-AR agonist CGP 12177 and the selective β3-AR agonist BRL 37344 fully relaxed the phenylephrine preconstricted MRA in a concentration-related manner (Figure 1a). In contrast, the β3-AR agonist CL 316243 failed to relax the phenylephrine precontracted vessel (Figure 1a). The pEC50 values and the maximum relaxation for all β-AR agonists used are displayed in Table 1. Salbutamol, CGP 12177 and BRL 37344 lay to the right 144, 100 and 263 times, respectively, in relation to isoprenaline.
Figure 1.
Concentration–response curves to several β-AR agonists in phenylephrine (a), serotonine (b) and U 46619 (c) precontracted MRA. Results are expressed as mean±s.e.m. The number of animals used is indicated in brackets. ***P<0.001 for all agonists versus isoprenaline by two-way ANOVA.
Table 1.
Relaxant effects of β-adrenoceptor agonist in rat mesenteric resistance arteries
| β-AR agonist | pEC50 | % Maximum response | n |
|---|---|---|---|
| Isoprenaline | 7.7±0.10 | 97.26±0.72 | 11 |
| CGP 12177 | 5.7±0.04*** | 96.20±0.87 | 10 |
| Salbutamol | 5.54±0.07*** | 96.20±0.87 | 12 |
| BRL 37344 | 5.28±0.07*** | 95.75±1.07 | 7 |
P<0.001 versus isoprenaline by unpaired Student's t-test.
U 46619 (1 nM–1 μM) and 5-HT (1–30 μM) contracted the MRA in a concentration-related manner. The pEC50 value obtained was 6.8±0.14 (n=8) for U 46619 and 5.20±0.2 (n=4) for 5-HT. The maximum contraction attained was 78±5.3 or 83±5.4% of 100 mM KCl for U 46619 and 5-HT, respectively. Isoprenaline was able to relax MRA preconstricted by either 5-HT (Figure 1b) or U 46619 (Figure 1c) in a concentration-related manner. When the vessel was contracted with U 46619, the values for the pEC50 and the maximum relaxation were similar to those obtained when the vessels were contracted with phenylephrine (Table 1). The midrange sensitivity but not the maximum relaxation to isoprenaline was slightly reduced with 5-HT precontracted vessels. Salbutamol, CGP12177A, BRL 37344 and CL 316243 failed to relax U 46619 or 5-HT precontracted vessels (Figure 1b and c).
Removal of endothelium partly inhibited the CRC to isoprenaline and papaverine (Figure 2) in phenylephrine precontracted vessels. Responses to isoprenaline in U 46619 were also similarly affected by endothelium removal (results not shown). L-NAME (30 μM) failed to modify the CRC to isoprenaline versus phenylephrine precontraction (Figure 2).
Figure 2.
Concentration–response curves to isoprenaline (top) and papaverine (bottom) in the presence and the absence of endothelium in phenylephrine precontracted MRA. The influence of L-NAME on isoprenaline response is also shown (top). Results are expressed as mean±s.e.m. The number of animals used is indicated in brackets. ***P<0.001 for isoprenaline and **P<0.01 for papaverine in the absence versus the presence of endothelium by two-way ANOVA.
Inhibition of β-AR-mediated vasodilatation
The CRC to isoprenaline (pEC50: 7.70±0.1; n=14) was shifted to the right by 0.1 μM propranolol (pEC50: 6.52±0.2; n=6; P<0.0001) and by 1 μM atenolol (pEC50: 7.1±0.08; n=5; P<0.01) with no reduction in the maximum relaxation (Figure 3a). However, ICI 118551 (0.1 μM) did not modify the CRC to isoprenaline (pEC50: 7.65±0.08, n=5; Figure 3a). Estimation of the magnitude of the shift from the EC50 values gave a 15- and a 3.9-fold shift for propranolol and atenolol, which corresponds to a pKB values of 8.17 (propranolol) and 7.47 (atenolol).
Figure 3.
Effect of different β-AR antagonists on isoprenaline-mediated vasodilatation (a), BRL 37344 (b) and CGP 12177 (c) on phenylephrine precontracted MRA. Results are expressed as mean±s.e.m. The number of animals used is indicated in brackets. ***P<0.001 for isoprenaline in presence versus absence of propranolol and for BRL 37344 and CGP 12177 in the presence versus absence of SR 59230A (0.3 μM); **P<0.01 for isoprenaline in presence versus absence of atenolol by two-way ANOVA.
The relaxations of phenylephrine-induced tone that were induced by BRL 37344 (Figure 3b) and CGP 12177 (Figure 3c) were shifted to the right by SR 59230A (0.1 and 0.3 μM), a selective β3-AR antagonist. The use of a higher concentration of SR 59230A (1 μM) was precluded because this compound drastically reduced the maximum contraction to phenylephrine. The nonselective β-AR antagonist propranolol (0.1 μM) had no effect on the CRC for BRL 37344 and CGP 12177 (results not shown).
Effect of SR 59230A, CGP 12177, BRL 37344 and CL 316243 on the CRC curves to phenylephrine
Phenylephrine (0.1–100 μM) contracted the MRA in a concentration-dependent manner (pEC50: 5.69±0.05; n=7). Preincubation with SR 59230A (Figure 4a), CGP 12177 (Figure 4b) and BRL 37344 (Figure 4c) shifted the CRC to phenylephrine to the right in a concentration-related manner. The estimated pKB was 7.1, 5.16 and 4.6 for SR 59230A, CGP 12177A and BRL 37344, respectively. In contrast, CL 316243 was without effect on the CRC to phenylephrine (Figure 4d).
Figure 4.
Concentration response curves for phenylephrine-induced contraction in the absence and presence of (a) SR 59230A, (b) CGP 12177, (c) BRL 37344 and (d) CL 316243 in MRA. Results are expressed as mean±s.e.m. The number of animals used is indicated in brackets. **P<0.001 for phenylephrine in presence versus absence of SR 59230A, CGP 12177A and BRL 37344 by two-way ANOVA.
β-AR agonist and antagonist capacity of the fluo-ligand BODIPY-TMR-CGP 12177
BODIPY-TMR-CGP 12177 failed to relax the phenylephrine precontracted MRA (Figure 5a). A slight relaxation was seen with the highest concentration used and can be ascribed to DMSO (Figure 5a). The CRC to isoprenaline (Figure 5b) was inhibited in a concentration-related manner by BODIPY-TMR-CGP 12177. Lower concentrations (1–10 nM) significantly shifted the CRC to isoprenaline to the right with no significant inhibition of maximum relaxation. However, the incubation with higher concentrations of BODIPY-TMR-CGP 12177 (0.1–1 μM) significantly decreased the maximum relaxation induced by this agonist. Incubation with BODIPY-TMR-CGP 12177 (1 μM; 30 min) followed by a 30 min washout abolished the CRC to isoprenaline (Figure 5c).
Figure 5.
Functional pharmacology of BODIPY-TMR-CGP 12177 in MRA precontracted with phenylephrine. (a) Concentration–response curves (CRC) to BODIPY-TMR-CGP 12177 and DMSO. (b) CRC to isoprenaline in the absence and presence of increasing concentrations of BODIPY-TMR-CGP 12177. (c) CRC to isoprenaline in the absence and presence of 1 μM BODIPY-TMR-CGP 12177 (30 min) followed by a washout period (every 5 min over 30 min). Results are expressed as mean±s.e.m. The number of animals used is indicated in brackets. ***P<0.001 for isoprenaline versus BODIPY-TMR-CGP by two-way ANOVA.
Binding-induced fluorescence of BODIPY-TMR-CGP 12177
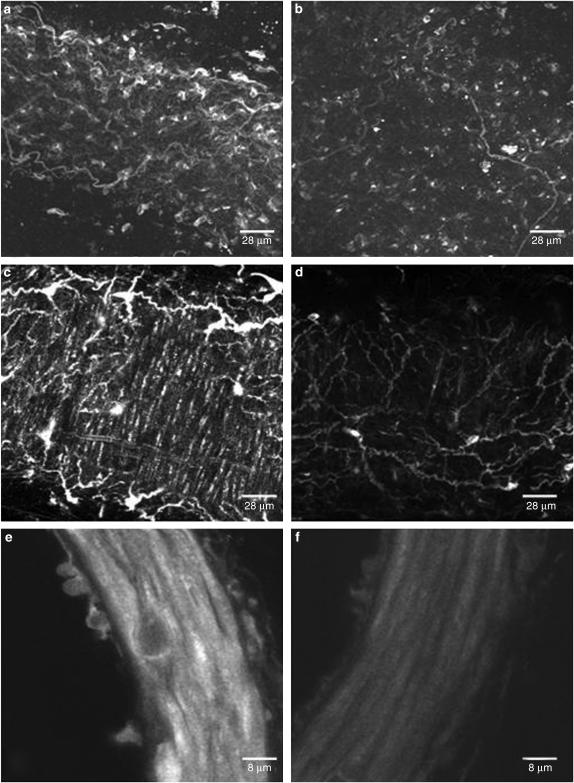
BODIPY-TMR-CGP 12177 did not exhibit significant fluorescence in solution. Therefore, experiments could be performed at equilibrium and without washing. BODIPY-TMR-CGP 12177 bound to adventitial (Figure 6a), smooth muscle (Figure 6c) and endothelial (Figure 6e) cells. The fluo-ligand bound to smooth muscle cells within the media of the MRA showing circumferential orientation of the cells (Figure 6c). Binding of BODIPY-TMR-CGP 12177 to endothelial cells could not be seen in whole (live) vessels. However, it was clearly visualised in fixed vessel rings (Figure 6e). This is possibly because β-ARs have low expression levels on the surface of endothelial cells and this is difficult to visualise when scanning through the entire thickness of the vessel wall. Incubation of the vessels with the irreversible β-AR antagonist BAAM decreased the fluorescence in adventitia (Figure 6b), media (Figure 6d) and endothelium (Figure 6f).
Figure 6.
Binding of BODIPY-TMR-CGP 12177 (1 μM) to the adventitial (a, b), smooth muscle (c, d) and endothelial (e, f) cells in the absence (left pannels) and presence (right pannels) of BAAM (10 μM). Smooth muscle derived fluorescence (c, d) is apparent as elongated structures running vertically in the images. Images were constructed by assembling serial (confocal) sections, of the vascular media, and are displayed as extended focus views using Metamorph image processing software.
Examination of smooth muscle binding was achieved using orthogonal (cross) sectioning. Within the vascular media, binding appeared to be both perinuclear and surface bound (Figure 7a inset). Resectioning the image volumes in the yz (Figure 7b) and xz (Figure 7c) axes reveals the cell profiles within the wall. In most cases, the fluorescence in individual smooth muscle cells appears either as a ‘ring' or ‘spot' (see arrows in Figure 7b), this is indicative of surface or intracellular binding, respectively.
Figure 7.
Orthogonal sectioning of a rat MRA in the presence of BODIPY-TMR-CGP 12177 (1 μM). (a) The image shows a single xy plane of a full data volume (image z-series). A magnification of one selected area shows CGP binding in a perinuclear region. (b) The yz section is equivalent to a virtual transverse section of the wall and shows the profile of medial smooth muscle cells. A ‘ring' of fluorescence (dashed arrow) is indicative of binding to the smooth muscle cell surface. Bright spots (full arrow) indicate intracellular binding. (c) The xz section is equivalent to a virtual transverse cross-section showing the convoluted internal elastic lamina and fluorescence binding in smooth muscle cells close to the adventitia and intima. Strong adventitial binding is apparent on both orthogonal sections. Calibration bar represents 50 μm.
In the adventitia, BODIPY-TMR-CGP 12177 binds to cells (probably fibrocytes) and elongated structures likely to be nerves (Figure 6c). The intensity of binding was reduced in the presence of BAAM (Figure 6d), indicating that these sites are β-ARs. Intense adventitial binding is also apparent when viewing the orthogonal sections in Figure 7b and c. Orthogonal sectioning did not reveal any endothelial binding, possibly due to the physical limitations of laser depth penetration and the relatively thin architecture of the endothelial cells. However, endothelial cells could be clearly visualised in the fixed cut ring sections shown in Figure 6e. Close inspection of the adventitial and endothelial cells in Figure 6a and e, respectively, shows perinuclear and thus probable intracellular binding. It is thus possible that failure to see binding on intact cells is due to the inability of the ligand to penetrate live endothelial cells.
The pattern of neuronal binding was examined by comparing the binding of the β-AR ligand (BODIPY-TMR-CGP 12177, 1 μM) with an α-AR ligand (QAPB, 1 μM) (Figure 8). In general, the two ligands bound to the same types of structures but the relative proportions differed. QAPB bound strongly to nerves at the adventitia-medial border, identified as sympathetic by their characteristic plexus arrangement and position (Figure 8, top left). BODIPY-TMR-CGP 12177 also bound to these nerves although colocalisation with QAPB was incomplete (Figure 8, bottom left panel), suggesting different phenotypes among the sympathetic nerve terminals. In the outer adventitial layer, there are neurone-like structures, which, although binding both ligands to some extent, displayed high density of BODIPY-TMR-CGP 12177 binding (Figure 8, bottom right panel). As well as showing differential distribution of the receptors, these data confirm the lack of signal crossover and the selectivity of binding of each fluo-ligand.
Figure 8.
Combined binding of QAPB and BODIPY-TMR-CGP 12177. Top panels show the binding of the fluorescent α-AR ligand (QAPB 1 μM; left) and BODIPY-TMR-CGP 12177 (1 μM; right) to a segment of rat MRA. The combination of both top panels (bottom left and right) shows the degree of colocalisation of the fluorescent ligands, which if coincident shows as yellow. Bottom left panel shows the colocalisation image of the two top panels (showing the inner most region of adventitia). Bottom right panel show a colocalisation image (at higher magnification) of the region outlined in the bottom left. This magnified view is composed of image planes comprising the inner and outer layers of adventitia, in order to incorporate the most superficial nerves. All images were collected using confocal microscopy and represent an ‘extended focus' view where several planes are merged. Calibration bar represents 50 μm.
Discussion
The present study shows that β-ARs that mediate vasodilatation in the MRA belong to the β1-AR subtype and are located intracellularly throughout the smooth muscle cell layer. Pharmacological evidence against a vasodilator effect of β2- and β3-ARs is reported. In addition, we have shown that the fluo-ligand BODIPY-TMR-CGP 12177 behaves as an irreversible β1-AR antagonist (Figure 4c) with no agonist action at this receptor subtype.
Previous studies have concluded that the main population of β-ARs responsible for vasodilatation in MRA is the β1-AR subtype (Graves & Poston, 1993; White et al., 2001). Nevertheless, the presence of the β2-AR subtype has been inferred, based on the effect of salbutamol (Zwaveling et al., 1996). The evidence from our present study and others is heavily against this. In tissues with a high proportion of β2-ARs (Schreurs et al., 1980; Granger et al., 1985; Priest et al., 1997), salbutamol has a pD2 value ranging from 7.0 to 8.3. The low pEC50 value (5.5) of salbutamol in the MRA (Zwaveling et al., 1996; White et al., 2001; Smits et al., 2002; present study), as in other blood vessels (Oriowo, 1994; Shafiei & Mahmoudian, 1997), in our opinion, is not consistent with stimulation of β2-ARs. We found that salbutamol produced relaxation of phenylephrine-induced tone, albeit 150 times less potent than isoprenaline. This was a false positive effect since salbutamol did not relax tone to other agents while isoprenaline did.
In summary, the presence of β1- rather than β2-ARs is based on several experimental observations: (i) the potency ratio between isoprenaline and salbutamol is more than 10 times greater than the usual potency ratio observed in tissues that express mainly β2-ARs (Arch et al., 1984; Priest et al., 1997); (ii) the estimated pKB values obtained for propranolol (8.17) and atenolol (6.6), a β1-AR selective antagonist, versus isoprenaline CRCs were similar to those previously reported in the rat MRA (White et al., 2001) and aorta (Doggrell & Henderson, 1998) and agree with the recently reported (Baker, 2005) pKD for both agonists at β1-ARs; (iii) ICI 118551, a highly selective β2-AR antagonist (Baker, 2005) did not modify the CRC to isoprenaline and (iv) no relaxation to salbutamol was found when the precontractile agent was U 46619 or 5-HT instead of phenylephrine.
We found ‘false positive' evidence for β3-ARs that can be explained by α-AR antagonist effects of some key β3-AR ligands, as demonstrated by Brahmadevara et al. (2004) in rat aorta. BRL 37344, a compound employed as a selective β3-AR agonist (Arch et al., 1984), and CGP 12177, an ‘atypical β-AR' partial agonist with β1- and β2-AR antagonistic properties (Mohell & Dicker, 1989), behaved as would be expected of full agonists in respect of relaxation of phenylephrine-induced tone. As potential β3-AR agonists, these compounds are less potent in MRA (present study) and in other blood vessels (Mohell & Dicker, 1989; Oriowo, 1994; 1995; Sooch & Marshall, 1997; Trochu et al., 1999; Brawley et al., 2000a; Brahmadevara et al., 2003; Matsushita et al., 2003) than in the colon (Bloom et al., 1992; Kaumann & Molenaar, 1996; Brahmadevara et al., 2003). In addition, CL 316243, a potent and selective β3-AR agonist (Kaumann & Molenaar, 1996; Manara et al., 1996; Baker, 2005) failed to relax phenylephrine preconstricted vessels, reflecting a similar observation in rat aorta (Brahmadevara et al., 2003).
In the present study, the CRC to BRL 37344 and CGP 12177 was antagonised by low concentrations of SR 59230A, a condition that seems to fit with the presence of β3-ARs (Manara et al., 1996). Brahmadevara et al. (2003) reported an inhibition by SR 59230A of the phenylephrine-induced tone in rat aorta suggesting an antagonism of α1-ARs. We found that phenylephrine CRC was shifted to the right by SR 59230A indicating antagonism of α1-ARs. CGP 12177 and BRL 37344 but not CL 12177A also exhibited α1-AR antagonism. These results are very similar to those observed in rat aorta (Brahmadevara et al., 2004) and intrapulmonary artery (Leblais et al., 2004). Note that in rat aorta, the α1-ARs are mainly α1D and in MRA there are mainly α1A. Therefore, these compounds are nonsubtype selective α1-AR antagonists.
It has been suggested that the effects of β-AR agonists differ according to the agent used to contract the vessel. In the present study, the ‘β3-AR agonists', BRL 37344 and CGP 12177, but not CL 316243, relaxed phenylephrine-induced tone via antagonism of α1-ARs. Consequently, their failure to relax contraction induced by U 46619 or 5-HT indicates an absence of β3-ARs. Similarly with salbutamol, low potency relaxation of phenylephrine-induced tone might have implied β2-ARs but failure to relax tone to other agents adds to the evidence for a lack of β2-ARs. To our knowledge, there is no contrary evidence in this vessel. In the main mesenteric artery, the presence of atypical β-ARs and the lack of β3-ARs have been reported (Kozlowska et al., 2003).
There is long-standing controversy over the possibility that the endothelium might influence β-AR-mediated vasorelaxation. This is not straightforward because there are several putative endothelial factors that can be released and can influence smooth muscle tone, and there is a further possibility that an action on smooth muscle can be transmitted chemically or electrically to endothelial cells, which, in turn, can release factors that influence the smooth muscle.
The general paradigm in resistance arteries is that endothelium-derived hyperpolarizing factor (EDHF) together with NO plays an important role on the response to vasodilators (Hwa et al., 1994; Shimokawa et al., 1996; Sekiguchi et al., 2002; Chauhan et al., 2003). With regard to the β-AR-mediated response, we first excluded an influence of NO in the action of isoprenaline by the absence of an effect of L-NAME. This is also consistent with a theoretical extrapolation of β-AR-mediated signalling, since it seems unlikely that activation of adenylyl cyclase, the (so far) ubiquitous mechanism for β-AR, would depolarise the endothelial cells and release endothelial factors. Nevertheless, we did find that the vasorelaxation by isoproterenol was attenuated by physical damage to the endothelium.
A decrease in the relaxation responses to β-AR agonists after removal of endothelium has been used to argue for the presence of endothelial β-ARs (Brawley et al., 2000b; Vanhoutte, 2001). However, endothelial cells can release several vasoactive factors, sometimes spontaneously and thus, when the endothelium is removed, the loss of basal release of endothelial factors could influence vascular tone. To test this concept, we studied the effects of a wide range of vasorelaxant agents (data not shown) in this preparation and consistently found that vasodilator effects were attenuated by endothelial destruction, even including such nonspecific agents as papaverine (Figure 2b). This suggests a nonspecific constitutive influence of the endothelium on the smooth muscle cells rather than an endothelial β-AR-mediated action. Thus, although we have demonstrated the presence of β-AR on endothelium using BODIPY-TMR-CGP 12177, we believe that the function of these receptors remains unknown. In contrast, the presence of a β-AR-mediated vasodilatation that survived endothelial destruction provides positive evidence for a vasodilator function for the β-AR that we visualised on vascular smooth muscle.
One objective of this study was to show the cellular distribution of β-ARs through the vessel wall since, surprisingly, this is not known in any detail. The fluo-ligand, BODIPY-TMR-CGP 12177, has been shown to be a long-acting β2-AR agonist in cells expressing β2-AR (Baker et al., 2003) but its other pharmacological properties have not been analysed. It is essential, when using a fluoligand, to know its affinity and selectivity for the receptors of interest (Daly & McGrath, 2003). In our hands, BODIPY-TMR-CGP 12177 was without effect on phenylephrine precontracted MRA indicating that, in this tissue, it does not behave as a β-AR agonist or as an α1-antagonist. Furthermore, since it has been shown to be a β2-AR agonist (Baker et al., 2003), we can reinforce the concept that this preparation does not respond to β2-AR agonists. Relaxation by isoprenaline was significantly inhibited by BODIPY-TMR-CGP 12177 in a concentration-dependent manner. These results, together with the blockade of isoprenaline-induced relaxation after incubation and wash out of 1 μm BODIPY-TMR-CGP 12177 (Figure 4a), indicate that this compound behaves as a slowly dissociating antagonist at β1-ARs (and in practical terms is irreversible). The absence of smooth muscle cell fluorescence induced by BODIPY-TMR-CGP 12177 in the presence of, or after incubation with, the irreversible β-AR antagonist BAAM shows that the binding is specific for β-ARs. Further evidence for the selectivity of BODIPY-TMR-CGP 12177 comes from its relative lack of colocalisation with the fluorescent ligand for α1-ARs (QAPB). Since BODIPY-TMR-CGP 12177 antagonised the vasodilator effect of isoprenaline at β1-ARs, the fluorescence indicates the presence of the functional β1-ARs on smooth muscle cells.
β1-ARs were found on smooth muscle cells throughout the vascular medial layer with no obvious bias according to depth of location. However, in some cases, a particularly high density of binding was observed in individual smooth muscle-like cells, which requires further study. This level of detail had not previously been available. It was also possible to take the resolution to the subcellular level. This showed that the β1-ARs were located in a general distribution across the surface of the cells. There was a bias towards the central portion of the cells that may simply reflect that this is the thickest part of the cell. Receptors were also present inside the cells, particularly in the perinuclear region. This configuration is not uncommon and can be seen for both α- and β-ARs as well as other receptor subtypes (Daly & McGrath, 2003). Agonist-induced cycling of β-AR in single cells is well documented (Von Zastrow & Kobilka, 1994). Therefore, it is not surprising that β-ARs are found at intracellular locations in vascular smooth muscle cells under normal conditions. QAPB is taken up into live cells by spontaneous endocytosis attached to α1A-AR (Pediani et al., 2005), so it is possible that this applies also to the β-AR ligand, explaining its intracellular location in live cells.
The binding of BODIPY-TMR-CGP 12177 to endothelial cells and adventitial cell bodies was susceptible to the irreversible β-AR antagonist BAAM. This is strong evidence for the presence of β-AR in these locations. The subtype is not known since the ligand has affinity for both β1-AR (present study) and β2-AR (Baker et al., 2003).
We discussed above our view that our functional pharmacological data produced no evidence in favour of a contribution of endothelial β-AR to the vasodilator action of isoprenaline. These receptors may, therefore, have another function. A long-standing literature suggests that endothelial β-AR may have a role in modulating vascular permeability (Zink et al., 1993; Allen & Coleman, 1995; Weneger et al., 1998). Thus, we can hypothesize that the functional role of endothelial β-ARs demonstrated with BODIPY-TMR-CGP 12177 would be related to vascular permeability rather than vasodilatation. The endothelial cells also have α-AR that can be demonstrated with the fluorescent ligand QAPB. In that case, an analysis using receptor knockout mice and selective antagonists showed that these receptors comprise both α1-AR and α2-AR (Shafaroudi et al., 2005). Since genetic manipulation is not available in the present rat study, it is safer to classify the receptors to which QAPB binds as α-AR without further subdivision.
The presence of β-AR in the adventitia is a new observation. We recently described the presence of α-AR in adventitial cells and on nerve terminals of mouse first-order mesenteric arteries (McGrath et al., 2005). The present observations extend this to rat third-order mesenteric arteries and show that these possess both α-AR and β-AR. The structures involved include freestanding fibrocyte/fibroblasts and two distinct types of nerve-like structure. One type of nerve is clearly the plexus of sympathetic nerve terminals that lies at the adventitia–medial interface. Here, the two-receptor types are present to some extent on most terminals and on a substantial population they are well colocalised. These presumably are the autoreceptors well known for modulation of transmitter release. The other structures consist of long processes, two or more of which are attached to their cell bodies. These lie further out in the adventitia and envelop the artery, running for considerable distances both around the circumference and along the length. These are possibly sensory nerves. Resistance arteries possess sensory nerves in the outer adventitia that may detect changes in the vessel's dimensions and take part in a local reflex adjustment (Scotland et al., 2004) involved in the ‘myogenic' response. If so, the presence of adrenoceptors, particularly β-AR, suggests adrenergic modulation of these reflexes and hence of autoregulation of blood flow.
In conclusion, in rat MRA, relaxation mediated by β-ARs is due mainly to the stimulation of β1-ARs present throughout the smooth muscle cell layer, whose population has a partial intracellular location. Evidence against the participation of β2- and β3-ARs is also reported. We propose that the focus of research on β-AR, as regards vascular resistance, should be on smooth muscle β1-ARs and should take their subcellular location into account. We also demonstrate the presence of adventitial, neuronal and endothelial β-ARs. This deserves further investigation.
Acknowledgments
This work was supported by the EU project VASCAN-2000 (QLG-CT-1999-00084) and Tenovus (Scotland). We are grateful to Mercè Martí (Servei de Microscopia, Universitat Autònoma de Barcelona) for technical assistance with confocal microscopy. We also thank M. Carmen Fernández Criado for the maintenance of the rat colonies at the Universidad Autónoma de Madrid. F.J.A. and S.M.R. are supported by Generalitat de Catalunya and MEC, respectively. EV was partly supported by a Salvador de Madariaga fellowship (MEC, Spain).
Abbreviations
- ARs
adrenoceptors
- CRC
concentration–response curve
- MRA
mesenteric resistance artery
References
- AKIMOTO Y., HORINOUCHI T., SHIBANO M., MATSUSHITA M., YAMASHITA Y., OKAMOTO T., YAMAKI F., TANAKA Y., KOIKE K. Nitric oxide (NO) primarily accounts for endothelium-dependent component of β-adrenoceptor-activated smooth muscle relaxation of mouse aorta in response to isoproterenol. J. Smooth Muscle Res. 2002;38:87–99. doi: 10.1540/jsmr.38.87. [DOI] [PubMed] [Google Scholar]
- ALLEN M.J., COLEMAN R.A. Beta2-adrenoceptors mediate a reduction in endothelial permeability in vitro. Eur. J. Pharmacol. 1995;274:7–15. doi: 10.1016/0014-2999(94)00689-5. [DOI] [PubMed] [Google Scholar]
- AMENTA F., COPPOLA L., GALLO P., FERRANTE A., FORLANI A., MONOPOLI A., NAPOLEONE P. Autoradiographic localization of β-adrenergic receptors in human large coronary arteries. Circ. Res. 1991;68:1591–1599. doi: 10.1161/01.res.68.6.1591. [DOI] [PubMed] [Google Scholar]
- ARCH J.R.S. β3-Adrenoceptor agonists: potential, pitfalls and progress. Eur. J. Pharmacol. 2002;440:99–107. doi: 10.1016/s0014-2999(02)01421-8. [DOI] [PubMed] [Google Scholar]
- ARCH J.R.S., AINSWORTH A.T., CAWTHORNE M.A., PIERCE V., SENITT M.V., THODY V.E., WILSON S. Atypical β-adrenoceptor brown adipocytes as a target for anti-obesity drugs. Nature. 1984;309:163–165. doi: 10.1038/309163a0. [DOI] [PubMed] [Google Scholar]
- ARCH J.R.S., KAUMANN A. β3 and atypical β-adrenoceptors. Med. Res. Rev. 1993;13:663–729. doi: 10.1002/med.2610130604. [DOI] [PubMed] [Google Scholar]
- BAKER J.G. The selectivity of β-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br. J. Pharmacol. 2005;144:317–322. doi: 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER J.G., HALL I.P., HILL S.J. Pharmacology and direct visualization of BODIPY-TMR-CGP: a long-acting fluorescent β2-adrenoceptor agonist. Br. J. Pharmacol. 2003;139:232–242. doi: 10.1038/sj.bjp.0705287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOOM J.D., DUTIA M.D., JOHNSON B.D., WISSNER A., BURNS M.G., LARGIS E.E., DOLAN J.A., CLAUS T.H. Disodium (R,R)-5-[2-[[2-(3-chlorophenyl)-2-hydroxymethyl]-amino]propyl]-1,3-benzodioxole-2,2-dicarboxylate (CL 316,243). A potent β-adrenergic agonist virtually specific for β3-adrenoceptors. A promising antidiabetic and antiobesity agent. J. Med. Chem. 1992;35:3081–3084. doi: 10.1021/jm00094a025. [DOI] [PubMed] [Google Scholar]
- BRAHMADEVARA N., SHAW A.M., MACDONALD A. Evidence against β3-adrenoceptors or low affinity state of β1-adrenoceptors mediating relaxation in rat isolated aorta. Br. J. Pharmacol. 2003;138:99–106. doi: 10.1038/sj.bjp.0705017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAHMADEVARA N., SHAW A.M., MACDONALD A. α1-Adrenoceptor antagonist properties of CGP 12177A and other β-adrenoceptor ligands: evidence against β3- or atypical β-adrenoceptors in rat aorta. Br. J. Pharmacol. 2004;142:781–787. doi: 10.1038/sj.bjp.0705840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAWLEY L., SHAW A.M., MACDONALD A. β1, β2 and atypical β-adrenoceptor-mediated relaxation in rat isolated aorta. Br. J. Pharmacol. 2000a;129:637–644. doi: 10.1038/sj.bjp.0703091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAWLEY L., SHAW A.M., MACDONALD A. Role of endothelium/nitric oxide in atypical β-adrenoceptor-mediated relaxation in rat isolated aorta. Eur. J. Pharmacol. 2000b;298:285–296. doi: 10.1016/s0014-2999(00)00319-8. [DOI] [PubMed] [Google Scholar]
- CHAUHAN S., RAHMAN A., NILSSON H., CLAPP L., MACALISTER R., AHLUWALIA A. NO contributes to EDHF-like responses in rat samll arteries: a role for NO stores. Cardiovasc. Res. 2003;57:207–216. doi: 10.1016/s0008-6363(02)00611-9. [DOI] [PubMed] [Google Scholar]
- CHRUSCINSKI A., BREDE M.E., MEINEL L., LOHSE M.J., KOBILKA B.K., HEIN L. Differential distribution of beta-adrenergic receptor subtypes in blood vessels of knockout mice lacking beta(1)- or beta(2) adrenergic receptors. Mol. Pharmacol. 2001;60:955–962. doi: 10.1124/mol.60.5.955. [DOI] [PubMed] [Google Scholar]
- DALY C.J., MCGRATH J.C. Fluorescent ligands, antibodies, and proteins for the study of receptors. Pharmac. Ther. 2003;100:101–118. doi: 10.1016/j.pharmthera.2003.08.001. [DOI] [PubMed] [Google Scholar]
- DOGGRELL S.A., HENDERSON C.J. The offset of β-adrenoceptor antagonism of the responses of the rat right ventricle to isoprenaline. J. Aut. Pharmacol. 1998;18:263–269. doi: 10.1046/j.1365-2680.1998.18592.x. [DOI] [PubMed] [Google Scholar]
- DUMAS M., DUMAS J.P., BARDOU M., ROCHETTE L., ADVENIER C., GIUDICELLI J.F. Influence of β-adrenoceptor agonists on the pulmonary circulation. Effects of a β3-adrenoceptor antagonist, SR 59230A. Eur. J. Pharmacol. 1998;348:223–228. doi: 10.1016/s0014-2999(98)00146-0. [DOI] [PubMed] [Google Scholar]
- GALITZKY J., LANGIN D., VERWAERDE P., MONTRASTRUC J.L., LAFONTAN M., BERLAN M. Lipolytic effects of conventional β3-adrenoceptor agonists and of CGP 12177 in rat and human fat cells: preliminary pharmacological evidence from a putative β4-adrenoceptor. Br. J. Pharmacol. 1997;122:1244–1250. doi: 10.1038/sj.bjp.0701523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGESCU A., PLUTEANU F., FLONTA M.L., BADILA E., DOROBANTU M., POPOV D. The cellular mechanisms involved in the vasodilator effect of nebivolol on the renal artery. Eur. J. Pharmacol. 2005;508:159–166. doi: 10.1016/j.ejphar.2004.11.043. [DOI] [PubMed] [Google Scholar]
- GRANGER S.E., HOLLINGWORTH M., WESTON A.H. A comparison of several calcium antagonists on uterine, vascular and cardiac muscles from the rat. Br. J. Pharmacol. 1985;85:225–262. doi: 10.1111/j.1476-5381.1985.tb08854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAVES J., POSTON L. Beta adrenoceptor agonist mediated relaxation of rat isolated resistance arteries: a role for endothelium and nitric oxide. Br. J. Pharmacol. 1993;18:631–637. doi: 10.1111/j.1476-5381.1993.tb12853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY D.W., MARSHALL I. Novel signal transduction pathway mediating endothelium-dependent β-adrenoceptor vasorelaxation in rat thoracic aorta. Br. J. Pharmacol. 1992;107:684–690. doi: 10.1111/j.1476-5381.1992.tb14507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUIMARAES S., MOURA D. Vascular adrenoceptors: an update. Pharmacol. Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- HWA J.J., GHIBAUDI L., WILLIAMS P., CHATTERJEE M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am. J. Physiol. 1994;266:H952–H958. doi: 10.1152/ajpheart.1994.266.3.H952. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., MOLENAAR P. Differences between the third cardiac β3-adrenoceptor and the colonic β3-adrenoceptor in the rat. Br. J. Pharmacol. 1996;118:2085–2098. doi: 10.1111/j.1476-5381.1996.tb15648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZLOWSKA H., SZYMSKA U., SCHLICKER E., MALINOWSKA B. Atypical β-adrenoceptors, different from β3-adrenoceptors and probably from the low affinity state of β1-adrenoceptors, relax the rat isolated mesenteric artery. Br. J. Pharmacol. 2003;140:3–12. doi: 10.1038/sj.bjp.0705421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDS A.M., ARNOLD A., McAULIFF J.P., LUDUENA F.P., BROWN T.G. Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967;214:597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- LEBLAIS V., POURAGEAUD F., IVORRA M.D., GUIBERT C., MARTHAN R., MULLER B. Role of α-adrenergic receptors in the effect of the β-adrenergic receptor ligands, CGP 12177, Bupranolol, and SR 59230A, on the contraction of rat intrapulmonary artery. J. Pharmacol. Exp. Ther. 2004;309:137–145. doi: 10.1124/jpet.103.061192. [DOI] [PubMed] [Google Scholar]
- MACDONALD A., MCLEAN M., MACAULEY L., SHAW A.M. Effects of propranolol and L-NAME on β-adrenoceptor-mediated reaxation in rat carotid artery. J. Aut. Pharmacol. 1999;19:145–149. doi: 10.1046/j.1365-2680.1999.00128.x. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., SCHLICKER E. Mediation of the positive chronotropic effect of CGP 12177 and cyapindolol in the pithed rat by atipycal β-adrenoceptors, different from β3-adrenoceptors. Br. J. Pharmacol. 1997;117:943–949. doi: 10.1111/j.1476-5381.1996.tb15285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGRATH J.C., DEIGHAN C., BRIONES A.M., SHAFAROUDI M.M., MCBRIDE M., ADLER J., ARRIBAS S., VILA E., DALY C.J. New aspects of vascular remodelling: the involvement of all vascular cell types. Exp. Physiol. 2005;90.4:469–475. doi: 10.1113/expphysiol.2005.030130. [DOI] [PubMed] [Google Scholar]
- MANARA L., BADONE D., BARONI M., BOCCARDI G., CECCHI R., CROCI T., GIUDICE A., GUZZI U., LANDI M., LE FUR G. Functional identification of rat atypical β-adrenoceptors by the first selective β3-antagonists, aryloxyypropanolamino-tetralines. Br. J. Pharmacol. 1996;117:435–442. doi: 10.1111/j.1476-5381.1996.tb15209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUSHITA M., HORINOUCHI T., TANAKA Y., TSURU H., KOIKE K. Characterization of β3-adrenoceptor-mediated relaxation in rat abdominal aorta smooth muscle. Eur. J. Pharmacol. 2003;482:235–244. doi: 10.1016/j.ejphar.2003.09.037. [DOI] [PubMed] [Google Scholar]
- MIQUEL M.R., SEGURA V., ALI Z., D'OCON M.P., MCGRATH J.C., DALY C.J. 3D image analysis of fluorescent drug binding. Mol. Imaging. 2005;4:1–13. doi: 10.1162/15353500200504172. [DOI] [PubMed] [Google Scholar]
- MOHELL N., DICKER A. The β-adrenergic radioligand [3H]CGP-12177, generally classified as an antagonist, is a thermogenic agonist in brown adipose tissue. Biochem. J. 1989;84:401–405. doi: 10.1042/bj2610401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLENAAR P., JONES C.R., MCMARTIN L.R., SUMMERS R.J. Autoradiographic localization and densitometric analysis of beta-1 and beta-2 adrenoceptors in the canine left anterior descending coronary artery. J. Pharmacol. Exp. Ther. 1988a;246:384–393. [PubMed] [Google Scholar]
- MOLENAAR P., MALTA E., JONES C.R., BUXTON B.F., SUMMERS R.J. Autoradiographic localization and function of β-adrenoceptors on the human internal mammary artery and saphenous vein. Br. J. Pharmacol. 1988b;95:225–233. doi: 10.1111/j.1476-5381.1988.tb16568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONCADA S., REES D.D., SCHULZ R., PALMER R.M.J. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NAKAI K., ITAKURA T., NAKA Y., NAKAKITA K., KAMEI I., IMAI H., YOKOTE H., KOMAI N. The distribution of adrenergic receptors in cerebral blood vessels: an autoradiographic study. Brain Res. 1986;381:148–152. doi: 10.1016/0006-8993(86)90703-1. [DOI] [PubMed] [Google Scholar]
- O'DONNELL S.R., WANSTALL J.C. Beta-1 and beta 2 adrenoceptor-mediated responses in preparations of pulmonary artery and aorta from young and aged rats. J. Pharmacol. Exp. Ther. 1984;228:733–738. [PubMed] [Google Scholar]
- ORIOWO M.A. Atypical β-adrenoceptors in the rat isolated common carotid artery. Br. J. Pharmacol. 1994;113:699–702. doi: 10.1111/j.1476-5381.1994.tb17049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORIOWO M.A. Different atypical β-adrenoceptors mediate isoproterenol-induced relaxation in vascular and non-vascular smooth muscles. Life Sci. 1995;56:PL269–PL275. doi: 10.1016/0024-3205(95)00076-3. [DOI] [PubMed] [Google Scholar]
- PEDIANI J.P., COLSTON J.F., CALDWELL D., MILLIGAN G., DALY C.J., MCGRATH J.C. β-arrestin dependent spontaneous α1a-adrenoceptor endocytosis causes intracellular transportation of α-blockers via recycling compartments. Molecular Pharmacology. 2005;67:992–1004. doi: 10.1124/mol.104.008417. [DOI] [PubMed] [Google Scholar]
- PRIEST R.M., HICKS D., WARD J.P.T. Noradrenaline, β-adrenoceptor-mediated vasorelaxation and nitric oxide in large and small pulmonary arteries of the rat. Br. J. Pharmacol. 1997;122:1375–1384. doi: 10.1038/sj.bjp.0701528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHREURS A.J.M., TERPSTRA G.K., RAAIJMAKERS J.A.M., NIJKAMP F.P. Effect of vaccination with Haemophilus influenza on adrenoceptor function of tracheal and parenchymal strips. J. Pharmacol. Exp. Ther. 1980;215:691–696. [PubMed] [Google Scholar]
- SCOTLAND R.S., CHAUHAN S., DAVIS C., DE FELIPE C., HUNT S., KABIR J., KOTSONIS P., OH U., AHLUWALIA A. Vanilloid receptor TRPV1, sensory C-fibers, and vascular autoregulation: a novel mechanism involved in myogenic constriction. Circ. Res. 2004;95:1027–1034. doi: 10.1161/01.RES.0000148633.93110.24. [DOI] [PubMed] [Google Scholar]
- SEKIGUCHI F., NAKAHIRA T., KAWATA K., SUNANO S.V. Responses to endothelium-derived factors and their interaction in mesenteric arteries from Wistar-Kyoto and stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2002;29:1066–1074. doi: 10.1046/j.1440-1681.2002.03778.x. [DOI] [PubMed] [Google Scholar]
- SHAFAROUDI M.M., MCBRIDE M., DEIGHAN C., WOKOMA A., MACMILLAN J., DALY C.J., MCGRATH J.C. Two ‘knockout' mouse models demonstrate that aortic vasodilatation is mediated. J. Pharmacol. Exp. Ther. 2005;314:804–810. doi: 10.1124/jpet.105.085944. [DOI] [PubMed] [Google Scholar]
- SHAFIEI M., MAHMOUDIAN M. Atypical beta-adrenoceptor of rat thoracic aorta. Gen. Pharm. 1997;32:557–562. doi: 10.1016/s0306-3623(98)00283-3. [DOI] [PubMed] [Google Scholar]
- SHEN Y.T., CERVONI P., CLAUS T., VATNE S.F. Differences in beta 3-adrenergic receptor cardiovascular regulation in conscious primates, rats and dogs. J. Pharmacol. Exp. Ther. 1996;278:1435–1443. [PubMed] [Google Scholar]
- SHIMOKAWA H., YASUTAKE H., FUJII K., OWADA M.K., NAKAIKE R., FUKUMOTO Y., TAKAYANAGI T., NAGAO T., EGASHIRA K., FUJISHIMA M., TAKESHITA A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J. Cardiovasc. Pharmacol. 1996;285:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- SMITS B.W., SIERO H.L.M., ELLENBROEK B.A., RIKSEN N.P., COOLS A.R., BORGGREVEN J.M.P.M., RONGEN G., RUSSEL F.G.M., SMITS P. Stress susceptibility as a determinant on the response to adrenergic stimuli on mesenteric resistance artery of the rat. J. Cardiovasc. Pharmacol. 2002;40:673–683. doi: 10.1097/00005344-200211000-00005. [DOI] [PubMed] [Google Scholar]
- SOOCH S., MARSHALL I. Atypical β-adrenoceptors in rat vasculature. Ann. NY Acad. Sci. 1997;812:211–212. doi: 10.1111/j.1749-6632.1997.tb48178.x. [DOI] [PubMed] [Google Scholar]
- STEINBERG S.F., JAFFE E.A., BILEZIKIAN J.P. Endothelial cells contain beta adrenoceptors. Naunyn Schmiedeberg's Arch. Pharmacol. 1984;325:310–313. doi: 10.1007/BF00504374. [DOI] [PubMed] [Google Scholar]
- TAGAYA E., TAMAOKI J., TAKEMURA H., ISONO K., NAGAI A. Atypical adrenoceptor-mediated relaxation of canine pulmonary artery through a cyclic adenosine monophosphate-dependent pathway. Lungs. 1999;177:321–332. doi: 10.1007/pl00007650. [DOI] [PubMed] [Google Scholar]
- TROCHU J.N., LEBLAIS V., RAUTUREAU Y., BÉVÉRELLI F., LE MAREC H., BERDEAUX A., GAUTHIER C. Beta 3-adrenoceptor stimulation induces vasorelaxation mediated essentially by endothelium-derived nitric oxide in rat thoracic aorta. Br. J. Pharmacol. 1999;128:69–76. doi: 10.1038/sj.bjp.0702797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANHOUTTE P.M. Endothelial adrenoceptors. J. Cardiovasc. Pharmacol. 2001;38:796–808. doi: 10.1097/00005344-200111000-00016. [DOI] [PubMed] [Google Scholar]
- VON ZASTROW M., KOBILKA B.K. Agonist dependent and independent steps in the mechanism of adrenergic receptor internalization. J. Biol. Chem. 1994;269:18448–18452. [PubMed] [Google Scholar]
- WENEGER J., ZINK S., ROSEN P., GALLA H.J. Use of electrochemical impedance measurements to monitor β-adrenergic stimulation of bovine aortic endothelial cells. Pflügers Arch. Eur. J. Physiol. 1998;437:925–934. doi: 10.1007/s004240050864. [DOI] [PubMed] [Google Scholar]
- WHITE R., BOTTRILL F.E., SIAU D., HILEY R. Protein kinase A-dependent and -independent effects of isoproterenol in rat isolated mesenteric artery: interactions with levocromakalim. J. Pharmacol. Exp. Ther. 2001;298:917–924. [PubMed] [Google Scholar]
- ZINK S., ROSEN P., SACKMANN B., LEMOINE H. Regulation by endothelial permeability by β-adrenoceptor agonists: contribution of β1 and β2-adrenoceptors. Biochim. Biophys. Acta. 1993;1178:286–298. doi: 10.1016/0167-4889(93)90206-5. [DOI] [PubMed] [Google Scholar]
- ZWAVELING I., WINKLER PRINS E.A., PFAFFENDORF M., VAN ZWIETEN P.A. The influence of hyperthyroidism on β-adrenoceptor-mediated relaxation of isolated small mesenteric arteries. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;353:438–444. doi: 10.1007/BF00261441. [DOI] [PubMed] [Google Scholar]