Abstract
Association between staphylococcal infection and pathogenesis of upper airways disease has been reported. This study aimed to investigate the mechanisms underlying the rat pulmonary inflammation induced by airway exposure to staphylococcal enterotoxin A (SEA).
SEA (0.3–10 ng trachea−1) caused dose-dependent neutrophil accumulation in BAL fluid, reaching maximal responses at 4 h (25-fold increase for 3 ng trachea−1). Significant accumulation of both lymphocytes and macrophages in BAL fluid was also observed at 4 h (2.1- and 1.9-fold increase, respectively, for 3 ng trachea−1). At later times (16 h), neutrophil counts in bone marrow (immature forms) and peripheral blood increased by 63 and 81%, respectively. SEA failed to directly induce chemotaxis and adhesion of isolated neutrophils.
Analysis of mRNA expression for iNOS, COX-2 and CINC-2 in lung tissue showed an upregulation of these enzymes, which paralleled elevated levels of LTB4, PGE2, TNF-α, IL-6 and NO2− in BAL fluid. Expression of CINC-1 was unchanged, whereas CINC-3 was reduced in SEA-treated rats. Incubation of isolated alveolar macrophages with SEA (3 μg ml−1) resulted in significant elevations of TNF-α and NO2− levels in the cell supernatants.
Dexamethasone (0.5 mg kg−1), celecoxib (3 mg kg−1) and compound 1400 W (5 mg kg−1) markedly reduced SEA-induced lung neutrophil influx and NO2− levels in BAL fluid. The lipoxygenase inhibitor AA-861 (100 μg kg−1) partly inhibited the neutrophil influx in SEA-treated rats without modifying the NO2− levels. None of these treatments reduced the number of mononuclear cells in BAL fluid (except of dexamethasone, which abolished the increased lymphocyte counts).
Our study shows that airways exposure to SEA results in marked neutrophil influx through mechanisms involving increased expressions of CINC-2, iNOS and COX-2, as well as enhanced production of NO, PGE2, LTB4, TNF-α and IL-6.
Keywords: Cytokine-induced neutrophil chemoattractant, enterotoxins, nitric oxide, cyclooxygenase-2, cytokines
Introduction
Staphylococcus aureus is one of the most common Gram-positive pathogens in cases of food poisoning and staphylococcus-associated toxic shock syndrome in humans and animals (Müller-Alouf et al., 2001), which often progress to sepsis and multiorgan dysfunction (Kotzin et al., 1993; Lowy, 1998). This pathogen secretes a family of 25–30 kDa exoproteins, which are classified into eight distinct immunological types, namely staphylococcal enterotoxin types A–E and G–I, and are referred to as superantigens due to their ability to stimulate T-lymphocyte proliferation in very low concentrations, resulting in fever, shock and death. They bind as intact molecules to the class II major histocompatibility complex (MHC) antigens expressed on professional antigen-presenting cells outside the peptide-binding groove and then sequentially bind the T-cell receptor (TcR) via the variable region of the TcR-chain (Proft & Fraser, 2003). A number of studies have demonstrated that the enteropathogenic events of staphylococcal enterotoxins involve massive release of both stored (histamine, serotonin) and newly generated inflammatory mediators (IL-1, IL-2, IL-6, IL-8, TNF-α and IFN-γ) from resident cells and infiltrating leukocytes (Scheuber et al., 1987; Marrack & Kappler, 1990; Micusan & Thibodeau, 1993; Tessier et al., 1998; Desouza et al., 2001). A neurogenic component, mediated by substance P, may also take part in inflammatory responses induced by staphylococcal enterotoxins in mice (Desouza & Ribeiro-DaSilva 1996; 1998; Linardi et al., 2000), monkeys and humans (Micusan & Thibodeau, 1993).
Acute lung inflammation is an important component of pulmonary allergic diseases, including bronchial asthma, which is associated with mucosal edema, prominent airways eosinophil infiltration and release of several inflammatory mediators (Barnes et al., 1998). Evidences suggest a link between bacterial organisms and exacerbation of asthma, where these organisms may be implicated in asthma pathogenesis (Kraft, 2000). S. aureus is reported as a common and predominating bacterium of the upper respiratory tract, and is believed to contribute to the disease pathogenesis of the respiratory tract through the secretion of its toxins known as superantigens (Kotzin et al., 1993). The presence of IgE antibodies to S. aureus enterotoxins was shown to correlate with the severity of eosinophilic inflammation in upper airway disease (Bachert et al., 2002a, 2002b; 2003; Rossi & Monasterolo, 2004). On the other hand, several studies report that nonallergic, IgE-independent bronchial asthma, can be provoked by virus and bacterial infections, but the mechanisms underlying this type of noneosinophilic asthma particularly those triggered by Gram-positive bacteria, have not been extensively investigated (Turner et al., 1995; Wenzel et al., 1999; Douwes et al., 2002). There is also limited information available on the local epithelial or mucosal effects caused by staphylococcal superantigens exposure. Experimental studies have shown that intravenous (i.v.) administration of staphylococcal enterotoxin type B in mice (Neumann et al., 1997) or type A (SEA) in rabbits (Miller et al., 1996; Peterson et al., 1999) produces an acute inflammatory lung injury characterized by granulocyte infiltration into the airways (Neumann et al., 1997). More recently, repetitive intranasal administration of staphylococcal enterotoxin B in mice has been shown to trigger an inflammatory response characterized by mucosal and airway recruitment of lymphocytes, eosinophils and neutrophils (Herz et al., 1999). However, the resulting pulmonary inflammation in response to airway exposure to SEA has not yet been investigated. Therefore, in this study, the rat airways were exposed to SEA in a dose- and time-dependent manner and the resulting pulmonary inflammation was investigated, focusing our attention on the expression and production of inflammatory mediators involved in the leukocyte accumulation in bronchoalveolar lavage (BAL) fluid. In an attempt to understand the pharmacological mechanisms underlying the SEA-induced airways inflammation, a number of pharmacological agents were tested regarding their ability to reduce the neutrophil influx into the airways.
Methods
Animal experimentation guidelines
The experimental protocols were approved by the Ethical Principles in Animal Research adopted by the Brazilian College for Animal Experimentation (COBEA). Male Wistar rats (250–300 g) were housed in temperature-controlled rooms and received water and food ad libitum until used.
Intratracheal injection of staphylococcal enterotoxin A (SEA)
Rats were anesthetized with pentobarbital sodium (50 mg kg−1, intraperitoneally (i.p.)) and intratracheally injected with SEA (0.3–10.0 ng trachea−1, 0.4 ml). Control animals received 0.4 ml of sterile phosphate-buffered saline (PBS) alone. Leukocyte counts in peripheral blood, BAL and bone marrow were assessed at selected times thereafter (4, 16, 24 and 48 h).
Leukocyte counts in BAL fluid
BAL was performed at 4–48 h after SEA (or PBS) intratracheal injection. Briefly, the trachea was exposed and cannulated with a polyethylene tube (1 mm diameter) connected to a syringe. The lungs were washed by flushing with PBS solution containing heparin (20 UI ml−1). The PBS buffer was instilled through the tracheal cannula as one 10-ml aliquot followed by three 5-ml aliquots. The fluid recovered after each aliquot instillation (approximately 20 ml) was combined and centrifuged (1000 × g for 10 min at 20°C). The cell supernatant was stored at −80°C and the cell pellet was ressuspended in 2 ml of PBS solution. Total cell counts were carried out done with an automated cell counter (CELL-DYN 1700), while differential counts were carried out on a minimum of 200 cells using cytospin preparation stained with May–Grünwald. The cells were classified as neutrophils, eosinophils, mast cells, lymphocytes and macrophages based on normal morphological criteria.
Leukocyte counts in peripheral blood and bone marrow
Blood samples were obtained from the abdominal artery after the SEA (or PBS) injection into the airways. Total cell counts were carried out with an automated cell counter (CELL-DYN 1700, U.S.A.), while differential counts were carried out on blood smears stained by the May–Grünwald method. The cells were classified as neutrophils, eosinophils, mast cells, lymphocytes and mononuclear based on normal morphological criteria.
For measurement of cells in bone marrow, femurs were removed from rats immediately after killing. The epiphyses were cut transversely and bone marrow cells were flushed out with PBS containing heparin (20 IU ml−1). Differential count was carried out on a minimum of 200 cells using cytospin preparation stained with Leishman. Results are expressed as the number of leukocytes per femur. Cells were classified as immature neutrophils (myeloblast, promyelocyte and myelocyte), mature neutrophils (metamyelocyte, band and mature), eosinophils, mast cells, lymphocytes and mononuclear cells based on normal morphological criteria.
Measurement of LTB4, TNF-α, IL-10, IL-6, PGE2 and NO2− in BAL
Rat LTB4, TNF-α, IL-10, IL-6 and PGE2 were measured in BAL fluid supernatant using commercially available enzyme-linked immunosorbent assays (ELISA) according to the manufacturers instructions for rat TNF-α, LTB4, IL-10, IL-6 and PGE2. Nitrite production in BAL fluid was quantified colorimetrically after the Griess reaction (Greenberg et al., 1995). Briefly, BAL fluid supernatant (100 μl) was reacted with an equal volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylenedihydrochloride, 2.5% phosphoric acid) in duplicate microtiter wells at room temperature. Chromophore absorbance at 450 nm was determined. Nitrite concentrations were calculated using sodium nitrite as a standard.
Pharmacological investigation with different drugs
The following drugs were used at the indicated doses and schedules of administration: (1) dexamethasone (0.5 mg kg−1) was administered i.p. 1 h before SEA exposition (Cunha & Ferreira, 1986); (2) the lipoxygenase inhibitor AA-861 (100 μg kg−1) was administered i.v. 15 min before SEA exposition (Filliatre et al., 2001); (3) the selective cyclooxygenase-2 (COX-2) inhibitor celacoxib (3 mg kg−1) was administered i.p. 1 h before SEA exposition (Filliatre et al., 2001); (4) the iNOS selective inhibitor compound 1400 W (5 mg kg−1) was administered i.v. immediately before SEA exposition (Parmentier et al., 1999).
Analysis of iNOS, COX-2, CINC-1, CINC-2 and CINC-3 gene expression by RT–PCR
Total RNA from whole lung was extracted by the Trizol reagent method, according to the manufacturers protocol (Life Technologies, GIBCO-BRL, U.S.A.). cDNA was synthesized from 15 μg of total RNA using Superscript II (Life Technologies), and the obtained material was stored at −20°C until use. PCR reactions were performed in a final volume of 50 μl, containing 5 μl of cDNA solution, 5 μl of 10 × PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, 2 U of Taq DNA polymerase (Life Technologies) and variable concentrations of each oligonucleotide primer pair (CINC-1: 1 μM, CINC-2: 2 μM, CINC-3: 3 μM, COX-2 and iNOS: 5 μM, glyceraldehyde-3-phosphate dehydrogenase (GAPDH)- internal control: 3 μM). The nucleotide sequences of the used primers are show in the table below:

Amplification cycle for CINCs was carried out with denaturation for 5 min at 94°C followed by 23 cycles of amplification consisting of a denaturation step 95°C for 1 min, a primer-annealing step at 56°C for 2 min and an extension step at 72°C for 3 min. After the last amplification cycle, samples were incubated at 72°C for 7 min for extension. In all, 33 cycles were performed for the amplification of the COX-2 (1 min of denaturation at 94°C, 2 min of annealing at 56°C and 1 min of extension at 72°C) and 30 cycles were performed for the iNOS (1 min of denaturation at 94°C, 45 s of annealing at 65°C and 1.5 min of extension at 72°C). Lungs samples obtained from rats 6 h after an lipopolysaccharide (LPS) injection (0.3 mg kg−1, i.v.) were run in parallel and served as positive controls for COX-2 and iNOS expression. PCR products were separated on 1.8% agarose gels containing ethidium bromide. Band fluorescence images were acquired and digitalized using a ChemiImager 5500 system (Alpha Scientific), and the band intensities were determined by densitometry using the equipment software. For each sample, the ratios between the densitometry value for each specific gene and the corresponding GAPDH were calculated and statistically analyzed for comparison among the experimental groups.
Isolation of peripheral blood neutrophils
Neutrophils were separated from rat peripheral blood in 3.13% (w v−1 sodium citrate (10 : 1) and obtained by Dextran sedimentation by Ficcol (1.077 g l−1) gradient. For each experiment, a pool of blood of five rats was used. After separation of monocytes and granulocytes by centrifuging at 400 × g for 30 min, the granulocyte layer was washed once in Eagle's minimum essential medium (MEM; pH 7.2) before performing a hypotonic lysis to disrupt the red cells. Cells were washed once again in MEM and resuspended in MEM/0.1% ovalbumin. Samples of cell suspension were used to determine total cell number using an improved Neubauer hemocytometer, and then cytospinned onto slides for a differentiation count. The final cell suspension contained 89% of neutrophils. Cell viability (>95%) was assessed by Trypan blue dye exclusion test.
Neutrophil adhesion assays in vitro
96-well plates were prepared by coating individual wells with 60 μl of serum (1 : 10 dilution in PBS) overnight at 4°C. Wells were then washed twice with PBS before blocking noncoated sites with 0.1% (w v−1) bovine serum albumin (BSA) for 60 min at 37°C. Wells were washed twice again with PBS before allowing plates to dry. Neutrophils (50 μl of 1 × 106 cells ml−1 in MEM/ovalbumin) were seeded onto the coated wells alone or with SEA and cells were allowed to adhere for 30 min at 37°C, 5% CO2. A positive control was run using the N-formyl-methionyl-leucyl-phenylalanine (fMLP; 10−5 M). Following incubation, nonadhered cells were washed twice with PBS. MEM (50 μl) was added to each well and varying concentrations of the original neutrophil cell suspension were added to empty wells to form a standard curve. Plates were then stored frozen overnight before measuring the myeloperoxidase (MPO) content of adherent cells (Bradley et al., 1982). Plates were defrosted on ice before extracting with hexadecytrimethyl-ammonium bromide (HTAB) in 50 mM potassium phosphate buffer, pH 6.0. In all, 20 μl of each well sample to be measured was mixed with 200 μl of o-dianisidine solution (0.167 mg ml−1 o-dianiidine dihydrochloride, 0.0005% hydrogen peroxide in 50 mM phosphate buffer, pH 6.0) immediately prior to reading change of absorbance at 460 nm over 5 min in a microplate (Multiscan MS, Labsystems, CA, U.S.A.). Adherence was calculated by comparing absorbance changes of unknowns to those of the standard curve.
Neutrophil chemotaxis assays in vitro
Neutrophil migration assays were performed using a 96-well chemotaxis chamber (NeuroProbe, MD, U.S.A.). In all, 25 μl of 8 × 106 cells ml−1 neutrophils (prepared in MEM containing 0.1% ovalbumin) were added to the upper compartment of the chamber and separated by a polycarbonate filter (5 μm pore) from the lower chamber containing 29 μl of MEM, SEA or fMLP (10−7 M). The chambers were incubated at 37°C in a 5% CO2 atmosphere for 120 min. The wells of the upper compartment were emptied by aspiration, and then disassembled. To detach adherent neutrophil from the filter, the microtiter plate with attached filter was centrifuged at 1200 r.p.m. for 5 min at room temperature (Somersalo et al. (1990), with modifications). The filter was carefully removed. The resulting material in each well was homogeneized, and transferred to another plate where the MPO assay was carried out, as described above. Migrated neutrophils were calculated by comparing absorbance changes of unknowns to those of the standard curve.
Measurement of TNF-α and NO2− production by alveolar macrophages in vitro
BAL fluid obtained from naïve rats was centrifuged (10 min, 470 × g) and cells recovered from pellets were pooled. The cell pellets were resuspended in culture medium (RPMI 1640) supplemented with BSA (0.1%), and transferred to 35-mm diameter tissue culture wells (1 ml well−1, six wells). They were allowed to adhere for 2 h at 37°C in a humidified atmosphere containing 5% CO2. Nonadherent alveolar macrophages were discarded, and the remaining cells corresponded to >95%. Cells were incubated for 4 h in the presence of either LPS (3 μg ml−1) or SEA (3 μg ml−1), and supernatant from each well was collected and stored at −70°C. The concentrations of TNF-α were assayed by commercially available enzyme-linked immunosorbent assays (ELISA) according to the manufacturer's instructions. The NO2− levels were quantified colorimetrically after the Griess reaction, as stated above.
Materials
Staphyloccocal enterotoxin A (SEA), minimum essential medium (MEM), Dextran, AA-861, N-formyl-L-methionyl-L-leucyl-phenylalanine (fMLP), hexadecytrimethyl-ammonium bromide (HTAB), lipopolysaccharide (LPS) from Escherichia coli, dexamethasone and Griess reagent were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.). N-(3-(aminomethyl)benzyl)acetamidine (1400 W) was purchased from Alexis (Nottingham, U.K.). Celecoxib was obtained from Laboratórios Pfizer Ltd (São Paulo, Brazil). Enzyme-linked immunosorbent assays (ELISA) for rat TNF-α were obtained from BD Biosciences (CA, U.S.A.), whereas ELISA for rat LTB4, PGE2, IL-6 and IL-10 were obtained from R&D Systems (MN, U.S.A.). The chemicals used in PCR were of the highest grade available locally.
Statistical analysis
Data are presented as the mean values±s.e.m. and were analyzed by analysis of variance (ANOVA) for multiple comparisons followed by Bonferroni test or Student's unpaired t-test where appropriate. In both cases, the level of significance was set at P<0.05.
Results
Intratracheal injection of SEA: total and differential leukocyte counts in BAL fluid
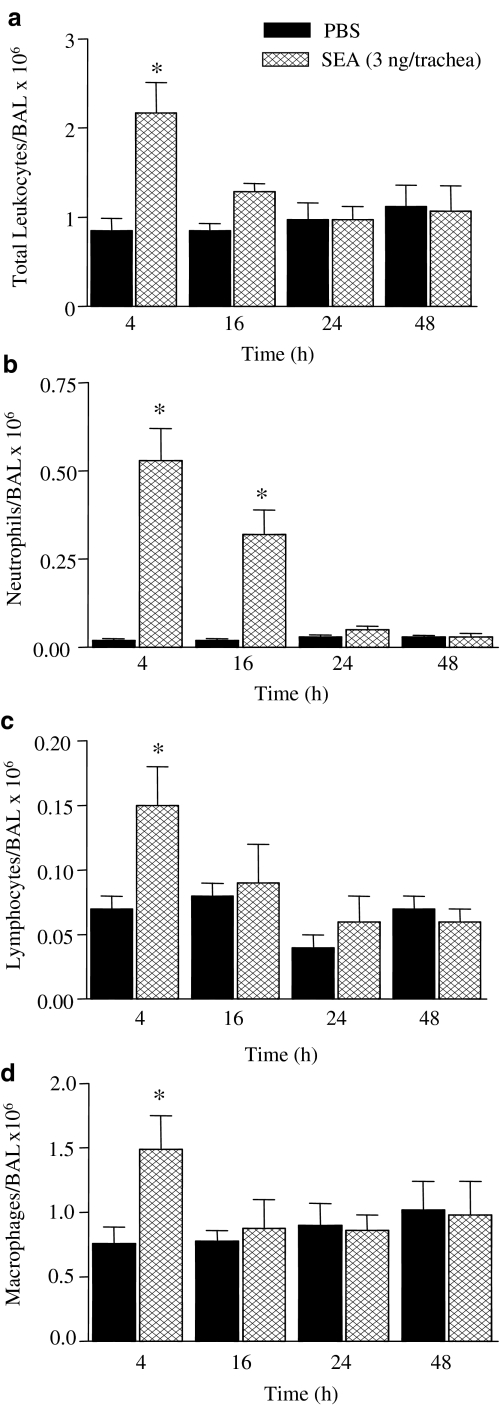
Figure 1 shows that intratracheal injection of SEA (3.0 ng trachea−1) caused a marked accumulation of total leukocytes in BAL fluid, as observed at 4 h post-SEA injection, returning to basal levels at 16 h thereafter. The neutrophil accumulation peaked at 4 h (25-fold increase), remaining markedly elevated (16-fold increase) at 16 h post-SEA injection, in comparison with the respective PBS group (P<0.05). At 24 and 48 h post-SEA injection, the number of neutrophils in BAL fluid was not changed compared with the PBS group. A significant elevation in the number of lymphocytes and macrophages were detected only 4 h post-SEA injection, with increases of 2.1- and 1.9-fold, respectively, in comparison with the PBS groups (P<0.05). Eosinophils were virtually absent in BAL fluid in all time-points evaluated.
Figure 1.
Time-course alterations in the total and differential leukocyte counts in BAL fluid of rats exposed to SEA. BAL fluid was obtained after intratracheal injections of SEA (3.0 ng trachea−1) at the indicated time-periods. Panels a–d show counts of total leukocytes, neutrophils, lymphocytes and macrophages, respectively. The black bars indicate the counts obtained with PBS alone. The data are the mean values±s.e.m. of six rats. *P<0.05 compared with respective PBS group.
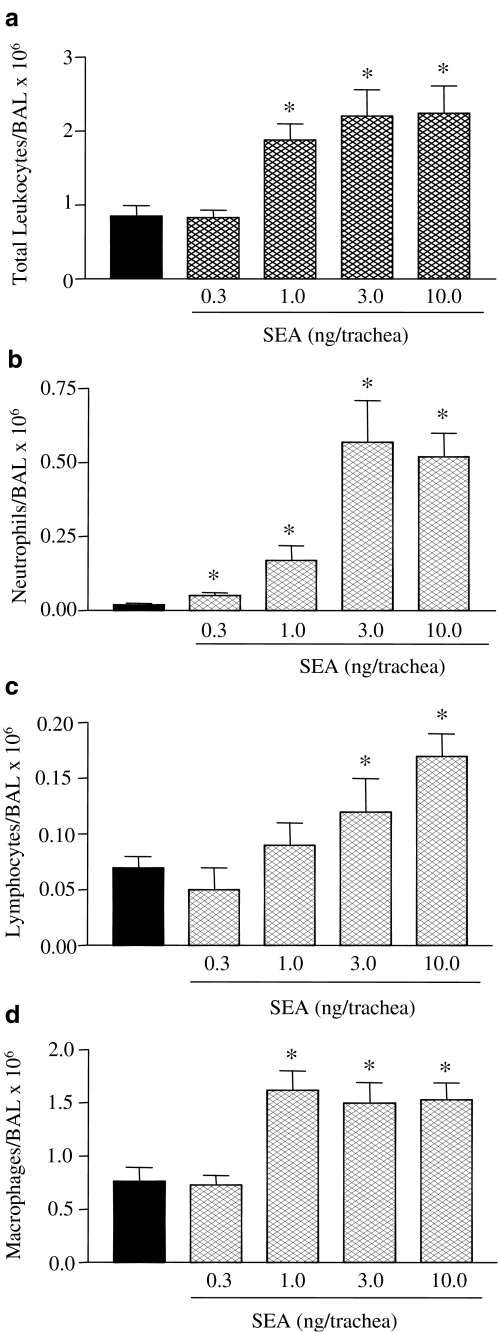
Intratracheal injection of different doses of SEA (0.3–10 ng trachea−1) resulted in a dose-dependent influx of total leukocytes and neutrophils in BAL fluid at 4 h, with maximal neutrophil responses obtained with 3.0 ng trachea−1 (Figure 2). Maximal accumulation of macrophages was observed with 1.0 ng of SEA, while for lymphocytes maximal response was observed with 10.0 ng (Figure 2).
Figure 2.
Dose-dependent alterations in the total and differential leukocyte counts in BAL fluid of rats exposed to SEA. The BAL fluid was obtained after intratracheal injection of SEA (4 h) at the indicated doses. Panels a–d show counts of total leukocytes, neutrophils, lymphocytes and macrophages, respectively. The black bars indicate the counts obtained with PBS alone. The data are the mean values±s.e.m. of six rats. *P<0.05 compared with respective PBS group.
To exclude the possibility that SEA-induced pulmonary cell influx is due to contamination with Gram-negative bacterial products such as LPS, a solution of SEA (7.5 ng ml−1) was incubated with the antibiotic polymyxin B (30 μg ml−1) for 30 min at 37°C prior to its intratracheal injection (3.0 ng trachea−1; n=5). At a dose that nearly abolishes the LPS-induced pulmonary cell influx, polymyxin B had no significant effect in the neutrophil, lymphocyte and macrophage influxes in response to SEA (0.80±0.30, 0.2±0.1 and 1.3±0.3 × 106 BAL−1, respectively) compared with untreated SEA (0.85±0.33, 0.16±0.03 and 1.49±0.21 × 106 BAL−1, respectively). In addition, reductions of 65, 31 and 27% (P<0.05) in the neutrophil, lymphocyte and macrophage influxes were observed when a SEA solution (7.5 ng ml−1) was boiled for 1 h, and then injected at the dose of 3.0 ng trachea−1.
Total and differential leukocyte counts in peripheral blood
At 4 h after intratracheal injection of SEA (0.3–10 ng trachea−1), no changes in total leukocytes and neutrophil counts in peripheral blood could be detected at any dose of SEA used, as compared with PBS (not shown; n=6). However, at later times (16 h), a significant increase (P<0.05) in total leukocytes and neutrophils was detected (3.0 ng trachea−1: 6.3±0.3 and 2.9±0.2 × 106 cells ml−1, respectively) compared with PBS (5.7±0.4 and 1.6±0.1 × 106 cells ml−1, respectively). The lymphocyte number in peripheral blood at 4 h post-SEA (3.0 ng trachea−1) injection (3.8±0.3 × 106 cells ml−1) was not significantly affected compared with PBS group (3.6±0.3 × 106 cells ml−1, respectively). No significant alterations on lymphocyte counts were observed at the other time-points (not shown; n=6). No eosinophils were detected in peripheral blood in any time evaluated.
Total and differential leukocyte counts in bone marrow
At 4 h post-SEA intratracheal injection (0.3–10 ng trachea−1), the number of mature or immature forms of neutrophils in bone marrow was not significantly affected in comparison with control animals (not shown, n=6). However, at later times (16 h), the number of immature neutrophils increased by 63% (P<0.05) in comparison with the PBS group (7.0±0.6 × 106 and 4.3±0.4 × 106 cells femur−1, respectively). The number of mature neutrophils did not change significantly between SEA (3.0 ng trachea−1) at 4, 16, 24 and 48 h (43.5±2.4, 49.5±4.5, 45.3±4.9 and 45.2±4.0 × 106 cells femur−1, respectively) and PBS (42.3±2.4 × 106 cells femur−1). The number of bone marrow lymphocytes was not significantly affected at 4 and 16 h post-SEA injection (not shown), but at 48 h a significant increase in lymphocyte counts could be detected (1.7±0.3 and 3.0±0.6 × 106 cells femur−1 for PBS and SEA, respectively; n=6).
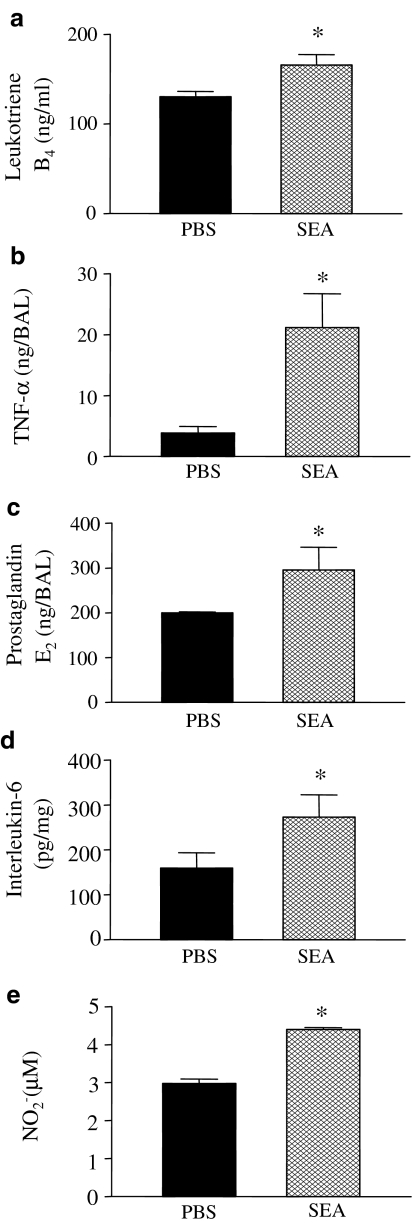
Measurements of PGE2, LTB4, NO2−, TNF-α, IL-6 and IL-10 levels in BAL fluid
The concentrations of LTB4, PGE2, TNF-α, IL-6 and IL-10 were measured in the BAL fluid from control and SEA (3.0 ng)-treated rats. As shown in Figure 3, the concentrations of LTB4, PGE2, NO2−, TNF-α and IL-6 were significantly higher after SEA instillation when compared with control groups. The IL-10 levels were not significantly altered between both groups (0.34±0.9 and 0.26±0.9 ng BAL−1 for control and SEA, respectively).
Figure 3.
Measurements of levels of LTB4 (panel a), TNF-α (panel b), PGE2 (panel c), IL-6 (panel d) and NO2− (panel e) levels in BAL fluid after rat airways exposition with SEA. Rats were intratracheally injected with PBS or SEA (3.0 ng trachea−1), and BAL fluid was colleted at 1 h (PGE2) or 4 h (LTB4, TNF-α, IL-6 and NO2−) thereafter. Results are mean values±s.e.m. from four animals. *P<0.05 compared with the respective PBS group (black bars).
Inducible NOS, COX-2 and CINC mRNA expression in the lung tissue
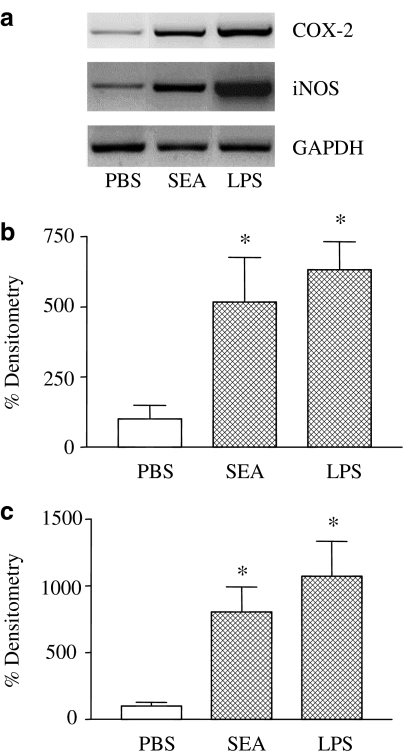
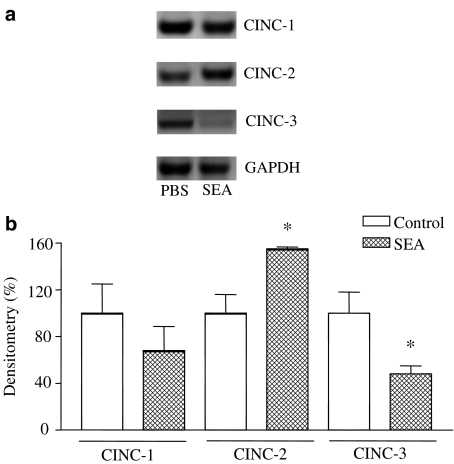
To determine whether iNOS and COX-2 are upregulated during SEA (3.0 ng trachea−1, 4 h)-induced lung inflammation, lung extracts were assessed for enzymes mRNA expression, using LPS (0.3 mg kg−1, i.v.)-treated rats as positive controls. Both COX-2 and iNOS expressions were significantly increased in both SEA- and LPS-treated rats (Figure 4). The CINC-1, CINC-2 and CINC-3 mRNA expressions were also evaluated in lung tissue of SEA (3 ng trachea−1, 4 h)-treated rats. Figure 5 shows that mRNA expression for CINC-1 did not differ significantly between control and treated rats, whereas CINC-2 mRNA expression was significantly increased (P<0.05) in the lung tissue of SEA-treated rats. On the other hand, the CINC-3 mRNA expression significantly decreased (P<0.05) in the lung tissue of SEA-treated rats compared with control animals (Figure 5).
Figure 4.
Representative RT–PCR amplification of mRNA for iNOS and COX-2 in lungs of rats exposed with SEA (panel a). Lung extracts were assessed for enzymes mRNA expression from PBS or SEA (3.0 ng trachea−1, 4 h)-treated rats. GAPDH was used as the internal standard. Positive controls were carried out using rats endovenously injected with LPS (0.3 mg kg−1, 12 h). In the panels above, the respective mRNA levels were estimated by densitometry of the bands (panel b, iNOS; panel c, COX-2). *P<0.05 compared with respective PBS groups.
Figure 5.
Representative RT–PCR amplification of mRNA for CINC-1, CINC-2 and CINC-3 in lungs of rats exposed with SEA (panel a). Lung extracts were assessed for enzyme mRNA expression from PBS- or SEA (3.0 ng trachea−1, 4 h)-treated rats. GAPDH was used as the internal standard. In the panel b, the mRNA levels estimated by densitometry of the bands. *P<0.05 compared with the respective PBS groups.
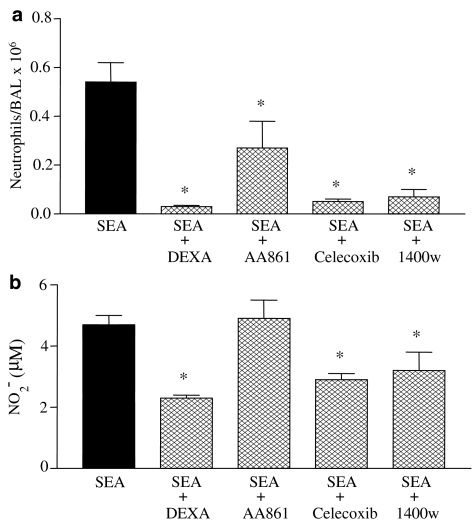
Pharmacological investigation with different drugs in the neutrophil and mononuclear cell counts, and NO2− levels in BAL fluid
For these experimental protocols, BAL fluid was examined at 4 h post-intratracheal injection of 3.0 ng trachea−1 of SEA. Figure 6 shows that pretreatment of rats with dexamethasone (0.5 mg kg−1, i.p.) nearly abolished both the SEA-induced neutrophil accumulation and increased levels of NO2− in BAL fluid. Significant inhibitory responses in neutrophil influx and increased NO2− levels were also observed with the COX-2 inhibitor celecoxib (3 mg kg−1, i.p.) and the selective iNOS inhibitor compound 1400W (5 mg kg−1, i.v.). The lipoxygenase inhibitor AA-861 (100 μg kg−1, i.v.) partly inhibited (P<0.05) the SEA-induced neutrophil influx in BAL, but had no significant effect in the NO2− levels (Figure 6). Additionally, none of these treatments reduced the number of mononuclear cells in BAL fluid, except of dexamethasone, which abolished the increased lymphocyte counts (not shown, n=4–6).
Figure 6.
The effect of different pharmacological agents in the neutrophil influx and NO2− levels in BAL fluid from rats exposed to SEA. Dexamethasone (DX, 0.5 mg kg−1), AA-861 (AA, 100 μg kg−1), celacoxib (3 mg kg−1) and 1400 W (5 mg kg−1) were administered as described in Methods. Neutrophil counts (panel a) and NO2− measurement (panel b) were performed at 4 h after SEA injection (3.0 ng trachea−1). The black bars indicate the counts obtained with SEA in untreated animals (Control). The data are the mean values±s.e.m. of 4–6 rats. *P<0.05 compared with the untreated group.
In vitro neutrophil chemotaxis and adhesion
Table 1 shows that SEA (0.01-1 ng well−1) was not able to directly induce neutrophil chemotaxis in vitro (37°C, 5% CO2) when compared with random chemotaxis (MEM). In the same experimental protocols, the chemoattractant fMLP (10−7 M) induced a significant neutrophil chemotaxis. Adhesion of neutrophils to serum-coated plates was significantly increased (P<0.05) when cells were seeded onto the coated wells with fMLP (10−5 M), but no neutrophil adhesion was observed when SEA (0.1–1.0 ng well−1) was used as stimulus (Table 1).
Table 1.
Rat neutrophil chemotaxis and adhesion in vitro induced by SEA
| Chemotaxis ( × 105/ml) | Adhesion (%) | ||
|---|---|---|---|
| MEM/Control | 7.13±0.62 | MEM/Control | 49.5±0.86 |
| fMLP (10−7 M) | 11.1±0.40* | fMLP (10−5 M) | 71.6±0.89* |
| SEA (0.01 ng well−1) | 4.90±0.64 | SEA (0.1 ng well−1) | 55.5±0.14 |
| SEA (0.10 ng well−1) | 6.50±0.40 | SEA (0.3 ng well−1) | 48.6±1.91 |
| SEA (1.00 ng well−1) | 8.00±0.51 | SEA (1.0 ng well−1) | 53.8±0.87 |
Neutrophils were isolated from rat peripheral blood in 3.13% (w v−1) sodium citrate (10 : 1) and obtained by dextran sedimentation using Ficol (1.077 g l−1) gradient (see Methods).
The myeloperoxidase (MPO) activity was measured, and results were expressed as absolute number of neutrophil that migrated through the filter (chemotaxis) or % neutrophil adhesion. The data represent the mean values±s.e.m. of three experiments (each in duplicate).
P<0.05 compared with respective MEM.
Measurement of TNF-α and NO2− levels in isolated alveolar macrophages
Isolated alveolar macrophages were incubated in vitro with SEA (3 μg ml−1), and at 4 h after incubation levels of TNF-α and NO2− were evaluated in the alveolar macrophage supernatants. Incubation of alveolar macrophages with LPS (3 μg ml−1) was used as a positive control. Data showed that TNF-α production was significantly increased (P<0.05) in response to SEA (4.4±0.1 ng ml−1, n=4) or LPS (4.8±0.05 ng ml−1, n=4), as compared with nontreated cells (3.1±0.2 ng ml−1). Similarly, the NO2− levels were increased in alveolar macrophages incubated with either SEA (24.4±3.6 μM; P<0.05) or LPS (51.6±3.8 μM; P<0.01), compared with untreated cells (15.9±3.4 μM).
Discussion
Our present study clearly shows that intratracheal instillation of SEA attracts leukocytes to the rat airways, preferentially neutrophils, by indirect mechanisms involving increased expressions of CINC-2, iNOS and COX-2, along with enhanced productions of NO2−, PGE2, LTB4, TNF-α and IL-6. Furthermore, our findings that eosinophils were virtually absent in all concentrations and time-points evaluated after SEA instillation indicate that pulmonary inflammation triggered by SEA mimics the noneosinophilic asthma, where infiltrated neutrophils rather than eosinophils and lymphocytes are postulated to underlie this inflammatory condition (Douwes et al., 2002). The neutrophil accumulation into the lungs after SEA instillation (4 h) was accompanied by a late increase in the number of neutrophils (16 h) in bone marrow (immature forms) and peripheral blood, which was not observed with lymphocytes and macrophages. Enterotoxins such as SEA can directly or indirectly trigger the production of granulocyte-macrophage colony-stimulation factor (GM-CSF), a glycoprotein known to induce proliferation and development of neutrophil progenitors in bone marrow (Sallerfors & Olofsson, 1992). Although our study did not attempt to assess GM-CSF production in bone marrow, it is tempting to suggest that increased number of immature neutrophils in bone marrow and mature forms in peripheral blood at 16 h post-SEA exposition reflects increased GM-CSF production by pulmonary or bone marrow stromal cells.
The expression of class II molecules is usually limited to specialized cells of the immune system, but they can also be expressed in different pulmonary cells, including epithelial cells and alveolar macrophages, where binding of staphylococcal enterotoxins (including SEA) to epitopes of MHC-class II molecules can trigger the release of inflammatory mediators and leukocyte accumulation (Müller-Alouf et al., 2001; Schramm & Thorlacius, 2003). Neutrophils do not express MHC class II molecules in normal conditions, and therefore may not directly interact with staphylococcal antigens (Gosselin et al., 1993). Accordingly, our findings showed that, in conditions where fMLP produced significant neutrophil chemotaxis and adhesion, SEA was unable to directly change these cell functions, confirming that indirect mechanisms are rather involved in SEA-induced lung neutrophil accumulation.
Alveolar macrophages are richly present in alveoli, distal airspaces and conducting airways, and have been considered the first line of defense against numerous agents. Direct effects of superantigens on macrophage activity have been documented, in particular the production of cytokines (Miller et al., 1996; Wrigth & Chapes, 1999). They produce both Th1 (TNF-α) and Th2 cytokines (IL-6 and IL-10), along with other inflammatory mediators, including nitric oxide and products of arachidonic acid metabolism such as PGE2 and LTB4. Moreover, it is well established that iNOS and COX-2 can be expressed in murine macrophages (J774.2) under stimulation with cytokines and Gram-negative bacterial toxins such as LPS (Swierkosz et al., 1995), but no studies have examined the expression of these enzymes in animals exposed to SEA. Our present study showed an increased number of macrophages in BAL fluid at 4 h post-SEA injection, and that was accompanied by enhanced expressions of CINC-2, COX-2 and iNOS in lung tissues, as well as by higher levels of NO2−, cytokines (IL-6 and TNF-α) and arachidonic acid products (PGE2 and LTB4) in BAL fluid. Furthermore, significant amounts of TNF-α and NO2− were also found in isolated alveolar macrophages incubated with SEA. It is likely therefore that SEA-induced neutrophil infiltration is, at least partly, mediated by alveolar macrophages. Additionally, in our study, the elevations of lymphocytes in BAL fluid of SEA-treated rats were smaller compared with those of neutrophils, which is in agreement with the air-pouch model in mice, where no T cells could be detected in inflammatory exudates of SEA-injected air-pouches (Tessier et al., 1998).
Cytokine networks between alveolar-capillary cell membranes are pivotal to initiation and propagation of the inflammatory response leading to pulmonary injury. The IL-8 family of chemokines plays a major role in neutrophil infiltration into inflamed tissues. In rats, the isomers CINC-1, CINC-2α, CINC-2β and CINC-3 have been considered the functional homologs of IL-8, and present similar ability to induce chemotaxis and to activate rat neutrophils both in vitro and in vivo (Watanabe et al., 1991; Nakagawa et al., 1998). Our data showed that CINC-2 mRNA expression is increased in the lung tissue of SEA-treated rats, indicating an important role for this cytokine in lung inflammation induced by airways exposure to SEA. This is in agreement with a previous study demonstrating a remarkable upregulation of CINC-2 mRNA expression (but not of CINC-1 and CINC-3) in the airways of Pseudomonas-infected rats, which suggested that CINC-2 acts as a major chemoattractant for neutrophils in this inflammatory condition (Amano et al., 2000). In our present study, the CINC-3 mRNA expression in the lung homogenates of SEA-exposed rats was clearly reduced, whereas CINC-1 mRNA expression was unchanged. While additional studies are required to elucidate this aspect, one may suggest that this reflects the time-course after SEA exposition. For instance, in LPS-stimulated rat peritoneal macrophages, expression of CINC-1 expression reaches a maximum at 12 h, while CINC-2 and CINC-3 expressions increase up to 24 h after LPS stimulation (Shibata et al., 2000a). Additionally, CINC-3 has been shown to inhibit neutrophil chemotaxis and calcium mobilization induced by CINC-1 and CINC-2 (Shibata et al., 2000b). Whether elevation of pulmonary CINC-2 levels downregulates CINC-3 expression remains to be elucidated.
The cytokine TNF-α produced by alveolar macrophages has been shown to exert critical roles in neutrophil influx induced by staphylococcal enterotoxins in the mice air-pouch (Tessier et al., 1998). In addition, incubation of human peripheral mononuclear cells with SEA in vitro induces the secretion of TNF-α that leads to endothelial damage (Fujisawa et al., 1998). Additionally, the pleiotropic cytokine IL-6 regulates inflammatory responses during Gram-positive bacterial infection (Hack et al., 1989), but the exact role of this cytokine in the lung pathophysiology is still unclear, since it may present both pro- and anti-inflammatory effects. In our present study, the exposure of rat airways to SEA significantly increased the levels of both TNF-α and IL-6 in BAL fluid, further supporting a major role for these two cytokines in SEA-induced lung inflammation. However, this study cannot ascertain if IL-6 acts to attenuate the SEA-induced lung inflammation, downregulating the TNF-α production and NF-κB signaling (Trepicchio et al., 1997). Of interest, in IL-6-deficient mice, IL-6 has been shown to downregulate the activation of the cytokine network in the lung of mice treated with the Gram-positive bacteria Streptococcus pneumoniae (van der Poll et al., 1997). Several evidences show that the cytokine IL-10 reduces the levels and expression of TNF-α by activated monocytes and/or macrophages, and this has been associated with clinical protection and reduction of lung pathology (Morrison et al., 2000). In our study, the marked elevations of TNF-α and IL-6 in BAL fluid were not accompanied by concomitant elevations in the IL-10 levels, suggesting that SEA-induced lung injury does not undergo downregulation by this cytokine. This contrasts with a previous study where staphylococcal enterotoxin B, injected i.p. in mice, was able to induce the release of significant amounts of plasma IL-10, and that the TNF-α levels were markedly elevated in IL-10-deficient mice (Haskó et al., 1998). In our hands, staphylococcal enterotoxin B, given intratracheally in rats, also elevated by 211% the IL-10 levels in BAL fluid (data not showed). Therefore, the findings that SEA is incapable to release IL-10 may explain its much higher potency to induce inflammation compared with Staphylococcal enterotoxin B (Tessier et al., 1998).
It has been shown that COX-2, via its product PGE2, modulates the iNOS pathway, and conversely the COX enzyme system contributes to iNOS gene induction and the resultant NO production. It is suggested that this crosstalk between iNOS and COX-2 represents an important mechanism by which the inflammatory responses can be amplified or attenuated (Goodwin et al., 1999). In our study, pretreatment of the animals with either the selective iNOS inhibitor 1400 W or the selective COX-2 inhibitor celecoxib markedly reduced SEA-induced neutrophil influx and NO2− levels into the airways, emphasizing the importance of both NO and/or PGE2, derived from iNOS and COX-2, respectively, to trigger SEA-induced pulmonary neutrophil accumulation. Consistent with this, high serum levels of nitrite and nitrate were detected in mice after i.p. injection of staphylococcal enterotoxin B (Florquin et al., 1994) and detectable expression of mRNA for iNOS was found in mouse endothelial cells treated with this enterotoxin (LeClaire et al., 1995).
Besides its well-established capacity to inhibit the arachidonic acid metabolism, glucocorticoids are also known to suppress gene expressions which are subject to NF-κB regulation (Adcock & Caramori, 2001), such as CINCs, TNF-α, iNOS and COX-2. Dexamethasone potently attenuates Gram-negative bacterial toxin-induced mediator expression, leukocyte recruitment and associated tissue injury during endotoxemia (Satoh et al., 2001; Schramm & Thorlacius, 2003), but the effects of dexamethasone on SEA-induced inflammatory responses have been neglected. Our findings that dexamethasone nearly abolished the SEA-induced neutrophil influx and NO2− levels into the airways strongly suggests that it is due to suppression of functions in SEA-activated cells, including inhibition of CINCs, iNOS and COX-2 expressions, and reductions of NO2−, TNF-α, IL-6 and PGE2 release in the lung tissue. In immune inflammation in mice, TNF-α induces in vivo peritoneal neutrophil migration via mechanisms involving release of LTB4 (Canetti et al., 2001). This may explain the moderate (but significant) increase in LTB4 levels in BAL fluid of SEA-treated rats and the partial inhibitory effects of the selective lypoxygenase inhibitor AA861 in SEA-induced neutrophil influx.
In conclusion, this study shows that airways exposition to SEA in rats mimics a nonallergic inflammation characterized by a large influx of neutrophils and absence of eosinophils in BAL fluid. The mechanisms underlying this neutrophil influx involve complex interactions of different pathways, resulting in increased expressions of CINC-2, iNOS and COX-2, as well as enhanced production of NO, PGE2, LTB4, TNF-α and IL-6. It is reasonable to suggest that elucidation of the key mediators in lung inflammation by SEA along with the discovery of specific inhibitory agents would make it possible to develop clinically effective anti-inflammatory therapy.
Acknowledgments
Ivani A. De Souza thanks Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support.
Abbreviations
- BAL
bronchoalveolar lavage
- CINC
cytokine-induced neutrophil chemoattractant
- COX-2
cyclooxygenase-2
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- IL-6
interleukin-6
- IL-10
interleukin-10
- iNOS
inducible nitric oxide synthase
- LTB4
leukotriene B4
- MEM
Eagle's minimum essential medium
- MPO
myeloperoxidase
- PBS
phosphate-buffered saline
- PGE2
prostaglandin E2
- SEA
staphylococcal enterotoxin type A
- TNF-α
tumoral necrosis factor-α
References
- ADCOCK I.M., CARAMORI G. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol. Cell. Biol. 2001;79:376–384. doi: 10.1046/j.1440-1711.2001.01025.x. [DOI] [PubMed] [Google Scholar]
- AMANO H., OISHI K., SONODA F., SENBA M., WADA A., NAKAGAWA H., NAGATAKE T. Role of cytokine-induced neutrophil chemoattractant-2 (CINC-2) in a rat model of chronic bronchopulmonary infections with Pseudomonasaeruginosa. Cytokine. 2000;12:1662–1668. doi: 10.1006/cyto.2000.0771. [DOI] [PubMed] [Google Scholar]
- BACHERT C., GEVAERT P., VAN CAUWENBERGE P. Staphylococcus aureus superantigens and airway disease. Curr. Allergy Asthma Rep. 2002a;2:252–258. doi: 10.1007/s11882-002-0027-9. [DOI] [PubMed] [Google Scholar]
- BACHERT C., GEVAERT P., VAN CAUWENBERGE P. Staphylococcus aureus enterotoxins: a key in airways disease. Allergy. 2002b;57:480–487. doi: 10.1034/j.1398-9995.2002.02156.x. [DOI] [PubMed] [Google Scholar]
- BACHERT C., GEVAERT P., HOWARTH P., HOLTAPPELS G., VAN CAUWENBERGE P., JOHANSSON S.G. IgE to Staphylococcus aureus enterotoxins in serum is related to severity of asthma. J. Allergy Clin. Immunol. 2003;111:1131–1132. [PubMed] [Google Scholar]
- BARNES P.J., CHUNG K.F., PAGE C.P. Inflammatory mediators of asthma: an update. Pharmacol. Rev. 1998;50:515–596. [PubMed] [Google Scholar]
- BRADLEY P.P., PRIEBAT D.A., CHRISTENSEN R.D., ROTHSTEIN G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- CANETTI C., SILVA J.S., FERREIRA S.H., CUNHA F.Q. Tumor necrosis factor-alpha and leukotriene B4 mediate the neutrophil migration in immune inflammation. Br. J. Pharmacol. 2001;134:1619–1628. doi: 10.1038/sj.bjp.0704403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA F.Q., FERREIRA S.H. The release of a neutrophil chemotactic factor from peritoneal macrophages by endotoxin: inhibition by glucocorticoids. Eur. J. Pharmacol. 1986;129:65–76. doi: 10.1016/0014-2999(86)90337-7. [DOI] [PubMed] [Google Scholar]
- DESOUZA I.A., RIBEIRO-DASILVA G. Resident macrophages modulate the neutrophil migration induced by staphylococcal enterotoxin B into mouse peritoneal cavity. J. Nat. Toxins. 1996;5:341–350. [Google Scholar]
- DESOUZA I.A., RIBEIRO-DASILVA G. Neutrophil migration induced by staphylococcal enterotoxin type A in mice: a pharmacological analysis. Eur. J. Pharmacol. 1998;363:189–195. doi: 10.1016/s0014-2999(98)00805-x. [DOI] [PubMed] [Google Scholar]
- DESOUZA I.A., HYSLOP S., FRANCO-PENTEADO C.F., RIBEIRO-DASILVA G. Mouse macrophages release a neutrophil chemotactic mediator following stimulation by staphylococcal enterotoxin type A. Inflamm. Res. 2001;50:206–212. doi: 10.1007/s000110050745. [DOI] [PubMed] [Google Scholar]
- DOUWES J., GIBSON P., PEKKANEN J., PEARCE N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57:643–648. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILLIATRE L.G., SAYAH S., LATOURNERIE V., RENAUD J.F., FINET M., HANF R. Cyclo-oxygenase and lipoxygenase pathways in mast cell dependent-neurogenic inflammation induced by electrical stimulation of the rat saphenous nerve. Br. J. Pharmacol. 2001;132:1581–1589. doi: 10.1038/sj.bjp.0703950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLORQUIN S., AMRAOUI Z., DUBOIS C., DECUYPER J., GOLDMAN M. The protective role of endogenously synthesized nitric oxide in staphylococcal enterotoxin B-induced shock in mice. J. Exp. Méd. 1994;180:1153–1158. doi: 10.1084/jem.180.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJISAWA N., HAYASHI S., KURDOWSKA A., NOBLE J.M., NAITOH K., MILLER E.J. Staphylococcal enterotoxin A-induced injury of human lung endothelial cells and IL-8 accumulation are mediated by TNF-α. J. Immunol. 1998;161:5627–5632. [PubMed] [Google Scholar]
- GOODWIN D.C., LANDINO L.M., MARNETT L.J. Effects of nitric oxide and nitric oxide-derived species on prostaglandin endoperoxide synthase and prostaglandin. FASEB J. 1999;13:1121–1136. doi: 10.1096/fasebj.13.10.1121. [DOI] [PubMed] [Google Scholar]
- GOSSELIN E.J., WARDWELL K., RIGBY W.F., GUYRE P.M. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J. Immunol. 1993;151:1482–1490. [PubMed] [Google Scholar]
- GREENBERG S.S., XIE J., SPITZER J.J., WANG J., LANCASTER J., GRISHHAM M.B., POWERS D.R., GILES T.D. Nitro containing L-arginine analogs interfere with assays for nitrate and nitrite. Life Sci. 1995;57:1949–1961. doi: 10.1016/0024-3205(95)02181-h. [DOI] [PubMed] [Google Scholar]
- HACK C.E., DE GROOT E.R., FELT-BERSMA R.J., NUIJENS J.H., STRACK VAN SCHIJNDEL R.J., EERENBERG-BELMER A.J., THIJS L.G., AARDEN L.A. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74:1704–1710. [PubMed] [Google Scholar]
- HASKÓ G., VIRÁG L., EGNACZYK G., SALZMAN A.L., SZABÓ C. The crucial role of IL-10 in the suppression of the immunological response in mice exposed to staphylococcal enterotoxin B. Eur. J. Immunol. 1998;28:1417–1425. doi: 10.1002/(SICI)1521-4141(199804)28:04<1417::AID-IMMU1417>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- HERZ U., RÜCKERT R., WOLLENHAUPT K., TSCHERNIG T., NEUNHAUS-STEINMETZ U., PABST R., RENZ H. Airway exposure to bacterial superantigen (SEB) induces lymphocyte-dependent airway inflammation associated with increased airway responsiveness – a model for non-allergic asthma. Eur. J. Pharmacol. 1999;29:1021–1031. doi: 10.1002/(SICI)1521-4141(199903)29:03<1021::AID-IMMU1021>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- KOTZIN B.L., LEUNG D.Y., KAPPLER J., MARRACK P. Superantigens and their potential role in human disease. Adv. Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- KRAFT M. The role of bacterila infections in asthma. Clin. Chest Med. 2000;21:301–313. doi: 10.1016/s0272-5231(05)70268-9. [DOI] [PubMed] [Google Scholar]
- LECLAIRE R.D., KELL W.M., SADIK R.A., DOWNS M.B., PARKER G.W. Regulation of staphylococcal enterotoxin B-elicited nitric oxide production by endothelial cells. Infect. Immun. 1995;63:539–546. doi: 10.1128/iai.63.2.539-546.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINARDI A., COSTA S.K.P., DASILVA G.R., ANTUNES A. Involvement of kinins, mast cells and sensory neurons on the plasma exudation and paw oedema induced by staphylococcal enterotoxin B in the mouse. Eur. J. Pharmacol. 2000;399:235–242. doi: 10.1016/s0014-2999(00)00375-7. [DOI] [PubMed] [Google Scholar]
- LOWY F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- MARRACK P., KAPPLER J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- MICUSAN V.V., THIBODEAU J. Superantigens of microbiol oringin. Semin. Immunol. 1993;5:3–11. doi: 10.1006/smim.1993.1002. [DOI] [PubMed] [Google Scholar]
- MILLER E.J., NAGAO S., CARR F.K., NOBLE J.M., COHEN A.B. Interleukin-8 (IL-8) is a major neutrophil chemotaxin from human alveolar macrophages stimulated with staphylococcal enterotoxin A (SEA) Inflamm. Res. 1996;45:386–392. doi: 10.1007/BF02252933. [DOI] [PubMed] [Google Scholar]
- MORRISON D.F., FOSS D.L., MURTAUGH M.P. Interleukin-10 gene therapy-mediated amelioration of bacterial pneumonia. Infect. Immun. 2000;68:4752–4758. doi: 10.1128/iai.68.8.4752-4758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÜLLER-ALOUF H., VARNOY C., SIMONET M., ALOUF J.E. Superantigen bacterial toxins: state of the art. Toxicon. 2001;39:1691–1701. doi: 10.1016/s0041-0101(01)00156-8. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA H., ANDO Y., TACANO K., SUNADA Y. Differential production of chemokines and their role in neutrophil infiltration in rat allergic inflammation. Int. Arch. Allergy Immunol. 1998;115:137–143. doi: 10.1159/000023893. [DOI] [PubMed] [Google Scholar]
- NEUMANN B., ENGELHARDT B., WAGNER H., HOLZAMANN B. Induction of acute inflammatory lung injury by staphylococcal enterotoxin B. J. Immunol. 1997;158:1862–1868. [PubMed] [Google Scholar]
- PARMENTIER S., BOHME G.A., LEROUET D., DAMOUR D., STUTZMANN J.M., MARGAILL I., PLOTKINE M. Selective inhibition of inducible nitric oxide synthase prevents ischaemic brain injury. Br. J. Pharmacol. 1999;127:546–552. doi: 10.1038/sj.bjp.0702549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON B.T., EDMUND J.M., MORRIS D. Neutrophil influx and migration in rabbit airways in response to staphylococcal enterotoxin A. Exp. Lung Res. 1999;25:41–54. doi: 10.1080/019021499270411. [DOI] [PubMed] [Google Scholar]
- PROFT T., FRASER J.D. Bacterial superantigens. Clin. Exp. Immunol. 2003;133:299–306. doi: 10.1046/j.1365-2249.2003.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSSI R.E., MONASTEROLO G. Prevalence of serum IgE antibodies to the Staphylococcal aureus enterotoxins (SAE, SEB, SEC, SED, TSST-1) in patients with persistent allergic rhinitis. Int. Arch. Allergy Immunol. 2004;133:261–266. doi: 10.1159/000076833. [DOI] [PubMed] [Google Scholar]
- SALLERFORS B., OLOFSSON T. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) secretion by adherent monocytes measured by quantitative immunoassays. Eur. J. Haematol. 1992;49:199–207. doi: 10.1111/j.1600-0609.1992.tb00047.x. [DOI] [PubMed] [Google Scholar]
- SATOH S., OISHI K., IWAGAKI A., SENBA M., AKAIKE T., AKIYAMA M., MUKAIDA N., ATSUSHIMA K.M., NAGATAKE T. Dexamethasone impairs pulmonary defense against Pseudomonas aeruginosa through suppressing iNOS gene expression and peroxynitrite production in mice. Clin. Exp. Immunol. 2001;126:266–273. doi: 10.1046/j.1365-2249.2001.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEUBER P.H., DENZLINGER C., WILKER D., BECK G., KEPPLER D., HAMMER D.K. Staphylococcal enterotoxin B as a non-immunological mast cell stimulus in primates: the role of endogenous cysteinyl leukotrienes. Int. Arch. Allergy Appl. Immunol. 1987;82:289–291. doi: 10.1159/000234209. [DOI] [PubMed] [Google Scholar]
- SCHRAMM R., THORLACIUS H. Staphylococcal enterotoxin B-induced acute inflammation is inhibited by dexamethasone: important role of CXC chemokines KC and macrophage inflammatory protein 2. Infect. Immun. 2003;71:2542–2547. doi: 10.1128/IAI.71.5.2542-2547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBATA F., KONISHI K., NAKAGAWA H. Identification of a common receptor for three types of rat cytokine-induced neutrophil chemoattractants (CINCS) Cytokine. 2000;12:1368–1373. doi: 10.1006/cyto.2000.0739. [DOI] [PubMed] [Google Scholar]
- SHIBATA F., SHIBATA Y., YOSHIMOTO Y., NAKAGAWA H. The expression of three types of CINCs by lipopolysaccharide-stimulated rat macrophages is inhibited similarly by anti-inflammatory steroids. Inflamm. Res. 2000a;49:80–85. doi: 10.1007/s000110050562. [DOI] [PubMed] [Google Scholar]
- SOMERSALO K., SALO O.P., BJORKSTEN F., MUSTAKALLIO K.K. A simplified boyden chamber assay for neutrophil chemotaxis based on quantitation of myeloperoxidase. Anal. Biochem. 1990;185:238–242. doi: 10.1016/0003-2697(90)90286-i. [DOI] [PubMed] [Google Scholar]
- SWIERKOSZ T.A., MITCHELL J.A., WARNER T.D., BOTTING R.M., VANE J.R. Co-induction of nitric oxide synthase and cyclo-oxygenase: interactions between nitric oxide and prostanoids. Br. J. Pharmacol. 1995;114:1335–1342. doi: 10.1111/j.1476-5381.1995.tb13353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESSIER P.A., NACCACHE P.H., DIENER K.R., GLADUE R.P., NEOTE K.A., CLARK-LEWIS I., MCCOLL S.R. Induction of acute inflammation in vivo by staphylococcal superantigens. II. Critical role for chemokines, ICAM-1 and TNF-α. J. Immunol. 1998;161:1204–1211. [PubMed] [Google Scholar]
- TREPICCHIO W.L., WANG L., BOZZA M., DORNER A.J. IL-11 regulates macrophage effector function through the inhibition of nuclear factor-kappaB. J. Immunol. 1997;159:5661–5670. [PubMed] [Google Scholar]
- TURNER M.O., HUSSACK P., SEARS M.R., DOLOVICH J., HARGREAVE F.E. Exacerbations of asthma without sputum eosinophilia. Thorax. 1995;50:1057–1061. doi: 10.1136/thx.50.10.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER POLL T., KEOGH C.V., GUIRAO X., BUURMAN W.A., KOPF M., LOWRY S.F. Interleukin-6 gene-deficient mice show impaired defence against pneumococcal pneumonia. J. Infect. Dis. 1997;176:439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- WATANABE K., KOIZUMI F., KURASHIGE Y., TSURUFUJI S., NAKAGAWA H. Rat CINC, a member of the interleukin-8 family, is a neutrophil-specific chemoattractant in vivo. Exp. Mol. Pathol. 1991;55:30–37. doi: 10.1016/0014-4800(91)90016-q. [DOI] [PubMed] [Google Scholar]
- WENZEL S.E., SCHWARTZ L.B., LANGMACK E.L., HALLIDAY J.L., TRUDEAU J.B., GIBBS R.L., CHU H.W. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am. J. Respir. Crit. Care. Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- WRIGTH A.D., CHAPES S.K. Cross-linking staphylococcal enterotoxin A bound to major histocompatibility complex class I is required for TNF-alpha secretion. Cell. Immunol. 1999;197:129–135. doi: 10.1006/cimm.1999.1571. [DOI] [PubMed] [Google Scholar]