Abstract
Ketamine shows, besides its general anaesthetic effect, a potent analgesic effect after spinal administration. We investigated the local anaesthetic-like action of ketamine and its enantiomers in Na+ and K+ channels and their functional consequences in dorsal horn neurones of laminae I–III, which are important neuronal structures for pain transmission receiving most of their primary sensory input from Aδ and C fibres.
Combining the patch-clamp recordings in slice preparation with the ‘entire soma isolation' method, we studied action of ketamine on Na+ and voltage-activated K+ currents. The changes in repetitive firing behaviour of tonically firing neurones were investigated in current-clamp mode after application of ketamine.
Concentration–effect curves for the Na+ peak current revealed for tonic block half-maximal inhibiting concentrations (IC50) of 128 μM and 269 μM for S(+) and R(−)-ketamine, respectively, showing a weak stereoselectivity. The block of Na+ current was use-dependent. The voltage-dependent K+ current (KDR) was also sensitive to ketamine with IC50 values of 266 μM and 196 μM for S(+) and R(−)-ketamine, respectively. Rapidly inactivating K+ currents (KA) were less sensitive to ketamine. The block of KDR channels led to an increase in action potential duration and, as a consequence, to lowering of the discharge frequency in the neurones.
We conclude that ketamine blocks Na+ and KDR channels in superficial dorsal horn neurones of the lumbar spinal cord at clinically relevant concentrations for local, intrathecal application. Ketamine reduces the excitability of the neurones, which may play an important role in the complex mechanism of its action during spinal anaesthesia.
Keywords: Electrophysiology, ion channels, pain, action potential, sensory neurones, pharmacology
Introduction
Ketamine, commonly used as a racemate of two enantiomers (R(−) and S(+)) for general anaesthesia, is a dissociative anaesthetic with unique actions in the central nervous system. It has been in clinical use for more than four decades. In addition, a series of clinical studies have shown potent analgesia after spinal administration of ketamine alone or in combination with opioids and α2-receptor agonists, in both animals and humans, suggesting that ketamine alters pain perception at the spinal level (Klimscha et al., 1998; Joo et al., 2000; Koinig et al., 2000; Horvath et al., 2001; Sator-Katzenschlager et al., 2001; Vranken et al., 2004). Dorsal horn neurones located in laminae I–III in the spinal cord are important neuronal structures for pain transmission receiving most of their primary sensory input from Aδ and C fibres. These neurones are a relevant pharmacological site of action for the antinociceptive effects of different drugs during spinal anaesthesia.
Ketamine acts on a variety of receptors, including nicotinic (Scheller et al., 1996), muscarinic (Hustveit et al., 1995), and opioid receptors (Finck & Ngai, 1982; Smith et al., 1987; Hustveit et al., 1995) and affects the NMDA receptor complex channel as a noncompetitive antagonist at the phencyclidine receptor site (Yamamura et al., 1990). Studies examining the effects of ketamine on ion channels have shown that ketamine blocks peripheral and central nervous system Na+ channels (Scheller et al., 1996), voltage sensitive K+ (Brau et al., 1997), and Ca2+ channels (Hirota & Lambert, 1996). The blockade of different types of voltage-gated Na+ channels during regional anaesthesia by local anaesthetic-like drugs has been studied extensively and is of eminent importance for suppressing pain transmission in peripheral nerve, dorsal root ganglion neurones, and dorsal horn neurones of the spinal cord. Voltage-gated K+ channels play a major role in modulation of firing pattern in spinal cord neurones (Safronov, 1999; Hess & El Manira, 2001; Olschewski et al., 2001; Melnick et al., 2004a, 2004b). Owing to their importance in regulating cellular excitability, K+ channels represent potential targets for anaesthetic action. In ketamine-induced analgesia, blockade of Na+ and K+ channels may also play a key role for the antinociceptive effect of the drug.
This study was designed to investigate the effects of ketamine and its enantiomers on voltage-gated ion currents and functional consequences in intact dorsal horn neurones firing tonically during spinal anaesthesia. According to cutaneous afferent input, most of the tonically firing neurones were shown to be either wide-dynamic-range or nociceptive-specific neurones (Lopez-Garcia & King, 1994). The experiments were performed on thin-slice preparations of rat spinal cord by means of the patch-clamp technique.
Methods
Preparation
Experiments were performed by means of the patch-clamp technique (Hamill et al., 1981) on 200 μm thin slices, cut from lumbar enlargements (L3–6) of the spinal cord of 4- to 16-day-old rats of both sexes (Olschewski et al., 2002). Animals were rapidly decapitated and the spinal cords were carefully cut out in ice-cold preparation solution bubbled with 95% O2–5% CO2. After removal of the pial membrane with fine forceps, the spinal cord was embedded in a preparation solution containing 2% agar cooled down to 39°C. To accelerate solidification of the agar, the beaker with the preparation was placed in ice-cold water. The agar block containing the lumbar enlargement of the spinal cord was cut out and glued to a glass stage fixed in the chamber of the tissue slicer. The spinal cord was sliced in ice-cold preparation solution under continuous bubbling. The slices were thereafter incubated for 1 h at 32°C. The procedures of animal decapitation have been reported to the local veterinarian authority and are in accordance with the German guidelines.
Solutions and drugs
Preparation solution contained (in mM): NaCl 115, KCl 5.6, CaCl2 2, MgCl2 1, glucose 11, NaH2PO4 1, NaHCO3 25 (pH 7.4 when bubbled with 95% O2–5% CO2). Experimental solution (low-Ca2+, high-Mg2+ solution) was obtained from preparation solution by setting [Ca2+] to 0.1 mM and [Mg2+] to 5 mM. Tetraethyl-ammonium (TEA) containing solution (TEA-solution) used for Na+ current recordings contained (in mM): NaCl 95, KCl 5.6, CaCl2 0.1, MgCl2 5, glucose 11, NaH2PO4 1, NaHCO3 25 and TEA-Cl 20 (pH 7.4 when bubbled with 95% O2–5% CO2). The study of K+ currents was carried out in Na+-free choline-Cl solution containing (in mM): choline-Cl 141, KCl 0.6, CaCl2 0.1, MgCl2 5, glucose 11, HEPES 10 (pH 7.4 adjusted with 5 mM KOH). Tetrodotoxin (TTX, 200 nM) was added to this solution to block voltage-gated Na+ channels. The experimental chamber with a volume of 0.4 ml was continuously perfused by external solution at a rate of 2–3 ml min−1. The pipette solution used for action potential recordings from intact neurones contained (in mM): NaCl 5, KCl 144.4, MgCl2 1, EGTA 3, HEPES 10 (pH 7.3 by 10.6 mM KOH). Internal solution for K+ current recording from isolated somata contained (high-Kin) (in mM): NaCl 5, KCl 144.4, MgCl2 1, EGTA 3, HEPES 10 (pH 7.3 by 10 mM NaOH). The Na+ currents were measured in isolated somata using pipette solution (high-Csin) (in mM): NaCl 5.8, CsCl 134, MgCl2 1, EGTA 3, HEPES 10 (pH 7.3 adjusted with 9.2 mM NaOH).
The S(+) and R(−) forms of ketamine were donated as HCl salt by Parke-Davis Gödecke (Freiburg, Germany) and were used in the study in a concentration range from 30 nM to 1 mM. TTX was obtained from Latoxan (Rosans, France). All other compounds were purchased from Sigma Chemical Company (St Louis, MO, U.S.A.). All drugs were dissolved in distilled water.
Electrophysiology
Current recording
Pipettes pulled from borosilicate glass tube (GC 150, Clark Electromedical Instruments, Pangbourne, U.K.) were fire-polished to give a final resistance of 3–4 MΩ for whole-cell recording. The patch-clamp amplifier was an EPC-7 (List, Darmstadt, Germany) and Axopatch 200B (Axon Instruments, Foster City, CA, U.S.A.). The effective corner frequency of the low-pass filter was 5 kHz and the frequency of digitization was 10 kHz in all whole-cell experiments. The data were stored and analysed using commercially available software (pCLAMP, Axon Instruments, Foster City, CA, U.S.A.). Transients and leakage currents were recorded and digitally subtracted offline in all experiments using averaged records with hyperpolarizing impulses (100 ms voltage steps from −80 to −120 mV) that activated no currents (correction file, Figure 1b). Offset potentials were nullified directly before formation of the seal. The small amplitude of currents recorded in somata (<500 pA) made series resistance compensation unnecessary. The amplitude of the capacitive transient was monitored and recorded. The input resistance and the series resistance of the somata were calculated from correction file recordings as described above. The neurones were rejected if these parameters varied larger than 20% during recording.
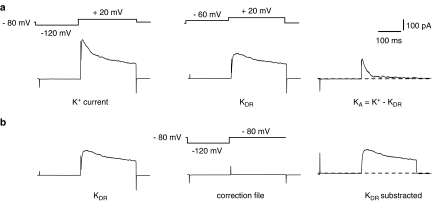
Figure 1.
Separation of a voltage-gated K+ current into inactivating A (KA) and delayed-rectifier (KDR) components in dorsal horn neurons. (a) All K+ current (KA+KDR) was activated by a voltage step to +20 mV after a 150-ms prepulse to −120 mV (left). The delayed-rectifier component of the K+ current (KDR) was activated by a voltage step to +20 mV after a 150-ms prepulse to −60 mV (middle). Inactivating A current was obtained by digital subtraction of delayed-rectifier current from total current: KA=K+−KDR. (b) Offline subtraction of leak and transient currents for calculation of KDR by hyperpolarizing impulses to −120 mV. Dotted lines indicate zero level.
All experiments were carried out at room temperature of 21–23°C.
Identification of dorsal horn neurones
The dorsal horn neurones were identified in spinal cord slices as multipolar cells with a soma (8–12 mm diameter) located in laminae I–III. In voltage-clamp mode, neurones were distinguished from glial cells on the basis of the magnitude of Na+ currents as described previously (Safronov et al., 1997). All neurones studied possessed a large Na+ current exceeding 1 nA, were able to generate action potentials and showed spontaneous synaptic activity.
‘Entire soma isolation' (ESI) method
A detailed description of the ESI method has been given elsewhere (Safronov et al., 1997; Olschewski et al., 2002). Briefly, in the whole-cell recording configuration, entire somata of dorsal horn neurones could easily be isolated from the slice by slow withdrawal of the recording pipette, leaving all or nearly all of their processes in the slice (Safronov et al., 1997). After recording from the neurone in the slice was completed, a slight suction was applied to the recording pipette and it was gently withdrawn until the connection between the soma and the slice was broken. The applied suction was similar to that needed to break the membrane into formation of whole-cell configuration. The suction was released immediately after completion of the isolation. The isolated neurone was classified as a soma if adjacent processes could not be seen either during the experiment or after, when the recording pipette was turned over. The isolated structure was classified as a soma+axon if it contained one 10–100 μm process and preserved more than 85% of the original Na+ current recorded in the slice before isolation. The neurone was considered as a soma+dendrite if at least one adjacent process was observed but the amplitude of Na+ current was in the range typical for isolated somata. For this study only isolated somata were used. Stable recordings could be obtained for 1 h and more. The isolation of the soma from a dorsal horn neurone in a spinal cord slice was monitored under infrared optics (Hamamatsu Photonics, Japan) as pictured in the previous work of Safronov et al. (1997). Changes in leakage current and membrane resting potential were monitored during isolation of the cell in experimental solution. The good physiological state of the isolated structures was confirmed by a considerable increase in their input resistances (reflecting a decrease in membrane leakage conductance), and by stable or even more negative membrane resting potentials (Safronov et al., 1997).
Separation of delayed-rectifier K+ currents (KDR)
Potassium currents were recorded in external choline-Cl solution. KDR were separated as described previously (Olschewski et al., 2002). Total K+ currents activated by depolarizing steps following a 150 ms prepulse to −120 mV consisted of both rapidly inactivating A-type and delayed-rectifier components (Figure 1a). A similar depolarization applied after 150 ms prepulse to −60 mV (at which A-type K+ channels are almost completely inactivated) elicited only a noninactivating component of K+ current, which was considered in the present study as a delayed-rectifier current. The amplitudes of the delayed-rectifier currents were measured after offline leakage subtraction at the beginning (10 ms) and at the end (200 ms) of the current (Figure 1b).
Statistical analysis and fitting
The normalized amplitudes of currents in concentration–effect curves were fitted by means of a nonlinear least-squares method with the equation: 1(1+(C(IC50)−1)h)−1 (equation 1), where C was the drug concentration, IC50 was the concentration giving a half-maximum effect and h was the Hill coefficient. The steady-state inactivation characteristic was fitted with Boltzmann function: 1(1+exp((E50−E)(k)−1)−1 (equation 2), where E50 is the potential at which a half-maximal conductance is activated and k is the steepness factor.
The present study is based on recordings from 39 intact neurones in the spinal cord slice and 52 isolated somata. Numerical values and the parameters obtained by fitting the data points using a non-linear least-squares procedure are given as mean±standard error (s.e.). Resting membrane potentials are given as mean±standard deviation (s.d.). Intergroup differences were assessed by a factorial analysis of variance with post hoc analysis with Fisher's least significant difference test. Student's paired t-test was used to compare the relative amplitudes of Na+ currents at the 30th pulse before and after 100 μM ketamine (phasic blockade). P-values <0.05 were considered significant.
Analysis of changes in repetitive firing behaviour
For each neurone, several corresponding pairs of recordings were subjected to a detailed analysis of changes in repetitive firing behaviour. Each pair contained one train of action potentials recorded in control and one in the blocker-containing solution, both activated by current pulses of the same strength. For each individual recording, the mean firing frequency was determined as f=(N−1)(ΔT)−1, where N was the number of complete spikes observed during current injection and ΔT was the time interval between the first and the last spikes in the train. For each pair, a ratio (k) between the mean firing frequencies in blocker-containing (fB) and control (fC) solutions was chosen as a measure of changes in firing behaviour, k=fB (fC)−1. The k values determined for at least five pairs of recordings were then averaged to give the k value for one neurone. The k values given in the text represent the mean values from at least five different neurones.
Results
Ketamine stereoselectively blocks Na+ current
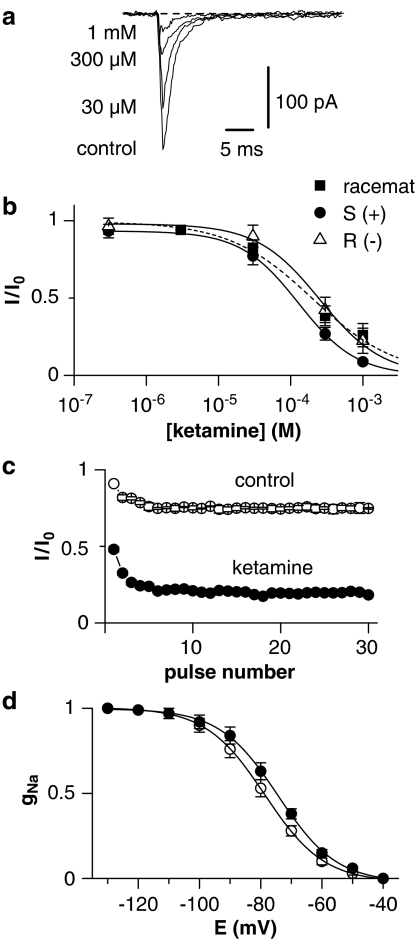
Na+ currents were recorded from isolated somata of dorsal horn neurones in external TEA-solution. The pipettes were filled with high-Csin solution. The peak Na+ currents remaining in the isolated somata after separation from the axon initial segment had a small amplitude of 150–300 pA (10–20% of original Na+ current measured in most intact dorsal horn neurones). Therefore, the voltage error caused by the resistance in series was small, avoiding problems with a poor space clamp of the neuronal membrane. Figure 2a illustrates an example of the dose-dependent inhibition of Na+ current by ketamine. Blockade was rapid in onset and readily reversible on washout. The IC50 values for ketamine and both enantiomers S(+)- and R(−)-ketamine are given in Table 1. S(+)-ketamine was significantly more potent than R(−)-ketamine.
Figure 2.
Tonic and phasic blockade of Na+ current by ketamine in somata. (a) Recordings of Na+ current in the control solution and in the presence of 100, 300, and 1000 μM ketamine. Holding potential was set to −80 mV and Na+ currents were activated by 50-ms voltage steps to −20 mV after a 50-ms prepulse to −120 mV. (b) Concentration dependence of the Na+ current suppression by ketamine and its enantiomers (8 somata). The data points were fitted using equation (1). IC50 values are given in Table 1. Error bars indicate s.e. if exceeding symbol size. (c) Normalized amplitudes of Na+ currents recorded in control solution and in the presence of 100 μM ketamine as a function of pulse number. The currents were activated by a 10-ms voltage step to −20 mV at a frequency of 10 Hz. Holding potential was −80 mV. Each current was normalized to the amplitude of the first Na+ current recorded in control solution (10 somata). (d) Effects of ketamine on Na+ currents assessed by shifts in the steady-state availability curve. Each symbol represents the mean normalized fractional current derived from at least five different experiments. The data points were fitted by equation (2).
Table 1.
IC50 values for ketamine and both enantiomers S(+)- and R(−)-ketamine
| Racemate | S(+)-ketamine | R(−)-ketamine | Stereopotency ratio R(−)/S(+) | ||||
|---|---|---|---|---|---|---|---|
| IC50 (μM) | Hill | IC50 (μM) | Hill | IC50 (μM) | Hill | ||
| Na+ current | 199±8 | 0.74 | 128±3 | 1.08 | 269±9* | 0.99 | 2.10 |
| KDR current (peak) | 424±9 | 0.72 | 323±2 | 0.79 | 363±19 | 0.72 | 1.12 |
| KDR current (tail) | 338±9 | 0.78 | 266±7 | 0.72 | 196±33 | 0.74 | 0.73 |
Number of neurons in the parentheses.
P<0.05 for difference from S(+)-ketamine.
Use-dependent or phasic block was defined as the additional reduction in Na+ current for the last pulse relative to the first pulse, assessed in a 10-Hz test train of 10-ms depolarizing pulses from −80 to −20 mV. Figure 2c shows the peak amplitudes of the Na+ currents in control and after addition of 100 μM ketamine normalized to the amplitude of the first current recorded in control solution (n=10). In the absence of ketamine, the amplitude of the 30th current was reduced to 0.82, probably due to the insufficient recovery of Na+ channels from slow inactivation. In the presence of 100 μM ketamine, the relative amplitude was reduced from 0.52 to 0.20 (P<0.001) at the 30th pulse.
Na+ channel blockers that induce use-dependent block are known to shift the inactivation curve of voltage-dependent Na currents in the hyperpolarizing direction. We tested this effect in five cells. Fitting equation (2) to the availability curves gave half-maximal availability potentials of −73.9±0.4 mV (k=9.6±0.3 mV) for control and −78.5±0.4 mV (k=9.4±0.4 mV) for 100 μM ketamine (Figure 2d).
Effects of ketamine on KA and KDR currents
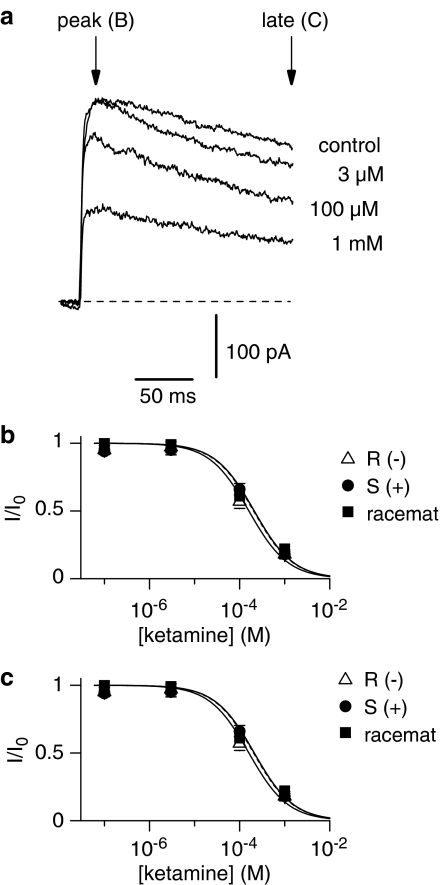
K+ currents were recorded from isolated somata of spinal dorsal horn neurones in external choline-Cl solution using pipettes filled with high-Kin solution. KDR currents were sensitive to externally applied ketamine at concentrations of 0.1 μM–1 mM (Figure 3a–c). The amplitudes of the delayed-rectifier currents were measured after offline leakage subtraction either at the beginning (10 ms, peak) or at the end (200 ms, late) of the current as indicated by arrows in Figure 3a. The dose–response curves were fitted with the Hill equation giving IC50 values for ketamine and both enantiomers S(+)- and R(−)-ketamine shown in Table 1.
Figure 3.
Inhibition of KDR currents by ketamine in somata. (a) KDR current in experimental solution and in the presence of 3 μM, 100 μM, and 1 mM ketamine. The currents were activated by a 250 ms voltage step to +20 mV following a 150 ms prepulse to –60 mV. Holding potential was –80 mV. (b and c) Concentration dependence of the KDR current suppression by ketamine and its enantiomers measured at the beginning (10 ms, peak) and at the end (200 ms, late) of the current as indicated by arrows (6 somata). The data points were fitted by the equation (1). The resulting IC50 values are given in Table 1. Error bars indicate s.e. if exceeding symbol size.
Next the sensitivity of fast inactivating KA currents to ketamine was studied. The potassium current traces in Figure 4a demonstrate the inhibitory effect of ketamine. The highest applied concentrations of local ketamine (1 mM) suppressed up to 60% of the current (Figure 4b). Blockade of KA channels was reversible.
Figure 4.
Effect of ketamine on KA currents in somata. (a) KA current in control solution and in the presence of 1 mM S(+)- and R(−)-ketamine. The currents were activated by a 250 ms voltage step to +40 mV following a 150 ms prepulse to –120 mV. Holding potential was –80 mV. The procedure of separating KA currents from other K+ currents is explained in the Methods section. (b) Summarized data showing relative KA current after application of ketamine (white bars), or R(−)-enantiomer (grey bars), or S(+)-enantiomer (black bars), elicited by the test potential at +40 mV. Values are mean±s.e.m. (n=7).
Action potentials
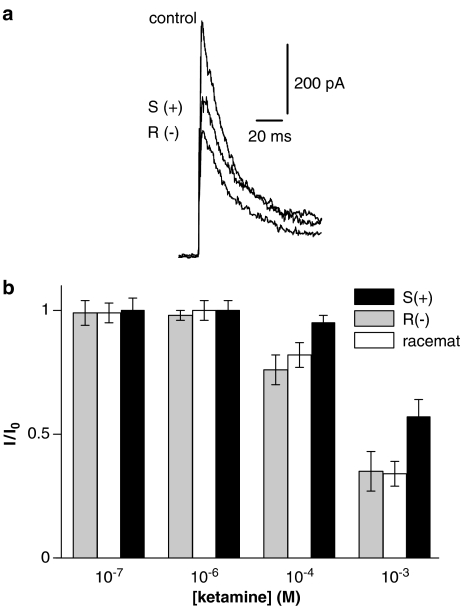
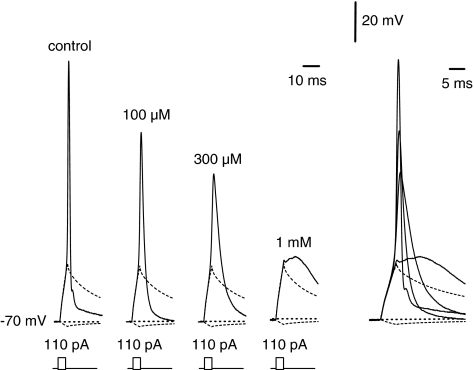
Current-clamp experiments were performed on intact tonically firing neurones of the spinal cord slice in experimental solution using pipettes filled with high-Kin solution. Intact neurones of the spinal cord showed a resting membrane potential of −65±6.8 mV (n=81). Single action potentials were elicited using a 10 ms depolarizing current pulse of increasing amplitude (Figure 5). The first changes in action potentials were seen at 30 μM ketamine (Table 2). The effects were more pronounced with increasing concentrations of ketamine. The application of 1 mM ketamine resulted in a complete disappearance of the action potentials. At this concentration the membrane response represented only the passive response to the stimulus current, as additionally shown in Figure 5. A detailed analysis of different action potential parameters is given in Table 2. The changes in repetitive firing behaviour were analysed in tonically firing dorsal horn neurones induced by 100 μM ketamine during a depolarizing current pulse of 500 ms length. Traces in Figure 6 demonstrate the inhibitory effect of ketamine. Application of ketamine led to a slight reduction in the amplitude of the first spike but resulted in a pronounced reduction in the firing frequency (k=0.74±0.08). The observed effects on the firing rates were completely reversible after washout.
Figure 5.
Changes of single action potentials by ketamine. Recordings of action potentials in control solution (left) and after application of 100 μM, 300 μM, and 1 mM ketamine (n=8). The membrane potential was adjusted to –70 mV by injecting a sustained current through the recording pipette. The impulse protocol is noticed below the corresponding traces. Right, all traces superimposed with a different time scale.
Table 2.
Effect of increasing concentrations of ketamine on properties of single action potentials (n=8)
| Overshoot (mV) | Duration (ms) | Max. positive slope (V s−1) | Max. negative slope (V s−1) | |
|---|---|---|---|---|
| Control | 47.5±3.0 | 1.6±0.1 | 124.1±8.6 | −100.8±3.7 |
| 30 μM | 35.2±2.6* | 2.2±0.1 | 99.4±7.7* | −73.8±4.7** |
| 100 μM | 19.0±2.7*** | 3.4±0.3*** | 61.4±5.0*** | −45.7±3.8*** |
| 300 μM | 3.1±1.1*** | 5.1±0.3*** | 38.6±3.3*** | −23.2±4.2*** |
Duration of action potentials is measured at the half-maximum amplitude {Madeja, 2000 7/id}. Positive and negative slope are the corresponding maximum values after differentiation of the action potentials. Significance levels are given as P<0.05 (*), P<0.01 (**) and P<0.001 (***) for differences from control.
Figure 6.
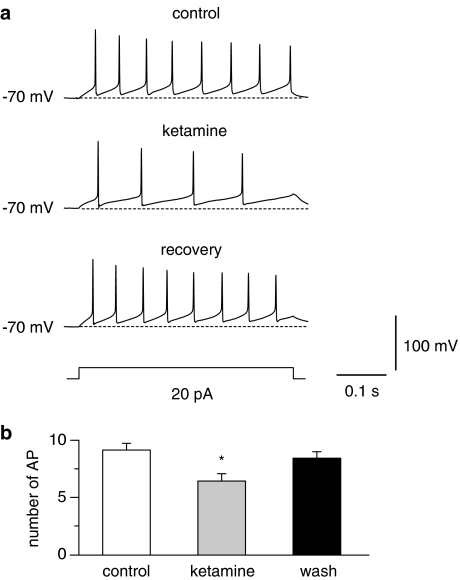
Reduction of firing frequencies by low concentrations of ketamine. (a) Series of action potentials were evoked by 500 ms current pulses. The maximum firing frequency in control solution was compared with that after application of 100 μM ketamine (n=7). The current pulse applied in the traces evoked the maximum firing frequency in control and ketamine containing solution. The effects of ketamine on series of action potentials are completely reversible. (b) Summarized data showing the changes in number of complete action potential spikes in the train observed during current injection (number of AP) in control and after application of 100 μM ketamine. Values are mean±s.e.m. (n=7). (*P<0.05 difference from control and from wash).
Discussion
In this study, we show complex effects of ketamine on nociceptive-specific dorsal horn neurones of the spinal cord, related to Na+ and K+ conductances. The balanced contribution of Na+ and KDR conductances, in particular, is important for generation and modulation of tonic firing in these neurones (Olschewski et al., 2001). The ketamine concentrations evoking these cellular effects correspond to the concentrations necessary for antinociceptive action during intrathecal application in clinical studies.
Dorsal horn neurones in laminae I–III that receive inputs from primary afferent fibres participate in central sensory transmission/modulation under physiological conditions and play key roles in the complex mechanisms of drug action during spinal anaesthesia. Extensive electrophysiological nociceptive studies have demonstrated an important role of voltage-gated Na+ channels in pain processing. Previous studies have shown that several anaesthetics interact with these ion channels in neuronal preparations. Experimentally, ketamine blocks Na+ channels in dorsal root ganglia, frog myelinated nerve fibres (Brau et al., 1997), and rat brain Na+ channels (Haeseler et al., 2003). We showed that ketamine blocks voltage-dependent sodium channels in a local anaesthetic-like manner, including tonic blockade, phasic blockade and shift of the steady-state inactivation curve toward negative voltage. IC50 values for tonic block on Na+ channels corresponded to the IC50 values reported for ketamine block of Na+ channels expressed in different neuronal tissues (Brau et al., 1997; Zhou & Zhao, 2000; Haeseler et al., 2003).
K+ channels play a major role in reducing the excitability of tissues such as the nervous system, where these channels set the resting membrane potential of neurones, dissipate excitatory influences and determine the duration and frequency of action potentials. The major K+ conductance in superficial dorsal horn neurones occurs through TEA-sensitive delayed-rectifier K+ channels (KDR). The TEA-insensitive fast-inactivating K+ current (KA) was strongly inactivated by resting membrane potential and it was also not activated by a subthreshold depolarization (Wolff et al., 1998). In our study ketamine blocked KDR more potently than KA. Interestingly, ketamine had no significant effect on voltage-gated K+ channels of cardiac parasympathetic neurone in clinical concentrations (Irnaten et al., 2002). In contrast, ketamine inhibited K+ currents in different type of cells (Benoit, 1995; Brau et al., 1997; Dreixler et al., 2000). This disparity in effects of ketamine on different cells could be explained by the heterogeneity of the K+ channels in different tissues.
Owing to its higher anaesthetic potency and lower risk of side effects, S(+)-ketamine might possess a better therapeutic efficacy compared with racemic ketamine. Only a weak stereoselectivity for blockade was demonstrated in rat brain IIa sodium channels by ketamine, with a stereopotency ratio R(−)/S(+) of 1.4 (Haeseler et al., 2003). Similar stereoselectivity of Na+ current to ketamine enantiomers was reported by Brau et al. (1997). Our results show a moderate stereoselectivity of Na+ current to ketamine enantiomers with a stereopotency ratio R(−)/S(+) of 2.1, similar to the stereoselective response of NMDA receptor currents in cultured hippocampal neurones (Zeilhofer et al., 1992). In a few studies, effects of ketamine stereoisomers were investigated on voltage-dependent and background K+ channels. However, stereoselective blockade by ketamine has only been demonstrated on mitochondrial and aortic ATP-sensitive K+ channels (Mullenheim et al., 2001; Dojo et al., 2002) and on a flickering background K+ channel in peripheral nerve fibres (Brau et al., 1997). Contrary to these findings, in superficial dorsal horn neurones, the K+ channels were not blocked stereoselectively by ketamine, at least not under our experimental conditions.
In superficial dorsal horn neurones the basic pattern of tonic firing is generated by voltage-gated Na+ and K+ channels, as demonstrated in our previous work (Olschewski et al., 2001). Moreover, Melnick et al. (2004b) showed recently that Ca2+-dependent conductances are unlikely to be responsible for the appearance of the basic form of tonic firing and provided further strong evidence for the role of the voltage-gated Na+ and KDR channels in dorsal horn neurones (Melnick et al., 2004b). Inhibition of Na+ and KDR channels by ketamine may contribute to the reduction of excitability in dorsal horn neurones by additive effects. First, at the concentration reported in our study, ketamine blocked Na+ channels that reduced the amplitude and the maximum rate of rise of a single action potential and increased the width of the single action potentials in a dose-dependent manner. Second, blockade of KDR by ketamine led to a decrease of the maximum negative slope of action potential, similar to TEA (Olschewski et al., 2001), providing support for the role of KDR channels in spike repolarization. This reduction is critical for generation of repetitive firing (Melnick et al., 2004b). We have previously shown that tonic firing can be generated with only about 20% of the available Na+ channels, but that at least 50% of the available KDR are necessary for the generation of sustained firing (Olschewski et al., 2001). Consequently, ketamine reduced the firing frequency of dorsal horn neurones at low concentrations without marked effects on single action potentials. This might be the most important effect of the substance when applied intrathecally as the firing frequency encodes the strength of sensory modalities in the nociceptive processing system.
Clinically, plasma concentrations of ketamine after an intravenous administration of 2 mg kg−1 generally reach 10–60 μM during general anaesthesia. When applied intrathecally, the ketamine dose used for anaesthesia varied between 0.1 and 0.7 mg kg−1 (Hawksworth & Serpell, 1998; Togal et al., 2004). Assuming that the volume of cerebrospinal fluid of the lumbar spinal cord for distribution after application of ketamine was 20 ml (Dennhardt & Konder, 1983; Biscoping, 1986), the initial concentration of ketamine in the lumbar region can be estimated to 1 mM (0.3 mg ml−1). Recently, Carpenter et al. (1998) determined the volume of lumbosacral cerebrospinal fluid using magnetic resonance images and calculated a volume of approximately 50 ml. Since this volume includes the sacral cerebrospinal fluid and the roots, the initial concentration of ketamine in the lumbar region for spinal anaesthesia can be estimated to 400–500 μM. Therefore, our experimental concentrations reducing firing rate were within the therapeutic range, at least during intrathecal administration. Moreover, IC50 values for ketamine on Na+ and KDR channels were in the same range IC50 values for ketamine on NMDA receptor channels (Wu & Johnson, 1996; Li et al., 2004).
In summary, our results provide new insight into the physiologic basis for the antinociceptive action of ketamine at the spinal cord level during intrathecal administration and show the first time direct evidences for modulation of firing frequency in neurones involved in pain transmission. Despite the fact that the anaesthetic effect of ketamine results from interaction with NMDA receptors, it appears that ketamine acts as an important inhibitory modulator of sensory information targeting Na+ and KDR channels in the spinal dorsal horn.
Acknowledgments
This study was supported in part by the Justus-Liebig-University Giessen and by the B. Braun-Stiftung. We thank Brigitte Agari and Otto Becker for excellent technical assistance and Mary Kay Steen-Müller for carefully reviewing the manuscript.
Abbreviations
- EGTA
ethylene glycol-bis(β-aminoethyl ether) N,N,N′,N′-tetraacetic acid
- ESI
entire soma isolation
- HEPES
N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid)
- IC50
half-maximal inhibiting concentration
- KDR
delayed-rectifier K+ currents
- TEA
tetraethyl-ammonium
- TTX
tetrodotoxin
References
- BENOIT E. Effects of intravenous anaesthetics on nerve axons. Eur. J. Anaesthesiol. 1995;12:59–70. [PubMed] [Google Scholar]
- BISCOPING J. Effect of glucose concentration in bupivacaine solutions on the distribution of local anesthetics in cerebrospinal fluid during spinal anesthesia. Reg. Anesth. 1986;9:9–14. [PubMed] [Google Scholar]
- BRAU M.E., SANDER F., VOGEL W., HEMPELMANN G. Blocking mechanisms of ketamine and its enantiomers in enzymatically demyelinated peripheral nerve as revealed by single-channel experiments. Anesthesiology. 1997;86:394–404. doi: 10.1097/00000542-199702000-00014. [DOI] [PubMed] [Google Scholar]
- CARPENTER R.L., HOGAN Q.H., LIU S.S., CRANE B., MOORE J. Lumbosacral cerebrospinal fluid volume is the primary determinant of sensory block extent and duration during spinal anesthesia. Anesthesiology. 1998;89:24–29. doi: 10.1097/00000542-199807000-00007. [DOI] [PubMed] [Google Scholar]
- DENNHARDT R., KONDER H. Blood and cerebrospinal fluid levels of bupivacaine in spinal anesthesia. Region. Anesth. 1983;6:72–75. [PubMed] [Google Scholar]
- DOJO M., KINOSHITA H., IRANAMI H., NAKAHATA K., KIMOTO Y., HATANO Y. Ketamine stereoselectively affects vasorelaxation mediated by ATP-sensitive K+ channels in the rat aorta. Anesthesiology. 2002;97:882–886. doi: 10.1097/00000542-200210000-00020. [DOI] [PubMed] [Google Scholar]
- DREIXLER J.C., JENKINS A., CAO Y.J., ROIZEN J.D., HOUAMED K.M. Patch-clamp analysis of anesthetic interactions with recombinant SK2 subtype neuronal calcium-activated potassium channels. Anesth. Analg. 2000;90:727–732. doi: 10.1097/00000539-200003000-00040. [DOI] [PubMed] [Google Scholar]
- FINCK A.D., NGAI S.H. Opiate receptor mediation of ketamine analgesia. Anesthesiology. 1982;56:291–297. doi: 10.1097/00000542-198204000-00011. [DOI] [PubMed] [Google Scholar]
- HAESELER G., TETZLAFF D., BUFLER J., DENGLER R., MUNTE S., HECKER H., LEUWER M. Blockade of voltage-operated neuronal and skeletal muscle sodium channels by S(+)- and R(−)-ketamine. Anesth. Analg. 2003;96:1019–1026. doi: 10.1213/01.ANE.0000052513.91900.D5. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HAWKSWORTH C., SERPELL M. Intrathecal anesthesia with ketamine. Region. Anesth. Pain Med. 1998;23:283–288. doi: 10.1016/s1098-7339(98)90056-6. [DOI] [PubMed] [Google Scholar]
- HESS D., EL MANIRA A. Characterization of a high-voltage-activated IA current with a role in spike timing and locomotor pattern generation. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5276–5281. doi: 10.1073/pnas.091096198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIROTA K., LAMBERT D.G. I.v. anaesthetic agents inhibit dihydropyridine binding to L-type voltage-sensitive Ca2+ channels in rat cerebrocortical membranes. Br. J. Anaesth. 1996;77:248–253. doi: 10.1093/bja/77.2.248. [DOI] [PubMed] [Google Scholar]
- HORVATH G., JOO G., DOBOS I., KLIMSCHA W., TOTH G., BENEDEK G. The synergistic antinociceptive interactions of endomorphin-1 with dexmedetomidine and/or S(+)-ketamine in rats. Anesth. Analg. 2001;93:1018–1024. doi: 10.1097/00000539-200110000-00044. [DOI] [PubMed] [Google Scholar]
- HUSTVEIT O., MAURSET A., OYE I. Interaction of the chiral forms of ketamine with opioid, phencyclidine, sigma and muscarinic receptors. Pharmacol. Toxicol. 1995;77:355–359. doi: 10.1111/j.1600-0773.1995.tb01041.x. [DOI] [PubMed] [Google Scholar]
- IRNATEN M., WANG J., CHANG K.S., ANDRESEN M.C., MENDELOWITZ D. Ketamine inhibits sodium currents in identified cardiac parasympathetic neurons in nucleus ambiguus. Anesthesiology. 2002;96:659–666. doi: 10.1097/00000542-200203000-00023. [DOI] [PubMed] [Google Scholar]
- JOO G., HORVATH G., KLIMSCHA W., KEKESI G., DOBOS I., SZIKSZAY M., BENEDEK G. The effects of ketamine and its enantiomers on the morphine- or dexmedetomidine-induced antinociception after intrathecal administration in rats. Anesthesiology. 2000;93:231–241. doi: 10.1097/00000542-200007000-00034. [DOI] [PubMed] [Google Scholar]
- KLIMSCHA W., HORVATH G., SZIKSZAY M., DOBOS I., BENEDEK G. Antinociceptive effect of the S(+)-enantiomer of ketamine on carrageenan hyperalgesia after intrathecal administration in rats. Anesth. Analg. 1998;86:561–565. doi: 10.1097/00000539-199803000-00023. [DOI] [PubMed] [Google Scholar]
- KOINIG H., MARHOFER P., KRENN C.G., KLIMSCHA W., WILDLING E., ERLACHER W., NIKOLIC A., TURNHEIM K., SEMSROTH M. Analgesic effects of caudal and intramuscular S(+)-ketamine in children. Anesthesiology. 2000;93:976–980. doi: 10.1097/00000542-200010000-00017. [DOI] [PubMed] [Google Scholar]
- LI J., MCROBERTS J.A., NIE J., ENNES H.S., MAYER E.A. Electrophysiological characterization of N-methyl-D-aspartate receptors in rat dorsal root ganglia neurons. Pain. 2004;109:443–452. doi: 10.1016/j.pain.2004.02.021. [DOI] [PubMed] [Google Scholar]
- LOPEZ-GARCIA J.A., KING A.E. Membrane properties of physiologically classified rat dorsal horn neurons in vitro: correlation with cutaneous sensory afferent input. Eur. J. Neurosci. 1994;6:998–1007. doi: 10.1111/j.1460-9568.1994.tb00594.x. [DOI] [PubMed] [Google Scholar]
- MELNICK I.V., SANTOS S.F., SAFRONOV B.V. Mechanism of spike frequency adaptation in substantia gelatinosa neurons of rat. J. Physiol. 2004a;559:383–395. doi: 10.1113/jphysiol.2004.066415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELNICK I.V., SANTOS S.F., SZOKOL K., SZUCS P., SAFRONOV B.V. Ionic basis of tonic firing in spinal substantia gelatinosa neurons of rat. J. Neurophysiol. 2004b;91:646–655. doi: 10.1152/jn.00883.2003. [DOI] [PubMed] [Google Scholar]
- MULLENHEIM J., FRASSDORF J., PRECKEL B., THAMER V., SCHLACK W. Ketamine, but not S(+)-ketamine, blocks ischemic preconditioning in rabbit hearts in vivo. Anesthesiology. 2001;94:630–636. doi: 10.1097/00000542-200104000-00017. [DOI] [PubMed] [Google Scholar]
- OLSCHEWSKI A., HEMPELMANN G., VOGEL W., SAFRONOV B.V. Suppression of potassium conductance by droperidol has influence on excitability of spinal sensory neurons. Anesthesiology. 2001;94:280–289. doi: 10.1097/00000542-200102000-00018. [DOI] [PubMed] [Google Scholar]
- OLSCHEWSKI A., WOLFF M., BRAU M.E., HEMPELMANN G., VOGEL W., SAFRONOV B.V. Enhancement of delayed-rectifier potassium conductance by low concentrations of local anaesthetics in spinal sensory neurones. Br. J. Pharmacol. 2002;136:540–549. doi: 10.1038/sj.bjp.0704754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAFRONOV B.V. Spatial distribution of NA+ and K+ channels in spinal dorsal horn neurones: role of the soma, axon and dendrites in spike generation. Prog. Neurobiol. 1999;59:217–241. doi: 10.1016/s0301-0082(98)00051-3. [DOI] [PubMed] [Google Scholar]
- SAFRONOV B.V., WOLFF M., VOGEL W. Functional distribution of three types of Na+ channel on soma and processes of dorsal horn neurones of rat spinal cord. J. Physiol. 1997;503:371–385. doi: 10.1111/j.1469-7793.1997.371bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATOR-KATZENSCHLAGER S., DEUSCH E., MAIER P., SPACEK A., KRESS H.G. The long-term antinociceptive effect of intrathecal S(+)-ketamine in a patient with established morphine tolerance. Anesth. Analg. 2001;93:1032–1034. doi: 10.1097/00000539-200110000-00047. [DOI] [PubMed] [Google Scholar]
- SCHELLER M., BUFLER J., HERTLE I., SCHNECK H.J., FRANKE C., KOCHS E. Ketamine blocks currents through mammalian nicotinic acetylcholine receptor channels by interaction with both the open and the closed state. Anesth. Analg. 1996;83:830–836. doi: 10.1097/00000539-199610000-00031. [DOI] [PubMed] [Google Scholar]
- SMITH D.J., BOUCHAL R.L., DESANCTIS C.A., MONROE P.J., AMEDRO J.B., PERROTTI J.M., CRISP T. Properties of the interaction between ketamine and opiate binding sites in vivo and in vitro. Neuropharmacology. 1987;26:1253–1260. doi: 10.1016/0028-3908(87)90084-0. [DOI] [PubMed] [Google Scholar]
- TOGAL T., DEMIRBILEK S., KOROGLU A., YAPICI E., ERSOY O. Effects of S(+) ketamine added to bupivacaine for spinal anaesthesia for prostate surgery in elderly patients. Eur. J. Anaesthesiol. 2004;21:193–197. doi: 10.1017/s0265021504003059. [DOI] [PubMed] [Google Scholar]
- VRANKEN J.H., VAN DER VEGT M.H., KAL J.E., KRUIS M.R. Treatment of neuropathic cancer pain with continuous intrathecal administration of S+-ketamine. Acta. Anaesthesiol. Scand. 2004;48:249–252. doi: 10.1111/j.0001-5172.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- WOLFF M., VOGEL W., SAFRONOV B.V. Uneven distribution of K+ channels in soma, axon and dendrites of rat spinal neurones: functional role of the soma in generation of action potentials. J. Physiol. 1998;509:767–776. doi: 10.1111/j.1469-7793.1998.767bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU Y.N., JOHNSON S.W. Pharmacological characterization of inward current evoked by N-methyl-D-aspartate in dopamine neurons in the rat brain slice. J. Pharmacol. Exp. Ther. 1996;279:457–463. [PubMed] [Google Scholar]
- YAMAMURA T., HARADA K., OKAMURA A., KEMMOTSU O. Is the site of action of ketamine anesthesia the N-methyl-D-aspartate receptor. Anesthesiology. 1990;72:704–710. doi: 10.1097/00000542-199004000-00021. [DOI] [PubMed] [Google Scholar]
- ZEILHOFER H.U., SWANDULLA D., GEISSLINGER G., BRUNE K. Differential effects of ketamine enantiomers on NMDA receptor currents in cultured neurons. Eur. J. Pharmacol. 1992;213:155–158. doi: 10.1016/0014-2999(92)90248-3. [DOI] [PubMed] [Google Scholar]
- ZHOU Z.S., ZHAO Z.Q. Ketamine blockage of both tetrodotoxin TTX-sensitive and TTX-resistant sodium channels of rat dorsal root ganglion neurons. Brain Res. Bull. 2000;52:427–433. doi: 10.1016/s0361-9230(00)00283-5. [DOI] [PubMed] [Google Scholar]