Abstract
We aimed to functionally characterize endothelin (ET) receptors in the rat carotid artery. mRNA and protein expressions of both ETA and ETB receptors, evaluated by reverse transcription–polymerase chain reaction (RT–PCR) and Western immunoblotting, were detected in carotid segments. Immunohistochemical assays showed that ETB receptors are expressed in the endothelium and smooth muscle cells, while ETA receptors are expressed only in the smooth muscle cells. In endothelium-denuded vessels, levels of ETB receptor mRNA were reduced.
Vascular reactivity experiments, using standard muscle bath procedures, showed that ET-1 induces contraction in endothelium-intact and -denuded carotid rings in a concentration-dependent manner. Endothelial removal enhanced ET-1-induced contraction. BQ123 and BQ788, selective antagonists for ETA and ETB receptors, respectively, produced concentration-dependent rightward displacements of the ET-1 concentration–response curves.
IRL1620, a selective agonist for ETB receptors, induced a slight vasoconstriction that was abolished by BQ788, but not affected by BQ123. IRL1620-induced contraction was augmented after endothelium removal.
ET-1 concentration dependently relaxed phenylephrine-precontracted rings with intact endothelium. The relaxation was augmented in the presence of BQ123, reduced in the presence of BQ788 and completely abolished after endothelium removal. IRL1620 induced vasorelaxation that was abolished by BQ788 and endothelium removal, but not affected by BQ123.
Preincubation of intact rings with NG-nitro-L-arginine methyl ester (L-NAME), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), indomethacin or tetraethylammonium (TEA) reduced IRL1620-induced relaxation. The combination of L-NAME, indomethacin and TEA completely abolished IRL1620-induced relaxation while sulfaphenazole did not affect this response. 4-aminopyridine (4-AP), but not apamin, glibenclamide or charybdotoxin, reduced IRL1620-induced relaxation.
The major finding of this work is that it firstly demonstrated functionally the existence of both ETA and ETB vasoconstrictor receptors located on the smooth muscle of rat carotid arteries and endothelial ETB receptors that mediated vasorelaxation via NO–cGMP pathway, vasodilator cyclooxygenase product(s) and the activation of voltage-dependent K+ channels.
Keywords: Carotid artery, ETB receptor, relaxation, endothelin-1
Introduction
Endothelin-1 (ET-1) is a 21-amino-acid peptide produced by the endothelium, belonging to a family of potent vasoconstrictors (Yanagisawa et al., 1988). The actions of ETs are mediated by two receptors named ETA and ETB. The ETA receptor is restricted to vascular smooth muscle and mediates vasoconstriction (Haynes & Webb, 1993). ETB receptors were initially described on vascular endothelium, mediating relaxation via production of nitric oxide (NO) (Hirata et al., 1993) and (or) prostacyclin (PGI2) (Filep et al., 1991). Some studies have shown that in addition to ETB receptors mediating vasorelaxation (Matsuda et al., 1993; Schilling et al., 1995) there are also ETB receptors causing contraction located on vascular smooth muscle (Ihara et al. 1991; Deng et al., 1995).
Although the functions of ET receptors are well characterized, it has been described that these receptors possess different distribution and functional importance depending on the species and blood vessel. In this line, Caló et al. (1996) demonstrated that the rabbit carotid artery is a pure ETA system, while the rabbit pulmonary artery contains contractile ETA and ETB receptors which contribute by approximately 20 and 80%, respectively, to the contraction induced by ETs. In addition, they also found that the rabbit jugular vein is a pure contractile ETB system. It has been demonstrated previously that human saphenous vein contains both endothelin ETA and ETB contractile receptors (White et al., 1994). ETs were also described to contract the human isolated umbilical artery via stimulation of an ETA receptor, while both ETA and ETB receptors mediate the contraction in the human umbilical vein (Bogoni et al., 1996). Similarly, ETA and ETB receptors located in the vascular smooth muscle were described to mediate the contractile effect induced by ET in the pig coronary artery (Elmoselhi & Grover, 1997).
Endothelial ETB receptor mediating relaxation was described in the isolated rat basilar artery (Schilling et al., 1995), rat aorta (Fujitani et al., 1993) and guinea-pig aorta (Matsuda et al., 1993). On the other hand, the selective agonists for ETB receptors alanine [1,3,11,15]ET-1 and sarafotoxin failed to induce relaxation in rat renal artery, further suggesting that ETB receptors mediating relaxation do not appear to be present in this vascular bed (Clark & Pierre, 1995).
The presence of ETA and ETB receptors mRNA was previously identified in the carotid artery of Sprague–Dawley rat (Wang et al., 1995; 1996). It was also noted by these authors that the levels of ETA and ETB receptors mRNA were elevated in rat carotid arteries after balloon angioplasty. Additionally, the presence of ETA receptors was demonstrated in the medial layer of rat carotid arteries by use of autoradiography (De Oliveira et al., 1995). The authors suggested that in addition to the ETA receptors a small amount of ETB receptors could be expressed in this vascular bed. Furthermore, Viswanathan et al. (1997) confirmed the presence of ETA receptors in the medial layer of rat carotid arteries and also verified that ETA receptor expression was increased in the media of these arteries after balloon angioplasty.
The endothelinergic system has been postulated to have a pathophysiological role in a wide range of diseases (for reviews, please refer to Rubanyi & Polokoff, 1994; D'Orléans-Juste et al., 2002). Thus, the study of the physiological expression and function of ET receptors may provide valuable information on the contribution of ETs to the arterial response to injuries. The function of ET receptors in blood vessels could have important implications for the design of ET receptor antagonists for use in vascular diseases in which ETs have been implicated. Although the expression of ET receptors has been described in rat carotid, to our knowledge, there are no reports describing the functionality of these receptors in this vascular bed. The aims of the present study were to attempt a functional characterization of the ET receptors in the rat carotid artery and also to investigate the mechanisms underlying ETB-induced relaxation. In addition, RT–PCR and Western immunoblotting were performed to detect the mRNA and protein expression of ETA and ETB receptors. Immunohistochemical assays were conducted to localize these receptors.
Methods
Reverse transcriptase–polymerase chain reaction
RT–PCR was performed in rat carotid arteries with intact or denuded endothelium. The endothelium was removed mechanically by gently rolling the lumen of the vessels on a thin wire. Endothelial removal was confirmed by RT–PCR for eNOS.
Total cell RNA was isolated from carotid arteries using Trizol Reagent (Gibco BRL, Life Technologies, Rockville, MD, U.S.A.). After DNA digestion (RQ1 DNAse RNAse-free, Promega Corporation, Madison, WI, USA), total RNA (20 ng per sample) was used for RT in the presence of an RNase inhibitor (RnasIn®, Promega Corporation), 200 U of Moloney murine leukemia virus RT (Gibco BRL) and 1 μg of oligo (dT)12–18 primer at 37°C for 60 min, according to manufacturer specifications. The cDNA products were isolated by phenol–chloroform extraction, precipitated with ethanol, resuspended in 120 μl TE (10 mM Tris-HCl and 1 mM EDTA, pH 7.5) and stored at −20°C until required for the PCR. PCR primers were designed on the basis of published rat cDNA sequences for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), ETA/ETB receptors, and are as follows: ETA, antisense primer CTGTGCTGCTCGCCCTTGTA, sense primer GAAGTCGTCCGTGGGCATCA (216-bp fragment); ETB, antisense primer CACGATGAGGACAATGAGAT, sense primer TTACAAGACAGCCAAAGACT (565-bp fragment); GAPDH, antisense primer CACCACCCTGTTGCTGTA, sense primer TATGATGACATCAAGAAGGTGG (219-bp fragment), eNOS left: TGACCCTCACCGATACAACA, right: CTGGCCTTCTGCTCATTTTC. GAPDH was used as an internal control for the coamplification. In order to identify the optimal amplification conditions, a series of pilot studies were performed using a thermal cycler with a temperature gradient in the annealing step (Eppendorf Mastercycler gradient, Eppendorf-Netheler-Hinz, Hamburg, Germany), various amounts of RT products from 2 to 200 ng RNA, and 20–35 cycles of PCR amplification. The following conditions were selected for PCR in a volume of 50 μl: RT products from 20 ng of RNA, 2.5 U Taq polymerase (Gibco BRL), 30 cycles for ETA receptor gene, 36 for ETB receptor gene, 26 cycles for eNOS gene, and 24 cycles for the GAPDH gene. Amplification was carried out using an initial denaturing cycle at 94°C for 5 min and the subsequent cycles as follows: denaturation, 30 s at 94°C; annealing, 30 s at 58°C (ETB), 66°C (ETA), 62°C (GAPDH) or 60°C (eNOS), and extension, 45 s at 72°C. PCR products (10 μl per lane) were electrophoresed using 1% agarose gel containing ethidium bromide (0.5 μg ml−1). The identity of cDNA products has been confirmed by DNA sequence analysis. The gel was subjected to ultraviolet light and photographed. The band intensities were measured using a software package (Kodak Digital Science, Eastman Kodak Company, New Haven, CT, U.S.A.) and the signals are reported relatively to the intensity of GAPDH amplification in each co-amplified sample.
Western immunoblotting
Total protein was extracted from carotid rings with endothelium. The Bradford assay was used to determine protein concentration. Denaturation of proteins was performed in 2 × Laemmli sample buffer by heating to 95°C for 5 min, cooled on ice and followed by a quick spin. Total protein (20 μg) was separated by electrophoresis on 10% SDS polyacrylamide gel and transferred to methanol-activated PVDF membrane (Amersham) in Tris-Glycine buffer containing 20% of methanol. Membranes were blocked on TBST with 8% nonfat dry milk and incubated with rabbit polyclonal antiserum (1 : 200) raised against rat ETBR (AER-002) and (1 : 100) rat ETAR (AER-001) (Alomone Labs). COX-1 was used as internal control and detected with rabbit polyclonal antiserum (1/750) (160109) (Cayman). As the second antibody, the donkey polyclonal antiserum against rabbit IgG coupled to horse-radish peroxidase (NA9340V) (Amersham) was used. Visualization of protein bands was carried out with the enhanced chemiluminescence's ECL detection system (Amersham). Densitometric analysis was performed with a densitometer (Gel Doc, Bio-Rad) to determine protein level.
Immunohistochemistry
Carotid arteries were fixed in Methacarn (60% methanol, 30% chloroform, and 10% acetic acid), and paraffin-embedded longitudinal sections (7 μm) were incubated with 3% H2O2 and a Pierce solution to block endogenous peroxidase and biotin, respectively. Then, sections were incubated (humidified box, 4°C) with primary polyclonal antibodies against rat ETA and ETB receptors (1 : 10 dilution; Alomone Labs Ltd – Jerusalem, Israel) and with a biotin-conjugated secondary anti-rabbit antibody (1 : 1000, Vector Laboratories Inc., Burlingame, CA, U.S.A.) and streptavidin-conjugated peroxidase (Vectastain ABC kit, Vector Laboratories Inc., Burlingame, CA, U.S.A.). Color was developed by the addition of DAB (Sigma). Sections were lightly stained in hematoxylin, dehydrated with alcohol and xylene, and scored by an independent observer unaware of the groups and treatments of the rats. To evaluate background reaction, procedures were also performed in sections incubated only with the secondary antibody (indirect technique) or in the absence of antibodies (direct technique).
Vascular reactivity studies
Male Wistar rats weighting between 400 and 450 g (100–120 days old) were anesthetized and killed by aortic exsanguination in accordance with standards and policies of the University of Sao Paulo's Animal Care and Use Committee.
The carotid artery was quickly removed, cleaned of adherent connective tissues and cut into 5-mm-length rings. Two stainless-steel stirrups were passed through the lumen of each ring. One stirrup was connected to an isometric force transducer (Letica Scientific Instruments) to measure tension in the vessels. The rings were placed in 5-ml organ chambers containing Krebs solution, pH 7.4, gassed with 95% O2/5% CO2, and maintained at 37°C. The composition of Krebs solution was as follows (mM): NaCl, 118.0; KCl, 4.7; KH2PO4, 1.2; MgSO4, 1.2; NaHCO3, 15.0; Glucose, 5.5; CaCl2, 2.5. The rings were stretched until an optimal basal tension of 1.0 g, which was determined by length–tension relationship experiments, and then were allowed to equilibrate for 60 min, with the bath fluid being changed every 15–20 min. In some rings, the endothelium was removed mechanically by gently rolling the lumen of the vessel on a thin wire. Endothelial integrity was assessed qualitatively by the degree of relaxation caused by acetylcholine (1 μM) in the presence of contractile tone induced by phenylephrine (0.1 μM). For studies of endothelium-intact vessels, the ring was discarded if relaxation with acetylcholine was not 80% or greater. For studies of endothelium-denuded vessels, the rings were discarded if there was any degree of relaxation.
Effects of endothelial removal on ET-1-induced contraction
To verify the influence of the endothelium on the contraction induced by ET-1, concentration–response curves for this peptide (10−12–3 × 10−8 mol l−1) were obtained in endothelium-intact and -denuded rings.
Effects of antagonists on ET-1-induced contraction
Initial studies showed that consecutive reproducible concentration–response curves for ET-1 could not be obtained (data not shown). The contraction induced by the peptide was much smaller following the construction of the first curve, further indicating tachyphylaxis. Therefore, for a given preparation, contractile responses to ET-1 could not be studied in the absence and subsequent presence of an antagonist. Hence, antagonists were added 30 min prior to the construction of the curve and the responses were compared with those observed in time-matched control experiments. Both the selective ETA (BQ123; Ihara et al., 1992) and ETB (BQ788; Ishikawa et al., 1994) receptor antagonists were tested. After incubation with the antagonists, concentration–response curves for ET-1 (10−12–10−7 mol l−1) were obtained. Four concentrations of BQ123 (0.01, 0.1, 1 and 3 μM) and three concentrations of BQ788 (0.3, 1 and 3 μM) were tested in endothelium-denuded rings to construct a Schild plot.
IRL1620-induced contraction
To verify whether the selective ETB receptor agonist IRL1620 (Takai et al., 1992), could induce contraction, cumulative concentration–response curves for this peptide (10−10–3 × 10−7 mol l−1) were obtained in endothelium-intact and denuded rings. The contractile responses of IRL1620 were also analyzed in the presence of BQ123 (3 μM) and BQ788 (3 μM).
ET-1 and IRL1620-induced relaxation
Endothelium-intact and -denuded rings were precontracted with phenylephrine, used at concentrations of 0.1 and 0.03 μM, respectively, to induce contractions of similar magnitude. After reaching a stable and sustainable contraction, ET-1 (10−14–3 × 10−11 mol l−1) or IRL1620 (10−10–3−10−8 mol l−1) were added cumulatively to the organ bath. In endothelium-intact rings, experiments were conducted in the absence and presence of BQ123 (3 μM) and BQ788 (3 μM). As would be expected owing to their ET receptor specificity, neither BQ123 nor BQ788 changed the resting tension of the tissues or the contractile response induced by phenylephrine.
The mechanisms underlying the relaxant effect induced by IRL1620 were studied in endothelium-intact rings. These mechanisms were evaluated by experiments performed in the presence of NG-nitro-L-arginine-methyl-ester (NO synthase inhibitor, L-NAME, 100 μM), indomethacin (cyclooxygenase inhibitor, 10 μM), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (guanylyl cyclase inhibitor, ODQ, 1 μM), sulfaphenazole (selective blocker of cytochrome P450 2C9, 10 μM; Bussemaker et al., 2003). The concentrations of the channel blockers tetraethylammonium chloride (nonselective K+ channel blocker, TEA, 10 mM), apamin (selective blocker of low-conductance Ca2+-activated channels, 1 μM), glibenclamide (selective blocker of ATP-sensitive K+ channels, 3 μM), charybdotoxin (selective blocker of large-conductance Ca2+-activated K+ channels, 0.1 μM) and 4-aminopyridine (selective blocker of voltage-dependent K+ channels, 4-AP, 1 mM) were used as described by Nelson & Quayle (1995). All drugs were incubated for 30 min before the experimental procedures. Relaxation was expressed as percentage change from the phenylephrine-contracted levels. Since we noted that L-NAME and ODQ enhanced phenylephrine-induced contraction, the rings with intact endothelium exposed to these compounds were precontracted with phenylephrine 0.03 μM, to induce a magnitude of contraction similar to that found in the intact rings not exposed to the inhibitors.
Drugs
The following drugs were used: phenylephrine hydrochloride, acetylcholine hydrochloride, ODQ, glibenclamide, 4-AP, ET-1 (Sigma, St Louis, MO, U.S.A.), L-NAME, TEA, sulfaphenazole (Sigma/RBI, Natick, MA, U.S.A.), indomethacin (Calbiochem, U.S.A.), apamin, BQ123, BQ788, IRL1620 (American Peptide Company, Sunnyvale, CA, U.S.A.) and charybdotoxin (Alomone Labs, Jerusalem, Israel). Glibenclamide and ODQ were prepared as stock solutions in ethanol and DMSO, respectively. Indomethacin was dissolved in Tris buffer (pH 8.4). The other drugs were dissolved in distilled water. The bath concentration of ethanol or DMSO did not exceed 0.5%, which was shown to have no effects per se on the basal tonus of the preparations or on the agonist-mediated contraction or relaxation.
Data analysis
Contractions were recorded as changes in the displacement (g) from baseline. Relaxation was expressed as percentage change from the phenylephrine-contracted levels. Agonist concentration–response curves were fitted using a nonlinear interactive fitting program (Graph Pad Prism 2.01; GraphPad Software Inc., San Diego, CA, U.S.A.). Agonist potencies and maximum response are expressed as pD2 (negative logarithm of the molar concentration of agonist producing 50% of the maximum response) and Emax (maximum effect elicited by the agonist), respectively. Concentration ratios (CRs) were determined from EC50 values in the presence and absence of the antagonists. The concentration–response curves to the agonist in the presence or absence of the antagonists were analyzed by plotting the negative logarithm of the ratio of concentrations of the agonist that produced the same effect (50% contraction) in the presence and absence of the antagonist minus 1[log(CR−1)] against the negative logarithm of the concentration of antagonists (i.e., Schild plot analysis; Arunlakshana & Schild, 1959). The intercept on the abscissa yields the pA2 value (negative logarithm of the concentration of antagonist that induces a two-fold rightward shift of the concentration–response curve to the agonist). Statistically significant differences were calculated by one-way analysis of variance (ANOVA) or Student's t-test. P<0.05 was considered as statistically significant.
Results
Effects of endothelium removal on ET-1-induced contraction
Cumulative concentrations of ET-1 induced contraction of both endothelium-intact and -denuded rat carotid arteries in a concentration-dependent manner (Figure 1). Removal of functional endothelium lead to an enhancement in the Emax values (0.63±0.02 g) when compared to intact rings (0.44±0.02 g) (P<0.05, Student's t-test). Conversely, no differences in the pD2 values between denuded (8.95±0.12) and intact rings (8.72±0.05) were found.
Figure 1.
Concentration–response curves for ET-1 obtained in isolated rat carotid rings in the presence (Endo+) and absence (Endo−) of endothelium. Values are means±s.e.m.; n=9 for Endo+ and n=6 for Endo− rings.
ETA and ETB receptor mRNA expression
The results obtained by RT–PCR show that rat carotid arteries with endothelium express mRNA for both ETA and ETB receptors. Similarly, mRNAs for ETA and ETB receptors were observed in endothelium-denuded carotid rings. However, endothelial denudation reduced the levels of mRNA for ETB receptors (Figure 2a). Endothelial denudation was confirmed by the absence of mRNA for eNOS (Figure 2b) and Von Willebrand factor (data not shown).
Figure 2.
Representative RT–PCR products of 20 ng total RNA extracted from carotid arteries of Wistar rats. The bar graphs show the relative absorbance values of ETA and ETB receptor bands in endothelium intact (Endo+) or denuded (Endo−) rings (a) and the relative absorbance values of eNOS bands (b). ETA and ETB values were normalized to the corresponding GAPDH values, used as internal standard. Results are reported as means±s.e.m. and are representative of three experiments.
Expression and localization of ETA and ETB receptors in the rat carotid artery
Western immunoblotting assays detected the protein expression of ETA and ETB receptors in the rat carotid. The expression of ETA receptors was not significantly different from that found for ETB receptors (Student's t-test) (Figure 3). The immunohistochemical studies revealed intense staining for ETA and ETB immunoreactivities in smooth muscle cells. In endothelial cells, positive immunostaining for ETB, but not for ETA, receptors was detected (Figure 4).
Figure 3.
Representative Western immunoblotting products of 20 μg total protein extracted from endothelium-intact carotid arteries of Wistar rats. The bar graphs show the relative absorbance values of ETA and ETB receptor bands. Values were normalized by the corresponding COX-1 bands, used as internal standard. Results are reported as means±s.e.m. and are representative of five experiments.
Figure 4.
Representative immunohistochemical photomicrographs of ETA (a) and ETB (b) receptors in rat carotid artery sections. Arrows indicate expression of ETA receptor in smooth muscle cells and ETB in both endothelial and smooth muscle cells.
Effects of antagonists on ET-1-induced contraction
BQ123 (0.1, 1 and 3 μM) produced concentration-dependent rightward displacements of the ET-1 concentration–response curves with reduction of the maximal response (Figure 5a). Schild analysis yielded pA2 value for BQ123 of 7.07±0.33 and a slope of 0.36±0.10, which was significantly different from the unity. Similarly, BQ788 (1 and 3 μM) displaced the curves for ET-1 to the right (Figure 5b). Schild analysis yielded pA2 values for BQ788 of 6.20±0.10 and a slope of 0.71±0.10, which was significantly different from unity. The combination of BQ123 and BQ788 resulted in a greater antagonism than that exerted by the antagonists when added individually. The pD2 of ET-1 concentration–response curves, as well as the Emax values observed in the absence and presence of BQ123 and BQ788, is given in Table 1.
Figure 5.
Concentration–response curves for ET-1 obtained in endothelim-denuded rat carotid rings in the absence or presence of different concentrations of BQ123 (a) or BQ788 (b). Values are means±s.e.m. Six preparations were used in each group.
Table 1.
Emax and pD2 values for ET1-induced contraction of endothelium-denuded rat carotid rings in the absence or presence of different concentrations of BQ123 and BQ788
| Groups | Emax (g) | pD2 |
|---|---|---|
| ET-1 | 0.63±0.02 | 8.95±0.12 |
| ET-1+BQ123 (0.01 μM) | 0.56±0.06 | 8.76±0.12 |
| ET-1+BQ123 (0.1 μM) | 0.49±0.05* | 8.46±0.13* |
| ET-1+BQ123 (1 μM) | 0.45±0.04* | 8.03±0.22** |
| ET-1+BQ123 (3 μM) | 0.37±0.03** | 8.15±0.08** |
| ET-1+BQ788 (0.3 μM) | 0.56±0.04 | 9.00±0.11 |
| ET-1+BQ788 (1 μM) | 0.50±0.07 | 8.59±0.08* |
| ET-1+BQ788 (3 μM) | 0.44±0.05* | 8.46±0.10* |
| ET-1+BQ123 (3 μM)+BQ788 (3 μM) | 0.08±0.02** | — |
Values are means±s.e.m. Six preparations were used in each group.
P<0.05 vs control (ET-1);
P<0.01 vs control (ANOVA).
IRL1620-induced contraction
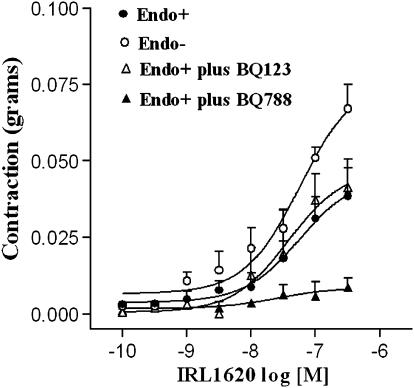
The cumulative application of the selective ETB agonist IRL1620 induced a small contraction of intact rings, which was blocked by BQ788 but not BQ123 (Figure 6). The contraction induced by IRL1620 was greater in endothelium-denuded than in intact rings. The pD2 of IRL1620, as well as the Emax values observed in the absence and presence of BQ123 and BQ788, are given in Table 2.
Figure 6.
Concentration–response curves for IRL1620 obtained in endothelium-denuded (Endo−) and endothelium-intact (Endo+) rat carotid rings in the absence or presence of BQ123 (3 μM) and BQ788 (3 μM). Values are means±s.e.m. of 5–7 independent preparations.
Table 2.
Emax and pD2 values for IRL1620-induced contraction of endothelium-denuded (Endo−) and endothelium-intact (Endo+) rat carotid in the absence or presence of BQ123 and BQ788
| Groups | Emax (g) | pD2 | n |
|---|---|---|---|
| Endo+ | 0.040±0.009 | 7.27±0.26 | 7 |
| Endo− | 0.067±0.008* | 8.03±0.21 | 6 |
| Endo+ plus BQ123 (3 μM) | 0.041±0.009 | 7.43±0.21 | 5 |
| Endo+ plus BQ788 (3 μM) | — | — | 5 |
Values are means±s.e.m. of n preparations.
P<0.05 vs Endo+ (ANOVA).
ET-1 and IRL1620-induced relaxation
Figure 7a shows that preincubation of the rings with BQ788 (Emax: 23.45±1.66%; n=5) reduced ET-1-induced relaxation (Emax: 50.30±2.51%; n=7) (P<0.01), while BQ123 enhanced the relaxation (Emax: 65.79±6.02%; n=8) (P<0.01). Denudation of the endothelium abolished the relaxation induced by ET-1 (Emax: 7.53±3.07%; n=5) (P<0.01). The pD2 values of ET-1 concentration–response curves in the presence of BQ788 (13.28±0.16) or BQ123 (13.26±0.19) were not significantly different from that found in the absence of the antagonists (12.85±0.36).
Figure 7.
Relaxation responses induced by ET-1 (a) and IRL1620 (b) on rat carotid rings precontracted with phenylephrine. The concentration–response curves for both agonists were obtained in endothelium-denuded (Endo−) and endothelium-intact rings (Endo+) in the absence or presence of BQ123 (3 μM) and BQ788 (3 μm). Values are means±s.e.m. of 5–8 independent preparations.
The relaxation induced by IRL1620 in endothelium-intact rings (Emax: 45.32±3.34%; n=8) was significantly reduced (P<0.01) in the presence of BQ788 (3 μM) (Emax: 10.93±3.96%; n=6), but not in the presence of BQ123 (3 μM) (Emax: 46.42±6.11%; n=5). Denudation of the endothelium abolished (P<0.01) the relaxation induced by IRL1620 (Emax: 2.35±3.80%; n=6). No differences were found between the pD2 values of endothelium-intact rings in the absence (pD2: 8.76±0.24) or presence of BQ123 (pD2: 9.08±0.14) (Figure 7b).
In order to verify the mechanisms underlying ETB-induced relaxation, endothelium-intact rings were exposed to a variety of drugs. When added alone, L-NAME or indomethacin reduced IRL1620-induced relaxation to a similar extent. The combination of these two compounds showed further suppression than that observed with either L-NAME or indomethacin alone. However, even when added together, these compounds were not able to abolish IRL1620-induced relaxation. TEA also reduced the relaxation induced by IRL1620. The combination of TEA, L-NAME and indomethacin completely abolished IRL1620-induced relaxation. Also, the guanylyl cyclase inhibitor ODQ reduced ETB-induced relaxation. However, sulfaphenazole, a selective blocker of CPY2C9, did not alter IRL1620-induced relaxation. Finally, the vasodilatory response induced by IRL1620 was reduced by 4-AP, whereas apamin, glibenclamide or charybdotoxin had no effect in this response (Figure 8). The mean pD2's of the relaxant IRL1620 concentration–response curves, as well as the Emax values, in the absence or presence of the previously mentioned inhibitors, are given in Table 3.
Figure 8.
Relaxation responses induced by IRL1620 on endothelium-intact rat carotid rings precontracted with phenylephrine. The concentration–response curves were obtained in the absence (control) or in the presence of L-NAME (100 μM), ODQ (1 μM), indomethacin (10 μM) (a), TEA (10 mM), sulfaphenazole (10 μM) (b), 4-AP (1 mM), glibenclamide (3 μM), apamin (1 μM) or charybdotoxin (0.01 μM) (c). The rings were pre-incubated with the drugs for 30 min. Values are means±s.e.m. of 6–8 independent preparations.
Table 3.
Emax and pD2 values for IRL1620-induced relaxation of endothelium-intact rat carotid in the absence (Control) or presence of different drugs
| Groups | Emax (% relaxation) | pD2 | n |
|---|---|---|---|
| Control | 45.52±3.34 | 8.76±0.24 | 8 |
| L-NAME (100 μM) | 26.29±5.07* | 9.01±0.16 | 7 |
| ODQ (1 μM) | 27.79±5.27* | 9.11±0.06 | 7 |
| INDO (10 nM) | 22.93±7.16* | 8.87±0.60 | 6 |
| L-NAME (100 μM)+INDO (10 μM) | 13.57±3.78** | 8.75±0.10 | 6 |
| TEA (10 mM) | 24.02±3.90* | 8.63±0.18 | 7 |
| L-NAME (100 μM)+INDO (10 μM)+TEA (10 mM) | 7.24±3.70** | — | 6 |
| Sulfaphenazole (10 μM) | 40.01±3.10 | 8.55±0.20 | 5 |
| 4-AP (1 mM) | 18.62±6.95** | 8.48±0.42 | 8 |
| Apamin (1 μM) | 41.25±1.15 | 9.07±0.20 | 6 |
| Glibenclamide (3 μM) | 42.90±3.88 | 8.91±0.26 | 7 |
| Charybdotoxin(0.1 μM) | 47.99±7.87 | 8.79±0.14 | 6 |
Values are means±s.e.m. of n preparations.
P<0.05 vs Endo+;
P<0.01 vs Endo+ (ANOVA).
Discussion
In the present study, mRNA expression of both ETA and ETB receptors was detected in carotid segments. Endothelial removal reduced mRNA expression of ETB, but not ETA, receptors, further indicating the presence of mRNA for ETB receptors in the vascular endothelium. By using Western immunoblotting, we demonstrated the protein expression of both ETA and ETB receptors in rat carotid artery with endothelium. Immunohistochemical assays showed that ETB receptors are expressed in the endothelium and smooth muscle cells of rat carotid, while ETA receptors are expressed exclusively in the smooth muscle cells.
A pharmacological characterization of the receptors mediating the contractile effects of ETs in endothelium-denuded rat carotid arteries has been attempted by use of the classical criteria recommended by Schild, namely the order of potency of agonists and the affinities of competitive antagonists. The selective antagonist for ETA receptors BQ123 produced a rightward displacement of ET-1-induced curves in a concentration-dependent manner. Interestingly, this effect was followed by a decrease in Emax, as described previously (Zellers et al., 1994; Bogoni et al., 1996; Dieye & Gairard, 2000), resulting in a Schild plot slope less than unity. Some possibilities have been postulated to explain this: (1) tachyphylaxis to the agonist, (2) equilibrium conditions between the antagonist and receptors have not been attained, (3) the interaction between the antagonist and receptors is not reversible, (4) the antagonist is not competitive and (5) heterogeneous receptor population are observed (Kenakin, 1981; 1992). We noted that two consecutive curves for ET-1 could not be obtained in the same tissue. Thus, stimulation with ET-1 was performed in parallel vessels to avoid tachyphylaxis. The interaction between the ET-1 antagonist and its receptors is reversible and BQ123 acts as a competitive antagonist (Ihara et al., 1992). Finally, BQ123 was left in contact with the carotids for 30 min, which is a period of incubation sufficient to achieve the equilibrium conditions (Ihara et al., 1992; Clark & Pierre, 1995). Thus, four of the five possibilities raised were excluded. Schild plots yielding slopes of less than unity have also been obtained with BQ123 in other tissues (Schoeffter et al., 1993; Riezebos et al., 1994), and such findings were interpreted as an indicative of receptor heterogeneity. In our experiments, the most plausible explanation for a slope less than unity is a heterogeneous population of ET receptors mediating contraction. In support of this proposal, BQ788, a selective antagonist for ETB receptors, produced a rightward displacement of the concentration–response curves for ET-1. Furthermore, a combination of BQ123 and BQ788 resulted in a greater antagonism than that exerted by the antagonists when added individually, further supporting the idea that ETA and ETB receptors are involved in ET-1-induced contraction.
IRL1620, a selective agonist for the ETB receptors (Takai et al., 1992), evoked contractions of rat carotid arteries, albeit in only approximately 70% of the preparations tested. BQ788, but not BQ123, inhibited IRL1620-induced contraction, confirming that the contraction is mediated by ETB receptors. Removal of the endothelium significantly enhanced IRL1620-induced contraction, further suggesting that the endothelium counteracts the contraction induced by ETB receptors. The reason(s) why IRL1620 did not contract all the arteries tested is unclear, albeit it is unlikely related to tissue damage, since phenylephrine added to the tissues at the end of the concentration–response curves to the ETB agonist produced a contractile response similar in magnitude to that obtained at the beginning of the experiment. This result is in line with previous observations of White et al. (1994) in human saphenous vein showing that the endothelin ETB receptor-selective agonists [Ala1,3,11,15]endothelin-1 and sarafotoxin S6c only produced contractions in 50% of the preparations tested. The authors suggested that this response occurs because the proportion of ETA and ETB receptors varies among vessels and/or along the length of an individual vessel. IRL1620 produced about 10% of the total contraction induced by ET-1 in both endothelium-intact and -denuded carotid rings. A small contraction induced by IRL1620 was also reported in rat (Sharifi & Schiffrin, 1996) and porcine (Elmoselhi & Grover, 1997) arteries. Our data indicate that in the rat carotid ET-1 acts predominantly on the ETA receptor to induce contraction, while ETB receptors play a minor role.
Apart from their potent vasoconstrictor responses, ETs have also been shown to produce endothelium-dependent relaxation (Matsuda et al., 1993; Zellers et al., 1994) via ETB receptors (Auguet et al., 1993; Fujitani et al., 1993). ET-1 induced endothelium-dependent relaxation that was increased in the presence of BQ123, but reduced by BQ788, indicating that ET-1-induced relaxation is mediated by endothelial ETB receptors. These data also suggest that ETA receptors in the smooth muscle cells counteract the relaxant response induced by the activation of endothelial ETB receptors. Relaxation induced by ET-1, in a similar concentration range of that employed in the present study, has been described previously (Zellers et al., 1994). Frelin & Guedin (1994) hypothesized, regarding the response of the ET-1-induced biphasic action (vasodilation followed by constriction), that ET-1 first stimulates endothelial ETB receptors and occupies all these receptors and then diffuses into the media to act on receptors on smooth muscle.
IRL1620-induced relaxation was not altered by BQ123, but reduced in the presence of BQ788, confirming that the endothelium-dependent relaxation induced by IRL1620 is mediated by ETB receptors. IRL1620 induced relaxation in a low concentration when compared to that necessary to induce contraction. A possible explanation for this observation is that ETB receptors found on the vascular smooth muscle possess far less affinity for ETB receptors selective agonists than the supersensitive receptor moiety located on the endothelium (Sokolovsky et al., 1992).
ETB receptors were described to mediate relaxation via production of NO (Hirata et al., 1993) and vasodilator cyclooxygenase product(s) (Filep et al., 1991). We found that L-NAME as well as indomethacin partially, but significantly, reduced ETB-mediated relaxation, further indicating that activation of NO synthase and vasodilator cyclooxygenase product(s) plays a role in ETB-mediating relaxation. The selective inhibitor of guanylyl cyclase enzyme, ODQ (Garthwaite et al., 1995), reduced the vasorelaxant action of IRL1620, confirming the involvement of the NO–cGMP pathway in ETB-mediated vasorelaxation, as observed previously (Fujitani et al., 1993). When L-NAME and indomethacin were simultaneously added, further additional inhibitory effect on IRL1620-induced relaxation was observed. However, the relaxant response was not completely abolished, indicating that factors unrelated to the production of vasodilator cyclooxygenase product(s) or NO synthase activation also participate in this response.
TEA, a nonselective blocker of K+ channels, significantly reduced IRL1620-induced relaxation. When added simultaneously, L-NAME, indomethacin and TEA completely abolished ETB-mediated relaxation, indicating that cyclooxygenase product(s), NO and K+ channels are involved in this response. Sulfaphenazole, a specific inhibitor of cytochrome P450 2C9 (CYP2C9) epoxygenase, a putative EDHF candidate (Archer et al., 2003), had no effect on IRL1620-induced relaxation, suggesting that CYP2C9 metabolites of arachidonic acid could not account for the ETB-mediated relaxation in this vascular bed. The lack of effect of sulfaphenazole is in agreement with the nonsignificant effect of apamin and charybdotoxin, because epoxyeicosatrienoic acids have been reported to exert their effects through activation of BKCa channels on smooth muscle cells (Archer et al., 2003).
We found that 4-AP, but not apamin, glibenclamide or charybdotoxin, reduced IRL1620-induced relaxation, indicating that the activation of KV channels plays a role in ETB-mediated relaxation. Although a rise in Ca2+ is known to occur upon activation of endothelial ETB receptors, Ca2+-activated K+ channels do not participate in this response in the rat carotid, which is in agreement with previous studies in rat isolated extrapulmonary and intrapulmonary arteries (Higashi et al., 1997), and in the perfused lung (Hasunuma et al., 1990; Muramatsu et al., 1999), where activation of KATP channels was described. Our data show that ETB-induced relaxation is reduced by the K+ channel blockers TEA and 4-AP, suggesting that ET-1 may cause membrane hyperpolarization by opening K+ channels as described previously in the rat mesenteric artery (Nakashima & Vanhoutte, 1993a, 1993b).
In summary, the present study shows that rat carotid artery contains a heterogeneous population of contractile ETA and ETB receptors located in the vascular smooth muscle and endothelial ETB receptors that mediated vasorelaxation via NO–cGMP pathway, vasodilator cyclooxygenase product(s) and the activation of KV channels. The study of the expression and function of ET receptors may provide valuable information on the contribution of ETs to the arterial response to injuries since the endothelinergic system has a pathophysiological role in a wide range of diseases.
Acknowledgments
We are most grateful to Professor Lusiane M. Bendhack (Faculty of Pharmaceutical Sciences-USP) for the kind gift of ODQ and Marcio A.F. de Godoy for helpful scientific discussion. Our thanks are also expressed to Mirian C.C. de Melo, Juliana A. Vercesi, Mayara S. Gomes and Eleni L.T. Gomes for their technical assistance. This work was supported by FAPESP (Brazil) and the Canadian Institutes for Health Research Sciences.
Abbreviations
- 4-AP
4-aminopyridine
- BQ123
c(DTrp–Dasp–Pro–Dval–Leu)
- BQ788
[N-cis-2,6-dimethyl-piperidinocarbonyl-L-γ-methylleucyl1-D-1methoxycarbonyltryptophanyl-D-norleucine]
- EDHF
endothelium-derived hyperpolarizing factor
- EDRF
endothelium-derived relaxing factor
- ET-1
endothelin-1
- IRL1620
{succinyl-[Glu9,Ala11,15]-ET-1(8–210}
- L-NAME
NG-nitro-L-arginine methyl ester
- NO
nitric oxide
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- TEA
tetraethylammonium
References
- ARCHER S.L., GRAGASIN F.S., WU X., WANG S., McMURTRY S., KIM D.H., PLATONOV M., KOSHAL A., HASHIMOTO K., CAMPBELL W.B., FALCK J.R., MICHELAKIS E.D. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BKCa channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:282–293. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUGUET M., DELLAFLOTTE S., CHABRIER P.E., BRAQUET P. Characterization of endothelin receptors mediating contraction and relaxation in rabbit saphenous artery and vein. Can. J. Physiol. Pharmacol. 1993;71:818–823. doi: 10.1139/y93-122. [DOI] [PubMed] [Google Scholar]
- BOGONI G., RIZZI A., CALO G., CAMPOBASSO C., D'ORLEANS-JUSTE P., REGOLI D. Characterization of endothelin receptors in the human umbilical artery and vein. Br. J. Pharmacol. 1996;119:1600–1604. doi: 10.1111/j.1476-5381.1996.tb16078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSSEMAKER E., POPP R., FISSLTHALER B., LARSON C.M., FLEMING I., BUSSE R., BRANDES R.P. Aged spontaneously hypertensive rats exhibit a selective loss of EDHF-mediated relaxation in the renal artery. Hypertension. 2003;42:562–568. doi: 10.1161/01.HYP.0000088852.28814.E2. [DOI] [PubMed] [Google Scholar]
- CALÓ G., GRATTON J.P., TÉLÉMAQUE S., D'ORLÉANS-JUSTE P., REGOLI D. Pharmacology of endothelins: vascular preparations for studying ETA and ETB receptors. Mol. Cell. Biochem. 1996;154:31–37. doi: 10.1007/BF00248458. [DOI] [PubMed] [Google Scholar]
- CLARK K.L., PIERRE L. Characterization of endothelin receptors in rat renal artery in vitro. Br. J. Pharmacol. 1995;114:785–790. doi: 10.1111/j.1476-5381.1995.tb13273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ORLÉANS-JUSTE P., LABONTÉ J., BKAILY G., CHOUFANI S., PLANTE M., HONORÉ J.C. Function of the endothelinB receptor in cardiovascular physiology and pathophysiology. Pharmacol. Ther. 2002;95:221–238. doi: 10.1016/s0163-7258(02)00235-8. [DOI] [PubMed] [Google Scholar]
- DE OLIVEIRA A.M., VISWANATHAN M., CAPSONI S., HEEMSKERK F.M.J., CORREA F.M.A., SAAVEDRA J.M. Characterization of endothelinA receptors in cerebral and peripheral arteries of the rat. Peptides. 1995;16:139–144. doi: 10.1016/0196-9781(94)00169-7. [DOI] [PubMed] [Google Scholar]
- DENG L.Y., LI J.S., SCHIFFRIN E.L. Endothelin receptor subtypes in resistance arteries from humans and rats. Cardiovasc. Res. 1995;29:532–535. [PubMed] [Google Scholar]
- DIEYE A.M., GAIRARD A. Endothelium and aortic contraction to endothelin-1 in the pregnant rat. Can. J. Physiol. Pharmacol. 2000;78:372–377. [PubMed] [Google Scholar]
- ELMOSELHI A.B., GROVER A.K. Endothelin contraction in pig coronary artery: receptor types and Ca2+-mobilization. Mol. Cell. Biochem. 1997;176:29–33. [PubMed] [Google Scholar]
- FILEP J., BATTISTINI B., COTÉ Y.P., BEAUDOIN A.R., SIROIS P. Endothelin-1-induced prostacyclin release from bovine aortic endothelial cells. Biochem. Biophys. Res. Commun. 1991;177:171–176. doi: 10.1016/0006-291x(91)91964-e. [DOI] [PubMed] [Google Scholar]
- FRELIN C., GUEDIN D. Why are circulating concentrations of endothelin-1 so low. Cardiovasc. Res. 1994;28:1613–1622. doi: 10.1093/cvr/28.11.1613. [DOI] [PubMed] [Google Scholar]
- FUJITANI Y., UEDA H., OKADA T., URADE Y., KARAKI H. A selective agonist of endothelin type B receptor, IRL1620, stimulates cyclic GMP increase via nitric oxide formation in rat aorta. J. Pharmacol. Exp. Ther. 1993;267:683–689. [PubMed] [Google Scholar]
- GARTHWAITE J., SOUTHAM E., BOULTON C.L., NIELSEN E.B., SCHMIDT K., MAYER B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- HASUNUMA K., RODMAN D.M., O'BRIEN R.F., MCMURTY I.F. Endothelin-1 causes pulmonary vasodilation in rats. Am. J. Physiol. 1990;259:H48–H54. doi: 10.1152/ajpheart.1990.259.1.H48. [DOI] [PubMed] [Google Scholar]
- HAYNES W.G., WEBB D.J. The endothelin family of peptides: local hormones with diverse roles in health and disease. Clin. Sci. 1993;84:485–500. doi: 10.1042/cs0840485. [DOI] [PubMed] [Google Scholar]
- HIGASHI T., ISHIZAKI T., SHIGEMORI K., YAMAMURA T., NAKAI T. Pharmacological characterization of endothelin-induced rat pulmonary arterial dilation. Br. J. Pharmacol. 1997;121:782–786. doi: 10.1038/sj.bjp.0701177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRATA Y., EMORI T., EGUCHI S., KANNO K., IMAI T., OHATA K., MARUMO F. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J. Clin. Invest. 1993;91:1367–1373. doi: 10.1172/JCI116338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IHARA M., NOGUCHI K., SAEKI T., FUKURUDA T., TSUCHIDA S., KIMURA S., FUKAMI T., ISHIKAWA K., NISHIKIBE M., YANO M. Biological profiles of highly potent novel endothelin antagonists selective for ETA receptor. Life Sci. 1992;50:247–255. doi: 10.1016/0024-3205(92)90331-i. [DOI] [PubMed] [Google Scholar]
- IHARA M., SAEKI T., FUNABASHI K., NAKAMICHI K., YANO M., FUKURODA T., MIYAJI M., IKEMOTO M. Two endothelin receptor subtypes in porcine arteries. J. Cardiovasc. Pharmacol. 1991;17 (Suppl. 7):S119–S121. doi: 10.1097/00005344-199100177-00031. [DOI] [PubMed] [Google Scholar]
- ISHIKAWA K., IHARA M., NOGUCHI K., MASE T., MINO N., SAEKI T., FUKURUDA T., FUKAMI T., OZAKI S., NAGASE T., NISHIKIBE M., YANO M. Biochemical and pharmacological profile of a newly-developed potent and selective endothelin B receptor antagonist, BQ-788. Proc. Natl. Acad. Sci. U.S.A. 1994;20:4892–4896. doi: 10.1073/pnas.91.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENAKIN T.P. The mesurement of antagonist potency and the importance of selective inhibition of agonist uptake processes. J. Pharmacol. Exp. Ther. 1981;219:112–120. [PubMed] [Google Scholar]
- KENAKIN T.P. Tissue response as a functional discriminator of receptor heterogeneity: effects of mixed receptor populations on Schild regressions. Mol. Pharmacol. 1992;41:699–707. [PubMed] [Google Scholar]
- MATSUDA H., BEPPU S., OHMORI F., YAMADA M., MIYATAKE K. Involvement of cyclo-oxygenase-generated vasodilating eicosanoid(s) in addition to nitric oxide in endothelin-1 induced endothelium-dependent vasorelaxation in guinea pig aorta. Heart Vessels. 1993;8:121–127. doi: 10.1007/BF01744796. [DOI] [PubMed] [Google Scholar]
- MURAMATSU M., OKA M., MORIO Y., SOMA S., TAKAHASHI H., FUKUCHI Y. Chronic hypoxia augments endothelin-B receptor-mediated vasodilation in isolated perfused rat lungs. Am. J. Physiol. 1999;276:L358–L364. doi: 10.1152/ajplung.1999.276.2.L358. [DOI] [PubMed] [Google Scholar]
- NAKASHIMA M., VANHOUTTE P.M. Endothelin-1 and -3 cause endothelium-dependent hyperpolarization in the rat mesenteric artery. Am. J. Physiol. 1993a;265:H2137–H2141. doi: 10.1152/ajpheart.1993.265.6.H2137. [DOI] [PubMed] [Google Scholar]
- NAKASHIMA M., VANHOUTTE P.M. Age-dependent decrease in endothelium-dependent hyperpolarizations to endothelin-3 in the rat mesenteric artery. J. Cardiovasc. Pharmacol. 1993b;22 (Suppl 8):S352–S354. doi: 10.1097/00005344-199322008-00092. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- RIEZEBOS J., WATTS I.S., VALLANCE P.J. Endothelin receptors mediating functional responses in human small arteries and veins. Br. J. Pharmacol. 1994;111:609–615. doi: 10.1111/j.1476-5381.1994.tb14780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBANYI G.M., POLOKOFF M.A. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol. Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- SCHILLING L., FEGER G.I., EHRENREICH H., WHAL M. Endothelin-3-induced relaxation of isolated rat basilar artery is mediated by an endothelial ETB-type endothelin receptor. J. Cereb. Blood Flow Metab. 1995;15:699–705. doi: 10.1038/jcbfm.1995.86. [DOI] [PubMed] [Google Scholar]
- SCHOEFFTER P., RANDRIANTSOA A., JOST B., BRUTTEL K. Comparative effects of the two endothelin ETA receptor antagonists, BQ123 and FR139317, on endothelin-1-induced contraction in guinea pig iliac artery. Eur. J. Pharmacol. 1993;241:165–169. doi: 10.1016/0014-2999(93)90198-q. [DOI] [PubMed] [Google Scholar]
- SHARIFI A.M., SCHIFFRIN E.L. Endothelin receptors mediating vasoconstriction in rat pressurized small arteries. Can. J. Physiol. Pharmacol. 1996;74:934–939. [PubMed] [Google Scholar]
- SOKOLOVSKY M., AMBAR I., GARLON R. A novel subtype of endothelin receptors. J. Biol. Chem. 1992;267:20551–20554. [PubMed] [Google Scholar]
- TAKAI M., UMEMURA K., YAMASAKI K., WATANABE T., FUJITANI Y., ODA K., URADE Y., INUI T., YAMAMURA T., OKADA T. A potent and specific agonist, Suc-[Glu9 Ala11,15]-endothelin-1 (8-21), IRL 1620, for ETB receptor. Biochem. Biophys. Res. Commun. 1992;184:953–959. doi: 10.1016/0006-291x(92)90683-c. [DOI] [PubMed] [Google Scholar]
- VISWANATHAN M., DE OLIVEIRA A.M., JOHREN O., SAAVEDRA J.M. Increased endothelin ET(A) receptor expression in rat carotid arteries after balloon injury. Peptides. 1997;18:247–255. doi: 10.1016/s0196-9781(96)00285-9. [DOI] [PubMed] [Google Scholar]
- WANG X., DOUGLAS S.A., FEUERSTEIN G.Z., OHLSTEIN E.H. Temporal expression of ECE-1, ET-1, ET-3, ETA, and ETB receptor mRNAs after balloon angioplasty in the rat. J. Cardiovasc. Pharmacol. 1995;26 (Suppl 3):S22–S25. [PubMed] [Google Scholar]
- WANG X., DOUGLAS S.A., LOUDEN C., VICKERY-CLARK L.M., FEUERSTEIN G.Z., OHLSTEIN E.H. Expression of endothelin-1, endothelin-3, endothelin-converting enzyme-1, and endothelin-A and endothelin-B receptor mRNA after angioplasty-induced neointimal formation in the rat. Circ. Res. 1996;78:322–328. doi: 10.1161/01.res.78.2.322. [DOI] [PubMed] [Google Scholar]
- WHITE D.G., GARRAT H., MUNDIN J.W., SUMNER M.J., VALLANCE P.J., WATTS I.S. Human saphenous vein contains both endothelin ETA and ETB contractile receptors. Eur. J. Pharmacol. 1994;257:307–310. doi: 10.1016/0014-2999(94)90144-9. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA M., KURIHARA K., KIMURA S., TOMOBE Y., KOBAYASHI M., MITSUI Y., GOTO K., MASAKI T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- ZELLERS T.M., MCCORMICK J., WU Y. Interaction among ET-1, endothelium-derived nitric oxide, and prostacyclin in pulmonary arteries and veins. Am. J. Physiol. 1994;267:H139–H147. doi: 10.1152/ajpheart.1994.267.1.H139. [DOI] [PubMed] [Google Scholar]