Abstract
S 21403 (mitiglinide) is a new drug for type 2 diabetes mellitus (T2DM). Its action on insulin release and biosynthesis was investigated in several experimental systems utilizing pancreas from normal and T2DM animals.
At high concentrations (10 μM), S 21403, like classical sulphonylurea, induced insulin release in the absence of glucose. In contrast, at therapeutic (0.1–1.0 μM) concentrations, S 21403 amplified insulin secretion glucose dose-dependently and with similar magnitude in normal and diabetic GK rat islets.
In perfused GK rat pancreas, S 21403 induced normal kinetics of insulin secretion including first-phase response.
The effect of S 21403 was strongly modulated by physiological factors. Thus, 0.1 μM adrenaline inhibited S 21403-induced insulin release. There was marked synergism between S 21403 and arginine in GK rat islets, combination of the two normalizing insulin secretion.
In primary islet cultures from normal rats or prediabetic Psammomys obesus, prolonged exposure to S 21403 did not induce further depletion of insulin stores under normal or ‘glucotoxic' conditions.
Proinsulin biosynthesis was not affected by 2-h exposure of rat or prediabetic P. obesus islets to 1 μM S 21403. Yet, 24-h exposure of rat islets to S 21403 resulted in 30% increase in proinsulin biosynthesis at 8.3 mM glucose.
Amplification by S 21403 of glucose-induced insulin secretion in diabetic GK β-cells with restoration of first-phase response, a strong synergistic interaction with arginine and marked inhibition by adrenaline, make it a prime candidate for successful oral antidiabetic agent.
Keywords: S 21403, mitiglinide, β-cell, type 2 diabetes, insulin release, insulin biosynthesis, oral antidiabetics
Introduction
In type 2, noninsulin-dependent diabetes mellitus (T2DM), both insulin resistance and deficient insulin secretion are present. Although the precise aetiologic role of the pancreatic vs peripheral defect is a matter of controversy, it is now increasingly accepted that T2DM does not develop unless insulin secretion is impaired (Cerasi, 1995; Ferrannini et al., 2003). At all stages of T2DM, first-phase insulin response to glucose is grossly deficient. However, also the global magnitude of the release is reduced, which progresses with the severity of the disease. Despite these limitations in the function of the diabetic β-cell, insulin release can still be sufficiently stimulated to allow improvement of the hyperglycaemia by insulin-releasing agents, such as sulphonylureas.
S 21403 (mitiglinide, KAD-1229), a succinic acid derivative, is a short-acting insulin secretagogue that does not belong to the sulphonylurea family (Ohnota et al., 1995). However, it shares with it the ability to close the β-cell ATP-dependent K+channels (K+ATP) (Reimann et al., 2001; Sunaga et al., 2001), thus depolarising the cell and eliciting calcium influx, which leads to exocytosis of insulin. Effects on K+ATP channels, shared with glucose, lead only to limited insulin secretion; the full physiological glucose effect requires additional actions distal to closure of K+ATP channels (involving the PKA and PKC pathways, as well as other unidentified mechanisms), which amplify the release up to its full magnitude (Henquin, 2004). Whether S 21403 shares with glucose such actions is not known.
The diabetic environment leads to depletion of the insulin content of the β-cell in many animal models of T2DM, as well as in human islets (Gadot et al., 1994; Marshak et al., 1999). Reduced insulin content is a further limiting factor for insulin secretion; therefore, inability of (pro)insulin biosynthesis to keep pace with insulin release may be an important contributing factor in the pathogenesis of T2DM.
In the present report, we describe in detail the insulin secretory action of S 21403 in islets derived from normal and T2DM animals under a variety of conditions. To this end, we used isolated, batch-incubated islets from normal Wistar rats, diabetic GK rats and prediabetic Psammomys obesus. We also generated in vitro chronic hyperglycaemic conditions (‘glucose toxicity') using cultured islets, and evaluated the role that chronic exposure to S 21403 may play in this context. Furthermore, in perfused pancreas preparations from normal as well as diabetic GK rats, we analysed the kinetic aspects of S 21403-induced insulin release. Sulphonylureas have been shown either to be neutral or inhibit insulin biosynthesis (Schatz et al., 1975; Hakan Borg & Andersson, 1981); we have also analysed the action of S 21403 on the insulin biosynthetic rate of islets.
Methods
Animals
For all the studies, permission was obtained from the institutional Animal Study Ethical Committees. In addition to normal Wistar rats, two animal models of T2DM were employed:
GK rats: The colony at the Karolinska Hospital exhibits hyperglycaemia from weaning. T2DM in this model is accompanied by deficient insulin output and hypoinsulinaemia, with normal peripheral sensitivity to insulin (Ostenson et al., 1993; Abdel-Halim et al., 1998). Animals remain moderately diabetic throughout life, with blood glucose levels in the 8–12 mM range.
P. obesus: The colony at the Hebrew University has been extensively characterised (Gadot et al., 1994; Donath et al., 1999; Leibowitz et al., 2001). In contrast to the GK rat, and in spite of the fact that its obesity is mild, this gerbil (nicknamed sand rat) is severely insulin resistant. When presented with a calorie-rich diet, it develops hyperglycaemia within 4–5 days (Nesher et al., 1999), reaching blood glucose levels around 15 mM, which may progress to severe ketotic diabetes after a few weeks (Shafrir & Ziv, 1998). Islet insulin content is rapidly depleted with the onset of hyperglycaemia (Donath et al., 1999).
Islet insulin secretion studies
Animals were anaesthetised with pentobarbital (100 mg kg−1, rats) or ketamine (200 mg kg−1, P. obesus), and the pancreas rapidly removed. When islets were used for batch incubations, the pancreas was digested with collagenase and islets separated from exocrine and other nonislet components by repetitive washes and handpicking under a stereomicroscope; they were then transferred to culture dishes containing RPMI 1640 medium and 10% calf serum, and incubated overnight at 37°C to reduce β-cell membrane damage induced during isolation. Thereafter, they were preincubated for 30 min at 37°C in Krebs–Ringer bicarbonate buffer (KRB) containing 3.3 mM glucose and 2 mg ml−1 bovine serum albumin (BSA), then three islets each were transferred to the experimental incubations in triplicate (60 min at 37°C) in 300 μl KRB containing the desired concentrations of glucose, S 21403, arginine or adrenaline (and combinations thereof). At the end of the incubation, the supernatant was saved at −20°C for insulin determination.
For long-term islet culture, the pancreas was digested by collagenase and islets isolated manually; all procedures thereafter were conducted under sterile conditions in a tissue culture hood. Islets were washed with RPMI 1640 containing 5.5 mM glucose and antibiotics (100 U ml−1 penicillin and 100 μg ml−1 streptomycin), followed by suspension in test medium with antibiotics and 10% foetal bovine serum (FBS). Approximately 50 islets suspended in 10 ml test medium were maintained for 1 week at 37°C under 5% CO2 in air in 100 mm bacteriological plates that do not allow cell attachment (Miniplast, Ein Shemer, Israel). In normal rat and prediabetic P. obesus islets, conditions that create a glucotoxic situation were tested, with and without the addition of 1 μM S 21403. In the case of rat islets, the control condition under chronic culture necessitates a relatively high glucose concentration (8.3 mM) to prevent β-cell apoptosis, whereas glucose toxicity was induced by medium containing 33 mM glucose (Hoorens et al., 1996; Van de Casteele et al., 2003). In P. obesus islets, glucose toxicity appears already at 8.3 mM glucose; therefore, cultures were maintained either at 5.5 (control) or 33 mM glucose for 1 week (Donath et al., 1999). At the end of the culture period, the islets were washed and preincubated for 1 h in KRB buffer with 1.7 mM glucose to obtain a basal state, then incubated at either 3.3 or 16.7 mM glucose in batches of five islets during 1 h to test insulin response capacity. At the end of incubations at 3.3 mM glucose, the islets were collected, disrupted by repeated freeze/thaw cycles and insulin extracted in acid-alcohol. To determine islet insulin content at the end of the culture period, insulin in the islet extract and the amount secreted during the 1-h incubation were added.
Proinsulin biosynthesis
To evaluate the short-term effect of S 21403 on proinsulin biosynthesis, islets were preincubated for 1 h at 37°C in KRB–BSA buffer containing 3.3 mM glucose, followed by 2-h incubation in KRB–BSA containing either 1.7 or 8.3 mM glucose with and without 1 μM S 21403. Groups of 25 islets were then pulsed with 25 μCi L-[4,5-3H]leucine (150 Ci mmol−1; Amersham, Aylesbury, U.K.) for 15 min at 37°C in the same incubation buffer. Leucine incorporation was terminated by the addition of 1 ml ice-cold glucose-free KRB–BSA buffer and rapid centrifugation. The islet pellet was suspended in 450 μl of immunoprecipitation buffer (0.2 M glycine, 0.1% RIA grade BSA, 0.5% NP-40, pH 8.8), and the incorporation of tritiated leucine into newly synthesized (pro)insulin determined in 50 μl aliquots by immunoprecipitation using anti-insulin serum (Sigma, Rehovot, Israel) and protein A Sepharose (Sigma). Each sample (50 μl) was pretreated with protein A Sepharose prior to immunoprecipitation with the anti-insulin serum to correct for nonspecific binding. This procedure was validated by HPLC (Gross et al., 1996). Aliquots were used to determine total protein biosynthesis by trichloroacetic acid precipitation, as described (Alarcon et al., 1993). A similar experimental protocol was used to determine the long-term action of S 21403, except that the islets were incubated in RPMI medium containing 10% FBS and 1.7 or 8.3 mM glucose with and without S 21403 for 22 h, followed by 2 h of incubation in KRB–BSA buffer containing the same glucose and S 21403 concentrations, and 15 min pulse, as above.
Pancreas perfusion
Rats were anaesthetised (pentobarbital, 100 mg kg−1), the aorta was canulated, and after separation from surrounding tissues, the isolated pancreas was placed in a 37°C chamber and perfused with KRB buffer through the aorta at the rate of 2.8 ml min−1. Pancreata (five to six per condition) were first perfused with KRB containing 3.3 mM glucose (−40 to −10 min); the effect of the basal glucose concentration was then investigated during the last 10 min (−20 to −10 min), followed by a 40-min perfusion (−10 to 30 min) with various glucose concentrations, as indicated in the individual experiments, without or with different concentrations of S 21403 (0.1, 1 or 10 μM) introduced between 0 and 30 min through a side canula linked to an infusion pump. The perfusate was collected from the portal vein at frequent intervals, and stored at −20°C for insulin assay.
Insulin assays
Insulin was measured by standard RIA. Rat insulin standard (Novo-Nordisk, Bagsvaerd, Denmark) was used for studies with Wistar and GK rats. The single insulin of P. obesus is closer to human than rat/mouse insulins (Kaiser et al., 1997); in RIA P. obesus insulin gives dilution curves that are parallel to human but not rat insulin standards (Gross et al., 1996), therefore the former was used (Novo-Nordisk). The intra- and interassay CV were 1.2 and 2.3–2.8%, respectively. The minimal detectable concentration was 3.9 μU ml−1 in the rat insulin assay and 22 pM in the P. obesus assay. Insulin secretion data were expressed as secretion rate (μU min−1) in experiments employing the perfused pancreas, and as cumulative secretion (μU islet−1) in the 1-h static incubations. The biological efficiency of P. obesus insulin is not known; therefore, results were expressed in molar rather than biological units.
Statistical analysis
Nonparametric Wilcoxon or Mann–Whitney rank test was used to determine significance where groups of data were compared. In the proinsulin biosynthesis studies, samples with and without S 21403 incubated under similar conditions were analysed by Student's t-test. Data were evaluated using the InStat statistical software from GraphPad Software, San Diego, CA, U.S.A. Significance level was set at P<0.05. Results are presented as mean±s.e.m.
Results
Modulation of glucose-induced insulin secretion by S 21403
Batch-incubated normal islets
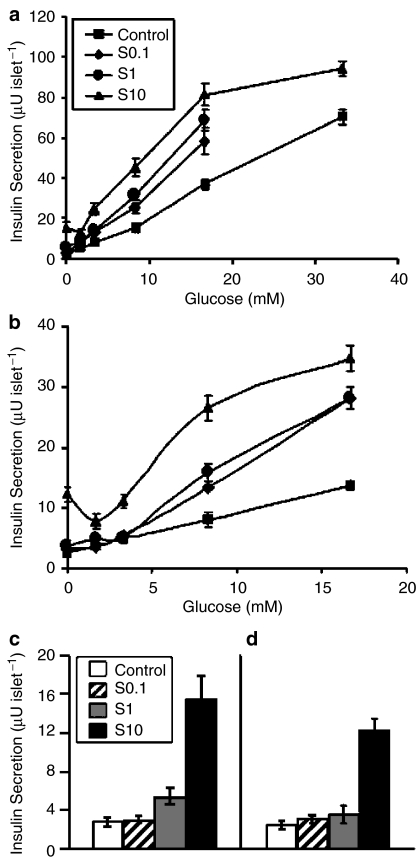
The interaction between glucose and S 21403 on insulin secretion during 60-min incubations is illustrated in Figure 1. In normal islets (Figure 1a and c), high concentrations of S 21403 (10 μM) acted like classical sulphonylurea, a highly significant effect on insulin secretion being registered in the absence of glucose (P<0.0001 vs control), or in the presence of subthreshold concentrations of the hexose (1.7 and 3.3 mM). In fact, the release induced by S 21403 in the absence of glucose (15.5±2.33 μU islet−1) was equivalent to the effect of 8.3 mM glucose (15.5±2.22 μU islet−1). At a near-maximally stimulatory glucose concentration (16.7 mM), the drug effect did not diminish, as is usually the case with sulphonylurea; instead, glucose-induced insulin secretion was markedly amplified (81.5±5.59 vs 37.4±2.47 μU islet−1 for S 21403 and control, respectively). The stimulatory effect of 10 μM S 21403 started to taper off only at the very high glucose concentration of 33 mM (94.1±3.5 vs 69.5±3.5 μU islet−1 for S 21403 and control, respectively). At lower S 21403 concentrations (0.1 and 1 μM), no significant effect was observed on insulin secretion in the absence or presence of 1.7 mM glucose (P=0.3–0.6). At basal glucose (3.3 mM), these concentrations of S 21403 had minimal effect on insulin release (12.6±2.54 vs 8.3±1.06 μU islet−1, P=0.3 for 0.1 μM S 21403; 14.0±1.72 vs 8.3±1.06 μU islet−1, P=0.001 for 1 μM S 21403). At stimulatory glucose concentrations (8.3 and 16.7 mM), S 21403 strongly amplified glucose-induced insulin secretion (1.6-fold for 0.1 μM S 21403 at both glucose concentrations; 2.0- and 1.8-fold for 1 μM S 21403 at 8.3 and 16.7 mM glucose, respectively).
Figure 1.
Interaction between glucose and S 21403 on insulin secretion. Islets of Langerhans isolated from normal Wistar rats (panels a and c) or diabetic GK rats (panels b and d) were incubated for 1 h in the absence or presence of various concentrations of glucose without (Control) or with the addition of 0.1, 1 or 10 μM of S 21403 (S). Panels c (Wistar rat) and d (GK rat) show islets incubated with different concentrations of S 21403 in the absence of glucose. Note difference of scale in Y-axis. Results are the mean of nine to 14 incubations in triplicate; the vertical bars show±s.e.m. (when not shown, the s.e.m. was smaller than the symbol).
Batch-incubated islets from diabetic GK rats
As expected, islets from diabetic GK rats had low insulin response to glucose (Figure 1b; note scale difference from Figure 1a). Nevertheless, the effect of S 21403 was otherwise similar to that observed in nondiabetic islets. Thus, 0.1 and 1 μM S 21403 had no effect on insulin secretion at 0–3.3 mM glucose (P=0.19–0.95), while 10 μM S 21403 was strongly stimulatory (Figure 1d; P=0.0038 to <0.0001). At stimulatory glucose (8.3 and 16.7 mM), all drug concentrations significantly amplified glucose-induced insulin secretion (P=0.015 to <0.0001). It is noteworthy that the amplification of glucose-induced insulin release by S 21403 in GK islets (1.6- to 3.3-fold according to incubation conditions) was quantitatively similar to that in normal Wistar islets (1.6- to 2.9-fold).
Perfused pancreas in normal rats
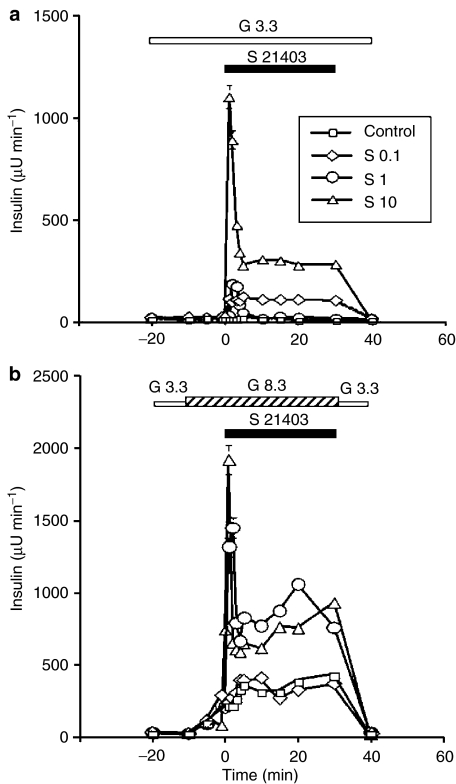
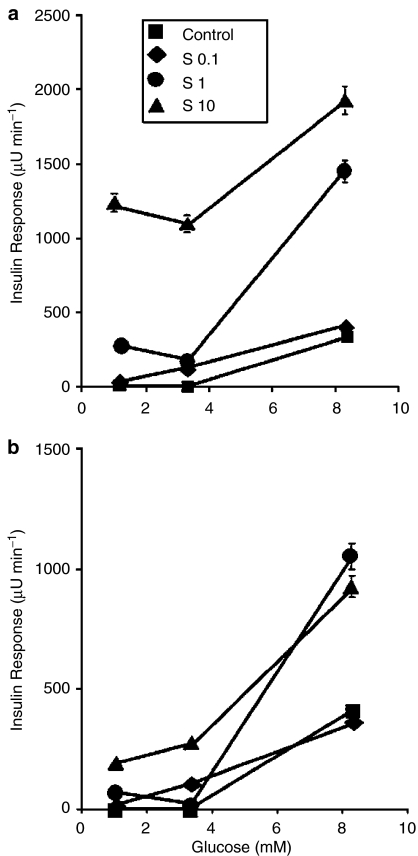
The kinetics of insulin release from the perfused rat pancreas stimulated by S 21403 under various ambient glucose concentrations is presented in Figure 2. At the nonstimulatory glucose concentrations of 1 mM (not shown) and 3.3 mM (Figure 2a), 0.1 μM S 21403 elicited no or minimal insulin release, while 1 and 10 μM S 21403 induced a moderate or substantial first-phase insulin response, respectively. A clear second-phase release was evident at the high S 21413 concentration of 10 μM. At 8.3 mM glucose, the effect of 0.1 μM S 21403 was not different from that of glucose alone (Figure 2b); in contrast, both 1 and 10 μM S 21403 strongly amplified glucose-induced first- and second-phase insulin responses. The glucose–S 21403 relationship is better visualised in the dose–response curves presented in Figure 3a and b. Peak first-phase insulin response (Figure 3a) induced by 8.3 mM glucose was 4.1-fold augmented by 1 μM S 21403; increasing drug concentration 10-fold (10 μM) had limited additional effect (5.5-fold; difference between peak values, P=0.1). More striking is the difference at lower glucose concentrations: the effect of 10 μM S 21403 at 1 mM glucose was as large as that of 1 μM drug at 8.3 mM glucose (Figure 3a). Second-phase insulin responses followed a similar pattern; 1 and 10 μM S 21403 had now similar amplifying effects on secretion induced by 8.3 mM glucose (Figure 3b). Plotting the area under the curve of first- and second-phase insulin responses, instead of the respective peak values, did not yield significantly different information (not shown).
Figure 2.
Effect of S 21403 on the kinetics of insulin release. The isolated pancreas of Wistar rats was perfused with 3.3 mM glucose throughout the experiment in panel a, while in panel b, the glucose concentration was raised to 8.3 mM between −10 and 30 min. Different μM concentrations of S 21403 (S) were added to the perfusate between 0 and 30 min. Control denotes perfusion with glucose alone. Mean±s.e.m. of five to six experiments for each condition; where not shown, the s.e.m. was smaller than the symbol.
Figure 3.
Modulation by S 21403 of the dose–response relationships for glucose-induced first-phase (panel a) and second-phase (panel b) peak insulin responses. Part of the data derived from the experiments illustrated in Figure 2. Control denotes perfusion with glucose alone, S stands for S 21403. Mean±s.e.m. of five to six experiments; where not shown, the s.e.m. was smaller than the symbol.
Perfused pancreas in GK diabetic rats
When pancreata of diabetic GK rats were perfused with 8.3 mM glucose for 30 min following a 30 min basal perfusion at 3.3 mM glucose, no significant insulin response was obtained (not shown). As seen in Figure 4a, 1 μM S 21403 added 10 min after the initiation of the 8.3 mM glucose perfusion induced a sharp but modest (∼100 μU min−1) peak of insulin release, which was not followed by second-phase response. At 10 μM, the drug elicited a marked and biphasic insulin response in the presence of 8.3 mM glucose (Figure 4b), similar to that induced by 8–10 mM glucose in the normal rat pancreas, except for the somewhat lower second-phase response.
Figure 4.
Induction of insulin secretion by S 21403 in pancreas isolated from diabetic GK rats. The pancreas was perfused with 8.3 mM glucose between −10 and 30 min. In panel a, 1 μM S 21403 was introduced between 0 and 30 min, while in panel b, the concentration of S 21403 was 10 μM. Mean±s.e.m. of five to six experiments; where not shown, the s.e.m. was smaller than the symbol.
Physiologic modulation of S 21403-induced insulin secretion
Stimulatory effects of arginine
Under physiological conditions, the β-cell is never subjected to the exclusive effect of glucose. Especially during meals, the pancreas is stimulated also by amino acids. To mimic these conditions, we evaluated the effect of arginine on S 21403-induced insulin secretion.
Table 1 presents the effect of 20 mM arginine on islets isolated from normal (Wistar) and diabetic (GK) rats. Arginine had a significantly weaker stimulatory effect on GK islets, indicating that the β-cell secretory defect in this diabetes model is not restricted to glucose. S 21403 (10 μM) had substantial effects on insulin release in both types of islets, with similar amplification of 4.5- and five-fold in GK and Wistar rat islets, respectively. In normal islets, simultaneous stimulation with arginine and S 21403 had only an additive effect; in contrast, a strong synergism was obtained between the two agents in GK islets, the insulin secretion rate induced by arginine+S 21403 being nearly four-fold that of arginine alone, or 2.7-fold of the sum of the effects of arginine and S 21403 used separately. Of interest in the context of therapeutic use, S 21403+arginine-induced insulin secretion in these diabetic islets was of the same magnitude as that registered in normal islets (P=0.47).
Table 1.
Effect of arginine on S 21403-induced insulin secretion from isolated islets
| Insulin secretion (μU islet−1 h−1) | Wistar rats | GK rats |
|---|---|---|
| No addition | 1.88±0.27 | 0.96±0.16 |
| Arginine (20 mM) | 35.1±2.51 | 10.7±0.53** |
| S 21403 (10 μM) | 9.4±0.66 | 4.5±0.49** |
| Arginine+S 21403 | 43.4±3.28* | 40.6±2.0§ |
Results of 11 to 14 incubations in triplicate. All incubations in the presence of 3.3 mM glucose (60 min); mean±s.e.m.
P=0.2 vs arginine alone.
P<0.0001 vs Wistar rats.
P<0.0001 vs arginine alone.
Inhibitory effect of adrenaline
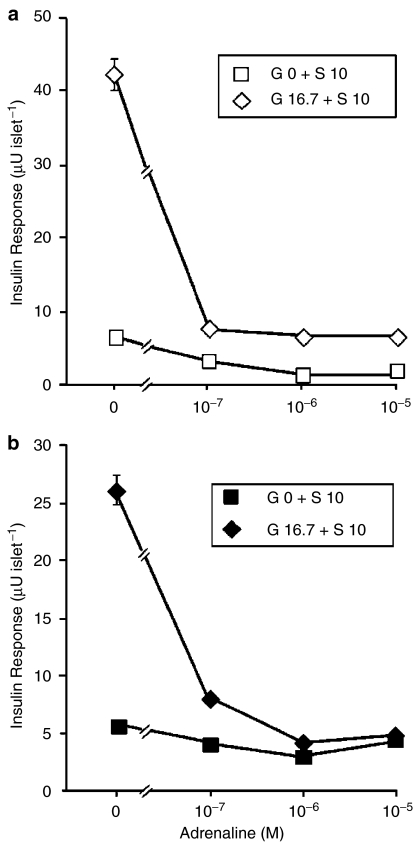
The ability of the physiological inhibitor of the β-cell, adrenaline, to suppress S 21403-induced insulin secretion was evaluated both in normal islets and islets isolated from diabetic GK rats. Figure 5a presents the results obtained in normal islets incubated for 1 h in the absence and presence of 16.7 mM glucose with 10 μM S 21403 and exposed to 10−7 to 10−5 M adrenaline. It is clearly seen that adrenaline is a strong inhibitor of S 21403-induced insulin release, maximal inhibition of 75–85% being observed with 10−7 to 10−6 M adrenaline, even in the absence of glucose; IC50 was 10−7 M at 0 glucose, and substantially below that concentration in the presence of 16.7 mM glucose. Thus, S 21403-induced insulin release seems to be very sensitive to inhibition by adrenaline in normal rat islets.
Figure 5.
Inhibition of the effect of S 21403 by adrenaline. Islets from normal Wistar rats (a) or diabetic GK rats (b) were incubated for 1 h with 10 μM S 21403 (S 10) and different concentrations of adrenaline in the absence (G 0) or the presence of 16.7 mM glucose (G 16.7). Mean±s.e.m. of five experiments in triplicate; where not shown, the s.e.m. was smaller than the symbol.
Similar experiments were performed in islets isolated from GK diabetic rats; results are presented in Figure 5b. Again, adrenaline markedly inhibited insulin secretion, especially in the presence of high glucose (83%); however, the sensitivity of the GK islet to the adrenergic agent seems lower, since the maximal inhibitory effect at high glucose was obtained with 10−6 vs 10−7 M adrenaline in GK and Wistar rat islets, respectively. Nevertheless, taking into consideration the high dose of S 21403 used, also in GK islets adrenaline seems to be quite efficient in suppressing insulin release.
Effect of chronic exposure to S 21403 and glucose on insulin secretion
To evaluate the chronic effect of S 21403, normal rat islets and islets isolated from prediabetic, normoglycaemic P. obesus were cultured, and exposed for 1 week to S 21403.
Normal islets
Table 2 shows that when rat islets were cultured for 1 week at the (for this system) physiological glucose concentration of 8.3 mM, their subsequent acute (1 h) responsiveness to 16.7 mM glucose was fully retained, with insulin secretion being stimulated more than 15-fold over the basal rate. Addition of 1 μM S 21403 to the medium during the 1-week culture did not deplete the islet insulin content, and the response to the subsequent 1-h glucose challenge was unmodified. Elevation of the culture glucose concentration to 33 mM had a dramatic effect on the β-cell: insulin content dropped by 60% (P=0.02), basal insulin release during the 1-h incubation rose 20-fold and the stimulatory effect of 16.7 mM glucose was obliterated. Under these glucotoxic conditions, the islets secreted more than 20% of their insulin content during 1 h compared to 7–8% at 16.7 mM glucose and 0.4% at 3.3 mM glucose in control cultures. Again, addition of 1 μM S 21403 to 33 mM glucose during the culture did not alter these findings (Table 2).
Table 2.
Chronic effects of S 21403 and glucose in normal rat islets
| Culture condition | G 8.3 | G 8.3+S | G 33 | G 33+S |
|---|---|---|---|---|
| Islet insulin contenta (μU islet−1) | 1515±269 | 1874±63.5 | 612±26* | 656±71.5** |
| Secretion at G 3.3b (μU islet−1 h−1) | 6.8±1 | 8.8±0.6 | 135.2±21 | 157.5±17.4 |
| Secretion at G 16.7b (μU islet−1 h−1) | 119.2±13.1 | 132.8±25.5 | 134±12 | 115.6±22.6 |
Results are mean±s.e.m. of four culture plates in glucose alone (G, 8.3 or 33 mM) or with the addition of 1 μM S 21403 (S) for 1 week.
Islet insulin content was determined after the culture period and following the 1-h acute incubation at 3.3 mM glucose; the values shown are islet content+the insulin secreted during the 1-h incubation.
The 1-h incubation in 3.3 or 16.7 mM glucose at the end of the culture period (see Methods).
P=0.016 vs G 8.3.
P=0.022 vs G 8.3+S.
Islets of prediabetic, normoglycaemic P. obesus
Table 3 presents the effect of a 1-week culture in the presence of 5.5 mM glucose; even under these conditions, the P. obesus islet insulin content was markedly reduced. On subsequent 1-h incubations, the insulin response to 16.7 mM glucose was only 50% higher than basal (NS). Adding 1 μM S 21403 to the culture improved somewhat this response (two-fold), which however failed to reach significance (P=0.07); the drug did not lead to further depletion of the islet insulin content. When P. obesus islets were cultured at 33 mM glucose, islet insulin content was diminished further by more than 50% compared to cultures at 5.5 mM glucose (P=0.0019), and the acute response to 16.7 mM glucose was obliterated. S 21403 improved somewhat the islet insulin content (P=0.0506), and the acute response to 16.7 mM glucose was significantly higher in absolute terms than that registered in cultures without S 21403 (P=0.0096); nevertheless, this was not higher than the basal secretion.
Table 3.
Chronic effects of S 21403 and glucose in islets from prediabetic P. obesus
| Culture condition | G 5.5 | G 5.5+S | G 33 | G 33+S |
|---|---|---|---|---|
| Islet insulin contenta (pmol islet−1) | 1.05±0.21 | 0.87±0.12 | 0.40±0.02* | 0.48±0.02** |
| Secretion at G 3.3b (pmol islet−1 h−1) | 0.092±0.024 | 0.057±0.001 | 0.124±0.009 | 0.0116±0.009 |
| Secretion at G 16.7b (pmol islet−1 h−1) | 0.133±0.026† | 0.118±0.028‡ | 0.032±0.003 | 0.052±0.004§ |
Results are mean±s.e.m. of four culture plates in glucose alone (G, 5.5 or 33 mM) or with the addition of 1 μM S 21403 (S) for 1 week.
Islet insulin content was determined after the culture period and following the 1-h acute incubation at 3.3 mM glucose; the values shown are islet content+the insulin secreted during the 1-h incubation.
The 1-h incubation in 3.3 or 16.7 mM glucose at the end of the culture period (see Methods).
P=0.0019 vs G 5.5.
P=0.0506 vs G 33.
P=0.3 vs G 3.3.
P=0.07 vs G 3.3 in G 5.5+S.
P=0.0096 vs G 16.7 in G 33.
Effect of S 21403 on proinsulin biosynthesis
Exposure of rat and P. obesus islets for 2 h to 8.3 mM glucose resulted in 14.6±1.28- and 10.5±2.38-fold stimulation of proinsulin biosynthesis relative to islets at 1.7 mM glucose (Figures 6a and 7a, respectively). Rat islets preserved their response to glucose (8.6±1.16-fold) under the longer incubation time of 24 h, whereas islets from P. obesus markedly reduced the response (1.8±0.22-fold) (Figures 6b and 7b). This was mostly due to increased biosynthesis at 1.7 mM glucose, whereas the stimulatory effect of glucose was preserved. While 1 μM S 21403 did not modify glucose-induced proinsulin biosynthesis at 2 h, it further improved the glucose responsiveness in rat islets following 24-h exposure (Figure 6b); thus, the effect of 8.3 mM glucose was augmented 1.5±0.13-fold (P<0.01), and a tendency for increase was also observed at 1.7 mM glucose (2.1±0.39-fold, P=0.08). No such effects could be detected in P. obesus islets (Figure 7a and b). No effect on total protein biosynthesis was observed in either rat or P. obesus islets exposed to 1 μM S 21403 for 2 or 24 h (not shown).
Figure 6.
Proinsulin biosynthesis in Wistar rat islets. Islets were incubated for 2 h (a) or 24 h (b) with 1.7 or 8.3 mM glucose (G 1.7 and G 8.3, respectively) in the absence (Control, open columns) or presence (Test, filled columns) of 1 μM S 21403. To evaluate the rate of proinsulin biosynthesis, leucine incorporation into islet insulin-like material is shown (see Methods for details). Mean±s.e.m. of three to four experiments, each using islets pooled from three to four rats.
Figure 7.
Proinsulin biosynthesis in islets from prediabetic, normoglycaemic P. obesus. Islets were incubated for 2 h (a) or 24 h (b) with 1.7 or 8.3 mM glucose (G 1.7 and G 8.3, respectively) in the absence (Control, open columns) or presence (Test, filled columns) of 1 μM S 21403. To evaluate the rate of proinsulin biosynthesis, leucine incorporation into islet insulin-like material is shown (see Methods for details). Mean±s.e.m. of 4 experiments, each using islets pooled from 2 animals.
Discussion
It is accepted that T2DM does not manifest itself unless insulin secretion decreases markedly. It is therefore logical from a pathophysiological viewpoint to use agents that stimulate the release of insulin, and this has indeed been standard policy since the 1950s when sulphonylureas were introduced. Over recent years, a new family of insulin releasers, including the pharmacological class of glinides, has been introduced to improve the pharmacodynamic profile obtained with sulphonylureas, their onset of action being more rapid and of shorter duration, thus better mimicking the physiological insulin response to meals (Owens et al., 2002; Standl & Fuchtenbusch, 2003). S 21403 is a new member of this family. Although these molecules bear no chemical resemblance to sulphonylureas, they elicit insulin release by a similar mechanism, that is, closure of the β-cell K+ATP channel, depolarization of the cell, influx of calcium and, hence, exocytosis of insulin (Reimann et al., 2001; Sunaga et al., 2001).
Closure of the K+ATP channel corresponds to the first step in stimulus–secretion coupling when a β-cell is challenged with glucose; therefore, sulphonylureas fail to show effect on insulin secretion when the β-cell is maximally stimulated with glucose. The S 21403 concentrations measured in diabetic patients range from 0.01 to 2 μM and from 0.02 to 3.9 μM at the therapeutic doses of 10 and 20 mg t.i.d., respectively (Breuel, 2004; Guillausseau, 2004). In the present study, S 21403 concentrations that were within the therapeutic range (0.1 and 1 μM) did not induce insulin secretion in the absence of glucose; a clear glucose-independent response was obtained only with 10 μM S 21403, which is well above maximal therapeutic concentrations (Breuel, 2004; Guillausseau, 2004). The amplificatory effect of S 21403 on glucose-induced insulin secretion (between 1.5- and five-fold) diminished with increasing glucose concentrations: the three- to five-fold stimulation observed at 3.3 mM glucose was reduced to approximately two-fold at 16.7 mM and only 1.4-fold at 33 mM glucose. Despite the observation of lack of significant stimulation of insulin release at subthreshold concentrations of glucose, the diminishing response at increasing glucose concentrations does not support a clear gain amplifier function for S 21403 (Henquin, 2004). Indeed, at high concentrations S 21403 (10 μM) did induce significant release of insulin in the absence of glucose, a common finding with sulphonylurea and in line with their action at the level of K+ATP channels. These observations were verified using the highly sensitive perfused rat pancreas preparation, and found pertinent both for first- and second-phase insulin secretion. The amplification of first-phase release by S 21403 was significantly more pronounced in our hands than previously reported (Gregorio et al., 2002). Despite the similar mechanisms involved, the kinetics of the action of S 21403 were extremely rapid; indeed, both the onset of the stimulation and its offset once the drug is removed from the perfusate were as rapid as those observed for glucose-induced biphasic insulin release. It is of interest to note that the fast reduction of insulin secretion when S 21403 was removed from the perfusate was not shared by common sulphonylurea agents (Gregorio et al., 2002) and could serve to limit the risk of hypoglycaemia.
In islets derived from lean, insulinopenic diabetic GK rats, S 21403 had quantitatively the same amplifying effect on glucose-induced insulin secretion as in islets from normal Wistar rats. Furthermore, in the perfused pancreas of GK rats, we obtained a normal biphasic insulin response when S 21403 was added to 8.3 mM glucose, which barely stimulates insulin secretion in this diabetes model (Ostenson et al., 1993). Admittedly, a high concentration of S 21403 (10 μM) was necessary to demonstrate normalized secretion kinetics; nevertheless, against the notoriously poor responsiveness of the GK rat pancreas, this is indeed an achievement.
Although for many reasons it may be convenient to evaluate the effect of insulinotropic drugs, for example, S 21403, in terms of interactions with glucose, in real life the β-cell is also strongly influenced by other nutrients like amino acids. In the present study, S 21403 and arginine had only additive effects on insulin secretion in normal islets. In islets from GK rats, in our hands arginine was a poor stimulator of insulin secretion. This is in variance to findings by Portha et al. (1991) and Hughes et al. (1994); we assume this to be related to differences between various GK rat colonies (CG Östenson, Stockholm, personal communication). In contrast, a strong synergistic interaction was observed in islets isolated from diabetic GK rats. We do not know the reason for this difference from the response of Wistar rat islets. GK islets in vivo are chronically exposed to mild hyperglycaemia, which could carry over a potentiating effect on subsequent incubations with drugs and nutrients (Cerasi, 1975; Nesher & Cerasi, 1987). However, in the present experiments islets were cultured overnight, and therefore it is unlikely that memories of in vivo conditions would persist. Of major interest is the finding that in the presence of S 21403 and arginine, insulin secretion in GK islets was of the same magnitude as secretion for normal Wistar islets. This again points to the marked insulin-releasing efficacy of S 21403 in this T2DM model.
A concern in the treatment of T2DM patients, particularly with long-acting sulphonylurea-like agents, is the risk for hypoglycaemia (Holstein & Egberts, 2003). We show in this study that S 21403 has several characteristics that predict low risk for hypoglycaemia, first and foremost its lack of significant insulin-releasing effect at low and basal glucose concentrations when used at near-therapeutic doses. Furthermore, insulin release from normal as well as GK diabetic islets in the presence of S 21403 was exquisitely sensitive to the inhibitory action of adrenaline, the main protector against hypoglycaemia, even in the absence of glucose and despite the fact that a high concentration (10 μM) of drug was used. Thus, the probability that S 21403 may continue to stimulate the secretion of insulin when blood glucose falls and the adrenergic system is activated should be minimal, which leads us to conclude that this drug should show a very low risk for hypoglycaemia. Nevertheless, it has to be borne in mind that in vivo many other factors not studied here (e.g. plasma half-life of the drug) are as important in determining the risk of hypoglycaemia. In vivo studies using nondiabetic Wistar and diabetic GK rats suggested that S 21403 (KAD-1229) could be a suitable agent for controlling postprandial hyperglycaemia, since it was able to suppress the increase in plasma glucose seen after a meal load up to 5 h after the meal (Ichikawa et al., 2002). The drug was also effective when administered orally to diabetic dogs (Misawa et al., 2001).
The chronic use of pharmacological insulin secretagogues may result in controversial effects on pancreatic insulin stores and the insulin biosynthetic rate, as observed in experimental animals (Andersson & Borg, 1980; Hakan Borg & Andersson, 1981; Portha & Serradas, 1991; Rustenbeck et al., 2001). We therefore tested the effect of 1-week exposure to S 21403 on islet insulin content, in normal islets as well as islets from normoglycaemic (prediabetic) P. obesus, under chronic conditions simulating normoglycaemia and hyperglycaemia. We could not demonstrate any deleterious action of chronic S 21403 exposure under these conditions. In fact, especially in islets of P. obesus, which are markedly prone to deplete their insulin stores when challenged for prolonged periods, chronic exposure to S 21403 seemed to reduce the loss of insulin and improve the acute insulin response to glucose. Further support for lack of negative chronic effects, and possibly the existence of positive effects on pancreatic insulin content, comes from the study of S 21403 action on proinsulin biosynthesis. We found no inhibition of the proinsulin biosynthetic rate whether rat or P. obesus islets were exposed acutely or during 24 h to S 21403. Indeed, at least in rat islets, both the basal and the glucose-stimulated proinsulin biosynthetic rate tended to augment by 50–100%. S 21403 is derived from succinic acid, which seems to be the main mediator of the effect of glucose on proinsulin biosynthesis (Alarcon et al., 2002); whether this fact is of importance remains to be investigated.
In conclusion, the demonstration here that S 21403 amplifies glucose-induced insulin secretion; that it has the ability to provoke normal biphasic insulin secretion from the pancreas of diabetic GK rats; that it is highly sensitive to the suppressive effect of adrenaline on insulin secretion; that it does not lead to depletion of insulin stores under chronic culture conditions and may even stimulate the biosynthesis of proinsulin, thus helping replete islet insulin; all indicate that S 21403 has the potential to become an excellent oral antidiabetic drug.
Acknowledgments
We are grateful to the technical assistance provided by the staff of our laboratories, especially to Yaffa Ariav for proinsulin biosynthesis studies. This work was supported by a grant from Servier, Paris.
Abbreviations
- BSA
bovine serum albumin
- FBS
foetal bovine serum
- KRB
Krebs–Ringer bicarbonate buffer
- T2DM
type 2 diabetes mellitus
References
- ABDEL-HALIM S.M., GUENIFI A., HE B., YANG B., MUSTAFA M., HOJEBERG B., HILLERT J., BAKHIET M., EFENDIC S. Mutations in the promoter of adenylyl cyclase (AC)-III gene, overexpression of AC-III mRNA, and enhanced cAMP generation in islets from the spontaneously diabetic GK rat model of type 2 diabetes. Diabetes. 1998;47:498–504. doi: 10.2337/diabetes.47.3.498. [DOI] [PubMed] [Google Scholar]
- ALARCON C., LINCOLN B., RHODES C.J. The biosynthesis of the subtilisin-related proprotein convertase PC3, but no that of the PC2 convertase, is regulated by glucose in parallel to proinsulin biosynthesis in rat pancreatic islets. J. Biol. Chem. 1993;268:4276–4280. [PubMed] [Google Scholar]
- ALARCON C., WICKSTEED B., PRENTKI M., CORKEY B.E., RHODES C.J. Succinate is a preferential metabolic stimulus-coupling signal for glucose-induced proinsulin biosynthesis translation. Diabetes. 2002;51:2496–2504. doi: 10.2337/diabetes.51.8.2496. [DOI] [PubMed] [Google Scholar]
- ANDERSSON A., BORG L.A. Effects of glipizide on the insulin production by isolated mouse pancreatic islets. Acta Endocrinol. (Copenhagen) 1980;239 (Suppl):37–41. [PubMed] [Google Scholar]
- BREUEL H.P.Safety, pharmacodynamics and pharmacokinetics of S 21403 following six repeated oral doses (2.5 mg t.i.d., 5 mg t.i.d., 10 mg t.i.d., 20 mg t.i.d., 30 mg t.i.d., 40 mg t.i.d.) in 70 type 2 diabetic patients. A 7 day double-blind controlled study versus placebo 2004. CL2-21403-004; Servier Internal Report No. NP07578
- CERASI E. Insulin deficiency and insulin resistance in the pathogenesis of type 2 diabetes: is a divorce possible. Diabetologia. 1995;38:992–997. doi: 10.1007/BF00400591. [DOI] [PubMed] [Google Scholar]
- CERASI E. Potentiation of insulin release by glucose in man. I. Quantitative analysis of the enhancement of glucose-induced insulin secretion by pretreatment with glucose in normal subjects. Acta Endocrinol. (Copenhagen) 1975;79:483–501. [PubMed] [Google Scholar]
- DONATH M.Y., GROSS D.J., CERASI E., KAISER N. Hyperglycemia-induced beta-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes. 1999;48:738–744. doi: 10.2337/diabetes.48.4.738. [DOI] [PubMed] [Google Scholar]
- FERRANNINI E., GASTALDELLI A., MIYAZAKI Y., MATSUDA M., PETTITI M., NATALI A., MARI A., DEFRONZO R.A. Predominant role of reduced beta-cell sensitivity to glucose over insulin resistance in impaired glucose tolerance. Diabetologia. 2003;46:1211–1219. doi: 10.1007/s00125-003-1169-6. [DOI] [PubMed] [Google Scholar]
- GADOT M., LEIBOWITZ G., SHAFRIR E., CERASI E., GROSS D.J., KAISER N. Hyperproinsulinemia and insulin deficiency in the diabetic Psammomys obesus. Endocrinology. 1994;135:610–616. doi: 10.1210/endo.135.2.8033810. [DOI] [PubMed] [Google Scholar]
- GREGORIO F., AMBROSI F., BOEMI M., CARLE F., FILIPPONI P. Effects of S 21403 on hormone secretion from isolated rat pancreas at different glucose concentrations. Eur. J. Pharmacol. 2002;456:141–147. doi: 10.1016/s0014-2999(02)02620-1. [DOI] [PubMed] [Google Scholar]
- GROSS D.J., LEIBOWITZ G., CERASI E., KAISER N. Increased susceptibility of islets from diabetes-prone Psammomys obesus to the deleterious effects of chronic glucose exposure. Endocrinology. 1996;137:5610–5615. doi: 10.1210/endo.137.12.8940391. [DOI] [PubMed] [Google Scholar]
- GUILLAUSSEAU P.J.Dose ranging study of S 21403 administered orally t.i.d. at the doses of 3 mg, 5 mg, 8 mg, 12 mg and 20 mg over 9 weeks in 300 type 2 diabetic patients 2004. CL2-21403-006; Servier Internal Report No. NP08322
- HAKAN BORG L.A., ANDERSSON A. Long-term effects of glibenclamide on the insulin production, oxidative metabolism and quantitative ultrastructure of mouse pancreatic islets maintained in tissue culture at different glucose concentrations. Acta Diabetol. Lat. 1981;18:65–83. doi: 10.1007/BF02056108. [DOI] [PubMed] [Google Scholar]
- HENQUIN J.C. Pathways in beta-cell stimulus-secretion coupling as targets for therapeutic insulin secretagogues. Diabetes. 2004;53 (Suppl 3):S48–S58. doi: 10.2337/diabetes.53.suppl_3.s48. [DOI] [PubMed] [Google Scholar]
- HOLSTEIN A., EGBERTS E.H. Risk of hypoglycaemia with oral antidiabetic agents in patients with Type 2 diabetes. Exp. Clin. Endocrinol. Diabetes. 2003;111:405–414. doi: 10.1055/s-2003-44287. [DOI] [PubMed] [Google Scholar]
- HOORENS A., VAN DE CASTEELE M., KLOPPEL G., PIPELEERS D. Glucose promotes survival of rat pancreatic beta cells by activating synthesis of proteins which suppress a constitutive apoptotic program. J. Clin. Invest. 1996;98:1568–1574. doi: 10.1172/JCI118950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES S.J., SUZUKI K., GOTO Y. The role of islet secretory function in the development of diabetes in the GK Wistar rat. Diabetologia. 1994;36:863–870. doi: 10.1007/BF00400940. [DOI] [PubMed] [Google Scholar]
- ICHIKAWA K., YAMATO T., OJIMA K., TSUJI A., ISHIKAWA K., KUSAMA H., KOJIMA K. Effect of KAD-1229, a novel hypoglycaemic agent, on plasma glucose levels after meal load in type 2 diabetic rats. Clin. Exp. Pharmacol. Physiol. 2002;29:423–427. doi: 10.1046/j.1440-1681.2002.03682.x. [DOI] [PubMed] [Google Scholar]
- KAISER N., BAILYES E.M., SCHNEIDER B.S., CERASI E., STEINER D.F., HUTTON J.C., GROSS D.J. Characterization of the unusual insulin of Psammomys obesus, a rodent with nutrition-induced NIDDM-like syndrome. Diabetes. 1997;46:953–957. doi: 10.2337/diab.46.6.953. [DOI] [PubMed] [Google Scholar]
- LEIBOWITZ G., FERBER S., APELQVIST A., EDLUND H., GROSS D.J., CERASI E., MELLOUL D., KAISER N. IPF1/PDX1 deficiency and beta-cell dysfunction in Psammomys obesus, an animal with type 2 diabetes. Diabetes. 2001;50:1799–1806. doi: 10.2337/diabetes.50.8.1799. [DOI] [PubMed] [Google Scholar]
- MARSHAK S., LEIBOWITZ G., BERTUZZI F., SOCCI C., KAISER N., GROSS D.J., CERASI E., MELLOUL D. Impaired beta-cell functions induced by chronic exposure of cultured human pancreatic islets to high glucose. Diabetes. 1999;48:1230–1236. doi: 10.2337/diabetes.48.6.1230. [DOI] [PubMed] [Google Scholar]
- MISAWA K., ICHIKAWA K., OJIMA K., HAMANO S., KTAMURA T., KOMATSU H. Effect of KAD-1229, a non-sulfonylurea hypoglycemic agent, on plasma glucose and insulin in streptozocin-induced diabetic dogs. Pharmacology. 2001;62:65–72. doi: 10.1159/000056073. [DOI] [PubMed] [Google Scholar]
- NESHER R., CERASI E. Biphasic insulin release as the expression of combined inhibitory and potentiating effects of glucose. Endocrinology. 1987;121:1017–1024. doi: 10.1210/endo-121-3-1017. [DOI] [PubMed] [Google Scholar]
- NESHER R., GROSS D.J., DONATH M.Y., CERASI E., KAISER N. Interaction between genetic and dietary factors determines β-cell function in Psammomys obesus, an animal model of type 2 diabetes. Diabetes. 1999;48:731–737. doi: 10.2337/diabetes.48.4.731. [DOI] [PubMed] [Google Scholar]
- OHNOTA H., KOBAYASHI M., KOIZUMI T., KATSUNO K., SATO F., AIZAWA T. In vitro insulinotropic action of a new non-sulfonylurea hypoglycemic agent, calcium (2s)-2-benzyl-3-(cis-hexahydro-2-isoindolinyl-carbonyl) propionate dihydrate (KAD-1229), in rat pancreatic B-cells. Biochem. Pharmacol. 1995;49:165–171. doi: 10.1016/s0006-2952(94)00484-6. [DOI] [PubMed] [Google Scholar]
- OSTENSON C.G., KHAN A., ABDEL-HALIM S.M., GUENIFI A., SUZUKI K., GOTO Y., EFENDIC S. Abnormal insulin secretion and glucose metabolism in pancreatic islets from the spontaneously diabetic GK rat. Diabetologia. 1993;36:3–8. doi: 10.1007/BF00399086. [DOI] [PubMed] [Google Scholar]
- OWENS D.R., COZMA L.S., LUZIO S.D. Early-phase prandial insulin secretion: its role in the pathogenesis of type 2 diabetes mellitus and its modulation by repaglinide. Diabetes Nutr. Metab. 2002;15:19–27. [PubMed] [Google Scholar]
- PORTHA B., SERRADAS P. Improvement in glucose-induced insulin secretion in diabetic rats after long-term gliclazide treatment: a comparative study using different models of non-insulin-dependent diabetes mellitus induced by neonatal streptozotocin. Am. J. Med. 1991;90:15S–21S. doi: 10.1016/0002-9343(91)90413-r. [DOI] [PubMed] [Google Scholar]
- PORTHA B., SERRADAS P., BAILBE D., SUZUKI K., GOTO Y., GIROIX M.H. Beta-cell insensitivity to glucose in the GK rat, a spontaneous nonobese model for type II diabetes. Diabetes. 1991;40:486–491. doi: 10.2337/diab.40.4.486. [DOI] [PubMed] [Google Scholar]
- REIMANN F., PROKS P., ASHCROFT F.M. Effects of mitiglinide (S 21403) on Kir6.2/SUR1, Kir6.2/SUR2A and Kir6.2/SUR2B types of ATP-sensitive potassium channel. Br. J. Pharmacol. 2001;132:1542–1548. doi: 10.1038/sj.bjp.0703962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSTENBECK I., WINKLER M., JORNS A. Desensitization of insulin secretory response to imidazolines, tolbutamide, and quinine. I. Secretory and morphological studies. Biochem. Pharmacol. 2001;62:1685–1694. doi: 10.1016/s0006-2952(01)00792-4. [DOI] [PubMed] [Google Scholar]
- SCHATZ H., NIERLE C., PFEIFFER E.F. (Pro-) insulin biosynthesis and release of newly synthesized (pro-) insulin from isolated islets of rat pancreas in the presence of amino acids and sulphonylureas. Eur. J. Clin. Invest. 1975;5:477–485. doi: 10.1111/j.1365-2362.1975.tb00480.x. [DOI] [PubMed] [Google Scholar]
- SHAFRIR R., ZIV E. Cellular mechanism of nutritionally induced insulin resistance: the desert rodent Psammomys obesus and other animals in which insulin resistance leads to detrimental outcome. J. Basic Clin. Physiol. Pharmacol. 1998;9:345–385. doi: 10.1515/JBCPP.1998.9.2-4.347. [DOI] [PubMed] [Google Scholar]
- STANDL E., FUCHTENBUSCH M. The role of oral antidiabetic agents: why and when to use an early-phase insulin secretion agent in Type II diabetes mellitus. Diabetologia. 2003;46 (Suppl 1):M30–M36. doi: 10.1007/s00125-002-0934-2. [DOI] [PubMed] [Google Scholar]
- SUNAGA Y., GONOI T., SHIBASAKI T., ICHIKAWA K., KUSAMA H., YANO H., SEINO S. The effects of mitiglinide (KAD-1229), a new anti-diabetic drug, on ATP-sensitive K+ channels and insulin secretion: comparison with the sulfonylureas and nateglinide. Eur. J. Pharmacol. 2001;431:119–125. doi: 10.1016/s0014-2999(01)01412-1. [DOI] [PubMed] [Google Scholar]
- VAN DE CASTEELE M., KEFAS B.A., CAI Y., HEIMBERG H., SCOTT D.K., HENQUIN J.C., PIPELEERS D., JONAS J.C. Prolonged culture in low glucose induces apoptosis of rat pancreatic beta-cells through induction of c-myc. Biochem. Biophys. Res. Commun. 2003;312:937–944. doi: 10.1016/j.bbrc.2003.11.013. [DOI] [PubMed] [Google Scholar]