Abstract
In the spontaneously hypertensive rat (SHR) and aging Wistar–Kyoto rats (WKY), acetylcholine releases an endothelium-derived contracting factor (EDCF) produced by endothelial cyclooxygenase-1, which stimulates thromboxane A2 receptors (TP receptors) on vascular smooth muscle. The purpose of the present study was to identify this EDCF by measuring changes in isometric tension and the release of various prostaglandins by acetylcholine.
In isolated aortic rings of SHR, U 46619, prostaglandin (PG) H2, PGF2α, PGE2, PGD2, prostacyclin (PGI2) and 8-isoprostane, all activate TP receptors of the vascular smooth muscle to produce a contraction (U 46619≫8-isoprostane=PGF2α=PGH2>PGE2=PGD2>PGI2). The contractions produced by PGH2 and PGI2 were fast and transient, mimicking endothelium-dependent contractions. PGI2 did not relax isolated aortic rings of WKY and SHR.
Acetylcholine evoked the endothelium-dependent release of thromboxane A2, PGF2α, PGE2, PGI2 and most likely PGH2 (PGI2≫PGF2α⩾PGE2>TXA2>8-isoprostane, PGD2). Dazoxiben abolished the production of thromboxane A2, but did not influence the endothelium-dependent contractions to acetylcholine.
The release of PGI2 was significantly larger in the aorta of SHR than in WKY, and the former was more sensitive to the contractile effect of PGI2 than the latter. The inhibition of PGI-synthase was associated with an increase in PGH2 spillover and the enhancement of acetylcholine-induced endothelium-dependent contractions.
Thus, in the aorta of SHR and aging WKY, the endothelium-dependent contractions elicited by acetylcholine most likely involve the release of PGI2 with a concomitant contribution of PGH2.
Keywords: Endothelium-dependent contractions, TP receptors, spontaneously hypertensive rat, prostaglandins, endoperoxide, prostacyclin
Introduction
Under several pathological conditions, such as hypertension, endothelium-dependent relaxations are impaired (Vanhoutte et al., 2005). In the spontaneously hypertensive rat (SHR), the endothelial dysfunction is attributed to the occurrence of a concomitant endothelium-dependent contraction mediated by a yet unidentified endothelium-derived contracting factor (EDCF; Lüscher & Vanhoutte, 1986). This endothelium-dependent contraction involves the production of reactive oxygen species, the activation of endothelial cyclooxygenase (COX)-1, the diffusion of EDCF and the subsequent stimulation of thromboxane A2 receptors (TP receptors) located on smooth muscle cells (Lüscher & Vanhoutte, 1986; Auch-Schwelk et al., 1990; Kato et al., 1990; Ge et al., 1995; Yang et al., 2002, 2003a; 2003b; 2004).
EDCF-mediated responses are observed not only in hypertension but also in diabetes, and probably reflect the premature aging of the blood vessel wall subjected to an exaggerated oxidative stress. The information available in humans confirms that EDCF-mediated responses contribute to the blunting of endothelium-dependent vasodilatations in aged subjects and essential hypertensive patients (Vanhoutte et al., 2005). The identification of EDCF could therefore provide new insights into the mechanism of endothelial dysfunction and potentially reveal new therapeutic targets. Various candidates have been proposed for the identity of EDCF in the SHR; they include the endoperoxides prostaglandin H2 (PGH2; Ge et al., 1995), prostacyclin (PGI2; Rapoport & Williams, 1996), thromboxane A2 (Taddei & Vanhoutte, 1993) and isoprostanes (Janssen, 2002).
The purpose of the present study was to identify the EDCF released by acetylcholine from the aortic rings of SHR and Wistar–Kyoto rats (WKY).
Methods
Experiments were performed on thoracic aortas from 1-year-old male SHR (402±6 g, n=102) and normotensive WKY (432±8 g, n=52), both from Charles River (L'Arbresles, France). The rats were anesthetized with pentobarbital sodium (50 mg kg−1, intraperitoneally) and the blood pressure was measured from the carotid artery (systolic blood pressure: 185±5 and 105±3 mmHg, in SHR and WKY, respectively; P<0.05). The aorta was then dissected free, excised and placed in cold modified Krebs–Ringer bicarbonate solution of the following composition (mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2SO4 1.2, NaHCO3 25.0, edetate calcium di-sodium 0.026, glucose 11.1 (control solution). In some aortas, the endothelium was removed from segments of various lengths by infusing a saponin solution (1 mg ml−1, for 20 s) that was subsequently flushed with control solution. Then the aorta was cut into rings (4–5 mm in length). In some rings the isometric tension was recorded, while in others the release of prostanoids was determined.
Isometric tension recording
The rings were suspended in organ chambers (20 ml), which contained control solution (37°C) aerated with 95% O2 and 5% CO2, and were connected to a force transducer in order to record isometric contraction. They were stretched progressively to reach the optimal point of their length–active tension relationship (approximately 2 g). Drug incubation time was 45 min for most of the experiments. Concentration–response curves were obtained in a cumulative manner. Each ring was exposed to only one set of cumulative concentration of each given agonist. Contractile responses were expressed as a percentage of the reference contraction to KCl (60 mM), performed for each individual ring at the beginning of the experiment. Relaxations were expressed as a percentage of the maximal relaxation elicited by papaverine (100 μM) in rings contracted with phenylephrine.
Release of prostaglandins
In order to measure the release of prostanoids, rings were placed in thermostated mini-chambers containing 1 ml of control solution (37°C) aerated with 95% O2 and 5% CO2. The equilibration time was 1 h during which the solution was changed every 15 min. The incubation period with drugs was 20 min and acetylcholine was applied for 10 min in the presence of the drugs. Each ring was exposed once and to a single concentration of acetylcholine. Then the aortic rings were removed and the mini-chambers were freeze-clamped in liquid nitrogen and stored at −80°C for further analysis. The rings were placed in a dry hot box (60°C for 48 h) and the dry weight was measured.
The prostaglandins were measured with the following EIA kits from Cayman Chemical (Ann Arbor, MI, U.S.A.), 6-keto prostaglandin F1α, thromboxane B2, prostaglandin E2, prostaglandin F2α, prostaglandin D2 MOX and 8-isoprostane. Undiluted 50 μl samples were dosed at the exception of the 6-keto prostaglandin F1α measurement, which required a systematic 50-time dilution in control solution and some samples which were subjected to a two-time dilution for the assessment of prostaglandin E2 and prostaglandin F2α. The various assays were performed as indicated by the manufacturer procedure booklet.
Additionally, the production of 8-isoprostane was assessed by mass spectrometry as previously described (Il'Yasova et al., 2004).
Drugs
Acetylcholine hydrochloride, indomethacin, isoproterenol, NG-nitro-L-arginine (L-NA), papaverine, phenylephrine, SnCl2 and tranylcypromine were obtained from Sigma (La Verpillère, France). Prostaglandin F2α (PGF2α), PGH2, prostaglandin E2 (PGE2), prostaglandin D2 (PGD2), prostaglandin I2 (PGI2), iloprost, 6-keto prostaglandin F1α (6-keto-PGF1α), 8-isoprostane, 9α,11α-azoprosta-5Z,13E-dien-1-oic acid (U 51605), 9,11-dideoxy-9α,11α-epoxymethano prostaglandin F2α (U 46619), N[-2-(cyclohexyloxy)-4-nitrophenyl]-methanesulfonamide (NS 398) and 2-[(1-oxopenytyl)oxy]-benzoic acid (valeryl salicylate) were purchased from Cayman Chemical Company (Ann Arbor, MI, U.S.A.). 3-[(6-amino-(4-chlorobenzensulfonyl)-2-methyl-5,6,7,8-tetrahydronapht]-1-yl)propionic acid (S 18886) and dazoxiben were synthesized at the Institut de Recherches Servier (Suresnes, France). Drug concentrations are expressed as final molar concentrations in the bath solution.
Data analysis
Data are expressed as means±s.e.m.; n refers to the number of rats from which the aortas were taken. The ED20 (concentration of agonist causing a contraction representing 20% of the reference contraction to 60 mM KCl, or causing a relaxation representing 20% of the reference relaxation to 100 μM papaverine) was calculated using the Michaelis–Menten equation and nonlinear regression that included all the data points. The apparent antagonist dissociation constants were determined according to the equation pKb=−log[Ant]/(dose ratio−1). [Ant] represents the concentration of the antagonist and dose ratio the ED50 of the agonist in the presence of the antagonist divided by the ED50 in the absence of the antagonist. Statistical analysis was performed by two-tailed Student's t-test for control and treatment comparisons, and by ANOVA1 or ANOVA2 analysis for multiple comparisons, followed by a Newman–Keuls or a Bonferroni post-hoc test, respectively, where appropriate. Differences were considered to be statistically significant when P was <0.05.
Results
Acetylcholine-induced endothelium-dependent contractions
In the presence of L-nitro-arginine, contractions in response to acetylcholine were observed in rings with, but not without, endothelium. They were transient and the maximal amplitude was observed for concentrations of acetylcholine ranging from 3 to 30 μM. They were usually smaller than the reference contraction to KCl (60 mM) (Figure 1).
Figure 1.
Tracings showing the transient contractions evoked by half-log cumulative addition of acetylcholine (10 nM–100 μM, top), PGI2 (100 nM–30 μM, middle) and PGH2 (1 nM–1 μM, bottom) in rings with endothelium of SHR aortas, in the presence of L-NA (100 μM). KCl (60 mM) represents the reference contraction.
Prostanoids-induced changes in tension
U 46619 (0.1 nM–1 μM), 8-isoprostane (0.1 nM–30 μM), PGF2α (1 nM–30 μM), PGH2 (1 nM–1 μM), PGE2, PGD2 and PGI2 (10 nM–30 μM) produced concentration-dependent contractions of aortic rings of both WKY and SHR (Figure 2). However, neither iloprost nor 6-keto-PGF1α, up to 30 μM evoked a significant contraction (data not shown). In SHR rings (in the presence or absence of the endothelium), the order of potency of the agonists was U 46619≫8-isoprostane=PGF2α=PGH2>PGE2=PGD2>PGI2, and was similar to that observed in aortas of the WKY: U 46619≫8-isoprostane>PGF2α=PGH2⩾PGE2=PGD2≫PGI2. The contractions elicited by PGI2 and PGH2 were transient (Figure 1), while those in response to U 46619, 8-isoprostane, PGD2, PGF2α and PGE2 (data not shown) were sustained. U 46619, PGI2 and PGH2 were significantly more potent in the SHR than in WKY (Figure 3). The contractions in response to all the prostanoids tested were potentiated by removal of the endothelium (Table 1) or by the presence of L-NA (100 μM, data not shown).
Figure 2.
Concentration–response curves to various prostaglandin analog in aortic rings without endothelium of WKY (top) and SHR (bottom). Data are shown as mean±s.e.m. of at least four different experiments.
Figure 3.
Concentration–response curves to U 46619 and PGF2α (top left), 8-isoprostane and PGE2 (bottom left) PGI2 (top right) and PGH2 (bottom, right) in aortic rings without endothelium of WKY and SHR. Data are shown as mean±s.e.m. of at least four different experiments.
Table 1.
Prostanoids-induced contractions in aortic rings with and without endothelium of WKY and SHR
| Prostaglandins | With endothelium−log (ED20) | Without endothelium−log (ED20) |
|---|---|---|
| U 46619 | ||
| WKY (n=4) | 7.67 | 8.34* |
| SHR (n=4–20) | 8.32# | 8.86*# |
| PGH2 | ||
| WKY (n=5) | 6.13 | 6.86* |
| SHR (n=5–9) | 6.16 | 7.24*# |
| PGF2α | ||
| WKY (n=4) | 5.59 | 6.80* |
| SHR (n=5) | 6.29 | 7.15* |
| 8-Isoprostane | ||
| WKY (n=6) | 6.24# | 6.86* |
| SHR (n=11) | 6.10 | 6.84* |
| PGE2 | ||
| WKY (n=5) | 5.80 | 6.20*# |
| SHR (n=5) | 5.89 | 6.27* |
| PGD2 | ||
| WKY (n=5) | 5.38 | 6.36* |
| SHR (n=5) | 5.74 | 6.24* |
| PGI2 | ||
| WKY (n=5) | <4.5 | 5.03* |
| SHR (n=8–14) | <4.5 | 5.53*# |
The ED20 is the concentration of agonist causing a contraction representing 20% of the reference contraction to KCl (60 mM); n indicates the number of animals from which tissues were taken. The statistical analysis was performed on the whole dose–response curves (ANOVA 2 followed by Bonferroni post-tests for paired or unpaired experiments). *Indicates a statistically significant difference between vessels with and without endothelium, while #indicates that the contractions in response to a given prostanoid were larger in the strain of rat which has been labeled. These two labelings do not necessarily indicate a statistically significant difference at the level of the ED20.
In SHR rings without endothelium, S 18886 (0.3–30 nM) produced a rightward shift of the concentration–response curves elicited by U 46619 and 8-isoprostane. In both cases, the slope of the Schild‘s plot was significantly different from unity, indicating that the antagonism was not competitive. The pKb values calculated with the lowest concentrations of S 18886 versus U 46619 and 8-isoprostane were similar, 9.3 and 9.6, respectively (n=4). At the concentration of 100 nM, S 18886 virtually abolished the contractions in responses to all the other prostanoids studied, that is PGE2, PGF2α, PGH2 and PGI2 (data not shown).
In phenylephrine-contracted rings of SHR and WKY, with and without endothelium, and in the presence or not of S 18886, PGI2 (up to 10 μM) and iloprost (up to 3 μM) did not produce a significant relaxation (data not shown). However, under the same experimental conditions, isoproterenol produced a concentration-dependent relaxation in both rings with and without endothelium (ED20: 7.6 and 6.5 and maximal relaxation in % of papaverine: 72.3±3.2 and 32.3±3.4; n=5, in SHR rings with and without endothelium, respectively).
Acetylcholine-induced release of prostaglandins
Acetylcholine (10 μM) evoked the release of 6-keto-PGF1α (stable metabolite of PGI2), thromboxane B2 (stable metabolite of thromboxane A2), PGE2 and PGF2α in the aorta of both WKY and SHR. This release was endothelium-dependent in both strains. The release of PGI2 was 10–100 times larger than that of the other prostaglandins. Furthermore, in contrast to that of thromboxane A2, PGE2 and PGF2α, the release of PGI2 was significantly larger in SHR than in WKY aortas (Figure 4). The release of 8-isoprostane, as assessed with the EIA kit, was within a similar range to that of thromboxane A2, that is small but measurable (<200 pg ml−1), and appeared both endothelium- and acetylcholine-dependent. However, these findings were not confirmed by direct measurement of 8-isoprostane by mass spectrometry (data not shown). PGD2 levels, as assessed with the EIA kit, were low, around the threshold for detection (<20 pg ml−1; data not shown).
Figure 4.
Basal and acetylcholine-dependent release of prostaglandins (PGI2, PGE2, thromboxane A2 and PGF2α) in aortic rings, with and without endothelium, of WKY and SHR. Data are shown as mean±s.e.m. of at least four different experiments. The * indicates a significant effect of acetylcholine and # a significant difference between WKY and SHR.
Thromboxane A2
In SHR aortic rings, dazoxiben (10 μM) abolished the acetylcholine-dependent release of thromboxane A2 without affecting that of PGI2 or PGE2. However, the endothelium-dependent contractions in response to acetylcholine (presence of L-NA, 100 μM) were not affected by the presence of the thromboxane synthase inhibitor (Figure 5).
Figure 5.
Effects of dazoxiben (10 μM) on the basal and acetylcholine-dependent release of thromboxane A2 (a), PGI2 (b) and PGE2 (c), as well as the acetylcholine-induced endothelium-dependent contraction in the aortic rings with endothelium of SHR (d). Contractile experiments were performed in the presence of L-NA (100 μM). Data are shown as mean±s.e.m. of at least five different experiments. The * indicates a significant effect of dazoxiben.
Prostacyclin
In both WKY and SHR aortas, acetylcholine induced a concentration- and endothelium-dependent release of PGI2 and contractions that were superimposable (Figure 6). The acetylcholine-induced endothelium-dependent release of PGI2 was dependent on the endothelial mass and half of the acetylcholine-induced endothelium-dependent release of PGI2 occurred within 5 min following stimulation with acetylcholine (data not shown).
Figure 6.
PGI2 release (left Y-axis) and acetylcholine-induced contraction (right Y-axis) in aortic rings with and without the endothelium of WKY (top) and SHR (bottom). Contractions were obtained in the presence of L-NA (100 μM). Data are shown as mean±s.e.m. of at least three different experiments.
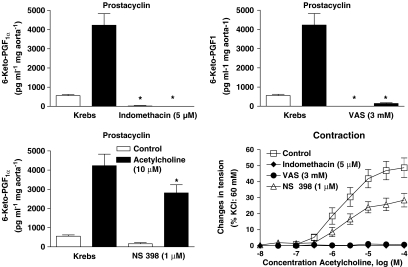
The acetylcholine-induced release of PGI2 was unaffected by the presence of 100 μM L-NA (4927±1882 and 3880±961 pg ml−1 mg aorta−1, n=6 in the absence and presence of L-NA, respectively) or 100 nM S 18886 (3768±462 and 4001±430 pg ml−1 mg aorta−1, n=7 in the absence and presence of S 18886, respectively). However, the preferential cycloxygenase-2 blocker, NS 398 (1 μM), produced a partial but statistically significant inhibition of both the basal and acetylcholine-stimulated production of PGI2, while the preferential COX-1 inhibitor, valeryl salicylate (3 mM), or the nonselective inhibitor, indomethacin (5 μM), abolished it (Figure 7).
Figure 7.
Effects of the COX inhibitors indomethacin (5 μM, top left), valeryl salycylate (3 mM, top right) and NS 398 (1 μM, bottom left), on the basal and acetylcholine-dependent release of PGI2, as well as on the acetylcholine-induced endothelium-dependent contractions (bottom right) in rings with endothelium of SHR aortas. Data are shown as mean±s.e.m. of at least six different experiments. The * indicates a significant effect of an inhibitor.
In the SHR aorta, tranylcypromine (100 μM), a putative inhibitor of the PGI-synthase did not significantly alter the acetylcholine-dependent production of either PGI2 (acetylcholine 10 μM: 3085±586 and 4116±366 pg ml−1 mg aorta−1, n=5 in the absence and presence of tranycylpromine, respectively) or PGE2 (data not shown). U 51605, a combined inhibitor of PGI- and thromboxane A2 synthases, produced a concentration-dependent inhibition of PGI2 release, which was statistically significant at each of the concentrations tested (0.5–10 μM), while the inhibition of thromboxane A2 production was statistically significant only at the two highest concentrations (3 and 10 μM; Figure 8). By contrast, U 51605 (0.5, 1, 3 and 10 μM) produced a statistically significant increase in acetylcholine-induced release of PGE2 and PGF2α (Figure 9).
Figure 8.
Effects of U 51605 at 0.5 μM (left) and at 1, 3 and 10 μM (right) on basal and acetylcholine-stimulated release of PGI2 (top) and thromboxane A2 (bottom) in isolated aortic rings with endothelium of SHR. Data are shown as mean±s.e.m. of at least five different experiments. As the experiments involving the various concentrations of U 51605 were not contemporary, the data shown in the graphs situated on the right-hand side are expressed in percentage of the control acetylcholine response. The * indicates a significant effect of acetylcholine and # a significant effect of U 51605.
Figure 9.
Effects of U 51605 at 0.5 μM (left) and at 1, 3 and 10 μM (right) on basal and acetylcholine-stimulated PGE2 (top) and PGF2α (bottom) release in aortic rings with endothelium of SHR. Data are shown as mean±s.e.m. of at least five different experiments. As the experiments involving the various concentrations of U 51605 were not contemporary, the data shown in the graphs situated on the right-hand side are expressed in percentage of the control acetylcholine response. The * indicates a significant effect of acetylcholine and # a significant effect of U 51605.
In the SHR aorta without endothelium, U 51605 (10 nM–10 μM) produced a concentration-dependent contraction (−log ED20: 7.29), which was abolished in the presence of S 18886 (100 nM; data not shown). Additionally, U 51605 (0.5–3 μM) produced a concentration-dependent noncompetitive inhibition of U 46619-induced contraction, which was statistically significant at each of the concentrations of U 51605 tested (pKb value calculated for U 51605 at the concentration of 0.5 μM: 6.6; Figure 10).
Figure 10.
Effects of U 51605 in SHR isolated aortic rings. Concentration-dependent inhibition of U 46619-induced contraction in isolated aortic rings without endothelium by U 51605 (0.5, 1 and 3 μM; top). Effects of U 51605 (0.5, 1 and 3 μM) on the endothelium-dependent contractions to acetylcholine (presence of L-NA: 100 μM, bottom). Data are shown as mean±s.e.m. of at least five different experiments.
Acetylcholine-induced endothelium-dependent contractions (10 nM–100 μM) were significantly potentiated by the presence of 0.5 μM U 51605, were not significantly affected by 1 μM U 51605 and were significantly inhibited by 3 μM U 51605 (Figure 10). In the SHR aorta with endothelium contracted with phenylephrine, U 51605, up to 3 μM, did not affect the endothelium-dependent relaxations induced by acetylcholine (data not shown).
Discussion
The present study demonstrates that, in the SHR aorta, PGI2 qualifies as one endothelium-derived contractile factor released by acetylcholine.
In the isolated aortic rings of SHR and WKY, the various prostaglandins studied, that is, U 46619, PGH2, PGF2α , PGE2, PGD2, PGI2 as well as 8-isoprostane, all activate the TP receptors on vascular smooth muscle to cause contraction, since the contractions were blocked by the specific TP receptor antagonist, S 18886 (Simonet et al., 1998).
The thromboxane A2 analog, U 46619 is by far the most potent vasoconstrictor among the various prostanoids studied. Acetylcholine induced a small but measurable endothelium-dependent release of thromboxane B2, the stable metabolite of thromboxane A2, which was fully and selectively blocked by the thromboxane synthase blocker, dazoxiben. Whether or not the generation of thromboxane A2 was truly of endothelial origin or from the transcellular metabolism of PGH2, by platelets adhering to the endothelium (Pfister et al., 2002) or by the smooth muscle cells themselves, is unknown. However, this production of thromboxane A2 did not contribute to the acetylcholine-induced endothelium-dependent contraction, since an effective concentration of dazoxiben did not influence the cholinergic response (see also: Lüscher & Vanhoutte, 1986; Koga et al., 1989; Auch-Schwelck et al., 1990; Kato et al., 1990).
8-isoprostane (8-epiPGF2α) is produced from the oxidative modification of polyunsaturated fatty acids via a free radical-catalyzed mechanism (Morrow et al., 1990). Under some circumstances, 8-isoprostane could be a direct product of COX or an indirect consequence of superoxide anion production by COX-mediated metabolism (Watkins et al., 1999). In both WKY and SHR aortic rings, 8-isoprostane was a potent constrictor. First EIA dosages were consistent with an acetylcholine-stimulated and endothelium-dependent release of this prostanoid, supporting the hypothesis that an isoprostane could be the EDCF released by acetylcholine (Janssen, 2002). However, the results of this dosage were not confirmed by mass spectrometry analysis, suggesting that the data provided by the EIA kit should be attributed to the cross-detection of another prostaglandin(s) species. This discrepancy in the measurement of 8-isoprostane with an immunoassay kit and by mass spectrometry has been previously reported (Il'Yasova et al., 2004). Therefore, in the SHR aorta, 8-isoprostane is not the EDCF released by acetylcholine.
PGI2 is generally described as an endothelium-derived vasodilator, which, by stimulating its receptor (PGI2 receptors or IP receptors) and activating adenylate cyclase, elevates intracellular cyclic-AMP concentration and produces smooth muscle relaxation (Wise & Jones, 1996). However, in WKY and SHR, neither PGI2 nor its stable analog iloprost was able to produce a relaxation. These results confirm earlier observations showing that IP receptor agonists cannot evoke relaxation in the aorta of these two strains of rats, at least when older than 15 weeks (Levy, 1980; Rapoport & Williams, 1996). In both WKY and SHR, the IP receptor gene expression decreases with age and, at any given age, is systematically less expressed in SHR than in WKY (Numaguchi et al., 1999). The present study supports the hypothesis of a dysfunction linked to the IP receptor itself, since the aorta from both strains did relax in response to isoproterenol, a β-adrenoceptor agonist which also evokes cyclic-AMP-dependent relaxation.
PGI2 evokes contractions in various vascular preparations including human coronary arteries (Pomerantz et al., 1978; Davis et al., 1980; Zhao et al., 1996). In smooth muscle cells of the guinea-pig carotid artery, PGI2, but not iloprost, induces TP receptor-dependent depolarization and firing of action potentials (Corriu et al., 2001). The present study confirms that in the aorta of both WKY and SHR, PGI2 produces also contractions via the activation of TP receptors (Levy, 1980; Williams et al., 1994; Zhao et al., 1996), and shows that it is more potent in SHR than in WKY.
Acetylcholine produced a concentration-dependent release of PGI2. This release was fully endothelium-dependent and was markedly larger than the release of any of the other prostaglandins (i.e. thromboxane A2, PGE2, PGF2α or PGD2). This observation is consistent with previous report indicating that in most blood vessels PGI2 is the principal metabolite of arachidonic acid, the endothelial cells being the predominant site of its synthesis (Moncada et al., 1976). The release of PGI2 was significantly larger in SHR than in WKY aortas (about two-fold) at any given concentration of acetylcholine tested. The acetylcholine-induced endothelium-dependent contraction and endothelium-dependent release of PGI2 were superimposable for both SHR and WKY. The time course of PGI2 release was rapid and compatible with the time course of endothelium-dependent contractions. Furthermore, the endothelium-independent contraction elicited by exogenously added PGI2 mimicked the endothelium-dependent contractions elicited by acetylcholine. These contractions were in both cases transient of small magnitude and virtually abolished by the presence of a functional NO-synthase. By contrast, contractions in response to U46619, 8-isoprostane, PGE2, PGF2α or PGD2 were sustained and slowly developing. Furthermore, if the endothelium-derived NO, a potent functional antagonist, produces a marked rightward shift of the concentration–response curves of these prostaglandins, it virtually abolishes the contractions to PGI2 and the endothelium-dependent contractions to acetylcholine. Therefore, the release of PGI2 could explain the endothelium-dependent contractions in response to acetylcholine. In both cases, the transient nature of the contraction can be due to the rapid degradation of PGI2 into its inactive metabolite 6-keto-PGF1α. Furthermore, the release of a weak agonist of the TP receptor, such as PGI2, may explain the relatively small amplitude of the endothelium-dependent contractions in response to acetylcholine.
The release of PGI2 was not affected by the inhibition of NO-synthase, consistent with an exclusive role of NO as a functional antagonist of the EDCF response (Yang et al., 2004), or by S 18886, the TP receptor antagonist, in agreement with the observation that the activation of the TP receptor mediates the effect of EDCF on smooth muscle cells, but does not contribute to the endothelial production of the factor (Yang et al., 2003b). The pattern of inhibition of PGI2 release by COX inhibitors was identical to that of the endothelium-dependent contractions, that is, partial inhibition by the preferential COX-2 inhibitor, NS 398, and complete inhibition either by the preferential COX-1 inhibitor, VAS, or the nonselective inhibitor, indomethacin (Yang et al., 2002). Whether the partial inhibition by the COX-2 inhibitor should be attributed to a contribution of this enzyme, which is expressed in the endothelium of aging and hypertensive rats (Heymes et al., 2000; Alvarez et al., 2005), or to an ancillary effect of NS 398 on COX-1 remains to be determined. Taken into conjunction, these results substantiate the suggestion of Rapoport and Williams (1996) that PGI2 is one of the EDCFs released by acetylcholine in the aorta of SHR and WKY.
However, Kato et al. (1990) as well as Ge et al. (1995) have proposed that PGH2 must be the EDCF released by acetylcholine. This proposal was based on the indirect measurement of PGH2 release and on the observation that the endoperoxide was a more potent contracting agent in SHR than in WKY. The present study confirms the latter observation and shows that the transient contractile response mimics the acetylcholine-induced endothelium-dependent contractions.
PGH2 is an unstable prostaglandin which is spontaneously or enzymatically transformed in the more stable isomer PGE2 and in the presence of mild reducing agents, such as SnCl2, is converted to PGF2α (Hamberg et al., 1974; Ge et al., 1995; Camacho et al., 1998). The amount of PGH2 can be estimated, theoretically, from the difference in PGF2α production in the absence and presence of SnCl2. In the present study, the presence of SnCl2 (1 mM) produced unspecific effects and did not allow a proper quantification of prostaglandin release or a proper recording of changes in isometric tension (unpublished observations). In endothelial cells, if the constitutive presence of the soluble PGE-synthase associated with COX-1 is debatable, the parallel induction of the membrane-bound form of PGE-synthase with COX-2 is well documented (Soler et al., 2000; Murakami et al., 2002). Since the induction of COX-2 has been suggested in the aorta of SHR and aging WKY (Heymes et al., 2000; Alvarez et al., 2005), the production of PGE2 might be of enzymatic origin. However, it cannot be excluded that the rate of formation of PGH2 exceeds its metabolism. If one assumes that, under the present experimental conditions, the entire production of PGE2 is a perfect surrogate for the endothelial production of PGH2, this production is roughly 30 times less than that of PGI2. Although PGH2 is approximately 30 times more potent than PGI2 in producing contraction, it is difficult to attribute an exclusive role to the endoperoxide in acetylcholine-induced endothelium-dependent contractions.
In the spontaneous hypertensive rat, the augmented responsiveness to endoperoxides, unlike the overexpression of COX-1, is already present in the aorta of prehypertensive animals (Iwama et al., 1992; Jameson et al., 1993; Ge et al., 1999). Thus, this hyper responsiveness may constitute a genetic platform for the disease. By contrast, the overexpression of COX probably reflects an adjustment to the chronic hypertensive process, resulting in premature aging of the endothelial cells. This interpretation is reinforced by the observations that endothelium-dependent contractions appear also in arteries of aging normotensive animals (Koga et al., 1989; Fujii et al., 1999; Heymes et al., 2000). The resulting massive increase in PGI2 production is associated with the disappearance of functional IP receptors leading to relaxation. Taken in conjunction, these observations suggest that both PGI2 and PGH2 can contribute to the acetylcholine-induced endothelium-dependent contractions.
In order to determine more precisely the contribution of PGI2 in the endothelium-dependent contractions evoked by acetylcholine, it was attempted to inhibit PGI synthase. The monoamine oxidase inhibitor and antidepressant tranylcypromine, at the concentration used in the present study, is often presented as a nonspecific inhibitor of this enzyme (Garcia-Cohen et al., 2000). However, under the present experimental conditions, tranylcypromine did not affect the production of PGI2 or PGE2, confirming previous observations in the same artery (Rapoport & Williams, 1996), and precluding its utilization. U 51605 is a stable analog of PGH2 and a partial agonist at TP receptors (Huzoor-Akbar et al., 1985; Mukhopadhyay et al., 1985), properties which were confirmed in the present study. U 51605 produced a concentration-dependent inhibition of PGI2 release and for the two highest concentrations tested also that of thromboxane A2, indicating that this compound is a preferential inhibitor of PGI synthase (Gorman et al., 1977; 1979). Since dazoxiben, the specific thromboxane synthase inhibitor, did not affect the acetylcholine-induced endothelium-dependent contractions, this compound could be considered as a useful tool to investigate the role of protacyclin in these contractions. Importantly, U 51605 did not interact with the endothelial muscarinic receptor since this drug did not affect the endothelium-dependent relaxation in response to acetylcholine.
U 51605 at 0.5 μM, the lowest concentration tested, significant blocked the TP receptor and produced a 50% inhibition of PGI2 release. Paradoxically, the inhibition of the release of PGI2, the putative EDCF and the concomitant blockade of TP receptors were associated with an enhancement of the endothelium-dependent contractions to acetylcholine. This paradox is only apparent since the inhibition of PGI2 release was compensated by a major increase in PGE2 and PGF2α production. In endothelial cells, the inhibition of PGI synthase consistently leads to an increase in PGE2 production (Zou et al., 1999; Bachschmid et al., 2003). Again, whether or not the release of PGE2 and PGF2α could be solely attributable to the nonenzymatic transformation of PGH2 or is partially linked to the activation of endothelial PG synthases and/or the transcellular metabolism of PGH2 by the underlying smooth muscle cells is unknown.
U 51605 at 1 μM did not potentiate, and at 3 μM inhibited the acetylcholine-induced endothelium-dependent contractions, most likely because of the overwhelming antagonistic properties of this compound toward TP receptors. Additionally, the fact that U 51605 does not produce a concentration-dependent increase in PGE2 and PGF2α production while the inhibition of PGI2 release was concentration-dependent may indicate other nonspecific properties of U 51605.
In conclusion, the endothelium-dependent contractions elicited by acetylcholine in the aorta of SHR and aging WKY most likely involve at least in part the release of PGI2. This conclusion is based on the following: (a) in WKY and SHR, PGI2 is a contracting but not a relaxing factor; (b) PGI2 is a more potent contracting agent in SHR than in WKY; (c) the contractions evoked by PGI2 mimic the endothelium-dependent contractions produced by acetylcholine both in terms of duration and amplitude; (d) the PGI2 and the endothelium-dependent contractions both involve activation of TP receptors; (e) PGI2 is the most abundant prostaglandin released by acetylcholine and is of endothelial origin; (f) the release of PGI2 is two times larger in SHR than in WKY; (g) the time course of the release of PGI2 is compatible with the time course of the observed endothelium-dependent contractions; (h) the release of PGI2 correlates with the amplitude of the endothelium-dependent contractions over the full concentration range of acetylcholine in both WKY and SHR; (i) the endothelium-dependent contractions and the release of PGI2 are affected similarly by COX inhibitors; and (j) the inhibition of PGI2 synthesis enhances the acetylcholine-induced endothelium-dependent contractions. Paradoxically, this observation also supports the hypothesis that PGI2 contributes to endothelium-dependent contractions, since the inhibition of PGI-synthase may enhance PGH2 spillover, a more potent TP receptor agonist than PGI2 itself. This hypothesis that PGI2 is an EDCF is in agreement with a recent study suggesting that PGI2 is the main factor accounting for endothelial dysfunction in the SHR aorta (Blanco-Rivero et al., 2005).
PGH2 most likely contributes also to the endothelium-dependent contractions evoked by acetylcholine. Most of the arguments developed above for a contribution of PGI2 apply also for PGH2. Nevertheless, although it is difficult to quantify exactly the extent of PGH2 production, the amount released in response to acetylcholine is 30–100 times less than that of PGI2. However, under conditions when the PGI-synthase activity is inhibited, either pharmacologically (U 51605) or under pathological conditions (for instance, following the peroxynitrate-dependent tyrosine nitration of the enzyme; Zou et al., 2002), the contribution of PGH2 will increase and so will the amplitude of the endothelium-dependent contractions.
Acknowledgments
We thank M. Gaudin and M. Germain for technical assistance. Dr J. Morrow was supported by NIH grants DK48831, GM15431, CA77839, RR00095.
Abbreviations
- COX
cyclooxygenase
- EDCF
endothelium-derived contracting factor(s)
- NOS
nitric oxide synthase
- L-NA
NG-nitro-L-arginine
- SHR
spontaneously hypertensive rat
- PGH2
prostaglandin H2
- PGI2
prostacyclin
- WKY
Wistar–Kyoto rats
References
- ALVAREZ Y., BRIONES A.M., BALFAGON G., ALONSO M.J., SALAICES M. Hypertension increases the participation of vasoconstrictor prostanoids from cyclooxygenase-2 in phenylephrine responses. J. Hypertens. 2005;23:767–777. doi: 10.1097/01.hjh.0000163145.12707.63. [DOI] [PubMed] [Google Scholar]
- AUCH-SCHWELK W., KATUSIC Z.S., VANHOUTTE P.M. Thromboxane A2 receptor antagonists inhibit endothelium-dependent contractions. Hypertension. 1990;15:699–703. doi: 10.1161/01.hyp.15.6.699. [DOI] [PubMed] [Google Scholar]
- BACHSCHMID M., THURAU S., ZOU M.H., ULLRICH V. Endothelial cell activation by endotoxin involves superoxide/NO-mediated nitration of prostacyclin synthase and thromboxane receptor stimulation. FASEB J. 2003;17:914–916. doi: 10.1096/fj.02-0530fje. [DOI] [PubMed] [Google Scholar]
- BLANCO-RIVERO J., CACHOFEIRO V., LAHERA V., ARAS-LOPEZ R., MARQUEZ-RODAS I., SALAICES M., XAVIER F.E., FERRE M., BALFAGON G. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension. 2005;46:107–112. doi: 10.1161/01.HYP.0000171479.36880.17. [DOI] [PubMed] [Google Scholar]
- CAMACHO M., LOPEZ-BELMONTE J., VILA L. Rate of vasoconstrictor prostanoids released by endothelial cells depends on cyclooxygenase-2 expression and prostaglandin I synthase activity. Circ. Res. 1998;83:353–365. doi: 10.1161/01.res.83.4.353. [DOI] [PubMed] [Google Scholar]
- CORRIU C., FELETOU M., EDWARDS G., WESTON A.H., VANHOUTTE P.M. Differential effects of Prostacyclin and Iloprost in the isolated carotid artery of the guinea-pig. Eur. J. Pharmacol. 2001;426:89–94. doi: 10.1016/s0014-2999(01)01203-1. [DOI] [PubMed] [Google Scholar]
- DAVIS K., GRINSBURG R., BRISTOW M., HARRISON D.C. Biphasic action of prostacyclin in the human coronary artery. Clin. Res. 1980;28:165A. [Google Scholar]
- FUJII K., ONAKA U., ABE I., FUJISHIMA M. Eicosanoids and membrane properties in arteries of aged spontaneously hypertensive rats. J. Hypertens. 1999;17:75–80. doi: 10.1097/00004872-199917010-00012. [DOI] [PubMed] [Google Scholar]
- GARCIA-COHEN E.-C., MARIN J., DIEZ-PICAZO L.D., BAENA A.B., SALAICES M., RODRIGUEZ-MARTINEZ M.A. Oxidative stress induced by tert-butyl hydroperoxide causes vasoconstriction in the aorta from hypertensive and aged rats, role of cyclooxygenase-2 isoform. J. Pharmacol. Exp. Ther. 2000;293:75–81. [PubMed] [Google Scholar]
- GE T., HUGHES H., JUNQUERO D.C., WU K.K., VANHOUTTE P.M., BOULANGER C.M. Endothelium-dependent contractions are associated with both augmented expression of prostaglandin H synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circ. Res. 1995;76:1003–1010. doi: 10.1161/01.res.76.6.1003. [DOI] [PubMed] [Google Scholar]
- GE T., VANHOUTTE P.M., BOULANGER C.M. Increased response to prostaglandin H2 precedes changes in PGF-synthase 1 expression in the SHR aorta. Acta Pharmacol. Sin. 1999;20:1087–1092. [PubMed] [Google Scholar]
- GORMAN R.R., BUNDY G.L., PETERSON D.C., SUN F.F., MILLER O.V., FITZPATRICK F.A. Inhibition of platelet thromboxane synthetase by 9,11-azoprosta-5,13-dienoic acid. Proc. Natl. Acad. Sci. U.S.A. 1977;74:4007–4011. doi: 10.1073/pnas.74.9.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORMAN R.R., HAMILTON R.D., HOPKINS N.K. Stimulation of human foreskin fibroblast adenosine 3′,5′-cyclic monophosphate levels by prostacyclin (prostaglandin I2) J. Biol. Chem. 1979;254:1671–1676. [PubMed] [Google Scholar]
- HAMBERG M., SVENSSON J., WAKABAYASHI T., SAMUELSSON B. Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proc. Natl. Acad. Sci. U.S.A. 1974;71:345–349. doi: 10.1073/pnas.71.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYMES C., HABIB A., YANG D., MATHIEU E., MAROTTE F., SAMUEL J.-L., BOULANGER C.M. Cyclo-oxygenase-1 and -2 contribution to endothelial dysfunction in ageing. Br. J. Pharmacol. 2000;131:804–810. doi: 10.1038/sj.bjp.0703632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUZOOR-AKBAR, MUKHOPADHYAY A., ANDERSON K.S., NAVRAN S.S., ROMSTEDT K., MILLER D.D., FELLER D.R. Antagonism of prostaglandin-mediated responses in platelets and vascular smooth muscle by 13-azaprostanoic acid analogues. Evidence for selective blockade of thromboxane A2 responses. Biochem. Pharmacol. 1985;34:641–647. doi: 10.1016/0006-2952(85)90258-8. [DOI] [PubMed] [Google Scholar]
- IL'YASOVA D., MORROW J.D., IVANOVA A., WAGENKNETCH L.E. Epidemiological marker for oxidant status, comparison of the ELISA and the gas chromatography/mass spectrometry assay for urine 2,3-dinor-5,6-dihydro-15-F2t-isoprostane. Ann. Epidemiol. 2004;14:793–797. doi: 10.1016/j.annepidem.2004.03.003. [DOI] [PubMed] [Google Scholar]
- IWAMA Y., KATO T., MURAMATSU M., ASANO H., SHIMIZU K., TOKI Y., MIYAZAKI Y., OKUMURA K., HASHIMOTO H., ITO T., SATAKE T. Correlation with blood pressure of the acetylcholine-induced endothelium-derived contracting factor in the rat aorta. Hypertension. 1992;19:326–332. doi: 10.1161/01.hyp.19.4.326. [DOI] [PubMed] [Google Scholar]
- JAMESON M., DAI F.X., LÜSCHER T., SKOPEC J., DIEDERICH A. Endothelium-derived contracting factors in resistance arteries of young spontaneously hypertensive rats before development of overt hypertension. Hypertension. 1993;21:280–288. doi: 10.1161/01.hyp.21.3.280. [DOI] [PubMed] [Google Scholar]
- JANSSEN L.J. Are endothelium-derived hyperpolarizating and contracting factors isoprostanes . Trends Pharmacol. Sci. 2002;23:59–62. doi: 10.1016/s0165-6147(02)01890-4. [DOI] [PubMed] [Google Scholar]
- KATO T., IWAMA Y., OKAMURA K., HASHIMOTO H., ITO T., SAKATE T. Prostaglandin H2 may be the endothelium-derived contracting factor released by acetylcholine in the aorta of the rat. Hypertension. 1990;15:475–481. doi: 10.1161/01.hyp.15.5.475. [DOI] [PubMed] [Google Scholar]
- KOGA T., TAKATA Y., KOBAYASHI K., TAKISHITA S., YAMASHITA Y., FUJISHIMA M. Age and hypertension promote endothelium-dependent contractions to acetylcholine in the rat aorta of the rat. Hypertension. 1989;14:542–548. doi: 10.1161/01.hyp.14.5.542. [DOI] [PubMed] [Google Scholar]
- LEVY J.V. Prostacyclin-induced contraction of isolated aortic strips from normal and spontaneously hypertensive rats (SHR) Prostaglandins. 1980;19:517–5250. doi: 10.1016/s0090-6980(80)80002-5. [DOI] [PubMed] [Google Scholar]
- LÜSCHER T.F., VANHOUTTE P.M. Endothelium-dependent contractions to acetylcholine in the aorta of spontaneously hypertensive rat. Hypertension. 1986;8:344–348. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- MONCADA S., GRYGLEWSKI R.J., BUNTING S., VANE J.R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- MORROW J.D., HILL K.E., BURK R.F., NANNOUR T.M., BADR K.F., ROBERTS II L.J. A series of prostaglandins F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical catalyzed mechanism. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUKHOPADHYAY A., NAVRAN S.S., AMIN H.M., ABDEL-AZIZ S.A., CHANG J., SOBER D.J., MILLER D.D., FELLER D.R. Effects of trimetoquinol analogs for antagonism of endoperoxide/thromboxane A2-mediated responses in human platelets and rat aorta. J. Pharmacol. Exp. Ther. 1985;232:1–9. [PubMed] [Google Scholar]
- MURAKAMI M., NAKATAMI Y., TANIOKA T., KUDO I. Prostaglandin E synthase. Prostagland. Other Lipid Mediat. 2002;68–69:383–399. doi: 10.1016/s0090-6980(02)00043-6. [DOI] [PubMed] [Google Scholar]
- NUMAGUCHI Y., HARADA M., OSANAI H., HAYASHI K., TOKI Y., OKAMURA K., ITO T., HAYAKAWA T. Altered gene expression of prostacyclin synthase and prostacyclin receptor in the thoracic aorta of spontaneously hypertensive rats. Cardiovasc. Res. 1999;41:682–688. doi: 10.1016/s0008-6363(98)00239-9. [DOI] [PubMed] [Google Scholar]
- PFISTER S.L., HUGHES M.J., ROSOLOWSKI M., CAMPBELL W.B. Role of contaminating platelets in thromboxane synthesis in primary culture of human umbilical vein endothelial cells. Prostagland. Other Lipid Med. 2002;70:39–49. doi: 10.1016/s0090-6980(02)00009-6. [DOI] [PubMed] [Google Scholar]
- POMERANTZ K., SINTEROSE A., RAMWELL P. The effect of prostacyclin on the human umbilical artery. Prostaglandins. 1978;15:1035–1044. doi: 10.1016/0090-6980(78)90046-1. [DOI] [PubMed] [Google Scholar]
- RAPOPORT R.M., WILLAMS S.P. Role of prostaglandins in acetylcholine-induced contraction of aorta from spontaneously hypertensive and Wistar–Kyoto rats. Hypertension. 1996;28:64–75. doi: 10.1161/01.hyp.28.1.64. [DOI] [PubMed] [Google Scholar]
- SIMONET S., DESCOMBES J.J., VALLEZ M.O., DUBUFFET T., LAVIELLE G., VERBEUREN T.J.S 18886, a new thromboxane (TP)-receptor antagonist is the active isomer of S 18204 in all species, except in the guinea-pig Recent Advances in Prostaglandin, Thromboxane, and Leukotriene Research 1998New York, U.S.A.: Plenum Press; 173–176.ed. Sinzinger et al., pp [DOI] [PubMed] [Google Scholar]
- SOLER M., CAMACHO M., ESCUDERO J.-R., INIGUEZ M.A., VILA L. Human vascular smooth muscle but not endothelial cells express prostaglandin E synthase. Circ. Res. 2000;87:504–507. doi: 10.1161/01.res.87.6.504. [DOI] [PubMed] [Google Scholar]
- TADDEI S., VANHOUTTE P.M. Endothelium-dependent contractions to endothelin in the rat aorta are mediated by thromboxane A2. J.Cardiovasc. Pharmacol. 1993;22:S328–S331. doi: 10.1097/00005344-199322008-00086. [DOI] [PubMed] [Google Scholar]
- VANHOUTTE P.M., FELETOU M., TADDEI S. Endothelium-dependent contractions in hypertension. Br. J. Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATKINS M.T., PATTON G.M., SOLER H.M., ALBADAWI H., HUMPHRIES D.E., EVANS J.E., KADOWAKI K. Synthesis of 8-epi-prostaglandinF2α by human endothelial cells, role of prostaglandin H2 synthase. Biochem. J. 1999;344:747–775. [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS S.P., DORN II G.W., RAPOPORT R.M. Prostaglandin I2 mediates contraction and relaxation of vascular smooth muscle. Am. J. Physiol. 1994;267:H796–H803. doi: 10.1152/ajpheart.1994.267.2.H796. [DOI] [PubMed] [Google Scholar]
- WISE H., JONES R.L. Focus on prostacyclin and its novel mimetics. Trends Pharmacol. Sci. 1996;17:17–21. doi: 10.1016/0165-6147(96)81565-3. [DOI] [PubMed] [Google Scholar]
- YANG D., FÉLÉTOU M., BOULANGER C.M., WU H.F., LEVENS N., ZHANG J.N., VANHOUTTE P.M. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br. J. Pharmacol. 2002;136:104–110. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG D., FÉLÉTOU M., LEVENS N., ZHANG J.N., VANHOUTTE P.M. A diffusible substance(s) mediates endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 2003b;41:143–148. doi: 10.1161/01.hyp.0000047651.45322.16. [DOI] [PubMed] [Google Scholar]
- YANG D., GLUAIS P., ZHANG J.-N., VANHOUTTE P.M., FÉLÉTOU M. NO and inactivation of the endothelium-dependent contracting factor released by acetylcholine in SHR. J. Cardiovasc. Pharmacol. 2004;43:815–820. doi: 10.1097/00005344-200406000-00011. [DOI] [PubMed] [Google Scholar]
- YANG D., LEVENS N., ZHANG J.N., VANHOUTTE P.M., FÉLÉTOU M. Specific potentiation of endothelium-dependent contractions in SHR by tetrahydrobiopterin. Hypertension. 2003a;41:136–142. doi: 10.1161/01.hyp.0000047669.93078.a7. [DOI] [PubMed] [Google Scholar]
- ZHAO Y.J., WANG J., TOD M.L., RUBIN L.J., YUAN X.J. Pulmonary vasoconstriction effects of prostacyclin in rats, potential role of thromboxane receptors. J. Appl. Physiol. 1996;81:2595–2603. doi: 10.1152/jappl.1996.81.6.2595. [DOI] [PubMed] [Google Scholar]
- ZOU M., JENDRAL M., ULLRICH V. Prostaglandin endoperoxide-dependent vasospasm in bovine coronary arteries after nitration of prostacyclin synthase. Br. J. Pharmacol. 1999;126:1283–1292. doi: 10.1038/sj.bjp.0702434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOU M.H., SHI C., COHEN R.A. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes. 2002;51:198–203. doi: 10.2337/diabetes.51.1.198. [DOI] [PubMed] [Google Scholar]