Abstract
The effectiveness of a selective endothelin receptor-A (ET-A) antagonist, A-127722 (approximately 10 mg kg−1 day−1 as 200 mg kg−1 powdered food), to reverse existing cardiac remodelling and prevent further remodelling was tested in deoxycorticosterone acetate (DOCA)-salt hypertensive rats.
Uninephrectomised rats (UNX) administered DOCA (25 mg every fourth day s.c.) and 1% NaCl in drinking water for 28 days developed hypertension (systolic blood pressure (BP): UNX 128±6 mmHg, DOCA-salt 182±5* mmHg; *P<0.05 vs UNX), left ventricular hypertrophy (UNX 1.99±0.06 mg kg−1 body wt, DOCA-salt 3.30±0.08* mg kg−1 body wt), decreased left ventricular internal diameter (UNX 6.69±0.18 mm, DOCA-salt 5.51±0.37* mm), an increased left ventricular monocyte/macrophage infiltration together with an increased interstitial collagen from 2.7±0.3 to 11.7±1.3%, increased passive diastolic stiffness (UNX 21.1±0.5, DOCA-salt 30.1±1.3*), prolongation of the action potential duration at 20 and 90% of repolarisation (APD20–UNX 6.8±1.1, DOCA-salt 10.1±1.5* ms; APD90–UNX 34.4±3.5 ms, DOCA-salt 64.3±10.4* ms) and vascular dysfunctions (2.6-fold decrease in maximal contractile response to noradrenaline, 3.5-fold decrease in maximal relaxation response to acetylcholine).
Administration of A-127722 for 14 days starting 14 days after surgery attenuated the increases in systolic BP (150±6** mmHg, **P<0.05 vs DOCA-salt), left ventricular wet weight (2.65±0.06** mg kg−1 body wt) and internal diameter (6.39±0.31** mm), prevented left ventricular monocyte/macrophage accumulation, attenuated the increased left ventricular interstitial collagen (7.6±1.3%**), reversed the increased passive diastolic stiffness (22.1±1.2**), attenuated the action potential duration prolongation (APD20 – 7.6±1.4**, APD90 – 41.5±6.9** ms) and normalised changes in vascular function.
ET-A receptor antagonism both reverses and prevents the cardiac and vascular remodelling in DOCA-salt hypertension and improves cardiovascular function.
Keywords: Endothelin, remodelling, hypertension, hypertrophy, collagen, rats
Introduction
Endothelin (ET)-1, a 21-amino-acid peptide released by endothelial cells, is a potent vasoconstrictor that may play a role in the pathogenesis of cardiovascular diseases as diverse as hypertension, chronic heart failure, ischaemic heart disease, atherosclerosis and chronic renal failure (Touyz & Schiffrin, 2003; Masaki, 2004). ET-1 activates ET-A and ET-B receptors; nonselective ET receptor antagonists such as bosentan attenuated left ventricular remodelling and improved survival in rats with coronary artery ligation (Mulder et al., 1997). However, early intervention with the high-affinity nonselective ET receptor antagonist, SB 209670, promoted left ventricular dilatation without altering infarct size or collagen composition (Øie et al., 2002). Using the same model, treatment with the selective ET-A antagonist, LU 135252 (30 mg kg−1 day−1), for 9 months initially lowered blood pressure (BP), improved cardiac function measured by echocardiography and decreased left ventricular collagen density but did not improve long-term survival (Mulder et al., 2002).
These potential therapeutic benefits of ET receptor antagonists were defined using rat models of cardiovascular disease, in particular the coronary artery ligation model of myocardial infarction and heart failure. Other studies with ET receptor antagonists have used the deoxycorticosterone acetate (DOCA)-salt hypertensive rat. These rats develop hypertension, cardiac hypertrophy and fibrosis together with overexpression of ET by vascular cells and cardiac endothelial cells, suggesting that ET plays an important role in these structural and functional changes (Schiffrin, 2001). In this model, administration of the ET-A receptor selective antagonist, A-127722 (30 mg kg−1 day−1), as a prevention protocol produced a small decrease in BP with no change in heart weight but normalised procollagen expression and prevention of cardiac collagen deposition (Ammarguellat et al., 2001). Further, the increase in fibronectin deposition, matrix metalloproteinase activity and upregulation of inflammatory mediators was reduced following ET-A receptor antagonism with BMS 182874 (40 mg kg−1 day−1), another selective ET-A receptor antagonist (Ammarguellat et al., 2002). This ET-A receptor selective antagonist reduced cardiac macrophage content and decreased expression of cell adhesion molecules in DOCA-salt hypertensive rats (Callera et al., 2004). DOCA-salt hypertensive rats also developed prolongation of the action potential duration (Momtaz et al., 1996); ET produced similar prolongation (Yorikane et al., 1991) that was blocked by the selective ET-A receptor antagonist, BQ-123 (Garjani et al., 1995). Endothelial dysfunction developed in DOCA-salt hypertensive rats and was prevented by administration of apocynin, an inhibitor of NADPH oxidase (Beswick et al., 2001), or the antioxidant, sesamin (Nakano et al., 2003).
Increases in reactive oxygen species, especially superoxide produced by NADPH oxidase, may underlie the development of hypertension and cardiovascular remodelling in DOCA-salt rats (Beswick et al., 2001; Li et al., 2003). Reactive oxygen species can initiate free radical chain reactions resulting in membrane and cell damage; this has been proposed as the cause of cardiovascular damage (Sawyer et al., 2002). The major source of superoxide production in the vasculature is NADPH oxidase (Li & Shah, 2004). The most likely mechanism for NADPH oxidase activation in DOCA-salt hypertensive rats is an increased ET-1 concentration acting through ET-A receptors since selective blockade of ET-A receptors reduced arterial superoxide formation and improved structure and function (Callera et al., 2003; Li et al., 2003; Pu et al., 2003).
This study has used a reversal protocol (oral administration of A-127722 for 2 weeks started 2 weeks after initiation of DOCA-salt hypertension) to determine whether antagonism of ET-A receptors reverses existing changes and possibly prevents further cardiovascular remodelling, thus improving cardiovascular function. We have used echocardiography to define cardiac function in vivo and the isolated Langendorff heart to define function ex vivo. Single-cell microelectrode studies on isolated left ventricular papillary muscles were used to determine changes in cardiac action potentials. Isolated thoracic aortic rings were used to examine endothelial dysfunction in DOCA-salt vessels. Collagen deposition was defined using laser confocal microscopy of picrosirius red-stained slices.
Methods
DOCA-salt hypertensive rats
Male Wistar rats (8–10-week-old) were obtained from the Central Animal Breeding House of The University of Queensland. All experimental protocols were approved by the Animal Experimentation Ethics Committee of The University of Queensland under the guidelines of the National Medical and Health Research Council of Australia. Rats were fed pelleted rat chow and were housed in 12-h light/dark conditions. Uninephrectomy was performed on all treated rats. The rats were anaesthetised with tiletamine (25 mg kg−1 i.p.) and zolazepam (25 mg kg−1 i.p.) (Zoletil®) together with xylazine (10 mg kg−1 i.p.) (Rompun®); a lateral abdominal incision was used to access the kidneys, and the left renal vessels and ureter were ligated. The left kidney was removed and weighed and the incision site sutured. Uninephrectomised rats (UNX rats) were given either no further treatment or 1% NaCl in the drinking water with subcutaneous injections of DOCA (25 mg in 0.4 ml dimethylformamide every fourth day) (DOCA-salt rats) (Dallemagne et al., 2000). Experiments were performed 14 or 28 days after surgery. After 14 days, rats were given A-127722 (200 mg kg−1 powdered food) for a further 14 days.
Assessment of physiological parameters
Food and water intake and body weights were measured daily for all rats. Systolic BP and heart rate were measured in a subgroup of rats lightly anaesthetised with Zoletil® (tiletamine 15 mg kg−1 i.p. with zolazepam 15 mg kg−1 i.p.) using a tail pulse transducer (MLT1010) and an inflatable tail cuff with a Capto SP844 physiological pressure transducer (MLT844/D) connected to a PowerLab data acquisition unit (ADInstruments, Sydney, Australia). Rats were killed with pentobarbitone (200 mg kg−1 i.p.). Blood was taken from the abdominal vena cava, centrifuged and the plasma frozen.
Echocardiography
Serial, in vivo left parasternal and left apical echocardiographic images of rats were obtained using the Hewlett Packard Sonos 5500 (12 MHz frequency fetal transducer) at an image depth of 3 cm using two focal zones (Brown et al., 2002). Rats were anaesthetised with Zoletil® (tiletamine 25 mg kg−1 i.p. with zolazepam 25 mg kg−1 i.p.) together with Rompun® (xylazine 10 mg kg−1 i.p.), which produces general anaesthesia in rats for 2–3 h. Left ventricular M-mode measurements at the level of the papillary muscles included left ventricular end-diastolic dimensions, left ventricular end-systolic dimensions, interventricular septum and posterior wall thicknesses and fractional shortening. Cardiac output, ejection fraction and left ventricular mass were derived from these values (Litwin et al., 1994). Pulsed-wave Doppler analyses of mitral valve inflows were used as estimates of diastolic function.
Isolated heart preparations
The nonrecirculating Langendorff heart preparation was used for isolated myocardial experiments (Brown et al., 1999). Briefly, rats were anaesthetised with pentobarbitone sodium (100 mg kg−1 i.p.). The right leg femoral vein was exposed and heparin (1000 IU) administered as an intravenous injection. The hearts were rapidly excised and placed in ice-cold modified Krebs–Henseleit solution, and the aorta was isolated and cannulated via the dorsal root. Retrograde perfusion was initiated at constant pressure (100 cm H2O) with modified Krebs–Henseleit buffer containing (in mM) NaCl 119.1, KCl 4.75, MgSO4 1.19, KH2PO4 1.19, NaHCO3 25.0, glucose 11.0 and CaCl2 2.16 maintained at 37°C and bubbled with 95% O2 and 5% CO2. A latex balloon catheter was inserted into the left ventricle for measurement of isovolumic left ventricular function via connection to a Capto SP844 physiological pressure transducer (MLT844/D) linked to a PowerLab recording system. Hearts were paced at 250 b.p.m. by attaching two electrodes to the surface of the right atria. End-diastolic pressure was initially set to 5 mmHg by balloon inflation and all hearts received an equilibration period of approximately 25 min. End-diastolic pressure was measured for 3 min at 5 mmHg increments beginning at 0 mmHg up to a maximum of 30 mmHg. Measurements of diastolic pressure and systolic pressure were made after 2 min of each 3 min recording for further calculation of diastolic stiffness and left ventricular developed pressure. Myocardial diastolic stiffness was defined by the stiffness constant (k, dimensionless), which is the slope of the linear relation between the tangent elastic modulus (E, dyne cm−2) and stress (σ, dyne cm−2) (Brown et al., 1999). To assess contractile function, maximal +dP/dt values were calculated at a diastolic pressure of 10 mmHg. At the end of the experiment, the atria were removed and the weight of the ventricles plus septum was recorded.
Microelectrode studies on isolated left ventricular papillary muscles
Rats were anaesthetised by CO2 inhalation and then killed by exsanguination. The thorax was opened quickly and the heart was removed. The left ventricular papillary muscles were quickly dissected in cold Tyrode physiological salt solution (in mM: NaCl 136.9, KCl 5.4, MgCl2·H2O 1.0, NaH2PO4·2H2O 0.4, NaHCO3 22.6, CaCl2·2H2O 1.8, glucose 5.5, ascorbic acid 0.3, Na2EDTA 0.05) bubbled with 95% O2 and 5% CO2. A stainless-steel hook was placed in one end of the papillary muscle. The muscle was placed in a 1.0 ml experimental chamber continuously perfused with gassed warm Tyrode solution at 3 ml min−1 (35±0.5°C) and was fixed at the other end with a small stainless steel pin embedded into a rubber base. The hook was attached to a modified sensor element (SensoNor AE801) connected to an amplifier (World Precision Instruments, Sarasota, FL, U.S.A.; TBM-4). The muscle was slowly stretched to maximum preload over 1 min. Contractions were induced by field stimulation (Grass SD-9) via electrodes on either side of the muscle (stimulation frequency 1 Hz, pulse width 0.5 ms; stimulus strength 20% above threshold).
After maximum preload was attained, the muscle was allowed to equilibrate for a further 45 min before impalement with a glass microelectrode (World Precision Instruments, filamented borosilicate glass, outer diameter 1.5 mm) that had a tip resistance of 5–15 mΩ when filled with 3 M KCl. The reference electrode was an Ag/AgCl electrode. A Cyto 721 electrometer (World Precision Instruments) was used to record bioelectrical activity. All signals were recorded via a PowerLab 4S data acquisition unit (ADInstruments, Sydney, Australia). Preparations that had a stable resting potential more negative than −60 mV were accepted. Continual impalement throughout an experiment was not always possible; however, if displacement occurred, then the results of a subsequent impalement were accepted provided the data fitted the criteria described above.
Isolated thoracic aortic rings
Thoracic aortic rings (approximately 4 mm in length) were suspended with a resting tension of 10 mN (Brown et al., 1991). Cumulative concentration–response curves were performed for noradrenaline and either acetylcholine or sodium nitroprusside in the presence of a submaximal contraction to noradrenaline. Maximal contraction was recorded as that produced by addition of isotonic potassium chloride (100 mM).
Collagen distribution by picrosirius red staining and laser confocal microscopy
All experimental animals had the major organs removed and weighed. From the organs removed, the left ventricle and septum underwent histological analysis. Tissues were initially fixed for 3 days in Telly's fixative (100 ml of 70% ethanol, 5 ml of glacial acetic acid and 10 ml of 40% formaldehyde) and then transferred into a prestain/fixative known as modified Bouin's fluid (85 ml of saturated picric acid, 5 ml glacial acetic acid and 10 ml of 40% formaldehyde) for 2 days. The samples were then dehydrated and embedded in paraffin wax. Thick sections (15 μm) were cut and placed on glass slides coated with Mayer's albumin solution (1 g powdered egg albumin, 50 ml glycerol, 50 ml distilled water), left to air dry for 2 days and then heated in an oven at 56°C for 1 h. Phosphomolybdic acid (0.2% in distilled water, 5 min) was then applied to reduce nonspecific binding of the stain to the section and then washed in distilled water. The collagen-selective picrosirius red stain (0.1% sirius red F3BA in saturated picric acid) was then used and allowed to incubate for 90 min. The sections were then washed, dehydrated and mounted in Depex with a coverslip. Image analysis of the stained sections took place on the laser scanning confocal microscope (BioRad MRC-1024 – Rhodamine/Texas red filter, 568 nm, emission 609 DF 32 by green excitation). Randomly assigned slides and sections were scanned representing the perivascular areas of the left ventricle. The images were taken with an objective lens of × 40 magnification and analysed for pixel intensity in a specified area of the section. The data were compiled by a software image-rendering program (IA-IP-Lab, Scanalytics Inc., Australia).
Immunofluorescence
For immunostaining, 5 μm thick sections were dewaxed and antigen retrieval was carried out by microwaving tissue sections in 0.01 M citrate buffer for 15 min followed by pepsin digestion (0.05 M) for a further 15 min at 37°C. Samples were then incubated for 20 min at room temperature in blocking solution (PBS containing 5% sheep serum and 1% bovine serum albumin) before application of primary antibodies for rat macrophages (ED1; Serotec mouse anti-rat ED1 diluted 1 : 15) or α-smooth muscle actin (α-SMA) (1A4; diluted 1 : 400) and incubation overnight at 4°C. Omission of primary antibodies, and staining with an irrelevant mouse immunoglobulin of the same isotype, served as negative controls. Following PBS washes, samples were incubated with IgG-fluorescein-conjugated secondary antibody (Chemicon, Temecula, CA, U.S.A.; diluted 1 : 200). Sections were counterstained with propidium iodide, mounted with gelvatol (polyvinyl alcohol/glycerol) mounting medium containing n-propyl gallate (5 mg ml−1) as an antifade agent and visualised with a Biorad MRC-1024 confocal laser scanning microscope using an objective lens of × 40 magnification. A zero to four grading scale was used to classify the extent of ED 1-positive monocyte/macrophage infiltration in the left ventricle: 0=no monocyte/macrophages present; 1=low levels of singular monocyte/macrophages located throughout the left ventricle; 2=moderate numbers of monocyte/macrophages located throughout the left ventricle; 3=large numbers of monocyte/macrophages located in groups throughout the left ventricle; 4=large numbers of monocyte/macrophages located as groups throughout the left ventricle and scar sites, and associated directly with areas of fibrosis. α-SMA was qualitatively assessed for spatial location in the left ventricle.
Data analysis
All results are given as mean±s.e.m. The negative log EC50 of the increase in force of contraction in mN was determined from the concentration giving half-maximal responses in individual concentration–response curves. These results were analysed by two-way analysis of variance followed by the Duncan test to determine differences between treatment groups and by paired or unpaired t-tests as appropriate; P<0.05 was considered significant.
Drugs
DOCA, heparin, noradrenaline, acetylcholine, sodium nitroprusside and α-SMA antibody were purchased from Sigma Chemical Company (St Louis, MO, U.S.A.) A-127722 (2(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-[[(dibutyl amino) carbonyl]methyl]-pyrrolidine-3-carboxylic acid) was provided by Abbott Pharmaceuticals (U.S.A.) and thoroughly mixed with ground rat food pellets to a final concentration of 200 mg kg−1. Noradrenaline, acetylcholine and sodium nitroprusside were dissolved in distilled water; DOCA was dissolved in dimethylformamide with mild heating.
Results
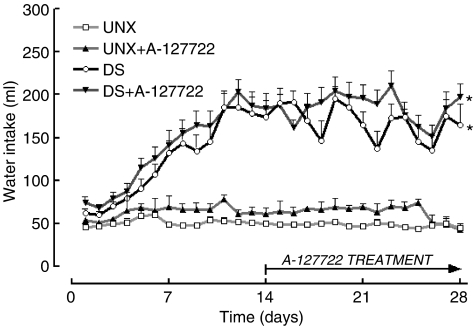
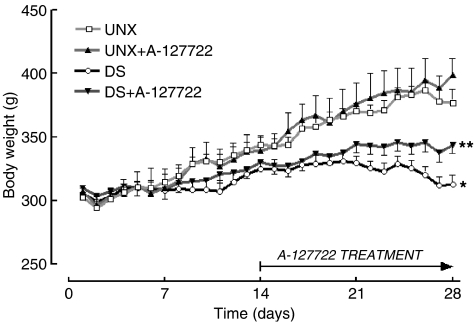
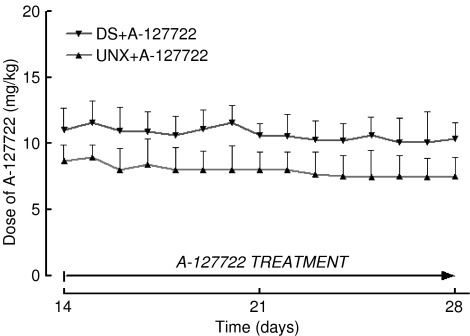
DOCA-salt hypertensive rats showed an increased water intake (Figure 1) but decreased body weight (Figure 2). Administration of A-127722 for 14 days starting 14 days after induction of hypertension failed to alter water intake (Figure 1); however, the final body weights were significantly improved (Figure 2). Intake of A-127722 calculated from daily food intake was not significantly different between the treated groups (UNX+A-127722, 8.9±0.7 mg kg−1 day−1; DOCA-salt+A-127722, 10.7±0.7 mg kg−1 day−1) (Figure 3). Systolic BP increased during DOCA-salt treatment while treatment with A-127722 prevented further increases in BP during the last 14 days (Table 1).
Figure 1.
Daily water intake measurements for UNX (n=21), UNX+A-127722 (n=16), DOCA-salt (n=36) and DOCA-salt+A-127722 (n=20) treated rats; *P<0.05 vs UNX rats.
Figure 2.
Daily body weight measurements for UNX (n=21), UNX+A-127722 (n=16), DOCA-salt (n=36) and DOCA-salt+A-127722 (n=20) treated rats; *P<0.05 vs UNX rats.
Figure 3.
Daily A-127722 dose for UNX+A-127722 (n=16) and DOCA-salt+A-127722 (n=20).
Table 1.
Physiological parameters
| Parameter | UNX (n=10) | UNX+A-127722 (n=8) | DOCA-salt 14 days (n=6) | DOCA-salt 28 days (n=10) | DOCA-salt+A-127722 28 days (n=8) |
|---|---|---|---|---|---|
| Whole animal | |||||
| Initial body weight (g) | 302±4 | 307±2 | 305±4 | 307±5 | 310±4 |
| Final body weight (g) | 376±11 | 399±13 | 325±6 | 312±8 | 344±7 |
| Heart rate (b.p.m.) | 390±12 | 382±19 | 401±23 | 397±17 | 368±20 |
| Systolic blood pressure (mmHg) | 128±6 | 127±3 | 158±3* | 182±5*# | 150±6** |
| Organ weights | |||||
| Left ventricular weight (mg g−1 body wt) | 1.99±0.06 | 2.20±0.05 | 2.48±0.05* | 3.30±0.08*# | 2.65±0.06**# |
| Right ventricular weight (mg g−1 body wt) | 0.56±0.02 | 0.54±0.04 | 0.56±0.02 | 0.65±0.03* | 0.63±0.03* |
| Kidney weight (mg g−1 body wt) | 4.98±0.11 | 4.65±0.08 | 7.76±0.33* | 10.90±0.40* | 10.03±0.55*# |
| Histology | |||||
| Thoracic aortic ring wall thickness (μm) | 192±7 | 196±8 | 236±4* | 312±5*# | 228±3** |
| Left ventricular perivascular collagen fraction (% area) | 27.9±4.4 | 32.3±3.9 | 37.8±2.1* | 38.9±2.8* | 32.7±1.9** |
| Left ventricular interstitial collagen fraction (% area) | 2.7±0.3 | 4.2±1.9 | 6.7±2.4* | 11.7±1.3*# | 7.6±1.3** |
| Kidney interstitial collagen fraction (% area) | 22.4±3.6 | 19.8±3.7 | 30.9±2.2* | 39.1±3.6* | 32.4±4.8* |
| Function | |||||
| Diastolic stiffness | 21.1±0.5 | 22.5±1.0 | 24.6±1.8* | 30.1±1.3*# | 22.1±1.2** |
| Maximum +dP/dt (mmHg s−1) | 1790±140 | 1630±100 | 1670±80 | 1380±170*# | 1440± 100* |
| Minimum −dP/dt (mmHg s−1) | 1490±70 | 1500±130 | 1500±110 | 1310±150 | 1250±110 |
| Echocardiography | |||||
| LVIDd (mm) | 6.69±0.18 | 7.17±0.16 | 6.29±0.16 | 5.51±0.37*# | 6.39±0.31** |
| LVPWd (mm) | 1.74±0.17 | 1.78±0.17 | 1.79±0.07 | 1.98±0.09 | 1.85±0.09 |
| E/A (early/atrial) mitral valve flow ratio | 1.92±0.02 | 1.88±0.07 | 1.82±0.27 | 1.64±0.10* | 1.88±0.10** |
| Ejection fraction (%) | 83.1±4.4 | 82.7±5.6 | 94.6±1.1* | 92.7±1.2* | 94.8±1.3* |
| Cardiac output (ml min−1) | 75.3±3.6 | 72.1±6.8 | 63.9±7.8 | 53.7±6.7* | 58.1±5.3* |
| Ascending aorta (m s−1) | 0.75±0.08 | 0.88±0.10 | 1.12±0.07* | 1.23±0.05* | 0.98±0.06** |
| Left ventricular mass (g) (Litwin et al., 1994) | 0.76±0.04 | 0.85±0.09 | 0.75±0.03 | 1.36±0.09*# | 0.99±0.08**# |
All values shown represent the mean±s.e.m.; LVIDd=left ventricular internal diameter in diastole; LVPWd=left ventricular posterior wall thickness in diastole.
P<0.05 vs UNX rats;
P<0.05 vs DOCA-salt rats.
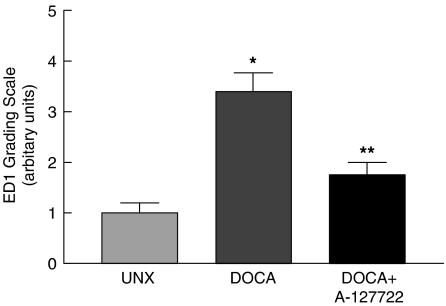
DOCA-salt rats showed an increasing degree of left ventricular hypertrophy during the study period as evidenced by left ventricular wet weights relative to body weight and left ventricular mass derived from echocardiography (Table 1). This increase in left ventricular mass was supported by a decrease in left ventricular internal diameter, indicating concentric cardiac hypertrophy (Table 1). Treatment from day 14 with A-127722 prevented further increases in these parameters (Table 1). In contrast, the increased weights of the right ventricle and remnant kidney were not affected by A-127722 treatment (Table 1). Thoracic aortic wall thicknesses and aortic blood flow velocities were increased by DOCA-salt treatment; A-127722 treatment prevented and reversed these changes (Table 1). DOCA-salt rats showed increased left ventricular perivascular and interstitial collagen following 2 weeks of hypertension with further increases occurring 4 weeks after induction (Table 1). Treatment from day 14 with A-127722 prevented this further increase (Table 1). As a functional measure of left ventricular remodelling, diastolic stiffness obtained from the isolated Langendorff heart preparation was significantly increased in the DOCA-salt hypertensive rat after 2 weeks and further increased after 4 weeks. This increase in cardiac stiffness was completely prevented and reversed by A-127722 treatment for the last 14 days (Table 1). ED1-positive monocyte/macrophages were found in the left ventricle of UNX rats in low numbers; there was a marked increase in DOCA-salt rats (Figure 4). These cells were usually found as clusters of cells located at scar sites and throughout the interstitium. Treatment with A-127722 significantly reduced macrophage infiltration within scar sites and almost completely prevented macrophage infiltration into the left ventricular interstitium of DOCA-salt rats.
Figure 4.
Immunohistochemical analysis of ED1 cells in left ventricular interstitium; *P<0.05 vs UNX rats; **P<0.05 vs DOCA-salt rats.
Echocardiographic assessment showed that A-127722 failed to alter the increased ejection fraction and decreased cardiac output observed in DOCA-salt hypertensive rats (Table 1). The lack of significant improvement in systolic function by A-127722 in vivo was supported by contractility studies on isolated Langendorff hearts. DOCA-salt hearts showed a decreased +dP/dt that was unaltered by A-127722 treatment (Table 1). Diastolic cardiac function as defined by the E/A (early/atrial) mitral valve flow ratio showed a restrictive pattern that was prevented by treatment with A-127722 (Table 1).
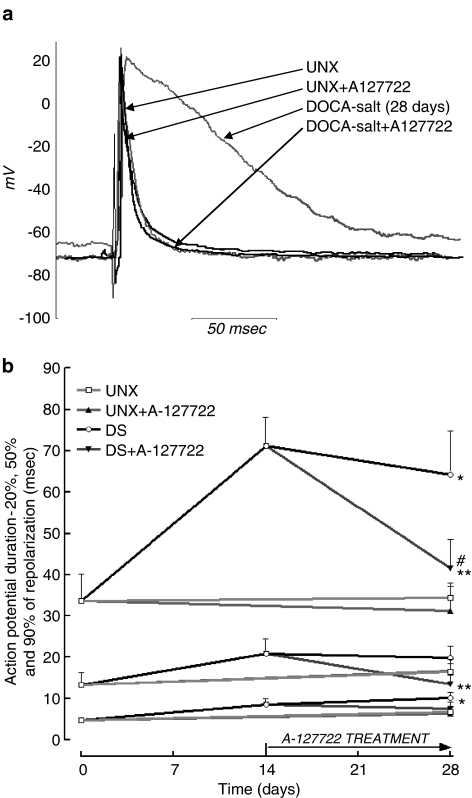
Left ventricular remodelling in DOCA-salt hearts resulted in a significant increase in action potential duration at 20 and 90% of repolarisation after 2 and 4 weeks. This was partially attenuated by A-127722 at 20% of repolarisation and significantly prevented and reversed at 50 and 90% of repolarisation (Figure 5).
Figure 5.
Representative action potentials (a) and action potential durations (b) at 20, 50 and 90% repolarisation in isolated papillary muscles from UNX (n=8), UNX+A-127722 (n=6), DOCA-salt (n=6 at 2 weeks, n=8 at 4 weeks) and DOCA-salt+A-127722 (n=8) treated rats; *P<0.05 vs UNX rats, **P<0.05 vs DOCA-salt rats (4 weeks), #P<0.05 vs DOCA-salt rats (2 weeks).
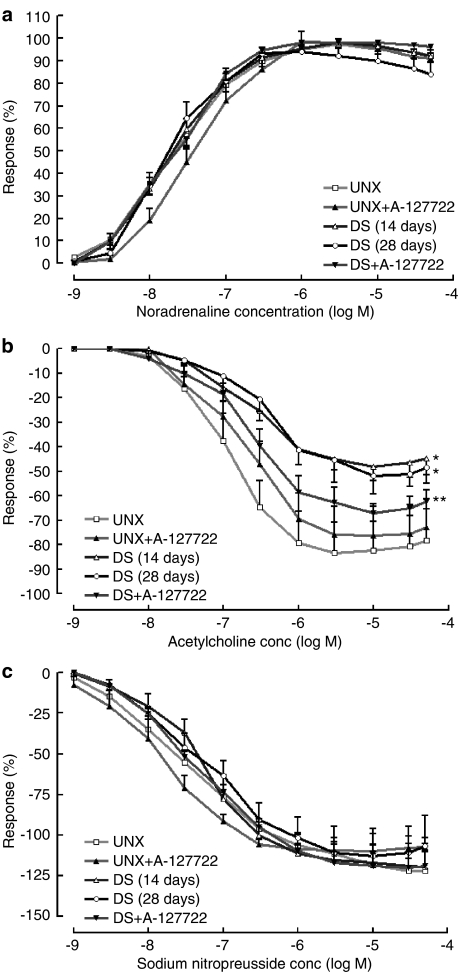
Isolated thoracic aortic rings from DOCA-salt rats showed a decreased absolute maximal contractile responses to noradrenaline and potassium chloride with marked endothelial dysfunction shown as markedly reduced relaxation responses to acetylcholine but unchanged relaxant responses to sodium nitroprusside (Figure 6). Treatment with A-127722 normalised these decreased responses (Figure 6).
Figure 6.
Cumulative concentration–response curves for (a) noradrenaline, (b) acetylcholine and (c) sodium nitroprusside in thoracic aortic rings from UNX (n=12), UNX+A-127722 (n=7), DOCA-salt (n=7 at 2 weeks, n=12 at 4 weeks) and DOCA-salt+A-127722 (n=8) treated rats; maximal contractile response to noradrenaline from UNX (9.9±1.9 mN), UNX+A-127722 (9.4±1.3 mN), DOCA-salt 2 weeks (8.9±1.3 mN), DOCA-salt 4 weeks (4.7±0.5*# mN) and DOCA-salt+A-127722 (8.8±1.6** mN); maximal contractile response to potassium chloride (100 mM) from UNX (13.9±4.2 mN), UNX+A-127722 (15.5±1.6 mN), DOCA-salt 2 weeks (10.5±3.8 mN), DOCA-salt 4 weeks (5.4±0.6* mN) and DOCA-salt+A-127722 (10.1±1.8** mN); maximal relaxant response to acetylcholine from UNX (5.2±1.9 mN), UNX+A-127722 (5.4±0.2 mN), DOCA-salt 2 weeks (2.9±0.8* mN), DOCA-salt 4 weeks (1.8±0.4* mN) and DOCA-salt+A-127722 (3.9±0.9** mN); maximal relaxant response to sodium nitroprusside from UNX (8.3±1.9 mN), UNX+A-127722 (9.1±1.5 mN), DOCA-salt 2 weeks (9.2±2.7 mN), DOCA-salt 4 weeks (6.1±1.8 mN) and DOCA-salt+A-127722 (9.9±2.3 mN); *P<0.05 vs UNX rats; **P<0.05 vs DOCA-salt rats, #P<0.05 vs DOCA-salt rats (2 weeks).
Discussion
Hypertension, hypertrophy, activation of inflammatory processes, cardiac fibrosis, increased cardiac stiffness, decreased cardiac function, action potential prolongation and endothelial dysfunction have been well described following induction of DOCA-salt hypertension in rats (Momtaz et al., 1996; Brown et al., 1999; Ammarguellat et al., 2001; 2002; Mirkovic et al., 2002; Nakano et al., 2003; Callera et al., 2004). An increased ET-1 concentration acting through ET-A receptors has been shown to be a major cause of the hypertension, hypertrophy and fibrosis since these changes could be attenuated or prevented by administration of the selective ET-A receptor antagonists, A-127722, BMS 182874 and LU 135252 (Ammarguellat et al., 2001; 2002; Mulder et al., 2002; Callera et al., 2004). ET-A and nonselective antagonists may decrease BP in normotensive rats following coronary artery ligation (Mulder et al., 2002; Øie et al., 2002), suggesting the blockade of endogenous ETs. Since A-127722 decreased systolic BP, monocyte/macrophage infiltration, interstitial and perivascular collagen deposition, cardiac stiffness, action potential prolongation and endothelial dysfunction in our study, our first major conclusion is that these changes in the DOCA-salt hypertensive rat are mediated predominantly by ET-A receptor activation.
In contrast to these consistent responses reported with selective ET-A receptor antagonists, the nonselective ET receptor antagonists, bosentan and SB 209670, either prevented or had no effect on cardiovascular remodelling (Mulder et al., 1997; Øie et al., 2002). ET-B receptor activation may be cardioprotective, for example, by limiting the size of the infarct following coronary artery occlusion and preventing a reduction in ET-B receptors in the ischaemic zone (Crockett et al., 2004). However, in DOCA-salt hypertensive rats, ET-1 acting through an upregulation of ET-B receptors was a potent stimulus for elevation of superoxide levels in sympathetic ganglia (Dai et al., 2004). This suggests that selective ET-A receptor antagonism is a better therapeutic intervention than nonselective ET receptor antagonism.
In the DOCA-salt hypertensive rat, the key mechanism for the induction of cardiac and vascular damage by ET-1 is likely to be the augmentation of vascular and cardiac superoxide production by NADPH oxidase by activation of ET-A receptors (Callera et al., 2003; Li et al., 2003; Pu et al., 2003). Selective blockade of ET-A receptors reduced arterial superoxide formation (Callera et al., 2003; Li et al., 2003) that was associated with improved vascular reactivity to acetylcholine (Callera et al., 2003) and decreased hypertrophic remodelling and extracellular matrix deposition (Pu et al., 2003). Extracellular matrix deposition is strongly linked to an increased ventricular stiffness (Weber et al., 1993). In addition, ET-1 induced cyclooxygenase-2 upregulation in rat endothelial cells that may contribute to superoxide generation; this upregulation was inhibited by the nonselective ET receptor antagonist, TAK044 (Sugiyama et al., 2004). ET-1-induced signalling events in vascular smooth muscle cells have been shown to critically depend on reactive oxygen species (Daou & Srivastava, 2004). Further, both hypertension and vascular production of superoxide were attenuated by the selective ET-A receptor antagonist, ABT-627 (5 mg kg−1 day−1) in ET-B receptor-deficient rats (Elmarakby et al., 2004). Suppression of superoxide formation by sesamin also markedly decreased the impaired vasodilator responses to acetylcholine in DOCA-salt hypertensive rats (Nakano et al., 2003). Thus, oxidative stress induced by ET-1 via ET-A receptors is likely to play an important role in both cardiac and endothelial dysfunction in the DOCA-salt hypertensive rat.
The same mechanism may be involved in the electrical remodelling in the DOCA-salt hypertensive rat heart. Reactive oxygen species such as superoxide mediated the prolongation of the action potential duration of the cytokine, tissue necrosis factor-α, by a functional depression of the delayed rectifier K+ current (Wang et al., 2004). ET-1 increased action potential duration in cardiac myocytes (Yorikane et al., 1991), probably due to an increased Ca2+ mobilisation and decreased K+ channel currents (Washizuka et al., 1997). Chronic treatment with the selective ET-A receptor antagonist, TA-0201 (approximately 1.3 mg kg−1 day−1), inhibited electrical remodelling including action potential duration prolongation and suppressed ventricular arrhythmias in cardiomyopathic hamster hearts (Matsumoto et al., 2002). Acute ET-A receptor blockade with BQ-123 (0.4 mg kg−1) reduced the incidence of ventricular tachycardia and fibrillation during the first 24 h after coronary artery ligation through a decrease in dispersal of repolarisation (Baltogiannis et al., 2005). Duru et al. (2001) have discussed the proposed mechanisms and therapeutic potential of ET antagonists as antiarrhythmic drugs. These results strongly suggest that administration of A-127722 as a selective ET-A receptor antagonist in our study reversed the prolongation of the action potential duration by suppressing the increased superoxide production following activation of ET-A receptors in the DOCA-salt hypertensive rat.
The second major conclusion of this study is that selective ET-A receptor antagonism can reverse some of the existing damage to the heart and blood vessels in addition to preventing further damage. Cardiac and vascular damage in the DOCA-salt hypertensive rat is clearly evident after 2 weeks and increases in the following 2 weeks. While administration of an ET-A antagonist has been shown to prevent cardiovascular damage when given from the initiation of DOCA-salt hypertension, our results show that the cardiovascular changes produced in the first 2 weeks can be reversed by ET-A receptor blockade during the next 2 weeks. In particular, the decreased interstitial collagen should improve both myocardial contraction and relaxation, while the decreased perivascular collagen should improve nutrient distribution and removal of waste products. We have shown previously using a similar reversal protocol that the anti-inflammatory compound, pirfenidone, reversed the cardiovascular changes in DOCA-salt hypertensive rats (Mirkovic et al., 2002) and in streptozotocin-diabetic rats (Miric et al., 2001).
Thus, an increased ET production is likely to be responsible for the cardiac and vascular changes in the DOCA-salt hypertensive rat probably through activation of superoxide production by NADPH oxidase. Further, selective blockade of ET-A receptors both reverses and prevents these cardiovascular changes and leads to an improved cardiac and vascular function.
Abbreviations
- A-127722
2(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-[[(dibutyl amino) carbonyl]methyl]-pyrrolidine-3-carboxylic acid
- APD20,50,90
action potential duration at 20, 50 or 90% of repolarisation
- DOCA
deoxycorticosterone acetate
- ET
endothelin
- UNX
uninephrectomied
References
- AMMARGUELLAT F., LAROUCHE I., SCHIFFRIN E.L. Myocardial fibrosis in DOCA-salt hypertensive rats. Effects of endothelin ETA receptor antagonism. Circulation. 2001;103:319–324. doi: 10.1161/01.cir.103.2.319. [DOI] [PubMed] [Google Scholar]
- AMMARGUELLAT F.Z., GANNON P.O., AMIRI F., SCHIFFRIN E.L. Fibrosis, matrix metalloproteinases, and inflammation in the heart of DOCA-salt hypertensive rats: role of ETA receptors. Hypertension. 2002;39:679–684. doi: 10.1161/hy0202.103481. [DOI] [PubMed] [Google Scholar]
- BALTOGIANNIS G.G., TSALIKAKIS D.G., MITSI A.C., HATZISTERGOS K.E., ELAIOPOULOS D., FOTIADIS D.I., KYRIAKIDES Z.S., KOLETTIS T.M. Endothelin receptor-A blockade decreases ventricular arrhythmias after myocardial infarction in rats. Cardiovasc. Res. 2005;67:647–654. doi: 10.1016/j.cardiores.2005.04.020. [DOI] [PubMed] [Google Scholar]
- BESWICK R.A., ZHANG H., MARABLE D., CATRAVAS J.D., HILL W.D., WEBB R.C. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension. 2001;37:781–786. doi: 10.1161/01.hyp.37.2.781. [DOI] [PubMed] [Google Scholar]
- BROWN L., CRAGOE E.J., JR, ABEL K.C., MANLEY S.W., BOURKE J.R. Amiloride analogues induce responses in isolated rat cardiovascular tissues by inhibition of Na+/Ca2+ exchange. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;344:220–224. doi: 10.1007/BF00167222. [DOI] [PubMed] [Google Scholar]
- BROWN L., DUCE B., MIRIC G., SERNIA C. Reversal of cardiac fibrosis in deoxycorticosterone acetate-salt hypertensive rats by inhibition of the renin–angiotensin system. J. Am. Soc. Nephrol. 1999;10:S143–S148. [PubMed] [Google Scholar]
- BROWN L., FENNING A., CHAN V., LOCH D., WILSON K., ANDERSON B., BURSTOW D. Echocardiographic assessment of cardiac structure and function in rats. Heart Lung Circ. 2002;11:167–173. doi: 10.1046/j.1444-2892.2002.00148.x. [DOI] [PubMed] [Google Scholar]
- CALLERA GE., MONTEZANO A.C., TOUYZ R.M., ZORN T.M.T., CARVALHO M.H.C., FORTES Z.B., NIGRO D., SCHIFFRIN E.L., TOSTES R.C. ETA receptor mediates altered leukocyte-endothelial cell interaction and adhesion molecules expression in DOCA-salt rats. Hypertension. 2004;43:872–879. doi: 10.1161/01.HYP.0000117296.30296.14. [DOI] [PubMed] [Google Scholar]
- CALLERA G.E., TOUYZ R.M., TEIXEIRA S.A., MUSCARA M.N., CARVALHO M.H.C., FORTES Z.B., NIGRO D., SCHIFFRIN E.L., TOSTES R.C. ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension. 2003;42:811–817. doi: 10.1161/01.HYP.0000088363.65943.6C. [DOI] [PubMed] [Google Scholar]
- CROCKETT T.R., GRAY G.A., KANE K.A., WAINWRIGHT C.L. Sarafotoxin 6c (S6c) reduces infarct size and preserves mRNA for the ETB receptor in the ischemic/reperfused myocardium of anesthetized rats. J. Cardiovasc. Pharmacol. 2004;44:148–154. doi: 10.1097/00005344-200408000-00002. [DOI] [PubMed] [Google Scholar]
- DAI X., GALLIGAN J.J., WATTS S.W., FINK G.D., KREULEN D.L. Increased O2•− production and upregulation of ETB receptors by sympathetic neurons in DOCA-salt hypertensive rats. Hypertension. 2004;43:1048–1054. doi: 10.1161/01.HYP.0000126068.27125.42. [DOI] [PubMed] [Google Scholar]
- DALLEMAGNE C., OOI S.-Y., BROWN L., GOBÉ G., ENDRE Z. Renal impairment in deoxycorticosterone acetate-salt hypertensive rats. Nephrology. 2000;5:255–264. [Google Scholar]
- DAOU G.B., SRIVASTAVA A.K. Reactive oxygen species mediate endothelin-1-induced activation of ERK1/2, PKB, and Pyk2 signaling, as well as protein synthesis, in vascular smooth muscle cells. Free Radic. Biol. Med. 2004;37:208–215. doi: 10.1016/j.freeradbiomed.2004.04.018. [DOI] [PubMed] [Google Scholar]
- DURU F., BARTON T., LÜSCHER T.F., CANDINAS R. Endothelin and cardiac arrhythmias: do endothelin antagonists have a therapeutic potential as antiarrhythmic drugs. Cardiovasc. Res. 2001;49:272–280. doi: 10.1016/s0008-6363(00)00263-7. [DOI] [PubMed] [Google Scholar]
- ELMARAKBY A.A., LOOMIS E.D., POLLOCK J.S., POLLOCK D.M. ETA receptor blockade attenuates hypertension and decreases reactive oxygen species in ETB receptor-deficient rats. J. Cardiovasc. Pharmacol. 2004;44 (Suppl. 1):S7–S10. doi: 10.1097/01.fjc.0000166205.66555.40. [DOI] [PubMed] [Google Scholar]
- GARJANI A., WAINWRIGHT C.L., ZEITLIN I.J., WILSON C., SLEE S.J. Effects of endothelin-1 and the ETA-receptor antagonist, BQ-123, on ischemic arrhythmias in anesthetized rats. J. Cardiovasc. Pharmacol. 1995;25:634–642. doi: 10.1097/00005344-199504000-00018. [DOI] [PubMed] [Google Scholar]
- LI J.-M., SHAH A.M. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am. J. Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- LI L., FINK G.D, WATTS S.W., NORTHCOTT C.A., GALLIGAN J.J., PAGANO P.J., CHEN A.F. Endothelin-1 increases vascular superoxide via endothelinA–NADPH oxidase pathway in low-renin hypertension. Circulation. 2003;107:1053–1058. doi: 10.1161/01.cir.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- LITWIN S.E., KATZ S.E., MORGAN J.P., DOUGLAS P.S. Serial echocardiographic assessment of left ventricular geometry and function after large myocardial infarction in the rat. Circulation. 1994;89:345–354. doi: 10.1161/01.cir.89.1.345. [DOI] [PubMed] [Google Scholar]
- MASAKI T. Historical review: endothelin. Trends Pharmacol. Sci. 2004;25:219–224. doi: 10.1016/j.tips.2004.02.008. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO Y., AIHARA H., YAMAUCHI-KOHNO R., REIEN Y., OGURA T., YABANA H., MASUDA Y., SATO T., KOMURO I., NAKAYA H. Long-tern endothelin A receptor blockade inhibits electrical remodelling in cardiomyopathic hamsters. Circulation. 2002;106:613–619. [PubMed] [Google Scholar]
- MIRIC G., DALLEMAGNE C., ENDRE Z., MARGOLIN S., TAYLOR S.M., BROWN L. Reversal of cardiac and renal fibrosis by pirfenidone and spironolactone in streptozotocin-diabetic rats. Br. J. Pharmacol. 2001;133:687–694. doi: 10.1038/sj.bjp.0704131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIRKOVIC S., SEYMOUR A.-M.L., FENNING A., STRACHAN A., MARGOLIN S.B., TAYLOR S.M., BROWN L. Attenuation of cardiac fibrosis by pirfenidone and amiloride in DOCA-salt hypertensive rats. Br. J. Pharmacol. 2002;135:961–968. doi: 10.1038/sj.bjp.0704539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOMTAZ A., COULOMBE A., RICHER P., MERCADIER J.-J., CORABOEUF E. Action potential and plateau ionic currents in moderately and severely DOCA-salt hypertrophied rat hearts. J. Mol. Cell. Cardiol. 1996;28:2511–2522. doi: 10.1006/jmcc.1996.0243. [DOI] [PubMed] [Google Scholar]
- MULDER P., BOUJEDAINI H., RICHARD V., HENRY J.-P., RENET S., MÜNTER K., THUILLEZ C. Long-term survival and hemodynamics after endothelin-A receptor antagonism and angiotensin-converting enzyme inhibition in rats with chronic heart failure. Monotherapy versus combination therapy. Circulation. 2002;106:1159–1164. doi: 10.1161/01.cir.0000027138.07524.38. [DOI] [PubMed] [Google Scholar]
- MULDER P., RICHARD V., DERUMEAUX G., HOGIE M., HENRY J.P., LALLEMAND F., COMPAQGNON P., MACÈ B., COMOY E., LETAC B., THUILLEZ C. Role of endogenous endothelin in chronic heart failure: effect of long-term treatment with an endothelin antagonist on survival, hemodynamics, and cardiac remodeling. Circulation. 1997;96:1976–1982. doi: 10.1161/01.cir.96.6.1976. [DOI] [PubMed] [Google Scholar]
- NAKANO D., ITOH C., ISHII F., KAWANISHI H., TAKAOKA M., KISO Y., TSURUOKA N., TANAKA T., MATSUMURA Y. Effects of sesamin on aortic oxidative stress and endothelial dysfunction in deoxycorticosterone acetate-salt hypertensive rats. Biol. Pharm. Bull. 2003;26:1701–1705. doi: 10.1248/bpb.26.1701. [DOI] [PubMed] [Google Scholar]
- ØIE E., YNDESTAD A., ROBINS S.P., BJØRNERHEIM R., ÅSBERG A., ATTRAMADAL H. Early intervention with a potent endothelin-A/endothelin-B receptor antagonist aggravates left ventricular remodelling after myocardial infarction in rats. Basic Res. Cardiol. 2002;97:239–247. doi: 10.1007/s003950200017. [DOI] [PubMed] [Google Scholar]
- PU Q., NEVES M.F., VIRDIS A., TOUYZ R.M., SCHIFFRIN E.L. Endothelin antagonism on aldosterone-induced oxidative stress and vascular remodelling. Hypertension. 2003;42:49–55. doi: 10.1161/01.HYP.0000078357.92682.EC. [DOI] [PubMed] [Google Scholar]
- SAWYER D.B., SIWIK D.A., XIAO L., PIMENTEL D.R., SINGH K., COLUCCI W.S. Role of oxidative stress in myocardial hypertrophy and failure. J. Mol. Cell. Cardiol. 2002;34:379–388. doi: 10.1006/jmcc.2002.1526. [DOI] [PubMed] [Google Scholar]
- SCHIFFRIN E.L. Role of endothelin-1 in hypertension and vascular disease. Am. J. Hypertens. 2001;14:83S–93S. doi: 10.1016/s0895-7061(01)02074-x. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA T., YOSHIMOTO T., SATO R., FUKAI N., OZAWA N., SHICHIRI M., KIRATA Y. Endothelin-1 induces cyclooxygenase-2 expression and generation of reactive oxygen species in endothelial cells. J. Cardiovasc. Pharmacol. 2004;43 (Suppl. 1):S332–S335. doi: 10.1097/01.fjc.0000166267.17174.0e. [DOI] [PubMed] [Google Scholar]
- TOUYZ R.M., SCHIFFRIN E.L. Role of endothelin in human hypertension. Can. J. Physiol. Pharmacol. 2003;81:533–541. doi: 10.1139/y03-009. [DOI] [PubMed] [Google Scholar]
- WANG J., WANG H., ZHANG Y., GAO H., NATTEL S., WANG Z. Impairment of HERG K(+) channel function by tumor necrosis factor-alpha: role of reactive oxygen species as a mediator. J. Biol. Chem. 2004;279:13289–13292. doi: 10.1074/jbc.C400025200. [DOI] [PubMed] [Google Scholar]
- WASHIZUKA T., HORIE M., WATANUKI M., SASAYAMA S. Endothelin-1 inhibits the slow component of cardiac delayed rectifier K+ currents via a pertussis toxin-sensitive mechanism. Circ. Res. 1997;81:211–218. doi: 10.1161/01.res.81.2.211. [DOI] [PubMed] [Google Scholar]
- WEBER K.T., BRILLA C.G., JANICKI J.S. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc. Res. 1993;27:341–348. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- YORIKANE R., KOIKE H., MIYAKE S. Electrophysiological effects of endothelin-1 on canine myocardial cells. J. Cardiovasc. Pharmacol. 1991;17:159–162. doi: 10.1097/00005344-199100177-00044. [DOI] [PubMed] [Google Scholar]