Abstract
Δ9-tetrahydrocannabivarin (THCV) displaced [3H]CP55940 from specific binding sites on mouse brain and CHO-hCB2 cell membranes (Ki=75.4 and 62.8 nM, respectively).
THCV (1 μM) also antagonized CP55940-induced stimulation of [35S]GTPγS binding to these membranes (apparent KB=93.1 and 10.1 nM, respectively).
In the mouse vas deferens, the ability of Δ9-tetrahydrocannabinol (THC) to inhibit electrically evoked contractions was antagonized by THCV, its apparent KB-value (96.7 nM) approximating the apparent KB-values for its antagonism of CP55940- and R-(+)-WIN55212-induced stimulation of [35S]GTPγS binding to mouse brain membranes.
THCV also antagonized R-(+)-WIN55212, anandamide, methanandamide and CP55940 in the vas deferens, but with lower apparent KB-values (1.5, 1.2, 4.6 and 10.3 nM, respectively).
THCV (100 nM) did not oppose clonidine, capsaicin or (−)-7-hydroxy-cannabidiol-dimethylheptyl-induced inhibition of electrically evoked contractions of the vas deferens.
Contractile responses of the vas deferens to phenylephrine hydrochloride or β,γ-methylene-ATP were not reduced by 1 μM THCV or R-(+)-WIN55212, suggesting that THCV interacts with R-(+)-WIN55212 at prejunctional sites.
At 32 μM, THCV did reduce contractile responses to phenylephrine hydrochloride and β,γ-methylene-ATP, and above 3 μM it inhibited electrically evoked contractions of the vas deferens in an SR141716A-independent manner.
In conclusion, THCV behaves as a competitive CB1 and CB2 receptor antagonist. In the vas deferens, it antagonized several cannabinoids more potently than THC and was also more potent against CP55940 and R-(+)-WIN55212 in this tissue than in brain membranes. The bases of these agonist- and tissue-dependent effects remain to be established.
Keywords: Δ9-Tetrahydrocannabivarin, Δ9-tetrahydrocannabinol, R-(+)-WIN55212, anandamide, methanandamide, CP55940, cannabinoids, mouse vas deferens, CB1 receptor antagonist, CB2 receptor antagonist
Introduction
Cannabis sativa is the natural source of a set of at least 66 oxygen-containing aromatic hydrocarbon compounds that are known collectively as phytocannabinoids (reviewed in ElSohly, 2002). This study focused on a little-investigated phytocannabinoid, the n-propyl analogue of Δ9-tetrahydrocannabinol (THC) (Figure 1), which was first detected in cannabis by Gill et al. (1970) and named Δ9-tetrahydrocannabivarin (THCV) by Merkus (1971). The initial objective of this research was to establish whether THCV can activate or block cannabinoid CB1 or CB2 receptors. Some of our experiments were performed with membranes prepared from healthy brain tissue, which is densely populated with CB1 but not CB2 receptors (reviewed in Howlett et al., 2002), or from Chinese hamster ovary (CHO) cells transfected with hCB2 receptors. These membranes were used to investigate the ability of THCV to displace [3H]CP55940 from CB1- and CB2-binding sites and to determine whether it behaves as a CB1 or CB2 receptor agonist or antagonist. Experiments were also carried out with the mouse isolated vas deferens, a tissue in which cannabinoid receptor agonists such as R-(+)-WIN55212, CP55940, THC and 2-arachidonoyl ethanolamide (anandamide) can inhibit electrically evoked contractions (Devane et al., 1992; Pertwee et al., 1995b). This they are thought to do by acting on prejunctional neuronal cannabinoid CB1 receptors to inhibit release of the contractile neurotransmitters, ATP, acting on postjunctional P2X purinoceptors, and noradrenaline, acting on postjunctional α1-adrenoceptors (von Kügelgen & Starke, 1991; Trendelenburg et al., 2000; see also Pertwee, 1997; Schlicker & Kathman, 2001). Experiments were also performed with (−)-7-hydroxy-cannabidiol-dimethylheptyl, a synthetic analogue of the phytocannabinoid, (−)-cannabidiol, that inhibits electrically evoked contractions of the mouse vas deferens through a mechanism that appears to operate prejunctionally and to be at least partly CB1 receptor-independent (Pertwee et al., 2005). Some of the results described in this paper have been presented to the British Pharmacological Society (Pertwee et al., 2004).
Figure 1.
Structures of THC and THCV.
Methods
The methods used comply with the U.K. Animals (Scientific Procedures) Act, 1986 and Associated Guidelines for the Use of Experimental Animals.
Drugs and chemicals
THCV was supplied by GW Pharmaceuticals (Porton Down, Wiltshire, U.K.), THC by the National Institute on Drug Abuse (Bethesda, MD, U.S.A.) and (−)-7-hydroxy-cannabidiol-dimethylheptyl by Professor R. Mechoulam (Hebrew University of Jerusalem, Israel). SR141716A and SR144528 were obtained from Sanofi-Aventis (Montpellier, France). Phenylephrine hydrochloride, β,γ-methyleneadenosine 5′-triphosphate (β,γ-methylene-ATP), anandamide and clonidine hydrochloride were purchased from Sigma-Aldrich (Poole, Dorset, U.K.), R-(+)-WIN55212 and CP55940 from Tocris (Bristol, U.K.) and capsaicin from Research Biochemicals International (Natick, MA, U.S.A.). Phenylephrine hydrochloride, β,γ-methylene-ATP and clonidine were dissolved in a 0.9% aqueous solution of NaCl (saline). R-(+)-WIN55212 was dissolved in a 50% (v v−1) solution of dimethyl sulphoxide (DMSO) in saline and all other drugs were dissolved in pure DMSO. Drugs were added to organ baths in a volume of 10 μl. For the binding experiments, [3H]CP55940 (168 Ci mmol−1), [3H]R-(+)-WIN55212 (40 Ci mmol−1) and [35S]GTPγS (1250 Ci mmol−1) were obtained from Perkin-Elmer Life Sciences Inc. (Boston, MA, U.S.A.). [3H]SR141716A (44 Ci mmol−1) was obtained from Amersham Biosciences U.K. Ltd (Little Chalfont, Buckinghamshire, U.K.), GTPγS and adenosine deaminase from Roche Diagnostic (Indianapolis, IN, U.S.A.) and GDP from Sigma-Aldrich.
CHO cells
CHO cells stably transfected with cDNA encoding human cannabinoid CB2 receptors (Bmax=72.6 pmol mg−1 protein) were maintained at 37°C and 5% CO2 in Dulbecco's Modified Eagles's medium (DMEM) nutrient mixture F-12 HAM supplemented with 2 mM L-glutamine, 10% foetal calf serum, 0.6% penicillin–streptomycin, hygromycin B (300 μg ml−1) and G 418 (600 μg ml−1). These CHO-hCB2 cells were passed twice a week using a nonenzymatic cell dissociation solution.
Membrane preparation
Binding assays with [3H]CP55940 and with [35S]GTPγS were performed with mouse whole brain membranes, prepared as described by Thomas et al. (2004), or with CHO-hCB2 cell membranes (Ross et al., 1999a). The hCB2 transfected cells were removed from flasks by scraping and then frozen as a pellet at −20°C until required. Before use in a radioligand-binding assay, cells were defrosted, diluted in 50 mM Tris-binding buffer (radioligand displacement assay) or GTPγS-binding buffer ([35S]GTPγS-binding assay), and homogenized with a 1 ml hand-held homogenizer. Protein assays were performed using a Bio-Rad Dc kit (Bio-Rad, Hercules, CA, U.S.A.).
Radioligand displacement assay
The assays were carried out with [3H]CP55940, Tris-binding buffer (50 mM Tris-HCl; 50 mM Tris-Base; 0.1% BSA), total assay volume 500 μl, using the filtration procedure described previously by Ross et al. (1999b). Binding was initiated by the addition of either the brain membranes (33 μg protein per well) or the transfected hCB2 cells (25 μg protein per well). All assays were performed at 37°C for 60 min before termination by addition of ice-cold Tris-binding buffer and vacuum filtration using a 24-well sampling manifold (cell harvester; Brandel Inc., Gaitherburg, MD, U.S.A.) and GF/B filters (Whatman, Maidstone, U.K.) that had been soaked in wash buffer at 4°C for at least 24 h. Each reaction well was washed six times with a 1.2 ml aliquot of Tris-binding buffer. The filters were oven-dried for 60 min and then placed in 5 ml of scintillation fluid (Ultima Gold XR, Packard). Radioactivity was quantified by liquid scintillation spectrometry. Specific binding was defined as the difference between the binding that occurred in the presence and absence of 1 μM unlabelled CP55940. The concentration of [3H]CP55940 used in our displacement assays was 0.7 nM. THCV was stored as a stock solution of 10 mM in DMSO, the vehicle concentration in all assay wells being 0.1% DMSO. The binding parameters for [3H]CP55940, determined by fitting data from saturation-binding experiments to a one-site saturation plot using GraphPad Prism, were 2336 fmol mg−1 protein (Bmax) and 2.31 nM (Kd) in mouse brain membranes (Thomas et al., 2004), and 72570 fmol mg−1 protein (Bmax) and 1.043 nM (Kd) in hCB2-transfected cells.
[35S]GTPγS-binding assay
The method for measuring agonist-stimulated [35S]GTPγS binding to cannabinoid CB1 receptors was adapted from the methods of Kurkinen et al. (1997) and Breivogel et al. (2001). The conditions used for measuring agonist-stimulated [35S]GTPγS binding to transfected cannabinoid CB2 receptors were adapted from those used by MacLennan et al. (1998) and Griffin et al. (1999). The assays were carried out with GTPγS-binding buffer (50 mM Tris-HCl; 50 mM Tris-Base; 5 mM MgCl2; 1 mM EDTA; 100 mM NaCl; 1 mM dithiothreitol; 0.1% BSA) in the presence of [35S]GTPγS and GDP, in a final volume of 500 μl. Binding was initiated by the addition of [35S]GTPγS to the wells. Nonspecific binding was measured in the presence of 30 μM GTPγS. The drugs were incubated in the assay for 60 min at 30°C. The reaction was terminated by a rapid vacuum filtration method using Tris-binding buffer as described previously, and the radioactivity was quantified by liquid scintillation spectrometry. The concentrations of [35S]GTPγS and GDP present in the assay varied depending on whether the assay was conducted with mouse brain or transfected cell membranes. However, the protein concentration was the same in all the [35S]GTPγS-binding assays (5 μg protein per well). When the assay was conducted with mouse brain membranes, 0.1 nM [35S]GTPγS and 30 μM GDP were present, whereas the corresponding concentrations present when the assay was conducted with transfected cell membranes were 0.7 nM and 320 μM, respectively. Additionally, mouse brain membranes were preincubated for 30 min at 30°C with 0.5 U ml−1 adenosine deaminase (200 U mg−1) to remove endogenous adenosine. Agonists and antagonists were stored as a stock solution of 1 or 10 mM in DMSO, the vehicle concentration in all assay wells being 0.11% DMSO.
Vas deferens experiments
Vasa deferentia were obtained from albino MF1 mice weighing 31–59 g. The tissues were mounted vertically in 4 ml organ baths. They were then subjected to electrical stimulation of progressively greater intensity, followed by an equilibration procedure in which they were exposed to alternate periods of stimulation (2 min) and rest (10 min) until contractions with consistent amplitudes were obtained (Thomas et al., 2004). These contractions were monophasic and isometric, and were evoked by 0.5 s trains of pulses of 110% maximal voltage (train frequency 0.1 Hz; pulse frequency 5 Hz; pulse duration 0.5 ms).
Except in our experiments with phenylephrine, all drug additions were made to the organ baths after the equilibration period and there was no washout between these additions. In most experiments, there was an initial application of a potential antagonist or its vehicle. This was followed 28 min later by a 2-min period of electrical stimulation, at the end of which the lowest of a series of concentrations of the twitch inhibitors, R-(+)-WIN55212, CP55940, THC, anandamide, (−)-7-hydroxy-cannabidiol-dimethylheptyl or clonidine, was applied. After a period of rest, the tissues were electrically stimulated for 2 min and then subjected to a further addition of twitch inhibitor. This cycle of drug addition, rest and 2 min stimulation was repeated so as to construct cumulative concentration–response curves. Only one concentration–response curve was constructed per tissue (Pertwee et al., 1996). Rest periods were 3 min for clonidine, 13 min for R-(+)-WIN55212, CP55940 and anandamide, 28 min for THC and THCV, and 58 min for (−)-7-hydroxy-cannabidiol-dimethylheptyl. Experiments were also performed with capsaicin. This drug was added at intervals of 3 min and the tissues were not rested from electrical stimulation between these additions. In some experiments, cumulative concentration–response curves for THCV were constructed without prior addition of any other compound, again using a cycle of drug addition, 28 min rest and 2 min stimulation.
In experiments with β,γ-methylene-ATP, no electrical stimuli were applied after the equilibration procedure. Log concentration–response curves of β,γ-methylene-ATP were constructed cumulatively without washout. THCV, WIN or drug vehicle was added 30 min before the first addition of β,γ-methylene-ATP, each subsequent addition of which was made immediately after the effect of the previous dose had reached a plateau (dose cycles of 1–2 min). Only one addition of phenylephrine was made to each tissue and this was carried out 30 min after the addition of THCV, WIN or drug vehicle.
Analysis of data
Values have been expressed as means and variability as s.e.m. or as 95% confidence limits. The concentration of THCV that produced a 50% displacement of radioligand from specific binding sites (IC50 value) was calculated using GraphPad Prism 4. Its dissociation constant (Ki-value) was calculated using the equation of Cheng & Prusoff (1973). Net agonist-stimulated [35S]GTPγS-binding values were calculated by subtracting basal binding values (obtained in the absence of agonist) from agonist-stimulated values (obtained in the presence of agonist) as detailed elsewhere (Ross et al., 1999a). Inhibition of the electrically evoked twitch response of the vas deferens has been expressed in percentage terms, and this has been calculated by comparing the amplitude of the twitch response after each addition of a twitch inhibitor with its amplitude immediately before the first addition of the inhibitor. Contractile responses to phenylephrine and β,γ-methylene-ATP have been expressed as increases in tension (g).
Values for EC50, maximal effect (Emax) and the s.e.m. or 95% confidence limits of these values have been calculated by nonlinear regression analysis using the equation for a sigmoid concentration–response curve (GraphPad Prism). The apparent dissociation constant (KB) values for antagonism of agonists by THCV in the vas deferens or [35S]GTPγS-binding assay have been calculated by Schild analysis from the concentration ratio, defined as the concentration of an agonist that elicits a response of a particular size in the presence of a competitive reversible antagonist at a concentration, B, divided by the concentration of the same agonist that produces an identical response in the absence of the antagonist. The methods used to determine the concentration ratio and apparent KB-values and to establish whether log concentration–response plots deviated significantly from parallelism are detailed elsewhere (Pertwee et al., 2002). Mean values have been compared using Student's two-tailed t-test for unpaired data or one-way analysis of variance (ANOVA) followed by Dunnett's test (GraphPad Prism). A P-value <0.05 was considered to be significant.
Results
Radioligand experiments
THCV displaced [3H]CP55940 from specific binding sites in mouse brain and CHO-hCB2 cell membranes in a manner that fitted significantly better to a one-site than a two-site competition curve (P<0.05; GraphPad Prism 4). Its mean Ki-values were 75.4 and 62.8 nM, respectively (Figure 2). THCV also displaced [3H]R-(+)-WIN55212 and [3H]SR141716A from specific binding sites in mouse brain membranes, its mean EC50 values with 95% confidence limits shown in brackets being 61.3 nM (48.6 and 77.3 nM; n=4–7) and 86.8 nM (63.8 and 118.1 nM; n=4–6), respectively. The corresponding EC50 value of THCV for displacement of [3H]CP55940 is 98.2 nM (69.6 and 138.6 nM; n=4–8).
Figure 2.
Displacement of [3H]CP55940 by THCV from specific binding sites in (a) mouse whole brain membranes and (b) CHO-hCB2 cell membranes. Each symbol represents the mean percent displacement±s.e.m. Mean Ki-values for this displacement with 95% confidence limits shown in brackets were calculated by the Cheng–Prusoff equation to be (a) 75.4 nM (53.4 and 106.3 nM; n=4–8) in mouse whole brain membranes and (b) 62.8 nM (52.5 and 75.3 nM; n=6) in CHO-hCB2 cell membranes.
The ability of CP55940 to stimulate [35S]GTPγS binding to mouse brain and CHO-hCB2 membranes was attenuated by THCV, which at 1 μM produced significant dextral shifts in the log concentration–response curves of this cannabinoid receptor agonist that did not deviate significantly from parallelism (Figure 3). The mean apparent KB-values for this antagonism are shown in Table 1, as are the mean apparent KB-values of SR141716A for antagonism of CP55940 in mouse brain membranes and of SR144528 for antagonism of CP55940 in the CHO-hCB2 cell membranes. At 1 μM, THCV also produced a significant parallel dextral shift in the log concentration–response curve of R-(+)-WIN55212 for stimulation of GTPγS binding to mouse brain membranes (see Table 1 for its apparent KB-value against R-(+)-WIN55212).
Figure 3.
The effect of 1 μM THCV on the mean log concentration–response curve of CP55940 for stimulation of [35S]GTPγS binding (a) to mouse whole brain membranes and (b) to CHO-hCB2 cell membranes. Each symbol represents the mean percentage increase in [35S]GTPγS binding±s.e.m. (n=6). Mean apparent KB-values of THCV for its antagonism of CP55940 have been calculated from these data and these values are listed in Table 1.
Table 1.
Mean apparent KB-values of THCV, SR141716A and SR144528 for antagonism of CP55940- or R-(+)-WIN55212-induced activation of [35S]GTPγS binding to mouse whole brain or CHO-hCB2 membranes
| Antagonist | Agonist | Membrane preparation | Mean apparent KB (nM) | 95% confidence limits (nM) | n |
|---|---|---|---|---|---|
| THCV (1000 nM) | CP55940 | Brain | 93.1 | 66.5 and 130.6 | 6 |
| THCV (1000 nM) | R-(+)-WIN55212 | Brain | 85.4 | 29.3 and 270.5 | 5 |
| SR141716A (10 nM) | CP55940 | Brain | 0.09 | 0.021 and 0.41 | 4 |
| THCV (1000 nM) | CP55940 | CHO-hCB2 | 10.1 | 5.0 and 20.5 | 6 |
| SR144528 (100 nM) | CP55940 | CHO-hCB2 | 0.49 | 0.26 and 0.85 | 6 |
Mean apparent KB-values were calculated by Schild analysis using data obtained with the antagonist concentrations shown.
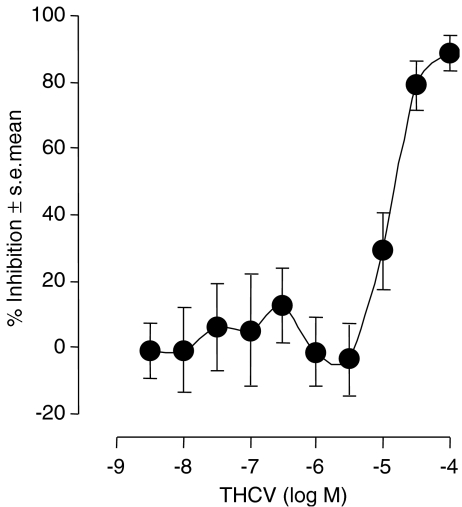
Micromolar concentrations of THCV inhibit evoked contractions of the vas deferens
THCV produced a concentration-related inhibition of electrically evoked contractions of the mouse isolated vas deferens with an EC50 of 12.7 μM (6.9 and 23.2 μM) (Figure 4). It is unlikely that this effect was CB1-receptor mediated as it was not attenuated by SR141716A at 100 nM (n=7; data not shown), a concentration that equals or exceeds concentrations of this CB1-selective antagonist found previously to antagonize established CB1 receptor agonists in the same bioassay (Pertwee et al., 1995b; Ross et al., 2001). At 32 μM, a concentration at which it produced a marked inhibition of electrically evoked contractions, THCV also attenuated contractile responses of the vas deferens to both the P2 receptor agonist β,γ-methylene-ATP, and the α1-adrenoceptor agonist, phenylephrine hydrochloride (Figure 5). In contrast, at 1 μM, a concentration at which it had no detectable inhibitory effect on electrically evoked contractions (Figure 4), THCV did not induce any significant reduction in the amplitude of contractions induced either by β,γ-methylene-ATP (n=8; data not shown) or by phenylephrine (Figure 5). These findings suggest that THCV inhibited electrically evoked contractions of the vas deferens, at least in part, by acting postjunctionally to block contractile responses to endogenously released ATP and noradrenaline.
Figure 4.
The mean log concentration–response curve of THCV in the mouse isolated vas deferens. Each symbol represents the mean value±s.e.m. for inhibition of electrically evoked contractions expressed as a percentage of the amplitude of the twitch response measured immediately before the first addition to the organ bath of THCV. Tissues were exposed to THCV concentrations ranging either from 3.2 nM to 10 μM (n=10) or from 1 to 100 μM (n=10).
Figure 5.
Upper panel: the effect of pretreatment with THCV on mean increases in tension of the mouse isolated vas deferens induced by β,γ-methylene ATP in the presence of DMSO (○) or 32 μM THCV (•). For the construction of log concentration–response curves, β,γ-methylene ATP was first added 30 min after DMSO or THCV (n=7 or 8). Lower panels: the effect of pretreatment with R-(+)-WIN55212 or THCV on mean increases in tension of the mouse isolated vas deferens induced by (a) 32 μM phenylephrine or (b) 3.2 μM phenylephrine. Additions of phenylephrine were made 30 min after DMSO (open columns), THCV or R-(+)-WIN55212 (n=8). In all panels, mean increases in tension are expressed in grams±s.e.m. The asterisks indicate significant differences between responses to β,γ-methylene ATP (unpaired t-test) or to phenylephrine (ANOVA followed by Dunnett's test) in the absence of added cannabinoids, and corresponding responses in the presence of R-(+)-WIN55212 or THCV (*P<0.05; ***P<0.001).
THCV behaves as a potent surmountable competitive antagonist of R-(+)-WIN55212 in the vas deferens
As shown in Figure 6, at concentrations well below those at which it inhibited electrically evoked contractions, THCV opposed R-(+)-WIN55212-induced inhibition of the twitch response in a manner that was concentration-related and not accompanied by any significant change in the maximum effect (Emax) of R-(+)-WIN55212 (P>0.05; ANOVA followed by Dunnett's test; n=6–9). The dextral shifts produced by THCV in the log concentration–response curve of R-(+)-WIN55212 do not deviate significantly from parallelism and yield a Schild plot with a slope that is not significantly different from unity (Figure 6). The mean apparent KB-value of THCV was calculated by the Tallarida method (see Pertwee et al., 2002) to be 1.5 nM (Table 2). At 1 μM, a concentration that markedly attenuated electrically evoked contractions (Figure 6), R-(+)-WIN55212 did not decrease the ability of β,γ-methylene-ATP (n=7 or 10; data not shown) or phenylephrine (Figure 5) to induce contractions of the vas deferens.
Figure 6.
Upper panel: the effect of pretreatment with THCV on the mean log concentration–response curve of R-(+)-WIN55212 in the mouse isolated vas deferens. Each symbol represents the mean value±s.e.m. for inhibition of electrically evoked contractions expressed as a percentage of the amplitude of the twitch response measured immediately before the first addition of R-(+)-WIN55212 to the organ bath. THCV or DMSO was added 30 min before the first addition of R-(+)-WIN55212, further additions of which were made at 15-min intervals. Each log concentration–response curve was constructed cumulatively without washout (n=6–9). Lower panel: Schild plot for antagonism of R-(+)-WIN55212 by 10–1000 nM THCV, in which values for log x−1 were calculated from the data shown in the upper panel (x=concentration ratio). The slope of this plot is 0.99±0.14 and this does not differ significantly from unity (P>0.05; one-sample t-test). The mean apparent KB-value of THCV for its antagonism of R-(+)-WIN55212 has been calculated from this slope by Schild analysis and is listed in Table 1.
Table 2.
Mean apparent KB-values of THCV for antagonism of drug-induced inhibition of electrically evoked contractions of the mouse isolated vas deferens
| THCV (nM) | Twitch inhibitor | Mean apparent KB of THCV (nM) | 95% confidence limits (nM) | n |
|---|---|---|---|---|
| 10–1000 | R-(+)-WIN55212 | 1.5 | 1.1 and 2.3 | 6–9 |
| 100 | Anandamide | 1.2 | 0.2 and 6.2 | 7 |
| 100 | Methanandamide | 4.6 | 1.5 and 11.6 | 12 |
| 100 | CP55940 | 10.3 | 3.8 and 31.7 | 14 |
| 1000 | THC | 96.7 | 15.4 and 978 | 10 |
| 100 | Clonidine | >100 | — | 8 |
| 100 | Capsaicin | >100 | — | 8 |
| 100 | 7-OH-CBD-DMH | >100 | — | 8 |
THCV is also a potent surmountable competitive antagonist of anandamide, methanandamide and CP55940 in the vas deferens
THCV was shown to antagonize anandamide at 10, 100 and 1000 nM (Figure 7), and methanandamide and CP55940 at 100 nM (Table 2). The dextral shifts produced by THCV in the log concentration–response curves of these twitch inhibitors did not deviate significantly from parallelism. The mean apparent KB-value for the antagonism of anandamide by 10 nM THCV with its 95% confidence limits shown in brackets is 1.4 nM (0.36 and 7.50 nM). Mean apparent KB-values for antagonism of anandamide, methanandamide and CP55940 by 100 nM THCV are listed in Table 2.
Figure 7.
The effect of pretreatment with THCV on the mean log concentration–response curves of anandamide (n=7 or 8) and clonidine (n=8) in the mouse isolated vas deferens. Each symbol represents the mean value±s.e.m. for inhibition of electrically evoked contractions expressed as a percentage of the amplitude of the twitch response measured immediately before the first addition of twitch inhibitor to the organ bath. THCV or DMSO was added 30 min before the first addition of anandamide or clonidine, further additions of which were made at 15- and 5-min intervals, respectively. Each log concentration–response curve was constructed cumulatively without washout.
THCV (100 nM) does not antagonize clonidine, capsaicin, (−)-7-hydroxy-cannabidiol-dimethylheptyl or THC in the vas deferens
At 100 nM, THCV did not reduce the ability of clonidine, capsaicin or (−)-7-hydroxy-cannabidiol-dimethylheptyl to inhibit electrically evoked contractions (Figure 7 and Table 2), indicating it to possess at least some degree of selectivity as an antagonist of twitch inhibitors in the vas deferens. Nor did 100 nM THCV antagonize the cannabinoid receptor agonist, THC (n=11; data not shown). However, at 1 μM, THCV did produce a significant dextral shift in the log concentration–response curve of THC that did not deviate significantly from parallelism (see Table 2 for its apparent KB-value against THC).
Discussion
Well-established mixed CB1/CB2 cannabinoid receptor ligands such as CP55940, R-(+)-WIN55212 and THC displace [3H]CP55940 from specific binding sites on membranes prepared from brain tissue or from cells transfected with CB2 receptors (reviewed in Pertwee, 1997; Howlett et al., 2002), and THCV was found to share this ability. The Ki-value of THCV determined from our experiments with mouse brain membranes (75.4 nM) presumably represents its Ki for the mouse CB1 receptor, as there is little evidence for the presence of a significant population of CB2 receptors in healthy brain tissue (reviewed in Howlett et al., 2002). This Ki-value is at least 1.8 times greater than reported CB1 Ki-values of its structural analogue, THC, determined in experiments with [3H]CP55940 using rat brain membranes (1.6–43 nM; see Pertwee, 1997). However, the hCB2 Ki-value of THCV (62.8 nM) is little different from hCB2 Ki-values of THC determined previously with [3H]CP55940 (reviewed in Pertwee, 1997).
THCV behaved as a reasonably potent competitive antagonist of CP55940, as indicated by the manner in which it antagonized the ability of this cannabinoid receptor agonist to stimulate [35S]GTPγS binding in experiments with mouse brain membranes (Figure 3). Results from additional [35S]GTPγS-binding assays conducted with mouse brain membranes indicated that THCV can also antagonize R-(+)-WIN55212, again in an apparently competitive manner. THCV exhibited similar potencies against CP55940 and R-(+)-WIN55212 in these experiments, suggesting that both agonists were competing with THCV for the same target. It is likely that this target was the cannabinoid CB1 receptor as results from the brain membrane experiments showed that the apparent KB-value of THCV for its antagonism of CP55940 (Table 1) did not deviate significantly from its corresponding Ki-value for displacement of [3H]CP55940 (Figure 2).
THCV also behaved as a competitive CB2 receptor antagonist, as measured by its ability to antagonize CP55940 in the [35S]GTPγS-binding assay when CHO-hCB2 cell membranes were used. Interestingly, its apparent KB-value in these experiments was significantly less than its apparent KB-value for the antagonism of CP55940-induced activation of [35S]GTPγS binding in mouse brain membranes (Table 2), indicating it to be more potent as a CB2 than a CB1 receptor antagonist. Unexpectedly, its hCB2 KB-value was also significantly lesser than its Ki-value for the displacement of [3H]CP55940 from CHO-hCB2 cell membranes (Figure 2). Why this should be remains to be established. It is noteworthy, however, that we also found the corresponding hCB2 KB-value of the established CB2-selective antagonist SR144528 (Table 1) to be significantly less than the mean Ki-value of this ligand for its displacement of [3H]CP55940 from CHO-hCB2 cell membranes. More specifically, we found this Ki-value with its 95% confidence limits shown in brackets to be 7.5 nM (5.7 and 10.0; n=6–8), a value that does not deviate significantly from the hCB2 Ki-value of SR144528 determined previously in this laboratory (Ross et al., 1999a). One possibility, that the potency of THCV as a hCB2 receptor antagonist was found to be so much greater than its affinity for hCB2 receptors because THCV binds more readily to these receptors in the presence of GTPγS-binding buffer (see Methods), is unlikely as we found THCV to be no more potent in displacing [3H]CP55940 from CHO-hCB2 cell membranes when this was determined using GTPγS-binding buffer (mean Ki=68.1 nM; 95% confidence limits=44.7 and 103.9 nM; n=5 or 6) instead of the Tris-binding buffer used in all our other binding experiments with radiolabelled cannabinoids (Figure 2).
Although THCV behaved as a cannabinoid receptor antagonist in the [35S]GTPγS-binding assays, it was found to share the ability of CB1 receptor agonists to inhibit electrically evoked contractions of the mouse isolated vas deferens (reviewed in Pertwee, 1997). It is likely, however, that the mechanism by which THCV inhibited these contractions is not the same as the mechanism that underlies the inhibition of electrically evoked contractions induced by THC or other established CB1 receptor agonists. Thus, while CB1 receptor agonists appear to act on CB1 receptors located on prejunctional neurones to produce this effect (reviewed in Pertwee 1997; Schlicker & Kathman, 2001), THCV probably acted in a CB1-receptor-independent manner as its EC50 for twitch inhibition (12.7 μM) greatly exceeded its Ki for CB1-binding sites and as it was not antagonized by the CB1-selective antagonist, SR141716A, when this was administered at a concentration (100 nM) found previously to oppose inhibition of electrically evoked contractions of the mouse vas deferens induced by CB1 receptor agonists (Pertwee et al., 1995b). THCV could well have produced its inhibitory effect on electrically evoked contractions of the vas deferens by acting postjunctionally to oppose the contractile effects of noradrenaline and ATP that were presumably being released in response to electrical stimulation (see Introduction). Thus, the amplitudes of both phenylephrine- and β,γ-methylene-ATP-evoked contractions of this tissue were reduced by THCV when this was administered at a concentration that attenuated electrically evoked contractions of the vas deferens (32 μM), but not when it was administered at a concentration that did not affect the amplitude of electrically evoked contractions (1 μM). Taken together, our brain membrane and vas deferens data suggest that while THCV has significant affinity for the CB1 receptor, it lacks detectable CB1 receptor efficacy. This is in line with the hypothesis that the length of the C-3 side chain of classical cannabinoids such as THC is an important determinant of the affinity or efficacy displayed by these ligands at CB1 receptors (reviewed in Razdan, 1986; Howlett et al., 2002). Our finding that THCV was less potent than THC as an inhibitor of electrically evoked contractions of the mouse vas deferens (Figure 4; Pertwee et al., 1995b), agrees with an earlier report that THCV shows less activity than THC as an inhibitor of electrically evoked contractions of the guinea-pig isolated ileum (Gill et al., 1970).
At concentrations below those at which it inhibited electrically evoked contractions of the mouse vas deferens, THCV opposed the abilities of the established CB1 receptor agonists, R-(+)-WIN55212, anandamide, methanandamide, CP55940 and THC, to inhibit the twitch response. This it did in a competitive, surmountable manner. For R-(+)-WIN55212, at least, it is likely that the interaction with THCV took place on prejunctional neurones rather than postjunctionally, as the amplitudes of β,γ-methylene-ATP- and phenylephrine-evoked contractions of the vas deferens were unaffected by a concentration of R-(+)-WIN55212 that did markedly inhibit electrically evoked contractions of this tissue preparation. THCV produced its antagonism of cannabinoids at concentrations that by themselves did not affect the amplitude of electrically evoked contractions, or indeed [35S]GTPγS binding to brain or CHO-hCB2 cell membranes (data not shown), suggesting it to be a neutral antagonist (reviewed in Pertwee, 2005a).
At 100 nM, a concentration at which it antagonized R-(+)-WIN55212, anandamide, methanandamide and CP55940 in the vas deferens, THCV produced no antagonism of clonidine or capsaicin. This is an indication that it possesses selectivity and also suggests that, at 100 nM at least, it was not acting against any of the cannabinoids we investigated by blocking α2-adrenergic or TRPV1 receptors. Nor did 100 nM THCV antagonize (−)-7-hydroxy-cannabidiol-dimethylheptyl, an analogue of cannabidiol that has been postulated to act at least in part through an as yet unidentified neuronal non-CB1 target in the vas deferens (Pertwee et al., 2005).
The apparent KB-value of THCV for its antagonism of THC in the vas deferens (Table 2) is close to its cannabinoid CB1 Ki-value (Figure 2) and to its apparent KB-values for antagonism of CP55940- or R-(+)-WIN55212-induced stimulation of [35S]GTPγS binding to mouse brain membranes. These findings are consistent with the hypothesis that THC and THCV were competing for CB1 receptors since there is good evidence that it is mainly CB1 receptors that mediate cannabinoid-induced inhibition of electrically evoked contractions of the mouse vas deferens (reviewed in Howlett et al., 2002). Moreover, reported CB1 Ki-values of the selective CB1 receptor antagonist, SR141716A (Howlett et al., 2002), also approximate to its apparent KB-value for antagonism of THC in the mouse vas deferens (Pertwee et al., 1995b). In contrast, the apparent KB-values of THCV for antagonism of R-(+)-WIN55212, anandamide, methanandamide and CP55940 were all significantly less than its cannabinoid CB1 Ki-value. In spite of these findings, however, it would be premature to conclude that THCV antagonized these other cannabinoids in a manner that was entirely CB1 receptor-independent. Thus, firstly the CB1 selective antagonist, SR141716A, antagonizes R-(+)-WIN55212 in the mouse vas deferens no less potently than it antagonizes THC (Pertwee et al., 1995b), and secondly Schlicker et al. (2003) have found that R-(+)-WIN55212 inhibits evoked noradrenaline release in the vasa deferentia obtained from CB1+/+ mice but not in vasa deferentia from CB1−/− mice, suggesting that R-(+)-WIN55212 might not inhibit electrically evoked contractions of mouse vasa deferentia that lack CB1 receptors. It is also noteworthy that although there is evidence that some central and peripheral neurones express non-CB1 receptors that can be activated by R-(+)-WIN55212 and/or anandamide (reviewed in Pertwee, 2005b), the available data suggest that these are not targets at which R-(+)-WIN55212, anandamide or CP55940 would be antagonized by THCV. Thus, some of these proposed targets are activated by capsaicin (Ross et al., 2001; Hájos & Freund, 2002) or clonidine (Molderings et al., 2002), while others appear not to be activated by CP55940 (Breivogel et al., 2001; Mang et al., 2001; Zygmunt et al., 2002). Yet, THCV produced no antagonism of clonidine or capsaicin in the vas deferens (see above), and was no less potent in antagonizing CP55940 than anandamide (Table 2).
The apparent KB-value of THCV for its antagonism of CP55940-induced activation of [35S]GTPγS binding to CHO-hCB2 cell membranes is essentially the same as its apparent KB-value for antagonism of CP55940 in the mouse vas deferens (Tables 1 and 2). This raises the possibility that it is cannabinoid CB2 receptors for which THCV competes with CP55940, and indeed with R-(+)-WIN55212, anandamide and methanandamide, in this tissue preparation. However, there are three reasons for rejecting this hypothesis. Firstly, Rinaldi-Carmona et al. (1998) found that the apparent KB-value of SR144528 for its antagonism of CP55940 in the mouse vas deferens (501 nM) greatly exceeded its hCB2 Ki-value. Secondly, we have found that SR144528 produces no significant antagonism of R-(+)-WIN55212 in the vas deferens (data not shown) even when administered at concentrations (0.1 or 1 μM) well above its apparent KB-value for antagonism of CP55940 in CHO-hCB2 cell membranes. Finally, while another CB2 receptor antagonist, AM630, has been found to resemble THCV in producing an antagonism of cannabinoid receptor agonists in mouse vasa deferentia that is agonist dependent, it differs from THCV in being more potent against THC than against either anandamide or R-(+)-WIN55212 (Pertwee et al., 1995a).
In conclusion, we have obtained evidence from competitive binding and GTPγS-binding experiments with mouse brain and CHO-hCB2 cell membranes that THCV is a competitive cannabinoid CB1 and CB2 receptor antagonist. In line with this hypothesis, THCV antagonized THC in the mouse isolated vas deferens, in a manner that suggested that it was competing with THC for CB1 receptors. At concentrations in the micromolar range, well above those at which it is capable of antagonizing THC in the vas deferens, THCV exhibited additional pharmacological actions, in this respect resembling the CB1 selective antagonist SR141716A which, at micromolar concentrations, also interacts with non-CB1 targets (reviewed in Pertwee, 2005a). In addition to THC, several other cannabinoids including R-(+)-WIN55212 and anandamide were antagonized by THCV in the mouse vas deferens. Unexpectedly however, the potency exhibited by THCV against these other cannabinoids in the vas deferens was far greater than the potency it exhibited as an antagonist of THC in this tissue preparation or indeed as a CB1 receptor antagonist in mouse brain membranes. Clearly, further experiments will be required to establish how THCV produces antagonism of cannabinoids that is both agonist- and tissue-dependent. Given the high potency that THCV exhibited as an antagonist of anandamide in the vas deferens, it will also be important to investigate the ability of this plant cannabinoid to modulate the tonic activity of the endocannabinoid system induced by endogenous release of anandamide or other endocannabinoids. In addition to providing a more complete characterization of this system, data from such experiments may point to the possible clinical applications for THCV or a related compound.
Acknowledgments
This investigation was supported by grants from GW Pharmaceuticals, the BBSRC and the National Institute on Drug Abuse (DA-9789). We thank Professor Raphael Mechoulam for (−)-7-hydroxy-cannabidiol-dimethylheptyl.
Abbreviations
- anandamide
arachidonoyl ethanolamide
- BSA
bovine serum albumin
- capsaicin
3-methoxy-4-hydroxy-benzyl-8-methyl-6-nonenamide
- CHO
Chinese hamster ovary
- CP55940
(−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- DMSO
dimethyl sulphoxide
- GDP
guanosine 5′-diphosphate
- GTPγS
guanosine-5′-O-(3-thiotriphosphate)
- methanandamide
R-(+)-arachidonoyl-1′-hydroxy-2′-propylamide
- β,γ-methylene-ATP
β,γ-methyleneadenosine 5′-triphosphate
- R-(+)-WIN55212
(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone
- SR141716A
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride
- SR144528
N-[(1S)-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide
- THC
Δ9-tetrahydrocannabinol
- THCV
Δ9-tetrahydrocannabivarin
References
- BREIVOGEL C.S., GRIFFIN G., DI MARZO V., MARTIN B.R. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol. Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- CHENG Y.-C., PRUSOFF W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- ELSOHLY M.A.Chemical constituents of Cannabis Cannabis and Cannabinoids. Pharmacology, Toxicology and Therapeutic Potential 2002New York: Haworth Press, Inc; 27–36.ed. Grotenhermen, F. & Russo, E. pp [Google Scholar]
- GILL E.W., PATON W.D.M., PERTWEE R.G. Preliminary experiments on the chemistry and pharmacology of cannabis. Nature. 1970;228:134–136. doi: 10.1038/228134a0. [DOI] [PubMed] [Google Scholar]
- GRIFFIN G., WRAY E.J., TAO Q., MCALLISTER S.D., RORRER W.K., AUNG M.M., MARTIN B.R., ABOOD M.E. Evaluation of the cannabinoid CB2 receptor-selective antagonist, SR144528: further evidence for cannabinoid CB2 receptor absence in the rat central nervous system. Eur. J. Pharmacol. 1999;377:117–125. doi: 10.1016/s0014-2999(99)00402-1. [DOI] [PubMed] [Google Scholar]
- HÁJOS N., FREUND T.F. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- HOWLETT A.C., BARTH F., BONNER T.I., CABRAL G., CASELLAS P., DEVANE W.A., FELDER C.C., HERKENHAM M., MACKIE K., MARTIN B.R., MECHOULAM R., PERTWEE R.G. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- KURKINEN K.M.A., KOISTINAHO J., LAITINEN J.T. [γ-35S]GTP autoradiography allows region-specific detection of muscarinic receptor-dependent G-protein activation in the chick optic tectum. Brain Res. 1997;769:21–28. doi: 10.1016/s0006-8993(97)00663-x. [DOI] [PubMed] [Google Scholar]
- MACLENNAN S.J., REYNEN P.H., KWAN J., BONHAUS D.W. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br. J. Pharmacol. 1998;124:619–622. doi: 10.1038/sj.bjp.0701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANG C.F., ERBELDING D., KILBINGER H. Differential effects of anandamide on acetylcholine release in the guinea-pig ileum mediated via vanilloid and non-CB1 cannabinoid receptors. Br. J. Pharmacol. 2001;134:161–167. doi: 10.1038/sj.bjp.0704220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERKUS F.W.H.M. Cannabivarin and tetrahydrocannabivarin, two new constituents of hashish. Nature. 1971;232:579–580. doi: 10.1038/232579a0. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G.J., BÖNISCH H., HAMMERMANN R., GÖTHERT M., BRÜSS M. Noradrenaline release-inhibiting receptors on PC12 cells devoid of α2- and CB1 receptors: similarities to presynaptic imidazoline and edg receptors. Neurochem. Int. 2002;40:157–167. doi: 10.1016/s0197-0186(01)00076-6. [DOI] [PubMed] [Google Scholar]
- PERTWEE R., GRIFFIN G., FERNANDO S., LI X., HILL A., MAKRIYANNIS A. AM630, a competitive cannabinoid receptor antagonist. Life Sci. 1995a;56:1949–1955. doi: 10.1016/0024-3205(95)00175-6. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005a;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G.Pharmacological actions of cannabinoids Cannabinoids, Handbook of Experimental Pharmacology 2005bHeidelburg: Springer-Verlag; 1–51.ed. Pertwee, R.G., Vol. 168, pp [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G., FERNANDO S.R., GRIFFIN G., RYAN W., RAZDAN R.K., COMPTON D.R., MARTIN B.R. Agonist–antagonist characterization of 6′-cyanohex-2′-yne-Δ8-tetrahydrocannabinol in two isolated tissue preparations. Eur. J. Pharmacol. 1996;315:195–201. doi: 10.1016/s0014-2999(96)00631-0. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G., GRIFFIN G., LAINTON J.A.H., HUFFMAN J.W. Pharmacological characterization of three novel cannabinoid receptor agonists in the mouse isolated vas deferens. Eur. J. Pharmacol. 1995b;284:241–247. doi: 10.1016/0014-2999(95)00318-f. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G., ROSS R.A., CRAIB S.J., THOMAS A. (−)-Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. Eur. J. Pharmacol. 2002;456:99–106. doi: 10.1016/s0014-2999(02)02624-9. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G., STEVENSON L.A., ROSS R.A., PRICE M.R., WEASE K.N., THOMAS A.The phytocannabinoid, THCV, potently antagonizes WIN55212-2 and anandamide in the mouse isolated vas deferens Proc. Br. Pharmacol. Soc. 2004. at
- PERTWEE R.G., THOMAS A., STEVENSON L.A., MAOR Y., MECHOULAM R. (−)-7-Hydroxy-4′-dimethylheptyl-cannabidiol activates a non-CB1, non-CB2, non-TRPV1 target in the mouse vas deferens in a cannabidiol-sensitive manner. Neuropharmacology. 2005;48:1139–1146. doi: 10.1016/j.neuropharm.2005.01.010. [DOI] [PubMed] [Google Scholar]
- RAZDAN R.K. Structure–activity relationships in cannabinoids. Pharmacol. Rev. 1986;38:75–149. [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J.-M., CASELLAS P., CONGY C., OUSTRIC D., SARRAN M., BOUABOULA M., CALANDRA B., PORTIER M., SHIRE D., BRELIÈRE J.-C., LE FUR G. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- ROSS R.A., BROCKIE H.C., STEVENSON L.A., MURPHY V.L., TEMPLETON F., MAKRIYANNIS A., PERTWEE R.G. Agonist–inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656 and AM630. Br. J. Pharmacol. 1999a;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS R.A., GIBSON T.M., BROCKIE H.C., LESLIE M., PASHMI G., CRAIB S.J., DI MARZO V., PERTWEE R.G. Structure–activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br. J. Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS R.A., GIBSON T.M., STEVENSON L.A., SAHA B., CROCKER P., RAZDAN R.K., PERTWEE R.G. Structural determinants of the partial agonist–inverse agonist properties of 6′-azidohex-2′-yne-Δ8-tetrahydrocannabinol at cannabinoid receptors. Br. J. Pharmacol. 1999b;128:735–743. doi: 10.1038/sj.bjp.0702836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLICKER E., KATHMAN M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol. Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., REDMER A., WERNER A., KATHMAN M. Lack of CB1 receptors increases noradrenaline release in vas deferens without affecting atrial noradrenaline release or cortical acetylcholine release. Br. J. Pharmacol. 2003;140:323–328. doi: 10.1038/sj.bjp.0705449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS A., ROSS R.A., SAHA B., MAHADEVAN A., RAZDAN R.K., PERTWEE R.G. 6″-Azidohex-2″-yne-cannabidiol: a potential neutral, competitive cannabinoid CB1 receptor antagonist. Eur. J. Pharmacol. 2004;487:213–221. doi: 10.1016/j.ejphar.2004.01.023. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG A.U., COX S.L., SCHELB V., KLEBROFF W., KHAIRALLAH L., STARKE K. Modulation of 3H-noradrenaline release by presynaptic opioid, cannabinoid and bradykinin receptors and β-adrenoceptors in mouse tissues. Br. J. Pharmacol. 2000;130:321–330. doi: 10.1038/sj.bjp.0703305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON KÜGELGEN I., STARKE K. Noradrenaline-ATP co-transmission in the sympathetic nervous system. Trends Pharmacol. Sci. 1991;12:319–324. doi: 10.1016/0165-6147(91)90587-i. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., ANDERSSON D.A., HÖGESTÄTT E.D. Δ9-tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. J. Neurosci. 2002;22:4720–4727. doi: 10.1523/JNEUROSCI.22-11-04720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]