Abstract
Inhibition of the type 5 phosphodiesterase and inhibition of Rho kinase are both effective in reducing pulmonary hypertension (PH). Here we investigate whether Rho kinase inhibition is involved in the beneficial effect of the type 5 phosphodiesterase inhibitor sildenafil on PH.
Chronic hypoxia-induced PH in rats is associated with an increase in RhoA activity in pulmonary artery that was maximal after 2 days (10.7±0.9-fold increase, n=6, P<0.001). The activity of Rho kinase assessed by measuring the level of myosin phosphatase target subunit 1 (MYPT1) phosphorylation was also increased (5.7±0.8-fold over control, n=8).
Chronic fasudil (30 mg kg−1 day−1; 14 days) and sildenafil (25 mg kg−1 day−1; 14 days) treatments reduced PH and pulmonary cardiovascular remodelling, and inhibited the MYPT1 phosphorylation in pulmonary artery from hypoxic rats by 82.3±3% (n=4) and by 76.6±2% (n=4), respectively.
The inhibitory effect of sildenafil (10 μM) on MYPT1 phosphorylation was demonstrated by the loss of actin stress fibres in vascular smooth muscle cells. However, in vitro kinase assays indicated that sildenafil had no direct inhibitory action on Rho kinase activity.
Sildenafil treatment induced increased RhoA phosphorylation and association to its cytosolic inhibitory protein, guanine dissociation inhibitor (GDI) in pulmonary artery.
We propose that sildenafil inhibits RhoA/Rho kinase-dependent functions in pulmonary artery through enhanced RhoA phosphorylation and cytosolic sequestration by GDI. The inhibition of intracellular events downstream of RhoA thus participates in the beneficial effect of sildenafil on PH.
Keywords: Rho proteins, signal transduction, phosphodiesterase, smooth muscle
Introduction
The pathogenesis of pulmonary hypertension (PH) is a complex and multifactorial process. Pathologic changes of pulmonary arteries, which involve endothelial dysfunction, endothelial and smooth muscle cell proliferation, and increased vasoconstriction, decrease the lumen area of the pulmonary microvasculature, causing fixed elevation of pulmonary resistance. Although these pathological features are common to all forms of human PH (Rabinovitch, 1997), the mechanisms responsible for this abnormal vascular proliferation are unknown. However, impairment of endothelial functions leading to an imbalance of vasodilator and vasoconstrictor influences is likely to play a central role in the initiation and progression of PH (Budhiraja et al., 2004). Drugs that improve the endothelial function or restore the altered balance of endothelium-derived vasoactive mediators such as endothelin-1 receptor antagonists and prostacyclin analogues are used to treat this disease with moderate success (Runo & Loyd, 2003). The NO/cGMP axis is also considered as a major target for the treatment of PH. Recently, the type 5 phosphodiesterase inhibitor sildenafil has been identified as a promising therapeutic agent for PH. The type 5 phosphodiesterase is the major cGMP-degrading phosphodiesterase in the pulmonary vasculature and is upregulated in PH (Maclean et al., 1997; Zhao et al., 2001; Murray et al., 2002; Sebkhi et al., 2003). Sildenafil reduces right ventricular hypertrophy in chronic hypoxic mice (Zhao et al., 2001), pulmonary arterial pressure (PAP) in chronic hypoxic rats (Sebkhi et al., 2003), and improves survival rate in rats with PH induced by monocrotaline injection (Itoh et al., 2004; Schermuly et al., 2004). Short-term studies in patients with PH suggest that sildenafil is an effective pulmonary vasodilator (Ghofrani et al., 2002; Michelakis et al., 2002).
On the other hand, recent pharmacological studies have suggested a role for the serine/threonine kinase Rho kinase in the development of PH. In vivo, intravenous or oral treatment with Rho kinase inhibitor (Y-27632 or fasudil) nearly normalizes the high pulmonary AP in chronically hypoxic rats, attenuates the development of chronic hypoxia-induced PH in mice, and reduces pulmonary arterial lesions in the model of monocrotaline-induced PH in rats (Abe et al., 2004; Fagan et al., 2004; Nagaoka et al., 2004). In addition, inhaled Y-27632 or fasudil causes sustained and selective pulmonary vasodilation in monocrotaline-induced PH and in spontaneous PH in fawn-hooded rats, as well as in chronically hypoxic rats (Nagaoka et al., 2005). Rho kinase is one of the main downstream effectors of the small G protein RhoA, which functions as a tightly regulated molecular switch that governs a wide range of cellular functions (Van Aelst & D'Souza-Schorey, 1997). In particular, Rho kinase phosphorylates the myosin phosphatase target subunit 1 (MYPT1) of smooth muscle myosin phosphatase at Thr-696, leading to the inhibition of its activity (Feng et al., 1999). This inhibition of smooth muscle myosin phosphatase activity is a primary mechanism of the Ca2+ sensitization of smooth muscle contraction (Feng et al., 1999; Somlyo and Somlyo, 2003). A large body of evidence has now been obtained regarding the important functions of RhoA in the vasculature, and RhoA has been shown to play a major role in the regulation of vascular cell processes such as actin cytoskeleton organization, contraction, gene expression, and differentiation (Somlyo & Somlyo, 2000; Mack et al., 2001). In vascular smooth muscle cells, RhoA has been shown to be regulated by the NO/cGMP pathways. RhoA is phosphorylated by cGMP-dependent protein kinase (PKG) (Sauzeau et al., 2000). This phosphorylation prevents the translocation of active GTP-bound RhoA to the membrane, which is an obligatory step for the activation of its downstream effectors. Activation of the NO/cGMP pathway thus leads to the inhibition of RhoA-dependent functions, including actin cytoskeleton organization, Ca2+ sensitization of the contraction, and gene transcription (Sauzeau et al., 2000; Gudi et al., 2002).
The purpose of this study was thus to investigate whether the beneficial effect of sildenafil on chronic hypoxia-induced PH in rats is mediated through the inhibition of RhoA-dependent pathways.
Methods
Animals
All experiments were conducted in accordance with institutional guidelines for the care and use of laboratory animals. Male Wistar rats (250 g) were used. The normoxic rats were housed in room air at a normal atmospheric pressure (760 mmHg). The hypoxic rats were housed in a hypobaric chamber at 380 mmHg (Vacucell 111 L, Medcenter, Munich, Germany) for 0–14 days. Sildenafil (25 mg kg−1 day−1) and fasudil (30 mg kg−1 day−1; LC Laboratories, Woburn, MA, U.S.A.) were administrated by gavage. At completion of the exposure to hypoxia or treatment, lungs and extralobar pulmonary arteries were removed. Tissues were then prepared as indicated for histologic or Western blot analyses.
Western blot analysis
Pulmonary arteries were harvested and de-endothelialized, then homogenized in NETF buffer (100 mM NaCl, 2 mM EGTA, 50 mM Tris-Cl, pH 7.4 and 50 mM NaF) containing 1% NP-40, 2 mM orthovanadate, protease inhibitors, and phosphatase inhibitor cocktail (Sigma). Nuclei and unlysed cells were removed by low-speed centrifugation at 10,000 × g for 15 min at 4°C. After determination of protein concentration in lysates from pulmonary artery samples, equal amounts of protein were loaded in each lane of polyacrylamide/SDS gels, which were then electrophoresed and transferred to nitrocellulose. Lysates were analysed by Western blot with mouse monoclonal antibodies against RhoA (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) or rabbit polyclonal anti-Rho guanine dissociation inhibitor (GDI) antibody (Upstate Biotechnology, Lake Placid, NY, U.S.A.). Equal loading was confirmed by reprobing the membrane with anti-β-actin antibody (Sigma, Saint Quentin Fallavier, France). When needed, cell fractionation was performed according to Dignam (1990).
Rho kinase inhibits myosin phosphatase activity by phosphorylation of MYPT1 on Thr-696 (Feng et al., 1999). Rho kinase activity in rat pulmonary artery samples was quantified by Western blot analysis for MYPT1 using a sheep polyclonal anti-MYPT1 antibody (Upstate, Euromedex, Munolsheim, France) and for phosphorylated MYPT1 using a rabbit polyclonal anti-phospho-MYPT1 (Thr-696) (Upstate).
Immunoreactive bands were visualized using horseradish peroxidase-conjugated secondary antibody and subsequent ECL detection (Amersham Pharmacia Biotech, Orsay, France), then quantified by densitometric analysis using QuantityOne (Biorad, Hercules, CA, U.S.A.).
RhoA activity
RhoA activity was assessed in de-endothelialized pulmonary artery samples by a pulldown assay using the Rho-binding domain of the Rho effector protein Rhotekin as described previously (Ren et al., 1999). cDNA encoding for the Rhotekin RBD was kindly provided by Dr Martin Schwartz (The Scripps Research Institute, La Jolla, CA, U.S.A.). Briefly, the pulmonary arteries were rapidly removed, rinsed and placed in ice-cold phosphate-buffered saline (PBS) and dissected on ice. Artery samples were then lysed in a lysis buffer (50 mM Tris, pH 7.2, 500 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, protease inhibitor cocktail (Sigma), 1 mM phenylmethylsulphonyl fluoride). After centrifugation at 12,000 × g at 4°C for 10 min, the extracts were incubated at 4°C for 45 min with glutathione-Sepharose 4B beads coupled with glutathione-S-transferase (GST)–rhotekin fusion protein to determine RhoA activity. Precipitated GTP-bound RhoA and total RhoA were then analysed by Western blotting using a mouse monoclonal anti-RhoA antibody. Immunoreactive bands were detected using ECL detection and quantified by densitometric analysis.
Pressure measurements
Rats were anaesthetized by intraperitoneal injection of 250 mg urethane. Mean PAP was measured by the insertion of a catheter connected to a pressure transducer into the right jugular vein, then through the right atrium and the right ventricle (RV) into the pulmonary artery. Pressure was then displayed on a HP 78353A recorder (Hewlett-Packard, Issy-Les-Moulineaux, France). Systemic arterial pressure (AP) was measured by another catheter inserted into the left femoral artery.
Histologic analysis
The left lung was sectioned, fixed in 4% paraformaldehyde in PBS, and processed for paraffin-embedded sections. Sections (10 μm) were stained with haematoxylin–eosin–saffron. A total of 15–20 vessels was examined in each section. Pulmonary arteries (50–150 μm in diameter) associated with an airway distal to the respiratory bronchiole were analysed. The relative wall thickness ((external diameter−internal diameter)/external diameter) was quantified using using Metamorph-Metaview software (Universal Imaging Co., West Chester, PA, U.S.A.) and normalized to values obtained under control conditions. These observations were made by experimenters ‘blinded' to conditions and treatments.
Actin staining
Smooth muscle cells from rat pulmonary artery were isolated by enzymatic dissociation and cultured in DMEM with 10% foetal calf serum (FCS), 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin. Secondary cultures were obtained by serial passages after the cells were harvested with 0.5 g l−1 trypsin and 0.2 g l−1 EDTA (trypsin–EDTA) and reseeded in fresh DMEM containing 10% FCS and antibiotics. After dissociation, myocytes at passage 2 were seeded on glass coverslips and cultured in DMEM with 10% FCS for 2 days. The cells were then washed and maintained in serum-free DMEM in the absence or presence of 10 μM sildenafil or fasudil for 0.5–12 h. Cells were then fixed for 30 min in 4% paraformaldehyde, permeabilized in 0.5% Triton X-100, and then rinsed in PBS. To quantify actin cytoskeleton organization, dual labelling of monomeric G-actin and polymerized F-actin was performed (Knowles & McCulloch, 1992). Cells were simultaneously stained with FITC-conjugated phalloidin (5 μg ml−1) to label F-actin and Texas red-labelled DNase I (10 μg ml−1) to localize monomeric G-actin, then washed in PBS. Coverslips were mounted on a glass slide and examined with a fluorescence microscope (Eclipse E-600, Nikon, Champigny-sur-Marne, France). The background fluorescence signal was estimated by collecting planes from areas of the slide without cells, and was electronically subtracted before analysis. Images were collected with a cool-SNAP camera (Princeton Instruments, Evry, France), stored, and analysed using Metamorph software (Universal Imaging, West Chester, PA, U.S.A.). For each area examined, images of FITC-phalloidin and Texas Red-DNase I fluorescence were collected. The time of measurements and image capturing and the image intensity gain at both wavelengths were optimally adjusted and kept constant. The ratio of fluorescence of FITC-phalloidin and Texas Red-DNase I (F- to G-actin ratio), used to quantify actin cytoskeleton organization, was calculated for at least 20 cells in each experimental condition and expressed as a percentage of the ratio obtained under control condition. A decrease in the F- to G-actin ratio was assumed to represent depolymerization of actin filaments.
Rho kinase assay
The kinase reaction was carried out in 50 μl kinase buffer (20 mM Tris, 25 mM β-glycerol phosphate, 1 mM EGTA, 0.1 mM sodium orthovanadate, 1 mM dithiothreitol, pH 7.5) containing 90 μM [γ-32P]ATP (2.2 mCi mmol−1), 50 μM S6 kinase substrate peptide, 20 mU Rho kinase (Upstate), without or with 10 μM of sildenafil or fasudil. After incubation for 30 min at 30°C with agitation, the reaction mixtures were spotted onto Whatman P81 paper. The paper was washed three times for 5 min with 0.75% phosphoric acid, once for 3 min with acetone. 32P incorporation into substrate was then determined by Cerenkov counting.
Coimmunoprecipitation
Pulmonary artery samples were lysed in NETF buffer containing 1% NP-40, 2 mM orthovanadate, protease inhibitors, and phosphatase inhibitor cocktail (Sigma). Samples were precleared with 40 μl of protein A-Sepharose beads (which have been washed in NETF buffer to give a 50% slurry), and immunoprecipitations were carried out with rabbit polyclonal anti-GDI antibody (Upstate) or anti-phosphoserine antibody (Zymed, CliniSciences, Montrouge, France) preadsorbed on protein A-Sepharose beads. The protein A-Sepharose-bound immune complexes were washed twice in NETF buffer containing NP-40 (1% w v−1) and once in NETF without detergent. Pellets from the immunoprecipitations were heated at 95°C for 5 min in 70 μl of Laemmli sample buffer for SDS–polyacrylamide gel electrophoresis and analysed by immunoblot for RhoA and/or GDI. Signals from immunoreactive bands were detected by ECL (Amersham) and quantified using QuantityOne (BioRad).
Statistical analysis
Values are expressed as means±s.e.m. Statistical analysis was performed by Student's t-test or ANOVA. P<0.05 was considered significant.
Results
Fasudil reduces chronic hypoxia-induced PH
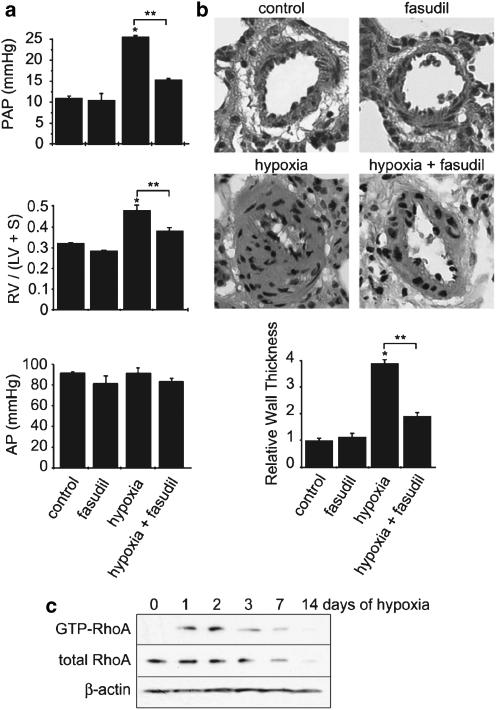
As classically described, rats chronically exposed to hypoxia (14 days) showed an increase in PAP associated with RV hypertrophy, assessed by the ratio of RV weight to left ventricle (LV) plus interventricular septum weight (RV/LV+S) (Figure 1a). Lung specimen from hypoxic rats demonstrated severe pulmonary arteriolar hyperplasia; the relative wall thickness of small pulmonary vessels was increased by more than 400% in hypoxic rats (Figure 1b). Although we cannot exclude that pulmonary vasoconstriction could contribute to this medial thickness, the increased nucleus number in the medial layer of pulmonary vessels indicated smooth muscle cell proliferation in hypoxic rats (Stenmark & McMurtry, 2005). To assess the effect of Rho kinase inhibition on these parameters, rats were treated with fasudil (30 mg kg−1 day−1, by gavage) during 15 days of hypobaric hypoxia. As shown in Figure 1, fasudil treatment strongly attenuated the rise in PAP and the right ventricular remodelling induced by chronic hypoxia (Figure 1a). Fasudil also significantly reduced pulmonary arterial remodelling in rats exposed to chronic hypoxia (Figure 1b). Fasudil treatment induced a slight but nonsignificant decrease in systemic AP (Figure 1a). These observations are thus consistent with the effects of Rho kinase inhibition previously reported in other PH models or with other pharmacological Rho kinase inhibitors (Abe et al., 2004; Fagan et al., 2004; Nagaoka et al., 2004; Hyvelin et al., 2005).
Figure 1.
Chronic hypoxia-induced PH is associated with RhoA activation and is reduced by Rho kinase inhibition. (a) PAP, right ventricular hypertrophy (RV/LV+S), and systemic AP determined in control rats (normoxia), rats chronically treated for 14 days with fasudil (30 mg kg−1 day−1), rats exposed to hypoxia for 14 days, and fasudil-treated rats exposed to hypoxia. (b) Sections of lung tissue and quantification of the relative thickness of the pulmonary artery wall in the different experimental conditions (*P<0.01 vs control, **P<0.01, n=5–10). (c) Amount of active GTP-bound RhoA in pulmonary arteries obtained from rats exposed to hypoxia for 0–14 days. Total RhoA and β-actin amounts were also assessed in each sample. Data shown are representative of four independent experiments.
Chronic hypoxia induces RhoA activation
To test whether the involvement of Rho kinase in the pathogenesis of hypoxia-induced PH was related to the activation of RhoA, we directly analysed RhoA activity by pulldown assay in the rat pulmonary artery. As shown in Figure 1c, exposure to hypobaric hypoxia induced an increase in RhoA activity that started the first day of exposure to hypoxia. The maximal RhoA activity (10.7±0.9-fold increase over control, n=6; P<0.001) was measured after 2 days of exposure to hypoxia. Then, after 3 days, the level of RhoA activity remained higher than that in normoxia (day 0), although the level of RhoA expression was decreased, as described previously (Sauzeau et al., 2003a). These results thus demonstrated that the development of chronic hypoxia-induced PH is associated with the activation of RhoA in pulmonary artery.
Sildenafil reduces chronic hypoxia-induced PH
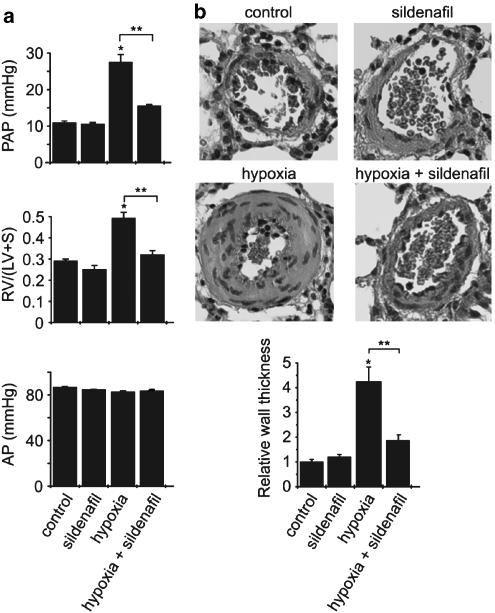
Recent studies have reported the beneficial effect of sildenafil in animal models of PH and in humans. Our results confirm that treatment with sildenafil resulted in a decrease in PAP (Figure 2a), and demonstrate that it also reduced the right ventricular hypertrophy (Figure 2a) and the pulmonary artery thickening in hypoxic rats (Figure 2b) without significant effect in normoxic animals (Figure 2). Sildenafil treatment had no effect on systemic AP (Figure 2a). To determine whether attenuation of chronic hypoxia-induced PH by sildenafil was associated with decreased RhoA activity, we measured the amount of GTP-bound RhoA in pulmonary artery from control and treated rats exposed to hypoxia for 2 days. As seen in Figure 3a, hypoxia-induced RhoA activation was not inhibited by sildenafil treatment; in contrast, both the amount of RhoA and the level of active RhoA are increased in sildenafil-treated hypoxic rats, in agreement with the positive regulation of PKG signalling and sildenafil on RhoA expression (Sauzeau et al., 2003a, 2003b).
Figure 2.
Sildenafil reduces chronic hypoxia induced-PH. (a) PAP, right ventricular hypertrophy (RV/LV+S), and systemic AP determined in control rats (normoxia), rats chronically treated for 14 days with sildenafil (25 mg kg−1 day−1), rats exposed to hypoxia for 14 days, and sildenafil-treated rats exposed to hypoxia. (b) Sections of lung tissue and quantification of the relative thickness of the pulmonary artery wall in the different experimental conditions (*P<0.01 vs control, **P<0.01, n=5–10).
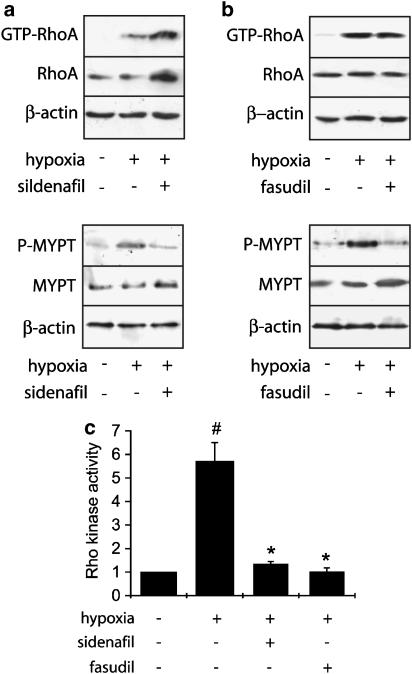
Figure 3.
Effect of sildenafil and fasudil on hypoxia-induced rise in RhoA and Rho kinase activity in rat pulmonary artery. RhoA activity has been analysed by pull-down assay and Rho kinase activity has been assessed by the extent of phosphorylation of MYPT1 in pulmonary artery from control rats, rats exposed to hypoxia for 2 days, and rats treated with sildenafil (25 mg kg−1 day−1) (a) or fasudil (30 mg kg−1 day−1) (b) and exposed to hypoxia for 2 days. Total RhoA, total MYPT1, and β-actin amounts were also assessed in each sample corresponding to proteins extracted from two pooled pulmonary arteries in each group. (c) Corresponding densitometric analyses. Rho kinase activity was quantified by the ratio of the amount of phosphorylated MYPT to total MYPT and expressed relative to the control considered as 1. The data presented are representative of four independent experiments (eight rats in each group) (#P<0.001 vs control; *P<0.001 vs hypoxia).
The target of RhoA, Rho kinase, phosphorylates the MYPT1 subunit of myosin light-chain phosphatase at the Thr-696 residue and this phosphorylation serves as a marker for Rho kinase activity (Feng et al., 1999). We therefore examined the activity of Rho kinase by measuring the level of MYPT1 phosphorylation in pulmonary artery from control and treated rats exposed to hypoxia for 2 days.
Inhibitory effect of Sildenafil treatment on hypoxia-induced increase in Rho kinase activity
In agreement with the observed increase in RhoA activity, analysis of MYPT1 phosphorylation indicated that exposure to hypoxia for 2 days induced Rho kinase activation (5.7±0.8-fold over control, n=8) in pulmonary artery (Figure 3). This hypoxia-induced Rho kinase activation was inhibited by sildenafil treatment (76.6±2%, n=4) (Figure 3a and c). The inhibitory effect of sildenafil on MYPT1 phosphorylation is similar to that obtained by treatment with fasudil (82.3±3%, n=4), which occurred without any effect on Rho activity and expression (Figure 3b and c).
Sildenafil inhibits RhoA/Rho kinase-dependent actin cytoskeleton organization without direct effect on Rho kinase activity
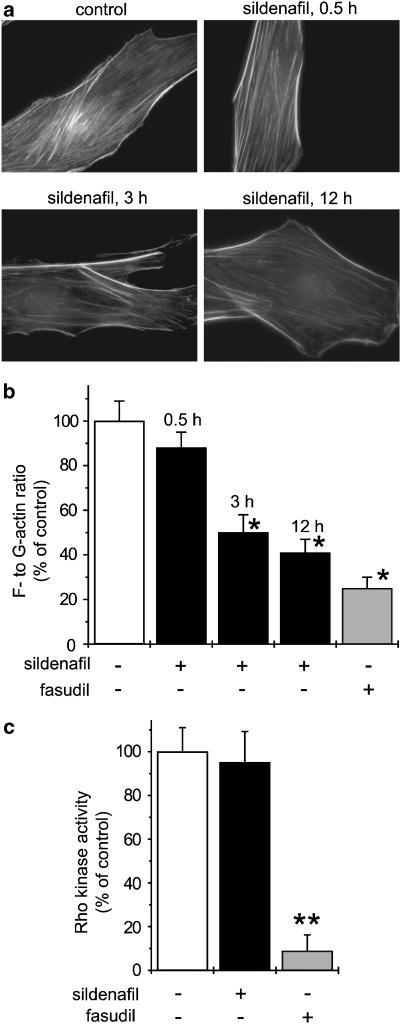
Actin stress fibre oganization in vascular smooth muscle cells is controlled by the RhoA/Rho kinase pathway through MYPT1 phosphorylation (Fukata et al., 2001). To confirm the inhibitory action of sildenafil on the RhoA/Rho kinase pathway, we assessed the effect of sildenafil treatment on actin cytoskeleton organization in smooth muscle cells. Sildenafil treatment (10 μM) for 0.5–12 h induced a decrease in actin stress fibre organization, thus confirming the inhibitory effect of sildenafil on cell functions downstream of RhoA/Rho kinase (Figure 4a and b). As expected, Rho kinase inhibition by fasudil (10 μM, 12 h) inhibited actin stress fibre organization in vascular smooth muscle cells (Figure 4b).
Figure 4.
Effect of sildenafil on actin stress fibre organization and Rho kinase activity. (a) Sildenafil (10 μM) induced a time-dependent inhibition of actin stress fibres visualized by F-actin staining using FITC-phalloidin. (b) Quantification of the time-dependent inhibitory effect of sildenafil on actin cytoskeleton organization by the ratio of F- to G-actin. The inhibitory effect of fasudil (10 μM, 12 h) is also shown. Results are expressed as a percentage of ratio of F- to G-actin in control cells. (c) Sildenafil (10 μM) had no effect on Rho kinase activity, which was strongly inhibited by fasudil (10 μM). Results are expressed as a percentage of Rho kinase activity in control conditions (*P<0.001 and **P<0.0001 vs control, n=4–6).
To address whether the inhibitory action of sildenafil involved a direct effect on Rho kinase, in vitro Rho kinase assay has been performed. Figure 4c shows that the Rho kinase activity was inhibited by ∼95% by 10 μM fasudil, but was not modified in the presence of 10 μM of sildenafil. This result thus provides evidence that sildenafil-induced inhibition of events downstream of Rho kinase such as MYPT1 phosphorylation or actin stress fibre formation was not due to the direct effect on the enzyme activity of Rho kinase.
Sildenafil increases phosphorylation of RhoA and its interaction with GDI
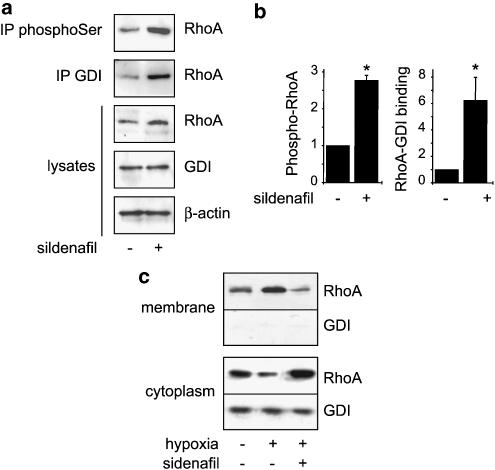
Phosphorylation of Ser-188 of RhoA by cAMP-dependent protein kinase or PKG has been shown to increase its association to the inhibitory regulating protein GDI independently of its GDP or GTP loading, thereby forcing the cytosolic location of RhoA and preventing the activation of its downstream effectors (Lang et al., 1996; Sauzeau et al., 2000; Sawada et al., 2001; Forget et al., 2002; Ellerbroek et al., 2003). The inhibitory action of sildenafil on Rho kinase-dependent phosphorylation, which is associated with a high level of GTP-bound RhoA, could thus result from an increased phosphorylation of RhoA by PKG and cytosolic sequestration through enhanced GDI association. To address this hypothesis, serine-phosphorylated proteins were immunoprecipitated from the pulmonary artery of control- and sildenafil-treated rats, and RhoA was detected with specific anti-RhoA antibodies. Figure 5 shows that serine-phosphorylated RhoA is detected under control condition. The amount of serine-phosphorylated RhoA immunoprecipitated from the pulmonary artery of sildenafil-treated rats was strongly increased compared to control (2.8±0.14-fold over control, n=3) (Figure 5a and b). This increase in serine-phosphorylated RhoA correlated with an increase in the amount of RhoA that coimmunoprecipitated with GDI (Figure 5a and b). These results thus provide evidence that inhibition of type 5 phosphodiesterase by sildenafil increases RhoA phosphorylation and its interaction with cytosolic GDI in pulmonary artery. To confirm that sildenafil induced relocalization of RhoA in the cytosol, we directly examined the subcellular distribution of RhoA and GDI in membrane and cytosolic fractions from the pulmonary artery of control and hypoxic rats. Results shown in Figure 5c confirm that hypoxia-induced RhoA activation is associated to membrane translocation of RhoA and that sildenafil treatment of hypoxic rat relocalized RhoA in the cytosolic fraction.
Figure 5.
Sildenafil increases RhoA phosphorylation and regulates RhoA association to GDI in vivo. (a) Protein samples were extracted from pooled pulmonary arteries of control rats (n=3) and sildenafil-treated rats (25 mg kg−1 day−1) (n=3). Serine-phosphorylated RhoA was detected by Western blotting with anti-RhoA antibody in protein extract immunoprecipitated with anti-phosphoserine antibody. RhoA binding to GDI was detected by Western blotting with anti-RhoA antibody in protein extract immunoprecipitated with anti-GDI andibody. RhoA expression was examined by Western blotting in total lysates. Western blotting with anti-GDI antibody shows the presence of similar amounts of GDI in the samples, and equal loading was confirmed by examination of β-actin expression. (b) Corresponding densitometric analyses. The amounts of phosphorylated RhoA and RhoA/Rho-GDI binding in treated rats are expressed relative to their respective controls considered as 1 (*P<0.01). (c) Subcellular distribution of RhoA and GDI was analysed by Western blotting in membrane and cytosolic fractions of control, hypoxic and sildenafil-treated hypoxic rats. Each sample corresponded to a pool of three pulmonary arteries. The data presented are representative of three independent experiments (nine rats in each group).
Discussion
Results of the present study demonstrate that the type 5 phosphodiesterase inhibitor sildenafil inhibits RhoA/Rho kinase-dependent functions in pulmonary artery. This inhibitory effect resulted from increased RhoA phosphorylation and association to the cytosolic inhibitory protein GDI. Our results thus suggest that the inhibition of intracellular events downstream of RhoA, such as Rho kinase activation, participates in the beneficial effect of sildenafil on PH.
Recent data from pharmacological studies have suggested a role for Rho kinase in the development of PH. In vivo treatment with Rho kinase inhibitor reduced the PAP and pulmonary arterial remodelling in rat and mice models of PH (Abe et al., 2004; Fagan et al., 2004; Jernigan et al., 2004; Nagaoka et al., 2004; 2005; Hyvelin et al., 2005). Our results, showing that the Rho kinase inhibitor fasudil prevented hypoxia-induced PH and cardiovascular remodelling in rats, are in agreement with these data and suggest that, independent of the cause of PH, activation of Rho kinase is a common point in downstream signalling and a critical component of the pathogenesis of PH. The beneficial effect of Rho kinase inhibitor on PH could be ascribed to multiple mechanisms, including its inhibitory effect on pulmonary vasoconstriction (Robertson et al., 2000; Wang et al., 2001; Nagaoka et al., 2004), the prevention of mechanical stress-induced expression of growth factors (Wilson et al., 1993), or the inhibition of Rho kinase-mediated mitogenic effects of serotonin (Liu et al., 2004) and Rho kinase-mediated inhibition of nitric oxide synthase (Takemoto et al., 2002).
RhoA is considered as the main upstream activator of Rho kinase. Here we show that exposure to hypoxia rapidly induced RhoA activation. The maximal activation of RhoA was detected after 2 days of exposure to hypoxia, in agreement with previous results showing a moderated increase in RhoA activity in pulmonary arteries from rats exposed to hypoxia for 4 weeks (Jernigan et al., 2004). The identification of upstream signalling pathway responsible for RhoA activation therefore constitutes an important question. As the RhoA/Rho kinase signalling seems to be a common component of different models of PH, it is conceivable that the impairment of endothelial functions and the subsequent imbalance of vasodilator and vasoconstrictor actions could be involved in its activation. In fact, the increased influence of serotonin and endothelin-1 reported in PH could account for the enhanced RhoA activity in pulmonary artery observed in hypoxic rat as both vasoconstrictor receptors could couple to RhoA signalling pathway (Giaid et al., 1993; Li et al., 1994; Frasch et al., 1999; Somlyo & Somlyo, 2000). On the other hand, the impaired production/bioavailability of NO induced by chronic hypoxia could also participate in the increased RhoA activity (Hampl & Herget, 2000). The NO/PKG pathway mediated RhoA phosphorylation, which led to its cytosolic sequestration through enhanced interaction to GDI and thus prevented activation of downstream effector target activation (Lang et al., 1996; Sauzeau et al., 2000; Ellerbroek et al., 2003). Accordingly, a decreased influence of NO/PKG signalling would reduce the phosphorylation and the cytosolic sequestration of RhoA and, thus, would increase the amount of available RhoA, able to activate downstream pathways such as Rho kinase activation and MYPT1 phosphorylation.
On the opposite, by promoting cytosolic sequestration of RhoA through enhanced association with GDI, sildenafil-mediated increase in RhoA phosphorylation prevents activation of downstream effectors, as shown by the decreased phosphorylation of MYPT1. Rho kinase-mediated MYPT1 phosphorylation and the subsequent inhibition of myosin phosphatase activity is a major mechanism of Ca2+ sensitization of smooth muscle contraction, and decreased MYPT1 phosphorylation is expected to be associated with Ca2+ desensitization (Somlyo & Somlyo, 2003). Accordingly, sildenafil should inhibit Ca2+ sensitization of smooth muscle contraction. In agreement with the present finding, our previous results indicated that sildenafil is indeed an inhibitor of Ca2+ sensitization of the contraction in arterial smooth muscle contraction (Sauzeau et al., 2003a, 2003b). Sildenafil-induced RhoA phosphorylation and subsequent inhibition of Rho kinase-mediated MYPT1 phosporylation is therefore involved in the relaxing effect of sildenafil. However, other mechanisms depending on PKG activity could contribute to this effect. PKG has been shown to phosphorylate MYPT1 at Ser-695 and, although this phosphorylation had no direct effect on myosin phosphatase activity, in vitro experiments using recombinant proteins have suggested that phosphorylation of Ser-695 could reduce phosphorylation of Thr-696, thereby limiting the inactivation of myosin phosphatase (Wooldridge et al., 2004).
By promoting GDI-mediated cytosolic sequestration of RhoA, sildenafil prevents activation of downstream effectors and could thus affect RhoA-mediated effects that are independent of Rho kinase. This suggests that drugs able to interfere with RhoA protein would have beneficial effects on PH. Statins represent such drugs. By inhibiting HMG-CoA reductase, statins prevent the synthesis of isoprenoid intermediates, including farnesyl- and geranylgeranyl-pyrophosphate. By this way, statins inhibit the isoprenylation of RhoA, which results in the accumulation of cytoplasmic RhoA and the inhibition of dowstream pathway (Liao & Laufs, 2005). Simvastatin treatment has been shown to potently attenuate chronic hypoxic PH in rats and inhibit vascular remodelling (Girgis et al., 2003). Furthermore, simvastatin rescues rats from fatal PH induced by monocrotaline (Nishimura et al., 2003). Although the activation of RhoA-dependent signalling pathways has not been analysed in these reports, these data support our hypothesis that drugs affecting RhoA should be effective on PH.
Acknowledgments
This work is supported by grants from INSERM, the Région Pays de la Loire, and the Association Française contre la Myopathie. Malvyne Rolli-Derkinderen was supported by a grant from the Groupe de Réflexion sur la Recherche Cardiovasculaire. We thank Dr Martin Schwartz for the gift of the plasmids encoding the Rhotekin RBD and N. Vaillant for technical assistance.
Abbreviations
- AP
arterial pressure
- GDI
guanine dissociation inhibitor
- LV
left ventricle
- MYPT1
myosin phosphatase target subunit 1
- PAP
pulmonary arterial pressure
- PH
pulmonary hypertension
- PKG
cGMP-dependent protein kinase
- RV
right ventricle
References
- ABE K., SHIMOKAWA H., MORIKAWA K., UWATOKU T., OI K., MATSUMOTO Y., HATTORI T., NAKASHIMA Y., KAIBUCHI K., SUEISHI K., TAKESHIT A. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ. Res. 2004;94:385–393. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- BUDHIRAJA R., TUDER R.M., HASSOUN P.M. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- DIGNAM J.D. Preparation of extracts from higher eukaryotes. Methods Enzymol. 1990;182:194–203. doi: 10.1016/0076-6879(90)82017-v. [DOI] [PubMed] [Google Scholar]
- ELLERBROEK S.M., WENNERBERG K., BURRIDGE K. Serine phosphorylation negatively regulates RhoA in vivo. J. Biol. Chem. 2003;278:19023–19031. doi: 10.1074/jbc.M213066200. [DOI] [PubMed] [Google Scholar]
- FAGAN K.A., OKA M., BAUER N.R., GEBB S.A., IVY D.D., MORRIS K.G., MCMURTRY I.F. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L656–L664. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- FENG J., ITO M., ICHIKAWA K., ISAKA N., NISHIKAWA M., HARTSHORNE D.J., NAKANO T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J. Biol. Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- FORGET M.A., DESROSIERS R.R., GINGRAS D., BELIVEAU R. Phosphorylation states of Cdc42 and RhoA regulate their interactions with Rho GDP dissociation inhibitor and their extraction from biological membranes. Biochem. J. 2002;361:243–254. doi: 10.1042/0264-6021:3610243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASCH H.F., MARSHALL C., MARSHALL B.E. Endothelin-1 is elevated in monocrotaline pulmonary hypertension. Am. J. Physiol. 1999;276:L304–L310. doi: 10.1152/ajplung.1999.276.2.L304. [DOI] [PubMed] [Google Scholar]
- FUKATA Y., AMANO M., KAIBUCHI K. Rho–Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol. Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- GHOFRANI H.A., WIEDEMANN R., ROSE F., SCHERMULY R.T., OLSCHEWSKI H., WEISSMANN N., GUNTHER A., WALMRATH D., SEEGER W., GRIMMINGER F. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002;360:895–900. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- GIAID A., YANAGISAWA M., LANGLEBEN D., MICHEL R.P., LEVY R., SHENNIB H., KIMURA S., MASAKI T., DUGUID W.P., STEWART D.J. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- GIRGIS R.E., LI D., ZHAN X., GARCIA J.G., TUDER R.M., HASSOUN P.M., JOHNS R.A. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H938–H945. doi: 10.1152/ajpheart.01097.2002. [DOI] [PubMed] [Google Scholar]
- GUDI T., CHEN J.C., CASTEEL D.E., SEASHOLTZ T.M., BOSS G.R., PILZ R.B. cGMP-dependent protein kinase inhibits serum-response element-dependent transcription by inhibiting rho activation and functions. J. Biol. Chem. 2002;277:37382–37393. doi: 10.1074/jbc.M204491200. [DOI] [PubMed] [Google Scholar]
- HAMPL V., HERGET J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol. Rev. 2000;80:1337–1372. doi: 10.1152/physrev.2000.80.4.1337. [DOI] [PubMed] [Google Scholar]
- HYVELIN J.M., HOWELL K., NICHOL A., COSTELLO C.M., PRESTON R.J., MCLOUGHLIN P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ. Res. 2005;97:185–191. doi: 10.1161/01.RES.0000174287.17953.83. [DOI] [PubMed] [Google Scholar]
- ITOH T., NAGAYA N., FUJII T., IWASE T., NAKANISHI N., HAMADA K., KANGAWA K., KIMURA H. A combination of oral sildenafil and beraprost ameliorates pulmonary hypertension in rats. Am. J. Respir. Crit. Care Med. 2004;169:34–38. doi: 10.1164/rccm.200303-346OC. [DOI] [PubMed] [Google Scholar]
- JERNIGAN N.L., WALKER B.R., RESTA T.C. Chronic hypoxia augments protein kinase G-mediated Ca2+ desensitization in pulmonary vascular smooth muscle through inhibition of RhoA/Rho kinase signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L1220–L1229. doi: 10.1152/ajplung.00196.2004. [DOI] [PubMed] [Google Scholar]
- KNOWLES G.C., MCCULLOCH C.A. Simultaneous localization and quantification of relative G and F actin content: optimization of fluorescence labeling methods. J. Histochem. Cytochem. 1992;40:1605–1612. doi: 10.1177/40.10.1527379. [DOI] [PubMed] [Google Scholar]
- LANG P., GESBERT F., DELESPINE-CARMAGNAT M., STANCOU R., POUCHELET M., BERTOGLIO J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 1996;15:510–519. [PMC free article] [PubMed] [Google Scholar]
- LI H., CHEN S.J., CHEN Y.F., MENG Q.C., DURAND J., OPARIL S., ELTON T.S. Enhanced endothelin-1 and endothelin receptor gene expression in chronic hypoxia. J. Appl. Physiol. 1994;77:1451–1459. doi: 10.1152/jappl.1994.77.3.1451. [DOI] [PubMed] [Google Scholar]
- LIAO J.K., LAUFS U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y., SUZUKI Y.J., DAY R.M., FANBURG B.L. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ. Res. 2004;95:579–586. doi: 10.1161/01.RES.0000141428.53262.a4. [DOI] [PubMed] [Google Scholar]
- MACK C.P., SOMLYO A.V., HAUTMANN M., SOMLYO A.P., OWENS G.K. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J. Biol. Chem. 2001;276:341–347. doi: 10.1074/jbc.M005505200. [DOI] [PubMed] [Google Scholar]
- MACLEAN M.R., JOHNSTON E.D., MCCULLOCH K.M., POOLEY L., HOUSLAY M.D., SWEENEY G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J. Pharmacol. Exp. Ther. 1997;283:619–624. [PubMed] [Google Scholar]
- MICHELAKIS E., TYMCHAK W., LIEN D., WEBSTER L., HASHIMOTO K., ARCHER S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation. 2002;105:2398–2403. doi: 10.1161/01.cir.0000016641.12984.dc. [DOI] [PubMed] [Google Scholar]
- MURRAY F., MACLEAN M.R., PYNE N.J. Increased expression of the cGMP-inhibited cAMP-specific (PDE3) and cGMP binding cGMP-specific (PDE5) phosphodiesterases in models of pulmonary hypertension. Br. J. Pharmacol. 2002;137:1187–1194. doi: 10.1038/sj.bjp.0704984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGAOKA T., FAGAN K.A., GEBB S.A., MORRIS K.G., SUZUKI T., SHIMOKAWA H., MCMURTRY I.F., OKA M. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2005;171:494–499. doi: 10.1164/rccm.200405-637OC. [DOI] [PubMed] [Google Scholar]
- NAGAOKA T., MORIO Y., CASANOVA N., BAUER N., GEBB S., MCMURTRY I., OKA M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L665–L672. doi: 10.1152/ajplung.00050.2003. [DOI] [PubMed] [Google Scholar]
- NISHIMURA T., VASZAR L.T., FAUL J.L., ZHAO G., BERRY G.J., SHI L., QIU D., BENSON G., PEARL R.G., KAO P.N. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation. 2003;108:1640–1645. doi: 10.1161/01.CIR.0000087592.47401.37. [DOI] [PubMed] [Google Scholar]
- RABINOVITCH M. Pulmonary hypertension: updating a mysterious disease. Cardiovasc. Res. 1997;34:268–272. doi: 10.1016/s0008-6363(97)00053-9. [DOI] [PubMed] [Google Scholar]
- REN X.D., KIOSSES W.B., SCHWARTZ M.A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON T.P., DIPP M., WARD J.P., AARONSON P.I., EVANS A.M. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br. J. Pharmacol. 2000;131:5–9. doi: 10.1038/sj.bjp.0703537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUNO J.R., LOYD J.E. Primary pulmonary hypertension. Lancet. 2003;361:1533–1544. doi: 10.1016/S0140-6736(03)13167-4. [DOI] [PubMed] [Google Scholar]
- SAUZEAU V., LE JEUNE H., CARIO-TOUMANIANTZ C., SMOLENSKI A., LOHMANN S.M., BERTOGLIO J., CHARDIN P., PACAUD P., LOIRAND G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- SAUZEAU V., ROLLI-DERKINDEREN M., LEHOUX S., LOIRAND G., PACAUD P. Sildenafil prevents change in RhoA expression induced by chronic hypoxia in rat pulmonary artery. Circ. Res. 2003a;93:630–637. doi: 10.1161/01.RES.0000093220.90027.D9. [DOI] [PubMed] [Google Scholar]
- SAUZEAU V., ROLLI-DERKINDEREN M., MARIONNEAU C., LOIRAND G., PACAUD P. RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J. Biol. Chem. 2003b;278:9472–9480. doi: 10.1074/jbc.M212776200. [DOI] [PubMed] [Google Scholar]
- SAWADA N., ITOH H., YAMASHITA J., DOI K., INOUE M., MASATSUGU K., FUKUNAGA Y., SAKAGUCHI S., SONE M., YAMAHARA K., YURUGI T., NAKAO K. cGMP-dependent protein kinase phosphorylates and inactivates RhoA. Biochem. Biophys. Res. Commun. 2001;280:798–805. doi: 10.1006/bbrc.2000.4194. [DOI] [PubMed] [Google Scholar]
- SCHERMULY R.T., KREISSELMEIER K.P., GHOFRANI H.A., YILMAZ H., BUTROUS G., ERMERT L., ERMERT M., WEISSMANN N., ROSE F., GUENTHER A., WALMRATH D., SEEGER W., GRIMMINGER F. Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am. J. Respir. Crit. Care Med. 2004;169:39–45. doi: 10.1164/rccm.200302-282OC. [DOI] [PubMed] [Google Scholar]
- SEBKHI A., STRANGE J.W., PHILLIPS S.C., WHARTON J., WILKINS M.R. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation. 2003;107:3230–3235. doi: 10.1161/01.CIR.0000074226.20466.B1. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol. 2000;522 Part 2:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- STENMARK K.R., MCMURTRY I.F. Vascular remodeling versus vasoconstriction in chronic hypoxic pulmonary hypertension: a time for reappraisal. Circ. Res. 2005;97:95–98. doi: 10.1161/01.RES.00000175934.68087.29. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO M., SUN J., HIROKI J., SHIMOKAWA H., LIAO J.K. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- VAN AELST L., D'SOUZA-SCHOREY C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- WANG Z., JIN N., GANGULI S., SWARTZ D.R., LI L., RHOADES R.A. Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am. J. Respir. Cell Mol. Biol. 2001;25:628–635. doi: 10.1165/ajrcmb.25.5.4461. [DOI] [PubMed] [Google Scholar]
- WILSON E., MAI Q., SUDHIR K., WEISS R.H., IVES H.E. Mechanical strain induces growth of vascular smooth muscle cells via autocrine action of PDGF. J. Cell Biol. 1993;123:741–747. doi: 10.1083/jcb.123.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLDRIDGE A.A., MACDONALD J.A., ERDODI F., MA C., BORMAN M.A., HARTSHORNE D.J., HAYSTEAD T.A. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of serine 695 in response to cyclic nucleotides. J. Biol. Chem. 2004;279:34496–34504. doi: 10.1074/jbc.M405957200. [DOI] [PubMed] [Google Scholar]
- ZHAO L., MASON N.A., MORRELL N.W., KOJONAZAROV B., SADYKOV A., MARIPOV A., MIRRAKHIMOV M.M., ALDASHEV A., WILKINS M.R. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation. 2001;104:424–428. doi: 10.1161/hc2901.093117. [DOI] [PubMed] [Google Scholar]