Abstract
The segment-specific actions of endothelin peptides and agonists have not been thoroughly investigated in the renal microcirculation. The current studies were performed to assess the relative contribution of ETA and ETB receptors to the renal pre- and postglomerular arteriolar responses to ET-1.
Experiments determined the effect of selective ETA (A-127722; 30 nM) and ETB (A-192621; 30 nM) receptor blockade, on arteriolar responses to ET-1 concentrations of 1 pM to 10 nM in rat kidneys using the isolated juxtamedullary nephron technique. Renal perfusion pressure was set at 110 mmHg.
Baseline afferent arteriolar diameter was similar in all groups and averaged 17.8±0.6 μm (n=14). In control experiments (n=6), ET-1 produced significant concentration-dependent decreases in arteriolar diameter, with 10 nM ET-1 decreasing diameter by 85±1%.
Selective blockade of ETA receptors (n=6) prevented ET-1-mediated vasoconstriction, except at concentrations of 1 and 10 nM. Similarly, the vasoconstrictor profile was right shifted during selective ETB receptor blockade (n=4). Combined ETA and ETB receptor blockade (n=5) completely abolished afferent arteriolar diameter responses to ET-1.
ETB selective agonists (S6c and IRL-1620) produced disparate responses. S6c produced a concentration-dependent vasoconstriction of afferent arterioles. In contrast, S6c produced a concentration-dependent dilation of efferent arterioles that could be blocked with an ETB receptor antagonist. IRL-1620, another ETB agonist, was less effective at altering afferent or efferent diameter and produced a small reduction in pre- and postglomerular arteriolar diameter.
These data demonstrate that both ETA and ETB receptors participate in ET-1-mediated vasoconstriction of afferent arterioles. ETB receptor stimulation provides a significant vasodilatory influence on the efferent arteriole. Furthermore, since selective ETA and ETB receptor antagonists abolished preglomerular vasoconstrictor responses at lower ET-1 concentrations, these data support a possible interaction between ETA and ETB receptors in the control of afferent arteriolar diameter.
Keywords: Endothelin receptors, renal microcirculation, IRL-1640, S6c
Introduction
Endothelin-1 (ET-1) is a powerful vasoconstrictor and mitogen involved in the pathophysiology of many cardiovascular diseases. The vasoconstrictor effects are primarily mediated by ETA receptors although, in some vascular beds, including the kidney, ETB receptors also contribute to the vasoconstrictor actions of exogenously administered ET-1 (Pollock, 2000). However, ETB receptors are better known for their ability to stimulate NO and prostaglandin release from the vascular endothelium to produce vasodilatation. At the whole kidney level, exogenous ET-1 produces decreases in blood flow without overt vasodilatation (Pollock et al., 2005). This vasoconstriction is mediated by a combination of ETA and ETB receptor activity (Pollock & Opgenorth, 1993; 1994). In fact, ETB receptor-specific agonists decrease renal blood flow. However, ETB receptor-specific antagonists also decrease renal blood flow and exaggerate ET-1-induced decreases in renal blood flow suggesting a tonic vasodilator influence of endogenous ET-1 (D'Orléans-Juste et al., 1994; Allcock et al., 1995; Pollock et al., 2005).
The factors governing the complex receptor-specific actions of ET-1, within the renal microcirculation, are not known. Recently, Just et al. (2004) have suggested that the balance of ETA and ETB receptor actions, at the whole kidney level, is not simply additive. Much of what we know about the segment-specific actions of ET-1 on the renal microcirculation comes from studies in the hydronephrotic kidney of the rat. Loutzenhiser et al. (1990) observed that ET-1 produces a less pronounced vasoconstriction in the efferent versus the afferent arteriole. The preglomerular vasoconstriction produced by systemic administration of ET-1 could be severely inhibited by the ETA selective antagonist, BQ-123 (Cavarape & Bartoli, 1998). In the same model, there is functional evidence of heterogeneous distribution of ETA and ETB receptors in the renal microcirculation (Endlich et al., 1996; Åkerman et al., 1998). Whole kidney experiments, however, are complicated by the fact that delivery of ET peptides via the circulation may not distribute evenly throughout the kidney, except at very high concentrations, due to the irreversible nature of ET binding to its receptors. In isolated afferent and efferent arterioles from the rabbit, vasoconstrictor responses to ET-1 and endothelin 3 (ET-3) suggest a predominance of ETA over ETB receptors in both segments (Edwards et al., 1990).
Previous studies have not performed a thorough assessment of receptor-specific actions of endothelin peptides in the pre- and postglomerular microcirculation with an intact vasculo-tubular unit. We speculate that site-specific actions may provide some explanations into the physiological actions of ETA and ETB receptors. The purpose of the present study was to determine receptor-dependent responses to ET-1, and related peptides, in the renal microcirculation. We used the blood perfused juxtamedullary nephron preparation to identify pre- and postglomerular actions of ET-1, endothelin 2 (ET-2), ET-3, Sarafotoxin (S6c) and IRL-1620. These studies were extended by confirming receptor-dependent contributions to changes in afferent and efferent arteriolar caliber using receptor selective antagonists.
Methods
All experiments were performed with approval from the Institutional Animal Care and Use Committee at the Medical College of Georgia.
In vitro blood perfused juxtamedullary nephron experiments
Videomicroscopy experiments were conducted in vitro using the blood perfused juxtamedullary nephron technique, as previously described (Inscho et al., 1998; Inscho & Cook, 2002). For each experiment, two male Sprague–Dawley rats (350–400 g) were anesthetized with sodium pentobarbital (40 mg kg−1; i.p.) and prepared for videomicroscopy experiments. Perfusate blood was collected and prepared as described (Inscho et al., 1991; 1992; 1995; 1996). Briefly, blood was collected from the nephrectomized blood donor rat into a heparinized (500 units) syringe. The plasma and erythrocyte fractions were separated and the leukocyte fraction was discarded. Plasma was filtered (0.2 μm) and combined with the erythrocytes to yield a hematocrit of approximately 33%. The reconstituted blood was filtered through a 5 μm nylon mesh.
The right renal artery of the kidney donor was cannulated and perfused with a Tyrode's buffer solution containing 52 g l−1 bovine serum albumin (Sigma Chemical Company, St Louis, MO, U.S.A.) and a complement of L-amino acids, as described (Inscho et al., 1991; 1992; 1995). The rat was exsanguinated into a heparinized syringe (500 units) via a carotid artery cannula and processed with blood collected from the blood donor rat. The perfused kidney was removed and sectioned along the longitudinal axis leaving the intact papilla on the dorsal two thirds portion of the kidney (Casellas & Navar, 1984). The papilla was reflected and the pelvic mucosa removed to expose the renal tubules, glomeruli and microvasculature of juxtamedullary nephrons.
After completing the dissection, the Tyrode's perfusate was replaced with the reconstituted blood. The blood perfusate was stirred continuously in a closed reservoir while being oxygenated with a 95% O2–5% CO2 gas mixture. Perfusion pressure was monitored by a pressure cannula located within the double barreled perfusion cannula and connected to a Statham P23Db pressure transducer linked to a polygraph recorder (Grass Instruments, Quincy, MA, U.S.A.). Perfusion pressure was fixed at 110 mmHg. The inner cortical surface of the kidney was superfused with warmed (37°C) Tyrode's buffer containing 10 g l−1 bovine serum albumin.
The perfusion chamber containing the kidney was mounted to the stage of a Nikon Optiphot-2UD microscope (Nikon Inc., Tokyo, Japan) equipped with a Zeiss water immersion objective (× 40). The tissue was transilluminated and viewed with a high-resolution Newvicon camera (NC-70, Dage-MTI, Michigan City, IN, U.S.A.). The video image was enhanced using an image processor (MFJ-1425, MFJ Enterprises Inc., Starkville, MS, U.S.A.) and displayed on a video monitor while being simultaneously recorded on videotape for later analysis. Vascular inside diameters were measured at a single site using a calibrated image shearing monitor (Model 901, Instrumentation for Physiology and Medicine, San Diego, CA, U.S.A.).
Experimental protocols
Arteriolar responses to endothelin agonists and antagonists were determined. Measurements of arteriolar diameter were made at 12 s intervals, and the sustained diameter was calculated from the average of measurements made during the final 2 min of each treatment period. Each protocol consisted of 6–12, 5-min periods. Each protocol began with a 5-min control period to ensure a stable vessel diameter and was followed by either exposure to endothelin peptides or agonists, or by administration of endothelin receptor antagonists followed by agonist stimulation. Owing to the slow washout time for endothelin peptides, only one set of dose–response curves was evaluated in each kidney.
Series 1: effect of endothelin peptides on afferent and efferent arteriolar diameter
Afferent and efferent arteriolar responses were determined for ET-1, ET-2 and ET-3. Endothelin-mediated responses were assessed in response to increasing agonist concentrations ranging from 1 pM to 10 nM. Arterioles were exposed to each agonist concentration for 5 min increments before the next solution change was initiated.
Series 2: effect of ETA and ETB receptor blockade on the afferent and efferent arteriolar response to ET-1
These experiments were performed to determine the effect of ETA and ETB receptor blockade on the afferent and efferent arteriolar vasoconstriction induced by ET-1. Control afferent diameter was determined prior to introduction of either an ETA antagonist (A-127722; 30 nM) or and ETB antagonist (A-192621; 30 nM). This concentration of antagonist has been shown to be selective for their respective receptors (Winn et al., 1996; Von Geldern et al., 1999; Wessale et al., 2002; Wu-Wong et al., 2002). After 20 min of exposure to the ET receptor blockers, the concentration–response relationship to ET-1 was determined. In a separate set of kidneys, the effect of combined ETA and ETB receptor blockade on the afferent arteriolar response to ET-1 was assessed. Experiments were performed as described above except that combined endothelin receptor blockade was imposed using pretreatment with ETA and ETB receptor antagonists.
Series 3: effect of endothelin ETB agonists on afferent and efferent arteriolar diameter
The effect of the selective ETB receptor agonists, S6c and IRL-1620, on afferent and efferent arteriolar diameter was assessed on separate groups of kidneys. S6c and IRL-1620 were administered at concentrations ranging from 1 pM to 10 nM, consistent with experiments using native endothelin peptides. The ability of an ETB receptor antagonist to block S6c-mediated effects was determined on a separate set of efferent arterioles.
Statistical analysis
Within treatment group comparisons were evaluated using a one-way analysis of variance for repeated measures to determine if arteriolar diameter changed as a result of the endothelin agonist treatment. Differences between group means, within each series, were determined using Newman–Kuels multiple range test. A two-way analysis of variance for repeated measures was used to determine if the magnitude of the afferent arteriolar response to an endothelin peptide was different from the efferent arteriolar response to the same peptide. Bonferroni post hoc analysis was performed to determine differences between vessel segments at each peptide concentration tested. P-values <0.05 (P<0.05) were considered to indicate statistically significant differences. All values are reported as the mean±s.e.
Materials
S6c, IRL-1620 and endothelin peptides were obtained from American Peptide, Inc. (Sunnyvale, CA, U.S.A.). Bovine serum albumin was purchased from Calbiochem Inc. (La Jolla, CA, U.S.A.). The endothelin receptor antagonists, A-127722 and A-192621, were kindly provided by Abbott Laboratories (Abbott Park, IL, U.S.A.). All other reagents were obtained from Sigma Chemical Co. (St Louis, MO, U.S.A.).
Results
Effect of endothelin peptides on afferent and efferent arteriolar diameter
Naturally occurring endothelin peptides exert varying degrees of selectivity for activating ETA and ETB receptors. In general, ETA receptors are activated by ET-1 and ET-2, whereas, ETB receptors are responsive to ET-1, ET-2 and ET-3 (Wessale et al., 2002; Wu-Wong et al., 2002). Therefore, initial studies focused on establishing the dose-dependent responses of juxtamedullary afferent and efferent arterioles to ET-1, ET-2 and ET-3. Baseline diameters were similar between each of the groups treated with ET-1, ET-2 and ET-3 (Table 1). ET-1 stimulated a marked, concentration-dependent vasoconstriction of both afferent and efferent arterioles (Figure 1a), however, the magnitude of the afferent arteriolar response to ET-1 was significantly greater than the efferent arteriolar response. The highest dose of ET-1 (10 nM) reduced afferent diameter by 84±1% compared to 67±5% for efferent arterioles. Significant vasoconstriction was evident at ET-1 concentrations as low as 1 and 10 pM for afferent and efferent arterioles, respectively.
Table 1.
Baseline diameters of afferent and efferent arterioles (μm)
| Agonist | Afferent arterioles | Efferent arterioles |
|---|---|---|
| ET-1 | 18.2±0.5 (n=21) | 22.0±0.8 (n=17) |
| ET-2 | 20.5±0.8 (n=5) | 22.8±1.2 (n=6) |
| ET-3 | 19.3±1.3 (n=6) | 23.0±0.7 (n=6) |
| S6c | 16.1±0.5 (n=6) | 19.8±0.6 (n=11) |
| IRL-1620 | 15.5±0.7 (n=6) | 20.9±1.3 (n=5) |
| ETA blockade (A127722) | 17.7±0.5 (n=6) | 21.1±1.6 (n=5) |
| ETB blockade (A192621) | 15.5±0.7 (n=6) | 20.2±1.3 (n=6) |
| ETA and ETB blockade | 19.5±0.9 (n=5) |
Figure 1.
Afferent and efferent arteriolar responses to increasing concentrations of ET-1 (a), ET-2 (b) and ET-3 (c). Endothelin peptides were applied over a range from 1 pM to 10 nM. Afferent and efferent arteriolar responses are depicted by the closed and open symbols, respectively. Each data point represents the mean vessel diameter in microns, measured at 12 s intervals, throughout the experimental period. Data are plotted as the percent of the respective control diameter. *Indicates a significant reduction in diameter compared to the control diameter (P<0.05). †Indicates a significant difference in the response of afferent arterioles compared to efferent arterioles (P<0.05).
ET-2 also evoked significant vasoconstriction of afferent and efferent arterioles but the concentration–response curve was shifted to the right (Figure 1b). ET-2 at 10 nM reduced afferent and efferent arteriolar diameter similarly by 74±3 and 68±11%, respectively. Afferent arterioles were slightly more responsive to ET-2 than efferent arterioles, as a significant reduction in afferent diameter was evident at an ET-2 concentration of 100 pM.
ET-3 binds selectively to ETB receptors at low concentrations, and in these studies, ET-3 also produced a concentration-dependent vasoconstriction of afferent arterioles (Figure 1c) but concentrations reaching 1 nM were required. The overall concentration–response profile was shifted further to the right compared to ET-1 or ET-2. The magnitude of the response exhibited by afferent arterioles was significantly greater than the efferent arteriolar response at ET-3 concentrations of 1 and 10 nM. ET-3 reduced afferent diameter by 84±2% compared to 51±6% for efferent arterioles.
Afferent and efferent arteriolar response to ET-1 during ETA and ETB receptor blockade
Administration of endothelin receptor antagonists had no significant effect on baseline afferent or efferent arteriolar diameter. Afferent arteriolar diameter averaged between 98 and 101% of the baseline diameter (Table 1) during ETA, ETB and combined ETA and ETB receptor blockade. Similarly, efferent arteriolar diameter averaged between 100 and 104% of the baseline diameter (Table 1).
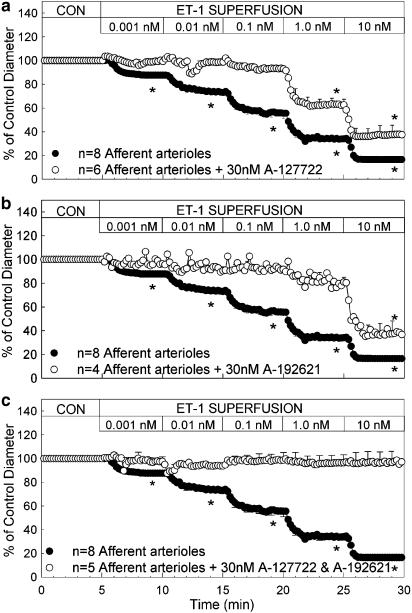
The effect of ETA and ETB receptor blockade on the afferent arteriolar response to ET-1 is illustrated in Figure 2. Blockade of ETA receptors with 30 nM A-127722 significantly attenuated ET-1-mediated afferent arteriolar vasoconstriction (Figure 2a). The concentration response profile to ET-1 is shifted to the right. The magnitude of the vasoconstriction is significantly attenuated at all concentrations tested. In the presence of the ETA antagonist, A-127722, 1 nM ET-1 was required to significantly reduce afferent arteriolar diameter. Similarly, pretreatment with the ETB receptor antagonist, A-192621, also shifted the concentration–response relationship markedly to the right. As illustrated in Figure 2b, 10 nM ET-1 was needed to produce a significant vasoconstriction compared to the 1 pM concentration that was effective under control conditions. During combined blockade of ETA and ETB receptors, by simultaneous treatment with A-127722 and A-192621, the afferent arteriolar vasoconstrictor response to ET-1 was completely blocked (Figure 2c). Afferent diameter averaged 19.0±0.5 μm during ETA and ETB receptor blockade alone and remained within 94 and 99% of the antagonist control diameter when exposed to ET-1 concentrations ranging from 1 pM to 10 nM.
Figure 2.
Effect of ETA, ETB and combined ETA and ETB receptor blockade on afferent arteriolar responses to increasing concentrations of ET-1. ET-1 was applied over a range from 1 pM to 10 nM. Control data (closed symbols) are taken from Figure 1 and are reproduced here for comparison to separate responses during ETA (30 nM A-127722; a), ETB (30 nM A-192621; b) or combined ETA and ETB receptor blockade (30 nM A-127722+30 nM A-192621; c). Each data point represents the mean vessel diameter in microns, measured at 12 s intervals, throughout the experimental period. Data are plotted as the percent of the respective control diameter during ETA or ETB receptor blockade alone. *Indicates a significant reduction in diameter compared to the control diameter (P<0.05).
The results obtained for efferent arterioles are shown in Figure 3. ETA receptor blockade prevented the ET-1-mediated vasoconstriction and revealed a significant and concentration-dependent vasodilatation with ET-1 concentrations between 1 and 100 pM (Figure 3a). As ET-1 concentrations reached 1 and 10 nM, the vasodilatory influence was overcome and reverted to a significant vasoconstriction. Thus, during ETA receptor blockade, the concentration–response profile to ET-1 was different from the relationship observed with control efferent arterioles.
Figure 3.
Effect of ETA and ETB receptor blockade on efferent arteriolar responses to increasing concentrations of ET-1. ET-1 was applied over a range from 1 pM to 10 nM. Control data (closed symbols) are taken from Figure 1 and are reproduced here for comparison to separate responses during ETA (30 nM A-127722; a) or ETB (30 nM A-192621; b) receptor blockade. Each data point represents the mean vessel diameter in microns, measured at 12 s intervals, throughout the experimental period. Data are plotted as the percent of the respective control diameter during ETA or ETB receptor blockade alone. *Indicates a significant reduction in diameter compared to the control diameter (P<0.05).
The effect of ETB receptor blockade on the efferent arteriolar responses to ET-1 is illustrated in Figure 3b. No vasodilatory response was evident in arterioles exposed to A-192621. ET-1 concentrations of 0.1, 1 and 10 nM significantly reduced efferent arteriolar diameter to 62±4, 30±43 and 28±1% of control diameter, respectively. In contrast, in control animals, with intact ETB receptors, these same concentrations of ET-1 reduced efferent arteriolar diameter to 74±3, 54±2 and 33±5% of the control diameter, respectively.
Effect of endothelin ETB agonists on afferent and efferent arteriolar diameter
To further explore the segmental differences in the arteriolar response to ET-1 during blockade of ETA receptors, we assessed the effect of ETB agonists on afferent and efferent arteriolar diameter. For these studies, we examined the segmental diameter responses to the ETB agonists, S6c and IRL-1620. Baseline afferent and efferent arteriolar diameters were similar between the S6c- and IRL-1620-treated groups (Table 1).
Figure 4a presents the afferent and efferent arteriolar responses to increasing concentrations of S6c. As shown, S6c produced a concentration-dependent afferent arteriolar vasoconstriction that was significant at concentrations from 10 pM to 10 nM. In contrast, S6c produced a concentration-dependent vasodilatation of efferent arterioles that was significant over the range of 100 pM–10 nM. Consequently, S6c produced a significant increase in efferent diameter compared to a significant decrease in afferent arteriolar diameter. Figure 4b depicts the effect of ETB receptor blockade on the efferent vasodilatory response evoked by S6c. Efferent arterioles were pretreated with 30 nM A-192621 for 20 min before being exposed to increasing concentrations of ET-1. ETB-receptor blockade eliminated the vasodilatation induced by S6c and revealed a modest, but significant, vasoconstriction.
Figure 4.
The effect of ETB receptor stimulation, with S6c, on afferent and efferent arteriolar diameter (a). S6c was applied over a range from 1 pM to 10 nM. Afferent and efferent arteriolar responses are depicted by the closed and open symbols, respectively. (b) Depicts the effect of ETB receptor blockade (30 nM A192621) on the efferent arteriolar diameter response to S6c. Control efferent arteriolar responses from panel a are presented by the closed symbols and responses from separate arterioles during ETB receptor blockade are shown by the open symbols. Each data point represents the mean vessel diameter in microns, measured at 12 s intervals, throughout the experimental period. Data are plotted as the percent of the respective control diameter. *Indicates a significant reduction in diameter compared to the control diameter (P<0.05).
Similar experiments performed using a different ETB agonist, IRL-1620 (1 pM–10 nM), yielded somewhat different results. IRL-1620 produced a modest, concentration-dependent vasoconstriction of afferent and efferent arterioles at concentrations of 0.01 nM and higher. The magnitude of the maximum reduction in arteriolar diameter averaged 24±3 and 28±5% for afferent and efferent arterioles compared to a 57±3% reduction in afferent diameter and a 17±7% increase in the diameter of efferent arterioles when exposed to S6c.
Discussion
Over 10 years ago, several labs established, at the whole kidney level, that both ETA and ETB receptor activation can produce vasoconstriction (Pollock & Opgenorth, 1993; 1994; Wellings et al., 1994; Seo & Luscher, 1995). However, a few reports have suggested that ETB receptors oppose these effects via endothelial-dependent vasodilatation (D'Orléans-Juste et al., 1994; Allcock et al., 1995). The current study represents one of the few characterizations of receptor-specific actions of endothelin peptides in the renal microcirculation. Studies where endothelin peptides are infused via the circulation could be limited by the fact that endothelin peptides bind irreversibly to their receptors, thus limiting the effective concentrations of peptides reaching different segments of the renal microvascular tree as it penetrates the kidney. Thus, whole kidney administration of endothelin peptides can only yield average information about the response of the renal circulation and does not provide site specific information regarding afferent and efferent arteriolar responsiveness. Using the blood-perfused juxtamedullary nephron preparation, we were able to identify a heterogeneous response to endothelin peptides and site-specific actions of endothelin receptor activation within the renal microcirculation. An advantage of using the juxtamedullary nephron method to localize, and identify, receptor-specific actions is that the entire circulation is exposed to the peptide at the same time. The major findings include (1) the afferent arteriole is more sensitive to the vasoconstrictor actions of ET-1 compared to the efferent arteriole; (2) ETA receptors mediate a large portion of the vasoconstrictor actions of ET-1 in both the afferent and efferent arteriole; (3) ETB receptor-mediated vasoconstriction is evident in the afferent arteriole, whereas ETB receptor-mediated vasodilatation is evident in the efferent arteriole.
These studies provide a comprehensive and direct determination of changes in afferent and efferent diameter in response to increasing concentrations of the major endothelin family peptides, in a blood perfused system, at physiological pressures and flows, and where normal vasculo-tubular associations are preserved. Edwards et al. (1990) reported that both ET-1 and ET-3 produce dose-dependent vasoconstriction of isolated rabbit afferent and efferent arterioles but they did not identify receptor-specific actions or potency differences between pre- and postglomerular vessels. Our studies support the contention that ET-1 and ET-2 have similar potencies, consistent with reports of similar relative affinities determined in receptor binding studies (DeLeon & Garcia, 1995) and calcium signaling studies (Schroeder et al., 2000; Fellner & Arendshorst, 2004). In isolated preglomerular smooth muscle cells, ET-1 and ET-2 stimulate marked increases in intracellular calcium concentration that were of similar magnitude and time course (Schroeder et al., 2000). Afferent arterioles exhibited significantly greater vasoconstriction to ET-1 than efferent arterioles. This observation is in general agreement with other studies using perfusion fixed rabbit kidneys (Denton et al., 2004) and hydronephrotic kidneys (Loutzenhiser et al., 1990; Cavarape & Bartoli, 1998), however, in those studies, efferent arterioles were nearly unresponsive to ET-1 administration. In other reports, afferent arterioles were less responsive or similarly responsive to ET compared to efferent arterioles (Lanese et al., 1992; Endlich et al., 1996). The reasons for these discrepancies are not clear considering that the studies included isolated arterioles, and blood perfused or buffer perfused hydronephrotic kidney preparations. ET-3 was less potent than ET-1 in both afferent and efferent arterioles. These results are consistent with a relative predominance of ETA receptors since ET-3 activates only ETB receptors at lower doses (Sakurai et al., 1992). Nevertheless, the maximum vasoconstriction produced by ET-1 and ET-3 was similar in both locations.
In previous studies in the blood perfused hydronephrotic kidney model, Endlich et al. (1996) noted that ETA blockade with BQ-123 did not alter baseline renal vascular diameters or glomerular blood flow. ETB blockade with BQ-788 increased glomerular blood flow and interlobular and afferent arteriolar diameter by 3–7%, but baseline diameters remained unchanged during endothelin receptor blockade with IRL-1038 (Endlich et al., 1996). In the current study, ETA or ETB receptor antagonists did not alter the baseline diameters of either pre- or postglomerular arterioles. These findings suggest a lack of endogenous endothelin activity in the microcirculation of the in vitro blood perfused juxtamedullary nephron. The explanation for the apparently contrasting findings is not clear at the present time, but could be related to unique features of the isolated perfused juxtamedullary nephron approach in which the renal tubules remain present, and functional, and where the renal nerves are cut reducing the influence of sympathetic nerve activity on renal vascular resistance. Renal tubules produce important modulators of microvascular function that would be absent in isolated arteriole and hydronephrotic preparations. Alternatively, it may reflect a unique characteristic of the juxtamedullary nephron population which receives only a small fraction of the total renal blood flow and thus, the response may be different from the main population of superficial and midcortical nephrons. Certainly at the whole kidney level, there is considerable evidence for endogenous ETA- and ETB-dependent tone since administration of receptor-specific antagonists will alter baseline blood flow (Pollock & Opgenorth, 1993; Matsuura et al., 1997; Just et al., 2004; Pollock et al., 2005). One study, which argues against reduced responsiveness of juxtamedullary nephrons, reports that juxtamedullary afferent arterioles of hydronephrotic kidneys exhibit greater responsiveness to ET-1 compared to outer cortical arterioles and that juxtamedullary efferent arterioles exhibit similar or enhanced responses to the ETB agonist (IRL-1620) compared to outer cortical vessels (Endlich et al., 1996).
Our results provide new insight into ETB-mediated renal vasodilatation, which has been difficult to visualize in previous studies, given the overwhelming potency of the vasoconstrictor response. First, blockade of ETA receptors allowed a small, but significant vasodilator response to ET-1 in the efferent arteriole, consistent with the idea that ETB-mediated vasoconstriction is not evident until high concentrations of ET-1 are encountered in this segment of the microcirculation. Secondly, ETB antagonism increased the vasoconstrictor response to ET-1, consistent with ETB-mediated vasodilatation that is apparent only with simultaneous ETA receptor activation. Just et al. (2004) have recently reported a somewhat similar phenomenon at the whole kidney level, and suggested that receptor crosstalk, or synergy, could occur within the renal circulation. Endlich, however, reported ET-1 only vasoconstricted efferent arterioles either before, or during selective ETA and ETB receptor blockade. He also noted that ETB receptor stimulation, with IRL-1620, also produced vasoconstriction of efferent arterioles (Endlich et al., 1996). However, in support of our observation of efferent dilatation by ET-1 during ETA receptor blockade, the ETB-selective agonist, S6c, produced a concentration-dependent vasodilatation in the efferent arteriole. This unique observation indicates that vasodilatory ETB receptors are expressed by the efferent arteriole and could modulate the efferent arteriolar response to endogenous endothelin peptides, however, it is not clear why S6c did not revert to a vasoconstrictor response at higher concentrations similar to the vasoconstriction observed with ET-1 during ETA receptor blockade.
A somewhat puzzling aspect of our observations is that both ETA and ETB receptor antagonists inhibited the ET-1-induced decreases in afferent arteriolar diameter produced by low doses of ET-1. The precise mechanism for this effect is not apparent, but could again be an indication of ETA and ETB receptor interaction, as previously suggested (Just et al., 2004). Furthermore, Harada et al. (2002) have provided evidence from binding studies that the ETB receptor does not independently recognize ET-1 without the aid of the ETA receptor. One could also speculate that blocking ETA receptors during ET-1 administration may result in both endothelial and vascular ETB receptor activation that could possibly result in no net change in diameter. However, we do not have sufficient information to know whether this may occur under the conditions of our experiments. Blocking ETB receptors purportedly would increase the vasoconstrictor actions of ET-1 through the ETA receptor, as shown in vivo, yet under these conditions, we did not see any change in arteriolar diameter. It is important to remember that the in vivo results provide an indication of the net balance of effects within the entire renal circulation.
Both A-127722 and A-192621 maintain a very high degree of selectivity at the concentrations used in the current study and actually have higher affinity for their respective receptors than ET-1 (Winn et al., 1996; Von Geldern et al., 1999; Wessale et al., 2002; Wu-Wong et al., 2002). Although we cannot completely rule it out, a lack of antagonist specificity is not a likely explanation for the complex response we have observed. In binding studies, the IC50 for A-127722 in 0.1 nM for the ETA receptor and 98 nM for the ETB receptor, indicating an 890-fold selectivity ratio (Winn et al., 1996). For A-192621, the IC50 is 8200 nM for the ETA receptor and 6.4 nM for the ETB receptor, a more than 1000-fold selectivity (Von Geldern et al., 1999). Even though antagonist crossover cannot be eliminated from possibility, the concentration of 30 nM used in the current study is clearly below the IC50 for the alternate receptor.
The situation in the efferent arteriole may be more straightforward, in that low doses of ET-1 produced vasoconstriction exclusively through ETA receptors, and ETB-dependent vasoconstriction is only evident at higher doses. The latter finding is consistent with the suggestion that vasoconstrictor ETB receptors have lower affinity for ET-1 in the renal circulation (Pollock, 2000).
Anecdotal observations suggest that activation of the ETB receptor, by the so-called ETB receptor agonists, does not occur in the same manner that ET-1 activates ETB receptors. For example, when infused into the renal artery of an intact rat, S6c can produce a rapid decline in renal blood flow, within the initial minute of infusion, followed by a slight recovery and stabilization of the renal vasoconstriction (Pollock et al., 2005). In contrast, ET-1 infusion produces a very slowly developing decrease in renal blood flow. When an ETA antagonist is added, in order to activate only ETB receptors, the decline in renal blood flow is attenuated, but still develops with a different time course from S6c. In the juxtamedullary preparation, S6c constricts the afferent arteriole and dilates the efferent arteriole, indicating differences in the functional activity of ETB-dependent endothelial dilator and smooth muscle constrictor mechanisms in afferent versus efferent arterioles. In isolated preglomerular smooth muscle cells, ET-3 and S6c did not evoke increases in intracellular calcium concentration, but modest increases were observed with IRL-1620 (Schroeder et al., 2000; Fellner & Arendshorst, 2004; Pollock et al., 2005). We also observed a very slight vasoconstrictor response to S6c during ETB receptor blockade that could indicate either (1) S6c may activate ETA receptors (although unlikely at these concentrations given the extremely low affinity S6c has for ETA receptors) or; (2) that the ETB antagonist may displace endogenous ET-1 that could be activating ETA receptors.
A potentially very important observation made in this study is that the ETB agonist, IRL-1620, had a similar potency, but clearly produced less vasoconstriction and no apparent vasodilatation, compared to S6c. This constitutes direct evidence that IRL-1620 does not activate ETB receptors in a fashion similar to ET-1 or S6c. These results agree with a previous report that there are fewer IRL-1620 binding sites in the kidney compared to ET-3 (Pollock et al., 2000). These binding studies used very low concentrations of ET-3 that should not result in significant binding of ET-3 to the ETA receptor, however, this possibility may still exist. The difference between ET-1, S6c and IRL-1620, in terms of ETB receptor-dependent effects, may be due to differences in binding characteristics, at least within the kidney. While this will need to be explored in the future, it does raise the question of the reliability of using IRL-1620 as an ETB agonist.
In general, these studies provide new information regarding the functional distribution of ETA and ETB receptors within the rat renal microcirculation. ETA receptor-mediated vasoconstriction appears to predominate at lower endothelin concentrations throughout the renal microcirculation while vasoconstrictor ETB receptors are active in the preglomerular vessels and vasodilator ETB receptors are more obvious in the efferent arteriole.
Acknowledgments
These studies were supported by NIH Grants HL74167 (E.W.I., J.D.I., D.M.P.), HL64776 (D.M.P.), DK38226 (J.D.I), and DK44628 (E.W.I.) and Established Investigator Awards (E.W.I, D.M.P. and J.D.I.) and a Grant-In-Aid from the American Heart Association (E.W.I.).
Abbreviations
- ET-1
endothelin 1
- ET-2
endothelin 2
- ET-3
endothelin 3
- S6c
Sarafotoxin
References
- ÅKERMAN K.E.O., NÄSMAN J., LUND P.E., SHARIATMADARI R., KUKKONEN J.P. Endogenous extracellular purine nucleotides redirect α2-adrenoceptor signaling. FEBS Lett. 1998;430:209–212. doi: 10.1016/s0014-5793(98)00664-4. [DOI] [PubMed] [Google Scholar]
- ALLCOCK G.H., WARNER T.D., VANE J.R. Roles of endothelin receptors in the regional and systemic vascular responses to ET-1 in the anaesthetized ganglion-blocked rat: use of selective antagonists. Br. J. Pharmacol. 1995;116:2482–2486. doi: 10.1111/j.1476-5381.1995.tb15099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASELLAS D., NAVAR L.G. In vitro perfusion of juxtamedullary nephrons in rats. Am. J. Physiol. Renal, Fluid Electrolyte Physiol. 1984;246:F349–F358. doi: 10.1152/ajprenal.1984.246.3.F349. [DOI] [PubMed] [Google Scholar]
- CAVARAPE A., BARTOLI E. Effects of BQ-123 on systemic and renal hemodynamic responses to endothelin-1 in the rat split hydronephrotic kidney. J. Hypertens. 1998;16:1449–1458. doi: 10.1097/00004872-199816100-00008. [DOI] [PubMed] [Google Scholar]
- DELEON H., GARCIA R. Characterization of endothelin receptor subtypes in isolated rat renal preglomerular microvessels. Regul. Pept. 1995;60:1–8. doi: 10.1016/0167-0115(95)00112-1. [DOI] [PubMed] [Google Scholar]
- DENTON K.M., SHWETA A., FINKELSTEIN L., FLOWER R.L., EVANS R.G. Effect of endothelin-1 on regional kidney blood flow and renal arteriole calibre in rabbits. Clin. Exp. Pharmacol. Physiol. 2004;31:494–501. doi: 10.1111/j.1440-1681.2004.04036.x. [DOI] [PubMed] [Google Scholar]
- D'ORLÉANS-JUSTE P., CLAING A., TELEMAQUE S., MAURICE M.C., YANO M., GRATTON J.P. Block of endothelin-1-induced release of thromboxane A2 from the guinea pig lung and nitric oxide from the rabbit kidney by a selective ETB receptor antagonist, BQ-788. Br. J. Pharmacol. 1994;113:1257–1262. doi: 10.1111/j.1476-5381.1994.tb17133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS R.M., TRIZNA W., OHLSTEIN E.H. Renal microvascular effects of endothelin. Am. J. Physiol. Renal, Fluid Electrolyte Physiol. 1990;259:F217–F221. doi: 10.1152/ajprenal.1990.259.2.F217. [DOI] [PubMed] [Google Scholar]
- ENDLICH K., HOFFEND J., STEINHAUSEN M. Localization of endothelin ETA and ETB receptor-mediated constriction in the renal microcirculation of rats. J. Physiol. (London) 1996;497:211–218. doi: 10.1113/jphysiol.1996.sp021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELLNER S.K., ARENDSHORST W.J. Endothelin A and B receptors of preglomerular vascular smooth muscle cells. Kidney Int. 2004;65:1810–1817. doi: 10.1111/j.1523-1755.2004.00579.x. [DOI] [PubMed] [Google Scholar]
- HARADA N., HIMENO A., SHIGEMATSU K., SUMIKAWA K., NIWA M. Endothelin-1 binding to endothelin receptors in the rat pituitary gland: possible formation of an ETA-ETB receptor heterodimer. Cell Mol. Neurobiol. 2002;2:207–226. doi: 10.1023/A:1019822107048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INSCHO E.W., CARMINES P.K., NAVAR L.G. Juxtamedullary afferent arteriolar responses to P1 and P2 purinergic stimulation. Hypertension. 1991;17:1033–1037. doi: 10.1161/01.hyp.17.6.1033. [DOI] [PubMed] [Google Scholar]
- INSCHO E.W., COOK A.K. P2 receptor-mediated afferent arteriolar vasoconstriction during calcium channel blockade. Am. J. Physiol. Renal Physiol. 2002;282:F245–F255. doi: 10.1152/ajprenal.0038.2001. [DOI] [PubMed] [Google Scholar]
- INSCHO E.W., COOK A.K., MUI V., MILLER J. Direct assessment of renal microvascular responses to P2-purinoceptor agonists. Am. J. Physiol. Renal Physiol. 1998;274:F718–F727. doi: 10.1152/ajprenal.1998.274.4.F718. [DOI] [PubMed] [Google Scholar]
- INSCHO E.W., COOK A.K., NAVAR L.G. Pressure-mediated vasoconstriction of juxtamedullary afferent arterioles involves P2-purinoceptor activation. Am. J. Physiol. Renal, Fluid Electrolyte Physiol. 1996;271:F1077–F1085. doi: 10.1152/ajprenal.1996.271.5.F1077. [DOI] [PubMed] [Google Scholar]
- INSCHO E.W., OHISHI K., COOK A.K., BELOTT T.P., NAVAR L.G. Calcium activation mechanisms in the renal microvascular response to extracellular ATP. Am. J. Physiol. Renal, Fluid Electrolyte Physiol. 1995;268:F876–F884. doi: 10.1152/ajprenal.1995.268.5.F876. [DOI] [PubMed] [Google Scholar]
- INSCHO E.W., OHISHI K., NAVAR L.G. Effects of ATP on pre- and postglomerular juxtamedullary microvasculature. Am. J. Physiol. Renal, Fluid Electrolyte Physiol. 1992;263:F886–F893. doi: 10.1152/ajprenal.1992.263.5.F886. [DOI] [PubMed] [Google Scholar]
- JUST A., OLSON A.J.M., ARENDSHORST W.J. Dual constrictor and dilator actions of ETB receptors in the rat renal microcirculation: interactions with ETA receptors. Am. J. Physiol. Renal Physiol. 2004;286:F660–F668. doi: 10.1152/ajprenal.00368.2003. [DOI] [PubMed] [Google Scholar]
- LANESE D.M., YUAN B.H., MCMURTRY I.F., CONGER J.D. Comparative sensitivities of isolated rat renal arterioles to endothelin. Am. J. Physiol. Renal Physiol. 1992;263:F894–F899. doi: 10.1152/ajprenal.1992.263.5.F894. [DOI] [PubMed] [Google Scholar]
- LOUTZENHISER R., EPSTEIN M., HAYASHI K., HORTON C. Direct visualization of effects of endothelin on the renal microvasculature. Am. J. Physiol. Renal, Fluid Electrolyte Physiol. 1990;258:F61–F68. doi: 10.1152/ajprenal.1990.258.1.F61. [DOI] [PubMed] [Google Scholar]
- MATSUURA T., MIURA K., EBARA T., YUKIMURA T., YAMANAKA S., KIM S., IWAO H. Renal vascular effects of the selective endothelin receptor antagonists in anaesthetized rats. Br. J. Pharmacol. 1997;122:81–86. doi: 10.1038/sj.bjp.0701349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK D.M. Renal endothelin in hypertension. Curr. Opin. Nephrol. Hypertension. 2000;9:157–164. doi: 10.1097/00041552-200003000-00010. [DOI] [PubMed] [Google Scholar]
- POLLOCK D.M., ALLCOCK G.H., KRISHNAN A., DAYTON B.D., POLLOCK J.S. Upregulation of endothelin B receptors in kidneys of DOCA-salt hypertensive rats. Am. J. Physiol. Renal Physiol. 2000;278:F279–F286. doi: 10.1152/ajprenal.2000.278.2.F279. [DOI] [PubMed] [Google Scholar]
- POLLOCK D.M., JENKINS J.M., COOK A.K., IMIG J.D., INSCHO E.W. L-type calcium channels in the renal microcirculatory response to endothelin. Am. J. Physiol. Renal. Physiol. 2005;288:F771–F777. doi: 10.1152/ajprenal.00315.2004. [DOI] [PubMed] [Google Scholar]
- POLLOCK D.M., OPGENORTH T.J. Evidence for endothelin-induced renal vasoconstriction independent of ETA receptor activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1993;264:R222–R226. doi: 10.1152/ajpregu.1993.264.1.R222. [DOI] [PubMed] [Google Scholar]
- POLLOCK D.M., OPGENORTH T.J. ETA receptor-mediated responses to endothelin-1 and big endothelin-1 in the rat kidney. Br. J. Pharmacol. 1994;111:729–732. doi: 10.1111/j.1476-5381.1994.tb14798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKURAI T., YANAGISAWA M., MASAKI T. Molecular characterization of endothelin receptors. Trends Pharmacol. Sci. 1992;13:103–108. doi: 10.1016/0165-6147(92)90038-8. [DOI] [PubMed] [Google Scholar]
- SCHROEDER A.C., IMIG J.D., LEBLANC E.A., PHAM B.T., POLLOCK D.M., INSCHO E.W. Endothelin-mediated calcium signaling in preglomerular smooth muscle cells. Hypertension. 2000;35:280–286. doi: 10.1161/01.hyp.35.1.280. [DOI] [PubMed] [Google Scholar]
- SEO B., LUSCHER T.F. ETA and ETB receptors mediate contraction to endothelin-1 in renal artery of aging SHR. Effects of FR139317 and bosentan. Hypertension. 1995;25:501–506. doi: 10.1161/01.hyp.25.4.501. [DOI] [PubMed] [Google Scholar]
- VON GELDERN T.W., TASKER A.S., SORENSEN B.K., WINN M., SZCZEPANKIEWICZ B.G., DIXON D.B., CHIOU W.J., WANG L., WESSALE J.L., ADLER A., MARSH K.C., NGUYEN B., OPGENORTH T.J. Pyrrolidine-3-carboxylic acids as endothelin antagonists. 4. Side chain conformational restriction leads to ET(B) selectivity. J. Med. Chem. 1999;42:3668–3678. doi: 10.1021/jm990170q. [DOI] [PubMed] [Google Scholar]
- WELLINGS R.P., CORDER R., WARNER T.D., CRISTOL J.P., THIEMERMANN C., VANE J.R. Evidence from receptor antagonists of an important role for ETB receptor-mediated vasoconstrictor effects of endothelin-1 in the rat kidney. Br. J. Pharmacol. 1994;111:515–520. doi: 10.1111/j.1476-5381.1994.tb14767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESSALE J.L., ADLER A., NOVOSAD E.I., CALZADILLA S.V., DAYTON B.D., MARSH K.C., WINN M., JAE H.S., VON GELDERN T.W., OPGENORTH T.J. Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: ex vivo and in vivo studies. Clin. Sci. (London) 2002;103 (Suppl. 48):112S–117S. doi: 10.1042/CS103S112S. [DOI] [PubMed] [Google Scholar]
- WINN M., VON GELDERN T.W., OPGENORTH T.J., JAE H.S., TASKER A.S., BOYD S.A., KESTER J.A., MANTEI R.A., BAL R., SORENSEN B.K., WU-WONG J.R., CHIOU W.J., DIXON D.B., NOVOSAD E.I., HERNANDEZ L., MARSH K.C. 2,4-Diarylpyrrolidine-3-carboxylic acids – potent ETA selective endothelin receptor antagonists. J. Med. Chem. 1996;39:1039–1048. doi: 10.1021/jm9505369. [DOI] [PubMed] [Google Scholar]
- WU-WONG J.R., DIXON D.B., CHIOU W.J., SORENSEN B.K., LIU G., JAE H.S., TASKER A.S., VON GELDERN T.W., WINN M., OPGENORTH T.J. Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: in vitro studies. Clin. Sci. (London) 2002;103 (Suppl. 48):107S–111S. doi: 10.1042/CS103S107S. [DOI] [PubMed] [Google Scholar]