Abstract
Most of the cannabinoids in Cannabis sativa L. have not been fully evaluated for their pharmacological activity. A publication in this issue presents evidence that a plant cannabinoid, Δ9-tetrahydrocannabivarin is a potent antagonist of anandamide, a major endogenous cannabinoid. It seems possible that many of the non-psychoactive constituents of this plant will be of biological interest.
Keywords: Anandamide, CB1 receptor antagonist, CB2 receptor antagonist, mouse vas deferens, Δ9-tetrahydrocannabinol, Δ9-tetrahydrocannabivarin, -(+)-(R)- WIN55212
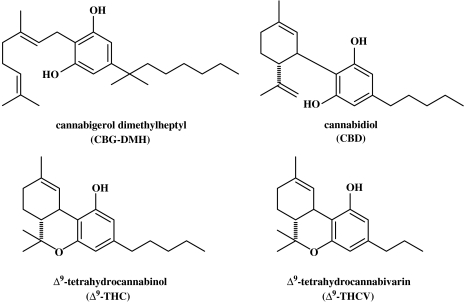
Cannabis sativa L. produces more than 60 terpeno-phenols that have not been detected in any other plant. One of these constituents, Δ9-tetrahydrocannabinol (THC) (Gaoni & Mechoulam, 1964) has been the object of thousands of publications, as it is by far the major psychoactive principle in marijuana and hashish. Cannabidiol (CBD), a nonpsychoactive component, has also been widely investigated due to its anti-inflammatory, antischizophrenic and antiepileptic properties (Pertwee, 2005). Surprisingly, the other plant cannabinoids have been mostly neglected. Cannabinoid acids, which are precursors of the neutral cannabinoids, such as THC and CBD, were shown to be antibiotic and were actually used for some time in veterinary medicine in Czechoslovakia about 50 years ago. Most of the other plant cannabinoids were assayed for possible psychoactivity. When none was found, interest in them waned (Figure 1).
Figure 1.
Structures of some cannabinoids mentioned above.
The discovery of the endocannabinoid system and the plethora of activities of the endocannabinoids raise the possibility that some of the plant cannabinoids may cause related effects. The best-known endocannabinoids, anandamide and 2-arachidonoyl glycerol, have been found to play a role not only in the central nervous system but also in most physiological systems that have been investigated – the immune, the cardiovascular, the reproductive, the respiratory, the skeletal systems, to name a few. Some of the activities are CB1/CB2 cannabinoid receptor-dependent, but many are not. Numerous additional receptors have been proposed (Howlett et al., 2002). Is it possible that some of the plant cannabinoids, which are not psychoactive (and presumably do not bind to the CB1 receptor), are also active in these systems?
Recently, there has been some renewed interest in the neglected plant cannabinoids. It is certainly not a renaissance yet. At a meeting of the International Cannabinoid Research Society, Maor et al. (2005) reported that the nonpsychoactive dimethylheptyl homolog of cannabigerol (CBG-DMH) has hypotensive and vasorelaxant properties. Vasorelaxation of rat abdominal aorta by CBG-DMH was pertussis toxin-sensitive and was not inhibited by a nitric oxide synthase inhibitor or by CB1/CB2 or vanilloid receptor antagonists. CBG-DMH also suppresses generation of nitric oxide and formation of tumor necrosis factor α by murine macrophages. The mechanism of the hypotensive effect is quite obscure. It may be related to that caused by abnormal-cannabidiol, a CBD isomer (Ho & Hiley, 2003), which was reported decades ago, as the effect of both compounds is inhibited by CBD. Interestingly, CBD does not inhibit the hypotension caused by THC. Does CBG-DMH, a plant cannabinoid derivative, cause hypotension via a new mechanism?
In this issue of British Journal of Pharmacology, Thomas et al. (2005) show that another of the neglected plant cannabinoids, tetrahydrocannabivarin (THCV), the propyl homolog of THC (Gill et al., 1970), is a potent antagonist of WIN55212 (WIN) and of anandamide. It exhibits at least some degree of selectivity as it is more potent in antagonizing these agonists in the vas deferens than in brain membranes. It is also more potent in antagonizing the inhibition by WIN and anandamide of electrically evoked contractions of the vas deferens (KB values of 1.5 and 1.2 nM, respectively) than in antagonizing the inhibition caused by THC (KB value of 97 nM).
THCV displaced [3H]CP55940 (a synthetic cannabinoid agonist) from specific CB1 binding sites on mouse brain and CHO-hCB2 cell membranes with a mean Ki of 75.4 and 62.8 nM, respectively. THCV also antagonized CP55940-induced stimulation of [35S]GTPγS binding to these membranes.
At 3–1000 nM, THCV did not inhibit electrically evoked contractions of mouse isolated vas deferens; however, concentrations of THCV in this range produced dextral shifts in the log concentration–response curves of WIN and anandamide for electrically evoked contractions. These shifts were not accompanied by a decrease in the maximal effect of either agonist. However at concentrations above 3 μM, THCV did reduce the contractile response of the vas deferens in a CB1 receptor antagonist (SR141716)-independent manner. Thus, THCV resembles this antagonist, which at high (micromolar) doses also interacts with non-CB1 targets.
THCV (100 nM) did not oppose the inhibition of electrically evoked contractions caused by either clonidine or capsaicin. Neither THCV nor WIN (both at 1 μM) altered the size of contractions induced by β,γ-methylene-ATP or phenylephrine. On this basis, the authors conclude that THCV interacts with WIN at prejunctional sites.
The discovery of the competitive CB1/CB2 receptor antagonistic properties of THCV poses numerous, yet unanswered, questions. In the original publication on THCV, Gill et al. (1970) reported that it is about five times less active than THC in producing a cataleptic effect in mouse and the time course of its action appears different. Is this effect CB1 mediated? Does THCV in marijuana, particularly in Pakistani hashish, where apparently it is found in higher concentrations than in marijuana used in Europe and the US, lower or enhance (or modify) THC action? Does THCV mimic the many other activities seen with SR141716? Assuming that its toxicity is low, as noted for most cannabinoids, can it serve as a drug in obesity or in nicotine dependence as Rimonabant (the generic name for SR141716)?
About 30 years ago, Paton and Pertwee (1973) commented on cannabis: ‘Nor does one readily find another substance so ‘contradictory', capable of taming yet producing aggressiveness, of both enhancing and depressing spontaneous activity, of being anticonvulsant yet generating epileptiform cortical discharges'. Is this ‘contradictory' behavior due, in part at least, to the presence of ‘contradictory' components such as THCV with agonist–antagonist properties? Such behavior by cannabinoids is not unexpected. Fride et al. (1995) found that very low doses of anandamide (0.0001–0.1 mg/kg), which had no effects when administered alone, partially or fully inhibited THC-induced effects. And, Bayewitch et al. (1996) reported that THC antagonizes the agonist-induced inhibition of adenylyl cyclase mediated by the CB2 receptor and concluded that THC constitutes a weak antagonist for this receptor under the conditions of their assays. A biphasic profile of action has been noted for numerous cannabinoids (see, e.g., Sulcova et al., 1998).
Many of the effects seen with anandamide, WIN and THC are not CB1-dependent (Howlett et al., 2002). As most of the plant cannabinoids do not bind to this receptor, it seems reasonable to evaluate these compounds for their activities in a wide array of assays. I sincerely believe that the plant cannabinoids are a neglected pharmacological treasure trove.
References
- BAYEWITCH M., RHEE M.-H., AVIDOR-REISS T., BREUER A., MECHOULAM R., VOGEL Z. Δ9-Tetrahydrocannabinol antagonizes the peripheral cannabinoid receptor-mediated inhibition of adenylyl cyclase. J. Biol. Chem. 1996;271:9902–9905. doi: 10.1074/jbc.271.17.9902. [DOI] [PubMed] [Google Scholar]
- FRIDE E., BARG J., LEVI R., SAYA D., HELDMAN R., MECHOULAM R., VOGEL Z. Low doses of anandamides inhibit pharmacological effects of Δ9-tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 1995;272:699–707. [PubMed] [Google Scholar]
- GAONI Y., MECHOULAM R. Isolation, structure and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964;86:1646–1647. [Google Scholar]
- GILL E.W., PATON W.D.M., PERTWEE R.G. Preliminary experiments on the chemistry and pharmacology of cannabis. Nature. 1970;228:134–136. doi: 10.1038/228134a0. [DOI] [PubMed] [Google Scholar]
- HO W.S., HILEY C.R. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br. J. Pharmacol. 2003;138:1320–1332. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWLETT A.C., BARTH F., BONNER T.I., CABRAL G., CASELLAS P., DEVANE W.A., FELDER C.C., HERKENHAM M., MACKIE K., MARTIN B.R., MECHOULAM R., PERTWEE R.G. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- MAOR Y., HOROWITZ M., GALLILY R., MECHOULAM R.Cannabigerol-dimethyl heptyl (CBG-DMH), a synthetic cannabinoid with hypotensive and vasorelaxant properties 2005 Symposium on the Cannabinoids 2005152Burlington, Vermont, International Cannabinoid Research Society, p
- PATON W.D.M., PERTWEE R.G.The pharmacology of cannabis in animals Marijuana, Chemistry, Pharmacology, Metabolism and Clinical Effects 1973New York: Academic Press Inc; 191–285.ed. MECHOULAM, R. pp [Google Scholar]
- PERTWEE R.G.Cannabidiol as a potential medicine Cannabinoids as Therapeutics 2005Basel: Birkhauser Verlag; 47–65.ed. MECHOULAM, R. pp [Google Scholar]
- SULCOVA E., MECHOULAM R., FRIDE E. Biphasic effects of anandamide. Pharmacol. Biochem. Behav. 1998;59:347–353. doi: 10.1016/s0091-3057(97)00422-x. [DOI] [PubMed] [Google Scholar]
- THOMAS A., STEVENSON L.A., WEASE K.N., PRICE M.R., BAILLIE G., ROSS R.A., PERTWEE R.G.Evidence that the plant cannabinoid Δ9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist Br. J. Pharmacol 2005146917–926.this issue [DOI] [PMC free article] [PubMed] [Google Scholar]