Abstract
Trypsin-like serine proteinases trigger signal transduction pathways through proteolytic cleavage of proteinase-activated receptors (PARs) in many tissues. Three members, PAR-1, PAR-2 and PAR-4, are trypsin substrates, as trypsinolytic cleavage of the extracellular N terminus produces receptor activation. Here, the ability of the three human pancreatic trypsin isoforms (cationic trypsin, anionic trypsin and mesotrypsin (trypsin IV)) as recombinant proteins was tested on PARs.
Using fura 2 [Ca2+]i measurements, we analyzed three human epithelial cell lines, HBE (human bronchial epithelial), A549 (human pulmonary epithelial) and HEK (human embryonic kidney)-293 cells, which express functional PAR-1 and PAR-2. Human mesotrypsin failed to induce a PAR-mediated Ca2+ response in human epithelial cells even at high concentrations. In addition, mesotrypsin did not affect the magnitude of PAR activation by subsequently added bovine trypsin. In HBE cells, which like A549 cells express high PAR-2 levels with negligible PAR-1 levels (<11%), half-maximal responses were seen for both cationic and anionic trypsins at about 5 nM. In the epithelial cells, mesotrypsin did not activate PAR-2 or PAR-1, whereas both anionic and cationic trypsins were comparable activators.
We also investigated human astrocytoma 1321N1cells, which express PAR-1 and some PAR-3, but no PAR-2. High concentrations (>100 nM) of mesotrypsin produced a relatively weak Ca2+ signal, apparently through PAR-1 activation. Half-maximal responses were observed at 60 nM mesotrypsin, and at 10–20 nM cationic and anionic trypsins.
Using a desensitization assay with PAR-2-AP, we confirmed that both cationic and anionic trypsin isoforms cause [Ca2+]i elevation in HBE cells mainly through PAR-2 activation. Desensitization of PAR-1 with thrombin receptor agonist peptide in 1321N1 cells demonstrated that all three recombinant trypsin isoforms act through PAR-1.
Thus, the activity of human cationic and anionic trypsins on PARs was comparable to that of bovine pancreatic trypsin. Mesotrypsin (trypsin IV), in contrast to cationic and anionic trypsin, cannot activate or disable PARs in human epithelial cells, demonstrating that the receptors are no substrates for this isoenzyme. On the other hand, mesotrypsin activates PAR-1 in human astrocytoma cells. This might play a role in protection/degeneration or plasticity processes in the human brain.
Keywords: Protease-activated receptor (PAR), anionic and cationic trypsin, mesotrypsin, epithelial cells, astrocytoma cells
Introduction
The digestive proteinase trypsin can also activate specific cell surface receptors, so called proteinase-activated receptors (PARs), which belong to a family of G protein-coupled receptors (Dery et al., 1998). The physiological significance of PARs in different tissues has been summarized in several reviews (Macfarlane et al., 2001; Howell et al., 2002; Vergnolle et al., 2003; Wang & Reiser, 2003). A novel mechanism of receptor activation was revealed with the discovery of these receptors. PARs are cleaved by a serine protease at a specific site on the N-terminal extension protruding into the extracellular space. The newly exposed N terminus itself acts as a tethered ligand to activate the receptor through interaction with a site on extracellular loop 2. This activation leads to numerous intracellular events, such as stimulation of phospholipase C (PLC)-catalyzed hydrolysis of polyphosphoinositides, resulting in the formation of inositol 1,4,5-trisphosphate (InsP3), mobilization of intracellular Ca2+ and generation of diacylglycerol, the endogenous activator of protein kinase C, and activation of the mitogen-activated protein kinase and other kinases (reviewed in Nystedt et al., 1995; Wang & Reiser, 2003).
Four PARs have been identified, with distinct N-terminal cleavage sites and tethered ligand pharmacology. Several synthetic PAR-activating peptides (AP) were developed for PAR-1, PAR-2 and PAR-4 on the basis of their tethered ligand sequences. Surprisingly, even peptides of 5 or 6 amino acids were able to activate the PARs. PAR-2 seems to be activated physiologically mainly by trypsin (Nystedt et al., 1995). PAR-1 and PAR-4 are also activated by trypsin, although these two PARs are considered predominantly as thrombin receptors (Vouret-Craviari et al., 1995; Blackhart et al., 1996; Xu et al., 1998). To date no attempts have been undertaken to explore whether the various isoforms of trypsin differ in their ability to activate PARs.
The human pancreas secretes two major trypsinogen isoforms, cationic trypsinogen (PRSS1) and anionic trypsinogen (PRSS2) and a third less abundant isoform mesotrypsinogen (PRSS3) (Rinderknecht et al., 1984). The almost complete resistance of mesotrypsin to polypeptide trypsin inhibitors was recognized as its most remarkable property. The sequence of the cDNA, which encodes human PRSS3 (Nyaruhucha et al., 1997), the crystal structure of mesotrypsin (Katona et al., 2002), and in vitro mutagenesis established that the evolutionary substitution of the highly conserved Gly198 (Gly193 in the chymotrypsin numbering system) by Arg is responsible for blockage of the interaction of mesotrypsin with inhibitors and protein substrates.
The physiological role of mesotrypsin has been controversial since its discovery. Recently, the digestive degradation of trypsin inhibitors was suggested as a predominant function of mesotrypsin (Szmola et al., 2003). In addition to the pancreas, mesotrypsin was also identified in the human brain and in epithelial cell lines as trypsin IV (Wiegand et al., 1993; Takeuchi et al., 1999; Cottrell et al., 2004). Its possible protective or degenerative role in these tissues has already been discussed (Minn et al., 1998; Szmola et al., 2003). PARs are known to be involved in processes of cell and tissue protection or degeneration (Ossovskaya & Bunnett, 2004; Rohatgi et al., 2004). Therefore, elucidation of the capacity of mesotrypsin to activate these receptors will contribute to our understanding of its physiological significance.
Using RT–PCR, immunocytochemistry and cytosolic Ca2+ measurements, we have shown functional expression of PAR-2 in the human airway epithelial cell lines HBE (human bronchial epithelial) and A549 and demonstrated the activation of PAR-2 by commercial trypsin from bovine pancreas (Ubl et al., 2002). Here, we report our results of testing PAR activation in human epithelial cell lines HBE, A549 and human embryonic kidney (HEK)-293 as well as in the human astrocytoma cell line 1321N1, by different isoforms of human recombinant trypsin. We did not detect a significant difference between the PAR-activating capacity of bovine trypsin and the cationic or anionic isoforms of human trypsin. Surprisingly, mesotrypsin was completely ineffective with respect to PAR activation or PAR receptor disabling in epithelial cells expressing both PAR-1 and PAR-2. These results stand in contrast to a recently published report claiming that trypsin IV (mesotrypsin) might be an agonist of PAR-2 (Cottrell et al., 2004).
Methods
Materials
The cell culture media Dulbecco's modified Eagle's medium (DMEM) and DMEM-Ham's nutrient mixture F-12 (1 : 1), fetal calf serum (FCS), and antibiotics (penicillin, kanamycin, gentamicin and streptomycin) were obtained from Biochrom KG (Berlin, Germany). Pituitary extract was from GIBCO-BRL and insulin–transferrin–sodium selenite (ITS) solution was from Roche Diagnostics (Mannheim, Germany). Fura-2-acetoxymethylester (AM) was purchased from Molecular Probes (MoBiTec, Göttingen, Germany). The synthetic thrombin receptor agonist peptide (TRag, Ala-parafluorPhe-Arg-Cha-homoArg-Tyr-NH2) was from Neosystems Laboratoire (Strasbourg, France) and human PAR-2-AP (SLIGKV) from Bachem (Weil am Rhein, Germany). Hydrocortisone, 3,5,3′-triiodothyronine (T3), epidermal growth factor (EGF), cholera toxin, enteropeptidase and trypsin from bovine pancreas were from Sigma (Taufkirchen, Germany), and fluorogenic substrates from Bachem (Heidelberg, Germany).
Trypsin preparations
Cloning, expression, purification and functional properties of recombinant human PRSS1, PRSS2 and PRSS3 were described in previous references (Sahin-Tóth, 2000; Kukor et al., 2003; Szmola et al., 2003). PRSS1 and PRSS2 preparations were lyophilized, while mesotrypsinogen preparations were stored in 50 mM HCl. Trypsinogen preparations were freshly activated by 1.5 U ml−1 (200 ng ml−1) porcine intestinal enteropeptidase (Sigma) in 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 10 mM CaCl2 with 0.01% bovine serum albumin (BSA) at 37°C for 30 min. The resulting trypsin activity was measured by hydrolysis of the fluorochromic substrate Z-Gly-Pro-Arg-AMC (Bachem) in an assay mixture containing 25 μM substrate, 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM CaCl2, 0.01% BSA and 20–30 nM trypsin (activated trypsinogens or bovine trypsin standard) in a final volume of 200 μl at 25°C. AMC fluorescence was recorded at 360 nm (excitation)/440 nm (emission) wavelength settings using a Safire fluorescence reader (Tecan, Crailsheim, Germany). Trypsin activity was expressed in units. One unit corresponds to the liberation of one μmole AMC per min at 25°C. Activated trypsinogens were standardized against a commercial bovine trypsin preparation (Sigma) with a specific activity of 195 units mg−1 of protein.
Specific activities of recombinant trypsins (in units mg−1 of protein) were for PRSS1, PRSS2, and PRSS3 159.9±54.0, 104.1±22.5 and 362.7±126.0, respectively. These data show a somewhat higher catalytic activity of mesotrypsin to the peptide substrate Z-Gly-Pro-Arg-AMC, which also corresponds to the kinetic data shown previously (Szmola et al., 2003).
Cell cultures
The HBE cell line was kindly provided by Dr T. Meyer and Professor Dr L. Pott (Institut für Physiologie, Ruhr-Universität Bochum, Germany). HBE cells were cultured in DMEM-Hams's F-12 (1 : 1) supplemented with 50 μg ml−1 gentamicin, 50 μg ml−1 kanamycin, 10 μg ml−1 ITS, 1 μM hydrocortisone, 3.75 μg ml−1 pituitary extract, 25 ng ml−1 EGF, 30 nM T3 and 10 ng ml−1 cholera toxin. 1321N1 and A549 cells were cultured in DMEM supplemented with 10% FCS and 100 μg ml−1 penicillin and streptomycin and kept at 37°C in a humidified atmosphere of 5% (A549 cells) and 10% (1321N1 cells) CO2. HEK-293 cells were cultured in DMEM-Hams's F-12 (1 : 1) supplemented with 10% FCS and 100 μg ml−1 penicillin and streptomycin and kept at 37°C in a humidified atmosphere of 5% CO2. For the experiments, the cells were grown on round cover slips (22 mm diameter) placed in Petri dishes (60 mm diameter) for 3–4 days reaching 50–80% confluence, corresponding approximately to 1 × 106 cells per dish.
Ca2+ measurements
The cytosolic Ca2+ activity ([Ca2+]i) was measured using the Ca2+ sensitive fluorescent dye fura-2-AM. For dye loading, the cells grown on a coverslip were placed in 1 ml HEPES-buffered saline (HBS) (buffer composition in mM: 145 NaCl, 5.4 KCl, 1 MgCl2, 1.8 CaCl2, 25 glucose, 20 HEPES, pH 7.4 adjusted with tris(hydroxymethyl)-aminomethane)) for 30 min at 37°C, supplemented with 2 μM fura-2-AM. Loaded cells were transferred into a perfusion chamber with a bath volume of about 0.2 ml and mounted on an inverted microscope (Zeiss, Axiovert 135, Jena, Germany). During the experiments, the cells were continuously superfused with medium heated to 37°C. The perfusion system was combined with a 6-port valve (Thomachrom, Type RH 0112) from Reichelt (Heidelberg, Germany) to allow the switch between solutions containing different agents to be tested. Single-cell fluorescence measurements of [Ca2+]i were performed using an imaging system from TILL Photonics GmbH (Munich, Germany). Cells were excited alternately at 340 and 380 nm for 25–75 ms at each wavelength with a rate of 0.33 Hz and the resultant emission collected above 510 nm. Images were stored on a personal computer and subsequently the changes in fluorescence ratio (F340 nm/F380 nm=ratio) were determined from selected regions of interest covering a single cell.
RNA preparation and RT–PCR
Total RNA was isolated from A549, HBE, HEK-293 and 1321N1 cells with the RNeasy Kit (Qiagen, Hilden, Germany). The isolation included DNAse treatment. Reverse transcription was carried out with 1 μg of each RNA using oligo(dT) primer with the Omniscript kit (Qiagen) in a final volume of 20 μl according to manufacturer's recommendations. Of this solution, 1 μl was then amplified using Hotstar Mastermix kit (Qiagen) and the following primer pairs: PAR-1 (accession number M62424; position 254–669) sense 5′-CGCCTGCTTCAGTCTGTGCGGC-3′, antisense 5′-GGCCAGGTGCAGCATGTACACC-3′, PAR-2 (accession number NM_005242; position 191–532) sense 5′-GCCATCCTGCTAGCAGCCTCTC-3′, antisense 5′-GATGACAGAGAGGAGGTCAGCC-3′, PAR-3 (accession number U92971; position 197–649), sense 5′-TTGTCAGAGTGGCATGGAA-3′, antisense 5′-TGGCCCGGCACAGGACCTCTC-3′, PAR-4 (accession number AF080214; position 71-494) sense 5′-CAGCGTCTACGACGAGAGCGG-3′, antisense 5′-CACTGAGCCATACATGTGACCAT-3′ and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for internal control (accession number BC020308; position 285-860) sense 5′-TCCAAAATCAAGTGGGGCGATGCT-3′, antisense 5′-ACCACCTGGTGCTCAGTGTAGCCC-3′. The usage of intron-flanking primers excludes any possibility of genomic DNA amplification. PCR conditions were as follows: denaturation for 15 min at 95°C, 35 cycles at 94°C for 30 s, 60°C (PAR1-3 and GAPDH) or 64°C (PAR-4) for 90 s, 72°C for 1 min and elongation at 72°C for 10 min.
PAR-3 and PAR-4 PCR were confirmed with clones containing the full-length DNA. Plasmid pBJ containing the complete cDNA of human PAR-4 was a generous gift from Dr S. Couglin (San Francisco, U.S.A.). The hPAR3-GFP clone was generated as follows. cDNA was prepared from human fibroblasts (Sokolova et al., 2005) by RT reaction using oligo(dT) primers with the Omniscript kit. The PAR-3 fragment was amplified by PCR (30 cycles: 30 s at 94°C, 1 min 51°C, 2 min 72°C and 10 min at 72°C) using a primer pair flanking the entire coding region (designed with published human PAR-3 sequence) and XhoI/HindIII restriction sites (underlined); (sense: 5′-GTCATCCTCGAGAAAATGAAAGCCCTC-3′, anitisense 5′-ATTTCACTAAAGCTTTTTTGTAAGGTAAGC-3′). The PCR product (length 1140 bp) was purified, digested with XhoI /HindIII restriction enzymes and ligated into pEGFP-N1 vector (BD Biosciences Clontech, Germany). The DNA specificity was confirmed in all cases by sequencing.
PCR products were analyzed by Tris-borate-EDTA agarose (1%) gel electrophoresis and visualized with ethidium bromide (10 mg ml−1). Documentation was performed by using Gel Doc™EQ system with Quantity One software (Bio-Rad, München, Germany).
Real-time RT–PCR analysis
cDNA was generated from 1 μg of total RNA with iScript™ cDNA synthesis kit (BioRad) in a final volume of 20 μl according to the manufacturer's protocol. Real-time PCR was performed in the iCycler (Bio-Rad) in 25 μl reaction volume using iQ™SYBR®Green Supermix (BioRad), as described by the manufacturer. Amplification specificity of PCR products was confirmed by melting curve analysis and agarose gel electrophoresis. All mRNA measurements were normalized to the GAPDH mRNA level. Increase (n-fold transcription) was determined from a preset threshold value of the number of cycles.
Results
Expression of PAR isoforms in human epithelial and astrocytoma cells
Total mRNA of HBE, A549, HEK-293 and 1321N1 cells was extracted to determine the mRNA expression of PARs by RT–PCR. Primers for GAPDH were used as an internal control of RT–PCR analysis. A549 cells express three of the four PAR subtypes: PCR products corresponding to PAR-1 (415 bp), PAR-2 (341 bp) and PAR-3 (452 bp) were found (data not shown). Expression of PAR-1 and PAR-2 and no PAR-3 was readily apparent in HBE and HEK-293 cells, whereas 1321N1 cells expressed PAR-1 and PAR-3. A PCR fragment corresponding to PAR-4 was not detected in all the cells tested although the appearance of the PAR-4 PCR signal was unambiguously demonstrated using a plasmid containing full-length DNA of human PAR-4 (data not shown).
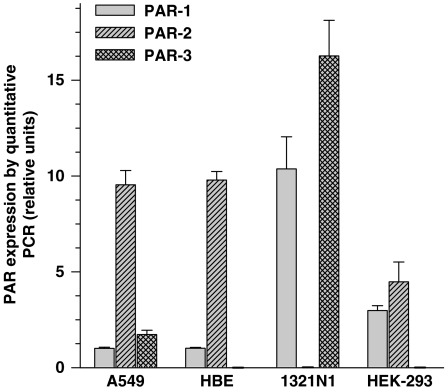
We have used quantitative real-time RT–PCR to estimate the levels of PAR expression in the epithelial cell lines and in 1321N1 cells. The data presented in Figure 1 are the means obtained with two different cultures of each cell line. Amounts of PAR transcripts were calculated by normalization to the housekeeping gene GAPDH. The results are expressed relative to the PAR-1 level in A549 cells, corrected for the level of GAPDH expression. The PAR-2 transcript level was 9–10-fold higher than PAR-1 level in both A549 and HBE cells. In HEK-293 cells, PAR-1 and PAR-2 mRNA levels showed comparable expression, which was 3–4-fold higher than the reference value. Finally, relative expression levels of PAR-1 and PAR-3 in 1321N1 cells were prominently higher, exceeding the PAR-1 level in A549 cells by 10 and 16 times, respectively.
Figure 1.
Expression of proteinase-activated receptors in human cell lines: A549, HBE, HEK-293, 1321N1. PAR expression level in the cell lines determined by real-time RT–PCR. All mRNA measurements were normalized to the GAPDH mRNA level. The values given are means of triplicate measurement data±s.e. PAR expression levels are expressed relative to the PAR-1 mRNA expression in A549 cells, which was arbitrarily chosen as reference value of 1.
Characteristics of Ca2+ responses via PAR activation
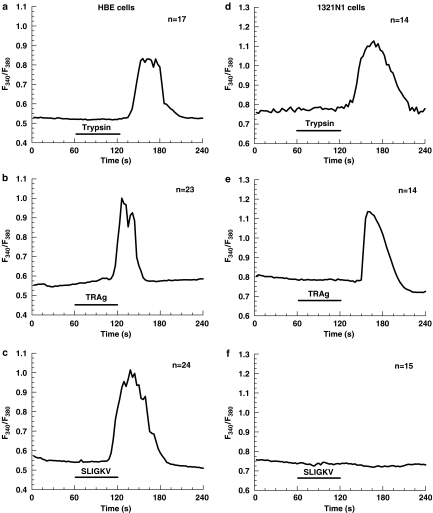
To confirm the functional expression of PARs after identifying the presence of their mRNA in the cells, we performed [Ca2+]i measurements in fura 2-AM-loaded cells stimulated with either bovine trypsin or synthetic PAR-APs, TRag and SLIGKV (PAR2-AP), which activate PAR-1 and PAR-2, respectively. Short-term application of trypsin at a concentration of 10 nM induced a transient rise of [Ca2+]i in HBE cells (Figure 2a), as well as in HEK-293 and A549 cells (data not shown). Trypsin at 10 nM concentration is known from our previous experiments to produce near half-maximal amplitude of Ca2+ response to PAR-2 activation in A549 and HBE cells (Ubl et al., 2002). Both receptor-APs also elicited Ca2+ responses in epithelial cells. The efficacy of peptide-induced activation was more than 100 times lower for TRag and 10 000 times lower for PAR-2 AP (Figure 2b and c) as compared to the proteolytic activation of PARs by trypsin. This difference in efficacy between activation by protease and peptide is well known (Ubl et al., 1998; 2002; Ossovskaya & Bunnett, 2004).
Figure 2.
Ca2+ responses elicited in HBE (left) and 1321N1 (right) cells by: 10 nM and 50 nM commercial bovine trypsin (a and d, respectively), 1 μM PAR-1-AP TRAg (b and e), 100 μM PAR-2-AP SLIGKV (c and f). The cells were exposed to the agents as indicated by the respective bars. The changes of the intracellular calcium concentration ([Ca2+]i) in fura-2-AM loaded cells indicated by the change in the fluorescence ratio (F340 nm/F380 nm) were measured. The traces are the mean of the indicated number (n) of single cells measured in one experiment and are representative for at least three different experiments. The delay in the onset of the response is caused by the superfusion system.
1321N1 astrocytoma cells demonstrated lower susceptibility to be activated by bovine trypsin as compared to the epithelial cells: there was only a weak elevation of [Ca2+]i when the cells were treated with 10 nM trypsin (data not shown). To cause significant Ca2+ responses in these cells 50 nM trypsin were required (Figure 2d). Taking into account the ability of trypsin to activate both PAR-1 and PAR-2 and the absence of PAR-2 in 1321N1 cells, one can conclude that trypsin induces [Ca2+]i signaling in the human astrocytoma cells through PAR-1. The functionality of PAR-1 expression in 1321N1 cells was confirmed with TRag (Figure 2e), whereas PAR-2-AP was completely ineffective (Figure 2f).
Thus, the previously demonstrated link between functional expression of PAR-1 and PAR-2 and cytosolic Ca2+ release in A549 and HBE cells was confirmed in our present experiments and extended with respect to HEK-293 cells and 1321N1 cells. This allowed us to use [Ca2+]i measurements in these epithelial cells and astrocytoma cells as a test system to explore the ability of different trypsin isoforms to activate PARs.
Activation of PARs in epithelial and astrocytoma cells by human trypsin isoforms
The cells were exposed to recombinant trypsins for 1 min, which is sufficient time to produce the peak amplitude of Ca2+ responses for any given concentration of PAR agonists (our previous observation). The cells were then superfused for 2–3 min with control buffer to allow the Ca2+ signal to decrease, to restore the Ca2+ baseline. We tested all three human trypsin isoforms. The purity and homogeneity of the samples of recombinant human PRSS1, PRSS2 and PRSS3 was confirmed as described before (Sahin-Tóth, 2000; Kukor et al., 2003; Szmola et al., 2003).
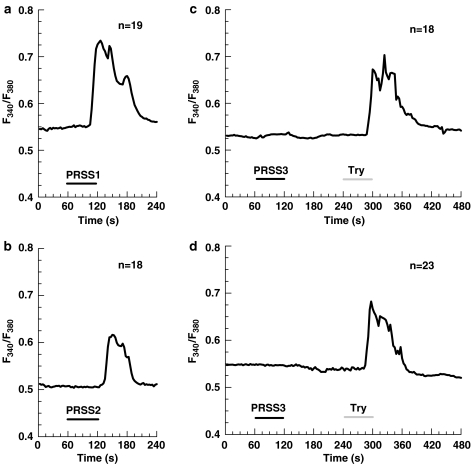
Figure 3 represents typical Ca2+ responses elicited in A549 cells by the same concentration (10 nM) of cationic trypsin (Figure 3a), anionic trypsin (Figure 3b) and the failure of mesotrypsin to elevate [Ca2+]i (Figure 3c). Similar results were obtained with HBE and HEK-293 cells (data not shown). Control experiments in which the cells were exposed to the trypsinogen-activating enzyme enteropeptidase using the same protocol showed that the enteropeptidase itself was not able to cause any Ca2+ response.
Figure 3.
Ca2+ responses in A549 cells produced by different isoforms of human recombinant trypsin: 10 nM cationic trypsin (PRSS1) (a), 10 nM anionic trypsin (PRSS2) (b), 10 nM mesotrypsin (PRSS3) followed by 10 nM bovine trypsin (c), and 400 nM mesotrypsin (PRSS3) followed by 10 nM bovine trypsin (d). The cells were exposed to the agents as indicated by the respective bars. The traces are the mean of the indicated number (n) of single cells measured in one experiment and are representative for at least three different experiments. The delay in the onset of the response is caused by the superfusion system.
From these experiments, it became apparent that cationic and anionic trypsin cause [Ca2+]i elevation comparable to that produced by the application of commercial bovine trypsin, whereas mesotrypsin was completely ineffective. To make certain that higher mesotrypsin concentrations would not be able to activate [Ca2+]i signaling, we tried to stimulate the cells with 400 nM mesotrypsin which is approximately four-fold higher than the saturating concentration of trypsin for PAR-2 activation in human lung epithelial cells (Ubl et al., 2002). Again, we failed to see any response to mesotrypsin in A549 cells (Figure 3d).
To find out whether mesotrypsin is able to influence the PAR responsiveness, we have applied additional pulses of commercial bovine trypsin, 3 min after mesotrypsin applications. The amplitude of the subsequent Ca2+ responses to bovine trypsin was not affected (Figure 3c and d), suggesting that the mesoform of trypsin was ineffective with respect to both PAR activation and also PAR receptor disabling. Thus, we conclude that mesotrypsin, in contrast to cationic and anionic trypsin isoforms, cannot activate or disable PARs in human epithelial cells.
We further characterized the ability of the respective trypsin isoform to cause Ca2+ responses in HBE cells. The data for the concentration dependence of the response amplitude (peak change of the ratio above basal level) allowed us to determine that the effectiveness of cationic trypsin was similar to that of commercial bovine trypsin in HBE cells, which had been already characterized before (Ubl et al., 2002). Anionic trypsin was 20–30% less effective than cationic trypsin (data not shown). Analysis of the concentration–effect curves revealed similar parameters for both trypsin isoforms, which coincide with that of bovine trypsin: EC50 value of about 5 nM and the response maximum at about 50 nM (Ubl et al., 2002).
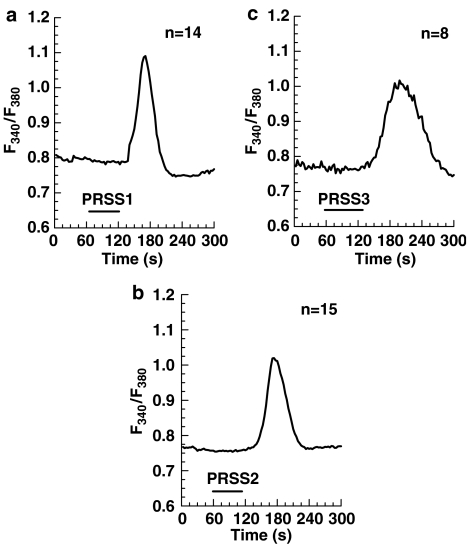
As Ca2+ responses in 1321N1 astrocytoma cells were induced with 50 nM bovine trypsin, we used the same concentration of the trypsin isoforms to test their activity in these cells. Cationic and anionic trypsin displayed an ability comparable to that of bovine trypsin to induce cytosolic Ca2+ release (Figure 4a and b), whereas mesotrypsin only slightly affected the [Ca2+]i level in the cells (data not shown). A total of 400 nM mesotrypsin produced a distinguishable Ca2+ response in 1321N1 cells (Figure 4c), which was comparable to that of 50 nM anionic trypsin. As the 1321N1 cells do not express PAR-2, mesotrypsin seems to be an activator of PAR-1; however, with somewhat weaker activity than the two other trypsin isoforms.
Figure 4.
Ca2+ responses in 1321N1 cells produced by different isoforms of human recombinant trypsin: 50 nM cationic trypsin (PRSS1) (a), 50 nM anionic trypsin (PRSS2) (b), 400 nM mesotrypsin (PRSS3) (c). The cells were exposed to the agents as indicated by the respective bars. The traces are the mean of the indicated number (n) of single cells measured in one experiment and are representative for at least three different experiments. The delay in the onset of the response is caused by the superfusion system.
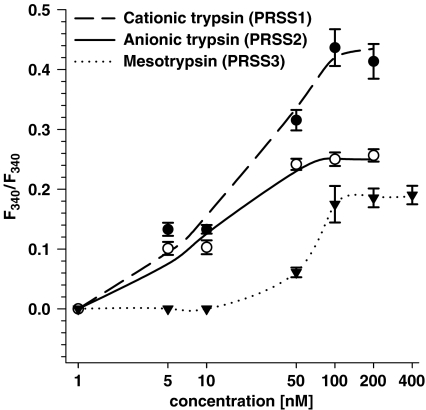
The effectiveness of different trypsin isoforms with respect to the activation of cytosolic Ca2+ release in 1321N1 cells was analyzed within the concentration range of 1–400 nM (Figure 5). Lower effectiveness of both cationic and anionic trypsin isoforms as compared to that in HBE cells is evident from the analysis of the concentration response curves. The EC50 values of these isoforms in 1321N1 cells were determined as 20 and 10 nM, respectively. Similar to the epithelial cells, cationic trypsin-produced effects in 1321N1 cells were more pronounced than those induced by anionic trypsin. Mesotrypsin was clearly effective in 1321N1 cells, in contrast to the epithelial cell lines. As shown in Figure 5, effects of mesotrypsin became apparent when its concentration was higher than 10 nM. The EC50 value of mesotrypsin in 1321N1 cells was found to be 60 nM, which is 3–5-fold higher than that of PRSS1 and PRSS2. The reasons for the difference in the maximal responses seen with the three trypsin isoforms still has to be elucidated. Considering the higher specific activity of PRSS3, this difference in effectiveness might even be somewhat higher.
Figure 5.
Concentration–effect curves for recombinant human trypsin isoforms in human astrocytoma 1321N1 cells. The cells were stimulated for 1 min with varying concentrations of the proteases and the resulting change in the ratio of the fura 2 fluorescence was recorded. The amplitude values of the Ca2+ responses are given as means±s.e. from a minimum of 50 single cells measured in at least three different experiments (in some cases error bars are smaller in size than the symbols used).
We wanted to ascertain that recombinant human trypsin isoforms used in our experiments cause [Ca2+]i signaling through the activation of PARs, mainly through the PAR-2 activation in the epithelial cells (as it was established in the epithelial cell lines with bovine pancreas trypsin before, Ubl et al., 2002) and through PAR-1 activation in 1321N1 cells (as suggested by the current experiments). Therefore, we tested whether Ca2+ responses produced by trypsin isoforms would be influenced by PAR desensitization after treatment of the cells with the corresponding PAR-AP. The results of the desensitization assays with PAR-2-AP in HBE cells and with TRag (specific PAR-1-AP) in 1321N1 cells are presented in the Figure 6a and b, respectively.
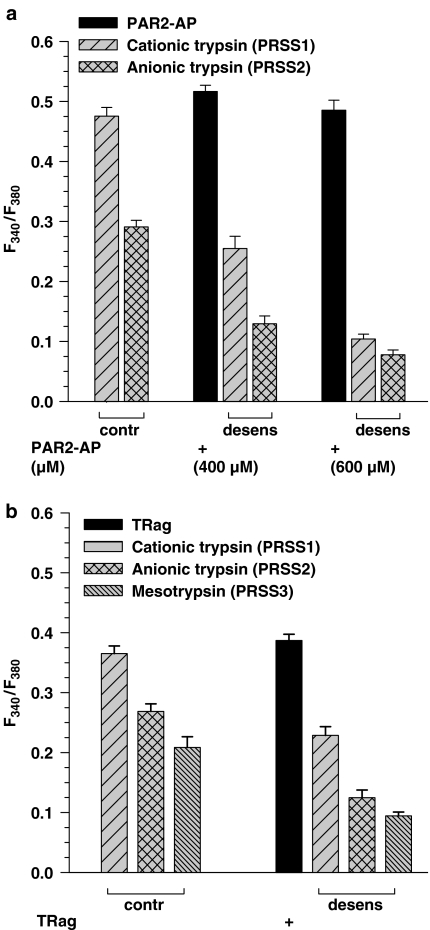
Figure 6.
Desensitization of Ca2+ responses in HBE (a) and 1321N1 (b) cells produced by the different isoforms of human recombinant trypsin after prestimulation of the cells with corresponding PAR-AP for 1 min. After 3 min washing, the cells were exposed for 1 min to a test concentration of the indicated trypsin isoforms. Control cells were not exposed to the peptide before the protease addition (left group of columns). HBE cells were treated with 400 or 600 μM PAR-2-AP, followed by the application of 10 nM of cationic or anionic trypsins (a). 1321N1 cells were treated with 10 μM TRag, followed by the application of 50 nM cationic or anionic trypsins or 400 nM mesotrypsin (b). The amplitude values of the Ca2+ responses are given as means±s.e.m. from a minimum of 50 single cells measured in at least three different experiments with the experimental protocol presented.
Cytosolic Ca2+ release produced by cationic or anionic trypsins after treatment of the HBE cells with high concentrations of PAR-2-AP was significantly decreased as compared to the control measurements. After PAR-2 desensitization with 600 μM of PAR-2-AP, the effect of both PRSS1 and PRSS2 was lowered four to five times (Figure 6a). Similarly, desensitization of PAR-2 with trypsin isoforms reduced the following response to 100 μM PAR 2-AP to 40% of the control response (data not shown). These results confirm the notion that the proteases cause [Ca2+]i elevation in HBE cells mainly through PAR-2 activation.
Stimulation of PAR-1 in 1321N1 cells by 10 μM of the receptor-specific agonist peptide TRag before the applications of PRSS1, PRSS2 and PRSS3 resulted in approximately 50% decrease of Ca2+ responses produced by these trypsin isoforms (Figure 6b). Again, disarming PAR-1 with 400 nM mesotrypsin, resulted in a decrease of the response to 0.5 μM TRag to 40% of control (data not shown). These results provide clear evidence that mesotrypsin, like anionic and cationic trypsins, mediates its effects in the astrocytoma cells through PAR-1.
Discussion
In this study we tried to extend the knowledge about trypsin-mediated PAR activation by investigating the ability of different human trypsin isoforms to activate human PARs. We have previously identified and characterized PAR subtypes in human lung epithelial cells and lung fibroblasts (Ubl et al., 2002; Sokolova et al., 2005). Here, we used [Ca2+]i measurements in the human epithelial HBE, A549 and HEK-293 cell lines, which were shown to express PAR-1 and PAR-2 (with predominant expression of PAR-2, which exceeded PAR-1 expression 9–10-fold). In addition, human astrocytoma 1321N1 cells expressing functional PAR-1 and no PAR-2 also served as test systems for the activation of these PAR subtypes. We analyzed PAR-1 and PAR-2 activation by recombinant human cationic and anionic trypsin isoforms and mesotrypsin in comparison to commercial bovine trypsin. The ability of the human trypsin isoforms to activate Ca2+ signaling directly through the interaction with PARs was confirmed in desensitization assays. Our results clearly demonstrate that mesotrypsin failed to produce Ca2+ responses in epithelial cells even at high concentrations, whereas anionic and cationic trypsins showed typical activity comparable to that of bovine trypsin.
Astrocytoma cells were found to be less sensitive to trypsin and needed higher trypsin concentration to produce distinguishable Ca2+ responses than epithelial cells. Taking into account the lack of PAR-2 in these cells, one can suggest that trypsin initiates the signaling through PAR-1. This was confirmed by the results of desensitization assay in 1321N1 cells, in which responses to trypsin isoforms were significantly diminished after PAR-1 desensitization with TRag. Like in epithelial cells, anionic and cationic trypsin isoforms displayed an activity in 1321N1 cells similar to commercial trypsin. In contrast to epithelial cells, high concentrations of mesotrypsin produced significant Ca2+ responses in astrocytoma cells, suggesting that it can be considered as a potential activator of PAR-1 in brain cells. Such difference between cells from different tissue origin can be due to specific properties of the receptor in the particular tissue. At present several possibilities have been suggested concerning the state of PARs: either mutations or glycosylation, which were both shown to be able to influence receptor activation (Nanevicz et al., 1995; Compton et al., 2000; 2001).
In addition to the activating cleavage, there is also the possibility of proteolytic digestion of PARs elsewhere in the N terminus. Such cleavage may prevent receptor activation by amputating or destroying the tethered ligand, depending upon the site where the additional cleavage occurs. Chymotrypsin is known to disable PAR-1 (Vouret-Craviari et al., 1995), cathepsin G can disable PAR-1 and PAR-2 (Molino et al., 1995; Dulon et al., 2003; Sokolova et al., 2005), and bacterial metalloprotease thermolysin was shown by us to disable PAR-2 in HBE and A549 cells (Ubl et al., 2002). We have tested the possibility for mesotrypsin to act as a disabling protease. Our experiments showed that this was not the case, because even high concentrations of mesotrypsin did not influence the subsequent PAR activation by bovine trypsin in the epithelial cells.
The decreased ability or even inability of mesotrypsin to cleave PARs is fully consistent with available structural and functional data on this trypsin isoform. Analysis of the cloned cDNA, the crystal structure and functional characterization of recombinant mesotrypsin confirmed that the presence of an arginine residue in the place of the highly conserved Gly198 is responsible for destabilizing enzyme-inhibitor and enzyme-substrate complexes (Nyaruhucha et al., 1997; Katona et al., 2002; Szmola et al., 2003). Arg198 occupies an extended conformation and fills the S2′ subsite of the molecule. The positively charged side chain results in a steric clash with the P2′ side chains of inhibitors and contributes to the strong clustering of positive charges around the primary specificity pocket of mesotrypsin. As a consequence, mesotrypsin exhibits resistance to polypeptide trypsin-inhibitors and poorly cleaves most polypeptide substrates. In this context, the defective activation of PARs by mesotrypsin is in agreement with previous observations demonstrating that mesotrypsin cannot activate trypsinogen, chymotrypsinogen or proelastase, and degrades trypsinogens at a diminished rate (Szmola et al., 2003). As to the biological function of mesotrypsin, the recent study by Szmola et al. (2003) demonstrated that mesotrypsin rapidly hydrolyzes the reactive site of trypsin inhibitors. This finding suggested a physiological role for mesotrypsin in the digestive degradation of dietary trypsin inhibitors (Szmola et al., 2003).
The data on the inefficacy of mesotrypsin with respect to PAR activation in epithelial cells presented here are not in line with the recently reported results of Bunnett and coworkers (Cottrell et al., 2004). These authors produced lysates of CHO cells expressing trypsinogen IV, which were subsequently treated with enteropeptidase to generate enzymatic activity. However, in their study, the active enzyme was not characterized in detail. The lysates elicited weak Ca2+ responses in epithelial cell lines expressing PAR-2 and PAR-4. On the basis of this observation as well as the detection of trypsinogen IV and enteropeptidase mRNA in different cells of epithelial origin, the conclusion was put forward that trypsin IV (mesotrypsin) might be a potential agonist of PAR-2 and PAR-4 in epithelial tissues. From our data, however, we conclude that mesotrypsin does not seem to be a potent activator of PAR-2 in epithelial cells.
An alternatively spliced form of PRSS3 (trypsinogen IV) was found to be expressed in the human brain (Wiegand et al., 1993). It was shown that trypsinogen IV expression in mouse neurons leads to a massive increase of glial fibrillar acidic protein expression in astrocytes as well as to the lack of amyloid deposits in trypsinogen IV transgenic mice (Minn et al., 1998). These results together with mesotrypsin's resistance to polypeptide trypsin inhibitors, which are present in the brain as well (Osterwalder et al., 1996), suggest a special physiological role of this trypsin isoform in the human brain. Recently, it was recognized that neuronal PARs play roles in neurogenic inflammation and neurodegenerative processes, as well as in nociception (reviewed in Vergnolle et al., 2003; Rohatgi et al., 2004). PAR-1 localization and function were explored not only in rodent but also in human brain (Junge et al., 2004). The possible involvement of mesotrypsin in these processes in the brain through the PARs system can be supported by our data demonstrating the potency of mesotrypsin to activate PAR-1 in human astrocytoma cells. If the knowledge on this new PAR-1 agonist in the brain would be extended, mesotrypsin could be considered as another signaling molecule in the brain acting via PAR-1, in addition to thrombin (Striggow et al., 2000). Our findings could imply involvement of mesotrypsin in protection/degeneration or plasticity processes in the human brain for which serine proteases are known to be important (Yoshida & Shiosaka, 1999; Vergnolle et al., 2003; Rohatgi et al., 2004).
In summary, from our results we conclude that PAR-1 and PAR-2 are not substrates for mesotrypsin in human epithelial cells. According to our results on human astrocytoma cells, this trypsin isoform may be a potential activator of PAR-1 in the brain. In contrast to cationic and anionic trypsin isoforms, mesotrypsin cannot activate or disable the receptors at reasonable concentrations and cannot be directly involved in physiological regulatory processes, which are due to proteolytic cleavage of PARs. However, mesotrypsin possibly takes part in protection/degeneration processes in human brain, in which PAR-1 participates.
Acknowledgments
This study was supported by a Grant from Deutsche Forschungsgemeinschaft (Re 563/11-1), RUS 176/99 from BMBF, and Land Sachsen-Anhalt to GR and by NIH Grant DK58088 to M.S.-T. We thank Dr T. Wartmann for help with preparing the active recombinant trypsins.
Abbreviations
- AM
acetoxymethylester
- AP
activating peptide
- BSA
bovine serum albumin
- DMEM
Dulbecco's modified Eagle's medium
- EGF
epidermal growth factor
- GAPDH
glyceraldehyde-3-phosphate dehydroxygenase
- HBE cells
human bronchial epithelial cells
- HEK cells
human embryonic kidney cells
- InsP3
inositol 1,4,5-trisphosphate
- PAR
proteinase-activated receptor
- PLC
phospholipase C
- TRag
thrombin receptor agonist peptide
References
- BLACKHART B.D., EMILSSON K., NGUYEN D., TENG W., MARTELLI A.J., NYSTEDT S., SUNDELIN J., SCARBOROUGH R.M. Ligand cross-reactivity within the protease-activated receptor family. J. Biol. Chem. 1996;271:16466–16471. doi: 10.1074/jbc.271.28.16466. [DOI] [PubMed] [Google Scholar]
- COMPTON S.J., CAIRNS J.A., PALMER K.J., AL-ANI B., HOLLENBERG M.D., WALLS A.F. A polymorphic protease-activated receptor 2 (PAR2) displaying reduced sensitivity to trypsin and differential responses to PAR agonists. J. Biol. Chem. 2000;275:39207–39212. doi: 10.1074/jbc.M007215200. [DOI] [PubMed] [Google Scholar]
- COMPTON S.J., RENAUX B., WIJESURIYA S.J., HOLLENBERG M.D. Glycosylation and the activation of proteinase-activated receptor 2 (PAR(2)) by human mast cell tryptase. Br. J. Pharmacol. 2001;134:705–718. doi: 10.1038/sj.bjp.0704303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTTRELL G.S., AMADESI S., GRADY E.F., BUNNETT N.W. Trypsin IV: A novel agonist of protease-activated receptors 2 and 4. J. Biol. Chem. 2004;279:13532–13539. doi: 10.1074/jbc.M312090200. [DOI] [PubMed] [Google Scholar]
- DERY O., CORVERA C.U., STEINHOFF M., BUNNETT N.W. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am. J. Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- DULON S., CANDE C., BUNNETT N.W., HOLLENBERG M.D., CHIGNARD M., PIDARD D. Proteinase-activated receptor-2 and human lung epithelial cells: disarming by neutrophil serine proteinases. Am. J. Respir. Cell. Mol. Biol. 2003;28:339–346. doi: 10.1165/rcmb.4908. [DOI] [PubMed] [Google Scholar]
- HOWELL D.C., LAURENT G.J., CHAMBERS R.C. Role of thrombin and its major cellular receptor, protease-activated receptor-1, in pulmonary fibrosis. Biochem. Soc. Trans. 2002;30:211–216. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- JUNGE C.E., LEE C.J., HUBBARD K.B., ZHANG Z., OLSON J.J., HEPLER J.R., BRAT D.J., TRAYNELIS S.F. Protease-activated receptor-1 in human brain: localization and functional expression in astrocytes. Exp. Neurol. 2004;188:94–103. doi: 10.1016/j.expneurol.2004.02.018. [DOI] [PubMed] [Google Scholar]
- KATONA G., BERGLUND G.I., HAJDU J., GRÁF L., SZILÁGYI L. Crystal structure reveals basis for the inhibitor resistance of human brain trypsin. J. Mol. Biol. 2002;315:1209–1218. doi: 10.1006/jmbi.2001.5305. [DOI] [PubMed] [Google Scholar]
- KUKOR Z., TÓTH M., SAHIN-TÓTH M. Human anionic trypsinogen: properties of autocatalytic activation and degradation and implications in pancreatic diseases. Eur. J. Biochem. 2003;270:2047–2058. doi: 10.1046/j.1432-1033.2003.03581.x. [DOI] [PubMed] [Google Scholar]
- MACFARLANE S.R., SEATTER M.J., KANKE T., HUNTER G.D., PLEVIN R. Proteinase-activated receptors. Pharmacol. Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- MINN A., SCHUBERT M., NEISS W.F., MÜLLER-HILL B. Enhanced GFAP expression in astrocytes of transgenic mice expressing the human brain-specific trypsinogen IV. Glia. 1998;22:338–347. doi: 10.1002/(sici)1098-1136(199804)22:4<338::aid-glia3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- MOLINO M., BLANCHARD N., BELMONTE E., TARVER A.P., ABRAMS C., HOXIE J.A., CERLETTI C., BRASS L.F. Proteolysis of the human platelet and endothelial cell thrombin receptor by neutrophil-derived cathepsin G. J. Biol. Chem. 1995;270:11168–11175. doi: 10.1074/jbc.270.19.11168. [DOI] [PubMed] [Google Scholar]
- NANEVICZ T., ISHII M., WANG L., CHEN M., CHEN J., TURCK C.W., COHEN F.E., COUGHLIN S.R. Mechanisms of thrombin receptor agonist specificity. Chimeric receptors and complementary mutations identify an agonist recognition site. J. Biol. Chem. 1995;270:21619–21625. doi: 10.1074/jbc.270.37.21619. [DOI] [PubMed] [Google Scholar]
- NYARUHUCHA C.N., KITO M., FUKUOKA S.I. Identification and expression of the cDNA-encoding human mesotrypsin(ogen), an isoform of trypsin with inhibitor resistance. J. Biol. Chem. 1997;272:10573–10578. doi: 10.1074/jbc.272.16.10573. [DOI] [PubMed] [Google Scholar]
- NYSTEDT S., LARSSON A.K., ABERG H., SUNDELIN J. The mouse proteinase-activated receptor-2 cDNA and gene. Molecular cloning and functional expression. J. Biol. Chem. 1995;270:5950–5955. doi: 10.1074/jbc.270.11.5950. [DOI] [PubMed] [Google Scholar]
- OSSOVSKAYA V.S., BUNNETT N.W. Protease-activated receptors: contribution to physiology and disease. Physiol. Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- OSTERWALDER T., CONTARTESE J., STOECKLI E.T., KUHN T.B., SONDEREGGER P. Neuroserpin, an axonally secreted serine protease inhibitor. EMBO J. 1996;15:2944–2953. [PMC free article] [PubMed] [Google Scholar]
- RINDERKNECHT H., RENNER I.G., ABRAMSON S.B., CARMACK C. Mesotrypsin: a new inhibitor-resistant protease from a zymogen in human pancreatic tissue and fluid. Gastroenterology. 1984;86:681–692. [PubMed] [Google Scholar]
- ROHATGI T., SEDEHIZADE F., REYMANN K.G., REISER G. Protease-activated receptors in neuronal development, neurodegeneration, and neuroprotection: thrombin as signaling molecule in the brain. Neuroscientist. 2004;10:501–512. doi: 10.1177/1073858404269955. [DOI] [PubMed] [Google Scholar]
- SAHIN-TÓTH M. Human cationic trypsinogen. Role of Asn-21 in zymogen activation and implications in hereditary pancreatitis. J. Biol. Chem. 2000;275:22750–22755. doi: 10.1074/jbc.M002943200. [DOI] [PubMed] [Google Scholar]
- SOKOLOVA E., GRISHINA Z., BÜHLING F., WELTE T., REISER G. Protease-activated receptor-1 in human lung fibroblasts mediates a negative feedback downregulation via prostaglandin E2. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L793–L802. doi: 10.1152/ajplung.00343.2004. [DOI] [PubMed] [Google Scholar]
- STRIGGOW F., RIEK M., BREDER J., HENRICH NOACK P., REYMANN K.G., REISER G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2264–2269. doi: 10.1073/pnas.040552897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZMOLA R., KUKOR Z., SAHIN-TÓTH M. Human mesotrypsin is a unique digestive protease specialized for the degradation of trypsin inhibitors. J. Biol. Chem. 2003;278:48580–48589. doi: 10.1074/jbc.M310301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI T., SHUMAN M.A., CRAIK C.S. Reverse biochemistry: use of macromolecular protease inhibitors to dissect complex biological processes and identify a membrane-type serine protease in epithelial cancer and normal tissue. Proc. Natl. Acad. Sci. U.S.A. 1999;96:11054–11061. doi: 10.1073/pnas.96.20.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UBL J.J., GRISHINA Z.V., SUKHOMLIN T.K., WELTE T., SEDEHIZADE F., REISER G. Human bronchial epithelial cells express PAR-2 with different sensitivity to thermolysin. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L1339–L1348. doi: 10.1152/ajplung.00392.2001. [DOI] [PubMed] [Google Scholar]
- UBL J.J., VÖHRINGER C., REISER G. Co-existence of two types of [Ca2+]i-inducing protease-activated receptors (PAR-1 and PAR-2) in rat astrocytes and C6 glioma cells. Neuroscience. 1998;86:597–609. doi: 10.1016/s0306-4522(97)00686-6. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N., FERAZZINI M., D'ANDREA M.R., BUDDENKOTTE J., STEINHOFF M. Proteinase-activated receptors: novel signals for peripheral nerves. Trends Neurosci. 2003;26:496–500. doi: 10.1016/S0166-2236(03)00208-X. [DOI] [PubMed] [Google Scholar]
- VOURET-CRAVIARI V., GRALL D., CHAMBARD J.C., RASMUSSEN U.B., POUYSSEGUR J., VAN OBBERGHEN-SCHILLING E. Post-translational and activation-dependent modifications of the G protein-coupled thrombin receptor. J. Biol. Chem. 1995;270:8367–8372. doi: 10.1074/jbc.270.14.8367. [DOI] [PubMed] [Google Scholar]
- WANG H., REISER G. Thrombin signaling in the brain: the role of protease-activated receptors. Biol. Chem. 2003;384:193–202. doi: 10.1515/BC.2003.021. [DOI] [PubMed] [Google Scholar]
- WIEGAND U., CORBACH S., MINN A., KANG J., MÜLLER-HILL B. Cloning of the cDNA encoding human brain trypsinogen and characterization of its product. Gene. 1993;136:167–175. doi: 10.1016/0378-1119(93)90460-k. [DOI] [PubMed] [Google Scholar]
- XU W.F., ANDERSEN H., WHITMORE T.E., PRESNELL S.R., YEE D.P., CHING A., GILBERT T., DAVIE E.W., FOSTER D.C. Cloning and characterization of human protease-activated receptor 4. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA S., SHIOSAKA S. Plasticity-related serine proteases in the brain (review) Int. J. Mol. Med. 1999;3:405–409. doi: 10.3892/ijmm.3.4.405. [DOI] [PubMed] [Google Scholar]