Abstract
Protein kinase C (PKC) may contribute to enhanced contractile responses of arteries from streptozotocin-diabetic rats to stimulation of G-protein coupled receptors. This was investigated by comparing the effects of PKC inhibitors on contractile responses of mesenteric arteries from diabetic and age-matched control rats to noradrenaline (NA) and endothelin-1 (ET-1). The effects of NA and ET-1 on the distribution of three isoforms of PKC implicated in contraction were also determined. In addition, the effect of NA on phosphorylation of CPI-17, a substrate for PKC, was investigated.
Contractile responses of endothelium-denuded arteries from diabetic rats to NA were enhanced, but were normalized by PKC inhibition. In contrast, contractile responses to ET-1 were not significantly different, and were blocked to a similar extent by PKC inhibition, in arteries from control and diabetic rats.
NA produced only a small increase in particulate levels of PKCɛ in control arteries (to 125±8% of levels in untreated arteries), but a significant increase in particulate PKCα (to 190±22%) and a much greater increase in particulate PKCɛ (to 230±19%) in arteries from diabetic rats. ET-1 increased particulate PKCα and ɛ to a similar extent in arteries from control and diabetic rats.
NA significantly enhanced CPI-17 phosphorylation from a basal level of 22±10 to 71±7% of total in arteries from diabetic rats, and this was prevented by PKC inhibition. NA had no detectable effect on CPI-17 phosphorylation in arteries from control rats.
These data suggest that NA-induced activation of PKC and CPI-17, its downstream target, is selectively enhanced in arteries from diabetic rats, and mediates the enhanced contractile responses to this agonist.
Keywords: Vascular smooth muscle, α-adrenoceptor, contraction, PKC, CPI-17, diabetes, G-protein coupled receptor, noradrenaline, endothelin-1
Introduction
Cardiovascular complications are recognized to be the major cause of morbidity and mortality associated with diabetes mellitus, and the underlying reasons for this are the subject of intense investigation. Among the hemodynamic changes that occur are vasoconstriction and altered blood flow, which have been proposed to be related in part to abnormal vascular reactivity (Koya & King, 1998). This possibility has been extensively investigated in arteries from rats treated with streptozotocin (STZ), which induces a condition resembling poorly controlled type I diabetes. Although the results of these studies have not all been in agreement, we and others have consistently found that contractile responses of arteries from rats with well-established diabetes to stimulation of α1-adrenoceptors are enhanced (MacLeod, 1985; Abebe et al., 1990; Inazu et al., 1991; Taylor et al., 1994). Similarly, contractile responses to stimulation of other G-protein coupled receptors (GPCRs), including endothelin (ET) receptors, have also been reported to be increased in arteries from diabetic rats (Hattori et al., 1999; Tickerhoof et al., 2003). Furthermore, previous studies from this lab have demonstrated that the increased contractile responses of arteries from diabetic rats to α1-adrenoceptor stimulation result from a change in the signal transduction process downstream from the receptor (Weber & MacLeod, 1997).
Two processes are believed to contribute to contractile responses of vascular smooth muscle to stimulation of GPCRs: an increase in [Ca2+]i levels and an increase in Ca2+ sensitivity. Recently, we reported that the enhanced maximum contractile response of mesenteric arteries from diabetic rats to noradrenaline (NA) in the presence of extracellular Ca2+ was not associated with a corresponding greater increase in [Ca2+]i (Chow et al., 2001), suggesting that there is a relatively greater increase in Ca2+ sensitivity in response to NA in diabetic than in control mesenteric arteries. Both α1-adrenoceptors and ET receptors (Sokolovsky, 1995) couple to phospholipase C (PLC), generally via pertussis toxin (PTX)-insensitive G-proteins of the Gq/11 family, resulting in breakdown of phosphotidylinositol 4,5-bisphosphate (PIP2) and production of diacylglycerol (DAG), the endogenous activator of protein kinase C (PKC). PKC has been proposed to induce Ca2+ sensitization by a number of different mechanisms. One is by the inhibitory phosphorylation of thin filament-associated proteins, caldesmon and calponin, which can interfere with the binding between actin and myosin (Pohl et al., 1997; Sohn et al., 2001). PKC can also activate CPI-17 (PKC-potentiated inhibitor protein of 17 kDa), which when phosphorylated becomes a potent myosin light chain phosphatase (MLCP) inhibitor (Eto et al., 1997). This leads to enhanced phosphorylation of myosin light chain (MLC) by myosin light chain kinase (MLCK), resulting in Ca2+ sensitization. Phosphorylation of CPI-17 has been suggested to be the major mechanism of Ca2+ sensitization by PKC under physiological conditions (Somlyo & Somlyo, 2003). We have previously reported that NA stimulation of the phosphoinositide pathway is enhanced in mesenteric arteries from diabetic rats (Abebe & MacLeod, 1991; 1992). Therefore, we hypothesized that the increase in Ca2+ sensitivity of mesenteric arteries from diabetic rats in response to NA may result from increased activation of PKC and its downstream target, CPI-17. Based on the results of pharmacological studies with PKC inhibitors, PKC has also been suggested to be involved in enhanced vasoconstrictor responses to ET-1 in aorta and coronary arteries from STZ-diabetic rats (Hattori et al., 1999; Tickerhoof et al., 2003).
The PKC superfamily has been shown to include 12 isoforms, three of which, PKC α, δ and ɛ, have been implicated in Ca2+ sensitization and contraction in response to stimulation of GPCR in vascular smooth muscle (Lee et al., 1999; Eto et al., 2001). Although diabetes-induced increases in the expression and/or activity of some PKC isoforms, in particular PKCβ2, have been demonstrated in various cardiovascular tissues (Koya & King, 1998; Meier & King, 2000), the effect of diabetes on agonist-induced changes in PKC isoforms involved in Ca2+ sensitization has not been reported. In the present investigation, the role of the PKC pathway in vasoconstrictor responses of mesenteric arteries from diabetic rats to NA and ET-1 was investigated. We first determined the effects of two structurally distinct PKC inhibitors on contractile responses to these agonists. Since activation of PKC has been shown to involve its translocation to isoform-specific binding sites at cell membranes (Taggart et al., 1999), changes in the subcellular distribution of the isoforms is often used as an index of their activation. Therefore, the effect of a maximum concentration of NA and ET-1 on levels of PKC α, δ and ɛ in the cytosol and particulate fractions of mesenteric arteries from control and diabetic rats was also investigated. Finally, we compared the effects of NA in the absence and presence of the PKC inhibitor, calphostin C, on the phosphorylation of CPI-17 in mesenteric arteries from control and diabetic rats.
Methods
Male Wistar rats weighing between 150 and 175 g were obtained from the University of British Columbia Animal Care Unit and were housed and treated in accordance with the guidelines of the Canadian Council on Animal Care. Diabetes was induced by injection of 60 mg kg−1 STZ into the lateral tail vein of rats lightly anesthetized with halothane. Control rats received the citrate buffer vehicle. STZ-treated rats with blood glucose levels of 13 mmol l−1 or greater, measured with an Ames glucometer 1 week after injection, were considered diabetic and were kept for experiments. After 12–14 weeks, animals were weighed and given an overdose of sodium pentobarbital. Blood was collected by cardiac puncture for later assay of plasma insulin and glucose levels. The superior mesenteric artery was excised, placed in Krebs solution (composition in mM: NaCl 124, KCl 4.7, NaHCO3 25, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2 and dextrose 11.5) at room temperature and cleaned of fat and connective tissue. The artery was then cut into 4 mm segments. The endothelium was removed from each segment by rubbing the internal lumen of the vessel gently against a thin wire. The rings were then suspended in isolated tissue baths using triangular hooks, one of which was attached to a fixed tissue support, while the other was connected to a Grass FT.03 force displacement transducer which in turn was attached to a Grass polygraph (Model 7E, Grass Instruments Co., Quincy, MA, U.S.A.). The rings were equilibrated for 90 min under 1 × g resting tension in Krebs solution continuously aerated with 95% O2/5% CO2 and maintained at a temperature of 37°C. In some rings, cumulative concentration–response curves to NA or ET-1 were obtained. In these preparations, the loss of the endothelium was first confirmed by demonstrating their inability, when precontracted with 3 μM phenylephrine, to relax in response to 0.1 μM acetylcholine. The tissues were then washed for 1 h and cumulative concentration–response curves to NA and ET-1 were obtained in the presence and absence of Ro-318220 (3-[1-[3-(amidinothio)propyl]-1H-indol-3-yl]-3-(1-methyl-1H-indol-3-yl) maleimide methanesulfonate (Calbiochem, San Diego, CA, U.S.A.), 3 μM for 30 min, or calphostin C (Sigma Chemical Co., St Louis, MO, U.S.A.), 3 μM for 30 min.
Western blotting
Western blotting for PKC isoforms
Mesenteric arteries from control and diabetic animals were cleaned, denuded and frozen in liquid nitrogen for the measurement of total solubilizable levels of PKC isoforms. The arteries were powdered, sonicated in EGTA-detergent buffer (composition: Tris HCl 20 mM, mercaptoethanol 50 mM, EGTA 5 mM, EDTA 2 mM, NaF 10 mM, AEBSF 1 mM, leupeptin 25 μg ml−1, aprotinin 2 μg ml−1, NP40 1%, SDS 0.1% and deoxycholic acid 0.5%), and then centrifuged at 100,000 × g for 1 h. The supernatant was collected and used as total solubilizable protein.
To investigate the effect of agonist-induced stimulation on PKC isoforms in the cytosol and particulate fraction, following the equilibration period, artery rings from control and diabetic animals were treated with 30 μM NA, 0.03 μM ET-1 or remained untreated. Once the peak sustained contractile response was reached (5 min in the case of NA and 7 min for ET-1), the rings were quickly rinsed in ice-cold Krebs solution, blotted, frozen in liquid nitrogen and stored at −70°C. The frozen rings were powdered, sonicated in EGTA buffer (without detergents) and then centrifuged at 100,000 × g for 1 h. The supernatant was retained as the cytosol fraction and the pellets were resuspended in the EGTA-detergent buffer. Following centrifugation at 100,000 × g for 1 h, the supernatant was collected and used as the detergent-soluble particulate fraction. The protein content in the different fractions was determined using the Bradford assay.
In preliminary experiments, the efficiency of detergent extraction of PKC isoforms was determined. None of the PKC isoforms were detected in the supernatant on re-extraction of the final pellet with EGTA-detergent buffer. When the final pellet was analyzed, approximately 15–25% of the total PKC isoform content was found to remain in the insoluble fraction. However, there were no significant differences between arteries from control and diabetic rats, or between untreated and agonist-treated preparations in the amount of any of the isoforms remaining in the insoluble fraction.
Equal amounts of protein were subjected to SDS–PAGE on 11% polyacrylamide gels and the resolved proteins were electrophoretically transferred to a nitrocellulose membrane. Membranes were reversibly stained with Ponceau and were then blocked with 5% nonfat milk/0.05% Tween-TBS and incubated with the appropriate PKC isoform-specific (rabbit polyclonal, 1 : 500, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, U.S.A.) primary antibody overnight at 4°C. Actin was used as an internal control and membranes were incubated with actin primary antibody (goat polyclonal, 1 : 200, Santa Cruz Biotechnology, Inc.) in the manner described above. In preliminary experiments, each of the antibodies for PKCα, β2, δ and ɛ were tested against the purified standard for each isoform, and no crossreactivity was detected. The same dilution of each PKC isoform antibody and the same amount of protein were used for all the control and diabetic samples. These were found in preliminary experiments to give signals in the linear range of the optical density vs protein concentration curve. Immune complexes were detected following incubation of membranes with horseradish peroxidase-conjugated anti-rabbit (for PKC) or anti-goat (for actin) secondary antibody (Santa Cruz Biotechnology, Inc.) for 2 h at room temperature (1 : 20,000 in 3% nonfat milk) using an enhanced chemiluminescence detection kit (Amersham Pharmacia, Piscataway, NJ, U.S.A.). Band intensity was analyzed by densitometry and normalized for actin on the same membrane. Total actin levels and actin in the cytosol and particulate fractions were not significantly different in mesenteric arteries from control and diabetic rats (Figure 1a and b). Treatment with NA or ET-1 (Figure 1b) did not significantly affect cytosol or particulate levels of actin in either animal group. As an alternative to actin, some of the membranes were analyzed by the method described by Ping et al. (1997), using the most predominant band on the Ponceau-stained membrane. The results corrected by this method and by actin were found to be similar.
Figure 1.
(a) Total levels of actin in control and diabetic arteries (n=5 in each group). (b) Soluble and particulate levels of actin in control (C) and diabetic (D) arteries in the absence (UT) and presence of ET-1 (0.03 μM for 7 min, n=11). Each bar represents mean±s.e.m.
Western blotting for CPI-17
Artery rings from control and diabetic animals were divided into three groups. The first group remained untreated, the second was treated with 30 μM NA for 5 min, and the third was treated with 30 μM calphostin C for 35 min, with 30 μM NA being added for the last 5 min. The rings were then frozen by immersion in acetone containing 10% trichloroacetic acid (TCA) and 10 mM dithiothreitol (DTT) cooled with dry ice, and stored at −70°C (Woodsome et al., 2001). Frozen tissues were gradually warmed to −20°C, then 4°C and finally washed several times with ice-cold acetone to remove the TCA. The tissues were ground in a motor-driven glass homogenizer (Kontes) in EGTA buffer containing DTT 20 mM and 0.5% SDS. Following centrifugation at 10,000 × g for 15 min, the supernatant was collected and equal amounts of protein were subjected to SDS–PAGE on 16% polyacrylamide gels. The resolved proteins were electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane. Membranes were blocked with 5% nonfat milk/0.05% Tween-TBS overnight and then incubated with the appropriate anti-CPI-17 (0.2 μg ml−1) or anti-phospho-CPI-17 (Thr38, 2 μg ml−1) (rabbit polyclonal, Upstate, Charlottesville, VA, U.S.A.) primary antibody for 3 h at room temperature. Actin was used as an internal control and membranes were incubated with actin primary antibody as described above. Membranes were then incubated with horseradish peroxidase-conjugated anti-rabbit (1 : 12,500 for total CPI-17 and 1 : 7575 for anti-phospho-CPI-17 in 3% nonfat milk) or anti-goat (for actin, 1 : 20,000 in 3% nonfat milk) secondary antibody (Santa Cruz Biotechnology, Inc.) for 2 h at room temperature. Levels of phosphorylated CPI-17 were expressed as a percent of total CPI-17 levels in each group.
Statistical analyses
All data are presented as the mean±s.e.m. NA and ET-1 concentration–response curves were analyzed by nonlinear regression using GraphPad Prism version 4.00 (GraphPad Software, San Diego, CA, U.S.A.) for calculation of pD2 (−log EC50) values and maximum responses (Rmax). In Western blotting experiments, total levels of PKC isoforms in arteries from diabetic rats were calculated relative to levels of the same isoform in control arteries. Cytosolic and particulate levels of PKC isoforms following NA and ET-1 treatment were calculated relative to levels of the same isoform in the corresponding fraction from untreated control arteries. Statistical significance was evaluated by one-way or two-way ANOVA followed by Newman–Keuls post hoc test for multiple comparisons, using NCSS (NCSS, Kaysville, UT, U.S.A.). A P<0.05 was considered statistically significant.
Results
Some characteristics of rats used in the present investigation are shown in Table 1. Diabetic rats weighed significantly less than their corresponding controls, and had much lower serum insulin levels, associated with significant hyperglycemia. STZ-treated rats also exhibited other symptoms of diabetes including polydypsia, polyuria and increased food intake. However, there was no significant difference in the cross-sectional areas of arteries from the two groups of animals.
Table 1.
Characteristics of control and diabetic rats
| Body weight (g) | Blood glucose (mmol l−1) | Serum insulin (ng ml−1) | Mesenteric artery cross-sectional area (g mm−2) | |
|---|---|---|---|---|
| Control (n=29) | 581.2±54.8 | 7.9±0.5 | 7.1±0.5 | 0.32±0.01 |
| Diabetic (n=32) | 365.5±62.6* | 22.0±1.6* | 0.5±0.1* | 0.30±0.03 |
Values represent mean±s.e.m. of number of animals in brackets.
P<0.05 compared to control value (one-way ANOVA).
Effect of PKC inhibitors on contractile responses to NA and ET-1
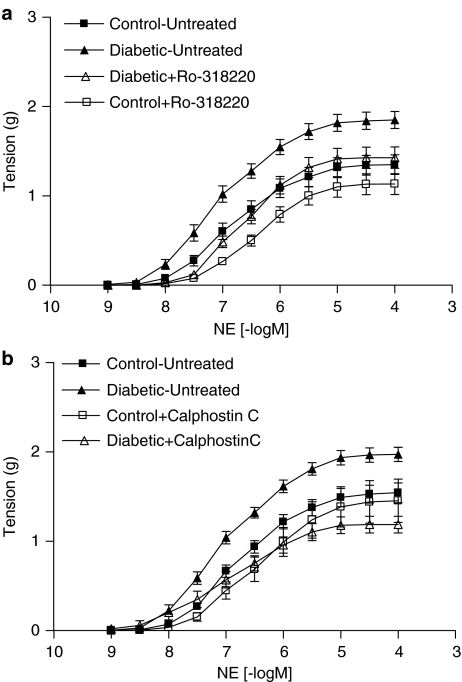
Consistent with our previous reports, mesenteric arteries from diabetic rats exhibited enhanced contractile responses to NA (Figure 2a and b). Both the maximum contractile response (Rmax) and the sensitivity (pD2 or –log EC50) of mesenteric arteries from diabetic rats to NA were significantly greater than the corresponding values obtained in mesenteric arteries from control rats (Table 2). At the same time, there was no change in contractile responses of mesenteric arteries from diabetic rats to depolarization with 0.1 M KCl in the presence of 1 μM phentolamine (0.7±0.1 g) compared to control (0.6±0.1 g).
Figure 2.
Cumulative concentration–response curves to NA in mesenteric arteries from control and diabetic rats in the absence and presence of 3 μM Ro-318220 (a, n=13) and calphostin C (b, n=8). Each point represents mean±s.e.m.
Table 2.
NA, ET-1 and PdBu Rmax and pD2 values in mesenteric arteries from control and diabetic rats
| Control | Diabetic | |||
|---|---|---|---|---|
| Rmax | pD2 | Rmax | pD2 | |
| g | –log EC50 | g | −log EC50 | |
| Noradrenaline | ||||
| Untreated (13) | 1.35±0.10 | 6.84±0.1 | 1.86±0.09* | 7.13±0.13* |
| Ro-318220 | 1.15±0.09 | 6.40±0.06** | 1.44±0.09** | 6.63±0.04** |
| Untreated (8) | 1.51±0.09 | 6.81±0.06 | 1.93±0.08* | 7.11±0.08* |
| Calphostin C | 1.43±0.23 | 6.47±0.09** | 1.16±0.09** | 6.88±0.09*,** |
| Endothelin | ||||
| Untreated (4) | 1.57±0.21 | 8.16±0.14 | 1.92±0.34 | 8.24±0.17 |
| Ro-318220 | 0.76±0.12** | 7.97±0.08 | 0.67±0.16** | 7.97±0.05 |
| Untreated (5) | 1.66±0.11 | 8.43±0.19 | 1.94±0.20 | 8.27±0.11 |
| Calphostin C | 0.82±0.19** | 8.08±0.15 | 0.69±0.12** | 8.07±0.18 |
| Phorbol 12,13 dibutyrate | ||||
| Untreated (8) | 2.13±0.27 | 7.28±0.09 | 1.97±0.29 | 7.32±0.11 |
| Ro-318,220 | ND | ND | ND | ND |
Mesenteric arteries were isolated from control and diabetic rats and cumulative concentration response curves to NA, ET-1 and PdBu were constructed in the absence and presence of PKC inhibitors, Ro-318220, 3 μM for 30 min, and calphostin C, 3 μM for 30 min. Number in parentheses represents number of animals. ND, not detectable.
P<0.05 compared to corresponding control value,
P<0.05 compared to corresponding untreated value (two-way ANOVA followed by Newman–Keuls post hoc test).
Incubation of mesenteric arteries with either 3 μM Ro-318220 or 3 μM calphostin C for 30 min had no significant effect on the contractile responses to 0.1 M KCl plus 1 μM phentolamine. In the presence of Ro-318220, responses of arteries from control rats to KCl plus phentolamine were 99±8% and those from diabetic rats were 95±4% of responses in untreated arteries, while in the presence of calphostin C, responses of arteries from control and diabetic rats were 90±5 and 95±2%, respectively, of those in untreated arteries. In mesenteric arteries from control rats, neither Ro-318220 nor calphostin C had a significant effect on the NA Rmax, but both inhibitors produced a shift in the NA concentration–response curve, resulting in a significant decrease in the NA pD2 value (Figure 2a and b, Table 2). Ro-318220 and calphostin C had a greater inhibitory effect on contractile responses of mesenteric arteries from diabetic rats to NA, producing a significant decrease in both the pD2 value and Rmax to NA. In the presence of Ro-318220, there was no significant difference in NA pD2 values or Rmax between arteries from control and diabetic animals (Figure 2a, Table 2). In the presence of calphostin C, the NA pD2 value in arteries from diabetic rats remained greater than that in control arteries (Figure 2b, Table 2). However, calphostin C abolished the significant difference in the NA Rmax between arteries from the two groups of animals.
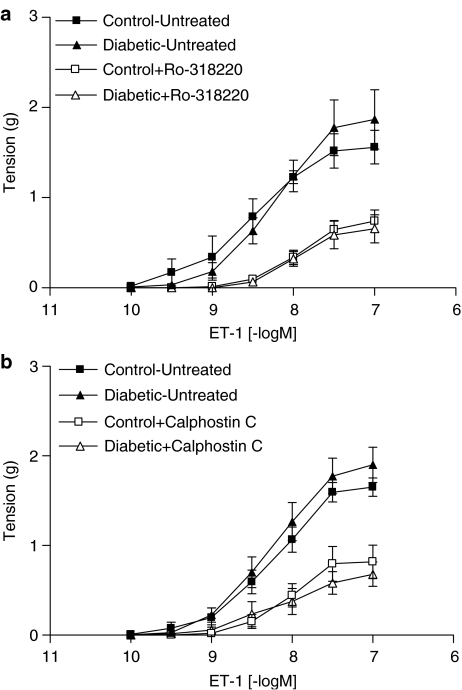
In contrast to NA, neither the maximum contractile response nor the sensitivity of mesenteric arteries from diabetic rats to ET-1 were significantly different from control (Figure 3a and b, Table 2). Incubation with Ro-318220 resulted in marked attenuation of maximum responses to ET-1 in arteries from both control and diabetic rats, without affecting the ET-1 pD2 value (Figure 3a, Table 2). Similar results were obtained with calphostin C (Figure 3b), which significantly decreased the ET-1 Rmax in arteries from both control and diabetic rats (Table 2) without altering the sensitivity to this agonist. There was no significant difference between arteries from control and diabetic rats in response to ET-1 in the presence of either antagonist.
Figure 3.
Cumulative concentration–response curves to ET-1 in mesenteric arteries from control and diabetic rats in the absence and presence of 3 μM Ro-318220 (a, n=4) and calphostin C (b, n=5). Each point represents mean±s.e.m.
Contractile responses of mesenteric arteries from control and diabetic rats to direct activation of PKC with phorbol 12,13 dibutyrate (PdBu) were also compared. Responses to PdBu, which were completely abolished by Ro-318220, were not significantly different between arteries from control and diabetic rats (Table 2).
Effect of diabetes on levels of PKC isoforms in mesenteric arteries
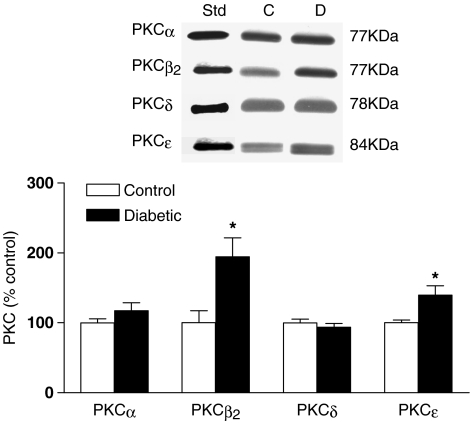
PKCα, β2, δ and ɛ were all detected in mesenteric arteries from both control and diabetic rats (Figure 4). No significant differences in levels of PKCα or PKCδ were detected between arteries from control and diabetic rats. However, levels of PKCβ2 and PKCɛ in arteries from diabetic rats were increased to 195±26.9 and 140±13.2%, respectively, of levels in control arteries.
Figure 4.
Total levels of PKCα, β2, δ and ɛ in untreated mesenteric arteries from control and diabetic rats. A representative Western blot of each isoform, together with a purified standard (Std) of the isoform is shown, together with a graph showing the mean±s.e.m. of data obtained from five different preparations. Open and filled bars represent arteries from control and diabetic animals, respectively. Results were expressed as a percent of levels of the isoform in control mesenteric arteries. *P<0.05 compared to the corresponding control (one-way ANOVA).
Effect of NA and ET-1 on cytosolic and particulate levels of PKC isoforms in mesenteric arteries
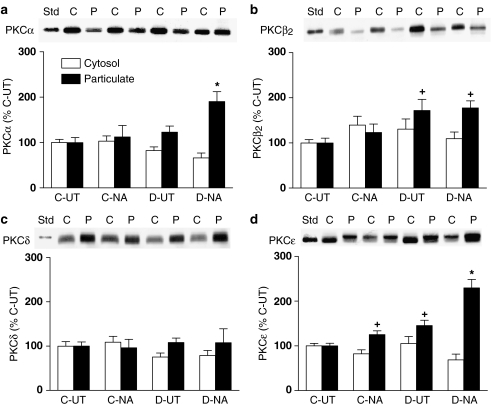
Treatment with 30 μM NA for 5 min, the time required for the sustained contractile response to this agonist to reach its maximum in arteries from both control and diabetic rats, had no significant effect on cytosolic or particulate levels of PKCα in control arteries, but resulted in a marked increase in levels of PKCα in the particulate fraction of mesenteric arteries from diabetic rats to 190±22% of levels in untreated control arteries (Figure 5a). NA treatment had no detectable effect on particulate levels of PKCβ2 (Figure 5b) or PKCδ (Figure 5c) in mesenteric arteries from either control or diabetic rats. However, NA did result in a small but significant increase of PKCɛ in the particulate fraction of control mesenteric arteries, to 125±8% of those in untreated arteries (Figure 5d). Particulate levels of PKCɛ in untreated arteries from diabetic rats were significantly greater than those in untreated control arteries, and treatment with NA resulted in a further significant increase in PKCɛ particulate levels, to 230±19% of control. The NA-induced increase in PKCɛ in the particulate fraction was significantly greater in mesenteric arteries from diabetic rats than in corresponding control arteries. In general, levels of PKC isoforms in the cytosolic fraction decreased in parallel to the NA-induced increases in particulate levels, but the changes were less marked than those in the particulate fraction, and did not reach statistical significance. This may have arisen in part because a greater proportion of PKC is found in the cytosol under resting conditions, in which case a relatively small decrease in cytosol levels will result a more marked increase in levels in the particulate fraction.
Figure 5.
The distribution of PKCα (a), PKCβ2 (b) PKCδ (c) and PKCɛ (d) in mesenteric arteries from control (C) and diabetic (D) rats in the absence (UT) and presence of NA (30 μM for 5 min). A representative Western blot showing cytosolic (C) and particulate (P) fractions is shown, together with a graph showing the mean±s.e.m. of data obtained from five different preparations. The first band on each blot represents the purified standard of the isoform (Std). Cytosolic (open bars) and particulate (closed bars) levels of PKC isoforms following NA treatment were calculated relative to levels of the same isoform in the corresponding fraction from untreated control arteries. *P<0.05 compared to all the other corresponding groups; +P<0.05 compared to the corresponding control untreated group (two-way ANOVA followed by Newman–Keuls post hoc test).
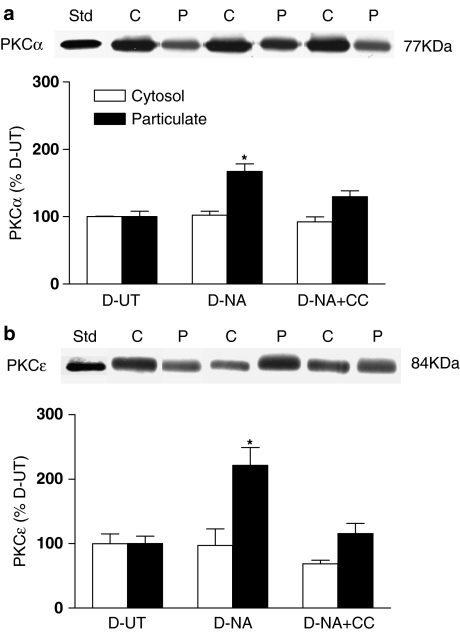
The effect of treatment of arteries from diabetic animals with calphostin C (3 μM for 30 min) on NA-induced changes in particulate levels of PKCα and PKCɛ was also determined. Calphostin C completely prevented the NA-induced increase in particulate levels of both isoforms (Figure 6). No significant changes in cytosolic levels of PKCα or PKCɛ were detected in the presence of NA alone or with calphostin C.
Figure 6.
The distribution of PKCα (a) and PKCɛ (b) in mesenteric arteries from diabetic rats (D) in the UT, in the presence of NA (30 μM for 7 min) and in the presence of NA plus calphostin C (NA+CC, 3 μM calphostin C for 35 min with 30 μM NA being added for the last 5 min). A representative Western blot showing cytosolic (C) and particulate (P) fractions is shown, together with a graph showing the mean±s.e.m. of data obtained from three different preparations. The first band on each blot represents the purified standard of the isoform (Std). Cytosolic (open bars) and particulate (filled bars) levels of PKC isoforms were calculated relative to levels of the same isoform in the corresponding fraction from untreated diabetic arteries. *P<0.05 compared to all other corresponding groups.
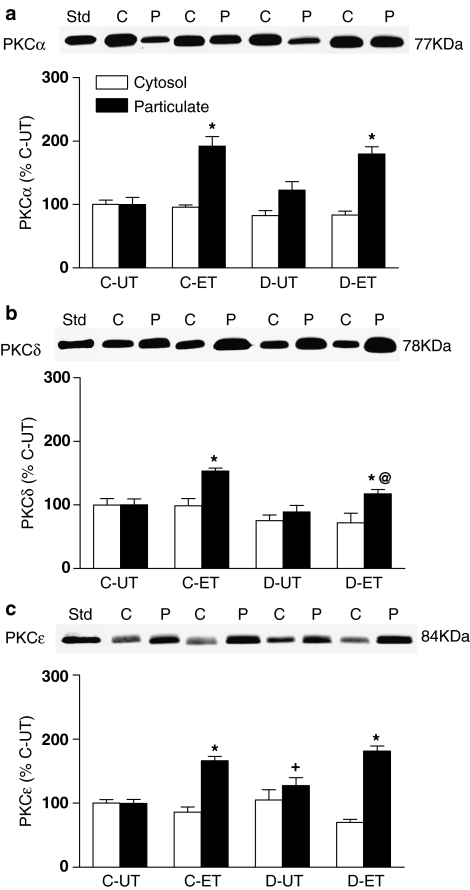
Treatment with 0.03 μM ET-1 for 7 min, the time required for the sustained contractile response to this agonist to reach its maximum, resulted in similar increases in particulate levels of PKCα in mesenteric arteries from both control and diabetic rats. Particulate levels of PKCα were increased by ET-1 to 192±15% in control and to 180±12% in diabetic arteries of levels in untreated control arteries (Figure 7a). Like NA, ET-1 had no significant effect on soluble or particulate levels of PKCβ2 (data not shown). However, ET-1 produced significant increases in particulate levels of PKCδ in arteries from both control and diabetic animals (Figure 7b). The ET-1-induced increase in particulate PKCδ was significantly less in diabetic (117±7%) than in control (153±5%) arteries. In mesenteric arteries from control rats, ET-1 treatment resulted in a significant increase in levels of PKCɛ in the particulate fraction, to 166±7% of levels in the untreated control (Figure 7c). A similar increase in particulate levels of PKCɛ in response to ET-1, to 181±8% of control, was also detected in mesenteric arteries from diabetic rats. As was found with NA, the ET-1-induced increases in particulate levels of PKC isoforms were not associated with significant decreases in their levels in the cytosolic fraction.
Figure 7.
The distribution of PKCα (a), PKCδ (b) and PKCɛ (c) in mesenteric arteries from control (C) and diabetic rats (D) in the absence (UT) and presence of ET-1 (0.03 μM for 7 min.). A representative Western blot showing cytosolic (C) and particulate (P) fractions is shown, together with a graph showing the mean±s.e.m. of data obtained from five different preparations. The first band on each blot represents the purified standard of the isoform (Std). Cytosolic (open bars) and particulate (closed bars) levels of PKC isoforms following ET-1 treatment were calculated relative to levels of the same isoform in the corresponding fraction from untreated control arteries. *P<0.05 compared to the corresponding untreated group; +P<0.05 compared to the corresponding control untreated group; @P<0.05 compared to the corresponding control ET-1-treated group (two-way ANOVA followed by Newman–Keuls post hoc test).
Effect of NA on phosphorylation of CPI-17
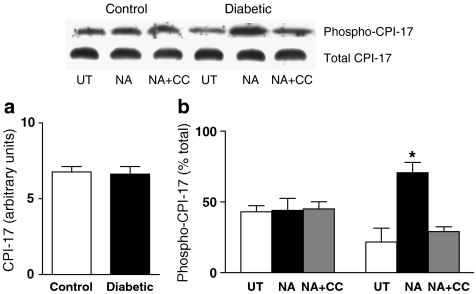
Since PKC-mediated CPI-17 phosphorylation has been suggested to increase smooth muscle contractility, we investigated whether the increased particulate levels of PKCα and ɛ and contractility in response to NA in mesenteric arteries from diabetic rats was associated with increased phosphorylation of this downstream target. No significant differences in total protein levels of CPI-17 were detected between mesenteric arteries from control and diabetic rats (Figure 8a). NA had no significant effect on the phosphorylation of CPI-17 in control arteries (Figure 8b). Levels of phosphorylated CPI-17 were lower in untreated arteries from diabetic than control animals, although this difference was not statistically significant. However, NA produced a significant increase in CPI-17 phosphorylation in mesenteric arteries from diabetic rats, and in the presence of NA, levels of phosphorylated CPI-17 were significantly greater in arteries from diabetic than control animals. The NA-induced increase in CPI-17 phosphorylation in mesenteric arteries from diabetic rats was blocked by the same concentration of calphostin C (3 μM) that normalized the contractile response of diabetic arteries to NA (Figure 8b), and prevented the NA-induced increase in particulate levels of PKCα and ɛ (Figure 6).
Figure 8.
Total levels of CPI-17 in control and diabetic arteries (a). Levels of phosphorylated CPI-17 in mesenteric arteries from control (C) and diabetic (D) rats in the untreated state (UT), in the presence of NA (30 μM for 5 min) and in the presence of NA plus calphostin C (NA+CC, 3 μM calphostin C for 35 min with 30 μM NA being added for the last 5 min) (b). A representative Western blot showing levels of total and phosphorylated CPI-17 is shown, together with a graph showing the mean±s.e.m. of data obtained from three different preparations. Levels of phosphorylated CPI-17 were expressed as a percent of total CPI-17 levels in each group. *P<0.05 compared to C-NA, C-NA+CC, D-UT and D-NA+CC (two-way ANOVA followed by Newman–Keuls post hoc test).
Discussion
The results of the present investigation demonstrate that the enhanced reactivity of mesenteric arteries from diabetic rats to NA is blocked by selective inhibition of PKC, and is associated with increased particulate levels of PKCα and ɛ, as well as greater PKC-mediated phosphorylation of CPI-17. These data suggest that increased activation of the PKC/CPI-17 pathway mediates the enhanced contractile response to NA in mesenteric arteries from diabetic rats. On the other hand, although PKC appears to contribute to a greater extent to ET-1 than to NA-induced contractions in control mesenteric arteries, no evidence was found for enhanced participation of PKC in the response to ET-1 in mesenteric arteries from diabetic rats.
Basal levels of PKC isoforms
It is now well established that increased levels and activity of specific isoforms of PKC occur in the cardiovascular system in diabetes, and evidence suggests that this may contribute to the cardiovascular dysfunction associated with this condition (Koya & King, 1998). The isoform that has most frequently been found to be altered in the vasculature is PKCβ2. For instance, an increase in total PKC activity in the particulate fraction was found to be associated with increased particulate levels of PKCβ2 but not PKCα in aorta from STZ-diabetic rats (Inoguchi et al., 1992). Increased total levels of PKCβ2 were recently reported in coronary arteries (Tickerhoof et al., 2003) and in small mesenteric arteries from diabetic rats (Wigg et al., 2004). Consistent with these studies, we detected a two-fold increase in total PKCβ2 levels in superior mesenteric arteries from diabetic rats. There was also a smaller but significant increase in PKCɛ levels, but no change in expression of PKCα or δ in arteries from diabetic rats. This contrasts with a recent report of increased expression of PKCα and δ, but not PKCɛ, in coronary arteries from diabetic rats (Tickerhoof et al., 2003). Whether this reflects a difference due to the longer duration of diabetes in the present study, or to differences between arteries is not clear.
The precise mechanisms that lead to hyperglycemia-induced increases in the expression and/or activity of PKC isoforms are not clear. One of the major mechanisms proposed to lead to increased PKC activation in arteries from diabetic rats is enhanced de novo synthesis of DAG, resulting from glucose being directly metabolized to DAG (Inoguchi et al., 1994). It has been suggested that upregulation of PKC could also result from the hyperglycemia-induced increase in the synthesis of DAG, which might enhance transcription and translation of PKC genes (Guo et al., 2003). Alternatively, the persistent activation of PKC, associated with its translocation to the cell membrane, could result in depletion of cytosolic PKC, triggering an increase in PKC synthesis (Guo et al., 2003). The reasons for the differential upregulation of the PKC isoforms in diabetic tissues are also not clear but might be explained by differences in protein stability, in rates of synthesis and degradation or in susceptibility to post-translational alterations such as phosphorylation (Tickerhoof et al., 2003). Other mechanisms proposed to lead to increased activity of PKC in mesenteric arteries from diabetic rats include accumulation of advanced glycosylation end-products and increased activation of the renin–angiotensin system (Osicka et al., 2001).
Role of PKC in GPCR-mediated responses in mesenteric arteries from control rats
The results of this investigation suggest that PKC may play a more prominent role in the contractile response to ET-1 than to NA in mesenteric arteries from control rats. Treatment of arteries with the nonisoform selective PKC inhibitors Ro-318220 and calphostin C produced a decrease in the NA pD2 value but had no effect on the NA Rmax, whereas both antagonists reduced the ET-1 Rmax by more than 50%. Ro-318220 is a potent and selective PKC inhibitor belonging to the group of inhibitors known as bisindolylmaleimides, that inhibit PKC by competing with ATP binding at the catalytic site (Davis et al., 1989). Ro-318200 appears to be more selective than its parent compound, staurosporine (Davis et al., 1989) and unlike the latter does not appear to inhibit MLCK (Mobley et al., 1994). Calphostin C, on the other hand, inhibits PKC by interacting with the regulatory domain, competing at the binding site for DAG and phorbol esters (Kobayashi et al., 1989). At the concentrations used, neither Ro-318220 nor calphostin C had any effect on the contractile response to K+-depolarization, suggesting that their inhibitory effects were not due nonspecific actions. At the same time, Ro-318220 abolished the contractile response of mesenteric arteries to PdBu, suggesting that it is effectively blocking PKC activity. Our observations that the contractile response to NA was associated with only a small increase in particulate levels of PKCɛ, with no change in PKCα or δ, and was not associated with a detectable increase in phosphorylation of CPI-17, a proposed downstream mediator of the Ca2+ sensitizing effects of PKC, are consistent with a minor role for PKC in the NA response. It is possible that other pathways, such as RhoA/Rho kinase (Altmann et al., 2003), participate to a greater extent in α-adrenoceptor-mediated contraction in control mesenteric arteries. On the other hand, the much greater increase in particulate levels of all three PKC isoforms by ET-1 is consistent with the results of the inhibitor studies, and suggests that under normal circumstances, the ET-1 signaling pathway may be more effectively coupled to PKC.
Role of PKC in GPCR-mediated responses in mesenteric arteries from diabetic rats
While PKC may not contribute to a large extent to the contractile response of control mesenteric arteries to NA, it appears to mediate the enhanced contractile response of mesenteric arteries from diabetic rats to this agonist, since both Ro-318220 and calphostin C produced greater inhibition of NA-induced contractions in diabetic than control arteries, and normalized the significant difference in NA Rmax values found in the absence of antagonist. The enhanced contractile response to NA was also associated with increased particulate levels of PKCα as well as with a greater increase in particulate PKCɛ levels than in mesenteric arteries from control rats, which were blocked following treatment with calphostin C. This suggests that the enhanced contractile response to NA may be mediated by increased activation of these isoforms. This may result from increased production of DAG, since we have previously found that the breakdown of PIP2 and the production of inositol 1,4,5 trisphosphate (IP3) and phosphatidic acid, a phosphorylated product of DAG, are enhanced in response to the same concentration of NA in aorta and mesenteric arteries from diabetic rats (Abebe & MacLeod, 1991; 1992).
In contrast to the results in control arteries, treatment of arteries from diabetic rats with NA produced an almost three-fold increase in CPI-17 phosphorylation and this increase was also blocked by calphostin C. These data suggest that the significantly greater contractile responses to NA in diabetic arteries arise via PKC-dependent phosphorylation and activation of CPI-17. While all PKC isoforms have been shown to bind to CPI-17 in vitro, PKCα, δ and ɛ have the greatest ability to phosphorylate and activate it (Eto et al., 2001; Zemlickova et al., 2004). It is not clear how activated PKC in the membrane fraction can phosphorylate CPI-17, which is located in the cytosol. However, a number of possibilities have been suggested. One is that CPI-17 lies in close proximity to the plasma membrane where activated PKC is present and can readily phosphorylate it. Once phosphorylated, CPI-17 then diffuses to the myofilaments to inhibit MLCP (Eto et al., 2001). Alternatively CPI-17 may shuttle between membrane-bound active PKC and MLCP (Ruegg, 1999). It is also possible that activated PKC in the membrane is in equilibrium with cytosolic PKC, and the amount of activated PKC in the cytosolic fraction is sufficient to cause CPI-17 phosphorylation (Ruegg, 1999).
The receptor through which NA produces these actions on PKC and CPI-17 was not specifically examined in the present investigation. However, we have previously established that the enhanced contractile response of mesenteric arteries from diabetic rats to NA can be detected in the presence of a β-blocker and blockers of both neuronal and extraneuronal uptake (Harris & MacLeod, 1988), suggesting that alterations in these processes are not responsible for the increased NA response. Furthermore, we have also demonstrated that both the enhanced contractile response and the increase in inositol phosphate production in response to NA in mesenteric arteries from diabetic rats arise from stimulation of α1-adrenoceptors, based on pharmacological studies with selective antagonists (Abebe et al., 1990; Abebe & MacLeod, 1992). Additional evidence that the effects of NA arise from stimulation of α1-receptors comes from our observation that phenylephrine, which is known to have greater selectivity for α1 than for α2 or β receptors, also produces both enhanced contractile responses and increased particulate levels of PKCα and ɛ in mesenteric arteries from diabetic compared to control rats (Mueed and MacLeod, unpublished observations).
In contrast to NA, there was no evidence for an enhancement of the contractile response to ET-1 in mesenteric arteries from diabetic rats, nor was there any indication that the contractile response of diabetic mesenteric arteries to ET-1 was more dependent on PKC than that of control arteries, since both Ro-318220 and calphostin C produced a similar magnitude of block of contractile responses to ET-1 and ET-1 produced a similar increase in particulate levels of PKCα and ɛ in mesenteric arteries from control and diabetic animals. The reason that PKCδ levels in the particulate fraction were actually lower in response to ET-1 in arteries from diabetic than control rats is not known, but since the contractile response to this agonist was unaffected, the other two isoforms may play a more important role in this process. The lack of increase in the contractile response of mesenteric arteries from diabetic rats to ET-1 is not consistent with previous reports in aorta and coronary arteries of diabetic rats, which indicated that vasoconstrictor responsiveness to this agonist was increased, and that the increase could be blocked by inhibition of PKC (Hattori et al., 1999; Tickerhoof et al., 2003). However, it is in agreement with a report that contractile responses of endothelium-denuded mesenteric arteries from diabetic rats to ET-1 are unchanged (Arikawa et al., 2001). Differences in the effects of diabetes on contractile responses to stimulation of GPCR in different arteries have previously been reported (James & Hodgson, 1995). Although the reason for this is not known, these results demonstrate that the upregulation of PKCβ2 and ɛ in mesenteric arteries from diabetic rats is not associated with a generalized increase in the ability of GPCR agonists to activate PKC. Rather, it appears the activation of PKC by agonists is not enhanced unless the production of DAG itself is enhanced, and this appears to occur as a result of a selective change in the pathway coupling α1-adrenoceptors to phosphoinositide hydrolysis. This change appears to be due to the diabetic state, since enhanced contractile responses to NA were normalized by treatment of diabetic rats with insulin (MacLeod, 1985).
Despite the increased basal levels of PKCβ2 and ɛ, there was also no generalized increase in contractile responses of mesenteric arteries from diabetic rats to direct activation of PKC with PdBu. Although this is somewhat surprising, there is no evidence that PKCβ2, the isoform most strongly upregulated, participates in calcium sensitization or contraction, and we found no evidence that agonists elevate particulate levels of this isoform in either control or diabetic arteries. This is not the case for PKCɛ. However, it is possible that the effects of the relatively small increase in PKCɛ in mesenteric arteries from diabetic rats cannot be detected due to the activation of all DAG-dependent PKC isoforms (including those that are unchanged) in response to PdBu.
The smooth muscle cell plays an important role in maintaining and regulating vasomotor tone, and abnormalities in vascular reactivity have been implicated as potential contributors to the cardiovascular complications of diabetes. In this study, we have shown that inhibitors of PKC normalized the enhanced contractile responses of the diabetic arteries to NA, and demonstrate for the first time, to our knowledge, that α-adrenoceptor-mediated increase in particulate levels of specific PKC isoforms and phosphorylation of CPI-17 are also enhanced in arteries from diabetic animals. These data are consistent with a contribution of increased activation of PKC to the enhanced contractile responses of arteries from diabetic rats to stimulation of α-adrenoceptors. This appears to result from a selective change in α-adrenoceptor-mediated signalling in arteries from diabetic animals, since similar changes were not seen in response to ET-1.
Acknowledgments
This work was supported by a grant-in-aid from the Heart and Stroke Foundation of B.C. and Yukon. Irem Mueed is the recipient of the Rx&D/CIHR Health Research Foundation Graduate Research Scholarship.
Abbreviations
- ET-1
endothelin-1
- GPCR
G-protein coupled receptors
- KCl
potassium chloride
- PdBu
phorbol (12,13) dibutyrate
- PIP2
phosphatidylinositol biphosphate
- PKC
protein kinase C
- PLC
phospholipase C
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- MLCP
myosin light chain phosphatase
- NA
noradrenaline
- STZ
streptozotocin
- UT
untreated
References
- ABEBE W., MACLEOD K.M. Enhanced arterial contractility to noradrenaline in diabetic rats is associated with increased phosphoinositide metabolism. Can J. Physiol. Pharmacol. 1991;69:355–361. doi: 10.1139/y91-054. [DOI] [PubMed] [Google Scholar]
- ABEBE W., MACLEOD K.M. Augmented inositol phosphate production in mesenteric arteries from diabetic rats. Eur. J. Pharmacol. 1992;225:29–36. doi: 10.1016/0922-4106(92)90035-t. [DOI] [PubMed] [Google Scholar]
- ABEBE W., HARRIS K.H., MACLEOD K.M. Enhanced contractile responses of arteries from diabetic rats to a1-adrenoceptor stimulation in the absence and presence of extracellular calcium. J. Cardiovasc. Pharmacol. 1990;16:239–248. doi: 10.1097/00005344-199008000-00010. [DOI] [PubMed] [Google Scholar]
- ALTMANN C., STEENPASS V., CZYBORRA P., HEIN P., MICHEL M.C. Comparison of signalling mechanisms involved in rat mesenteric microvessel contraction by noradrenaline and sphingosylphosphorylcholine. Br. J. Pharmacol. 2003;138:261–271. doi: 10.1038/sj.bjp.0705028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARIKAWA E., VERMA S., DUMONT A.S., MCNEILL J.H. Chronic bosentan treatment improves renal artery vascular function in diabetes. J. Hypertens. 2001;19:803–812. doi: 10.1097/00004872-200104000-00018. [DOI] [PubMed] [Google Scholar]
- CHOW W.L., ZHANG L., MACLEOD K.M. Noradrenaline-induced changes in intracellular Ca(2+) and tension in mesenteric arteries from diabetic rats. Br. J. Pharmacol. 2001;134:179–187. doi: 10.1038/sj.bjp.0704221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS P.D., HILL C.H., KEECH E., LAWTON G., NIXON J.S., SEDGWICK A.D., WADSWORTH J., WESTMACOTT D., WILKINSON S.E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989;259:61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- ETO M., KITAZAWA T., YAZAWA M., MUKAI H., ONO Y., BRAUTIGAN D.L. Histamine-induced vasoconstriction involves phosphorylation of a specific inhibitor protein for myosin phosphatase by protein kinase C alpha and delta isoforms. J. Biol. Chem. 2001;276:29072–29078. doi: 10.1074/jbc.M103206200. [DOI] [PubMed] [Google Scholar]
- ETO M., SENBA S., MORITA F., YAZAWA M. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: its specific localization in smooth muscle. FEBS Lett. 1997;410:356–360. doi: 10.1016/s0014-5793(97)00657-1. [DOI] [PubMed] [Google Scholar]
- GUO M., WU M.H., KOROMPAI F., YUAN S.Y. Upregulation of PKC genes and isozymes in cardiovascular tissues during early stages of experimental diabetes. Physiol. Genomics. 2003;12:139–146. doi: 10.1152/physiolgenomics.00125.2002. [DOI] [PubMed] [Google Scholar]
- HARRIS K.H., MACLEOD K.M. Influence of the endothelium on contractile responses of arteries from diabetic rats. Eur. J. Pharmacol. 1988;153:55–64. doi: 10.1016/0014-2999(88)90587-0. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., KAWASAKI H., KANNO M. Increased contractile responses to endothelin-1 and U46619 via a protein kinase C-mediated nifedipine-sensitive pathway in diabetic rat aorta. Res. Commun. Mol. Pathol. Pharmacol. 1999;104:73–80. [PubMed] [Google Scholar]
- INAZU M., SAKAI Y., HOMMA I. Contractile responses and calcium mobilization in renal arteries of diabetic rats. Eur. J. Pharmacol. 1991;203:79–84. doi: 10.1016/0014-2999(91)90793-p. [DOI] [PubMed] [Google Scholar]
- INOGUCHI T., BATTAN R., HANDLER E., SPORTSMAN J.R., HEATH W., KING G.L. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc. Natl. Acad. Sci. U.S.A. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOGUCHI T., XIA P., KUNISAKI M., HIGASHI S., FEENER E.P., KING G.L. Insulin's effect on protein kinase C and diacylglycerol induced by diabetes and glucose in vascular tissues. Am. J. Physiol. 1994;267:E369–E379. doi: 10.1152/ajpendo.1994.267.3.E369. [DOI] [PubMed] [Google Scholar]
- JAMES G.M., HODGSON W.C. Attenuated 5-HT2 receptor-mediated responses in hindquarters of diabetic rats. Eur. J. Pharmacol. 1995;294:109–115. doi: 10.1016/0014-2999(95)00524-2. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI E., NAKANO H., MORIMOTO M., TAMAOKI T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- KOYA D., KING G.L. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–866. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- LEE Y.H., KIM I., LAPORTE R., WALSH M.P., MORGAN K.G. Isozyme-specific inhibitors of protein kinase C translocation: effects on contractility of single permeabilized vascular muscle cells of the ferret. J. Physiol. 1999;517 (Part 3):709–720. doi: 10.1111/j.1469-7793.1999.0709s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEOD K.M. The effect of insulin treatment on changes in vascular reactivity in chronic, experimental diabetes. Diabetes. 1985;34:1160–1167. doi: 10.2337/diab.34.11.1160. [DOI] [PubMed] [Google Scholar]
- MEIER M., KING G.L. Protein kinase C activation and its pharmacological inhibition in vascular disease. Vasc. Med. 2000;5:173–185. doi: 10.1177/1358836X0000500307. [DOI] [PubMed] [Google Scholar]
- MOBLEY PL, HEDBERG K., BONIN L., CHEN B., GRIFFITH O.H. Decreased phosphorylation of four 20-kDa proteins precedes staurosporine-induced disruption of the actin/myosin cytoskeleton in rat astrocytes. Exp. Cell Res. 1994;214:55–66. doi: 10.1006/excr.1994.1233. [DOI] [PubMed] [Google Scholar]
- OSICKA T.M., YU Y., LEE V., PANAGIOTOPOULOS S., KEMP B.E., JERUMS G. Aminoguanidine and ramipril prevent diabetes-induced increases in protein kinase C activity in glomeruli, retina and mesenteric artery. Clin. Sci. (London) 2001;100:249–257. [PubMed] [Google Scholar]
- PING P., ZHANG J., QIU Y., TANG X.L., MANCHIKALAPUDI S., CAO X., BOLLI R. Ischemic pre-conditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ. Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- POHL J., WINDER S.J., ALLEN B.G., WALSH M.P., SELLERS J.R., GERTHOFFER W.T. Phosphorylation of calponin in airway smooth muscle. Am. J. Physiol. 1997;272:L115–L123. doi: 10.1152/ajplung.1997.272.1.L115. [DOI] [PubMed] [Google Scholar]
- RUEGG J.C. Smooth muscle: PKC-induced Ca2+ sensitisation by myosin phosphatase inhibition. J. Physiol. 1999;520 (Part 1):3. doi: 10.1111/j.1469-7793.1999.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOHN U.D., CAO W., TANG D.C., STULL J.T., HAEBERLE J.R., WANG C.L., HARNETT K.M., BEHAR J., BIANCANI P. Myosin light chain kinase- and PKC-dependent contraction of LES and esophageal smooth muscle. Am. J. Physiol. Gastrointest Liver Physiol. 2001;281:G467–G478. doi: 10.1152/ajpgi.2001.281.2.G467. [DOI] [PubMed] [Google Scholar]
- SOKOLOVSKY M. Endothelin receptor subtypes and their role in transmembrane signaling mechanisms. Pharmacol. Ther. 1995;68:435–471. doi: 10.1016/0163-7258(95)02015-2. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- TAGGART M.J., LEE Y.H., MORGAN K.G. Cellular redistribution of PKCalpha, rhoA, and ROKalpha following smooth muscle agonist stimulation. Exp. Cell Res. 1999;251:92–101. doi: 10.1006/excr.1999.4565. [DOI] [PubMed] [Google Scholar]
- TAYLOR P.D., OON B.B., THOMAS C.R., POSTON L. Prevention by insulin treatment of endothelial dysfunction but not enhanced noradrenaline-induced contractility in mesenteric resistance arteries from streptozotocin-induced diabetic rats. Br. J. Pharmacol. 1994;111:35–41. doi: 10.1111/j.1476-5381.1994.tb14020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TICKERHOOF M.M., FARRELL P.A., KORZICK D.H. Alterations in rat coronary vasoreactivity and vascular protein kinase C isoforms in Type 1 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2694–H2703. doi: 10.1152/ajpheart.00394.2003. [DOI] [PubMed] [Google Scholar]
- WEBER L.P., MACLEOD K.M. Influence of streptozotocin diabetes on the alpha-1 adrenoceptor and associated G proteins in rat arteries. J. Pharmacol. Exp. Ther. 1997;283:1469–1478. [PubMed] [Google Scholar]
- WIGG S.J., TARE M., FORBES J., COOPER M.E., THOMAS M.C., COLEMAN H.A., PARKINGTON H.C., O'BRIEN R.C. Early vitamin E supplementation attenuates diabetes-associated vascular dysfunction and the rise in protein kinase C-beta in mesenteric artery and ameliorates wall stiffness in femoral artery of Wistar rats. Diabetologia. 2004;47:1038–1046. doi: 10.1007/s00125-004-1411-x. [DOI] [PubMed] [Google Scholar]
- WOODSOME T.P., ETO M., EVERETT A., BRAUTIGAN D.L., KITAZAWA T. Expression of CPI-17 and myosin phosphatase correlates with Ca2+ sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J. Physiol. 2001;535:553–564. doi: 10.1111/j.1469-7793.2001.t01-1-00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEMLICKOVA E., JOHANNES F.J., AITKEN A., DUBOIS T. Association of CPI-17 with protein kinase C and casein kinase I. Biochem. Biophys. Res. Commun. 2004;316:39–47. doi: 10.1016/j.bbrc.2004.02.014. [DOI] [PubMed] [Google Scholar]