Abstract
N-terminal labelled fluorescent BODIPY®-NPY peptide analogues were tested in Y1, Y2, Y4 and Y5 receptor-binding assays performed in rat brain membrane preparations and HEK293 cells expressing the rat Y1, Y2, Y4 and Y5 receptors.
BODIPY®TMR/FL-[Leu31, Pro34]NPY/PYY were able to compete for specific [125][Leu31, Pro34]PYY-binding sites with an affinity similar to that observed for the native peptide at the Y1 (Ki=1–6 nM), Y2 (Ki>1000 nM), Y4 (Ki=10 nM) and Y5 (Ki=1–4 nM) receptor subtypes.
BODIPY®FL-PYY(3–36) was able to compete for specific Y2 (Ki=10 nM) and Y5 (Ki=30 nM) binding sites, but had almost no affinity in Y1 and Y4 assays.
BODIPY®FL-hPP was able to compete with high affinity (Ki; 1 and 15 nM) only in Y4 and Y5 receptor-binding assays.
BODIPY®TMR-[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP and BODIPY®TMR-[hPP(1–17), Ala31, Aib32]NPY were potent competitors only on specific Y5-binding sites (Ki=0.1–0.6 nM).
As expected, these fluorescent peptides inhibited forskolin-induced cAMP accumulation, demonstrating that they retained their agonist properties.
When tested in confocal microscopy imaging, fluorescent Y1 and Y5 agonists internalized in a time-dependent manner in Y1 and Y5 transfected cells, respectively.
These results demonstrate that BODIPY®-conjugated NPY analogues retain their selectivity, affinity and agonist properties for the Y1, Y2, Y4 and Y5 receptor subtypes, respectively. Thus, they represent novel tools to study and visualize NPY receptors in living cells.
Keywords: BODIPY®NPY analogues, NPY receptors, rat brain, receptor-binding assays, cAMP assays, cell imaging, confocal microscopy
Introduction
Neuropeptide Y (NPY), a 36-amino-acid peptide isolated from porcine brain (Tatemoto, 1982), shares high sequence homology with peptide YY (PYY) and the pancreatic polypeptides (PPs) (Tatemoto et al., 1982). NPY is one of the most abundant peptides found in the central nervous system (CNS) (Chronwall et al., 1985; De Quidt & Emson, 1986) and is believed to be involved in several physiological functions such as circadian rhythms, feeding and sexual behaviours, moods and memory, as well as neuroendocrine and cardiovascular controls (for reviews, see Inui, 1999; Vezzani et al., 1999; Dumont et al., 2000b; 2002; Kask et al., 2002; Redrobe et al., 2002; Kalra & Kalra, 2004; Carvajal et al., 2005).
The biological effects of NPY and related peptides are mediated by the activation of specific plasma membrane receptors. Five NPY receptor subtypes have been cloned and designated as Y1, Y2, Y4, Y5 and y6 (Michel et al., 1998). They all belong to the type 1 G-protein-coupled receptors superfamily, and each one possesses a distinct pharmacological profile (Dumont et al., 2002). The Y1 subtype is preferentially activated by NPY, PYY and [Leu31, Pro34]-substituted analogues, while C-terminal fragments bind with much lower affinities (Herzog et al., 1992; Larhammar et al., 1992). In contrast, the Y2 subtype exhibits high affinity for NPY, PYY and C-terminal fragments such as NPY(2–36), NPY(3–36), PYY(3–36), NPY(13–36) and PYY(13–36), whereas [Leu31,Pro34]-substituted analogues are less potent (Gerald et al., 1995; Gehlert et al., 1996a). The Y4 subtype displays very high affinity for PPs (Bard et al., 1995; Lundell et al., 1995) and [Leu31, Pro34]PYY (Gehlert et al., 1996b), while the Y5 subtype is characterized by its ability to bind NPY, PYY, [Leu31, Pro34]-substituted analogues, human PP and long C-terminal fragments such as NPY(3–36) and PYY(3–36) (Gerald et al., 1996). Recently, a number of antagonists have been developed for the Y1 (BIBO3304, J-104870, G1264879A, LY357897 and J-115814), Y2 (BIIE0246 and JNJ-5207787) and Y5 (CGP71683A, NPY5RA, GW438014A and L-152,804) receptors (for reviews, see Berglund et al., 2003a; Dumont et al., 2004).
NPY receptor subtypes have been characterized and their differential distribution investigated in the brain using various radioligands, including [125I][Leu31,Pro34]PYY, [125I]PYY(3–36), [125I]hPP, [125I]GR231118 and [125I][cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP (Dumont et al., 2000b; 2002; 2004). However, the use of radioligands does not easily allow for discrete cellular localization and, most importantly, for investigation in live cells of processes triggered by ligand–receptor interaction (internalization, trafficking, sequestration and recycling). While receptor antibodies represent important tools for detailed cellular localization, their application is limited in living cells (necessity of developing extracellular site-directed antibodies, assessment that the ligand–receptor–antibody and ligand–receptor complexes use identical internalization and trafficking pathways, etc.). Fluorescent peptide ligands represent an attractive alternative. However, a critical issue relates to the preservation of ligand affinity, selectivity and agonist properties. Fluorescent ligands have been developed for a variety of peptide receptors, including the opioids (Gaudriault et al., 1997; Arttamangkul et al., 2000), neurotensin (Faure et al., 1994; 1995), substance P (Bennett & Simmons, 2001), somatostatin (Nouel et al., 1997) and growth hormone-releasing hormone (Veyrat-Durebex et al., 2005) using a variety of chromophores. The development of BODIPY® fluorophores has generated much interest mainly because (a) their spectral characteristics are often superior to those of conventional dyes, (b) they are relatively nonpolar, (c) the chromophore lacks ionic charge, (d) they are insensitive to solvent polarity and pH changes and (e) they exist in numerous wavelength versions (McGrath et al., 1996).
Cyamin and fluorescein have been used to label various peptides, including NPY, [Pro34]NPY and [Ahx(5–24)]NPY (Ingenhoven & Beck-Sickinger, 1997; Fabry et al., 2000). However, high background was observed with these fluorescent NPY analogues in addition to the high pH sensitivity of these dyes and rapid photobleaching. To circumscribe these problems, NPY and PYY analogues bearing a single BODIPY® molecule at the N-terminus were developed here and characterized in Y1, Y2, Y4 and Y5 receptor preparations. We have already reported that BODIPY®TMR-[Leu31, Pro34]NPY can be internalized in a time-dependent manner as do radiolabelled probes in human embryonic kidney (HEK293) cells transfected with the rat Y1 receptor cDNA (Pheng et al., 2003). In the present study, using competition-binding experiments and cAMP assays, we demonstrate that BODIPY® derivatives of NPY, [Leu31, Pro34]NPY, [Leu31, Pro34]PYY, PYY(3–36), hPP and the Y5 receptor agonists, [hPP(1–17), Ala31,Aib32]NPY and [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP (Cabrele et al., 2000; 2001) maintain their selectivity, high affinity and agonist properties for the targeted NPY receptor subtype. In addition, cell imaging using confocal microscopy reveals that these fluorescent probes can be used to visualize NPY receptors in living cells.
Methods
Materials
Male (200–250 g) Sprague–Dawley CD rats were obtained from Charles River Canada (St-Constant, QC, Canada) and kept on a 12-h light–dark cycle (light on at 0700 h) in temperature- and humidity-controlled rooms. Animals were fed standard laboratory chow and had free access to food and tap water. Animal protocols were approved by the McGill University Animal Care Committee in compliance with guidelines of the Canadian Council of Animal Care. All animals were killed between 0900 and 1030 h.
NPY receptor-transfected cells
HEK293 cells were obtained from Dr S.H. Shen Biotechnology Research Institute (Montreal, QC, Canada) and maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% foetal calf serum (FCS) with antibiotics–antimycotics (penicillin G sodium, 100 U ml−1; streptomycin sulphate, 100 μg ml−1; amphotericin B, 0.25 μg ml−1). Cultured cells were transfected with either of the rat Y1, Y2, Y4 or Y5 receptor cDNA using a calcium phosphate method (Tong et al., 1995). Briefly, 125 μl of 2.5 M calcium phosphate was added to 1.125 ml water containing 50 μg of either rat Y1, Y2, Y4 or Y5 receptor cDNA, which was previously inserted in expressing pcDNA3 (Y2 and Y4) and pTR5-DC-GFP/TK/hygro (Y1 and Y5) vectors, and was slowly mixed with 1.25 ml 2 × HEPES buffer at pH 7.05 and left at room temperature for 20 min. The mixture was added to a 150 mm dish containing HEK293 cells at 30% confluence and returned to the incubator. The medium was changed the next morning. Transiently transfected HEK293 cells (Y2 and Y4) were used 48–72 h after transfection, while stably transfected HEK293 cells (Y1 and Y5) were kept at −80°C (10% DMSO in FCS) until use. Stably transfected HEK293 cells expressing Y1 and Y5 subtypes cells were cultured in a 150-mm dish containing DMEM supplemented with 10% FCS, antibiotics–antimycotics, doxycycline (25 μg ml−1) and hygromycin (125 μg ml−1). Cells transfected with this vector are GFP-positive and levels of GFP protein expression were used as an indicator of Y1 and Y5 receptor expression levels. In these cells, both proteins (GFP and NPY receptor) are transcribed in opposite directions under the control of a bidirectional promoter stimulated in the presence of doxycycline. SK-N-MC cells were purchased from the American Tissue Culture Collection (ATCC) (Manassa, VA, U.S.A.) and plated in a 150-mm dish containing DMEM, 10% FCS containing antibiotic-antimycotic substances. Those cells are endogenously expressing Y1 receptors.
Membrane preparations from transfected cells and brain tissues
At confluence, the transient (Y2 and Y4) or stably (Y1 and Y5) transfected HEK293 cells were washed with Krebs Ringer phosphate (KRP) buffer, pH 7.4, and scraped. Detached cells were then centrifuged at 400 g for 10 min and the pellet was washed with KRP buffer (pH 7.4) of the following composition: NaCl (120 mM), KCl (4.7 mM), CaCl2 (2.2 mM), KH2PO4 (1.2 mM), MgSO4 (1.2 mM), dextrose (5.5 mM) and NaHCO3 (25 mM), recentrifuged twice, and resuspended in 8 ml of KRP buffer, pH 7.4 (1–2 × 106 cells ml−1) and used for receptor-binding assays.
Rat brain membranes were prepared as described previously (Dumont et al., 1995). Briefly, rats were killed by decapitation and their brains rapidly removed and homogenized in KRP buffer at pH 7.4 using a Brinkman polytron (at setting 6 for 15–20 s). Homogenates were centrifuged at 49,000 × g for 20 min, supernatants discarded and pellets washed, resuspended and recentrifuged twice. Pellets were then resuspended in KRP to get a final protein concentration of 2–6 mg ml−1.
Radioligand receptor-binding assays
All binding assays were initiated by adding 100 μl of membrane or cell preparations in a final volume of 500 μl of KRP containing 0.1% (w v−1) bovine serum albumin (BSA), 0.05% (w v−1) bacitracin, 30–35 pM of radiolabelled peptides in the presence and absence of various competitors at concentrations ranging from 10−12 to 10–6 M. In rat brain membrane preparations, NPY receptors were investigated using [125I][Leu31, Pro34]PYY for the Y1, Y4 and Y5 subtypes and [125I]GR231118 for Y1-like receptors, [125I]PYY(3–36) for the Y2-like subtype, [125I]hPP for Y4- and Y5-like sites (Dumont et al., 1995; 2005; Dumont & Quirion, 2000) while Y5-like binding was also performed using [125I][Leu31, Pro34]PYY in the presence of 1 μM BIBO3304 (Dumont et al., 1998). [125I]GR231118 and [125I][Leu31, Pro34]PYY were also used in HEK293 cells expressing the rat Y1 or Y5 receptor, respectively. Binding in HEK293 cells expressing the rat Y2 or Y4 receptor was performed using [125I]PYY(3–36) and [125I]hPP, respectively. Nonspecific binding was determined in the presence of 1 μM of the same unlabeled peptide, that is, [Leu31, Pro34]PYY, PYY(3–36), GR231118 or hPP (specific binding representing 75–90% of total binding). Following a 2-h incubation, the binding reaction was terminated by rapid filtration through Schleicher and Schuell #32 glass filters (previously soaked in 1.0% polyethyleneimine) using a cell harvester filtering apparatus (Brandel Instruments, Gaithersburg, MD, U.S.A.). Filters were rinsed three times with 3 ml of cold KRP and the radioactivity remaining on filters was quantified using a gamma counter with 85% efficiency (Packard Instruments).
Cyclic AMP assay
Cyclic AMP accumulation was determined in HEK293 cells expressing either the rat Y1, Y2, Y4 or Y5 receptor. At confluence, cells were washed with Krebs buffer and resuspended in 5 ml Krebs buffer (pH 7.4). In all, 100 μl of cell suspension (150,000 cells) was preincubated in a final volume of 0.5 ml of Krebs containing 0.1% BSA and 0.1 mM 3-isobutyl-1-methylxanthine at 37°C for 30 min, and then incubated in the presence of 10 μM forskolin and increasing concentrations of peptides (0.1–1000 nM) for 30 min at 37°C (Gerald et al., 1996; Cabrele et al., 2000). Incubation was stopped by adding 100 μl of 1 M HCl. Cell lysis was performed by incubation for 3 min at 100°C, followed by centrifugation. Then, 0.5 ml of the supernatant was lyophilized and cAMP determined using a cAMP radioimmunoassay (Amersham, Mississauga, ON, Canada) performed according to the manufacturer's instructions.
Visualization of fluorescent probes
Transfected HEK293 cells and SK-N-MC cells (endogenously expressing Y1 receptors) were plated as described above, except that they were cultured in a 150-mm petri dish on polylysine-coated glass coverslips. At 80% confluence, cells were washed twice with KRP buffer and incubated in fresh KRP supplemented with 0.1% BSA, 0.05% bacitracin and 1, 5 or 20 nM fluorescent peptides at 37°C for 5, 15 and 45 min. Nonspecific binding was determined in the presence of 1 μM nonfluorescent parent peptide. At the end of the incubation period, cells were rapidly washed twice in cold KRP and air-dried (Pheng et al., 2003). Preliminary experiments using cells fixed with 4% paraformaldehyde give similar results and cellular integrity (data not shown). Labelled cells were examined under a Nikon confocal microscope system (PCM200) equipped with argon (488 nm) and HeNe (543 nm) lasers. For BODIPY®FL peptides and GFP the argon laser (488/510 nm) was used, while for BODIPY®TMR, we used the HeNe laser (543/580 nm). Specificity of the fluorescent signal detected was confirmed with the disappearance of the fluorescent signal with the other laser. All pictures were captured using identical parameters in order to prevent any false-positive results, and were saved as tiff files.
Data analysis
All binding experiments were repeated two to five times, each in triplicate, and results expressed as percentage of specific binding. Cyclic AMP assays were performed three times, each in duplicate. Confocal microscope visualization was performed on three different experiments. IC50 values (i.e. concentration of unlabelled peptide required to compete for 50% of specific binding of the radioligand) of the various analogues used in our study were calculated from the competition-binding assays data using the GraphPad Prism software (GraphPad Software Inc., San Diego, CA, U.S.A.) with a fit to one- to two-site competition curves. Ki values were determined according to the equation of Cheng and Prusoff (Ki=IC50/(1+C/KD)) (Cheng & Prusoff, 1973). As all binding experiments were performed at concentrations of radioligand equal to or below 0.1 KD of the radiolabelled probe, this usually generates Ki values similar to IC50. EC50 values represent the concentrations of the agonist that were able to inhibit 50% of 10 μM forskolin-induced cyclic AMP accumulation, calculated from a concentration–response curve and analysed using GraphPad Prism sigmoid curve function. Student's t-test was used to assess statistical differences between native and BODIPY®-conjugated NPY analogues (P<0.05 being considered significant).
Reagents
NPY, [Leu31, Pro34]NPY, [Leu31, Pro34]PYY, PYY(3–36) and hPP were synthesized by one of us (AF) as described previously (Forest et al., 1990), while [hPP(1–17), Ala31, Aib32]NPY and [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP were generously provided by Dr Annette Beck-Sickenger (University of Leipzig, Germany). High-quality N-terminus-labelled BODIPY®TMR and BODIPY®FL-peptides were used. BODIPY®TMR-NPY, BODYPY®TMR-PYY(3–36), BODIPY®TMR-[Leu31, Pro34]NPY, BODIPY®TMR-[hPP(1–17), Ala31, Aib32]NPY and BODIPY®TMR-[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP were synthesized by Perkin-Elmer (Advanced BioConcept, Montreal, QC, Canada). BODIPY®FL-[Leu31, Pro34]PYY, BODIPY®FL-PYY(3–36) and BODIPY®FL-hPP were synthesized by one of us (PG) (Veyrat-Durebex et al., 2005). ((R)-N-[[4-(aminocarbonylaminomethyl)-phenyl]methyl]-N2-(diphenylacetyl)-argininamide trifluoro-acetate), known as BIBO3304, was provided by Boehringer Ingelheim (Germany), while homodimeric Ile–Glu–Pro–Dpr–Tyr–Arg–Leu–Arg–Tyr–CONH2 (firstly known as 1229U91, GW1229 and now GR231118) was a gift from Glaxo Wellcome (Research Triangle Park, NC, U.S.A.). BSA and Iodine-125 were obtained from MP Biochemical/ICN (Montreal, QC, Canada) and bacitracin was purchased from Sigma/Aldrich (St-Louis, MI, U.S.A.). Schleicher and Schuell #32 glass filters were obtained from VWR International (Montréal, QC, Canada). All tissue culture media, antibiotics and reagents were obtained from Gibco-BRL (Burlington, ON, Canada). The rat Y1, Y2, Y4 and Y5 receptor cDNA were generously provided by Dr H. Herzog (Sidney, Australia), The expression vectors, pcDNA3 and pTR5-DC-GFP/TK/hygro were obtained from Invitrogen (San Diego, CA, U.S.A.) and Dr Dick D. Moose (Biotechnology Institute, Montreal, QC, Canada), respectively. All other chemicals were of analytical grade and obtained from Fisher Scientific (Montreal, QC, Canada) or Sigma/Aldrich (St-Louis, MI, U.S.A.).
Radiolabelling of [Leu31,Pro34]PYY, PYY(3–36), hPP and GR231118 was performed using the chloramine T method as described previously (Dumont et al., 1995; Dumont & Quirion, 2000). The specific activity was assumed to be of theoretical value (2000 Ci mmol−1).
Results
The ability of various BODIPY®-conjugated NPY-related peptides to compete against NPY Y1, Y2, Y4 and Y5 receptors was evaluated in HEK293 cells transfected with the Y1, Y2, Y4 or Y5 receptor cDNA and in rat brain homogenates. As shown in Table 1, the addition of the chromophore BODIPY®TMR at the N-terminus of NPY and [Leu31, Pro34]NPY had little effect on the ability of BODIPY®TMR-NPY and BODIPY®TMR-[Leu31, Pro34]NPY to compete against specific [125I]GR231118 binding in a Y1 receptor-binding assay. In fact, these two fluorescent peptides competed against specific [125I]GR231118 binding with Ki values slightly higher or lower to those observed for the nonfluorescent probe in HEK293 cells transfected with the Y1 receptor cDNA: NPY (Ki=4.8 nM), BODIPY®TMR-NPY (Ki=15 nM), [Leu31, Pro34]NPY (Ki=2.4 nM) and BODIPY®TMR-[Leu31, Pro34]NPY (Ki=0.8 nM) (Table 1). The addition of BODIPY®FL at the N-terminus of [Leu31, Pro34]PYY resulted in a lower Ki value in this receptor-binding assay (Ki of 0.4 vs 6.1 nM for the fluorescent peptide) (Table 1). Additionally and as seen for the nonfluorescent corresponding peptides, BODIPY®FL-PYY(3–36), BODIPY®FL-hPP, BODIPY®TMR-[hPP(1–17), Ala31, Aib32]NPY and BODIPY®-TMR[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP were much less potent or inactive in competing against specific [125I]GR231118 binding in HEK293 cells transfected with the rat Y1 receptor cDNA (Table 1).
Table 1.
Competition-binding parameters of BODIPY®-labelled and native peptides of the NPY family in various NPY Y1 receptor assays
| Y1 HEK293-transfected cells | Rat brain Ki (nM) | ||
|---|---|---|---|
| Competitors | [125I]GR231118 Ki (nM) | [125I][Leu31, Pro34]PYY | [125I]GR231118 |
| NPY | 4.8±1 (5)a | 2.1±0.8 (5) | 48±10 (5) |
| BODIPY®TMR-NPY | 15±4 (4)* | 5.3±2.4 (4) | 85±25 (5) |
| [Leu31, Pro34]NPY | 2.4±0.6 (5) | 2.4±1.1 (5) | 37±6 (5) |
| BODIPY®TMR-[Leu31, Pro34]NPY | 0.8±0.3 (4)* | 2.9±0.7 (4) | 55±9 (5) |
| [Leu31, Pro34]PYY | 0.4±0.1 (5) | 2.3±1.1 (5) | 24±5 (5) |
| BODIPY®FL-[Leu31, Pro34]PYY | 6.1±1.5 (4) * | 35±5 (5)* | 73±15 (5)* |
| PYY(3–36) | 150±35 (3) | 140±40 (4) | >1000 (3) |
| BODIPY®FL-PYY(3–36) | 380±70 (3)* | 100±25 (3) | >1000 (2) |
| hPP | 180±65 (3) | 32±10 (5) | >1000 (3) |
| BODIPY®FL-hPP | >500 (3)* | >500 (3)* | >1000 (2) |
| [hPP(1–17), Ala31, Aib32]NPY | >1000 (2) | >1000 (4) | >1000 (3) |
| BODIPY®TMR-[hPP(1–17), Ala31, Aib32]NPY | >1000 (2) | >1000 (3) | >1000 (2) |
| [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP | >1000 (2) | >1000 (4) | >1000 (3) |
| BODIPY®TMR-[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP | >1000 (2) | >1000 (3) | >1000 (2) |
Data are the mean±s.e.m. of two to five determinations, each performed in triplicate.
Number in parenthesis represents the number of determinations. Ki represents the concentration of competitor needed to inhibit 50% specifically bound [125I][Leu31, Pro34]PYY or [125I]GR231118.
P<0.05, fluorescent vs nonfluorescent peptide.
The respective profiles of BODIPY®TMR-NPY, BODIPY®TMR-[Leu31, Pro34]NPY, BODIPY®FL-[Leu31, Pro34]PYY, BODIPY®FL-PYY(3–36), BODIPY®FL-hPP, BODIPY®TMR-[hPP(1–17), Ala31, Aib32]NPY and BODIPY®-TMR[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP in a Y2 receptor-binding assay using transfected HEK293 cells is shown in Table 2. The addition of BODIPY® in the N-terminal region of NPY and PYY(3–36) resulted in some changes in apparent affinities. NPY and PYY(3–36) competed against specific [125I]PYY(3–36) binding with Ki's of 3.8 and 0.6 nM, respectively, while BODIPY®TMR-NPY and BODIPY®FL-PYY(3–36) had a Ki of 10 nM (Table 2). As expected, fluorescent [Leu31, Pro34]NPY, [Leu31, Pro34]PYY, hPP, [hPP(1–17), Ala31, Aib32]NPY and [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP were much less potent in competing against specific [125I]PYY(3–36) binding, as also seen for the nonfluorescent homologues (Table 2).
Table 2.
Competition-binding parameters of BODIPY®-labelled and native peptides of the NPY family in various NPY Y2 receptor assays
| Y2 HEK293-transfected cells | Rat brain [125I]PYY(3–36) | |
|---|---|---|
| Competitors | Ki (nM) | Ki (nM) |
| NPY | 3.8±1.6 (3)a | 2.9±1.0 (5) |
| BODIPY®TMR-NPY | 10±4 (3)* | 16±6 (4)* |
| [Leu31, Pro34]NPY | >1000 (3) | 200±45 (5) |
| BODIPY®TMR-[Leu31, Pro34]NPY | >1000 (3) | >1000 (3) |
| [Leu31, Pro34]PYY | 650±110 (3) | 165±30 (5) |
| BODIPY®FL-[Leu31, Pro34]PYY | >1000 (3) | >1000 (3) |
| PYY(3–36) | 0.6±0.2 (3) | 1.2±0.3 (5) |
| BODIPY®FL-PYY(3–36) | 10±3 (3)* | 17±5 (4)* |
| hPP | >1000 (3) | >1000 (3) |
| BODIPY®FL-hPP | >1000 (3) | >1000 (2) |
| [hPP(1–17), Ala31, Aib32]NPY | >1000 (3) | >1000 (3) |
| BODIPY®TMR-[hPP(1–17), Ala31, Aib32]NPY | >1000 (3) | >1000 (2) |
| [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP | >1000 (3) | >1000 (3) |
| BODIPY®TMR-[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP | >1000 (3) | >1000 (2) |
Data are the mean±s.e.m. of two to five determinations, each performed in triplicate.
Number in parenthesis represents the number of determinations. Ki represents the concentration of competitor needed to inhibit 50% specifically bound [125I]PYY(3–36).
P<0.05, fluorescent vs nonfluorescent peptide.
BODIPY®FL-[Leu31, Pro34]PYY, BODIPY®FL-PYY(3–36) and BODIPY®FL-hPP were also tested in a Y4 receptor-binding assay. In Y4 receptor-transfected HEK293 cells, only [Leu31, Pro34]PYY, BODIPY®FL-[Leu31, Pro34]PYY, hPP and BODIPY®FL-hPP were able to compete against specific [125I]hPP binding with Ki values in the nM range, while NPY, PYY(3–36), BODIPY®TMR-NPY and BODIPY®FL-PYY(3–36) were much less potent (Table 3).
Table 3.
Competition-binding parameters of BODIPY®-labelled and native peptides of the NPY family in various NPY Y4 receptor assays
| Y4 HEK293-transfected cells | Rat brain [125I]hPP | |
|---|---|---|
| Competitors | Ki (nM) | Ki (nM) |
| NPY | 140±30 (3)a | 10.0±1.5 (5) |
| BODIPY®TMR-NPY | 875±180 (3)* | 56.0±12.0 (4)* |
| [Leu31, Pro34]PYY | 5.0±1 (3) | 2.0±0.6 (5) |
| BODIPY®FL-[Leu31, Pro34]PYY | 10±3 (3) | 1.0±0.4 (4) |
| PYY(3–36) | 350±120 (3) | 2.0±0.5 (5) |
| BODIPY®FL-PYY(3–36) | >1000 (3) | 2.0±0.3 (4) |
| hPP | 5.0±1 (3) | 0.6±0.2 (5) |
| BODIPY®FL-hPP | 10±3 (3) | 3.0±0.6 (4)* |
Data are the mean±s.e.m. of three to five determinations, each performed in triplicate.
Number in parenthesis represents the number of determinations. Ki represents the concentration of competitor needed to inhibit 50% specifically bound [125I]hPP.
P<0.05, fluorescent vs nonfluorescent peptide.
The ability of native and BODIPY®-conjugated peptide analogues to compete against specific [125I][Leu31, Pro34]PYY binding in HEK293 cells transfected with the rat Y5 receptor cDNA was evaluated next. [hPP(1–17), Ala31, Aib32]NPY, [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP and their BODIPY®-conjugated analogues were the most potent analogues to compete against specific [125I][Leu31, Pro34]PYY binding with Ki values in the low nM range (Table 4). Additionally, and as previously reported on the Y5 receptor subtype, NPY, [Leu31, Pro34]NPY, [Leu31, Pro34]PYY, PYY(3–36) and hPP were able to compete against specific [125I][Leu31, Pro34]PYY binding with Ki values between 1 and 25 nM (Table 4). The addition of BODIPY® at the N-terminus of these peptides had no or little effect on their ability to compete against specific [125I][Leu31, Pro34]PYY/Y5 binding, except for BODIPY®FL-hPP (Table 4).
Table 4.
Competition-binding parameters of BODIPY®-labelled and native peptides of the NPY family in various NPY Y5-like receptor assays
| Y5 HEK293-transfected cells | Rat brain | |
|---|---|---|
| Competitors | Ki (nM) | Ki (nM) |
| NPY | 1.3±0.4 (3)a | 4.0±1.9 (5) |
| BODIPY®TMR-NPY | 2.0±0.8 (3) | 2.9±1.5 (4) |
| [Leu31, Pro34]NPY | 5.2±0.3 (3) | 7.7±2.8 (5) |
| BODIPY®TMR-[Leu31, Pro34]NPY | 1.3±0.4 (3)* | 2.7±0.3 (4) |
| [Leu31, Pro34]PYY | 3.2±0.8 (3) | 3.5±0.9 (5) |
| BODIPY®FL-[Leu31, Pro34]PYY | 3.0±0.7 (3) | 4.0±1.0 (4) |
| PYY(3–36) | 25±6 (3) | 14±2 (5) |
| BODIPY®FL-PYY(3–36) | 30±5 (3) | 12.0±1.0 (4) |
| hPP | 1.6±0.4 (3) | 2.0±0.7 (5) |
| BODIPY®FL-hPP | 15±3 (3)* | 26±10 (4)* |
| [hPP(1–17), Ala31, Aib32]NPY | 0.6±0.2 (3) | 0.7±0.2 (5) |
| BODIPY®TMR-[hPP(1–17), Ala31, Aib32]NPY | 0.4±0.1 (3) | 0.6±0.1 (4) |
| [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP | 0.1±0.04 (3) | 0.15±0.03 (5) |
| BODIPY®TMR-[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP | 0.1±0.03 (3) | 0.2±0.1 (4) |
Data are the mean±s.e.m. of three to five determinations, each performed in triplicate.
Number in parenthesis represents the number of determinations. Ki represents the concentration of competitor needed to inhibit 50% specifically bound [125I][Leu31, Pro34]PYY in the presence of BIBO3304 in order to mask Y1 receptors.
P<0.05, fluorescent vs nonfluorescent peptide.
Using HEK293 cells transfected with the rat Y1, Y2 Y4 or Y5 receptor cDNA, the agonist property of BODIPY®-conjugated analogues was investigated next by measuring their ability to inhibit forskolin-induced cAMP accumulation. NPY inhibited forskolin-stimulated cAMP accumulation with EC50 values of 5, 10 and 19 nM in cells transfected with the rat Y1, Y2 or Y5 receptor cDNA, respectively (Table 5). The BODIPY®-conjugated analogue, BODIPY®TMR-NPY, was able to inhibit forskolin-stimulated cAMP accumulation with EC50 values of 40, 65 and 25 nM in Y1, Y2 and Y5 assays, respectively (Table 5). BODIPY®FL-[Leu31, Pro34]PYY displayed similar potency as the nonconjugated peptide to inhibit cAMP accumulation in Y5-transfected cells (Table 5), while being somewhat less potent in Y4 and Y1 assays (Table 5). BODIPY®FL-PYY(3–36) was less potent than PYY(3–36) in cells transfected with the rat Y2 receptor cDNA to inhibit forskolin-induced cAMP production (Table 5), while it had a similar potency in a Y5 assay (Table 5). BODIPY®-hPP was somewhat less potent than hPP to inhibit forskolin-induced cAMP accumulation in Y4- and Y5-transfected cells, while conjugated BODIPY®TMR Y5 agonists such as BODIPY®TMR-[hPP(1–17), Ala31, Aib32]NPY and BODIPY®TMR-[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP were as potent as their homologues in cells transfected with the rat Y5 receptor cDNA (Table 5). All fluorescent peptides tested in HEK293 cells transfected with either the rat Y1, Y2, Y4 or Y5 receptor cDNA were able to inhibit forskolin-induced cAMP accumulation at maximal levels similar to those observed for the nonfluorescent homologues (data not shown). In fact, all BODIPY®TMR and BODIPY®FL peptides maintained their full agonist properties.
Table 5.
Potencies of various BODIPY®-labelled and native peptides of the NPY family in HEK293 cells transfected with either of the rat Y1, Y2, Y4 or Y5 receptor cDNA to inhibit forskolin-induced cAMP accumulation
| cAMP assay in HEK293-transfected cells (EC50) (nM) | ||||
|---|---|---|---|---|
| Peptides | Y1 | Y2 | Y4 | Y5 |
| NPY | 5±1 (3)a | 10±2 (3) | ND | 19±2 (3) |
| BODIPY®TMR-NPY | 40±5 (3)* | 65±15 (3)* | ND | 25±3 (3) |
| [Leu31, Pro34]NPY | ND | ND | ND | 30±5 (3) |
| BODIPY®TMR-[Leu31, Pro34]NPY | ND | ND | ND | 20±4 (3) |
| [Leu31, Pro34]PYY | 3.0±0.6 (3) | ND | 35±4 (3) | 15±4 (3) |
| BODIPY®-FL[Leu31, Pro34]PYY | 32±8 (3)* | ND | 80±16 (3)* | 12±3 (3) |
| PYY(3–36) | ND | 7±2 (3) | ND | 50±6 (3) |
| BODIPY®FL-PYY(3–36) | ND | 45±9 (3)* | ND | 60±8 (3) |
| hPP | ND | ND | 7±2 (3) | 9±3 (3) |
| BODIPY®FL-hPP | ND | ND | 15±3 (3) | 60±15 (3)* |
| [hPP(1–17), Ala31, Aib32]NPY | ND | ND | ND | 35±4 (3) |
| BODIPY®TMR-[hPP(1–17), Ala31, Aib32]NPY | ND | ND | ND | 40±5 (3) |
| [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP | ND | ND | ND | 22±3 (3) |
| BODIPY®TMR-[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP | ND | ND | ND | 30±5 (3) |
Data are the mean±s.e.m. of three determinations, each performed in duplicate.
Number in parenthesis represents the number of determinations. EC50 represents the concentration of agonist needed to inhibit 50% of cAMP accumulation induced by forskolin. ND, not determined.
P<0.05, fluorescent vs nonfluorescent peptide.
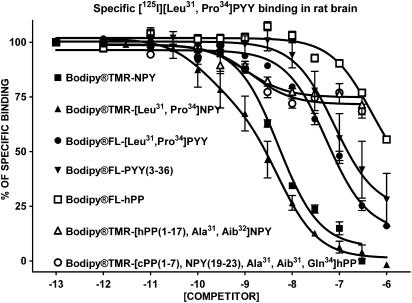
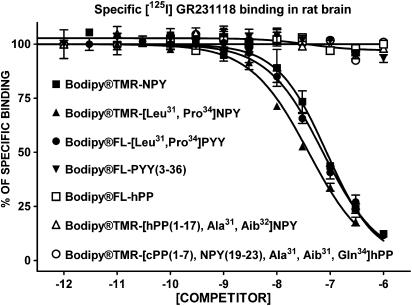
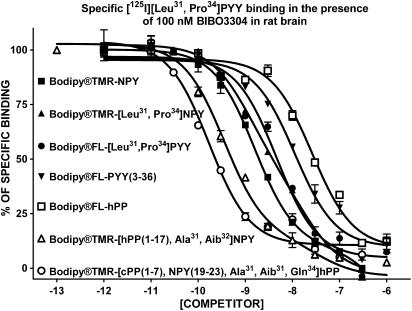
The apparent affinity of native and BODIPY®-conjugated peptides for specific [125I][Leu31, Pro34]PYY (labelling Y1, Y4 and Y5 subtypes), [125I]GR231118 (Y1 and Y4 subtypes), [125I]PYY(3–36) (Y2 and Y5 subtypes), [125I]hPP (Y4 and Y5 subtypes) and [125I][Leu31, Pro34]PYY in the presence of the Y1 blocker BIBO3304 (Y4 and Y5 subtypes) binding in rat brain homogenates was investigated next. As shown in Figure 1, BODIPY®TMR-NPY and BODIPY®TMR-[Leu31, Pro34]NPY are the most potent competitors against specific [125I][Leu31, Pro34]PYY binding in rat brain homogenates. On the other hand, and as previously reported for Y5-related molecules such as CGP71683A (Dumont et al., 2000a) and the Y5 peptide agonists [hPP(1–17), Ala31, Aib32]NPY and [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP (Dumont et al., 2005), BODIPY®TMR-conjugated Y5 peptides were able to compete for only 30% of specific sites recognized by [125I][Leu31, Pro34]PYY in rat brain membrane homogenates (Figure 1). We also evaluated the competition-binding profile of these peptides against specific [125I]GR231118 binding, another Y1 receptor-binding assay (Dumont & Quirion, 2000). GR231118 having Y1 antagonist properties, it can recognize Y1 receptors in various affinity states, contrasting with the radiolabelled agonist probe [125I][Leu31, Pro34]PYY, which mostly binds to the high, affinity state (Dumont & Quirion, 2000). Under these binding assay conditions, BODIPY®TMR-NPY, BODIPY®TMR-[Leu31, Pro34]NPY and BODIPY®FL-[Leu31, Pro34]PYY were the only analogues that were able to compete against specific [125I]]GR231118 binding in rat brain (Figure 2) but with Ki values 3–20 times lower than those observed when using [125I][Leu31, Pro34]PYY (Table 1). All other peptides, including native and conjugated BODIPY® peptides such as PYY(3–36), hPP, [hPP(1–17), Ala31, Aib32]NPY and [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP, were basically inactive in this assay (Figure 2 and Table 1).
Figure 1.
Competition-binding profiles of BODIPY®-labelled peptides against specific [125I][Leu31,Pro34]PYY-binding sites (Y1-, Y4- and Y5-like). Data are the mean±s.e.m. of two to five determinations, each performed in triplicate.
Figure 2.
Competition-binding profiles of BODIPY®-labelled peptides against specific [125I]GR231118-binding sites (Y1-like). Data are the mean±s.e.m. of two to five determinations, each performed in triplicate.
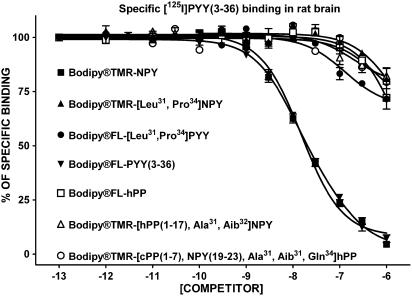
In a Y2 receptor-binding assay using rat brain membrane homogenates, adding a BODIPY® in the N-terminal region of NPY and PYY(3–36) resulted in lower Ki values (Figure 1 and Table 2) in accordance with data obtained in HEK293-Y2 receptor-transfected cells (Table 2). NPY and PYY(3–36) competed against specific [125I]PYY(3–36) binding with Ki's of 2.9 and 1.2 nM, respectively, while BODIPY®TMR-NPY and BODIPY®FL-PYY(3–36) had values of 16 and 17 nM, in rat brain homogenates (Table 2). Fluorescent [Leu31, Pro34]NPY, [Leu31, Pro34]PYY, hPP, [hPP(1–17), Ala31, Aib32]NPY and [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP were mostly inactive, as well as the nonfluorescent homologues in competing against specific [125I]PYY(3–36) binding in rat brain homogenates (Figure 3 and Table 2).
Figure 3.
Competition-binding profiles of BODIPY®-labelled peptides against specific [125I]PYY(3–36)-binding sites (Y2-like). Data are the mean±s.e.m. of two to five determinations, each performed in triplicate.
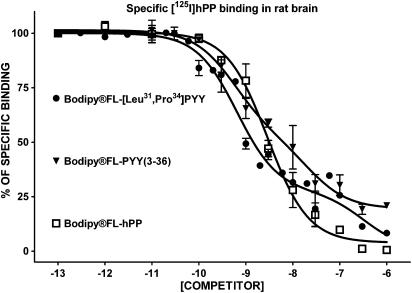
The addition of BODIPY® in the N-terminal region did not induce major changes in the affinity of various analogues to compete against specific [125I]hPP binding in rat brain membrane homogenates (Figure 4 and Table 3). Native and fluorescent peptides including BODIPY®FL-[Leu31, Pro34]PYY, BODIPY®FL-PYY(3–36) and BODIPY®FL-hPP at concentrations of 1–3 nM inhibited 50% of specific [125I]hPP binding in this preparation (Table 3) while NPY and BODIPY®TMR-NPY were able to compete against specific [125I]hPP binding with Ki values of 10 and 56 nM, respectively (Table 3). The competition-binding curves clearly revealed that [125I]hPP recognizes a heterogeneous population of sites in the rat brain (Figure 4), in accordance with earlier results (Dumont et al., 2005).
Figure 4.
Competition-binding profiles of BODIPY®-labelled peptides against specific [125I]hPP-binding sites (Y4- and Y5-like). Data are the mean±s.e.m. of two to five determinations, each performed in triplicate.
The ability of native and BODIPY®-conjugated peptide analogues to compete against specific [125I][Leu31, Pro34]PYY binding resistant to a saturating concentration of BIBO3304 was determined next. This assay condition was reported to represent binding to the Y5 receptor subtype in rat brain membrane homogenates (Dumont et al., 1998). [hPP(1–17), Ala31, Aib32]NPY, [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP and their BODIPY®-conjugated analogues were the most potent analogues competing against specific [125I][Leu31, Pro34]PYY/BIBO3304-insensitive sites in rat brain membrane homogenates, with Ki values of 0.15–0.7 nM (Table 4). Additionally, all analogues having significant Y5 receptor affinity, such as NPY, [Leu31, Pro34]NPY, [Leu31, Pro34]PYY, PYY(3–36) and hPP, were able to compete against specific [125I][Leu31, Pro34]PYY/BIBO3304-insensitive sites, with Ki values between 2 and 12 nM (Figure 5 and Table 4). The addition of BODIPY® at the N-terminus of these peptides had no or little effect on their apparent affinity, except for BODIPY®FL-hPP (Table 4).
Figure 5.
Competition-binding profiles of BODIPY®-labelled peptides against specific [125I][Leu31,Pro34]PYY/BIBO3304-insensitive binding sites (Y4- and Y5-like). Data are the mean±s.e.m. of two to five determinations, each performed in triplicate.
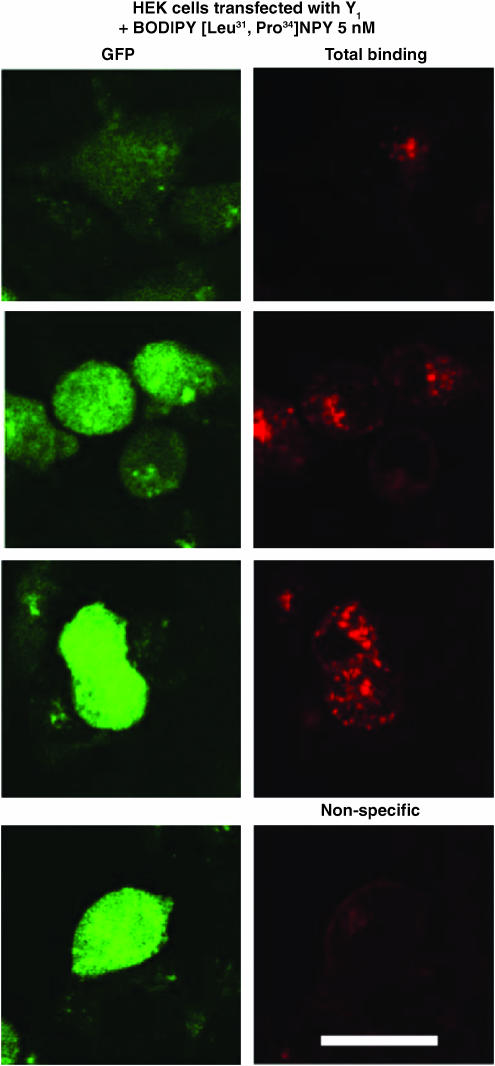
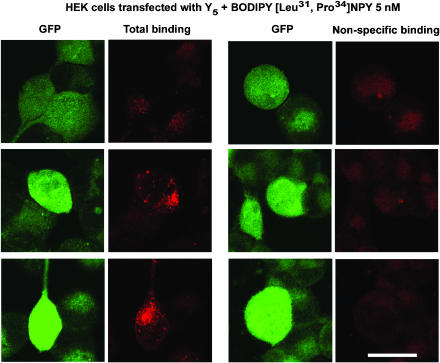
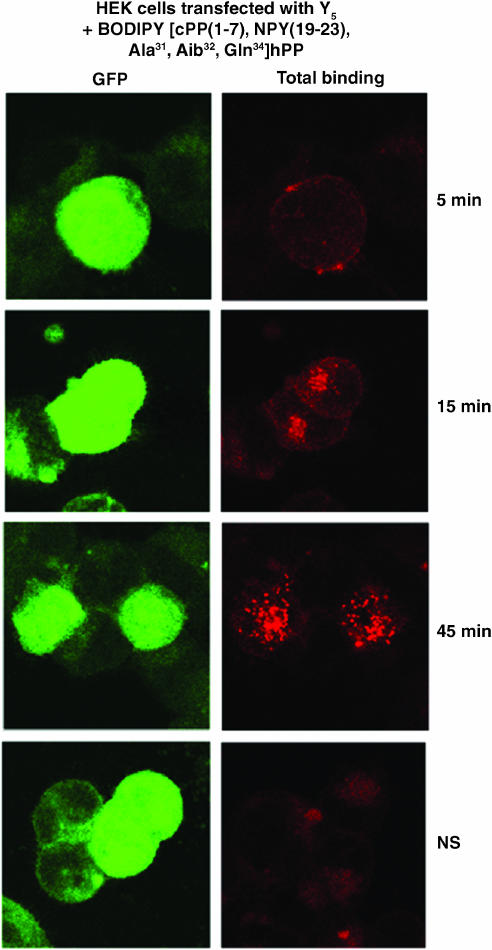
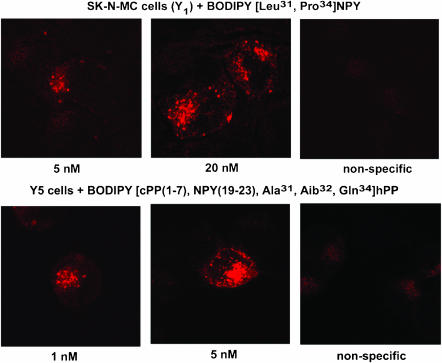
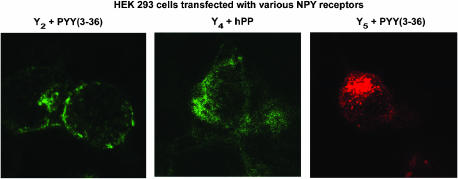
Finally, we investigated the potential usefulness of these fluorescent agonists to visualize NPY receptors in HEK293 cells transfected with the rat Y1, Y2, Y4 or Y5 receptor cDNA, as well as in SK-N-MC cells. HEK293 cells were transfected with the pTR5-DC-GFP/TK/hygro vector bearing the Y1 or Y5 receptor cDNA. This construct will then express both GFP and the receptor proteins. As shown in Figures 6 and 7, GFP expression varied from cell to cell. Similarly, HEK293 cells transfected with the Y1 or Y5 receptor cDNA in the presence of 5 nM BODIPY®TMR-[Leu31, Pro34]NPY for 45 min at 37°C show that levels of Y1 (Figure 6) and Y5 (Figure 7) receptor expression vary between cells. In fact, cells expressing low levels of GFP (green) show lower levels of signal detected with the fluorescent probe (red), while high levels of GFP expression are associated with strong BODIPY®TMR-[Leu31, Pro34]NPY signals (Figures 6 and 7). BODIPY®TMR-[Leu31, Pro34]NPY signal disappeared in the presence of 1 μM [Leu31, Pro34]NPY (Figures 6 and 7). Time dependency was investigated next, as previously reported for Y1-transfected HEK293 cells (Pheng et al., 2003). HEK293 cells transfected with the Y5 receptor cDNA and expressing similar levels of Y5 receptors (as shown by GFP expression, Figure 8; green signal) revealed that signals obtained with 5 nM BODIPY®TMR-[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP increased over time (Figure 8; red signal). Cells incubated at 37°C in the presence of fluorescent probes for 5 min show that BODIPY®TMR-[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP fluorescent signals are mostly observed at the surface, with limited signal seen intracellularly. Longer incubation time leads to increased fluorescent signals (Figure 8). Signal disappeared in the presence of 1 μM [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP, demonstrating the specificity of the fluorescent signal observed with this probe (Figure 8). Additionally, in HEK293 cells transfected with the rat Y1 or Y5 receptor cDNA, fluorescence is mostly seen intracellularly after a 45-min incubation, suggesting that BODIPY®TMR-[Leu31, Pro34]NPY (Figures 6 and 7) and BODIPY®TMR-[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP (Figure 8) are internalized. In SK-N-MC/Y1-expressing cells, the fluorescent signal is clearly concentration-dependent. SK-N-MC cells incubated with 5 nM BODIPY®TMR-[Leu31, Pro34]NPY for 45 min at 37°C demonstrated a lower signal than cells incubated with 20 nM BODIPY®TMR-[Leu31, Pro34]NPY (Figure 9). Similar results were obtained using 1 and 5 nM BODIPY®TMR-[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP in HEK293 cells transfected with the rat Y5 receptor cDNA (Figure 9). Positive fluorescent signals were also observed in HEK293 cells transfected with the rat Y2, Y4 or Y5 receptor cDNA incubated with 20 nM BODIPY®FL-PYY(3–36), BODIPY®FL-hPP or BODIPY®TMR-PYY(3–36) for 45 min at 37°C, respectively (Figure 10). As control, nontransfected HEK293 cells incubated in the presence of fluorescent peptides failed to reveal any significant fluorescent signal (data not shown), confirming the absence of endogenously expressed NPY receptors in those cells and the specificity of the probes under study. In cells expressing Y1 (Figures 6 and 9) and Y5 (Figures 7 and 8 and 9) receptors, a punctate pattern of fluorescence was observed and distributed throughout the cytoplasm, the nucleus being negative. This pattern may be different in HEK293 cells transfected with the rat Y2 and Y4 receptor cDNA (Figure 10). For example, in Y2-transfected cells most of the fluorescent signal is seen at the level of the plasma membrane (Figure 10), while in Y4 transfected cells the signal is more diffuse (Figure 10).
Figure 6.
Visualization of BODIPY®TMR-[Leu31, Pro34]NPY in HEK293 cells expressing the rat Y1 receptor incubated with 5 nM BODIPY®TMR-[Leu31, Pro34]NPY for 45 min at 37°C. Nonspecific binding represents signal obtained in the presence of 1 μM [Leu31, Pro34]NPY incubated under the same conditions with the fluorescent probe. The peptide is represented in red following HeNe laser (excitation 543 nm/emission 580 nm), and GFP-positive cells expressing low, moderate and high levels of GFP are represented in green following argon laser (excitation 488 nm/emission 510 nm). Scale bar 10 μm. All images were taken using the same setting.
Figure 7.
Visualization of BODIPY®TMR-[Leu31, Pro34]NPY in HEK293 cells expressing the rat Y5 receptor incubated with 5 nM BODIPY®TMR-[Leu31, Pro34]NPY for 45 min at 37°C. Nonspecific binding represents signal obtained in the presence of 1 μM [Leu31, Pro34]NPY incubated under the same conditions with the fluorescent probe. The peptide is represented in red following HeNe laser (excitation 543 nm/emission 580 nm), and GFP-positive cells expressing low, moderate and high levels of GFP are represented in green following argon laser (excitation 488 nm/emission 510 nm). Scale bar 10 μm. All images were taken using the same setting.
Figure 8.
Visualization of BODIPY®TMR-[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP in HEK293 cells expressing the rat Y5 receptor incubated for 5, 15 and 45 min at 37°C. Nonspecific binding represents signal obtained in the presence of 1 μM [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP incubated for 45 min with the fluorescent probe. The peptide is represented in red following HeNe laser (excitation 543 nm/emission 580 nm), and GFP-positive cells are represented in green following argon laser (excitation 488 nm/emission 510 nm). Scale bar 10 μm. All images were taken using the same setting.
Figure 9.
Visualization of 5 and 20 nM BODIPY®TMR-[Leu31, Pro34]NPY in SK-N-MC cells and 1 and 5 nM BODIPY®TMR-[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP in HEK293 cells expressing the rat Y5 receptor. Cells were incubated with fluorescent probe for 45 min at 37°C. Nonspecific binding represents signal obtained in the presence of 1 μM native peptide incubated with the highest concentration of the fluorescent probe. Scale bar 10 μm. All images were taken using the same setting.
Figure 10.
Visualization of BODIPY®FL-PYY(3–36), BODIPY®FL-hPP and BODIPY®TMR-PYY(3–36) in HEK293 cells expressing the rat Y2, Y4 or Y5 receptor cDNA, respectively. Y2-expressing cells were incubated with 20 nM BODIPY®FL-PYY(3–36) (excitation 488 nm/emission 510 nm), Y4 cells were incubated with 20 nM BODIPY®FL-hPP (excitation 488 nm/emission 510 nm), and Y5 cells with 5 nM BODIPY®TMR-PYY(3–36) (excitation 543 nm/emission 580 nm) for 45 min at 37°C. Nonspecific binding represents signal obtained in the presence of 1 μM native peptide incubated under the same conditions with the fluorescent probe. Scale bar 10 μm. All images were taken using the same setting.
Discussion
In the present study, we investigated the ability of several BODIPY®-labelled analogues of the NPY family to bind and activate the Y1, Y2, Y4 or Y5 receptor subtypes. Receptor-binding assays were performed in HEK293 cells transfected with either the rat Y1, Y2, Y4 or Y5 receptor cDNA as well as in rat brain membrane homogenates. Agonist properties were evaluated by measuring the capacity of these analogues to inhibit forskolin-induced cAMP production in transfected cells. As expected, BODIPY®TMR-[Leu31, Pro34]NPY and BODIPY®FL-[Leu31, Pro34]PYY were almost inactive on the Y2 subtype (as for the nonfluorescent peptides), while they maintained their ability to bind and stimulate the Y1, Y4 and Y5 receptors. BODIPY®FL-PYY(3–36) was found to be devoid of activity on the Y1 and Y4 receptor subtypes, but retained its affinity and activity for the Y2 and Y5 receptors. These data are identical to those reported for [Leu31, Pro34]NPY/PYY and PYY(3–36) (Michel et al., 1998). Furthermore and as seen for hPP, BODIPY®FL-hPP conserved similar characteristics as those reported for the nonconjugated peptide (Walker et al., 1997; Dumont et al., 1998). BODIPY®FL-hPP behaves as a potent agonist on the Y4 and Y5 receptors, while having much lower affinities for the Y1 and Y2 receptor subtypes. The addition of the fluorochome BODIPY®TMR in the N-terminal region of Y5 receptor agonists, such as [hPP(1–17), Ala31, Aib32]NPY and [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP (Cabrele et al., 2000; 2001), generated fluorescent probes that maintained their affinities and agonist activities for the Y5 subtype. Overall, conjugated BODIPY®FL and BODIPY®TMR fluorescent probes had comparatively similar or slightly lower affinities and potencies than parent peptides. They also conserved their ligand selectivity profile for the various NPY receptor subtypes. Moreover, these probes can be used to visualize NPY receptor subtypes as demonstrated here in HEK293 cells transfected with NPY receptors and SK-N-MC cells endogenously expressing Y1 receptors.
Many attempts have been made to conjugate fluorescent molecules to receptor ligands, in order to develop better tools to investigate ligand–receptor interaction, localization as well as internalization and cellular trafficking processes. One of the major issues is to ensure that fluorescent ligands bound to the receptor will not dissociate or diffuse from the binding site. One approach is to use fluorescent probes that possess very high affinity for the receptor under study (McGrath et al., 1996). Several chromophores are presently available and each one has different properties, including charge, hydrophobicity and size, which might have an impact on the affinity, potency and agonist or antagonist properties (McGrath et al., 1996).
Various studies have demonstrated that fluorescent ligands are useful probes to study peptide ligand–receptor interaction using, for example, N-alpha-fluoresceinyl thiocarbamyl (FTC)-[Glu1]neurotensin (Faure et al., 1994); BODIPY®FL, BODIPY®TR or Alexa Fluor 488 opioid peptides (Gaudriault et al., 1997; Arttamangkul et al., 2000); N-hydroxy-succinide-fluorescein, BODIPY®FL or BODIPY® 576/589 somatostatin (Nouel et al., 1997); Alexa 488, BODIPY®FL, fluorescein, Oregon Green 488 or tetramethylrhodamine-conjugated substance P (Bennett & Simmons, 2001) and 5-carboxyfluorescein, succinimidyl ester growth hormone-releasing hormone (Veyrat-Durebex et al., 2005). Studies that have evaluated different fluorescent dyes have generally shown that the BODIPY® dye represents one of the most appropriate fluorescent probes resulting in a fluorescent ligand that usually conserved its pharmacological properties (Arttamangkul et al., 2000; Bennett & Simmons, 2001). Other advantages of BODIPY® dyes include very high fluorescent yields, pH insensitivity and long commercially available wavelengths. The conjugation of BODIPY® moieties to peptides generally occurs via the formation of an amide bond at either N-α- or N-ɛ-amino groups or less frequently at a modified C-terminus (Gaudriault et al., 1997). For NPY, it has been reported that the C-terminal region is highly critical for receptor recognition and activation (Schwartz et al., 1990; Beck-Sickinger et al., 1994). Additionally, the C-terminus moiety is identical for NPY, PYY and PPs in all mammals (Larhammar, 1996), suggesting that the C-terminus tyrosine amide moiety may bind in a pocket formed by hydrophobic amino acids of transmembrane domains 1, 2, 6 and 7 at least for the Y1 receptor subtype (Walker et al., 1994). Therefore, the conjugation of BODIPY® dyes was performed under controlled conditions to favour N-terminus acylation (Veyrat-Durebex et al., 2005).
A critical issue was to establish whether the addition of the BODIPY® moiety at the N-terminus region of various NPY analogues will affect their ligand selectivity profile, affinity and agonist properties. Our results demonstrated that BODIPY®TMR-NPY was able to compete against specific Y1, Y2 and Y5 sites with similar or slightly lower affinity as compared to the nonfluorescent peptide. Moreover, BODIPY®TMR-NPY was able to inhibit forskolin-stimulated cAMP accumulation, demonstrating that the addition of a BODIPY® moiety in the N-terminal region did not affect its agonist properties. Additionally, and as seen for [Leu31, Pro34]NPY and [Leu31, Pro34]PYY (Michel et al., 1998; Dumont et al., 2002), BODIPY®TMR-[Leu31, Pro34]NPY and BODIPY®FL-[Leu31, Pro34]PYY were potent competitors in Y1, Y4 and Y5 receptor-binding assays, but not in Y2 assays. These analogues were also able to inhibit forskolin-induced cAMP accumulation with similar or slightly lower potency as compared to the nonfluorescent analogues. Competition-binding experiments have also shown that BODIPY®FL-PYY(3–36) was able to compete with high affinity for Y2 and Y5, but not for Y1 and Y4 sites. This profile is highly similar to that reported for PYY(3–36) itself (Michel et al., 1998; Dumont et al., 2002). BODIPY®FL-PYY(3–36) retained its agonist property, as demonstrated by its ability to inhibit forskolin-induced cAMP accumulation. The PP analogue BODIPY®FL-hPP displayed similar properties as the nonfluorescent peptide. Indeed, BODIPY®FL-hPP was a potent competitor in Y4 and Y5 receptor-binding assays, while being almost inactive in Y1 and Y2 assays. Finally, the selective Y5 receptor agonists [hPP(1–17), Ala31, Aib32]NPY, [cPP(1–7) and NPY(19–23), Ala31, Aib32, Gln34]hPP conjugated with BODIPY®TMR were as potent as the nonfluorescent analogues in Y5 receptor-binding and functional assays, while being much less potent in Y1, Y2 and Y4 assays (Cabrele et al., 2000; 2001). These data clearly demonstrate that BODIPY® analogues of NPY, [Leu31, Pro34]NPY, [Leu31, Pro34]PYY, PYY(3–36), hPP, [hPP(1–17), Ala31, Aib32]NPY and [cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP retained their selectivity profile, affinity and agonist activities for the various classes of NPY receptors under study. Moreover, receptor-binding assays demonstrated that BODIPY®-conjugated NPY analogues can be used to tag NPY receptors in rat brain homogenates, a tissue that expresses heterogeneous populations of NPY sites (Dumont et al., 2004; 2005). The fact that the rat brain expresses Y1, Y2, Y4, Y5 and possibly other not fully characterized NPY receptors (Dumont et al., 2005) could explain apparent differences in Ki values obtained in HEK293-transfected cells as compared with those observed in rat brain homogenates for the Y1 and Y4 receptors. Furthermore, G-protein-coupled receptors, including all NPY receptor subtypes, exist under multiple affinity states (Perez & Karnik, 2005). It is well known that certain agonists are able to distinguish between the affinity states of a given receptor subtype, while antagonists often bind with similar affinity to various receptor conformations. Additionally, the proportion of receptors in a given affinity state may be different from cell to cell, and from tissue to tissue. This could also explain differences observed in Ki values, especially for those obtained using, for example, [125I][Leu31,Pro34]PYY (agonist) and [125I]GR231118 (antagonist or partial agonist), since at concentrations of radioligands used in the present study, the agonist will mostly bind to the high-affinity state, while a radiolabelled antagonist may target most affinity states.
These various fluorescent probes can be used to visualize NPY receptors in transfected cells or cell lines endogenously expressing a specific receptor subtype. Previous studies in HEK293 cells transfected with the rat Y1 receptor cDNA have demonstrated that in cells incubated with sucrose or phenylarsine oxide prior to the addition of BODIPY®, TMR-[Leu31, Pro34]NPY resulted in the inhibition of the internalization of the probe, most of the fluorescent signals remaining at the plasma membrane (Pheng et al., 2003). Furthermore, receptor internalization processes have been investigated for the Y1, Y2, Y4 and Y5 receptor subtypes using either radiolabelled probes or FRET techniques (Fabry et al., 2000; Parker et al., 2002a, 2002b; 2003; Berglund et al., 2003b; Pheng et al., 2003). Overall, these results have shown that Y1 and Y5 receptors are rapidly internalized, while Y4 receptors are internalized at a slower rate and the Y2 subtype does not or is only very slowly internalized. Moreover, HEK293 cells transfected with EGFP-Y1 receptors revealed that, at time 0, fluorescent receptors are located at the cell surface with the fluorescent signal increasing in the cytoplasm, in a time-dependent manner, when cells are incubated in the presence of NPY or PYY (Gicquiaux et al., 2002). In the present study, we have shown that levels of NPY receptor protein expression varied from cell to cell in HEK293-transfected cells with the Y1 or Y5 receptor cDNA, as observed by GFP expression and BODIPY-peptide signals. These data are in accordance to those reported for HEK293 cells transfected with the rat Y1 receptor cDNA (Tong et al., 1995). Additionally, signals obtained with fluorescent probes are time- and concentration-dependent. Furthermore, after 45 min in the presence of fluorescent probe, most of the fluorescent signal was found inside cells incubated with BODIPY®TMR-[Leu31, Pro34]NPY in Y1-transfected and endogenously expressing (SK-N-MC cells) cells, as well as with BODIPY®TMR-[Leu31, Pro34]NPY, BODIPY®TMR-[cPP(1–7), NPY(19–23), Ala31, Aib32, Gln34]hPP and BODIPY®TMR-PYY(3–36) in Y5-transfected cells, suggesting that these fluorescent probes are internalized, in agreement with previous studies on the internalization of Y1 and Y5 receptors (Fabry et al., 2000; Parker et al., 2002a, 2002b, 2003; Berglund et al., 2003b; Pheng et al., 2003).
In summary, BODIPY® can be conjugated to the N-terminus of various NPY, PYY and PP analogues without inducing significant loss of affinity, agonist properties and receptor selectivity. These tools should prove most useful to investigate in detail NPY receptor pharmacokinetics in living neuronal and non-neuronal cells.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research (CIHR) to R. Quirion, P. Gaudreau and A. Fournier. P. Gaudreau and A. Fournier are recipients of a scholarship ‘Chercheur-boursier national' from ‘Fonds de la Recherche en Santé du Québec'.
Abbreviations
- BIBO3304
(R)-N-[[4-(aminocarbonylaminomethyl)-phenyl]methyl]-N2-(diphenylacetyl)-argininamide trifluoroacetate
- BSA
bovine serum albumin
- GR231118
homodimeric Ile–Glu–Pro–Dpr–Tyr–Arg–Leu–Arg–Tyr–CONH2
- HEK293
human embryonic kidney cells
- hPP
human pancreatic polypeptide
- NPY
neuropeptide Y
- PYY
peptide YY
References
- ARTTAMANGKUL S., ALVAREZ-MAUBECIN V., THOMAS G., WILLIAMS J.T., GRANDY D.K. Binding and internalization of fluorescent opioid peptide conjugates in living cells. Mol. Pharmacol. 2000;58:1570–1580. doi: 10.1124/mol.58.6.1570. [DOI] [PubMed] [Google Scholar]
- BARD J.A., WALKER M.W., BRANCHEK T.A., WEINSHANK R.L. Cloning and functional expression of a human Y4 subtype receptor for pancreatic polypeptide, neuropeptide Y, and peptide YY. J. Biol. Chem. 1995;270:26762–26765. doi: 10.1074/jbc.270.45.26762. [DOI] [PubMed] [Google Scholar]
- BECK-SICKINGER A.G., WIELAND H.A., WITTNEBEN H., WILLIM K.D., RUDOLF K., JUNG G. Complete L-alanine scan of neuropeptide Y reveals ligands binding to Y1 and Y2 receptors with distinguished conformations. Eur. J. Biochem. 1994;225:947–958. doi: 10.1111/j.1432-1033.1994.0947b.x. [DOI] [PubMed] [Google Scholar]
- BENNETT V.J., SIMMONS M.A. Analysis of fluorescently labeled substance P analogs: binding, imaging and receptor activation. BMC. Chem. Biol. 2001;1:1. doi: 10.1186/1472-6769-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGLUND M.M., HIPSKIND P.A., GEHLERT D.R. Recent developments in our understanding of the physiological role of PP-fold peptide receptor subtypes. Exp. Biol. Med. (Maywood.). 2003a;228:217–244. doi: 10.1177/153537020322800301. [DOI] [PubMed] [Google Scholar]
- BERGLUND M.M., SCHOBER D.A., STATNICK M.A., MCDONALD P.H., GEHLERT D.R. The use of bioluminescence resonance energy transfer (BRET2) to study neuropeptide Y receptor agonist in. J. Pharmacol. Exp. Ther. 2003b;306:147–156. doi: 10.1124/jpet.103.051227. [DOI] [PubMed] [Google Scholar]
- CABRELE C., LANGER M., BADER R., WIELAND H.A., DOODS H.N., ZERBE O., BECK-SICKINGER A.G. The first selective agonist for the neuropeptide Y Y5 receptor increases food intake in rats. J. Biol. Chem. 2000;275:36043–36048. doi: 10.1074/jbc.M000626200. [DOI] [PubMed] [Google Scholar]
- CABRELE C., WIELAND H.A., LANGER M., STIDSEN C.E., BECK-SICKINGER A.G. Y-receptor affinity modulation by the design of pancreatic polypeptide/neuropeptide Y chimera led to Y5-receptor ligands with picomolar affinity. Peptides. 2001;22:365–378. doi: 10.1016/s0196-9781(01)00339-4. [DOI] [PubMed] [Google Scholar]
- CARVAJAL C., DUMONT Y., QUIRION R.Neuropeptide Y: role in emotion and alcohol dependence Curr. Drug Targets CNS Neurol. Disord 2005(in press) [DOI] [PubMed]
- CHENG Y., PRUSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CHRONWALL B.M., DIMAGGIO D.A., MASSARI V.J., PICKEL V.M., RUGGIERO D.A., O'DONOHUE T.L. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience. 1985;15:1159–1181. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- DE QUIDT M.E., EMSON P.C. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system – I. Radioimmunoassay and chromatographic characterisation. Neuroscience. 1986;18:527–543. doi: 10.1016/0306-4522(86)90056-4. [DOI] [PubMed] [Google Scholar]
- DUMONT Y., CADIEUX A., DOODS H., FOURNIER A., QUIRION R. Potent and selective tools to investigate neuropeptide Y receptors in the central and peripheral nervous systems: BIB03304 (Y1) and CGP71683A (Y5) Can. J. Physiol. Pharmacol. 2000a;78:116–125. [PubMed] [Google Scholar]
- DUMONT Y., CHABOT J.G., QUIRION R. Receptor autoradiography as mean to explore the possible functional relevance of neuropeptides: Focus on new agonists and antagonists to study natriuretic peptides, neuropeptide Y and calcitonin gene-related peptides. Peptides. 2004;25:365–391. doi: 10.1016/j.peptides.2004.01.013. [DOI] [PubMed] [Google Scholar]
- DUMONT Y., FOURNIER A., QUIRION R. Expression and characterization of the neuropeptide Y Y5 receptor subtype in the rat brain. J. Neurosci. 1998;18:5565–5574. doi: 10.1523/JNEUROSCI.18-15-05565.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMONT Y., FOURNIER A., ST PIERRE S., QUIRION R. Characterization of neuropeptide Y binding sites in rat brain membrane preparations using [125I][Leu31, Pro34]peptide YY and [125I]peptide YY3–36 as selective Y1 and Y2 radioligands. J. Pharmacol. Exp. Ther. 1995;272:673–680. [PubMed] [Google Scholar]
- DUMONT Y., JACQUES D., ST PIERRE J.A., TONG Y., PARKER R., HERZOG H., QUIRION R.Neuropeptide Y, peptide YY and pancreatic polypeptide receptor proteins and mRNAs in mammalian brains Handbook of Chemical Neuroanatomy, Vol 16 Peptide Receptors, Part 1 2000bLondon, U.K.: Elsevier; 375–475.ed. Quirion, R., Bjorklund, A. & Hokfelt, T. [Google Scholar]
- DUMONT Y., MOYSE E., FOURNIER A., QUIRION R. Evidence for the existence of an additional class of neuropeptide Y receptor sites in the rat brain. J. Pharmacol. Exp. Ther. 2005;315:99–108. doi: 10.1124/jpet.105.089300. [DOI] [PubMed] [Google Scholar]
- DUMONT Y., QUIRION R. 125I]- GR231118: a high affinity radioligand to investigate neuropeptide Y Y1 and Y4 receptors. Br. J. Pharmacol. 2000;129:37–46. doi: 10.1038/sj.bjp.0702983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMONT Y., REDROBE J.P., QUIRION R.Neuropeptide Y receptors Understanding G Protein-Coupled Receptors and their Role in the CNS 2002Oxford, U.K.: Oxford University Press; 372–401.ed. Pangalos, M.N. & Davies, C.H. [Google Scholar]
- FABRY M., LANGER M., ROTHEN-RUTISHAUSER B., WUNDERLI-ALLENSPACH H., HOCKER H., BECK-SICKINGER A.G. Monitoring of the internalization of neuropeptide Y on neuroblastoma cell line SK-N-MC. Eur. J. Biochem. 2000;267:5631–5637. doi: 10.1046/j.1432-1327.2000.01631.x. [DOI] [PubMed] [Google Scholar]
- FAURE M.P., ALONSO A., NOUEL D., GAUDRIAULT G., DENNIS M., VINCENT J.P., BEAUDET A. Somatodendritic internalization and perinuclear targeting of neurotensin in the mammalian brain. J. Neurosci. 1995;15:4140–4147. doi: 10.1523/JNEUROSCI.15-06-04140.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAURE M.P., GAUDREAU P., SHAW I., CASHMAN N.R., BEAUDET A. Synthesis of a biologically active fluorescent probe for labeling neurotensin receptors. J. Histochem. Cytochem. 1994;42:755–763. doi: 10.1177/42.6.8189037. [DOI] [PubMed] [Google Scholar]
- FOREST M., MARTEL J.C., ST PIERRE S., QUIRION R., FOURNIER A. Structural study of the N-terminal segment of neuropeptide tyrosine. J. Med. Chem. 1990;33:1615–1619. doi: 10.1021/jm00168a014. [DOI] [PubMed] [Google Scholar]
- GAUDRIAULT G., NOUEL D., DAL FARRA C., BEAUDET A., VINCENT J.P. Receptor-induced internalization of selective peptidic mu and delta opioid ligands. J. Biol. Chem. 1997;272:2880–2888. doi: 10.1074/jbc.272.5.2880. [DOI] [PubMed] [Google Scholar]
- GEHLERT D.R., BEAVERS L.S., JOHNSON D., GACKENHEIMER S.L., SCHOBER D.A., GADSKI R.A. Expression cloning of a human brain neuropeptide Y Y2 receptor. Mol. Pharmacol. 1996a;49:224–228. [PubMed] [Google Scholar]
- GEHLERT D.R., GACKENHEIMER S.L., SCHOBER D.A., BEAVERS L., GADSKI R., BURNETT J.P., MAYNE N., LUNDELL I., LARHAMMAR D. The neuropeptide Y Y1 receptor selective radioligand, [125I][Leu31, Pro34]peptide YY, is also a high affinity radioligand for human pancreatic polypeptide 1 receptors. Eur. J. Pharmacol. 1996b;318:485–490. doi: 10.1016/s0014-2999(96)00797-2. [DOI] [PubMed] [Google Scholar]
- GERALD C., WALKER M.W., CRISCIONE L., GUSTAFSON E.L., BATZL-HARTMANN C., SMITH K.E., VAYSSE P., DURKIN M.M., LAZ T.M., LINEMEYER D.L., SCHAFFHAUSER A.O., WHITEBREAD S., HOFBAUER K.G., TABER R.I., BRANCHEK T.A., WEINSHANK R.L. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature. 1996;382:168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- GERALD C., WALKER M.W., VAYSSE P.J., HE C., BRANCHEK T.A., WEINSHANK R.L. Expression cloning and pharmacological characterization of a human hippocampal neuropeptide Y/peptide YY Y2 receptor subtype. J. Biol. Chem. 1995;270:26758–26761. doi: 10.1074/jbc.270.45.26758. [DOI] [PubMed] [Google Scholar]
- GICQUIAUX H., LECAT S., GAIRE M., DIETERLEN A., MELY Y., TAKEDA K., BUCHER B., GALZI J.L. Rapid internalization and recycling of the human neuropeptide Y Y1 receptor. J. Biol. Chem. 2002;277:6645–6655. doi: 10.1074/jbc.M107224200. [DOI] [PubMed] [Google Scholar]
- HERZOG H., HORT Y.J., BALL H.J., HAYES G., SHINE J., SELBIE L.A. Cloned human neuropeptide Y receptor couples to two different second messenger systems. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5794–5798. doi: 10.1073/pnas.89.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGENHOVEN N., BECK-SICKINGER A.G. Fluorescent labelled analogues of neuropeptide Y for the characterization of cells expressing NPY receptor subtypes. J. Recept. Signal. Transduct. Res. 1997;17:407–418. doi: 10.3109/10799899709036617. [DOI] [PubMed] [Google Scholar]
- INUI A. Neuropeptide Y feeding receptors: are multiple subtypes involved. Trends Pharmacol. Sci. 1999;20:43–46. doi: 10.1016/s0165-6147(99)01303-6. [DOI] [PubMed] [Google Scholar]
- KALRA S.P., KALRA P.S. NPY – an endearing journey in search of a neurochemical on/off switch for appetite, sex and reproduction. Peptides. 2004;25:465–471. doi: 10.1016/j.peptides.2004.03.001. [DOI] [PubMed] [Google Scholar]
- KASK A., HARRO J., VON HORSTEN S., REDROBE J.P., DUMONT Y., QUIRION R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci. Biobehav. Rev. 2002;26:259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- LARHAMMAR D. Evolution of neuropeptide Y, peptide YY and pancreatic polypeptide. Regul. Pept. 1996;62:1–11. doi: 10.1016/0167-0115(95)00169-7. [DOI] [PubMed] [Google Scholar]
- LARHAMMAR D., BLOMQVIST A.G., YEE F., JAZIN E., YOO H., WAHLESTEDT C. Cloning and functional expression of a human neuropeptide Y/peptide YY receptor of the Y1 type. J. Biol. Chem. 1992;267:10935–10938. [PubMed] [Google Scholar]
- LUNDELL I., BLOMQVIST A.G., BERGLUND M.M., SCHOBER D.A., JOHNSON D., STATNICK M.A., GADSKI R.A., GEHLERT D.R., LARHAMMAR D. Cloning of a human receptor of the NPY receptor family with high affinity for pancreatic polypeptide and peptide YY. J. Biol. Chem. 1995;270:29123–29128. doi: 10.1074/jbc.270.49.29123. [DOI] [PubMed] [Google Scholar]
- MCGRATH J.C., ARRIBAS S., DALY C.J. Fluorescent ligands for the study of receptors. Trends Pharmacol. Sci. 1996;17:393–399. doi: 10.1016/s0165-6147(96)40004-9. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., BECK-SICKINGER A., COX H., DOODS H.N., HERZOG H., LARHAMMAR D., QUIRION R., SCHWARTZ T., WESTFALL T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- NOUEL D., GAUDRIAULT G., HOULE M., REISINE T., VINCENT J.P., MAZELLA J., BEAUDET A. Differential internalization of somatostatin in COS-7 cells transfected with SST1 and SST2 receptor subtypes: a confocal microscopic study using novel fluorescent somatostatin derivatives. Endocrinology. 1997;138:296–306. doi: 10.1210/endo.138.1.4834. [DOI] [PubMed] [Google Scholar]
- PARKER M.S., LUNDELL I., PARKER S.L. Internalization of pancreatic polypeptide Y4 receptors: correlation of receptor intake and affinity. Eur. J. Pharmacol. 2002a;452:279–287. doi: 10.1016/s0014-2999(02)02339-7. [DOI] [PubMed] [Google Scholar]
- PARKER S.L., PARKER M.S., BUSCHAUER A., BALASUBRAMANIAM A. Ligand internalization by cloned neuropeptide Y Y(5) receptors excludes Y(2) and Y(4) receptor-selective peptides. Eur. J. Pharmacol. 2003;474:31–42. doi: 10.1016/s0014-2999(03)02039-9. [DOI] [PubMed] [Google Scholar]
- PARKER S.L., PARKER M.S., LUNDELL I., BALASUBRAMANIAM A., BUSCHAUER A., KANE J.K., YALCIN A., BERGLUND M.M. Agonist internalization by cloned Y1 neuropeptide Y (NPY) receptor in Chinese hamster ovary cells shows strong preference for NPY, endosome-linked entry and fast receptor recycling. Regul. Pept. 2002b;107:49–62. doi: 10.1016/s0167-0115(02)00094-0. [DOI] [PubMed] [Google Scholar]
- PEREZ D.M., KARNIK S.S. Multiple signaling states of G-protein-coupled receptors. Pharmacol. Rev. 2005;57:147–161. doi: 10.1124/pr.57.2.2. [DOI] [PubMed] [Google Scholar]
- PHENG L.H., DUMONT Y., FOURNIER A., CHABOT J.G., BEAUDET A., QUIRION R. Agonist- and antagonist-induced sequestration/internalization of neuropeptide Y Y(1) receptors in HEK293 cells. Br. J. Pharmacol. 2003;139:695–704. doi: 10.1038/sj.bjp.0705306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDROBE J.P., DUMONT Y., QUIRION R. Neuropeptide Y (NPY) and depression: from animal studies to the human condition. Life Sci. 2002;71:2921–2937. doi: 10.1016/s0024-3205(02)02159-8. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ T.W., FUHLENDORFF J., KJEMS L.L., KRISTENSEN M.S., VERVELDE M., O'HARE M., KRSTENANSKY J.L., BJORNHOLM B. Signal epitopes in the three-dimensional structure of neuropeptide Y. Interaction with Y1, Y2, and pancreatic polypeptide receptors. Ann. N.Y. Acad. Sci. 1990;611:35–47. doi: 10.1111/j.1749-6632.1990.tb48920.x. [DOI] [PubMed] [Google Scholar]
- TATEMOTO K. Neuropeptide Y: complete amino acid sequence of the brain peptide. Proc. Natl. Acad. Sci. U.S.A. 1982;79:5485–5489. doi: 10.1073/pnas.79.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATEMOTO K., CARLQUIST M., MUTT V. Neuropeptide Y – a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- TONG Y., DUMONT Y., SHEN S.H., HERZOG H., SHINE J., QUIRION R. Expression of the neuropeptide Y Y1 receptor in human embryonic kidney 293 cells: ligand binding characteristics, in situ hybridization and receptor autoradiography. Mol. Brain Res. 1995;34:303–308. doi: 10.1016/0169-328x(95)00176-s. [DOI] [PubMed] [Google Scholar]
- VEYRAT-DUREBEX C., POMERLEAU L., LANGLOIS D., GAUDREAU P. Internalization and trafficking of the human and rat growth hormone-releasing hormone receptor. J. Cell Physiol. 2005;203:335–344. doi: 10.1002/jcp.20233. [DOI] [PubMed] [Google Scholar]
- VEZZANI A., SPERK G., COLMERS W.F. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- WALKER M.W., SMITH K.E., BARD J., VAYSSE P.J., GERALD C., DAOUTI S., WEINSHANK R.L., BRANCHEK T.A. A structure–activity analysis of the cloned rat and human Y4 receptors for pancreatic polypeptide. Peptides. 1997;18:609–612. doi: 10.1016/s0196-9781(97)00070-3. [DOI] [PubMed] [Google Scholar]
- WALKER P., MUNOZ M., MARTINEZ R., PEITSCH M.C. Acidic residues in extracellular loops of the human Y1 neuropeptide Y receptor are essential for ligand binding. J. Biol. Chem. 1994;269:2863–2869. [PubMed] [Google Scholar]