Abstract
The effects of oral administration of the HMG-CoA reductase inhibitor, simvastatin (SV), on age-related endothelial dysfunction were investigated in the aorta of male Wistar rats.
Adult (12–14 weeks) and old (60–80 weeks) rats were treated daily for 12 weeks with either vehicle or SV (1 mg kg−1). In old rats, SV treatment did not significantly affect systolic blood pressure and LDL-cholesterol, but it reduced plasma cholesterol, triglycerides and oxidised LDL though it did not affect total antioxidant status.
SV improved endothelium-dependent relaxation to acetylcholine and A-23187 in vessels from aged, but not adult, rats. This effect was linked to a greater NO vasodilatation via an increased expression of endothelial NO-synthase. A mechanism sensitive to superoxide dismutase and catalase also accounts for enhanced endothelial vasodilatation.
Finally, SV did not affect the release of prostacyclin, but it inhibited the generation of thromboxane (TX) A2 from COX-2 isoform. The effect of the latter was sensitive to the Tp receptor antagonist, ICI-192,605.
The present study provides evidence that oral administration of SV improves endothelial dysfunction in the aorta from aged rats by mechanisms associated with enhanced NO vasodilatation, reduced release of TXA2 from cyclo-oxygenase, and increased antioxidant properties of the vessel wall. These data underscore a new therapeutic perspective for SV in age-related endothelial dysfunction.
Keywords: Simvastatin, ageing, nitric oxide, cyclo-oxygenase, endothelium, HMG-CoA reductase
Introduction
Ageing, independently of age-related diseases resulting from chronic exposure of the arteries to several risk factors, is characterised not only by reduced arterial compliance and alteration of the contractile properties of the vascular wall but also by endothelial dysfunction. Ageing is associated with endothelial dysfunction characterised as a progressive decline in endothelium-dependent vasodilatation in resistance and conductance arteries from both humans and different animal species (Egashira et al., 1993; Matz et al., 2000; Taddei et al., 2000). The mechanism underlying age-related dysfunction results from alteration in the equilibrium between the effect of relaxing and contracting factors released by the endothelium. This phenomenon includes progressive reduction of nitric oxide (NO)-mediated dilatation related to changes in expression and activity of endothelial NO-synthase (eNOS), increased production of oxygen-derived free radicals and increased release of cyclo-oxygenase (COX)-derived contracting factors (for a review; see Matz & Andriantsitohaina, 2003).
Simvastatin (SV) is an inhibitor of HMG-CoA reductase that belongs to lipid-lowering drugs. Recently, we reported that SV produces direct relaxation of conductance and small arteries of the rat through the mevalonate-sensitive pathway (Alvarez de Sotomayor et al., 2000). The endothelium-dependent relaxation to SV involves both NO and vasodilator eicosanoids by a mechanism sensitive to superoxide dismutase (SOD) and to tyrosine kinase inhibitors. An increase in endothelial cytosolic calcium involving the activation of Rho protein is essential for the action of SV (Alvarez de Sotomayor & Andriantsitohaina, 2001). Interestingly, SV is able to improve endothelial dysfunction associated with several cardiovascular diseases including hypercholesterolaemia and hypertension (Alvarez de Sotomayor et al., 1999; Carneado et al., 2002). Many experimental studies and clinical trials suggest that benefits observed using HMG-CoA reductase inhibitors appear to be greater than might be expected from changes in lipid metabolism, those benefits being explained by the ‘pleiotropic' effects of statins. Accordingly, it has recently been demonstrated that, with equal lowering of LDL-cholesterol by SV or ezetimibe, only SV improved endothelial function in patients with congestive heart failure (Landmesser et al., 2005). Several facts may contribute to explain the effect of HMG-CoA reductase inhibitors, including improvement of endothelial NO pathway, increased antioxidant defence and anti-inflammatory properties (Laufs et al., 1998; Carneado et al., 2002; Dichtl et al., 2003).
The present study was designed to test the hypothesis that SV is able to improve age-related endothelial dysfunction in the rat aorta. Furthermore, the mechanisms involved were examined, including its effect on NO and COX pathways.
Methods
Animals
Adult (12–14 weeks) and old (60–80 weeks) male Wistar rats were bred in our institute from genitors provided by Iffa-Credo (Lyon, France). All experiments were performed according to the guidelines for the ethical treatment of animals of the European Union. Animals were housed at 24±2°C with 60±20% relative humidity, on a 12,12 L,D cycle. Rats were fed a standard diet and water ad libitum, and were weekly weighed. Systolic blood pressure (SBP) was measured before the start and at the end of the treatment by tail-cuff method with a pressure meter (Niprem 645, Cibertec, Madrid, Spain). Old rats were randomly divided into two groups (n=15 in each group): the first group (67.89±5.67-weeks old) in which SV was given in a dose of 1 mg kg−1 body weight and the second group (65.15±6.85-weeks old) receiving the vehicle (0.5% carboxymethylcellulose, 10 ml kg−1 body weight). All substances were given by gavage. In the other series of experiments, two groups of six adult rats each received either SV (1 mg kg−1) or vehicle. The dose of SV used in the present study corresponded to the lowest dose that was effective in improving endothelial dysfunction in different models of experimental hypertension (Alvarez de Sotomayor et al., 1999; Carneado et al., 2002; Pérez-Guerrero et al., 2003). The treatments were administered daily during 12 weeks. The in vitro experiments were performed 3 days after withdrawing the treatments in order to study SV-induced long-term effect only. Animals were anaesthetised with pentobarbitone (60 mg kg−1) and blood was collected by intracardiac puncture for biochemical assays. At necropsy, no apparent pathology was noted in any animal.
Blood biochemical assays
Total antioxidant status (TAS), cholesterol, LDL-cholesterol, oxidised LDL, triglycerides and NO2+NO3 were measured in serum. TAS was assayed using the kit, TAS (Randox Lab, Barcelona, Spain) based on the method reported by Miller et al. (1993). Serum cholesterol, triglycerides and LDL concentration were measured with a CHOD-PAP® kit (Roche Diagnostics, Barcelona, Spain). Oxidised LDL was assessed using an enzyme immunoassay kit. Plasma NO2+NO3 was determined with a NO Colorimetric Assay® kit (Roche Diagnostics, Barcelona, Spain) (Green et al., 1982).
Arterial preparation and mounting
Thoracic aortas were carefully removed and cleaned of connective and fat tissue. Then, aortic segments (2–3 mm in length) were mounted on myographs filled with physiological salt solution (PSS) of the following composition (mM): NaCl 119, KCl 47, MgSO4 1.7, KH2PO4 1.18, NaHCO3 25, CaCl2 1.8 and glucose 11. The PSS was continuously kept at 37°C and gassed with 95% O2 and 5% CO2 at pH 7.4. Aortic rings were stretched to 2.5 g, which yielded a maximal contractile response in aortic rings from old rats (Matz et al., 2000). Mechanical activity was recorded isometrically by a force transducer (Pioden UF-1) coupled to a Powerlab® data acquisition system (AD-Instruments, Castle Hill, Australia). Challenges with 10−6 mol l−1 phenylephrine (Phe) were performed in aorta in order to test its maximal contractile capacity and to elicit reproducible contractile response. The presence of functional endothelium was assessed by the ability of acetylcholine (ACh, 10−6 mol l−1) to induce relaxation of precontracted vessels.
Relaxation experiments
Arteries were precontracted at 80% of their maximal contraction with Phe. The concentration of Phe was adjusted for each preparation, being 3 × 10−7 mol l−1. Precontaction values (being 1.84±0.17 and 2.04±0.31 g, n=6 for the aorta from adult and old rats, respectively) were not significantly altered by SV treatment (being 1.90±0.22 and 1.89±0.21 g, n=6 for aortic rings from adult and old rats, respectively). When the contraction reached a plateau, cumulative addition of vasodilator agents was performed (i.e., ACh from 3 × 10−8 to 10−5 mol l−1), calcium ionophore calcimycin (A-23187 from 3 × 10−9 to 10−5 mol l−1) and the NO donor, sodium nitroprusside (SNP from 3 × 10−10 to 10−6 mol l−1). Concentration–response curves to ACh were constructed in the absence or presence of indicated inhibitors: the NO synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME 3 × 10−4 mol l−1), the superoxide anion (SOD, 150 UI ml−1), the hydrogen peroxide scavengers (catalase, 1000 UI ml−1), the nonselective COX inhibitor, indomethacin (10−5 mol l−1), the selective COX-2 inhibitor, N-(2-cyclohexyloxy-4-nitrophenyl) methane-sulphonamide (NS-398, 10−6 mol l−1) and the TXA2/prostaglandin H2 Tp receptor antagonist, 4(z)-6-(2-o-chlorophenyl-4-o-hydroxyphenyl-1,3-dioxan-cis-5-yl) hexenoic acid (ICI-192,605, 10−6 mol l−1). All the inhibitors were used at a maximally active concentration (Matz et al., 2000) and were incubated with the tissue for 25 min before the precontraction with Phe.
Contraction to TX agonist
After the contractile capacity of the aorta with functional endothelium had been tested with Phe, concentration–response curves to the TX analogue, 9,11-di-deoxy-11α, 9α-epoxymethano-prostaglandin F2α (U46619) were constructed. The effect of U46619 was expressed as percentage of KCl (80 mM)-induced contraction.
Measurement of TXA2 and prostacyclin (PGI2)
Both TXA2 and PGI2 are instable molecules that are quickly converted to the TXB2 and 6-keto-PGF1, respectively. Therefore, assays of TXB2 and 6-keto-PGF1α were performed in intact aorta as described previously (Matz et al., 2000). Briefly, intact aortas were incubated in PSS at 37°C and bubbled with a 95% O2–5% CO2 gas mixture and stimulated with Phe (10−6 mol l−1 for aorta) and ACh (10−6 mol l−1) in order to stimulate the release of TXB2 and 6-keto-PGF1α in the medium. Concentrations of TXB2 and 6-keto-PGF1α were assessed by using competitive enzyme-immunoassay kits (Cayman Chemical Co., Ann Arbor, MI, U.S.A.) based on Pradelles method (Pradelles et al., 1985), and were expressed as pg mg−1 of dry tissue.
Western blotting of eNOS, COX-1 and COX-2
Aortic rings were homogenised in lysis buffer and 25 μg of protein fractions were loaded into 7% and 10% SDS–polyacrylamide gel to separate eNOS and COX-1 or COX-2, respectively. After electrophoresis, proteins were transferred onto nitrocellulose membrane. Immunostaining was achieved using specific monoclonal mouse anti-eNOS (Sigma-Aldrich), anti-COX-1 (Cayman Chemical) and anti-COX-2 (Calbiochem) antibodies, and reacted with peroxidase-conjugated antimouse antibodies. The blots were detected using an enhanced chemiluminescence assay (Pierce Chemical Company, Rockford, IL, U.S.A.) and evaluated by densitometry.
Drugs
SV was generously provided by MSD laboratories. ACh chloride, A-23187, catalase, indomethacin, L-NAME, Phe hydrochloride, SNP, SOD and U46619 were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.). ICI-192,605 and NS-398 from Tocris (Biogen Cientifica SL, Madrid, Spain).
Statistical analysis
Results are expressed as relaxation percent of the initial precontraction level of means±s.e.m. from n experiments, n represents the number of rats, which was at least equal to 6. Analysis of variance (ANOVA) and Tukey's multiple comparison test were used for statistical analysis. Differences were considered significant when P<0.05. Curves were fitted by a concentration–response nonlinear regression equation. Area under curve (AUC) was calculated from concentration–response curves in the absence or presence of L-NAME in order to evaluate the participation of the NO component of ACh-induced relaxation.
Results
Effect of SV on blood pressure, plasma cholesterol, TG and TAS in old rats (Table 1)
Table 1.
Body weight, SBP, plasma cholesterol, LDL-cholesterol, oxidised LDL, triglycerides, TAS, and nitrites+nitrates (NO2+NO3) from old rats treated with either placebo or simvastatin (1 mg kg−1)
| Placebo | Simvastatin (1 mg kg−1) | |
|---|---|---|
| Body weight (gm) | 676.25±15.80 | 702.86±20.79 |
| SBP (mmHg) | 134.14±16.67 | 135.31±28.47 |
| Cholesterol (mg dl−1) | 147.2±10.9 | 102.4±16.0* |
| LDL-cholesterol (mg dl−1) | 12.3±1.4 | 10.1±1.4 |
| Oxidised LDL (mU ml−1) | 23.8±1.6 | 18.7±1.3* |
| Triglycerides (mg dl−1) | 208.2±32.7 | 166.9±30.8* |
| TAS (U gm−1) | 905.5±53.1 | 921.8±50.9 |
| NO2+NO3 (μmol l−1) | 11.3±0.5 | 9.4±0.3 |
Data represented are mean±s.e.m. of n=10.
P<0.05 placebo versus simvastatin.
Body weight, SBP, plasma NO2+NO3 and LDL-cholesterol were not affected by treatment with SV at the dosage used. Plasma cholesterol and triglycerides were significantly reduced (P<0.05). Interestingly, SV significantly reduced oxidised LDL even though it did not affect TAS.
Endothelium-dependent and NO donor-induced relaxation
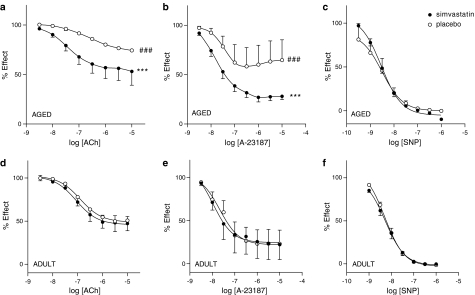
ACh produced relaxation in a concentration-dependent manner in aortic rings with endothelium, but it failed to produce relaxation in endothelium-denuded arteries (not shown). As expected, the endothelium-dependent response to ACh and the calcium ionophore A-23187, but the relaxation to the NO donor SNP, were significantly decreased in vessels from old versus adult rats showing age-related endothelial dysfunction (P<0.001; Figure 1a, b, d and e). In old rats, treatment with 1 mg kg−1 of SV significantly enhanced relaxation of the aorta in response to both ACh and A-23187 (P<0.001 versus placebo; Figure 1a and b), but it did not affect relaxations to SNP (Figure 1c). Interestingly, the relaxations to ACh and A-23187 were restored towards that obtained in aorta from adult rats (Figure 1d and e). In aorta from adult rats, SV treatment did not alter either the endothelium-dependent (ACh- and A-23187) or -independent (SNP) relaxations (Figure 1d–f). Since SV improved age-related endothelial dysfunction, all the following experiments have been performed in vessels from old rats.
Figure 1.
SV improves the endothelial function of aorta from aged (a–c, n=7), but not in those from adult (d–f, n=6), rats after 12 weeks of treatment. Concentration–response curves to ACh (a and d), A-23187 (b and e) and SNP (c and f) in aortic rings from placebo- and SV-treated rats ***P<0.001 placebo versus SV-treated rats. ###P<0.001 aged versus adult rats.
Effect of SV on endothelial NO pathways
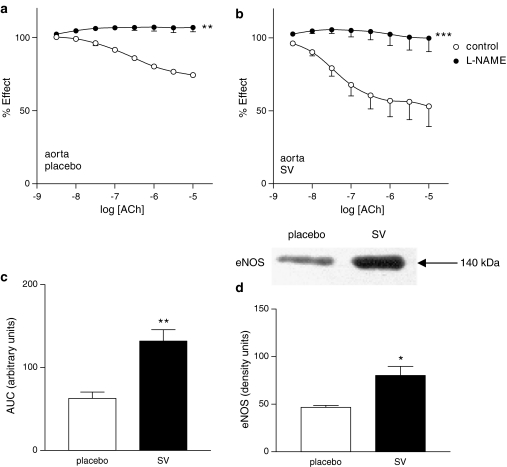
The NO synthase inhibitor L-NAME (3 × 10−4 mol l−1) almost completely abolished endothelium-dependent responses to ACh in aorta from old rats treated with either placebo or SV (Figure 2a and b). In order to compare the participation of NO in relaxation of arteries from placebo and SV-treated rats, the areas under the curves of ACh-induced response were analysed. As shown in Figure 2c, treatment with SV increased the component sensitive to the NO inhibitor of ACh-induced relaxation (P<0.01). Interestingly, the 140 kDa eNOS isoform was expressed in aorta and its expression was significantly enhanced in vessels from old rats treated with SV compared to those from rats treated with the vehicle.
Figure 2.
Concentration–response curves to ACh in aortic rings from placebo- (a) and SV-treated (b) rats in the absence and presence of L-NAME (3 × 10−4 mol l−1). **P<0.01; ***P<0.001 control versus curve in the presence of L-NAME. NO-mediated vasodilation expressed as difference between areas under the curve in the absence and presence of L-NAME (c). Data represented are mean±s.e.m. of n=7. **P<0.01 placebo versus SV. Representative Western blot of aortic eNOS and bars showing optic densitometry of n=4 blots of eNOS. (d) *P<0.05 placebo- versus SV-treated rats.
Effect of SV on endothelial COX pathways
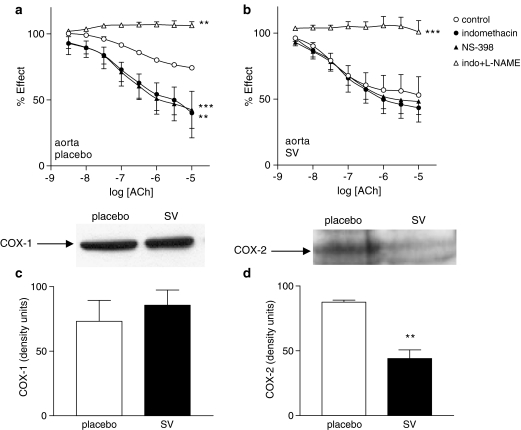
In aorta from placebo-treated rats, both the nonselective COX inhibitor, indomethacin (10−6 mol l−1) and the selective COX-2 inhibitor NS-398 (10−6 mol l−1) significantly increased relaxation in responses to ACh (Figure 3a; P<0.01 and 0.001, respectively).
Figure 3.
Concentration–response curves to ACh of aortic rings from placebo- (a) and SV-treated (b) rats in the absence and presence of indomethacin (10−5 mol l−1), NS-398 (10−6 mol l−1) or indomethacin (10−5 mol l−1) plus L-NAME (3 × 10−4 mol l−1). Data represented are mean±s.e.m. of n=7. *P<0.05; **P<0.01; ***P<0.001 control versus curve in the presence of inhibitor. Representative Western blot and bars showing optic densitometry of n=4 blots of aortic COX-1 (c) and COX-2 (d). **P<0.01 placebo versus SV.
In contrast, in aorta from SV-treated rats, ACh-induced relaxations were affected neither by indomethacin nor NS-398 (Figure 3b). In both vehicle- and SV-treated rats, ACh failed to produce relaxation of aortic rings in the presence of indomethacin plus L-NAME (Figure 3a and b). Furthermore, ACh was not able to induce contractile response in L-NAME-treated vessels (data not shown).
SV treatment did not modify the expression of COX-1 enzyme, but it significantly reduced the expression of COX-2 in the aorta (Figure 3c and d).
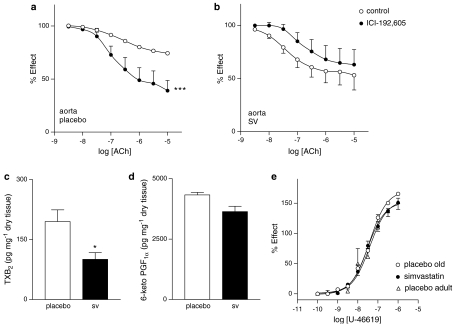
To evaluate the role of prostanoids acting on Tp receptor, relaxant response to the ACh of aortic rings was studied in the presence of ICI-192,605 (10−6 mol l−1). This inhibitor was able to significantly increase response to ACh in aortas from placebo- (P<0.001), but not in those from SV-treated rats (Figure 4a and b).
Figure 4.
Concentration–response curves to ACh in aortic rings from placebo- (a) and SV-treated (b) rats in the absence and presence of the Tp receptor antagonist ICI-192,605 (10−6 mol l−1). ***P<0.001 control versus curve in the presence of inhibitor. TXB2 (c) and PGF1α (d) released by aortic rings from placebo- (open bar) and SV-treated (closed bar). Data represented are mean±s.e.m. of n=7. *P<0.05 placebo versus SV-treated rats. Concentration–response curves to the TX receptor agonist U46619 in endothelium-intact aortic rings from adult (open triangle) and old rats treated with either placebo (open circle) or SV (filled circle) (e). Data represented are mean±s.e.m. of n=6.
Interestingly, assay of TXB2, the stable analogue of TXA2, showed that SV treatment reduced the release of this metabolite in aorta from old rats (Figure 4c), although the release of vasodilatory PGI2 using the assay of 6-keto-PGF1α was not affected by SV treatment (Figure 4d). Finally, the concentration–response curves to U46619, acting on Tp receptors, were not altered either by ageing or by SV treatment (Figure 4e). Altogether, these data suggest that SV treatment is associated with reduced release of TXB2, but not a downregulation of smooth muscle Tp receptors (i.e. receptor expression or post receptor signalling).
Role of oxygen-free radicals in the effect of SV
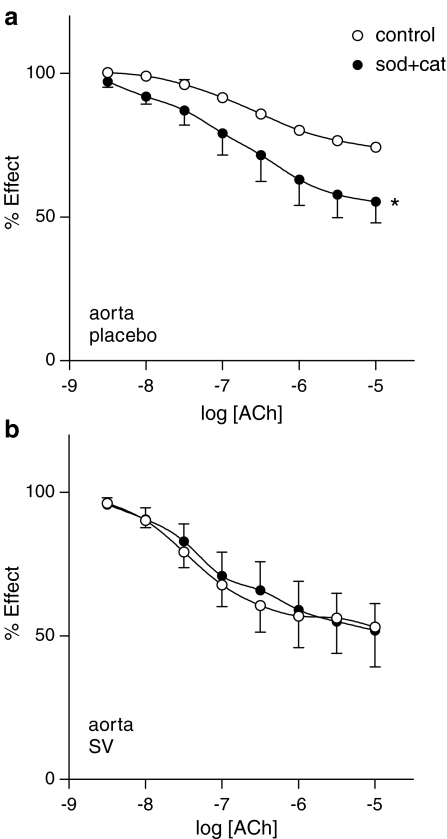
The effect of the oxygen-free radical scavenger, SOD (150 UI ml−1) plus catalase (1000 UI ml−1) was tested on endothelial responses to ACh. SOD plus catalase significantly enhanced ACh-induced endothelial relaxation in aorta from placebo-treated rats (P<0.05; Figure 5a). On the contrary, SOD plus catalase had no effect on ACh-induced relaxation of aortic rings taken from SV-treated rats (Figure 5b).
Figure 5.
Concentration–response curves to ACh in aortic rings from placebo- (a) and SV-treated (b) rats in the absence and presence of ROS scavengers SOD (150 UI ml−1) plus catalase (1000 UI ml−1). Data represented are mean±s.e.m. of n=7. *P<0.05; **P<0.01 control versus curve in the presence of inhibitor.
Discussion
The present study provides evidence that treatment with SV improved age-related endothelial dysfunction in aorta of the rat. The mechanisms involved enhanced endothelial NO vasodilatation through an increase of eNOS expression, decreased participation of TXA2 associated with decreased expression of the COX-2 isoform and enhanced antioxidant properties of the vessel wall.
SV treatment reduced both total plasma cholesterol and triglycerides without significant decrease in LDL-cholesterol. Interestingly, SV reduced oxidised LDL. Reduced plasma cholesterol and triglycerides probably play a role in the beneficial effect of SV. However, previous data from the literature, including ours, reported that HMG-CoA inhibitors are known to exert several effects without decreasing plasma total cholesterol or LDL cholesterol, including a decrease of macrophage accumulation in atherosclerotic lesions (Baetta et al., 2002), a preservation of the structure of coronary adventitia (Wilson et al., 2002) and, interestingly, an improvement of endothelial function (Pérez-Guerrero et al., 2003). Finally, with equal LDL-cholesterol lowering, SV, but not ezetimide, improved flow-dependent dilation in the radial artery of chronic heart failure patients (Landmesser et al., 2005). Thus, reduced or unaltered cholesterol concentrations have been reported depending on the model and the study design. In the present study, the improvement of age-related endothelial dysfunction is associated with reduced plasma cholesterol.
SV has been shown to improve endothelial function and vasomotion in spontaneously hypertensive rats (Alvarez de Sotomayor et al., 1999; Carneado et al., 2002) and in hypertension induced by chronic inhibition of NO by L-NAME (Pérez-Guerrero et al., 2003). Interestingly, SV did not have any effect on endothelial function in normotensive young rats although it increased plasma nitrite concentration (Pérez-Guerrero et al., 2003). With regard to ageing, controversial data have been reported. Administration of another lipid-lowering drug such as the lipoprotein lipase activator NO-1886 for 3 months enhances endothelium-dependent relaxation of aorta from aged rats (Kusunoki et al., 2002). In contrast, no improvement of age-related endothelial dysfunction has been reported with atorvastatin in old patients (Weverling-Rijnsburger et al., 2004) or with cerivastatin in rats (Mukai et al., 2002). Nevertheless, SV 1 mg kg−1 daily for 12 weeks was able to restore endothelial function in the present study. The low dose of SV used in the present study does not allow reaching a sufficient plasma concentration of SV able to stimulate endothelium-dependent relaxation. Also, it should be noted that experiments were carried out 3 days after the last dose of SV and the results are probably produced by the long-term effect of SV.
In accordance with our previous studies (Alvarez de Sotomayor et al., 1999; Carneado et al., 2002), the use of the same dose of SV (i.e. 1 mg kg−1) did not affect SBP (Table 1) even though it improved age-related endothelial dysfunction. In line with our results, Ichihara et al. (2005) reported that SV decreased serum cholesterol levels without affecting blood pressure in hyperlipidaemic hypertensive patients (whose blood pressure was insufficiently controlled by antihypertensive therapy). However, it should be noted that the hypotensive effect of statin in humans has been shown with higher doses of this drug and with mildly hypertensive patients (Glorioso et al., 1999). Also, there are reports in which statins improve control of blood pressure in hypertensive patients (Borghi et al., 2004).
SV treatment improved both ACh- and A-23187-induced endothelium-dependent relaxation. Thus, its effect was not due to a change in agonist receptor number or a default in agonist signal transduction. The most likely hypothesis is that SV acts at the level of either the generation (i.e. synthesis or release) of or response to endothelial factors with ageing. Also, a restoration of the balance between endothelial relaxant (NO and PGI2) and constricting (vasoconstrictor products from COX or reactive oxygen species (ROS)) factors can be advanced.
With regard to the endothelial NO pathway, the restoration of endothelial function by SV resulted in an enhanced endothelial NO component of the response to ACh. Some effects of HMG-CoA reductase inhibitors have been attributed to their ability to enhance eNOS expression, to improve eNOS mRNA stability and increase eNOS activity or NO availability (Laufs et al., 1998). Hence, HMG-CoA reductase inhibitors can reduce the caveolin content of endothelial cells and thus its association with eNOS, which enhances phosphorylation of eNOS by the complex heat shock protein 90-Akt (Brouet et al., 2001). In the present study, the improvement of endothelial NO relaxation was associated with an increased expression of eNOS in aorta taken from SV-treated old rats. An increase of smooth muscle sensitivity to NO was unlikely, since the relaxation induced by the NO donor SNP was not affected by SV.
Increased NO availability through a diminished NO breakdown by ROS or an increased antioxidant defence mechanism might contribute to the increased endothelial NO component of the response. The superoxide anions and hydrogen peroxide scavengers, SOD plus catalase, were able to enhance response to ACh in the aorta from placebo-treated old rats, suggesting the participation of ROS in the reduced endothelial NO component of the response. It is interesting to note that SOD plus catalase failed to affect response to ACh in the aorta taken from SV-treated rats. Thus, it might be possible that SV treatment enhances the antioxidant capacity of the vessel wall, resulting either from increased expression of Cu/Zn SOD and catalase or through another antioxidant enzyme. An antioxidant property of SV treatment has already been reported in the improvement of endothelial function in hypertensive animals (Carneado et al., 2002). Also, HMG-CoA reductase inhibitors can inhibit the gp91 phox-containing NADPH oxidase, a generator of superoxide anions, which are NO scavengers (Vecchione & Brandes, 2002). It is shown here that SV treatment was sufficient to blunt the potentiating effect of SOD plus catalase in aorta from old rats and this may partially contribute to the increased NO component of ACh-induced relaxation.
Another possible explanation of the restoration of endothelial function by SV is a decreased production of endothelium-derived contracting factors. In accordance with our previous study (Matz et al., 2000), we found that using both the nonselective inhibitor of the COX isoforms, indomethacin, and the reported specific inhibitor of COX-2, NS-398, were able to enhance ACh-induced relaxation in the aorta from placebo-treated old rats. Furthermore, increased expressions of COX-1 and COX-2 isoforms were found in these vessels. The nature of COX vasoconstrictor metabolites involved a mechanism sensitive to Tp receptor antagonist ICI-192,605, and was associated with an increased TXA2 production. These results suggest that endothelial TXA2 from COX-2 isoforms are involved in the reduced endothelial relaxation in aorta from old rats. SV did not affect contractile response after stimulation of Tp receptor by the Tp receptor agonist, U-46619. Altogether, these results support the hypothesis that the effect of SV is related to a decrease of TXA2 production, but not to an effect on either expression of Tp receptors or the downstream signalling pathway of smooth muscle. SV treatment abolished the participation of indomethacin- and NS-398-sensitive components of ACh-induced response. Furthermore, the Tp receptor antagonist ICI-192,605 was not able to potentiate ACh-induced relaxation, and the stimulation of TXA2 production in response to the same agonist was significantly reduced in the aorta from SV-treated rats. Thus, SV treatment counteracts the participation of endothelial TXA2 from COX isoforms in aorta from aged rats. This effect of SV had not previously been reported. The vasoactive product released through COX isoforms is shifted towards vasoconstrictor prostanoids with ageing. Here we found that SV reduced TXA2 production, but it did not affect the release of 6-keto-PGF1α. Finally, these effects of SV were associated with reduced expression of COX-2, but not COX-1, isoforms in aorta from aged rats. Taken together, SV treatment reduced the capacity of the aorta from old rats to release vasoconstrictor products from the COX-2 isoforms without affecting the release of the vasodilatory prostanoid, PGI2. The latter may participate in the restoration of endothelial dysfunction. These results were reinforced by the reports showing that HMG-CoA reductase inhibitor was able to reduce COX-2 expression in primary endothelial cells through the regulation of peroxisome proliferator-activated receptor-alpha (Inoue et al., 2000) and plaque macrophages (Cipollone et al., 2003). Further studies are required to determine the mechanisms by which SV affects the release of endothelial TXA2 from COX-2 isoforms in aorta from old rats. Finally, ACh failed to produce relaxation, in L-NAME-treated vessels after blockade of the COX pathway with indomethacin. Furthermore, we did not find any contractile response to ACh in the presence of L-NAME in aged rats. Thus, the release of endothelial TXA2 observed during ageing was able to counteract NO-induced relaxation but it might not be released in sufficient amount to induce contraction in the absence of NO. From these experiments, one can advance the hypothesis that both pathways, NO and prostanoids, may merge to a final common pathways.
In conclusion, we provide evidence that treatment with the HMG-CoA reductase inhibitor, SV, restored endothelial function in the aorta from aged rats. The results shed light on the potential therapeutic effect of SV in enhancing the endothelial NO vasodilatation linked to an increase expression of eNOS and in reducing the release of TXA2 from COX-2 without affecting the release of PGI2. Increased antioxidant capacity of the vessel wall may play a role in the beneficial effect of SV. Thus, treatment with SV may be useful in improving age-related endothelial dysfunction and may contribute to the explanation of some clinical features of this drug in old patients (Eaton et al., 2002).
Abbreviations
- AUC
area under curve
- COX
cyclo-oxygenase
- HMG-CoA
hydroxy-methyl-glutaryl coenzime A
- L-NAME
NG-nitro-L-arginine methyl ester
- LDL
low-density lipoprotein
- NO
nitric oxide
- NOS
nitric oxide synthase
- PGI2
prostacyclin
- PSS
physiological salt solution
- ROS
reactive oxygen species
- SBP
systolic blood pressure
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- SV
simvastatin
- TAS
total antioxidant status
- TG
triglycerides
- TX
thromboxane
References
- ALVAREZ DE SOTOMAYOR M., ANDRIANTSITOHAINA R. Simvastatin and Ca(2+) signaling in endothelial cells: involvement of rho protein. Biochem. Biophys. Res. Commun. 2001;280:486–490. doi: 10.1006/bbrc.2000.4144. [DOI] [PubMed] [Google Scholar]
- ALVAREZ DE SOTOMAYOR M., HERRERA M.D., MARHUENDA E., ANDRIANTSITOHAINA R. Characterization of endothelial factors involved in the vasodilatory effect of simvastatin in aorta and small mesenteric artery of the rat. Br. J. Pharmacol. 2000;131:1179–1187. doi: 10.1038/sj.bjp.0703668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALVAREZ DE SOTOMAYOR M., PÉREZ-GUERRERO C., HERRERA M.D., MARHUENDA E. Effects of chronic treatment with simvastatin on endothelial dysfunction in spontaneously hypertensive rats. J. Hypertens. 1999;17:769–776. doi: 10.1097/00004872-199917060-00008. [DOI] [PubMed] [Google Scholar]
- BAETTA R., CAMERA M., COMPARATO C., ALTANA C., EZEKOWITS M.D., TREMOLI E. Fluvastatin reduces tissue factor expression and macrophage accumulation in carotid lesions of cholesterol-fed rabbits in the absence of lipid lowering. Arterioscler. Thromb. Vasc. Biol. 2002;22:692–698. doi: 10.1161/01.atv.0000012802.69414.a8. [DOI] [PubMed] [Google Scholar]
- BORGHI C., DORMI A., VERONESI M., SANGIORGI Z., GADDI A., BRISIGHELLA HEART STUDY WORKING PARTY Association between different lipid-lowering treatment strategies and blood pressure control in the Brisighella Heart Study. Am. Heart J. 2004;148:285–292. doi: 10.1016/j.ahj.2004.02.003. [DOI] [PubMed] [Google Scholar]
- BROUET A., SONVEAUX P., DESSY C., MONIOTTE S., BALLIGAND J.L., FERON O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ. Res. 2001;89:866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
- CARNEADO J., ALVAREZ DE SOTOMAYOR M., PEREZ-GUERRERO C., JIMENEZ L., HERRERA M.D., PAMIES E., MARTIN-SANZ V., STIEFEL P., MIRANDA M., BRAVO L., MARHUENDA E. Simvastatin improves endothelial function in SHR rats through a superoxide dismutase mediated antioxidant effect. J. Hypertens. 2002;20:429–437. doi: 10.1097/00004872-200203000-00018. [DOI] [PubMed] [Google Scholar]
- CIPOLLONE F., FAZIA M., IEZZI A., ZUCCHELLI M., PINI B., DE CESARE D., UCCHINO S., SPIGONARDO F., BAJOCCHI G., BEI R., MURARO R., ARTESE L., PIATTELLI A., CHIARELLI F., CUCCURULLO F., MEZETTI A. Supression of the functionally coupled cyclooxygenase-2/prostaglandin E synthase as a basis of simvastatin-dependent plaque stabilization in humans. Circulation. 2003;107:1479–1485. doi: 10.1161/01.cir.0000056530.03783.81. [DOI] [PubMed] [Google Scholar]
- DICHTL W., DULAK J., FRICK M., ALBER H.F., SCHWARZACHER S.P., ARES M.P., NILSSON J., PACHINGER O., WEIDINGER F. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2003;23:58–63. doi: 10.1161/01.atv.0000043456.48735.20. [DOI] [PubMed] [Google Scholar]
- EATON C.B., LAPANE K.L., MURPHY J.B., HUME A.L. Effect of statin (HMG-Co-A-reductase inhibitor) use on 1-year mortality and hospitalization rates in older patients with cardiovascular disease living in nursing homes. J. Am. Geriatr. Soc. 2002;50:1389–1395. doi: 10.1046/j.1532-5415.2002.50360.x. [DOI] [PubMed] [Google Scholar]
- EGASHIRA K., INOU T., HIROOKA Y., KAI H., SUGIMACHI M., SUZUKI S., KUGA T., URABE Y., TAKESHITA A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;88:77–81. doi: 10.1161/01.cir.88.1.77. [DOI] [PubMed] [Google Scholar]
- GLORIOSO N., TROFFA C., FILIGHEDDU F., DETTORI F., SORO A., PARPAGLIA P.P., COLLATINA S., PAHOR M. Effect of the HMG-CoA reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia. Hypertension. 1999;34:1281–1286. doi: 10.1161/01.hyp.34.6.1281. [DOI] [PubMed] [Google Scholar]
- GREEN L.C., WAGNER D.A., GLOGOWSKI J., SKIPPER P.L., WISHNOK J.S., TANNENBAUM S.R. Analysis of nitrate and (15 N) nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- ICHIHARA A., HAYASHI M., KOURA Y., TADA Y., KANESHIRO Y.N., SARUTA T. Long-term effects of statins on arterial pressure and stiffness of hypertensives. J. Hum. Hypertens. 2005;19:103–109. doi: 10.1038/sj.jhh.1001786. [DOI] [PubMed] [Google Scholar]
- INOUE I., GOTO S., MIZOTANI K., AWATA T., MASTUNAGA T., KAWAI S., NAKAJIMA T., HOKARI S., KOMODA T., KATAYAMA S. Lipohilic HMG-CoA reductase inhibitor has an anti-inflammatory effect, reduction of mRNA levels for interleukin 1β, interleukin-6, cyclooxygenase-2, and p22phox by regulation of peroxisome proliferator-activated receptor α (PPARα) in primary endothelial cells. Life. Sci. 2000;67:863–876. doi: 10.1016/s0024-3205(00)00680-9. [DOI] [PubMed] [Google Scholar]
- KUSUNOKI M., TSUTSUMI K., HARA T., OGAWA H., NAKAMURA T., MIYATA T., SAKAKIBARA F., FUKUZAWA Y., SUGA T., KAKUMU S., NAKAYA Y. A lipoprotein lipase activator, NO-1886 prevents impaired endothelium-dependent relaxation of aorta caused by exercise in aged rats. Exp. Gerontol. 2002;37:891–896. doi: 10.1016/s0531-5565(02)00023-2. [DOI] [PubMed] [Google Scholar]
- LANDMESSER U., BAHLMANN F., MUELLER M., SPIEKERMANN S., KIRCHHOFF N., SCHULZ S., MANES C., DE GROOT K., FLISER D., FAULER G., MÄRZ W., DREXLER S. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation. 2005;111:2356–2363. doi: 10.1161/01.CIR.0000164260.82417.3F. [DOI] [PubMed] [Google Scholar]
- LAUFS U., LA FATA V., PLUTZKY J., LIAO J.K. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- MATZ R.L., ALVAREZ DE SOTOMAYOR M., SCHOTT C., STOCLET J.C., ANDRIANTSITOHAINA R. Vascular bed heterogeneity in age-related endothelial dysfunction with respect to NO and eicosanoids. Br. J. Pharmacol. 2000;131:303–311. doi: 10.1038/sj.bjp.0703568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATZ R.L., ANDRIANTSITOHAINA R. Age-related endothelial dysfunction: potential implications for pharmacotherapy. Drugs Aging. 2003;20:527–550. doi: 10.2165/00002512-200320070-00005. [DOI] [PubMed] [Google Scholar]
- MILLER N.J., RICE-EVANS C., DAVIES M.J., GOPINATHAN V., MILNET A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993;84:407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- MUKAI Y., SHIMOKAWA H., HIGASHI M., MORIKAWA K., MATOBA T., HIROKI J., KUNIHIRO I., TALUKDER H.M.A., TAKESHITA A. Inhibition of renin–angiotensin system ameliorates endothelial dysfunction associated with aging in rats. Arterioscler. Thromb. Vasc. Biol. 2002;22:1445–1450. doi: 10.1161/01.atv.0000029121.63691.ce. [DOI] [PubMed] [Google Scholar]
- PÉREZ-GUERRERO C., ALVAREZ DE SOTOMAYOR M., JIMENEZ L., HERRERA M.D., MAHUENDA E. Effects of simvastatin on vascular and endothelial function after chronic inhibition of nitric oxide synthase by L-NAME. J. Cardiovasc. Pharmacol. 2003;42:204–210. doi: 10.1097/00005344-200308000-00008. [DOI] [PubMed] [Google Scholar]
- PRADELLES P., GRASI J., MACLOUF J. Enzyme inmunoassays of eicosanoids using acetylcholine esterase as label: an alternative to radioinmunoassay. Anal. Chem. 1985;57:1170–1173. doi: 10.1021/ac00284a003. [DOI] [PubMed] [Google Scholar]
- TADDEI S., GALETTA F., VIRDIS A., GHIADONI L., SALVETTI G., FRANZONI F., GIUSTI C., SALVETTI A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- VECCHIONE C., BRANDES R.P. Withdrawal of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors elicits oxidative stress and induces endothelial dysfunction in mice. Circ. Res. 2002;91:173–179. doi: 10.1161/01.res.0000028004.76218.b8. [DOI] [PubMed] [Google Scholar]
- WEVERLING-RIJNSBURGER A.W.E., BLAUW G.J., MEINDERS A.E. Effect of atorvastatin on impaired vascular function in healthy old men. J. Clin. Pharm. Ther. 2004;29:157–164. doi: 10.1111/j.1365-2710.2004.00548.x. [DOI] [PubMed] [Google Scholar]
- WILSON S.H., HERRMANN J., LERMAN L.O., HOLMES D.R., NAPOLI C., RITMAN E.L., LERMAN A. Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation. 2002;105:415–418. doi: 10.1161/hc0402.104119. [DOI] [PubMed] [Google Scholar]