Abstract
Endothelial dysfunction plays a role in the development of atherosclerosis and diabetes-associated vascular disease and, in the streptozotocin (STZ)-induced apoE-deficient diabetic mouse, we report that, when compared to the citrate (CIT)-treated nondiabetic apoE-deficient control, acetylcholine (Ach)-mediated endothelium-dependent relaxation was reduced in the small mesenteric arteries (SMA) and the plaque-prone regions of the aorta from the STZ-diabetic mouse.
In the SMA the component of Ach-mediated relaxation that was attributed to nitric oxide (NO) from STZ-treated diabetic apoE-deficient mice was enhanced; however, the endothelium-derived hyperpolarizing factor (EDHF)-mediated component was reduced. The EDHF component was assessed by determining the component of the Ach-mediated response that was resistant to the combination of the NO synthase (NOS) inhibitor Nω-nitro-L-arginine methyl ester, cyclooxygenase inhibitor, indomethacin, and soluble guanylate cyclase inhibitor, ODQ, and inhibited by the combination of the intermediate conductance KCa (IKCa) inhibitor TRAM-34 and the small-conductance KCa (SKCa) inhibitor apamin.
Endothelial NOS was increased but SK2, SK3 and connexin (Cx) 37 mRNA expressions were significantly (P<0.05) decreased in the SMA from STZ-treated apoE-deficient mice compared to the CIT-treated controls. There was no difference in the IKCa expression or in Cx 40, 43 and 45 mRNA levels between STZ- and CIT-treated mice.
The microvasculature of STZ-induced apoE-deficient mice developed endothelial dysfunction, which may be linked to a decrease in the contribution of the EDHF component due to a decrease in SK2 and 3 and Cx 37 expression.
Keywords: Apamin, apoE-deficient, atherosclerosis, connexins, EDHF, endothelium, eNOS, diabetes, SKCa channels, streptozotocin, TRAM-34
Introduction
Diabetes-associated vascular complications are a major clinical problem and patients with diabetes have a mortality rate 3–4 times that of the general population (Haffner et al., 1998). Furthermore, diabetes is associated with accelerated atherogenesis, a two- to four-fold increase in the incidence of coronary artery disease, as well as a 10-fold increase in peripheral vascular diseases and up to 75% of patients diagnosed with diabetes ultimately dying from vascular disease (Haffner et al., 1998; Grundy et al., 1999, 2002; Laakso, 1999). Damage to the endothelium as well as endothelial dysfunction has long been recognized as initiating events in the development of atherosclerosis (Duncan, 1963; Ross & Glomset, 1973; Ross, 1993). Dysfunction of the endothelium plays an early prominent role in the development of cardiovascular disease and possibly precedes the development of diabetes itself (Vita et al., 1990; Haffner et al., 1998).
Endothelial dysfunction can simply be defined as a reduced endothelium-dependent vasodilator response to acetylcholine (Ach), and, although the endothelium plays many additional functions beyond the control of vascular tone, this reduced response to Ach serves as an important indicator of vascular dysfunction that implies a decreased bioavailability of nitric oxide (NO). It is well known that a reduction of NO has multiple effects on vascular homeostasis and cardiovascular health (Anderson, 1999; Verma & Anderson, 2002; Ding & Triggle, 2005).
Although the importance of endothelial dysfunction in the pathogenesis of cardiovascular disease has long been recognized (Anderson, 1999; Verma & Anderson, 2002), the cellular processes that lead to dysfunction are debated (De Vriese et al., 2000; Triggle et al., 2003; Andrews et al., 2005). On the one hand, diabetes-related hyperglycaemia has been shown to rapidly induce oxidative stress and endothelial cell dysfunction in cell culture (Nishikawa et al., 2000), as well as in invasive human studies (Kawano et al., 1999). On the other hand, diabetes-related dyslipidaemia has also been shown to be an important contributor to vascular dysfunction, as has been reported in familial hypercholesterolaemic patients before and after cholesterol reduction (Stadler et al., 1997), as well as postprandially in relation to remnant lipoprotein levels (Funada et al., 2002). In diabetes, triglycerides are typically raised and high-density lipoprotein (HDL) lowered (Grundy et al., 1999). Atherosclerosis is, however, associated with macrovascular disease and thus the role of dyslipidaemia versus hyperglycaemia in the pathogenesis of microvascular disease remains unclear and continues to be debated (Libby & Plutzky, 2002).
To better understand the link between diabetes and the development of cardiovascular disease, numerous animal models have been developed and transgenic mouse models offer many advantages (Coleman, 1982). Normal mice, however, do not develop atherosclerosis and thus, it may be argued, are unsuitable for comparative studies of the disease process in humans; however, with appropriate pharmacological and/or genetic manipulation, mice can be induced to mimic the human disease. In this regard, the apolipoprotein E (apoE)-deficient mouse has proved a useful model of human atherosclerosis and rapidly develops atherosclerotic lesions even when maintained on a normal chow diet (Meir & Leitersdorf, 2004). Overexpression of the apoE gene results in, dependent upon the promoter, a decrease in diet-induced hypercholesterolaemia and fatty streaks in the arterial wall (Harada et al., 1996). Crauwels et al. (2003) have reported that endothelial dysfunction was prominent on the plaque-rich regions in the aorta from the apoE-deficient mouse and plaques were predominantly found in the aortic root, the proximal segments and distal aorta segments with the central region of the aorta only minimally affected. Type I diabetes can also be induced in the apoE-deficient mouse with streptozotocin (STZ), and the current investigation was designed to determine whether endothelial dysfunction was exacerbated in the aorta and also whether dysfunction also developed in the microvasculature of the STZ-induced diabetic apoE-deficient mouse.
In the mouse aorta, endothelium-dependent relaxation is entirely mediated by NO (Huang et al., 1995) and a reduction in the bioavailability of NO is a common feature of diabetes that, likely, contributes, to the development of atherosclerosis. In the microvasculature there is, as in other species, a variable contribution of endothelium-dependent hyperpolarizing factor, EDHF (Chataigneau et al., 1999; Waldron et al., 1999; Ding et al., 2000). Although the nature of EDHF remains unknown (McGuire et al., 2001; Busse et al., 2002; Vanhoutte, 2004) and may be purely electrical coupling via myo-endothelial gap junctions (Sandow, 2004), the EDHF pathway may serve an important role in the microvasculature and provide the cellular basis for conducted vasodilatation (Takano et al., 2004). Of importance for the present study is that myo-endothelial gap junctions have been shown to play an important role in mediating the EDHF response in mouse small mesenteric arteries (SMA) (Dora et al., 2003).
Changes in the contribution of EDHF in disease states potentially may have important pathophysiological consequences (Feletou & Vanhoutte, 2004). Decreases in the contribution of EDHF to endothelium-dependent relaxation have been frequently reported in STZ-treated rats (Fukao et al., 1997; De Vriese et al., 2000; Wigg et al., 2001; Matsumoto et al., 2003, 2004), but in type II diabetic mice no change in the overall contribution of EDHF is apparent (Pannirselvam et al., 2002). In most vascular beds, the EDHF component of endothelium-dependent relaxation is inhibited by the combination of the small-conductance KCa (SKCa) blocker, apamin, and the nonspecific intermediate-conductance KCa (IKCa) blocker, charybdotoxin (Garland & Plane, 1996; McGuire et al., 2001), or the selective IKCa blocker, TRAM-34 (Wulff et al., 2000). In the current study, we thus sought answers to two questions: first, is endothelium-dependent relaxation compromised in the aorta of STZ-treated apoE-deficient mice, and is the endothelial dysfunction associated with the ‘plaque-prone' regions of the aorta – as described by Crauwels et al. (2003)? Second, is the contribution of EDHF to endothelium-dependent relaxation in SMA from STZ-treated apoE-deficient mice also compromised and does diabetes affect expression of the KCa channels and connexins (Cx)?
Methods
Experimental procedures
Animals
ApoE-deficient mice were purchased from the Heart Research Institute, NSW, Australia. Mice were assigned into age-matched groups. apoE-deficient mice, 6-week old, were injected 55 mg kg−1 day−1 STZ or vehicle (citrate buffer) (CIT) intraperitoneal over 5 consecutive days. Mice were monitored for glucosuria 2 weeks after STZ injection to assess the induction of diabetes, and only animals that tested positive were continued in the study. Mice were killed at 12–16 weeks after STZ or vehicle treatment. Animals with blood glucose levels above 20 mmol l−1 were used. The blood was taken at the time of decapitation, blood was collected in a centrifuged tube with 25 μl of heparin and stored in the fridge, the blood samples were analysed the following day for cholesterol and triglycerides and HDL/LDL. All procedures were approved by the RMIT University Animal Ethics Committee.
Plasma levels of total cholesterol and triglycerides HDL and LDL were measured enzymatically (CHOD-PAP and GPO-PAP, respectively, Roche, Basel, Switzerland), performed by Mayne Health Dorevitch Pathology (Victoria, Australia). Blood glucose levels were measured by the MediSense®2 Blood Glucose Testing System (MediSense Australia Pty. Ltd, Victoria, Australia) at the time of killing.
Myograph
First-order mesenteric arteries (SMA) were removed and placed in Krebs' solution (composition, mM: NaCl, 120; NaHCO3, 25; KCl, 4.8; NaH2PO4, 1.2; MgSO4, 1.2; Glucose, 11.0; CaCl2, 1.8) bubbled with 95% O2 and 5% CO2. Arteries were cut into 2 mm rings and mounted on a Mulvany–Halpern myograph as described previously (Mulvany & Halpern, 1977). Tissues were stretched to a set tension of 2 mN. All experiments were performed at 37°C. Tissues were maximally contracted with 120 mM high-potassium salt solution.
Phenylephrine (PE) cumulative concentration–response curves (0.1–30 μM) were performed on all tissues. The concentration of PE required to produce a 70% response was used to contract the vessels for subsequent vasorelaxation curves. Typically, PE produced a contraction that was stable for at least 20 min and only tissues in which the PE contraction was stable were used for the subsequent studies of Ach-mediated relaxation. Concentration–response curves to Ach (0.01–10 μM) were performed on all tissues and then repeated either alone or after a 15-min incubation with Nω-nitro-L-arginine methyl ester (L-NAME) (100 μM), indomethacin (Indo) (10 μM) and ODQ (10 μM), or after 30-min incubation with: L-NAME, Indo, ODQ plus apamin (10 μM) or L-NAME, Indo, ODQ, TRAM-34 (1 μM), and apamin. Concentration–response curves (0.1–30 μM) were performed up to a maximum of 4 times on each tissue. Time-controls for the maintenance of PE-induced tone and subsequent Ach-mediated relaxations revealed no significant difference between four sequential relaxation–response curves, confirming the reproducibility of Ach response curves.
Aortic ring segments of 3 mm in length (ID 800 μm) were systematically sectioned from the ascending thoracic aorta. Following the description by Crauwels et al. (2003), two aortic segments per mouse were dissected 3 mm from the origin of the left subclavian artery towards the diaphragm (the first segment being the ‘plaque-prone' and the second the ‘plaque-resistant'). Each segment was mounted through the lumen on two parallel 200-μm stainless steel pins in a Mulvany–Halpern myograph for isometric force recording. Each vessel preparation was gradually stretched according to the normalization procedures of Mulvany & Halpern (1977) to determine optimum resting tension (14–18 mN) and was equilibrated for 1 h before commencement of experiments. Preparations were contracted with PE (0.2 μM) and allowed to stabilize before constructing cumulative concentration–response curves to the endothelium-dependent vasodilator (Ach, 1 nM–10 μM) and the endothelium-independent vasodilator sodium nitroprusside (SNP, 1 nM–10 μM). Studies of Ach-mediated relaxation in the presence of L-NAME, Indo, ODQ or K-channel blockers were not performed in the aorta.
Real-time reverse transcription–PCR assay
The entire mesenteric artery arcade was homogenized and total RNA was isolated using Trizol reagent (Invitrogen) according to the manufacturer's instructions. The RNA yield was measured by absorbance at 260 nm. The first-strand cDNA was synthesized by Superscript II reverse-transcriptase with oligo(dT)12–18 primer from 1 μg of total RNA. To ensure that the primers were specific and efficient, a number of tests were performed. Primers were initially analysed by performing positive control and negative control PCR reactions with SYBR-green (Qiagen) at eight annealing temperatures, ranging from 52 to 62°C. Melt curve analysis was used to visualize primer specificity by revealing the presence or absence of primer dimers. For further verification, 1.0 μl of the PCR reaction was analysed on an Agilent Technologies 2100 Bioanalyzer using a DNA 500 LabChip kit. Sequencing was performed on products from successful reactions. PCR efficiency was determined by performing real-time PCR on serial dilutions of mouse brain cDNA. QRT–PCR was performed in duplicate using the following primer sequences:
eNOS, sense 5′-CAACGCTACCACGAGGACATT-3′ and antisense 5′-CTCCTGCAAAGAAAAGCTCTGG-3′; SK1, sense 5′-GTGAAGATTGAACAAGGGAAGG-3′and antisense 5′-TGCCTCCAACTCCTCCTG-3′;SK2, sense 5′-ACCATCAGACAGCAGCAAAGGG-3′and antisense 5′-GACCGCCGCCTCCTGGAC-3′;SK3, sense 5′-GCCAACTCTACCGCCATC-3′and antisense 5′-GGCTGTGGAACTTGGAGAG-3′;IKCa, sense 5′-ATGCTCCTGCGTCTCTAC-3′and antisense 5′-GAAGCGGACTTGGTTGAG-3′;β-actin, sense 5′-ACGGCCAGGTCATCACTATTG-3′and antisense 5′-CCAAGAAGGAAGGCTGGAAAAGA-3′;Cx37, sense 5′-AGGCAGGCTTCCTCTATGGC-3′and antisense 5′-AGACATAGCAGTCCACGATGTG-3′;Cx40, sense 5′-GAGGCCCACGGAGAAGAATG-3′and antisense 5′-TGGTAGAGTTCAGCCAGGCT-3′;Cx43, sense 5′-ACAAGGTCCAAGCCTACTCCA-3′and antisense 5′-CCCCAGGAGCAGGATTCTGA-3′;Cx45, sense 5′-CGGGCTGTGAGAATGTCTGC-3′and antisense 5′-CAGGTACATCACAGAGGGAGTTG-3′.
The amplification was carried out in 50 μl of reaction mixture containing 25 μl of iQ SYBR Green Supermix (Bio-Rad), 2 μl of the first-strand cDNA products, 100 nM each of the sense and antisense primers. For each set of primers, a no template control was included. The reactions were carried out on a 96-well plate, and a PCR amplification protocol was followed (95°C for 5 min and 40 cycles of amplification at 95°C for 30 s, 62°C (eNOS); 58.2°C (KCa); 60.1°C (Cx) for 30 s and 72°C for 30 s) using an iCycler iQ machine (Bio-Rad). Post-amplification melting curve analysis was performed to show a single amplification product without contamination. Electrophoresis analysis on a 2% agarose gel was also carried out for quality control. The fold relative to β-actin for the mRNA of interest was calculated using the 2−ΔCT method (Livak & Schmittgen, 2001).
Drugs
PE, Ach, Indo, apamin, ODQ and L-NAME were purchased from Sigma-Aldrich (Sydney, NSW, Australia). TRAM-34 was a gift from Dr Heike Wulff – Department of Medical Pharmacology and Toxicology, University of California, Davis, CA 95616, U.S.A. PE, Ach and L-NAME were dissolved in distilled water, while TRAM-34 and ODQ were dissolved in 100% DMSO and Indo was dissolved in 1% sodium carbonate.
Data analysis
Data are expressed as pD2 values and pD2 is defined as the negative logarithm to base 10 of the EC50 values. In all experiments, n equals the number of animals. Relaxation is expressed as percentage of PE-induced tone (plateau phase)±s.e.m. The significance of differences between mean values was calculated by Student's t-test. Statistical significance of differences between the means of data groups was performed using ANOVA for curve analysis. Significance was assumed if P<0.05.
Results
Metabolic analysis
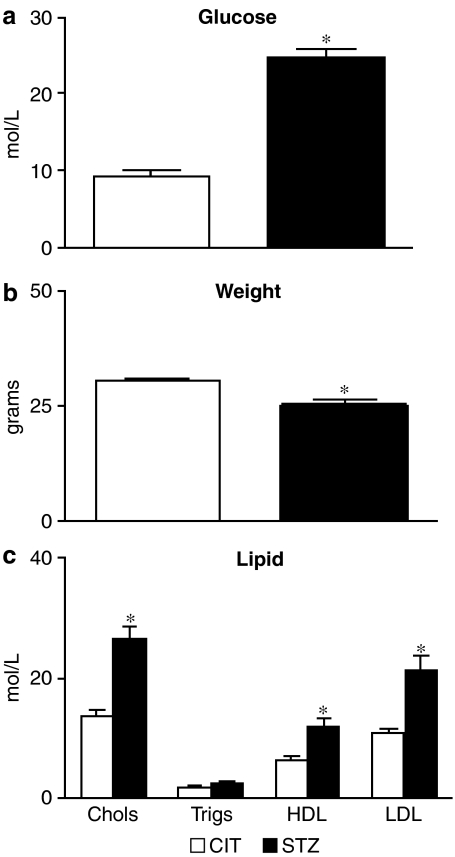
Blood glucose levels, total cholesterol, HDL and LDL levels were significantly greater in STZ-treated mice than CIT-treated mice (P<0.05, unpaired Student's t-test, n=8). Triglyceride levels for the CIT-treated mice were not significantly different from the STZ-treated group (P>0.05, unpaired t-test, n=8) (Figure 1).
Figure 1.
Plasma glucose (a), weights (b) and lipid (c) profile from CIT- and STZ-treated apoE-deficient mice taken at the time of killing.
Myograph – aorta
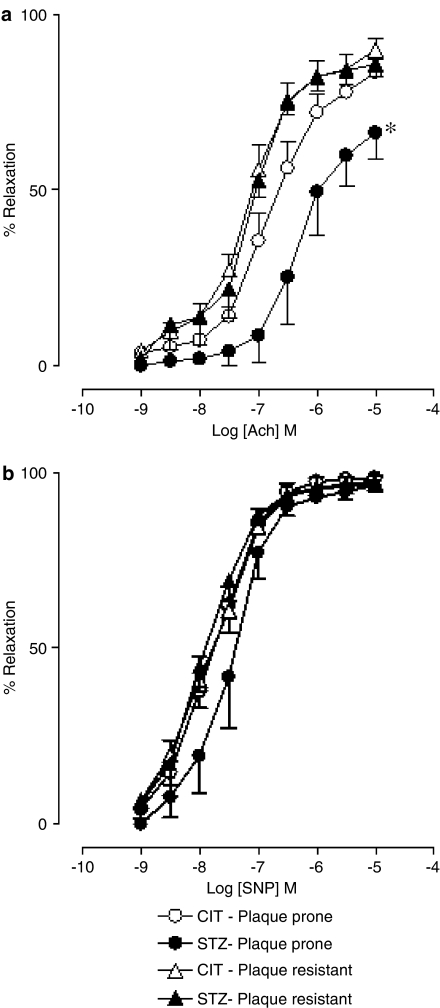
Relaxation responses to Ach were significantly (P<0.05, ANOVA) reduced in the plaque-prone aortic segments from STZ-treated apoE-deficient mice, compared to those in CIT-treated apoE-deficient mice (Figure 2a; STZ group: pD2=6.2±0.2; Emax=66.3±7.6%; n=4; CIT group: pD2=6.8±0.1; Emax=83.6±3.8%; n=6). In contrast, responses to Ach were not impaired in neighbouring plaque-resistant segments in STZ-treated mice (Figure 2a; STZ group: pD2=7.1±0.1; Emax=85.8±3.5%; n=4; CIT group: pD2=7.1±0.1; Emax=89.9±3.3%; n=7). SNP responses remained similar in all the segments studied (Figure 2b).
Figure 2.
Ach (a) and sodium nitroprusside (SNP, b) concentration–response curves in the absence of inhibitors in the aorta from CIT- and STZ-treated apoE-deficient mice in plaque-prone and -resistant segments.
Myograph – SMA
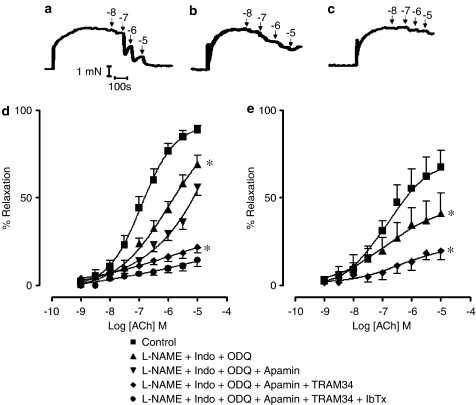
In endothelium-intact first-order SMA from CIT-treated (nondiabetic) apoE-deficient mice contracted with PE (1 μM), Ach induced a concentration-dependent relaxation, as shown in the representative traces (Figure 3a), with a maximum response of 88.6±2.7%. The representative traces, with a 30-min interval between each trace, illustrate Ach-induced relaxation in the presence of the NOS inhibitor, L-NAME (100 μM), the cyclooxygenase inhibitor, Indo (10 μM), and soluble guanylate cyclase inhibitor, ODQ (10 μM) (Figure 3b), and in the presence of the combination of L-NAME, Indo ODQ, the SKCa inhibitor apamin, and the combination of the putatively specific IKCa inhibitor TRAM-34 (Wulff et al., 2000) (Figure 3c). The presence of L-NAME, Indo and ODQ significantly reduced the maximal relaxation to Ach (69.2±4.7%) (P<0.05) (Figure 3d). In the presence of L-NAME, Indo, ODQ, TRAM-34 and apamin, the maximal relaxation to Ach was significantly reduced to 21.6±3.5% (P<0.05) (Figure 3d). However, the addition of apamin alone, without TRAM-34, produced no additional inhibition of Ach-mediated relaxation to that seen in the presence of L-NAME, Indo and ODQ (Figure 3d). Similarly, the addition of iberotoxin produced no further inhibition to that seen in the presence of TRAM-34 and apamin (Figure 3d). We have demonstrated that there is no time-dependent decrease of responses to Ach by the reproducibility of a series of four Ach-mediated relaxation–response curves (data not shown). We have also demonstrated that a contraction to PE was stable for 20 min in an SMA preparation from a CIT-treated apoE-deficient mouse (data not shown).
Figure 3.
Representative traces of Ach-mediated relaxation in the absence of inhibitors (a), in the presence of L-NAME, Indo and ODQ (b) and in the presence of the combination of L-NAME, Indo, ODQ, apamin and TRAM-34 (c) with a 30-min interval between each trace. Concentration–response curves to Ach in the SMA from CIT- (d) and STZ- (e) treated apoE-deficient mice in the absence or presence of L-NAME, Indo and ODQ (d, e), in the presence of apamin (d) or combination of TRAM-34 and apamin (d, e), as well as L-NAME, Indo, ODQ, apamin and TRAM-34 with iberiotoxin (IbTx) (d). *Significantly different from control responses (P<0.05), n=4–9.
In STZ-treated apoE-deficient mouse SMA contracted with PE, Ach also induced a concentration-dependent relaxation with a maximum relaxation of 71.5±7.5%, which was significantly decreased compared to the Ach relaxation in CIT-treated SMA (P<0.05) (Figure 3e). In addition, L-NAME (100 μM), Indo (10 μM) and ODQ (10 μM) significantly inhibited the maximal relaxation to Ach (P<0.05; Figure 3e) and the combination of TRAM-34 and apamin (in the presence of L-NAME, Indo and ODQ) also significantly reduced the maximal relaxation (P<0.05) (Figure 3e).
Real-time PCR analyses
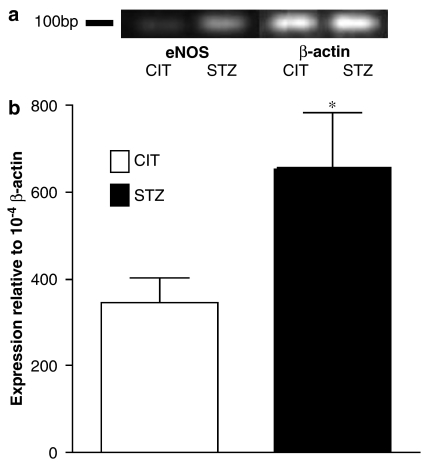
eNOS mRNA expression was examined by real-time PCR using β-actin as internal control (Figure 4a). There was a 1.9-fold increase of eNOS mRNA expression in STZ-treated apoE-deficient mouse mesenteric arteries compared to expression in CIT-treated apoE-deficient mice (P<0.05) (Figure 4b).
Figure 4.
mRNA expression of eNOS in CIT- and STZ-treated apoE-deficient mouse mesenteric arteries by the real-time PCR method. (a) Representative gel. (b) Analytical data of expression ratio. Values are expressed as the relative ratio to β-actin. *Significantly different (P<0.05), n=5.
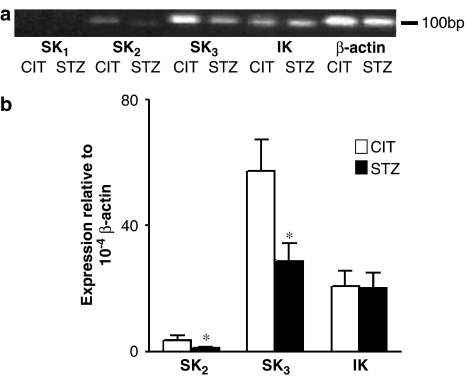
As reflected by the RT–PCR data, SK3 is the predominant isoform in apoE-deficient mouse mesenteric arteries, but both SK2 and SK3 are significantly decreased in STZ-treated apoE-deficient mouse mesenteric arteries comparing to that in CIT-treated apoE-deficient mouse mesenteric arteries (P<0.05) (Figure 5a and b). We did not detect SKCa1 expression in either CIT- or STZ-treated apoE-deficient mouse mesenteric arteries (Figure 5a). No significant difference was noted in IKCa expression in mesenteric arteries when CIT-treated was compared to STZ-treated apoE-deficient mice.
Figure 5.
mRNA expression of SK2, SK3 and IK in CIT- and STZ-treated apoE-deficient mouse mesenteric arteries by real-time PCR method. (a) Representative gel. (b) Analytical data of expression ratio. Values are expressed as the relative ratio to β-actin. *Significantly different (P<0.05), n=5.
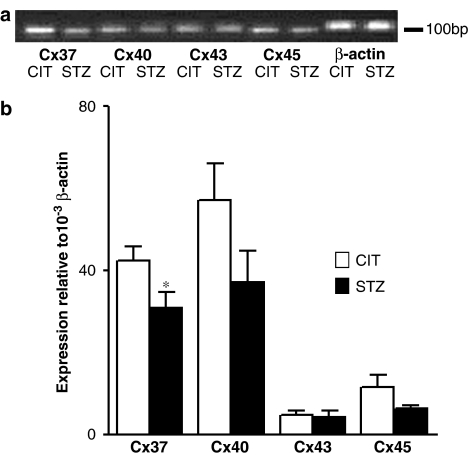
Cx 37 mRNA was decreased 23.8±3.4% in STZ-treated apoE-deficient mouse mesenteric arteries compared to CIT-treated controls (P<0.05). However, Cx 40, 43 and 45 mRNA levels were not significantly changed (P>0.05) (Figure 6a and b).
Figure 6.
mRNA expression of Cx 37, 40, 43 and 45 in CIT- and STZ-treated apoE-deficient mouse mesenteric arteries by real-time PCR method. (a) Representative gel. (b) Analytical data of expression ratio. Values are expressed as the relative ratio to β-actin. *Significantly different (P<0.05), n=5.
Discussion
The important findings from this study are that the induction of diabetes by STZ in the apoE-deficient mouse results in hyperglycaemia, elevated cholesterol and endothelial dysfunction in the SMA and plaque-prone areas of the aorta. In addition, in the SMA, endothelial dysfunction is associated with a reduced contribution of the EDHF component of the endothelium-dependent response to Ach, as well as a reduction in the expression of Cx37, SK2 and 3. Although a number of investigators have reported that the induction of diabetes in the apoE-deficient mouse provides a useful animal model of diabetic hyperlipidaemia (for instance, see Yamamoto et al., 1995), we believe that the current study is the first to describe endothelial dysfunction in this model.
In a vessel- and species-dependent manner, endothelium-dependent relaxation to Ach is made up of contributions from NO, prostacyclin and the putative EDHF (McGuire et al., 2001; Vanhoutte, 2004). In the mouse aorta NO appears to be the sole endothelial-derived mediator, whereas in the SMA both NO and EDHF are important (Huang et al., 1995; Waldron et al., 1999). In the SMA from the CIT-treated apoE-deficient (control) mouse, the addition of L-NAME, ODQ and Indo significantly reduced the maximal relaxation response to Ach by approximately 20%. In the STZ-treated apoE-deficient mouse, the effects of the L-NAME/Indo/ODQ combination were similar, with the data demonstrating an approximately 30% reduction of the NO component. Thus, despite a 1.9-fold increase in the expression of eNOS in the SMA from the STZ-treated apoE-deficient diabetic mouse, there was a reduction in endothelium-dependent relaxation to Ach. The L-NAME/Indo/ODQ-insensitive component, presumably, reflects the contribution of the putative EDHF. Interestingly, it was the L-NAME/Indo/ODQ-insensitive component that was reduced in the SMA of STZ-treated mice, indicating that the contribution of EDHF is reduced in STZ-induced diabetes – at least in the SMA (Figure 3b).
The relative contribution of EDHF to endothelium-dependent relaxation in a type 1 mouse model of diabetes differs from that which we report for the db/db mouse model of type 2 diabetes; in the SMA, endothelium-dependent relaxation is entirely mediated by EDHF, despite no change in eNOS mRNA expression (Pannirselvam et al., 2005). Collectively, these data suggest that the microvascular disease associated with both type I and II diabetes is linked to changes in the contribution of EDHF-mediated pathways in mouse microvasculature; however, in type I diabetes the EDHF-mediated component of endothelium-dependent relaxation is reduced, whereas in type II diabetes the contribution of EDHF is maintained.
As noted in the Introduction, it is the contribution of EDHF to endothelium-dependent relaxation in the microcirculation that is reduced in mesenteric arteries from STZ-treated rats (Fukao et al., 1997; Wigg et al., 2001; Matsumoto et al., 2003, 2004) and the renal vasculature (De Vriese et al., 2000). Thus, our data from mice are consistent with those reported for the STZ-induced diabetic rat and that, in the SMA, STZ-induced diabetes results in endothelial dysfunction that can be linked to a reduced contribution of EDHF. Studies of endothelium-dependent relaxation in larger blood vessels, such as the aorta, have, however, reported a reduction in the NO-mediated component (Kamata et al., 1999). Indeed, our study also demonstrated that endothelial function was also reduced in the aorta of the STZ-treated apoE-deficient mice, and this was correlated with the presence of plaque; these data are entirely in agreement with the publication from Crauwels et al. (2003) that described endothelial dysfunction only in the plaque-prone areas of the aorta from apoE-deficient mice. It should be stressed, however, that the apoE-deficient mice in the study by Crauwels et al. (2003) were 18 months of age, as compared to 12–16 weeks in the present study, and endothelial dysfunction was more dramatic in the older animals and amounted to a 75% reduction of the response to Ach in plaque-prone area, as well as a reduction in relaxation to the endothelium-independent vasodilator, NO. Thus, changes in vascular smooth muscle function are also apparent in older apoE-deficient mice.
The advantages of studying the STZ-treated apoE-deficient mouse as a preferred model to the STZ-induced diabetic rat was first reported by Yamamoto et al. (1995) and, in particular, they stressed that hypercholesterolaemia was not stably observed without dietary modification in the rat, but that stable hypercholesterolaemia without dietary modification could be obtained in the STZ-induced diabetic apoE-deficient mouse. Endothelial function has not been previously reported in the STZ-diabetic apoE-deficient mouse model, but has been extensively studied in the STZ-diabetic rat. Endothelial function in the STZ-diabetic rat aorta has been reported to show a triphasic change – enhanced at 1-week post-STZ, unaltered at 1–2 weeks and impaired at 8 weeks (Pieper, 1999). Kobayashi & Kamata (2001) reported a decrease in aortic endothelial function 9 weeks after STZ treatment and this was accompanied by an increase in oxidative stress, but no change in mRNA eNOS expression. In contrast in rats treated for 2 weeks with STZ, aortic eNOS mRNA and NADPH oxidase activation was increased (seven-fold increase in gp91phox mRNA) and endothelial dysfunction was associated with a reduction in the bioavailability of NO as measured by electron spin resonance (Hink et al., 2001). An elevated expression of eNOS may not necessarily be beneficial, as Ozaki et al. (2002) and Kawashima & Yokoyama (2004) report that the overexpression of eNOS in the apoE-deficient mouse leads to accelerated atherogenesis. Furthermore, an uncoupled eNOS may serve as an NADPH oxidase and result in the overproduction of superoxide anions (•O2−), and this may further exacerbate endothelial dysfunction. •O2− can oxidize tetrahydrobiopterin, an important cofactor in the regulation of NOS, and this will lead to an ‘uncoupled eNOS', which will then synthesize •O2− rather than NO (Pannirselvam et al., 2003; Alp & Channon, 2004). Thus, our finding that eNOS expression is elevated in the STZ-mouse SMA may reflect a pathophysiological event that contributes, via enhanced •O2− production, to microvascular disease.
Our data indicate that both SK2 and SK3 mRNA for the apamin-sensitive KCa channels are expressed in the mouse SMA. Western blot data from porcine coronary endothelial cells have also indicated that SK3 protein is abundant in samples of endothelium compared to whole arteries; SK2 protein was only present in whole artery nuclear fractions and immunofluorescent labelling for SK3 was highly expressed at the plasmalemma of endothelial cells, but not in smooth muscle (Burnham et al., 2002). The functional significance of the SK2 channel with the nuclear membrane is unknown at this time. The SK3 channel is thought to be essential for mediating the EDHF component of endothelium-dependent relaxation, and this is supported by data from the rat carotid artery (Eichler et al., 2003); interestingly we report that the expression of SK3 mRNA was reduced in the SMA of the STZ-diabetic mouse, as was the EDHF component of endothelium-dependent relaxation to Ach. In our study no change in IKCa expression was noted, and this may reflect the greater significance of the apamin-sensitive SKCa component in the determination of the EDHF-mediated hyperpolarization event. Two published studies support this conclusion: First, Crane et al. (2003) reported that, in rat SMA, TRAM-34 alone did not affect hyperpolarization to Ach, but, in combination with apamin, the hyperpolarization was completely abolished – suggesting that Ach-mediated endothelium-dependent hyperpolarization of vascular smooth muscle can be attributed to SKCa channels. Second, in the rat SMA the activation of the thromboxane receptor resulted in a differential block of the SKCa, as determined by a loss of smooth muscle hyperpolarization evoked with the SKCa activator 100 riluzole, but that due to the IKCa activator 1-EBIO was unaffected (Crane & Garland, 2004). The role of the apamin-sensitive SKCa channel in the mouse SMA in determining endothelium-dependent relaxation would appear to be different than in the rat SMA, as, for example, in the mouse SMA the Ach-mediated response cannot be entirely attributed to EDHF (Ding et al., 2000). Furthermore, apamin alone does not inhibit either the hyperpolarization or the relaxation mediated by the endothelium-dependent vasodilator peptide, SLIGRL-NH2 (McGuire et al., 2004). Nonetheless, changes in the expression of SK3 channels in the endothelium of a transgenic mouse have been associated with altered arterial tone and blood pressure, and these data reflect, presumably, the importance of SK3 channels in the regulation of resistance artery tone (Taylor et al., 2003). Our data that associate a reduction of the EDHF component of endothelium-dependent relaxation to Ach in SMA from STZ-induced diabetic apoE-deficient mice with a reduction in SK2 and SK3 KCa channels are also supportive of the importance of SKCa channels in determining the EDHF response.
Myoendothelial gap junctions play an important role in mediating the EDHF response in mouse SMA (Dora et al., 2003); in the hypertensive state, both the EDHF response and Cx 37 expression are decreased in the mesenteric artery from the spontaneously hypertensive rat (Rummery & Hill, 2004) and ovariectomy has been shown to reduce Cx 40 and 43 expression, as well as the EDHF component of endothelium-dependent relaxation to Ach in the rat SMA (Nawate et al., 2005). We found a decrease in the contribution of EDHF and Cx 37 in the SMA and mesenteric artery arcade, respectively, from the apoE-deficient STZ-diabetic mice and, thus, Cx 37 may also play a critical role in the mesenteric circulation for the mediation of EDHF. We did not detect a change in Cx 40, 43 or 45 mRNA expression; these data, however, must be interpreted carefully as it was not possible, due to the low abundance in the tissue, to determine protein expression, nor did we determine whether the number or distribution of myoendothelial gap junctions was altered in the STZ-diabetic animals. Additional studies with high-resolution electron microscopy would be valuable. Interestingly, although we did not detect differences in Cx 40 expression in SMA from the apoE versus the STZ-apoE, it has been shown that the phenotype of the Cx 40 knockout mouse presents with a profound reduction in Ach-mediated vasodilatation in cremaster arterioles (de Wit et al., 2000), and in the rat SMA application of a Cx 40 antibody has been shown to block EDHF-mediated vasodilatation (Mather et al., 2005). As stressed by Rummery & Hill (2004), more definitive support for a strong correlation between endothelial dysfunction, blood pressure and Cx expression awaits the use of mice, wherein Cx expression can be rapidly manipulated though an appropriate inducible system.
In conclusion, our studies of the micro- (SMA) vasculature of the STZ-diabetic apoE-deficient mouse reveal that endothelial function is depressed at 12–16 weeks. In the microvasculature, endothelial dysfunction can be linked to a decrease in the contribution of the EDHF component and this was reflected by a decrease in SK2, 3 and Cx 37 expression, but not Cx 40, 43 or 45, and a paradoxical increase in eNOS mRNA. In the macrovasculature (aorta), endothelial dysfunction was only significant in the plaque-prone area. Further studies are required to elucidate the importance of these changes in the contribution of the EDHF pathway, SKCa and Cx expression to diabetes-related microvascular disease and determine whether the enhanced expression of eNOS mRNA reflects a compensatory protective or pathophysiological process.
Acknowledgments
We acknowledge the financial support of a Research Infrastructure Grant from RMIT to HD, JR and CRT, a Faculty of Life Sciences Research Grant from RMIT to JM. WBW was supported by a Canadian Institutes of Health Research (CIHR) Group Grant awarded to the Smooth Muscle research Group at the University of Calgary, as well as Canadian Diabetes Association and CIHR operating grants to CRT. We also thank Yianfen Jiang and Zhong Jian Cheng (University of Calgary), Elisa Young and Christopher Hill (RMIT) for their assistance with experiments.
Abbreviations
- Ach
acetylcholine
- apoE
apolipoprotein E
- CIT
vehicle (citrate buffer)
- Cx
connexin
- EDHF
endothelium-derived hyperpolarizing factor
- eNOS
endothelial nitric oxide synthase
- IKCa
intermediate-conductance calcium-activated potassium channels
- Indo
indomethacin
- L-NAME
Nω-nitro-L-arginine methyl ester hydrochloride
- NO
nitric oxide
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PE
phenylephrine
- SKCa
small-conductance calcium-activated potassium channels
- SMA
small mesenteric arteries
- STZ
streptozotocin
- TRAM-34
1-[(2-chlorophenyl)(diphenyl)methyl]-1H-pyrazole
References
- ALP N.J., CHANNON K.M. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- ANDERSON T.J. Assessment and treatment of endothelial dysfunction in humans. J. Am. Coll. Cardiol. 1999;34:631–638. doi: 10.1016/s0735-1097(99)00259-4. [DOI] [PubMed] [Google Scholar]
- ANDREWS K.L., PANNIRSELVAM M., ANDERSON T.J., JENKINS A.J., TRIGGLE C.R., HILL M.A. The vascular endothelium in diabetes: a practical target for drug treatment. Expert Opin. Ther. Targets. 2005;9:101–117. doi: 10.1517/14728222.9.1.101. [DOI] [PubMed] [Google Scholar]
- BURNHAM M.P., BYCHKOV R., FELETOU M., RICHARDS G.R., VANHOUTTE P.M., WESTON A.H., EDWARDS G. Characterization of an apamin-sensitive small-conductance Ca(2+)-activated K(+) channel in porcine coronary artery endothelium: relevance to EDHF. Br. J. Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSSE R., EDWARDS G., FELETOU M., FLEMING I., VANHOUTTE P.M., WESTON A.H. EDHF: bringing the concepts together. Trends Pharmacol. Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- CHATAIGNEAU T., FELETOU M., HUANG P.L., FISHMAN M.C., DUHAULT J., VANHOUTTE P.M. Acetylcholine-induced relaxation in blood vessels from endothelial nitric oxide synthase knockout mice. Br. J. Pharmacol. 1999;126:219–226. doi: 10.1038/sj.bjp.0702300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN D.L. Diabetes–obesity syndromes in mice. Diabetes. 1982;31 (Suppl 1):1–6. doi: 10.2337/diab.31.1.s1. [DOI] [PubMed] [Google Scholar]
- CRANE G.J., GALLAGHER N., DORA K.A., GARLAND C.J. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J. Physiol. 2003;553:183–189. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE G.J., GARLAND C.J. Thromboxane receptor stimulation associated with loss of SKCa activity and reduced EDHF responses in the rat isolated mesenteric artery. Br. J. Pharmacol. 2004;142:43–50. doi: 10.1038/sj.bjp.0705756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAUWELS H.M., VAN HOVE C.E., HOLVOET P., HERMAN A.G., BULT H. Plaque-associated endothelial dysfunction in apolipoprotein E-deficient mice on a regular diet. Effect of human apolipoprotein AI. Cardiovasc. Res. 2003;59:189–199. doi: 10.1016/s0008-6363(03)00353-5. [DOI] [PubMed] [Google Scholar]
- DE VRIESE A.S., VAN DE VOORDE J., BLOM H.J., VANHOUTTE P.M., VERBEKE M., LAMEIRE N.H. The impaired renal vasodilator response attributed to endothelium-derived hyperpolarizing factor in streptozotocin-induced diabetic rats is restored by 5-methyltetrahydrofolate. Diabetologia. 2000;43:1116–1125. doi: 10.1007/s001250051502. [DOI] [PubMed] [Google Scholar]
- DE WIT C., ROOS F., BOLZ S.S., KIRCHHOFF S., KRUGER O., WILLECKE K., POHL U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ. Res. 2000;86:649–655. doi: 10.1161/01.res.86.6.649. [DOI] [PubMed] [Google Scholar]
- DING H., KUBES P., TRIGGLE C.R. Potassium- and acetylcholine-induced vasorelaxation in mice lacking endothelial nitric oxide synthase. Br. J. Pharmacol. 2000;129:1194–1200. doi: 10.1038/sj.bjp.0703144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DING H., TRIGGLE C.R. Endothelial cell dysfunction and the vascular complications associated with type II diabetes: assessing the health of the endothelium. Vasc. Health Risk Manage. 2005;1:55–71. doi: 10.2147/vhrm.1.1.55.58939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORA K.A., SANDOW S.L., GALLAGHER N.T., TAKANO H., RUMMERY N.M., HILL C.E., GARLAND C.J. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J. Vasc. Res. 2003;40:480–490. doi: 10.1159/000074549. [DOI] [PubMed] [Google Scholar]
- DUNCAN L.E. Evolution of the Atherosclerotic Plaque 1963Chicago: University of Chicago Press; ed. Jones, R.J. [Google Scholar]
- EICHLER I., WIBAWA J., GRGIC I., KNORR A., BRAKEMEIER S., PRIES A.R., HOYER J., KOHLER R. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br. J. Pharmacol. 2003;138:594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELETOU M., VANHOUTTE P.M. EDHF: new therapeutic targets. Pharmacol. Res. 2004;49:565–580. doi: 10.1016/j.phrs.2003.10.017. [DOI] [PubMed] [Google Scholar]
- FUKAO M., HATTORI Y., KANNO M., SAKUMA I., KITABATAKE A. Alterations in endothelium-dependent hyperpolarization and relaxation in mesenteric arteries from streptozotocin-induced diabetic rats. Br. J. Pharmacol. 1997;121:1383–1391. doi: 10.1038/sj.bjp.0701258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUNADA J., SEKIYA M., HAMADA M., HIWADA K. Postprandial elevation of remnant lipoprotein leads to endothelial dysfunction. Circ. J. 2002;66:127–132. doi: 10.1253/circj.66.127. [DOI] [PubMed] [Google Scholar]
- GARLAND C.J., PLANE F. Endothelium-Derived Hyperpolarizing Factor. New York: Harwood Academic Publishers; 1996. pp. 173–179. [Google Scholar]
- GRUNDY S.M., BENJAMIN I.J., BURKE G.L., CHAIT A., ECKEL R.H., HOWARD B.V., MITCH W., SMITH S.C., JR, SOWERS J.R. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- GRUNDY S.M., HOWARD B., SMITH S., JR, ECKEL R., REDBERG R., BONOW R.O. Prevention conference VI: Diabetes and cardiovascular disease: executive summary: conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation. 2002;105:2231–2239. doi: 10.1161/01.cir.0000013952.86046.dd. [DOI] [PubMed] [Google Scholar]
- HAFFNER S.M., LEHTO S., RONNEMAA T., PYORALA K., LAAKSO M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- HARADA K., SHIMANO H., ISHIBASHI S., YAMADA N. Transgenic mouse and gene therapy. Diabetes. 1996;45 (Suppl 3):S129–S132. doi: 10.2337/diab.45.3.s129. [DOI] [PubMed] [Google Scholar]
- HINK U., LI H., MOLLNAU H., OELZE M., MATHEIS E., HARTMANN M., SKATCHKOV M., THAISS F., STAHL R.A., WARNHOLTZ A., MEINERTZ T., GRIENDLING K., HARRISON D.G., FORSTERMANN U., MUNZEL T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- HUANG P.L., HUANG Z., MASHIMO H., BLOCH K.D., MOSKOWITZ M.A., BEVAN J.A., FISHMAN M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- KAMATA K., NAKAJIMA M., SUGIURA M. Effects of superoxide dismutase on the acetylcholine-induced relaxation response in cholesterol-fed and streptozotocin-induced diabetic mice. J. Smooth Muscle Res. 1999;35:33–46. doi: 10.1540/jsmr.35.33. [DOI] [PubMed] [Google Scholar]
- KAWANO H., MOTOYAMA T., HIRASHIMA O., HIRAI N., MIYAO Y., SAKAMOTO T., KUGIYAMA K., OGAWA H., YASUE H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J. Am. Coll. Cardiol. 1999;34:146–154. doi: 10.1016/s0735-1097(99)00168-0. [DOI] [PubMed] [Google Scholar]
- KAWASHIMA S., YOKOYAMA M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004;24:998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI T., KAMATA K. Effect of chronic insulin treatment on NO production and endothelium-dependent relaxation in aortae from established STZ-induced diabetic rats. Atherosclerosis. 2001;155:313–320. doi: 10.1016/s0021-9150(00)00583-9. [DOI] [PubMed] [Google Scholar]
- LAAKSO M. Hyperglycemia as a risk factor for cardiovascular disease in type 2 diabetes. Prim. Care. 1999;26:829–839. doi: 10.1016/s0095-4543(05)70133-0. [DOI] [PubMed] [Google Scholar]
- LIBBY P., PLUTZKY J. Diabetic macrovascular disease: the glucose paradox. Circulation. 2002;106:2760–2763. doi: 10.1161/01.cir.0000037282.92395.ae. [DOI] [PubMed] [Google Scholar]
- LIVAK K.J., SCHMITTGEN T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- MATHER S., DORA K.A., SANDOW S.L., WINTER P., GARLAND C.J. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ. Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO T., KOBAYASHI T., KAMATA K. Alterations in EDHF-type relaxation and phosphodiesterase activity in mesenteric arteries from diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H283–H291. doi: 10.1152/ajpheart.00954.2002. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO T., WAKABAYASHI K., KOBAYASHI T., KAMATA K. Diabetes-related changes in cAMP-dependent protein kinase activity and decrease in relaxation response in rat mesenteric artery. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1064–H1071. doi: 10.1152/ajpheart.00069.2004. [DOI] [PubMed] [Google Scholar]
- MCGUIRE J.J., DING H., TRIGGLE C.R. Endothelium-derived relaxing factors: a focus on endothelium-derived hyperpolarizing factor(s) Can. J. Physiol. Pharmacol. 2001;79:443–470. [PubMed] [Google Scholar]
- MCGUIRE J.J., HOLLENBERG M., TRIGGLE C.R. Hyperpolarization of vascular smooth muscle by the activation of endothelial proteinase-activated receptor 2 in murine small mesenteric arteries. Can. J. Physiol. Pharmacol. 2004;82:1103–1112. doi: 10.1139/y04-121. [DOI] [PubMed] [Google Scholar]
- MEIR K.S., LEITERSDORF E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler. Thromb. Vasc. Biol. 2004;24:1006–1014. doi: 10.1161/01.ATV.0000128849.12617.f4. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NAWATE S., FUKAO M., SAKUMA I., SOMA T., NAGAI K., TAKIKAWA O., MIWA S., KITABATAKE A. Reciprocal changes in endothelium-derived hyperpolarizing factor- and nitric oxide-system in the mesenteric artery of adult female rats following ovariectomy. Br. J. Pharmacol. 2005;144:178–189. doi: 10.1038/sj.bjp.0706091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIKAWA T., EDELSTEIN D., BROWNLEE M. The missing link: a single unifying mechanism for diabetic complications. Kidney Int. 2000;77 (Suppl.):S26–S30. doi: 10.1046/j.1523-1755.2000.07705.x. [DOI] [PubMed] [Google Scholar]
- OZAKI M., KAWASHIMA S., YAMASHITA T., HIRASE T., NAMIKI M., INOUE N., HIRATA K., YASUI H., SAKURAI H., YOSHIDA Y., MASADA M., YOKOYAMA M. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J. Clin. Invest. 2002;110:331–340. doi: 10.1172/JCI15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANNIRSELVAM M., SIMON V., VERMA S., ANDERSON T.J., TRIGGLE C.R. Chronic oral supplementation with sepiapterin prevents endothelial dysfunction and oxidative stress in small mesenteric arteries from diabetic (db/db) mice. Br. J. Pharmacol. 2003;140:701–706. doi: 10.1038/sj.bjp.0705476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANNIRSELVAM M., VERMA S., ANDERSON T.J., TRIGGLE C.R. Cellular basis of endothelial dysfunction in small mesenteric arteries from spontaneously diabetic (db/db−/−) mice: role of decreased tetrahydrobiopterin bioavailability. Br. J. Pharmacol. 2002;136:255–263. doi: 10.1038/sj.bjp.0704683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANNIRSELVAM M., WIEHLER W.B., ANDERSON T.J., TRIGGLE C.R. Enhanced vascular reactivity of small mesenteric arteries from diabetic mice is associated with enhanced oxidative stress and cyclooxygenase products. Br. J. Pharmacol. 2005;144:953–966. doi: 10.1038/sj.bjp.0706121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIEPER G.M. Enhanced, unaltered and impaired nitric oxide-mediated endothelium-dependent relaxation in experimental diabetes mellitus: importance of disease duration. Diabetologia. 1999;142:204–213. doi: 10.1007/s001250051140. [DOI] [PubMed] [Google Scholar]
- ROSS R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- ROSS R., GLOMSET J.A. Atherosclerosis and the arterial smooth muscle cell: proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973;190:1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- RUMMERY N.M., HILL C.E. Vascular gap junctions and implications for hypertension. Clin. Exp. Pharmacol. Physiol. 2004;31:659–667. doi: 10.1111/j.1440-1681.2004.04071.x. [DOI] [PubMed] [Google Scholar]
- SANDOW S.L. Factors, fiction and endothelium-derived hyperpolarizing factor. Clin. Exp. Pharmacol. Physiol. 2004;31:563–570. doi: 10.1111/j.1440-1681.2004.04048.x. [DOI] [PubMed] [Google Scholar]
- STADLER R.W., IBRAHIM S.F., LEES R.S. Peripheral vasoactivity in familial hypercholesterolemic subjects treated with heparin-induced extracorporeal LDL precipitation (HELP) Atherosclerosis. 1997;128:241–249. doi: 10.1016/s0021-9150(96)05998-9. [DOI] [PubMed] [Google Scholar]
- TAKANO H., DORA K.A., SPITALER M.M., GARLAND C.J. Spreading dilatation in rat mesenteric arteries associated with calcium-independent endothelial cell hyperpolarization. J. Physiol. 2004;556:887–903. doi: 10.1113/jphysiol.2003.060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR M.S., BONEV A.D., GROSS T.P., ECKMAN D.M., BRAYDEN J.E., BOND C.T., ADELMAN J.P., NELSON M.T. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ. Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- TRIGGLE C.R., HOLLENBERG M., ANDERSON T.J., DING H., JIANG Y., CERONI L., WIEHLER W.B., NG E.S., ELLIS A., ANDREWS K., MCGUIRE J.J., PANNIRSELVAM M. The endothelium in health and disease – a target for therapeutic intervention. J. Smooth Muscle Res. 2003;39:249–267. doi: 10.1540/jsmr.39.249. [DOI] [PubMed] [Google Scholar]
- VANHOUTTE P.M. Endothelium-dependent hyperpolarizations: the history. Pharmacol. Res. 2004;49:503–508. doi: 10.1016/j.phrs.2003.11.015. [DOI] [PubMed] [Google Scholar]
- VERMA S., ANDERSON T.J. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105:546–549. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- VITA J.A., TREASURE C.B., NABEL E.G., MCLENACHAN J.M., FISH R.D., YEUNG A.C., VEKSHTEIN V.I., SELWYN A.P., GANZ P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- WALDRON G.J., DING H., LOVREN F., KUBES P., TRIGGLE C.R. Acetylcholine-induced relaxation of peripheral arteries isolated from mice lacking endothelial nitric oxide synthase. Br. J. Pharmacol. 1999;128:653–658. doi: 10.1038/sj.bjp.0702858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIGG S.J., TARE M., TONTA M.A., O'BRIEN R.C., MEREDITH I.T., PARKINGTON H.C. Comparison of effects of diabetes mellitus on an EDHF-dependent and an EDHF-independent artery. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H232–H240. doi: 10.1152/ajpheart.2001.281.1.H232. [DOI] [PubMed] [Google Scholar]
- WULFF H., MILLER M.J., HANSEL W., GRISSMER S., CAHALAN M.D., CHANDY K.G. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO K., SHIMANO H., SHIMADA M., KAWAMURA M., GOTODA T., HARADA K., OHSUGA J., YAZAKI Y., YAMADA N. Overexpression of apolipoprotein E prevents development of diabetic hyperlipidemia in transgenic mice. Diabetes. 1995;44:580–585. doi: 10.2337/diab.44.5.580. [DOI] [PubMed] [Google Scholar]