Abstract
Neuronal nicotinic ACh receptors (nAChRs) readily desensitize in the presence of an agonist. However, when the agonist is applied for minutes, hours or days, it is unclear how extensive desensitization is, how long it persists after agonist removal and whether nAChRs consequently change their pharmacological properties.
These issues were explored with electrophysiological studies of native receptors of voltage-clamped human neuroblastoma SH-SY5Y cells. Puffer pulses of nicotine (1 mM)-evoked inward currents partly antagonized by methyllycaconitine (MLA; 10 nM) or α-conotoxin MII (MII; 10 nM), suggesting contribution by α7 and α3 subunit containing receptors, respectively. Nicotine-evoked currents desensitized with 150 ms time constant and fully recovered after a few s washout.
Although the current induced by 10 min application of nicotine (10 μM) decayed to baseline indicating complete desensitization, puffer applications of maximally effective doses of nicotine still generated small responses (22% of control). Similar responses to puffer-applied nicotine were observed when nicotine was chronically incubated for 8 or 48 h. On nicotine washout, cells recovered their response amplitude within 5 min and then increased it (about 50% of untreated controls) after 30 min without altering response kinetics or sensitivity to MLA and MII.
The present results suggest that native nAChRs of SH-SY5Y cells preserved a degree of responsiveness during chronic application of nicotine, and that they rapidly recovered on washout to generate larger responses without changes in kinetics or pharmacology. These data indicate strong compensatory mechanisms to retain nicotinic receptor function during long-term exposure to nicotine.
Keywords: Acetylcholine, receptor upregulation, α-conotoxin MII, metyllicaconitine
Introduction
Although nicotinic acetylcholine receptors (nAChRs) were the subject of the first analytical study of receptor desensitization (Katz & Thesleff, 1957), this process remains incompletely understood in terms of molecular changes underlying the receptor transition to the desensitized state (Quick & Lester, 2002; Lester et al., 2004). Since brain nAChRs are important for physiological neurotransmission (Wonnacott, 1997; Jones et al., 1999; Paterson & Nordberg, 2000; Dajas-Bailador & Wonnacott, 2004) and are subjected to operational modification in a number of neurodegenerative diseases (Maelicke et al., 2000; Paterson & Nordberg, 2000; Picciotto & Zoli, 2002), it seems useful to clarify how desensitization might shape receptor properties over short and long terms.
One salient aspect of desensitization of neuronal nAChRs is its time course (Giniatullin et al., 2005). The initial work by Katz & Thesleff (1957) on muscle nAChRs described a process with fast onset, complete suppression of receptor responses and rapid recovery after wash. This scheme is suitable to account for physiological processes when, owing to repeated neuronal activity, a rapid build up of ACh might be expected to take place to evoke nAChR desensitization transiently (Lester & Dani, 1995). Nevertheless, how much sustained agonist exposure can affect nAChR function remains unclear despite a plethora of studies on this subject. This issue is relevant to understand changes in nAChRs due to tobacco smoking, the basis for nicotine tolerance and withdrawal, or the consequences of chronic treatments with nicotinic cholinomimetics (Picciotto & Zoli, 2002).
Using a variety of models including expression systems for recombinant nAChRs, most molecular biology and biochemical investigations have indicated upregulation of neuronal nAChRs after hours or days of exposure to nicotine (Peng et al., 1997; Ke et al., 1998; Wang et al., 1998; Warpman et al., 1998; Fenster et al., 1999; Buisson & Bertrand, 2001; Kawai & Berg, 2001; Ridley et al., 2001). To reach such a conclusion, protocols normally involved long incubation in nicotine-containing solution and subsequent washout with control buffer. Curiously though, very few studies have directly addressed the question of what happens to nAChRs in the continuous chronic presence of nicotine, whether they can somehow preserve a certain degree of responsiveness, and how rapidly receptors can regain their full sensitivity after agonist washout. For instance, 0.1 μM nicotine applied for >8 h to α4β2 receptors expressed in HEK293 cells leaves a small residual response with good recovery on washout (Buisson & Bertrand, 2001), while a limited radioactive Rb+ flux due to α4β2 and α4β4 receptor activity has been found in expression systems following 1 h exposure to nicotine (Gentry et al., 2003). These data point to the need to study how native nAChRs modify their responsiveness during chronic agonist application, the time course of these changes and of any subsequent recovery.
SH-SY5Y cells are human neuroblastoma elements possessing native nAChRs (Peng et al., 1997; Ke et al., 1998; Warpman et al., 1998) containing combinations of α3, α5, α7 and β2, β4 subunits analogous to those normally found on autonomic ganglion neurons. For such reason SH-SY5Y cells have frequently been used as a model to investigate long-term alterations in nAChRs in the presence of cholinergic ligands (Peng et al., 1997; Warpman et al., 1998; Ridley et al., 2001). Nevertheless, there are no electrophysiological studies addressing this issue at single cell level.
Using SH-SY5Y cells, the present patch clamp study focused on the function of nAChRs under conditions known to induce their long-term perturbation. We wished to explore two main questions: (1) whether sustained agonist application led to complete loss of receptor sensitivity and how efficiently such receptors might recover from it; (2) to understand whether chronic agonist application led to compensatory changes in receptor function.
Methods
Cell culture
Human neuroblastoma SH-SY5Y cells, kindly provided by Dr C. Gotti (CNR Institute of Neuroscience, Milan, Italy), were maintained in culture in RPMI medium supplemented with 10% fetal calf serum, penicillin/streptomycin at 37°C in a 5% CO2/95% O2 sterile atmosphere. For the experiments, cells were plated on 35 mm polylysine (5 mg ml−1)-coated dishes. Chronic treatment with 10 μM nicotine was started by adding this drug to the culture medium on the second day of cell culture, and lasted 8 or 48 h with regular medium replacements.
Electrophysiological recording
In control experiments SH-SY5Y cells were continuously superfused with standard solution containing (in mM): NaCl 152, KCl 5, MgCl2 1, CaCl2 2, glucose 10, HEPES 10; pH was adjusted to 7.4 with NaOH. This concentration of extracellular Ca2+ appears to be optimal to observe reliable nicotine-induced currents on SH-SY5Y cells and their parent chromaffin cells (Gerzanich et al., 1995; Khiroug et al., 1997; Wang et al., 1998). Fast nicotinic currents were routinely induced with pressure application (20 psi; varying duration) of 1 mM nicotine with the puffer pipette placed 30–50 μm away from the patched cell (Di Angelantonio & Nistri, 2001). Nicotine and receptor antagonists were also applied by a fast superfusion system (Rapid Solution Changer RSC-200, BioLogic Science Instruments, Grenoble, France; Sokolova et al., 2004). For construction of dose–response plots nicotine concentrations were applied sequentially at 1–5 min interval (5 min for 1 mM nicotine).
To ensure reproducibility of results across cell populations either acutely (10 min) or chronically (8 or 48 h) treated with bath-applied nicotine, protocols to measure agonist or antagonist sensitivity, desensitization (and recovery from it) were standardized in terms of test doses of the puffer-applied agonist delivered at fixed time interval. To quantify recovery of AChR-mediated responses after chronic application of nicotine, we first recorded cells (without superfusing them) in the culturing medium containing 10 μM nicotine and measured their response to the standard nicotine puffer delivery. Thereafter, we continued to apply standard test pulses at fixed intervals after replacing the culture medium with control solution. Control cells were left in standard medium for the same time as those treated with chronic application of nicotine and patch clamped to provide reference data.
Patch pipettes had a resistance of 3–5 MΩ when filled (in mM) with CsCl 130; HEPES 20; MgCl2 1, Mg2ATP3 3; EGTA 10; pH was adjusted to 7.2 with CsOH. Cells were voltage clamped at −70 mV. Currents were filtered at 1 kHz and acquired with pCLAMP 8.0 software (Axon Instruments, Foster City, CA, U.S.A.).
All chemicals, including enzymes for cell culture, were from Sigma (Milan, Italy); culture mediums were obtained from Gibco BRL (Life Technologies, Milan, Italy), while α-conotoxin MII (MII) was from Tocris (Bristol, U.K.).
Data analysis
All data are presented as mean±s.e.m. (n=number of cells) with statistical significance assessed with paired t-test (for parametric data) or Mann–Whitney rank sum test (for nonparametric data). For concentration response curves a sigmoid function from Origin software (version 6.0) was used (Sokolova et al., 2004). A value of P<0.05 was accepted as indicative of statistically significant difference.
Results
Pharmacological properties of nAChRs in control conditions
The initial experiments characterized the pharmacological properties of nAChRs in untreated SH-SY5Y cells. Superfusion of nicotine (2 s pulse) produced inward currents as shown in the examples of Figure 1a (inset) which peaked and decayed before the end of the drug application. The concentration–peak amplitude response curve had 43 μM EC50 value and 1.7 Hill coefficient (Figure 1a). At 1 mM concentration, nicotine produced a maximal current of −169±14 pA (n=6) which fell rapidly to baseline and was followed by a rebound current on wash, presumably indicating a degree of open channel block by large concentrations of agonist (Maconochie & Knight, 1992; Drapeau & Legendre, 2001; Uteshev et al., 2002).
Figure 1.
Properties of native nAChRs expressed on SH-SY5Y cells. (a) Concentration–response curve for nicotine on control cells (n=8 cells). Insets show typical currents evoked by 100 μM or 1 mM concentrations applied by superfusion. Note rebound current at the end of 1 mM nicotine application. (b) 1 mM nicotine puffer pulse–response curve (n=6 cells). Insets demonstrate averaged currents induced by 50 or 200 ms pulses with rebound current after the 200 ms application. (c) Examples of currents elicited by 50 ms nicotine pulse at different membrane potentials as indicated in the scheme below with voltage steps from −70 mV holding potential. (d) Current–voltage (I–V) relation for nicotine pulse (50 ms) responses recorded from control cells (n=7).
Since the rapid current decay was presumably due to receptor desensitization, we performed additional control experiments to find out if nicotine, focally applied to the recorded cell via a puffer pipette, yielded a different response profile. Varying the puffer pulse duration allowed construction of pulse–response curves (Di Angelantonio & Nistri, 2001) as shown in Figure 1b with a maximal response of −159±15 pA (n=78) produced by 50 ms pulses. Even though the 50 ms current responses decayed to baseline with a monoexponential time constant (τ=150±13 ms; n=19), this phenomenon probably comprised a mixture of processes including receptor deactivation as well as fast desensitization. The latter was likely a major contributor to these responses because, at least on chromaffin cells (parents of SH-SY5Y cells), desensitization is rather fast (Maconochie & Knight, 1992). Furthermore, the response to a second 50 ms pulse of nicotine applied shortly after was readily inhibited (see example in Figure 2a), indicating that desensitization was occurring within this time frame. In keeping with a role of desensitization in currents evoked even by relatively short pulses of nicotine, is the observation that longer (2 s) pulses produced fading currents during the agonist application with a time constant value (τ=148±26 ms; n=10) very similar to the one after 50 ms pulses. Note that responses to long pulses were followed by a rebound current (see inset to Figure 1b for the 200 ms pulse of nicotine) in accordance with the data obtained with 1 mM application of nicotine via rapid superfusion (Figure 1a, inset). The reversal potential for nicotine-evoked currents was 6.8±2.0 mV (see Figure 1c and d).
Figure 2.
Recovery from desensitization and antagonist sensitivity of nAChRs on control SH-SY5Y cells. (a) Quantification of recovery from desensitization investigated with paired-pulse protocol on untreated cells. Ordinate provides amplitude of second response in each pair as per cent of the first one (n=8 cells). Insets show typical pairs of currents induced by 50 ms pulses of nicotine (arrowheads) applied at different time intervals. (b) Top, examples of averaged currents evoked by 50 ms pulses of nicotine before or during antagonist application. To aid comparison records are superimposed. Bottom, histograms with average data on antagonist sensitivity indicating that nAChRs of control cells have limited sensitivity to MLA (selective α7 antagonist; n=11), MII (selective α3/α6 antagonist; n=8) and DHβE (that at 1 μM concentration is preferentially selective for β2-containing receptors; n=5). A large concentration (100 μM) of DHβE, which exerts broad spectrum nAChR antagonism, strongly decreases nicotine responses (n=5). **P<0.01; *P<0.05.
By varying the time between pulses, it was possible to measure recovery from desensitization. As the shortest interval which could be used for pulse delivery without overlapping current responses was 1 s, at this time point the second current had already recovered to 61±7% of the first one (n=7), indicating prompt reattainment of nAChR-mediated responses despite their virtually complete current decay (Figure 2a).
Antagonist sensitivity of nAChRs was tested with methyllycaconitine (MLA) or MII (selective antagonists of α7- or α3-containing subunits, respectively; Dwoskin & Crooks, 2001; Nicke et al., 2004), which are expressed by SH-SY5Y cells (Lukas et al., 1990; Peng et al., 1997; Wang et al., 1998; Warpman et al., 1998). Either drug applied at 10 nM concentration for 5–10 min was moderately effective in blocking nicotine-induced currents (Figure 2b). As shown in Figure 2, a high concentration (100 μM) of dihydro-β-erythroidine (DHβE; at this concentration lacking subunit-specific effects) largely blocked nicotine-induced currents (n=5). At 1 μM concentration DHβE (a specific antagonist of α4β2 and α3β2 receptors; Dwoskin & Crooks, 2001) did not produce any change in the current amplitude (95±1%, n=5, Figure 2b, dashed line column).
Overall, these data suggest, under our experimental conditions, potent expression of nAChRs which underwent rapid, strong desensitization with quick recovery. nAChRs were probably heterogeneous in view of the limited block of subunit-selective antagonists.
Desensitization produced by continuous application of nicotine
In these experiments, 10 μM nicotine was continuously superfused (10 min) to produce desensitization of nAChRs, while responsiveness of these receptors was assessed with 50 ms puffer applications of 1 mM nicotine (see Figure 3a, left). As shown in the example of Figure 3a (right), nicotine (10 μM; filled bar) produced first a peak current (−163 pA), which then gradually decayed with a noisy baseline (presumably representing multiple channel openings; Maconochie & Knight, 1992). In seven out of 10 cells the current evoked by bath-applied nicotine decayed completely, while in the remaining three cells a very small residual current could be recorded up to the end of the 10 min application (−4.0±0.6 pA). Figure 3b presents averaged data on the development of desensitization to puffer pulses of nicotine in the continuous presence of bath-applied nicotine. The depression of nicotine-mediated responses followed a biexponential time course (first-time constant τ1=6±2 s; second-time constant τ2=96±30 s; n=10), which left a puffer pulse current amounting to 6±2% of control at 490 s from the start of bath application of nicotine. The biphasic nature of desensitization accords with previous reports of fast and slow nAChR desensitization (Maconochie & Knight, 1992; Khiroug et al., 1997). Recovery of puffer currents from nicotine (10 μM)-induced desensitization had gradual onset (see sample trace in Figure 3c) and biphasic time course (Figure 3d; τ1=14±6 s and τ2=277±120 s; n=7–10 cells) to attain a mean amplitude value of −170±18 pA.
Figure 3.
Desensitization properties of nAChRs to short-term nicotine application. (a) Left, current recorded in response to nicotine pulse (50 ms; arrowhead) in control conditions; right, sustained current evoked by superfusion (filled horizontal bar) of 10 μM nicotine during which test pulses of 1 mM nicotine (50 ms) are applied. The first test pulse is applied after 10 s from the start of 10 μM nicotine and then repeated at 1 min interval. The current evoked by continuous application of nicotine gradually fades back to baseline (not shown). (b) Plot of inhibition of responses to test pulses of nicotine applied every 60 s starting 10 s from the beginning of sustained 10 μM nicotine application (n=12). (c) Rapid return of responses to pulses of nicotine at the end of 10 μM nicotine bath application. The first pulse of nicotine is applied 10 s after washout of bath-applied nicotine. Different cell from (a). (d) Time course of return of nicotine pulse (50 ms)-induced currents during washout after 10 min nicotine application (10 μM); n=7–10.
Desensitization produced by chronic application of nicotine
The next series of experiments was designed for two purposes. First, to find out if, during rather prolonged exposure to nicotine, nAChRs preserved a degree of response. This issue was explored by measuring if there was any significant fast inward current elicited by pulses (50 ms) of nicotine. Second, it seemed interesting to study whether, after prolonged nicotine-dependent desensitization, nAChRs could recover their full sensitivity.
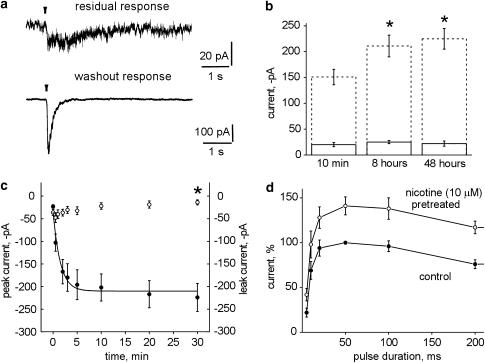
SH-SY5Y cells were chronically treated with nicotine (10 μM) for either 8 or 48 h, then patch-clamped and tested without washing out the nicotine-containing culture medium (control cells were plated and time matched like nicotine-treated cells). The majority of patched cells (90%) surprisingly responded to nicotine pulses with inward currents (see one example in Figure 4a, top) of −25±3 pA amplitude (n=26) for 8 h nicotine pretreatment, or −22±5 pA amplitude (n=24) for 48 h nicotine pretreatment. Thus, responses to test pulses of nicotine had the same amplitude regardless of the length of nicotine exposure (10 min, 8 h or 48 h; see solid columns in Figure 4b). The residual response was blocked (48±9%; n=6) by DHβE (100 μM). Likewise, MLA inhibited (28±7%; n=7) this response as much as MII did (25±8%; n=8). When cells were treated for 48 h with 1 mM nicotine, there was no response to 50 ms puffer pulses of nicotine (n=7).
Figure 4.
Long-term changes in nicotine-induced currents. (a) Examples of currents recorded in response to test pulse (50 ms) of 1 mM nicotine in culture solution containing 10 μM nicotine for 48 h (top) or after 30 min washout (bottom; same cell). (b) Solid lines in the bar chart show average amplitude of currents recorded in the continuous presence of nicotine (10 μM) applied for 10 min, 8 or 48 h in response to test pulse (50 ms) of nicotine (1 mM). Data are from 15 to 64 cells. Dashed columns represent average data of current amplitude after washout of nicotine applied for times indicated below (data are from 26 to 78 cells). Asterisks indicate statistically significant increase in the amplitude of responses from pretreated cells (P<0.05). (c) Time course of simultaneously recorded recovery in amplitude of test nicotine currents (50 ms; filled circles) and baseline leak current (open circles) during 30 min of washout after 48 h nicotine (10 μM) pretreatment; n=6. (d) Plots of current amplitude (as per cent of response to 50 ms of time-matched untreated cells) evoked by pulses of nicotine of varying duration. Open circles refer to cells treated for 8 h with 10 μM nicotine (n=6) while filled circles refer to time-matched untreated controls (n=8).
Standard solution was then used to washout the nicotine-containing culture medium for at least 30 min, and the amplitude of the responses to repeated test puffer pulses of nicotine (50 ms, 1 mM) was measured. Cells regained quickly their ability to generate robust inward currents as exemplified in Figure 4a, bottom. On average, after washing for 30 min, nicotine was applied for 8 or 48 h; the amplitude of responses to nicotine pulses was significantly larger (P<0.05) than that of the nicotine currents of untreated cells (Figure 4b, dashed columns). Note, however, that the τ value of current decay after 50 ms nicotine pulses was 156±16 ms (n=12), that is virtually the same as in untreated controls. When cells were exposed for 48 h to 1 mM nicotine (i.e. protocol used by Peng et al., 1997; Ke et al., 1998; Wang et al., 1998), and then washed for 30 min with standard solution, the amplitude of their responses to nicotine pulses was −201±20 pA (n=32), a value similar to the peak amplitude (−225±20 pA, n=20) recorded with a similar protocol based on the use of 10 μM nicotine exposure. Continuous presence of nicotine, therefore, produced upregulation of nicotinic receptors of SH-SY5Y cells in accordance with previous biochemical studies of the same cells (Peng et al., 1997; Ridley et al., 2001).
Figure 4c shows that the return of strong nAChR sensitivity following chronic desensitization was relatively fast, so that 90 s after the start of nicotine washout from the culture, the current amplitude (filled symbols) was already 50% of the value reached at steady state (30 min washout period). The increased amplitude of nicotine currents was not associated with a change in the leak current (open symbols in Figure 4c). Figure 4d plots the nicotine current amplitude as a function of increasing pulse duration after 8 h treatment with 10 μM nicotine or time-matched controls. It is clear that the two plots differed in the maximum amplitude.
We then tested if nAChRs upregulated by chronic nicotine treatment changed their functional properties. The reversal potential (6.7±1.8 mV; n=5) of 8 h treated cells was not significantly different from that of control cells, suggesting that the larger responses were not due to increased driving force for the ionic currents. Once the maximal enhancement of current amplitude had reached steady-state level, the τ value for current decay with 2 s pulses of nicotine was 139±12 ms (n=9), a result similar to the one of control-untreated cells. Recovery from desensitization induced by paired pulses of test nicotine applications was also very similar to the recovery of untreated cells (63±7% at 1 s interval; n=6; P>0.05 versus control). When we tested the sensitivity of upregulated receptors to antagonists, the blocking action by MLA, MII or DHβE (Figure 5a and b) was not significantly different from the one observed on control cells (see Figure 2b). Thus, long-term pretreatment with 10 μM nicotine did not apparently lead to preferential upregulation of subunits sensitive to MLA, MII or DHβE.
Figure 5.
Antagonist sensitivity of upregulated nAChRs. (a) After washout of 8 h nicotine (10 μM) application, typical currents are recorded in response to test pulses of nicotine (50 ms) in control solution or in the presence of 10 nM MLA, 10 nM MII, or 100 μM DHβE. (b) Histograms to quantify the extent of antagonist block of nicotine test pulses after 8 h nicotine (10 μM) treatment; **P<0.01; *P<0.05; n=7–20.
Discussion
The principal finding of the present study is the electrophysiological demonstration that nAChRs of SH-SY5Y cells readily desensitized, yet preserved a small degree of responsiveness even in the continuous presence of chronically applied nicotine. On agonist washout, upregulation of nAChR function appeared with no change in sensitivity to subunit-selective antagonists.
AChR profile of SH-SY5Y cells
These cells have been extensively used in previous studies concerned with nAChR changes due to long-term treatment with an agonist (Peng et al., 1997; Warpman et al., 1998; Ridley et al., 2001), though, to date, no single-cell electrophysiological data have been provided. Such cells express native nAChR subunits (α3, α5, α7 and β2, β4; Peng et al., 1997; Ke et al., 1998; Warpman et al., 1998) commonly found on mammalian neurons, making them a suitable model to examine the properties of neuronal AChRs which, on the vast majority of neurons, are a heterogeneous population. Additionally, because nAChR upregulation largely depends on the type of expression system used (Kuryatov et al., 1997; Paterson & Nordberg, 2000; Nelson et al., 2003) and the amount of expressed receptors before applying nicotine (Nashmi et al., 2003), studying native receptors offers certain experimental advantages. Finally, because nAChR plasticity might depend on the trafficking and coupling of various subunits (Nelson et al., 2003; Sallette et al., 2004; Darsow et al., 2005), investigating native neurons provides an important complement to more molecularly oriented studies of recombinant receptors, which can rarely replicate the native receptor population. Of course, one limitation of studies based on heterogeneous, native receptors is the difficulty to quantify the precise contribution by various receptor subunits to the overall changes in agonist responses.
In the present functional study on native receptors, the nicotine EC50 value was 43 μM, in keeping with the contribution by a heterogeneous receptor population to the observed current, and near the EC50 values for α3- or α7-containing receptors in such cells (Peng et al., 1997). We obtained comparable pharmacological results with either fast superfusion or puffer application of nicotine in accordance with previous studies (Di Angelantonio & Nistri, 2001), suggesting the suitability of puffer agonist applications to investigate nAChRs on these cells because of ease and rapidity of agonist delivery. A fraction of nAChRs probably included α7 ones in view of the partial blocking action of low concentrations of MLA, while a small fraction comprised α3-containing receptors sensitive to the MII (the toxin-sensitive α6 subunit is not apparently expressed by SH-SY5Y cells; Peng et al., 1997; Ke et al., 1998; Warpman et al., 1998). Since a low concentration of DHβE could not significantly affect nicotine-induced currents, it seems likely that α3β2 (or α4β2) receptors highly sensitive to DHβE (Dwoskin & Crooks, 2001) were a very small minority of the global, functional nAChR population. Nicotinic currents reversed near 0 mV, thus confirming that their open channels had the permeability expected for nAChRs (Jones et al., 1999).
Desensitization properties of nAChRs
Desensitization of nicotine-evoked currents developed rapidly with fast recovery. The speed of these processes might have outlined a preferential role of α3 subunit containing receptors (Quick & Lester, 2002), although our results with MII did not support this notion. A complementary protocol to study desensitization (Katz & Thesleff, 1957) relied on continuously applied, low (i.e. several times less than the EC50 value) concentration (10 μM) of nicotine that generated an initial current with subsequent decay to baseline. However, testing receptor sensitivity with a large dose of nicotine demonstrated that cells exposed to nicotine for 10 min retained a small response and recovered after washout. Perhaps these receptors belonged to the α3β2 type which has been found to generate incomplete desensitization when expressed in oocytes (Fenster et al., 1997).
nAChR sensitivity during chronic exposure to nicotine
Our goal was to understand whether chronic nicotine application could functionally upregulate native nAChRs. To this end it seemed necessary to understand first if nAChRs were fully desensitized by long agonist exposure, because some theories state that strong desensitization is a necessary requirement for upregulation, though this remains controversial (see Paterson & Nordberg, 2000; Buisson & Bertrand, 2002) and there are no electrophysiological data concerning nAChRs of SH-SY5Y cells under such conditions. As far as other nAChRs are concerned, there are limited functional data for α3β2 and α4β2 receptors expressed in oocytes (Hsu et al., 1996), and α4β2 receptors expressed in HEK293 cells (Buisson & Bertrand, 2001), all showing small residual responses following long-term exposure to agonist.
One interesting observation of the present study was that a small, yet clearly measurable response to nicotine remained in SH-SY5Y cells treated for 8 or 48 h with 10 μM nicotine with pharmacological characteristics analogous to control responses. This result suggests that there was a small number of receptors less prone to desensitization (Fenster et al., 1997; Olale et al., 1997; Paterson & Nordberg, 2000). Note, however, that with chronic application of a large concentration (1 mM) of nicotine no residual current was apparent, in keeping with the cyclic model of receptor operation comprising multiple desensitized states with their occurrence probability dependent on the agonist concentration (Katz & Thesleff, 1957; Paradiso & Steinbach, 2003). Washing out the chronically applied nicotine solution was not followed by an outward shift in leak current. This observation is not incompatible with the possibility that some nAChRs can be persistently activated by ambient ACh (Lester, 2004): if some nAChRs were tonically active, their number was just too small to contribute to the cell conductance at rest.
Upregulation of nAChRs
After long-term exposure to nicotine and subsequent washout, previous radioactive ligand-binding studies have shown very large upregulation of many AChR subtypes including α4β2 and α3β4 (Fenster et al., 1999; Gentry et al., 2003), α3β2 (Wang et al., 1998) and α7 (Molinari et al., 1998; Kawai & Berg, 2001) ones in various cells, comprising SH-SY5Y ones (Warpman et al., 1998; Ridley et al., 2001). There are, however, considerable discrepancies concerning the subtype predominantly enhanced. Furthermore, nAChRs of expression systems are more strongly upregulated than the same native receptors (Wang et al., 1998) and most of the upregulated subunits are intracellular (Peng et al., 1997). Since there is no change in mRNA of SH-SY5Y cells for any receptor subunit after chronic treatment with nicotine (Peng et al., 1997; Ke et al., 1998), it is likely that post-transcriptional mechanisms govern upregulation of nAChRs.
Functional studies give results not readily reconciled with binding data. In fact, population studies of intracellular Ca2+ levels in SH-SY5Y cells have shown that chronic nicotine treatment actually decreases responses to nicotine itself (Ridley et al., 2002). Likewise, radioactive Rb+ fluxes mediated by nAChRs of analogous cells are largely and persistently depressed (Ke et al., 1998). In either case measured responses are, however, a function of changes in membrane potential that was not controlled. Electrophysiological investigations with recombinant nAChRs expressed by single cells at constant membrane potential do show receptor upregulation after chronic nicotine administration (Molinari et al., 1998; Buisson & Bertrand, 2001), though the extent of enhancement is clearly less than the one found with binding experiments.
The present study is, therefore, the first electrophysiological report that native nAChRs of single SH-SY5Y cells were equi-effectively upregulated by a long-lasting application of 10 μM or 1 mM nicotine. Since these concentrations of nicotine evoked very different activation of nAChRs, yet similar degree of desensitization, we suggest that desensitization was an important contributor to receptor upregulation. Our observations are therefore in accordance with current theories stating that ‘the rationale behind nAChR upregulation is thought to lie in their rapid desensitization and consequent inactivation following chronic agonist exposure, putatively resulting in a deficit in cholinergic function, which is then counteracted by an increase in receptor number' (Paterson & Nordberg, 2000). Upregulation merely increased the maximum amplitude of the nicotine dose–response plot without altering sensitivity to antagonists (indicating that there was no preferential increase in certain receptor subunits), the current kinetics, desensitization time constant, or reversal potential.
Mechanisms underlying nAChR upregulation
To date, mechanistic interpretations of receptor upregulation stem from recombinant receptor studies. Thus, as far as α4β2 nAChRs are concerned, one theory predicts that, in control conditions, receptors exist in two interconvertible states (one with high affinity for nicotine, and the other one with low affinity for nicotine). Chronic exposure to nicotine would stabilize a large fraction of receptors in the high-affinity state (without neosynthesis of new receptors); consequently, upregulated receptors show higher single channel conductance and mediate larger responses (Buisson & Bertrand, 2002; Vallejo et al., 2005). Another hypothesis proposes that chronically applied nicotine converts pre-existing pools of immature nAChR subunits into high-affinity receptors by favouring specific intersubunit interactions to generate a distinct receptor stoichiometry (with lower ratio between α and β subunits) producing larger responses to agonists (Nelson et al., 2003; Sallette et al., 2004). In the case of α7 receptors, upregulation is suggested to be due to overexpression of surface receptors (without changes in their opening probability) mediated by phosphorylation (Cho et al., 2005). In all these cases, it is apparent that enhanced receptor activity is caused by post-translational mechanisms.
Our present data on a heterogeneous population of native receptors show that there was a simple upward shift in the nicotine dose–response curve without change in sensitivity to subunit-selective antagonists. The simplest interpretation is that the effect was due to a larger surface number of functional nAChRs after chronic exposure to nicotine without preferential enhancement of receptor subtypes.
Although nAChR upregulation may be a model to understand some of the effects due to chronic administration of nicotine, it is probable that the complex processes of nicotine tolerance and withdrawal additionally involve alterations in gene expression (Dunckley & Lukas, 2003) and regulatory protein kinases (Dajas-Bailador et al., 2002).
Acknowledgments
This work was supported by a PRIN grant from MIUR to A.N. We thank Dr Cecilia Gotti (CNR Institute of Neuroscience, Milan, Italy) for her kind supply of SH-SY5Y cells and Dr Massimo Righi for cell culture support.
Abbreviations
- ACh
acetylcholine
- DHβE
dihydro-β-erythroidine
- HEK293 cells
human embryonic kidney cells
- MII
α-conotoxin MII
- MLA
methyllycaconitine
- nAChR
nicotinic acetylcholine receptors
- SH-SY5Y cells
human neuroblastoma cells
References
- BUISSON B., BERTRAND D. Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. J. Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUISSON B., BERTRAND D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol. Sci. 2002;23:130–136. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- CHO C.H., SONG W., LEITZELL K., TEO E., MELETH A.D., QUICK M.W., LESTER R.A. Rapid upregulation of α7 nicotinic acetylcholine receptors by tyrosine dephosphorylation. J. Neurosci. 2005;25:3712–3723. doi: 10.1523/JNEUROSCI.5389-03.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAJAS-BAILADOR F., WONNACOTT S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol. Sci. 2004;25:317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- DAJAS-BAILADOR F.A., SOLIAKOV L., WONNACOTT S. Nicotine activates the extracellular signal-regulated kinase 1/2 via the α7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. J. Neurochem. 2002;80:520–530. doi: 10.1046/j.0022-3042.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- DARSOW T., BOOKER T.K., PINA-CRESPO J.C., HEINEMANN S.F. Exocytic trafficking is required for nicotine-induced up-regulation of α4β2 nicotinic acetylcholine receptors. J. Biol. Chem. 2005;280:18311–18320. doi: 10.1074/jbc.M501157200. [DOI] [PubMed] [Google Scholar]
- DI ANGELANTONIO S., NISTRI A. Calibration of agonist concentrations applied by pressure pulses or via rapid solution exchanger. J. Neurosci. Methods. 2001;110:155–161. doi: 10.1016/s0165-0270(01)00437-x. [DOI] [PubMed] [Google Scholar]
- DRAPEAU P., LEGENDRE P. Neuromuscular transmission on the rebound. Receptors Channels. 2001;7:491–496. [PubMed] [Google Scholar]
- DUNCKLEY T., LUKAS R.J. Nicotine modulates the expression of a diverse set of genes in the neuronal SH-SY5Y cell line. J. Biol. Chem. 2003;278:15633–15640. doi: 10.1074/jbc.M210389200. [DOI] [PubMed] [Google Scholar]
- DWOSKIN L.P., CROOKS P.A. Competitive neuronal nicotinic receptor antagonists: a new direction for drug discovery. J. Pharmacol. Exp. Ther. 2001;298:395–402. [PubMed] [Google Scholar]
- FENSTER C.P., RAINS M.F., NOERAGER B., QUICK M.W., LESTER R.A. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J. Neurosci. 1997;17:5747–5759. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENSTER C.P., WHITWORTH T.L., SHEFFIELD E.B., QUICK M.W., LESTER R.A. Upregulation of surface α4β2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J. Neurosci. 1999;19:4804–4814. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENTRY C.L., WILKINS L.H., Jr, LUKAS R.J. Effects of prolonged nicotinic ligand exposure on function of heterologously expressed, human α4β2- and α4β4-nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2003;304:206–216. doi: 10.1124/jpet.102.041756. [DOI] [PubMed] [Google Scholar]
- GERZANICH V., PENG X., WANG F., WELLS G., ANAND R., FLETCHER S., LINDSTROM J. Comparative pharmacology of epibatidine: a potent agonist for neuronal nicotinic acetylcholine receptors. Mol. Pharmacol. 1995;48:774–782. [PubMed] [Google Scholar]
- GINIATULLIN R., NISTRI A., YAKEL J.L. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- HSU Y.N., AMIN J., WEISS D.S., WECKER L. Sustained nicotine exposure differentially affects α3β2 and α4β2 neuronal nicotinic receptors expressed in Xenopus oocytes. J. Neurochem. 1996;66:667–675. doi: 10.1046/j.1471-4159.1996.66020667.x. [DOI] [PubMed] [Google Scholar]
- JONES S., SUDWEEKS S., YAKEL J.L. Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci. 1999;22:555–561. doi: 10.1016/s0166-2236(99)01471-x. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J. Physiol. 1957;138:63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAI H., BERG D.K. Nicotinic acetylcholine receptors containing α7 subunits on rat cortical neurons do not undergo long-lasting inactivation even when up-regulated by chronic nicotine exposure. J. Neurochem. 2001;78:1367–1378. doi: 10.1046/j.1471-4159.2001.00526.x. [DOI] [PubMed] [Google Scholar]
- KE L., EISENHOUR C.M., BENCHERIF M., LUKAS R.J. Effects of chronic nicotine treatment on expression of diverse nicotinic acetylcholine receptor subtypes. I. Dose- and time-dependent effects of nicotine treatment. J. Pharmacol. Exp. Ther. 1998;286:825–840. [PubMed] [Google Scholar]
- KHIROUG L., GINIATULLIN R., SOKOLOVA E., TALANTOVA M., NISTRI A. Imaging of intracellular calcium during desensitization of nicotinic acetylcholine receptors of rat chromaffin cells. Br. J. Pharmacol. 1997;122:1323–1332. doi: 10.1038/sj.bjp.0701518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURYATOV A., GERZANICH V., NELSON M., OLALE F., LINDSTROM J. Mutation causing autosomal dominant nocturnal frontal lobe epilepsy alters Ca2+ permeability, conductance, and gating of human α4β2 nicotinic acetylcholine receptors. J. Neurosci. 1997;17:9035–9047. doi: 10.1523/JNEUROSCI.17-23-09035.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER H.A., DIBAS M.I., DAHAN D.S., LEITE J.F., DOUGHERTY D.A. Cys-loop receptors: new twists and turns. Trends Neurosci. 2004;27:329–336. doi: 10.1016/j.tins.2004.04.002. [DOI] [PubMed] [Google Scholar]
- LESTER RA. Activation and desensitization of heteromeric neuronal nicotinic receptors: implications for non-synaptic transmission. Bioorg. Med. Chem. Lett. 2004;14:1897–1900. doi: 10.1016/j.bmcl.2004.02.081. [DOI] [PubMed] [Google Scholar]
- LESTER R.A., DANI J.A.J. Acetylcholine receptor desensitization induced by nicotine in rat medial habenula neurons. J. Neurophysiol. 1995;74:195–206. doi: 10.1152/jn.1995.74.1.195. [DOI] [PubMed] [Google Scholar]
- LUKAS R.J., AUDHYA T., GOLDSTEIN G., LUCERO L. Interactions of the thymic polypeptide hormone thymopoietin with neuronal nicotinic α-bungarotoxin binding sites and with muscle-type, but not ganglia-type, nicotinic acetylcholine receptor ligand-gated ion channels. Mol. Pharmacol. 1990;38:887–894. [PubMed] [Google Scholar]
- MACONOCHIE D.J., KNIGHT D.E. A study of the bovine adrenal chromaffin nicotinic receptor using patch clamp and concentration-jump techniques. J. Physiol. 1992;454:129–153. doi: 10.1113/jphysiol.1992.sp019257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAELICKE A., SCHRATTENHOLZ A., SAMOCHOCKI M., RADINA M., ALBUQUERQUE E.X. Allosterically potentiating ligands of nicotinic receptors as a treatment strategy for Alzheimer's disease. Behav. Brain. Res. 2000;113:199–206. doi: 10.1016/s0166-4328(00)00214-x. [DOI] [PubMed] [Google Scholar]
- MOLINARI E.J., DELBONO O., MESSI M.L., RENGANATHAN M., ARNERIC S.P., SULLIVAN J.P., GOPALAKRISHNAN M. Up-regulation of human α7 nicotinic receptors by chronic treatment with activator and antagonist ligands. Eur. J. Pharmacol. 1998;347:131–139. doi: 10.1016/s0014-2999(98)00084-3. [DOI] [PubMed] [Google Scholar]
- NASHMI R., DICKINSON M.E., MCKINNEY S., JAREB M., LABARCA C., FRASER S.E., LESTER H.A. Assembly of α4β2 nicotinic acetylcholine receptors assessed with functional fluorescently labeled subunits: effects of localization, trafficking, and nicotine-induced upregulation in clonal mammalian cells and in cultured midbrain neurons. J. Neurosci. 2003;23:11554–11567. doi: 10.1523/JNEUROSCI.23-37-11554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON M.E., KURYATOV A., CHOI C.H., ZHOU Y., LINDSTROM J. Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Mol. Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- NICKE A., WONNACOTT S., LEWIS R.J. Conotoxins as tools for the elucidation of structure and function of neuronal nicotinic acetylcholine receptor subtypes. Eur. J. Biochem. 2004;271:2305–2319. doi: 10.1111/j.1432-1033.2004.04145.x. [DOI] [PubMed] [Google Scholar]
- OLALE F., GERZANICH V., KURYATOV A., WANG F., LINDSTROM J. Chronic nicotine exposure differentially affects the function of human α3, α4, and α7 neuronal nicotinic receptor subtypes. J. Pharmacol. Exp. Ther. 1997;283:675–683. [PubMed] [Google Scholar]
- PARADISO K.G., STEINBACH J.H. Nicotine is highly effective at producing desensitization of rat α4β2 neuronal nicotinic receptors. J. Physiol. 2003;553:857–871. doi: 10.1113/jphysiol.2003.053447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATERSON D., NORDBERG A. Neuronal nicotinic receptors in the human brain. Prog. Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- PENG X., GERZANICH V., ANAND R., WANG F., LINDSTROM J. Chronic nicotine treatment up-regulates α3 and α7 acetylcholine receptor subtypes expressed by the human neuroblastoma cell line SH-SY5Y. Mol. Pharmacol. 1997;51:776–784. doi: 10.1124/mol.51.5.776. [DOI] [PubMed] [Google Scholar]
- PICCIOTTO M.R., ZOLI M. Nicotinic receptors in aging and dementia. J. Neurobiol. 2002;53:641–655. doi: 10.1002/neu.10102. [DOI] [PubMed] [Google Scholar]
- QUICK M.W., LESTER R.A. Desensitization of neuronal nicotinic receptors. J. Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- RIDLEY D.L., PAKKANEN J., WONNACOTT S. Effects of chronic drug treatments on increases in intracellular calcium mediated by nicotinic acetylcholine receptors in SH-SY5Y cells. Br. J. Pharmacol. 2002;135:1051–1059. doi: 10.1038/sj.bjp.0704508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIDLEY D.L., ROGERS A., WONNACOTT S. Differential effects of chronic drug treatment on α3* and α7 nicotinic receptor binding sites, in hippocampal neurones and SH-SY5Y cells. Br. J. Pharmacol. 2001;133:1286–1295. doi: 10.1038/sj.bjp.0704207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALLETTE J., BOHLER S., BENOIT P., SOUDANT M., PONS S., LE NOVERE N., CHANGEUX J.P., CORRINGER P.J. An extracellular protein microdomain controls up-regulation of neuronal nicotinic acetylcholine receptors by nicotine. J. Biol. Chem. 2004;279:18767–71875. doi: 10.1074/jbc.M308260200. [DOI] [PubMed] [Google Scholar]
- SOKOLOVA E., SKORINKIN A., FABBRETTI E., MASTEN L., NISTRI A., GINIATULLIN R. Agonist-dependence of recovery from desensitization of P2X3 receptors provides a novel and sensitive approach for their rapid up or downregulation. Br. J. Pharmacol. 2004;141:1048–1058. doi: 10.1038/sj.bjp.0705701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTESHEV V.V., MEYER E.M., PAPKE R.L. Activation and inhibition of native neuronal alpha-bungarotoxin-sensitive nicotinic ACh receptors. Brain Res. 2002;948:33–46. doi: 10.1016/s0006-8993(02)02946-3. [DOI] [PubMed] [Google Scholar]
- VALLEJO Y.F., BUISSON B., BERTRAND D., GREEN W.N. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J. Neurosci. 2005;25:5563–5572. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG F., NELSON M.E., KURYATOV A., OLALE F., COOPER J., KEYSER K., LINDSTROM J. Chronic nicotine treatment up-regulates human α3β2 but not α3β4 acetylcholine receptors stably transfected in human embryonic kidney cells. J. Biol. Chem. 1998;273:28721–28732. doi: 10.1074/jbc.273.44.28721. [DOI] [PubMed] [Google Scholar]
- WARPMAN U., FRIBERG L., GILLESPIE A., HELLSTROM-LINDAHL E., ZHANG X., NORDBERG A. Regulation of nicotinic receptor subtypes following chronic nicotinic agonist exposure in M10 and SH-SY5Y neuroblastoma cells. J. Neurochem. 1998;70:2028–2037. doi: 10.1046/j.1471-4159.1998.70052028.x. [DOI] [PubMed] [Google Scholar]
- WONNACOTT S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]