Abstract
Caffeic acid phenethyl ester (CAPE), an active component of propolis from honeybee hives (honeybee resin), has anti-inflammatory, anti-carcinogenic and anti-bacterial properties. This study was designed to investigate the anti-inflammatory effects of CAPE on Helicobacter pylori-induced NF-κB and AP-1 in the gastric epithelial cell line AGS.
Electrophoretic mobility shift assay was used to measure NF-κB- and AP-1-DNA binding activity. Western blotting was used to detect IκB-α and COX-2 expression in AGS cells cocultured with H. pylori. The antiproliferative effect of CAPE was measured by MTT assay.
Our results showed that caffeic phenethyl ester inhibits H. pylori-induced NF-κB and AP-1 DNA-binding activity in a dose (0.1–25 μg ml−1∼0.35–88 μM) and time- (15–240 min) dependent manner in AGS cells. Maximum inhibition by CAPE was observed at concentrations of 25 μg ml−1 (∼88 μM) CAPE prevented H. pylori- and cytokine-induced degradation of IκB-α protein.
Pretreatment of AGS cells with CAPE also blocked cytokine- and mitogen-induced NF-κB and AP-1 expression. Furthermore, CAPE suppressed H. pylori-induced cell proliferation and production of the cytokines TNF-α and IL-8. In addition, CAPE blocked H. pylori-induced COX-2 expression.
The inhibition of such transcription by CAPE could result in suppression of many genes during H. pylori-induced inflammation, and also provide new insights into the anti-cancer and anti-inflammatory properties of CAPE.
Keywords: CAPE, H. pylori, NF-κB, AP-1, COX-2, gastric epithelial cells
Introduction
Honey is a traditional remedy for dyspepsia and there have been a number of reports suggesting that honey can inhibit the growth of Helicobacter pylori (Ali et al., 1991; Osato et al., 1999), the causative agent of gastric ulcer and cancer (Graham, 1989; Nomura et al., 1991), and of other pathogenic organisms. However, the molecular mechanisms for its antibacterial effects are unclear. Caffeic acid phenethyl ester (CAPE), an active component of propolis from honeybee hives (honeybee resin), has been reported to have anti-inflammatory, anti-carcinogenic and immunomodulatory properties (Grunberger et al., 1988).
CAPE has many biological and pharmacological effects, including antioxidant properties and tumour cell cytotoxicity. Various investigators have demonstrated an anti-inflammatory action for CAPE both in vitro and in vivo (Michaulart et al., 1999; Orban et al., 2000). CAPE has been reported to be a specific inhibitor of nuclear factor-kappa B (NF-κB), which may account for some of its anti-inflammatory properties (Natarajan et al., 1996; Fitzpatrick et al., 2001). NF-κB resides in the cytoplasm in an inactive form as a heterodimer consisting of p50 and p65 (RelA) subunits complexed to the inhibitory molecule IκB, which prevents the migration of the heterodimer to the nucleus. Following a range of stimuli in many cell types, NF-κB translocates to the nucleus and binds to its specific DNA site and subsequently upregulates gene expression (Kopp & Ghosh, 1995; Barens & Karin, 1997). CAPE also alters the redox state, induces apoptosis, suppresses lipid peroxidation and displays antioxidant activity (Kimura et al., 1985; Chiao et al., 1995; Laranjinha et al., 1995). CAPE has also been shown to inhibit the growth of different types of transformed cells (Guarini et al., 1992; Su et al., 1994; Burke et al., 1995).
The transcription factor NF-κB plays a central role in regulating various host responses during the inflammatory process. Other transcription factors such as activator protein-1 (AP-1) also play an important role in the regulation of cellular functions, including proliferation and apoptosis, and may work in concert with NF-κB to elicit the inflammatory response during the infection. Direct contact between H. pylori and gastric epithelial cells induces NF-κB (Keates et al., 1997; Maeda et al., 2000), AP-1 (Meyer-ter-Vehn et al., 2000) and COX-2 expression (Kim et al., 2001) in gastric epithelial cells. The activation of such transcription factors by microbial pathogens including H. pylori determines the outcome of the cellular innate immune defence. Therefore, exploitation of the mechanisms causing activation represents an important field of potential therapeutic intervention that may be relevant to several inflammatory disease states.
The aim of our study was to examine the effect of CAPE on the transcription factors NF-κB and AP-1 activities during H. pylori infection of gastric epithelial cells. CAPE inhibited H. pylori-induced NF-κB, AP-1 and COX-2 expression in AGS cells. Furthermore, cytokine levels of tumour necrosis factor-α (TNF-α) and IL-8 were significantly reduced in CAPE-treated cells. Our findings demonstrate that CAPE modulates H. pylori-induced NF-κB and AP-1 activities in gastric epithelial cells and also downregulates the production of the cytokines.
Methods
Materials
NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′) and AP-1 (c-Jun) (5′-CGCTTGATGAGTCAGCCGGAA-3′) consensus oligonucleotides were obtained from Promega Corp. (Madison, WI, U.S.A.). Polyclonal antibodies to p65 and IκB-α, NF-κB supershift antibodies (anti-p50 (sc-114X), anti-p65 (sc-109X) anti-c-Rel (sc-70X)) and AP-1 supershift antibodies (anti-Fra-1, anti-c-Fos, anti-c-Jun and anti-Jun D) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Polyclonal COX-2 antibody was purchased from Cayman Chemical Company (Ann Arbor, MI, U.S.A.). [γ32P]ATP (35 pmol, 3000 Ci mmol−1) was from Amersham International (Aylesbury, U.K.). Poly(dI-dC) was obtained from Pharmacia (Biosystems, Milton Keynes, U.K.). CAPE, ascorbic acid (sodium salt), N-acetylcysteine (NAC), TNF-α, phorbol 12-myristate 13-acetate (PMA) and β-actin monoclonal antibody were obtained from Sigma (Poole, Dorset, U.K.). IL-8 and TNF-α enzyme-linked immunosorbant assay (ELISA) kits were from Pharmingen.
CAPE was dissolved in 50% ethanol; ascorbic acid (sodium salt) was dissolved in phosphate-buffered saline (PBS, pH 7.4) and NAC in dimethylsulphoxide (DMSO). All reagents were prepared immediately before each experiment. Appropriate dilutions of CAPE and other agents were made in cell culture medium just prior to use. The final amount of DMSO and ethanol was 0.1% ethanol/DMSO used in untreated controls.
Cell culture
The gastric epithelial cell line AGS was obtained from the European Collection of Animal Cell Cultures (ECACC) (Porton Down, Salisbury, U.K.). AGS cells were grown in RPMI 1640 medium supplemented with 10% filtered foetal calf serum (FCS), 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 2 mM L-glutamine. AGS cells were removed from flasks by trypsin/EDTA treatment and seeded at a density 5 × 105 cells ml−1 for experiments. As HuT 78 cells, a T-cell line derived from a human Sezary lymphoma (ECACC) contains high levels of constitutive NF-κB (O'Connell et al., 1995); these cells (1 × 106) were used as a positive control in electrophoretic mobility shift assay.
H. pylori culture
H. pylori reference strain NCTC 11638 obtained from the National Collection of Type Cultures (Colindale, U.K.) was used in this study. The bacteria were grown in a microaerobic humidified atmosphere on 7% lysed horse blood Columbia agar at 37°C. After 48–72 h, bacteria were harvested in PBS (pH 7.4) containing 8 mM Na2HPO4, 1.5 mM KH2PO4, 137 mM NaCl and 2.7 mM KCl or RPMI 1640 medium without antibiotics and re-suspended to a concentration of 6 × 108 colony-forming units (CFU) ml−1 using the McFarland standard kit and used immediately.
Cell culture treatments
Confluent AGS cells were preincubated with various amounts of CAPE for various periods of time followed by stimulation with a freshly prepared suspension of H. pylori (6 × 108 CFU ml−1), the cytokine TNF-α (20 ng ml−1)) or the phorbol ester PMA (20 ng ml−1) for 2 h, as shown in figure legends. The ratio of H. pylori to AGS cells is 100 : 1 and uninfected cells were used as a control in each experiment.
Total cell extract preparation
Cells were collected by centrifugation at 1400 r.p. m. for 5 min. The pellet of cells was re-suspended in lysis buffer containing 20 mM Tris-HCl (pH 7.5), 1% (w v−1) sodium dodecyl sulphate (SDS), 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 0.5 mM phenylmethylsulphonylfluoride (PMSF) and leupeptin (10 μg ml−1), and then the cells were solubilized by boiling for 5 min.
Western blot analysis
Equivalent amounts of cell lysates were resolved by electrophoresis through polyacrylamide gels using 10% separating gels according to the method of Laemmli (1970). Proteins were electrotransferred onto PVDF membrane using a semidry blotting apparatus (Atto). Blots were blocked with 5% (w v−1) dried skim milk in PBS for 1 h at room temperature and then incubated for 1 h at room temperature with the appropriate primary antibody (anti-IκB-α or anti-COX-2 at a dilution of 1 : 1000). A β-actin monoclonal antibody was used at a dilution of 1 : 5000 to ensure equal protein loading. Blots were then incubated with appropriate secondary antibody (at a dilution of 1 : 1000) for 1 h at room temperature. Immunodetection was performed by enhanced chemiluminescence.
Nuclear extract preparation
Nuclear extracts were prepared from AGS cells as described previously (Osborn et al., 1989). Briefly, AGS cells were washed twice in ice-cold PBS. The cells were pelleted by centrifugation at 1400 r.p.m. for 5 min and washed once in (1 ml) buffer A (10 mM Hepes (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM PMSF and 0.5 mM dithiothreitol (DTT) and centrifuged at 10,000 r.p.m. for 10 min. The pellet of cells was then resuspended in buffer A (20 μl) containing 0.1% (v v−1) NP-40 for 10 min on ice and lysed cells were centrifuged at 10,000 r.p.m. for 10 min. The supernatant was discarded and the nuclear pellet was extracted with (15 μl) buffer C (20 mM Hepes (pH 7.9), 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% (w v−1) glycerol and 0.5 mM PMSF) for 15 min on ice. After incubation, the nuclei were centrifuged at 10,000 r.p.m. for 10 min and the supernatant was diluted with 4 v of buffer D (10 mM Hepes (pH 7.9), 50 mM KCl, 0.2 mM EDTA, 25% (w v−1) glycerol and 0.5 mM PMSF). The nuclear extracts were used immediately or stored at −70°C until required. The protein concentration was determined on nuclear extracts by the method of Bradford (1979).
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts (4 μg protein) were incubated with 10,000 c.p.m. of NF-κB or AP-1 that had been previously labelled with (γ32P)ATP (10 mCi mmol−1) at the 5′-ends with T4 polynucleotide kinase. The assay was performed in 20 μl of binding buffer (10 mM Tris (pH 7.5), 4% (w v−1) glycerol, 5 mM DTT, 1 mM EDTA, 100 mM NaCl and 0.1 mg ml−1 nuclease-free BSA) in the presence of 2 μg poly(dI-dC) as non-specific competitor. The reaction mixture was then incubated for 30 min at room temperature after the addition of the probe DNA. The binding reaction was terminated using a loading dye (0.25% bromophenol, 0.25% xylene cyenol, 30% (w v−1) glycerol in deionized water) prior to electrophoretic separation of the DNA–protein complexes on 5% polyacrylamide gels that had been pre-electrophoresed for 30 min at 80 V. Gels were run at 150 V for 1–2 h at room temperature. After electrophoresis, the gels were dried and autoradiographed at −70°C for 24–36 h with intensifying screens.
Immunofluorescence
For immunofluorescence analysis, AGS cells grown on eight-well permanox chamber slides (Nunc, Naperville, IL, U.S.A.) were pretreated with CAPE (25 μg ml−1) for 1 h, followed by 2 h stimulation with H. pylori (6 × 108 CFU ml−1) at 37°C. The slides were gently washed with sterile PBS, fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 in PBS for 10 min. The slides were incubated with primary antibody anti-p65 for 1 h at room temperature, washed three times with 0.1% Tween 20 in PBS, followed by 30 min incubation with FITC-conjugated secondary antibody. Coverslips were mounted and images were acquired on Nikon TE 300 inverted microscope equipped with Leica DC-100 colour digital camera. Confocal microscopy was carried out on Axiovert × 100 TV microscope (Zeiss, Germany), using a Bio-Rad MRC 1024 confocal attachment (Hertfordshire, U.K.).
Cell proliferation
The antiproliferative effect of CAPE on gastric epithelial cells was determined by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay. AGS cells (1 × 105 cells ml−1) were incubated with CAPE (25 μg ml−1) for 1 h and then stimulated with either H. pylori (6 × 108 CFU ml−1) for an additional 8 h. To the cultured cells, 20 μl of freshly prepared MTT solution was added to each well and the plates were incubated for 4 h at 37°C. The absorbance of these wells was read at 492 nm using an ELISA plate reader. MTT assay was repeated three times in triplicate with similar results and the results are presented as the mean±standard deviation (s.d.).
Evaluation of cytokine production
AGS cells were seeded in 96-well plates (1 × 105 cells in 200 μl) overnight at 37°C. AGS cells were incubated with freshly harvested H. pylori (6 × 108 CFU ml−1) for 6 h in the presence or absence of a 1 h pretreatment with CAPE (25 μg ml−1). At the end of treatment, cell supernatants were collected and assayed for IL-8 and TNF-α production by ELISA according to the manufacturer instructions (Pharmingen). The minimum detectable dose of TNF-α was 2 pg ml−1 and 0.8 pg ml−1 for IL-8. All samples and controls were performed in triplicate and the average of the readings was taken. Recombinant human TNF-α and IL-8 standards as provided by the manufacturer were used to calculate the cytokine concentrations in each test sample. Cytokine concentrations are expressed as pg ml−1.
Statistical analysis
Data are expressed as the mean±s.d. values from at least three independent experiments with similar results. The Student's t-test or analysis of variance (ANOVA) was used to assess the statistical significance of the difference. P-values less than 0.05 (P<0.05) were considered statistically significant.
Results
H. pylori induces NF-κB DNA-binding activity in AGS cells
NF-κB consists of two subunits (p50/p65), which are bound to the inhibitor IκB and thus sequestered in the cytoplasm in resting cells. Upon activation, IκB is degraded and NF-κB translocated into the nucleus, where it binds to the promoter region of various genes including COX-2 and proinflammatory cytokines such as TNF-α and IL-8, and activates their transcription. It is well known that H. pylori induces NF-κB in gastric epithelial cells (Keates et al., 1997; Maeda et al., 2000). Exposure of the gastric epithelial cell line AGS to H. pylori (6 × 108 CFU ml−1) for 2 h induces NF-κB DNA-binding activity in these cells (Figure 1a). Competition assays with × 10- and × 100-fold molar excess of cold NF-κB oligonucleotide confirmed the specificity of NF-κB DNA complex induced by H. pylori. Figure 1a demonstrates that the addition of × 100-fold molar excess of cold NF-κB oligonucleotide completely abolished NF-κB DNA-complex formation (Figure 1a), whereas the addition of × 100-fold molar excess of cold AP-1 oligonucleotide had no effect on the formation of this induced DNA complex. Supershift studies were performed to identify the composition of the NF-κB DNA complex induced by H. pylori using antibodies directed against various NF-κB subunits (p50, p65 and c-Rel). The reaction mixture containing nuclear extracts were preincubated with anti-p50, anti-p65 or anti-c-Rel for 30 min prior to gel electrophoresis. Antibodies to p50 and p65 induced a supershift of the NF-κB–DNA complex, which confirms the presence of both p50 and p65 in the NF-κB DNA complex, while anti-c-Rel had no effect in the supershift assay (Figure 1b). The addition of × 100-fold molar excess of NF-κB oligonucleotide completely abolished NF-κB DNA-complex formation.
Figure 1.
H. pylori induces NF-κB DNA-binding activity in AGS cells. AGS cells were incubated with H. pylori (6 × 108 CFU ml−1) for 2 h and nuclear extracts were prepared and NF-κB DNA-binding activity was analysed by gel shift assay as described under Methods section. (a) Competition assay for the specificity of H. pylori-induced NF-κB DNA binding was performed using × 10- and × 100-fold molar excesses of cold NF-κB oligonucleotide or × 100-fold molar excess of cold AP-1 oligonucleotide. (b) Supershift assay was performed on the same nuclear extracts prepared from H. pylori-treated AGS cells after 30 min incubation with or without 0.5 μl of rabbit antisera to p50; lane 3, p65; lane 4, c-Rel; lane 5 and × 100-fold molar excess of cold NF-κB; lane 6. Nuclear extract from HuT 78 cells, which contain high levels of constitutive NF-κB, was used as a positive control (pc); lane 7. A representative gel of three independent experiments with similar results is shown.
CAPE inhibits H. pylori induced NF-κB DNA-binding activity in AGS cells
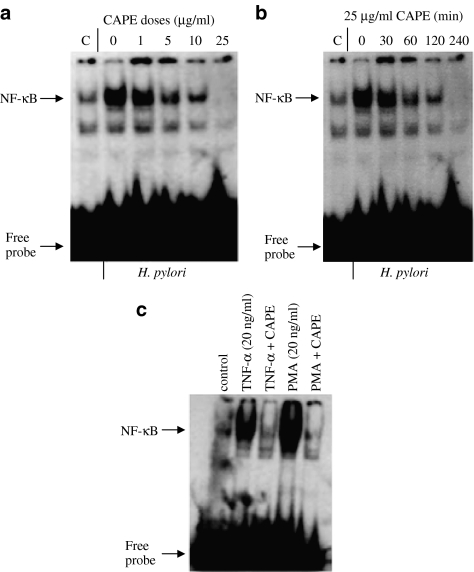
We tested whether CAPE could affect H. pylori-induced NF-κB DNA-binding activity in AGS cells. AGS cells were pretreated with different amounts of CAPE for different periods of time. Pretreatment of AGS cells with CAPE inhibited H. pylori-induced NF-κB DNA binding in a dose- and time-dependent manner. Maximum inhibition of NF-κB was observed with ⩾10 μg ml−1 of CAPE (Figure 2a). Time-course experiments showed that inhibition of NF-κB by CAPE was observed as early as 30 min (Figure 2b). We also examined the effects of CAPE on NF-κB activation in response to TNF-α and PMA. Figure 2c demonstrates that CAPE was able to block to TNF-α- and PMA-induced NF-κB DNA-binding activity in AGS cells.
Figure 2.
CAPE inhibits H. pylori-induced NF-κB DNA-binding activity. AGS cells were pretreated with different amounts of CAPE (1–25 μg ml−1) for 1 h (a) or AGS cells were exposed to 25 μg ml−1 CAPE for different periods of time ranging from 30 min to 4 h (b), before coculture of AGS cells with H. pylori (6 × 108 CFU ml−1) for a further 2 h. (c) AGS cells were preincubated with CAPE (25 μg ml−1) for 1 h, followed by a 2 h treatment with TNF-α (20 ng ml−1) or PMA (20 ng ml−1). At the end of incubation, nuclear extracts were prepared and gel shift assays for NF-κB DNA-binding activity were performed with radiolabelled NF-κB (as described under Methods section). Experiments were performed three times with similar results and a representative gel is shown.
CAPE prevents IκB-α degradation and p65 translocation
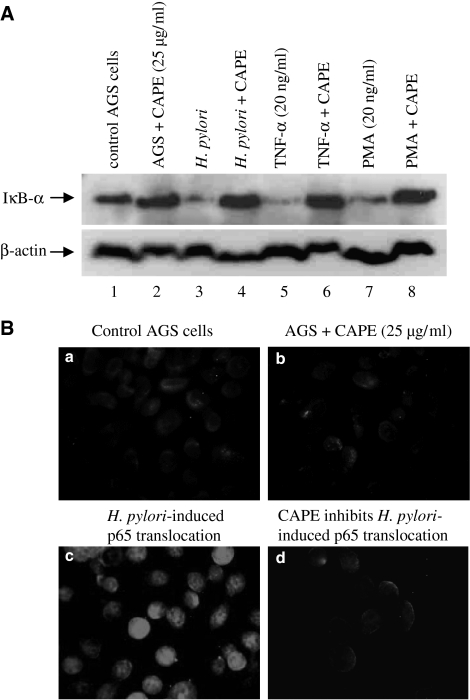
We further assessed the degradation of IκB-α in the cytosol by Western blot analysis and the nuclear translocation of the p65 subunit of NF-κB by immunofluorescence. Western blottting with antibody against IκB-α showed that CAPE prevented the degradation of IκB-α induced by H. pylori, TNF-α and PMA (Figure 3A). The increase in IκB-α levels was coincident with the observed inhibition of NF-κB DNA-binding activity. The IκB-α immunoblot was stripped and re-probed with anti-β-actin antibody to demonstrate equal protein loading. Furthermore, immunofluorescence was performed to determine the subcellular localization of p65 in AGS cells in response to CAPE. CAPE had no effect on the translocation of p65 in control AGS cells (Figure 3Bb) compared to untreated AGS cells (Figure 3Ba). H. pylori induced p65 translocation in AGS cells (Figure 3Bc) and CAPE pretreatment inhibited H. pylori-induced p65 translocation (Figure 3Bd).
Figure 3.
CAPE prevents IκB-α degradation and p65 translocation. (A) IκB-α immunoblotting. AGS cells were pretreated with CAPE (25 μg ml−1) for 1 h followed by 2 h stimulation with H. pylori (6 × 108 CFU ml−1), TNF-α (20 ng ml−1) or PMA (20 ng ml−1) for a further 2 h. Total cell extracts were prepared, separated by 10% polyacrylamide gels, blotted onto PVDF membrane and probed with anti-IκB-α antibody (as described under Methods). The immunoblot was stripped and re-probed for β-actin to ensure equal protein loading. (B) p65 Immunofluorescence. AGS cells grown on eight-well chamber slides were pretreated with CAPE (25 μg ml−1) for 1 h followed by 2 h coculture with H. pylori (6 × 108 CFU ml−1). The slides were fixed in 4% paraformaldehyde for 2 h at room temperature and permeabilized with 0.3% Triton X-100 in PBS. The slides were incubated with anti-p65 for 1 h at room temperature, followed by 30 min incubation with FITC-conjugated anti-rabbit antibody. Cells were visualized by fluorescence microscopy. Each experiment was repeated three times and one result is shown. (a) control AGS cells; (b) AGS cells+CAPE (25 μg ml−1), (c) H. pylori (6 × 108 CFU ml−1), (d) H. pylori+CAPE.
H. pylori induces AP-1 DNA-binding activity in AGS cells
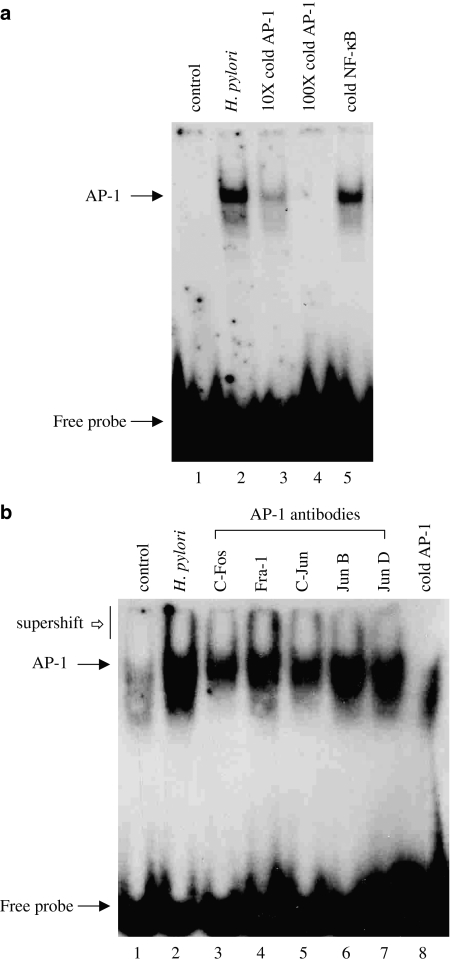
The transcription factor AP-1, which regulates the transcription of several genes involved in proliferation and apoptosis, is also activated during H. pylori infection (Meyer-ter-Vehn et al., 2000). Exposure of AGS cells to H. pylori (6 × 108 CFU ml−1) for 2 h induced AP-1 DNA-binding activity (Figure 4a). Competition assays with × 10- and × 100-fold molar excess of cold AP-1 oligonucleotide confirmed the specificity of AP-1 DNA complex induced by H. pylori, whereas the addition of × 100-fold molar excess of cold AP-1 oligonucleotide completely abolished AP-1 DNA-complex formation (Figure 4a). On the other hand, the addition of × 100-fold molar excess of cold NF-κB oligonucleotide had no effect on this induced DNA complex. Supershift studies were performed to identify the composition of the AP-1 DNA complex induced by H. pylori using antibodies directed against various AP-1 subunits (c-Fos, c-Jun, JunB and JunD). Supershift assay identified the presence of c-Fos, c-Jun and JunD, but not Fra-1 and JunB, in the H. pylori-induced AP-1–DNA complex, as judged by a decrease in the intensity of H. pylori-induced AP-1–DNA complex (Figure 4b).
Figure 4.
H. pylori induces AP-1 DNA-binding activity in AGS cells. AGS cells were incubated with H. pylori (6 × 108 CFU ml−1) for 2 h and nuclear extracts were prepared and AP-1 DNA-binding activity was analysed by gel shift assay as described under Methods section. (a) Competition assay for the specificity of H. pylori-induced AP-1 DNA-binding was performed using × 10- and × 100-fold molar excesses of cold AP-1 oligonucleotide or × 100-fold molar excess of cold NF-κB oligonucleotide. (b) Supershift assay was performed on the same nuclear extracts prepared form H. pylori-treated AGS cells after 30 min incubation with or without 0.5 μl of rabbit antisera to c-Fos; lane 3, Fra-1; lane 4, c-Jun; lane 5, JunB; lane 6, JunD; lane 7 and × 100-fold molar excess of cold AP-1 oligonucleotide; lane 8. A representative gel of three independent experiments with similar results is shown.
CAPE inhibits H. pylori induced AP-1 DNA-binding activity in AGS cells
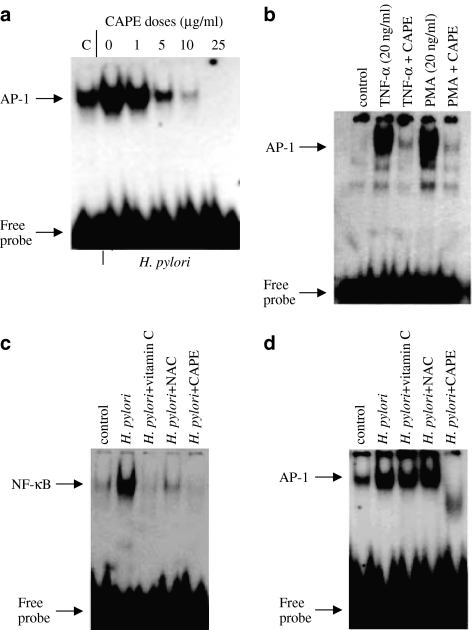
In order to investigate whether CAPE affects AP-1 DNA-binding activity in response to H. pylori, AGS cells were preincubated with various amounts of CAPE ranging between 1 and 25 μg ml−1 for 1 h before coculture with H. pylori for further 2 h. Figure 5a shows that CAPE inhibits H. pylori-induced AP-1 DNA binding in AGS cells. The dose-dependent inhibition of AP-1 binding by CAPE was similar to that seen for NF-κB DNA-binding with maximal inhibition seen at 10–25 μg ml−1 CAPE. Moreover, CAPE was also blocked TNF-α- and PMA-induced AP-1 DNA binding (Figure 5b).
Figure 5.
CAPE inhibits H. pylori-induced AP-1 DNA-binding activity. (a) AGS cells were pretreated with different amounts of CAPE between 1 and 25 μg ml−1 (a) for 1 h, and then AGS cells were cocultured with H. pylori (6 × 108 CFU ml−1) for a further 2 h. (b) AGS cells were preincubated with CAPE (25 μg ml−1) for 1 h, followed by a 2 h treatment with TNF-α (20 ng ml−1) or PMA (20 ng ml−1). Effect of vitamin C and NAC on H. pylori-induced NF-κB and AP-1 DNA-binding activity (c, d). AGS cells were pretreated with ascorbic acid (20 mM), NAC (20 mM) or CAPE (25 μg ml−1; ∼88 μM) for 1 h, followed by H. pylori (6 × 108 CFU ml−1) for 2 h. Nuclear extracts were prepared and assayed for NF-κB or AP-1 DNA-binding activity by EMSA. A representative gel is shown from three different experiments, with similar results.
Effect of vitamin C and NAC on H. pylori induced NF-κB and AP-1 DNA binding
To investigate the effect of CAPE in comparison to other antioxidants, AGS cells were preincubated with vitamin C (20 mM), NAC (20 mM) or CAPE (25 μg ml−1; ∼88 μM) for 1 h, washed and stimulated with H. pylori for 2 h. CAPE was as effective as vitamin C and NAC in inhibiting H. pylori-induced NF-κB DNA-binding activity (Figure 5c). Interestingly, neither vitamin C nor NAC blocked H. pylori-induced AP-1 DNA-binding activity in AGS cells, whereas CAPE inhibited H. pylori-induced AP-1 DNA-binding activity (Figure 5d).
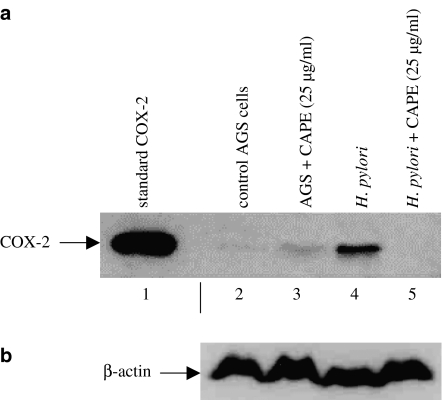
CAPE inhibits H. pylori induced COX-2 expression
COX-2 expression is regulated by both transcription factors NF-κB and AP-1, and induced by H. pylori infection (Kim et al., 2001). Pretreatment of AGS cells with CAPE (25 μg ml−1) completely inhibited H. pylori-induced COX-2 expression (Figure 6). The COX-2 immunoblot was stripped and re-probed with anti-β-actin antibody to demonstrate equal protein loading.
Figure 6.
Effect of CAPE on H. pylori-induced COX-2 expression. (a) AGS cells were pretreated with CAPE (25 μg ml−1) for 1 h, followed by 2 h coculture with H. pylori (6 × 108 CFU ml−1). Total cell extracts were prepared, separated by 10% polyacrylamide gels, blotted onto PVDF membrane and probed with anti-COX-2 antibody. Each experiment was repeated three times with similar results and a representative gel is shown. Lane 1 shows the standard COX-2 (72 kDa). (b) The immunoblot was stripped and re-probed for β-actin to ensure equal protein loading.
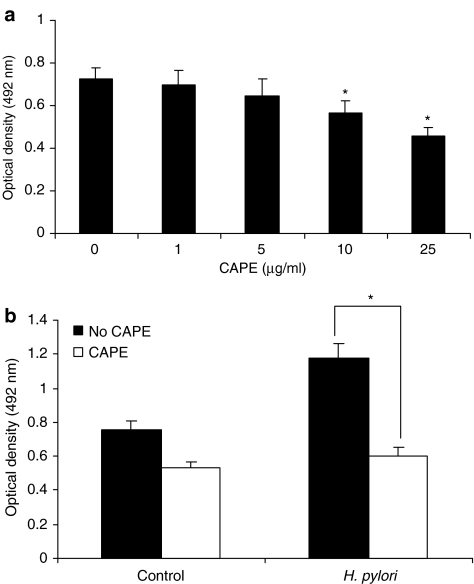
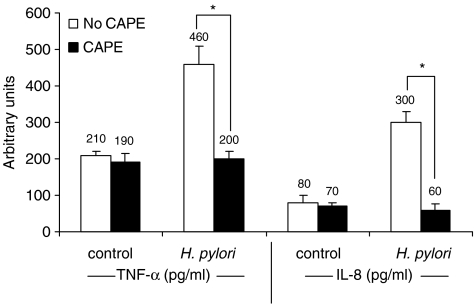
Effect of CAPE on proliferation and cytokine production
To determine whether CAPE affects cell proliferation, we treated AGS cells with different amounts of CAPE ranging from 1 μg ml−1 to 25 μg ml−1 for 24 h. CAPE induced a decrease in cell number in a dose-dependent manner (Figure 7a). The concentration of CAPE ⩽10 μg ml−1 had no significant cytotoxic effect on AGS cells. The viability of AGS cells was significantly (P<0.05) decreased by CAPE at concentrations ⩾10 μg ml−1, as determined by MTT assay. Pretreatment of AGS cells with CAPE (25 μg ml−1) for 1 h significantly (P<0.05) suppressed H. pylori-induced AGS cell proliferation after 24 h incubation (Figure 7b). We have further assessed whether CAPE treatment affects the expression of proinflammatory cytokines TNF-α and IL-8 during culture of AGS cells with H. pylori. H. pylori enhanced the production of IL-8 and TNF-α after 24 h incubation and CAPE (25 μg ml−1) pretreatment significantly (P<0.05) inhibited the production of these cytokines (Figure 8).
Figure 7.
Effect of CAPE on cell proliferation. (a) AGS cells (1 × 105 cells ml−1) were exposed to different amounts of CAPE (1–25 μg ml−1) for 24 h and cell number was evaluated by MTT assay. (b) AGS cells were incubated with CAPE (25 μg ml−1) for 1 h followed by 24 h stimulation with H. pylori (6 × 108 CFU ml−1). To the cultured cells, 20 μl of freshly prepared MTT solution was added to each well and the plates were incubated for 4 h at 37°C. The absorbance of these wells was read at 492 nm using an ELISA plate reader. Each experiment was repeated three times in triplicate per treatment group with similar results. The symbol (*) shows the statistical significance of CAPE treatment vs untreated AGS cells (P<0.05).
Figure 8.
Effect of CAPE on cytokine production. Confluent AGS cells (1 × 106 cells ml−1) were pretreated with 0–25 μg ml−1 CAPE for 1 h before stimulation with H. pylori (6 × 108 CFU ml−1) for 24 h. Cell supernatants were then collected and assayed for TNF-α and IL-8 production by ELISA technique. Each experiment was repeated three times with similar results and results are presented as the mean±s.d.
Discussion
The pharmacologically active molecules in the propolis are flavanoids and phenolic acid and their esters. These compounds, including CAPE, have multiple effects on bacteria, viruses and fungi. There have been a number of reports suggesting that honey has an inhibitory effect on H. pylori in vitro (Ali et al., 1991; al Somal et al., 1994). CAPE is one of the major components of propolis and CAPE has a strong antioxidant activity higher than that for galangin (Russo et al., 2002). The precise mechanisms of physiological and pharmacological properties responsible for the anti-inflammatory effect of honey and its product CAPE are yet unclear. In this study, we have demonstrated for the first time that CAPE, a major component of honey, modulates H. pylori-induced NF-κB, AP-1 DNA-binding activity and COX-2 expression in gastric epithelial cells. In addition, CAPE reduced TNF-α and IL-8 levels and suppressed the proliferative response of AGS cells to H. pylori.
The regulation of gene expression by transcription factors such as NF-κB and AP-1 is fundamental to the phenotype of all cells engaged in inflammatory processes. NF-κB and AP-1 are responsible for the expression of wide range of cytokines, enzymes and cell adhesion molecules, and therefore play an important role in the pathogenesis of several diseases including H. pylori infection. The redox regulation of NF-κB and related proteins has received increased attention because this protein controls the inducible expression of a wide range of genes involved in inflammatory and immune responses (Kopp & Ghosh, 1995; Barens & Karin, 1997). Treatment of gastric epithelial cells with CAPE inhibited NF-κB, AP-1 and COX-2 activation by H. pylori. Consistent with this observation, Natarajan et al. (1996) have demonstrated that CAPE inhibits the activation of NF-κB induced by a wide variety of agents. CAPE was also shown to be a potent inhibitor of cytokine- and mitogen-induced NF-κB and AP-1 in AGS cells.
Many antioxidants, including cysteine, metal chelators, dithiocarbamates, quinone derivatives, vitamin E, vitamin C and α-lipoic acid, suppress activation of NF-κB in response to diverse stimuli (Meyer et al., 1993; Bowie & O'Neill, 2000a, 2000b). In this study, we have demonstrated that CAPE is significantly more effective than the antioxidants vitamin C and NAC in inhibiting NF-κB DNA-binding activity in response to H. pylori. Inhibition of NF-κB activation by CAPE required concentrations in the micromolar range, whereas inhibition by vitamin C and NAC required relatively high concentrations in the millimolar range, 10 and 20 mM, respectively. CAPE (10 μg ml−1; ∼88 μM) is 227 times more potent as an inhibitor of NF-κB compared to vitamin C or NAC. We have also shown that, although vitamin C and NAC inhibit NF-κB, neither were effective inhibitors of AP-1 DNA binding. This observation is particularly interesting in that CAPE also inhibited AP-1 activation. Therefore, it is unlikely that the potent inhibitory effect of CAPE on NF-κB and AP-1 DNA-binding activity is solely due to its antioxidant properties.
The exact mechanism whereby CAPE inhibits NF-κB activation is not known. In this study, we found that pretreatment of gastric epithelial cells with CAPE upregulated IκB-α levels and prevented nuclear translocation of NF-κB/p65 in H. pylori-treated AGS cells. NF-κB presents in an inactive state in the cytosol bound to the inhibitory IκB protein. H. pylori infection of gastric epithelial cells results in phosphorylation and degradation of the IκB, thus allowing nuclear translocation of NF-κB. Our data demonstrated that control gastric cells (AGS) had high levels of IκB-α and no active p65. When AGS cells pretreated with CAPE, there was upregulation of IκB-α levels, which subsequently prevented p65 from translocation to the nucleus. Treatment of AGS cells with H. pylori or cytokines was associated with lower levels of IκB-α in the cytosol, as shown by immunoblotting, and increased levels of p65 in the nucleus, as shown by immunofluorescence. NF-κB activation involves phosphorylation and degradation of IκB, and protein kinase C has been shown to be involved in NF-κB activation by a variety of agents (Domínguez et al., 1993; Kopp & Ghosh, 1995; Barens & Karin, 1997). The inhibition of IκB-α degradation appears to be the initial step in NF-κB activation. Consistent with this, Bowie & O'Neill (2000b) have demonstrated that treatment of the endothelial cells ECV304 with vitamin C blocked IL-1- and TNF-mediated degradation and phosphorylation of IκB-α, due to inhibition of IKKinase (IKK) activation. The inhibition of TNF-induced IKK activation was mediated by p38 MAPK, as treatment of cells with vitamin C led to a rapid and sustained activation of p38 MAPK.
In addition to many biological and pharmacological activities of CAPE in blocking NF-κB and AP-1 activation and cytokine production, we found that CAPE treatment (25 μg ml−1) also resulted in a decrease in cell number of AGS cells. In rat macrophage and colonic epithelial cell lines, it has been shown that CAPE is an effective inducer of apoptosis in macrophages, as opposed to colonic epithelial cells (Fitzpatrick et al., 2001). In this regard, other investigators have also reported that CAPE can preferentially induce apoptosis, depending on the cell type treated with this compound (Chiao et al., 1995). The reasons for a selective effect on apoptosis are not fully understood, but may be related to the inherent redox state of a particular cell type (Chiao et al., 1995; Orban et al., 2000). In one study, in HL-60 cells, CAPE rapidly entered cells and induced DNA fragmentation and morphological changes typical of apoptosis. Moreover, treatment with CAPE caused rapid activation of caspase-3, downregulation of Bcl-2 expression, and upregulation of Bax expression (Chen et al., 2001).
The NF-κB pathway is a key mediator of genes involved in cellular proliferation, apoptosis and cytokine production. NF-κB activates the expression of various genes involved in the host immune and inflammatory response, including cytokine genes such as IL-1β, IL-2, IL-6, IL-8 and TNF-α, adhesion molecule genes and genes coding for acute-phase proteins (Barens & Karin, 1997; Yamamoto & Gaynor, 2001). Colonization of gastric epithelial cells with H. pylori induces NF-κB (Keates et al., 1997; Münzenmaier et al., 1997) and results in increased production of the proinflammatory cytokines TNF-α, IL-1, IL-6 and IL-8 (Aihara et al., 1997; Yamaoka et al., 1997), all of which are regulated by NF-κB. Significantly, CAPE inhibited H. pylori-induced IL-8 and TNF-α production by AGS cells in addition to H. pylori-induced AGS proliferation. Taken together, these observations indicate a potentially novel therapeutic use of CAPE for the treatment of the effects of H. pylori infection. This is supported by the finding that CAPE inhibited H. pylori-induced COX-2 expression in AGS cells. H. pylori infection is known to be associated with increased levels of COX-2 expression in gastric epithelial cells (Kim et al., 2001). The inhibition of COX-2 expression by CAPE is likely to contribute, in part, to both its anti-inflammatory and potentially chemopreventive activity.
In summary, the results presented in this study demonstrated that CAPE inhibited H. pylori-induced NF-κB, AP-1 and COX-2 expression in gastric epithelial cells. CAPE also inhibited cytokine-induced NF-κB and AP-1 expression. Our findings provide an insight into the molecular mechanisms for the anti-inflammatory and immunomodulatory activities of CAPE in relationship to H. pylori infection and other inflammatory disease states. Future studies are needed to clarify the molecular mechanisms by which CAPE inhibits H. pylori-mediated activation of NF-κB, AP-1 and COX-2 expression in gastric epithelial cells and to identify additional targets in gene regulation.
Acknowledgments
This work was supported in part by the Higher Education Authority Programme for Research in Third Level Institutions (PRTLI 3).
Abbreviations
- AP-1
activator protein-1
- CAPE
caffeic acid phenethyl ester
- NAC
N-acetylcysteine
- NF-κB
nuclear factor-kappa B
- PMA
phorbol 12-myristate 13-acetate
- TNF-α
tumour necrosis factor-α
References
- AIHARA M., TSUCHIMOTO D., TAKIZAWA H., AZUMA A., KILKUCHI M., MUKAIDA N., MATUUSHIMA K. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line, MKN 45. Infect. Immun. 1997;65:3218–3224. doi: 10.1128/iai.65.8.3218-3224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL SOMAL N., COLEY K.E., MOLAN P.C., HANCOCK B.M. Susceptibility of Helicobacter pylori to the antibacterial activity of manuka honey. J. R. Soc. Med. 1994;87:9–12. doi: 10.1177/014107689408700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALI A.T., CHOWDHURY M.N., AL HUMAYYD M.S. Inhibitory effect of natural honey on Helicobacter pylori. Trop. Gastroenterol. 1991;12:139–143. [PubMed] [Google Scholar]
- BARENS P.J., KARIN M. Nuclear factor-kappa B: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- BOWIE A.G., O'NEILL L.A.J. Oxidative stress and nuclear factor-κB activation: a reassessment of the evidence in the light of recent discoveries. Biochem. Pharmacol. 2000a;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- BOWIE A.G., O'NEILL L.A.J. Vitamin C inhibits NF-κB activation by TNF via the activation of p38 mitogen-activated protein kinase. J. Immunol. 2000b;165:7180–7188. doi: 10.4049/jimmunol.165.12.7180. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1979;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BURKE T.R., Jr, FESEN M.R., MAZUMDER A., WANG J., CAROTHERS A.M., GRUNBERGER D., DRISCOL J., KOHN K., POMMIER Y. Hydroxylated aromatic inhibitors of HIV-1 integrase. J. Med. Chem. 1995;38:4171–4178. doi: 10.1021/jm00021a006. [DOI] [PubMed] [Google Scholar]
- CHEN Y.J., SHIAO M.S., HSU M.L., TSAI T.H., WANG S.Y. Effect of caffeic acid phenethyl ester, an antioxidant from propolis, on inducing apoptosis in human leukemic HL-60 cells. J. Agric. Food Chem. 2001;49:5615–5619. doi: 10.1021/jf0107252. [DOI] [PubMed] [Google Scholar]
- CHIAO C., CAROTHERS A.M., GRUNBERGER D., SOLOMON G., PRESTON G.A., BARRETT J.C. Apoptosis and altered redox state induced by caffeic acid phenethyl ester (CAPE) in transformed rat fibroblast cells. Cancer Res. 1995;55:3576–3583. [PubMed] [Google Scholar]
- DOMÍNGUEZ I., SANZ L., AREZANA-SEISDEDOS F., DIAZ-MECO M.T., VIRELIZIER J.L., MOSCAT J. Inhibition of protein kinase C zeta subspecies blocks the activation of an NF-kappa B-like activity in Xenopus laevis oocytes. Mol. Cell Biol. 1993;13:1290–1295. doi: 10.1128/mcb.13.2.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZPATRICK L.R., WANG J., LE T. Caffeic acid phenethyl ester, an inhibitor of nuclear factor-κB, attenuates bacterial peptidoglycan polysaccharide-induced colitis in rats. JPET. 2001;299:915–920. [PubMed] [Google Scholar]
- GRAHAM D.Y. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989;96:615–625. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- GRUNBERGER D., BANERJEE R., EISINGER K., OLTZ K., EFROS E.M., CALDWELL M., ESTEVEZ V., NAKANISHI K. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia. 1988;44:230–232. doi: 10.1007/BF01941717. [DOI] [PubMed] [Google Scholar]
- GUARINI L., SU Z.-Z., ZUCKER S., LIN J., GRUNBERGER D., FISHER P.B. Growth inhibition and modulation of antigenic phenotype in human melanoma and glioblastoma multiforme cells by caffeic acid phenethyl ester (CAPE) Cell Mol. Biol. 1992;38:513–527. [PubMed] [Google Scholar]
- KEATES S., HITTI Y.S., UPTON M., KELLY C.P. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology. 1997;113:1099–1109. doi: 10.1053/gast.1997.v113.pm9322504. [DOI] [PubMed] [Google Scholar]
- KIM J.S., KIM J.M., JUNG H.C., SONG I.S. Expression of cyclooygenase-2 in human neutophils activated by Helicobacter pylori water-soluble proteins: possible involvement of NF-kappaB and MAP kinase signalling pathway. Digest. Dis. Sci. 2001;46:2277–2284. doi: 10.1023/a:1011939704802. [DOI] [PubMed] [Google Scholar]
- KIMURA Y., OKUDA H., OKUDA T., HATANO T., AGATA I., ARICHI S. Studies on the activities of tannins and related compounds from medicinal plants and drugs. VII. Effects of extracts of leaves of Artemisia species, and caffeic acid and chlorogenic acid on lipid metabolic injury in rats fed peroxidized oil. Chem. Pharm. Bull. 1985;33:2028–2034. doi: 10.1248/cpb.33.2028. [DOI] [PubMed] [Google Scholar]
- KOPP E.B., GHOSH S. NF-κB and Rel proteins in innate immunity. Adv. Immunol. 1995;58:1–27. doi: 10.1016/s0065-2776(08)60618-5. [DOI] [PubMed] [Google Scholar]
- LAENNLI U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;297:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LARANJINHA J., VIERIA O., MADEIRA V., ALMEIDA L. Two related phenolic antioxidants with opposite effects on vitamin E content in low density lipoproteins oxidized by ferrylmyoglobin: consumption vs regeneration. Arch. Biochem. Biophys. 1995;323:373–381. doi: 10.1006/abbi.1995.0057. [DOI] [PubMed] [Google Scholar]
- MAEDA S., YOSHIDA H., OGURA K., MITSUNO Y., HIRATA Y., YAMAJI Y., AKANUMA M., SHIRATORI Y., OMATA M. H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology. 2000;119:97–108. doi: 10.1053/gast.2000.8540. [DOI] [PubMed] [Google Scholar]
- MEYER M., SCHRECK R., BAEUERLE P.A. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells. EMBO J. 1993;12:2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER-TER-VEHN T., COVACCI A., KIST M., PAHI H.L. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-fos and c-jun. J. Biol. Chem. 2000;275:16064–16072. doi: 10.1074/jbc.M000959200. [DOI] [PubMed] [Google Scholar]
- MICHAULART P., MASFERER J.L., CAROTHERS A.M., SUBBARAMAIAH K., ZWEIFEL B.S., KOBOLDT C., METRE J.R., GRUNBERGER D., SACKS P.G., TANABE T., DANNENBERG A.J. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999;59:2347–2352. [PubMed] [Google Scholar]
- MÜNZENMAIER A., LANGE C., GLOCKER E., COVACCI A., MORAN A., BERESWILL S., BAEUERLE A.P., KIST M., PAHI H.L. A secreted/shed product of Helicobacter pylori activates transcription factor nuclear factor-kappa B. J. Immunol. 1997;159:6140–6147. [PubMed] [Google Scholar]
- NATARAJAN K., SINGH S., BURKE T.R., Jr, GRUNBERGER D., AGGARWAL B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOMURA A., STEMMERMANN G.N., CHYOU P.H., KATO I., PEREZ-PEREZ G.I., BLASER M.J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N. Engl. J. Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- O'CONNELL M.A., CLEERE R., LONG A., O'NEILL L.A., KELLEHER D. Cellular proliferation and activation of NF kappa B are induced by autocrine production of tumor necrosis factor alpha in the human T lymphoma line HuT 78. J. Biol. Chem. 1995;270:7399–7404. doi: 10.1074/jbc.270.13.7399. [DOI] [PubMed] [Google Scholar]
- ORBAN Z., MITSIADES N., BURKE T.R., Jr, TSOKOS M., CHROUSOS G.P. Caffeic acid phenethyl ester induces leukocyte apoptosis, modulates nuclear factor-kappa B and suppresses acute inflammation. Neuroimmunomodulation. 2000;7:99–105. doi: 10.1159/000026427. [DOI] [PubMed] [Google Scholar]
- OSATO M.S., REDDY S.G., GRAHAM D.Y. Osmotic effect of honey on growth and viability of Helicobacter pylori. Digest Dis. Sci. 1999;44:462–464. doi: 10.1023/a:1026676517213. [DOI] [PubMed] [Google Scholar]
- OSBORN L., KUNKEL S., NABEL G.J. Tumor necrosis factor alpha and interleukin-1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSO A., LONGO R., VANELLA A. Antioxidant activity of propolis: role of caffeic acid phenethyl ester and galangin. Fitoterapia. 2002;73 (Suppl 1):S21–S29. doi: 10.1016/s0367-326x(02)00187-9. [DOI] [PubMed] [Google Scholar]
- SU Z.-Z., LIN J., GRUNBERGER D., FISHER P.B. Growth suppression and toxicity induced by caffeic acid phenethyl ester (CAPE) in type 5 adenovirus-transformed rat embryo cells correlate directly with transformation progression. Cancer Res. 1994;54:1865–1870. [PubMed] [Google Scholar]
- YAMAMOTO Y., GAYNOR R.B. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Invest. 2001;107:135–141. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAOKA Y., KITA M., KODAMA T., SAWI N., KASHIMA K., IMANSHI J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut. 1997;41:442–451. doi: 10.1136/gut.41.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]