Abstract
Macrophages express several P2X and P2Y nucleotide receptors and display the phenomenon of ATP-induced P2X7-dependent membrane permeabilization, which occurs through a poorly understood mechanism. Several P2 receptors are known to be coupled to the activation of mitogen-activated protein kinases (MAPKs) and Ca2+ signaling.
Here, we use macrophages to investigate the phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) by nucleotides and the involvement of MAPKs and intracellular Ca2+ concentration in ATP-induced membrane permeabilization.
Short-term (5 min) pre-exposure to oxidized ATP (oATP), a P2X7 antagonist that does not inhibit P2X7-associated inward currents or membrane permeabilization, inhibits the activation of ERK1/2 by ATP, ADP, the P2X7 agonist 2′-3′-O-(4-benzoylbenzoyl)-ATP (BzATP), but not by UTP and UDP. We conclude that macrophages display several P2Y receptors coupled to the ERK1/2 pathway and that oATP antagonizes the action of purine nucleotides, possibly binding to P2X7 and/or other purine-binding P2Y receptors.
We also show that BzATP and ATP activate ERK1/2 by two different pathways since ERK1/2 activation by BzATP, but not by ATP, is blocked by the tryrosine kinase inhibitor, genistein, and the Src protein kinase inhibitor, tyrphostin. However, the activation of ERK1/2 by ATP is blocked by the protein kinase C (PKC) inhibitor, chelerythrine chloride. Under the same conditions, membrane permeabilization is not blocked by genistein, tyrphostin, or chelerythrine chloride, indicating that tyrosine kinase, Src protein kinase, and PKC are not required for pore opening.
Membrane permeabilization is independent of ERK1/2 activation since chelerythrine, or short-term exposure to oATP or PD98059, efficiently block ERK1/2 activation without inhibiting membrane permeabilization. In addition, membrane permeabilization is not inhibited by SB203580 and SB202190, two inhibitors of p38 MAPK, nor by intracellular BAPTA, which blocks ATP-induced Ca2+ signals.
These results suggest that multiple P2 receptors lead to ERK1/2 activation, that ligation of the same receptors by agonists with different affinities can lead to differential stimulation of separate pathways, and that MAPKs and intracellular Ca2+ fluxes are independent of P2X7-associated pore formation.
Keywords: P2 receptor, P2X7, ERK1/2, MAP kinase, macrophage, permeabilization, pore, ATP
Introduction
Since 1929, the pharmacological and immunological properties of extracellular nucleotides and nucleosides have attracted much attention due to their ability to modulate proliferation, differentiation, cell death, glioma formation, and cytokine maturation and secretion (Ralevic & Burnstock, 1998). Many of the effects of extracellular nucleotides are mediated by P2 receptors. They comprise two groups: P2Y receptors, a G-protein-coupled family of receptors comprised of nine members (P2Y1,2,4,6,11–15); and P2X, a family of ligand-gated cation channels comprised of seven members (P2X1–7) (Ralevic & Burnstock, 1998; North, 2002; Burnstock & Knight, 2004).
Besides opening a small ion channel selective for Na+, K+, and Ca2+, P2X7 receptors are also associated with the opening of a nonselective pore that allows the passage of molecules of up to 900 Da, induction of apoptosis and necrosis, cytokine release, killing of intracellular pathogens, and membrane blebbing (Steinberg et al., 1987; Coutinho-Silva & Persechini, 1997; Lammas et al., 1997; Coutinho-Silva et al., 1999; 2003; Di Virgilio et al., 2001; Morelli et al., 2001; North, 2002; Verhoef et al., 2003).
The amino-acid sequence of P2X7 receptors differs from other members of the P2X family by the presence of a longer C-terminal tail comprising approximately 240 amino acids that is thought to be involved in signal transduction (Surprenant et al., 1996; Kim et al., 2001; Saunders et al., 2003). However, although different signaling pathways are triggered by P2X7 receptors, the specific pathways that lead to each of the P2X7-dependent responses are still poorly understood (Kim et al., 2001; North, 2002). In particular, ATP-induced pore formation has been described as a phenomenon depending solely on the P2X7 molecule and/or as a consequence of the engagement of other molecules coupled to P2X7 by a yet unknown signaling pathway (Surprenant et al., 1996; Coutinho-Silva & Persechini, 1997; North, 2002). The latter possibility is supported by results from patch-clamping experiments suggesting that ATP-induced nonselective pore opening in macrophages requires an intracellular signal (Coutinho-Silva & Persechini, 1997; Persechini et al., 1998). Kim et al. (2001) subsequently showed that dephosphorylation of P2X7 residue 343Tyr is required for opening of the P2X7-associated cation channel, raising the possibility that tyrosine kinases may be involved in pore formation. Consistent with this possibility, several studies have implicated tyrosine kinases (Bronte et al., 1996; Kim et al., 2001; Hung & Sun, 2002), Rho-dependent kinase (Morelli et al., 2003; Verhoef et al., 2003; Pfeiffer et al., 2004), and MAPKs (Hu et al., 1998; Hide et al., 2000; Humphreys et al., 2000; Denlinger et al., 2001; Panenka et al., 2001; Aga et al., 2002; Bradford & Soltoff, 2002; Parvathenani et al., 2003; Gendron et al., 2003a; Pfeiffer et al., 2004) in P2X7-depending signaling and/or cell responses.
Identification of the signal transduction pathways and cellular functions associated with P2X7 and other P2 receptors have been hampered by the lack of specific pharmacological tools available to study different members of this receptor family (Ralevic & Burnstock, 1998; Jacobson et al., 2002; North, 2002). In particular, most of published results on the activation of mitogen-activated protein kinases (MAPKs) by P2X7 receptors have relied on the use of 2′-3′-O-(4-benzoylbenzoyl)-ATP (BzATP) (used as specific agonist) and/or oxidized ATP (used as a specific antagonist), two drugs now known to bind to other P2 receptors and to display other effects independently of P2 receptors. Among these are the non-P2-related inhibition of ATP-induced cytokine release by oxidized ATP (Beigi et al., 2003) and the recently identified effects of BzATP on all P2X receptors (Jacobson et al., 2002) and some P2Y receptors such as P2Y1 and P2Y11 (Boyer et al., 1996; Vigne et al., 1999; Vartian & Boehm, 2001; Jacobson et al., 2002; White et al., 2003). These properties are particularly relevant when studying primary cells such as macrophages and monocytes that express several P2X and P2Y receptor subtypes (Albuquerque et al., 1993; Dubyak & El-Moatassim, 1993; Jin et al., 1998; Ralevic & Burnstock, 1998; Adrian et al., 2000; Di Virgilio et al., 2001; Santiago-Perez et al., 2001; Warny et al., 2001; North, 2002; Bowler et al., 2003; Coutinho-Silva et al., 2005), many of them coupled to MAPK pathways (Dangelmaier et al., 2000; Lenz et al., 2000; Santiago-Perez et al., 2001; Burnstock, 2002).
Nonetheless, recent studies using recombinant P2X7 receptors transfected into cells that do not express other endogenous P2 receptors have demonstrated that P2X7 receptors mediate activation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) (Gendron et al., 2003b; Amstrup & Novak, 2003). However, the relevance of MAPKs for the induction of membrane permeabilization still remains unclear. While inhibitors of p38 but not of ERK1/2 activation block ATP-induced dye uptake in the monocytic cell line THP-1 (Donnelly-Roberts et al., 2004), and ERK1/2 plays no role in pore formation in primary thymocytes (Auger et al., 2005), it was recently reported that inhibitors of either p38 or ERK1/2 inhibit dye uptake and the formation of large pores in intraperitoneal murine macrophages and 2BH4 cells (Faria et al., 2004).
In order to reconcile these differences and to further characterize the effects of different ligand on P2-dependent signal transduction, we used intraperitoneal murine macrophages to study the activation of ERK1/2 by several agonists of P2 receptors, the involvement of tyrosine kinases and protein kinase C (PKC) in ERK1/2 activation, and the involvement of these pathways together with intracellular Ca2+ signaling in P2X7-associated ATP-induced membrane permeabilization. The possible effect of BzATP and oxidized ATP on ERK1/2 activation by P2 receptors other than P2X7 was also investigated.
Methods
Materials
Adenosine, ADP, ATP, UDP, UTP, BzATP, periodate-oxidized ATP (oATP), genistein, tyrphostin, chelerythrine chloride, 2-mercaptoethanol, Tween 20, ethidium bromide, HEPES, dimethyl sulfoxide (DMSO), sodium dodecyl sulfate (SDS), and RPMI 1640 medium were purchased from Sigma-Aldrich (St Louis, MO, U.S.A.). PD98059 was obtained from Biomol Research Laboratories (Plymouth Meeting, PA, U.S.A.); SB203580 and SB202190 were from Calbiochem (San Diego, CA, U.S.A.). Fetal bovine serum, penicillin, and streptomycin were obtained from Gibco/BRL (Grand Island, NY, U.S.A.). Thioglycollate medium was from Difco (Detroit, MI, U.S.A.). NaCl, MgCl2, methanol, Trizma-base, glacial acetic acid, formic acid, chloroform, glycine, and glycerol were from Reagen (Rio de Janeiro, RJ, Brazil). Polyvinylidene difluoride (PVDF) membranes and Enhanced Chemiluminescence (ECL-Plus) kit were from Amersham Pharmacia Biotech (São Paulo, SP, Brazil). Kodak X-OMAT film was purchased from Kodak (Rio de Janeiro, RJ, Brazil). Nonfat dry milk was obtained from Molico (Rio de Janeiro, RJ, Brazil). Anti-p44/p42 MAPK (Thr202/Tyr204) monoclonal antibody (anti-phospho-ERK1/2 antibody) and anti-ERK antibody were purchased from Cell Signaling Technology Inc. (Bervely, MA, U.S.A.). Horseradish peroxidase-conjugated anti-mouse-IgG was from Amersham Pharmacia Biosciences. Anti-rabbit IgG was from Sigma; BAPTA, Fura-2, and probenicide were from Molecular Probes (Eugene, OR, U.S.A.).
Animals
Female Swiss–Webster mice weighing 30 g, 8–12-week old, were obtained from the animal facilities of the Instituto de Microbiologia Paulo de Goes of the Federal University of Rio de Janeiro (Rio de Janeiro, RJ, Brazil). Animal handling was performed according to the guidelines for animal use in scientific experiments of the Instituto de Biofisica Carlos Chagas Filho of the Federal University of Rio de Janeiro.
Cell isolation and culture
Most experiments were performed with thioglycollate-elicited macrophages obtained from the intraperitoneal cavity of Swiss–Webster mice, collected 4 days after thioglycollate injection, as described (Coutinho-Silva & Persechini, 1997). In brief, cells were washed three times in PBS and kept on ice at a concentration of 106 cells ml−1 until used either in the flow cytometry-based membrane-permeabilization assays (see below) or plated in 35 mm culture dishes or glass coverslips in order to obtain adherent macrophages for use in ERK-activation assays, electrophysiological recordings, intracellular calcium measurements, or fluorescence microscopy-based permeabilization assays (see below). To obtain adherent macrophages, cells were first added to the culture dishes at a concentration of 6 × 106 cells dish−1 in 2 ml (ERK-activation assays) or 2 × 105 cells dish−1 in a central spot with 200 μl (other assays) in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 g l−1 sodium bicarbonate, 0.3 mg l−1 L-glutamine, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin at 37°C in a humidified atmosphere containing 5% CO2. Nonadherent cells were then removed after 2 h and the macrophages were kept for 4 days under the same culture conditions. Some experiments were performed using the murine macrophage-like cell line J774.A1 (American Type Culture Collection, Rockeville, MD, U.S.A.), harvested on 60 mm2 culture flasks in RPMI 1640 supplemented with 10% fetal bovine serum. The cultures were expanded every 3–4 days under standard culture conditions. Before the assay, J774.A1 cells were detached by adding 1 mg ml−1 trypsin for 3 min at 37°C and washed and kept in ice-cold PBS until use.
ERK-activation assays
Dishes containing 4-day-old macrophage cultures were gently washed twice in prewarmed RPMI medium buffered with 20 mM HEPES without serum (RPMI/HEPES) and kept for 15 min at 37°C. Nucleotides and/or other drugs were dissolved in the same medium, prewarmed at the same temperature, and then added to the culture dishes. Unless otherwise specified, cells were kept at 37°C for 5 min in the presence of the drugs. The medium was gently drained, 100 μl of lysis buffer (1 M Tris, 20% glycerol, 10% SDS, and 20% 2-mercaptoethanol, pH 6.8) was added and the cells were kept on ice for 10 min. The cell lysates were then harvested by scraping, boiled at 100°C for 10 min, and centrifuged in a micro centrifuge for 10 min at 14,000 r.p.m. The supernatant was collected and stored at −20°C until use.
Anti-ERK Western blots
Cell extracts (50 μg of protein per lane) were separated by electrophoresis on a 9% SDS–PAGE, and transferred to a PVDF membrane using standard protocols. Membranes were then incubated with a blocking buffer consisting of TTBS (50 mM Tris, 200 mM NaCl, 0.1% Tween 20, pH 7.6) containing 5% nonfat dry milk for 1 h at room temperature and then incubated with anti-phospho-ERK1/2-specific antibody (diluted 1:2000). After three rinses in TTBS for 5 min each, membranes were incubated for 1 h with horseradish peroxidase-conjugated anti-IgG (1 : 5000) diluted in blocking buffer. Membranes were then washed by gently shaking three times for 5 min in TTBS, and bound antibodies were detected by ECL-plus detection kit according to the manufacturer's instructions. The protein concentration in the extracts was determined by the Bradford assay (Bradford, 1976), using bovine serum albumin as standard. Equal loading of gels was confirmed by staining the gel immediately after electrophoresis and again at the end of all immune-staining steps with Ponceau Rouge S (Sigma) using a 0.2% solution in TCA. In selected experiments, we have also confirmed total ERK1/2 loading by striping the anti-phosfo-ERK and restaining with anti-ERK antibody (Promega Corporation, 1 : 5000 dilution). Primary antibody striping was performed by incubating membranes for 30 min at 50°C in 62.5 mM Tris-HCl buffer, pH 6.8, containing 2% SDS and 0.78% 2-mercaptoethanol, followed by two washes of 10 min in Tris-Buffered Saline plus Tween-20 (TBST).
Permeabilization assays
Unless otherwise specified, ATP-induced membrane permeabilization was measured by detecting ethidium bromide uptake using a flow cytometer (FACSCalibur cytometer, Becton, Dickinson and Co., Franklin Lakes, NJ, U.S.A.). In brief, freshly isolated macrophages or J774.A1 cells (106 ml−1) were removed from ice, prewarmed at 37°C for 5 min, treated with the indicated drug for 5–30 min at 37°C, and then incubated with ATP (0.1, 1, and 5 mM) or BzATP (300 μM or 1 mM) for 10 min in the presence of the drugs. Ethidium bromide (2 μM final concentration) was added during the last 5 min of incubation and the intensity of dye uptake was immediately determined by flow cytometry using an excitation wavelength of 488 nm and an emission wavelength of either 590 or 670 nm. At least 5000 data points were collected for each sample. The results were analyzed using the WinMDI program (Multiple Document Interface Flow Cytometry Application, version 2.8, by Joseph Trotter, The Scripps Research Institute, La Jolla, CA, U.S.A.). In some experiments, adherent macrophages of 3–5 day-old cultures were used for the permeabilization assays. In this case, dye uptake was determined using a fluorescence microscope (Axiovert 100, Karl Zeiss, Oberkochen, Germany) equipped with a HBO lamp and appropriate filters. In permeabilization assays using BAPTA-loaded cells, freshly isolated or adherent macrophages were prepared as described below in ‘Intracellular calcium measurements'.
Electrophysiology
Thioglycollate-elicited macrophages were plated in 35 mm plastic culture dishes for 3–5 days as described above. Before the experiment, the culture medium was exchanged for an extracellular solution containing, in mM: 135 NaCl, 5 KCl, 1 MgCl2, and 10 Na-HEPES, pH 7.4. Ionic currents were recorded in whole cell configuration, using an EPC-7 amplifier (List Electronic, Darmstadt, Germany) according to standard patch-clamping techniques (Hamill et al., 1981). Gigaohm seals were formed after offset potential compensation, using heat-polished micropipettes of 5–10 MΩ filled with an intrapipette solution (in mM: 135 KCl, 5 NaCl, 2 MgCl2, 0.1 K-EGTA, and 10 K-HEPES, pH 7.4). ATP was applied onto the cell surface by pneumatic injection, using a second micropipette filled with 5 mM ATP dissolved in extracellular solution and connected to a PPM-2 pneumatic pump (NeuroPhore BH-2 system, List). Data were collected using pClamp and Fetchex software, version 6.0, and a Digidata 1200 interface (Axon Instruments, U.S.A.) and plotted using Origin software (Microcal Inc., U.S.A., version 4.0).
Intracellular calcium measurements
Thioglycollate-elicited intraperitoneal murine macrophages plated on glass coverslips for 3–5 days were loaded with 5 μM Fura 2-AM and/or 10 μg ml−1 BAPTA for 30 min at room temperature in HEPES-buffered culture medium containing 2.5 mM probenicide. The cells were then washed and accommodated in a three-compartment superfusion chamber whose base was formed by the coverslip containing the cells. The central chamber containing the cells had a volume of 200 μl, and was perfused at a rate of 1 ml min−1. The perfusion solution contained, in mM: 135 NaCl, 5 KCl, 1 MgCl2, 10 Na-HEPES, pH 7.4; and either 1 mM CaCl2 or 1 mM EGTA. This solution was preheated to reach 37°C at the perfusion chamber. Intracellular calcium concentrations of groups of 20–40 cells were monitored continuously at 37°C with the use of a fluorescence photometer (Photon Technology, Princeton, NJ, U.S.A.). Fura-2 was excited alternately at 340 and 380 nm, and the emission at 510 nm was measured. The ratio measurement, which is proportional to the intracellular calcium concentration, was determined every 100 ms. ATP application was via continuous perfusion of the same solution containing the indicated concentrations of the drug (Bisaggio et al., 2001).
Statistical analysis and controls
Each experiment was performed at least twice in triplicate with a different group of mice. Data were analyzed using GraphPad InsTat software (GraphPad Software Inc., version 3.0) by Tukey test, considered significant at P<0.05.
Results
Activation of ERK1/2 by nucleotides and ATP-induced membrane permeabilization
Addition of 5 mM ATP to thioglycollate-elicited macrophages caused a transient increase in ERK1/2 phosphorylation (Figure 1). The intensity of phosphorylation reached a maximum approximately 5 min after exposure to ATP and returned to background levels after 30 min. Although we have observed ERK1/2 activation at ATP doses as low as 0.1 mM in some experiments, reproductive bands were observed only at 1 mM and above, and the maximum effect was observed at 5 mM (data not shown).
Figure 1.
Extracellular ATP induces activation of ERK1/2 in macrophages. Western blot against phospho-ERK 1 and 2 (ERK1/2-P, arrows) was performed in macrophage cultures treated without or with 5 mM ATP for 5, 10, 15, and 30 min, as indicated. Western blots were developed as described in Methods.
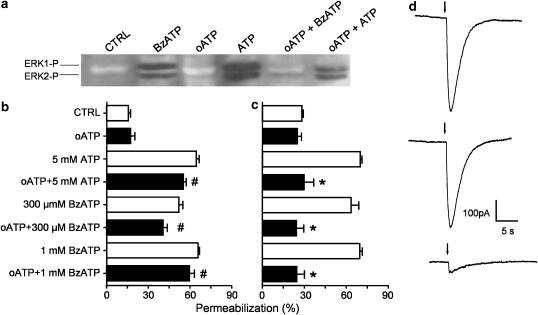
The requirement for high doses of ATP suggests that P2X7 receptors are involved (North, 2002). However, in preliminary experiments to investigate this possibility, we observed that, although BzATP was at least 10-fold more efficient (100–500 μM) than ATP at inducing maximal membrane permeabilization (data not shown), as expected for this P2X7 agonist (Jacobson et al., 2002; North, 2002), the activation of ERK1/2 required doses higher than 300 μM and was best observed at 1 mM (Figure 2 and data not shown). To further identify the possible receptors involved, we investigated the effects of oATP on ERK1/2 activation by ATP and BzATP in macrophages. We observed that a 5 min pre-exposure of the cells to oATP (300 μM) was sufficient to inhibit ERK1/2 phosphorylation by ATP and BzATP (Figure 2a). This effect was not expected for a P2X7-associated response since periods of preincubation smaller than 1 h are not effective in blocking ATP-induced membrane permeabilization (Murgia et al., 1993). However, in agreement with previously published results, we observed that a short-term preincubation period was not effective in inhibiting ATP- or BzATP-mediated membrane permeabilization (Figure 2b), in contrast to a 2 h period (Figure 2c). We further characterized the lack of inhibitory effect of short-term exposure of oATP by measuring P2X7-associated inward currents induced by ATP, which were inhibited only after long-term exposure to oATP (Figure 2d).
Figure 2.
ERK1/2 activation by ATP and BzATP, but not membrane permeabilization, is blocked by short-term exposure to oATP. (a) Western blots were performed with antibodies against phospho-ERK1/2 using macrophage cultures preincubated in the presence or absence of 300 μM oATP for 5 min, at 37°C. Then, 1 mM BzATP or 5 mM ATP were added for an additional 10 min, as indicated. Ethidium bromide was added during the last 5 min of incubation. Control macrophages (CTRL) were exposed to ethidium bromide in the absence of any drugs. (b, c) Permeabilization assay (ethidium bromide uptake) performed by flow cytometry in freshly isolated intraperitoneal macrophages. Cells were preincubated with or without 300 μM oATP for either 5 min (b) or 2 h (c) and then exposed to different concentrations of either BzATP or ATP for 10 min. Ethidium bromide was added 5 min after ATP. (d) Representative recordings of whole-cell inward currents obtained with three macrophages after pneumatic injections of ATP (arrows) before (upper recording) or 5 min (middle recording) and 2 h (lower recording) after the addition of 300 μM oATP. Data in b and c represent the mean %±s.e.m. of a representative experiment performed in triplicate. #P>0.05; *P<0.001 represents the significance of the differences between the permeabilization induced by ATP as compared to ATP plus drug.
No effect of MAPK on ATP-induced membrane permeabilization
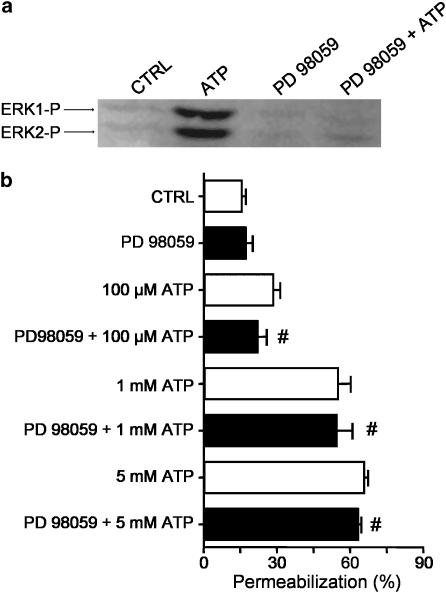
The above data demonstrate that 5 min pre-exposure to oATP inhibits ERK1/2 phosphorylation without inhibiting P2X7-dependent membrane permeabilization, suggesting that ERK1/2 phosphorylation is not required for pore formation. Consistent with this observation, a 5 min preincubation period of adherent macrophages with 10 μM of PD98059, a specific inhibitor of MEK, is sufficient to inhibit ATP-induced phosphorylation of ERK1/2 (Figure 3a), but the same inhibitor does not have a significant effect on ATP-induced membrane permeabilization in freshly isolated macrophages (Figure 3b) or adherent macrophages (data not shown).
Figure 3.
ERK1/2 phosphorylation is not required for ATP-induced membrane permeabilization in macrophages. (a) Western blot was performed with antibodies against phospho-ERK1/2 using macrophage cultures preincubated in the presence or absence of 10 μM PD98059 for 5 min at 37°C. Then, 1 mM BzATP or 5 mM ATP was added for an additional 5 min as indicated. Control macrophages (CTRL) were not exposed to any drugs. (b) Permeabilization assay (ethidium bromide uptake) performed by flow cytometry with freshly isolated intraperitoneal macrophages. Cells were preincubated with or without 10 μM PD98059 for 5 min at 37°C and then exposed to different concentrations of ATP for 10 min. Ethidium bromide was added 5 min after ATP. Data represent the mean %±s.e.m. of a representative experiment performed in triplicate. #P>0.05 represents the significance of the differences between the permeabilization induced by ATP as compared to ATP plus drug.
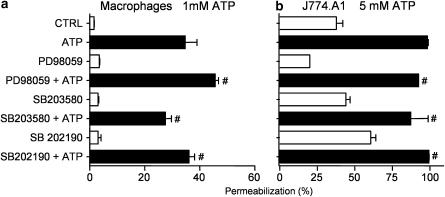
Recently, it has been shown that besides ERK1/2, p38 is activated by P2X7 in macrophages (Donnelly-Roberts et al., 2004; Pfeiffer et al., 2004). However, it is less clear whether MAPKs may be involved in ATP-induced membrane permeabilization. Using THP-1 macrophages, Donnelly-Roberts et al. (2004) reported that the p38 inhibitor SB202190 inhibits permeabilization induced by ATP, but the p38 inhibitor SB203580 and the MEK inhibitor PD98059 did not. In contrast, Faria et al. (2004) proposed that all three inhibitors are effective in inhibiting permeabilization in thioglycollate-elicited murine macrophages. We therefore investigated the possible effects of the three drugs using different doses and preincubation periods. Using either freshly isolated or adherent primary macrophages or the macrophage-like J774.A1 cell line, we found no differences in ATP-induced dye uptake in the presence of PD98059 (10, 30, and 100 μM), SB203580 (1, 10, and 100 μM), or SB202190 (1 and 10 μM) at pre-exposure times of 5 or 30 min (Figure 4 and data not shown).
Figure 4.
ERK1/2 and p38 are not required for ATP-induced membrane permeabilization in macrophages. Permeabilization assays (ethidium bromide uptake) were performed by flow cytometry in freshly isolated intraperitoneal macrophages (a) and J774.A1 cells (b). Cells were preincubated with or without PD98059 (30 μM), SB20358 (10 μM), and SB202190 (10 μM) for 30 min at 37°C and then exposed to 1 mM (a) or 5 mM (b) ATP for 10 min. Ethidium bromide was added 5 min after ATP. Data represent the mean %±s.e.m. of a representative experiment performed in triplicate. #P>0.05 represents the significance of the differences between the permeabilization induced by ATP as compared to ATP plus drug.
Activation of macrophage ERK1/2 by different P2 receptors
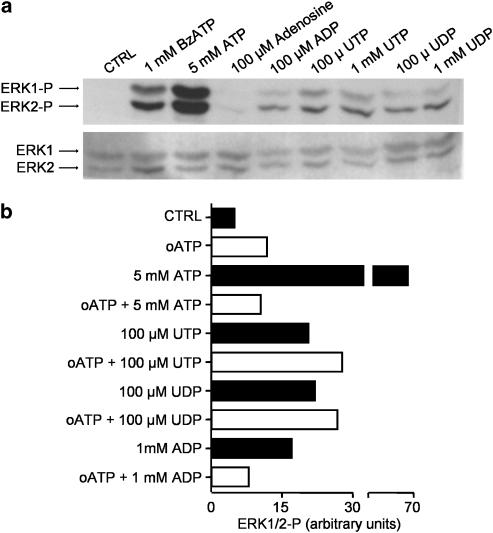
The above results prompted us to investigate whether other P2 and/or P1 receptors may be involved in the activation of ERK1/2 in macrophages. Thus, we investigated the effects of UTP, UDP, ADP, and adenosine on the phosphorylation of ERK1/2 in the presence or absence of oATP (Figure 5). UTP, UDP, and ADP induced ERK activation, while adenosine had no effect (Figure 5a). However, only the effects of ATP, BzATP, and ADP were blocked by oATP (Figures 5b and 2a). These data imply that several P2Y receptors (those that bind to UTP, UDP, and ADP) and possibly one or more P2X receptors, but not P1 receptors, are coupled to ERK1/2 activation in macrophages.
Figure 5.
ERK1/2 activation by different nucleotides and blockade by oATP. (a) Western blot was performed with antibodies against phospho-ERK1/2 (upper blot) and total ERK1/2 (lower blot) using macrophage cultures exposed to the indicated concentrations of BzATP, ATP, adenosine, ADP, UTP, or UDP for 5 min. (b) Densitometry analysis of a representative Western blot performed against phospho-ERK1/2 using macrophage cultures preincubated in the presence or absence of 300 μM oATP for 5 min at 37°C and then in the presence or absence of the indicated concentrations of ATP, UTP, UDP, or ADP for an additional 5 min. Band densities are expressed in arbitrary units. Control macrophages (CTRL) were not exposed to any drugs.
Involvement of two different intracellular pathways in activation of macrophage ERK1/2 by P2 receptors
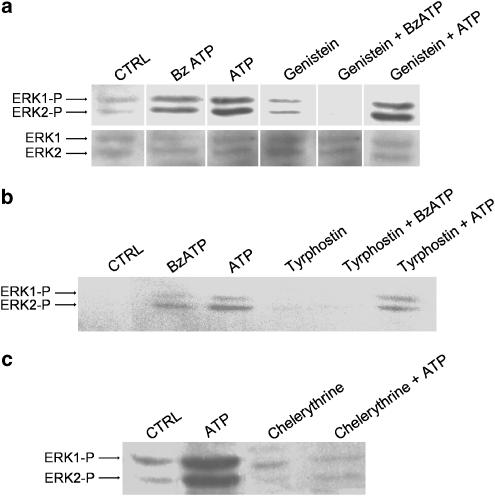
We next investigated possible upstream mechanisms that could link P2 ligation by ATP to ERK 1/2 activation. In most cases, MAPK pathways require a tyrosine kinase receptor to initiate the phosphorylation process (Dong et al., 2002). Alternatively, ERK could be activated via a PKC-dependent pathway, as already described for the ADP receptor, P2Y1 (Ralevic & Burnstock, 1998). We thus pretreated macrophages with the general tyrosine kinase inhibitor, genistein, for 5 min before exposure to either BzATP or ATP (Figure 6a). Unexpectedly, we found that genistein inhibited BzATP-induced but not ATP-induced ERK1/2 activation. Tyrphostin, an Src protein kinase inhibitor, also inhibited BzATP, but not ATP-induced ERK1/2 activation (Figure 6b). These data suggest that BzATP activates MAPK through a classical pathway recruiting tyrosine kinases, but ATP-induced activation may involve other pathways. In line with this possibility, we observed that chelerythrine chloride, an inhibitor of PKC, completely blocked activation of ERK1/2 by ATP (Figure 6c).
Figure 6.
Effect of inhibitors of protein kinases on ERK1/2 activation by BzATP and ATP in macrophages. Western blots were performed with antibodies against phospho-ERK1/2 (a–c) and total ERK 1/2 (lower blot in a) using macrophage cultures preincubated in the presence or absence of a kinase inhibitor for 5 min at 37°C and then 1 mM BzATP or 5 mM ATP mM was added for an additional 5 min. Control macrophages (CTRL) were not exposed to any drugs. The inhibitors used were (a) 10 μM genistein, (b) 25 μM tyrphostin, or (c) 10 μM chelerythrine chloride.
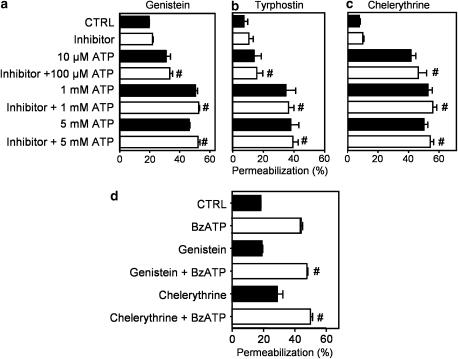
We also investigated the possible effects of genistein (Figure 7a and d), tyrphostin (Figure 7b), and chelerythrine (Figure 7c and d) on the phenomenon of P2X7-associated membrane permeabilization and found no effect of these drugs on the uptake of ethidium bromide by macrophages. These results further demonstrate that ERK1/2 phosphorylation and ATP- and BzATP-induced permeabilization are two independent events.
Figure 7.
Effect of inhibitors of protein kinase on ATP- and BzATP-induced membrane permeabilization in macrophages. The permeabilization assay (ethidium bromide uptake) was performed by flow cytometry in freshly isolated intraperitoneal macrophages. Cells were preincubated for 5 min (a–c) or 30 min (d) with or without 10 μM genistein (a and d), 25 μM tyrphostin (b), or 10 μM chelerythrine chloride (c and d), and then exposed to different concentrations of ATP (a–c) or 300 μM BzATP for 10 min. Ethidium bromide was added 5 min after ATP. Data represent the mean %±s.e.m. of a representative experiment performed in triplicate. #P>0.05 represents the significance of the differences between the permeabilization induced by nucleotide (ATP or BzATP) as compared to nucleotide plus drug.
Intracellular Ca2+ signaling is not required for ATP-induced membrane permeabilization
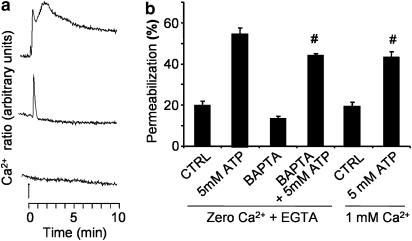
Most P2 receptors induce an increase in the free intracellular Ca2+ concentration due to either influx from the extracellular medium or release of intracellular Ca2+ stocks (Greenberg et al., 1988; Ralevic & Burnstock, 1998; North, 2002). Since the increase in free intracellular Ca2+ concentration is associated with the activation of a variety of signaling cascades, and macrophages display several P2 receptors that can induce intracellular Ca2+ signals (Coutinho-Silva et al., 2005), we decided to investigate whether intracellular Ca2+ signaling is required for membrane permeabilization in macrophages (Figure 8). We first showed that the ATP-induced intracellular Ca2+ concentration increase in adherent macrophages is partially diminished in the absence of extracellular Ca2+ and completely blocked by the intracellular calcium chelator, BAPTA (Figure 8a). Under the same experimental conditions, freshly isolated intraperitoneal macrophages (Figure 8b) and adherent macrophages (data not shown) displayed no significant alterations in ATP-induced membrane permeabilization.
Figure 8.
ATP-induced permeabilization in macrophages does not require intracellular Ca2+ signals. (a) The free intracellular Ca2+ concentration (fura-2 fluorescence ratio) was measured in adherent murine macrophages. ATP was added at a final concentration of 5 mM (arrow) and maintained in the medium for 10 min by continuous perfusion (calibrated bar at the bottom). Upper trace: cells were not loaded with BAPTA and the extracellular medium contained 1 mM CaCl2. Middle trace: cells were not loaded with BAPTA and the extracellular medium contained 1 mM EGTA and no CaCl2. Lower trace: cells were loaded with BAPTA and the extracellular medium contained 1 mM EGTA and no CaCl2. (b) The permeabilization assay (ethidium bromide uptake) was performed by flow cytometry in freshly isolated intraperitoneal macrophages. The concentrations of extracellular calcium and EGTA are indicated on the x-axis. The fluorescence ratio is proportional to the intracellular calcium concentration and was measured as described in Methods. Representative data of a single experiment are shown in (a). The experiments were repeated at least three times with similar results. Means±s.e.m. from a representative experiment performed in triplicate are shown in (b). (#)P>0.05 represents the significance of the differences between the permeabilization induced by ATP zero Ca2+ plus EGTA (second bar from the left) as compared to BAPTA (fourth bar from the left) and ATP 1 mM Ca2+ (last bar in the right).
Discussion
We had originally proposed that P2X7 activation may require intracellular signals to induce the formation of large nonselective pores based on results from electrophysiological recordings of the pores by cell-attached patch clamping (Coutinho-Silva & Persechini, 1997; Persechini et al., 1998). However, despite evidence indicating that P2X7 receptors are coupled to more than one signaling pathway, including phospholipase D and MAPKs (El-Moatassim & Dubyak, 1992; Amstrup & Novak, 2003; Gendron et al., 2003b), neither the intracellular mechanism of pore formation nor the molecular components of the pore have been identified (North, 2002). This may be partially due to the expression of multiple P2 receptor subtypes together with P2X7 in the cells studied, and the use of drugs such as BzATP and oATP that can stimulate other P2 receptors and non-P2 targets. In this study, we therefore investigated the activation of ERK1/2 by nucleotides and drugs targeting different receptors in intraperitoneal murine macrophages and J774.A1 cells and the pathways involved in ATP-induced P2X7-dependent membrane permeabilization.
Our results showed that ERK1/2 is activated by short-term (5 min) exposure to BzATP, ATP, UTP, ADP, and UDP, implying that multiple P2 receptor subtypes are expressed in macrophages and are coupled to this pathway. Moreover, while trying to characterize the contribution of P2X7 receptors, we observed that short-term (5 min) pre-exposure to oATP, a condition that does not inhibit P2X7-associated currents or P2X7-associated membrane permeabilization, can block the activation of ERK1/2 by ATP, BzATP, and ADP, but not by UTP and UDP. The incubation time required for oATP to inhibit ERK1/2 activation is surprisingly short, which has consequences for our understanding of both the mechanism of action of oATP and the signal transduction pathway leading to pore formation. First, it is likely that, at short periods of exposure, oATP acts as a competitive antagonist rather than as a covalent ligand of a P2 receptor. However, we cannot exclude the possibility that oATP may also enter the cell and somehow block ERK activation by BzATP, ATP, and ADP, but not UTP and UDP. Second, the observation that oATP inhibits ERK1/2 activation by ATP and BzATP without inhibiting either P2X7-associated currents or membrane permeabilization reinforces the conclusion that ERK1/2 activation plays no role in pore formation.
Our results also demonstrate that genistein, tyrphostin, chelerythrine, and oATP all block ERK1/2 activation but not ATP-induced membrane permeabilization, suggesting that pore formation does not require tyrosine kinase, Src protein kinase, PKC, or ERK1/2 activation. The lack of involvement of ERK1/2 was further confirmed by directly inhibiting ERK1/2 phosphorylation with PD98059, which does not affect pore formation in freshly isolated macrophages, adherent macrophage cultures, or J774.A1 cells. Finally, we have extended these results to other MAPKs by showing that neither SB202190 nor SB203580, two inhibitors of p38, were able to block ATP-mediated permeabilization. These findings also raise new questions regarding the signal transduction pathways involved in triggering P2X7-associated pore formation. Using THP-1 macrophages, Donnelly-Roberts et al. (2004) showed that the p38 inhibitor SB202190, but not the p38 inhibitor SB203580 or the MEK inhibitor PD98059, inhibits permeabilization. However, Faria et al. (2004) proposed that all three inhibitors can inhibit permeabilization in thioglycollate-elicited murine macrophages. In contrast, Auger et al. (2005) found that ERK1/2 activation is not required for P2X7-associated membrane permeabilization in thymocytes.
The reason for these discrepancies is currently not clear, but our studies with ERK1/2 inhibitors, the P2X7 antagonist oATP, and the differential effects of the P2X7 agonist ATP and BzATP are all consistent with the lack of coupling between ERK1/2 activation and P2X7-dependent pore formation. In addition, it is now becoming clear that membrane permeabilization is a more complicated phenomenon than initially thought, as suggested by a recent report (Jiang et al., 2005) showing that N-methyl-D-glucamine and YO-PRO-1 may utilize different pathways to enter P2X7-transfected HEK cells.
While searching for signal transduction pathways that could be involved in ATP-induced pore formation, we also demonstrated that membrane permeabilization does not require intracellular Ca2+ signaling. It was previously shown that extracellular Ca2+ is not required for pore formation, since P2X7-dependent membrane permeabilization is, on the contrary, potentiated by the absence of extracellular Mg2+ and Ca2+ (Steinberg & Silverstein, 1987). Since the permeabilization pore is large enough to permit passage of EGTA, EDTA, and Ca2+, it is reasonable to assume that the use of EGTA to chelate extracellular Ca2+ could also lead to the depletion of intracellular Ca2+ due to either efflux of Ca2+ or influx of EGTA. In agreement with this hypothesis, only a brief peak of intracellular Ca2+ is observed in macrophages exposed to ATP in the absence of extracellular Ca2+, as shown in Figure 8 and by others (Steinberg & Silverstein, 1987; Greenberg et al., 1988). The use of BAPTA allowed us to show that permeabilization occurs even in the absence of the short intracellular Ca2+ spike supplied by intracellular stocks of Ca2+.
Our data demonstrated that oATP can inhibit ERK1/2 activation by ATP and BzATP under conditions that do not block either P2X7-dependent pore formation or P2X7-associated inward currents. However, the macrophage P2 receptor(s) targeted by oATP during short-term exposure is (are) not known. Since ERK1/2 activation by UTP and UDP is not affected by oATP, none of the pyrimidine receptors (P2Y2, P2Y4, and P2Y6) seem to be involved. BzATP and ATP are agonists for all P2X receptors described to date (Jacobson et al., 2002). However, the high doses of ATP and BzATP required to activate ERK1/2 in macrophages indicates that P2X1–6 are not coupled to ERK1/2 activation under our experimental conditions. Instead, the high doses of agonist required suggest that P2X7 receptors may be involved – a possibility that cannot be excluded by the fact that short-term treatment with oATP does not inhibit ATP-induced membrane permeabilization, since ERK1/2 activation could be activated and blocked independently of cation channel opening and pore formation. In this context, it is worthwhile to note that recombinant P2X7 receptors truncated at the N-terminal lose their ability to activate ERK1/2 without decreasing Ca2+ influx (Amstrup & Novak, 2003), while truncation at the C-terminal, a condition known to abrogate membrane permeabilization but not cation channel opening (Kim et al., 2001), does not affect ERK1/2 activation. Taken together, our data suggest that oATP may inhibit ERK1/2 activation by interfering with one or more P2 receptors that bind to ATP, BzATP, and/or ADP. The best candidate receptors are P2X7 and the ADP-binding P2Y receptors (P2Y1, P2Y11, P2Y12).
The interaction of oATP with other mediators in the pathway leading to ERK1/2 activation by purine nucleotides should also be considered. Since oATP was first introduced to study P2 receptors, it has been widely used as an inhibitor of ATP-induced membrane permeabilization, presumably by covalently binding to the P2X7 receptor (Murgia et al., 1993; Di Virgilio et al., 2001). This reagent binds covalently to lysine residues in ATP-binding proteins and its effect therefore requires a preincubation period of over 1 h (Murgia et al., 1993). However, the use of oATP is complicated by the recent demonstration that it can inhibit the secretion of IL-8 stimulated by TNF-α independently of P2 receptors (Beigi et al., 2003). Although oATP is no longer thought to have an effect on the P2Y2 receptor (Murgia et al., 1993; Beigi et al., 2003), blockade of P2Y1 after long periods of exposure to oATP has been reported (Beigi et al., 2003). Our results indicate that oATP can also have significant antagonistic effects after short-term exposure, raising new possibilities for the use of this drug in the study of signal transduction by P2 receptors.
Quite unexpectedly, our data showed that ATP and BzATP are coupled to ERK1/2 activation through different pathways. Both a blocker of tyrosine kinases and a blocker of Src protein kinases inhibited the activation of ERK1/2 by BzATP, but not by ATP. These results could be due to the different affinities of BzATP and ATP for P2 receptors (Ralevic & Burnstock, 1998; Jacobson et al., 2002; North, 2002), but are also consistent with the possibility that BzATP and ATP may bind to superimposing but not identical receptor subtypes and that ATP may bind to more of these receptors than BzATP. In agreement with the latter interpretation, our data show that ERK1/2 phosphorylation is induced more efficiently by ATP than by BzATP (e.g. Figures 2a and 5a). In addition, ecto-nucleotidases present on the macrophage surface may also produce ADP and activate ERK1/2 through ADP-binding P2Y receptors (e.g. Figure 5). Moreover, chelerythrine chloride, an inhibitor of PKC, strongly inhibits the activation of ERK1/2 by ATP, indicating that PKC is possibly upstream from all pathways linking P2 receptors to ERK1/2 activation, in agreement with published results for P2X7 and other P2 receptors (Neary et al., 1999; Bradford & Soltoff, 2002; Hung & Sun, 2002; Gendron et al., 2003b). However, it should be noted that even at 100 μM, the intensity of the phopho-ERK1/2 bands induced by ADP remained much smaller than the one induced by ATP. Thus, although it is clear that an ADP-sensitive P2Y receptor can induce ERK1/2 phosphorylation, it seems unlikely that such a high dose of ADP could be induced by ecto-nucleotidases in 5 min and therefore it is improbable that ATP effects could be due only to an ADP-sensitive P2Y receptor. Further experiments are needed to clarify this point, which could also be addressed through the use of phospholipase C (PLC) inhibitors. Nonetheless, experiments with these drugs should be interpreted carefully, since it has been shown recently (Takenouchi et al., 2005) that U73122, a PLC inhibitor, can block ATP-induced membrane permeabilization in microglia through a PLC-independent mechanism.
In conclusion, multiple intracellular signaling pathways are triggered by ATP, BzATP, and other nucleotides in macrophages. It is tempting to speculate that cell responses such as proliferation, cell death, and cytokine secretion will depend on the pattern of binding of each agonist to the different P2 receptors, as has been shown for serial binding of microbial ligands to Toll-like receptors (Weiss et al., 2004; Napolitani et al., 2005), and on the behavior of ecto-nucleotidases present on the surface of these cells. Finally, our data suggest that, although MAPKs are activated by multiple P2 receptors, the protein kinases are not involved in ATP-induced P2X7-dependent membrane permeabilization.
Acknowledgments
We thank Dr Rodrigo da Cunha Bisaggio for helpful discussions and Vandir da Costa and Maria Leite Eduardo for technical assistance. This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Programa de Núcleos de Excelência (PRONEX-CNPq), and University of California.
Abbreviations
- BzATP
2′-3′-O-(4-benzoylbenzoyl)-ATP
- DMSO
dimethyl sulfoxide
- ERK1/2
extracellular signal-regulated kinases 1 and 2
- MAPK
mitogen-activated protein kinase
- oATP
periodate-oxidized ATP
- PKC
protein kinase C
- PVDF
polyvinylidene difluoride
- SDS
sodium dodecyl sulfate
References
- ADRIAN K., BERNHARD M.K., BREITINGER H., OGILVIE A. Expression of purinergic receptors (ionotropic P2X1-7 and metabotropic P2Y1-11) during myeloid differentiation of HL60 cells. Biochim. Biophys. Acta. 2000;1492:127–138. doi: 10.1016/s0167-4781(00)00094-4. [DOI] [PubMed] [Google Scholar]
- AGA M., JOHNSON C.J., HART A.P., GUADARRAMA A.G., SURESH M., SVAREN J., BERTICS P.J., DARIEN B.J. Modulation of monocyte signaling and pore formation in response to agonists of the nucleotide receptor P2X(7) J. Leukocyte Biol. 2002;72:222–232. [PubMed] [Google Scholar]
- ALBUQUERQUE C., OLIVEIRA S.M., COUTINHO-SILVA R., OLIVEIRA-CASTRO G.M., PERSECHINI P.M. ATP- and UTP-induced currents in macrophages and macrophage-polykaryons. Am. J. Physiol. 1993;265:C1663–C1673. doi: 10.1152/ajpcell.1993.265.6.C1663. [DOI] [PubMed] [Google Scholar]
- AMSTRUP J., NOVAK I. P2X7 receptor activates extracellular signal-regulated kinases ERK1 and ERK2 independently of Ca2+ influx. Biochem. J. 2003;374:51–61. doi: 10.1042/BJ20030585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUGER R., MOTTA I., BENIHOUD K., OJCIUS D.M., KANELLOPOULOS J.M. A role for mitogen-activated protein kinase ERK1/2 activation and non-selective pore formation in P2X7 receptor-mediated thymocyte death. J. Biol. Chem. 2005;280:28142–28151. doi: 10.1074/jbc.M501290200. [DOI] [PubMed] [Google Scholar]
- BEIGI R.D., KERTESY S.B., AQUILINA G., DUBYAK G.R. Oxidized ATP (oATP) attenuates proinflammatory signaling via P2 receptor-independent mechanisms. Br. J. Pharmacol. 2003;140:507–519. doi: 10.1038/sj.bjp.0705470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISAGGIO R.C., NIHEI O.K., PERSECHINI P.M., SAVINO W., ALVES L.A. Characterization of P2 receptors in thymic epithelial cells. Cell. Mol. Biol. 2001;47:19–31. [PubMed] [Google Scholar]
- BOWLER J.W., BAILEY R.J., NORTH R.A., SURPRENANT A. P2X4, P2Y1 and P2Y2 receptors on rat alveolar macrophages. Br. J. Pharmacol. 2003;140:567–575. doi: 10.1038/sj.bjp.0705459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER J.L., ROMERO-AVILA T., SCHACHTER J.B., HARDEN T.K. Identification of competitive antagonists of the P2Y1 receptor. Mol. Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- BRADFORD M.D., SOLTOFF S.P. P2X7 receptors activate protein kinase D and p42/p44 mitogen-activated protein kinase (MAPK) downstream of protein kinase C. Biochem. J. 2002;366:745–755. doi: 10.1042/BJ20020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRONTE V., MACINO B., ZAMBON A., ROSATO A., MANDRUZZATO S., ZANOVELLO P., COLLAVO D. Protein tyrosine kinases and phosphatases control apoptosis induced by extracellular adenosine 5′-triphosphate. Biochem. Biophys. Res. Commun. 1996;218:344–351. doi: 10.1006/bbrc.1996.0060. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Purinergic signaling and vascular cell proliferation and death. Arterioscler. Thromb. Vasc. Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., KNIGHT G.E. Cellular distribution and functions of P2 receptor subtypes in different systems. Int. Rev. Citoles. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- COUTINHO-SILVA R., OJCIUS D.M., GÓRECKI D.C., PERSECHINI P.M., BISAGGIO R.C., MENDES A.N., MARKES J., BURNSTOCK G., DUNN P.M. Multiple P2X and P2Y receptor subtypes in mouse J774, spleen and peritoneal macrophages. Biochem. Pharmacol. 2005;69:641–655. doi: 10.1016/j.bcp.2004.11.012. [DOI] [PubMed] [Google Scholar]
- COUTINHO-SILVA R., PERSECHINI P.M. P2Z purinoceptor-associated pores induced by extracellular ATP in macrophages and J774 cells. Am. J. Physiol. 1997;273:C1793–C1800. doi: 10.1152/ajpcell.1997.273.6.C1793. [DOI] [PubMed] [Google Scholar]
- COUTINHO-SILVA R., PERSECHINI P.M., BISAGGIO R.C., PERFETTINI J.-L., SÁ-NETO A.C.T., KANELLOPOULOS J.M., MOTTA-LY I., DAUTRY-VARSAT A., OJCIUS D.M. P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am. J. Physiol. 1999;276:C1139–C1147. doi: 10.1152/ajpcell.1999.276.5.C1139. [DOI] [PubMed] [Google Scholar]
- COUTINHO-SILVA R., STAHL L., RAYMOND M.-N., JUNGAS T., VERBEKE P., BURNSTOCK G., DARVILLE T., OJCIUS D.M. Inhibition of chlamydial infectious activity due to P2X7R-dependent phospholipase D activation. Immunity. 2003;19:403–412. doi: 10.1016/s1074-7613(03)00235-8. [DOI] [PubMed] [Google Scholar]
- DANGELMAIER C., JIN J., DANIEL J.L., SMITH J.B., KUNAPULI S.P. The P2Y1 receptor mediates ADP-induced p38 kinase-activating factor generation in human platelets. Eur. J. Biochem. 2000;267:2283–2289. doi: 10.1046/j.1432-1327.2000.01235.x. [DOI] [PubMed] [Google Scholar]
- DENLINGER L.C., FISETTE P.L., SOMMER J.A., WATTERS J.J., PRABHU U., DUBYAK G.R., PROCTOR R.A., BERTICS P.J. The nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J. Immunol. 2001;167:1871–1876. doi: 10.4049/jimmunol.167.4.1871. [DOI] [PubMed] [Google Scholar]
- DI VIRGILIO F., CHIOZZI P., FERRARI D., FALZONI S., SANZ J.M., MORELLI A., TORBOLI M., BOLOGNESI G., BARICORDI O.R. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- DONG C., DAVIS R.J., FLAVELL R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- DONNELLY-ROBERTS D.L., NAMOVIC M.T., FALTYNEK C.R., JARVIS M.F. Mitogen-activated protein kinase and caspase signaling pathways are required for P2X7 receptor (P2X7R)-induced pore formation in human THP-1 cells. J. Pharmacol. Exp. Ther. 2004;308:1053–1061. doi: 10.1124/jpet.103.059600. [DOI] [PubMed] [Google Scholar]
- DUBYAK G.R., EL-MOATASSIM C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- EL-MOATASSIM C., DUBYAK G.R. A novel pathway for the activation of phospholipase-D by P(2z) purinergic receptors in BAC1.2F5 macrophages. J. Biol. Chem. 1992;267:23664–23673. [PubMed] [Google Scholar]
- FARIA R.X., DE FARIAS F.P., ALVES L.A. Are second messengers crucial for opening the pore associated with P2X7 receptor. Am. J. Physiol. Cell Physiol. 2004;288:C260–C271. doi: 10.1152/ajpcell.00215.2004. [DOI] [PubMed] [Google Scholar]
- GENDRON F.P., CHALIMONIUK M., STROSZNAJDER J., SHEN S., GONZALEZ F.A., WEISMAN G.A., SUN G.Y. P2X7 nucleotide receptor activation enhances IFN gamma-induced type II nitric oxide synthase activity in BV-2 microglial cells. J. Neurochem. 2003a;87:344–352. doi: 10.1046/j.1471-4159.2003.01995.x. [DOI] [PubMed] [Google Scholar]
- GENDRON F.P., NEARY J.T., THEISS P.M., SUN G.Y., GONZALEZ F.A., WEISMAN G.A. Mechanisms of P2X7 receptor-mediated ERK1/2 phosphorylation in human astrocytoma cells. Am. J. Physiol. Cell Physiol. 2003b;284:C571–C581. doi: 10.1152/ajpcell.00286.2002. [DOI] [PubMed] [Google Scholar]
- GREENBERG S., DI VIRGILIO F., STEINBERG T.H., SILVERSTEIN S.C. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J. Biol. Chem. 1988;263:10337–10343. [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Eur. J. Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HIDE I., TANAKA M., INOUE A., NAKAJIMA K., KOHSAKA S., INOUE K., NAKATA Y. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J. Neurochem. 2000;75:965–972. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- HU Y., FISETTE P.L., DENLINGER L.C., GUADARRAMA A.G., SOMMER J.A., PROCTOR R.A., BERTICS P.J. Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitric-oxide synthase expression in RAW 264.7 macrophages. J. Biol. Chem. 1998;273:27170–27175. doi: 10.1074/jbc.273.42.27170. [DOI] [PubMed] [Google Scholar]
- HUMPHREYS B.D., RICE J., KERTESY S.B., DUBYAK G.R. Stress-activated protein kinase/JNK activation and apoptotic induction by the macrophage P2X7 nucleotide receptor. J. Biol. Chem. 2000;275:26792–26798. doi: 10.1074/jbc.M002770200. [DOI] [PubMed] [Google Scholar]
- HUNG A.C., SUN S.H. The P2X(7) receptor-mediated phospholipase D activation is regulated by both PKC-dependent and PKC-independent pathways in a rat brain-derived type-2 astrocyte cell line, RBA-2. Cell Signal. 2002;14:83–92. doi: 10.1016/s0898-6568(01)00230-3. [DOI] [PubMed] [Google Scholar]
- JACOBSON K.A., KING B.F., BURNSTOCK G. Pharmacological characterization of P2 (nucleotide) receptors. Cell Transm. 2002;16:1–23. [Google Scholar]
- JIANG L.H., RASSENDREN F., MACKENZIE A.B., ZHANG Y.H., SURPRENANT A., NORTH R.A. N-methyl-D-glucamine and propidium dyes utilize different permeation pathways at rat P2X7 receptors. Am. J. Physiol Cell Physiol. 2005;289:C1295–C1302. doi: 10.1152/ajpcell.00253.2005. [DOI] [PubMed] [Google Scholar]
- JIN J.G., DASARI V.R., SISTARE F.D., KUNAPULI S.P. Distribution of P2Y receptor subtypes on haematopoietic cells. Br. J. Pharmacol. 1998;123:789–794. doi: 10.1038/sj.bjp.0701665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM M., JIANG L.H., WILSON H.L., NORTH R.A., SURPRENANT A. Proteomic and functional evidence for a P2X(7) receptor signalling complex. EMBO J. 2001;20:6347–6358. doi: 10.1093/emboj/20.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMMAS D.A., STOBER C., HARVEY C.J., KENDRICK N., PANCHALINGAM S., KUMARARATNE D.S. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- LENZ G., GOTTFRIED C., LUO Z., AVRUCH J., RODNIGHT R., NIE W.J., KANG Y., NEARY J.T. P(2Y) purinoceptor subtypes recruit different MEK activators in astrocytes. Br. J. Pharmacol. 2000;129:927–936. doi: 10.1038/sj.bjp.0703138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORELLI A., CHIOZZI P., CHIESA A., FERRARI D., SANZ J.M., FALZONI S., PINTON P., RIZZUTO R., OLSON M.F., DI VIRGILIO F. Extracellular ATP causes ROCK I-dependent bleb formation in P2X7-transfected HEK293 cells. Mol. Biol. Cell. 2003;14:2655–2664. doi: 10.1091/mbc.02-04-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORELLI A., FERRARI D., BOLOGNESI G., RIZZUTO R., DI VIRGILIO F. Proapoptotic plasma membrane pore: P2X7 receptor. Drug Dev. Res. 2001;52:571–578. [Google Scholar]
- MURGIA M., HANAU S., PIZZO P., RIPPA M., DI VIRGILIO F. Oxidized ATP an irreversible inhibitor of macrophage purinergic P2Z receptor. J. Biol. Chem. 1993;268:8199–8203. [PubMed] [Google Scholar]
- NAPOLITANI G., RINALDI A., BERTONI F., SALLUSTO F., LANZAVECCHIA A. Selected toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEARY J.T., KANG Y., BU Y., YU E., AKONG K., PETERS C.M. Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway. J. Neurosci. 1999;19:4211–4220. doi: 10.1523/JNEUROSCI.19-11-04211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- PANENKA W., JIJON H., HERX L.M., ARMSTRONG J.N., FEIGHAN D., WEI T., YONG V.W., RANSOHOFF R.M., MACVICAR B.A. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J. Neurosci. 2001;21:7135–7142. doi: 10.1523/JNEUROSCI.21-18-07135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARVATHENANI L.K., TERTYSHNIKOVA S., GRECO C.R., ROBERTS S.B., ROBERTSON B., POSMANTUR R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J. Biol. Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- PERSECHINI P.M., BISAGGIO R.C., ALVES-NETO J.L., COUTINHO-SILVA R. Extracellular ATP in the lymphohematopoietic system: P2Z purinoceptors and membrane permeabilization. Braz. J. Med. Biol. Res. 1998;31:25–34. doi: 10.1590/s0100-879x1998000100004. [DOI] [PubMed] [Google Scholar]
- PFEIFFER Z.A., AGA M., PRABHU U., WATTERS J.J., HALL D.J., BERTICS P.J. The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J. Leukocyte Biol. 2004;75:1173–1182. doi: 10.1189/jlb.1203648. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- SANTIAGO-PEREZ L.I., FLORES R.V., SANTOS-BERRIOS C., CHORNA N.E., KRUGH B., GARRAD R.C., ERB L., WEISMAN G.A., GONZALEZ F.A. P2Y(2) nucleotide receptor signaling in human monocytic cells: activation, desensitization and coupling to mitogen-activated protein kinases. J. Cell Physiol. 2001;187:196–208. doi: 10.1002/jcp.1063. [DOI] [PubMed] [Google Scholar]
- SAUNDERS B.M., FERNANDO S.L., SLUYTER R., BRITTON W.J., WILEY J.S. A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of mycobacteria. J. Immunol. 2003;171:5442–5446. doi: 10.4049/jimmunol.171.10.5442. [DOI] [PubMed] [Google Scholar]
- STEINBERG T.H., NEWMAN A.S., SWANSON J.A., SILVERSTEIN S.C. ATP4− permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J. Biol. Chem. 1987;262:8884–8888. [PubMed] [Google Scholar]
- STEINBERG T.H., SILVERSTEIN S.C. Extracellular ATP4− promotes cation fluxes in the J774 mouse macrophage cell line. J. Biol. Chem. 1987;262:3118–3122. [PubMed] [Google Scholar]
- SURPRENANT A., RASSENDREN F., KAWASHIMA E., NORTH R.A., BUELL G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- TAKENOUCHI T., OGIHARA K., SATO M., KITANI H. Inhibitory effects of U73122 and U73343 on Ca(2+) influx and pore formation induced by the activation of P2X7 nucleotide receptors in mouse microglial cell line. Biochim. Biophys. Acta. 2005;1726:177–186. doi: 10.1016/j.bbagen.2005.08.001. [DOI] [PubMed] [Google Scholar]
- VARTIAN N., BOEHM S. P2Y receptor-mediated inhibition of voltage-activated Ca(2+) currents in PC12 cells. Eur. J. Neurosci. 2001;13:899–908. doi: 10.1046/j.1460-9568.2001.01461.x. [DOI] [PubMed] [Google Scholar]
- VERHOEF P.A., ESTACION M., SCHILLING W., DUBYAK G.R. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 beta release. J. Immunol. 2003;170:5728–5738. doi: 10.4049/jimmunol.170.11.5728. [DOI] [PubMed] [Google Scholar]
- VIGNE P., HECHLER B., GACHET C., BREITTMAYER J.P., FRELIN C. Benzoyl ATP is an antagonist of rat and human P2Y1 receptors and of platelet aggregation. Biochem. Biophys. Res. Commun. 1999;256:94–97. doi: 10.1006/bbrc.1999.9558. [DOI] [PubMed] [Google Scholar]
- WARNY M., ABOUDOLA S., ROBSON S.C., SEVIGNY J., COMMUNI D., SOLTOFF S.P., KELLY C.P. P2Y(6) nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J. Biol. Chem. 2001;276:26051–26056. doi: 10.1074/jbc.M102568200. [DOI] [PubMed] [Google Scholar]
- WEISS D.S., RAUPACH B., TAKEDA K., AKIRA S., ZYCHLINSKY A. Toll-like receptors are temporally involved in host defense. J. Immunol. 2004;172:4463–4469. doi: 10.4049/jimmunol.172.7.4463. [DOI] [PubMed] [Google Scholar]
- WHITE P.J., WEBB T.E., BOARDER M.R. Characterization of a Ca2+ response to both UTP and ATP at human P2Y11 receptors: evidence for agonist-specific signaling. Mol. Pharmacol. 2003;63:1356–1363. doi: 10.1124/mol.63.6.1356. [DOI] [PubMed] [Google Scholar]