Abstract
Activated β2-adrenoceptors are rapidly desensitized by phosphorylation of Ser262 by protein kinase A (PKA) and of Ser355,356 by G-protein-coupled receptor kinase (GRK). We sought to determine whether the phosphorylation and subsequent dephosphorylation of these sites had similar kinetics and requirements for receptor endocytosis.
The phosphorylation of the PKA and GRK sites were measured using antibodies that recognize phosphoserine 262 and phosphoserine 355,356. Endocytosis in stably transfected HEK293 cells was blocked by inducible expression of dominant-negative dynamin-1 K44A or by treatment with hypertonic sucrose.
The phosphorylation of the GRK site Ser355,356 during a 10 μM isoprenaline treatment rapidly reached a steady state, and the extent of kinetics of phosphorylation were unaffected by dynamin-1 K44A expression, and minimally by hypertonic sucrose.
In contrast, phosphorylation of the PKA site Ser262 during a 10 μM isoprenaline treatment peaked after 2 min and then rapidly declined, while inhibition of endocytosis enhanced and prolonged phosphorylation. Treatment with 300 pM isoprenaline, a concentration too low to provoke endocytosis, also resulted in prolonged PKA site phosphorylation.
The dephosphorylation of these sites was measured after removal of agonist. Significant dephosphorylation of phosphoserines 262 and 355,356 was observed under conditions of very low endocytosis, however dephosphorylation of the GRK site was greater if antagonist was present after removal of agonist.
The results indicate that the kinetics of β2-adrenoceptor GRK and PKA site phosphorylation are distinct and differently affected by endocytosis, and that receptor dephosphorylation can occur either at the plasma membrane or in internal compartments.
Keywords: β2-Adrenoceptor, endocytosis, phosphorylation, dephosphorylation, GRK, PKA
Introduction
A characteristic feature of signaling by G-protein-coupled receptors (GPCRs) is their very rapid desensitization following activation by ligands. β2-Adrenoceptor signaling is rapidly attenuated by protein kinases acting on numerous specific serines and threonines within intracellular receptor domains. These kinases include GPCR kinases, or GRKs (Benovic et al., 1986) and protein kinases A (PKA) and C (Lohse et al., 1990a; Yuan et al., 1994). Phosphorylated receptors are partially uncoupled from G protein, and fully uncoupled when phosphorylated receptors bind with high affinity to β-arrestin (Lohse et al., 1990b). β-Arrestin also binds to clathrin and clathrin adaptor proteins, thereby facilitating rapid receptor endocytosis (Goodman et al., 1996; Laporte et al., 1999).
Internalized receptors traffic through early endosomes, localizing with transferrin receptors (Von Zastrow & Kobilka, 1992) and rab5a (Moore et al., 1995) before returning to the cell surface (recycling) and coupling again to G protein (Yu et al., 1993). Studies of 32P-labeled β2-adrenoceptors implicate a role for endocytosis and for an endosomal protein-phosphatase 2A-like enzyme in receptor dephosphorylation (Krueger et al., 1997). Inhibition of endocytosis with concanavalin A or hypertonic sucrose reduces receptor dephosphorylation, as does the inhibition of protein phosphatases with calyculin A (Pippig et al., 1995). Inhibiting clathrin-mediated receptor endocytosis by expression of a dominant-negative mutant dynamin-1 leads to an increase in β2-adrenoceptor phosphorylation, and a decrease in dephosphorylation (Zhang et al., 1997). Conversely, stimulation of β2-adrenoceptor endocytosis by β-arrestin overexpression increases net receptor dephosphorylation (Zhang et al., 1997). All of these studies utilized the 32P content of receptors as an index of phosphorylation. This method could be improved upon if it were possible to distinguish the phosphorylation of receptors by PKA versus GRK.
Antibodies that recognize specific phosphoserine residues of the β2-adrenoceptor have proven valuable in measuring the kinetics of receptor phosphorylation by GRK and PKA individually (Tran et al., 2004). Using such antibodies, it is possible to determine the requirements for the phosphorylation and dephosphorylation of the β2-adrenoceptor sites phosphorylated by PKA and GRK individually. In this study, we compared the kinetics of agonist-induced phosphorylation of Ser262 and Ser355,356, and examined a role for endocytosis in the dephosphorylation at each site.
Methods
Cells and reagents
EcR293 cells were obtained from Invitrogen (Carlsbad, CA, U.S.A.) and cultured in Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum, in the presence of 200 μg ml−1 Zeocin. The 12β6 cells are a Geneticin-resistant derivative of HEK293 that express 2.0–3.3 pmol mg−1 of hemagglutinin epitope tag (HA)-β2-adrenoceptors, and were a gift of B. Kobilka (Palo Alto, CA, U.S.A.) (Von Zastrow & Kobilka, 1992). The12β6 line was cultured in DMEM with 10% fetal bovine serum, in the presence of 200 μg ml−1 Geneticin. Rabbit polyclonal antibodies to the β2-adrenoceptor carboxyl-terminus (α-CT) and to β2-adrenoceptor phosphoserines 355,356 (α-p355,356) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). A mouse monoclonal antibody to β2-adrenoceptor phosphoserine 262 (α-p262) was prepared as described earlier (Tran et al., 2004). Ponasterone, Zeocin and Geneticin were purchased from Invitrogen, hygromycin B from Roche Applied Science (Indianapolis, IN, U.S.A.) and isoprenaline from ICN Biochemicals (Irvine, CA, U.S.A.). [3H]CGP-12177 was obtained from Perkin-Elmer Life and Analytical Sciences (Boston, MA, U.S.A.). All other reagents were from Sigma Chemical Co. (St Louis, MO, U.S.A.), unless otherwise noted.
DNA constructs
A cDNA encoding a β2-adrenoceptor with an N-terminal FLAG epitope (FLAG β2-adrenoceptor; a gift from B. Kobilka, Palo Alto, CA, U.S.A.) was subcloned into the pcDNA3.1 vector for stable transfection of the EcR293 cell line. A human HA epitope-tagged-dynamin-1 K44A mutant cDNA construct was a gift from S. Schmid (La Jolla, CA, U.S.A.). The HA-dynamin-1 K44A mutant cDNA was subcloned into the vector pIND-Hygro (Invitrogen) for inducible expression in the EcR293 cells stably expressing FLAG-β2-adrenoceptors.
Inducible expression of HA-tagged human dynamin-1 K44A
The EcR293 cell line, derived from HEK293, was previously engineered to express a chimeric steroid receptor, VgRXR, for controlled expression of genes (No et al., 1996). We have used this system previously to express rab GTPases and GRKs (Rosenfeld et al., 2001; Millman et al., 2003; Moore et al., 2004). The plasmid pcDNA3.1:FLAG-β2AR was first transfected into EcR293 cells and a clone resistant to Geneticin (400 μg ml−1) was selected. The receptor number in this clone, designated EcR-βAR, was 1.2 pmol mg−1 protein. In a second transfection, EcR-βAR was transfected with pIND-Hygro:HA-dynamin-1 K44A, and clones resistant to hygromycin B (200 μg ml−1) were screened for ponasterone-inducible expression of HA-dynamin-1 K44A by immunoblotting and immunofluorescence microscopy. Two independent clones were examined, and both exhibited similar behavior. The data shown were obtained using the line designated EcR-βAR-dynKA. After 72 h of ponasterone treatment, >75% of the cells were positive for expression of HA-dynamin-1 K44A expression, as assessed by immunofluorescence. Cells that were positive for HA-dynamin-1 showed no internal receptors after treatment with 10 μM isoprenaline for 20 min (data not shown).
Measurement of β2-adrenoceptor endocytosis
To inhibit endocytosis, EcR-βAR-dynKA cells were grown in 24-well clusters in the presence of 5 μM ponasterone or vehicle alone for 72 h prior to the addition of 10 μM isoprenaline for varying times. Alternatively, 12β6 cells were grown in 24-well clusters for 24 h, then the medium was removed and replaced with Hank's balanced salt solution (HBSS) or HBSS with 0.5 M sucrose for 30 min at 37°C prior to the addition of 10 μM isoprenaline for varying times. After incubation with agonist, the cells were chilled, washed three times with cold DMEM with 10 mM HEPES, pH 7.4 (DMEM-H), and then incubated in DMEM-H on ice for 60 min with the hydrophilic radioligand, [3H]CGP-12177 (6 nm), which selectively binds surface β2-adrenoceptors (Staehelin et al., 1983). The excess label was aspirated, and the cells washed twice with cold DMEM-H. The cells were lysed with 0.1% sodium dodecyl sulfate, 0.1% NP-40 and the lysates assayed by scintillation spectroscopy. Nonspecific binding by [3H]CGP-12177, assessed by the addition of 1 μM propranolol, was <2%. The fraction of receptors left on the cell surface (compared with no agonist control) was plotted versus time of agonist exposure. To calculate the first-order rate constant for endocytosis (ke), we performed nonlinear regression of internalization curves as described by us previously (Morrison et al., 1996; Moore et al., 1999a, 1999b). The expression of HA-dynamin K44A had no effect upon the KD for binding of [3H]CGP-12177 (data not shown).
Measurement of β2-adrenoceptor phosphorylation and dephosphorylation by immunoblotting
EcR-βAR-dynKA cells were grown in six-well clusters in the presence of 5 μM ponasterone or vehicle alone for 72 h prior to the addition of isoprenaline (300 pM or 10 μM) for varying times. Alternatively, 12β6 cells were grown in six well clusters for 24 h, then the medium was removed and replaced with HBSS or HBSS with 0.5 M sucrose for 30 min at 37oC prior to the addition of 10 μM isoprenaline for varying times. The cells were chilled, washed two times with cold PBS and then harvested in 300 μl of lysis buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 2 mM ethylenediaminetetraacetic acid, 1% Triton X-100, 20 mM Na4P2O7. 10H2O, 50 mM NaF, 0.2 mM NaVO3, 0.1 μM okadaic acid and Complete® Mini protease inhibitor (Roche Applied Sciences, Indianapolis, IN, U.S.A.)). The lysates were rotated for 30 min at 4oC and centrifuged at 13,000 × g for 15 min. Equivalent protein loads from the supernatants were electrophoresed and blotted onto Immobilon membranes, and then probed with α-p355,356 at 1 : 500 or α-p262 at 1 : 1000. Bound α-p355,356 antibodies were detected using a goat-anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody. For the α-p262, a biotinylated goat-anti-mouse secondary antibody was used, followed by binding to streptavidin HRP. Bound antibody complexes were detected using the SuperSignal chemiluminescence system from Pierce (Milwaukee, WI, U.S.A.). The blots were then stripped and probed with α-CT at 1 : 1000 to visualize total β2-adrenoceptors. Control experiments showed no background signal remaining after the stripping procedure. Blots were imaged using a FluoroChem 8800 with a 12-bit charge-coupled device (CCD) camera having a dynamic range of 1000. No saturation (blooming) of the CCD chip was observed during the imaging of blots. The signals given with the α-p355,356 or α-p262 antibodies were first corrected for total β2-adrenoceptor levels obtained with the α-CT antibody and then either plotted as the fraction or percentage of the maximum level of phosphorylation, or fold over basal, as indicated in the figure legends.
For dephosphorylation, EcR-βAR-dynKA cells grown for 72 h in the presence of 5 μM ponasterone or vehicle were treated with 300 pM or 10 μM isoprenaline for the indicated times, then washed three times and incubated at 37°C in medium containing 3 μM propranolol before chilling and harvesting as described above. Alternatively, 12β6 cells were treated with hypertonic sucrose as described above before being treated with 300 pM or 10 μM isoprenaline for the indicated times. The cells were then washed three times and incubated in HBSS or HBSS with 0.5 M sucrose containing 3 μM propranolol before chilling and harvesting for immunoblotting as described above.
Results
Inhibition of endocytosis
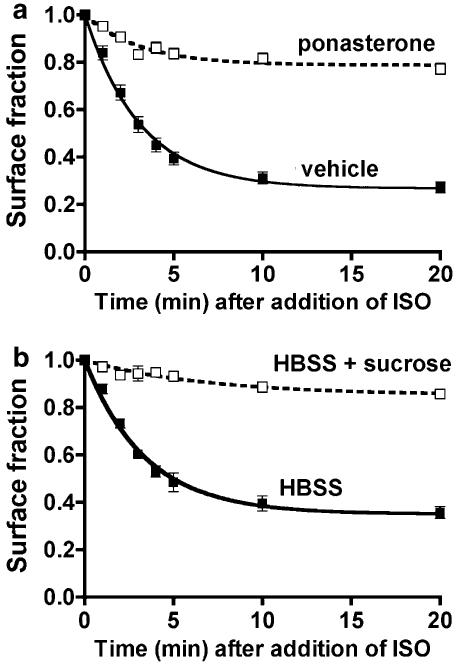
To investigate the role of endocytosis in the phosphorylation and dephosphorylation of the β2-adrenoceptor at Ser262 and Ser355,356, we employed two manipulations to inhibit endocytosis and two independent isolates of the HEK293 line expressing β2-adrenoceptors. By these means, we intended to reduce artifacts due to the experimental interventions, and clonal variation among lines of HEK293. First, a cell line was constructed that stably expresses FLAG-β2-adrenoceptors and inducibly expresses human HA-dynamin-1 K44A (designated EcR-βAR-dynKA). The inducible system employs a transcriptional promoter element that is activated by the binding of the chimeric steroid receptor Vg-RXR to ecdysone analogs (No et al., 1996). Induction of human HA-dynamin-1 K44A caused a reduction of the agonist-induced endocytic rate constant from 0.23±0.01 to 0.07±0.01 min−1, and the surface fraction remaining after 20 min was 27.2±2.3% for uninduced versus 77.2±2.3% for induced cells (Figure 1a). The second method utilized an independent line of HEK293 cells that express HA-β2-adrenoceptors (von Zastrow et al., 1992) and treatment with hypertonic sucrose to inhibit endocytosis (Daukas & Zigmond, 1985; Moore et al., 1995). This intervention reduced the endocytic rate constant from 0.19±0.01 to 0.02±0.004 min−1 and the surface fraction remaining after 20 min was 35.6±2.5% for untreated versus 86.7±1.5% for treated cells (Figure 1b). Using immunofluorescence microscopy with antibody to the β2-adrenoceptor C-terminus, no internal receptors were detectable after 15 min of agonist treatment after either manipulation (data not shown).
Figure 1.
Inhibition of β2-adrenoceptor endocytosis. Cells were grown in 24-well clusters, then isoprenaline (10 μM) was added to triplicate wells for 1–20 min before the clusters were quickly chilled. The monolayers were washed and then incubated on ice with 6 nM [3H]CGP-12177 in DMEM-H for 60 min. The label was aspirated and cells were washed and lysed for scintillation counting. The fraction of receptors left on the surface (relative to no agonist) is plotted as a function of time. The curves were modeled as described in Morrison et al. (1996). (a) The 12β6 cells were treated with 0.5 M sucrose in HBSS or HBSS alone for 30 min prior to and during exposure to agonist (N=2). (b) EcR-βAR-dynKA cells were treated with 5 μM ponasterone or with vehicle for 72 h prior to the addition of agonist (N=9).
Phosphorylation and dephosphorylation of the β2-adrenoceptor GRK site Ser355,356 in the presence or absence of endocytosis
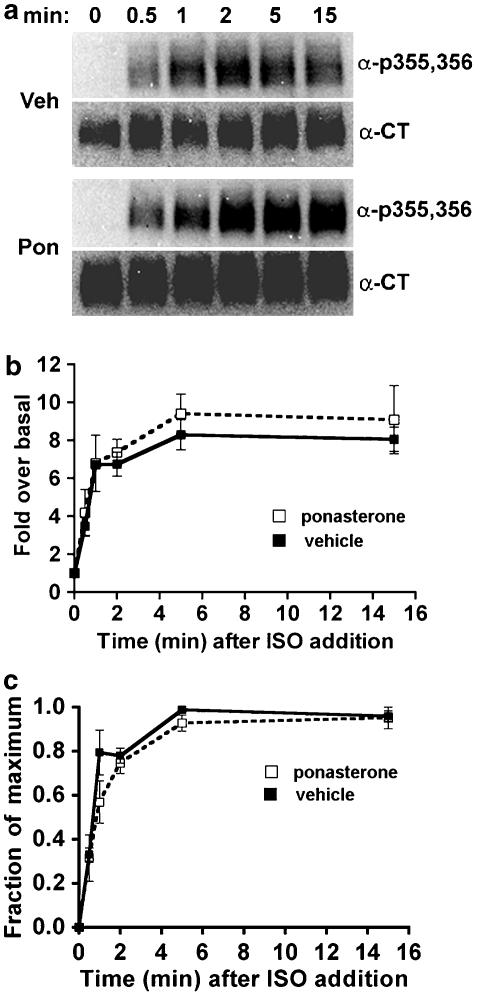
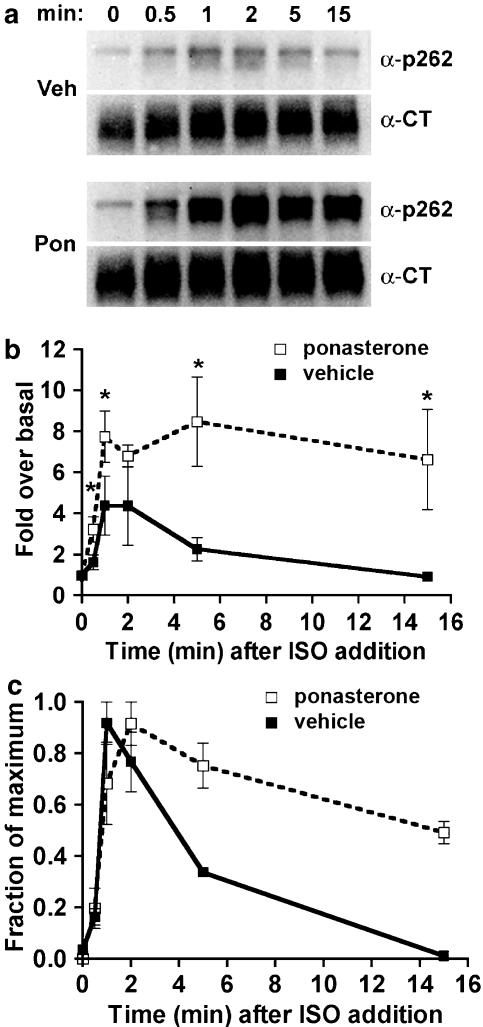
EcR-βAR-dynKA cells were grown in the presence of ponasterone to induce the expression of dynamin-1 K44A (to inhibit endocytosis) or with vehicle alone for 72 h, then 10 μM isoprenaline was added for various times before the cells were chilled and lysed. Extracts were prepared for immunoblotting as described in the Methods. A polyclonal antibody that recognizes the phosphoserine residues 355,356 on the β2-adrenoceptor C-terminal tail was used to detect phosphorylation of this site, as described earlier (Tran et al., 2004). The signal obtained with this antibody on the immunoblot was normalized to the signal obtained using an antibody that detects total receptors. This was necessary because the expression of dynamin-1 K44A consistently increased the total receptor number, as assessed by radioligand binding (by 44±12%, N=8), possibly due to inhibition of normal receptor turnover. The normalized data were plotted initially as fold over basal to determine the effect of inhibiting endocytosis on the steady-state level of Ser355,356 phosphorylation. In addition, after normalizing the antiphosphoserine 355,356 signal to the total receptor signal, we plotted all data as a fraction of maximum phosphorylation. When corrected for increased total receptor numbers, the phosphorylation of Ser355,356 was consistently 8–10-fold over basal both in the absence and presence of endocytosis (Figure 2b). If inhibiting endocytosis by dynamin-1 K44A expression reduces β2-adrenoceptor dephosphorylation, we would expect the rate of Ser355,356 phosphorylation to increase during continuous agonist exposure. There was no effect of expressing dynamin-1 K44A on the rate or extent of phosphorylation of Ser355,356 (Figure 2b).
Figure 2.
Phosphorylation of β2-adrenoceptors at the GRK site Ser355,356 after dominant-negative dynamin inhibition of endocytosis. EcR-βAR-DynKA cells were grown for 72 h in the presence of 5 μM ponasterone or vehicle alone. Isoprenaline was added to 10 μM for varying times, and then the cells were chilled and harvested in lysis buffer as described in the Methods. Equivalent protein was loaded in each lane for polyacrylamide gel electrophoresis (PAGE), then immunoblotted using the antibody against phosphoserine 355,356 (α-p355,356) and visualized by chemiluminescence. After stripping the blot, it was probed with antibody to the β2-adrenoceptor C-terminus (α-CT) to normalize phosphoreceptor levels to total receptors. (a) Representative blots; (b) data plotted as fold over basal and (c) data plotted as fraction of maximum phosphorylation (N=3).
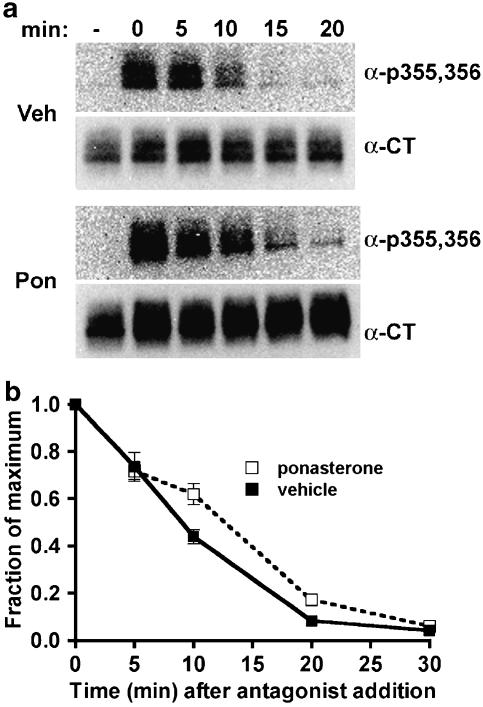
To determine whether expression of dynamin-1 K44A altered phosphoserine 355,356 dephosphorylation, agonist was added for 5 min to achieve steady-state phosphorylation, and then washed out and incubation continued in the presence of the hydrophobic antagonist propranolol. The addition of this hydrophobic antagonist reduces the effects of any residual agonist that is left after the washing step, and should rapidly stop phosphorylation of receptors at the plasma membrane and of intracellular receptors as well. The rate of dephosphorylation of phosphoserine 355,356 was not significantly changed by dynamin-1 K44A expression (Figure 3).
Figure 3.
Dephosphorylation of β2-adrenoceptors at the GRK site Ser355,356 after dominant-negative dynamin inhibition of endocytosis. EcR-βAR-dynKA cells were grown for 72 h in the presence of 5 μM ponasterone or vehicle alone. Isoprenaline was added to 10 μM for 5 min, and then the cells were rapidly washed and the incubation continued at 37oC in fresh medium with 3 μM propranolol. At various times, the cells were chilled and harvested in lysis buffer as described in the Methods. Equivalent protein was loaded in each lane for PAGE, then immunoblotted using the antibody against phosphoserine 355,356 (α-p355,356) and visualized by chemiluminescence. After stripping the blot, it was probed with antibody to the β2-adrenoceptor C-terminus (α-CT) to normalize phosphoreceptor levels to total receptors. (a) Representative blots and (b) data plotted as fraction of maximum phosphorylation (N=3).
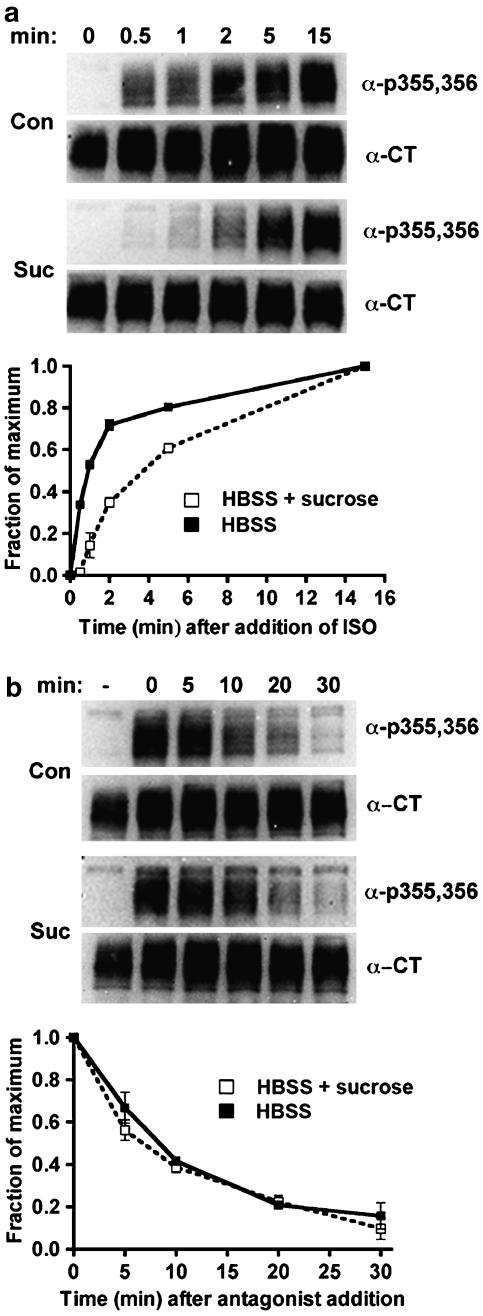
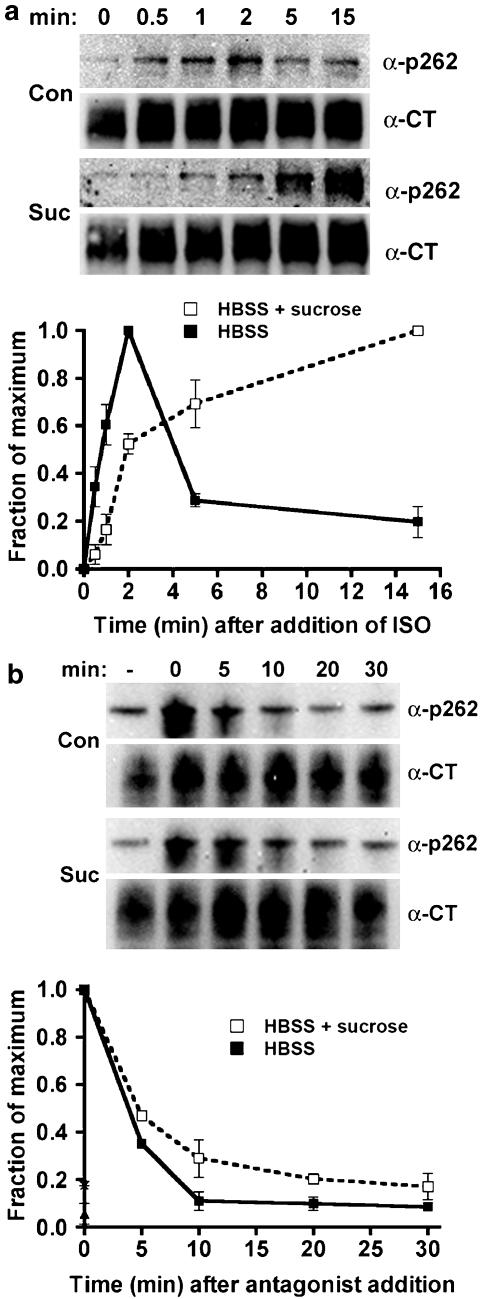
To substantiate the findings obtained by expressing HA-dynamin-1 K44A, we used hypertonic sucrose to inhibit endocytosis. This treatment blocks receptor-mediated endocytosis by preventing the formation of functional coated pits at a stage prior to the clustering of receptors (Heuser & Anderson, 1989), but prior to the stage inhibited by dynamin-1 K44A expression (Damke et al., 1994). In addition, we utilized an independently derived HEK293 cell line that expresses HA-tagged β2-adrenoceptors at levels similar to the EcR-βAR-dynKA line. Hypertonic sucrose slowed the rate of approach to steady-state phosphorylation of Ser355,356 (Figure 4a), which could indicate a reduced phosphorylation rate, or an increased dephosphorylation rate. After the addition of antagonist, the rate of dephosphorylation of phosphoserine 355,356 was the same in the presence or absence of hypertonic sucrose, with a t1/2 of ∼10 min (Figure 4b), suggesting that this experimental manipulation slows the phosphorylation of receptors at Ser355,356. The experimental variation between experiments was too great to determine changes in fold phosphorylation over basal. Nevertheless, the results are qualitatively similar to those obtained with expression of the dominant-negative dynamin.
Figure 4.
Phosphorylation and dephosphorylation of β2-adrenoceptors at the GRK site Ser355,356 after hypertonic sucrose treatment. The 12β6 cells were grown for 24 h, then treated with HBSS alone or HBSS+0.5 M sucrose at 37oC for 30 min. (a) Phosphorylation. Isoprenaline was added to 10 μM for varying times, and then the cells were chilled and harvested in lysis buffer as described in the Methods. (b) Dephosphorylation. After 5 min of 10 μM isoprenaline treatment, the cells were washed three times with medium, and then incubated for varying times in HBSS or HBSS+0.5 M sucrose containing 3 μM propranolol prior to harvest. For both (a) and (b), equivalent protein was loaded in each lane for PAGE, then immunoblotted using the antibody against phosphoserine 355,356 (α-p355,356) and visualized by chemiluminescence. After stripping the blot, it was probed with antibody to the β2-adrenoceptor C-terminus (α-CT) to normalize phosphoreceptor levels to total receptors. At the top of each panel are shown representative blots, with the data plotted as a fraction of maximum phosphorylation from three experiments shown in the lower panels (N=3).
The influence of agonist exposure times and antagonist presence during washout of agonist
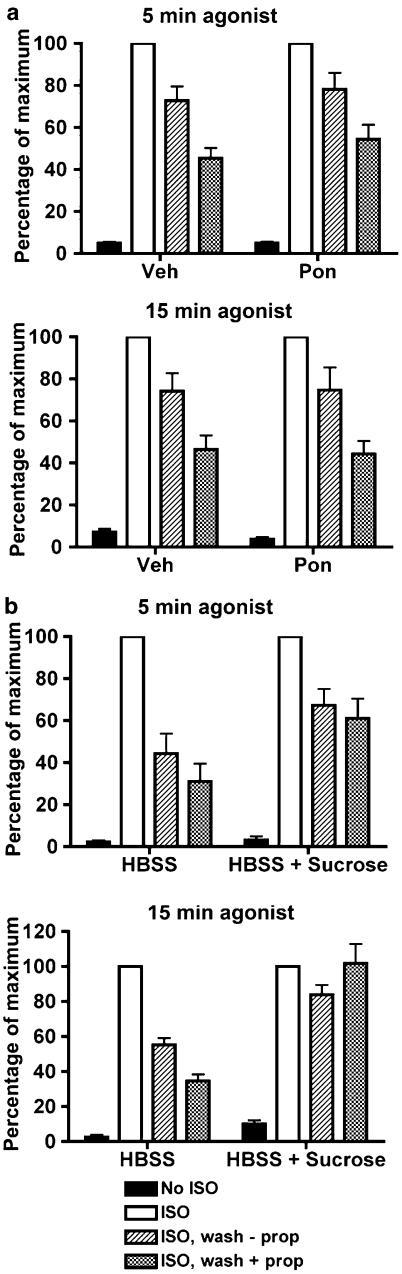
To determine the effect of agonist exposure times on the dephosphorylation of phosphoserine 355,356, we evaluated the extent of dephosphorylation after a 5 and a 15 min agonist treatment of EcR-βAR-dynKA cells (not induced with ponasterone) followed by agonist washout and antagonist addition for 30 min. After either duration of agonist exposure, the extent of receptor dephosphorylation was the same in the presence antagonist. Consistent with previous data, the expression of dynamin-1 K44A had no effect on the extent of phosphoserine 355,356 dephosphorylation (Figure 5a).
Figure 5.
Dephosphorylation of β2-adrenoceptors at the GRK site Ser355,356 after varying times of agonist exposure in the presence and absence of antagonist. (a) EcR-βAR-dynKA cells were grown for 72 h in the presence of 5 μM ponasterone or vehicle alone. Isoprenaline was added to 1 μM for either 5 min or 15 min, then the cells were washed extensively and incubated for 30 min in the presence or absence of 1 μM propranolol. The cells were chilled and harvested in lysis buffer, and Ser355,356 phosphorylation measured as described in the Methods. The levels of β2-adrenoceptor phosphorylation normalized to total receptor levels are shown as the percentage of maximum phosphorylation obtained after 5 or 15 min of agonist exposure. Values are the means±s.e.m. (N=3), where each experiment was performed in duplicate. *Significantly different (P<0.05, Student's two-tailed t-test) plus propranolol versus no propranolol. (b) HEK293 cells stably expressing HA-β2ARs (12β6 cells) were treated with HBSS or HBSS with 0.5 M sucrose for 30 min at 37oC, then with isoprenaline (1 μM) for either 5 or 15 min. The cells were washed extensively and incubated for 30 min in the presence or absence of 1 μM propranolol, then chilled and harvested in lysis buffer and Ser355,356 phosphorylation measured as described in the Methods. The levels of β2-adrenoceptor phosphorylation normalized to total receptor levels are shown as the percentage of maximum phosphorylation obtained after 5 or 15 min of agonist exposure. The error bars show the ranges of two determinations.
In other GPCR systems, β-arrestin is thought to inhibit the dephosphorylation of serines phosphorylated by GRK (Palczewski et al., 1989). Antagonist addition or rapid washout of agonist by perfusion causes the accelerated loss of β-arrestin binding to β2-adrenoceptors and other GPCRs (Santini et al., 2002; Krasel et al., 2005), and this could facilitate receptor dephosphorylation compared with a washout in the absence of antagonist. To test this hypothesis, we measured receptor dephosphorylation in EcR-βAR-dynKA cells after washout of agonist in the absence of antagonist. By 30 min after washout of agonist, the dephosphorylation of phosphoserine 355,356 was less extensive in the absence of antagonist than in its presence, and this difference was observed whether or not there was expression of dynamin-1 K44A (Figure 5a).
Similar studies were performed using hypertonic sucrose treatment of 12β6 cells. With this manipulation, increasing time of agonist exposure (from 5 to 15 min) rendered the dephosphorylation of phosphoserine 355,356 much more sensitive to the inhibition of endocytosis (Figure 5b).
Phosphorylation and dephosphorylation at the β2-adrenoceptor PKA site at Ser262 after stimulation with high agonist concentration
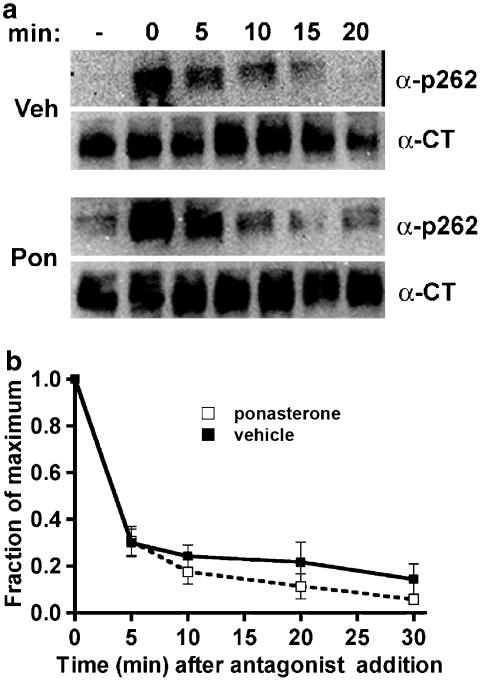
A monoclonal antibody that recognizes phosphoserine 262 on the β2-adrenoceptor intracellular loop 3 was developed previously (Tran et al., 2004), and used here to measure specifically the phosphorylation and dephosphorylation of β2-adrenoceptors by PKA. Using EcR-βAR-dynKA cells that were induced with ponasterone to express dynamin-1 K44A compared with cells treated with vehicle alone, we first examined the phosphorylation by PKA of receptors after treatment with a high agonist concentration (10 μM), where phosphorylation by GRK also occurs (Tran et al., 2004). Under these conditions, phosphorylation of the PKA site Ser262 rapidly increases (t1/2 of 1–2 min), and then rapidly declines (Figure 6c). The inhibition of endocytosis by dynamin-1 K44A expression dramatically changed these kinetics and caused a persistence of receptor phosphorylation, as well as a greater fold phosphorylation over baseline (Figure 6c). To determine whether the dephosphorylation of the phosphoserine 262 occurred when endocytosis was inhibited, the cells were grown in ponasterone or in vehicle alone, then treated with agonist for 2 min, and washed and incubated in the presence of the antagonist propranolol. Inhibiting endocytosis by dynamin-1 K44A expression did not change the dephosphorylation of phosphoserine 262 compared with control cells (Figure 7). Although the experimental variation between experiments was too great to determine changes in fold over basal, hypertonic sucrose inhibition of endocytosis produced results qualitatively similar to the expression of dynamin-1 K44A with respect to the overall kinetics (Figure 8). These changes in Ser262 phosphorylation were not observed in EcR-βAR cells (i.e. not transfected with HA-dynamin-1 K44A) that were treated with ponasterone, thus ruling out artifactual effects of the inducer itself (data not shown).
Figure 6.
Phosphorylation of β2-adrenoceptors at the PKA site Ser262 after dominant-negative dynamin inhibition of endocytosis. EcR-βAR-dynKA cells were grown for 72 h in the presence of 5 μM ponasterone or vehicle alone. Isoprenaline was added to 10 μM for varying times, and then the cells were chilled and harvested in lysis buffer as described in the Methods. Equivalent protein was loaded in each lane for PAGE, then immunoblotted using the antibody against phosphoserine 262 (α-p262) and visualized by chemiluminescence. After stripping the blot, it was probed with antibody to the β2-adrenoceptor C-terminus (α-CT) to normalize phosphoreceptor levels to total receptors. (a) Representative blots (b) data plotted as fold over basal and (c) data plotted as fraction of maximum phosphorylation. *P<0.05 (Student's two-tailed t-test) (N=4).
Figure 7.
Dephosphorylation of β2-adrenoceptors at the PKA site Ser262 after dominant-negative dynamin inhibition of endocytosis. EcR-βAR-dynKA cells were grown for 72 h in the presence of 5 μM ponasterone or vehicle alone. Isoprenaline was added to 10 μM for 5 min, and then the cells were rapidly washed and the incubation continued at 37°C in fresh medium 3 μM propranolol. At various times, the cells were chilled and harvested in lysis buffer as described in the Methods. Equivalent protein was loaded in each lane for PAGE, then immunoblotted using the antibody against phosphoserine 262 (α-p262) and visualized by chemiluminescence. After stripping the blot, it was probed with antibody to the β2-adrenoceptor C-terminus (α-CT) to normalize phosphoreceptor levels to total receptors. (a) Representative blots and (b) data plotted as fraction of maximum phosphorylation (N=3).
Figure 8.
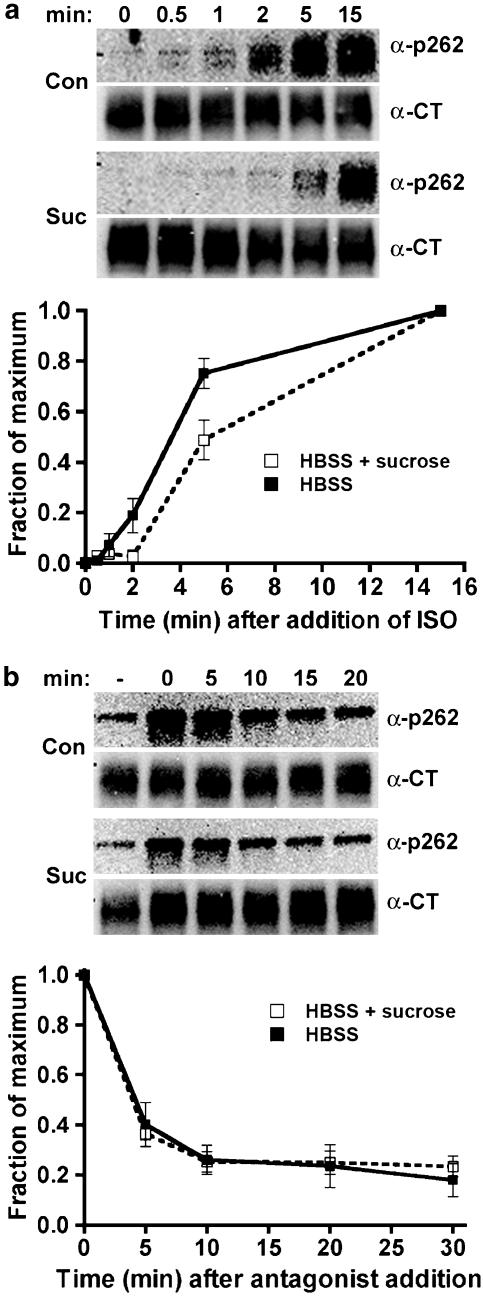
Phosphorylation and dephosphorylation of β2-adrenoceptors at the PKA site Ser262 after hypertonic sucrose treatment. The 12β6 cells were grown for 24 h, then treated with HBSS alone or HBSS+0.5 M sucrose at 37oC for 30 min. (a) Phosphorylation. Isoprenaline was added to 10 μM for varying times, and then the cells were chilled and harvested in lysis buffer as described in the Methods. (b) Dephosphorylation. After 2 min of 10 μM isoprenaline treatment, the cells were washed three times with medium, and then incubated for varying times in HBSS or HBSS+0.5 M sucrose containing 3 μM propranolol prior to harvest. For both (a) and (b), equivalent protein was loaded in each lane for PAGE, then immunoblotted using the antibody against phosphoserine 262 (α-p262) and visualized by chemiluminescence. After stripping the blot, it was probed with antibody to the β2-adrenoceptor C-terminus (α-CT) to normalize phosphoreceptor levels to total receptors. At the top of each panel are shown representative blots, with the data quantified as the fraction of maximum phosphorylation from three experiments shown in the lower panels. N=3 (a) and N=2 (b).
Phosphorylation and dephosphorylation at the PKA site Ser262 after stimulation with low agonist concentration
Receptor endocytosis is not detectable after treatment with very low agonist concentrations, nor is there phosphorylation by GRK (Tran et al., 2004) or recruitment of β-arrestin (Zhang et al., 1999). However, phosphorylation of the PKA site Ser262 still occurs (Tran et al., 2004). The 12β6 cells were treated for varying times with 300 pM isoprenaline, then extracts were immunoblotted to detect phosphorylation of PKA site Ser262 (Figure 9a). Phosphorylation at the GRK site Ser355,356 was not detected under these conditions, nor was there any detectable receptor endocytosis as assessed by immunofluorescence (Tran et al., 2004) (data not shown). Phosphorylation of the PKA site Ser262 after treatment with 300 pM isoprenaline did not exhibit the rapid increase and then decrease characteristic of the kinetics observed after treatment with 10 μM isoprenaline (compare with Figure 6). Instead, there was persistent phosphorylation similar to that observed at high agonist concentration when endocytosis is blocked. Hypertonic sucrose slowed the kinetics of phosphorylation somewhat, suggesting a nonspecific effect of this treatment. In addition, the rate of phosphorylation after treatment with 300 pM isoprenaline appeared to be slower than that observed at 10 μM, with a t1/2 of ∼2 min. Dephosphorylation took place with rapid kinetics when agonist was washed away and replaced with antagonist (Figure 9b), and this was minimally affected by hypertonic sucrose treatment.
Figure 9.
Phosphorylation and dephosphorylation of β2-adrenoceptors at the PKA site Ser262 after treatment with low agonist concentration in the presence of hypertonic sucrose. 12β6 cells were grown for 24 h, then treated with HBSS alone or HBSS+0.5 M sucrose at 37°C for 30 min. (a) Phosphorylation. Isoprenaline was added to 300 pM for varying times, and then the cells were chilled and harvested in lysis buffer as described in the Methods. (b) Dephosphorylation. After 15 min of 300 pM isoprenaline treatment, the cells were washed three times with medium, then incubated for varying times in HBSS or HBSS+0.5 M sucrose containing 3 μM propranolol prior to harvest. For both (a) and (b), equivalent protein was loaded in each lane for PAGE, then immunoblotted using the antibody against phosphoserine 262 (α-p262) and visualized by chemiluminescence. After stripping the blot, it was probed with antibody to the β2-adrenoceptor C-terminus (α-CT) to normalize phosphoreceptor levels to total receptors. At the top of each panel are shown representative blots, with the data quantified as the fraction of maximum phosphorylation from three experiments shown in the lower panels (N=3).
Discussion
The major finding of this study is that β2-adrenoceptor dephosphorylation monitored after the addition of antagonist can occur at the plasma membrane. First, after stimulation of receptors at a low (300 pM) concentration of isoprenaline that fully activates PKA site phosphorylation, but not phosphorylation by GRK, β-arrestin binding or endocytosis (Zhang et al., 1999; Tran et al., 2004), receptor dephosphorylation proceeds rapidly upon washout of agonist and the addition of antagonist (Figure 9). Consistent with this result, we also found no effect of blockade of internalization on the dephosphorylation of the PKA site after stimulation with 10 μM isoprenaline (Figure 4b), even though the kinetics of phosphorylation were altered (Figure 4a) (see Discussion below).
Second, we observed that following stimulation with a high agonist concentration, dephosphorylation of phosphoserines 355,356 in the presence of antagonist still occurred when endocytosis was severely reduced using two independent methods of inhibition (Figures 3, 4 and 6). Consistent with the small effect of the endocytosis blockers on dephosphorylation after antagonist addition, we also found that the rate of approach to steady-state phosphorylation by GRK was unaffected by reducing the rates of endocytosis (Figure 2).
In contrast to the lack of change in the kinetics of phosphorylation of the GRK site during inhibition of endocytosis, the phosphorylation of the PKA site was markedly changed: the phosphorylation did not show the rapid rise to a peak and decline, but rather was actually increased and more persistent (Figure 6). The qualitative pattern of the kinetics of phosphorylation of the PKA site under these conditions actually resembled the kinetics of phosphorylation upon stimulation with low agonist concentration where there is no detectable phosphorylation by GRK, β-arrestin recruitment or endocytosis (Figure 9). A possible explanation for these observations is that at high agonist concentrations, β-arrestin binding rapidly precludes PKA site phosphorylation unless antagonist is added, thus prolonging PKA site phosphorylation, whereas at low agonist concentrations, there is no recruitment of β-arrestin and therefore PKA phosphorylation is unimpaired.
How do our results compare with several previous studies showing a marked effect of endocytosis inhibitors on dephosphorylation? These studies showed that when agonist is washed away without adding antagonist, inhibiting β2-adrenoceptor endocytosis increases receptor phosphorylation as assessed by total 32P incorporation, and reduces total receptor dephosphorylation (Pippig et al., 1995; Zhang et al., 1997). Our data suggest that this increased total 32P incorporation was due to an increase in the phosphorylation of Ser262 (Figure 6), a result that cannot be discerned without the use of site-specific antiphosphoserine antibodies. Thus, our results regarding the dephosphorylation of phosphoserine 262 in the presence of antagonist after a 10 μM isoprenaline stimulation can be reconciled with previous findings.
It should be further noted that the measurement of phosphoserine 262 dephosphorylation in the absence of antagonist is complicated by the difficulty of rapidly washing away all agonist. During agonist exposure, cells take up fluid phase by pinocytosis and then release this material (Veithen et al., 1998) following the washout. We have found that the carryover of agonist following pretreatments of cells is readily detected in adenylyl cyclase assays of washed membrane preparations (data not shown). Thus, in the absence of antagonist to fully reverse receptor activation, and to compete with trace agonist levels, the phosphorylation of Ser262 persists after extensive washout (data not shown). Our data indicate, however, that there is significant dephosphorylation of phosphoserines 355,356 after washout of agonist without the addition of antagonist (Figure 5a) after 15 min of agonist exposure, whether or not there was expression of dynamin-1 K44A, supporting the idea that receptor dephosphorylation occurs at the plasma membrane (when dynamin-1 K44A is expressed) as well as in control conditions when endocytosis has reached steady state.
Finally, it should be noted that one commonly used method to inhibit endocytosis, hypertonic sucrose, produced variable results in our hands. Phosphorylation of Ser355,356 was slowed (Figure 4), as was phosphorylation of Ser262 at low agonist concentration (Figure 9). This is likely due to the effect of hypertonic sucrose in causing the formation of small nonfunctional clathrin microcages, and the inhibition of receptor clustering (Heuser & Anderson, 1989). Evidence from other GPCRs indicates that clustering precedes phosphorylation and may be required for it to occur efficiently (Xue et al., 2004). Finally, we note that hypertonic sucrose was better able to inhibit dephosphorylation if the initial agonist exposure time was increased from 5 to 15 min (Figure 5b). Although it is difficult to interpret this observation, it may explain some of the differences between this and previously published studies.
Extending the argument that dissociation of β-arrestin is needed before phosphatases can act upon receptors at phosphoserine 355,356, it follows that in the absence of antagonist addition to promote rapid dissociation of β-arrestin, β2-adrenoceptors for the most part require endocytosis to escape from β-arrestin binding prior to the action of phosphatases as has been shown in several studies (Pippig et al., 1995; Zhang et al., 1997). That antagonist or rapid washout by perfusion causes the rapid dissociation of β-arrestin from receptors (Santini et al., 2002; Krasel et al., 2005) has been well documented. Taken together, these results suggest that dephosphorylation may occur either at the plasma membrane if agonist is rapidly removed or following endocytosis, both of which should lead to β-arrestin dissociation.
Most of our studies were performed using addition of the antagonist propranolol to rapidly inactivate receptors. It could be argued that the use of antagonist to rapidly eliminate activation by agonist does not mimic in vivo events. Are there scenarios in vivo that would be mimicked by addition of propranolol? One such situation could occur in the nervous system at synapses, where rapid removal of catecholamines by reuptake, diffusion and catabolism has been amply demonstrated. In that regard, again, Krasel et al. (2005) mimicked rapid washout by superfusion in studies of single cell β-arrestin-β2-adrenoceptor interactions using fluorescence resonance energy transfer. We conclude that there is every reason to expect that rapid removal of agonist stimulation by antagonist or superfusion is not only important, but perhaps more physiological than simply performing repeated washouts that take substantially more time to block receptor activation.
We did not observe any effect of endocytosis inhibition on β2-adrenoceptor desensitization or resensitization of adenylyl cyclase activity after treatment with high agonist concentration (data not shown). This is not surprising because phosphorylation of Ser355,356 by GRK and subsequent dephosphorylation of that site is unaffected by inhibiting endocytosis (Figures 2 and 3). Desensitization via phosphorylation by GRK and binding by β-arrestin accounts for most desensitization of β2-adrenoceptors (an increase in EC50 of 10–12-fold), whereas desensitization mediated by phosphorylation of Ser262 increases the EC50 only by 2–3-fold (Yuan et al., 1994). The dramatic changes in the phosphorylation of Ser262 that we observe in the presence of high agonist concentration after inhibiting endocytosis (Figure 6) may indeed increase desensitization, but the effect could be small and thus very hard to detect in the presence of massive desensitization mediated by GRK-β-arrestin under these conditions. In addition, small but significant levels of agonist are carried through the membrane preparations used for adenylyl cyclase measurements (due in part to pinocytic uptake), and this causes a high baseline cyclase activity (data not shown), thus making it more difficult to detect small changes in EC50. Finally, our cell lines express a sufficient number of well-coupled receptors for there to be significant receptor reserve, thus only a small fraction of dephosphorylated receptors would be needed to fully activate adenylyl cyclase. This is particularly true in our stably transfected HEK293 cell lines, where the EC50 for adenylyl cyclase activation is 1–5 nM, in contrast to transiently transfected systems, where the EC50's are much higher (Zhang et al., 1997).
There is diverse information from other GPCR systems regarding the requirement for endocytosis in receptor dephosphorylation. Inhibition of endocytosis by expression of dynamin-1 K44A attenuates the dephosphorylation of CXCR2 receptors (Yang et al., 1999) and blocks the association of receptors with protein phosphatase 2A (Fan et al., 2001). In contrast, chemical inhibitors of internalization (i.e., concanavalin A or hypertonic sucrose) do not block dopamine D1 receptor dephosphorylation (Gardner et al., 2001), while hypertonic sucrose slowed the dephosphorylation κ-opioid receptors (McLaughlin et al., 2003). The dephosphorylation of rhodopsin does not require endocytosis and protein phosphatase 2A subunits translocate to the membrane during dark recovery of rhodopsin (Brown et al., 2002). In this respect, it should be noted that the binding of visual arrestin to rhodopsin blocks rhodopsin dephosphorylation, and that rhodopsin does not undergo endocytosis (Palczewski et al., 1989). Thus, there is evidence in the literature for the occurrence of dephosphorylation both at the plasma membrane and within internal compartments.
Phosphatases are associated with β2-adrenoceptors at the plasma membrane in a more or less constitutive manner via A-kinase-anchoring proteins (AKAPs). These proteins are molecular scaffolds that coordinate the locations of PKA, protein kinase C and other signaling molecules (Michel & Scott, 2002). One AKAP that associates with β2-adrenoceptors is gravin, and this association is increased by receptor activation (Shih et al., 1999; Lin et al., 2000). PKA and C, and protein phosphatase 2A are recruited by gravin (Lin et al., 2000; Tao et al., 2003). Other studies have shown that the β2-adrenoceptor is constitutively bound to the scaffold protein AKAP79 in a complex that also contains protein phosphatase 2B (calcineurin) and PKA (Fraser et al., 2000; Cong et al., 2001). Thus, the dephosphorylation of β2-adrenoceptors could be mediated by some of the many factors that are constitutively present at the plasma membrane or recruited upon receptor activation in addition to the dephosphorylation that occurs after endocytosis. Further work will be needed to determine the contributions of these factors to β2-adrenoceptor phosphorylation and dephosphorylation dynamics for both the PKA and GRK sites.
Acknowledgments
This work was supported by grants from the National Institutes of Health GM031208 (RBC) and HL50047 (BJK) and from the American Heart Association, Texas Affiliate 0455072Y (BJK). We thank H.O. Awwad for assistance with this study.
Abbreviations
- α-p355,356
rabbit polyclonal antibodies to β2-adrenoceptor phosphoserine 355,356
- α-p262
mouse monoclonal antibodies to β2-adrenoceptor phosphoserine 262
- AKAP
A-kinase-anchoring protein
- CCD
charge-coupled device
- DMEM
Dulbecco's modified Eagle's medium
- DMEM-H
DMEM with 10 mM HEPES, pH 7.4
- GPCR
G-protein-coupled receptor
- GRK
GPCR kinase
- HA
hemagglutinin epitope tag
- HBSS
Hank's-buffered saline solution
- HRP
horseradish peroxidase
- PAGE
polyacrylamide gel electrophoresis
- PKA
protein kinase A
References
- BENOVIC J.L., STRASSER R.H., CARON M.G., LEFKOWITZ R.J. Adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc. Natl. Acad. Sci. U.S.A. 1986;83:2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN B.M., CARLSON B.L., ZHU X., LOLLEY R.N., CRAFT C.M. Light-driven translocation of the protein phosphatase 2A complex regulates light/dark dephosphorylation of phosducin and rhodopsin. Biochemistry. 2002;41:13526–13538. doi: 10.1021/bi0204490. [DOI] [PubMed] [Google Scholar]
- CONG M., PERRY S.J., LIN F.T., FRASER I.D., HU L.A., CHEN W., PITCHER J.A., SCOTT J.D., LEFKOWITZ R.J. Regulation of membrane targeting of the G protein-coupled receptor kinase 2 by protein kinase A and its anchoring protein AKAP79. J. Biol. Chem. 2001;276:15192–15199. doi: 10.1074/jbc.M009130200. [DOI] [PubMed] [Google Scholar]
- DAMKE H., BABA T., WARNOCK D.E., SCHMID S.L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAUKAS G., ZIGMOND S.H. Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J. Cell Biol. 1985;101:1673–1679. doi: 10.1083/jcb.101.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAN G.H., YANG W., SAI J., RICHMOND A. Phosphorylation-independent association of CXCR2 with the protein phosphatase 2A core enzyme. J. Biol. Chem. 2001;276:16960–16968. doi: 10.1074/jbc.M009292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER I.D., CONG M., KIM J., ROLLINS E.N., DAAKA Y., LEFKOWITZ R.J., SCOTT J.D. Assembly of an A kinase-anchoring protein-β-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr. Biol. 2000;10:409–412. doi: 10.1016/s0960-9822(00)00419-x. [DOI] [PubMed] [Google Scholar]
- GARDNER B., LIU Z.F., JIANG D., SIBLEY D.R. The role of phosphorylation/dephosphorylation in agonist-induced desensitization of D1 dopamine receptor function: evidence for a novel pathway for receptor dephosphorylation. Mol. Pharmacol. 2001;59:310–321. doi: 10.1124/mol.59.2.310. [DOI] [PubMed] [Google Scholar]
- GOODMAN JR., O.B, KRUPNICK J.G., SANTINI F., GUREVICH V.V., PENN R.B., GAGNON A.W., KEEN J.H., BENOVIC J.L. β-Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- HEUSER J., ANDERSON R.G.W. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRASEL C., BUNEMANN M., LORENZ K., LOHSE M.J. β-Arrestin binding to the β2-adrenergic receptor requires both receptor phosphorylation and receptor activation. J. Biol. Chem. 2005;280:9528–9535. doi: 10.1074/jbc.M413078200. [DOI] [PubMed] [Google Scholar]
- KRUEGER K.M., DAAKA Y., PITCHER J.A., LEFKOWITZ R.J. The role of sequestration in G protein-coupled receptor resensitization Regulation of β 2-adrenergic dephosphorylation by vesicular acidification. J. Biol. Chem. 1997;272:5–8. doi: 10.1074/jbc.272.1.5. [DOI] [PubMed] [Google Scholar]
- LAPORTE S.A., OAKLEY R.H., ZHANG J., HOLT J.A., FERGUSON S.S., CARON M.G., BARAK L.S. The β2-adrenergic receptor/β-arrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN F., WANG H., MALBON C.C. Gravin-mediated formation of signaling complexes in β2-adrenergic receptor desensitization and resensitization. J. Biol. Chem. 2000;275:19025–19034. doi: 10.1074/jbc.275.25.19025. [DOI] [PubMed] [Google Scholar]
- LOHSE M.J., BENOVIC J.L., CARON M.G., LEFKOWITZ R.J. Multiple pathways of rapid β2-adrenergic receptor desensitization. Delineation with specific inhibitors. J. Biol. Chem. 1990a;265:3202–3209. [PubMed] [Google Scholar]
- LOHSE M.J., BENOVIC J.L., CODINA J., CARON M.G., LEFKOWITZ R.J. β-Arrestin: a protein that regulates β-adrenergic function. Science. 1990b;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- MCLAUGHLIN J.P., XU M., MACKIE K., CHAVKIN C. Phosphorylation of a carboxyl-terminal serine within the κ-opioid receptor produces desensitization and internalization. J. Biol. Chem. 2003;278:34631–34640. doi: 10.1074/jbc.M304022200. [DOI] [PubMed] [Google Scholar]
- MICHEL J.J., SCOTT J.D. AKAP mediated signal transduction. Annu. Rev. Pharmacol. Toxicol. 2002;42:235–257. doi: 10.1146/annurev.pharmtox.42.083101.135801. [DOI] [PubMed] [Google Scholar]
- MILLMAN E.E., ROSENFELD J.L., VAUGHAN D., NGUYEN J., DAI W., ALPIZAR-FOSTER E., FRIEDMAN J., CLARK R.B., KNOLL B.J., MOORE R.H. Endosome sorting of β2-adrenoceptors is GRK5 independent. Br. J. Pharmacol. 2003;141:277–284. doi: 10.1038/sj.bjp.0705504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE R.H., HALL H.S., ROSENFELD J.L., DAI W., KNOLL B.J. Specific changes in β2-adrenoceptor trafficking kinetics and intracellular sorting during downregulation. Eur. J. Pharmacol. 1999a;369:113–123. doi: 10.1016/s0014-2999(99)00055-2. [DOI] [PubMed] [Google Scholar]
- MOORE R.H., MILLMAN E.E., ALPIZAR-FOSTER E., DAI W., KNOLL B.J. Rab11 regulates the recycling and lysosome targeting of β2-adrenergic receptors. J. Cell Sci. 2004;117:3107–3117. doi: 10.1242/jcs.01168. [DOI] [PubMed] [Google Scholar]
- MOORE R.H., SADOVNIKOFF N., HOFFENBERG S., LIU S., WOODFORD P., ANGELIDES K., TRIAL J., CARSRUD N.D.V., DICKEY B.F., KNOLL B.J. Ligand-stimulated β2-adrenergic receptor internalization via the constitutive endocytic pathway into rab5-containing endosomes. J. Cell Sci. 1995;108:2983–2991. doi: 10.1242/jcs.108.9.2983. [DOI] [PubMed] [Google Scholar]
- MOORE R.H., TUFFAHA A., MILLMAN E.E., HALL H.S., DAI W., DICKEY B.F., KNOLL B.J. Agonist-induced sorting of human β2-adrenergic receptors to lysosomes during downregulation. J. Cell Sci. 1999b;112:329–338. doi: 10.1242/jcs.112.3.329. [DOI] [PubMed] [Google Scholar]
- MORRISON K.J., MOORE R.H., CARSRUD N.D.V., MILLMAN E.E., TRIAL J., CLARK R.B., BARBER R., TUVIM M., DICKEY B.F., KNOLL B.J. Repetitive endocytosis and recycling of the β2-adrenergic receptor during agonist-induced steady-state redistribution. Mol. Pharmacol. 1996;50:692–699. [PubMed] [Google Scholar]
- NO D., YAO T.P., EVANS R.M. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALCZEWSKI K., MCDOWELL J.H., JAKES S., INGEBRITSEN T.S., HARGRAVE P.A. Regulation of rhodopsin dephosphorylation by arrestin. J. Biol. Chem. 1989;264:15770–15773. [PubMed] [Google Scholar]
- PIPPIG S., ANDEXINGER S., LOHSE M.J. Sequestration and recycling of β2-adrenergic receptors permit receptor resensitization. Mol. Pharmacol. 1995;47:666–676. [PubMed] [Google Scholar]
- ROSENFELD J.L., MOORE R.H., ZIMMER K.-P., ALPIZAR-FOSTER E., DAI W., ZARKA M.N., KNOLL B.J. Lysosome proteins are redistributed during expression of a GTP-hydrolysis defective rab5a. J. Cell Sci. 2001;114:4499–4508. doi: 10.1242/jcs.114.24.4499. [DOI] [PubMed] [Google Scholar]
- SANTINI F., GAIDAROV I., KEEN J.H. G protein-coupled receptor/arrestin3 modulation of the endocytic machinery. J. Cell Biol. 2002;156:665–676. doi: 10.1083/jcb.200110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIH M., LIN F., SCOTT J.D., WANG H.Y., MALBON C.C. Dynamic complexes of β2-adrenergic receptors with protein kinases and phosphatases and the role of gravin. J. Biol. Chem. 1999;274:1588–1595. doi: 10.1074/jbc.274.3.1588. [DOI] [PubMed] [Google Scholar]
- STAEHELIN M., SIMONS P., JAEGGI K., WIGGER N. CGP-12177 A hydrophilic β-adrenergic receptor radioligand reveals high affinity binding of agonists to intact cells. J. Biol. Chem. 1983;258:3496–3502. [PubMed] [Google Scholar]
- TAO J., WANG H.Y., MALBON C.C. Protein kinase A regulates AKAP250 (gravin) scaffold binding to the β2-adrenergic receptor. EMBO J. 2003;22:6419–6429. doi: 10.1093/emboj/cdg628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAN T.M., FRIEDMAN J., QUNAIBI E., BAAMEUR F., MOORE R.H., CLARK R.B. Characterization of agonist stimulation of cAMP-dependent protein kinase and G protein-coupled receptor kinase phosphorylation of the β2-adrenergic receptor using phosphoserine-specific antibodies. Mol. Pharmacol. 2004;65:196–206. doi: 10.1124/mol.65.1.196. [DOI] [PubMed] [Google Scholar]
- VEITHEN A., AMYERE M., VAN DER SMISSEN P., CUPERS P., COURTOY P.J. Regulation of macropinocytosis in v-Src-transformed fibroblasts: cyclic AMP selectively promotes regurgitation of macropinosomes. J. Cell Sci. 1998;111:2329–2335. doi: 10.1242/jcs.111.16.2329. [DOI] [PubMed] [Google Scholar]
- VON ZASTROW M., KOBILKA B.K. Ligand-regulated internalization and recycling of human β2-adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J. Biol. Chem. 1992;267:3530–3538. [PubMed] [Google Scholar]
- XUE M., VINES C.M., BURANDA T., CIMINO D.F., BENNETT T.A., PROSSNITZ E.R. N-formyl peptide receptors cluster in an active raft-associated state prior to phosphorylation. J. Biol. Chem. 2004;279:45175–45184. doi: 10.1074/jbc.M407053200. [DOI] [PubMed] [Google Scholar]
- YANG W., WANG D., RICHMOND A. Role of clathrin-mediated endocytosis in CXCR2 sequestration, resensitization, and signal transduction. J. Biol. Chem. 1999;274:11328–11333. doi: 10.1074/jbc.274.16.11328. [DOI] [PubMed] [Google Scholar]
- YU S.S., LEFKOWITZ R.J., HAUSDORFF W.P. β Adrenergic receptor sequestration – a potential mechanism of receptor resensitization. J. Biol. Chem. 1993;268:337–341. [PubMed] [Google Scholar]
- YUAN N., FRIEDMAN J., WHALEY B.S., CLARK R.B. cAMP-dependent protein kinase and protein kinase C consensus site mutations of the β-adrenergic receptor. Effect on desensitization and stimulation of adenylyl cyclase. J. Biol. Chem. 1994;269:23032–23038. [PubMed] [Google Scholar]
- ZHANG J., BARAK L.S., ANBORGH P.H., LAPORTE S.A., CARON M.G., FERGUSON S.S. Cellular trafficking of G protein-coupled receptor/β-arrestin endocytic complexes. J. Biol. Chem. 1999;274:10999–11006. doi: 10.1074/jbc.274.16.10999. [DOI] [PubMed] [Google Scholar]
- ZHANG J., BARAK L.S., WINKLER K.E., CARON M.G., FERGUSON S.S.G. A central role for β-arrestins and clathrin-coated vesicle-mediated endocytosis in β2-adrenergic receptor resensitization. Differential regulation of receptor resensitization in two distinct cell types. J. Biol. Chem. 1997;272:27005–27014. doi: 10.1074/jbc.272.43.27005. [DOI] [PubMed] [Google Scholar]