Abstract
This study investigated several mechanisms involved in the vasorelaxant effects of (−)-epigallocatechin-3-gallate (EGCG).
EGCG (1 μM–1 mM) concentration dependently relaxed, after a transient increase in tension, contractions induced by noradrenaline (NA, 1 μM), high extracellular KCl (60 mM), or phorbol 12-myristate 13-acetate (PMA, 1 μM) in intact rat aortic rings. In a Ca2+-free solution, EGCG (1 μM–1 mM) relaxed 1 μM PMA-induced contractions, without previous transient contraction. However, EGCG (1 μM–1 mM) did not affect the 1 μM okadaic acid-induced contractions. Removal of endothelium and/or pretreatment with glibenclamide (10 μM), tetraethylammonium (2 mM) or charybdotoxin (100 nM) plus apamin (500 nM) did not modify the vasorelaxant effects of EGCG. In addition, EGCG noncompetitively antagonized the contractions induced by NA (in 1.5 mM Ca2+-containing solution) and Ca2+ (in depolarizing Ca2+-free high KCl 60 mM solution).
In rat aortic smooth muscle cells (RASMC), EGCG (100 μM) reduced increases in cytosolic free Ca2+ concentration ([Ca2+]i) induced by angiotensin II (ANG II, 100 nM) and KCl (60 mM) in 1.5 mM CaCl2-containing solution and by ANG II (100 nM) in the absence of extracellular Ca2+.
In RASMC, EGCG (100 μM) did not modify basal generation of cAMP or cGMP, but significantly reversed the inhibitory effects of NA (1 μM) and high KCl (60 mM) on cAMP and cGMP production.
EGCG inhibited the enzymatic activity of all the cyclic nucleotide PDE isoenzymes present in vascular tissue, being more effective on PDE2 (IC50∼17) and on PDE1 (IC50∼25).
Our results suggest that the vasorelaxant effects of EGCG in rat aorta are mediated, at least in part, by an inhibition of PDE activity, and the subsequent increase in cyclic nucleotide levels in RASMC, which, in turn, can reduce agonist- or high KCl concentration-induced increases in [Ca2+]i.
Keywords: (−)-Epigallocatechin-3-gallate, cyclic nucleotide PDE, cAMP, cGMP, cytosolic free Ca2+ concentration, fura-2, rat aorta, vascular smooth muscle cells, vasorelaxation
Introduction
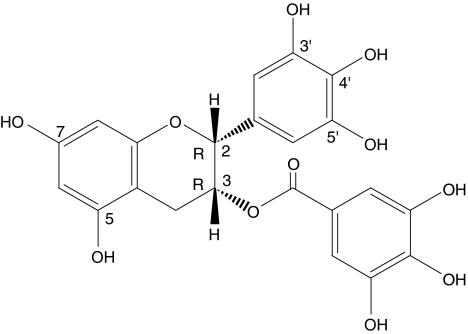
(−)-Epigallocatechin-3-gallate (EGCG, Figure 1) represents the major compound of a subclass of flavonoids, the catechin derivatives or flavan-3-ols in which tea infusions, one of the most consumed beverages in the world, is very rich (Graham, 1992). A wide variety of beneficial cardiovascular properties have been described for EGCG in the recent years. One of the most prominent is its antioxidant activity (Hertog et al., 1997; Álvarez et al., 2002) and also the ability to inhibit proliferation of vascular cells, which counteracts angiogenesis and vascular tumours, is frequently referred to Fassina et al. (2004). Other remarkable properties attributed to EGCG are antiatherogenic activities (Chyu et al., 2004; Ludwig et al., 2004), inhibition of vascular smooth muscle cell hypertrophy and inhibition of platelet aggregation (Deana et al., 2003; Zheng et al., 2004).
Figure 1.
Chemical structure and absolute stereochemistry of (−)-epigallocatechin-3-gallate.
EGCG has been considered mainly responsible for the vasorelaxant effect of green tea extract in isolated rat aorta (Chen et al., 2000), even though other authors have recently reported that this catechin is not associated to the green tea extract-induced vasorelaxation (Lim et al., 2003). The vasorelaxant effects of EGCG have been related to the inhibition of Ca2+ influx in smooth muscle cells by some authors (Huang et al., 1998), while others have described an endothelium-dependent mode of action either related to the stimulation of endothelial NO synthase (NOS) (Lorenz et al., 2004) or attributed to an increase in the production of prostacyclin (Mizugaki et al., 2000).
On the other hand, Shen et al. (2003) have reported that EGCG exerts direct contractile effects in rat aorta similar to those induced by an extract of tea polyphenols and Sanae et al. (2002) have found that this flavanol potentiates the contractile response to phenylephrine in endothelium-intact rat aorta. More recently, we have described a transient, Ca2+-dependent contractile response of EGCG in rat aorta (Álvarez-Castro et al., 2004), our own results suggesting that the opposite vascular effects of this catechin (contractile and relaxant) seemed to be due to a biphasic behaviour, more than to a dual concentration-selective mechanism.
In view of these contradictory reports, the present work was aimed to understand the effects of EGCG on vascular smooth muscle further in order to shed more light on the mechanisms of the vasorelaxant action of this catechin on isolated rat aortic rings. In cultured rat aortic smooth muscle cells (RASMC), we evaluated, for the first time, the effects of EGCG on the production of cAMP and cGMP, and on the agonist- or high KCl-induced increases in cytosolic free Ca2+ concentration ([Ca2+]i). We have also evaluated, again for the first time, the effect of EGCG on PDE (PDE1–5) enzymatic activity.
Methods
Animals
The animals used throughout this study (Male Wistar–Kyoto (WKY) rats (Iffa-Credo), purchased from Criffa (Barcelona, Spain)) were housed, cared for and acclimatized (before the experiments) as indicated previously (Orallo et al., 2002).
Ethical approval
All experiments were carried out in accordance with European regulations on the protection of animals (Directive 86/609), the Spanish Real Decreto 223/1988 and Orden Ministerial October/13/1989 and/or the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). In addition, all experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Santiago de Compostela (Spain), and conducted humanely.
Contraction–relaxation studies in isolated rat thoracic aorta rings
Vascular rings were prepared from aortae of male WKY rats (250–300 g) as described elsewhere (Orallo, 1997). The composition of the Krebs' solution was (mM): NaCl 119, KCl 4.7, CaCl2·2H2O 1.5, MgSO4·7H2O 1.2, KH2PO4 1.2, NaHCO3 25, EDTA-Na2 0.03, L-(+)-ascorbic acid 0.6 and glucose 11.
Rat aortic rings were equilibrated at a resting tension of 2 g for at least 1 h. Thereafter, isometric contractions were induced by the addition of noradrenaline (NA, 1 μM) or KCl (60 mM, instead of the equivalent amount of NaCl in order to maintain the osmolarity constant). Once the contraction stabilized, a single concentration of acetylcholine (1 μM) was added to the bath in order to assess the endothelial integrity of the preparations. Endothelium was considered to be intact when this drug elicited a vasorelaxation >50% of the maximal contraction obtained in vascular rings precontracted with NA or KCl. The absence of acetylcholine relaxant action in the vessels indicated the total removal of endothelial cells. After assessing the integrity of the endothelium, vascular tissues were allowed to recuperate for 1 h, during which the physiological solution was replaced every 15 min, before any experiment protocol was started.
Vasorelaxant activity of EGCG in precontracted rat aortic rings
Once the presence or absence of functional endothelium was verified and after the equilibration period, isometric contractions induced by NA (1 μM), phorbol 12-myristate 13-acetate (PMA, 1 μM), okadaic acid (OA, 1 μM) or high extracellular KCl concentration (60 mM) were obtained. In experiments with 60 mM KCl, the NaCl concentration was reduced accordingly in order to maintain constant osmolarity. When contraction of the tissue in response to the corresponding vasoconstrictor agent had stabilized (after approximately 20 min for NA and KCl or 60 min for PMA and OA), cumulative increasing concentrations of EGCG were added to the bath at 30–40 min intervals (the time needed to obtain steady-state relaxation).
In order to quantify the level of contractile function recovery for NA and KCl after treatment with EGCG, the aortic rings were maintained in the resting state over at least 1 h (Krebs' solution replaced every 15 min) before eliciting a new contraction with NA (1 μM) or KCl (60 mM).
For experiments in calcium-free medium, aorta preparations were equilibrated for 60 min in 1.5 mM Ca2+-containing solution and then in a Ca2+-free medium for 20–30 min before a PMA contraction was elicited (Ca2+-free solution had the same composition as that of Krebs solution, except that CaCl2 was replaced by 0.5 mM EGTA). When the contraction in response to PMA reached a stable plateau, cumulative concentration–relaxation curves for EGCG were constructed as indicated above.
In some experiments, 20 min before initiating the above experimental protocols, tetraethylammonium (TEA, 2 mM), glibenclamide (GB, 10 μM) or charybdotoxin (ChTX, 100 nM) plus apamin (500 nM) were included in the bath, in order to analyse the effects of these drugs on the vasorelaxation induced by EGCG.
Effects of EGCG on cumulative concentration–effect curves for NA and Ca2+ in isolated rat aortic rings
For obtaining NA cumulative concentration–response curves, progressively higher concentrations (0.3 nM–1 μM) were applied when a steady-state level had been reached for the preceding concentration. For constructing Ca2+ concentration–response curves, tissues were preincubated for 30 min in Ca2+-free depolarizing Krebs bicarbonate solution (containing 50 mM of KCl instead of the equivalent amount of NaCl, in order to maintain osmolarity) and calcium chloride (10 μM–10 mM) was then added to the bath in a stepwise manner.
To evaluate the actions of EGCG (30, 100 and 300 μM), arterial segments were preincubated with this compound for 30 min. Preincubation with EGCG was carried out before the third concentration–response curve to NA or Ca2+, since second and third curves were almost identical in control experiments. Only one concentration of EGCG was used per aortic ring.
Cell culture
RASMC were isolated from the medial layer of thoracic aorta of male WKY rats as described previously (Orallo, 1997). For calcium imaging studies, cells were maintained in culture and then seeded in glass coverslips according to the protocol detailed in Álvarez-Castro et al. (2004). For determination of cAMP and cGMP production in RASMC, cells were prepared according to Vidrio et al. (2003).
Determination of total cAMP and cGMP production in RASMC
The potential effects of EGCG on basal cAMP and cGMP levels and on NA- and KCl-induced decrease in cAMP and cGMP production were evaluated following the general methods detailed in Vidrio et al. (2003) and Orallo et al. (2004). Briefly, RASMC (passages 4–10) were seeded onto 96-well flat-bottom microtitre plates and the experiments started after an equilibration period of 12 h at 37°C and 5% CO2. To investigate actions on basal cAMP and cGMP formation, the RASMC were incubated for 20 min with EGCG (100 μM). At the end of incubation, the lysis reagent dodecyltrimethylammonium bromide (0.5% (w v−1)) was added to each well under continuous shaking to completely break down the cells and to stop cellular enzymatic reactions.
In another set of experiments, the potential influence of the EGCG (100 μM) on the decrease of cGMP and cAMP production induced by NA (1 μM) or high KCl (60 mM) was studied by preincubating the cells with this catechin for 10 min, followed by the addition of NA or KCl and by finalization of cellular enzymatic reactions 10 min later. For determination of cGMP production, the acetylation reagent 1 : 2 (v v−1) acetic anhydride/triethylamine was added to the different wells and total cGMP content of 50 μl aliquots from each acetylated sample was estimated with a commercially available cGMP enzymeimmunoassay (EIA) system using a Titertek Multiscan PLUS MKII multiwell plate reader (Lab Systems Inc., Vienna, U.S.A.).
For measurement of cAMP production, no prior acetylation was required. Therefore, total cAMP content in 100-μl aliquots from each nonacetylated sample was estimated with a cAMP enzyme immunoassay system, using a multiwell plate reader (see above).
In some tests, the validity of the method was checked by measuring the influence of 3-isobutyl-1-methylxanthine (IBMX, 10 μM, a well-known nonselective reference inhibitor of PDEs) on basal cAMP and cGMP levels or on NA- and high KCl-induced decrease in cyclic nucleotide biosynthesis.
Isolation and determination of PDE isoform activity
PDE1, PDE3–5 were isolated by anion exchange chromatography from a bovine aortic smooth muscle cytosol fraction as per Lugnier et al. (1986) and stored until use in small aliquots (200 μl) at −80°C. PDE2 was isolated from human platelets as per Kameni Tcheudji et al. (2001), and stored until use at −80°C in small aliquots.
PDE activities were measured by radioenzymatic assay as described previously (Keravis et al., 1980) at a substrate concentration of 1 μM cAMP or 1 μM cGMP in the presence of 10,000 c.p.m. [3H]-cAMP or [3H]-cGMP as tracers. The buffer solution comprised 50 mM Tris-HCl (pH 7.5), 2 mM magnesium acetate and 50 mg bovine serum albumin. PDE1 activity was assayed at 1 μM cGMP in the basal state (1 mM EGTA) or in calmodulin (CaM)-activated state (18 nM CaM with 10 μM CaCl2). PDE2 activity was evaluated at 1 μM cAMP+1 mM EGTA in the basal state (without cGMP) and in the activated state (in the presence of 5 μM cGMP). PDE3 and PDE4 activities were assayed at 1 μM cAMP+1 mM EGTA. To prevent reciprocal crosscontamination (possible interferences) between PDE3 and PDE4, all PDE3 assays were carried out in the presence of 50 μM rolipram, and all PDE4 assays in the presence of 50 μM cGMP. PDE5 activity was assayed at 1 μM cGMP+1 mM EGTA.
To investigate the effects of EGCG on PDE activities, it was added to the corresponding test tubes 5 min before initiating the enzymatic reaction, which was subsequently continued for 30 min.
To study the effects of EGCG on the enzymatic activity of PDE1 and PDE3–5 isoforms, we used bovine aorta, (a) in view of the difficulty of purifying sufficient quantities of the different PDEs from rat aorta, and (b) on the assumption that we can extrapolate the results from cow to rat since the PDEs isolated from the medial layer of rat aorta and bovine aorta show similar sensitivities to a number of drugs (Lugnier et al., 1986; Komas et al., 1991). In addition, to study the possible effects of EGCG on the enzymatic activity of PDE2, we used human platelets in place of bovine and rat aorta since PDE2 is not present in the medial layer of these arteries.
Measurement of [Ca2+]i
The effects of angiotensin II (ANG II, 100 nM) or high extracellular concentration of KCl (60 mM) on [Ca2+]i in RASMC were determined as previously described in detail (Álvarez-Castro et al., 2004). For incubation periods, EGCG was added in volumes of 2–20 μl to a final incubation volume of 2 ml of bathing solution. All procedures and experiments were performed at room temperature to minimize compartmentalization and cell extrusion of the fluorescent dye.
Data presentation and statistical analysis
Unless otherwise specified, results shown in the text, tables and figures are expressed as mean±s.e.m. Means were compared by the one-way analysis of variance followed by the Dunnett's post hoc test.
In the experiments carried out in precontracted rat aortic rings, contractile responses to vasoconstrictor agents are expressed as a percentage of the maximal contraction (Emax=100%) produced by the corresponding vasoconstrictor agent before the addition of EGCG. In these experiments, sigmoidal concentration–response curves for the vasorelaxant effects of EGCG were fitted using the program Origin™ 5.0 (Microcal Software Inc., Northampton, U.S.A.), with estimation of IC50 values (i.e. concentrations inducing 50% relaxation) for NA-, high KCl- and PMA-induced contractions.
Contractile responses to cumulative concentrations of NA or CaCl2 (in the presence or absence of EGCG) are expressed as a percentage of the maximal contraction (Emax=100%) reached in the control concentration–response curves. These curves were analysed by a sigmoidal curve-fitting analysis program (Origin™ 5.0) and the pD2 value for NA (negative log10 of the molar concentration of agonist required to elicit 50% of maximum effect (EC50)) was calculated.
EGCG pD′2 values (negative log10 of the molar concentration of antagonist required to induce a 50% depression of the maximal contraction induced by NA or by CaCl2) were estimated, using a linear fitting analysis program (Origin™ 5.0), as the X-intercept of the regression of log (CR-1) on log [EGCG], where CR is the ratio of the maximal control response produced by NA or CaCl2 to the maximum effect of these vasoconstrictor agents in the presence of EGCG. This regression was performed using data obtained with different concentrations of EGCG, which inhibited the maximal contractile effect induced by NA or CaCl2 between 20 and 80%.
In the experiments designed to determine the possible effects of EGCG on cyclic nucleotides production in RASMC, the amount of cGMP or cAMP formed was expressed as fmol of cGMP or cAMP per well. Each well contained approximately 50,000 cells.
In the experiments performed to study the possible effects of EGCG on PDE isoform enzymatic activity, the concentration of EGCG that caused 50% inhibition of substrate hydrolysis (IC50) was estimated by nonlinear regression analysis (GraphPad Prism software, San Diego, CA, U.S.A.) from the concentration–response curves obtained in each case with six different concentrations of EGCG.
The [Ca2+]i was calculated as detailed previously (Álvarez-Castro et al., 2004). Basal [Ca2+]i was determined by averaging resting [Ca2+]i values measured for 10 s on cells from different preparations. [Ca2+]i plateau in the response to ANG II was determined by averaging [Ca2+]i values measured between 60–180 s after the applications of ANG II on cells from different preparations. Only data obtained from cells that responded to the Ca2+ ionophore ionomycin (500 nM) at the end of the experiments were used.
Drugs, chemicals and radioisotopes
ANG II acetate salt, acetylcholine chloride, apamin from bee venom, (+)-ascorbic acid, bovine serum albumin, cAMP, cGMP, CaM, dimethylsulphoxide (DMSO), recombinant ChTX, EGCG, GB, IBMX, ionomycin, (−)-NA bitartrate, OA, pluronic F-127, PMA and TEA hydrochloride were purchased from Sigma-Aldrich (Alcobendas, Spain and L'Isle d'Abeau Chesnes, France). Rolipram was a generous gift from Schering (Berlin, Germany).
Fura-2 AM (fura-2 acetoxymethyl ester) was from Molecular Probes (Madrid, Spain). Acetic anhydride/triethylamine, cAMP EIA system, cGMP enzyme immunoassay system, dodecyl trimethylammonium bromide, [3H]-cAMP (42 Ci mmol−1) and [3H]-cGMP (15 Ci mmol−1) were from Amersham Pharmacia Biotech Biosciences (Barcelona, Spain and Saclay, France); [3H]-cAMP and [3H]-cGMP were further purified by thin-layer chromatography on 60F254 silica gel plates (20 cm × 20 cm) from Merck KGaA (Darmstadt, Germany) with isopropanol (70 ml) : NH4OH (15 ml) : H2O (15 ml).
Other specific chemicals and materials used in the different tests were purchased from suppliers indicated in the corresponding references provided in the Method sections. All other chemicals, including the reagents used in the preparation of the different solutions, were of the best quality available commercially.
Appropriate dilutions of the above drugs were prepared every day immediately before use in deionized water from the following concentrated stock solutions kept at −20°C: acetylcholine (10 mM), ANG II (1 mM), apamin (5 mM), ChTX (100 μM), EGCG (100 mM), IBMX (100 mM), NA (100 mM) and TEA (100 mM) in deionized water; GB (100 mM), ionomycin (1 mM), OA (100 mM) and PMA (100 mM) in DMSO. Sodium bisulphite (0.2% (w v−1)) was added to the NA stock solution to prevent oxidation. Fura-2-AM (5 μM) was prepared daily in physiological buffer containing 0.005% pluronic F-127 and 0.1% DMSO. Final concentration of DMSO never exceeded 0.01% in the experiments.
In all assays, neither deionized water (Milli-Q®, Millipore, Spain and France) nor appropriate dilutions of the vehicle used (DMSO) had significant pharmacological effects.
Studies involving light-sensitive compounds (EGCG and PMA) were carried out in the dark. For imaging experiments, appropriate precautionary measures were taken throughout the procedure to avoid degradation of light-sensitive compounds and extensive photobleaching due to the photosensitivity of the fura-2 molecule.
Results
Effects of EGCG on precontracted rat aortic rings
In 1.5 mM Ca2+-containing solution, NA (1 μM), OA (1 μM), PMA (1 μM) and high extracellular KCl concentration (60 mM) all caused slow and sustained contraction of the rat isolated aortic rings, with or without endothelium. PMA (1 μM) also contracted rat aorta in Ca2+-free medium with equal effectiveness. The maximal tensions reached are shown in Table 1. The maximal tension induced by KCl was significantly higher than that induced by NA, PMA and OA (n=10, P<0.01). The presence of functional endothelium significantly reduced the maximal contractions induced by all vasoconstrictor agents (Table 1, n=10, P<0.01). These contractile effects were maintained without significant tension changes in control rings for at least 90 min. DMSO (0.14 mM–42 mM) had no significant effects on NA-, OA-, PMA- and high KCl-induced contractions, in either endothelium-denuded or intact rat aortic rings (n=5, P>0.05).
Table 1.
Maximal tension values (mg) induced by NA (1 μM), OA (1 μM), PMA (1 μM, in 1.5 mM Ca2+-containing or Ca2+-free medium) or high extracellular KCl concentration (60 mM) in intact and endothelium-denuded rat aortic rings in the absence and presence of GB (10 μM), TEA (2 mM) or ChTX (100 nM) plus apamin (500 nM)
| Control | +GB | +TEA | ChTX+apamin | |
|---|---|---|---|---|
| With endothelium | ||||
| NA | 2264±131* | 2146±178* | 2359±171* | 2293±182* |
| OA | 2113±126* | 2158±162* | 2241±184* | 2171±173* |
| PMA (0 mM Ca2+) | 1874±98* | 1950±141* | 2011±154* | 2043±160* |
| PMA | 1941±109* | 1878±111* | 2105±169* | 1933±125* |
| KCl | 3372±163*,# | 3260±261*,# | 3351±265*,# | 3289±271*,# |
| Without endothelium | ||||
| NA | 3087±175 | 3120±239 | 3174±248 | 3056±223 |
| OA | 2833±145 | 2780±206 | 2921±223 | 2987±210 |
| PMA (0 mM Ca2+) | 2684±149 | 2750±194 | 2791±234 | 2801±205 |
| PMA | 2721±152 | 2823±111 | 2851±215 | 2772±179 |
| KCl | 3827±195# | 3798±272# | 3921±293# | 4002±265# |
Each value is the mean±s.e.m. from ten (control) or five (GB, TEA or ChTX plus apamin) experiments. Levels of statistical significance:
P<0.01 versus the corresponding values in endothelium-denuded rat aortic rings;
P<0.05 with respect to the corresponding maximal tensions induced by NA (1 μM) in intact or endothelium-denuded aortic rings.
Addition of EGCG (1–30 μM) on the sustained response induced by any of the vasoconstrictor agents used in 1.5 mM Ca2+-containing solution produced a transient concentration-dependent potentiation of the contraction which reverted until reaching the initial values of the response in 15–30 min. The magnitudes of these potentiations were independent of the vasoconstrictor used and their maximal values were ∼30% of the initial contraction.
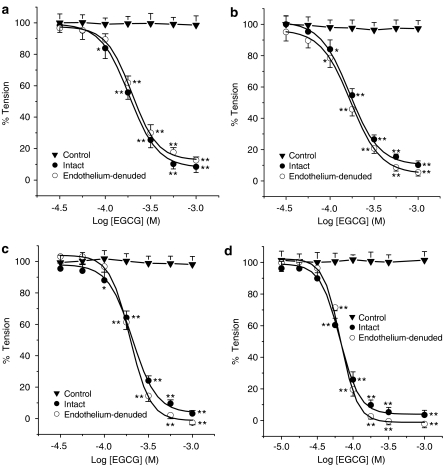
Higher concentrations of EGCG (30 μM–1 mM) showed a biphasic mode of action: first, potentiations of the contractile response similar to those described above were observed followed by, secondly, a concentration-dependent vasorelaxant effect. In these conditions, EGCG totally relaxed the NA-, PMA- and KCl-induced contractions in rat aortic rings in 1.5 mM Ca2+-containing solution (Figure 2). The differences between the IC50 values obtained for contractions induced by these vasoconstrictor agents were not significant (Table 2, n=5, P>0.05).
Figure 2.
Cumulative concentration–relaxation curves for EGCG (1 μM–1 mM) in intact and endothelium-denuded rat thoracic aortic rings precontracted with NA (1 μM, panel a), PMA (1 μM) in 1.5 mM Ca2+-containing solution (panel b) and in Ca2+-free medium (panel d) or high extracellular KCl concentration (60 mM, panel c). Each point represents the mean value±s.e.m. (indicated by vertical bars) from five experiments. Level of statistical significance: *P<0.05 or **P<0.01 with respect to the maximal tension (Emax=100%).
Table 2.
IC50 values (μM) for EGCG-induced vasorelaxation in intact and endothelium-denuded rat aortic rings precontracted with NA (1 μM), PMA (1 μM, in 1.5 mM Ca2+-containing or Ca2+-free medium) or high extracellular KCl (60 mM) in the absence (control) and presence of GB (10 μM), TEA (2 mM) or ChTX (100 nM) plus apamin (500 nM)
| Control | GB | TEA | ChTX+apamin | |
|---|---|---|---|---|
| With endothelium | ||||
| NA | 191.8±13.0 | 183.3±15.1 | 200.0±14.1 | 192.3±12.2 |
| PMA (0 mM Ca2+) | 65.6±5.1* | 58.7±4.5* | 62.7±5.1* | 66.4±3.6* |
| PMA | 178.5±12.2 | 173.7±14.5 | 185.5±14.7 | 187.6±15.0 |
| KCl | 201.2±14.9 | 189.7±13.3 | 207.7±16.7 | 191.0±12.8 |
| Without endothelium | ||||
| NA | 208.3±14.3 | 187.8±14.9 | 203.7±14.3 | 205.4±11.2 |
| PMA (0 mM Ca2+) | 67.0±5.2* | 57.3±4.0* | 63.4±5.3* | 58.1±8.3* |
| PMA | 171.6±13.8 | 177.4±14.2 | 182.4±13.1 | 186.1±14.6 |
| KCl | 193.2±15.1 | 200.1±14.7 | 202.7±15.0 | 200.3±12.9 |
Each value is the mean±s.e.m. from five experiments.
P<0.01 with respect to the corresponding value obtained with NA.
Mechanical removal of endothelium did not significantly modify the vasorelaxant effects of EGCG (Figure 2). The corresponding IC50 values are not significantly different in the presence or in the absence of endothelium (Table 2, n=5, P>0.05).
EGCG (30 μM–1 mM) and IBMX (0.1–100 μM) did not affect the contractile response induced by OA (1 μM) in either denuded or intact rat aorta (data not shown).
When the contraction by PMA was induced in Ca2+-free medium, the concentration-dependent vasorelaxation induced by EGCG (1 μM–1 mM) occurred without previous enhancement of tension. The IC50 obtained in this case for EGCG was significantly lower than that observed against NA, KCl and PMA in 1.5 mM Ca2+-containing solution (Table 2).
Preincubation of endothelium-containing and endothelium-denuded aortic rings with GB (10 μM), TEA (2 mM) or ChTX (100 nM) plus apamin (500 nM) did not significantly affect the magnitude of NA-, OA-, PMA- or high KCl-induced sustained contraction (Table 1, n=5, P>0.05). In addition, pretreatment of rat aorta rings with these K+ channel blockers had no effect on the concentration–relaxation curves for EGCG. The corresponding IC50 values do not present significant differences with respect those obtained in the absence of GB, TEA or ChTX plus apamin (Table 2, n=5, P>0.05).
Contraction recovery after treatment with EGCG (see experimental protocol in the Methods section) was of 46.2±3.8% (n=5) for the aortic rings where NA was used and of only 4.3±0.3% (n=5) for those where KCl was employed. The recovery in control rings (without EGCG treatment) was of ∼100%.
Effects of EGCG on cumulative concentration–effect curves for NA and Ca2+
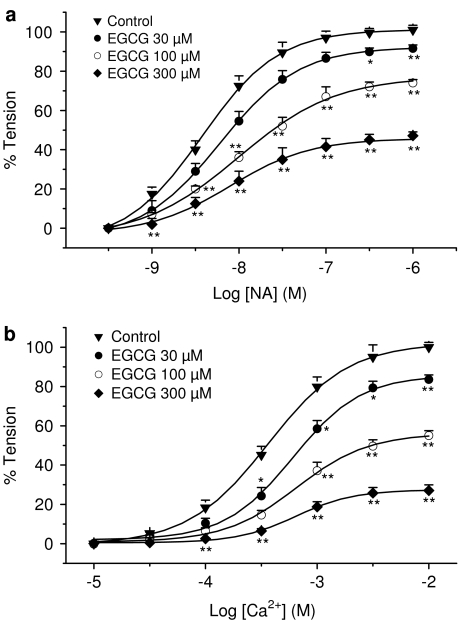
Cumulative addition of NA (0.3 nM–1 μM) in 1.5 mM Ca2+-containing solution or CaCl2 (10 μM–10 mM) in depolarizing (60 mM K+) Ca2+-free medium produced, in both cases, a concentration-dependent contraction of intact rat aortic rings. Maximum tension values reached were 3521±184 and 4428±224 mg, respectively, and pD2 value for NA was 8.08±0.37 (n=10), while EC50 for CaCl2 was 371±28 μM (n=5).
Preincubation with different concentrations of EGCG (30, 100 and 300 μM) for 30 min shifted to the right the concentration–response curves for NA and CaCl2, with a decrease of the maximum response in both cases (Figure 3). The corresponding pD′2 values for EGCG against NA and CaCl2 were 3.63±0.28 and 3.91±0.32, respectively, while the slope for the Schild regression did not differ from unit in any case (1.08±0.06 and 1.12±0.09, respectively, n=5, P>0.05).
Figure 3.
Cumulative concentration–response curves for NA (panel a) and for CaCl2 (panel b) in endothelium-intact rat thoracic aortic rings in the absence (control) or in the presence of different concentrations of EGCG. Each point represents the mean value±s.e.m. (indicated by vertical bars) from 10 (control with NA) or five (control with CaCl2 and EGCG-treated rings) experiments. Level of statistical significance: *P<0.05 or **P<0.01 with respect to control curve.
Effects of EGCG on cAMP and cGMP production in RASMC
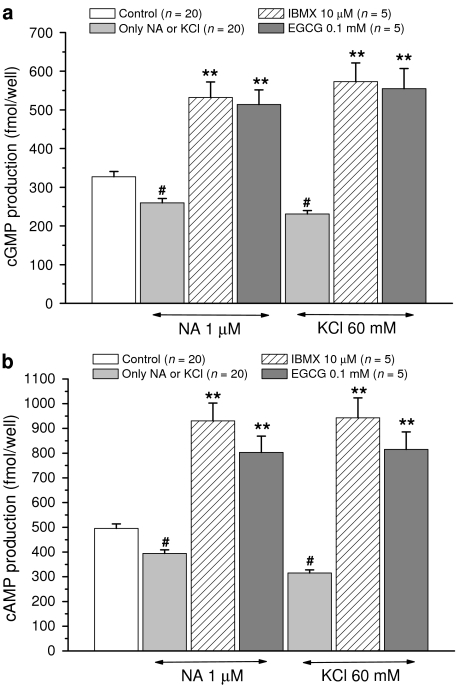
In control RASMC, basal cytosolic cGMP and cAMP production was 327.0±13.8 and 495.5±18.3 fmol well−1 (n=20), respectively. These values were unaffected by pretreatment with 100 μM EGCG (325.7±26.2 and 489.6±40.7) or 10 μM IBMX (330.8±27.0 and 498.6±38.9) (n=5, P>0.05).
NA (1 μM) and high KCl (60 mM) caused a significant decrease (n=20, P<0.05 with respect to basal value) in basal production of cGMP and cAMP in cultured RASMC (see Figure 4). EGCG (100 μM) and IBMX (10 μM), however, significantly reversed the inhibitory effects of NA (1 μM) and high KCl (60 mM) on cyclic nucleotide synthesis in cultured RASMC (Figure 4).
Figure 4.
Effects of EGCG (100 μM) and IBMX (10 μM) on the decrease of cGMP (panel a) and cAMP (panel b) production induced by NA (1 μM) or high KCl (60 mM) in cultured RASMC. Each point represents the mean value±s.e.m. (indicated by vertical bars) from the number of separate experiments shown within parentheses (in each separate experiment, the corresponding sample was assayed in duplicate). Levels of statistical significance: #P<0.05 and **P<0.01 with respect to control values.
Effects of EGCG on PDE activities
Table 3 reports the effects of EGCG on the main PDE isoforms participating in cardiovascular regulation. This catechin inhibited PDE1–5, the order of potency being PDE2∼PDE1>PDE4∼PDE5>PDE3, which means that PDE3 was the less sensitive isoform to the inhibitory effects of EGCG (IC50=91±10 μM) and that PDE1 and PDE2 were preferentially inhibited (IC50∼25 and ∼17 μM, respectively). PDE1 and PDE2 were inhibited with equal effectiveness in basal and stimulated state, suggesting that EGCG inhibitory effect is CaM- or cGMP-independent for PDE1 or PDE2, respectively.
Table 3.
IC50 values (μM) for the inhibitory effects of EGCG on the enzymatic activity of PDE isoforms isolated from bovine aortic medial layer (PDE1, PDE3–5) or human platelets (PDE2)
| Isoform | PDE1 | PDE2 | PDE3 | PDE4 | PDE5 | ||
|---|---|---|---|---|---|---|---|
| Substrate | cGMP | cAMP | cAMP | cAMP | cGMP | ||
| −CaM | +CaM | −cGMP | +cGMP | ||||
| EGCG | 29±5 (n=6) | 23±6 (n=5) | 17±7 (n=4) | 18±3 (n=3) | 91±10 (n=5) | 48±4 (n=5) | 56±7 (n=3) |
Results are means±s.e.m. of n determinations at 1 μM substrate concentration.
Effects of EGCG on ANG II- or KCl-induced [Ca2+]i increases levels in RASMC
In 1.5 mM Ca2+ external solution, the mean basal [Ca2+]i level in RASMC was 87.14±2.03 nM (n=47), and was unchanged throughout the experimental time course.
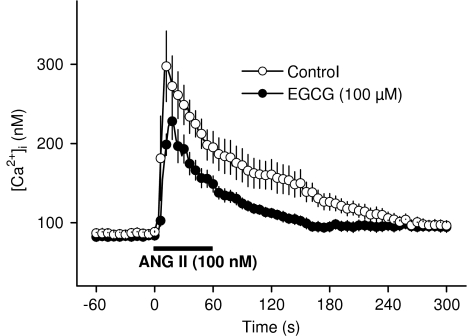
Application of ANG II (100 nM) induced a biphasic increase in [Ca2+]i with a peak of 297.54±44.60 nM and a plateau of 177.91±17.12 nM (n=14), which was maintained for at least 4 min (Figure 5).
Figure 5.
Inhibition of ANGII-induced increase in [Ca2+]i after 30 min pretreatment of RASMC with EGCG. Data are mean±s.e.m. from 14 and 15 cells, respectively.
Preincubation of the cells with EGCG (30, 100 μM) for 30 min caused a transient increase in [Ca2+]i that returned to basal values within ∼15 min (data not shown). For more details on the EGCG-induced increase in [Ca2+]i in RASMC, see Álvarez-Castro et al. (2004).
A 30 min preincubation of the RASMC with EGCG (100 μM) significantly reduced ANG II-induced increase in [Ca2+]i (229.13±17.82 nM and 132.60±15.07 nM for the peak and plateau values, respectively, n=15, P<0.05 with respect to control values, Figure 5). EGCG (30 μM) was without effect (290.22±32.01 nM and 185.24±19.09 nM for the peak and plateau values, respectively, n=18, P>0.05 with respect to control values).
In the absence of external Ca2+, application of ANG II (100 nM) only induced a transient increase in [Ca2+]i of 245.34±33.21 nM (n=16) that returned to basal values within ∼2 min. In these conditions, a 30 min preincubation of the cells with 100 μM EGCG significantly reduced ANG II-induced increase in [Ca2+]i (189.09±18.23 nM, n=17, P<0.05 with respect to control values). EGCG (30 μM) was without effect (223.01±26.80 nM, n=14, P>0.05 with respect to control values).
In a 1.5 mM Ca2+-containing external solution, KCl (60 mM) induced a sustained rise in [Ca2+]i without transient component that was maintained for at least 5 min (maximal [Ca2+]i value: 354.35±21.66 nM, n=16). A 30 min preincubation of the cells with 100 μM EGCG significantly reduced KCl-induced increase in [Ca2+]i (233.34±19.12 nM, n=15, P<0.05 with respect to control value). EGCG (30 μM) was without effect (368.34±31.22 nM, n=12, P>0.05 with respect to control value). In the absence of extracellular Ca2+, addition of KCl had no effect on [Ca2+]i, suggesting that the KCl-induced increase in [Ca2+]i occurs via transmembrane Ca2+ channels rather than the release of Ca2+ from intracellular stores.
Discussion
This study investigated for the first time several mechanisms involved in the vasorelaxant effects of EGCG.
At concentrations within the same range at which this catechin (Huang et al., 1998) or similar flavonoids have effects in vitro on vascular smooth muscle (Ibarra et al., 2002), in our experiments EGCG concentration-dependently relaxed with almost equal effectiveness and after a transient increase in tension the contractions induced by NA and high extracellular KCl concentration (60 mM) in rat aortic rings. This inhibitory effect of EGCG seems to be quite perdurable since recovery of the contractile function of EGCG-treated aortic rings was only ∼50% for NA and almost null for high KCl.
Likewise, EGCG noncompetitively antagonized the contractile response to cumulative addition of NA (in 1.5 mM Ca2+-containing bath solution) or Ca2+ (in Ca2+-free, 60 mM K+ solution), rightwardly displacing the corresponding concentration–effect curves with a decrease of the maximal response. If we accept the classical explanation for induction of contraction by NA and KCl, these results suggest that this natural compound may act by an intracellular and/or an extracellular mechanism (see below).
In the same way as other nonselective PDE inhibitors (Noguera et al., 2001d), the vasorelaxant effects of EGCG were endothelium-independent. The superoxide radicals (O2•−) scavenger activity of EGCG (Álvarez et al., 2002) could be implicated in an endothelium-dependent effect, since O2•− are principally responsible for the degradation of the endothelial-derived relaxing factor, NO• (Furchgott, 1999). However, these scavenger properties are possibly not enough to induce endothelium-dependent relaxation, as with ascorbic acid, which did not relax precontracted endothelium-containing vascular tissues (Huang et al., 1998).
These results apparently agree with those previously reported (Huang et al., 1998; Chen et al., 2000). In contrast, Lorenz et al. (2004) found that EGCG-induced vasodilation in rat aortic rings is abolished by pretreatment with NG-nitro-L-arginine methyl esther (a NOS inhibitor) and that EGCG increased NOS activity in bovine aortic endothelial cells. Also, Mizugaki et al. (2000) have detected an EGCG-stimulated elevation in the prostacyclin production in this type of cells in culture, suggesting the implication of some endothelium-dependent relaxing factors in the vasorelaxant action of EGCG. However, these latter results disagree with Sanae et al. (2002), who reported that EGCG potentiates the contractile response to phenylephrine in endothelium intact rat aorta.
The fact that the IC50 and pD′2 for EGCG against the contractions induced by NA and high extracellular KCl or Ca2+ were almost identical suggests that the vasorelaxant activity of this catechin in rat aorta may be via effects of one or both of the following types, whether direct or indirect (e.g., mediated by an increase in cytosolic cAMP and cGMP levels, possibly via nonselective inhibition of PDEs; Orallo, 1996):
Intracellular effects, through (among others) mechanisms such as an action on the contractile apparatus, for example, via a reduction in myosin light chain (MLC) phosphorylation, an increase in MLC dephosphorylation, an inhibition of some of the steps of the contractile effect induced by PKC activation or an inhibition of agonist-mediated release of Ca2+ from intracellular stores.
A nonselective effect of EGCG on the cell membrane, involving (among other mechanisms) the opening of K+ channels and/or the blockade of Ca2+ influx through transmembrane Ca2+ channels (see below).
In the present study, OA-induced sustained contraction of rat aorta was not attenuated by EGCG and IBMX, an agent widely used as a nonselective inhibitor of PDE1–5 isoforms (for the corresponding IC50 values of IBMX on PDE activities see Stoclet et al., 1995; Noguera et al., 2001). It has been previously reported that OA, a toxin isolated from marine black sponges of the genus Halichondoria, causes a sustained contraction in rat aorta, not associated with an increase in intracellular calcium concentration (Wakabayashi et al., 1995). This contraction seems to be due to the inhibition of myosin phosphatase isoforms and a subsequent marked increase in the degree of phosphorylation of MLC (OA inhibits dephosphorylation of phosphorylated MLC; Karaki et al., 1997; Knapp et al., 2000). The observed absence of effect of EGCG on OA-induced contraction indicates that this catechin does not act directly on the contractile machinery (e.g., by increasing MLC dephosphorylation or by reducing MLC phosphorylation).
Our results contrast, in part, with those reported by Kitano et al. (1997), who found that EGCG can counteract the inhibitory effects of OA on protein phosphatase 2A purified from bovine brain. This effect seems to be mediated by the incorporation of OA into the aggregates formed by EGCG in buffer solution, a phenomenon that seems less likely to occur under our experimental conditions, where the tissue is present.
The hypothesis that the vasorelaxant activity of EGCG may be due to an inhibition of some of the mechanisms involved in the contractile effect induced by PKC activation is supported by the results obtained in the presence of PMA.
The mechanism by which phorbol esters induce contraction in vascular smooth muscle has not yet been clarified. However, it has been reported that, in rat aorta, high concentrations of these compounds (including PMA) induce the activation of a number of PKC isoforms (Liou & Morgan, 1994; Malarkey et al., 1996). This PKC activation in turn induces (among other responses) an increase in the Ca2+ sensitivity of the contractile proteins (Jiang & Morgan, 1987; Andrea & Walsh, 1992) and, therefore, sustained contraction (which does not seem to be associated with any change in the intracellular free Ca2+ concentration or in MLC phosphorylation; see, e.g., Jiang & Morgan, 1987; Sato et al., 1992).
As previously reported for a number of PDE inhibitors (see, e.g., Itoh et al., 1993), in this study EGCG completely relaxed the contractions, with almost equal effectiveness, induced by PMA (in 1.5 mM Ca2+-containing solution) and those elicited by high KCl and NA (see above), suggesting that its vasorelaxant effects may be due, at least in part, to a direct inhibition of PKC enzymatic activity and/or to an inhibition of some of the mechanisms involved in the contractile process triggered by the activation of this kinase.
In this connection, we have no data about the direct action of EGCG on PKC isoforms present in rat aorta, but Kitano et al. (1997) have described an inhibitory effect of this catechin on the activity of PKC purified from bovine brain.
In Ca2+-free medium, EGCG also completely relaxed the contraction induced by PMA but with a IC50 significantly lower than that obtained in calcium containing bath solution, possibly because the contractile response of EGCG is not possible in the absence of external Ca2+ (Álvarez-Castro et al., 2004). In fact, the IC50 shown in these conditions are within the same range at which similar flavonoids exhibit vascular activity (Duarte et al., 1993; Herrera et al., 1996), while the IC50 for the relaxant effect of EGCG in 1.5 mM Ca2+-containing solution is closer to that obtained for baicalein (Chen et al., 1999), a flavonoid that exerts both contractile and relaxant effects in phenylephrine-, U46619- or high-K+-contracted rat mesenteric arteries.
Our results do not agree with those obtained by Huang et al. (1998), who found that EGCG did not significantly inhibit the sustained contraction induced by PMA in Ca2+-free solution on rat mesenteric artery. Several possible explanations for these differences could be given by the experimental conditions used in each case. This means: (a) PKC isoforms could be different from aorta to mesenteric artery; (b) we used a lower concentration of PMA; and (c) they employed only a single dose of EGCG (300 μM) in the relaxant experiments while we used a cumulative-concentration range (30 μM–1 mM).
The effect of EGCG on the cell membrane of rat aorta smooth muscle does not seem to be due to the opening of K+ channels, since:
Cromakalim and other ATP-sensitive K+ channel agonists, unlike EGCG (present study) and a number of nonselective PDE inhibitors (Noguera et al., 2001; Orallo et al., 2004), do not inhibit the contractions induced by high extracellular KCl (greater than about 30 mM) in vascular smooth muscle (Edwards & Weston, 1995).
The vasorelaxant effects of EGCG were unaffected by pretreatment with GB (a selective inhibitor of ATP-sensitive K+ channels), ChTX plus apamin (selective inhibitors of intermediate- and small-conductance Ca2+-activated K+ channels, respectively) or TEA (a nonselective blocker of large-conductance Ca2+-activated K+ channels and other K+ channels). Similar results have been previously reported by Huang et al. (1998).
The hypothesis that the vasorelaxant activity of EGCG may be mediated, at least in part, by a decrease of agonist- or high KCl-induced increase in [Ca2+]i (e.g., via inhibition of Ca2+ release from intracellular stores and/or Ca2+ influx through transmembrane Ca2+ channels) is supported by the experiments with fura-2.
Several PDE inhibitors have been described to decrease agonist-induced increases in [Ca2+]i in RASMC (Ohoka et al., 1990; Eckly-Michel et al., 1997). Similarly, in our experiments, when 100 μM EGCG was administered 30 min prior to ANG II or high extracellular KCl, it was able to inhibit the peak and the plateau phases of the [Ca2+]i elevation induced by both contractile agents in RASMC. At this concentration, EGCG also reduced significantly ANG II-induced increases in [Ca2+]i in the absence of extracellular Ca2+. These results suggest that the reduction of transmembrane Ca2+ influx and/or agonist-induced release of intracellular Ca2+ on these cells may contribute to the vasorelaxant action of EGCG, although these effects do not seem to be important for lower concentrations (30 μM) of the catechin.
It is likely that the inhibitory effects of EGCG on some of the steps of the contractile process triggered by PKC activation and on the ANG II- and high KCl-induced increases in [Ca2+]i are mediated, at least in part, by an increase in cytoplasmatic cAMP and cGMP levels (via inhibition of PDE activity) and subsequent activation of PKA and PKG, for five reasons:
As shown for the first time in this study, EGCG, like IBMX (a nonselective inhibitor of several PDE isoforms), had no significant effect on basal production of cAMP and cGMP, possibly due to the low PDEs activity in resting conditions (for a review see, e.g., Polson & Strada, 1996). However, both EGCG and IBMX significantly reversed the decrease of cGMP and cAMP generation induced by NA (1 μM) and high KCl (60 mM) in cultured RASMC.
cAMP and cGMP have been reported to relax phorbol dibutyrate-induced rat aorta contractions in Ca2+-containing and Ca2+-free physiological solutions, respectively (Ahn et al., 1997), and to inhibit a number of PKC effects (e.g., the increase in the Ca2+ sensitivity of the actin–myosin system (Bonnevier et al., 2004).
An increase in cytoplasmatic cAMP and cGMP levels can decrease Ca2+ influx in RASMC, possibly by phosphorylation of transmembrane Ca2+ channels or associated regulatory proteins (Orlov et al., 1996; Liu et al., 1997; Minowa et al., 1997), and inhibit Ca2+ release from intracellular stores (Orallo, 1996).
It has been previously reported that EGCG increases PKA activity in bovine aortic endothelial cells (Lorenz et al., 2004) and that a number of cAMP and cGMP analogues (which activate PKA and PKG, respectively) mimic the effects of the above-mentioned catechin, that is, they concentration-dependently inhibit the contractions induced by a number of vasoconstrictor agents in rat aorta (see, e.g., Ahn et al., 1997; Teede et al., 2001).
In the same way as other similar flavonoids (Ko et al., 2004; Orallo et al., 2004; for a review see Middleton et al., 2000), EGCG inhibited PDE1 and PDE5 (which in smooth muscle vessels mainly hydrolyse cGMP), PDE2 (which hydrolyses both cAMP and cGMP), PDE3 and PDE4 (which basically hydrolyse cAMP). In this connection, PDE1 plays a more relevant role than PDE5 in the biotransformation of cGMP in rat aorta without endothelium, while PDE3 seems to be more important than PDE4 in the degradation of cAMP in the same tissue (Noguera et al., 2001; Orallo et al., 2004). Thus, EGCG-induced cyclic nucleotide increases in smooth muscle cells could occur mainly by inhibition of PDE1 and PDE3 activities.
The fact that EGCG inhibits PDEs activity with IC50 values remarkably lower than the IC50 values for EGCG-induced vasorelaxation in intact and endothelium-denuded rat aortic rings (using 1.5 mM Ca2+-containing solution) may be due, at least in part, to the contractile effect elicited by this catechin in rat aorta (Álvarez-Castro et al., 2004). In line with this, the IC50 values for EGCG-induced vasorelaxation of PMA-induced contractions (in Ca2+-free medium) are very close to the IC50 required for inhibiting PDE activity, mainly PDE3–5 (see above and the Results section).
To summarize, in this study EGCG has been characterized as an agent with a sustained, endothelium-independent vasorelaxant activity. The vasorelaxant action seems to be mediated by an increase in cytosolic cAMP and cGMP concentrations (via the inhibition of various PDE isoforms), which can decrease Ca2+ influx and release of Ca2+ from intracellular stores in RASMC, although a possible direct inhibition of PKC cannot be discarded.
Bearing in mind these pharmacological properties and under the assumption that EGCG exhibits a similar behaviour in human blood vessels and increases cAMP and cGMP contents, it can be concluded that the natural plant flavonoid EGCG may have interesting potential as a structural model for the design and subsequent development of new PDE-inhibitory drugs, which could be useful for improving the pharmacological treatment of diseases (e.g., some cardiovascular pathologies) involving overactivity of PDEs.
Acknowledgments
This work was supported in part by grants from the Spanish Ministerio de Ciencia y Tecnología (SAF2002-0245, RYC2002-10), the Xunta de Galicia (PGIDIT02BTF20301PR and PGIDIT05BTF20302PR), Spain and the Centre National de la Recherche Scientifique, France.
Abbreviations
- ANG II
angiotensin II
- [Ca2+]i
cytosolic free Ca2+ concentration
- CaM
calmodulin
- ChTX
charybdotoxin
- DMSO
dimethylsulphoxide
- EGCG
(−)-epigallocatechin-3-gallate
- GB
glibenclamide
- IBMX
3-isobutyl-1-methylxanthine
- MLC
myosin light chain
- NA
noradrenaline
- OA
okadaic acid
- PMA
phorbol 12-myristate 13-acetate
- RASMC
rat aortic smooth muscle cells
- TEA
tetraethylammonium
References
- AHN H.Y., CHANG K.C., CHUNG M.H., KIM M.S., MORELAND R.S. Cyclic AMP and cyclic GMP relax phorbol ester-induced contractions of rat aorta by different mechanisms. Life Sci. 1997;60:2333–2340. doi: 10.1016/s0024-3205(97)00289-0. [DOI] [PubMed] [Google Scholar]
- ÁLVAREZ E., LEIRO J., ORALLO F. Effect of (−)-epigallocatechin-3-gallate on respiratory burst of rat macrophages. Int. Immunopharmacol. 2002;2:849–855. doi: 10.1016/s1567-5769(02)00032-2. [DOI] [PubMed] [Google Scholar]
- ÁLVAREZ-CASTRO E., CAMPOS-TOIMIL M., ORALLO F. Epigallocatechin-3-gallate induces contraction of the rat aorta by a calcium influx-dependent mechanism. Naunyn-Schmiedeberg's Arch. Pharmacol. 2004;369:496–506. doi: 10.1007/s00210-004-0923-8. [DOI] [PubMed] [Google Scholar]
- ANDREA J.E., WALSH M.P. Protein kinase C of smooth muscle. Hypertension. 1992;20:585–595. doi: 10.1161/01.hyp.20.5.585. [DOI] [PubMed] [Google Scholar]
- BONNEVIER J., FASSLER R., SOMLYO A.P., SOMLYO A.V., ARNER A. Modulation of Ca2+ sensitivity by cyclic nucleotides in smooth muscle from protein kinase G-deficient mice. J. Biol. Chem. 2004;279:5146–5151. doi: 10.1074/jbc.M306532200. [DOI] [PubMed] [Google Scholar]
- CHEN Z.Y., LAW W.I., YAO X.Q., LAU C.W., HO W.K., HUANG Y. Inhibitory effects of purified green tea epicatechins on contraction and proliferation of arterial smooth muscle cells. Acta Pharmacol. Sin. 2000;21:835–840. [PubMed] [Google Scholar]
- CHEN Z.Y., SU Y.L., LAU C.W., LAW W.I., HUANG Y. Endothelium-dependent contraction and direct relaxation induced by baicalein in rat mesenteric artery. Eur. J. Pharmacol. 1999;374:41–47. doi: 10.1016/s0014-2999(99)00291-5. [DOI] [PubMed] [Google Scholar]
- CHYU K.Y., BABBIDGE S.M., ZHAO X., DANDILLAYA R., RIETVELD A.G., YANO J., DIMAYUGA P., CERCEK B., SHAH P.K. Differential effects of green tea-derived catechin on developing versus established atherosclerosis in apolipoprotein E-null mice. Circulation. 2004;109:2448–2453. doi: 10.1161/01.CIR.0000128034.70732.C2. [DOI] [PubMed] [Google Scholar]
- DEANA R., TURETTA L., DONELLA-DEANA A., DONA M., MARIA B.A., DE MICHIEL L., GARBISA S. Green tea epigallocatechin-3-gallate inhibits platelet signalling pathways triggered by both proteolytic and non-proteolytic agonists. Thromb. Haemost. 2003;89:866–874. [PubMed] [Google Scholar]
- DUARTE J., PÉREZ V.F., UTRILLA P., JIMÉNEZ J., TAMARGO J., ZARZUELO A. Vasodilatory effects of flavonoids in rat aortic smooth muscle. Structure–activity relationships. Gen. Pharmacol. 1993;24:857–862. doi: 10.1016/0306-3623(93)90159-u. [DOI] [PubMed] [Google Scholar]
- ECKLY-MICHEL A., MARTIN V., LUGNIER C. Involvement of cyclic nucleotide-dependent protein kinases in cyclic AMP-mediated vasorelaxation. Br. J. Pharmacol. 1997;122:158–164. doi: 10.1038/sj.bjp.0701339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., WESTON A.H. Pharmacology of the potassium channel openers. Cardiovasc. Drugs Ther. 1995;9 (Suppl 2):185–193. doi: 10.1007/BF00878465. [DOI] [PubMed] [Google Scholar]
- FASSINA G., VENE R., MORINI M., MINGHELLI S., BENELLI R., NOONAN D.M., ALBINI A. Mechanisms of inhibition of tumor angiogenesis and vascular tumor growth by epigallocatechin-3-gallate. Clin. Cancer Res. 2004;10:4865–4873. doi: 10.1158/1078-0432.CCR-03-0672. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F. Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide. Biosci. Rep. 1999;19:235–251. doi: 10.1023/a:1020537506008. [DOI] [PubMed] [Google Scholar]
- GRAHAM H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- HERRERA M.D., ZARZUELO A., JIMENEZ J., MARHUENDA E., DUARTE J. Effects of flavonoids on rat aortic smooth muscle contractility: structure–activity relationships. Gen. Pharmacol. 1996;27:273–277. doi: 10.1016/0306-3623(95)02010-1. [DOI] [PubMed] [Google Scholar]
- HERTOG M.G., FESKENS E.J., KROMHOUT D. Antioxidant flavonols and coronary heart disease risk. Lancet. 1997;349:699. doi: 10.1016/S0140-6736(05)60135-3. [DOI] [PubMed] [Google Scholar]
- HUANG Y., ZHANG A., LAU C.W., CHEN Z.Y. Vasorelaxant effects of purified green tea epicatechin derivatives in rat mesenteric artery. Life Sci. 1998;63:275–283. doi: 10.1016/s0024-3205(98)00273-2. [DOI] [PubMed] [Google Scholar]
- IBARRA M., PÉREZ-VIZCAÍNO F., COGOLLUDO A., DUARTE J., ZARAGOZA-ARNÁEZ F., LÓPEZ-LÓPEZ J.G., TAMARGO J. Cardiovascular effects of isorhamnetin and quercetin in isolated rat and porcine vascular smooth muscle and isolated rat atria. Planta Med. 2002;68:307–310. doi: 10.1055/s-2002-26752. [DOI] [PubMed] [Google Scholar]
- ITOH H., KUSAGAWA M., SHIMOMURA A., SUGA T., ITO M., KONISHI T., NAKANO T. Ca2+-dependent and Ca2+-independent vasorelaxation induced by cardiotonic phosphodiesterase inhibitors. Eur. J. Pharmacol. 1993;240:57–66. doi: 10.1016/0014-2999(93)90545-s. [DOI] [PubMed] [Google Scholar]
- JIANG M.J., MORGAN K.G. Intracellular calcium levels in phorbol ester-induced contractions of vascular muscle. Am. J. Physiol. 1987;253:H1365–H1371. doi: 10.1152/ajpheart.1987.253.6.H1365. [DOI] [PubMed] [Google Scholar]
- KAMENI TCHEUDJI J.F., LEBEAU L., VIRMAUX N., MAFTEI C.G., COTE R.H., LUGNIER C., SCHULTZ P. Molecular organization of bovine rod cGMP–phosphodiesterase 6. J. Mol. Biol. 2001;310:781–791. doi: 10.1006/jmbi.2001.4813. [DOI] [PubMed] [Google Scholar]
- KARAKI H., OZAKI H., HORI M., MITSUI-SAITO M., AMANO K., HARADA K., MIYAMOTO S., NAKAZAWA H., WON K.J., SATO K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- KERAVIS T.M., WELLS J.N., HARDMAN J.G. Cyclic nucleotide phosphodiesterase activities from pig coronary arteries. Lack of interconvertibility of major forms. Biochim. Biophys. Acta. 1980;613:116–129. doi: 10.1016/0005-2744(80)90198-9. [DOI] [PubMed] [Google Scholar]
- KITANO K., NAM K.Y., KIMURA S., FUJIKI H., IMANISHI Y. Sealing effects of (−)-epigallocatechin gallate on protein kinase C and protein phosphatase 2A. Biophys. Chem. 1997;65:157–164. doi: 10.1016/s0301-4622(96)02254-5. [DOI] [PubMed] [Google Scholar]
- KNAPP J., BOKNIK P., LINCK B., LUSS H., MULLER F.U., PETERTONJES L., SCHMITZ W., NEUMANN J. Cantharidin enhances norepinephrine-induced vasoconstriction in an endothelium-dependent fashion. J. Pharmacol. Exp. Ther. 2000;294:620–626. [PubMed] [Google Scholar]
- KO W.C., SHIH C.M., LAI Y.H., CHEN J.H., HUANG H.L. Inhibitory effects of flavonoids on phosphodiesterase isozymes from guinea pig and their structure–activity relationships. Biochem. Pharmacol. 2004;68:2087–2094. doi: 10.1016/j.bcp.2004.06.030. [DOI] [PubMed] [Google Scholar]
- KOMAS N., LUGNIER C., STOCLET J.C. Endothelium-dependent and independent relaxation of the rat aorta by cyclic nucleotide phosphodiesterase inhibitors. Br. J. Pharmacol. 1991;104:495–503. doi: 10.1111/j.1476-5381.1991.tb12457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIM D.Y., LEE E.S., PARK H.G., KIM B.C., HONG S.P., LEE E.B. Comparison of green tea extract and epigallocatechin gallate on blood pressure and contractile responses of vascular smooth muscle of rats. Arch. Pharm. Res. 2003;26:214–223. doi: 10.1007/BF02976833. [DOI] [PubMed] [Google Scholar]
- LIOU Y.M., MORGAN K.G. Redistribution of protein kinase C isoforms in association with vascular hypertrophy of rat aorta. Am. J. Physiol. 1994;267:C980–C989. doi: 10.1152/ajpcell.1994.267.4.C980. [DOI] [PubMed] [Google Scholar]
- LIU H., XIONG Z., SPERELAKIS N. Cyclic nucleotides regulate the activity of L-type calcium channels in smooth muscle cells from rat portal vein. J. Mol. Cell. Cardiol. 1997;29:1411–1421. doi: 10.1006/jmcc.1997.0379. [DOI] [PubMed] [Google Scholar]
- LORENZ M., WESSLER S., FOLLMANN E., MICHAELIS W., DUSTERHOFT T., BAUMANN G., STANGL K., STANGL V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J. Biol. Chem. 2004;279:6190–6195. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- LUDWIG A., LORENZ M., GRIMBO N., STEINLE F., MEINERS S., BARTSCH C., STANGL K., BAUMANN G., STANGL V. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochem. Biophys. Res. Commun. 2004;316:659–665. doi: 10.1016/j.bbrc.2004.02.099. [DOI] [PubMed] [Google Scholar]
- LUGNIER C., SCHOEFFTER P., LE BEC A., STROUTHOU E., STOCLET J.C. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem. Pharmacol. 1986;35:1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- MALARKEY K., MCLEES A., PAUL A., GOULD G.W., PLEVIN R. The role of protein kinase C in activation and termination of mitogen-activated protein kinase activity in angiotensin II-stimulated rat aortic smooth-muscle cells. Cell Signal. 1996;8:123–129. doi: 10.1016/0898-6568(95)02036-5. [DOI] [PubMed] [Google Scholar]
- MIDDLETON E, JR., KANDASWAMI C., THEOHARIDES T.C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- MINOWA T., MIWA S., KOBAYASHI S., ENOKI T., ZHANG X.F., KOMURO T., IWAMURO Y., MASAKI T. Inhibitory effect of nitrovasodilators and cyclic GMP on ET-1-activated Ca2+-permeable nonselective cation channel in rat aortic smooth muscle cells. Br. J. Pharmacol. 1997;120:1536–1544. doi: 10.1038/sj.bjp.0701059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIZUGAKI M., ISHIZAWA F., YAMAZAKI T., HISHINUMA T. Epigallocatechin gallate increase the prostacyclin production of bovine aortic endothelial cells. Prostaglandins Other Lipid Mediat. 2000;62:157–164. doi: 10.1016/s0090-6980(00)00060-5. [DOI] [PubMed] [Google Scholar]
- NOGUERA M.A., IVORRA M.D., LUGNIER C., D'OCÓN P. Role of cyclic nucleotide phosphodiesterase isoenzymes in contractile responses of denuded rat aorta related to various Ca2+ sources. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:612–619. doi: 10.1007/s002100100397. [DOI] [PubMed] [Google Scholar]
- OHOKA M., HONDA M., MORIOKA S., ISHIKAWA S., NAKAYAMA K., YAMORI Y., MORIYAMA K. Effects of E-1020, a new cyclic AMP-specific phosphodiesterase inhibitor, on cyclic AMP and cytosolic free calcium of cultured vascular smooth muscle cells. Jpn. Circ. J. 1990;54:679–687. doi: 10.1253/jcj.54.679. [DOI] [PubMed] [Google Scholar]
- ORALLO F. Regulation of cytosolic calcium levels in vascular smooth muscle. Pharmacol. Ther. 1996;69:153–171. doi: 10.1016/0163-7258(95)02042-x. [DOI] [PubMed] [Google Scholar]
- ORALLO F. Study of the in vivo and in vitro cardiovascular effects of a hydralazine-like vasodilator agent (HPS-10) in normotensive rats. Br. J. Pharmacol. 1997;121:1627–1636. doi: 10.1038/sj.bjp.0701314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORALLO F., ÁLVAREZ E., BASARAN H., LUGNIER C. Comparative study of the vasorelaxant activity, superoxide-scavenging ability and cyclic nucleotide phosphodiesterase-inhibitory effects of hesperetin and hesperidin. Naunyn-Schmiedeberg's Arch. Pharmacol. 2004;370:452–463. doi: 10.1007/s00210-004-0994-6. [DOI] [PubMed] [Google Scholar]
- ORALLO F., ÁLVAREZ E., CAMIÑA M., LEIRO J.M., GÓMEZ E., FERNÁNDEZ P. The possible implication of trans-resveratrol in the cardioprotective effects of long-term moderate wine consumption. Mol. Pharmacol. 2002;61:294–302. doi: 10.1124/mol.61.2.294. [DOI] [PubMed] [Google Scholar]
- ORLOV S.N., TREMBLAY J., HAMET P. cAMP signaling inhibits dihydropyridine-sensitive Ca2+ influx in vascular smooth muscle cells. Hypertension. 1996;27:774–780. doi: 10.1161/01.hyp.27.3.774. [DOI] [PubMed] [Google Scholar]
- POLSON J.B., STRADA S.J. Cyclic nucleotide phosphodiesterases and vascular smooth muscle. Annu. Rev. Pharmacol. Toxicol. 1996;36:403–427. doi: 10.1146/annurev.pa.36.040196.002155. [DOI] [PubMed] [Google Scholar]
- SANAE F., MIYAICHI Y., KIZU H., HAYASHI H. Effects of catechins on vascular tone in rat thoracic aorta with endothelium. Life Sci. 2002;71:2553–2562. doi: 10.1016/s0024-3205(02)02080-5. [DOI] [PubMed] [Google Scholar]
- SATO K., HORI M., OZAKI H., TAKANO-OHMURO H., TSUCHIYA T., SUGI H., KARAKI H. Myosin phosphorylation-independent contraction induced by phorbol ester in vascular smooth muscle. J. Pharmacol. Exp. Ther. 1992;261:497–505. [PubMed] [Google Scholar]
- SHEN J.Z., ZHENG X.F., WEI E.Q., KWAN C.Y. Green tea catechins evoke a phasic contraction in rat aorta via H2O2-mediated multiple-signalling pathways. Clin. Exp. Pharmacol. Physiol. 2003;30:88–95. doi: 10.1046/j.1440-1681.2003.03796.x. [DOI] [PubMed] [Google Scholar]
- STOCLET J.C., KERAVIS T., KOMAS N., LUGNIER C. Cyclic nucleotide phosphodiesterases as therapeutic targets in cardiovascular diseases. Exp. Opin. Invest. Drugs. 1995;4:1081–1100. [Google Scholar]
- TEEDE H., VAN DER Z.A., MAJEWSKI H. Gender differences in protein kinase G-mediated vasorelaxation of rat aorta. Clin. Sci. (London) 2001;100:473–479. [PubMed] [Google Scholar]
- VIDRIO H., FERNÁNDEZ G., MEDINA M., ÁLVAREZ E., ORALLO F. Effects of hydrazine derivatives on vascular smooth muscle contractility, blood pressure and cGMP production in rats: comparison with hydralazine. Vasc. Pharmacol. 2003;40:13–21. doi: 10.1016/s1537-1891(02)00312-9. [DOI] [PubMed] [Google Scholar]
- WAKABAYASHI I., SAKAMOTO K., HATAKE K. Inhibitory effects of cadmium ion on extracellular Ca2+-independent contraction of rat aorta. Eur. J. Pharmacol. 1995;293:133–140. doi: 10.1016/0926-6917(95)00009-7. [DOI] [PubMed] [Google Scholar]
- ZHENG Y., SONG H.J., KIM C.H., KIM H.S., KIM E.G., SACHINIDIS A., AHN H.Y. Inhibitory effect of epigallocatechin 3-O-gallate on vascular smooth muscle cell hypertrophy induced by angiotensin II. J. Cardiovasc. Pharmacol. 2004;43:200–208. doi: 10.1097/00005344-200402000-00006. [DOI] [PubMed] [Google Scholar]