Abstract
The aim of this investigation was to determine if the human proton-coupled amino-acid transporter 1 (hPAT1 or SLC36A1) is responsible for the intestinal uptake of the orally-administered antiepileptic agent 4-amino-5-hexanoic acid (vigabatrin).
The Caco-2 cell line was used as a model of the human small intestinal epithelium. Competition experiments demonstrate that [3H]GABA uptake across the apical membrane was inhibited by vigabatrin and the GABA analogues trans-4-aminocrotonic acid (TACA) and guvacine, whereas 1-(aminomethyl)cyclohexaneacetic acid (gabapentin) had no affect.
Experiments with 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF)-loaded Caco-2 cells demonstrate that apical exposure to vigabatrin and TACA induce comparable levels of intracellular acidification (due to H+/amino-acid symport) to that generated by GABA, suggesting that they are substrates for a H+-coupled absorptive transporter such as hPAT1.
In hPAT1 and mPAT1-expressing Xenopus laevis oocytes [3H]GABA uptake was inhibited by vigabatrin, TACA and guvacine, whereas gabapentin failed to inhibit [3H]GABA uptake.
In Na+-free conditions, vigabatrin and TACA evoked similar current responses (due to H+/amino-acid symport) in hPAT1-expressing oocytes under voltage-clamp conditions to that induced by GABA (whereas no current was observed in water-injected oocytes) consistent with the ability of these GABA analogues to inhibit [3H]GABA uptake.
This study demonstrates that hPAT1 is the carrier responsible for the uptake of vigabatrin across the brush-border membrane of the small intestine and emphasises the therapeutic potential of hPAT1 as a delivery route for orally administered, clinically significant GABA-related compounds.
Keywords: Amino acid transport, proton-coupled transport, PAT1, SLC36, SLC36A1, GABA, vigabatrin, gabapentin, Caco-2, human intestinal epithelium
Introduction
The initial absorption barrier for any orally delivered agent is the brush-border membrane of the human small intestinal epithelium. The transmembrane transport of many nutrients and drug molecules across this membrane occurs via carrier-mediated transport mechanisms which can be Na+-coupled, H+-coupled or ion-independent. Previously, we have characterised, using the human intestinal epithelial cell line Caco-2 grown as confluent monolayers on permeable filters, a H+-coupled amino-acid transporter (system PAT) which is localised specifically to the apical membrane of these human enterocytes (Thwaites et al., 1993b; 1995c; Chen et al., 2003). System PAT transports a broad spectrum of substrates including small zwitterionic amino acids (alanine, proline and glycine) (Thwaites et al., 1993b; 1995c), β-amino acids (β-alanine and taurine) (Thwaites et al., 1993a; Thwaites & Stevens, 1999), osmolytes (betaine) (Thwaites et al., 1995c; Boll et al., 2003), proline analogues (including L-azetidine-2-carboxylic acid, cis-4-hydroxy-L-proline and 3,4-dehydro-D,L-proline) (Metzner et al., 2004) and a number of orally delivered neuromodulatory agents including D-serine (used in the treatment of schizophrenia) (Thwaites et al., 1995a, 1995c; Tsai et al., 1998), D-cycloserine (used as an orally delivered antibiotic and in the treatment of schizophrenia) (Ranaldi et al., 1994; Thwaites et al., 1995a; 2000; Evins et al., 2002) and γ-aminobutyric acid (GABA) and GABA analogues (including nipecotic acid and isonipecotic acid) (Thwaites et al., 2000; Metzner et al., 2004). The cDNA encoding the human proton-coupled amino-acid transporter (hPAT1, SLC36A1) was cloned from a Caco-2 cell cDNA library and, when expressed in isolation in heterologous cell types (Xenopus laevis oocytes or human retinal pigment epithelial (HRPE) cells), displays the same functional properties and substrate specificity as the endogenous system PAT (namely pH-dependent, Na+-independent, low-affinity, high-capacity transport) (Thwaites et al., 1993a; 1995c; Chen et al., 2003).
System PAT-like (Na+-independent, pH-dependent) amino-acid transport has also been demonstrated in rat small intestine (Anderson et al., 2004). Immunofluorescence studies, using a PAT1-specific antibody, localised PAT1 exclusively to the brush-border membrane of Caco-2 cell monolayers, and rat and human small intestine (Chen et al., 2003; Anderson et al., 2004). System PAT (and the hPAT1 clone) represent the high-capacity imino acid carrier, which has been described in rat small intestine over several decades and which has an identical substrate specificity to system PAT/hPAT1 (Munck, 1966; Munck et al., 1994; Anderson et al., 2004). The direct link between PAT1 and the imino acid carrier was not made until recently because of the apparent differences in ion dependency between studies in oocytes (where transport is Na+-independent) compared with those in intact epithelia, for example, rat small intestine or Caco-2 cell monolayers (where transport can be Na+-dependent). The apparent Na+-dependence of this H+-driven transport system (system PAT/PAT1/imino acid carrier) in intact epithelia is due to a functional coupling with the Na+/H+ exchanger NHE3 to maintain the H+-electrochemical gradient during transport, which is required for optimal absorption of PAT1 substrates to occur (Thwaites et al., 1999; Anderson et al., 2004; Anderson & Thwaites, 2005). The presence of this multifunctional absorptive transport system in the small intestine, where the H+-gradient is relevant physiologically due to the ‘acid microclimate' at the mucosal surface (Rawlings et al., 1987; McEwan et al., 1988; Daniel et al., 1989), provides a potential route for nutrient, osmolyte and drug absorption.
The neutral amino acid GABA acts as the principal inhibitory neurotransmitter in the mammalian central nervous system (Patsalos, 1999). A decrease in GABA levels, and thus perturbations in overall brain activity, is associated with the etiology of numerous neurological disorders such as anxiety, pain and epilepsy (reviewed in Wong et al., 2003). Several pharmacological approaches to modulating GABAergic function have been investigated in humans including direct activation of the GABA-receptor by specific agonists (Krogsgaard-Larsen, 1981), inhibition of rapid GABA uptake by both neuronal and glial cell bodies (Krogsgaard-Larsen et al., 1987; Krogsgaard-Larsen, 1988) or inhibition of GABA metabolism (Bolton et al., 1989).
Owing to the central inhibitory nature of GABAergic neurotransmission and its relationship with neurological disorders, the ability to deliver orally, clinically-active, GABA-related agents is of significant therapeutic interest. One close structural analogue of GABA is 4-amino-5-hexanoic acid or γ-vinyl GABA (vigabatrin, (brand name Sabril, Aventis)). Vigabatrin binds covalently to the active site of GABA-transaminase (GABA-T), the enzyme responsible for the catabolism of GABA. Binding irreversibly, vigabatrin inactivates GABA-T in a concentration-dependent manner and subsequently induces a universal increase in extracellular GABA levels in the brain (Preece et al., 1994; Petroff et al., 1996). Vigabatrin has been used extensively as adjunctive treatment for partial epilepsy and is also effective as a monotherapy for treatment of infantile spasms (West syndrome), although use is now restricted due to irreversible visual field defects associated with treatment (Patsalos & Duncan, 1994; Patsalos, 1999; Hadjikoutis et al., 2005).
Gabapentin (1-(aminomethyl)cyclohexaneacetic acid (brand name Neurontin, Pfizer)), also a synthetic analogue of GABA, is used clinically as adjunctive therapy for partial seizures (Bryans & Wustrow, 1999). Its mode of action remains unclear. However, it has been suggested that binding to GABAB receptors negatively coupled to voltage-dependent calcium channels may account for the anticonvulsant properties of gabapentin (Bertrand et al., 2001).
Following oral administration, both vigabatrin and gabapentin are rapidly absorbed with bioavailabilities of 80–90% and 60%, respectively (Loscher et al., 1993; Patsalos, 1999). It has been suggested that gabapentin absorption is Na+-independent and may be mediated by a L-type amino-acid transporter and/or system b0,+ (Stewart et al., 1993; Piyapolrungroj et al., 2001; Uchino et al., 2002). However, the route of oral absorption of vigabatrin remains undetermined. We have demonstrated clearly that hPAT1 is the nutrient carrier involved in the absorptive transport of GABA across the apical surface of the human small intestinal epithelium (Thwaites et al., 2000; Anderson et al., 2004). The purpose of this investigation was to determine if hPAT1 is responsible for the transport of these GABA-related therapeutic agents across the intestinal brush-border membrane, which is an essential stage in facilitating the high oral bioavailability of many clinically administered, anticonvulsant agents. In order to determine the structural limitations of hPAT1 for transport of antiepileptic drugs (AED), other known GABA analogues that are not used clinically (including trans-4-aminocrotonic acid (TACA) and guvacine) were included for comparison. Identification of the route of oral absorption and the nature of the nutrient carriers involved (if any) in the intestinal transport of vigabatrin and gabapentin could prove important for future antiepileptic drug design.
Methods
Materials
4-Amino-n-[2,3-3H]butyric acid (GABA) (81 Ci mmol−1), L-[2,3-3H]proline (43 Ci mmol−1), [2-3H]glycine (16 Ci mmol−1) and D-[14C]mannitol (59 mCi mmol−1) were from Amersham Biosciences (Little Chalfont, U.K.). [3-3H(N)β-Alanine (50 Ci mmol−1) was from American Radiolabeled Chemicals (St Louis, U.S.A.). [14C]Gly-Sar (56.7 mCi mmol−1) was from Cambridge Research Biochemicals (Stockton-on-Tees, U.K.). The acetoxy-methyl ester of 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) was from Invitrogen Ltd. (Paisley, U.K.). Cell culture plasticware was from Corning-Costar Ltd. (High Wycombe, U.K.) and all cell culture media and supplements were from Sigma-Aldrich Ltd. (Poole, U.K.). Vigabatrin, gabapentin, guvacine and TACA were all purchased from Tocris (Avonmouth, U.K.). FastTrack 2.0 Kit and TOPO TA Cloning kit were from Invitrogen. Omniscript Reverse Transcriptase was from Qiagen (Crawley, U.K.). Expand High Fidelity PCR System was purchased from Roche Diagnostics (Lewes, U.K.) and mMESSAGE mMACHINE Kit was from Ambion (Huntingdon, U.K.). All other chemicals were from VWR Ltd./Sigma-Aldrich (Poole, U.K.) and were of the highest quality available.
Apical amino acid uptake in Caco-2 cell monolayers
Caco-2 cells (passage number 108–124) were cultured and prepared as confluent monolayers, as described previously (Thwaites et al., 1993a, 1993b; 2000). Cell confluence was estimated by microscopy and determination of transepithelial electrical resistance (RT) measured at 37°C. [3H]GABA (0.5 μCi ml−1; 20–100 μM) uptake across the apical membrane of Caco-2 cell monolayers was measured over 5–15 min at 37°C, as described previously (Thwaites et al., 1993a, 1993b; 2000). Uptake was measured in the absence of external Na+, at an apical pH of 5.5 (basolateral pH 7.4) and in the presence/absence of various compounds (see figure legends for details). Cell monolayer-associated radiolabel was determined by scintillation counting. Transepithelial apical-to-basal (Ja–b) [3H]GABA (0.5 μCi ml−1, 20 μM) transport was measured over 90 min in Na+-free conditions (apical pH 5.5 or pH 7.4, basolateral pH 7.4) and at apical pH 5.5 in the presence of cold GABA, vigabatrin, isonipecotic acid and nipecotic acid (all 10 mM). [14C]Mannitol (0.5 μCi ml−1, 20 μM) was used to estimate passive paracellular transport under all experimental conditions tested.
Intracellular pH (pHi) measurements
Caco-2 cell monolayers were loaded with the pH sensitive dye BCECF, and changes in pHi were measured by microspectrofluorimetry, as described previously (Thwaites et al., 1993a, 1993b; 2000). The ability of GABA and GABA analogues (10 mM) to induce intracellular acidification in Caco-2 cell monolayers was tested by addition of each compound (at pH 5.5, Na+-free) to either the apical bathing solution (with basolateral pH 7.4, Na+-free) or the basolateral bathing solution (with apical pH 7.4, Na+-free). All solutions were preheated to 37°C. The rate of change of intracellular pH (ΔpHi min−1), due to a change in the composition of the superfusate, was calculated by linear regression (Photon Counter System 4.7, Newcastle Photometric Systems, U.K.) by comparison of the linear portions of the trace over 30–50 s (15–25 data points) periods before and after the change in composition (Thwaites et al., 2000). Example traces are presented as a change in intracellular pH (ΔpHi) over time.
Molecular cloning and in vitro transcription of hPAT1
Total RNA was isolated from Caco-2 cells using FastTrack 2.0 Kit (Invitrogen). RNA (1 μg) was used to synthesise cDNA with random hexamer oligonucleotides (1 μM) and Omniscript RT (Qiagen). The gene specific oligonucleotide primers for PCR (5′-CAGATGCTCCAGCTG-3′ (sense), 5′-GGAGAGAGATGAGAAAGAAGC-3′ (antisense)) were designed to the 5′ and 3′-untranslated regions (UTR) of hPAT1 (identified by alignment of two hPAT1 sequences (GenBank accession numbers NM_078483 and AF516142)). PCR was performed using Expand High Fidelity Polymerase (Roche) and 10 PCR cycles were carried out for 15 s at 94°C, 30 s at 60°C and 2 min at 72°C. In all, 20 cycles were subsequently performed at 94°C for 15 s, 60°C for 30 s, 72°C for 2 min with cycle elongation of 5 s for each cycle. PCR yielded a product of 1.91 kb, as expected from the relative positions of the primers. The product was then cloned into the pCR2.1-TOPO vector (Invitrogen) and construct fidelity was confirmed by DNA sequencing (Molecular Biology Unit, University of Newcastle upon Tyne, U.K.). Linearised hPAT1 plasmid DNA was used as a template for in vitro transcription with mMESSAGE mMACHINE Kit (Ambion).
Functional expression of hPAT1 and mPAT1 in Xenopus laevis oocytes
X. laevis oocytes were prepared and uptake experiments performed, as described previously (Anderson et al., 2004). Briefly, individual stage V/VI oocytes were injected with 50 nl of water (control), hPAT1 (1 μg μl−1) or mouse PAT1 (mPAT1, 1 μg μl−1) (Boll et al., 2002; 2003) cRNA and incubated for 2–3 days in Barth's solution at 18°C. [3H]GABA (5 μCi ml−1; 20–100 μM) uptake was measured in the presence/absence of various compounds at room temperature (22°C) over 40 min (Na+-free, pH 5.5). The oocytes were lysed in 10% SDS before scintillation counting.
Measurement of substrate-induced currents using the two-electrode voltage-clamp technique
Two-electrode voltage-clamp measurements were performed using X. laevis oocytes expressing hPAT1 (clamped at −60 mV), as described previously (Anderson et al., 2004). The currents induced following addition of various compounds (10 mM, Na+-free, pH 5.5, 60 s) to the bathing solution of hPAT1 or water-injected oocytes were determined by subtraction of current measured 20 s before the addition of the compound from that 60 s after addition of the compound. Results are presented both as representative traces and as mean substrate-induced current change (following subtraction of current produced by water-injected control oocytes under identical experimental conditions) and are expressed as a percentage of current evoked by 10 mM GABA.
Statistical analysis
Data are mean±s.e.m. or s.d., as appropriate. Statistical comparisons of mean were made using one-way analysis of variance (ANOVA) using the Bonferroni multiple comparisons post-test or a Student's t-test, as appropriate. Curve fitting was performed using GraphPad Prism version 3.0.
Results
Vigabatrin inhibition of hPAT1-mediated amino acid uptake in Caco-2 cell monolayers
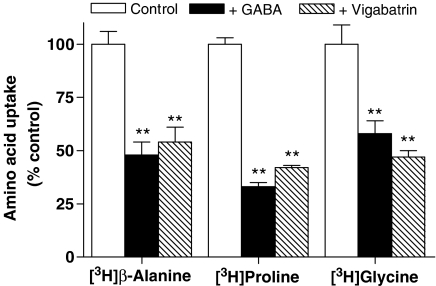
pH-dependent, Na+-independent, PAT1-mediated [3H]β-alanine, [3H]proline and [3H]glycine (all 20 μM) uptake across the apical membrane of Caco-2 cell monolayers was measured (apical pH 5.5, basolateral pH 7.4, Na+-free buffers). The presence of either 10 mM GABA or 10 mM vigabatrin at the apical membrane significantly inhibited the uptake of all three PAT1 substrates (Figure 1) (all P<0.01 versus control) suggesting that vigabatrin transport is via a Na+-independent pH-dependent uptake mechanism shared with the PAT1 substrates β-alanine, proline and glycine. In contrast, apical uptake of the dipeptide [14C]Gly-Sar via the human intestinal proton-coupled di/tripeptide transporter (hPepT1) was unaffected (P>0.05 versus control) by the presence of either GABA or vigabatrin demonstrating that vigabatrin, like GABA, is not absorbed via hPepT1 (data not shown).
Figure 1.
The effects of GABA and vigabatrin on amino acid uptake across the apical membrane of Caco-2 cell monolayers. Apical uptake of [3H]β-alanine, [3H]proline or [3H]glycine (all 20 μM) (5 min, apical pH 5.5, basolateral pH 7.4, Na+-free solutions) in Caco-2 cell monolayers was measured in the absence (open columns) or presence of unlabelled GABA (filled columns) or vigabatrin (hatched columns) (both 10 mM). Data are mean±s.e.m. (n=11): **P<0.01 versus control.
In Na+-free conditions, the transcellular transepithelial apical-to-basal [3H]GABA flux (Ja–b) (after subtraction of the paracellular component estimated by [14C]mannitol flux) was significantly increased (P<0.001) from 621±7 pmol cm−2 (90 min)−1 (n=11) to 1048±35 pmol cm−2 (90 min)−1 (n=11) when apical pH was decreased from pH 7.4 to pH 5.5. This pH-dependent [3H]GABA Ja–b is equivalent to the net flux of GABA (Jnet), as determined previously (Thwaites et al., 2000). The pH-dependent [3H]GABA Ja–b is reduced (down to levels measured in the absence of a transepithelial pH gradient) by 10 mM unlabelled GABA (486±9 pmol cm−2 (90 min)−1 (n=11)), vigabatrin (499±7 pmol cm−2 (90 min)−1 (n=12)), isonipecotic acid (577±9 pmol cm−2 (90 min)−1 (n=11)) and nipecotic acid (606±9 pmol cm−2 (90 min)−1 (n=11)) (all P<0.001 versus control, pH 5.5 alone).
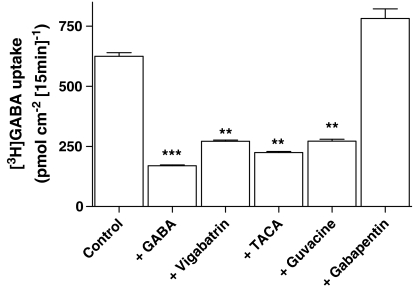
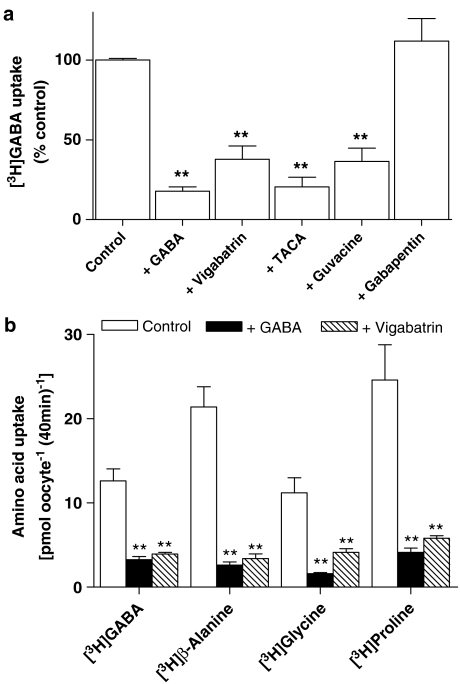
To determine whether other GABA derivatives are also likely to be substrates for hPAT1, [3H]GABA (100 μM) uptake across the apical membrane of Caco-2 cell monolayers was measured in the presence of vigabatrin, TACA, guvacine or gabapentin (all 10 mM). [3H]GABA uptake was significantly reduced by vigabatrin, TACA and guvacine (all P<0.01 versus control) suggesting that these GABA analogues are substrates for hPAT1 (Figure 2). In contrast, the AED gabapentin had no inhibitory effect on [3H]GABA uptake (P>0.05 versus control). In a separate series of experiments, [3H]GABA uptake (Na+-free, pH 5.5, at a lower substrate concentration of 20 μM GABA) across the brush-border membrane over 5 min was reduced from 104±4 pmol cm−2 (n=18) to 27±2 pmol cm−2 (n=18), 42±2 pmol cm−2 (n=18) and 39±1 pmol cm−2 (n=5) in the presence of (all 10 mM) cold GABA, vigabatrin and 4-amino-5-hexynoic acid (AHA or γ-acetylenic GABA), respectively, suggesting that AHA is also a hPAT1 substrate.
Figure 2.
The effects of GABA-related compounds on [3H]GABA uptake across the apical membrane of Caco-2 cell monolayers. Apical [3H]GABA (100 μM) uptake (15 min, apical pH 5.5, basolateral pH 7.4, Na+-free solutions) in Caco-2 cell monolayers was measured in the presence or absence (control) of unlabelled GABA, vigabatrin, TACA, guvacine or gabapentin (all 10 mM). Data are mean±s.e.m (n=6–12): ***P<0.001 or **P<0.01 both versus control.
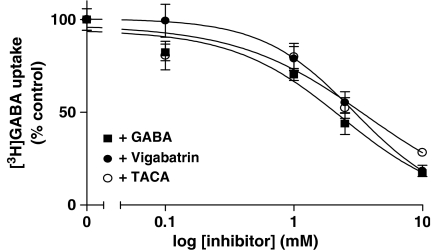
Figure 3 demonstrates that vigabatrin (IC50 2.9±1.2 mM) and TACA (IC50 3.6±1.3 mM) both inhibit [3H]GABA uptake across the apical membrane of Caco-2 cell monolayers in a concentration-dependent manner with a similar affinity to that measured using unlabelled GABA as the competitor (IC50 2.4±1.2 mM).
Figure 3.
Concentration-dependent inhibition of [3H]GABA uptake across the apical membrane of Caco-2 cells by GABA and related compounds. Apical [3H]GABA (20 μM) uptake (15 min, apical pH 5.5, basolateral pH 7.4, Na+-free solutions) in Caco-2 cell monolayers was measured in the absence and presence (all 0–10 mM) of unlabelled GABA (filled squares), vigabatrin (filled circles) or TACA (open circles). Data are the mean±s.e.m. (n=17–35). Non carrier-mediated uptake, measured in the presence of excess β-alanine (30 mM), was subtracted from total uptake to determine carrier-mediated uptake.
pHi measurements
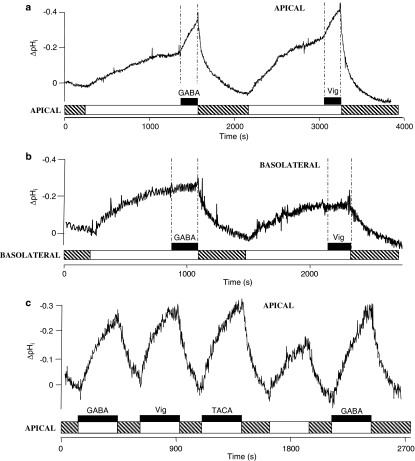
Competition experiments alone cannot determine whether a compound is simply an inhibitor of a transporter or a transported substrate. H+/vigabatrin cotransport was confirmed by measurement of substrate-coupled H+-flow across the apical membrane of BCECF-loaded Caco-2 cell monolayers (Figure 4). When the apical superfusate is changed from a pH 7.4 Na+-containing solution to a pH 5.5 Na+-free solution, it is clear that there is a marked intracellular acidification (Figure 4). Once the rate of this acidification due to the presence of apical pH 5.5 alone slowed (ΔpHi min−1 0.012±0.004, n=8), GABA (10 mM) was added to the apical superfusate, which led to an acceleration (P<0.001 versus pH 5.5 alone) in the rate of acidification (ΔpHi min−1 0.073±0.007, n=8) (Figure 4a). Similarly, when vigabatrin (10 mM) was added to the apical superfusate, the rate of acidification increased (P<0.001) from ΔpHi min−1 0.015±0.002 (n=8) to ΔpHi min−1 0.055±0.004 (n=8) (Figure 4a). In contrast when the manoeuvre was repeated at the basolateral surface of the BCECF-loaded Caco-2 cell monolayers the rate of acidification was not increased when either GABA (ΔpHi min−1 0.018±0.010 (n=7) in pH 5.5 alone and ΔpHi min−1 0.013±0.004 (n=7) after addition of GABA) or vigabatrin (ΔpHi min−1 0.010±0.004 (n=7) in pH 5.5 alone and ΔpHi min−1 0.011±0.003 (n=7) after addition of vigabatrin) was added to the basolateral superfusate (Figure 4b, both P>0.05), demonstrating that the H+/GABA or vigabatrin cotransport mechanism, like hPAT1, is confined to the apical surface of these intestinal enterocytes. As shown in Figure 4c, the initial rate of acidification across the apical membrane of BCECF-loaded Caco-2 cells is similar following apical exposure to GABA, vigabatrin or TACA (all 10 mM, pH 5.5, Na+-free solutions). The rate of intracellular acidification observed after addition of GABA to the apical superfusate is 198.7±7.5% (n=7) of that observed after superfusion of the pH 5.5 buffer alone; similar observations are made with vigabatrin (180.6±16.8% (n=6)) and TACA (171.4±11.0% (n=6)) (all P<0.01 versus pH 5.5 alone). Thus the ability of vigabatrin and other GABA-derivatives to induce ΔpHi is matched by their ability to inhibit [3H]GABA uptake, suggesting that they undergo H+/substrate cotransport via hPAT1.
Figure 4.
Intracellular pH measurements in BCECF-loaded Caco-2 cell monolayers. (a) The apical surface of the BCECF-loaded Caco-2 cell monolayers were superfused with a Na+-containing pH 7.4 solution (hatched bar). At the indicated point on the diagram the solution was replaced with Na+-free pH 5.5 solution (open bar) and subsequently either GABA or vigabatrin (both 10 mM; filled bars) were added to the apical superfusate for 200 s. Replacement of the apical superfusate with a pH 7.4 Na+-containing buffer (hatched bar) allows pHi to recover back towards baseline. The basolateral superfusate was pH 7.4 (Na+-free) throughout. A single representative trace of eight. (b) The basolateral surface of the BCECF-loaded Caco-2 cell monolayers were superfused with a Na+-containing pH 7.4 solution (hatched bar). At the indicated point on the diagram the solution was replaced with Na+-free pH 5.5 solution (open bar) and subsequently either GABA or vigabatrin (both 10 mM; filled bars) were added to the basolateral superfusate for 200 s. Replacement of the basolateral superfusate with a pH 7.4 Na+-containing buffer (hatched bar) allows pHi to recover back towards baseline. The apical superfusate was pH 7.4 (Na+-free) throughout. A single representative trace of seven. (c) The effects on pHi of sequential addition of pH 5.5 (Na+-free) alone (open bar) or GABA, vigabatrin and TACA (all 10 mM; filled bars) in a Na+-free pH 5.5 buffer. Basolateral pH 7.4, Na+-free under all conditions. Where indicated on the figure a Na+-containing pH 7.4 solution was superfused across the apical surface to allow the cells to recover (hatched bars). A single trace representative of six.
PAT1-mediated amino acid uptake in PAT1-expressing oocytes
In order to confirm that hPAT1 is the endogenous transporter responsible for the uptake of vigabatrin in Caco-2 cell monolayers measurements were made using PAT1-expressing oocytes. To enable direct comparison, transport was measured under identical experimental conditions to those used in the Caco-2 cell studies (extracellular pH 5.5, Na+-free incubation buffers). Under control conditions, [3H]GABA uptake (20 μM) into hPAT1-expressing oocytes is seven-fold greater than that in water-injected oocytes (P<0.001). Excess GABA, vigabatrin, TACA and guvacine all significantly inhibited [3H]GABA uptake (P<0.01), whereas gabapentin had no effect (P>0.05) (Figure 5a). In separate experiments AHA, GABA and vigabatrin (all 10 mM) all reduced (all P<0.001 versus control) hPAT1-mediated [3H]GABA uptake from 15.6±1.9 nmol oocyte−1 (40 min)−1 (n=18) to 1.4±0.3 nmol oocyte−1 (40 min)−1 (n=18), 2.3±0.2 nmol oocyte−1 (40 min)−1 (n=19) and 2.0±0.3 nmol oocyte−1 (40 min)−1 (n=20), respectively. Similar observations are made using mPAT1-expressing oocytes where GABA and vigabatrin (both 10 mM) markedly inhibited uptake of the PAT1 substrates [3H]GABA, [3H]β-alanine, [3H]glycine and [3H]proline (P<0.01 versus control) (Figure 5b).
Figure 5.
The effects of GABA and related compounds on amino acid uptake in PAT1-expressing oocytes. (a) [3H]GABA (20 μM) uptake (pH 5.5, Na+-free) via hPAT1 was measured in the absence (control) or presence of unlabelled GABA, vigabatrin, TACA, guvacine or gabapentin (all 10 mM). Data are expressed as a percentage control ([3H]GABA uptake in the absence of unlabelled compounds) after subtraction of uptake in water-injected oocytes under identical experimental conditions. Data are mean±s.e.m. (n=18–20): **P<0.01 versus control. (b) [3H]GABA, [3H]β-alanine, [3H]glycine, [3H]proline (all 20 μM) uptake (pH 5.5, Na+-free) via mPAT1 was measured in the absence (open columns) or presence of GABA (filled columns) or vigabatrin (hatched columns) (both 10 mM). Data are expressed as mean±s.e.m. (n=10) following subtraction of uptake into water-injected oocytes under identical experimental conditions: **P<0.01 versus control.
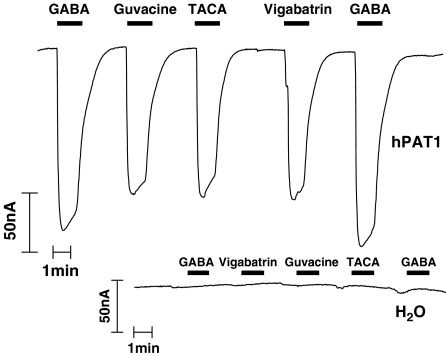
The ability of vigabatrin and other GABA-related compounds to induce inward current (due to electrogenic H+/substrate cotransport) in hPAT1-expressing oocytes was determined. Figure 6 demonstrates that extracellular exposure of hPAT1-expressing oocytes to GABA, vigabatrin, TACA and guvacine is associated with a large inward current not observed in water-injected oocytes. The current responses to vigabatrin (75.7±4.0% of the GABA response, mean±s.d., n=3), TACA (73.2±2.6%, n=3) and guvacine (87.6±3.8%, n=3) are similar to that induced by GABA alone (all P>0.05 versus GABA, all P<0.001 versus water). Thus, the ability of these GABA analogues to inhibit radiolabelled [3H]GABA uptake is similar to their ability to induce inward current, demonstrating that GABA, vigabatrin, TACA and guvacine are all substrates for transport via hPAT1.
Figure 6.
Current changes induced by GABA and related compounds in hPAT1-expressing oocytes. Single representative trace (of three) showing current changes in hPAT1 and water-injected oocytes (insert) following exposure (as indicated by bars) to GABA, vigabatrin, TACA or guvacine (all 10 mM, pH 5.5, Na+-free).
Discussion
The H+-coupled amino-acid transporter (system PAT) at the brush-border membrane of mammalian intestinal epithelia transports a broad range of amino acids and orally delivered therapeutic compounds (Thwaites et al., 1993a, 1993b; 1994; 1995a, 1995b, 1995c; Thwaites & Stevens, 1999; Thwaites et al., 2000; Boll et al., 2003; Metzner et al., 2004). cDNAs representing system PAT have been isolated from rat brain (LYAAT1) (Sagne et al., 2001), mouse (mPAT1) (Boll et al., 2002) and human intestine (hPAT1) (Chen et al., 2003). Studies using Caco-2 cell monolayers and PAT1-expressing X. laevis oocytes have used a combination of radiotracer flux studies and measurements of substrate-coupled H+-influx (using BCECF-loaded Caco-2 cell monolayers) or inward current (using the two-electrode voltage clamp technique in oocytes) to determine the structural requirements that allow interaction of a substrate with PAT1 (Thwaites et al., 1995c; Boll et al., 2003). By analysis of the transport and relative affinity of a large array of test compounds, the two major conformational limitations of the PAT1 active centre appear to be the size of the amino acid side chain and the length of the backbone (Boll et al., 2003). Such insight into the restrictions in the PAT1 binding site enables potential PAT1 substrates to be identified and highlights the potential of PAT1 as a route for oral delivery of therapeutic compounds.
Previous investigations have demonstrated that GABA and GABA analogues (nipecotic acid, isonipecotic acid, β-aminobutyric acid and 3-amino-1-propanesulphonic acid) can undergo rapid transport across the apical membrane of intestinal epithelial (Caco-2) cell monolayers via PAT1 (Thwaites et al., 2000). Therefore, the aim of this study was to determine if the orally delivered GABA analogues vigabatrin and gabapentin, used in the treatment of partial and focal epilepsy, and the nonclinically administered GABA derivatives TACA, guvacine and AHA are substrates for the PAT1 carrier.
We have shown through radiolabelled uptake experiments (Figure 1) that the GABA analogue vigabatrin inhibits uptake of known PAT1 substrates across the apical membrane of Caco-2 cell monolayers. Additional competition experiments verify that vigabatrin transport is concentration-dependent with a similar affinity to that obtained with other PAT1 substrates (Figures 2 and 3). The exclusive apical localisation of vigabatrin-induced H+-influx further supports the hypothesis that vigabatrin uptake is mediated via hPAT1 (Figure 4). In order to confirm that hPAT1 is the endogenous transporter responsible for the uptake of vigabatrin in Caco-2 cell monolayers, vigabatrin transport was investigated in oocytes injected with hPAT1 or mPAT1 cRNA. Competition experiments confirmed that vigabatrin inhibits uptake of GABA and other known PAT1 substrates in PAT1-expressing oocytes (Figure 5). Two-electrode voltage-clamp measurements, using hPAT1-expressing oocytes, confirm that vigabatrin transport is associated with an inward current (H+/vigabatrin cotransport) not observed in water-injected oocytes (Figure 6). In agreement with the proposed model of the PAT1 binding site, the linear structures of vigabatrin, TACA, and AHA and guvacine, where the α-carbon and imino group are incorporated into the heterocyclic structure, enables interaction with the PAT1 binding pocket. In contrast, the large side chain on the β-carbon of gabapentin predictably excludes this compound from PAT1 binding. Therefore, this study supports the hypothesis that one of the major restrictions for PAT1-binding is the size of the amino-acid side chain.
Although direct measurement of transepithelial transport of vigabatrin is necessary to verify that vigabatrin exits the basolateral membrane of these intestinal epithelial cells, previous investigations with other PAT1 substrates, namely β-alanine, L-alanine, α-methylaminoisobutyric acid, proline, GABA and glycine (Thwaites et al., 1993a, 1993b; 1994; 1995a, 1995b, 1995c; 2000), when considered alongside the high oral bioavailability of vigabatrin, suggest that transepithelial transport occurs. Potential efflux systems localised to the basolateral membrane of the small intestine include system L (CD98/LAT2, SLC3A2/SLC7A8) which exchanges neutral amino acids (Rossier et al., 1999; Bauch et al., 2003), system y+L (CD98/y+LAT1, SLC3A2/SLC7A7), which exchanges neutral amino acids for intracellular cationic amino acids in the presence of sodium (Kanai et al., 2000; Bauch et al., 2003), or system asc (CD98/asc1, SLC3A2/SLC7A10), which is a Na+-independent, high-affinity neutral amino-acid exchanger (Wagner et al., 2001). However, the specific carrier system(s) involved in vigabatrin transport across the basolateral membrane has yet to be determined.
Gabapentin absorption by rat and rabbit brush-border membrane vesicles (BBMV) is sodium-independent and greater in duodenal and ileal BBMV compared with jejunal BBMV (Piyapolrungroj et al., 2001). It has been suggested that the intestinal uptake of gabapentin across the brush-border membrane may be mediated by system b0,+ (rBAT/b0,+AT, SLC3A1/SLC7A9), which transports neutral and dibasic amino acids in a sodium-independent manner (Wagner et al., 2001). In a rat intestinal everted ring preparation, gabapentin uptake shared an inhibition profile with L-phenylalanine, suggesting that system L may play a role in basolateral transport of gabapentin (Stewart et al., 1993). However, further investigation into the route of gabapentin intestinal absorption is required.
Any orally delivered compound used to treat a CNS disorder (e.g. epilepsy) must cross several distinct barriers before reaching the target site of action. These barriers include the small intestinal epithelium (both apical and basolateral membranes), the blood–brain barrier (BBB) and, in the case of a drug with an intracellular site of action, the neuronal and/or glial cell membrane. The various transport mechanisms responsible for movement of vigabatrin across these membranes are not known. However, vigabatrin does inhibit carnitine uptake in the human placenta probably via the organic cation transporter OCTN2 (SLC22A5) (Wu et al., 2004). OCTN2 is expressed at the BBB (Berezowski et al., 2004). Vigabatrin also inhibits GABA transport via the Na+-dependent GABA transporter GAT1 (SLC6A1) (Eckstein-Ludwig et al., 1999) which may represent a route for vigabatrin influx into both neurones and glial cells. The role of PAT1 in vigabatrin transport in neural tissues is not clear as PAT1 is found mainly in lysosomes in neurones (Sagne et al., 2001; Agulhon et al., 2003) although functional PAT1-like expression has also been detected at the plasma membrane of cultured hippocampal neurones (Wreden et al., 2003).
The present study demonstrates that vigabatrin is a substrate for the H+-coupled amino-acid transporter hPAT1, which is expressed at the brush-border membrane of the human small intestinal epithelium. The high capacity of hPAT1 partially explains the high oral bioavailability of vigabatrin (80–90%) and emphasises the therapeutic significance of hPAT1 as a route for the oral absorption of clinically administered GABA-related compounds.
Acknowledgments
This study was supported by an MRC Career Establishment grant (G9801704) and BBSRC project grant (13/D17277) to DTT. KMG was supported by a BBSRC PhD studentship. mPAT1 cDNA was a gift from Dr M. Boll (Freising-Weihenstephan, Germany).
Abbreviations
- AED
anti-epileptic drugs
- AHA
4-amino-5-hexynoic acid or γ-acetylenic GABA
- BCECF
2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein
- BBB
blood–brain barrier
- BBMV
brush-border membrane vesicles
- GABA
γ-aminobutyric acid
- gabapentin
1-(aminomethyl)cyclohexaneacetic acid
- GABA-T
GABA-transaminase
- HRPE
human retinal pigment epithelia
- MCT
monocarboxylate transporter
- OAT
organic anion transporter
- OCT
organic cation transporter
- PAT
H+-coupled amino acid transporter
- PepT1
H+-coupled di/tripeptide transporter
- pHi
intracellular pH
- TACA
trans-4-aminocrotonic acid
- vigabatrin
4-amino-5-hexanoic acid or γ-vinyl GABA
References
- AGULHON C., ROSTAING P., RAVASSARD P., SAGNE C., TRILLER A., GIROS B. Lysosomal amino acid transporter LYAAT-1 in the rat central nervous system: an in situ hybridization and immunohistochemical study. J. Comp. Neurol. 2003;462:71–89. doi: 10.1002/cne.10712. [DOI] [PubMed] [Google Scholar]
- ANDERSON C.M.H., GRENADE D.S., BOLL M., FOLTZ M., WAKE K.A., KENNEDY D.J., MUNCK L.K., MIYAUCHI S., TAYLOR P.M., CAMPBELL F.C., MUNCK B.G., DANIEL H., GANAPATHY V., THWAITES D.T. H+/amino acid transporter 1 (PAT1) is the imino acid carrier: an intestinal nutrient/drug transporter in human and rat. Gastroenterology. 2004;127:1410–1422. doi: 10.1053/j.gastro.2004.08.017. [DOI] [PubMed] [Google Scholar]
- ANDERSON C.M.H., THWAITES D.T. Indirect regulation of the intestinal H+-coupled amino acid transporter hPAT1 (SLC36A1) J. Cell. Physiol. 2005;204:604–613. doi: 10.1002/jcp.20337. [DOI] [PubMed] [Google Scholar]
- BAUCH C., FORSTER N., LOFFING-CUENI D., SUMMA V., VERREY F. Functional cooperation of epithelial heteromeric amino acid transporters expressed in madin-darby canine kidney cells. J. Biol. Chem. 2003;278:1316–1322. doi: 10.1074/jbc.M210449200. [DOI] [PubMed] [Google Scholar]
- BEREZOWSKI V., MIECZ D., MARSZALEK M., BROER A., BROER S., CECCHELLI R., NALECZ K.A. Involvement of OCTN2 and B0,+ in the transport of carnitine through an in vitro model of the blood–brain barrier. J. Neurochem. 2004;91:860–872. doi: 10.1111/j.1471-4159.2004.02752.x. [DOI] [PubMed] [Google Scholar]
- BERTRAND S., NG G.Y., PURISAI M.G., WOLFE S.E., SEVERIDT M.W., NOUEL D., ROBITAILLE R., LOW M.J., O'NEILL G.P., METTERS K., LACAILLE J.C., CHRONWALL B.M., MORRIS S.J. The anticonvulsant, antihyperalgesic agent gabapentin is an agonist at brain gamma-aminobutyric acid type B receptors negatively coupled to voltage-dependent calcium channels. J. Pharmacol. Exp. Ther. 2001;298:15–24. [PubMed] [Google Scholar]
- BOLL M., FOLTZ M., ANDERSON C.M.H., OECHSLER C., KOTTRA G., THWAITES D.T., DANIEL H. Substrate recognition by the mammalian proton-dependent amino acid transporter PAT1. Mol. Membr. Biol. 2003;20:261–269. doi: 10.1080/0968768031000100759. [DOI] [PubMed] [Google Scholar]
- BOLL M., FOLTZ M., RUBIO-ALIAGA I., KOTTRA G., DANIEL H. Functional characterization of two novel mammalian electrogenic proton-dependent amino acid cotransporters. J. Biol. Chem. 2002;277:22966–22973. doi: 10.1074/jbc.M200374200. [DOI] [PubMed] [Google Scholar]
- BOLTON J.B., RIMMER E., WILLIAMS J., RICHENS A. The effect of vigabatrin on brain and platelet GABA-transaminase activities. Br. J. Clin. Pharmacol. 1989;27:35S–42S. doi: 10.1111/j.1365-2125.1989.tb03459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANS J.S., WUSTROW D.J. 3-substituted GABA analogs with central nervous system activity: a review. Med. Res. Rev. 1999;19:149–177. doi: 10.1002/(sici)1098-1128(199903)19:2<149::aid-med3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- CHEN Z., FEI Y.J., ANDERSON C.M.H., WAKE K.A., MIYAUCHI S., HUANG W., THWAITES D.T., GANAPATHY V. Structure, function and immunolocalization of a proton-coupled amino acid transporter (hPAT1) in the human intestinal cell line Caco-2. J. Physiol. 2003;546:349–361. doi: 10.1113/jphysiol.2002.026500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANIEL H., FETT C., KRATZ A. Demonstration and modification of intervillous pH profiles in rat small intestine in vitro. Am. J. Physiol. 1989;257:G489–G495. doi: 10.1152/ajpgi.1989.257.4.G489. [DOI] [PubMed] [Google Scholar]
- EVINS A.E., AMICO E., POSEVER T.A., TOKER R., GOFF D.C. D-Cycloserine added to risperidone in patients with primary negative symptoms of schizophrenia. Schizophr. Res. 2002;56:19–23. doi: 10.1016/s0920-9964(01)00220-1. [DOI] [PubMed] [Google Scholar]
- ECKSTEIN-LUDWIG U., FEI J., SCHWARZ W. Inhibition of uptake, steady-state currents, and transient charge movements generated by the neuronal GABA transporter by various anticonvulsant drugs. Br. J. Pharmacol. 1999;128:92–102. doi: 10.1038/sj.bjp.0702794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HADJIKOUTIS S., MORGAN J.E., WILD J.M, SMITH P.E.M. ocular complications of neurological therapy. Eur. J. Neurol. 2005;12:499–507. doi: 10.1111/j.1468-1331.2005.01025.x. [DOI] [PubMed] [Google Scholar]
- KANAI Y., FUKASAWA Y., CHA S.H., SEGAWA H., CHAIROUNGDUA A., KIM D.K., MATSUO H., KIM J.Y., MIYAMOTO K., TAKEDA E., ENDOU H. Transport properties of a system y+L neutral and basic amino acid transporter. Insights into the mechanisms of substrate recognition. J. Biol. Chem. 2000;275:20787–20793. doi: 10.1074/jbc.M000634200. [DOI] [PubMed] [Google Scholar]
- KROGSGAARD-LARSEN P. Gamma-aminobutyric acid agonists, antagonists, and uptake inhibitors. Design and therapeutic aspects. J. Med. Chem. 1981;24:1377–1383. doi: 10.1021/jm00144a001. [DOI] [PubMed] [Google Scholar]
- KROGSGAARD-LARSEN P. GABA synaptic mechanisms: stereochemical and conformational requirements. Med. Res. Rev. 1988;8:27–56. doi: 10.1002/med.2610080103. [DOI] [PubMed] [Google Scholar]
- KROGSGAARD-LARSEN P., FALCH E., LARSSON O.M., SCHOUSBOE A. GABA uptake inhibitors: relevance to antiepileptic drug research. Epilepsy Res. 1987;1:77–93. doi: 10.1016/0920-1211(87)90012-x. [DOI] [PubMed] [Google Scholar]
- LOSCHER W., FASSBENDER C.P., GRAM L., GRAMER M., HORSTERMANN D., ZAHNER B., STEFAN H. Determination of GABA and vigabatrin in human plasma by a rapid and simple HPLC method: correlation between clinical response to vigabatrin and increase in plasma GABA. Epilepsy Res. 1993;14:245–255. doi: 10.1016/0920-1211(93)90049-d. [DOI] [PubMed] [Google Scholar]
- MCEWAN G.T.A., DANIEL H., FETT C., BURGESS M.N., LUCAS M.L. The effect of Escherichia coli STa enterotoxin and other secretagogues on mucosal surface pH of rat small intestine in vivo. Proc. R. Soc. Lond. B. 1988;234:219–237. doi: 10.1098/rspb.1988.0045. [DOI] [PubMed] [Google Scholar]
- METZNER L., KALBITZ J., BRANDSCH M. Transport of pharmacologically active proline derivatives by the human proton-coupled amino acid transporter hPAT1. J. Pharmacol. Exp. Ther. 2004;309:28–35. doi: 10.1124/jpet.103.059014. [DOI] [PubMed] [Google Scholar]
- MUNCK B.G. Amino acid transport by the small intestine of the rat. The existence and specificity of the transport mechanism of imino acids and its relation to the transport of glycine. Biochim. Biophys. Acta. 1966;120:97–103. doi: 10.1016/0926-6585(66)90281-0. [DOI] [PubMed] [Google Scholar]
- MUNCK B.G., MUNCK L.K., RASMUSSEN S.N., POLACHE A. Specificity of the imino acid carrier in rat small intestine. Am. J. Physiol. 1994;266:R1154–R1161. doi: 10.1152/ajpregu.1994.266.4.R1154. [DOI] [PubMed] [Google Scholar]
- PATSALOS P.N. New antiepileptic drugs. Ann. Clin. Biochem. 1999;36:10–19. doi: 10.1177/000456329903600102. [DOI] [PubMed] [Google Scholar]
- PATSALOS P.N., DUNCAN J.S. New antiepileptic drugs. A review of their current status and clinical potential. CNS Drugs. 1994;2:40–47. [Google Scholar]
- PETROFF O.A., BEHAR K.L., MATTSON R.H., ROTHMAN D.L. Human brain gamma-aminobutyric acid levels and seizure control following initiation of vigabatrin therapy. J. Neurochem. 1996;67:2399–2404. doi: 10.1046/j.1471-4159.1996.67062399.x. [DOI] [PubMed] [Google Scholar]
- PIYAPOLRUNGROJ N., LI C., BOCKBRADER H., LIU G., FLEISHER D. Mucosal uptake of gabapentin (neurontin) vs. pregabalin in the small intestine. Pharm. Res. 2001;18:1126–1130. doi: 10.1023/a:1010970809090. [DOI] [PubMed] [Google Scholar]
- PREECE N.E., JACKSON G.D., HOUSEMAN J.A., DUNCAN J.S., WILLIAMS S.R. Nuclear magnetic resonance detection of increased cortical GABA in vigabatrin-treated rats in vivo. Epilepsia. 1994;35:431–436. doi: 10.1111/j.1528-1157.1994.tb02456.x. [DOI] [PubMed] [Google Scholar]
- RANALDI G., ISLAM K., SAMBUY Y. D-Cycloserine uses an active transport mechanism in the human intestinal cell line Caco 2. Antimicrob. Agents Chemother. 1994;38:1239–1245. doi: 10.1128/aac.38.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAWLINGS J.M., LUCAS M.L., RUSSELL R.I. Measurement of jejunal surface pH in situ by plastic pH electrode in patients with coeliac disease. Scand. J. Gastroenterol. 1987;22:377–384. doi: 10.3109/00365528709078608. [DOI] [PubMed] [Google Scholar]
- ROSSIER G., MEIER C., BAUCH C., SUMMA V., SORDAT B., VERREY F., KUHN L.C. LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J. Biol. Chem. 1999;274:34948–34954. doi: 10.1074/jbc.274.49.34948. [DOI] [PubMed] [Google Scholar]
- SAGNE C., AGULHON C., RAVASSARD P., DARMON M., HAMON M., EL MESTIKAWY S., GASNIER B., GIROS B. Identification and characterization of a lysosomal transporter for small neutral amino acids. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7206–7211. doi: 10.1073/pnas.121183498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART B.H., KUGLER A.R., THOMPSON P.R., BOCKBRADER H.N. A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm. Res. 1993;10:276–281. doi: 10.1023/a:1018951214146. [DOI] [PubMed] [Google Scholar]
- THWAITES D.T., ARMSTRONG G., HIRST B.H., SIMMONS N.L. D-Cycloserine transport in human intestinal epithelial (Caco-2) cells: mediation by a H+-coupled amino acid transporter. Br. J. Pharmacol. 1995a;115:761–766. doi: 10.1111/j.1476-5381.1995.tb14998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THWAITES D.T., BASTERFIELD L., MCCLEAVE P.M.J., CARTER S.M., SIMMONS N.L. Gamma-aminobutyric acid (GABA) transport across human intestinal epithelial (Caco-2) cell monolayers. Br. J. Pharmacol. 2000;129:457–464. doi: 10.1038/sj.bjp.0703069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THWAITES D.T., FORD D., GLANVILLE M., SIMMONS N.L. H+/solute-induced intracellular acidification leads to selective activation of apical Na+/H+ exchange in human intestinal epithelial cells. J. Clin. Invest. 1999;104:629–635. doi: 10.1172/JCI7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THWAITES D.T., MCEWAN G.T.A., BROWN C.D.A., HIRST B.H., SIMMONS N.L. L-Alanine absorption in human intestinal Caco-2 cells driven by the proton electrochemical gradient. J. Membr. Biol. 1994;140:143–151. doi: 10.1007/BF00232902. [DOI] [PubMed] [Google Scholar]
- THWAITES D.T., MCEWAN G.T.A., BROWN C.D.A., HIRST B.H., SIMMONS N.L. Na+-independent, H+-coupled transepithelial β-alanine absorption by human intestinal Caco-2 cell monolayers. J. Biol. Chem. 1993a;268:18438–18441. [PubMed] [Google Scholar]
- THWAITES D.T., MCEWAN G.T.A., COOK M.J., HIRST B.H., SIMMONS N.L. H+-coupled (Na+-independent) proline transport in human intestinal (Caco-2) epithelial cell monolayers. FEBS Lett. 1993b;333:78–82. doi: 10.1016/0014-5793(93)80378-8. [DOI] [PubMed] [Google Scholar]
- THWAITES D.T., MCEWAN G.T.A., HIRST B.H., SIMMONS N.L. H+-coupled α-methylaminoisobutyric acid transport in human intestinal Caco-2 cells. Biochim. Biophys. Acta. 1995b;1234:111–118. doi: 10.1016/0005-2736(94)00268-t. [DOI] [PubMed] [Google Scholar]
- THWAITES D.T., MCEWAN G.T.A., SIMMONS N.L. The role of the proton electrochemical gradient in the transepithelial absorption of amino acids by human intestinal Caco-2 cell monolayers. J. Membr. Biol. 1995c;145:245–256. doi: 10.1007/BF00232716. [DOI] [PubMed] [Google Scholar]
- THWAITES D.T., STEVENS B.C. H+-zwitterionic amino acid symport at the brush-border membrane of human intestinal epithelial (CACO-2) cells. Exp. Physiol. 1999;84:275–284. [PubMed] [Google Scholar]
- TSAI G., YANG P., CHUNG L.C., LANGE N., COYLE J.T. D-Serine added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry. 1998;44:1081–1089. doi: 10.1016/s0006-3223(98)00279-0. [DOI] [PubMed] [Google Scholar]
- UCHINO H., KANAI Y., KIM D.K., WEMPE M.F., CHAIROUNGDUA A., MORIMOTO E., ANDERS M.W., ENDOU H. Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol. Pharmacol. 2002;61:729–737. doi: 10.1124/mol.61.4.729. [DOI] [PubMed] [Google Scholar]
- WAGNER C.A., LANG F., BROER S. Function and structure of heterodimeric amino acid transporters. Am. J. Physiol. 2001;281:C1077–C1093. doi: 10.1152/ajpcell.2001.281.4.C1077. [DOI] [PubMed] [Google Scholar]
- WONG C.G., BOTTIGLIERI T., SNEAD O.C.R. GABA, gamma-hydroxybutyric acid, and neurological disease. Ann. Neurol. 2003;54:S3–S12. doi: 10.1002/ana.10696. [DOI] [PubMed] [Google Scholar]
- WREDEN C.C., JOHNSON J., TRAN C., SEAL R.P., COPENHAGEN D.R., REIMER R.J., EDWARDS R.H. The H+-coupled electrogenic lysosomal amino acid transporter LYAAT1 localizes to the axon and plasma membrane of hippocampal neurons. J. Neurosci. 2003;23:1265–1275. doi: 10.1523/JNEUROSCI.23-04-01265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU S.P., SHYU M.K., LIOU H.H., GAU C.S., LIN C.J. Interaction between anticonvulsants and human placental carnitine transporter. Epilepsia. 2004;45:204–210. doi: 10.1111/j.0013-9580.2004.29603.x. [DOI] [PubMed] [Google Scholar]