Abstract
The antifungal antibiotic clotrimazole (CLT) shows therapeutic effects on cancer, sickle cell disease, malaria, etc. by inhibiting membrane intermediate-conductance Ca2+-activated K+ channels (IKCa). However, it is unclear whether this drug would affect human cardiac K+ currents. The present study was therefore designed to investigate the effects of CLT on transient outward K+ current (Ito1), and ultra-rapid delayed rectifier K+ current (IKur) in isolated human atrial myocytes, and cloned hERG channel current (IhERG) and recombinant human cardiac KCNQ1/KCNE1 channel current (IKs) expressed in HEK 293 cells.
It was found that CLT inhibited Ito1 with an IC50 of 29.5 μM, accelerated Ito1 inactivation, and decreased recovery of Ito1 from inactivation. In addition, CLT inhibited human atrial IKur in a concentration-dependent manner (IC50=7.6 μM).
CLT substantially suppressed IhERG (IC50=3.6 μM), and negatively shifted the activation conductance of IhERG. Moreover, CLT inhibited IKs (IC50=15.1 μM), and positively shifted the activation conductance of the current.
These results indicate that the antifungal antibiotic CLT substantially inhibits human cardiac repolarization K+ currents including Ito1, IKur, IhERG, and IKs. However, caution is recommended when correlating the observed in vitro effects on cardiac ion currents to the clinical relevance.
Keywords: Clotrimazole, human cardiac repolarization K+ currents, transient outward K+ current, ultra-rapidly delayed rectifier K+ current, hERG channel, recombinant human cardiac KCNQ1/KCNE1
Introduction
Clotrimazole (CLT), a member of the imidazole family, is topically used for the treatment of mycoses (Weuta, 1974; Rodrigues et al., 1987; Yoshida & Aoyama, 1987). The antifungal effect is related to its inhibition of fungal sterol 14α-demethylase, a microsomal cytochrome P450-dependent enzyme (Rodrigues et al., 1987; Yoshida & Aoyama, 1987). In addition, it was reported that CLT had therapeutic effects in sickle cell disease (Brugnara et al., 1996), secretory diarrhea, cancer, etc. These effects are related to the blockade of the intermediate-conductance Ca2+-activated potassium channel (IKCa) in human erythrocytes, colonic epithelium, and many tumor cells (Alvarez et al., 1992; Brugnara et al., 1996; Rufo et al., 1997; Vandorpe et al., 1998; Jensen et al., 1999; Khanna et al., 1999; Wulff et al., 2000). CLT was found also to block L-type Ca2+ channel current (ICa.L) in cardiac myocytes (Xiao et al., 1998; Thomas et al., 1999), voltage-gated K+ currents in mouse pancreatic β-cells (Welker & Drews, 1997), and several types of cloned Kv channels expressed in mammalian cell lines (Wulff et al., 2000).
Although systemic administration is suggested, limited information is available in the literature as to whether CLT affects human cardiac repolarization K+ currents. The present study was therefore designed to investigate the effects of CLT on transient outward K+ current (Ito1), ultra-rapid delayed rectifier K+ current (IKur) in human atrial myocytes, and cloned hERG channel current (IhERG) and recombinant human cardiac KCNQ1/KCNE1 channel current (IKs) expressed in HEK 293 cells with whole-cell patch and/or perforated patch techniques.
Methods
Preparation of human atrial myocytes
Atrial myocytes were isolated from specimens of human right atrial appendage obtained from patients (52.4±2.7 years old; range from 24 to 75 years old) undergoing coronary artery bypass grafting. The procedure for obtaining the tissue was approved by the Ethics Committee of the University of Hong Kong, and a written consent was obtained from patients. All atrial specimens were grossly normal at the time of cardiac surgery, and all patients were free of supraventricular tachyarrhythmias and symptomatic congestive heart failure. After excision, the samples were quickly immersed in oxygenated, nominally Ca2+-free cardioplegic solution for transport to the laboratory. Atrial myocytes were enzymatically dissociated with a modified procedure as described previously (Du et al., 2004). Briefly, the myocardial tissue was minced with a sharp blade and then placed in a 15-ml tube containing 10 ml of the Ca2+-free Tyrode solution (36°C), gently agitated by continuous bubbling with 100% O2. After 15 min (5 min at a time in fresh solutions), the chunks were incubated for 50 min in a similar solution containing 150–200 U ml−1 collagenase (CLS II, Worthington Biochemical, Freehold, NJ, U.S.A.), 1.2 U ml−1 protease (type XXIV, Sigma Chemical, St Louis, MO, U.S.A.), and 1 mg ml−1 bovine serum albumin (Sigma). Subsequently, the supernatant was discarded and the chunks were re-incubated in a fresh enzyme solution with the same composition, but without protease. Microscope examination of the medium was performed every 5–10 min to determine the number and the quality of the isolated cells. When the yield appeared to be maximal, the chunks were suspended in a high K+ medium and gently blown with a pipette. The isolated myocytes were kept at room temperature in the medium for at least 1 h before study.
A small aliquot of the solution containing the isolated cells was placed in an open perfusion chamber (1 ml) mounted on the stage of an inverted microscope. Myocytes were allowed to adhere to the bottom of the chamber for 5–10 min and were then superfused at 2–3 ml min−1 with Tyrode solution. Only quiescent, rod-shaped cells showing clear cross-striations were selected for experiments.
Cell culture and gene transfection
A previous study had reported that human cardiac KCNQ1/KCNE1 channels transiently expressed in HEK 293 cells demonstrated the typical characteristics of IKs (Zhang et al., 2001). After co-transfecting hKCNQ1/pCEP4 and hKCNE1/pALTER-Max vectors (provided by Dr G.N. Tseng, Virginia Commonwealth University) into HEK 293 cells using Lipofectamine 2000™ (Invitrogen, Carlsbad, CA, U.S.A.) according to the instruction of the manufacturer, the cell line stably expressing the recombinant human cardiac KCNQ1/KCNE1 channels was selected in 200 μg ml−1 hygromycin (Sigma-Aldrich, St Louis, MO, U.S.A.), and was maintained in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum and 100 μg ml−1 hygromycin. The vector of hERG/pcDNA3 provided by Dr Gail Robertson (University of Wisconsin-Madison, WI, U.S.A.) was transfected transiently into HEK 293 cells using Lipofectamine 2000™.
Solution and drugs
Ca2+-free cardioplegic solution for transport of specimens contained (in mM): KH2PO4 50, MgSO4 8.0, adenosine 5.0, HEPES 10, glucose 140, mannitol 100, and taurine 10, with pH adjusted to 7.3 with KOH. The standard Tyrode's solution contained (in mM): NaCl 140, KCl 5.0, MgCl2 1.0, CaCl2 1.0, NaH2PO4 0.33, HEPES 5.0, glucose 10, with pH adjusted to 7.4 with NaOH. For the atrial tissue wash, Ca2+ was omitted. High-K+ storage medium contained (in mM): KCl 10, K-glutamate 120, KH2PO4 10, MgSO4 1.8, taurine 10, HEPES 10, EGTA 0.5, glucose 20, and mannitol 10, with pH adjusted to 7.3 with KOH. The pipette solution contained (in mM): KCl 20, K-aspartate 110, MgCl2 1.0, HEPES 10, EGTA 5.0, and GTP 0.1, Na2-phosphocreatine 5.0, Mg2-ATP 5.0, with pH adjusted to 7.2 with KOH. For Ito1 and IKur determination, BaCl2 (200 μM) and CdCl2 (200 μM) were added to the superfusion to block IK1 and ICa. Atropine (1.0 μM) was used to minimize possible acetylcholine-activated K+ current (IK,Ach) contamination during the current recording.
CLT (Sigma-Aldrich) was prepared as 50 mM stock solutions in dimethyl sulfoxide (DMSO, Sigma-Aldrich) and added to the bath solution at the indicated final concentrations. The DMSO concentration in the perfusion was <0.2% (v v−1) and caused no effect on the membrane currents. Experiments were conducted at room temperature (21–22°C).
Data acquisition and analysis
The whole-cell and/or perforated patch-clamp techniques were used as described previously. Briefly, borosilicate glass electrodes (1.2 mm OD) were pulled with a Brown-Flaming puller (model P-97, Sutter Instrument Co., Novato, CA, U.S.A.) and had tip resistances of 2–3 MΩ when filled with pipette solution. A 3-M KCl-agar salt bridge was used as reference electrode. Liquid junction potentials were compensated before the pipette touched the cell. After a gigaseal was obtained, the cell membrane was ruptured by gentle suction to establish the whole-cell configuration to record Ito1, IKur, and IhERG. Perforated patch configuration was used to record IKs to minimize rundown of the current. The pipette solution included 200 μg ml−1 amphotericin B (Sigma-Aldrich), and omitted GTP, ATP, and EGTA. The series resistance was electrically compensated to minimize voltage errors. The membrane currents were recorded with an EPC-10 amplifier and Pulse software (HEKA, Lambrecht, Germany). Command pulses were generated by a 12-bit digital-to-analog converter controlled by Pulse software. Current signals were low-pass filtered at 5 kHz and stored on the hard disk of an IBM computer.
Values are presented as mean±s.e. Nonlinear curve fitting was performed using Pulsefit (HEKA) and/or Sigmaplot (SPSS Science, Chicago, IL, U.S.A.). Paired and/or unpaired Student's t-tests were used to evaluate the statistical significance of differences between two group means. ANOVA was used for multiple groups. Values of P<0.05 were considered statistically significant.
Results
Effect of CLT on Ito1
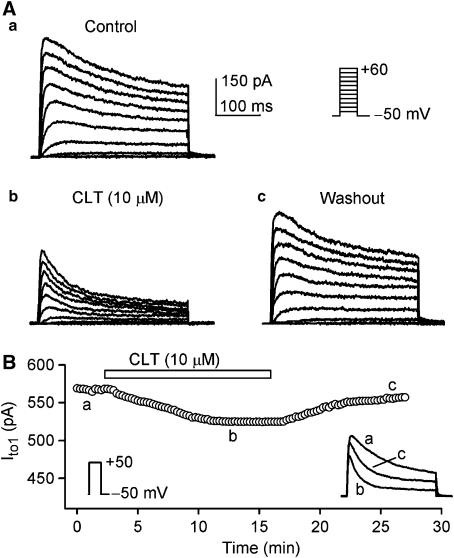
Figure 1A shows voltage-dependent Ito1 elicited by 300-ms voltage steps from −50 to between −40 and +60 mV (as shown in the inset) in a representative human atrial myocyte during control, in the presence of CLT, and after the drug washout. Ito1 was substantially inhibited by the application of 10 μM CLT, and the effect was partially reversed by washout (10 min).
Figure 1.
Effects of CLT on Ito1 in human atrial myocytes. (A) Voltage-dependent Ito1 traces (capacitance compensated) recorded at 0.2 Hz in a representative myocyte with 300-ms voltage steps from −50 to between −40 and +60 mV (inset) during control (a), in the presence of 10 μM CLT (b), or after washout (c). (B) Time course of Ito1 recorded in a typical experiment in the absence and presence of 10 μM CLT.
Figure 1B illustrates the time-dependent effect of CLT on Ito1 activated by a 300-ms voltage step (the left inset). The current measured was from peak to the ‘quasi'-steady-state level. CLT at 10 μM gradually inhibited Ito1, and the effect reached a steady-state level within 10 min. The inhibition was partially reversed by washout. The original Ito1 traces at the corresponding time points are shown in the right inset of the panel.
Results from Figure 1 indicate that the sustained current (i.e. IKur) is also substantially reduced when Ito1 is inhibited by CLT. Although the Ito1 was measured from peak to the 'quasi'-steady-state level, it might be confounded by effects from other currents. We have recently found that verapamil inhibits IKur without reduction of Ito1 amplitude, while it induces an increase of measured Ito1 in human atrial myocytes (Gao et al., 2004). Therefore, verapamil at 10 μM was used to separate Ito1 from IKur as described below.
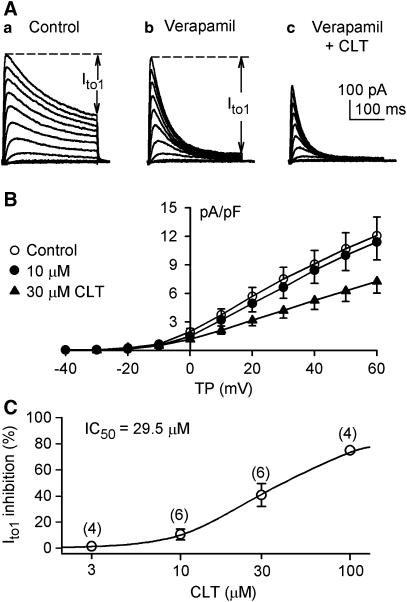
Figure 2A displays voltage-dependent Ito1 recorded in a representative myocyte with an identical protocol as shown in Figure 1A during control, in the presence of 10 μM verapamil, and co-presence of verapamil with 30 μM CLT. Ito1 amplitude was actually increased by the application of verapamil to inhibit IKur as described previously (Gao et al., 2004). CLT at 30 μM substantially suppressed Ito1. Figure 2B shows the I–V relationships of Ito1 in six cells in the presence of 10 μM verapamil to inhibit IKur (control), and after the application of 10 and 30 μM CLT. CLT significantly decreased Ito1 at test potentials from 0 to +50 mV (P<0.05 or 0.01 vs control). Figure 2C illustrates the concentration-response relationship for the inhibition of Ito1 by CLT. Data were fitted to the Hill equation:
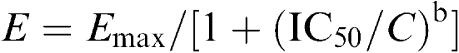 |
where E is the effect at concentration C, Emax is the maximal effect, IC50 is the concentration for half-maximal inhibitory effect, and b is Hill coefficient. The IC50 (at +50 mV) for the inhibition of Ito1 by CLT was 29.5 μM, and the Hill coefficient was 1.7.
Figure 2.
Effects of verapamil and CLT on Ito1. (A) Ito1 traces recorded in a representative myocyte with the same voltage protocol as shown in the inset of Figure 1A during control (a), in the presence of 10 μM verapamil for 10 min (b), in the co-presence of verapamil and 30 μM clotrmazole (CLT) for 10 min (c). Verapamil induced an increase of measured Ito1 by selectively inhibiting IKur. (B) I–V relationships of Ito1 in the presence of 10 μM verapamil (control), co-presence of verapamil and 10 or 30 μM CLT (10 min for each concentration). CLT inhibited Ito1 in a concentration-dependent manner (n=6, P<0.05 or 0.01 at 0–+60 mV vs control). (C) Concentration–response relationship for the inhibition of Ito1 by CLT. Symbols are mean data at +50 mV, and solid line is the best-fit to Hill equation. IC50 was 29.5 μM, Hill coefficient was 1.7, and Emax was 74.9%. The numbers in parentheses are numbers of experiments.
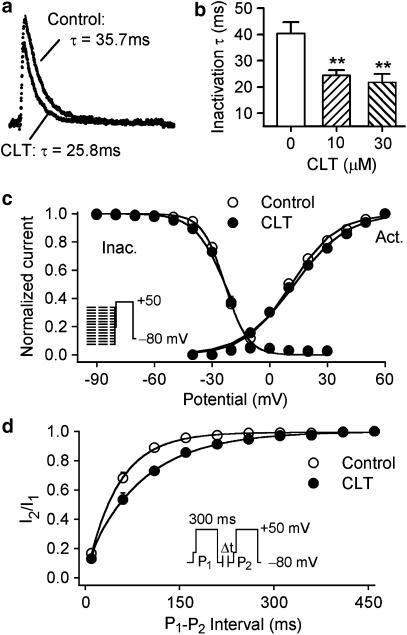
Figure 3a shows Ito1 traces recorded in a representative cell with a 300-ms voltage step from −50 to +50 mV in the presence of 10 μM verapamil (to inhibit IKur, control) and in the co-presence of verapamil and 10 μM CLT. Ito1 was well fitted to a mono-exponential function in the absence (control) and presence of CLT with the time constants shown. Figure 3b summarizes the averaged time constants, and the time constant of Ito1 inactivation was reduced from 40.3±4.2 ms in control to 24.5±1.9 and 21.2±3.2 ms (n=6, P<0.01 vs control), respectively, with 10 and 30 μM CLT. These results are consistent with the high-affinity open-channel block causing rapid current decay.
Figure 3.
Effects of CLT on the kinetics of Ito1. (a) Ito1 traces recorded from a representative cell upon a 300-ms voltage step from −50 to +50 mV in the presence of 10 μM verapamil (control) and co-presence of verapamil and 10 μM CLT. Raw data (points) of Ito1 were fitted to a mono-exponential function (solid lines, superimposed with raw data) with time constants shown. (b) Mean values of time constants at +50 mV during control, in the presence of 10 and 30 μM CLT. The time constant was reduced by the application of 10 and 30 μM CLT (n=6, **P<0.01 vs control). (c) Normalized voltage-dependent variables for Ito1 activation (Act.) and inactivation (Inact.) were fitted to Boltzmann distribution: y=1/{1+exp[(Vm−V0.5)/S], where Vm is the membrane potential, V0.5 is the midpoint, and S is the slope. V0.5 for activation conductance of Ito1 was 10.9±1.0 mV for control and 12.1±1.3 mV for 10 μM CLT (n=6, P=NS), while S was 12.5±1.0 and 13.8±1.7 mV for control and CLT, respectively (P=NS). For inactivation, V0.5 and S were −23.3±0.4 and −5.9±0.5 mV during control, −23.5±0.6 and −7.9±0.6 mV with 10 μM CLT (n=6, P=NS). (d) Recovery of Ito1 from inactivation was determined by 300-ms paired pulses from −80 to +50 mV after a 30-ms step of −40 mV (to inactivate INa) with varying P1 and P2 interval (inset). Data were fitted to bi-exponential functions. Recovery time constants (τ1 and τ2) of Ito1 were decreased by the application of 10 μM CLT (n=6, P<0.01).
Figure 3c displays voltage-dependent activation and inactivation of Ito1 evaluated in the absence and presence of 10 μM CLT with pretreatment with 10 μM verapamil. Voltage-dependent activation was determined from the I–V relationship for each cell in Figure 2B as described previously (Gao et al., 2005). Voltage-dependent inactivation was determined with the protocol as shown in the left inset (with 1-s conditioning pulses from voltages between −100 and +30 mV, followed by a 20-ms pulse to −40 mV to inactivate Na+ current and then a 300-ms test pulse to +50 mV). Data were fit to a Boltzmann distribution to obtain the half-activation or inactivation voltage (V0.5) and the slope (S). The mean data for activation and inactivation in the absence and presence of 10 μM CLT are displayed in Figure 3c. V0.5 for activation conductance of Ito1 was 10.9±1.0 mV in control, and 12.1±1.3 mV in CLT (n=6, P=NS), while S was 12.5±1.0 and 13.8±1.7 mV, respectively, for control and 10 μM CLT (P=NS). V0.5 and S of Ito1 inactivation were −23.3±0.4 and −5.9±0.5 mV during control, and −23.5±0.6 and −7.9±0.6 mV with 10 μM CLT (n=6, P=NS).
Recovery of Ito1 from inactivation was analyzed with a paired-pulse protocol, as shown in the inset of Figure 3d. The recovery curves were fitted to bi-exponential functions with the time constants τ1 and τ2 of 0.56±0.1 and 50.5±1.1 ms in control, and 4.7±0.4 and 89.2±6.9 ms after 10 μM CLT (n=6, P<0.01 vs control). The results indicate that recovery of Ito1 from inactivation was decreased by CLT.
CLT on IKur
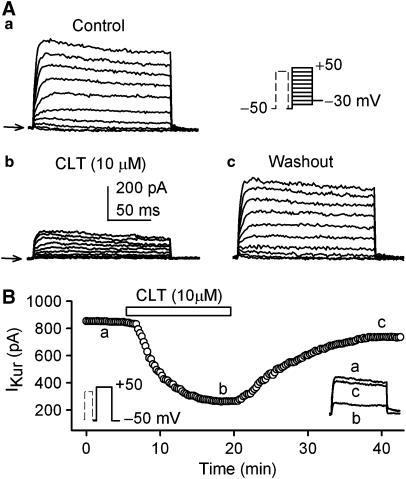
IKur was recorded using a 100-ms prepulse to +40 mV to inactivate Ito1, followed by a 150-ms test pulse from −50 to between −40 and +50 mV, then to −30 mV as described previously (Gao et al., 2004; 2005). Figure 4A illustrates voltage-dependent IKur traces recorded in a representative cell with the voltage protocol as shown in the inset in the absence and presence of CLT. CLT at 10 μM substantially decreased both IKur and tail current, and the effect was reversed by washout. Figure 4B displays the time-dependent effect of CLT on IKur recorded in a typical experiment with the voltage protocol shown in the left inset. IKur was measured from zero level to the current at the end of the voltage step. IKur was gradually decreased by CLT, and the effect partially recovered upon washout. The original IKur traces at the corresponding time points are shown in the right inset of the panel.
Figure 4.
Effect of CLT on IKur. (A) Representative voltage-dependent IKur (capacitance compensated) recorded at 0.2 Hz in a typical experiment with a 100-ms prepulse to +40 mV to inactivate Ito1, followed by 150-ms test pulses from −50 to between −40 and +50 mV after a 10-ms interval, then to −30 mV (as shown in the inset) under control conditions (a), in the presence of 10 μM CLT (b). IKur was substantially suppressed by the application of CLT, and the effect was significantly recovered by washout of the drug for 20 min (c). (B) Time-dependent effects of 10 μM CLT on IKur elicited by a 150-ms voltage step from −50 to +50 mV (as shown in the left inset) delivered every 10 s. The original IKur traces at the corresponding time points are shown in the right inset.
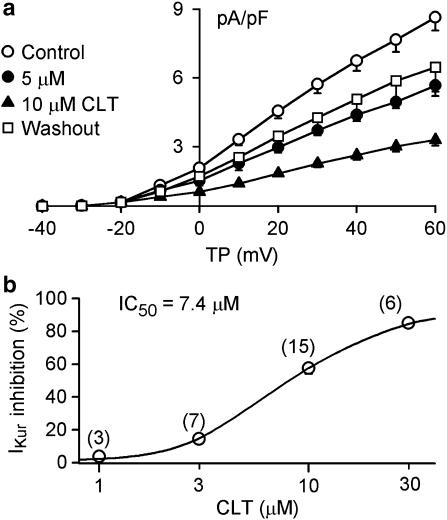
Figure 5a shows the I–V relationships of IKur in the absence and presence of 5 and 10 μM CLT. IKur was substantially inhibited by CLT. At +50 mV, IKur was inhibited by 26.5±4.9 and 57.7±3.3% with 5 and 10 μM CLT (n=10, P<0.01 vs control), respectively, and the effect partially recovered on washout. No voltage-dependent effect was observed (data not shown). Figure 5b illustrates the concentration–response relationship for inhibition of IKur by CLT at +50 mV. The IC50 was 7.4 μM with a Hill coefficient of 1.8.
Figure 5.
Concentration-dependent effect of CLT on IKur. (a) I–V relationships of IKur during control, in the presence of CLT at 5 and 10 μM, and after washout. CLT inhibited IKur in a concentration–dependent manner, and the effect was partially reversed by 77.1% upon drug washout. (b) Concentration–response relationship of IKur inhibition by CLT at +50 mV. Symbols are the mean values of inhibiting effect in cells exposed to different concentrations. Solid lines are the best-fit Hill equation. IC50 was 7.4 μM, b was 1.8, and Emax was 87.1%. The numbers in parentheses are numbers of experiments.
CLT on IhERG
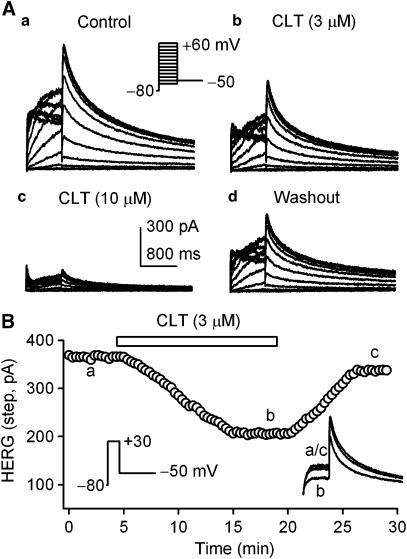
Figure 6A shows voltage-dependent IhERG traces recorded in a HEK 293 cell expressing hERG channels with 1-s voltage steps from −80 to between −60 and +60 mV, then back to −50 mV as shown in the inset at 0.1 Hz, in the absence and presence of CLT. The step IhERG (IhERG.step) and tail current (IhERG.tail) were substantially suppressed by the application of 3 and 10 μM CLT, and the effect was partially reversed by washout. Figure 6B illustrates the time-dependent effect of CLT on IhERG.step (measured from zero current to the level at the end of voltage step) recorded in a typical experiment by the voltage protocol shown in the left inset. The current was gradually suppressed by 3 μM CLT, and the effect significantly recovered upon washout. The original IhERG tracings at the corresponding time points are shown in the right inset.
Figure 6.
Effects of CLT on IhERG. (A) Voltage-dependent IhERG traces recorded at 0.1 Hz in a HEK 293 cell expressing hERG channels with 1-s voltage steps from −80 to between −60 and +60 mV, then back to −50 mV as shown (inset) under control conditions (a), in the presence of 3 (b) or 10 (c) μM CLT, and washout (d). IhERG was substantially inhibited by CLT (10 min exposure) at all voltages, and the effect partially recovered upon washout for 10 min. (B) Time-dependent effect of 3 μM CLT on the step IhERG recorded in a typical experiment with the voltage protocol shown in the left inset. The original current traces at the corresponding time points are shown in the right inset.
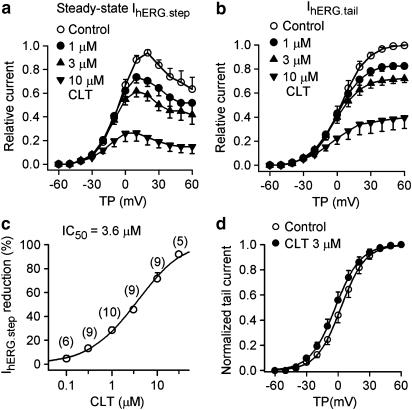
Figure 7 illustrates the concentration and voltage dependence of CLT effects on IhERG. Figure 7a and b displays the I–V relationships of relative IhERG.step and IhERG.tail in the absence and presence of 1, 3, and 10 μM CLT. The IhERG.step and IhERG.tail in the presence of CLT were relative to the maximum IhERG.step and IhERG.tail during control. Both IhERG.step and IhERG.tail were reduced with 1 and 3 μM CLT at potentials of +10 to +60 mV (n=9, P<0.01 vs control), and 10 μM CLT at −10 to +60 mV (P<0.01 vs control). Figure 7c shows the concentration–response relationship for inhibition of IhERG.step by CLT at +30 mV. The IC50 was 3.6 μM, and the Hill coefficient was 0.99. In addition, the IC50 for IhERG.tail was 6.4 μM with a Hill coefficient of 1.03.
Figure 7.
Concentration- and voltage-dependent effects of CLT on IhERG. (a) I–V relationships of step IhERG (IhERG.step) with 1, 3 and 10 μM CLT relative to the maximum current during control. (b) I–V relationships of IhERG tail (IhERG.tail) with 1, 3, and 10 μM CLT relative to the maximum current during control. (c) Concentration–response relationship of IhERG.step block by CLT at +30 mV. Symbols are mean values of inhibiting action exposed to different concentrations. Solid lines are best-fit Hill equation. For blockade of IhERG.step, IC50 was 3.6 μM, and b was 0.99, Emax was 93%. The numbers in parentheses are numbers of experiments. (d) Activation conductance of IhERG was fitted to Boltzmann distribution. V0.5 was negatively shifted with 3 μM CLT by 4.8 mV (from 2.8±1.8 to −2.0±1.7 mV, P<0.05), while S was not significantly altered (10.6±0.6 for control, and 11.2±0.5 for CLT, P=NS).
Voltage dependence of IhERG activation was determined by normalizing tail current. The activation curves were fitted to a Boltzmann distribution. V0.5 of the activation was negatively shifted by 4.8 mV (from 2.8±1.8 to −2.0±1.7 mV, P<0.05) with 3 μM CLT, while S was not significantly altered (10.6±0.6 for control and 11.2±0.5 for CLT, P=NS).
CLT on IKs
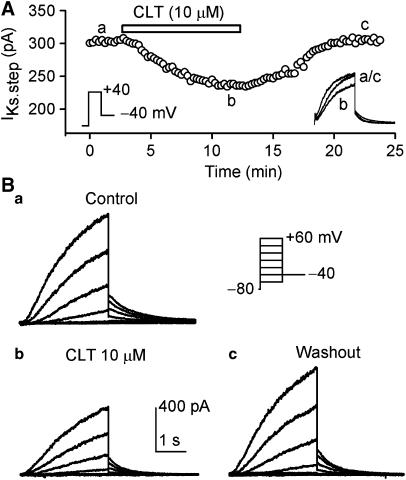
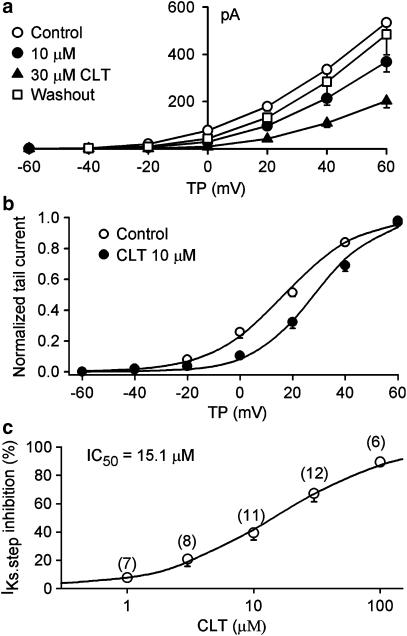
Figure 8A shows the time-dependent effect of CLT on IKs recorded with perforated configuration by a 3-s voltage step from −80 to +40 mV, then to −40 mV (left inset) in a representative HEK 293 cell stably expressing human recombinant IKs (KCNQ1/KCNE1). The voltage step-activated IKs (IKs.step) was measured from the significant activation of time-dependent current to the level at the end of depolarization step. The current was gradually decreased by 10 μM CLT, and reached a steady-state level within 8 min, and the effect recovered upon washout. The original IKs traces at the corresponding time points are shown in the right inset. Figure 8B displays voltage-dependent IKs recorded by the voltage protocol shown in the inset at 0.1 Hz (20-mV increment) in the absence and presence of CLT. IKs was substantially decreased by the application of 10 μM CLT, and the inhibitory effect was partially reversed by washout.
Figure 8.
Effects of CLT on IKs stably expressed in HEK 293 cells. (A) Time-dependent effect of 10 μM CLT on IKs-step recorded in a typical experiment with 3-s voltage step from −80 to +40 mV, then back to −40 mV as shown in the left inset. The original IKs traces at corresponding time points are shown in the right inset. (B) Voltage-dependent IKs traces recorded in a representative cell with the voltage protocol shown in the inset during control (a), in the presence of 10 μM CLT (b) and after washout (c). IKs was substantially inhibited by CLT (10 min exposure) at all voltages, and the effect recovered upon drug washout for 10 min.
Figure 9a shows the I–V relationships of IKs step current in the absence and presence of CLT. IKs was substantially inhibited at potentials of 0 to +60 mV by the application of 10 and 30 μM CLT (n=11, P<0.01 at −20 to +60 mV vs control). The effect was reversed by washout. Figure 9b displays the voltage dependence of IKs activation determined by normalizing IKs tail current in the absence and presence of CLT. V0.5 of IKs activation was positively shifted by 10.4 mV (from 19.8±1.2 mV in control to 30.2±2.3 mV in CLT, n=10, P<0.01 vs control) with 10 μM CLT, while S was not affected (14.3±0.8 mV in control and 12.1±0.9 mV in CLT, P=NS). Figure 9c illustrates the concentration–response relationship of CLT for inhibiting IKs. IC50 of CLT for IKs.step was 15.1 μM, Hill coefficient was 0.92. In addition, the measured IC50 for IKs.tail was 11.7 μM, with a coefficient of 0.9.
Figure 9.
Concentration-dependent effect of CLT on IKs. (a) I–V relationships of IKs.step under control conditions, in the presence of 10, and 30 μM CLT, and washout. CLT inhibited IKs with increasing concentration, and the effect was reversed by 77% upon washout. (b) Activation conductance of IKs in the absence and presence of 10 μM CLT was fitted to Boltzmann distribution. V0.5 of IKs activation was positively shifted by 10.4 mV with 10 μM CLT (from 19.8±1.2 mV in control to 30.2±2.3 mV in CLT, n=10, P<0.01 vs control), while S was not affected (14.3±0.8 in control and 12.1±0.9 in CLT, P=NS). (c) Concentration–response relationship of IKs-step inhibition by CLT at +40 mV. Symbols are the mean values of inhibiting effect. Solid lines are the best-fit Hill equation. IC50 was 15.1 μM, b was 0.92, and Emax was 89%. The numbers in parentheses are numbers of experiments.
Discussion
CLT is a widely used imidazole antimycotic in the treatment of patients with systemic and/or topical mycosis. The antimycotic effect is attributed to its inhibition of cytochrome P450 enzyme. CLT exerts its fungicidal effect by inhibiting fungal sterol 14α-demethylase, a microsomal cytochrome P450-dependent enzyme (Rodrigues et al., 1987; Yoshida & Aoyama, 1987). Recently, CLT was found to have therapeutic effects in sickle cell disease, and cancers (Brugnara et al., 1996; Brugnara, 2003), and has been used as a potential immunosuppressant for the treatment of autoimmune disorders, such as rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis (Jensen et al., 2002). In addition, CLT was reported to be a promising antimalarial agent suitable for clinical study (Tiffert et al., 2000). Moreover, CLT was found to prevent experimental restenosis after angioplasty (Kohler et al., 2003). These later effects are generally believed to be based on its selective blockade of the intermediate-conductance Ca2+-activated K+ channels (IKCa) in different types of cells.
CLT was also reported to inhibit other ionic channels, including Ito1 in mouse ventricular myocytes (Hernandez-Benito et al., 2001), voltage-gated K+ currents in smooth muscle cells from rabbit portal vein, and cloned Kv1.5 channel expressed in HEK 293 cells (Iftinca et al., 2001), ICa.L in guinea pig (Thomas et al., 1999), and rat (Xiao et al., 1998) ventricular myocytes. The IC50 of CLT for reducing ICa.L was 3.5 μM in rat ventricular myocytes. The present study provides additional information that CLT inhibits human cardiac repolarization K+ currents, including Ito1 and IKur in human atrial myocytes, and cloned IhERG and IKs expressed in HEK 293 cells. Taken together, the order of IC50 for different cardiac ion channel currents was ICa.L (3.5 μM)<IhERG (3.6 μM)<IKur (7.4 μM)<IKs (15.1 μM)<Ito1 (29.5 μM).
Ito1 plays an important role in human atrial repolarization. CLT significantly inhibited Ito1 in myocytes isolated from human atrium (Figures 1 and 2), and increased the inactivation of Ito1, suggesting an open-channel blocking action. CLT at 10 μM had no effect on voltage-dependent activation and inactivation of Ito1 (Figure 3). These properties are consistent with those observed in mouse ventricular myocytes (Hernandez-Benito et al., 2001). In addition, CLT significantly slowed the recovery of Ito1 from inactivation in human atrial myocytes.
IKur is reported to be present in the atrium, but not in the ventricle of human heart (Li et al., 1996), and is believed to be encoded by Kv1.5 (Feng et al., 1997). In the present study, IKur was inhibited by CLT in a concentration-dependent manner, with an IC50 of 7.4 μM in human atrial myocytes (Figure 5). The IC50 observed in the present study was higher than that observed in cloned rabbit portal vein Kv1.5 expressed in HEK cells (Kd=2 μM) (Iftinca et al., 2001), but close to that in cloned hKv1.5 expressed in HEK 293 cells (Kd=8.0 μM) (Wulff et al., 2000). However, sustained K+ current was not affected by CLT in mouse ventricular myocytes (Hernandez-Benito et al., 2001), suggesting a genuine difference between IKur/hKv1.5 and the sustained K+ current in mouse ventricular cells. The inhibition of Ito1 and IKur in human atrial myocytes may be beneficial for inhibiting supraventricular arrhythmias.
However, the present study demonstrated that CLT inhibited cloned IhERG expressed in HEK 293 cells with an IC50 of 3.6 μM, showing a close similarity to IhERG inhibition by another imadazole antimycotic (i.e. miconazole, IC50=2.1 μM) in HEK 293 cells (Kikuchi et al., 2005), and a higher sensitivity, compared with that of IhERG expressed in Xenopus oocytes by ketoconazole (IC50=49 μM) (Dumaine et al., 1998). It is well known that blockade of IKr (i.e. hERG channel) and/or IKs channels may cause long QT syndrome and Torsade de Pointe, which trigger life-threatening ventricular arrhythmias (Roden, 2004). The IC50 of CLT for blocking IhERG was 3.6 μM, which is close to clinical blood plasma concentrations required to act as antisickling agent (1–2 μM) (Brugnara et al., 1995; Rifai et al., 1995). CLT also inhibited IKs with an IC50 of 15.1 μM, close to that observed for IKs channels expressed in CHO cells (IC50=11.2 μM). Moreover, CLT shifted the activation conductance of the current to more positive potentials. These effects of CLT, at concentrations which are in the same range used to induce antiproliferative action (Benzaquen et al., 1995; Raicu et al., 2000; Smith et al., 2000) on cardiac IhERG and IKs would suggest a potential cardiac side effect; however, there are no case reports suggesting that CLT would induce QT prolongation of the ECG, although other imidazole antifungals (i.e. ketoconazole and miconazole) were reported to induce QT prolongation by blocking hERG channels (Dumaine et al., 1998; Wassmann et al., 1999; Kikuchi et al, 2005). However, since the most common use of this drug is as a topical cream for the treatment of fungal infections, systemic exposure to the agent would not be expected. Thus, for this clinical application, the effects on cardiac ion channels described herein may be irrelevant.
On the other hand, when CLT was used systematically as an antisickling agent, the blood plasma concentration of the compound would reach micromolar levels (Brugnara et al., 1995); however, it was shown to be 99% bound by plasma (Rifai et al., 1995). The 1% free CLT may not induce torsadogenic effect. In addition, CLT had an IC50 of 3.5 μM for ICa.L suppression (Xiao et al., 1998), similar to that (3.6 μM) of hERG inhibition. Such a profile is similar to the ICa.L channel blocker verapamil. Verapamil significantly blocks IhERG (Zhang et al., 1999), but has no torsadogenic effect (Redfern et al., 2003), which may be the case for CLT; however, this requires further investigation. Several potential uses of systemic administration for CLT are suggested (Tiffert et al., 2000; Jensen et al., 2002; Brugnara, 2003); further clinical studies are required to determine the efficacious dose or plasma drug level for each individual disease that needs a higher dose of CLT.
In summary, the present study provides the novel information that CLT significantly inhibits human cardiac repolarization potassium currents, including Ito1, IKur, IhERG, and IKs in a concentration-dependent manner in isolated cells. However, caution is recommended when extrapolating the observed in vitro effects on cardiac ion currents to the clinical relevance.
Acknowledgments
The study was supported in part by grants from Sun Chieh Yeh Heart Foundation, and CRCG of the University of Hong Kong. We thank Ms Haiying Sun for excellent technical assistance, Professor Tak-Ming Wong in the Department of Physiology for his substantial support, and Dr Heather J. Ballard for the critical reading of the manuscript.
Abbreviations
- hERG
human ether-á-go-go-related K+ channel
- IhERG
hERG channel current
- IKs
slowly activating delayed rectifier K+ current
- IKur
ultra-rapid delayed rectifier K+ current
- Ito1
transient outward K+ current
- IC50
the concentration for half-maximal inhibitory effect
References
- ALVAREZ J., MONTERO M., GARCIA-SANCHO J. High affinity inhibition of Ca(2+)-dependent K+ channels by cytochrome P-450 inhibitors. J. Biol. Chem. 1992;267:11789–11793. [PubMed] [Google Scholar]
- BENZAQUEN L.R., BRUGNARA C., BYERS H.R., GATTON-CELLI S., HALPERIN J.A. Clotrimazole inhibits cell proliferation in vitro and in vivo. Nat. Med. 1995;1:534–540. doi: 10.1038/nm0695-534. [DOI] [PubMed] [Google Scholar]
- BRUGNARA C. Sickle cell disease: from membrane pathophysiology to novel therapies for prevention of erythrocyte dehydration. J. Pediatr. Hematol. Oncol. 2003;25:927–933. doi: 10.1097/00043426-200312000-00004. [DOI] [PubMed] [Google Scholar]
- BRUGNARA C., ARMSBY C.C., SAKAMOTO M., RIFAI N., ALPER S.L., PLATT O. Oral administration of clotrimazole and blockade of human erythrocyte Ca(++)-activated K+ channel: the imidazole ring is not required for inhibitory activity. J. Pharmacol. Exp. Ther. 1995;273:266–272. [PubMed] [Google Scholar]
- BRUGNARA C., GEE B., ARMSBY C.C., KURTH S., SAKAMOTO M., RIFAI N., ALPER S.L., PLATT O.S. Therapy with oral clotrimazole induces inhibition of the Gardos channel and reduction of erythrocyte dehydration in patients with sickle cell disease. J Clin. Invest. 1996;97:1227–1234. doi: 10.1172/JCI118537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DU X.L., GAO Z., LAU C.P., CHIU S.W., TSE H.F., BAUMGARTEN C.M., LI G.R. Differential effects of tyrosine kinase inhibitors on volume-sensitive chloride current in human atrial myocytes: evidence for dual regulation by Src and EGFR kinases. J. Gen. Physiol. 2004;123:427–439. doi: 10.1085/jgp.200409013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMAINE R., ROY M.L., BROWN A.M. Blockade of HERG and Kv1.5 by ketoconazole. J. Pharmacol. Exp. Ther. 1998;286:727–735. [PubMed] [Google Scholar]
- FENG J., WIBLE B., LI G.R., WANG Z., NATTEL S. Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes. Circ. Res. 1997;80:572–579. doi: 10.1161/01.res.80.4.572. [DOI] [PubMed] [Google Scholar]
- GAO Z., LAU C.P., CHIU S.W., LI G.R. Inhibition of ultra-rapid delayed rectifier K+ current by verapamil in human atrial myocytes. J. Mol. Cell. Cardiol. 2004;36:257–263. doi: 10.1016/j.yjmcc.2003.11.003. [DOI] [PubMed] [Google Scholar]
- GAO Z., SUN H., CHIU S.W., LAU C.P., LI G.R. Effects of diltiazem and nifedipine on transient outward and ultra-rapid delayed rectifier potassium currents in human atrial myocytes. Br. J. Pharmacol. 2005;144:595–604. doi: 10.1038/sj.bjp.0706113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNANDEZ-BENITO M.J., MACIANSKIENE R., SIPIDO K.R., FLAMENG W., MUBAGWA K. Suppression of transient outward potassium currents in mouse ventricular myocytes by imidazole antimycotics and by glybenclamide. J. Pharmacol. Exp. Ther. 2001;298:598–606. [PubMed] [Google Scholar]
- IFTINCA M., WALDRON G.J., TRIGGLE C.R., COLE W.C. State-dependent block of rabbit vascular smooth muscle delayed rectifier and Kv1.5 channels by inhibitors of cytochrome P450-dependent enzymes. J. Pharmacol. Exp. Ther. 2001;298:718–728. [PubMed] [Google Scholar]
- JENSEN B.S., HERTZ M., CHRISTOPHERSEN P., MADSEN L.S. The Ca2+-activated K+ channel of intermediate conductance: a possible target for immune suppression. Expert Opin. Ther. Targets. 2002;6:623–636. doi: 10.1517/14728222.6.6.623. [DOI] [PubMed] [Google Scholar]
- JENSEN B.S., ODUM N., JORGENSEN N.K., CHRISTOPHERSEN P., OLESEN S.P. Inhibition of T cell proliferation by selective block of Ca(2+)-activated K(+) channels. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10917–10921. doi: 10.1073/pnas.96.19.10917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHANNA R., CHANG M.C., JOINER W.J., KACZMAREK L.K., SCHLICHTER L.C. hSK4/hIK1, a calmodulin-binding KCa channel in human T lymphocytes. Roles in proliferation and volume regulation. J. Biol. Chem. 1999;274:14838–14849. doi: 10.1074/jbc.274.21.14838. [DOI] [PubMed] [Google Scholar]
- KIKUCHI K., NAGATOMO T., ABE H., KAWAKAMI K., DUFF H.J., MAKIELSKI J.C., JANUARY C.T., NAKASHIMA Y. Blockade of HERG cardiac K+ current by antifungal drug miconazole. Br. J. Pharmacol. 2005;144:840–848. doi: 10.1038/sj.bjp.0706095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHLER R., WULFF H., EICHLER I., KNEIFEL M., NEUMANN D., KNORR A., GRGIC I., KAMPFE D., SI H., WIBAWA J., REAL R., BORNER K., BRAKEMEIER S., ORZECHOWSKI H.D., REUSCH H.P., PAUL M., CHANDY K.G., HOYER J. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation. 2003;108:1119–1125. doi: 10.1161/01.CIR.0000086464.04719.DD. [DOI] [PubMed] [Google Scholar]
- LI G.R., FENG J., YUE L., CARRIER M., NATTEL S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ. Res. 1996;78:689–696. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- RAICU M., FLOREA S., COSTACHE G., POPOV D., SIMIONESCU M. Clotrimazole inhibits smooth muscle cell proliferation and has a vasodilator effect on resistance arteries. Fundament. Clin. Pharmacol. 2000;14:477–485. doi: 10.1111/j.1472-8206.2000.tb00430.x. [DOI] [PubMed] [Google Scholar]
- REDFERN W.S., CARLSSON L., DAVIS A.S., LYNCH W.G., MACKENZIE I., PALETHORPE S., SIEGL P.K., STRANG I., SULLIVAN A.T., WALLIS R., CAMM A.J., HAMMOND T.G. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- RIFAI N., SAKAMOTO M., LAW T., PLATT O., MIKATI M., ARMSBY C.C., BRUGNARA C. HPLC measurement, blood distribution, and pharmacokinetics of oral clotrimazole, potentially useful antisickling agent. Clin. Chem. 1995;41:387–391. [PubMed] [Google Scholar]
- RODEN D.M. Drug-induced prolongation of the QT interval. N. Engl. J. Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- RODRIGUES A.D., GIBSON G.G., IOANNIDES C., PARKE D.V. Interactions of imidazole antifungal agents with purified cytochrome P-450 proteins. Biochem. Pharmacol. 1987;36:4277–4281. doi: 10.1016/0006-2952(87)90670-8. [DOI] [PubMed] [Google Scholar]
- RUFO P.A., MERLIN D., RIEGLER M., FERGUSON-MALTZMAN M.H., DICKINSON B.L., BRUGNARA C., ALPER S.L., LENCER W.I. The antifungal antibiotic, clotrimazole, inhibits chloride secretion by human intestinal T84 cells via blockade of distinct basolateral K+ conductances. Demonstration of efficacy in intact rabbit colon and in an in vivo mouse model of cholera. J. Clin. Invest. 1997;100:3111–3120. doi: 10.1172/JCI119866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH M.A., ZHANG W., NAZIRUDDIN B., COOPER J.D., PATTERSON G.A., MOHANAKUMAR T. Clotrimazole inhibits lung fibroblast proliferation in vitro: implications for use in the prevention and treatment of obliterative bronchiolitis after lung transplantation. Transplantation. 2000;70:1263–1267. doi: 10.1097/00007890-200010270-00027. [DOI] [PubMed] [Google Scholar]
- THOMAS G.P., KARMAZYN M., ZYGMUNT A.C., ANTZELEVITCH C., NARAYANAN N. The antifungal antibiotic clotrimazole potently inhibits L-type calcium current in guinea-pig ventricular myocytes. Br. J. Pharmacol. 1999;126:1531–1533. doi: 10.1038/sj.bjp.0702475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIFFERT T., GINSBURG H., KRUGLIAK M., ELFORD B.C., LEW V.L. Potent antimalarial activity of clotrimazole in in vitro cultures of Plasmodium falciparum. Proc. Natl. Acad. Sci. U.S.A. 2000;97:331–336. doi: 10.1073/pnas.97.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDORPE D.H., SHMUKLER B.E., JIANG L., LIM B., MAYLIE J., ADELMAN J.P., DE FRANCESCHI L., CAPPELLINI M.D., BRUGNARA C., ALPER S.L. cDNA cloning and functional characterization of the mouse Ca2+-gated K+ channel, mIK1. Roles in regulatory volume decrease and erythroid differentiation. J. Biol. Chem. 1998;273:21542–21553. doi: 10.1074/jbc.273.34.21542. [DOI] [PubMed] [Google Scholar]
- WASSMANN S., NICKENIG G., BOHM M. Long QT syndrome and torsade de pointes in a patient receiving fluconazole. Ann. Intern. Med. 1999;131:797. doi: 10.7326/0003-4819-131-10-199911160-00034. [DOI] [PubMed] [Google Scholar]
- WELKER S., DREWS G. Imidazole antimycotics affect the activity of various ion channels and insulin secretion in mouse pancreatic B-cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:543–550. doi: 10.1007/pl00005089. [DOI] [PubMed] [Google Scholar]
- WEUTA H. Clinical studies with oral clotrimazole. Postgrad. Med. J. 1974;50 (Suppl 1):45–48. [PubMed] [Google Scholar]
- WULFF H., MILLER M.J., HANSEL W., GRISSMER S., CAHALAN M.D., CHANDY K.G. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIAO Y.F., HUANG L., MORGAN J.P. Cytochrome P450: a novel system modulating Ca2+ channels and contraction in mammalian heart cells. J. Physiol. (London) 1998;508:777–792. doi: 10.1111/j.1469-7793.1998.777bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA Y., AOYAMA Y. Interaction of azole antifungal agents with cytochrome P-45014DM purified from Saccharomyces cerevisiae microsomes. Biochem. Pharmacol. 1987;36:229–235. doi: 10.1016/0006-2952(87)90694-0. [DOI] [PubMed] [Google Scholar]
- ZHANG S., RAJAMANI S., CHEN Y., GONG Q., RONG Y., ZHOU Z., RUOHO A., JANUARY C.T. Cocaine blocks HERG, but not KvLQT1+minK, potassium channels. Mol. Pharmacol. 2001;59:1069–1076. doi: 10.1124/mol.59.5.1069. [DOI] [PubMed] [Google Scholar]
- ZHANG S., ZHOU Z., GONG Q., MAKIELSKI J.C., JANUARY C.T. Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ. Res. 1999;84:989–998. doi: 10.1161/01.res.84.9.989. [DOI] [PubMed] [Google Scholar]