Abstract
Much attention has focused on tachykinin receptors as therapeutic targets for neuropsychiatric disorders, although their expressional distributions in the primate central nervous system (CNS) remain unclear. We cloned the genes encoding the NK-1 and NK-3 tachykinin receptors (referred to as rmNK-1 and rmNK-3) from the rhesus monkey (Macaca mulatta) brain and examined their pharmacological profiles and regional distributions in the CNS.
The deduced rmNK-1 amino-acid sequence differed by only two amino acids from the human NK-1 (hNK-1). The deduced rmNK-3 amino-acid sequence was two amino acids shorter than human NK-3 (hNK-3), with a seven-amino-acid difference in sequence.
Ligand binding studies revealed that the affinity of rmNK-1 to substance P (SP) was comparable to that of hNK-1 in cell lines that expressed individual receptors stably. Nonpeptide antagonists had similar effects on the binding of rmNK-1 and hNK-1. Affinity of rmNK-3 for NKB was stronger than for SP and the IC50 value was comparable with that of hNK-3. Ca2+ imaging showed that activations of both rmNK-1 and rmNK-3 by specific ligands, SP and senktide, induced increased intracellular Ca2+ in cell lines that stably expressed individual primate tachykinin receptors.
The amounts of rmNK-1 and rmNK-3 mRNAs were quantitatively determined in the monkey CNS. The expression of rmNK-1 was observed in all of the cortical and subcortical regions, including the hippocampus and the amygdala. The putamen contained the most NK-1 mRNA in the brain, with less rmNK-3 mRNA found in the cortex compared to rmNK-1 mRNA. In the monkey hippocampus and amygdala, rmNK-1 mRNA was present at markedly higher concentrations than rmNK-3 mRNA.
The present results provide an insight into the distinct physiological nature and significance of the NK-1 and NK-3 tachykinin systems in the primate CNS. These findings are indispensable for establishing model systems in the search for a subtype-specific tachykinin receptor agonist and antagonist for the treatment of neuropsychiatric disorders.
Keywords: Amygdala, CNS, hippocampus, neurokinin B, NK-1, NK-3, primate, quantitative PCR, substance P, tachykinin receptor
Introduction
Substance P (SP), neurokinin A (NKA) and neurokinin B (NKB) are tachykinin family peptides that share the C-terminal amino-acid sequence, Phe-X-Gly-Leu-Met-NH2. Tachykinins are widely distributed throughout the central and the peripheral nervous systems where they are believed to act as neurotransmitters or neuromodulators (Otsuka & Yoshioka, 1993). Within the brain, SP-immunoreactive cells are distributed in the amygdala, the hypothalamus, the hippocampus and the striatum in rodents (Ribeiro-da-Silva & Hökfelt, 2000) and primates (Hayashi, 1992; Jakab et al., 1996), which regions are considered to be involved in emotion, learning and memory, and motor control.
The biological action of the tachykinins is mediated through three subtypes of receptors, NK-1, NK-2 and NK-3, belonging to the G-protein-coupled receptor family. SP has a high affinity for NK-1, whereas NKA and NKB have been observed to bind preferentially to NK-2 and NK-3, respectively (Nakanishi et al., 1990; Otsuka & Yoshioka, 1993). While NK-1 and NK-3 are known to be distributed in the brains of rodents and their expression patterns have been extensively studied (Dietl & Palacios, 1991; Nakaya et al., 1994; Ding et al., 1996; Shughrue et al., 1996; Taoka et al., 1996; Mileusnic et al., 1999), fewer studies of NK-1 distribution in the primate brain have been undertaken (Parent et al., 1995; Jakab et al., 1996; Caberlotto et al., 2003). As regards NK-3, even the existence of the NK-3 receptor still remains controversial in the primate central nervous system (CNS). Autoradiographic analysis by Dietl & Palacios (1991) using eledoisin as a radioactive-ligand revealed no significant NK-3 binding in the monkey and human, whereas immunohistochemical analyses demonstrated the occurrence of NK-3 in the cortex and the hypothalamus of the human (Mileusnic et al., 1999; Koutcherov et al., 2000; Tooney et al., 2000).
Recently, it was reported that antagonists of NK-1 exhibited the antidepressant and anxiolytic activity associated with the NK-1 receptor in rodents and the human (Kramer et al., 1998; Boyce et al., 2001; Stout et al., 2001; Carrasco & Van de Kar, 2003), and the role of tachykinin systems in emotion and cognition have subsequently constituted a major focus of recent research (Longmore et al., 1997; Holmes et al., 2003). However, the development of nonpeptide NK-1 antagonists has revealed the occurrence of species differences in their affinity to NK-1 receptors, with pharmacological profiles of NK-1 in the rat and the mouse differing from those found in the guinea-pig and the human. These findings imply that limitations exist regarding the studies performed to date in rodents. Consequently, further research of tachykinin receptors in the non-human primate brain would be useful for elucidating the role of the tachykinin system in higher functions such as emotion and cognition, as well as for developing new drugs against neuropsychiatric disorders.
We therefore cloned NK-1 and NK-3 cDNAs of the rhesus monkey and investigated their pharmacological profiles as well as the distribution of their mRNAs in various regions of the brain.
Methods
Animals
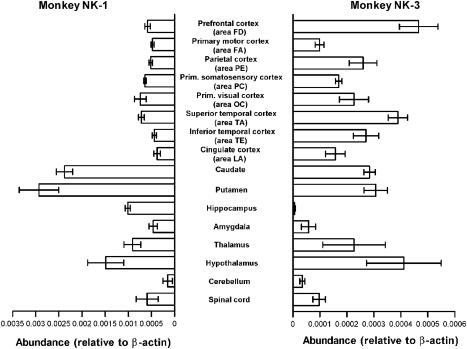
The experimental procedures used in this study were approved by the Institutional Committee on Laboratory Animals (Approval Number 15-09, Nippon Medical School; The Cooperation Research Program (Number 23), Primate Research Institute, Kyoto University) and executed in accordance with The Guide for the Care and Use of Laboratory Animals established by the NIH (1996) and The Guide for the Care and Use of Laboratory Primates established by The Primate Research Institute, Kyoto University (1986). Three 5-year-old male rhesus monkeys (Macaca mulatto) were used. All of the monkeys were pretreated with ketamine hydrochloride (10 mg kg−1 i.m.), deeply anesthetized with pentobarbital sodium (Nembutal; 25 mg kg−1 i.p.) and killed by bloodletting from the carotid artery. The dissection of the brain was performed on crushed ice by the methods described by Brown et al. (1979) and Hayashi & Oshima (1986). Cortical subdivisions were determined according to the nomenclature of von Bonin & Bailey (1947). In total, 16 regions of the CNS shown in Figure 4 were removed and stored at −80°C until use.
Figure 4.
Distribution of rmNK-1 and rmNK-3 mRNAs in the monkey CNS. Quantitative RT–PCR was used to measure transcript levels of rmNK-1 (left) and rmNK-3 (right) in 16 regions of the CNS. Tissue samples were obtained from three rhesus monkeys. Transcript levels were normalized to β-actin. Data are shown as means±s.e.m.
Molecular cloning of monkey NK-1 and NK-3 receptors
We extracted total RNA from the amygdala of a male monkey brain using ISOGEN (Nippon Gene, Japan), according to the manufacturer's instructions. In total, 10 μg of total RNA were reverse-transcribed using Superscript II reverse transcriptase (Invitrogen, U.S.A.). PCR reactions were performed with the first strand cDNA and primers based on the sequences for human NK-1 (hereafter referred to as hNK-1; Accession No. M81797) or NK-3 (hNK-3; Accession No. M89473) receptors using Ampli Taq Gold DNA polymerase (Applied Biosystems, U.S.A.) at 95°C for 10 min, 35 cycles at 95°C for 15 s, 60°C (for NK-1) or 55°C (for NK-3) for 30 s and 72°C for 30 s, and one cycle at 72°C for 10 min. The forward and reverse primer pairs were 5′-GACCTCTCCCCAAACATCTCCACTAACACC-3′ and 5′-CTAGGAGAGCACATTGGAGGAGAAGCTGAA-3′ for NK-1, and 5′-TGGCGCATCGCGCTCTGGTC-3′ and 5′-TGTTGTTTGGGACCTTCTGGC-3′ for NK-3, respectively. The expected PCR products of 1197 (NK-1) and 485 bps (NK-3) were isolated as possible partial fragments containing the monkey NK-1 (referred to hereafter as rmNK-1) and NK-3 (rmNK-3) sequences and their sequences were verified using a dye-termination cycle-sequencing-ready reaction kit (Applied Biosystems: ABI, U.S.A.) and an ABI 377 sequencer. To obtain the full-length open reading frame for rmNK-1 and rmNK-3, 3′- and 5′-rapid amplification of cDNA ends (RACE) was performed using a SMART RACE cDNA amplification kit (BD Biosciences Clontech, U.S.A.) and gene-specific primers based on the partial sequences of rmNK-1 and rmNK-3. Total RNA was extracted from the prefrontal lobe of a male monkey brain using ISOGEN and poly-A+ RNA was then purified using Oligotex-MAG (TaKaRa, Japan). First-strand cDNA was synthesized from 500 ng of poly-A+ RNA and was used for 3′- and 5′-RACEs. 3′- and 5′-RACE products were subcloned into the pGEM-T Easy vector (Promega, U.S.A.) and sequenced.
Establishment of cell lines expressing tachykinin receptors
To obtain cell lines expressing tachykinin receptors, CHO-K1 and HEK293 cells were transfected with monkey and human NK-1 or NK-3 cDNA subcloned into pcDNA3 using FuGENE6 (Roche, Germany). cDNAs of hNK-1 (Takahashi et al., 1992) and hNK-3 (Aramori et al., 1994) were gifts from Professor S. Nakanishi (Osaka Bioscience Institute, Japan) and Astellas Pharma Inc. (Japan), respectively. Cell clones that stably expressed each of the tachykinin receptors were selected and maintained in culture medium (Ham's F12 medium with 10% fetal bovine serum for CHO-K1 and DMEM and 10% horse serum for HEK293) containing 500 μg ml−1 G418 (Gibco, U.S.A.).
Radioligand binding assay
CHO-K1 cells stably expressing monkey and human NK-1 or NK-3 were seeded into 24-well plates at a cell density of 1 × 105 cells ml−1 and cultured for 48 h. Thereafter, a binding assay was carried out following a previous report (Gerard et al., 1991). Briefly, the cells were washed twice with cold wash buffer (50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 3 mM MnCl2, and 0.02% BSA) before being incubated with 0.1 nM radioligands (125I-labelled SP or 125I-labelled NKB; Perkin Elmer, U.S.A.) with unlabelled tachykinin-receptor agonists (SP, NKB; Peptide Institute, Japan) or antagonists (L733060 or WIN51708; Sigma, U.S.A.) in the same buffer containing protease inhibitors for 60 min at 4°C. The cells were then washed three times with cold wash buffer and lysed with 0.2 N NaOH before receptor-bound radioactive ligands were determined by γ-counting.
Measurement of intracellular Ca2+ mobilization
Transfected HEK293 cells were tested for mobilization of intracellular Ca2+ with 1 μM SP (Sigma, U.S.A.) for NK-1, or senktide (Calbiochem, Germany) for NK-3 receptor activation. The cells were re-plated onto poly-D-lysine-coated culture dishes (35 mm in diameter). After 24 h, the cells were stained with Fluo-3 AM cell-permeable Ca2+ indicator (Molecular Probes, U.S.A.) for 2 h and washed twice with the serum-free culture medium. SP or senktide was puff-applied (5-s duration) onto the cells through a glass pipette (tip diameter of 1–2 μm) controlled by a picospritzer (PV830, WPI, U.S.A.). Digital fluorescence imaging was performed with a Fluoview confocal microscope system (Olympus, Japan) mounted on the microscope. Fluorescence was excited using a 488 nm wavelength argon laser and emissions were detected using a 515 nm barrier filter. Fluorescence time courses were then recorded in frame scan mode (800 × 600 pixels), and images were sampled at 2 s intervals. Changes in intracellular Ca2+ concentration were expressed as percent of increase in the fluorescence intensity over resting levels (F/F0) × 100.
Quantitative analysis of NK-1 and NK-3 receptor mRNAs in the CNS
Samples of dissected brain tissues (40–350 mg) were broken up into pieces on dry ice and were ground to powder using a Cryopress (Microtech, Japan) in liquid nitrogen. Total RNA was extracted using ISOGEN as described above. Quantification of each mRNA was performed using TaqMan PCR with a GeneAmp 5700 sequence detection system (ABI). Forward and reverse primers and TaqMan probes for NK-1, NK-3, and β-actin (GenBank Accession No. AY497558) of the rhesus monkey as shown in Table 1 were designed using the Primer Express software version 2.0 (ABI). For quantification, cDNA sequences for NK-1, NK-3 or β-actin were inserted into pGEM-T easy vector, and then the plasmids were serially diluted to concentrations of 1.0 × 102–1.0 × 108 molecules reaction−1 tube for use as standards. All PCR reactions using the standards and the samples were performed in triplicate at 50°C for 2 min, 95°C for 10 min and 40 cycles at 95°C for 15 s and 60°C for 1 min.
Table 1.
List of primer sequences and TaqMan probes used for TaqMan-PCR
| Gen Bank Accession No. | Forward primer sequence | TaqMan probe sequence | Reverse primer sequence | |
|---|---|---|---|---|
| Monkey NK1 | AB180074 | TCAGACCTCTCCGCAAACATCT | ACTAACACCTCGGAACCCAATCAGTTCGTG | GACAATTTGCCAGGCTGGTT |
| Monkey NK3 | AB180075 | CCATTGCGGTGGACAGGTA | ATGGCTATTATTGATCCCCTGAAACCCAGA | TTGGTTGCTGTGGCAGACA |
| Monkey β-actin | AY497558 | ACGAGGCCCAGAGCAAGAG | CATTCTCACCCTGAAGTACCCCATCGAG | TCGTCCCAGTTGGTGACGAT |
Results
Molecular cloning of monkey NK-1 and NK-3 receptors
The deduced amino-acid sequences for the NK-1 and NK-3 receptors translated from the cloned mRNAs (GenBank Accession No. AB180074 for NK-1 and No. AB180075 for NK-3) are shown in Figure 1. Both the NK-1 and NK-3 monkey receptors were highly homologous to those found in the human, with the NK-1 receptor differing from that of the human by only two amino acids (alanine at 13 and valine at 406, Figure 1a). Two amino acids, valine at residue 116 and isoleucine at 290, both of which are thought to be important for species selectivity of the NK-1 receptor, were conserved in the human and the monkey. The C-terminus of the rmNK-3 sequence was two amino acids shorter than the human form. The NK-3 receptor of the human and the monkey were observed to differ at seven amino acids, five of which were distributed in the deduced intracellular C-terminal tail (Figure 1b).
Figure 1.
(a) Comparison of deduced amino-acid sequences of monkey and human NK-1. (b) Comparison of deduced amino-acid sequences of monkey and human NK-3. Amino-acid sequences were almost identical between the human and monkey, differing only at those amino acids indicated by boxes. Numbers in parentheses indicate amino-acid positions relative to the initiator methionine residue. Sequences were submitted to the DDBJ; monkey NK-1 (AB180074), NK-3 (AB180075).
Pharmacological profiles of rmNK-1 and rmNK-3
To investigate the pharmacological profiles of the rmNK-1 and rmNK-3 receptors, we established cell lines capable of stably expressing human and monkey NK-1 and NK-3 receptors and compared the ligand binding profiles of the two species.
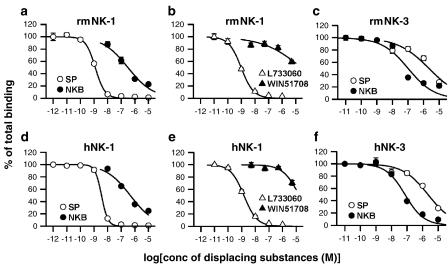
Nontransfected cells did not exhibit any detectable ligand binding. In CHO-K1 cells expressing rmNK-1, Scatchard analyses of 125I-labelled SP binding by displacement with SP and NKB indicated IC50s of 1.20±0.09 nM and 0.34±0.10 μM, respectively (Figure 2a). hNK-1 receptors also showed similar IC50s for both ligands (SP, 3.78±0.44 nM; NKB, 0.40±0.07 μM; Figure 2d). Selectivity of two NK-1 antagonists, L733060 and WIN51708, was then examined in CHO-K1 cells transfected with rmNK-1 and hNK-1; L733060 is reported to have a higher affinity for hNK-1 than for rat or mouse NK-1, while WIN51708 is thought to be selective for rat and mouse NK-1 (Stout et al., 2001). As shown in Figure 2b, rmNK-1 exhibited IC50 of 0.95±0.09 nM for L733060, which was similar to that observed in hNK-1 (1.46±0.18 nM, Figure 2e), but both rmNK-1 and hNK-1 exhibited lower affinity for WIN51708. In CHO-K1 cells expressing rmNK-3, 125I-labelled NKB binding was strongly displaced by NKB (IC50, 0.12±0.03 μM, Figure 2c), but weakly by SP (IC50, 2.45±0.66 μM, Figure 2c). These IC50 values were comparable with those obtained in CHO-K1 cells expressing hNK-3 (NKB, 75.2±7.64 nM; SP, 2.21±0.35 μM; Figure 2f).
Figure 2.
Comparison of the binding affinity of ligands to NK-1 and NK-3 receptors in the monkey and human based on the displacement of radioligands. Displacement of 125I-labelled SP by unlabelled SP or NKB in CHO-K1 cells stably expressing rmNK-1 (a) and hNK-1 (d). Displacement of 125I-SP by NK-1 antagonists L733060 or WIN51708 in the CHO-K1 ells stably expressing rmNK-1 (b) and hNK-1 (e). Displacement of 125I-labelled NKB by unlabelled NKB or SP in the CHO-K1 cells stably expressing rmNK-3 (c) and hNK-3 (f). Open circles indicate unlabelled SP, closed circles indicate unlabelled NKB, open triangles indicate L733060, closed triangles indicate WIN51708. Data from triplicate experiments are shown as means±s.e.m.
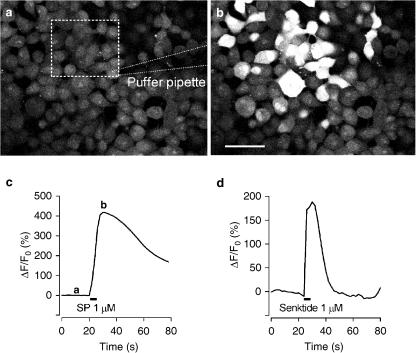
We sought to determine whether the cloned rmNK-1 and rmNK-3 receptors elicited physiological responses to individual agonists in HEK293 cell lines expressing rmNK-1 and rmNK-3 using Ca2+ imaging. The application of SP and senktide at 1 μM in cell lines transfected with rmNK-1 and rmNK-3 receptors, respectively, brought about a significant increase in intracellular Ca2+ (Figure 3).
Figure 3.
Intracellular Ca2+ mobilization induced by the NK-1 agonist, SP, or the NK-3 agonist, senktide. (a) A view of a subsample of HEK-293 cells stably expressing rmNK-1 incubated with a calcium indicator, Fluo-3 AM, before puff-application of SP. The square area surrounded by a dashed line is the region of interest (ROI). (b) The same view of (a) taken when fluorescence reached a peak just after SP application. Scale bar for (a) and (b) is 50 μm. (c) Time course of the change in fluorescence induced by SP in the ROI. The baseline was fixed relative to the fluorescent level before SP application shown in (a). (d) Time course of change in fluorescence induced by senktide in the HEK-293 cells stably expressing rmNK-3.
Distributions of rmNK-1 and rmNK-3 mRNAs in the CNS
The amounts of rmNK-1 and rmNK-3 mRNAs were quantified in 16 regions of the rhesus monkey CNS using the TaqMan PCR system (Figure 4). To normalize transcript levels, we simultaneously measured the amount of β-actin mRNA in the same samples and calculated the relative amount of each tachykinin receptor mRNA to β-actin mRNA. The expression of β-actin mRNA was similar in all of the samples (approximately 5 × 107 mRNA molecules μg−1 total RNA). Similar concentrations of rmNK-1 mRNA were found in all of the cortical regions. However, of the regions examined, expression of rmNK-1 mRNA was most apparent in the putamen. The hippocampus and the amygdala were found to contain amounts of rmNK-1 mRNA similar to those of the cortices, while rmNK-3 mRNA was expressed in the cortices at levels less than those of rmNK-1 mRNA. In the caudate and the putamen, NK-3 mRNA expression was one-tenth that of NK-1, and the expression levels of NK-3 mRNA were two and one orders of magnitude smaller than that of NK-1 mRNA in the hippocampus and the amygdala, respectively.
Discussion
Based on the findings of studies on the distribution and pathophysiological roles of the tachykinin receptors performed primarily in rodents, it is anticipated that tachykinin receptor antagonists will have several therapeutic applications involving the central and the peripheral nervous systems. However, increasing evidence suggests several differences in the central tachykinin systems of rodents and humans. Nonpeptide NK-1 antagonists clearly revealed species difference in their affinities for NK-1 receptors (Fong et al., 1992; Stout et al., 2001), with some antagonists being sensitive to rat and mouse NK-1 while others were sensitive to guinea-pig and human NK-1. In addition, even NK-1 antagonists with a high affinity for guinea-pig and human NK-1 failed to alleviate the pain state in humans in spite that animal experiments clearly showed pain-relieving effects of those drugs (Hill, 2000). Consequently, accumulating evidence on the mechanisms associated with the tachykinin receptors of a non-human primate such as the monkey may be helpful for increasing our understanding of tachykinin systems in the human.
In the present study we cloned genes of rmNK-1 and rmNK-3 from the rhesus monkey and revealed that the deduced amino-acid sequences for both receptors were, as expected, highly homologous to those of the human. The rmNK-1 receptor exhibited greater homology to hNK-1 than the rmNK-3 receptor did to the hNK-3. The amino-acid residues at 116 and 290 of the hNK-1 receptor, which have been reported to be critical in determining the ligand binding affinity for human-type NK-1 (Fong et al., 1992), were also conserved in the rmNK-1 receptor. Differences in the deduced sequences for NK-3 receptors from the monkey and the human were mostly observed in the C-terminal region. This finding suggests that the desensitization behaviour of the NK-3 receptor may differ between both primates because the C-terminus is thought to be responsible for the receptor desensitization (Nakanishi et al., 1990).
The pharmacological profiles of rmNK-1 and rmNK-3 confirmed that both receptors have functional similarities that are analogous to hNK-1 and hNK-3, respectively. The order of affinity for rmNK-1 was SP>NKB and for rmNK-3 NKB>SP, with the similar IC50s to those of the human. Binding studies using two specific NK-1 receptor antagonists also revealed the same order of affinity for rmNK-1 as for hNK-1. Regarding intracellular signalling in response to the ligands, Ca2+ imaging suggested that rmNK-1 and rmNK-3 both possibly act through G proteins to elevate cytoplasmic Ca2+ concentrations, which is consistent with results obtained for human tachykinin receptors (Aramori et al., 1994).
The amount of rmNK-1 mRNA observed throughout the cerebral cortices and the subcortical regions, including the hippocampus and the amygdala, were almost comparable. This widespread distribution of NK-1 mRNA in the monkey CNS is consistent with similar findings in NK-1 mRNA (Caberlotto et al., 2003), NK-1 immunoreactivity (Parent et al., 1995; Tooney et al., 2000) and SP binding (Dietl & Palacios, 1991) in the human. In addition, the reported distribution of the endogenous ligand seems to overlap with that of NK-1 mRNA in the present study. Intensely labelled cells positively expressing PPT-A mRNA were found in most subregions of the neocortex as well as the caudate and the putamen in the human and the monkey (Hurd et al., 1999). SP immunoreactivity was also observed in the cortex and the striatum in the monkey (Hayashi, 1992; Jakab et al., 1996). Of all regions examined, rmNK-1 mRNA was most abundant in the striatum, corroborating previous observations using positron emission tomography (Bergström et al., 2000), in situ hybridization (Caberlotto et al., 2003) and immunohistochemistry (Parent et al., 1995; Jakab et al., 1996). The intense expression of rmNK-1 supports the hypothesis that the tachykinin system is critical to physiological functions, such as locomotor activity, associated with the basal ganglia. rmNK-1 mRNA was expressed in substantial amount in the hippocampus and the amygdala, which are central to the processing of emotional information such as fear and anxiety (Maren, 2001). Consequently, in conjunction with previously published results in humans, the presence of NK-1 in the limbic system might support the rationale for developing new NK-1 antagonists as antidepressants and anxiolytics (Kramer et al., 1998; Boyce et al., 2001; Stout et al., 2001; Carrasco & Van de Kar, 2003).
Overall expression of rmNK-3 mRNA was lower than that of rmNK-1. Interestingly however, the amount of rmNK-3 mRNA in the prefrontal cortex was relatively high, implying that drugs affecting the NK-3 receptor might be promising as potential candidates for treating schizophrenia as has been postulated previously (Kamali, 2001). NK-3 mRNA and its specific radioligand binding have been observed in the rat hippocampus and amygdala (Ding et al., 1996; Shughrue et al., 1996; Saffroy et al., 2003), but the present study revealed that levels of rmNK-3 mRNA expression were surprisingly lower in the monkey hippocampus and amygdala compared to other regions. Using quantification methods similar to those employed in this study, we previously found that NK-3 mRNA was contained in a relatively large amount in the rat limbic system (data not shown). These results imply that data on the role of NK-3 in anxiety and fear obtained in studies conducted using a rodent model (Ribeiro & De Lima, 1998) should be carefully interpreted when extrapolated to primates. However, relative to in situ hybridization, the RT–PCR method adopted in this study has limitations regarding the accuracy with which it can be used to infer the subregional distribution of tachykinin receptor mRNA. Histological studies could provide further insights into the functional significance of individual subregions of the brain.
In conclusion, the pharmacological profiles and overall mRNA distribution of NK-1 and NK-3 receptors were found to be comparable between the monkey and human. Studies on tachykinin systems using monkey brain may be thus beneficial for understanding tachykinin-related behaviours in humans and contribute to the development of new therapeutic drugs for neuropsychiatric disorders.
Acknowledgments
We thank Professor Emeritus M. Otsuka (Tokyo Medical Dental University) for critical reading of the manuscript, K. Shimizu and T. Mori for sampling of the monkey brains, Professor S. Nakanishi (Osaka Bioscience Institute, Japan) for providing human NK-1 cDNA, Dr I. Aramori (Astellas Pharma Inc., Japan) for human NK-3 cDNA, Dr H. Sugihara (Department of Medicine, Nippon Medical School) for advice regarding the binding assay and Ms Y. Hyodo for administrative assistance. This study was supported by The Pharmacological Research Foundation, Tokyo (to H.S.), The Cooperation Research Program (Project Number 23) from the Primate Research Institute, Kyoto University (to H.S.), a Grant-in-Aid for Science Research (C) (Project No. 16590208) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to H.S.).
Abbreviations
- CNS
central nervous system
- NKA
neurokinin A
- NKB
neurokinin B
- PCR
polymerase chain reaction
- RACE
rapid amplification of cDNA ends
- SP
substance P
References
- ARAMORI I., MORIKAWA N., ZENKOH J, O'DONNELL N., IWAMI M., KOJO H., NOTSU Y., OKUHARA M., ONO S., NAKANISHI S. Subtype- and species-selectivity of a tachykinin receptor antagonist, FK888, for cloned rat and human tachykinin receptors. Eur. J. Pharmacol. 1994;269:277–281. doi: 10.1016/0922-4106(94)90098-1. [DOI] [PubMed] [Google Scholar]
- BERGSTRÖM M., FASTH K.-J., KILPATRICK G., WARD P., CABLE K.M., WIPPERMAN M.D., SUTHERLAND D.R., LÅNGSTRÖM B. Brain uptake and receptor binding of two [11C] labelled selective high affinity NK1-antagonists, GR203040 and GR205171 – PET studies in rhesus monkey. Neuropharmacology. 2000;39:664–670. doi: 10.1016/s0028-3908(99)00182-3. [DOI] [PubMed] [Google Scholar]
- BOYCE S., SMITH D., CARLSON E., HEWSON L., RIGBY M., O'DONNELL R., HARRISON T., RUPNIAK N.M.J. Intra-amygdala injection of the substance P (NK1 receptor) antagonist L-760735 inhibits neonatal vocalisations in guinea-pigs. Neuropharmacology. 2001;41:130–137. doi: 10.1016/s0028-3908(01)00051-x. [DOI] [PubMed] [Google Scholar]
- BROWN R.M., CRANE A.M., GOLDMAN P.S. Regional distribution of monoamines in the cerebral cortex and subcortical structures of the rhesus monkey: concentrations and in vivo synthesis rates. Brain Res. 1979;168:133–150. doi: 10.1016/0006-8993(79)90132-x. [DOI] [PubMed] [Google Scholar]
- CABERLOTTO L., HURD Y.L., MURDOCK P., WAHLIN J.P., MELOTTO S., CORSI M., CARLETTI R. Neurokinin 1 receptor and relative abundance of the short and long isoforms in the human brain. Eur. J. Neurosci. 2003;17:1736–1746. doi: 10.1046/j.1460-9568.2003.02600.x. [DOI] [PubMed] [Google Scholar]
- CARRASCO G.A., VAN DE KAR L.D. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- DIETL M.M., PALACIOS J.M. Phylogeny of tachykinin receptor localization in the vertebrate central nervous system: apparent absence of neurokinin-2 and neurokinin-3 binding sites in the human brain. Brain Res. 1991;539:211–222. doi: 10.1016/0006-8993(91)91623-9. [DOI] [PubMed] [Google Scholar]
- DING Y.-Q., SHIGEMOTO R., TAKADA M., OHISHI H., NAKANISHI S., MIZUNO N. Localization of the neuromedin K receptor (NK3) in the central nervous system of the rat. J. Comp. Neurol. 1996;364:290–310. doi: 10.1002/(SICI)1096-9861(19960108)364:2<290::AID-CNE8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- FONG T.M., YU H., STRADER C.D. Molecular basis for the species selectivity of the neurokinin-1 receptor antagonists CP-96,345 and RP67580. J. Biol. Chem. 1992;267:25668–25671. [PubMed] [Google Scholar]
- GERARD N.P., GARRAWAY L.A., EDDY R.L., JR, SHOWS T.B., IIJIMA H., PAQUET J.-L., GERARD C. Human substance P receptor (NK-1): Organization of the gene, chromosome localization, and functional expression of cDNA clones. Biochemistry. 1991;30:10640–10646. doi: 10.1021/bi00108a006. [DOI] [PubMed] [Google Scholar]
- HAYASHI M. Ontogeny of some neuropeptides in the primate brain. Prog. Neurobiol. 1992;38:231–260. doi: 10.1016/0301-0082(92)90021-6. [DOI] [PubMed] [Google Scholar]
- HAYASHI M., OSHIMA K. Neuropeptides in cerebral cortex of macaque monkey (Macaca fuscata fuscata): regional distribution and ontogeny. Brain Res. 1986;364:360–368. doi: 10.1016/0006-8993(86)90848-6. [DOI] [PubMed] [Google Scholar]
- HILL R. NK1 (substance P) receptor antagonists – why are they not analgesic in humans. Trends Pharmacol. Sci. 2000;21:244–246. doi: 10.1016/s0165-6147(00)01502-9. [DOI] [PubMed] [Google Scholar]
- HOLMES A., HEILIG M., RUPNIAK N.M.J., STECKLER T., GRIEBEL G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol. Sci. 2003;24:580–588. doi: 10.1016/j.tips.2003.09.011. [DOI] [PubMed] [Google Scholar]
- HURD Y.L., KELLER E., SOTONYI P., SEDVALL G. Preprotachykinin-A mRNA expression in the human and monkey brain: an in situ hybridization study. J. Comp. Neurol. 1999;411:56–72. doi: 10.1002/(sici)1096-9861(19990816)411:1<56::aid-cne5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- JAKAB R.L., HAZRATI L.-N., GOLDMAN-RAKIC P. Distribution and neurochemical character of substance P receptor (SPR)-immunoreactive striatal neurons of the macaque monkey: accumulation of SP fibers and SPR neurons and dendrites in ‘striocapsules' encircling striosomes. J. Comp. Neurol. 1996;369:137–149. doi: 10.1002/(SICI)1096-9861(19960520)369:1<137::AID-CNE10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- KAMALI F. Osanetant Sanofi-Synthélabo. Curr. Opin. Invest. Drugs. 2001;2:950–956. [PubMed] [Google Scholar]
- KOUTCHEROV Y., ASHWELL K.W.S., PAXINOS G. The distribution of the neurokinin B receptor in the human and rat hypothalamus. Neuroreport. 2000;11:3127–3131. doi: 10.1097/00001756-200009280-00018. [DOI] [PubMed] [Google Scholar]
- KRAMER M.S., CUTLER N., FEIGHNER J., SHRIVASTAVA R., CARMAN J., SRAMEK J.J., REINES S.A., LIU G., SNAVELY D., WYATT-KNOWLES E., HALE J.J., MILLS S.G., MACCOSS M., SWAIN C.J., HARRISON T., HILL R.G., HEFTI F., SCOLNICK E.M., CASCIERI M.A., CHICCHI G.G., SADOWSKI S., WILLIAMS A.R., HEWSON L., SMITH D., CARLSON E.J., HARGREAVES R.J., RUPNIAK N.M.J. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- LONGMORE J., HILL R.G., HARGREAVES R.J. Neurokinin-receptor antagonists: pharmacological tools and therapeutic drugs. Can. J. Physiol. Pharmacol. 1997;75:612–621. doi: 10.1139/cjpp-75-6-612. [DOI] [PubMed] [Google Scholar]
- MAREN S. Neurobiology of pavlovian fear conditioning. Annu. Rev. Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- MILEUSNIC D., LEE J.M., MAGNUSON D.J., HEJNA M.J., KRAUSE J.E., LORENS J.B., LORENS S.A. Neurokinin-3 receptor distribution in rat and human brain: an immunohistochemical study. Neuroscience. 1999;89:1269–1290. doi: 10.1016/s0306-4522(98)00349-2. [DOI] [PubMed] [Google Scholar]
- NAKANISHI S., OHKUBO H., KAKIZUKA A., YOKOTA Y., SHIGEMOTO R., SASAI Y., TAKUMI T. Molecular characterization of mammalian tachykinin receptors and a possible epithelial potassium channel. Recent Prog. Horm. Res. 1990;46:59–84. doi: 10.1016/b978-0-12-571146-3.50007-9. [DOI] [PubMed] [Google Scholar]
- NAKAYA Y., KANEKO T., SHIGEMOTO R., NAKANISHI S., MIZUNO N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J. Comp. Neurol. 1994;347:249–274. doi: 10.1002/cne.903470208. [DOI] [PubMed] [Google Scholar]
- OTSUKA M., YOSHIOKA K. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- PARENT A., CICCHETTI F., BEACH T.G. Striatal neurones displaying substance P (NK1) receptor immunoreactivity in human and non-human primates. Neuroreport. 1995;6:721–724. doi: 10.1097/00001756-199503270-00004. [DOI] [PubMed] [Google Scholar]
- RIBEIRO S.J., DE LIMA T.C.M. Naloxone-induced changes in tachykinin NK3 receptor modulation of experimental anxiety in mice. Neurosci. Lett. 1998;258:155–158. doi: 10.1016/s0304-3940(98)00880-5. [DOI] [PubMed] [Google Scholar]
- RIBEIRO-DA-SILVA A., HÖKFELT T. Neuroanatomical localisation of Substance P in the CNS and sensory neurons. Neuropeptides. 2000;34:256–271. doi: 10.1054/npep.2000.0834. [DOI] [PubMed] [Google Scholar]
- SAFFROY M., TORRENS Y., GLOWINSKI J., BEAUJOUAN J.-C. Autoradiographic distribution of tachykinin NK2 binding sites in the rat brain: comparison with NK1 and NK3 binding sites. Neuroscience. 2003;116:761–773. doi: 10.1016/s0306-4522(02)00748-0. [DOI] [PubMed] [Google Scholar]
- SHUGHRUE P.J., LANE M.V., MERCHENTHALER I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J. Comp. Neurol. 1996;372:395–414. doi: 10.1002/(SICI)1096-9861(19960826)372:3<395::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- STOUT S.C., OWENS M.J., NEMEROFF C.B. Neurokinin1 receptor antagonists as potential antidepressants. Annu. Rev. Pharmacol. Toxicol. 2001;41:877–906. doi: 10.1146/annurev.pharmtox.41.1.877. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI K., TANAKA A., HARA M., NAKANISHI S. The primary structure and gene organization of human substance P and neuromedin K receptors. Eur. J. Biochem. 1992;204:1025–1033. doi: 10.1111/j.1432-1033.1992.tb16724.x. [DOI] [PubMed] [Google Scholar]
- TAOKA M., SONG S.-Y., KUBOTA M., MINEGISHI A., YAMAKUNI T., KONISHI S. Increased level of neurokinin-1 tachykinin receptor gene expression during early postnatal development of rat brain. Neuroscience. 1996;74:845–853. doi: 10.1016/0306-4522(96)00198-4. [DOI] [PubMed] [Google Scholar]
- TOONEY P.A., AU G.G., CHAHL L.A. Tachykinin NK1 and NK3 receptors in the prefrontal cortex of the human brain. Clin. Exp. Pharmacol. Physiol. 2000;27:947–949. doi: 10.1046/j.1440-1681.2000.03367.x. [DOI] [PubMed] [Google Scholar]
- VON BONIN G, BAILEY P. The Neocortex of Macaca mulatta. Urbana, IL: The University of Illinois Press; 1947. [Google Scholar]