Abstract
Notch signaling, which is crucial to metazoan development, requires endocytosis of Notch ligands, such as Delta and Serrate. Neuralized is a plasma membrane-associated ubiquitin ligase that is required for neural development and Delta internalization. Neuralized is comprised of three domains that include a C-terminal RING domain and two neuralized homology repeat (NHR) domains. All three domains are conserved between organisms, suggesting that these regions of Neuralized are functionally important. Although the Neuralized RING domain has been shown to be required for Delta ubiquitination, the function of the NHR domains remains elusive. Here we show that neuralized1, a well-characterized neurogenic allele, exhibits a mutation in a conserved residue of the NHR1 domain that results in mislocalization of Neuralized and defects in Delta binding and internalization. Furthermore, we describe a novel isoform of Neuralized and show that it is recruited to the plasma membrane by Delta and that this is mediated by the NHR1 domain. Finally, we show that the NHR1 domain of Neuralized is both necessary and sufficient to bind Delta. Altogether, our data demonstrate that NHR domains can function in facilitating protein–protein interactions and in the case of Neuralized, mediate binding to its ubiquitination target, Delta.
INTRODUCTION
The Notch (N) signaling pathway is crucial to development in both vertebrates and invertebrates (reviewed in Justice and Jan, 2002). N signal transduction plays critical roles in processes such as lateral inhibition, boundary formation and cell lineage decisions (reviewed in Bray, 1998). In the Drosophila embryonic nervous system, the N pathway is involved in inhibiting neural cell fates (reviewed in Baker, 2000). If the N pathway is defective, then lateral inhibition fails to occur resulting in a neurogenic phenotype, specifically hypertrophy of the nervous system at the expense of nonneural tissue. Mutations in several genes have been found to give rise to this neurogenic phenotype and many of these encode key components of the N signaling pathway (Corbin et al., 1991; Portin and Rantanen, 1991; Hartenstein et al., 1992). Examples include the N receptor and its ligands Delta (Dl) and Serrate (Ser), as well as other modulators of the pathway such as Neuralized (Neur). neur is expressed in embryonic neural tissue and in regions of larval imaginal discs that will give rise to adult sense organs (Boulianne et al., 1991). Like mutations in N and Dl, mutations in neur result in embryonic lethal, neurogenic phenotypes (Lehmann et al., 1983). Mosaic analysis also indicates that neur is required for the development of the adult peripheral nervous system, including the eye and bristle sense organs (Yeh et al., 2000; Lai and Rubin, 2001a, 2001b).
neur encodes a peripheral membrane protein that exhibits E3 ubiquitin ligase activity (Yeh et al., 2000, 2001; Lai and Rubin, 2001a; Pavlopoulos et al., 2001). The Neur protein consists of three conserved domains; two neuralized homology repeat (NHR) domains and a carboxyl terminal RING domain. We have previously demonstrated that the Neur RING domain is necessary and sufficient for E3 ubiquitin ligase activity in vitro and that mutation of a conserved cysteine residue within the RING domain abolishes this function (Yeh et al., 2001). Protein ubiquitination plays an important role in regulating protein trafficking and degradation. In the case of integral membrane proteins, monoubiquitination serves as a signal for endocytosis (reviewed in Hicke and Dunn, 2003). Neur subcellular localization and its E3 ligase activity suggest that it plays a role in ubiquitination at the plasma membrane, likely targeting N signaling components for internalization.
In larval mitotic clones with reduced neur function, endocytosis of Dl is defective, resulting in reduced N signaling (Deblandre et al., 2001; Lai et al., 2001; Pavlopoulos et al., 2001). Moreover, several studies have shown that Neur binds to and ubiquitinates membrane-bound Dl, targeting it for endocytosis (Deblandre et al., 2001; Lai et al., 2001; Pavlopoulos et al., 2001; reviewed in Lai, 2002). Dl endocytosis in signal sending cells has been shown to promote N activation; however, the mechanism involved is unclear (Parks et al., 2000; Itoh et al., 2003; Wang and Struhl, 2004; Le Borgne et al., 2005a). One model suggests that Dl internalization with the N extracellular domain may unmask a N cleavage site required for signaling (Parks et al., 2000). Other models suggest that Dl endocytosis and recycling serve to activate the ligand either by clustering Dl, allowing post-translational modifications to its extracellular domain, or allowing Dl to interact with factors that increase its binding affinity for N (Hicks et al., 2002; Le Borgne and Schweisguth, 2003; Emery et al., 2005; reviewed in Chitnis, 2006a). In addition to Neur, Mind bomb, another Dl-targeting ubiquitin ligase, has been shown to play an integral role in N ligand endocytosis during development (Itoh et al., 2003; Lai et al., 2005; Le Borgne et al., 2005b; Pitsouli and Delidakis, 2005; Wang and Struhl, 2005). Liquid facets, an endocytic epsin, promotes and enhances the efficiency of Dl endocytosis and is thought to mediate Dl signaling by targeting N ligands into a select endocytic pathway (Overstreet et al., 2003, 2004; Wang and Struhl, 2004, 2005).
The conserved Neur RING domain is required for Dl internalization, but not Dl binding (Pavlopoulos et al., 2001; Pitsouli and Delidakis, 2005), suggesting that another region of Neur is mediating a protein–protein interaction with Dl. Neur exhibits two conserved NHR domains with unknown function. Proteins with NHR domains (also known as NEUZ domains) can be found in vertebrates and invertebrates, but not viruses, bacteria, fungi, or plants and include the β-catenin regulator OzzE3, Drosophila Bluestreak and Lung Inducible Neuralized-related C3HC4 RING protein (LINCR). Although the cellular role of the NHR domain is unknown, they tend to be clustered, each protein containing from two to six NHR domains (Ponting et al., 2001; Doerks et al., 2002). Partial deletion of the Neur NHR1 domain abrogates binding to Dl (Lai et al., 2001), but it is unclear whether or not the NHR domain is sufficient for the interaction to take place.
Here, we show that a point mutation in a highly conserved residue of the NHR domain results in altered Neur subcellular localization, defective Dl binding, and reduced N signaling. We also demonstrate that a novel cytoplasmic isoform of Neur is recruited to the plasma membrane by Dl and that the NHR1 domain of Neur is both necessary and sufficient to interact with Dl, indicating the NHR domain is a protein–protein interaction module. Taken together, our work demonstrates that the NHR domain is sufficient for protein–protein interactions, that mutation of this domain in Neur disrupts Dl binding, and that the function of Neur in Dl trafficking and N signaling is mediated by its NHR1 domain.
MATERIALS AND METHODS
Plasmid Construction
The pMT-DeltaWT-NdeMYC construct used in S2 cell transfections (Klueg et al., 1998) was obtained from the Drosophila Genome Resource Center (DGRC). The existence of two neur transcripts was confirmed by RT-PCR using total RNA extracted from Drosophila S2 cells and the Superscript II RT-PCR kit (Invitrogen, Burlington, Ontario, Canada). The cDNA encoding the novel isoform, NeurPC, was amplifed using a similar RT-PCR approach and cloned into the KpnI site of pBluescript. The cDNA encoding NeurPA has been previously described (Yeh et al., 2000). Using PCR, both isoform cDNAs were cloned into the KpnI and XhoI sites of pAc5.1/V5-His (Invitrogen) for constitutive expression in S2 cells. The resulting plasmids are pV5-NeurPC and pV5-NeurPA. Kozak sequences were engineered into 5′ primers.
To obtain pV5-NeurG167E, the Quikchange II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) was used, with pV5-NeurPA as a template, to introduce the G-to-A transition at the codon encoding Gly167, resulting in a Glu residue at this position.
Plasmids expressing V5-tagged Neur truncations were constructed via PCR using pV5-NeurPC as a template and were cloned into pAc5.1/V5-His. pV5-NeurNHR1 includes the portion of cDNA encoding amino acid residues 9–195, pV5-NeurNHR1-ΔF175 encodes amino acid residues 9–174, and pV5-NeurΔNHR1 encodes amino acids 173–672. Amino acid residues are arbitrarily in reference to NeurPC (GenPept NP_731310) because these regions are common to both isoforms.
To create transgenic lines UAS-NeurPC, UAS-NeurPA, and UAS-NeurG167E, both wild-type and mutant versions of V5-Neur were amplified via PCR and cloned into the KpnI site of pUAST. UAS constructs were then injected into w1118 embryos and transgenic lines obtained. Expression of all transgenes was performed at 25°C.
Drosophila Genetics
scabrousGAL4 (sca537.4) is described by FlyBase (Klaes et al., 1994). P{da-GAL4.w[-]}3 (8641), P{UAS-GFP.S65T}T2 (1521), and w1118 (3605) lines were obtained from the Bloomington Stock Center. UAS-NeurPC, UAS-NeurPA, and UAS-NeurG167E were generated in this study. All Bloomington stock numbers are indicated in parentheses. The neur1/TM3, Sb line (4222) is maintained by our laboratory and is available from the Bloomington Stock Center. Sequencing of this mutant allele was performed as previously described (Yeh et al., 2001).
Cell Culture
S2 cells were transfected using Cellfectin (Invitrogen). For every transfection, 2–3 μg of plasmid DNA was used. pMT-DeltaWT-NdeMYC expression was induced with 0.5 mM CuSO4 for 12–16 h. All assays were conducted at room temperature.
Immunostaining
S2 cells were stained using standard procedures. Briefly, cells were fixed with 3% paraformaldehyde and washed in PBS, and nonspecific interactions were blocked with 5% goat serum (diluted in 0.1% Triton and PBS [PBS-T]). Incubation with primary and secondary antibodies followed, with washes performed using PBS-T. Salivary glands, larval imaginal discs, and embryos were stained using standard procedures (Yeh et al., 2000).
All antibodies were diluted in 5% goat serum in PBS-T. Neur proteins were detected using mouse anti-V5 (Invitrogen, 1:1000). myc-Dl was detected using rabbit anti-myc (Upstate Biotechnology, Lake Placid, NY, 1:500). Endogenous Dl was detected with guinea pig anti-DlICD (Klueg et al., 1998) and was a gift of M. Muskavitch and K. Klueg (DGRC). Antibodies detecting endosomal markers were used as follows: guinea pig anti-Hrs (a gift of H. Bellen, 1:500), rabbit anti-Rab5 (a gift of M. González-Gaitán, 1:50), and rat anti-Rab11 (a gift of R. Cohen, 1:2000). Mouse anti-phosphotyrosine (BD Biosciences, San Diego, CA) was used at 1:1000. FITC-conjugated rabbit anti-HRP (Jackson ImmunoResearch, West Grove, PA) was used at 1:1000. DAPI was used at 1:5000. Cy3 and Alexa488 secondary antibodies were used at 1:1000. Samples were mounted in Dako Mounting Medium (DakoCytomation, Fort Collins, CO), and images were obtained using a Zeiss LSM510 META laser scanning confocal microscope (Zeiss, Thornwood, NY) or a Leica DMRA2 fluorescent microscope (Leica, Deerfield, IL).
Western Analysis and Coimmunoprecipitation
For Western analysis using fly tissues, adults were homogenized in RIPA buffer (1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 0.15 M NaCl, 0.01 M sodium phosphate, pH 7.2) supplemented with protease inhibitors (Roche, Indianapolis, IN). Lysates were centrifuged at 10,000 × g for 20 min and supernatants were analyzed. Neur proteins were detected using mouse anti- V5 (Invitrogen, 1:5000). β-tubulin was used as a loading control (Developmental Studies Hybridoma Bank [DSHB], 1:1000).
For coimmunoprecipitations, S2 cells were transfected with either no DNA, pMT-DeltaWT-NdeMYC alone, or pMT-DeltaWT-NdeMYC with the indicated V5-tagged Neur protein. Dl expression was induced with CuSO4 as described above. Cell lysates were made using RIPA as a lysis buffer supplemented with protease inhibitors. Lysates were precleared for 2 h and incubated for 12–16 h with protein G-Sepharose beads (Sigma, St. Louis, MO) and 1.6 μg of mouse anti-V5. Beads were then washed with lysis buffer and resuspended in standard protein sample buffer. All procedures were carried out at 4°C. For Western analysis, V5-Neur proteins were detected with mouse anti-V5 (1:5000) and coimmunoprecipitated myc-Dl was detected using rabbit anti-myc (1:1000). For analyzing experimental input, myc-Dl was detected using mouse anti-myc (DSHB, 1:30), and β-tubulin was used as a loading control (DSHB, 1:1000).
RESULTS
The Lethal, Neurogenic Allele neur1 Exhibits a Mutation in an Absolutely Conserved Glycine Residue of NHR1
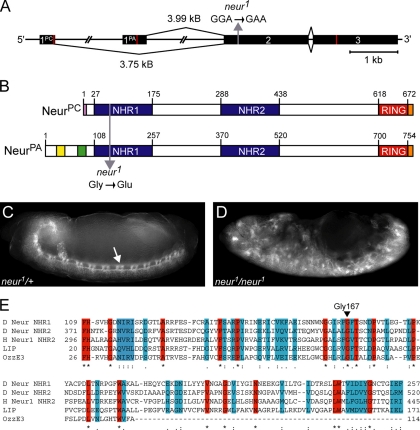
Neur is conserved from nematodes to humans and analysis of Neur proteins reveals conservation of three main regions/domains. These include the C-terminal RING domain, which we have previously shown to be required for ubiquitin ligase activity in vitro (Yeh et al., 2001) and two NHR domains of unknown function. Northern analysis indicates that neur produces two major transcripts (4 and 3.7 kb) at embryonic, larval, and adult stages of development (Boulianne et al., 1991; data not shown). The transcripts produced were initially thought to be a result of differential polyadenylation; however, the Berkley Drosophila Genome Project predicts neur ESTs upstream of the known genomic locus (Stapleton et al., 2002). We have determined that there are indeed two unique isoforms produced by neur, a result of alternative first exons (Figure 1A). The novel isoform, Neur-PC (NeurPC, GenPept NP_731310), is produced from a 3.75-kb transcript (GenBank NM_169256) and the well-characterized isoform Neur-PA (NeurPA, GenPept NP_476652) is produced from a 3.99-kb transcript (GenBank NM_057304). The sequences of these unique transcripts were confirmed by RT-PCR using total RNA isolated from Drosophila Schneider (S2) cells (data not shown). The neur transcripts only differ in their first exons, which include the translational start codons. As a result, the Neur proteins produced differ at their N-termini (Figure 1B). NeurPC is essentially an N-terminal truncation of NeurPA; the two NHR domains and the C-terminal RING domain are present in both isoforms. The unique 90 amino acid N-terminus of NeurPA contains a short glutamine/histidine-rich region (shown in yellow) and a short lysine/arginine-rich region (shown in green, Figure 1B).
Figure 1.
The neurogenic allele neur1 contains a mutation resulting in the subsitution of a conserved Gly residue of NHR1 with a Glu. (A) The neur locus produces two transcripts with unique first exons. The second exon mutation present in the neur1 allele is indicated with a gray arrow. Red lines indicate the ATG start sites and translational STOP codons. (B) The resulting protein isoforms differ at their N-termini. NHR domains (blue) and the RING domain (red) are present in both isoforms. NeurPA unique regions include the glutamine/histidine-rich region (yellow) and the lysine/arginine-rich region (green). NeurPC exhibits an eight amino acid unique region (pink). For ectopic expression V5 epitope tags are located at the carboxyl termini (orange). The G167E mutation present in neur1 is indicated with a gray arrow (residue numbering is in reference to NeurPA). (C and D) Embryos from neur1/TM3, Sb were collected and stained with FITC-conjugated anti-HRP, which labels the CNS. neur1 heterozygous embryos (C) and neur1/neur1 homozygous mutant embryos (D) are shown. The CNS of heterozygous embryos is indistinguishable from wild-type and is indicated by the arrow in C. neur1/neur1 mutant embryos exhibit a neurogenic phenotype consisting of excess neural tissue at the expense of epidermis (D). (E) A multiple sequence alignment of NHR domains reveals highly conserved residues (red) and residues with semiconserved substitutions (blue). Gly167, the amino acid residue affected in neur1, is one of the highly conserved residues and is indicated by the arrowhead. Sequence alignment was generated using ClustalW (http://www.ebi.ac.uk/clustalw/). Representative NHR domains are from Drosophila Neuralized (D Neur, GenPept NP_476652, residue numbering is in reference to NeurPA), human Neuralized-1 (H Neur1, GenPept NP_004201), mouse LINCR (LIP, GenPept NP_700457), and mouse OzzE3 (GenPept Q9D0S4).
Mutations in neur, like other neurogenic Drosophila genes such as N and Dl, were originally described as mutations causing hypertrophy of the CNS accompanied by epidermal defects (Lehmann et al., 1983). This original phenotypic analysis included the neur1 loss-of-function allele generated via ethyl methanesulfonate (EMS) mutagenesis. As expected, immunodetection using FITC-conjugated anti-HRP, which labels the surface of neurons, reveals excess neural tissue in neur1 homozygous embryos (Figure 1D) compared with neur1 heterozygous embryos, which are indistinguishable from wild type (Figure 1C, arrow indicates CNS). In addition to embryonic phenotypes, neur1 mutant clones exhibit excess sense organ precursors at the larval stage, indicating a defect in lateral inhibition and N signaling during development of the adult peripheral nervous system (Pavlopoulos et al., 2001). We have determined that this allele contains a mutation in a highly conserved residue of the NHR1 domain. Sequence analysis of the open reading frame (ORF) of the neur1 mutant allele reveals a G-to-A transition in the second exon (Figure 1A). This was the only location that the neur1 sequence differed from the wild-type ORF sequence. At the protein level, the neur1 mutation results in the substitution of Gly167 with a Glu (Figure 1B), causing a nonconservative amino acid substitution and will be referred to as NeurG167E (residue numbering is in reference to NeurPA).
Gly167 is located in the most N-terminal NHR domain (NHR1) of Neur and is conserved in all Neur protein sequences determined to date, including homologues from at least 15 different species ranging from nematodes to humans (Figure 1E, arrowhead). This conservation suggests that Gly167 is important in Neur function. In addition to being conserved in Neur homologues, Gly167 is also conserved in NHR domains from functionally unrelated proteins. The primary protein sequences of various NHR domains were compared, and a sample multiple sequence alignment (Figure 1E) reveals several highly conserved residues (shown in red), including Gly167. Sequence analysis of over 200 NHR domains, all found in eukaryotic organisms, reveals absolute conservation of Gly167, implicating this residue as important to NHR domain structure or function. Taken together, this suggests that a mutation in NHR1 abolishes Neur activity in vivo, resulting in defective N signaling and a neurogenic phenotype.
Since Neur plays a primary role during development in facilitating Dl endocytosis, it is likely that Dl trafficking is affected in neur1 mutants. Consistent with this model, others have shown that the neur1 allele exhibits defects in Dl trafficking. For example, Dl is uniformly localized at the cell membrane in neur1/neur1 mutant embryos (Morel et al., 2003), and Dl internalization is defective in neur1 mutant clones in larval eye discs and late pupal wings (Pavlopoulos et al., 2001). Therefore, we conclude that the defects in Dl trafficking and the neurogenic phenotype of the neur1 allele are a result of reduced Neur function due to a defective NHR1 domain.
The G167E Mutation in NHR1 Increases Protein Localization to HRS-positive Endosomes, at the Expense of Plasma Membrane Localization
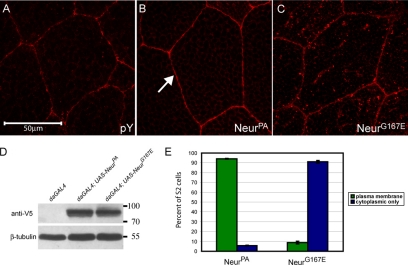
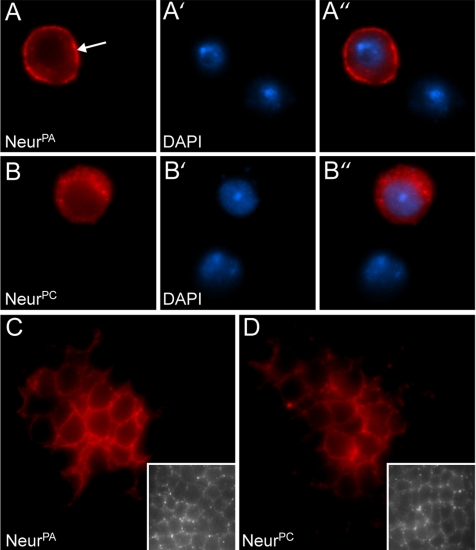
Since neurogenic embryos display vast neural and epidermal defects, we wanted to analyze the effects of the G167E mutation on Neur subcellular localization in wild-type tissue. We and others have previously reported that NeurPA exhibits predominantly plasma membrane localization when ectopically expressed in Drosophila tissues (Yeh et al., 2000; Lai and Rubin, 2001a). Because NeurPA localization has been well characterized, we focused mainly on the effects of G167E on the NeurPA isoform. To do this, we created transgenes capable of expressing either wild-type NeurPA or the mutant NeurG167E using the GAL4/UAS system (Brand and Perrimon, 1993). Proteins were C-terminally tagged with the V5 epitope, and subcellular localization was analyzed in the larval salivary gland using the scabrousGAL4 (scaGAL4) enhancer trap. We have previously shown that C-terminal epitope tags do not interfere with Neur function and can be used to rescue neurogenic phenotypes (Yeh et al., 2000). As expected, we find that V5-NeurPA localizes predominantly to the plasma membrane, with some cytoplasmic staining (Figure 2B, arrow indicates plasma membrane staining) comparable to a plasma membrane marker, anti-phosphotyrosine (Figure 2A). In contrast, V5-NeurG167E is present in many more cytoplasmic puncta than wild type (Figure 2C). In larval eye-antenna and leg discs (data not shown), embryonic neural tissue (Supplementary Figure 1, C and D) and larval wing discs (see Figure 4, C and D) NeurG167E is also predominantly localized to cytoplasmic puncta, and plasma membrane localization is reduced compared with NeurPA. To confirm that the differences seen in subcellular localization are not due to differentially expressed transgenes, we analyzed protein expression from the NeurPA and NeurG167E transgenes using the ubiquitous daughterlessGAL4 (daGAL4) driver (Figure 2D). The wild-type and mutant proteins are expressed at similar levels; moreover, because soluble fractions were analyzed, the mutant protein is not simply misfolding and forming aggregates in vivo. To quantify subcellular localization on a cell-to-cell basis we used Drosophila cell culture (Figure 2E). V5-tagged versions of NeurPA and NeurG167E were expressed in S2 cells under control of the actin promoter. Similar to in vivo, V5-NeurG167E is predominately localized to cytoplasmic puncta in S2 cells (Supplementary Figure 1, B to B″) compared with the plasma membrane localization of V5-NeurPA (Supplementary Figure 1, A to A″; plasma membrane localization is indicated by the arrow in A). However, ∼8.8% of cells expressing V5-NeurG167E exhibited some plasma membrane localization (quantified in Figure 2E). We conclude from this data that although the G167E mutation does not abolish plasma membrane localization, it is reduced, and NeurG167E favors a cytoplasmic punctate subcellular localization.
Figure 2.
NeurG167E exhibits increased localization to cytoplasmic puncta compared with wild-type NeurPA. (A) Salivary gland cells stained with anti-phosphotyrosine, a plasma membrane marker (shown in red). (B and C) Staining for V5-tagged NeurPA (B) or NeurG167E (C) in larval salivary glands (shown in red). Transgenes are expressed using scaGAL4. As expected, NeurPA exhibits predominantly plasma membrane localization as indicated by the arrow in B. NeurG167E is present in many more cytoplasmic puncta than wild type (C). (D) Western analysis of adult lysates of the genotype indicated. Neur proteins are detected using anti-V5. β-tubulin is shown as a loading control. The neur transgenes are expressed at comparable levels. (E) Quantification of subcellular localization in S2 cells. NeurPA is localized to the plasma membrane in >90% of cells. In contrast, NeurG167E is localized to the plasma membrane in <10% of cells and is predominantly cytoplasmic (>90%). For each construct, analysis was done in triplicate (n = 3) with a sample size of 100. Error bars, SE.
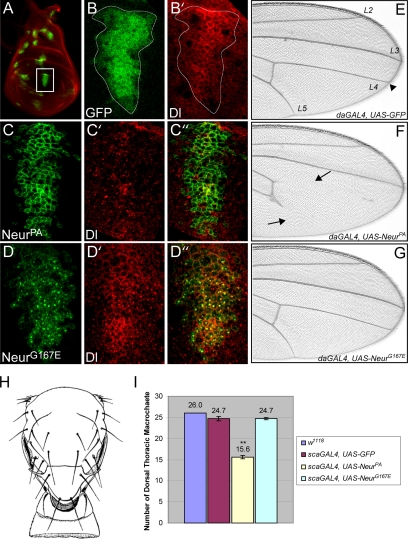
Figure 4.
A mutation in NHR1 prevents NeurG167E from increasing Dl internalization or disrupting N-dependent tissue development. (A) Expression of UAS-GFP in the wing disc (shown in green) using the scaGAL4 enhancer trap labels proneural regions. Dl staining (shown in red) is used to visualize the disc. (B and B′) A higher magnification of the presumptive wing vein tissue (white box in A). scaGAL4 expression boundaries are shown by GFP expression (shown in green, B). Dl staining is in red (B′). In this tissue, Dl is localized to the plasma membrane and in cytoplasmic puncta (B). (C and D). Same region as outlined in B when either V5-NeurPA (C) or V5-NeurG167E (D) is expressed (shown in green). Dl staining is shown in red (C′ and D′). Overlays are shown in C″ and D″. Colocalization is shown in yellow. All images were obtained from the same plane in the apical part of the wing disc. NeurPA is localized to the plasma membrane (C) and increases Dl internalization resulting in reduced levels of Dl at the plasma membrane (C′). NeurPA and Dl show little colocalization (C″). It should be noted that in other parts of the wing disc, Dl did maintain some plasma membrane localization, but was still reduced. In contrast, NeurG167E is predominantly localized to cytoplasmic puncta (D) and does not reduce plasma membrane levels of Dl (D′). NeurG167E and Dl exhibit colocalization in cytoplasmic puncta (D″). (E–G) Distal regions of adult wings expressing GFP (E), NeurPA (F), or NeurG167E (G) using the ubiquitous driver daGAL4. In wild-type wings, veins extend to the wing margin (arrowhead in E). When NeurPA is expressed, wing veins are truncated (arrows in F). In contrast, wing veins are unaffected when NeurG167E is expressed (G). (H) The adult dorsal thorax exhibits 26 large bristles, known as macrochaetes (adapted from Ferris, 1950). (I) Quantification of the number of macrocheates in flies expressing GFP, NeurPA, or NeurG167E compared with wild-type (w1118). Expression of NeurPA affects sense organ determination and results in reduced numbers of dorsal thoracic macrocheates compared with wild type and GFP controls. In contrast, NeurG167E does not alter the number of macrocheates compared with controls. Ten flies for each genotype were analyzed (n = 10). Error bars, SE. The asterisks indicate statistical significance (**p < 0.001).
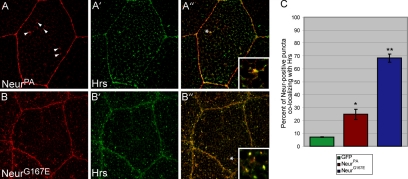
We next wanted to determine the identity of these cytoplasmic puncta using a candidate approach. Because Neur is critical to endocytic events in the N pathway, the NeurG167E mutant protein could be preferentially localized to a subset of endosomal compartments. To address this we analyzed the ability of V5-NeurPA and V5-NeurG167E to colocalize with various endocytic markers in salivary gland cells, including Rab5 (early endosomes), Rab11 (recycling endosomes), and Hrs (sorting endosomes, multivesicular body). Neither NeurPA nor NeurG167E were found to colocalize with Rab5 or Rab11 (data not shown). However, both proteins exhibited colocalization with Hrs, although at different levels. Hrs contains a FYVE domain involved in binding to phosphatidyl-inositol-3-phosphate and regulates inward budding of endosomal membranes and MVB formation (Mao et al., 2000; Lloyd et al., 2002). Although NeurPA protein is predominantly localized to the plasma membrane, some wild-type protein is found in cytoplasmic puncta (Figure 3A, arrowheads). Hrs exhibits a punctate staining in salivary gland cells (Figure 3A′). Although the majority of NeurPA-positive puncta did not colocalize with Hrs, every cell analyzed exhibited a low level of colocalization (a typical example of colocalization is shown in Figure 3A″ and inset). As a control, we analyzed the degree of colocalization between Hrs and GFP and found little colocalization (quantified in Figure 3C and data not shown), suggesting the bona fide presence of V5-NeurPA in Hrs-positive endosomes. Interestingly, the majority of NeurG167E protein is found in Hrs-positive endosomes (Figure 3, B–B″). To quantify this colocalization we analyzed the percentage of Neur-positive cytoplasmic puncta that were also Hrs-positive in salivary gland cells (Figure 3C). Approximately 24.7% of cytoplasmic puncta containing NeurPA were also positive for the endosomal marker Hrs. This was significantly more than the GFP control (p < 0.05), which exhibited only 7.1% colocalization. Puncta positive for NeurG167E were also positive for Hrs ∼68.4% of the time, in contrast to wild-type NeurPA (p < 0.001). Colocalization analysis in S2 cells yielded similar results (Supplementary Figure 2, A–A″ and B–B″), with NeurG167E colocalizing with Hrs in significantly more cells than wild type (Supplementary Figure 2C). These data show that the G167E mutation in NHR1 causes an increase in Hrs-positive subcellular localization, at the expense of plasma membrane localization. Moreover, this is not a novel phenotype because wild-type protein does colocalize with Hrs, albeit at lower levels. Taken together, this suggests that NeurG167E may have reduced function at the plasma membrane, resulting in defective Dl endocytosis.
Figure 3.
The G167E mutation increases Neur localization to Hrs-containing endosomes in vivo. (A and B) Staining for V5-tagged NeurPA (A) or NeurG167E (B) is shown in red, whereas staining for the endosomal marker Hrs (A′ and B′) is shown in green. Overlays are shown in A″ and B″ and colocalization is shown in yellow. Insets are digital magnifications of regions within each cell as indicated by the asterisks. V5-NeurPA, which is predominantly localized to the plasma membrane, exhibits a low level of cytoplasmic staining as indicated by the arrowheads in A. Some of these cytoplasmic puncta colocalize with Hrs as shown in A″. V5-NeurG167E, which exhibits many more cytoplasmic puncta than wild-type (B), also colocalizes with Hrs to a much higher degree (B″). (C) Quantification of colocalization between Hrs and GFP, NeurPA, or NeurG167E. As a control, the extent of colocalization between GFP and Hrs was determined. This low level of colocalization (∼7%) is considered baseline. NeurPA colocalizes with Hrs to a much higher extent than baseline (∼24%) and is statistically significant, indicating the bona fide presence of wild-type Neur in Hrs endosomes. NeurG167E exhibits a much higher degree of colocalization with Hrs than wild type (just below 70%). This increase is statistically significant compared with wild type. For each genotype, four cells from different salivary glands were sectioned throughout and vesicles counted (n = 4). Error bars, SE. The asterisks indicate statistical significance (*p < 0.05, **p < 0.001).
NeurG167E Colocalizes with Vesicular Dl In Vivo But Fails To Increase Dl Endocytosis or Disrupt N-dependent Tissue Development
Overexpression of wild-type Neur increases Dl internalization, resulting in a decrease in plasma membrane Dl (Lai et al., 2001; Pavlopoulos et al., 2001). To address the ability of NeurG167E to alter Dl subcellular localization, we overexpressed either NeurPA or NeurG167E in proneural regions and presumptive wing vein tissues using the scaGAL4 driver. The resulting pattern of expression in the larval wing imaginal discs can be visualized by driving UAS-GFP and is shown in Figure 4A. Because it is one of the larger patches of expression, we have focused our analysis on the region of the wing disk that will give rise to the L3 wing vein (white box in Figure 4A; outlined in Figure 4B). Endogenous Dl in this region exhibits both plasma membrane and vesicular localization (Figure 4B′). V5-NeurPA, as before, is primarily localized to the plasma membrane (Figure 4C) and reduces the amount of Dl found at the cell surface (Figure 4C′). Very little colocalization is seen (Figure 4C″). In contrast, NeurG167E exhibits predominantly cytoplasmic localization (Figure 4D) and is unable to reduce plasma membrane levels of Dl (Figure 4D′). This shows that the G167E mutation reduces Neur function at the plasma membrane, and as a consequence, alters Dl trafficking.
Interestingly, although NeurG167E and Dl do not colocalize at the plasma membrane, the vesicular form of Dl and NeurG167E show colocalization (Figure 4D″). Dl has been shown to be internalized into vesicles with the extracellular domain of N (Parks et al., 2000), and a subset of these vesicles, thought to mark an active Dl signal, are Hrs positive (Morel et al., 2003). Although NeurG167E does not function to increase Dl internalization, given its colocalization with vesicular Dl, it may be able to affect Dl signaling events after internalization. To address this, we analyzed tissues that require the N signal for their normal development. Wild-type wings exhibit a characteristic pattern of wing veins (Figure 4E) and require the N signaling pathway to specify vein and intervein tissue (Huppert et al., 1997). Induction of the N signal via overexpression of either Dl or the intracellular domain of N in the wing results in wing vein truncations (Huppert et al., 1997). Neur overexpression in the developing wing leads to wing vein abnormalities (Yeh et al., 2000; Lai and Rubin, 2001a). Specifically, ectopic expression of NeurPA in the wing results in truncations of the L4 and L5 wing veins with nearly 100% penetrance (Figure 4F). Truncations of the L2 and L3 wing veins are seen less often (data not shown). This phenotype suggests that the increase in Dl endocytosis caused by NeurPA overexpression is resulting in increased Dl signaling. In contrast, NeurG167E is unable to affect Dl signaling in the wing because overexpression of NeurG167E does not affect vein development, and wings are indistinguishable from wild type (Figure 4G).
Although the wing serves as a highly sensitive tissue to analyze N signaling, a caveat to our analysis is that neur mitotic clones in the wing give rise only to mild wing vein and margin defects (Yeh et al., 2000; Lai and Rubin, 2001a). In contrast, neur clones in the notal portion of the wing disc result in severe bristle phenotypes (Yeh et al., 2000). Additionally, neur expression is highest in the developing sense organ precursors during larval development (Boulianne et al., 1991; Yeh et al., 2000). For these reasons, we also analyzed the effects of NeurG167E on the development of the bristle sense organs on the thorax of the fly. The dorsal thorax of the adult fly exhibits 26 large mechanosensory bristles, or macrocheates, which are found in a stereotypical pattern (Figure 4H). To analyze the effects of NeurG167E in bristle formation, we quantified the number of macrocheates present on the dorsal thorax of adults. Similar to the results obtained in the wing, NeurPA overexpression increases N signaling, resulting in the reduction in the number of macrocheates due to increased inhibition of the sense organ precursor cell fate (Figure 4I). This decrease in macrochaete number was significantly different from the controls (p < 0.001), which included flies expressing GFP as a control. Ectopic expression of NeurG167E did not reduce the number of macrocheates present (Figure 4I), again suggesting that the mutant protein cannot affect Dl signaling even after internalization.
We conclude that although NeurG167E can still colocalize in intracellular vesicles with endogenous Dl, it does not affect downstream Dl signaling activity. This suggests that the two proteins are simply present in the same compartment of unknown identity and that the NHR1 mutation present in NeurG167E may be affecting the ability of Neur to bind to Dl.
The G167E Mutation in the Neur NHR1 Domain Disrupts Dl Binding
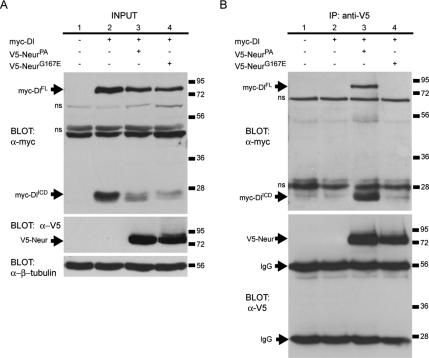
Our data thus far suggest that the G167E mutation in NHR1 may perturb Neur function in Dl trafficking by preventing protein–protein interactions. NeurPA has been shown to form a complex with Dl both in vivo and in cell culture (Lai et al., 2001; Pitsouli and Delidakis, 2005). We wanted to analyze the ability of NeurG167E to bind to Dl. To address this, we used immunoprecipitation of V5-tagged Neur proteins from S2 cells cotransfected with myc-tagged Dl and assayed the ability of Dl to coimmunoprecipitate with the various Neur proteins. In S2 cells expressing Dl, both the full-length version of the protein (DlFL) and its intracellular domain (DlICD) are detected (Figure 5A, top blot, lane 2). When Dl is coexpressed with either NeurPA or NeurG167E, both forms are present, albeit at slightly lower levels (Figure 5A, top blot, lanes 3 and 4). When Neur proteins are expressed in S2 cells, similar levels of both NeurPA and NeurG167E are observed in the input lysates (Figure 5A, middle blot, lanes 3 and 4). Additionally, the Neur proteins are also immunoprecipitated at comparable levels (Figure 5B, bottom blot, lanes 3 and 4). As expected, DlFL and DlICD are only coimmunoprecipitated in the presence of wild-type NeurPA (Figure 5B, top blot, lane 3). Note the inability of any Dl proteins to bind to NeurG167E (Figure 5B, top blot, lane 4). This data shows that the G167E mutation in NHR1 disrupts Neur binding to Dl, either directly or indirectly, and suggests that NHR1 may be crucial to the formation of this complex.
Figure 5.
The G167E mutation in the Neur NHR1 domain disrupts binding to Dl. (A) Western blots showing experimental input. Top, anti-myc labels Dl full-length (myc-DlFL) and the Dl intracellular domain (myc-DlICD) in lysates from transfected S2 cells (lanes 2–4). Lysate from untransfected cells is analyzed in lane 1. Middle, anti-V5 labels experimental input from lysates containing V5-NeurPA or V5-NeurG167E. Bottom, β-tubulin is used as a loading control. Nonspecific bands are indicated (ns). (B) Western analysis of coimmunoprecipitation assays. Top, myc-DlFL and myc-DlICD are only coimmunoprecipitated with wild-type NeurPA (lane 3). Dl does not coimmunoprecipitate in the absence of any V5-tagged Neur protein (lane 2) and does not coimmunoprecipitate with NeurG167E (lane 4). Nonspecific bands are indicated (ns). Bottom, V5-NeurPA (lane 3) and V5-NeurG167E (lane 4) are immunoprecipitated at similar levels. Anti-V5 also labels the immunoglobulin heavy and light chains (IgG) as indicated.
Dl-dependent Plasma Membrane Recruitment of Cytoplasmic Neur Is Mediated by NHR1
As mentioned earlier, the novel Neur isoform, NeurPC, differs from the well-characterized NeurPA isoform in that its N-terminus is truncated. To address whether the unique NeurPA N-terminus has a role in Neur function, we analyzed the localization of the Neur isoforms in S2 cells. A V5 epitope-tagged version of NeurPC was constructed and constitutively expressed in S2 cells, and subcellular localization was compared with V5-NeurPA. As described earlier, NeurPA is predominantly localized to the plasma membrane in S2 cells (Figure 6, A–A″; arrow in A indicates plasma membrane staining). In contrast, NeurPC, which lacks the glutamine/histidine- and lysine/arginine-rich regions found in NeurPA, exhibits staining predominantly in the cytoplasm (Figure 6, B–B″). The extent of plasma membrane localization for each isoform was quantified in S2 cells. NeurPA exhibits plasma membrane staining in 94.2 ± 0.5% of cells, whereas NeurPC does not exhibit plasma membrane localization and is present in cytoplasmic puncta in 100% of cells (n = 3, sample size = 100). Together, this demonstrates that the unique N-terminus of NeurPA is required for plasma membrane localization in S2 cells and that the novel isoform, NeurPC, exhibits cytoplasmic localization.
Figure 6.
The Neur isoforms are differentially localized in S2 cells but not in vivo. (A and B) V5-tagged NeurPA (A) and NeurPC (B) proteins were constitutively expressed in S2 cells. Cells were stained with anti-V5 (shown in red, A and B) and the nuclear marker DAPI (shown in blue, A′ and B′). Channel overlays are shown in A″ and B″. Note that NeurPA exhibits predominantly plasma membrane localization, indicated by the arrow in A. In contrast, NeurPC exhibits cytoplasmic localization (B). (C and D) V5-tagged NeurPA (C) and NeurPC (D) transgenes were expressed in proneural regions using scaGAL4. Third larval instar wing discs were stained with anti-V5 (shown in red). Embryonic localization of the Neur isoforms was also analyzed (C and D, insets). In both cases, the Neur isoforms exhibit predominantly plasma membrane localization.
To compare the subcellular localizations of NeurPC and NeurPA in Drosophila tissues, we expressed V5-tagged transgenes, similar to those used in S2 cell culture assays, via scaGAL4 in the developing embryonic neurectoderm and in proneural clusters found in larval imaginal discs, both tissues that endogenously express neur (Boulianne et al., 1991). As expected, NeurPA is localized to the plasma membrane, as demonstrated by immunostaining of the proneural region, which will give rise to the dorsocentral bristles (Figure 6C). Surprisingly, in these same wing disc regions, NeurPC, which is cytoplasmic in S2 cells, also exhibits plasma membrane localization (Figure 6D). The NeurPC and NeurPA plasma membrane localization is also observed in other developmental contexts, such as the eye-antenna and leg imaginal discs (data not shown) and in embryos (Figure 6, C and D, insets). This similarity in localization suggests that the NeurPA N-terminus is dispensable for plasma membrane localization in vivo and that a factor absent in S2 cells may be recruiting Neur to the plasma membrane in vivo.
Because Neur plays a key role in Dl endocytosis and Dl is not expressed in S2 cells, we hypothesized that Dl could be this missing factor. To address this, we performed cotransfection assays in S2 cells using V5-tagged NeurPC and myc-tagged Dl. Dl is normally localized to the plasma membrane when expressed in S2 cells (Fehon et al., 1990). On cotransfection with Dl, NeurPC, which is predominantly localized to cytoplasmic puncta in S2 cells, is recruited to the plasma membrane and colocalizes with Dl (Figure 7, B–B″, and compare Figure 7B′ to 6B). This demonstrates that the expression of Dl in S2 cells is sufficient for NeurPC plasma membrane localization. Additionally, since NeurPC lacks the N-terminus responsible for NeurPA plasma membrane localization, another region of Neur must be involved in Dl-mediated plasma membrane recruitment.
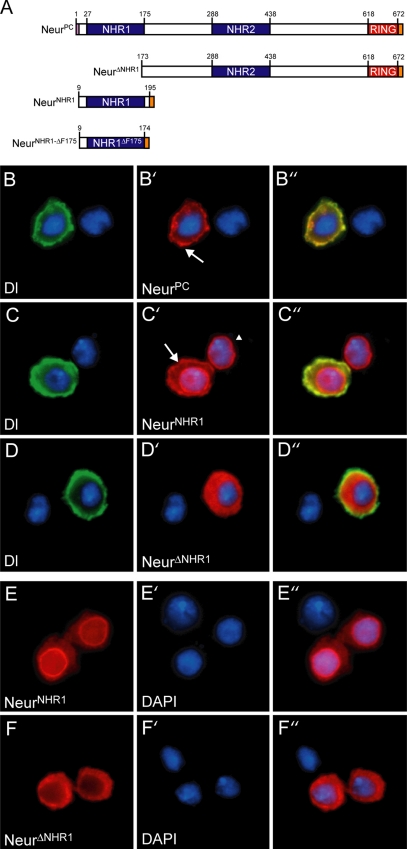
Figure 7.
Dl mediates plasma membrane recruitment of NeurPC via NHR1. (A) Various V5-tagged Neur truncations used in this study. (B, C, and D). S2 cells were cotransfected with the Neur construct indicated (shown in red, B′, C′, and D′) and myc-tagged Dl (shown in green, B, C, and D). Overlays are shown in B″, C″, and D″ and colocalization is indicated in yellow. Dl is able to recruit NeurPC to the membrane as shown by the arrow in B′. NHR1 is sufficient for plasma membrane recruitment by Dl as indicated by the arrow in C′. The cell to the right in C′ (indicated by the arrowhead) is singly transfected with NeurNHR1 and does not exhibit plasma membrane localization. Dl is unable to recruit NeurΔNHR1 to the plasma membrane (D′). (E and F) S2 cells were singly transfected with the Neur construct indicated (shown in red, E and F). Cells were stained with DAPI to visualize the nucleus (shown in blue, E′ and F′). Overlays are shown in E″ and F″. NeurNHR1 exhibits predominantly cytoplasmic and nuclear envelope localization (E) and NeurΔNHR1 is cytoplasmic (F).
To determine which region(s) of Neur is required for Dl-mediated membrane recruitment, we constructed several V5-tagged Neur deletion constructs for use in S2 cell culture (Figure 7A, note residue numbering is in reference to NeurPC). Deletion of the entire N-terminus, including NHR1 (NeurΔNHR1), results in a protein with cytoplasmic localization (Figure 7, F–F″). NeurΔNHR1 maintains its cytoplasmic distribution even in the presence of plasma membrane Dl (Figure 7, D–D″, and compare Figure 7, F″ to D′), showing that NHR1 is necessary for membrane recruitment by Dl. This suggests that either NHR1 is sufficient for membrane recruitment or that NHR1 must act together with one of the other conserved domains, such as NHR2, to mediate Dl-dependent membrane localization. To address this, we constructed a V5-tagged version of the Neur NHR1 domain (NeurNHR1). NeurNHR1 exhibits both cytoplasmic and nuclear envelope localization in S2 cells (Figure 7, E–E″). Interestingly, cotransfection with Dl results in NeurNHR1 membrane recruitment (Figure 7, C–C″, arrow), demonstrating that NHR1 is sufficient for Dl-mediated plasma membrane localization in S2 cells. The NeurNHR1 construct includes both N-terminal (residues 9–27) and C-terminal (residues 176–195) flanking residues (residue positions are in reference to NeurPC). These flanking residues are unlikely to play a role in plasma membrane recruitment for the following reasons. C-terminal flanking residues 176–195 are present in NeurΔNHR1, which exhibits cytoplasmic localization; therefore, they are not sufficient for membrane recruitment. In the case of the N-terminal flanking residues, a construct lacking the last conserved residue of the NHR1 domain, F175 (NeurNHR1ΔF175), but including residues 9–27 (Figure 7A), is not recruited to the plasma membrane by Dl (data not shown). Taken together, our data demonstrate that the NHR1 domain plays a crucial role in NeurPC plasma membrane recruitment by Dl and suggests that NHR1 may be mediating a protein–protein interaction between Neur and Dl.
The NHR1 Domain of Neur Is Necessary and Sufficient for Dl Binding
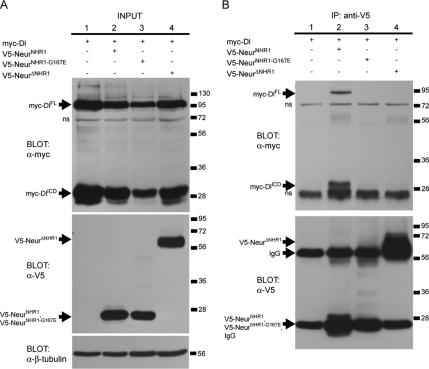
If Dl binding is mediated by the Neur NHR1 domain, then NHR1 would be expected to be both necessary and sufficient for the interaction to take place. Other groups have shown that partial deletion of NHR1 results in reduced Delta binding (Lai et al., 2001). However, it is unclear from these experiments whether NHR1 is sufficient for complex formation or whether it acts in tandem with NHR2 to mediate protein–protein interactions with Dl. To address this, we used similar coimmunoprecipitation approaches as described earlier using V5-NeurNHR1, V5-NeurΔNHR1, or V5-NeurNHR1-G167E, a V5-tagged version of the NHR1 domain exhibiting the point mutation present in the neur1 allele. S2 cells were cotransfected with one of the V5-tagged Neur proteins and myc-tagged Dl. As before, Dl is present as both DlFL and DlICD in all cases (Figure 8A, top blot, lanes 1–4). The immunoprecipitated Neur truncated proteins have molecular weights similar to the heavy and light chains of the antibody used (Figure 8B, bottom blot, lanes 2–4), but the input blot demonstrates that they are expressed at comparable levels (Figure 8A, middle blot, lanes 2–4). DlFL and DlICD are coimmunoprecipitated in the presence of V5-NeurNHR1 (Figure 8B, top blot, lane 2) showing that the NHR1 domain is sufficient for binding to Dl. Dl is not coimmunoprecipitated in the presence of the NHR1 mutant V5-NeurNHR1-G167E (Figure 8B, top blot, lane 3) or when NHR1 is deleted (Figure 8B, top blot, lane 4). Taken together, this data shows that the G167E mutation disrupts the interaction between NHR1 and Dl and that the Neur NHR1 domain is both necessary and sufficient for Dl binding.
Figure 8.
The Neur NHR1 domain is both necessary and sufficient for binding to Dl. (A) Western blots showing experimental input. Top, anti-myc detects myc-DlFL and myc-DlICD (lanes 1–4). Middle, V5-tagged Neur truncations are expressed at comparable levels and are detected by anti-V5 (lanes 2–4). Bottom, β-tubulin is used as a loading control. (B) Western analysis of coimmunoprecipitation assays. Top, myc-DlFL and myc-DlICD only coimmunoprecipitate with a wild-type NHR1 domain (lane 2). Note the absence of Dl with the immunoprecipitation of a mutated NHR1 domain (lane 3) or with a protein lacking NHR1 (lane 4). Nonspecific bands are indicated (ns). Bottom, staining with anti-V5 labels Neur proteins and IgG bands. V5-NeurΔNHR1 has a molecular weight similar to the heavy IgG band (lane 4). V5-NeurNHR1 and V5-NeurNHR1-G167E have molecular weights similar to the light IgG bands (lanes 2 and 3).
DISCUSSION
In this study we show that the NHR1 domain of Neur is both necessary and sufficient for binding to the N ligand Dl. Additionally, NHR1 is also necessary and sufficient for Dl-dependent plasma membrane localization of a cytoplasmic form of Neur. This demonstrates that the function of the NHR domain is to facilitate protein–protein interactions and, in the case of Neur, to mediate interaction with its ubiquitination target. This interaction is lost when NHR1 is mutated at a conserved residue, as in the lethal, neurogenic neur1 allele. As a result of a defective NHR1 domain, the mutant NeurG167E is unable to bind and internalize Dl and N signaling is disrupted.
Our analysis includes the identification of a novel Neur isoform in Drosophila. The two Neur isoforms, termed NeurPC and NeurPA, are a result of two transcripts that differ only in their first exons, suggesting they may be a result of developmentally regulated promoters. Northern analysis indicates that both transcripts are expressed at embryonic, larval and adult stages of development and both transcripts are expressed in S2 cells. At the protein level, NeurPC is essentially an N-terminal truncation of NeurPA, which exhibits a unique 90 amino acid N-terminus. The unique N-terminus of NeurPA includes a glutamine/histidine-rich region and a lysine/arginine-rich region, which may play a role in plasma membrane localization of NeurPA in S2 cells. These putative plasma membrane–conferring regions are absent in NeurPC and as a result, NeurPC is a cytoplasmic protein in S2 cells. Interestingly, NeurPC is recruited to the plasma membrane in S2 cells by Dl and exhibits plasma membrane localization in vivo; however, the functional relevance of this is unclear. Consistent with our analysis, membrane recruitment of NeurPC, both in vivo and in S2 cells, is defective when the G167E mutation is present (data not shown). The role of the NeurPA N-terminus in vivo and the different functions, if any, of the isoforms is an area of further analysis.
We have shown that the well-characterized neur1 allele exhibits a mutation in NHR1, altering Gly167 to a Glu. The glycine residue affected is conserved in Neur homologues and NHR domains from unrelated proteins. This conservation suggests that this residue is important to NHR function and/or structure. The G167E mutation could be altering NHR domain folding and resulting in a nonfunctional domain. Alternatively, the mutation could be altering the ability of the NHR domain to form protein–protein interactions with certain targets. We have demonstrated that the G167E mutation abolishes binding to Dl. Consistent with this, an NHR1 domain with the G167E mutation is no longer recruited to the membrane in S2 cells (data not shown). Interestingly, although interactions with Dl are disrupted, the NeurG167E protein is still able to localize to Hrs-positive endosomes. Because NeurG167E still contains a wild-type NHR2 domain, it remains possible that endosomal recruitment occurs via NHR2. However, our data show that NeurΔNHR1, a protein that includes NHR2, exhibits diffuse cytoplasmic localization, unlike the punctate endosomal localization of NeurG167E. This suggests that the G167E mutation in NHR1 may cause Neur to maintain some protein–protein interactions at the expense of other interactions, such as Dl. As a result, Dl is not internalized normally and N signaling is affected.
Recently, other Neur-binding proteins have been identified. In addition to Dl, Neur has also been shown to bind to and regulate endocytosis of Ser, another N ligand (Pitsouli and Delidakis, 2005). Although the Neur RING domain is not required for Ser binding, it is unclear whether or not either of the NHR domains is involved. In the embryo, neur activity is thought to be regulated by Bearded-related proteins such as twin of m4 (Tom; Bardin and Schweisguth, 2006; De Renzis et al., 2006; reviewed in Chitnis, 2006b). Tom was originally identified as a Neur-binding protein in a global yeast two-hybrid interaction analysis (Giot et al., 2003) and acts to antagonize Neur function in the embryo (De Renzis et al., 2006). Both Dl and Tom have been found to coimmunoprecipitate with Neur, and Tom inhibits binding between Dl and Neur (Lai et al., 2001; Bardin and Schweisguth, 2006). Interestingly, the NHR1 domain of Neur was found to be required for binding to Tom (Bardin and Schweisguth, 2006). Taken together with our analysis, this suggests that the NHR1 domain may have a role in regulating Neur activity by serving as a competitive binding site for both Dl and Tom. What remains to be elucidated is whether or not these interactions are direct. In the case of Tom, yeast two-hybrid analysis suggests that its interaction with Neur is direct (Bardin and Schweisguth, 2006). However, similar experiments with Dl, either full-length or its intracellular domain, fail to show a positive yeast two-hybrid result with Neur (C. Commisso, unpublished results). This suggests that the interaction between Neur and Dl may be indirect or that it requires a post-translational modification that does not take place in yeast.
Mind bomb (Mib) is a second RING domain-containing ubiquitin ligase that targets N ligands for internalization (Itoh et al., 2003; Lai et al., 2005; Le Borgne et al., 2005b; Pitsouli and Delidakis, 2005; Wang and Struhl, 2005). Neur and Mib can functionally replace each other, suggesting they have similar roles in N signaling (Lai et al., 2005; Le Borgne et al., 2005b; Wang and Struhl, 2005). Like Neur, Mib exhibits a unique repeated sequence in its N-terminus termed the Mib repeat that is required for Dl binding (Itoh et al., 2003; Lai et al., 2005). The Mib repeats and Neur NHR domains both seem to be important in binding to Delta and thereby serve a similar function. However, Mib repeats have very low homology to NHR domains. This is intriguing considering the functional homology between the two proteins.
Previous studies have established that nonautonomous N ligand endocytosis is required to activate N in signal-receiving cells. In addition to Neur, recent studies have also implicated the endocytic protein Epsin in N ligand endocytosis and signaling (Overstreet et al., 2004; Wang and Struhl, 2004, 2005) as well as Rab11-positive recycling endosomes (Emery et al., 2005; Jafar-Nejad et al., 2005). We did not observe colocalization between Neur and Rab11; however, some Neur-containing vesicles are Hrs-positive, a marker of the sorting endosome. Whether Neur plays a role in Dl trafficking after endocytosis is unclear; however, because Dl and NeurG167E are present in the same vesicular compartment and Dl binding is abolished, any postendocytic regulation of Dl trafficking involving Neur would likely be NHR1-dependent.
In addition to Neur, several other proteins have been shown to have NHR domains. For example, OzzE3 (the mammalian homologue of Drosophila CG3894-PA) exhibits at least two partial NHR domains and is a SOCS-box–containing E3 ubiquitin ligase that regulates β-catenin degradation during muscle development (Nastasi et al., 2004). Drosophila bluestreak (also known as CG6451) is conserved in vertebrates and encodes a protein with at least six NHR domains that is involved in oskar mRNA localization during egg development (Ruden et al., 2000). Another NHR-domain–containing protein known as LINCR is involved in the lung response to inflammation (Smith et al., 2002; Smith and Herschman, 2004; Hu et al., 2005). Although other NHR domains have not been studied in detail, our analysis suggests that they too may be important in mediating protein–protein interactions for ubiquitination targets.
By analyzing and understanding the function of NHR domains in Neur we gain insight into the relationship between Dl trafficking and N signaling. Moreover, because NHR domains are present in other proteins that play integral and diverse roles in development, we can also further understand mechanisms behind general signal transduction.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. William S. Trimble and other members of the Boulianne lab for critically reading the manuscript and for helpful discussions, especially Michael Garroni and Lara Skwarek. We also thank James Hughes and Oxana Gluscencova for expert assistance in generating fly transgenic lines. We are particularly grateful to Dr. M. Muskavitch, Dr. K. Klueg, Dr. H. Bellen, Dr. M. Gonzalez-Gaitan, Dr. R. Cohen, Dr. J. Brill, Dr. D. Rotin, the DGRC, the DSHB, and the Bloomington Stock Center for reagents. This work was supported by grants to G.L.B. from the Natural Sciences and Engineering Research Council of Canada. C.C. was supported by a Canadian Graduate Scholarship Doctoral Award administered by the Canadian Institutes of Health Research. G.L.B. is a Canada Research Chair in Molecular and Developmental Neurobiology.
Abbreviations used:
- da
daughterless
- Dl
Delta
- Hrs
hepatocyte responsive serum phosphoprotein
- N
Notch
- Neur
Neuralized
- NHR
Neuralized homology repeat
- RING
really interesting new gene
- S2
Schneider
- sca
scabrous
- Ser
Serrate.
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-08-0753 on October 25, 2006.
REFERENCES
- Baker N. E. Notch signaling in the nervous system. Pieces still missing from the puzzle. Bioessays. 2000;22:264–273. doi: 10.1002/(SICI)1521-1878(200003)22:3<264::AID-BIES8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Bardin A. J., Schweisguth F. Bearded family members inhibit Neuralized-mediated endocytosis and signaling activity of Delta in Drosophila. Dev. Cell. 2006;10:245–255. doi: 10.1016/j.devcel.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Boulianne G. L., de la Concha A., Campos-Ortega J. A., Jan L. Y., Jan Y. N. The Drosophila neurogenic gene neuralized encodes a novel protein and is expressed in precursors of larval and adult neurons. EMBO J. 1991;10:2975–2983. doi: 10.1002/j.1460-2075.1991.tb07848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Bray S. Notch signalling in Drosophila: three ways to use a pathway. Semin. Cell Dev. Biol. 1998;9:591–597. doi: 10.1006/scdb.1998.0262. [DOI] [PubMed] [Google Scholar]
- Chitnis A. Why is delta endocytosis required for effective activation of notch? Dev. Dyn. 2006a;235:886–894. doi: 10.1002/dvdy.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis A. B. Keeping single minded expression on the straight and narrow. Mol. Cell. 2006b;21:450–452. doi: 10.1016/j.molcel.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Corbin V., Michelson A. M., Abmayr S. M., Neel V., Alcamo E., Maniatis T., Young M. W. A role for the Drosophila neurogenic genes in mesoderm differentiation. Cell. 1991;67:311–323. doi: 10.1016/0092-8674(91)90183-y. [DOI] [PubMed] [Google Scholar]
- De Renzis S., Yu J., Zinzen R., Wieschaus E. Dorsal-ventral pattern of Delta trafficking is established by a Snail-Tom-Neuralized pathway. Dev. Cell. 2006;10:257–264. doi: 10.1016/j.devcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Deblandre G. A., Lai E. C., Kintner C. Xenopus neuralized is a ubiquitin ligase that interacts with XDelta1 and regulates Notch signaling. Dev. Cell. 2001;1:795–806. doi: 10.1016/s1534-5807(01)00091-0. [DOI] [PubMed] [Google Scholar]
- Doerks T., Copley R. R., Schultz J., Ponting C. P., Bork P. Systematic identification of novel protein domain families associated with nuclear functions. Genome Res. 2002;12:47–56. doi: 10.1101/gr.203201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery G., Hutterer A., Berdnik D., Mayer B., Wirtz-Peitz F., Gaitan M. G., Knoblich J. A. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Fehon R. G., Kooh P. J., Rebay I., Regan C. L., Xu T., Muskavitch M. A., Artavanis-Tsakonas S. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990;61:523–534. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- Ferris G. F. External morphology of the adult. In: Demerec M., editor. Biology of Drosophila. New York: Hafner Publishing; 1950. pp. 368–419. [Google Scholar]
- Giot L., et al. A protein interaction map of Drosophila melanogaster. Science. 2003;302:1727–1736. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- Hartenstein A. Y., Rugendorff A., Tepass U., Hartenstein V. The function of the neurogenic genes during epithelial development in the Drosophila embryo. Development. 1992;116:1203–1220. doi: 10.1242/dev.116.4.1203. [DOI] [PubMed] [Google Scholar]
- Hicke L., Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- Hicks C., Ladi E., Lindsell C., Hsieh J. J., Hayward S.D., Collazo A., Weinmaster G. A secreted Delta1-Fc fusion protein functions both as an activator and inhibitor of Notch1 signaling. J. Neurosci. Res. 2002;68:655–667. doi: 10.1002/jnr.10263. [DOI] [PubMed] [Google Scholar]
- Hu Y., Nguyen T. T., Bui K. C., Demello D. E., Smith J. B. A novel inflammation-induced ubiquitin E3 ligase in alveolar type II cells. Biochem. Biophys. Res. Commun. 2005;333:253–263. doi: 10.1016/j.bbrc.2005.05.102. [DOI] [PubMed] [Google Scholar]
- Huppert S. S., Jacobsen T. L., Muskavitch M. A. Feedback regulation is central to Delta-Notch signalling required for Drosophila wing vein morphogenesis. Development. 1997;124:3283–3291. doi: 10.1242/dev.124.17.3283. [DOI] [PubMed] [Google Scholar]
- Itoh M., et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Jafar-Nejad H., Andrews H. K., Acar M., Bayat V., Wirtz-Peitz F., Mehta S. Q., Knoblich J. A., Bellen H. J. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev. Cell. 2005;9:351–363. doi: 10.1016/j.devcel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Justice N. J., Jan Y. N. Variations on the Notch pathway in neural development. Curr. Opin. Neurobiol. 2002;12:64–70. doi: 10.1016/s0959-4388(02)00291-x. [DOI] [PubMed] [Google Scholar]
- Klaes A., Menne T., Stollewerk A., Scholz H., Klambt C. The Ets transcription factors encoded by the Drosophila gene pointed direct glial cell differentiation in the embryonic CNS. Cell. 1994;78:149–160. doi: 10.1016/0092-8674(94)90581-9. [DOI] [PubMed] [Google Scholar]
- Klueg K. M., Parody T. R., Muskavitch M. A. Complex proteolytic processing acts on Delta, a transmembrane ligand for Notch, during Drosophila development. Mol. Biol. Cell. 1998;9:1709–1723. doi: 10.1091/mbc.9.7.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E. C. Protein degradation: four E3s for the notch pathway. Curr. Biol. 2002;12:R74–R78. doi: 10.1016/s0960-9822(01)00679-0. [DOI] [PubMed] [Google Scholar]
- Lai E. C., Deblandre G. A., Kintner C., Rubin G. M. Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev. Cell. 2001;1:783–794. doi: 10.1016/s1534-5807(01)00092-2. [DOI] [PubMed] [Google Scholar]
- Lai E. C., Roegiers F., Qin X., Jan Y. N., Rubin G. M. The ubiquitin ligase Drosophila Mind bomb promotes Notch signaling by regulating the localization and activity of Serrate and Delta. Development. 2005;132:2319–2332. doi: 10.1242/dev.01825. [DOI] [PubMed] [Google Scholar]
- Lai E. C., Rubin G. M. neuralized functions cell-autonomously to regulate a subset of notch-dependent processes during adult Drosophila development. Dev. Biol. 2001a;231:217–233. doi: 10.1006/dbio.2000.0124. [DOI] [PubMed] [Google Scholar]
- Lai E. C., Rubin G. M. Neuralized is essential for a subset of Notch pathway-dependent cell fate decisions during Drosophila eye development. Proc. Natl. Acad. Sci. USA. 2001b;98:5637–5642. doi: 10.1073/pnas.101135498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R., Bardin A., Schweisguth F. The roles of receptor and ligand endocytosis in regulating Notch signaling. Development. 2005a;132:1751–1762. doi: 10.1242/dev.01789. [DOI] [PubMed] [Google Scholar]
- Le Borgne R., Remaud S., Hamel S., Schweisguth F. Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS Biol. 2005b;3:e96. doi: 10.1371/journal.pbio.0030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R., Schweisguth F. Notch signaling: endocytosis makes delta signal better. Curr. Biol. 2003;13:R273–R275. doi: 10.1016/s0960-9822(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Lehmann R., Jiménez F., Dietrich U., Campos-Ortega J. A. On the phenotype and development of mutants of early neurogenesis in Drosophila melanogaster. Roux's Arch. Dev. Biol. 1983;192:62–74. doi: 10.1007/BF00848482. [DOI] [PubMed] [Google Scholar]
- Lloyd T. E., Atkinson R., Wu M. N., Zhou Y., Pennetta G., Bellen H. J. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 2002;108:261–269. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]
- Mao Y., Nickitenko A., Duan X., Lloyd T. E., Wu M. N., Bellen H., Quiocho F. A. Crystal structure of the VHS and FYVE tandem domains of Hrs, a protein involved in membrane trafficking and signal transduction. Cell. 2000;100:447–456. doi: 10.1016/s0092-8674(00)80680-7. [DOI] [PubMed] [Google Scholar]
- Morel V., Le Borgne R., Schweisguth F. Snail is required for Delta endocytosis and Notch-dependent activation of single-minded expression. Dev. Genes Evol. 2003;213:65–72. doi: 10.1007/s00427-003-0296-x. [DOI] [PubMed] [Google Scholar]
- Nastasi T., et al. Ozz-E3, a muscle-specific ubiquitin ligase, regulates beta-catenin degradation during myogenesis. Dev. Cell. 2004;6:269–282. doi: 10.1016/s1534-5807(04)00020-6. [DOI] [PubMed] [Google Scholar]
- Overstreet E., Chen X., Wendland B., Fischer J. A. Either part of a Drosophila epsin protein, divided after the ENTH domain, functions in endocytosis of delta in the developing eye. Curr. Biol. 2003;13:854–860. doi: 10.1016/s0960-9822(03)00326-9. [DOI] [PubMed] [Google Scholar]
- Overstreet E., Fitch E., Fischer J. A. Fat facets and Liquid facets promote Delta endocytosis and Delta signaling in the signaling cells. Development. 2004;131:5355–5366. doi: 10.1242/dev.01434. [DOI] [PubMed] [Google Scholar]
- Parks A. L., Klueg K. M., Stout J. R., Muskavitch M. A. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;127:1373–1385. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- Pavlopoulos E., Pitsouli C., Klueg K. M., Muskavitch M. A., Moschonas N. K., Delidakis C. neuralized encodes a peripheral membrane protein involved in delta signaling and endocytosis. Dev. Cell. 2001;1:807–816. doi: 10.1016/s1534-5807(01)00093-4. [DOI] [PubMed] [Google Scholar]
- Pitsouli C., Delidakis C. The interplay between DSL proteins and ubiquitin ligases in Notch signaling. Development. 2005;132:4041–4050. doi: 10.1242/dev.01979. [DOI] [PubMed] [Google Scholar]
- Ponting C. P., Mott R., Bork P., Copley R. R. Novel protein domains and repeats in Drosophila melanogaster: insights into structure, function, and evolution. Genome Res. 2001;11:1996–2008. doi: 10.1101/gr.198701. [DOI] [PubMed] [Google Scholar]
- Portin P., Rantanen M. Interaction of master mind, big brain, neuralized and Notch genes of Drosophila melanogaster as expressed in adult morphology. Hereditas. 1991;114:197–200. doi: 10.1111/j.1601-5223.1991.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Ruden D. M., Sollars V., Wang X., Mori D., Alterman M., Lu X. Membrane fusion proteins are required for oskar mRNA localization in the Drosophila egg chamber. Dev. Biol. 2000;218:314–325. doi: 10.1006/dbio.1999.9583. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Herschman H. R. Targeted identification of glucocorticoid-attenuated response genes: in vitro and in vivo models. Proc. Am. Thorac. Soc. 2004;1:275–281. doi: 10.1513/pats.200402-017MS. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Nguyen T. T., Hughes H. J., Herschman H. R., Widney D. P., Bui K. C., Rovai L. E. Glucocorticoid-attenuated response genes induced in the lung during endotoxemia. Am. J. Physiol. Lung. Cell Mol. Physiol. 2002;283:L636–L647. doi: 10.1152/ajplung.00496.2001. [DOI] [PubMed] [Google Scholar]
- Stapleton M., et al. A Drosophila full-length cDNA resource. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-12-research0080. RESEARCH0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Struhl G. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development. 2004;131:5367–5380. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- Wang W., Struhl G. Distinct roles for Mind bomb, Neuralized and Epsin in mediating DSL endocytosis and signaling in Drosophila. Development. 2005;132:2883–2894. doi: 10.1242/dev.01860. [DOI] [PubMed] [Google Scholar]
- Yeh E., Dermer M., Commisso C., Zhou L., McGlade C. J., Boulianne G. L. Neuralized functions as an E3 ubiquitin ligase during Drosophila development. Curr. Biol. 2001;11:1675–1679. doi: 10.1016/s0960-9822(01)00527-9. [DOI] [PubMed] [Google Scholar]
- Yeh E., Zhou L., Rudzik N., Boulianne G. L. Neuralized functions cell autonomously to regulate Drosophila sense organ development. EMBO J. 2000;19:4827–4837. doi: 10.1093/emboj/19.17.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.