Abstract
The mechanism of protein quality control and elimination of misfolded proteins in the cytoplasm is poorly understood. We studied the involvement of cytoplasmic factors required for degradation of two endoplasmic reticulum (ER)-import–defective mutated derivatives of carboxypeptidase yscY (ΔssCPY* and ΔssCPY*-GFP) and also examined the requirements for degradation of the corresponding wild-type enzyme made ER-import incompetent by removal of its signal sequence (ΔssCPY). All these protein species are rapidly degraded via the ubiquitin–proteasome system. Degradation requires the ubiquitin-conjugating enzymes Ubc4p and Ubc5p, the cytoplasmic Hsp70 Ssa chaperone machinery, and the Hsp70 cochaperone Ydj1p. Neither the Hsp90 chaperones nor Hsp104 or the small heat-shock proteins Hsp26 and Hsp42 are involved in the degradation process. Elimination of a GFP fusion (GFP-cODC), containing the C-terminal 37 amino acids of ornithine decarboxylase (cODC) directing this enzyme to the proteasome, is independent of Ssa1p function. Fusion of ΔssCPY* to GFP-cODC to form ΔssCPY*-GFP-cODC reimposes a dependency on the Ssa1p chaperone for degradation. Evidently, the misfolded protein domain dictates the route of protein elimination. These data and our further results give evidence that the Ssa1p-Ydj1p machinery recognizes misfolded protein domains, keeps misfolded proteins soluble, solubilizes precipitated protein material, and escorts and delivers misfolded proteins in the ubiquitinated state to the proteasome for degradation.
INTRODUCTION
Newly synthesized proteins must fold into their native three-dimensional structures and maintain this state throughout their lifetime. Molecular chaperones facilitate the initial folding of proteins to their native form, as well as the assembly of multiprotein complexes. Translocation of proteins into the endoplasmic reticulum (ER) or into mitochondria and their folding also relies on molecular chaperones associated with these cellular compartments (Caplan et al., 1992; Parsell and Lindquist, 1993; Hartl, 1996; Frydman, 2001; Hartl and Hayer-Hartl, 2002; Anken et al., 2005; Mayer and Bukau, 2005). Molecular chaperones are involved not only in the folding of proteins but also in their quality control. This includes recognition of misfolding, prevention of protein aggregation, and facilitation of refolding of partially unfolded proteins due to stresses (Goldberg, 2003; Kleizen and Braakman, 2004). Terminally misfolded proteins have to be recognized and eliminated. This process is essential to all cells. Misfolding leads to the exposure of hydrophobic patches in proteins, which may cause their aggregation in the aqueous cellular environment. This may result in the formation of toxic protein precipitates, which are associated with severe diseases such as Alzheimer's, Parkinson, or Creutzfeldt-Jakob disease in humans or bovine spongiform encephalopathy (BSE) in cattle (Kopito, 2000; Dobson, 2003; Goldberg, 2003; Barral et al., 2004).
Selective protein degradation via the ubiquitin–proteasome system is a major pathway conserved throughout eukaryotic evolution (Hochstrasser, 1996; Varshavsky, 1997; Hershko and Ciechanover, 1998; Wolf and Hilt, 2004). Ubiquitination of proteins is mediated by three consecutive reactions: ubiquitin activation via an E1 enzyme, ubiquitin conjugation via E2 enzymes, and the action of ubiquitin protein ligases, E3's, which mediate the selection of substrate and initiate its ubiquitination. Quality control and degradation of secretory proteins (ERQD) as well as of cytoplasmic proteins is under intensive study (Plemper et al., 1997; Sommer and Wolf, 1997; Brodsky and McCracken, 1999; Kostova and Wolf, 2003; Hirsch et al., 2004; McClellan et al., 2005b; Schafer and Wolf, 2005; Bukau et al., 2006). Cytoplasmic degradation is pertinent not only to proteins native to the cytoplasm, but also to secretory proteins that fail to fold properly. Misfolded secretory proteins are recognized in the ER, prevented from continuing along the secretory pathway, retrotranslocated to the cytoplasmic side of the ER, polyubiquitinated, and delivered to the proteasome for degradation. This mechanism for delivering misfolded ER proteins to the proteasome makes use of a trimeric AAA-ATPase complex consisting of Cdc48p-Ufd1p-Npl4p and of two UBA-UBL-domain proteins, Dsk2p and Rad23p, which are able to dock to the proteasome (Hartmann-Petersen and Gordon, 2004; Elsasser and Finley, 2005). Such a mechanism ensures that misfolded soluble or membrane bound secretory proteins are not released into the cytoplasm, where aggregation would occur, but are escorted instead in a protein bound form to the proteasome for elimination (Medicherla et al., 2004).
This broadly accepted view was in part inferred from experiments using the misfolded ER-lumenal model substrate CPY* (Finger et al., 1993; Hiller et al., 1996; Schafer and Wolf, 2006). Studies of CPY* processing and degradation have been more recently extended to two of its membrane bound derivatives, CT* and CTG*, carrying the ER lumenal CPY* module, a transmembrane domain (CT*), or, in addition, the green fluorescent protein GFP (CTG*) (Taxis et al., 2003). During our studies on the delivery mechanism of these misfolded ER model substrates to the proteasome, we also studied the degradation requirements of the cytoplasmically located CPY* derivative ΔssCPY*-GFP. This protein lacks a signal sequence directing it to the ER. Thus, in contrast to ER lumen misfolded proteins like CPY*, which makes a round trip from cytoplasm to ER and back, ΔssCPY*-GFP is made and remains in the cytoplasm. ΔssCPY*-GFP was also rapidly degraded via the proteasome but did not require the Cdc48p-Ufd1p-Npl4p AAA-ATPase complex nor the UBA-UBL proteins Dsk2p and Rad23p (Medicherla et al., 2004). This pointed to a completely different recognition and delivery mechanism for this misfolded ER import defective secretory protein. Recently it has been found in mammalian cells that the efficiency of protein compartmentalization into the secretory pathway is far from perfect. Because of inefficient signal sequence recognition, inefficient translocation into the ER, and leaky ribosomal scanning, the efficiency of segregation to the ER was shown to vary considerably (Levine et al., 2005). This raises the question of the fate of these remnant proteins mislocalized to the cytoplasm. It was the aim of this study to unravel the agents that recognize misfolded cytoplasmically located proteins and deliver them to the proteasome.
MATERIALS AND METHODS
Yeast Strains and Plasmids
Yeast strains used in this study are summarized in Table 1. All other methods for yeast manipulation and genetic experiments were carried out using standard methods (Guthrie and Fink, 1991; Ausubel et al., 1992). The SNL1 gene in W303–1C was disrupted by PCR amplification of the Δsnl1::KANR fragment from strain BY4743 (EUROSCARF, Frankfurt, Germany) using the primer pairs SNL1 5′ Primer (GACGAATATAAGGTCAAAAGCTTCA) and SNL1 3′ Primer (TTTATTTTGGTATGATTTTAGGCGA). Correct integration of the disrupted DNA was confirmed by PCR analysis and Southern blotting. The identity of DNA fragments generated by PCR was verified by sequencing. Detailed cloning strategies are available on request. The plasmid pRS316-ΔssCPY*-GFP is described previously (Medicherla et al., 2004). To generate the plasmid pZK116 expressing ΔssCPY*, the signal sequence was removed from the CPY* allele in plasmid pRS316-CPY* (Kostova and Wolf, 2005) by a QuickChange–based (Stratagene, La Jolla, CA) PCR-mutagenesis approach. The DNA of cytoplasmically localized, N-terminally GFP fused CPY* (ΔssGC*) was cloned in two steps. First, the SphI restriction site of the plasmid was introduced to the end of the CPY promoter in pZK116 by QuickChange (Stratagene)-based PCR mutagenesis, yielding pZK116m. Then the PCR-amplified 0.7-kb GFP DNA fragment prepared from plasmid pRS316-ΔssCPY*-GFP as template was cloned into the SphI restriction site of pZK116m, generating plasmid pRS316-ΔssGFP-CPY*. The PCR-amplified 0.75-kb DNA fragment of GFPuv from p416-PADH-GFPuv as template (Hoyt et al., 2003) was cloned into pRS316-ΔssCPY*-GFP between the HpaI and EcoRI restriction sites, yielding plasmid pRS316-ΔssCPY*-GFPuv. The PCR-amplified 1.2-kb partial fragment of PRC1 from pYEP13/PRC1, which encodes wild-type CPY was used as template and inserted into pZK116 between the Bsu36I and EcoRI restriction sites, generating pRS316-ΔssCPY. The 3.4-kb ORF, which encodes ΔssCPY*-GFP from plasmid pRS316-ΔssCPY*-GFP was subcloned into the 2μ plasmid pRS426 between the ClaI and EcoRI restriction sites, leading to overexpression of ΔssCPY*-GFP. The PCR-amplified 0.5-kb DNA fragment of GFPuv-cODC or GFPuv-cODC-C441A from p416PADH-GFPuv425cODC or p416PADH-GFPuv425cODC-C441A as templates (Hoyt et al., 2003) were cloned into respective sites of pRS316-ΔssCPY*-GFPuv between the MluI and EcoRI restriction sites, yielding pRS316-ΔssCPY*-GFPuv-cODC or pRS316-ΔssCPY*-GFPuv-cODC-C441A, respectively.
Table 1.
Yeast strains used in this study
| Name | Genotype | Source |
|---|---|---|
| YWO1 | Matα ura3-52 leu2-3,2-112 his3 Δ200 lys2-801 trp1-1 | Seufert and Jentsch (1990) |
| YWO23 | Matα ura3-52 leu2-3,2-112 his3 Δ200 lys2-801 trp1-1 Δubc4::HIS3 Δubc5::LEU2 | Seufert and Jentsch (1990) |
| YPH499Y | Mata ura3-52 leu2-1 his3Δ200 trp1-63 lys2-801 ade2-101 prc1-1 | Hiller et al. (1996) |
| CMY762Y | Mata cim3-1 ura3-52 leu2-1 his3 Δ200 prc1-1 | Hiller et al. (1996) |
| W303-1C | Matα ade2-1 ura3-1 his3-11,15 leu2-3,112 trp1-1 can1-100 prc1-1 | Knop et al. (1996) |
| YPK002 | W303-1C Δsnl1::KANR | This study |
| YPD5 | W303-1C Δydj1-2::HIS3 LEU2::ydj1-151 | Taxis et al. (2003) |
| YPD21 | Matα his3-11, 15 leu2-3, 112 ura3-52 trp1-81 lys2 prc1-1 Δssa2::LEU2 Δssa3::TRP1 Δssa4::LYS2 | Taxis et al. (2003) |
| YPD22 | YPD21 Δssa2::LEU2 Δssa3::TRP1 Δssa4::LYS2 ssa1-45 | Taxis et al. (2003) |
| YCT397 | Mata leu2-3,112 ura3-52 ade1-100 his4-519 prc1-1 | Jarosch et al. (2002) |
| YCT415 | YCT397 ufd1-1 | Jarosch et al. (2002) |
| W303-1B | Matα ade2-1 ura3-1 his3-11,15 leu2-3,112 trp1-1 can1-100 | Chiang and Schekman (1991) |
| AGC14 | Matα ade2-1 ura3-1 his3-11,15 leu2-3,112 trp1-1 can1-100 Δhsp26::LEU2 Δhsp42::HygBR | Cashikar et al. (2005) |
| MHY501 | Matα his3-200 leu2-3,112 ura3-52 lys2-801 trp1-1 | Swanson et al. (2001) |
| MHY1631 | MHY501 Δssm4/doa10::HIS3 | Swanson et al. (2001) |
| MHY1669 | MHY501 Δhrd1/der3::LEU2 | Swanson et al. (2001) |
| MHY1703 | MHY501 Δhrd1/der3::LEU2 Δssm4/doa10::HIS3 | Swanson et al. (2001) |
| YRH023 | W303-1C Δhsp104::KANR | Taxis et al. (2003) |
| YRH030 | W303-1c Δsti1-1::HIS3 | Taxis et al. (2003) |
| YRH050 | W303-1C Δhsc82::KANR hsp82G170D | Taxis et al. (2003) |
| Y406-C | Matα ura3-52 leu2-3,112 his3-11,15 lys2 trp1-1 prc1-1 | Deak (1998) |
| Y420-C | Y406-C Δssb1::LEU2 Δssb2::HIS3 | Deak (1998) |
| BY4743 | Matα/a his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 lys2Δ0/LYS2, MET15/met15Δ0, ura3Δ0/ura3Δ0 | EUROSCARF |
| BY474Δsse1 | BY4743 Δsse1::kanMX4/Δsse1::kanMX4 | EUROSCARF |
Antibodies
Polyclonal anti-rabbit CPY (Knop et al., 1993), polyclonal anti-rabbit GFP antibodies (Molecular Probes, Eugene, OR) were used for immunoprecipitation of ΔssCPY*-GFP* and its derivates. Monoclonal anti-mouse CPY (Molecular Probes), polyclonal anti-rabbit GFP antibodies were diluted 1:10,000 for immunodetection. Monoclonal anti-mouse ubiquitin antibody (BabCO, Richmond, CA) was used at 1:2000 dilution for immunodetection.
Pulse-Chase Analysis
Pulse-chase experiments using cells expressing CPY* or CPY fusion proteins, respectively, cell breakage in buffer containing urea, and SDS were performed as described previously (Hiller et al., 1996; Taxis et al., 2003). Temperature-sensitive strains were grown at 25°C and shifted to restrictive temperature for labeling with 200 μCi of 35S-Met at 37°C for 20 min. Cells were chased with excess of unlabeled chase media for the times indicated in the respective figure legends.
Cycloheximide Decay Experiments
Cells were grown in synthetic complete medium. Temperature-sensitive strains were shifted to restrictive temperature of 37°C for 60 min. Cycloheximide was added (0.5 mg/ml), and 2 OD600 of cells were taken at the indicated time points. Cell extracts were prepared by alkaline lysis and subjected to SDS-PAGE followed by immunodetection (Hiller et al., 1996; Taxis et al., 2003).
Solubility Assay
Cells expressing ΔssCG* were grown at 30°C and shifted to 37°C for 60 min before assay. Twenty OD600 of yeast cells were harvested, washed once with four volumes of 20 mM sodium azide, and resuspended in 1 ml of ice-cold sorbitol lysis buffer (0.7 M sorbitol, 50 mM Tris-HCl, pH 7.5, 1 mM PMSF, 1 μg/ml pepstatin-A). Subsequently, all material was kept on ice, and cells were lysed with glass beads in ice-cold sorbitol lysis buffer. Lysates were precleared by centrifugation at 500 × g for 5 min at 4°C. Total protein (T) was precipitated from 400 μl of lysate with TCA (11% final concentration). Total protein (T) was solubilized with 60 μl of urea buffer (40 mM Tris-HCl, pH 6.8, 8 M urea, 5% SDS, 100 mM EDTA, pH 8, 200μg/ml bromophenol blue, 1.5% beta mercaptoethanol). In addition 400 μl of lysate was spun in a Beckman T110 rotor (Fullerton, CA) at 130,000 × g for 30 min at 4°C. The supernatant was subjected to TCA precipitation and treated as soluble protein (S). The pellet of the 130,000 × g centrifugation step was washed once with sorbitol lysis buffer followed by solubilization with 60 μl of urea buffer as described above. Equal amounts of solubilized protein were analyzed by SDS-PAGE followed by immunoblotting. Immunoblots were analyzed with anti-CPY or anti-PGK. Resolubilization of aggregated ΔssCG* was tested as follows: After temperature shift of cells to 37°C for 1 h, cycloheximide was added to a final concentration of 0.5 mg/ml. Twenty OD600 of cells were taken at the indicated time points, and the solubility assay was performed as stated above.
Fluorescence Microscopy
Cells overexpressing ΔssCPY*-GFP or harboring an empty plasmid were grown at 30°C and shifted to 37°C for 60 min before viewing fluorescence in living cells. Cells were collected by centrifugation, washed once, and resuspended in fresh SC medium. The suspension, 2.2 μl, was dropped onto a 76 × 26-mm microscopy slide, covered with a coverslip, and subjected to immediate viewing. Fluorescence microscopy was performed with an Axioplan microscope equipped with a 100× oil-immersion objective (Carl Zeiss, Thornwood, NY) and GFP filter.
Ubiquitination of ΔssCG*
Fifty OD600 of yeast cells overexpressing ΔssCPY*-GFP or harboring an empty plasmid were grown at 25°C and shifted to 37°C for 60 min before analysis. Cells were washed once with ice-cold washing buffer (20 mM sodium azide, 2 mM PMSF, 20 mM NEM) and incubated for 10 min on ice. Cells were resuspended in ice-cold IP buffer (50 mM Tris-HCl, pH 7.5, 190 mM NaCl, 1.25% Triton X-100, 6 mM EDTA, 2 mM PMSF, 20 mM NEM), and 500 μl of 0.5-mm glass beads were added. Cells were lysed by five pulses of 1-min duration in a Mini-bead beater, with cooling on ice between pulses. Lysates were cleared by centrifugation (130,000 × g, 30 min at 4°C), immunoprecipitated with anti-GFP, fractionated, and analyzed using anti-ubiquitin or anti-CPY.
RESULTS
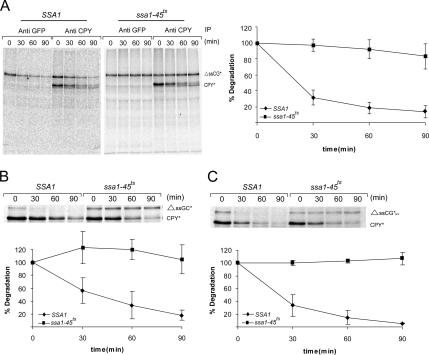
Because degradation of the cytoplasmically localized substrate ΔssCPY*-GFP(ΔssCG*) by the proteasome did not require any of the cytoplasmic helper components of the ERAD pathway (Medicherla et al., 2004), we searched for different chaperones that might be involved in its elimination. We reasoned that, as found for misfolded ER proteins, recognition, unfolding, escort, and delivery machineries must exist to deliver misfolded cytoplasmic proteins to the proteasome for degradation. Previous in vivo experiments in yeast had indicated that the Hsp40 cofactor of the Hsp70 chaperone Ssa1, Ydj1p, promotes the degradation of some short-lived and abnormal proteins (Lee et al., 1996), thus suggesting the requirement for Hsp70. We therefore assessed whether the Hsp70 chaperone machinery of the Ssa class had a crucial role in the degradation of ΔssCPY*-GFP (ΔssCG*). We tested the requirement for the Hsp70 Ssa chaperones by comparing the properties of two strains, both of which lack three of the four Ssa proteins (Ssa2p, Ssa3p, Ssa4p). In ssa1-45ts cells Ssa1 is present as a temperature-sensitive allele, whereas in isogenic SSA1 cells the gene is present as a wild-type copy (Becker et al., 1996; Taxis et al., 2003). As can be seen in Figure 1A, degradation of ΔssCG* progresses with a half-life of 20–30 min in SSA1 cells. Degradation of ΔssCG* is nearly completely abolished in ssa1-45ts cells under restrictive conditions. A similar almost complete dependence on Ssa1 for ΔssCG* degradation is observed using antibodies directed against either CPY or GFP for immunoprecipitation (Figure 1A). As expected, degradation of endogenously expressed CPY*, which is retrotranslocated from the ER lumen to the cytoplasm (Hiller et al., 1996), is not affected by the absence of Ssa1p. To test whether the position of the strongly folded GFP domain within ΔssCG* had any effect on the degradation pattern and whether its context influenced the Ssa1p-dependence of degradation, we constructed ΔssGFP-CPY* (ΔssGC*), carrying GFP N-terminally fused to signal sequence deleted CPY*. As can be seen in Figure 1B, ΔssGC* is degraded nearly as rapidly as ΔssCG*, and lack of an active Ssa apparatus blocks degradation of this substrate as well. Also, fusion of a variant of GFPuv that fluoresces more brightly than wild-type GFP at the C-terminus of ΔssCPY* does not affect the half life of ΔssCG* degradation (Figure 1C).
Figure 1.
The Hsp70 chaperone machinery of Ssa1p is required for the degradation of cytoplasmically localized misfolded proteins. Pulse-chase analysis was done in SSA1 and ssa1-45ts cells. Cells expressing the substrates were lysed at the indicated times, and proteins were immunoprecipitated with anti CPY (A–C) or anti GFP (A), separated by SDS-PAGE, and analyzed using a PhosphoImager and ImagerQuaNT (Amersham Bioscience). Plotted data represent the mean values of three independent experiments. Substrates: A: ΔssCG*; B: ΔssGC*; C: ΔssCG*uv. The ERQD substrate CPY* served as a control.
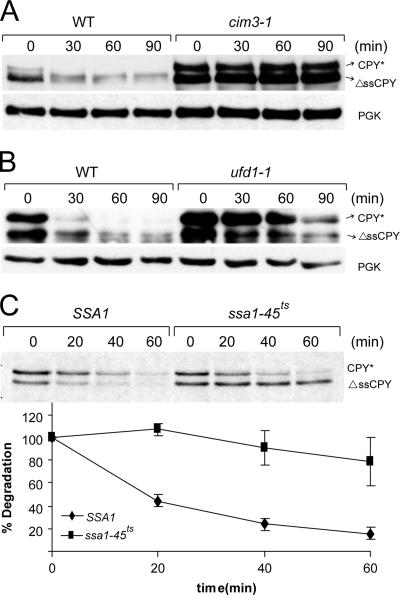
In vitro studies had shown that the 26S proteasome is unable to degrade the GFP moiety of certain fusion proteins, because of its strongly folded structure (Liu et al., 2003). It was therefore possible that Ssa1p was only required for unfolding of the GFP moiety of ΔssCPY*-GFP (ΔssCG*) to allow its degradation by the proteasome in vivo. We constructed a CPY* protein without signal sequence, ΔssCPY*. It is an ER import-incompetent CPY* species that due to mutation (G255R) is misfolded. As previously published for ΔssCPY*-GFP (Medicherla et al., 2004), the signal sequence deletion causes ΔssCPY* to be located in the cytosol (data not shown). This protein is rapidly degraded by the proteasome: elimination of ΔssCPY* is severely disrupted in the proteasome mutant cim3-1 (Figure 2A). We have previously shown (Medicherla et al., 2004) that the elimination of cytosolic ΔssCPY*-GFP does not require the trimeric Cdc48p-Ufd1p-Npl4p complex. Testing the requirement of this trimeric complex for degradation in ufd1-1 mutant shows that Cdc48p-Ufd1p-Npl4p is also not involved in the proteasomal elimination process of ΔssCPY* (Figure 2B). As can be seen in Figure 2C, ΔssCPY* is rapidly degraded in SSA1 but not in ssa1-45ts mutant cells under restrictive conditions. These experiments indicate that the Ssa machinery is needed for the degradation of misfolded proteins of the cytoplasm.
Figure 2.
Degradation of misfolded and ER import incompetent CPY* is dependent on the proteasome and Ssa1p but not on the Cdc48-Ufd1-Npl4 complex. Cycloheximide decay experiments were performed in the proteasomal mutant cim3-1 (A) and in ufd1-1 cells (B) expressing ΔssCPY*. Cycloheximide was added (t = 0 min), and samples were collected at the indicated time points and subjected to SDS-PAGE, followed by immunoblotting. Immunoblots were analyzed with anti-CPY and anti-PGK as a loading control. Pulse-chase analysis in SSA1 and ssa1-45ts cells (C) was performed and analyzed as described in the legend to Figure 1. The ERQD substrate CPY* served as a control.
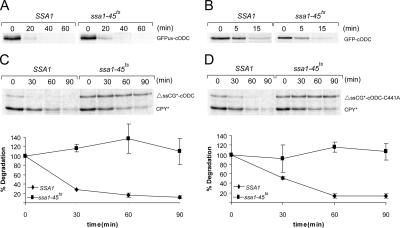
For elucidation if proteasomal degradation of the strongly folded GFP domain is indeed independent of Ssa helper proteins we tested the degradation of GFP linked to the C-terminal 37 amino acids of mouse ornithine decarboxylase (cODC). This 37 amino acid C-terminal sequence has been shown to be a ubiquitin-independent transferable element, one with the capacity to direct diverse proteins to proteasomal degradation (Hoyt et al., 2003; Zhang et al., 2003, 2004). We tested the Ssa1 dependency of degradation of the fusion proteins GFPuv-cODC and GFP-cODC. The GFP-cODC proteins are rapidly degraded, regardless of the Ssa status of the cell turnover is similar in SSA1 and ssa1-45ts mutant cells, whether under permissive or restrictive conditions of incubation (Figure 3, A and B). These experiments indicate that in the cellular environment, there must be means to unfold the GFP domain for degradation that do not depend on the Ssa machinery. Interestingly, degradation of a fusion protein consisting of ΔssCPY* and GFPuv-cODC (ΔssCG*-cODC) is again dependent on Ssa1p, as is ΔssCG* (Figure 3C). It has been reported that the C-terminal 37 amino acids of ODC represent a critical signal for rapid ODC degradation and that a mutation of Cys441 to Ala441 in this sequence causes a significant stabilization of ODC or of proteins to which cODC is attached (Hoyt et al., 2003). However, the Cys441 to Ala441 mutation in ΔssCG*-cODC-C441A did not lead to stabilization but directed this protein to a form of degradation that relied on the Ssa1 protein (Figure 3D). Obviously, Ssa1p-directed degradation of the ΔssCPY* moiety of the protein dominates over the Ssa1p-independent cODC-directed degradation in the fusion protein.
Figure 3.
Ubiquitin-independent degradation of GFP-cODC does not require Ssa1p activity, but its fusion to ΔssCPY* makes the process Ssa1p dependent. Pulse-chase analysis was done in SSA1 and ssa1-45ts cells expressing GFPuv-cODC (A), GFP-cODC (B), ΔssCG*-cODC (C), and ΔssCG*-cODC-C441A (D).
It has been shown that the import of secretory proteins into the ER can be faulty (Levine et al., 2005). Because the intracellular mislocalization of proteins may lead to severe defects, we were also interested in the question of how wild-type secretory proteins that fail to advance into the ER are handled by the cell's cytosol. We chose mislocalized but otherwise wild-type carboxypeptidase yscY (CPY) for this analysis. Using a multicopy plasmid for expression Blachly-Dyson and Stevens (1987) found ∼90% of signal sequence deleted CPY in the cytosol and ∼10% in the ER. We constructed a signal sequence deleted CPY (ΔssCPY) and expressed it from a single-copy plasmid. We found ΔssCPY, like ΔssCPY*, to be solely located in the cytosol (data not shown). The fact that in contrast to Blachly-Dyson and Stevens (1987) we did not find a small portion of ΔssCPY in the ER may be due to the different expression conditions. We analyzed the fate of ΔssCPY. The mislocalized and presumably misfolded ΔssCPY is rapidly degraded; its turnover is performed by the proteasome, as evidenced by the stabilization conferred by the proteasomal cim3-1 mutant (Figure 4A). As is true for the mutated CPY species, degradation of ΔssCPY is independent of the trimeric Cdc48p-Ufd1p-Npl4p complex required for elimination of misfolded ER proteins (Figure 4B). However, elimination of ΔssCPY does require an intact Ssa1 protein (Figure 4C). The fate and chaperone dependence of the cytoplasmically mislocalized wild-type CPY species is similar to that of its mutated counterpart in the cytoplasmic environment. In the reducing environment of the cytoplasm, folding of CPY is likely to be defective due to disturbed formation of disulphide bonds (Endrizzi et al., 1994; Jamsa et al., 1994).
Figure 4.
The fate of the cytoplasmically mislocalized wild-type CPY is similar to its mutated counterpart. Cycloheximide decay experiments (A and B) and pulse-chase analysis (C) were performed as described in the legend to Figure 2.
Hsp70 chaperones function in a complex with cochaperones of the Hsp40 family, which modulate the substrate specificity of the Hsp70s (Cheetham and Caplan, 1998; Johnson and Craig, 2001; Rudiger et al., 2001; Fan et al., 2003). In a previous study, we had shown that for degradation of the ERQD substrate CTG* the help of the Hsp40 cochaperones Hdj1p, Cwc23p, and Jid1p is required (Taxis et al., 2003). However, for the degradation of cytoplasmic ΔssCG*, none of these Hsp40 cochaperones are needed (data not shown). In contrast, the Hsp70 cochaperone Ydj1p has a strong influence on degradation of ΔssCG*, as well as ΔssCPY* and ΔssCPY: degradation of all three cytosolic model substrates is considerably slowed in ydj1-151ts mutant cells under restrictive conditions (Figure 5, A–C). Ydj1p is not required for any of the ERQD substrates derived from CPY* (Taxis et al., 2003). It can be concluded that the CQD (cytoplasmic quality control and degradation) substrates ΔssCG*, ΔssCPY*, ΔssCPY, and the ERQD substrate CTG* have different cochaperone requirements.
Figure 5.
The Hsp70 cochaperone Ydj1p promotes the degradation of cytoplasmically localized misfolded proteins. Pulse-chase analysis was performed in wild-type (WT) and ydj1-151ts cells expressing ΔssCG* (A), ΔssCPY* (B), and ΔssCPY (C).
Another class of Hsp70 chaperones, the Ssb members, are ribosome associated and involved in the folding of newly synthesized polypeptide chains (Pfund et al., 1998, 2001). We tested a strain defective in this chaperone family (Δssb1Δssb2) and found that they are dispensable for degradation of ΔssCG* (Figure 6A). We also tested whether components of the Hsp90 chaperones were involved in degradation of ΔssCG*. The yeast Hsp90 chaperone family consists of two proteins, Hsc82p and Hsp82p. They are associated with the cochaperone Sti1p/HOP, which is also an activator of the Ssa1 proteins (Nathan et al., 1997; Wegele et al., 2003). The Hsp90 chaperones Hsc82p and Hsp82p are not required for degradation of ΔssCG* (Figure 6B). Consequently, the Hsp70/Hsp90 cochaperone Sti1p/HOP has no effect on the degradation of ΔssCG* (Figure 6C). It has been suggested that another major cytoplasmic chaperone, Hsp104, works together with the Hsp70s of the Ssa family and binds in an ATP-dependent manner to the Ssa1p-Ydj1p complex to unfold proteins (Parsell and Lindquist, 1993; Parsell et al., 1994; Glover and Lindquist, 1998; Lum et al., 2004). ER-associated degradation of CTG* requires both Ssa and Hsp104 chaperones (Taxis et al., 2003). However, Hsp104p is not required for elimination of ΔssCG* (Figure 6E). We were further interested in the involvement of the Hsp110 chaperone Sse1p in elimination of ΔssCG*. The protein is a component of the Hsp90 chaperone complex and mediates degradation of misfolded VHL (McClellan et al., 2005a). No function of Sse1p in ΔssCG* degradation can be observed (Figure 6D). Two small heat-shock proteins, Hsp26 and Hsp42 are ubiquitous molecular chaperones that protect yeast cells from a variety of cellular stresses. In vitro they have been found to bind to unfolded proteins to form large cocomplexes and by this prevent their aggregation (Haslbeck et al., 1999, 2004; Cashikar et al., 2005). We tested the involvement of Hsp26 and Hsp42 in degradation of ΔssCG*. As can be seen Figure 6F, degradation of ΔssCG* was not affected by the absence of Hsp26 and Hsp42. Recently, BAG domain proteins were shown to interact with Hsp70 chaperones as a nucleotide exchange factor in the cytosol of higher eukaryotic cells. In mammalian cells, together with the E3 ligase CHIP, they are known to be partners in a degradative Hsp70 complex (Esser et al., 2004). There exists a BAG-1 homologue in yeast, Snl1p, which functionally interacts with Hsp70 chaperones (Sondermann et al., 2001, 2002). However, no alteration of degradation of ΔssCG* is seen in SNL1 deletion mutant cells (Figure 6G).
Figure 6.
The Hsp70 Ssb class; the Hsp90 complex, Hsp104, Hsp110; small heat shock proteins Hsp26, Hsp42; and the yeast Bag1 homologue, Snl1p, are not involved in the degradation of ΔssCG*. Pulse-chase analysis was done in Δssb1Δssb2 (A), Δhsc82hsp82G170D (B), Δhsp104 (E), Δhsp26Δhsp42 (F), and Δsnl1 (G) cells expressing ΔssCG*, and cycloheximide decay experiments were performed in sti1-1 (C) and Δsse1 (D) cells expressing ΔssCG* as described in the legend to Figure 2. PGK and CPY were served as a loading control.
We tested whether the Ssa machinery has any function in keeping misfolded ΔssCG* in the soluble state in the cytoplasm. When testing wild-type cells harboring all four Ssa chaperones (Ssa1p, Ssa2p, Ssa3p, Ssa4p), most of the ΔssCG* protein is found in the soluble state, and this does not change when cells are shifted from 30 to 37°C (Figure 7B). As can be seen in Figure 7A, when SSA1 cells containing solely Ssa1p are transferred from 30 to 37°C, the ΔssCG* material in the pellet increases, indicating aggregation of the misfolded protein material with increased temperature. The amount of precipitated ΔssCG* in SSA1 cells varied somewhat in different experiments (data not shown). Apparently, in the absence of Ssa2, Ssa3, and Ssa4 the single Ssa1 species is functioning at or beyond its limits in keeping misfolded protein soluble under heat stress. However, analyzing the amount of soluble and precipitated cellular protein material in vitro may not be fully informative of the solubility properties of ΔssCG*, because in vitro conditions (buffer, salt, protein concentration, etc.) are very different from the cellular environment. We therefore analyzed the solubility of ΔssCG* in the different strains by fluorescence microscopy, thus visualizing the distribution pattern of the GFP moiety of the protein. As can be seen in Figure 6C, no precipitated ΔssCG* material can be seen at 37°C in wild-type cells containing all four Ssa species, regardless of whether ΔssCG* was expressed from a single-copy (data not shown) or multicopy plasmid (Figure 7C). In contrast, at 37°C some punctuated fluorescent dots, indicating precipitated material, are visible in cells containing only Ssa1p, substantiating the in vitro finding. Nevertheless, the misfolded protein is rapidly degraded in SSA1 cells at 37°C (Figures 1, A–C). A dramatic increase in such precipitated fluorescent material appears under the restrictive conditions of 37°C in the ssa1-45 and ydj1-151 mutant cells. Under the restrictive conditions of 37°C in ssa1-45ts mutant cells, we see most of the misfolded ΔssCG* material in the pellet (Figure 7A), and degradation is completely blocked (Figures 1, A and C). The behavior of ΔssCG* in the ydj1-151ts mutant mirrors the behavior of this substrate in the ssa1-45ts mutant. Under permissive conditions a significant fraction of ΔssCG* is soluble, whereas at restrictive conditions a major part of the protein is found in the pellet fraction precipitated in cells (Figures 7, A and B). We have shown that ΔssCG* is nearly completely degraded in SSA1 cells at 37°C (Figure 1A) despite the fact that under these conditions ΔssCG* partly precipitates (Figure 7A). This indicates that Ssa1p may have the capacity to resolubilize the precipitated material for degradation under the conditions tested. We tested resolubilization of ΔssCG* in SSA1 and ssa1-45ts cells in a cycloheximide decay experiment at 37°C (Figure 7D). As can be seen, within 30 min of cycloheximide treatment the amount of ΔssCG* material increases in SSA1 cells but thereafter nearly completely disappears in the total fraction and in the pellet within 90 min. In ssa1-45ts cells the precipitated material persists, whereas GFP-cODC carrying the 37 amino acid targeting sequence of ODC for the proteasome is rapidly degraded by the enzyme (Hoyt et al., 2003 and Figures 3A and 7E). GFP carrying the mutated version of the proteasomal-targeting sequence (GFP-cODC-C441A) is not eliminated by the proteasome (Hoyt et al., 2003). Indeed, the GFP-cODC-C441A protein accumulates in SSA1 cells (Figure 7E). However, in contrast to ΔssCG* (Figure 7C) the accumulated material does not show any sign of aggregation.
Figure 7.
Ssa1p and its cochaperone Ydj1p are required for rescue of aggregated ΔssCG*. Cells expressing ΔssCG* were grown at 30°C and shifted to 37°C for 60 min before the solubility assay. The solubility of ΔssCG* was assessed in SSA1, ssa1-45ts (A), wild-type W303-1C (SSA1, SSA2, SSA3, SSA4), and ydj1-151ts strains (B). The same amount of total (T), supernatant (S), and pellet (P) fraction was analyzed via SDS-PAGE and immunoblot. Immunoblots were analyzed with CPY antibody and PGK antibody as a control. The fluorescence of ΔssCG* was analyzed in living cells (C) as described in Material and Methods. The cells harboring overexpressed ΔssCG* or an empty plasmid were grown at 30°C and shifted to 37°C for 60 min before analysis. All the cells were visualized by fluorescence microscopy using equal exposure times and conditions. Resolubilization of aggregated ΔssCG* was assessed in SSA1 and ssa1-45ts cells (D). After temperature shift of cells to 37°C for 1 h, cycloheximide was added to a final concentration of 0.5 mg/ml to block further protein synthesis. Twenty OD600 of cells were taken at the indicated time points and treated as indicated for the above solubility assay. Immunoblot of Sec61p served as control. Three independent experiments gave similar results. The fluorescence of GFP-cODC and GFP-cODC-C414A were analyzed in SSA1 cells at 37°C as stated above (E).
With few exceptions, like ODC and the cyclin-dependent kinase inhibitor p21 (Sheaff et al., 2000; Verma and Deshaies, 2000; Liu et al., 2003; Hoyt and Coffino, 2004), ubiquitination of substrates is required before their elimination via the proteasome (Heinemeyer et al., 1991; Pickart, 2001; Wolf and Hilt, 2004). Several groups have shown that in mammalian cells a CHIP-associated Hsp70 chaperone complex triggers ubiquitination of its protein clients and mediates proteasomal degradation (Connell et al., 2001; Demand et al., 2001; Jiang et al., 2001; Murata et al., 2001). We searched for ubiquitinated ΔssCG* material in mutant and wild-type cells, under the experimental design of Figure 7, and analyzed the soluble fraction of the respective cell extracts. The buffer used for solubilization (Figure 7, sorbitol, or Figure 8, Tris/HCl) did not alter the experimental result (data not shown). Although we find clearly similar amounts of ubiquitinated ΔssCG* in wild-type and mutant cells at 25°C (Figure 8A), conditions that do not induce the mutant character, we see a considerably changed ubiquitin pattern of ΔssCG* material at 37°C, which leads to the expression of the mutant phenotype of ssa1-45ts and ydj1-151ts cells. Interestingly, considerably more ubiquitinated ΔssCG* can be found in ssa1-45ts and ydj1-151ts under restrictive conditions compared with WT (SSA1, SSA2, SSA3, SSA4) and SSA1 cells (Figure 8B), despite the fact that the mutant cells show much less soluble ΔssCG* material (Figure 7). This might indicate that ΔssCG* in the SSA1 and wild-type cells is completely degraded, whereas degradation of the ubiquitinated material is retarded in the mutant cells.
Figure 8.
The state of ubiquitinated misfolded proteins in wild-type, SSA1, ssa1-45ts, and ydj1-151ts cells at the different temperature of 25 and 37°C. The cells harboring overexpressed ΔssCG* or an empty plasmid (control) were grown at 25°C (A) and shifted to 37°C (B) for 60 min before analysis. Cell extracts were immunoprecipitated with anti-GFP antibody, separated by SDS-PAGE, followed by immunoblotting, and analyzed with anti-ubiquitin or anti-CPY antibodies.
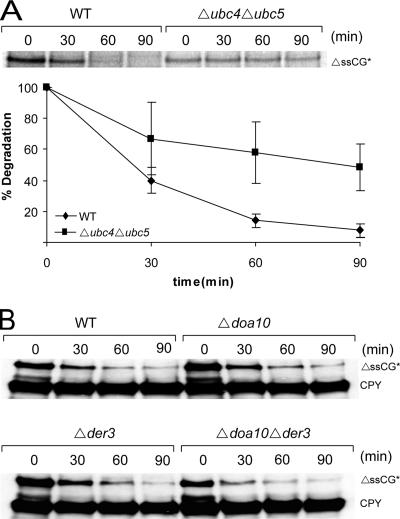
We were also interested in the components of the ubiquitination machinery in the degradation pathway. At present there are 13 ubiquitin-conjugating enzymes known to exist in yeast. As can be seen Figure 9A, deletion of the ubiquitin-conjugating enzymes Ubc4p and Ubc5p leads to a considerable stabilization of ΔssCG*, indicating involvement of Ubc4p and Ubc5p in the degradation of this misfolded cytoplasmic protein. Because degradation is not completely halted in the ubc4/ubc5 double deletion mutant, an overlapping E2 activity must be present for ubiquitination of ΔssCG*. In mammalian cells, CHIP has been discovered as an important E3 ligase involved in degradation of proteins in the cytoplasm (Connell et al., 2001; Demand et al., 2001; King et al., 2001; Murata et al., 2001; Cyr et al., 2002; Esser et al., 2004). In yeast cells no CHIP orthologue has been found yet. However, there are a multitude of E3 ligases present in yeast cells. Besides its involvement in degradation of several ERQD substrates, the ER membrane–located E3 ligase Doa10p is required for degradation of Deg1-GFP, a cytoplasmically and nuclear localized substrate (Swanson et al., 2001; Huyer et al., 2004; Ravid et al., 2006). However, degradation of ΔssCG* is independent of the function of the E3 ligase Doa10p (Figure 9B). Degradation of ΔssCG* did also not require the second ER membrane–located E3 ligase Der3/Hrd1p (Figure 9B).
Figure 9.
Degradation of ΔssCG* requires the E2 proteins Ubc4p and Ubc5p but not the E3 ligases Doa10p and Der3p. Pulse-chase analysis was done in Δubc4Δubc5 mutant cells (A) and cycloheximide decay experiments were performed in Δdoa10Δder3 cells (B) as described in the legend to Figure 2. CPY served as a loading control.
DISCUSSION
Misfolded proteins of the ER are eliminated by proteasomal degradation in the cytosol. After detection, retrotranslocation, and ubiquitination at the cytosolic surface of the ER, they are channelled to the proteasome via the trimeric AAA-ATPase complex Cdc48p-Ufd1p-Npl4p and the UBA-UBL domain proteins Dsk2p and Rad23p (Brodsky and McCracken, 1999; Kostova and Wolf, 2003; Hirsch et al., 2004; Medicherla et al., 2004). Understanding of this mechanism, to a large extent, had been elaborated by using what turned out during time to be a model substrate for studying the ER-associated degradation process, mutated vacuolar carboxypeptidase yscY (CPY*) (Hiller et al., 1996; Schafer and Wolf, 2006). We had shown that degradation of a cytoplasmically localized derivative of CPY* devoid of the signal sequence required for ER import (ΔssCPY*-GFP) did not depend on the Cdc48p-Ufd1p-Npl4p, Dsk2p and Rad23p pathway for proteasomal degradation (Medicherla et al., 2004). It became therefore our aim to understand the mechanism of degradation of misfolded proteins in the cytoplasm.
We therefore sought to determine the components that are required for elimination of ΔssCG* in the cytoplasm. As can be seen in Figure 1, degradation of ΔssCG* requires the Hsp70 chaperone Ssa1p. Recent in vitro experiments had shown that the 26S proteasome is unable to unfold the strongly folded GFP moiety of several fusion proteins tested (Liu et al., 2003). We constructed a signal-sequence–deleted, cytoplasmically localized ΔssCPY* molecule devoid of the GFP domain to inquire if unfolding of that domain is responsible for the Ssa1p requirement. Surprisingly, ΔssCPY* degradation also depended on Ssa1p function (Figure 2C), clearly indicating that this Hsp70 species has a more general function in the degradation of cytoplasmically located misfolded proteins. The present finding that degradation of ΔssCPY* and ΔssCPY also require Ssa1p points to the fact that the role of this chaperone is not limited to unfolding, but serves additional purposes. Degradation of GFP fused to the C-terminal 37 amino acids of ornithine decarboxylase (GFP-cODC) without the aid of Ssa1p implies that the proteasome has other means to unfold GFP (Figure 3, A and B). A C441A mutation in the C-terminal 37-amino acid tail of ODC abolishes degradation of the fusion protein GFP-cODC-C441A (Hoyt et al., 2003). The 37-amino acid stretch of cODC, whether wild type or mutated is not recognized as a misfolded protein domain by the cell (Hoyt et al., 2003), and therefore the fate of GFP-cODC is independent of Ssa1p. Interestingly, fusion of mutated ΔssCPY* to GFP-cODC (ΔssCG*-cODC) reimposes a dependence of the Ssa1 chaperone for degradation (Figure 3C). Also, mutation of cODC does not lead to stabilization of ΔssCG*-cODC-C441A (Figure 3D). Thus Ssa1p seems to function in the recognition of the misfolded ΔssCPY* domain of the fusion protein; its misfolded status dictates the route of elimination.
It has recently been shown that the in vivo efficiency of signal sequence-mediated protein segregation into the secretory pathway varies tremendously, ranging from >95% to <60% in mammalian cells (Levine et al., 2005). Remnant secretory proteins thus find themselves entrapped in the cytoplasm. Because mislocalized proteins may be harmful to the cell, the fate of these proteins is of high interest. The usefulness of mutated CPY variants in defining degradation pathways impelled a test of the fate of wild-type CPY remaining in the cytoplasm. As are ΔssCG* and ΔssCPY*, ER import incompetent wild-type CPY is rapidly degraded by the proteasome (Figure 4A), indicating an altered structure that is recognized by the cytoplasmic proteolysis system. We reason that proper folding of the enzyme is most likely defective because of disturbed formation of disulphide bonds (Endrizzi et al., 1994; Jamsa et al., 1994) in the reducing environment of the cytoplasm, compared with the oxidative environment of the ER in which CPY normally assumes its native and active form. As shown for ΔssCG* (Medicherla et al., 2004), glycosylation of the enzyme is also likely to be absent in the cytoplasm. Thus the cell is easily able to eliminate mislocalized secretory proteins, which cannot fold efficiently in the cytoplasmic environment, in this way avoiding their unwanted presence in the cytoplasm.
All three cytoplasmically localized CPY derivatives, whether mutated (ΔssCG*, ΔssCPY*) or wild-type (ΔssCPY), required the Hsp70 chaperone Ssa1p for elimination. While our work was in progress McClellan et al. (2005) reported the requirement of Ssa1p for degradation of misfolded von Hippel Lindau (VHL) tumor suppressor protein in the yeast cytoplasm. We therefore conclude that the need for Ssa1p is likely to be a general feature of degradation of misfolded proteins in the cytoplasm. A crucial role for Hsp70 function in the degradation of different substrates has also been shown in mammalian cells (for review see Esser et al., 2004). The functional requirement of Ssa1p for substrate recognition does not seem to be limited to ubiquitin-dependent substrates only. It has been reported that overexpression of the molecular chaperones Hsp70 and Hsp40 facilitate degradation of α-synuclein, which is natively disordered and degraded by the proteasome in the absence of ubiquitin modification (Tofaris et al., 2001; Muchowski and Wacker, 2005).
In contrast to degradation of the ERQD substrate CTG*, which, along with Ssa1p, is dependent on the Hsp40 cochaperones Hdj1p, Cwc23p, and Jid1p but not Ydj1p (Taxis et al., 2003), elimination of the CQD substrate ΔssCG* instead depends on the cochaperone Ydj1p and is independent of the other three cochaperones. Degradation of ΔssCPY* and ΔssCPY, too, is dependent on Ydj1p (Figure 5). In their work on the degradation of misfolded VHL tumor suppressor protein in the yeast cytoplasm, McClellan et al. (2005) reported the Hsp70 cochaperone Sti1/HOP to be required for degradation of VHL. They also reported the necessity of the Hsp90 chaperone system for elimination of misfolded VHL. In addition, the participation of the Hsp110 chaperone Sse1p was found for degradation of misfolded VHL. Ydj1p was not required for elimination of misfolded VHL (McClellan et al., 2005a). Surprisingly, except for Ssa1p, the requirement of factors required for elimination of the three cytosolic substrates tested in our work differs completely from the factors reported by McClellan et al. (2005) for degradation of VHL. Neither the Hsp90 family of chaperones nor the Hsp110 chaperone Sse1p is required for degradation of ΔssCG* (Figure 6, B and D). Although the cochaperone Sti1p/HOP is necessary for degradation of misfolded VHL (McClellan et al., 2005a), this factor is not involved in ΔssCG* degradation (Figure 6C). In contrast, the Hsp40 cochaperone Ydj1p is an important factor in ΔssCG* as well as ΔssCPY* and ΔssCPY elimination (Figure 5). Although McClellan et al. (2005) show only a minor portion of insoluble misfolded VHL in cells devoid of the Hsp70 cochaperone Sti1/HOP, the situation concerning ΔssCG* is again different.
In vitro analysis shows that in wild-type cells harboring all four Hsp70 species of the Ssa type (Figure 7B, WT) the majority of ΔssCG* is found in the soluble fraction of cells grown either at 30 or 37°C. As expected, the fluorescence of ΔssCG* is distributed throughout the cytoplasm of these cells in vivo (Figure 7C). In contrast, in vitro analysis at 30°C of SSA1 or ssa1-45ts cells harboring only one functional Ssa-species shows that the insoluble portion of ΔssCG* increases, indicating that one Ssa-species is at its limits in keeping the misfolded protein soluble. At 37°C the insolubility of ΔssCG* increases in SSA1 cells, and nearly all ΔssCG* material is insoluble in ssa1-45 cells, which lack Ssa1p activity at this temperature (Figure 7A). Similar results have been observed for ΔssCPY* and ΔssCPY (data not shown). This behavior is reflected in vivo when analyzing the fluorescence of ΔssCG* (Figure 7C). The fact that less aggregated ΔssCG* material is seen in the fluorescence images compared with the solubility assay in vitro may be due to the presence of oligomeric ΔssCG* species in vivo, which under in vitro conditions form insoluble precipitates. It is interesting to note that degradation of ΔssCG* is rapid and nearly complete in SSA1 cells at 37°C, indicating that the precipitated material is susceptible to degradation (Figure 1). It has been shown that the Hsp70 chaperone machinery is able to remodel and disaggregate protein aggregates in vitro (Zietkiewicz et al., 2006). Here we show that Ssa1 is able to resolubilize precipitated ΔssCG* material in vivo (Figure 7D). We also tested the involvement of Hsp104 and the small heat-shock proteins Hsp26 and Hsp42 in the degradation process of ΔssCG*. Surprisingly none of them exhibited any effect (Figure 6, E and F). Cells defective in the activity of the Hsp40 cochaperone Ydj1p also show increasing amounts of ΔssCG* aggregates (Figure 7, B and C). Degradation of ΔssCG* is not completely blocked in ydj1-151ts cells at the nonpermissive temperature of 37°C (Figure 5). The most likely explanation for this behavior is that Ssa1p is active without Ydj1p and that this cochaperone only augments the capacity of Hsp70 chaperone to disaggregate oligomeric and insoluble precipitates. The absence of Ydj1p dependency of misfolded VHL degradation may be due to the fact that this protein remains soluble in the cytoplasm and does not form aggregates (McClellan et al., 2005a). The Hsp40 cochaperones have a conserved J-domain, which is proposed to interact with Hsp70, and have been shown to exhibit a protective function in experimental model protein aggregation (Schaffar et al., 2004; Muchowski and Wacker, 2005; Novoselova et al., 2005). This implies that Ydj1p cannot be only some “specificity factor” for protein recognition, but rather represents an Ssa1p-linked activity enhancer. After substrate solubilization Ssa1p is obviously able to perform the additional tasks of keeping the substrate soluble and delivering it to the proteasome. The discovery that the neuronal Hsc70 cochaperone Hsj1p can act as a neuronal shuttling factor for sorting of chaperone clients to the proteasome supports this idea (Westhoff et al., 2005).
When comparing the protein quality control process in the two major folding compartments of the cells, the cytoplasm and the ER, it is obvious that similar mechanisms operate. As found for the Hsp70 class of Ssa-chaperones in the cytoplasm (Hartl and Hayer-Hartl, 2002; Deuerling and Bukau, 2004), the major Hsp70 protein of the ER, BiP in mammalian cells (Sitia and Braakman, 2003) or Kar2p in yeast, is required for protein folding (Simons et al., 1995). In case folding is not successful, Kar2p is necessary to prevent proteins from aggregation and keep soluble misfolded proteins of the ER in the soluble state (Nishikawa et al., 2001), to finally allow their retrotranslocation into the cytoplasm and degradation by the proteasome (Plemper et al., 1997; Brodsky et al., 1999). These functions of Kar2p are also dependent on cochaperones (Nishikawa et al., 2001). As shown here and elsewhere (McClellan et al., 2005a), Ssa1p together with its cochaperones seems to have parallel functions in the cytoplasm.
Central agents of CQD seem to be the Hsp70 chaperone Ssa1p (Figures 1 and 2 and McClellan et al., 2005), the ubiquitin-conjugating enzymes Ubc4p and Ubc5p (Figure 9 A and McClellan et al., 2005), and the proteasome (Figure 2 and McClellan et al., 2005). The ubiquitin protein ligase (E3) that functions in this system remains to be identified. We analyzed a subset of known ubiquitin protein ligases (E3's) Doa10p, Der3p (Figure 9B), Rsp5p, Hul5p, Ufd4p, and the SCF complex (data not shown). None of these ligases is involved in the degradation of the model substrate ΔssCG* in the cytoplasm. This suggests the involvement of a novel E3 in degradation process of the misfolded proteins in the cytoplasm.
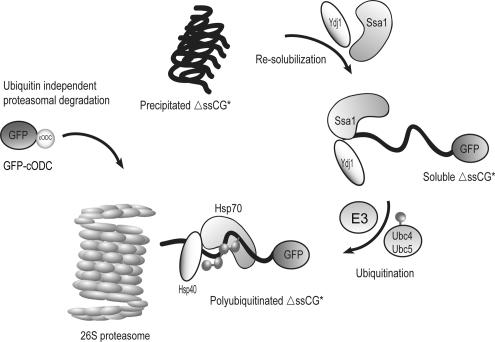
Our experiments show that the Hsp90 family of chaperones is not invariably needed for degradation of misfolded proteins (Figure 6B). In the case of degradation of misfolded VHL, Hsp90 action may be uniquely required to generate a specific conformation of this substrate, one that can subsequently be recognized by an ubiquitin ligase involved in quality control. The specific cochaperone required for Ssa1p-dependent ubiquitin–proteasome degradation of misfolded cytoplasmic proteins may depend on the function Ssa1p has to fulfill in this process. Because only the soluble form of ΔssCG* can be degraded by the proteasome, we consider the polyubiquitinated ΔssCG* material in wild-type and SSA1 cells at 37°C to be the steady state level of resolubilized and not yet degraded ΔssCG* (Figure 8B). Compared with wild-type and Ssa1p-proficient cells, a considerably greater amount of ubiquitinated soluble ΔssCG* material can be found in ssa1-45ts and ydj1-151ts cells under these restrictive conditions (Figure 8B), despite the fact that much less soluble ΔssCG* material is present in the mutant cells (Figures 7, A and B). From this one may conclude that ΔssCG* material ubiquitinated before the temperature shift of cells to 37°C may remain undegraded in the ubiquitinated state in the ssa1-45ts cells or less well degraded in the ydj1-151ts mutant after the temperature shift, because of inactivation of the chaperone proteins. The fact that polyubiquitinated protein material accumulates in ssa1-45ts mutant cells at the restrictive temperature of 37°C, despite the presence of an active proteasome (Figure 8B) indicates that Ssa1p may have a function beyond solubilization of precipitated protein material or keeping misfolded proteins soluble. We conclude that Ssa1p is likely to have several functions. Ssa1p can unfold proteins (Taxis et al., 2003), recognize misfolded protein domains (Figure 3), solubilize (and keep soluble) aggregated misfolded proteins (Figures 1A and 7D), and escort and deliver misfolded cytoplasmic proteins to the proteasome for degradation (Figure 10). The finding of an interaction of Ssa1p with the 26S proteasome (Verma et al., 2000; Coffino, P., and Maxwell, R. A., unpublished data) substantiates the validity of this last conclusion.
Figure 10.
Model of protein quality control in the cytoplasm. See text for details.
ACKNOWLEDGMENTS
We thank S. Lindquist, E. A. Craig, J. L. Brodsky, and J. Buchner for yeast strains, plasmids, and reagents. We are grateful to members of Wolf group and especially Dr. A. Schaefer for helpful discussions. This work was supported by grants from the German-Israeli Foundation for Scientific Research and Development (GIF), Jerusalem, the Deutsche Akademische Austauschdienst (DAAD), Bonn, the Fonds der Chemischen Industrie, Frankfurt, the EU Network of Excellence RUBICON, and the Deutsche Forschungsgemeinschaft (DFG), Bonn, to D. H. W. and by grants from the National Institutes of Health No. GM45335 and GM074760 to P. C.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-04-0338) on October 25, 2006.
REFERENCES
- Anken E., Braakman I., Craig E. Versatility of the endoplasmic reticulum protein folding factory. Crit. Rev. Biochem. Mol. Biol. 2005;40:191–228. doi: 10.1080/10409230591008161. [DOI] [PubMed] [Google Scholar]
- Ausubel F. M., Kingston R. E., Seidman F. G., Struhl K., Moore D. D., Brent R., Smith F. A. New York: Greene; 1992. Current Protocols in Molecular Biology. [Google Scholar]
- Barral J. M., Broadley S. A., Schaffar G., Hartl F. U. Roles of molecular chaperones in protein misfolding diseases. Semin. Cell Dev. Biol. 2004;15:17–29. doi: 10.1016/j.semcdb.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Becker J., Walter W., Yan W., Craig E. A. Functional interaction of cytosolic hsp70 and a DnaJ-related protein, Ydj1p, in protein translocation in vivo. Mol. Cell Biol. 1996;16:4378–4386. doi: 10.1128/mcb.16.8.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky J. L., McCracken A. A. ER protein quality control and proteasome-mediated protein degradation. Semin. Cell Dev. Biol. 1999;10:507–513. doi: 10.1006/scdb.1999.0321. [DOI] [PubMed] [Google Scholar]
- Brodsky J. L., Werner E. D., Dubas M. E., Goeckeler J. L., Kruse K. B., McCracken A. A. The requirement for molecular chaperones during endoplasmic reticulum-associated protein degradation demonstrates that protein export and import are mechanistically distinct. J. Biol. Chem. 1999;274:3453–3460. doi: 10.1074/jbc.274.6.3453. [DOI] [PubMed] [Google Scholar]
- Bukau B., Weissman J., Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Caplan A. J., Cyr D. M., Douglas M. G. YDJ1p facilitates polypeptide translocation across different intracellular membranes by a conserved mechanism. Cell. 1992;71:1143–1155. doi: 10.1016/s0092-8674(05)80063-7. [DOI] [PubMed] [Google Scholar]
- Cashikar A. G., Duennwald M., Lindquist S. L. A chaperone pathway in protein disaggregation. Hsp26 alters the nature of protein aggregates to facilitate reactivation by Hsp104. J. Biol. Chem. 2005;280:23869–23875. doi: 10.1074/jbc.M502854200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham M. E., Caplan A. J. Structure, function and evolution of DnaJ: conservation and adaptation of chaperone function. Cell Stress Chaperones. 1998;3:28–36. doi: 10.1379/1466-1268(1998)003<0028:sfaeod>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang H. L., Schekman R. Regulated import and degradation of a cytosolic protein in the yeast vacuole. Nature. 1991;350:313–318. doi: 10.1038/350313a0. [DOI] [PubMed] [Google Scholar]
- Connell P., Ballinger C. A., Jiang J., Wu Y., Thompson L. J., Hohfeld J., Patterson C. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat. Cell Biol. 2001;3:93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- Cyr D. M., Hohfeld J., Patterson C. Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem. Sci. 2002;27:368–375. doi: 10.1016/s0968-0004(02)02125-4. [DOI] [PubMed] [Google Scholar]
- Deak P. Stuttgart: University of Stuttgart; 1998. Mechanistische Untersuchungen zur ER-Degradation con CPY in Saccharomyces cerevisiae, Diploma thesis. [Google Scholar]
- Demand J., Alberti S., Patterson C., Hohfeld J. Cooperation of a ubiquitin domain protein and an E3 ubiquitin ligase during chaperone/proteasome coupling. Curr. Biol. 2001;11:1569–1577. doi: 10.1016/s0960-9822(01)00487-0. [DOI] [PubMed] [Google Scholar]
- Deuerling E., Bukau B. Chaperone-assisted folding of newly synthesized proteins in the cytosol. Crit. Rev. Biochem. Mol. Biol. 2004;39:261–277. doi: 10.1080/10409230490892496. [DOI] [PubMed] [Google Scholar]
- Dobson C. M. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- Elsasser S., Finley D. Delivery of ubiquitinated substrates to protein-unfolding machines. Nat. Cell Biol. 2005;7:742–749. doi: 10.1038/ncb0805-742. [DOI] [PubMed] [Google Scholar]
- Endrizzi J. A., Breddam K., Remington S. J. 2.8-A structure of yeast serine carboxypeptidase. Biochemistry. 1994;33:11106–11120. doi: 10.1021/bi00203a007. [DOI] [PubMed] [Google Scholar]
- Esser C., Alberti S., Hohfeld J. Cooperation of molecular chaperones with the ubiquitin/proteasome system. Biochim. Biophys. Acta. 2004;1695:171–188. doi: 10.1016/j.bbamcr.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Fan C. Y., Lee S., Cyr D. M. Mechanisms for regulation of Hsp70 function by Hsp40. Cell Stress Chaperones. 2003;8:309–316. doi: 10.1379/1466-1268(2003)008<0309:mfrohf>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger A., Knop M., Wolf D. H. Analysis of two mutated vacuolar proteins reveals a degradation pathway in the endoplasmic reticulum or a related compartment of yeast. Eur. J. Biochem. 1993;218:565–574. doi: 10.1111/j.1432-1033.1993.tb18410.x. [DOI] [PubMed] [Google Scholar]
- Frydman J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 2001;70:603–647. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- Glover J. R., Lindquist S. Hsp104, Hsp70, and Hsp 40, a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Fink G. R. Guide to Yeast Genetics and Molecular Biology: Methods in Enzymology. Vol. 194. San Diego, CA: Academic Press; 1991. [PubMed] [Google Scholar]
- Hartl F. U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Hartmann-Petersen R., Gordon C. Protein degradation: recognition of ubiquitinylated substrates. Curr. Biol. 2004;14:R754–R756. doi: 10.1016/j.cub.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Haslbeck M., Braun N., Stromer T., Richter B., Model N., Weinkauf S., Buchner J. Hsp42 is the general small heat shock protein in the cytosol of Saccharomyces cerevisiae. EMBO J. 2004;23:638–649. doi: 10.1038/sj.emboj.7600080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslbeck M., Walke S., Stromer T., Ehrnsperger M., White H. E., Chen S., Saibil H. R., Buchner J. Hsp26, a temperature-regulated chaperone. EMBO J. 1999;18:6744–6751. doi: 10.1093/emboj/18.23.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeyer W., Kleinschmidt J. A., Saidowsky J., Escher C., Wolf D. H. Proteinase yscE, the yeast proteasome/multicatalytic-multifunctional proteinase: mutants unravel its function in stress induced proteolysis and uncover its necessity for cell survival. EMBO J. 1991;10:555–562. doi: 10.1002/j.1460-2075.1991.tb07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hiller M. M., Finger A., Schweiger M., Wolf D. H. ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science. 1996;273:1725–1728. doi: 10.1126/science.273.5282.1725. [DOI] [PubMed] [Google Scholar]
- Hirsch C., Jarosch E., Sommer T., Wolf D. H. Endoplasmic reticulum-associated protein degradation-one model fits all? Biochim. Biophys. Acta. 2004;1695:215–223. doi: 10.1016/j.bbamcr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M. Protein degradation or regulation: Ub the judge. Cell. 1996;84:813–815. doi: 10.1016/s0092-8674(00)81058-2. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., Coffino P. Ubiquitin-free routes into the proteasome. Cell Mol. Life Sci. 2004;61:1596–1600. doi: 10.1007/s00018-004-4133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt M. A., Zhang M., Coffino P. Ubiquitin-independent mechanisms of mouse ornithine decarboxylase degradation are conserved between mammalian and fungal cells. J. Biol. Chem. 2003;278:12135–12143. doi: 10.1074/jbc.M211802200. [DOI] [PubMed] [Google Scholar]
- Huyer G., Piluek W. F., Fansler Z., Kreft S. G., Hochstrasser M., Brodsky J. L., Michaelis S. Distinct machinery is required in Saccharomyces cerevisiae for the endoplasmic reticulum-associated degradation of a multispanning membrane protein and a soluble luminal protein. J. Biol. Chem. 2004;279:38369–38378. doi: 10.1074/jbc.M402468200. [DOI] [PubMed] [Google Scholar]
- Jamsa E., Simonen M., Makarow M. Selective retention of secretory proteins in the yeast endoplasmic reticulum by treatment of cells with a reducing agent. Yeast. 1994;10:355–370. doi: 10.1002/yea.320100308. [DOI] [PubMed] [Google Scholar]
- Jarosch E., Taxis C., Volkwein C., Bordallo J., Finley D., Wolf D. H., Sommer T. Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nat. Cell Biol. 2002;4:134–139. doi: 10.1038/ncb746. [DOI] [PubMed] [Google Scholar]
- Jiang J., Ballinger C. A., Wu Y., Dai Q., Cyr D. M., Hohfeld J., Patterson C. CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J. Biol. Chem. 2001;276:42938–42944. doi: 10.1074/jbc.M101968200. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Craig E. A. An essential role for the substrate-binding region of Hsp40s in Saccharomyces cerevisiae. J. Cell Biol. 2001;152:851–856. doi: 10.1083/jcb.152.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King F. W., Wawrzynow A., Hohfeld J., Zylicz M. Co-chaperones Bag-1, Hop and Hsp40 regulate Hsc70 and Hsp90 interactions with wild-type or mutant p53. EMBO J. 2001;20:6297–6305. doi: 10.1093/emboj/20.22.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleizen B., Braakman I. Protein folding and quality control in the endoplasmic reticulum. Curr. Opin. Cell Biol. 2004;16:343–349. doi: 10.1016/j.ceb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Knop M., Finger A., Braun T., Hellmuth K., Wolf D. H. Der1, a novel protein specifically required for endoplasmic reticulum degradation in yeast. EMBO J. 1996;15:753–763. [PMC free article] [PubMed] [Google Scholar]
- Knop M., Schiffer H. H., Rupp S., Wolf D. H. Vacuolar/lysosomal proteolysis: proteases, substrates, mechanisms. Curr. Opin. Cell Biol. 1993;5:990–996. doi: 10.1016/0955-0674(93)90082-2. [DOI] [PubMed] [Google Scholar]
- Kopito R. R. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- Kostova Z., Wolf D. H. For whom the bell tolls: protein quality control of the endoplasmic reticulum and the ubiquitin-proteasome connection. EMBO J. 2003;22:2309–2317. doi: 10.1093/emboj/cdg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostova Z., Wolf D. H. Importance of carbohydrate positioning in the recognition of mutated CPY for ER-associated degradation. J. Cell Sci. 2005;118:1485–1492. doi: 10.1242/jcs.01740. [DOI] [PubMed] [Google Scholar]
- Lee D. H., Sherman M. Y., Goldberg A. L. Involvement of the molecular chaperone Ydj1 in the ubiquitin-dependent degradation of short-lived and abnormal proteins in Saccharomyces cerevisiae. Mol. Cell Biol. 1996;16:4773–4781. doi: 10.1128/mcb.16.9.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine C. G., Mitra D., Sharma A., Smith C. L., Hegde R. S. The efficiency of protein compartmentalization into the secretory pathway. Mol. Biol. Cell. 2005;16:279–291. doi: 10.1091/mbc.E04-06-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. W., Corboy M. J., DeMartino G. N., Thomas P. J. Endoproteolytic activity of the proteasome. Science. 2003;299:408–411. doi: 10.1126/science.1079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum R., Tkach J. M., Vierling E., Glover J. R. Evidence for an unfolding/threading mechanism for protein disaggregation by Saccharomyces cerevisiae Hsp104. J. Biol. Chem. 2004;279:29139–29146. doi: 10.1074/jbc.M403777200. [DOI] [PubMed] [Google Scholar]
- Mayer M. P., Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol. Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan A. J., Scott M. D., Frydman J. Folding and quality control of the VHL tumor suppressor proceed through distinct chaperone pathways. Cell. 2005a;121:739–748. doi: 10.1016/j.cell.2005.03.024. [DOI] [PubMed] [Google Scholar]
- McClellan A. J., Tam S., Kaganovich D., Frydman J. Protein quality control: chaperones culling corrupt conformations. Nat. Cell Biol. 2005b;7:736–741. doi: 10.1038/ncb0805-736. [DOI] [PubMed] [Google Scholar]
- Medicherla B., Kostova Z., Schaefer A., Wolf D. H. A genomic screen identifies Dsk2p and Rad23p as essential components of ER-associated degradation. EMBO Rep. 2004;5:692–697. doi: 10.1038/sj.embor.7400164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski P. J., Wacker J. L. Modulation of neurodegeneration by molecular chaperones. Nat. Rev. Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- Murata S., Minami Y., Minami M., Chiba T., Tanaka K. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep. 2001;2:1133–1138. doi: 10.1093/embo-reports/kve246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D. F., Vos M. H., Lindquist S. In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc. Natl. Acad. Sci. USA. 1997;94:12949–12956. doi: 10.1073/pnas.94.24.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S. I., Fewell S. W., Kato Y., Brodsky J. L., Endo T. Molecular chaperones in the yeast endoplasmic reticulum maintain the solubility of proteins for retrotranslocation and degradation. J. Cell Biol. 2001;153:1061–1070. doi: 10.1083/jcb.153.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoselova T. V., Margulis B. A., Novoselov S. S., Sapozhnikov A. M., van der Spuy J., Cheetham M. E., Guzhova I. V. Treatment with extracellular HSP70/HSC70 protein can reduce polyglutamine toxicity and aggregation. J. Neurochem. 2005;94:597–606. doi: 10.1111/j.1471-4159.2005.03119.x. [DOI] [PubMed] [Google Scholar]
- Parsell D. A., Kowal A. S., Singer M. A., Lindquist S. Protein disaggregation mediated by heat-shock protein Hsp104. Nature. 1994;372:475–478. doi: 10.1038/372475a0. [DOI] [PubMed] [Google Scholar]
- Parsell D. A., Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Pfund C., Huang P., Lopez-Hoyo N., Craig E. A. Divergent functional properties of the ribosome-associated molecular chaperone Ssb compared with other Hsp70s. Mol. Biol. Cell. 2001;12:3773–3782. doi: 10.1091/mbc.12.12.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfund C., Lopez-Hoyo N., Ziegelhoffer T., Schilke B. A., Lopez-Buesa P., Walter W. A., Wiedmann M., Craig E. A. The molecular chaperone Ssb from Saccharomyces cerevisiae is a component of the ribosome-nascent chain complex. EMBO J. 1998;17:3981–3989. doi: 10.1093/emboj/17.14.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart C. M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- Plemper R. K., Bohmler S., Bordallo J., Sommer T., Wolf D. H. Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature. 1997;388:891–895. doi: 10.1038/42276. [DOI] [PubMed] [Google Scholar]
- Ravid T., Kreft S. G., Hochstrasser M. Membrane and soluble substrates of the Doa10 ubiquitin ligase are degraded by distinct pathways. EMBO J. 2006;25:533–543. doi: 10.1038/sj.emboj.7600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudiger S., Schneider-Mergener J., Bukau B. Its substrate specificity characterizes the DnaJ co-chaperone as a scanning factor for the DnaK chaperone. EMBO J. 2001;20:1042–1050. doi: 10.1093/emboj/20.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer A., Wolf D. H. Yeast genomics in the elucidation of endoplasmic reticulum (ER) quality control and associated protein degradation (ERQD) In: Deshaies R. J., editor. Methods in Enzymology. Vol. 399. Academic Press; 2005. pp. 459–468. [DOI] [PubMed] [Google Scholar]
- Schafer A., Wolf D. H. CPY* and the power of yeast genetics in the elucidation of quality control and associated protein degradation of endoplasmic reticulum. Curr. Top. Microbiol. Immunol. 2006;300:41–56. doi: 10.1007/3-540-28007-3_3. [DOI] [PubMed] [Google Scholar]
- Schaffar G., Breuer P., Boteva R., Behrends C., Tzvetkov N., Strippel N., Sakahira H., Siegers K., Hayer-Hartl M., Hartl F. U. Cellular toxicity of polyglutamine expansion proteins: mechanism of transcription factor deactivation. Mol. Cell. 2004;15:95–105. doi: 10.1016/j.molcel.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Seufert W., Jentsch S. Ubiquitin-conjugating enzymes UBC4 and UBC5 mediate selective degradation of short-lived and abnormal proteins. EMBO J. 1990;9:543–550. doi: 10.1002/j.1460-2075.1990.tb08141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaff R. J., Singer J. D., Swanger J., Smitherman M., Roberts J. M., Clurman B. E. Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination. Mol. Cell. 2000;5:403–410. doi: 10.1016/s1097-2765(00)80435-9. [DOI] [PubMed] [Google Scholar]
- Simons J. F., Ferro-Novick S., Rose M. D., Helenius A. BiP/Kar2p serves as a molecular chaperone during carboxypeptidase Y folding in yeast. J. Cell Biol. 1995;130:41–49. doi: 10.1083/jcb.130.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R., Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–894. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- Sommer T., Wolf D. H. Endoplasmic reticulum degradation: reverse protein flow of no return. FASEB J. 1997;11:1227–1233. doi: 10.1096/fasebj.11.14.9409541. [DOI] [PubMed] [Google Scholar]
- Sondermann H., Ho A. K., Listenberger L. L., Siegers K., Moarefi I., Wente S. R., Hartl F. U., Young J. C. Prediction of novel Bag-1 homologs based on structure/function analysis identifies Snl1p as an Hsp70 co-chaperone in Saccharomyces cerevisiae. J. Biol. Chem. 2002;277:33220–33227. doi: 10.1074/jbc.M204624200. [DOI] [PubMed] [Google Scholar]
- Sondermann H., Scheufler C., Schneider C., Hohfeld J., Hartl F. U., Moarefi I. Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science. 2001;291:1553–1557. doi: 10.1126/science.1057268. [DOI] [PubMed] [Google Scholar]
- Swanson R., Locher M., Hochstrasser M. A conserved ubiquitin ligase of the nuclear envelope/endoplasmic reticulum that functions in both ER-associated and Matalpha2 repressor degradation. Genes Dev. 2001;15:2660–2674. doi: 10.1101/gad.933301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxis C., Hitt R., Park S. H., Deak P. M., Kostova Z., Wolf D. H. Use of modular substrates demonstrates mechanistic diversity and reveals differences in chaperone requirement of ERAD. J. Biol. Chem. 2003;278:35903–35913. doi: 10.1074/jbc.M301080200. [DOI] [PubMed] [Google Scholar]
- Tofaris G. K., Layfield R., Spillantini M. G. alpha-synuclein metabolism and aggregation is linked to ubiquitin-independent degradation by the proteasome. FEBS Lett. 2001;509:22–26. doi: 10.1016/s0014-5793(01)03115-5. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. The ubiquitin system. Trends Biochem. Sci. 1997;22:383–387. doi: 10.1016/s0968-0004(97)01122-5. [DOI] [PubMed] [Google Scholar]
- Verma R., Deshaies R.J. A proteasome howdunit: the case of the missing signal. Cell. 2000;101:341–344. doi: 10.1016/s0092-8674(00)80843-0. [DOI] [PubMed] [Google Scholar]
- Wegele H., Haslbeck M., Reinstein J., Buchner J. Sti1 is a novel activator of the Ssa proteins. J. Biol. Chem. 2003;278:25970–25976. doi: 10.1074/jbc.M301548200. [DOI] [PubMed] [Google Scholar]
- Westhoff B., Chapple J. P., van der Spuy J., Hohfeld J., Cheetham M. E. HSJ1 is a neuronal shuttling factor for the sorting of chaperone clients to the proteasome. Curr. Biol. 2005;15:1058–1064. doi: 10.1016/j.cub.2005.04.058. [DOI] [PubMed] [Google Scholar]
- Wolf D. H., Hilt W. The proteasome: a proteolytic nanomachine of cell regulation and waste disposal. Biochim. Biophys. Acta. 2004;1695:19–31. doi: 10.1016/j.bbamcr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Zhang M., MacDonald A. I., Hoyt M. A., Coffino P. Proteasomes begin ornithine decarboxylase digestion at the C terminus. J. Biol. Chem. 2004;279:20959–20965. doi: 10.1074/jbc.M314043200. [DOI] [PubMed] [Google Scholar]
- Zhang M., Pickart C. M., Coffino P. Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate. EMBO J. 2003;22:1488–1496. doi: 10.1093/emboj/cdg158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietkiewicz S., Lewandowska A., Stocki P., Liberek K. Hsp70 chaperone machine remodels protein aggregates at the initial step of Hsp70-Hsp100-dependent disaggregation. J. Biol. Chem. 2006;281:7022–7029. doi: 10.1074/jbc.M507893200. [DOI] [PubMed] [Google Scholar]