Abstract
Macrophages are an important source of vascular endothelial growth factor (VEGF). Adenosine A2A receptor (A2AR) agonists with Toll-like receptor (TLR) 2, 4, 7, and 9 agonists synergistically induce macrophage VEGF expression. We show here using VEGF promoter-luciferase reporter constructs that the TLR4 agonist Escherichia coli lipopolysaccharide (LPS) and the A2AR agonists NECA and CGS21680 synergistically augment VEGF transcription in macrophages and that the HRE in the VEGF promoter is essential for this transcription. We examined whether LPS and/or NECA induce HIF-1α expression. HIF-1α mRNA levels were increased in LPS-treated macrophages in an NF-κB–dependent manner; NECA strongly increased these levels in an A2AR-dependent manner. LPS induced luciferase expression from a HIF-1α promoter-luciferase construct in an A2AR-independent manner. Further stimulation with NECA did not increase HIF-1α promoter activity, indicating that the A2AR-dependent increase in HIF-1α mRNA is post-transcriptional. LPS/NECA treatment also increased HIF-1α protein and DNA binding levels. Deletion of putative NF-κB–binding sites from the VEGF promoter did not affect LPS/NECA-induced VEGF promoter activity, suggesting that NF-κB is not directly involved in VEGF transcription. Taken together, these data indicate that LPS/NECA-induced VEGF expression involves transcriptional regulation of the VEGF promoter by HIF-1α through the HRE. HIF-1α is transcriptionally induced by LPS and post-transcriptionally up-regulated in an A2AR-dependent manner.
INTRODUCTION
Macrophages play a key role in induction of angiogenesis, which is crucial for wound healing, fibroproliferative responses, and solid tumor development (Crowther et al., 2001). When stimulated, macrophages secrete an array of cytokines and growth factors, including the potent angiogenic factor vascular endothelial growth factor (VEGF). VEGF is an endothelial cell–specific mitogen and plays an important role in vascular development and angiogenesis during embryogenesis, wound healing, solid tumor growth, and certain chronic fibroproliferative inflammatory diseases (Ferrara and Davis-Smyth, 1997). Macrophages are exquisitely sensitive to their microenvironment and produce VEGF in a tightly regulated manner (Crowther et al., 2001).
Macrophages produce elevated levels of VEGF in response to a variety of stimuli, including hypoxia and endotoxin (lipopolysaccharide [LPS]) together with interferon-γ (IFN-γ), and growth factors, and cytokines such as TGF-α, TGF-β, IL-1β, and IL-6 (Goldman et al., 1993; Pertovaara et al., 1994; Levy et al., 1995; Cohen et al., 1996; Gille et al., 1997; Xiong et al., 1998). Other stimuli such as hydrogen peroxide and nitric oxide (NO) have also been implicated in VEGF up-regulation (Kimura et al., 2000; Cho et al., 2001). We have shown previously that VEGF expression by murine macrophages is synergistically up-regulated by Escherichia coli LPS acting through Toll-like receptor, TLR4, receptors with adenosine acting through A2A receptors (A2ARs; Leibovich et al., 2002). The synergy between A2AR agonists and E. coli LPS that results in the up-regulation of VEGF production is not limited to TLR4, but can also be induced by TLR2, 7, and 9 agonists (Pinhal-Enfield et al., 2003). In this study we have investigated further the mechanism of this increased VEGF gene expression in murine macrophages by LPS (a TLR4 agonist) with 5′-N-ethyl-carboxamidoadenosine (NECA), or 2-[p-(2-carboxylethyl)-phenylethyl amino]-5′-N-ethyl-carboxamido-adenosine (CGS21680) (A2AR agonists). Using a VEGF promoter-luciferase reporter construct transfected into RAW 264.7 cells, we demonstrate that a strong synergistic activation of the VEGF promoter is induced by coactivation of TLR4 and A2AR, but not of either receptor alone. Subsequently, we studied the cis-element(s) essential for this transcriptional activation by constructing a series of luciferase reporter constructs containing the VEGF promoter with specific deletions of individual known cis-elements. The hypoxia response element (HRE) of the VEGF promoter was found to be vital for the LPS/NECA-induced transcription. The HRE is a region of the promoter involved in the induction of VEGF by hypoxia and is the binding site for hypoxia inducible factor-1 (HIF-1). HIF-1 is a dimeric transcription factor consisting of HIF-1α and HIF-1β (ARNT) (Liu et al., 1995; Forsythe et al., 1996; Damert et al., 1997; Ema et al., 1997). HIF-1β is stable and constitutively expressed. Although HIF-1α is constitutively expressed, it is extremely unstable under normoxic conditions and is stabilized by hypoxia (Guillemin and Krasnow, 1997). Increased steady state levels of HIF-1α protein have been reported to be induced, even under normoxic conditions, by signaling pathways other than hypoxia (Zelzer et al., 1998; Kimura et al., 2000; Laughner et al., 2001; Jung et al., 2003a, 2003b, 2003c; Blouin et al., 2004; Frede et al., 2006). For example, HIF-1α has been found to be induced in tumor cell lines by a variety of growth factors and hormones, including insulin, insulin-like growth factor, angiotensin II, and epidermal growth factor (Zelzer et al., 1998; Feldser et al., 1999; Richard et al., 2000; Zhong et al., 2000). In these studies, increased HIF-1α protein levels were generally the result of increased translation of HIF-1α mRNA or increased stabilization of HIF-1α protein. Because the synergistic activation of VEGF promoter constructs by TLR4 and A2AR ligation was found to require the presence of the HRE in the VEGF promoter, we tested the hypothesis that this ligation triggers a signaling cascade that leads to increased expression of HIF-1α, and to increased binding of HIF-1 to the HRE, resulting in increased transcription of the VEGF gene.
MATERIALS AND METHODS
Reagents
E. coli LPS was purchased from Sigma Chemical Co. (St. Louis, MO). NECA and 2-[p-(2-carboxylethyl)-phenylethyl amino]-5′-N-ethyl-carboxamido-adenosine (CGS21680) were purchased from Sigma Chemical Co. Panepoxydone, SN 50, and BAY11-7085 were purchased from Axxora (San Diego, CA). ZM241385 was purchased from Tocris-Cookson (Bristol, United Kingdom).
Animals
C57Bl/6J mice (female, 7–8 wk) were purchased from Jackson Laboratories (Bar Harbor, ME). The animal experimentation protocols were approved by the New Jersey Medical School (NJMS) animal care and use committee (IACUC).
Cell Culture
Murine peritoneal macrophages were harvested from C57Bl/6J mice (7–8 wk) as described previously (Xiong et al., 1998). Mice were injected intraperitoneally with 2.5 ml thioglycolate broth, and 4 d later peritoneal macrophages were harvested. RAW 264.7 (murine macrophage-like cell line) cells were purchased from American Type Culture Collection (Manassas, VA). The cells were cultured as a monolayer in RPMI 1640 medium (Cellgro, Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS; Gemini Bio-Products, Calabasas, CA), 2 mM l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Irvine Scientific, Santa Ana, CA). The cells were incubated at 37°C in a humidified incubator in 5% CO2 and 95% air. All experiments were performed at a concentration of 1 × 106 cells/ml medium. Cells were plated 20–24 h before stimulation. The medium used for stimulation contained 1% FBS, and cells were stimulated with LPS (100 ng/ml), NECA (1 μM), a combination of LPS and NECA, or hypoxia (1% O2). For incubation of cells under hypoxic conditions, cultures were placed in a hypoxia chamber (Billups-Rothenberg, Del Mar, CA). The chamber was filled with a gas mixture of 1% O2, 5% CO2, and 94% N2. The sealed chamber was then placed in a 37°C incubator. Conditioned media were harvested 24 h after stimulation and stored at −20°C.
VEGF and TNF-α Assays
VEGF and TNF-α levels in the macrophage-conditioned media were assayed using Quantikine M murine VEGF and TNF-α ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol. The assays were linear in the range of 7.8 to 500 pg/ml for VEGF and 23.4 to 1500 pg/ml for TNF-α. Samples were assayed in duplicate and results are expressed as means ± SD.
MTT Assay
Viability of the cells was assessed by MTT (methyl thiazole tetrazolium) assay as described previously (Denizot and Lang, 1986).
Extraction of RNA and Real-Time PCR
Mouse peritoneal macrophages were plated in 60-mm dishes (4 × 106 cells/dish). After overnight incubation, cells were treated with various reagents as indicated in the figure legends. RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol for isolation of total RNA from animal cells. RNA was quantified using SYBR Green II (Sigma) according to the manufacturer's instructions. Real-time quantitative PCRs were performed by means of TaqMan technology and ABI 7500 Real-time PCR system (Applied Biosystems, Foster City, CA).
cDNA was synthesized from total RNA using TaqMan Reverse Transcription Reagents (Applied Biosystems) following the manufacturer's instructions. Real-time PCR was performed in an ABI Prism 7500 Sequence Detector (Columbia, MD) using 1/10th volume of each cDNA reaction and TaqMan Universal PCR Master Mix. The TaqMan Gene Expression Assay for cyclophilin D (Mm00835365_g1) was purchased from Applied Biosystems. Primers and probes for mouse VEGF and HIF-1α mRNA were designed using Applied Biosystems' Primer Express 2.0 software and synthesized at the Molecular Resource Facility at NJMS-UMDNJ (Newark, NJ). 5′-ends of the probes were labeled with the fluorescent dye, 6-carboxy fluorescene (FAM), and 3′-ends were coupled to the quencher molecule, Black Hole Quencher dye-1 (BHQ-1). Sequences of primers and probes are presented in Table 1. The real-time PCR reactions were carried out using the manufacturer's protocol for absolute quantification. After initial denaturation at 95°C for 10 min, reactions were subjected to 40 cycles of PCR, each cycle consisting of 15 s at 95°C, followed by 60 s at 60°C. For each sample, gene expression levels of VEGF and HIF-1α were normalized to that of endogenous cyclophilin D, and data were calculated as fold expression relative to the average of the untreated control group.
Table 1.
Primers and probes used in TaqMan real-time PCR
| Target | Primers and probes | |
|---|---|---|
| VEGF | Forward primer: | 5′-TGCACCCACGACAGAAGGA-3′ |
| Reverse primer: | 5′-GGCAGTAGCTTCGCTGGTAGAC-3′ | |
| Probe: | 5′-AGAAGTCCCATGAAGTGATCAAG-3′ | |
| HIF-1α | Forward primer: | 5′-CTCAGAGGAAGCGAAAAATGGA-3′ |
| Reverse primer: | 5′-CAGTCACCTGGTTGCTGCAAT-3′ | |
| Probe: | 5′-CTCCCTTTTTCAAGCAGCAGGAATTGGAA-3′ |
Plasmids
The pGL3-basic (promoterless) luciferase vector and phRL-TK (Renilla luciferase reporter plasmid) were purchased from Promega (Madison, WI). pHRE-Luc (a plasmid with four HREs in tandem, cloned in the pGL3-promoter vector) was kindly provided by Dr. Sean Colgan (Harvard Medical School, Boston, MA). The pHXN1aLuc plasmid containing the murine HIF1α exon I.2 promoter in the pGL3-basic vector was a kind gift of Dr. Roland H. Wenger (University of Zurich, Switzerland; Wenger et al., 1998).
Construction of Mouse VEGF Promoter Deletion Luciferase Constructs
A 1.3-kb fragment (−963 to +404) containing the promoter region of the mouse VEGF gene (GenBank TM/EMBL databank accession number U41393; Shima et al., 1996) was cloned into the pGL3-basic vector. This construct was designated as pVEGF-Luc. Specific cis-acting elements were then deleted from pVEGF-Luc by inverse PCR, using two primers in inverted tail-to-tail directions to amplify the entire pVEGF-Luc plasmid, excluding the region to be deleted. For each deletion, a specific primer pair flanking the site to be deleted was designed (Table 2). The amplified plasmid was then recircularized and grown in E. coli strain XL10-Gold (Stratagene, La Jolla, CA). The VEGF promoter insert with the desired deletion was then excised from the amplified plasmid and religated into the original pGL3-basic vector, to ensure that the plasmid and luciferase gene remained error-free. The resulting constructs are schematically depicted in Figure 1 and were designated as pVEGF(-HRE) Luc (−917 to −893 deleted), pVEGF(-AP-1) Luc (−881 to −872 deleted), pVEGF(-AP-2) Luc (−778 to −695 deleted), pVEGF(-NF-κB) Luc (−222 to −107 deleted), and pVEGF(-Sp1) Luc (−77 to −55 deleted). All sequences were confirmed by automated sequencing using 3130xl Genetic Analyzer (Applied Biosystems), to ensure that no unanticipated mutations were introduced by the cloning procedure.
Table 2.
PCR primers used to delete specific cis-elements from the murine VEGF promoter
| Deletion | PCR primers |
|---|---|
| HRE | For: 5′-CACTCCCCGCCACTGACTAA-3′ |
| Rev: 5′-TGCACTGTGTAGTCTGGCAGAGC-3′ | |
| AP-1 | For: 5′-TCCAGAACTCCACTTCCCGTT-3′ |
| Rev: 5′-AGACGACCTGTGGAAACCCACGTA-3′ | |
| AP-2 | For: 5′-GCTTCCGAGGTCAAACACGC-3′ |
| Rev: 5′-TGAATGGGATCCTCTGGGAAG-3′ | |
| NF-κB | For: 5′-AAAGGCGGTGCCTGGTCTCA-3′ |
| Rev: 5′-GGGAATCAGGGGAGACAGGAGAGTGAGG-3′ | |
| Sp1 | For: 5′-GCTTGGGGGTGGAGCTAGATTTCC-3′ |
| Rev: 5′-AGCGGTCTGGTGGAGCCAGGCA-3′ |
Figure 1.
Schematic representation of the mouse VEGF promoter-luciferase reporter construct and its derivative plasmids containing deletions of specific cis-elements from the VEGF promoter.
Transient Transfections and Reporter Assays
All plasmids were prepared using PhoenIX Midiprep Kit (Qbiogene, Carlsbad, CA) and were electrophoresed to confirm that they were in the supercoiled form. RAW 264.7 cells were transiently transfected using Superfect (Qiagen, Valencia, CA) as described previously (Ramanathan et al., 2003). Cells were cotransfected with the Renilla luciferase vector, phRL-TK (Promega), in order to normalize for transfection efficiency. After 18-h incubation, the transfectants were resuspended in fresh medium and plated into six-well plates at a density of 0.625 × 106 cells/well (0.0625 × 106 cells/cm2). The cells were allowed to adhere for 6–7 h at 37°C. The medium was then changed to RPMI 1640 with 1% FBS, and the cells were stimulated as follows: LPS (100 ng/ml), NECA (1 μM), CGS21680 (1 μM), LPS/NECA, LPS/CGS21680, or hypoxia. In some cases, ZM241385 (1 μM), an inhibitor of A2AR signaling, was added to the media. After 22–24-h incubation, cells were lysed with passive lysis buffer (Promega) and luciferase assays were performed using the Dual-luciferase Assay Kit (Promega) following the manufacturer's protocol. Luciferase light units were measured using Lmax Luminescence Microplate Reader (Molecular Devices, Sunnyvale, CA) using dual injector system. Firefly luciferase light units were normalized to Renilla luciferase light units, to normalize for transfection efficiency. The results reported are representative of at least three independent experiments.
Preparation of Nuclear Extracts
Murine peritoneal macrophages or RAW264.7 cells were plated in 100-mm dishes. After overnight incubation, the medium was changed to RPMI 1640 with 1% FBS, and the cells were stimulated as described above for 12 h. The nuclear extracts were prepared as described previously (Pinhal-Enfield et al., 2003) and stored at −80°C until use. The protein concentrations were determined by the Bradford method using the Bio-Rad protein assay dye reagent (Bio-Rad Laboratories, Hercules, CA).
Western Blot Analyses
Nuclear extracts (50 μg) were resolved using SDS-PAGE. After transfer onto nitrocellulose membranes, HIF-1α protein was detected using rabbit polyclonal antibody NB 100-479 from Novus Biologicals (Littleton, CO). The blots were developed using an enhanced chemifluoroescence (ECF) system (GE Healthcare, Piscataway, NJ) and visualized using a Typhoon 9410 variable mode imager (GE Healthcare). To control for sample loading, blots were also stained with a rabbit polyclonal antibody to nucleoplasmin (Cell Signaling Technology, Beverly, MA).
Trans-AM HIF-1α Assay
Activation of HIF-1α was quantified using an ELISA-based assay kit (Trans-AM HIF-1; Active Motif, Carlsbad, CA). The assay was performed following the manufacturer's instructions with some important modifications. Nuclear protein (25 μg) was incubated in a 96-well plate coated with an oligonucleotide containing the HIF-1–binding site. The presence of the HIF-1 transcription complex was evaluated with a 1:5000 dilution of an anti HIF-1α mAb (NB 100-123) from Novus Biologicals instead of the antibody from the kit, because the supplied antibody recognized human but not mouse HIF-1α. HIF-1 binding was then detected by incubation with an HRP-conjugated secondary antibody and substrate. Results are expressed as the fold increase of the absorbance at 450 nm over control conditions.
RESULTS
Coligation of TLR4 and A2ARs Increases Steady State Levels of VEGF mRNA
Previously, we have reported that LPS with NECA or CGS21680 synergistically up-regulate VEGF expression in macrophages (Leibovich et al., 2002). To understand the mechanism of this up-regulation, murine peritoneal macrophages were stimulated with LPS, NECA, a combination of LPS and NECA, or hypoxia for 4, 8, 12, and 16 h. Total RNA was harvested and reverse-transcribed, and cDNA was used to perform TaqMan real-time PCR analysis for the quantification of VEGF mRNA levels. Both LPS and NECA, when added individually, failed to enhance VEGF mRNA levels (Figure 2), whereas LPS/NECA treatment caused a significant and persistent induction of VEGF gene expression. This induction was apparent by 8 h (6-fold), reached peak levels at 12 h (9.7-fold), and increased only slightly thereafter (10.2-fold at 16 h). Because hypoxia has been shown in numerous studies (Levy et al., 1995; Liu et al., 1995; Shima et al., 1995; Wang et al., 1995; Forsythe et al., 1996; Xiong et al., 1998; Fukumura et al., 2001; Ramanathan et al., 2003) to induce VEGF gene expression, total RNA extracted from macrophages incubated under hypoxic conditions was also analyzed to compare with the level of induction by LPS/NECA. Hypoxic induction of VEGF mRNA was rapid, reaching 5.3-fold by 4 h, increasing to 12.7- and 13.7-fold by 8 and 12 h, respectively. At 16 h the VEGF mRNA level in hypoxic sample decreased to 9.6-fold.
Figure 2.
Coligation of TLR4 and A2ARs increases steady state levels of VEGF mRNA. Murine peritoneal macrophages were treated with LPS (100 ng/ml), NECA (1 μM), a combination of LPS and NECA, or hypoxia for 4, 8, 12, or 16 h. At the end of the incubation period, total RNA was isolated and subjected to TaqMan real-time PCR analysis as described in Materials and Methods. VEGF mRNA levels were normalized to the corresponding levels of endogenous cyclophilin D mRNA, and data were calculated as fold expression relative to the untreated control group. Hypoxia, a known inducer of VEGF expression, served as a positive control. Results reported are means ± SD for duplicate samples from at least three independent experiments.
Synergistic Up-Regulation of VEGF Gene Expression by LPS and NECA Occurs at the Transcriptional Level via the HRE within the VEGF Promoter
To determine whether synergistic up-regulation of VEGF gene expression by LPS and NECA occurs at the transcriptional level, RAW 264.7 cells were transiently transfected with pVEGF-Luc, a luciferase reporter plasmid containing the full-length VEGF promoter, and transfectants were treated with LPS, NECA, a combination of LPS and NECA, or hypoxia. There was a 15.4-fold induction of luciferase expression driven by the full-length VEGF promoter in cells treated with LPS together with NECA, whereas LPS or NECA alone did not cause a significant increase (Figure 3A). This clearly indicates that synergistic up-regulation of VEGF expression by LPS and NECA occurs at the transcriptional level. Cells incubated under hypoxia, a known inducer of VEGF gene transcription, showed 11.2-fold induction of VEGF promoter activity.
Figure 3.
Synergistic up-regulation of VEGF gene expression by LPS and NECA occurs at the transcriptional level and requires the hypoxia response element (HRE) within the VEGF promoter. RAW 264.7 cells were transiently transfected with (A) pVEGF-Luc, a luciferase reporter constructed by cloning a 1.3-kb fragment (−963 to +404) containing the promoter region of the mouse VEGF gene into the promoterless luciferase vector (pGL3-basic), (B) pVEGF-Luc or a series of luciferase reporter constructs each containing a VEGF promoter fragment lacking an individual cis-acting element, or (C) pHRE-Luc or pGL3-promoter vector. Cells were cotransfected with Renilla luciferase reporter plasmid phRL-TK to normalize for transfection efficiency. Transfectants were stimulated with LPS (100 ng/ml), NECA (1 μM), a combination of LPS and NECA, or hypoxia. After 24-h incubation, cells were lysed and assayed for firefly and Renilla luciferase activities. Hypoxia, a known inducer of VEGF expression, served as a positive control. Data were calculated as fold induction relative to the untreated control group. Results reported here are means ± SD for duplicate samples from at least three independent experiments.
To investigate which cis-element(s) within the VEGF promoter are involved in the transcriptional activation of VEGF gene expression by LPS/NECA, RAW 264.7 cells were transiently transfected with a series of VEGF promoter-luciferase reporter constructs in which major cis-acting elements were individually deleted as described in Materials and Methods. Deletion of the HRE resulted in drastic (>87%) down-regulation of VEGF promoter activity induced not only by hypoxia but also by LPS/NECA (Figure 3B). Deletion of the AP-1 binding site caused 22 and 39% decrease in VEGF promoter activity induced by LPS/NECA and hypoxia, respectively. Deletion of AP-2 binding sites resulted in 25 and 55% reduction in VEGF promoter activity induced by LPS/NECA and hypoxia, respectively. Deletion of the NF-κB binding sites, on the other hand, had only a slight effect on the LPS/NECA inducible activity, whereas hypoxic induction was slightly increased. Finally, deletion of Sp1 elements down-regulated the LPS/NECA- and hypoxia-induced VEGF promoter activity by 64 and 38%, respectively. Taken together, these data suggest that the HRE region is vital for the synergistic activation of the VEGF promoter by LPS and NECA. AP-1 and AP-2 binding sites were found to be involved in but not sufficient for, the hypoxic induction of VEGF transcription, and Sp1 binding sites were also required to achieve full transcriptional activation of the VEGF promoter by LPS and NECA. Surprisingly, deletion of the NF-κB binding sites had no effect on activation of the VEGF promoter by either LPS/NECA or hypoxia.
To confirm further the activation of the HRE by LPS/NECA, RAW 264.7 cells were transiently transfected with pHRE-Luc, an HRE reporter construct containing four HREs in tandem, cloned into the pGL3-promoter vector (Figure 3C). The HRE-driven luciferase activity was strongly augmented by hypoxia (94.9-fold), as well as LPS/NECA (67.32-fold). LPS alone caused a much lower (26-fold) induction, whereas NECA alone did not significantly induce HRE-driven luciferase activity. Cells transfected with parental pGL3-promoter vector showed only slight induction of luciferase activity by LPS, NECA, LPS/NECA, and hypoxia.
Induction of HIF-1α mRNA Expression in Macrophages Treated with Agonists of TLR4 and A2AR
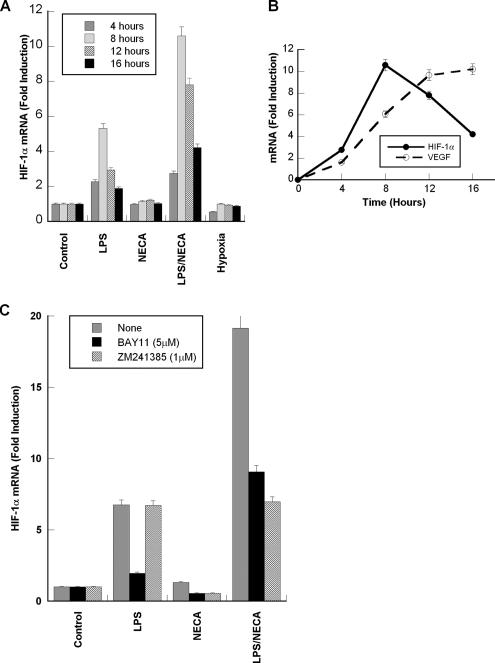
Activation of the HRE in the VEGF promoter by LPS/NECA suggested that the HIF-1 transcription factor might be critical for the regulation of VEGF expression, and that HIF-1α gene expression might be enhanced under these conditions. We therefore measured the levels of HIF-1α mRNA transcripts in these cells by TaqMan real-time PCR. Total RNA was extracted from macrophages treated with LPS, NECA, LPS/NECA, or hypoxia for 4, 8, 12, and 16 h. Real-time PCR analyses showed that neither NECA nor hypoxia had any significant effect on HIF-1α mRNA levels, whereas LPS treatment caused a modest 2.2-fold induction by 4 h, which reached a transient 5.3-fold induction at 8 h (Figure 4A). Treatment with LPS/NECA on the other hand, induced a strong and persistent up-regulation of HIF-1α gene expression, which peaked by 8 h (10.6-fold induction). The time course of HIF-1α and VEGF gene expression (Figure 4B) clearly shows that the peak induction of HIF-1α gene expression precedes the maximum induction of VEGF gene expression by LPS and NECA.
Figure 4.
LPS and NECA treatment of macrophages increases the steady state levels of HIF-1α mRNA. (A) Murine peritoneal macrophages were treated with LPS (100 ng/ml), NECA (1 μM), a combination of LPS and NECA, or hypoxia for 4, 8, 12, or 16 h. Total RNA was then isolated and subjected to TaqMan real-time PCR analysis as described in Materials and Methods. HIF-1α mRNA levels were normalized to the corresponding levels of endogenous cyclophilin D mRNA, and data were calculated as fold expression relative to the untreated control group. (B) Time course of VEGF and HIF-1α mRNA induction after the treatment of macrophages with LPS and NECA. (C) Murine peritoneal macrophages were treated with LPS (100 ng/ml), NECA (1 μM), a combination of LPS and NECA, or hypoxia for 12 h with or without BAY11-7085 (5 μM), or ZM241385 (1 μM). Total RNA was then isolated and subjected to TaqMan real-time PCR analysis as described above. Results reported are means ± SD for duplicate samples from at least three independent experiments.
To determine the role of A2AR signaling in the LPS- and NECA-induced up-regulation of HIF1α mRNA expression, macrophages were treated with LPS, NECA, or LPS/NECA for 12 h in the presence or absence of the A2AR antagonist ZM241385 (1 μM). ZM241385 had no effect on LPS-induced HIF-1α mRNA levels, but decreased the LPS/NECA-induced HIF-1α mRNA expression to the levels induced by LPS alone (Figure 4C). To determine the role of NF-κB in the induction of HIF-1α mRNA expression, macrophages were treated as described above in the presence or absence of the NF-κB inhibitor BAY11-7085 (5 μM). BAY11-7085 strongly reduced LPS-induced HIF-1α mRNA levels; however, the LPS/NECA-induced HIF-1α mRNA levels were only partially reduced. These results suggest that LPS induces HIF-1α expression in an NF-κB–dependent, A2AR-independent manner, whereas NECA acts in an NF-κB–independent and A2AR-dependent manner to increase mRNA levels.
Increased HIF-1α Expression Induced by LPS and NECA Involves Both Transcriptional and Post-Transcriptional Regulation
To determine whether the up-regulation of HIF-1α gene expression by LPS and NECA occurs at the transcriptional level, RAW 264.7 cells were transiently transfected with pHXN1aLuc, a luciferase reporter plasmid containing the murine HIF-1α exon I.2 promoter (Wenger et al., 1998), and transfectants were treated with LPS, NECA, a combination of LPS and NECA, or hypoxia. There was a 2.5-fold induction of luciferase expression driven by the HIF-1α promoter in cells treated with LPS, whereas NECA alone did not induce luciferase expression (Figure 5). Cells treated with LPS/NECA expressed luciferase at levels comparable to that of LPS alone. This indicates that up-regulation of HIF-1α mRNA expression by LPS occurs at the transcriptional level. ZM241385 did not affect LPS-, NECA-, or LPS/NECA-induced HIF-1α exon I.2 promoter activity. These results indicate that the A2AR-mediated increase in HIF-1α mRNA expression in LPS/NECA treated cells determined by RT-PCR is not transcriptional, but is regulated at the post-transcriptional level. Cells incubated under hypoxia did not show any induction of HIF-1α promoter activity.
Figure 5.
Induction of the HIF-1α exon I.2 promoter by LPS and NECA. RAW264.7 cells were transfected with pHXN1aLuc, a luciferase reporter plasmid containing the murine HIF-1α exon I.2 promoter. Cells were cotransfected with Renilla luciferase reporter plasmid phRL-TK to normalize for transfection efficiency. Transfectants were treated with LPS (100 ng/ml), NECA (1 μM), a combination of LPS and NECA, or hypoxia for 20 h. In some cases, cells were treated with the A2AR antagonist ZM241385 (1 μM). Cells were lysed and assayed for firefly and Renilla luciferase activities. Data were calculated as fold induction relative to the untreated control group. Results reported here are means ± SD for duplicate samples from at least three independent experiments.
HIF-1α Protein and DNA-binding Activity Are Increased in Macrophages Treated with Agonists of TLR4 and A2AR
Murine peritoneal macrophages and RAW264.7 were treated with LPS, NECA, LPS/NECA, or hypoxia, and nuclear extracts were prepared as described in Materials and Methods. Protein, 50 μg, from each of these extracts was electrophoresed on SDS-PAGE gels, transferred to nitrocellulose membranes, and stained with a rabbit polyclonal antibody to HIF-1α. Figure 6A shows a typical Western blot of HIF-1α in macrophage extracts. HIF-1α levels were low in both untreated and NECA-treated extracts. Higher levels of HIF-1α were detected in LPS-treated extracts, and even higher levels were found in LPS/NECA-treated extracts. The highest levels of HIF-1α were found in extracts of hypoxic macrophages. To confirm equal loading of protein in each well, blots were probed with an antibody to nucleoplasmin.
Figure 6.
Treatment of macrophages with LPS and NECA leads to increased HIF-1α DNA binding activity. RAW 264.7 cells were treated with LPS (100 ng/ml) (L), NECA (1 μM) (N), a combination of LPS and NECA (L/N), or hypoxia (H). After incubation for 12 h, nuclear extracts were prepared. (A) For Western blot analysis, 50 μg protein were electrophoresed on SDS-PAGE, transferred to nitrocellulose membranes, and immunostained with a rabbit anti-HIF-1α polyclonal antibody. As a loading control, blots were immunostained with a rabbit anti-nucleoplasmin (NPM) antibody. (B) HIF-1α DNA-binding activity was quantified as described in Materials and Methods. HIF-1α bound to HIF-1–specific double-stranded oligonucleotides was detected by an ELISA-based assay using an mAb against HIF-1α. Hypoxia-induced samples were included as a positive control. Results are expressed as the fold increase of the absorbance at 450 nm over untreated control. Results reported here are means ± SD for duplicate samples from at least three independent experiments.
DNA-binding activity of HIF-1α in the nuclear extracts was measured by TransAM HIF-1α assay. LPS alone caused only a slight (1.2-fold) induction, whereas NECA actually down-regulated HIF-1α-DNA–binding activity (Figure 6B). LPS/NECA and hypoxia caused 1.7- and 4.5-fold induction of HIF-1α-DNA–binding activity, respectively. This indicates that LPS/NECA induces expression of an activated form of HIF-1α that is capable of transcriptional activation.
Effects of the A2AR Antagonist ZM241385 and the NF-κB Inhibitor BAY11-7085 on VEGF Promoter Activity
RAW 264.7 cells were transiently transfected with pVEGF-Luc or pHRE-Luc. The transfectants were treated with LPS, NECA, LPS/NECA, or hypoxia, with or without BAY11-7085. Treatment with the A2AR antagonist ZM241385 was used to confirm the specific involvement of A2AR signaling in the synergistic activation of the VEGF promoter and the HRE by LPS/NECA. BAY11-7085 (5 μM) caused a 23 and 19% decrease in LPS/NECA-induced VEGF promoter activity and HRE-driven luciferase activity, respectively (Figure 7, A and B). Hypoxic induction of the luciferase activity was not down-regulated by BAY11-7085. ZM241385 caused a strong down-regulation of LPS/NECA-induced VEGF promoter–driven (by 68%) and HRE-driven (by 70%) luciferase activity.
Figure 7.
LPS/NECA-induced activation of the VEGF promoter-luciferase construct and the HRE reporter construct is blocked by the A2AR antagonist ZM241385, but not by the NF-κB inhibitor BAY11-7085. RAW 264.7 cells were transiently transfected with (A) pVEGF-Luc or (B) pHRE-Luc. Cells were cotransfected with Renilla luciferase reporter plasmid phRL-TK to normalize for the transfection efficiency. Transfectants were stimulated with LPS (100 ng/ml), NECA (1 μM), a combination of LPS and NECA, or hypoxia either in the presence or absence of BAY11-7085 (5 or 10 μM) or ZM241385 (1 μM). After 24-h incubation, cells were lysed and assayed for firefly and Renilla luciferase activities. Hypoxia, a known inducer of VEGF expression, served as a positive control. Data were calculated as fold induction relative to the untreated control group. Results reported here are means ± SD for duplicate samples from at least three independent experiments.
Effects of NF-κB Inhibitors on TNF-α and VEGF Expression Induced by Agonists of TLR4 and A2AR in Macrophages
Deletion of the NF-κB binding sites from the VEGF promoter had little effect on its activation by LPS/NECA, suggesting that direct binding of NF-κB to the VEGF promoter is not required for VEGF expression. To determine whether NF-κB activation plays a role in LPS/NECA-induced expression of VEGF, macrophages were treated with TLR4 and A2AR agonists in the presence or absence of NF-κB inhibitors, such as panepoxydone, SN 50, and BAY11-7085. Because TNF-α is a known target for NF-κB–induced gene expression, we also examined the effects of these inhibitors on TNF-α expression. Treatment with the specific A2AR antagonist ZM241385 was included to determine the role of A2AR signaling. Although panepoxydone and SN 50 down-regulated LPS-induced TNF-α expression (data not shown), BAY11-7085 was found to be most effective in down-regulating LPS-induced TNF-α expression without adversely affecting cell viability, as assessed using MTT assays. At a concentration of 5 μM, BAY11-7085 strongly inhibited LPS-induced TNF-α levels (60%; Figure 8A). VEGF expression induced by LPS/NECA, was not as strongly decreased (28%) by BAY11-7085 (Figure 8B). These results suggest that NF-κB contributes to, but is less crucial for the induction of VEGF expression by this pathway than to the expression of TNF-α. ZM241385 completely inhibited the LPS/NECA-induced, but not the hypoxia-induced, VEGF expression and prevented NECA-mediated down-regulation of LPS-induced TNF-α expression, demonstrating the specificity of the NECA response to A2ARs.
Figure 8.
Inhibitor of NF-κB BAY11-7085 down-regulates LPS-induced TNF-α expression but not LPS/NECA-induced VEGF expression in murine peritoneal macrophages. Murine peritoneal macrophages were stimulated with LPS (100 ng/ml), NECA (1 μM), a combination of LPS and NECA, or hypoxia either in the presence or absence of BAY11-7085 (1 or 5 μM) or ZM241385 (1 μM). After 24-h incubation, conditioned media were harvested and assayed for (A) TNF-α and (B) VEGF protein levels by ELISAs. MTT assays were used to normalize the cytokine levels to cell viability. Data were calculated as fold induction relative to the untreated control group. Results reported here are means ± SD for duplicate samples from at least three independent experiments.
DISCUSSION
As mediators of innate immune responses, macrophages can secrete an array of proinflammatory cytokines such as TNF-α, IL-1, and IL-12 via stimulation of TLRs, which act as pattern recognition receptors for various conserved structural features of bacteria, viruses, and fungi (Aderem and Ulevitch, 2000; Means et al., 2000; Underhill and Ozinsky, 2002; Akira and Takeda, 2004). During wound healing, macrophages play a key role in induction of angiogenesis by secreting the potent angiogenic factor VEGF in response to various stimuli (Ferrara et al., 1998; Nissen et al., 1998; Xiong et al., 1998; Perez-Ruiz et al., 1999; Crowther et al., 2001). Hypoxic induction of VEGF gene expression is mediated by the transcription factor HIF-1. HIF-1 consists of a dimer of HIF-1β (ARNT), which is constitutively expressed and HIF-1α, which is normally rapidly degraded under normoxic conditions (Forsythe et al., 1996). We have shown previously that costimulation of macrophages with agonists of TLRs 2, 4, 7, or 9, and A2ARs synergistically up-regulates VEGF expression, whereas down-regulating expression of inflammatory cytokines such as TNF-α and IL-12 (Pinhal-Enfield et al., 2003). We have termed this alternate pathway of macrophage activation an “angiogenic switch.” In this study, we show that this synergistic induction of the VEGF gene expression involves transcriptional activation of the VEGF gene through induction of HIF-1α gene expression. Using a VEGF promoter-luciferase reporter, we show a strong induction of VEGF promoter activity by LPS (TLR4 agonist) and NECA or CGS21680 (A2AR agonists). We have reported previously using a murine VEGF promoter reporter construct and a series of its 5′-end sequential deletions, that the HRE plays an important role in the induction of VEGF promoter activity by hypoxia as well as by LPS with IFN-γ (Ramanathan et al., 2003). In the present study, we demonstrate that the HRE is also crucial for the induction of VEGF promoter activity by the combined action of LPS and NECA.
Because the HRE is at the 5′-end of the murine VEGF promoter and was absent from all the deletion constructs in our prior study (Ramanathan et al., 2003), we were previously unable to address the potential importance of the promoter elements downstream of the HRE. Therefore, in this study, we engineered a series of VEGF promoter-reporter constructs in which major cis-acting elements were individually deleted, while retaining the HRE (Figure 2B). Deletion of the HRE abrogated the induction of the VEGF promoter by LPS and NECA, indicating that the HRE region of the VEGF promoter is essential for its transcriptional activation by LPS and NECA. In confirmation, treatment with LPS and NECA was also shown to induce reporter activity from a vector containing only the HRE elements as enhancer (pHRE-Luc).
We investigated the mechanism of LPS/NECA-induced activation of the HRE under normoxic conditions. Several nonhypoxic stimuli have been shown to strongly increase HIF-1 activation under normoxic conditions in a cell-specific manner, thereby increasing the transcription of genes that are primarily induced by hypoxia. These stimulants include cytokines such as IFN-γ, IL-1β, and TNF (Albina et al., 2001; Haddad and Land, 2001; Jung et al., 2003a, 2003b; Scharte et al., 2003; Frede et al., 2005; Varney et al., 2005), growth factors (Feldser et al., 1999; Gorlach et al., 2001; Shih and Claffey, 2001; Tacchini et al., 2001; Fukuda et al., 2002), and hormones such as thrombin, thrombopoietin, and angiotensin (Gorlach et al., 2001; Page et al., 2002; Kirito et al., 2005). Most of these studies report induction of HIF-1α through an increase in protein levels rather than increased levels of HIF-1α mRNA. Increase in HIF-1α protein levels were found to be due to increased translation rather than to increased stabilization of HIF-1α protein. Richard et al. (1999) demonstrated an increase in the transcriptional activity of HIF-1 through p42/p44 MAPK-mediated phosphorylation (Richard et al., 1999). However there are few reports demonstrating increased transcription of the HIF-1α gene leading to increased protein levels. Blouin et al. (2004) showed that in a macrophage-derived cell model, HIF-1α mRNA levels were markedly increased after LPS stimulation via activation of diacylglycerol-sensitive forms of PKC (Blouin et al., 2004). Recently, LPS has also been shown to induce HIF-1α expression in human monocytes, in a p44/p42 MAPK and NF-κB–dependent manner (Frede et al., 2006).
We examined the possibility that treatment with LPS and NECA might result in increased HIF-1α expression in macrophages. Real-time PCR analyses showed that HIF-1α mRNA levels were increased transiently in LPS-treated cells, whereas LPS/NECA-treated macrophages showed a stronger and more persistent increase in HIF-1α mRNA levels (Figure 4A). This increase preceded the induction of VEGF gene expression by LPS/NECA, an induction that was not observed in macrophages treated with LPS alone. The LPS-induced increase in HIF-1α mRNA levels was mediated transcriptionally. RAW264.7 cells transfected with a HIF-1α exon I.2 promoter luciferase construct (pHXN1aLuc) showed a significant basal level of luciferase expression. LPS treatment of these cells resulted in a marked increase in luciferase expression in an NF-κB–dependent manner. NECA did not alter either basal or LPS-induced luciferase expression. However, as noted above, NECA with LPS resulted in a significant increase in HIF-1α mRNA levels. This indicates that NECA does not transcriptionally induce HIF-1α expression, but rather post-transcriptionally stabilizes the LPS-induced HIF-1α mRNA. The A2AR antagonist ZM241385 blocks the NECA-induced increase in HIF-1α mRNA in LPS-treated cells, bringing these levels down to those observed in cells treated with LPS alone. These results indicate that the LPS-induced transcriptional up-regulation of HIF-1α is independent of A2AR signaling, but that the subsequent stabilization of HIF-1α mRNA induced by NECA is a critical post-transcriptional A2AR-dependent event. The mechanism by which the A2AR agonist stabilizes HIF-1α mRNA is not clear.
Levels of HIF-1α protein and HIF-1α DNA-binding activity were also increased in cells treated with LPS and NECA. However, both HIF-1α protein levels and DNA-binding activity were not as strongly elevated as those induced by hypoxia, even though the induction of VEGF expression by LPS/ NECA was as strong or stronger than that induced by hypoxia. This raises the possibility that other, as yet unidentified factors that promote VEGF gene transcription via binding to the HRE might be involved in the up-regulation of VEGF expression. Because the VEGF promoter contains a region with putative NF-κB–binding sites, we further investigated the possible role of NF-κB in induction of VEGF gene expression by LPS/NECA. Deletion of the VEGF promoter region containing the putative NF-κB–binding sites had little effect on the LPS/NECA induction of luciferase expression in transfected RAW264.7 cells. Although the NF-κB inhibitor BAY11-7085 strongly suppressed LPS-induced TNF-α expression, LPS/NECA-induced VEGF expression was only mildly decreased in the presence of this inhibitor. Also, BAY11-7085 had little effect on activation of the intact VEGF promoter by LPS/NECA. Together, these results suggest that activation of NF-κB does not play a direct role in up-regulation of VEGF expression by this pathway.
Although, our data clearly suggest a role for HIF-1α–mediated transcriptional activation of the VEGF gene, the exact mechanism of stabilization of HIF-1α under these nonhypoxic conditions remains to be explored. Using a macrophage-derived cell model, Blouin et al. (2004) have shown that LPS treatment of a macrophage cell line results in induction of hypoxia-inducible genes via nonhypoxic induction of HIF-1α through transcriptional mechanisms (Blouin et al., 2004). Chun et al. (2003) have also reported that phorbol ester stimulates the nonhypoxic induction of a novel human HIF-1α isoform that lacks exon 11 and is thereby stabilized under normoxic conditions. In the present study, using primary murine peritoneal macrophages, we show that LPS alone was not enough to cause the induction of VEGF gene transcription via the HRE, although it caused a significant induction of HIF-1α gene expression. Costimulation of LPS-treated cells with NECA induced HIF-1α mRNA levels that were twice that in cells treated with LPS alone, and the increased levels of HIF-1α mRNA persisted for at least 16 h. This clearly indicated that ligation of A2ARs contributes to the synergistic induction of both HIF-1α as well as to VEGF gene expression. It remains to be explored if additional regulatory mechanisms play a role in the induction and activation of HIF-1α by this pathway.
Our studies suggest that HIF-1 activation in macrophages is induced by the synergistic action of TLR agonists such as LPS, together with adenosine A2AR agonists, in a hypoxia-independent manner. The presence of TLR agonists together with high levels of extracellular adenosine is likely to occur in numerous disease states. Understanding the mechanisms by which signaling pathways from TLR agonists and A2AR agonists interact to regulate HIF-1 and VEGF expression may lead to novel therapeutic strategies for the treatment of fibroproliferative diseases.
ACKNOWLEDGMENTS
The reporter plasmid pHRE-Luc was kindly provided by Dr. Sean Colgan (Harvard Medical School, Boston, MA). The reporter plasmid pHXN1aLuc was kindly provided by Prof. Roland H. Wenger (University of Zurich, Switzerland). This work was supported by a grant from the U.S. Public Health Service (RO1-GM-068636).
Abbreviations used:
- NECA
5′-N-ethyl-carboxamidoadenosine
- CGS21680
2-[p-(2-carboxylethyl)-phenylethyl amino]-5′-N-ethyl-carboxamido-adenosine
- LPS
lipopolysaccharide
- HRE
hypoxia response element
- HIF
hypoxia inducible factor
- MTT
methyl thiazole tetrazolium.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-07-0596 on October 25, 2006.
REFERENCES
- Aderem A., Ulevitch R. J. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Albina J. E., Mastrofrancesco B., Vessella J. A., Louis C. A., Henry W. L., Jr, Reichner J. S. HIF-1 expression in healing wounds: HIF-1alpha induction in primary inflammatory cells by TNF-alpha. Am J. Physiol. Cell Physiol. 2001;281:C1971–C1977. doi: 10.1152/ajpcell.2001.281.6.C1971. [DOI] [PubMed] [Google Scholar]
- Blouin C. C., Page E. L., Soucy G. M., Richard D. E. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1alpha. Blood. 2004;103:1124–1130. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- Cho M., Hunt T. K., Hussain M. Z. Hydrogen peroxide stimulates macrophage vascular endothelial growth factor release. Am J. Physiol. Heart Circ. Physiol. 2001;280:H2357–H2363. doi: 10.1152/ajpheart.2001.280.5.H2357. [DOI] [PubMed] [Google Scholar]
- Chun Y. S., Lee K. H., Choi E., Bae S. Y., Yeo E. J., Huang L. E., Kim M. S., Park J. W. Phorbol ester stimulates the nonhypoxic induction of a novel hypoxia-inducible factor 1alpha isoform: implications for tumor promotion. Cancer Res. 2003;63:8700–8707. [PubMed] [Google Scholar]
- Cohen T., Nahari D., Cerem L. W., Neufeld G., Levi B. Z. Interleukin 6 induces the expression of vascular endothelial growth factor. J. Biol. Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- Crowther M., Brown N. J., Bishop E. T., Lewis C. E. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J. Leukoc. Biol. 2001;70:478–490. [PubMed] [Google Scholar]
- Damert A., Ikeda E., Risau W. Activator-protein-1 binding potentiates the hypoxia-induciblefactor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem. J. 1997;327(Pt 2):419–423. doi: 10.1042/bj3270419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Ema M., Taya S., Yokotani N., Sogawa K., Matsuda Y., Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc. Natl. Acad. Sci. USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldser D., Agani F., Iyer N. V., Pak B., Ferreira G., Semenza G. L. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59:3915–3918. [PubMed] [Google Scholar]
- Ferrara N., Chen H., Davis-Smyth T., Gerber H. P., Nguyen T. N., Peers D., Chisholm V., Hillan K. J., Schwall R. H. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat. Med. 1998;4:336–340. doi: 10.1038/nm0398-336. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Forsythe J. A., Jiang B. H., Iyer N. V., Agani F., Leung S. W., Koos R. D., Semenza G. L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frede S., Freitag P., Otto T., Heilmaier C., Fandrey J. The proinflammatory cytokine interleukin 1beta and hypoxia cooperatively induce the expression of adrenomedullin in ovarian carcinoma cells through hypoxia inducible factor 1 activation. Cancer Res. 2005;65:4690–4697. doi: 10.1158/0008-5472.CAN-04-3877. [DOI] [PubMed] [Google Scholar]
- Frede S., Stockmann C., Freitag P., Fandrey J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. Biochem. J. 2006;396:517–527. doi: 10.1042/BJ20051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R., Hirota K., Fan F., Jung Y. D., Ellis L. M., Semenza G. L. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J. Biol. Chem. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- Fukumura D., Xu L., Chen Y., Gohongi T., Seed B., Jain R. K. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001;61:6020–6024. [PubMed] [Google Scholar]
- Gille J., Swerlick R. A., Caughman S. W. Transforming growth factor-alpha-induced transcriptional activation of the vascular permeability factor (VPF/VEGF) gene requires AP-2-dependent DNA binding and transactivation. EMBO J. 1997;16:750–759. doi: 10.1093/emboj/16.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman C. K., Kim J., Wong W. L., King V., Brock T., Gillespie G. Y. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol. Biol. Cell. 1993;4:121–133. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach A., Diebold I., Schini-Kerth V. B., Berchner-Pfannschmidt U., Roth U., Brandes R. P., Kietzmann T., Busse R. Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: role of the p22(phox)-containing NADPH oxidase. Circ. Res. 2001;89:47–54. doi: 10.1161/hh1301.092678. [DOI] [PubMed] [Google Scholar]
- Guillemin K., Krasnow M. A. The hypoxic response: huffing and HIFing. Cell. 1997;89:9–12. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- Haddad J. J., Land S. C. A non-hypoxic, ROS-sensitive pathway mediates TNF-alpha-dependent regulation of HIF-1alpha. FEBS Lett. 2001;505:269–274. doi: 10.1016/s0014-5793(01)02833-2. [DOI] [PubMed] [Google Scholar]
- Jung Y., Isaacs J. S., Lee S., Trepel J., Liu Z. G., Neckers L. Hypoxia-inducible factor induction by tumour necrosis factor in normoxic cells requires receptor-interacting protein-dependent nuclear factor kappa B activation. Biochem. J. 2003a;370:1011–1017. doi: 10.1042/BJ20021279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y. J., Isaacs J. S., Lee S., Trepel J., Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003b;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- Jung Y. J., Isaacs J. S., Lee S., Trepel J., Neckers L. Microtubule disruption utilizes an NFkappa B-dependent pathway to stabilize HIF-1alpha protein. J. Biol. Chem. 2003c;278:7445–7452. doi: 10.1074/jbc.M209804200. [DOI] [PubMed] [Google Scholar]
- Kimura H., Weisz A., Kurashima Y., Hashimoto K., Ogura T., D'Acquisto F., Addeo R., Makuuchi M., Esumi H. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood. 2000;95:189–197. [PubMed] [Google Scholar]
- Kirito K., Fox N., Komatsu N., Kaushansky K. Thrombopoietin enhances expression of vascular endothelial growth factor (VEGF) in primitive hematopoietic cells through induction of HIF-1alpha. Blood. 2005;105:4258–4263. doi: 10.1182/blood-2004-07-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughner E., Taghavi P., Chiles K., Mahon P. C., Semenza G. L. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol. Cell. Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., et al. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin. Am. J. Pathol. 2002;160:2231–2244. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A. P., Levy N. S., Wegner S., Goldberg M. A. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J. Biol. Chem. 1995;270:13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- Liu Y., Cox S. R., Morita T., Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ. Res. 1995;77:638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- Means T. K., Golenbock D. T., Fenton M. J. Structure and function of Toll-like receptor proteins. Life Sci. 2000;68:241–258. doi: 10.1016/s0024-3205(00)00939-5. [DOI] [PubMed] [Google Scholar]
- Nissen N. N., Polverini P. J., Koch A. E., Volin M. V., Gamelli R. L., DiPietro L. A. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 1998;152:1445–1452. [PMC free article] [PubMed] [Google Scholar]
- Page E. L., Robitaille G. A., Pouyssegur J., Richard D. E. Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J. Biol. Chem. 2002;277:48403–48409. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- Perez-Ruiz M., et al. Vascular endothelial growth factor production in peritoneal macrophages of cirrhotic patients: regulation by cytokines and bacterial lipopolysaccharide. Hepatology. 1999;29:1057–1063. doi: 10.1002/hep.510290416. [DOI] [PubMed] [Google Scholar]
- Pertovaara L., Kaipainen A., Mustonen T., Orpana A., Ferrara N., Saksela O., Alitalo K. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J. Biol. Chem. 1994;269:6271–6274. [PubMed] [Google Scholar]
- Pinhal-Enfield G., Ramanathan M., Hasko G., Vogel S. N., Salzman A. L., Boons G. J., Leibovich S. J. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. Am. J. Pathol. 2003;163:711–721. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan M., Giladi A., Leibovich S. J. Regulation of vascular endothelial growth factor gene expression in murine macrophages by nitric oxide and hypoxia. Exp. Biol. Med. (Maywood) 2003;228:697–705. doi: 10.1177/153537020322800608. [DOI] [PubMed] [Google Scholar]
- Richard D. E., Berra E., Gothie E., Roux D., Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J. Biol. Chem. 1999;274:32631–32637. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- Richard D. E., Berra E., Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J. Biol. Chem. 2000;275:26765–26771. doi: 10.1074/jbc.M003325200. [DOI] [PubMed] [Google Scholar]
- Scharte M., Han X., Bertges D. J., Fink M. P., Delude R. L. Cytokines induce HIF-1 DNA binding and the expression of HIF-1-dependent genes in cultured rat enterocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G373–G384. doi: 10.1152/ajpgi.00076.2002. [DOI] [PubMed] [Google Scholar]
- Shih S. C., Claffey K. P. Role of AP-1 and HIF-1 transcription factors in TGF-beta activation of VEGF expression. Growth Factors. 2001;19:19–34. doi: 10.3109/08977190109001073. [DOI] [PubMed] [Google Scholar]
- Shima D. T., Deutsch U., D'Amore P. A. Hypoxic induction of vascular endothelial growth factor (VEGF) in human epithelial cells is mediated by increases in mRNA stability. FEBS Lett. 1995;370:203–208. doi: 10.1016/0014-5793(95)00831-s. [DOI] [PubMed] [Google Scholar]
- Shima D. T., Kuroki M., Deutsch U., Ng Y. S., Adamis A. P., D'Amore P. A. The mouse gene for vascular endothelial growth factor. Genomic structure, definition of the transcriptional unit, and characterization of transcriptional and post-transcriptional regulatory sequences. J. Biol. Chem. 1996;271:3877–3883. doi: 10.1074/jbc.271.7.3877. [DOI] [PubMed] [Google Scholar]
- Tacchini L., Dansi P., Matteucci E., Desiderio M. A. Hepatocyte growth factor signalling stimulates hypoxia inducible factor-1 (HIF-1) activity in HepG2 hepatoma cells. Carcinogenesis. 2001;22:1363–1371. doi: 10.1093/carcin/22.9.1363. [DOI] [PubMed] [Google Scholar]
- Underhill D. M., Ozinsky A. Toll-like receptors: key mediators of microbe detection. Curr. Opin. Immunol. 2002;14:103–110. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- Varney M. L., Olsen K. J., Mosley R. L., Singh R. K. Paracrine regulation of vascular endothelial growth factor—a expression during macrophage-melanoma cell interaction: role of monocyte chemotactic protein-1 and macrophage colony-stimulating factor. J. Interferon Cytokine Res. 2005;25:674–683. doi: 10.1089/jir.2005.25.674. [DOI] [PubMed] [Google Scholar]
- Wang G. L., Jiang B. H., Rue E. A., Semenza G. L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger R. H., Rolfs A., Spielmann P., Zimmermann D. R., Gassmann M. Mouse hypoxia-inducible factor-1alpha is encoded by two different mRNA isoforms: expression from a tissue-specific and a housekeeping-type promoter. Blood. 1998;91:3471–3480. [PubMed] [Google Scholar]
- Xiong M., Elson G., Legarda D., Leibovich S. J. Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. Am. J. Pathol. 1998;153:587–598. doi: 10.1016/S0002-9440(10)65601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelzer E., Levy Y., Kahana C., Shilo B. Z., Rubinstein M., Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H., Chiles K., Feldser D., Laughner E., Hanrahan C., Georgescu M. M., Simons J. W., Semenza G. L. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]