Abstract
E-cadherin forms calcium-dependent homophilic intercellular adhesions between epithelial cells. These contacts regulate multiple aspects of cell behavior, including the organization of intercellular tight junctions (TJs). To distinguish between the roles of E-cadherin in formation versus maintenance of junctions, Madin-Darby canine kidney (MDCK) cells were depleted of E-cadherin by RNA interference. Surprisingly, reducing E-cadherin expression had little effect on the protein levels or localization of adherens junction (AJ) or TJ markers. The cells underwent morphological changes, as the normally flat apical surface swelled into a dome. However, apical–basal polarity was not compromised, transmembrane resistance was normal, and zonula occludin protein 1 dynamics at the TJs were unchanged. Additionally, an E-cadherin/Cadherin-6 double knockdown also failed to disrupt established TJs, although β-catenin was lost from the cell cortex. Nevertheless, cells depleted of E-cadherin failed to properly reestablish cell polarity after junction disassembly. Recovery of cell–cell adhesion, transepithelial resistance, and the localization of TJ and AJ markers were all delayed. In contrast, depletion of α-catenin caused long-term disruption of junctions. These results indicate that E-cadherin and Cadherin-6 function as a scaffold for the construction of polarized structures, and they become largely dispensable in mature junctions, whereas α-catenin is essential for the maintenance of functional junctions.
INTRODUCTION
The cadherins are a large family of transmembrane glycoproteins that form homophilic, calcium-dependent interactions with neighboring cells (Takeichi, 1988; Gumbiner, 2000; Nollet et al., 2000). Cadherin family members include type I cadherins (E-, N-, P-, and R-cadherin), type II cadherins (Cadherin-6 and VE-cadherin), desmosomal cadherins (desmocollins and desmogleins), and a large subfamily of cadherin-like molecules (Nollet et al., 2000). The predominant epithelial isoform, E-cadherin, localizes to the lateral membrane of differentiated epithelia, providing the structural foundation for adherens junctions (AJs), a multiprotein complex that links cell–cell contacts to the actin cytoskeleton and various signaling molecules (Perez-Moreno et al., 2003). The extracellular domain is composed of five ectodomain modules (ECs), with the most membrane-distal module (EC1) mediating binding with the E-cadherin on the adjacent cell (Boggon et al., 2002). Calcium ions bind between the EC domains to promote a rod-like conformation required for transinteractions (Gumbiner, 1996; Patel et al., 2006). The cytoplasmic tail of E-cadherin binds to the armadillo repeat protein β-catenin, an important target of the Wnt signaling pathway and a cofactor for TCF/LEF-mediated transcription (Gavard and Mege, 2005). The β-catenin in turn binds α-catenin, which interacts with actin, as well as several actin-binding proteins: vinculin, formins, α-actinin, zonula occludin protein (ZO)-1, and afadin (Bershadsky, 2004). Cell–cell adhesions also contain desmosomes, which link cell contacts to intermediate filaments as well as nectin-based, calcium-independent adhesions, which are linked to actin (Takai and Nakanishi, 2003; Yin and Green, 2004).
Assembly of adhesions de novo is a highly organized, hierarchical process (Adams and Nelson, 1998; Braga, 2002; Jamora and Fuchs, 2002). During cell junction biogenesis, cadherin-based adhesions form rapidly, recruit actin, and stimulate phosphatidylinositol 3-kinase and the activities of the small GTPases Rac1 and Cdc42 (Yap and Kovacs, 2003). These events enhance E-cadherin recruitment to cell–cell contact sites, strengthen the cadherin–catenin complex, and promote actin reorganization into belts around the cell periphery (Vasioukhin and Fuchs, 2001). Importantly, stable epithelial adhesions require the F-actin network, because treatment with latruculin A or cytochalasin D causes loss of AJs as well as desmosomes and tight junctions (TJs) (Shen and Turner, 2005).
E-cadherin engagement promotes epithelial apical–basal polarization, including the formation of TJs (Gumbiner et al., 1988). TJs are the most apical junction complex and provide a barrier to pericellular diffusion (gate function) as well as a diffusion barrier between apical and basal–lateral membrane compartments (fence function) (Matter and Balda, 2003). TJs are composed of transmembrane proteins occludin, and claudin, which anchor scaffolding proteins, including ZO-1, ZO-2, and ZO-3, at the cell periphery (Gonzalez-Mariscal et al., 2003). These proteins in turn bind actin and regulatory complexes such as the PAR proteins and the apical Crb complex. In a poorly understood process, TJ proteins are initially recruited to cadherin contacts when cells touch, possibly through α-catenin–mediated recruitment of ZO-1 (Muller et al., 2005), and only later move away to form the TJ.
Regulation of cell adhesions through cadherins is essential for embryonic development, and coordinated adhesion restructuring is necessary during epithelial-to-mesenchymal transitions (D'Souza-Schorey, 2005). This remodeling can occur through regulated transport of cadherin–catenin-containing vesicles to and from the membrane. Cadherin endocytosis is a potent mechanism for junction remodeling and involves the ARF6 complex, Scr-mediated ubiquitination, or p120 catenin-regulated degradation (Davis et al., 2003; Bryant and Stow, 2004). Loss of cadherin-based adhesions is a hallmark of carcinogenesis and correlates with tumor progression. In many epithelial-derived tumors, E-cadherin expression is down-regulated, leading to loss of cell adhesions, increased proliferation, and tumor invasiveness (Takeichi, 1991; Van Aken et al., 2001; Cowin et al., 2005). Other examples of adhesion loss include E-cadherin EC domain mutants, or disruption of the link to actin through catenins (Shimoyama et al., 1992). Not surprisingly, E-cadherin knockout mice are embryonic lethal, failing to develop past the trophoectoderm, 32-cell stage (Larue et al., 1994; Ohsugi et al., 1997). Postnatal lethality in mice with a conditional E-cadherin knockout in skin is due to failure of the epidermis to prevent water loss (Tinkle et al., 2004; Tunggal et al., 2005). Although illustrative of the importance of E-cadherin in cell–cell contacts, the functions of E-cadherin has remained unclear in these models, because of either residual maternal transcript or compensation by other cadherin family members. Particularly, it is uncertain whether these defects arise from loss of the ability to maintain proper junctions or to form them in the first place.
To distinguish between the roles of cadherins in the initial formation versus maintenance of junctions, we suppressed E-cadherin expression in Madin-Darby canine kidney (MDCK) cells by RNA interference (RNAi). This resulted in a reduction in E-cadherin levels without observable increase in related cadherin isoform expression. Surprisingly, E-cadherin knockdown had little effect on the protein levels or localization of either TJ or AJ proteins in confluent MDCK monolayers, and cell polarity was maintained. Even the codepletion of E-cadherin with Cadherin-6, which led to a marked loss of β-catenin from the cell cortex, did not seem to disrupt TJs. In contrast, cells depleted of E-cadherin were unable to repolarize properly after TJ disassembly. Depletion of α-catenin, in contrast, disrupted both the establishment and maintenance of junctions. These studies indicate that cadherins function as a scaffold for the establishment of cell polarity but that they are largely dispensable for the maintenance of preformed cell–cell junctions and apical/basal polarity.
MATERIALS AND METHODS
Cell Culture and Transfection
MDCK T23 and MDCK T23 yellow fluorescent protein (YFP)-ZO-1 cells were cultured in DMEM with 10% fetal calf serum (FCS) and 100 U/ml penicillin-streptomycin (Invitrogen, Carlsbad, CA). MDCK T23 cells were nucleofected (Amaxa Biosystems, Gaithersburg, MD) with 2.5 × 106 cells per transfection, by using 3 μg of DNA, (2 μg of pSuper plus 1 μg of pKseYFP (Venus, a superenhanced yellow fluorescent protein) or pKmRFP (monomeric red fluorescent protein) as a transfection marker. Experiments were performed 2–3 d posttransfection.
Immunostaining and Antibodies
Cells were fixed in either 4% paraformaldehyde-phosphate-buffered saline (PFA-PBS) or methanol/acetone as described previously (Qin et al., 2005). PFA-fixed cells were permeabilized with 0.5% Triton X (TX)-100 for 10 min. All samples were blocked in 10% FCS-PBS for 1 h before staining. Primary antibodies used were mouse anti-E-cadherin and anti-β-catenin (1:500; BD Biosciences, San Jose, CA), mouse anti-IQGAP (1:100; Upstate Biotechnology Charlottesville, VA), rabbit anti-green fluorescent protein (GFP) (1:200; Invitrogen), rabbit anti-α-catenin (1:2000; Sigma-Aldrich, St. Louis, MO), rabbit anti-Claudin 1 (1:20; Zymed Laboratories, South San Francisco, CA), mouse anti-gp135 (1:300; clone 3F21D8), and mouse anti-ZO-1 (1:100; Zymed Laboratories). Mouse anti-Na+/K+-ATPase H6 (1:250) was a gift from Michael Caplan (Yale University, New Haven, CT). Par3 (1:100) and Pals1 (1:50) antibodies were produced in rabbits against recombinant proteins (Cocalico Biologicals, Reamstown, PA). Alexa 594-phalloidin (1:50; Invitrogen) was used to visualize F-actin. Secondaries were goat anti-mouse or anti-rabbit antibodies conjugated to Alexa 488, Alexa 594, and 7-amino-4-methylcoumarin-3-acetic acid (AMCA) (1:1000; Invitrogen). Images were captured on a TE200 widefield microscope (Nikon, Tokyo, Japan) using a 60× water immersion lens (1.2 numerical aperture [NA]) and a Orca charge-coupled device camera (Hamamatsu, Bridgewater, NJ) or for confocal images a 510 Meta/FCS laser-scanning confocal microscope (Carl Zeiss, Thornwood, NY), by using a 100× oil immersion lens (1.3 NA). Widefield images were processed using the Adobe Photoshop 7.0 Levels tool (Adobe Systems, Mountain View, CA) to enhance contrast. Confocal image stacks were processed using Volocity software (Improvision, Warwick, United Kingdom).
Western Blots and Immunoprecipitations
Whole cell lysates were collected by adding SDS sample buffer or, for Cadherin-6 lysates, a 1% SDS buffer (1% SDS [wt/vol], 10 mM Tris, pH 7.5, and 1 mM EDTA), and boiled for 10 min before analysis by SDS-PAGE. Immunoprecipitations were performed as follows: cells were lysed in lysis buffer (0.5% Triton X-100, 25 mM Tris, pH 7.4, 300 mM sucrose, 25 mM NaF, 150 mM NaCl, 2 mM Na3VO4, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride) and centrifuged for 10 min at 10,000 × g. Supernatants were immunoprecipitated (anti-β-catenin, GammaBind-Plus Sepharose beads; GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) for 1 h at 4°C. Samples were washed three times in lysis buffer with 500 mM NaCl and once with lysis buffer then eluted in SDS sample buffer. Primary antibodies were rabbit anti-β-tubulin (1:100; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-ZO-1 (1:1000; Zymed Laboratories), mouse anti-E-cadherin and β-catenin (1:1000; BD Biosciences), rabbit anti-α-catenin (1:2000; Sigma-Aldrich), rabbit anti-Pals1 (1:1000) and mouse anti-Ran (1:5000; BD Transduction Laboratories, Lexington, KY), and mouse anti-Rac (1:1000; Upstate Biotechnology). Rabbit anti-Pan-cadherin and rabbit anti-Cadherin-6 were gifts from Barry Gumbiner (Department of Cell Biology, University of Virginia School of Medicine) and James Nelson (Biological Sciences, Stanford University School of Medicine, Stanford, CA), respectively. Quantitative Westerns were performed using an Odyssey detector (LI-COR, Lincoln, NE). Secondary antibodies for Odyssey detection included Alexa Fluor IRDye 800-conjugated goat anti-mouse (Rockland, Gilbertsville, PA) and Alexa Fluor 680-conjugated goat anti-rabbit (Invitrogen).
Constructs
Canine E-cadherin, Cadherin-6, and α-catenin sequences from the Broad Institute Reference Dog Assembly Boxer library were screened for candidate small interfering RNA primers by using rational design criteria (Reynolds et al., 2004). Oligonucleotides for short hairpin RNA (shRNA) are as follows: E-cadherin primer A sense strand, 5′-gatccccGGACGTGGAAGATGTGAATttcaagagaATTCACATCTTCCACGTCCtttttggaaa-3′ and antisense, 5′-agcttttccaaaaaGGACGTGGAAGATGTGAATtctcttgaaATTCACATCTTCCACGTCCggg-3′;primer B sense strand, 5′gatccccGTCTAACAGGGACAAAGAAttcaagagaTTCTTTGTCCCTGTTAGACtttttggaaa-3′ and antisense, 5′-agcttttccaaaaaGTCTAACAGGGACAAAGAAtctcttgaaTTCTTTGTCCCTGTTAGACggg-3′. Cadherin-6 primers are as follows: sense strand, 5′-gatccccGCGGCTACAGTCAGAATTAttcaagagaTAATTCTGACTGTAGCCGCtttttggaaa-3′ and antisense, agcttttccaaaaaGCGGCTACAGTCAGAATTAtctcttgaaTAATTCTGACTGTAGCCGCggg-3′. Primers designed for α-catenin are as follows: primer 1 sense strand, 5′-gatccccTGAACAAGACTTAGGAATAttcaagagaTATTCCTAAGTCTTGTTCAtttttggaaa-3′ and antisense, 5′-agcttttccaaaaaTGAACAAGACTTAGGAATAtctcttgaaTATTCCTAAGTCTTGTTCAggg-3′; primer 2 sense strand, 5′-gatccccGGCTAACAGAGACCTGATAttcaagagaTATCAGGTCTCTGTTAGCCtttttggaaa-3′ and antisense, 5′-agcttttccaaaaaGGCTAACAGAGACCTGATAtctcttgaaTATCAGGTCTCTGTTAGCCggg-3′. Annealed primers were cloned using BglII and HindIII restriction sites into pSuper, as described previously (Brummelkamp et al., 2002). A sequence targeting luciferase (pSLuc) was used as a negative control (Malliri et al., 2004).
Transepithelial Resistance (TER) Measurements
Calcium switch and TER were performed as described previously (Gao et al., 2002). MDCK T23 cells were nucleofected as described above with control or E-cadherin pSuper plasmids. Cells were then plated onto 0.4-μm pore, 12-mm Transwell filters (Corning Glassworks, Corning, NY) at 5.5 × 105 cells per filter. Resistance measurements were performed 2–3 d later, and values listed are the average of three trials with background resistance subtracted.
Fluorescence Recovery after Photobleaching (FRAP)
An MDCK stable line expressing YFP-ZO-1 was established by cotransfection of pKYFP-ZO-1 and a vector expressing a hygromycin resistance gene, pHyg (McNeil et al., 2006). Cells were then selected by hygromycin and screened for expression of pKYFP-ZO-1. For FRAP assays, cells were nucleofected with pKmRFP as a transfection marker and either control or E-cadherin pSuper plasmid. After 48 h, cells were subjected to photobleaching and imaged by confocal microscopy (Meta LSM510; Carl Zeiss), by using a 100× oil lens (1.3 NA). Cells were imaged with a 488-nm laser line at 75% power, 0.1% transmission, and 543-nm laser line at 59% transmission. For photobleaching, regions of interest (ROIs) were selected between two adjacent transfected cells (marked with monomeric red fluorescent protein [mRFP]), and bleached with the 488-nm laser line, at 75% power, 100% transmission for two iterations. Recovery within the ROI was monitored for 400 s. For each sample, two images were recorded prebleach, and postbleach images are represented as a percentage of total YFP-ZO-1 in the cell. For all images, mean intensities were adjusted for bleaching during imaging.
RESULTS
Knockdown of E-Cadherin in MDCK Cells
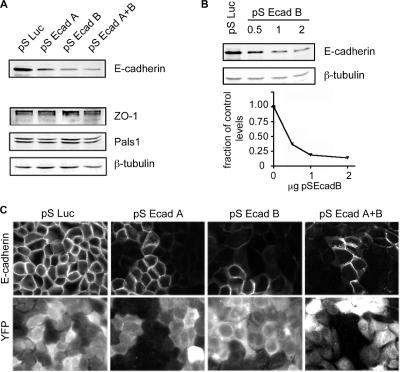
To investigate the role of E-cadherin in cell polarity, we depleted E-cadherin from MDCK cells by RNAi. Two shRNAs, pSEcad-A and -B, were created against canine E-cadherin sequences. Cells were transiently transfected with pSuper plasmids expressing either the E-cadherin target sequences or, as a control, shRNAs complementary to the Luciferase gene (pSLuc). Efficiency of knockdown was assayed by immunoblots of cell lysates 2 d posttransfection (Figure 1A). Both shRNAs depleted E-cadherin in MDCK cells, with pSEcad-B exhibiting most effective. In subsequent experiments, cells referred to as knockdown (KD) cells were transfected with pSEcad-B or a mixture of pSEcad-A and -B. Figure 1A also shows that protein levels of ZO-1 and Pals1 are similar between knockdown and control lysates. β-tubulin was used as a protein loading control. pSEcad vector concentrations were titrated to optimize E-cadherin suppression (Figure 1B). By quantitative immunoblot, E-cadherin depletion to 15–20% of control values was achieved with 1–2 μg of plasmid. Importantly, the immunoblots were from transient transfections in which the efficiency of transfection was estimated to be 70–80%. This suggests that gene silencing in transfected cells approaches 100%. Efficient E-cadherin suppression in transfected cells was confirmed by immunofluorescence (Figure 1C). MDCK cells were transfected with the shRNAs plus pK-YFP as a transfection marker. Images were obtained after 48 h and selected to include both transfected and nontransfected cells. Little or no E-cadherin was detected in the transfected cells.
Figure 1.
E-cadherin knockdown in MDCK cells. E-cadherin shRNAs were generated against canine E-cadherin sequence and expressed in MDCK cells. Control samples expressed shRNAs against luciferase (pSLuc). (A). Immunoblot of cell lysates 2 d posttransfection. β-tubulin was used as a loading control. (B). Western blot of cell lysates from control cells, and cells transfected with increasing amounts of pS-EcadB. The bottom panel shows quantitative measurements of E-cadherin depletion, adjusted for differences in gel loading. (C) Immunofluorescence staining of fixed control and KD cells. E-cadherin is silenced in cells coexpressing the YFP marker.
Cell–Cell Contracts Are Maintained in E-Cadherin Knockdown Cells
To determine the effects of E-cadherin knockdown on cell polarity, we analyzed TJ and AJ marker proteins by immunofluorescence. MDCK cells were transfected in suspension then plated onto Lab-Tek slides at sufficient density to immediately form a confluent monolayer. Cells were fixed and stained after 48 h, the time point at which we observed maximum E-cadherin knockdown (Figure 3A). This protocol allows the reformation of cell–cell junctions (complete by ∼5 h after trypsinization; Chen and Macara, 2005) before any significant loss of E-cadherin and enables us to ask whether E-cadherin is required for maintenance of cell polarity after mature cell–cell contacts have formed.
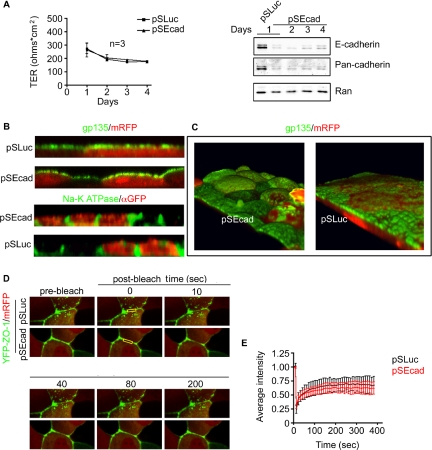
Figure 3.
TJs are not disrupted in cells depleted of E-cadherin. (A) TER was measured up to 4 d posttransfection. Results are plotted for control (■) and knockdown cells (▴), as means of three independent experiments after background subtraction. Below is a representative Western blot of Cadherin expression over this time period. (B) Cell polarity is maintained in cells depleted of E-cadherin. MDCK cells grown on filters were fixed and stained 2 d posttransfection. Cells were imaged by confocal microscopy with 20 × 1 μm z-sections. Assembled images are viewed in cross-section, stained with the apical marker gp135 (green), or the basal–lateral marker Na+/K+ATPase (green). mRFP or YFP marks transfected cells. (C) 3-D–assembled confocal sections of control or E-cadherin knockdown cells. Apical surfaces are stained with gp135 (green), with mRFP as a transfection marker. (D) FRAP analysis of TJ stability in YFP-ZO-1 MDCK stable cell line. Confocal sections of cells expressing YFP-ZO-1 are shown before and at various time points after photobleaching. mRFP expression marks transfected cells and boxed areas at time point 1 indicate bleached region. (E) Quantification of FRAP in control (n = 6) and knockdown cells (n = 5).
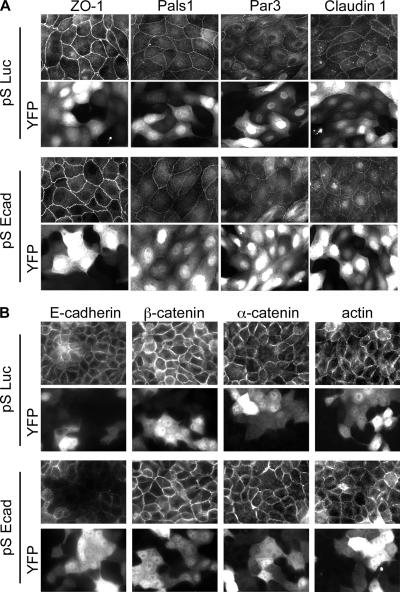
In differentiated epithelia, ZO-1, Pals1, Par3, and the transmembrane protein claudin1 all localize to the apical TJ complex (Gonzalez-Mariscal et al., 2003). Unexpectedly, loss of E-cadherin had no effect on this localization (Figure 2A). Both control and knockdown cells (marked by YFP expression) showed TJ marker proteins at cell–cell contacts. Remarkably, E-cadherin silencing also failed to disrupt peripheral localization of AJ proteins (Figure 2B). Despite a reduction of E-cadherin in transfected cells to almost undetectable levels, adherens components β-catenin and α-catenin still localized to cell–cell contacts (Figure 2B), and cortical actin bundles were still localized to the cell periphery.
Figure 2.
Cell–cell contacts are maintained in cells depleted of E-cadherin. Immunofluorescence staining of MDCK cells fixed 2 d posttransfection with E-cadherin pSEcad or pSLuc shRNAs. YFP expression is a transfection marker. (A) TJ marker proteins ZO-1, Pals1, Par3, and the transmembrane protein Claudin1 localize to cell–cell contacts in control and knockdown cells. (B) Adherens junction proteins E-cadherin, β-catenin, α-catenin, and actin localization at cell–cell contacts in both control and knockdown cells.
E-Cadherin–depleted Cells Retain Apical–Basal Polarity
Tight junctions seem intact in the knockdown cells, but they do they function properly? To answer this question, we measured the pericellular permeability of control and knockdown cells by TER. Resistance was measured over 4 d posttransfection (Figure 3A). An immunoblot, of cell lysates over this period (Figure 3A, right), with E-cadherin and pan-cadherin antibodies shows cadherin levels were lowest at 2 d posttransfection. Strikingly, depletion had no apparent effect on pericellular permeability as measured by TER. Therefore, epithelial gate function was preserved in the E-cadherin knockdown cells.
Another measure of TJ integrity is whether cells can maintain distinct apical and basal membrane surfaces. To determine whether fence function is maintained, MDCK cells were grown to confluence on filters and assayed by immunofluorescence for the apical marker gp135, or the lateral marker Na+/K+ ATPase (Figure 3B). mRFP was introduced to identify cells depleted of E-cadherin. The images shown are cross-sections of compiled confocal layers. In both control and knockdown cells gp135 was confined to the apical surface and Na+/K+ATPase was limited to the lateral surface. Taken together, these data suggest that endogenous levels of E-cadherin are not required for the maintenance of cell polarity. We noticed, however, that the apical surfaces of cells lacking E-cadherin were bulbous rather than being flat, as in the control cells (Figure 3B). The domed shapes of these cells were particularly evident in three-dimensional (3-D) reconstruction of confocal sections (Figure 3C). Control cells consistently have flat apical surfaces. The reason for this morphological change remains unclear. The level of gp135, an apical marker, was not increased relative to total cell protein or basolateral markers by E-cadherin silencing (data not shown), suggesting that the apical/lateral surface ratio was not increased. Moreover, changing the osmotic pressure of the media in the apical compartment of the filter did not reverse the effect, showing that the domed shape of the cells was not caused by a defect in cellular osmotic regulation (data not shown).
Previously, we had reported that suppression of E-cadherin by RNAi in MDCK cells increased cell migration and promoted a more fibroblast-like phenotype in subconfluent cells (Qin et al., 2005). Increased junction remodeling may be required for motile cells and this could be reflected in increased turnover of junction proteins. These considerations led us to ask whether TJ dynamics were altered by E-cadherin depletion. FRAP was used to assess this possibility. MDCK cells stably expressing a YFP–ZO-1 construct were subjected to photobleaching and imaged by confocal microscopy (Figure 3E). The YFP-ZO1 fusion behaves similarly to endogenous protein, because it localizes to TJs and is capable of compensating functionally for ZO1 depletion by RNAi (McNeil et al., 2006). This cell line was transfected with pSEcad or control plasmid and imaged 48 h posttransfection. Regions within the junctions between two adjacent transfected cells were selected for bleaching (yellow box Figure 3D), and average intensity was monitored during recovery (Figure 3E). In both control and E-cadherin–depleted cells, YFP ZO-1 fluorescence intensities recovered from photobleaching to ∼50% of initial values, indicating that both mobile and immobile populations of ZO-1 exist within the TJ. In both cases, the half-time for recovery of the mobile fraction was ∼50 s. These results show that E-cadherin levels are not important for ZO-1 turnover at the membranes of polarized MDCK cells and that TJ stability is probably not regulated by the dynamics of proteins in these junctions.
E-Cadherin–depleted Cells Exhibit Increased Calcium Sensitivity
Cell–cell contacts in MDCK cells are composed of both calcium-dependent and calcium-independent adhesions. Importantly, neither type of junction can be maintained when cells are incubated in media containing <10 μM extracellular calcium (Gonzalez-Mariscal et al., 1990). To determine the requirement for E-cadherin in junction maintenance during calcium withdrawal, YFP-ZO-1–expressing MDCK cells were imaged by time-lapse microscopy in either 2 or 100 μM extracellular calcium. After ∼30-min incubation in 2 μM calcium, control and KD cells both began to lose junctions, and YFP-ZO-1 accumulated near remaining cell–cell contacts as punctate dots (Figure 4A). By 60 min, YFP-ZO-1 was no longer localized at the junction, instead showing up as a hazy ring in the cytoplasm. Previous studies have shown MDCK cells can maintain junctions in calcium concentrations as low as 100 μM (Gonzalez-Mariscal et al., 1990). Strikingly, this concentration was sufficient to displace YFP-ZO-1 from cell–cell contacts in E-cadherin–depleted cell but not in control cells, indicating an increased sensitivity to extracellular calcium concentration. A similar sensitivity was observed when cells, incubated in 100 μM calcium for 15 min, were fixed and stained with phalloidin to observe actin structures (Figure 4B). In E-cadherin–depleted cells, actin was released from the cortex and occurred as a ring in the cytoplasm, whereas the untransfected and control cells retained cortical actin structures. Interestingly, cortical actin disassociation occurred at a time when most of the YFP-ZO-1 was still retained at the junctions (Figure 4A, 30 min). This result shows that E-cadherin is required for the attachment of the actin ring to the cortex in low calcium.
Figure 4.
Increased calcium sensitivity in E-cadherin–depleted cells. (A) YFP-ZO-1 becomes mislocalized at 100 μM extracellular calcium in cells depleted of E-cadherin. MDCK T23 cells stably expressing YFP-ZO-1 were transfected with vectors for mRFP plus pSLuc or pSEcad shRNAs. After 2 d, cells were switched to medium containing either 2 or 100 μM calcium (t = 0), and YFP-ZO-1 localization was monitored for up to 60 min. (B) MDCK cells expressing control (pSLuc) or E-cadherin shRNA along with YFP, were incubated in medium containing 100 μM calcium for 15 min. Fixed cells were stained with phalloidin to visualize actin structures. (C) E-cadherin depletion causes loss of TER at 100 μM calcium. Control (pS) and E-cadherin knockdown cells (KD) were plated on Transwell filters and allowed to grow to confluence. Cells were then incubated in medium containing 2 or 100 μM calcium. TER was measured for 60 min.
As an alternative approach, MDCK T23 cells were incubated in media with either 2 or 100 μM calcium, and junction integrity was measured by TER (Figure 4C). Again, 2 μM calcium media disrupted cell–cell contacts in both control and E-cadherin suppressed cultures. The rate of resistance loss was similar between the two samples. Incubation with 100 μM calcium, however, caused a slight drop in resistance in control cultures, but decreased the resistance of E-cadherin–suppressed cells in a manner similar to incubation with 2 μM calcium.
E-Cadherin–β-Catenin Complex Is Disrupted in E-Cadherin Knockdown Cells
By quantitative Western blot analysis, a reduction of E-cadherin by 85% (±5%), caused a drop of only 40% (±8%) in β-catenin, and α-catenin remained essentially unchanged (Figure 5A). E-cadherin binds β-catenin in a 1:1 stoichiometric complex (Ozawa and Kemler, 1992; Huber and Weis, 2001). Therefore, one explanation for β-catenin stability and for its continued peripheral localization in E-cadherin depleted cells is that E-cadherin exists in large excess over β-catenin, and the reduction in E-cadherin by RNAi would then be insufficient to disrupt the complex. To test for this possibility, MDCK cell lysates were subjected to serial immunodepletion with anti-E-cadherin antibody. The resulting supernatants were blotted for E-cadherin, β-catenin, and β-tubulin (Figure 5B). By the third round of immunoprecipitation, supernatants were almost completely devoid of E-cadherin, yet a considerable fraction of the β-catenin remained in the supernatant. To control for disassociation of the complex over the course of the experiment, MDCK cell lysates were immunoprecipitated after either 1- or 5-h incubations on ice. The E-cadherin–β-catenin complex remained stable during this period (data not shown). If we assume that the β-catenin normally associated with E-cadherin is destroyed in the knockdown cells, we can calculate that there is roughly a twofold excess of β-catenin over E-cadherin in MDCK cells. Most of this β-catenin is at the lateral cell cortex in confluent monolayers (Figure 2B). Also, in day 2 lysates most of the E-cadherin occurs in the Triton X-100–soluble fraction, and this fraction does not change with depletion of E-cadherin by RNAi (Figure 5C). Additionally, when the supernatants were immunoprecipitated for β-catenin, the amount of E-cadherin bound to β-catenin was found to be at similar ratios to the protein levels in cell lysates. Together, these data indicate that there is an excess of β-catenin in MDCK cells relative to E-cadherin. Thus, the continued stability of β-catenin in cells depleted of E-cadherin cannot be due to binding to residual E-cadherin.
Figure 5.
β-catenin is in excess of E-cadherin in MDCK cells. (A) Western blot analysis of whole cell lysates 2 d posttransfection with pSLuc or pSEcad vectors. Lysates were blotted for E-cadherin, β-catenin, and α-catenin to determine relative protein abundances. (B) β-catenin is in excess of E-cadherin in MDCK cells. TX-100 lysates from untransfected cells were analyzed by immunoblot after serial E-cadherin immunodepletion. (C) MDCK cells were lysed in a TX-100 lysis buffer, and samples of the insoluble (pellet) and soluble (sup) fractions were analyzed by immunoblot. The fraction of β-catenin in the insoluble pool does not change in cells after E-cadherin depletion. Soluble lysate fractions were immunoprecipitated with β-catenin antibody or mouse nonimmune antibody (NIIgG) as a control. Samples were blotted for β-catenin. (D) Immunoblot of cell lysates from control (pSLuc) or pS-Ecad–transfected cells. Lysates were blotted for E-cadherin and Pan-cadherin. β-tubulin is a loading control. (E) Whole cell lysates of control (pSLuc) and pSEcad cells blotted for Cadherin-6, E-cadherin, and β-tubulin.
β-Catenin Is Stabilized by Cadherin-6
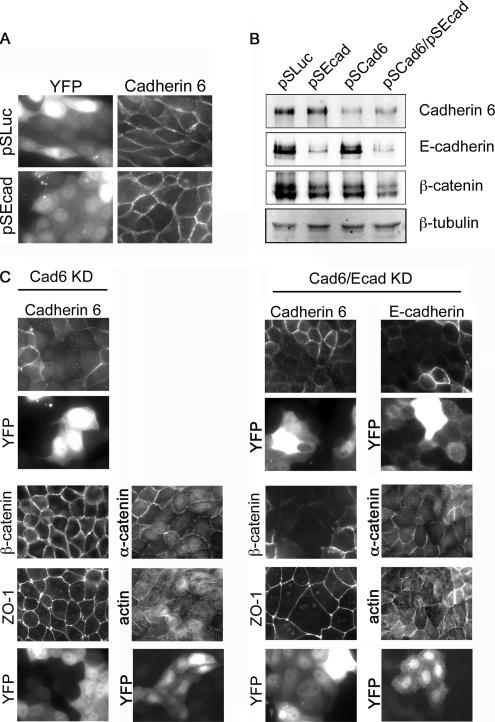
Free β-catenin will be degraded or imported into the nucleus. Therefore, the surplus protein is presumably bound to other cadherin family members or to α-catenin. In conditional E-cadherin knockout mice, an increased expression of P-cadherin is thought to compensate for the loss of the E-cadherin (Tinkle et al., 2004; Tunggal et al., 2005). MDCK cells express several classical cadherins, although most are expressed at low levels (Geiger et al., 1990). We first asked whether these cadherins are up-regulated in E-cadherin–depleted MDCK cells, by blotting lysates with a pan-cadherin antibody, which detects the highly conserved cytoplasmic domain of classical cadherins. However, the reduction in band intensities in pan-cadherin immunoblots was similar to that for the E-cadherin blot (Figure 5E), suggesting that no up-regulation occurs, and confirming that the majority of type I cadherin in MDCK cells is the E isoform. Cadherin-6 (K-cadherin), a type II atypical cadherin, is also expressed in MDCK cells, although at low levels (Stewart et al., 2000). However, Cadherin-6 expression was not induced by E-cadherin knockdown (Figure 5E). Nonetheless, it seemed possible that Cadherin-6 contributes to cell–cell adhesions and β-catenin stability. To test this possibility, we immunostained MDCK cells, and observed that Cadherin-6 was retained at cell–cell contacts in E-cadherin-depleted cells (Figure 6A). But to what extent does Cadherin-6 stabilize cell–cell contacts in the absence of E-cadherin? To address this question, shRNAs were produced to target the canine Cadherin-6 sequence. These shRNAs efficiently silenced Cadherin-6 expression, as shown both by immunoblots of whole cell lysates (Figure 6B, lanes 3 and 4), and by immunofluorescence staining with anti-Cadherin-6 antibody (Figure 6C). (Whole cell lysates were processed in the absence of β-ME, as described in Materials and Methods, which may account for β-catenin resolving as a double band). Cadherin-6 depletion alone had no visible effect on β-catenin localization, yet when E-cadherin was also suppressed, β-catenin was almost entirely lost from the cell periphery, and the overall level of the protein was substantially reduced. Defects were also seen in the localization of α-catenin and actin structures at the cell cortex. Strikingly, however, ZO-1 remained at the cell junctions in both the Cadherin-6 single knockdown and the Cadherin-6/E-cadherin double knockdown (Figure 6C).
Figure 6.
Simultaneous suppression of Cadherin-6 and E-cadherin causes mislocalization of β-catenin, yet TJs are not disrupted. (A) Immunofluorescence of Cadherin-6 in MDCK cells fixed 2 d after transfection with E-cadherin (pSEcad) or pSLuc shRNA vectors. YFP is the transfection marker. (B) Immunoblot analysis of cell lysates from MDCK cells transfected with pSLuc or pSEcad, Cadherin-6 shRNA vector (pSCad6), or both pSEcad plus pSCad6. (C) Immunofluorescence images of MDCK cells depleted of Cadherin-6 or of both E-cadherin and Cadherin-6. Cells were fixed and stained with anti-Cadherin-6 or anti-E-cadherin to confirm cadherin suppression in transfected cells. Cellular localization of ZO-1, β-catenin, α-catenin, and actin is shown for both Cadherin-6 and double knockdown conditions. Transfected cells express YFP.
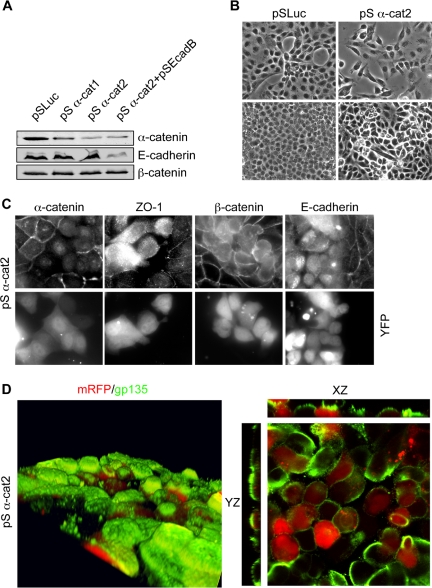
α-Catenin Is Required for Cell Junction Stability
One mechanism that might provide cell junction stability in cadherin-depleted cells is the continuing function of α-catenin. In addition to being a component of the cadherin–β-catenin complex, α-catenin interacts with the actin network at AJs. Also, α-catenin binds to Afadin, which is linked to nectin-based cell–cell adhesions (Miyoshi and Takai, 2005). Could these complexes serve to stabilize adhesions in the absence of cadherins? To answer this question, we depleted MDCK cells of α-catenin by RNAi. Two shRNAs were generated against canine α-catenin sequence and tested for protein suppression 2 d after transfection. Primer 2 reduced α-catenin expression as much as 80% compared with controls (Figure 7A). This reduction of α-catenin levels did not alter the stability of β-catenin as assessed by Western blot. Nor did it further reduce β-catenin levels when E-cadherin was also depleted by RNAi. Therefore, α-catenin levels do not contribute to β-catenin protein stability.
Figure 7.
Suppression of α-catenin disrupts confluent MDCK cells. (A) Immunoblot of cell lysates 2 d posttransfection with control, α-catenin and/or E-cadherin shRNA vectors. (B) DIC image of MDCK cells at low confluence (top) or high confluence (bottom) transfected with either control or α-catenin shRNA vectors. (C) Immunofluorescence images of α-catenin–depleted MDCK cells stained for α-catenin, ZO-1, β-catenin, and E-cadherin. YFP was a transfection marker. (D) Recompiled confocal sections of α-catenin–depleted cells grown on filters. Cells were fixed and stained for the apical marker gp135 (green). mRFP was used as a transfection marker (red). The right-hand panels are cross-sections of the same image.
What effect does α-catenin suppression have on MDCK cells? Normally, MDCK cells plated at low densities form tight colonies. Differential interference contrast (DIC) imaging revealed that cells depleted of α-catenin failed to form colonies and retained a mesenchymal phenotype (Figure 7B). A similar effect was seen with E-cadherin depletion (Qin et al., 2005). Interestingly, by immunofluorescence, cells transfected with α-catenin shRNA2 exhibited severe defects both in their TJs and AJs, as determined by staining for ZO-1, β-catenin, and E-cadherin (Figure 7C). All three proteins exhibited diffuse cytoplasmic staining in transfected cells. Additionally, cells depleted of α-catenin were rounded and lost cell–cell contacts, particularly when adjacent to other transfected cells. Even when grown on filters, transfected cells remained rounded and failed to maintain cell–cell adhesions (Figure 7D). Distinct apical and lateral surfaces were not maintained, because gp135 staining was seen along the lateral walls (Figure 7D, right). Overall, loss of α-catenin causes a much more severe defect in epithelial adhesion and cell polarity than does the loss of E-cadherin, and these effects are not caused by instability of E-cadherin or β-catenin.
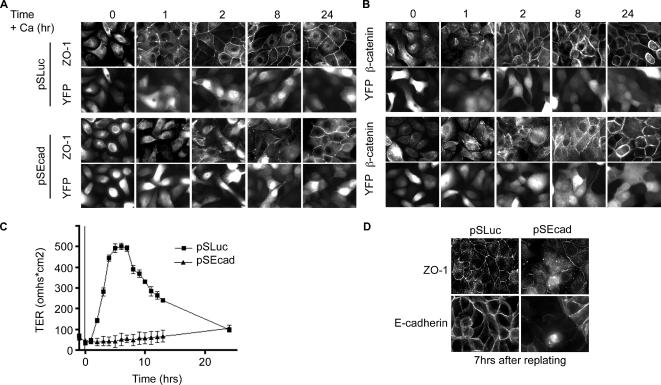
E-Cadherin Depletion Disrupts Cell Polarization in Response to Calcium Switch
To examine the effects of E-cadherin depletion on junction formation, MDCK cells were subjected to calcium switch. After readdition of calcium to the medium, control cells establish TJs within the first hour, as indicated both by the accumulation of the TJ marker ZO-1 and the initial increase in TER (Figures 8, A and C). However, for cells depleted of E-cadherin, ZO-1 localization remained incomplete even 8 h postcalcium switch. Nevertheless, after 24 h the knockdown cells had relatively normal TJs. This disruption was not specific to calcium switch. When cells were trypsinized and replated, control cells formed normal junctions by 7 h, but knockdown cells were still disrupted (Figure 8D). Additionally, TJ recovery was monitored by TER measurements postcalcium switch (Figure 8C). TER recovery was severely reduced, although samples did establish normal resistance after 24 h. Recruitment of β-catenin to cell adhesions was also delayed in E-cadherin–depleted cells (Figure 8B).
Figure 8.
TJ biogenesis is disrupted in cells depleted of E-cadherin. E-cadherin knockdown cells fail to polarize properly after calcium switch. (A) Immunofluorescence of MDCK cells 2 d after transfection with control shRNA and YFP. (B) E-cadherin–depleted cells fixed and stained 2 d after transfection. Cells were stained for ZO-1 and β-catenin. YFP marks transfected cells. (C) Average TER after calcium switch for control (■) and knockdown cells (▴). (D) Trypsinized and replated cells were analyzed by immunofluorescence for TJ formation. Cells were fixed 7 h after replating and stained for E-cadherin and ZO-1.
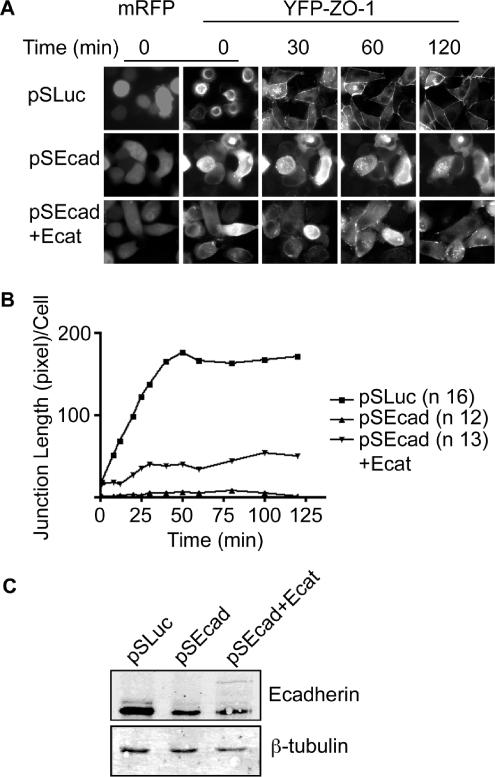
Cell Polarization Defects Can Be Partially Rescued with E-Cadherin–α-Catenin Fusion Protein
To further investigate the effects of cadherin depletion on epithelial polarization, cells were imaged by time-lapse video microscopy after calcium switch (Figure 9A), by using a stable YFP-ZO-1 cell line. Figure 9A shows pSuper-transfected cells, marked with mRFP, at various time points after readdition of calcium. In control cells, before readdition of calcium (time 0), YFP-ZO-1 is located in both the cytoplasm and in apical ring structures (see Supplemental Video 1). Previously, we and others have described these rings as containing actin, occludin, and other apical and TJ markers (Ivanov et al., 2006; McNeil et al., 2006). Strikingly, in samples with reduced cadherin levels, YFP-ZO-1 seems entirely cytoplasmic (Figure 9A, middle, and Supplementary Video 2). After the addition of calcium, YFP-ZO-1 accumulated along cell–cell contacts in control cells as puncta and developed into a continuous band around the cell perimeter (Figure 9, A and B). This process was completed between 30–60 min after addition of normal media. In contrast, very little YFP-ZO-1 localized to cell–cell contacts in E-cadherin–depleted cells. Several puncta were observed, but they failed to develop into mature ZO-1 bands. To show that disruption of cell–cell junction biogenesis was due to loss of E-cadherin, we performed rescue experiments. Although we were unable to observe rescue with a wild-type E-cadherin–GFP fusion (which was expressed at subendogenous levels), we were able to achieve partial rescue with an E-cadherin–α-catenin fusion. This construct was also expressed at very low levels (Figure 9C), but it significantly increased the accumulation of YFP-ZO-1 at the cell periphery. The junctions seemed to mature and strengthen along cell–cell contacts but, as in knockdown cells, they were transient (Figure 9A, bottom, and Supplementary Video 3). These data were quantified by measuring the length of YFP-ZO-1 accumulation at cell–cell contacts per transfected cell (Figure 9B).
Figure 9.
E-cadherin knockdown is partially rescued by E-cadherin–α-catenin fusion protein. (A) Live cell time-lapse imaging of YFP-ZO-1 MDCK cells after calcium switch. Cells were transfected with vectors for control or E-cadherin shRNAs or the E-cadherin–α-catenin fusion protein (Ecat). mRFP marks transfected cells. Videos are available in Supplementary Material. (B) Quantification of ZO-1 length as a measure of TJ recovery (pixels per cell). Average lengths are recorded up to 120 min after calcium switch for control cells (■) E-cadherin depleted cells (▴) and depleted cells plus an E-cadherin–α-catenin fusion protein (▾). (C) Immunoblot of cell lysates from YFP-ZO-1 cells transfected with pSLuc, pSEcadB, or pSEcadB vectors plus the E-cadherin–α-catenin fusion protein.
DISCUSSION
The E-cadherin–catenin complex plays pivotal roles in the construction of epithelia. Knockout mouse models and genetic analysis in Drosophila have demonstrated that it is essential from early embryogenesis through the later stages of organogenesis (Larue et al., 1994; Tepass et al., 1996; Ohsugi et al., 1997; Tinkle et al., 2004; Tunggal et al., 2005). In addition to providing adhesion between adjacent cells, E-cadherin, like the integrins, mediates outside-in signaling, and, during the initial stages of cell–cell contact, E-cadherin engagement initiates the activation of Rho family GTPases, actin network restructuring, and recruitment of TJ proteins to the plasma membrane (Ando-Akatsuka et al., 1999; Braga, 2002; Bershadsky, 2004). Expression of Rho mutants compromises apical–basal polarization and can disrupt the formation of both AJs and TJs (Braga et al., 1997; Jou and Nelson, 1998). There is also evidence for inside-out signaling, because perturbation of actin dynamics can disrupt E-cadherin–mediated adhesions (Jaffe et al., 1990; Angres et al., 1996). But once the junctions have formed, is E-cadherin still required? Cell–cell contacts are strengthened by other types of intercellular linkages that form after the initial transdimerization of E-cadherin. Cadherin-6 is present at low levels in MDCK cells and might provide adhesions between cells. Desmosomes provide tensile strength to epithelial sheets. Nectins, junctional adhesion molecules, claudins, and occludin all provide additional linkages that might stabilize adhesions.
Contrary to established ideas, recent data show that the cortical actin ring, which forms early after the initial E-cadherin contacts, is not coupled to E-cadherin through the catenins, because α-catenin cannot bind to actin when associated with E-cadherin through β-catenin; and α-catenin dimers bound to actin cannot associate with β-catenin (Drees et al., 2005; Yamada et al., 2005). It is conceivable, therefore, that even if E-cadherin is essential for the initial organization of cortical actin, it is not required after maturation of the AJs. Animal models have provided ambiguous evidence concerning this issue. For example, a conditional knockout of E-cadherin in skin did not result in the loss of epithelial adhesions, but this was attributed, at least in the basal layer, to a compensatory up-regulation of P-cadherin (Tinkle et al., 2004; Tunggal et al., 2005). In the early embryo, the effects of an E-cadherin knockout are not fully penetrant. Around one-half the embryos form blastocysts, and some cells possess apparently normal AJs, with β-catenin present at the cell cortex (Larue et al., 1994; Ohsugi et al., 1997). Again, this variability has been attributed to the presence of residual maternal protein. However, it is also possible that junctions, once formed, might persist even in the absence of E-cadherin, so long as the cells are not mechanically stressed.
To address this issue, we used shRNAs to silence E-cadherin gene expression in MDCK epithelial cells. When plated at high density, these cells begin to form junctions within 1–2 h, and they are polarized after 24 h, but depletion of the endogenous E-cadherin occurs very slowly, and it is not complete for 48 h, long after the junctions have formed. This temporal delay provides a way to ask whether preformed junctions require E-cadherin function. We estimate that our shRNAs can reduce E-cadherin in transfected cells by >90%; yet these cells remain polarized, and they have intact, functional TJs. Surprisingly, β-catenin and actin also remain at the cortex. We considered that this retention might result from there being a large excess of E-cadherin over β-catenin in MDCK cells, so that even after knockdown there would be sufficient to bind and stabilize the β-catenin. However, this proved not to be the case. To the contrary, we estimate that MDCK cells contain about a twofold excess of β-catenin over E-cadherin.
We also wondered whether other members of the cadherin family might compensate for the loss of E-cadherin. The drop in cadherin measured by immunoblot with a Pan-cadherin antibody was similar to that detected using an antibody specific for E-cadherin, consistent with previous data showing that E-cadherin is the major type I isoform present in MDCK T23 cells (Stewart et al., 2000). Nonetheless, the type II cadherin, Cadherin-6, is expressed in MDCK cells, so to determine the contribution of this isoform to β-catenin and TJ stability at cell contacts, both cadherins were depleted from the cells. Interestingly, the double knockdown caused a substantial loss of β-catenin as measured by immunoblots of cell lysates, and an almost complete disappearance from cell–cell junctions. Defects in α-catenin and actin localization were also observed, although they were not as dramatic as the displacement of β-catenin. Yet, TJs seemed intact, as measured by ZO-1 immunofluorescence. Therefore, TJs once formed are stable even in the absence of functional AJs. Importantly, this stability does not result from the static nature of TJs, because FRAP analysis shows that at least 50% of the ZO-1 at junctions is dynamic, with a half-life of ∼30 s.
The loss of E-cadherin had a reproducible effect on the morphology of the epithelial cells. As we showed previously, at low density they seem more fibroblastic than the control cells and migrate more rapidly, consistent with reduced adhesiveness (Qin et al., 2005). Moreover, the apical surfaces become domed. This phenotype was not caused by increased apicalization, nor by an osmotic imbalance (data not shown). Rather, we suggest that doming results from reduced adhesiveness between cells, which reduces the contractile tension exerted by the cortical actin ring. A similar phenotype can be induced by the addition of blebbistatin, which inhibits myosin (Chen and Macara, 2005; data not shown).
The effects of silencing E-cadherin expression were more profound during the assembly of junctions after a calcium switch. Calcium depletion triggered the rapid loss of TJs and caused rounding in knockdown cells, presumably because it releases desmosomal attachments and those of other cadherins present at the lateral membranes. When incubated in low calcium media, normal cells retain an apical ring that contains actin, ZO-1, and E-cadherin. However, this ring was absent in cells depleted of E-cadherin, and the ZO-1 was diffuse through the cytoplasm. When calcium was added back to the medium, the ZO-1 did not accumulate at cell contacts, and TJ assembly was severely delayed. Clearly, stable E-cadherin–mediated attachments are essential to enable TJ and other cell–cell contacts to form and mature.
Interestingly, silencing of α-catenin expression disrupted cell–cell junctions even in polarized monolayers. The cells remained rounded, β-catenin and E-cadherin were dispersed from the cortex into the cytoplasm, and TJs were lost. This result is consistent with animal model studies, in which a conditional α-catenin knockout showed a more severe phenotype than the equivalent E-cadherin knockout (Vasioukhin et al., 2001). One pivotal function of α-catenin is likely the formation and maintenance of the cortical actin ring, either through direct binding to actin, or through associations with vinculin and other actin-binding proteins. Overall, our data support a model in which E-cadherin acts as a critical adhesive scaffold between cells early in junction assembly, but once stable junctions have matured its adhesive and signaling functions become largely dispensable.
Supplementary Material
ACKNOWLEDGMENTS
We thank Barry Gumbiner for the Ecad-α cat fusion vector and Pan-cadherin antibody, James Nelson for the Cadherin-6 antibody, Michael Caplan for the Na+/K+ ATPase antibody, Atsushi Miyawaki for the seYFP construct, and Roger Tsien for the mRFP construct. We also thank the members of the Macara laboratory for helpful suggestions. This work was supported by Grant GM-70902 from the Department of Health and Human Services, National Institutes of Health.
Abbreviations used:
- AJ
adherens junction
- MDCK
Madin-Darby canine kidney
- TJ
tight junction
- YFP
yellow fluorescent protein
- ZO
zonula occludin protein.
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-05-0471) on November 8, 2006.
REFERENCES
- Adams C. L., Nelson W. J. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr. Opin. Cell Biol. 1998;10:572–577. doi: 10.1016/s0955-0674(98)80031-8. [DOI] [PubMed] [Google Scholar]
- Ando-Akatsuka Y., Yonemura S., Itoh M., Furuse M., Tsukita S. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J. Cell Physiol. 1999;179:115–125. doi: 10.1002/(SICI)1097-4652(199905)179:2<115::AID-JCP1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Angres B., Barth A., Nelson W. J. Mechanism for transition from initial to stable cell-cell adhesion: kinetic analysis of E-cadherin-mediated adhesion using a quantitative adhesion assay. J. Cell Biol. 1996;134:549–557. doi: 10.1083/jcb.134.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky A. Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol. 2004;14:589–593. doi: 10.1016/j.tcb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Boggon T. J., Murray J., Chappuis-Flament S., Wong E., Gumbiner B. M., Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- Braga V. M. Cell-cell adhesion and signalling. Curr. Opin. Cell Biol. 2002;14:546–556. doi: 10.1016/s0955-0674(02)00373-3. [DOI] [PubMed] [Google Scholar]
- Braga V. M., Machesky L. M., Hall A., Hotchin N. A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J. Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp T. R., Bernards R., Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Bryant D. M., Stow J. L. The ins and outs of E-cadherin trafficking. Trends Cell Biol. 2004;14:427–434. doi: 10.1016/j.tcb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Chen X., Macara I. G. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat. Cell Biol. 2005;7:262–269. doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- Cowin P., Rowlands T. M., Hatsell S. J. Cadherins and catenins in breast cancer. Curr. Opin. Cell Biol. 2005;17:499–508. doi: 10.1016/j.ceb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C. Disassembling adherens junctions: breaking up is hard to do. Trends Cell Biol. 2005;15:19–26. doi: 10.1016/j.tcb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Davis M. A., Ireton R. C., Reynolds A. B. A core function for p120-catenin in cadherin turnover. J. Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees F., Pokutta S., Yamada S., Nelson W. J., Weis W. I. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Joberty G., Macara I. G. Assembly of epithelial tight junctions is negatively regulated by Par6. Curr. Biol. 2002;12:221–225. doi: 10.1016/s0960-9822(01)00663-7. [DOI] [PubMed] [Google Scholar]
- Gavard J., Mege R. M. Once upon a time there was beta-catenin in cadherin-mediated signalling. Biol. Cell. 2005;97:921–926. doi: 10.1042/BC20040531. [DOI] [PubMed] [Google Scholar]
- Geiger B., Volberg T., Ginsberg D., Bitzur S., Sabanay I., Hynes R. O. Broad spectrum pan-cadherin antibodies, reactive with the C-terminal 24 amino acid residues of N-cadherin. J. Cell Sci. 1990;97:607–614. doi: 10.1242/jcs.97.4.607. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Betanzos A., Nava P., Jaramillo B. E. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Contreras R. G., Bolivar J. J., Ponce A., Chavez De Ramirez B., Cereijido M. Role of calcium in tight junction formation between epithelial cells. Am. J. Physiol. 1990;259:C978–C986. doi: 10.1152/ajpcell.1990.259.6.C978. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Stevenson B., Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J. Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B. M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. M. Regulation of cadherin adhesive activity. J. Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A. H., Weis W. I. The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. 2001;105:391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- Ivanov A. I., McCall I. C., Babbin B., Samarin S. N., Nusrat A., Parkos C. A. Microtubules regulate disassembly of epithelial apical junctions. BMC Cell Biol. 2006;7:12. doi: 10.1186/1471-2121-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe S. H., Friedlander D. R., Matsuzaki F., Crossin K. L., Cunningham B. A., Edelman G. M. Differential effects of the cytoplasmic domains of cell adhesion molecules on cell aggregation and sorting-out. Proc. Natl. Acad. Sci. USA. 1990;87:3589–3593. doi: 10.1073/pnas.87.9.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C., Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat. Cell Biol. 2002;4:E101–E108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- Jou T. S., Nelson W. J. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J. Cell Biol. 1998;142:85–100. doi: 10.1083/jcb.142.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L., Ohsugi M., Hirchenhain J., Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc. Natl. Acad. Sci. USA. 1994;91:8263–8267. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliri A., van Es S., Huveneers S., Collard J. G. The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J. Biol. Chem. 2004;279:30092–30098. doi: 10.1074/jbc.M401192200. [DOI] [PubMed] [Google Scholar]
- Matter K., Balda M. S. Functional analysis of tight junctions. Methods. 2003;30:228–234. doi: 10.1016/s1046-2023(03)00029-x. [DOI] [PubMed] [Google Scholar]
- McNeil E., Capaldo C. T., Macara I. G. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby Canine Kidney epithelial cells. Mol. Biol. Cell. 2006;17:1922–1932. doi: 10.1091/mbc.E05-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi J., Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv. Drug Deliv. Rev. 2005;57:815–855. doi: 10.1016/j.addr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Muller S. L., Portwich M., Schmidt A., Utepbergenov D. I., Huber O., Blasig I. E., Krause G. The tight junction protein occludin and the adherens junction protein α-catenin share a common interaction mechanism with ZO–1. J. Biol. Chem. 2005;280:3747–3756. doi: 10.1074/jbc.M411365200. [DOI] [PubMed] [Google Scholar]
- Nollet F., Kools P., van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J. Mol. Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- Ohsugi M., Larue L., Schwarz H., Kemler R. Cell-junctional and cytoskeletal organization in mouse blastocysts lacking E-cadherin. Dev. Biol. 1997;185:261–271. doi: 10.1006/dbio.1997.8560. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Kemler R. Molecular organization of the uvomorulin-catenin complex. J. Cell Biol. 1992;116:989–996. doi: 10.1083/jcb.116.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. D., Ciatto C., Chen C. P., Bahna F., Rajebhosale M., Arkus N., Schieren I., Jessell T. M., Honig B., Price S. R., Shapiro L. Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell. 2006;124:1255–1268. doi: 10.1016/j.cell.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M., Jamora C., Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- Qin Y., Capaldo C., Gumbiner B. M., Macara I. G. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol. 2005;171:1061–1071. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A., Leake D., Boese Q., Scaringe S., Marshall W. S., Khvorova A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- Shen L., Turner J. R. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol. Biol. Cell. 2005;16:3919–3936. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama Y., Nagafuchi A., Fujita S., Gotoh M., Takeichi M., Tsukita S., Hirohashi S. Cadherin dysfunction in a human cancer cell line: possible involvement of loss of alpha-catenin expression in reduced cell-cell adhesiveness. Cancer Res. 1992;52:5770–5774. [PubMed] [Google Scholar]
- Stewart D. B., Barth A. I., Nelson W. J. Differential regulation of endogenous cadherin expression in Madin-Darby canine kidney cells by cell-cell adhesion and activation of β-catenin signaling. J. Biol. Chem. 2000;275:20707–20716. doi: 10.1074/jbc.M000467200. [DOI] [PubMed] [Google Scholar]
- Takai Y., Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J. Cell Sci. 2003;116:17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Tepass U., Gruszynski-DeFeo E., Haag T. A., Omatyar L., Torok T., Hartenstein V. shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev. 1996;10:672–685. doi: 10.1101/gad.10.6.672. [DOI] [PubMed] [Google Scholar]
- Tinkle C. L., Lechler T., Pasolli H. A., Fuchs E. Conditional targeting of E-cadherin in skin: insights into hyperproliferative and degenerative responses. Proc. Natl. Acad. Sci. USA. 2004;101:552–557. doi: 10.1073/pnas.0307437100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunggal J. A., Helfrich I., Schmitz A., Schwarz H., Gunzel D., Fromm M., Kemler R., Krieg T., Niessen C. M. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 2005;24:1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aken E., De Wever O., Correia da Rocha A. S., Mareel M. Defective E-cadherin/catenin complexes in human cancer. Virchows Arch. 2001;439:725–751. doi: 10.1007/s004280100516. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V., Bauer C., Degenstein L., Wise B., Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of α-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V., Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr. Opin. Cell Biol. 2001;13:76–84. doi: 10.1016/s0955-0674(00)00177-0. [DOI] [PubMed] [Google Scholar]
- Yamada S., Pokutta S., Drees F., Weis W. I., Nelson W. J. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap A. S., Kovacs E. M. Direct cadherin-activated cell signaling: a view from the plasma membrane. J. Cell Biol. 2003;160:11–16. doi: 10.1083/jcb.200208156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T., Green K. J. Regulation of desmosome assembly and adhesion. Semin. Cell Dev. Biol. 2004;15:665–677. doi: 10.1016/j.semcdb.2004.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.