Abstract
α4β1 and α4β7 integrins are preferentially expressed on eosinophils and mononuclear leukocytes and play critical roles in their recruitment to inflammatory sites. We investigated the effects of TR14035, a small molecule, α4β1/α4β7 dual antagonist, in a rat model of allergic asthma.
Actively sensitized rats were challenged with aerosol antigen or saline on day 21, and the responses evaluated 24 and 48-h later. TR14035 (3 mg kg−1, p.o.) was given 1-h before and 4-h after antigen or saline challenge.
Airway hyper-responsiveness to intravenous 5-hydroxytryptamine was suppressed in TR14035-treated rats. Eosinophil, mononuclear cell and neutrophil counts, and eosinophil peroxidase and protein content in the bronchoalveolar lavage fluid (BALF) were decreased in TR14035-treated rats. Histological study showed a marked reduction of lung inflammatory lesions by TR14035.
At 24-h postchallenge, antigen-induced lung interleukin (IL)-5 mRNA upregulation was suppressed in TR14035-treated rats. By contrast, IL-4 levels in BALF were not significantly affected by TR14035 treatment.
IL-4 selectively upregulates vascular cell adhesion molecule-1 (VCAM-1), which is the main endothelial ligand of α4 integrins. Intravital microscopy within the rat mesenteric microcirculation showed that 24-h exposure to 1 μg per rat of IL-4 induced a significant increase in leukocyte rolling flux, adhesion and emigration. These responses were decreased by 48, 100 and 99%, respectively in animals treated with TR14035.
In conclusion, TR14035, by acting on α4β1 and α4β7 integrins, is an orally active inhibitor of airway leukocyte recruitment and hyper-responsiveness in animal models with potential interest for the treatment of asthma.
Keywords: α4 integrins, allergic asthma, airway hyper-responsiveness, leukocyte
Introduction
Allergic airway inflammation is characterized by airway and peribronchial leukocyte accumulation that consists of eosinophils and lymphocytes. It is widely accepted that lymphocytes, through the production of Th2 cytokines such as interleukin (IL)-4, IL-5 and IL-13, mediate the recruitment and activation of eosinophils (O'Byrne et al., 2004). In allergic airway diseases, eosinophils have been hypothesized to secrete toxic granule proteins and other inflammatory mediators that contribute to the hallmark features of asthma (Kay, 2005).
Leukocyte migration into the lung requires the interaction between surface-adhesion receptors on leukocyte and counter-structures on pulmonary endothelium. The α4 integrin subunit (CD49d) associates with either the β1 (CD29) or the β7 subunit to form the integrin heterodimers very late antigen-4 (VLA-4; α4β1; CD49d/CD29) and α4β7 (Berlin et al., 1993; Carlos & Harlan, 1994). VLA-4 is expressed at significant levels on all circulating leukocytes except neutrophils, which display low level expression but functionally active. VLA-4 binds to vascular cell adhesion molecule-1 (VCAM-1; CD106), a member of the Ig gene superfamily, that is expressed on cytokine-activated endothelial cells, and to the CS-1 subdomain of human fibronectin. α4β7 is expressed on a subset of T and B cells, natural killer, and eosinophils. It binds to the mucosal vascular adressin (MAdCAM-1), a member of the Ig and mucin-like families of adhesion molecules, as well as to VCAM-1 and fibronectin (Berlin et al., 1993; Erle et al., 1994).
One approach to inhibit infiltration of eosinophils into tissues is by interfering with cell adhesion molecules that mediate the rolling and the firm adhesion of these cells prior to their emigration to the extravascular tissue (Lobb et al., 1996; Wardlaw, 1999). In this context, VCAM-1, the main endothelial ligand of α4 integrins can be upregulated in the airways following antigen challenge (Bentley et al., 1993; Ohkawara et al., 1995). Thus, monoclonal antibodies (mAbs) directed against α4 integrins have been proved to inhibit not only the migration of eosinophils into the airways but also allergen-induced airway hyper-responsiveness in different animal models of asthma including guinea-pigs (Pretolani et al., 1994; Kraneveld et al., 1997), rats (Rabb et al., 1994; Richards et al., 1996), rabbits (Gascoigne et al., 2003), sheep (Abraham et al., 1994) and mice (Henderson et al., 1997; Kanehiro et al., 2000, 2001; Borchers et al., 2001a, 2001b). More recently, different companies have developed small molecules that antagonize α4 integrin function and demonstrated that these compounds can also inhibit leukocyte infiltration and airway hyper-responsiveness in different in vivo animal models of pulmonary inflammation (Abraham et al., 1997; 2000; Kudlacz et al., 2002; Huryn et al., 2004). Furthermore, some of these compounds are currently undergoing clinical trials for the treatment of asthma (Vanderslice et al., 2004).
In the present study, we have evaluated the effect of a synthetic small molecule, that is a dual α4β1 and α4β7 antagonist, TR14035 (Sircar et al., 2002), in a rat model of allergic asthma. This compound was proved to be effective blocking α4β7-dependent adhesion of lymphocytes to high endothelial venules (Egger et al., 2002), and was reported to have reached phase I clinical trial for asthma (Vanderslice et al., 2004). In addition, since IL-4 causes VCAM-1 upregulation without affecting the expression of intercellular adhesion molecule-1 (ICAM-1) or E-selectin, we have employed intravital microscopy to test the effect of TR14035 on IL-4-induced leukocyte–endothelial cell interactions in the mesenteric microvasculature of the rat.
Methods
Animal model and experimental groups
Male Brown Norway rats (250–300 g) were supplied by B&K Universal (Barcelona, Spain) and kept at room temperature of 22°C under a 12 h phase light–dark cycle and fed on A04 pellets (Panlab, Barcelona, Spain). Drinking water was freely available. The experimental protocols were approved by the institutional ethics committee and comply with Spanish and European community regulations for use of laboratory animals.
Animals were actively sensitized as previously outlined (Blesa et al., 2003). Sensitized animals were randomly distributed into negative control (vehicle+saline; NS), positive control (vehicle+antigen; SZ) and drug-treated (NS+TR14035, SZ+TR14035) groups. TR14035 (3 mg kg−1) was administered orally by gavage at 1 h before and 4 h after challenge with antigen or saline. In an additional group of experiments, TR14035 was orally administered at 10 mg kg−1 with the same schedule. TR14035 was solved in NaOH 0.5 N and diluted as appropriate in phosphate-buffer saline. The oral route was selected as convenient in the clinical setting. The dose level and schedule were based on pilot studies in this laboratory.
Animals were anesthetized and instrumented for measurement of lung resistance (RL) and dynamic compliance (Cdyn) as previously outlined (Suchankova et al., 2005). Unanesthetized rats were exposed to ovalbumin (1% in saline) or saline aerosol for 15 min. The time course of airway hyper-responsiveness in antigen exposed Brown Norway rats has been previously examined (Elwood et al., 1992) and the response at 24 h was selected on this basis. 5-Hydroxytryptamine (5-HT) was used to assess airways hyper-responsiveness since it produces a direct and reproducible bronchoconstrictor response in rats (Pauwels et al., 1985). Thus, airway responsiveness was determined in anesthetized animals from dose–response curves to 5-HT (5–50 μg kg−1, i.v.). After measurement of airway responsiveness (24 h post-challenge) or in additional experiments at 48 h after challenge with antigen, animals were killed with an overdose of sodium thiopental. Then, bronchoalveolar cells were collected in two successive lavages using 6 ml aliquots of sterile saline at room temperature instilled and recovered through a polyethylene tracheal cannula. Cell suspensions were concentrated by low-speed centrifugation, and the cell pellet resuspended. Total cell counts were made in a haemocytometer. Differential cell counts were determined from cytospin preparations by counting 300 cells stained with May–Grünwald–Giemsa. Because the yield of the instilled fluid was equivalent in all experimental groups (⩾85%), the results are expressed as absolute cell counts per ml of lavage fluid. The cell-free supernatant was used for biochemical determinations. The levels of free eosinophil peroxidase in the supernatant from bronchoalveolar lavage fluid (BALF) was determined as a marker of eosinophil activation, according to a previously described method (Strath et al., 1985) with modifications (Pons et al., 2000), and the results expressed as the optical density measured at 490 nm in a Microplate Autoreader (EL309, Bio-Tek Instruments). Total protein concentration was measured by a standard technique (BCA protein assay reagent kit; Pierce, Rockford, U.S.A.). IL-4 was measured by enzyme-immunoassay kit (Diaclone Research, Besançon, France).
For histological studies, lung was perfused and tissue blocks placed in formalin, cut into 4-μm-thick serial sections, and stained with haematoxylin–eosin or with toluidine blue to identify inflammatory cells as previously outlined (Serrano-Mollar et al., 2003).
The IL-5 mRNA transcripts were measured by real-time quantitative reverse transcriptase–polymerase chain reaction (RT–PCR). The method used for obtaining quantitative data of relative gene expression was the comparative Ct method (ΔΔCt method) as described by the manufacturer (PE-ABI PRISM 7700 Sequence Detection System; Perkin-Elmer Applied Biosystems; Foster City, CA, U.S.A.) and previously reported (Sanz et al., 2005). Glyceraldehyide 3-phosphate dehydrogenase (GAPDH) was chosen as the endogenous control gene. Lung tissue samples were obtained at 24 h postchallenge as an appropriate time point to measure antigen-induced IL-5 mRNA upregulation in this model (Huang et al., 2001; Underwood et al., 2002). IL-5 mRNA was measured since no ELISA kit for rat IL-5 is available, assuming that increases in IL-5 mRNA expression will translate into changes in protein (Underwood et al., 2002). Total RNA was extracted from lung tissue homogenates by using TriPure Isolation Reagent (Roche Applied Science, Indianapolis, IN, U.S.A.). Reverse transcription of RNA to generate cDNA was carried out by using TaqMan Reverse Transcription Reagents (Applied Biosystems) and PCR was performed by using TaqMan Universal PCR Mix (Applied Biosystems) as indicated by the manufacturer. TaqMan primer-probe sets for the following gene were obtained from Applied Biosystems (Taqman Assay-on-Demand™ Gene Expression Products): IL-5 (Rn01459975_m1). The PCR primer for rat GAPDH was designed using the Primer Express software (PE Biosystems, Morrisville, NC, U.S.A.) according to the published rat GAPDH cDNA sequence (GenBank NM17008) as previously reported (Blesa et al., 2003).
Intravital microscopy
Male Brown Norway rats (250–300 g) were fasted for 20–24 h prior to experiments with free access to water. The animals were anesthetized with sodium pentobarbital (65 mg kg−1, i.p.). A tracheotomy was performed to facilitate breathing and the right jugular vein was cannulated for intravenous administration of additional anesthetic as required. The right carotid artery was cannulated to monitor systemic arterial blood pressure through a pressure transducer (Spectramed Stathan P-23XL) connected to a recorder (GRASS RPS7C8B, Quincy, MA, U.S.A.).
A midline abdominal incision was made and a segment of the mid-jejunal mesentery exteriorized and carefully placed on an optically clear viewing pedestal to allow transillumination of a 3 cm2 segment of the mesenteric microvasculature. The temperature of the pedestal was maintained at 37°C. The exposed intestine was continuously superfused with a bicarbonate buffer saline (BBS, pH 7.4, 2 ml min−1, 37°C) and covered with a BBS-soaked gauze to prevent evaporation. Mesenteric microcirculation was observed through an orthostatic microscope (Nikon Optiphot-2, SMZ1, Badhoevedorp, The Netherlands) with a × 20 objective lens (Nikon SLDW) and a × 10 eyepiece as previously described (Sanz et al., 2002). A video camera (Sony SSC-C350P, Koeln, Germany) mounted on the microscope projected the image onto a color monitor (Sony Trinitron PVM-14N2E) and the images were captured on videotape (Sony SVT-S3000P) with superimposed time and date for subsequent playback analysis. The final magnification of the image on the monitor was × 1300.
Single unbranched mesenteric venules with diameters ranging between 25 and 40 μm were studied. Venular diameter (Dv) was measured on-line using a video caliper (Microcirculation Research Institute, Texas A&M University, College Station, TX, U.S.A.). Centerline red blood cell velocity (Vrbc) was also measured on-line with an optical Doppler velocimeter (Microcirculation Research Institute, Texas A&M University). Venular blood flow was calculated from the product of mean red blood cell velocity (Vmean=Vrbc 1.6−1) and microvascular cross-sectional area, assuming cylindrical geometry. Venular wall shear rate (γ) was calculated based on the Newtonian definition: γ=8 × (Vmean/Dv) s−1, in which Dv is venular diameter (House & Lipowsky, 1987).
The number of rolling, adherent and emigrated leukocytes was determined off-line during playback analysis of videotaped images. Rolling leukocyte flux was determined by counting the number of leukocytes rolling passing a fixed reference point in the microvessel per min. The same reference point was used throughout the experiment as leukocytes may roll for only a section of the vessel before rejoining the blood flow or becoming firmly adherent. Vwbc was determined by measuring the time required for a leukocyte to traverse a distance of 100 μm along the length of the venule and was expressed as μm s−1. A leukocyte was considered to be adherent to venular endothelium if it remained stationary for a period equal to or exceeding 30 s. Adherent cells were expressed as the number per 100 μm length of venule. Leukocyte emigration was expressed as the number of white blood cells per microscopic field.
Experimental protocol
Animals were first sedated with ether and later i.p. injected with 5 ml of saline or a 5 ml solution of recombinant rat IL-4 (1 μg rat−1, equivalent to ∼5000 U of IL-4/animal). In a previous study, this dose of IL-4 caused significant eosinophil accumulation in the rat skin (Sanz et al., 1998). In each experimental group, 24 h after the i.p injection of the agent under investigation, measurements of leukocyte rolling flux, velocity, adhesion, emigration, mean arterial blood pressure (MABP), Vrbc, shear rate and diameter were obtained and recorded for 5 min. In another set of experiments, TR14035 (3 mg kg−1) was administered orally by gavage at 1 h before and 4 h after saline or IL-4 i.p. injection and responses were evaluated 24 h after the administration of the stimuli. To determine the effect of TR14035 on levels of circulating leukocytes, blood samples were taken from the rats once the experiment using intravital microscopy was completed.
Statistical analysis
Data are presented as mean±s.e.m. of n experiments. Statistical analysis of data was carried out by analysis of variance (ANOVA) followed by Bonferroni test (GraphPad Software Inc, San Diego, CA, U.S.A.). Significance was accepted when P<0.05.
Materials
TR14035 (N-(2,6)-dichlorobenzoyl)-(L)-4-(2′6′-bis-methoxyphenyl)phenylalanine) was synthesized by Uriach (Barcelona, Spain) as previously outlined (Sircar et al., 2002). Recombinant rat IL-4 was from PeproTech, London, U.K. Other drugs were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.).
Results
Effect of TR14035 on antigen-induced airway hyper-responsiveness and inflammation
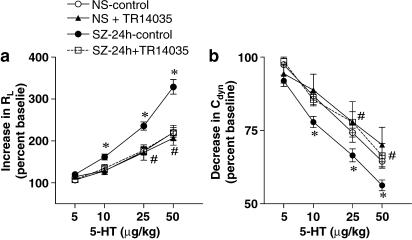
TR14035 was tested in an experimental model of allergic asthma in Brown Norway rats. At 24 h post-antigen challenge, untreated sensitized rats exhibited augmented airway responses to 5-HT compared with untreated saline-challenged animals (Figure 1). No significant differences in airway responses to 5-HT were found between untreated and TR14035-treated animals challenged with saline. By contrast, administration of TR14035 to antigen-challenged rats supressed airway hyperer-reponsiveness to 5-HT (Figure 1).
Figure 1.
The oral treatment with TR14035 (3 mg kg−1, 1 h before and 3 h after antigen challenge), an α4β1/α4β7 dual antagonist, produced a significant decrease of the airways hyper-responsiveness to 5-hydroxytryptamine (5-HT) in an experimental model of allergic asthma in Brown Norway rats. The airway responses to intravenous 5-HT are expressed as increases in lung resistance (RL) (a) and decreases in lung compliance (Cdyn) (b) obtained 24 h after challenge with antigen or saline. The experimental groups are: saline challenge without drug treatment (i.e. negative control; NS), antigen challenge without drug treatment (i.e. positive control; SZ), saline challenge in TR14035-treated rats (NS+TR14035), and antigen challenge in TR14035-treated rats (SZ+TR14035). Points are mean±s.e.m. of seven animals in each group except studies at 48 h which were carried out in five animals. *P<0.05 NS vs SZ, #P<0.05 SZ vs SZ+TR14035.
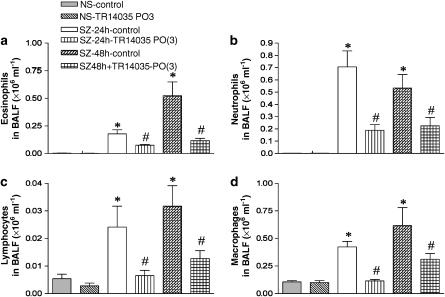
To asses the effects of TR14035 on the development of allergic inflammation after ovalbumin challenge, inflammatory cells in BALF were measured. At 24 h after challenge, significant increases in the number of total cells as well as eosinophils, neutrophils, lymphocytes and macrophages were found in the BALF of untreated animals exposed to antigen compared with the counts in saline-challenged rats (Figure 2). Again, no differences in the number of these cells were found in the BALF of untreated and TR14035-treated saline-challenged animals. When the dual α4β1/α4β7 antagonist was administered to allergen-exposed rats, eosinophil, neutrophil, lymphocyte and macrophage infiltration in the BALF was reduced by 64, 66, 88 and 95% respectively, compared with the infiltration found in the untreated group. Additional experiments at 48 h postantigen challenge also demonstrated the ability of TR14035 to decrease cell counts in BALF (Figure 2).
Figure 2.
The oral treatment with TR14035 (3 mg kg−1, 1 h before and 3 h after antigen challenge), an α4β1/α4β7 dual antagonist, produced a significant decrease of the cell counts for eosinophils (a), neutrophils (b), lymphocytes (c) and macrophages (d) in bronchoalveolar lavage fluid (BALF) in an experimental model of allergic asthma in Brown Norway rats. The cell counts were obtained 24 and 48 h after challenge with antigen or saline in the experimental groups outlined in Figure 1. Total cell counts were 0.11±0.01, 0.11±0.02, 1.28±0.18*, 1.71±0.40*, 0.42±0.10# and 0.67±0.13# × 106 cells ml−1 for NS, NS+TR14035, SZ-24 h, SZ-48 h, SZ-24 h+TR14035, and SZ-48 h+TR14035, respectively. Data are mean±s.e.m. of seven animals in each group. *P<0.05 vs NS; #P<0.05 vs the corresponding SZ group.
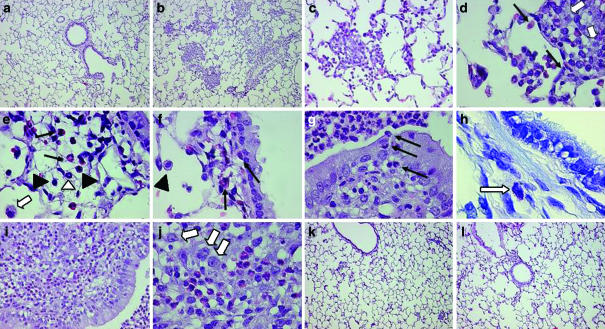
Light microscopic evaluation of lung tissues collected from saline-challenged rats either untreated or treated with TR14035 showed occasional mild inflammatory changes with very few eosinophils. Lungs from sensitized rats challenged with antigen and examined 24 and 48 h later showed multiple areas of inflammation in which blood vessels and bronchioles were surrounded by eosinophils, scattered lymphocytes and some macrophages, neutrophils and mast cells. Pulmonary lesions in antigen-challenged sensitized rats treated with TR14035 showed a marked decrease in the number and intensity of the clusters of leukocytic inflammatory lesions (Figure 3).
Figure 3.
Representative photomicrographs of lung histology in nonsensitized animals (a), sensitized animals at 24 h (b–h) and 48 h (i, j) postantigen challenge, and sensitized animals orally treated with the α4β1/α4β7 dual antagonist TR14305 (3 mg kg−1 given 1 h before and 3 h after antigen challenge) at 24 h (k) and 48 h (l) after challenge with antigen in an experimental model of allergic asthma in Brown Norway rats. All lung sections were stained with haematoxylin–eosin except panel h that shows a toluidine blue staining. At 24 h after antigen challenge, a marked peribronchial and perivascular infiltration of inflammatory cells extending into the adjacent pulmonary alveoli was evident in vehicle-treated rats (b–c). Eosinophils (closed arrows) are shown in inflammatory foci (d–f) and in the epithelium (g). Macrophages (open arrows), scattered lymphocytes (closed triangles), neutrophils (open triangle) and mastocytes (indicated by arrow in panel h) are also found in the inflammatory lesions. Lung tissue demonstrates severe inflammatory reactions at 48 h after antigen challenge (i, j). Treatment with TR14035 produced a marked inhibition of inflammatory cell infiltration in the peribronchial and perivascular areas and pulmonary alveoli at 24 and 48 h after antigen challenge (k, l). Magnification of panels × 10 (a, b, k, l), × 40 (c, i) and × 100 (d–h, j).
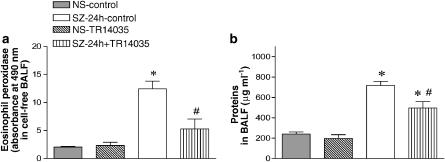
The antigen-induced augmentation of EPO activity in BALF measured at 24 h postovalbumin challenge is considered a marker of eosinophil activation. Consistent with the results encountered for eosinophils in BALF, the augmented EPO in untreated antigen-challenged animals was also significantly reduced in TR14035-treated rats by 69% (Figure 4).
Figure 4.
The oral treatment with TR14035 (3 mg kg−1, 1 h before and 3 h after antigen challenge), an α4β1/α4β7 dual antagonist, produced a significant decrease of eosinophil peroxidase activity (a) and proteins (b) in bronchoalveolar lavage fluid (BALF) in an experimental model of allergic asthma in Brown Norway rats. Values were obtained at 24 h after challenge with antigen or saline. Experimental groups are as indicated in Figure 1. Columns are mean±s.e.m. of seven animals in each group. *P<0.05 vs NS; #P<0.05 vs SZ.
Protein levels in BALF were significantly increased at 24 h post-antigen challenge in the antigen-challenged group, and treatment with TR14035 decreased this response by 46% (Figure 4). Administration of TR14035 to saline-exposed animals did not modify BALF protein content. These results suggest that the airway microvascular leakage after antigen-challenge in sensitized animals is in part related to the infiltration of inflammatory cells, since blockade of α4-integrin function reduced this response.
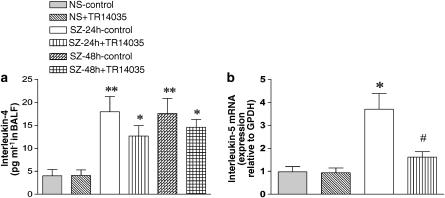
In addition, we determined the IL-5 mRNA in lung tissue and the concentration of IL-4 in BALF. After sensitization and challenge with antigen, an increase in IL-5 mRNA and IL-4 protein was observed. Treatment with TR14035 decreased significantly the upregulated IL-5 mRNA but decrease in IL-4 protein failed to achieve statistical significance (Figure 5). TR14035 failed also to decrease IL-4 protein in BALF collected at 48 h postantigen challenge. In additional studies with sensitized rats treated with a higher dose of TR14035 (10 mg kg−1), no significant decrease of IL-4 protein was found at 24 h postantigen challenge (25.4±6 vs 19.1±3.8 pg ml−1 in the absence and presence of TR14035, respectively; n=5 in each group, P>0.05). TR14035 administration had no effect on lung IL-5 mRNA or BALF IL-4 protein in saline-challenged animals (Figure 5).
Figure 5.
Effect of oral treatment with TR14035 (3 mg kg−1, 1 h before and 3 h after antigen challenge), an α4β1/α4β7 dual antagonist, on antigen-induced increase of interleukin-4 concentration in bronchoalveolar lavage fluid (BALF) (a) and interleukin-5 mRNA transcript in lung tissue (b) in a model of allergic asthma in Brown Norway rats. Determination of interleukin-4 levels in BALF was done by enzyme-immunoassay, and quantification of the mRNA levels of interleukin-5 was measured by real-time quantitative RT–PCR using the comparative Ct method (ΔΔCt method) and expressed as relative to GAPDH. The Ct values for GAPDH were similar in the different samples, thus confirming the value of this housekeeping gene as endogenous control. Data are shown for the following experimental groups: saline challenge without drug treatment (i.e. negative control; NS), antigen challenge without drug treatment (i.e. positive control; SZ), saline challenge in TR14035-treated rats (NS+TR14035), and antigen challenge in TR14035-treated rats (SZ+TR14035). Measurements are made at 24 and 48 h after challenge with antigen as indicated. Columns are mean±s.e.m. of seven (NS and SZ at 24 h for IL-4) and five (SZ at 48 h for IL-4 and SZ at 24 h for IL-5) animals in each group. *P<0.05 NS vs SZ, #P<0.05 SZ vs SZ+TR14035.
Intravital microscopy in rat mesentery
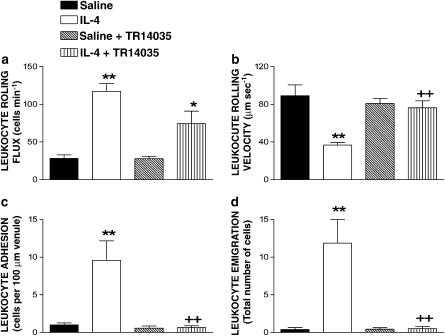
Since TR14035 did not significantly affect the production of IL-4 in antigen-challenged animals, it is likely that this dual α4β1/α4β7 antagonist exerts part of its anti-inflammatory activity by inhibiting the interaction of α4-integrins with VCAM-1. IL-4 causes P-selectin expression through a transcriptionally dependent mechanism with kinetics that are delayed compared with the action of other cytokines such as TNF-α and VCAM-1 upregulation (Yao et al., 1996). To investigate such possibility, intravital microscopy was used to examine leukocyte trafficking in the mesentery as leukocyte–endothelial cell interactions would be expected to precede the tissue accumulation of leukocytes. Figure 6 shows the effect of TR14035 on IL-4-induced leukocyte–endothelial cell interactions. After 24 h of i.p. injection of 1 μg per rat of IL-4, significant increases in leukocyte rolling flux, adhesion and emigration, and significant decreases in the Vwbc were detected compared to values obtained in the untreated saline group. Treatment with TR14035 decreased IL-4-induced leukocyte rolling flux by 48%, but this effect did not reach statistical significance (Figure 6a). In contrast, it significantly increased the reduction in the Vwbc elicited by IL-4 (Figure 6b). Leukocyte adhesion and emigration were abolished by the dual α4β1/α4β7 antagonist treatment after 24 h exposure to IL-4 (100 and 99% inhibition, respectively; Figure 6c and d). None of these treatments had significant effects on circulating leukocyte counts, MABP and shear rate (Table 1).
Figure 6.
Effect of the oral treatment with TR14035 (3 mg kg−1, 1 h before and 3 h after antigen challenge), an α4β1/α4β7 dual antagonist, on IL-4-induced leukocyte rolling flux (a), leukocyte rolling velocity (b), leukocyte adhesion (c) and leukocyte emigration (d) in Brown Norway rat mesenteric postcapillary venules. Parameters were measured 24 h after i.p. injection of 5 ml of saline or 5 ml of IL-4 (1 μg/rat) in the following experimental groups: untreated rats exposed to saline (negative control); TR14035 treated rats exposed to saline; untreated rats exposed to IL-4 (positive control); and IL-4-exposed rats treated with TR14035. Data are mean±s.e.m. of 7–8 animals per group; *P<0.05 or **P<0.01 compared to negative control; +P<0.05 or ++P<0.01 compared to positive control.
Table 1.
Haemodynamic parameters in vehicle and TR14035-treated animals after 24 h saline and 5 ml of IL-4 (1 μg rat−1) exposure
| Leukocyte counts (cells μl−1) | MABP (mmHg) | Shear rate (s−1) | |
|---|---|---|---|
| Treatment | 24 h | 24 h | 24 h |
| Saline | 3956±719 | 109.8±3.7 | 585.2±51.6 |
| TR14035+saline | 2887±743 | 110.9±5.4 | 428.4±76.0 |
| IL-4 | 3998±979 | 113.1±5.5 | 455.5±78.0 |
| TR14035+IL-4 | 3060±808 | 107.2±5.6 | 483.0±64.2 |
Values are mean±s.e.m. of 7–8 animals in each group. No significant changes among the different groups were observed.
Discussion
TR14035 is an N-benzoyl-L-biphenylalanine derivative endowed with a potent dual inhibitory activity on α4β1 and α4β7 integrins with IC50 values of 87 and 7 nM, respectively (Sircar et al., 2002). The potency of this compound for blocking the binding of human and rodent α4β1 and α4β7 integrins to soluble ligands was similar in both animal species (Egger et al., 2002). In the present study, we have evaluated the effects of the oral administration of TR14035 in an animal model of allergic asthma.
Antigen-induced airway inflammation has been studied extensively in animal models. In particular, Brown Norway rats produce high levels of IgE in response to active immunization (Pauwels et al., 1979), develop early and late airway responses (Martin et al., 1993), and airway hyper-responsiveness and increases of eosinophil, lymphocyte and neutrophil counts in BALF (Elwood et al., 1992). In this study, oral administration of TR14035 to sensitized Brown Norway rats suppressed airway hyper-responsiveness to 5-HT and reduced the number of eosinophils, neutrophils, lymphocytes and macrophages in BALF. In addition, protein content and eosinophil peroxidase were diminished. In parallel to these beneficial effects, TR14035 also improved the inflammatory lesions assessed by histological examination of lung sections. However, although IL-4 levels in BALF tended to be reduced by the administration of the dual α4β1/α4β7 antagonist, differences failed to reach statistical significance.
Neutralizing antibodies against α4-integrins ameliorate various aspects of late phase allergic airway responses such as inflammatory cell infiltration and hyper-responsiveness (Abraham et al., 1994; Foster, 1996; Kanehiro et al., 2000; Kudlacz et al., 2002). These effects have made α4β1/VCAM-1 interaction an attractive target for the development of orally active, small molecule antagonists (Huryn et al., 2004). In this context, several α4β1 antagonists have been effective in inhibiting these responses in animal models of asthma (Abraham et al., 1997; 2000; Kudlacz et al., 2002). In keeping with these results, our study showed that the oral administration of TR14035 markedly reduced the augmented lung resistance and dynamic compliance responses to intravenous 5-HT, as well as the eosinophil accumulation in BALF at 24 h after allergen challenge. Despite these consistent findings, treatment with an anti-VLA-4 antibody (Henderson et al., 1997) or a VLA-4 antagonist (Koo et al., 2003) prevented eosinophil recruitment but did not suppress allergen-induced airway hyper-responsiveness. Conversely, Rabb et al. (1994) reported a decrease in airway hyper-responsiveness without reduced pulmonary leukocyte infiltration after antigen challenged in sensitized rats receiving an anti-VLA-4 antibody.
In the present study, the maximum inhibition of BALF eosinophil infiltration was ∼64%. The lack of total inhibition of eosinophil influx by TR14035 is in agreement with previous observations with an α4 integrins antibody or α4β1 antagonists tested in a murine model of allergen-induced pulmonary inflammation (Kudlacz et al., 2002), and suggests that cell adhesion molecule interactions other than α4β1/VCAM-1 pathway are involved in the eosinophil trafficking from the blood vessel to the extravascular space. In this regard, administration of antibodies against both β2 and α4 integrins have been shown to cause a total inhibition of eosinophil infiltration in a rat model of allergic pulmonary inflammation (Schneider et al., 1999). Indeed, ICAM-1, the main endothelial ligand for β2 integrins, is clearly upregulated at sites of allergic inflammation (Montefort et al., 1992).
Eosinophils also express α4β7 integrin (Erle et al., 1994; Walsh et al., 1996), but its role in allergic asthma has been scarcely studied. The inhibition of BAL eosinophilia by selective antagonist of α4β1 integrin (Kudlacz et al., 2002) is of similar magnitude (∼60%) than that produced by α4β1/α4β7 dual antagonists. Therefore, it appears that this cell adhesion molecule do not play an important role in eosinophil infiltration into the airways. However, although VCAM-1 is more effective than MAdCAM-1 in supporting eosinophil rolling, β1 and β7 mAbs inhibit eosinophil rolling on VCAM-1 and fibronectin most efficiently in combination (Sriramarao et al., 2000). Furthermore, full inhibition of eosinophil adhesion to fibronectin has been reported to require the combination of β1 and β7 mAbs (Matsumoto et al., 1997), and also, in vitro studies suggest that α4β7 integrin may be important for eosinophil survival (Meerschaert et al., 1999).
On the other hand, we have observed a significant increase in neutrophil influx in sensitized Brown Norway rats after antigen-challenge. Interestingly, oral treatment with TR14935 markedly reduced (69%) the number of these cells in the BALF 24 h after antigen challenge. Consistent with our observations, in a sheep model of allergic inflammation, another group found increases in the number of infiltrating neutrophils, which were decreased by the administration of BIO-1211, an α4β1 integrin antagonist (Abraham et al., 2000). These results are not surprising since rat neutrophils express functional α4- and β1-integrins (Davenpeck et al., 1998), which would explain this observation.
Another finding was the capability of TR14035 treatment of inhibiting EPO activity in the BALF of antigen-challenged sensitized rats. TR14035 inhibited eosinophil number and EPO to a similar extent (64 and 69%, respectively); therefore, we cannot assure that TR14035 is inhibiting eosinophil activation since the reduction in EPO is likely a consequence of the reduced eosinophil numbers. Nevertheless, an inhibition of eosinophil activation by dual α4β1/α4β7 antagonists has been previously reported (Abraham et al., 2000; Koo et al., 2003). It is possible that the engagement of α4-integrins with VCAM-1 and other activation signals on eosinophils triggers their degranulation. In fact, treatment with an α4-integrin mAb, HP1/2, reduced antigen-, platelet activating factor- and IL-5-induced eosinophil peroxidase release (Abraham et al., 1994; Milne & Piper, 1995; Kraneveld et al., 1997).
Also relevant is the decrease of protein content in BALF of allergen-challenged sensitized animals treated with TR14035. This result is in contrast with a previous report of increased lung plasma leakage after administration of an anti-α4 mAb in a rat model of pulmonary inflammation (Taylor et al., 1997). However, other studies in humans and guinea-pigs indicate an association between eosinophil accumulation and plasma exudation (Walls et al., 1991; Collins et al., 1993) that is related to the release of a variety of mediators including sulphidopeptide leukotrienes (Goldie & Pedersen, 1995; Kanehiro et al., 2000). Therefore, the antiexudative effect of TR14035 found in our study may be related to the reduced eosinophil infiltration and activation produced by this antagonist in this experimental model.
In the present study, oral administration of TR14035, in addition to promote a clear reduction in eosinophil influx, also decreased the lymphocyte infiltration. This finding is consistent with the inhibition of lung IL-5 mRNA upregulation elicited by TR14035 treatment observed in this study, which is in agreement with the reported decrease in IL-5 mRNA in BALF cells from rats treated with TA-2, an α4 mAb (Ramos-Barbon et al., 2001). Despite this finding, the dual α4β1/α4β7 antagonist TR14035 (3 and 10 mg kg−1) failed to significantly diminish IL-4 levels in the BALF of antigen-challenged sensitized animals. IL-4 is released from Th2 lymphocytes and macrophages, and also by mast cells following IgE-crosslinking (Yamaguchi et al., 1997), which might explain the lack of effect of TR14035 on mediator release after antigen challenge in our model. Similar observations have been reported for the systemic administration of anti-α4 mAb in murine models of asthma (Henderson et al., 1997; Kanehiro et al., 2000; 2001).
IL-4 upregulates VCAM-1 in vitro and in vivo without affecting the expression of ICAM-1 or E-selectin (Yao et al., 1996; Sanz et al., 1998; Hickey et al., 1999). In our study, we have demonstrated by intravital microscopy that oral treatment with TR14035 inhibits IL-4 function since leukocyte adhesion and emigration were nearly abrogated in animals treated with this antagonist.
Collectively, these data provide strong evidence that the α4 adhesion pathway participates in pathophysiologic responses associated with the prolonged inflammatory events that follow antigen challenge in the allergic Brown Norway rat model. This is the first report that shows that oral treatment with a dual α4β1/α4β7 antagonist inhibits most of the characteristic features of allergic pulmonary inflammation. Thus, these results support the potential benefit of antiadhesion therapy in the treatment of pulmonary allergic diseases such as asthma.
Acknowledgments
The present study was supported by research grants SAF 2002-01482 (MJS), SAF2002-04667 (JC) and SAF2003-07206-C02-01 (EJM) from CICYT (Ministry of Education and Science, Spanish Government) and Groups-03/166 and ‘Network-CTIAE/C/03/116' and project GV04B72 from Regional Government (Generalitat Valenciana). YNAN was supported by a grant from Spanish Ministry of Education and Science. The help of Dr M. Cerdá of the Department of Pathology of the University Clinic Hospital with the histological readouts and the technical assistance of Pedro Santamaria are gratefully acknowledged.
Abbreviations
- Dv
venular diameter
- 5-HT
5-hydroxytryptamine or serotonin
- ICAM-1
intercellular adhesion molecule-1
- IL-4
interleukin 4
- IL-5
interleukin-5
- IL-13
interleukin-13
- mAb
monoclonal antibody
- MABP
mean arterial blood pressure
- MAdCAM-1
mucosal addressin cell adhesion molecule-1
- PAF
platelet activating factor
- VCAM-1
vascular cell adhesion molecule-1
- VLA-4
very late antigen-4
- Vmean
mean red blood cell velocity
- Vrbc
centerline red blood cell velocity
- Vwbc
leukocyte rolling velocity
References
- ABRAHAM W.M., AHMED A., SIELCZAK M.W., NARITA M., ARRHENIUS T., ELICES M.J. Blockade of late-phase airway responses and airway hyperresponsiveness in allergic sheep with a small-molecule peptide inhibitor of VLA-4. Am. J. Respir. Crit. Care Med. 1997;156:696–703. doi: 10.1164/ajrccm.156.3.9609039. [DOI] [PubMed] [Google Scholar]
- ABRAHAM W.M., GILL A., AHMED A., SIELCZAK M.W., LAUREDO I.T., BOTINNIKOVA Y., LIN K.C., PEPINSKY B., LEONE D.R., LOBB R.R., ADAMS S.P. A small-molecule, tight-binding inhibitor of the integrin alpha(4)beta(1) blocks antigen-induced airway responses and inflammation in experimental asthma in sheep. Am. J. Respir. Crit. Care Med. 2000;162:603–611. doi: 10.1164/ajrccm.162.2.9911061. [DOI] [PubMed] [Google Scholar]
- ABRAHAM W.M., SIELCZAK M.W., AHMED A., CORTES A., LAUREDO I.T., KIM J., PEPINSKY B., BENJAMIN C.D., LEONE D.R., LOBB R.R., WELLER P.F. Alpha 4-integrins mediate antigen-induced late bronchial responses and prolonged airway hyperresponsiveness in sheep. J. Clin. Invest. 1994;93:776–787. doi: 10.1172/JCI117032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENTLEY A.M., DURHAM S.R., ROBINSON D.S., MENZ G., STORZ C., CROMWELL O., KAY A.B., WARDLAW A.J. Expression of endothelial and leukocyte adhesion molecules interacellular adhesion molecule-1, E-selectin, and vascular cell adhesion molecule-1 in the bronchial mucosa in steady-state and allergen-induced asthma. J. Allergy Clin. Immunol. 1993;92:857–868. doi: 10.1016/0091-6749(93)90064-m. [DOI] [PubMed] [Google Scholar]
- BERLIN C., BERG E.L., BRISKIN M.J., ANDREW D.P., KILSHAW P.J., HOLZMANN B., WEISSMAN I.L., HAMANN A., BUTCHER E.C. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- BLESA S., CORTIJO J., MATA M., SERRANO A., CLOSA D., SANTANGELO F., ESTRELA J.M., SUCHANKOVA J., MORCILLO E.J. Oral N-acetylcysteine attenuates the rat pulmonary inflammatory response to antigen. Eur. Respir. J. 2003;21:394–400. doi: 10.1183/09031936.03.00039602. [DOI] [PubMed] [Google Scholar]
- BORCHERS M.T., CROSBY J., FARMER S., SYPEK J., ANSAY T., LEE N.A., LEE J.J. Blockade of CD49d inhibits allergic airway pathologies independent of effects on leukocyte recruitment. Am. J. Physiol. Lung Cell Mol. Physiol. 2001a;280:L813–L821. doi: 10.1152/ajplung.2001.280.4.L813. [DOI] [PubMed] [Google Scholar]
- BORCHERS M.T., CROSBY J., JUSTICE P., FARMER S., HINES E., LEE J.J., LEE N.A. Intrinsic AHR in IL-5 transgenic mice is dependent on CD4(+) cells and CD49d-mediated signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2001b;281:L653–L659. doi: 10.1152/ajplung.2001.281.3.L653. [DOI] [PubMed] [Google Scholar]
- CARLOS T.M., HARLAN J.M. Leukocyte–endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- COLLINS D.S., DUPUIS R., GLEICH G.J., BARTEMES K.R., KOH Y.Y., POLLICE M., ALBERTINE K.H., FISH J.E., PETERS S.P. Immunoglobulin E-mediated increase in vascular permeability correlates with eosinophilic inflammation. Am. Rev. Respir. Dis. 1993;147:677–683. doi: 10.1164/ajrccm/147.3.677. [DOI] [PubMed] [Google Scholar]
- DAVENPECK K.L., STERBINSKY S.A., BOCHNER B.S. Rat neutrophils express alpha4 and beta1 integrins and bind to vascular cell adhesion molecule-1 (VCAM-1) and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) Blood. 1998;91:2341–2346. [PubMed] [Google Scholar]
- EGGER L.A., KIDAMBI U., CAO J., VAN RIPER G., MCCAULEY E., MUMFORD R.A., AMO S., LINGHAM R., LANZA T., LIN L.S., DE LASZLO S.E., YOUNG D.N., KOPKA I.E., TONG S., PIKOUNIS B., BENSON E., WARWOOD S., BARGATZE R.F., HAGMANN W.K., SCHMIDT J.A., DETMERS P.A. Alpha(4)beta(7)/alpha(4)beta(1) dual integrin antagonists block alpha(4)beta(7)-dependent adhesion under shear flow. J. Pharmacol. Exp. Ther. 2002;302:153–162. doi: 10.1124/jpet.302.1.153. [DOI] [PubMed] [Google Scholar]
- ELWOOD W., BARNES P.J., CHUNG K.F. Airway hyperresponsiveness is associated with inflammatory cell infiltration in allergic Brown-Norway rats. Int. Arch. Allergy Immunol. 1992;99:91–97. doi: 10.1159/000236340. [DOI] [PubMed] [Google Scholar]
- ERLE D.J., BRISKIN M.J., BUTCHER E.C., GARCIA-PARDO A., LAZAROVITS A.I., TIDSWELL M. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J. Immunol. 1994;153:517–528. [PubMed] [Google Scholar]
- FOSTER C.A. VCAM-1/alpha 4-integrin adhesion pathway: therapeutic target for allergic inflammatory disorders. J. Allergy Clin. Immunol. 1996;98:S270–S277. doi: 10.1016/s0091-6749(96)70075-1. [DOI] [PubMed] [Google Scholar]
- GASCOIGNE M.H., HOLLAND K., PAGE C.P., SHOCK A., ROBINSON M., FOULKES R., GOZZARD N. The effect of anti-integrin monoclonal antibodies on antigen-induced pulmonary inflammation in allergic rabbits. Pulm. Pharmacol. Ther. 2003;16:279–285. doi: 10.1016/S1094-5539(03)00069-5. [DOI] [PubMed] [Google Scholar]
- GOLDIE R.G., PEDERSEN K.E. Mechanisms of increased airway microvascular permeability: role in airway inflammation and obstruction. Clin. Exp. Pharmacol. Physiol. 1995;22:387–396. doi: 10.1111/j.1440-1681.1995.tb02028.x. [DOI] [PubMed] [Google Scholar]
- HENDERSON W.R., JR, CHI E.Y., ALBERT R.K., CHU S.J., LAMM W.J., ROCHON Y., JONAS M., CHRISTIE P.E., HARLAN J.M. Blockade of CD49d (alpha4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J. Clin. Invest. 1997;100:3083–3092. doi: 10.1172/JCI119863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HICKEY M.J., GRANGER D.N., KUBES P. Molecular mechanisms underlying IL-4-induced leukocyte recruitment in vivo: a critical role for the alpha 4 integrin. J. Immunol. 1999;163:3441–3448. [PubMed] [Google Scholar]
- HOUSE S.D., LIPOWSKY H.H. Leukocyte–endothelium adhesion: microhemodynamics in mesentery of the cat. Microvasc. Res. 1987;34:363–379. doi: 10.1016/0026-2862(87)90068-9. [DOI] [PubMed] [Google Scholar]
- HUANG T.J., ADCOCK I.M., CHUNG K.F. A novel transcription factor inhibitor, SP100030, inhibits cytokine gene expression, but not airway eosinophilia or hyperresponsiveness in sensitized and allergen-exposed rat. Br. J. Pharmacol. 2001;134:1029–1036. doi: 10.1038/sj.bjp.0704344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURYN D.M., KONRADI A.W., ASHWELL S., FREEDMAN S.B., LOMBARDO L.J., PLEISS M.A., THORSETT E.D., YEDNOCK T., KENNEDY J.D. The identification and optimization of orally efficacious, small molecule VLA-4 antagonists. Curr. Top. Med. Chem. 2004;4:1473–1484. doi: 10.2174/1568026043387467. [DOI] [PubMed] [Google Scholar]
- KANEHIRO A., IKEMURA T., MAKELA M.J., LAHN M., JOETHAM A., DAKHAMA A., GELFAND E.W. Inhibition of phosphodiesterase 4 attenuates airway hyperresponsiveness and airway inflammation in a model of secondary allergen challenge. Am. J. Respir. Crit. Care Med. 2001;163:173–184. doi: 10.1164/ajrccm.163.1.2001118. [DOI] [PubMed] [Google Scholar]
- KANEHIRO A., TAKEDA K., JOETHAM A., TOMKINSON A., IKEMURA T., IRVIN C.G., GELFAND E.W. Timing of administration of anti-VLA-4 differentiates airway hyperresponsiveness in the central and peripheral airways in mice. Am. J. Respir. Crit. Care Med. 2000;162:1132–1139. doi: 10.1164/ajrccm.162.3.9910100. [DOI] [PubMed] [Google Scholar]
- KAY A.B. The role of eosinophils in the pathogenesis of asthma. Trends. Mol. Med. 2005;11:148–152. doi: 10.1016/j.molmed.2005.02.002. [DOI] [PubMed] [Google Scholar]
- KOO G.C., SHAH K., DING G.J., XIAO J., WNEK R., DOHERTY G., TONG X.C., PEPINSKY R.B., LIN K.C., HAGMANN W.K., KAWKA D., SINGER I.I. A small molecule very late antigen-4 antagonist can inhibit ovalbumin-induced lung inflammation. Am. J. Respir. Crit. Care Med. 2003;167:1400–1409. doi: 10.1164/rccm.200207-696OC. [DOI] [PubMed] [Google Scholar]
- KRANEVELD A.D., VAN ARK I., VAN DER LINDE H.J., FATTAH D., NIJKAMP F.P., VAN OOSTERHOUT A.J. Antibody to very late activation antigen 4 prevents interleukin-5-induced airway hyperresponsiveness and eosinophil infiltration in the airways of guinea pigs. J. Allergy Clin. Immunol. 1997;100:242–250. doi: 10.1016/s0091-6749(97)70231-8. [DOI] [PubMed] [Google Scholar]
- KUDLACZ E., WHITNEY C., ANDRESEN C., DUPLANTIER A., BECKIUS G., CHUPAK L., KLEIN A., KRAUS K., MILICI A. Pulmonary eosinophilia in a murine model of allergic inflammation is attenuated by small molecule alpha4beta1 antagonists. J. Pharmacol. Exp. Ther. 2002;301:747–752. doi: 10.1124/jpet.301.2.747. [DOI] [PubMed] [Google Scholar]
- LOBB R.R., PEPINSKY B., LEONE D.R., ABRAHAM W.M. The role of alpha 4 integrins in lung pathophysiology. Eur. Respir. J. Suppl. 1996;22:104s–108s. [PubMed] [Google Scholar]
- MARTIN J.G., XU L.J., TOH M.Y., OLIVENSTEIN R., POWELL W.S. Leukotrienes in bile during the early and the late airway responses after allergen challenge of sensitized rats. Am. Rev. Respir. Dis. 1993;147:104–110. doi: 10.1164/ajrccm/147.1.104. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO K., STERBINSKY S.A., BICKEL C.A., ZHOU D.F., KOVACH N.L., BOCHNER B.S. Regulation of alpha 4 integrin-mediated adhesion of human eosinophils to fibronectin and vascular cell adhesion molecule-1. J. Allergy Clin. Immunol. 1997;99:648–656. doi: 10.1016/s0091-6749(97)70027-7. [DOI] [PubMed] [Google Scholar]
- MEERSCHAERT J., VRTIS R.F., SHIKAMA Y., SEDGWICK J.B., BUSSE W.W., MOSHER D.F. Engagement of alpha4beta7 integrins by monoclonal antibodies or ligands enhances survival of human eosinophils in vitro. J. Immunol. 1999;163:6217–6227. [PubMed] [Google Scholar]
- MILNE A.A., PIPER P.J. Role of the VLA-4 integrin in leucocyte recruitment and bronchial hyperresponsiveness in the guinea-pig. Eur. J. Pharmacol. 1995;282:243–249. doi: 10.1016/0014-2999(95)00340-q. [DOI] [PubMed] [Google Scholar]
- MONTEFORT S., ROCHE W.R., HOWARTH P.H., DJUKANOVIC R., GRATZIOU C., CARROLL M., SMITH L., BRITTEN K.M., HASKARD D., LEE T.H., HOLGATE S.T. Intercellular adhesion molecule-1 (ICAM-1) and endothelial leucocyte adhesion molecule-1 (ELAM-1) expression in the bronchial mucosa of normal and asthmatic subjects. Eur. Respir. J. 1992;5:815–823. [PubMed] [Google Scholar]
- O'BYRNE P.M., INMAN M.D., ADELROTH E. Reassessing the Th2 cytokine basis of asthma. Trends Pharmacol. Sci. 2004;25:244–248. doi: 10.1016/j.tips.2004.03.008. [DOI] [PubMed] [Google Scholar]
- OHKAWARA Y., YAMAUCHI K., MARUYAMA N., HOSHI H., OHNO I., HONMA M., TANNO Y., TAMURA G., SHIRATO K., OHTANI H. In situ expression of the cell adhesion molecules in bronchial tissues from asthmatics with air flow limitation: in vivo evidence of VCAM-1/VLA-4 interaction in selective eosinophil infiltration. Am. J. Respir. Cell Mol. Biol. 1995;12:4–12. doi: 10.1165/ajrcmb.12.1.7529029. [DOI] [PubMed] [Google Scholar]
- PAUWELS R., BAZIN H., PLATTEAU B., VAN DER STRAETEN M. The influence of antigen dose on IgE production in different rat strains. Immunology. 1979;36:151–157. [PMC free article] [PubMed] [Google Scholar]
- PAUWELS R., VAN DER STRAETEN M., WEYNE J., BAZIN H. Genetic factors in non-specific bronchial reactivity in rats. Eur. J. Respir. Dis. 1985;66:98–104. [PubMed] [Google Scholar]
- PONS R., SANTAMARIA P., SUCHANKOVA J., CORTIJO J., MORCILLO E.J. Effects of inhaled glaucine on pulmonary responses to antigen in sensitized guinea pigs. Eur. J. Pharmacol. 2000;397:187–195. doi: 10.1016/s0014-2999(00)00224-7. [DOI] [PubMed] [Google Scholar]
- PRETOLANI M., RUFFIE C., LAPA E SILVA J.R., JOSEPH D., LOBB R.R., VARGAFTIG B.B. Antibody to very late activation antigen 4 prevents antigen-induced bronchial hyperreactivity and cellular infiltration in the guinea pig airways. J. Exp. Med. 1994;180:795–805. doi: 10.1084/jem.180.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABB H.A., OLIVENSTEIN R., ISSEKUTZ T.B., RENZI P.M., MARTIN J.G. The role of the leukocyte adhesion molecules VLA-4, LFA-1, and Mac-1 in allergic airway responses in the rat. Am. J. Respir. Crit. Care Med. 1994;149:1186–1191. doi: 10.1164/ajrccm.149.5.8173758. [DOI] [PubMed] [Google Scholar]
- RAMOS-BARBON D., SUZUKI M., TAHA R., MOLET S., ISSEKUTZ T.B., HAMID Q., MARTIN J.G. Effect of alpha4-integrin blockade on CD4+ cell-driven late airway responses in the rat. Am. J. Respir. Crit. Care Med. 2001;163:101–108. doi: 10.1164/ajrccm.163.1.2001093. [DOI] [PubMed] [Google Scholar]
- RICHARDS I.M., KOLBASA K.P., HATFIELD C.A., WINTERROWD G.E., VONDERFECHT S.L., FIDLER S.F., GRIFFIN R.L., BRASHLER J.R., KRZESICKI R.F., SLY L.M., READY K.A., STAITE N.D., CHIN J.E. Role of very late activation antigen-4 in the antigen-induced accumulation of eosinophils and lymphocytes in the lungs and airway lumen of sensitized Brown Norway rats. Am. J. Respir. Cell Mol. Biol. 1996;15:172–183. doi: 10.1165/ajrcmb.15.2.8703473. [DOI] [PubMed] [Google Scholar]
- SANZ M.J., ALVAREZ A., PIQUERAS L., CERDA M., ISSEKUTZ A.C., LOBB R.R., CORTIJO J., MORCILLO E.J. Rolipram inhibits leukocyte-endothelial cell interactions in vivo through P- and E-selectin downregulation. Br. J. Pharmacol. 2002;135:1872–1881. doi: 10.1038/sj.bjp.0704644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANZ M.J., MARINOVA-MUTAFCHIEVA L., GREEN P., LOBB R.R., FELDMANN M., NOURSHARGH S. IL-4-induced eosinophil accumulation in rat skin is dependent on endogenous TNF-alpha and alpha 4 integrin/VCAM-1 adhesion pathways. J. Immunol. 1998;160:5637–5645. [PubMed] [Google Scholar]
- SANZ M.J., NABAH Y.N., CERDA-NICOLAS M., O'CONNOR J.E., ISSEKUTZ A.C., CORTIJO J., MORCILLO E.J. Erythromycin exerts in vivo anti-inflammatory activity downregulating cell adhesion molecule expression. Br. J. Pharmacol. 2005;144:190–201. doi: 10.1038/sj.bjp.0706021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER T., ISSEKUTZ T.B., ISSEKUTZ A.C. The role of alpha4 (CD49d) and beta2 (CD18) integrins in eosinophil and neutrophil migration to allergic lung inflammation in the Brown Norway rat. Am. J. Respir. Cell Mol. Biol. 1999;20:448–457. doi: 10.1165/ajrcmb.20.3.3207. [DOI] [PubMed] [Google Scholar]
- SERRANO-MOLLAR A., CLOSA D., PRATS N., BLESA S., MARTINEZ-LOSA M., CORTIJO J., ESTRELA J.M., MORCILLO E.J., BULBENA O. In vivo antioxidant treatment protects against bleomycin-induced lung damage in rats. Br. J. Pharmacol. 2003;138:1037–1048. doi: 10.1038/sj.bjp.0705138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRCAR I., GUDMUNDSSON K.S., MARTIN R., LIANG J., NOMURA S., JAYAKUMAR H., TEEGARDEN B.R., NOWLIN D.M., CARDARELLI P.M., MAH J.R., CONNELL S., GRIFFITH R.C., LAZARIDES E. Synthesis and SAR of N-benzoyl-L-biphenylalanine derivatives: discovery of TR-14035, a dual alpha(4)beta(7)/alpha(4)beta(1) integrin antagonist. Bioorg. Med. Chem. 2002;10:2051–2066. doi: 10.1016/s0968-0896(02)00021-4. [DOI] [PubMed] [Google Scholar]
- SRIRAMARAO P., DISCIPIO R.G., COBB R.R., CYBULSKY M., STACHNICK G., CASTANEDA D., ELICES M., BROIDE D.H. VCAM-1 is more effective than MAdCAM-1 in supporting eosinophil rolling under conditions of shear flow. Blood. 2000;95:592–601. [PubMed] [Google Scholar]
- STRATH M., WARREN D.J., SANDERSON C.J. Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J. Immunol. Methods. 1985;83:209–215. doi: 10.1016/0022-1759(85)90242-x. [DOI] [PubMed] [Google Scholar]
- SUCHANKOVA J., MATA M., CORTIJO J., MORCILLO E.J. Effects of bemiparin on airway responses to antigen in sensitized Brown-Norway rats. Eur. J. Pharmacol. 2005;507:261–271. doi: 10.1016/j.ejphar.2004.11.014. [DOI] [PubMed] [Google Scholar]
- TAYLOR B.M., KOLBASA K.P., CHIN J.E., RICHARDS I.M., FLEMING W.E., GRIFFIN R.L., FIDLER S.F., SUN F.F. Roles of adhesion molecules ICAM-1 and alpha4 integrin in antigen-induced changes in microvascular permeability associated with lung inflammation in sensitized Brown Norway rats. Am. J. Respir. Cell Mol. Biol. 1997;17:757–766. doi: 10.1165/ajrcmb.17.6.2697. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD S.L., HADDAD EL B., BIRRELL M.A., MCCLUSKIE K., PECORARO M., DABROWSKI D., WEBBER S.E., FOSTER M.L., BELVISI M.G. Functional characterization and biomarker identification in the Brown Norway model of allergic airway inflammation. Br. J. Pharmacol. 2002;137:263–275. doi: 10.1038/sj.bjp.0704865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDERSLICE P., BIEDIGER R.J., WOODSIDE D.G., BERENS K.L., HOLLAND G.W., DIXON R.A. Development of cell adhesion molecule antagonists as therapeutics for asthma and COPD. Pulm. Pharmacol. Ther. 2004;17:1–10. doi: 10.1016/j.pupt.2003.10.004. [DOI] [PubMed] [Google Scholar]
- WALLS A.F., RHEE Y.K., GOULD D.J., WALTERS C., ROBINSON C., CHURCH M.K., HOLGATE S.T. Inflammatory mediators and cellular infiltration of the lungs in a guinea pig model of the late asthmatic reaction. Lung. 1991;169:227–240. doi: 10.1007/BF02714157. [DOI] [PubMed] [Google Scholar]
- WALSH G.M., SYMON F.A., LAZAROVILS A.L., WARDLAW A.J. Integrin alpha 4 beta 7 mediates human eosinophil interaction with MAdCAM-1, VCAM-1 and fibronectin. Immunology. 1996;89:112–119. doi: 10.1046/j.1365-2567.1996.d01-713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLAW A.J. Molecular basis for selective eosinophil trafficking in asthma: a multistep paradigm. J. Allergy. Clin. Immunol. 1999;104:917–926. doi: 10.1016/s0091-6749(99)70069-2. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI M., LANTZ C.S., OETTGEN H.C., KATONA I.M., FLEMING T., MIYAJIMA I., KINET J.P., GALLI S.J. IgE enhances mouse mast cell Fc(epsilon)RI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J. Exp. Med. 1997;185:663–672. doi: 10.1084/jem.185.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAO L., PAN J., SETIADI H., PATEL K.D., MCEVER R.P. Interleukin 4 or oncostatin M induces a prolonged increase in P-selectin mRNA and protein in human endothelial cells. J. Exp. Med. 1996;184:81–92. doi: 10.1084/jem.184.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]