Abstract
As the beagle dog is a commonly used preclinical species to test the effects of new drugs on cardiac repolarisation and Purkinje fibres have become an established in vitro preparation to assess the effects of these new drugs on action potential duration (APD), the main aim of this study was therefore to evaluate the relative contribution of the inward (IK1) and slow delayed (IKs) rectifier cardiac K+ currents to action potential repolarisation in beagle Purkinje fibres under three different experimental conditions: (i) selective block of IK1 with BaCl2, (ii) selective block of IKs with (−) chromanol 293B under basal conditions and (iii) selective block of IKs during β-adrenoceptor stimulation. Furthermore, the dependence of this contribution on gender and pacing rate was investigated. Microelectrode techniques were employed to measure APD in Purkinje fibres from adult female and male dogs.
At stimulation rates of 3.33, 1.0 and 0.2 Hz, the degree of prolongation of APD evoked by BaCl2 (10 μM) was comparable in fibres from female and male dogs.
At the same stimulation rates, 10 μM (−) chromanol 293B did not change the APD in fibres from female and male dogs.
During β-adrenoceptor stimulation with 0.1 μM isoproterenol, an APD prolonging effect of (−) chromanol 293B was detected.
In the presence of isoproterenol, action potentials in fibres from male dogs get shorter when changing the stimulation rate from 1.0 to 0.2 Hz, while the opposite is seen in fibres from female dogs. This alteration was completely reversed by (−) chromanol 293B.
In conclusion, our findings confirm that β-adrenoceptor stimulation is one condition where there may be an increased role of IKs in action potential repolarisation. Gender differences in the autonomic modulation of IKs could be a contributing factor to the reported increased susceptibility of female hearts to arrhythmias.
Keywords: Gender, Purkinje fibre, action potential duration, slow delayed rectifier cardiac K+ current, inward rectfier cardiac K+ current, β-adrenoceptor stimulation
Introduction
Noncardiovascular drugs that block the rapid delayed rectifier K+ current (IKr) are associated with a delay in ventricular repolarisation, a prolongation of the QT interval and an increased risk of developing Torsades de Pointes (Redfern et al., 2003; Straus et al., 2005). Therefore, substantial prolongation of the QT interval, with or without documented arrhythmias, could be the basis for nonapproval of a new drug for human use or its approval with a warning/precautionary statement regarding the risk it carries (Anon, 2005a). As a result, noncardiovascular QT-prolonging drugs have been a major preoccupation of regulatory authorities and pharmaceutical industry since the mid-1990s. Consequently, emerging regulatory guidance defines and recommends electrophysiology studies prior to first administration of a new drug to humans (Anon, 1997; 2005b) and a mandatory ‘thorough QT/QTc' study (Anon, 2005a). In vitro electrophysiology follow-up studies employing Purkinje fibres can provide valuable information concerning the effect of a new drug on action potential parameters, particularly when combined with other risk factors (i.e. gender, extreme bradycardia and autonomic nervous system tone) (Anon, 2005b). Understandably, therefore, the contribution of IKr to action potentials in this preparation has been well explored using potent, selective pharmacological tools. In contrast, the effects of inward (IK1) and slow delayed (IKs) rectifier K+ current blockers have not been studied in as much depth.
IKr, IKs and IK1 currents contribute to ventricular action potential repolarisation (Tamargo et al., 2004). This is also supported by the fact that mutations in the genes encoding the proteins conducting IK1 (KCNJ2), IKr (KCNH2/KCNE2) and IKs (KCNQ1/KCNE1) have been identified in some types of the congenital long QT syndrome (Keating & Sanguinetti, 2001; Plaster et al., 2001; Chiang, 2004). Prolongation of action potential duration (APD) and the QT interval in mongrel dog and rabbit heart preparations (Biliczki et al., 2002; Lengyel et al., 2004) are consistent observations following selective block of IK1. In contrast, reports on the effect of IKs blockers are inconsistent. For example, IKs block does not significantly prolong APD in mongrel dog and rabbit heart preparations isolated (Purkinje cells (Han et al., 2001), ventricular myocytes (Volders et al., 2003), ventricular muscle or Purkinje fibres (Lengyel et al., 2001; Biliczki et al., 2002; Stengl et al., 2003; Volders et al., 2003; Lu et al., 2005)). In contrast, IKs blockers do prolong ventricular APD in both guinea pig and diseased human ventricular myocytes (Bosch et al., 1998), papillary muscles and guinea pig Langendorff-perfused hearts (Gogelein et al., 2000), and epicardial, M and endocardial cells recorded simultaneously from an arterially-perfused wedge of canine left ventricle (Shimizu & Antzelevitch, 1998). These data suggest species differences and also regional variations in the influence of IKs on APD (Varro et al., 2000; Zicha et al., 2003). Other complicating factors may be the influence of adrenoceptor stimulation and gender. Isoproterenol, a nonspecific β-adrenoceptor agonist, increased and accelerated IKs activation to promote an APD shortening that was reversed by IKs blockers (Han et al., 2001; Stengl et al., 2003; Volders et al., 2003; Lu et al., 2005). While it is well established that there are gender differences in cardiac electrophysiology (Abi-Gerges et al., 2004a), a recent study from our laboratory, comparing action potential parameters in Purkinje fibres from adult male and female beagle dogs, provided electrophysiological evidence pointing to gender-related differences in basal APD, reverse rate dependence and proarrhythmic events evoked by dofetilide, a highly selective blocker of IKr (Abi-Gerges et al., 2004b). Although it is clear that IK1 and IKs currents contribute to mongrel dog ventricular action potential repolarisation, the relative contribution, and the dependence of this on gender and β-adrenoceptor stimulation, is not well characterised in beagle dogs and requires further investigation.
Cardiac Purkinje cells are believed to be the source of early afterdepolarisations initiating Torsades de Pointes (Nattel & Quantz, 1988). Moreover, the conflicting results generated with the IKs blockers on APD during β-adrenoceptor stimulation in mongrel dog isolated Purkinje cells (Han et al., 2001) and rabbit Purkinje fibres (Lu et al., 2005) have raised a question as to whether β-adrenoceptor stimulated-IKs plays a potential role in action potential repolarisation in beagle dog Purkinje fibres. Since the beagle dog is a commonly used preclinical species to test the effects of new drugs on cardiac repolarisation (Gralinski, 2003) and isolated Purkinje fibres have become an established in vitro preparation to assess the effects of these new drugs on APD (Anon, 1997; 2005b), the main aim of this study was therefore to evaluate the relative contribution of IK1 and IKs to action potential repolarisation in beagle dog Purkinje fibres under three different experimental conditions: (i) selective block of IK1 with BaCl2, (ii) selective block of IKs with (−) chromanol 293B under basal conditions and (iii) selective block of IKs during β-adrenoceptor stimulation. Furthermore, the dependence of this contribution on gender and pacing rate was investigated.
Methods
Electrophysiological measurements
Alderley Park beagle dogs of either sex were used. The females weighed 9.38–13.97 kg and were 15–42 months old. The males weighed 9.02–14.93 kg and were 10–26 months old. All animals were maintained in accordance with the Guide for the Home Office Code and Practice for the Housing and Care of Animals used in Scientific Procedures. The procedures were authorized under a project licence granted under the Animals (Scientific Procedures) Act 1986.
Electrophysiological experiments were performed on isolated beagle Purkinje fibres. The experimental approach used is similar to that in our recent study (Abi-Gerges et al., 2004b). Briefly, Purkinje fibres were excised and each one was placed in a separate, custom-made glass recording chamber. The fibres were perfused with gassed (95% O2/5% CO2) Krebs buffer of the following composition (in mM): NaCl 118, KCl 4, MgCl2 1, CaCl2 1.8, NaHCO3 31, NaH2PO4 1.8, glucose 11. The preparations were fixed at the bottom of the recording chamber by small pins and continuously superfused with the gassed Krebs buffer at a rate of 5 ml min−1. The temperature in the recording chamber was maintained at 37.4±0.1°C. Purkinje fibres were stimulated at a rate of 1.0 Hz (for at least 45 min to allow the fibres to stabilize) through a DS2A stimulator (Digitimer Limited, Welwyn Garden City, U.K.) that was controlled by a TG1010 frequency pulse generator (Thurlby Thandar Instruments Ltd, Huntingdon, U.K.). The following stimulation parameters were used: stimulation voltage of twice the threshold for action potential generation, pulse width 2 ms, stimulation rates of 3.33, 1.0 and 0.2 Hz to mimic fast, normal and low pacing rates, respectively. Intracellular potentials were recorded with glass micropipettes filled with 3 M KCl, with tip resistances between 10 and 18 MΩ. The micropipette was connected to the headstage of a MultiClamp 700A amplifier (Axon Instruments Inc., Union City, CA, U.S.A.). Action potential signals were acquired by action potential software (Notocord HEM 3.4 or 3.5; Notocord Systems S.A., Croissy Sur Seine, France) at a sampling rate of 50 kHz. This software was set up to acquire, on line, the following action potential parameters: resting membrane potential (in mV), amplitude of action potential (in mV), maximum rate of depolarisation of the action potential (in V/s) and APD at 50, 70 or at 90% repolarisation (APD50, APD70 or APD90 in ms).
Experimental protocol
Following the stabilisation period, data acquisition was commenced providing action potentials were typical of those observed in beagle dog Purkinje fibres. Action potential stability was assessed in the first 5–10 min of recording in Krebs buffer at a stimulation rate of 1.0 Hz. Rate adaptation was investigated by changing the stimulation frequency in the following sequence; 1.0, 0.2, 1.0, 3.33 and 1.0 Hz ensuring steady state had been achieved at each frequency prior to changing to the next rate. To study the influence of either IK1 or IKs in the beagle dog Purkinje fibre cardiac action potential in conditions of fast, normal and low pacing rates, the same sequence was repeated following perfusion of the same fibre with Krebs buffer containing either 10 μM BaCl2 or 10 μM (−) chromanol 293B. Similar concentrations of BaCl2 and (−) chromanol 293B have shown to selectively block IK1 and IKs, respectively, in isolated mongrel dog cardiac tissue (Biliczki et al., 2002). To assess the potential role of β-adrenoceptor stimulation on the role of IKs in beagle dog action potential repolarisation, the stimulation rate sequence described above was repeated. This was carried out first in the presence of 0.1 μM isoproterenol and then with 0.1 μM isoproterenol plus 10 μM (−) chromanol 293B. These concentrations of isoproterenol and (−) chromanol 293B are similar to those used by Volders et al. (2003). Attempts were made to maintain the same impalement throughout each experiment. If, however, the impalement became dislodged, adjustment was attempted. If the action potential parameters of the re-established impalement deviated by less than 5% from the previous measurement, the experiment continued.
Drugs
(−)-[3R,4S]-chromanol 293B [(−) chromanol 293B] was purchased from Tocris Cookson Inc. (Bristol, U.K.) and dissolved in dimethylsulfoxide at a concentration of 10 mM. BaCl2 and isoproterenol were purchased from Sigma-Aldrich Company Ltd (Poole, U.K.) and dissolved in Krebs buffer at concentrations of 10 and 1 mM, respectively. Stocks of (−) chromanol 293B and BaCl2 were kept at −20°C until use. The stock of isoproterenol was prepared fresh at the start of each experiment. Stock solutions were diluted using Krebs buffer on the day of the experiment to give the desired final drug concentrations. All the other chemicals were purchased from Sigma-Aldrich.
Data analysis and statistics
In order to fully explore the data, comparisons were made in action potential parameters under conditions of ‘within' stimulation rate and also ‘across' stimulation rates when fibres were exposed to the treatments isoproterenol or isoproterenol plus (−) chromanol 293B. ‘Within stimulation rate' measurements made at 3.33, 1.0 or 0.2 Hz were expressed as a percentage change relative to control values in Krebs buffer alone under different experimental conditions. For example, for the 3.33 Hz ‘within stimulation rate' data the APD at 3.33 Hz with isoproterenol or isoproterenol plus (−) chromanol 293B was expressed relative to APD in Krebs buffer at 3.33 Hz. ‘Across stimulation rate' measurements for the analysis of isoproterenol or isoproterenol and (−) chromanol 293B effects were made at both 3.33 and 0.2 Hz and expressed as a percentage change relative to control values obtained at 1.0 Hz under the corresponding experimental condition. For example, the ‘across stimulation rate' data for isoproterenol were generated by expressing APD at 3.33 and 0.2 Hz in isoproterenol relative to APD in isoproterenol at 1.0 Hz. These measurements were made to assess how these experimental conditions affected rate adaptation of the action potential.
Results are expressed as mean±s.e.m. Differences were tested for statistical significance using the paired (two sample for means; same gender) and unpaired (two sample assuming unequal variances; gender differences) Student's t-test. A value of P<0.05 was considered significant.
Results
Effects of pacing rate, BaCl2 and (−) chromanol 293B on APD in beagle Purkinje fibres
The ‘rate adaptation' data obtained from Purkinje fibre experiments performed in our laboratory are collected in a database that is regularly updated after the completion of investigative studies. These data have demonstrated that under control conditions, relative to APD90 at a stimulation rate of 1.0 Hz, there was a significantly greater increase in APD90 at a stimulation rate of 0.2 Hz in fibres from female dogs. In contrast, the decrease in APD90 at a stimulation rate of 3.33 Hz relative to 1.0 Hz was the same in fibres from both sexes (Table 1). Moreover, we sought to determine whether IK1 or IKs block by 10 μM BaCl2 or 10 μM (−) chromanol 293B affected APD at baseline in fibres from female and male animals. Relative to control values, BaCl2, but not (−) chromanol 293B, produced a significant (P<0.01) prolongation of APD90 at stimulation rates of 3.33, 1.0 and 0.2 Hz in fibres from both sexes (Table 1). Similar data were obtained with APD50 (data not shown). Thus, IK1, but not unstimulated IKs, contributes to action potential repolarisation at baseline and our results point to an absence of gender differences in the response to IK1 block in beagle Purkinje fibres. In addition, BaCl2 or (−) chromanol 293B did not affect the resting membrane potential or the amplitude and maximum rate of depolarisation of the action potential in fibres from animals of either sex, and there was no difference between females and males for these parameters (data not shown).
Table 1.
Effects of pacing rate, BaCl2 and (−) chromanol 293B on APD90 in beagle dog Purkinje fibres
| Treatment | Stimulation rate (Hz) | Sex | % Change in APD90 | n | Number of animals |
|---|---|---|---|---|---|
| Krebs buffera | 3.33 | Male | −38.2±1.7 | 17 | 16 |
| Female | −36.9±2.0NS | 19 | 17 | ||
| 0.2 | Male | 11.0±1.2 | 31b | 24 | |
| Female | 16.2±1.3* | 32b | 28 | ||
| BaCl2 (10 μM)c | 3.33 | Male | 8.0±1.2 | 5 | 4 |
| Female | 7.0±0.9NS | 6 | 4 | ||
| 1.0 | Male | 12.2±3.0 | 5 | 5 | |
| Female | 11.4±1.9NS | 6 | 5 | ||
| 0.2 | Male | 18.6±4.0 | 5 | 5 | |
| Female | 14.7±3.9NS | 6 | 5 | ||
| (−) chromanol 293B (10 μM)c | 3.33 | Male | −0.6±1.2 | 4 | 4 |
| Female | −0.2±0.6NS | 4 | 4 | ||
| 1.0 | Male | −0.2±0.7 | 4 | 4 | |
| Female | 1.4±1.3NS | 4 | 4 | ||
| 0.2 | Male | 0.2±1.3 | 4 | 4 | |
| Female | 1.8±2.2NS | 4 | 4 |
APD90: the duration of action potential at 90% repolarisation. Data are expressed as mean±s.e.m.
Data are expressed as a per cent change relative to APD90 at 1.0 Hz in Krebs buffer.
Data were obtained in this study and Abi-Gerges et al. (2004a).
Data are expressed as a per cent change relative to APD90 at the same stimulation rate in Krebs buffer.
P>0.05
P<0.01 versus values from male animals.
Effect of (−) chromanol 293B on APD during β-adrenoceptor stimulation in beagle Purkinje fibres
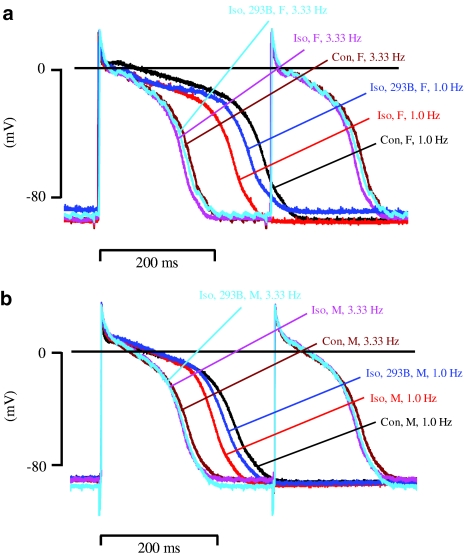
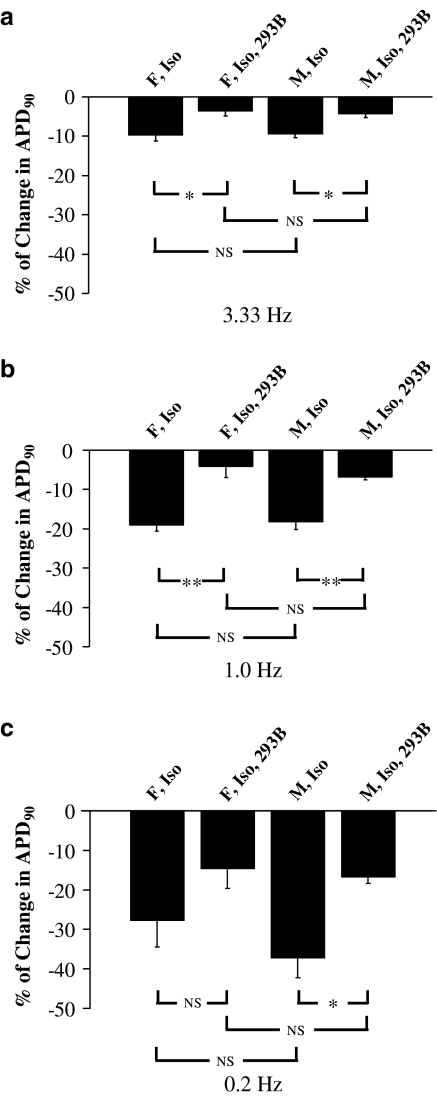
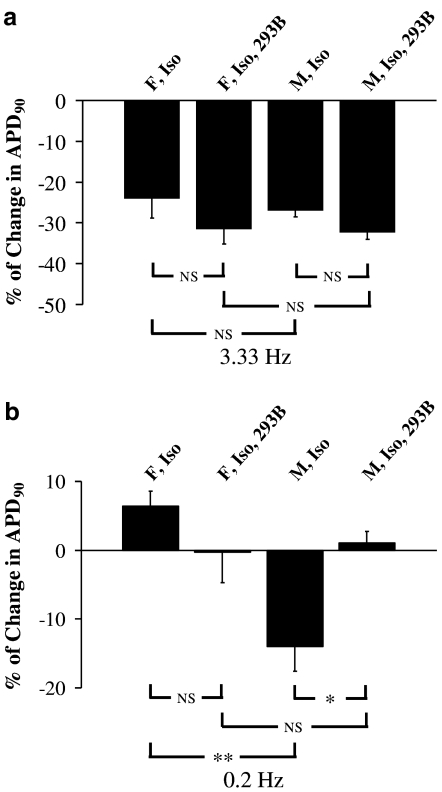
We sought to determine whether during β-adrenoceptor stimulation there was evidence for a role of IKs in beagle APD and whether its influence was gender dependent. To illustrate the effect of isoproterenol alone (0.1 μM) and in combination with (−) chromanol 293B (10 μM) at a given stimulation rate, a ‘within stimulation rate' analysis was carried out (see Methods). At a stimulation rate of 1.0 Hz, isoproterenol shortened APD90 in fibres from female and male animals. This shortening was significantly attenuated by (−) chromanol 293B (Figures 1, 2 and 3b). Similar observations were made with APD50 (Figures 1 and 2). Moreover, at a stimulation rate of 3.33 Hz, isoproterenol shortened APD90 in fibres from female and male animals (Figure 1), although the degree of shortening of APD90 at 3.33 Hz was less significant in both sexes compared to 1.0 Hz data (Figure 3a and b). In the presence of isoproterenol, (−) chromanol 293B slightly but not significantly reduced fast-pacing, rate-dependent shortening of APD90 in fibres of both sexes (Figure 3a). Similarly, isoproterenol-induced APD50 shortening was slightly but not significantly attenuated by (−) chromanol 293B (Figure 1). Furthermore, an ‘across stimulation rate' analysis (see Methods) showed that the ability of the action potential to adapt to an increase in the pacing rate with a decrease in APD90 was not significantly affected in the presence of isoproterenol alone or in combination with (−) chromanol 293B (Figure 4a).
Figure 1.
Effect of (−) chromanol 293B on APD during β-adrenoceptor stimulation in beagle dog Purkinje fibres. (a) and (b) show representative action potentials recorded in fibres from female (F) and male (M) animals under control conditions, in the presence of 0.1 μM isoproterenol (Iso) with and without 10 μM (−) chromanol 293B (293B) at stimulation rates of 3.33 and 1.0 Hz.
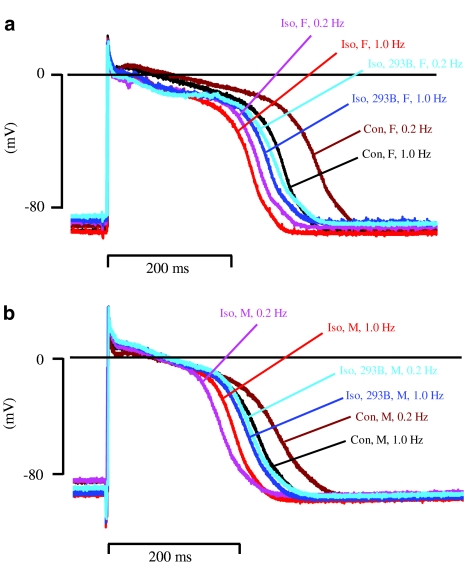
Figure 2.
Effect of (−) chromanol 293B on APD during β-adrenoceptor stimulation in beagle dog Purkinje fibres. (a) and (b) show representative action potentials recorded in fibres from female (F) and male (M) animals under control conditions, in the presence of 0.1 μM isoproterenol (Iso) with and without 10 μM (−) chromanol 293B (293B) at stimulation rates of 1.0 and 0.2 Hz.
Figure 3.
Effect of (−) chromanol 293B on APD during β-adrenoceptor stimulation in beagle dog Purkinje fibres – a ‘within stimulation rate' analysis (see Methods). (a–c) The mean per cent change in APD90 induced by isoproterenol (Iso) alone or when combined with 10 μM (−) chromanol 293B (293B) during stimulation rates of 3.33, 1.0 and 0.2 Hz in both female (F) and male (M) animals (n=4 for each gender), respectively. NSP>0.05, *P<0.05, **P<0.01.
Figure 4.
Effect of (−) chromanol 293B on rate adaptation of the action potential during β-adrenoceptor stimulation in beagle dog Purkinje fibres – an ‘across stimulation rate' analysis (see Methods). (a) The mean per cent change in APD90 induced by isoproterenol (Iso) alone or when combined with 10 μM (−) chromanol 293B (293B) at a stimulation rate of 3.33 Hz in both female and male animals (n=4 for each gender). NSP>0.05. (b) The mean per cent change in APD90 induced by Iso alone or when combined with 10 μM 293B at a stimulation rate of 0.2 Hz in both F and M animals (n=4 for each gender). NSP>0.05, *P<0.05, **P<0.01.
Finally, we evaluated the role of IKs during β-adrenoceptor stimulation at a low pacing rate. Figure 2 shows examples of the effects of isoproterenol on APD at both 1.0 and 0.2 Hz stimulation rate (same fibres as in Figure 1). When the stimulation rate was changed from 1.0 to 0.2 Hz in the presence of isoproterenol, there was a marked shortening in APD90 only in fibres from male animals (Figure 2). Relative to the control values at 0.2 Hz, a ‘within stimulation rate' analysis shows that isoproterenol shortened APD90 in fibres from female and male animals (Figure 3c). This change was attenuated by (−) chromanol 293B (10 μM) in both genders, although this attenuation was only statistically significant in fibres from male animals (Figure 3c). Similar observations were made with APD50 (Figure 2). Moreover, while the degree of change of APD90 evoked by isoproterenol at 0.2 Hz shows a tendency for a gender difference, this is not the case when isoproterenol and (−) chromanol 293B are present (Figure 3c). Additionally, and in contrast to a high pacing rate, an ‘across stimulation rate' analysis shows that in the presence of isoproterenol action potential in fibres from male dogs get shorter when changing the stimulation rate from 1.0 to 0.2 Hz, while the opposite is seen in fibres from female dogs. Most importantly, (−) chromanol 293B completely reversed this alteration (Figure 4b). Similar results were obtained with APD50 (Figure 2). Conversely, at stimulation rates of 3.33, 1.0 or 0.2 Hz, isoproterenol alone and in combination with (−) chromanol 293B, did not significantly alter the maximum rate of depolarisation or amplitude of the action potential or the resting membrane potential in fibres from female and male animals, and there were no differences between females and males for these other parameters (data not shown).
Discussion
There are four findings from this study of Purkinje fibres from beagle dogs: (i) the degree of APD prolongation evoked by BaCl2 was the same in fibres from males as it was in fibres from females, (ii) under basal conditions, (−) chromanol 293B did not change APD in fibres from either sex at any stimulation rate, (iii) in the presence of isoproterenol, within each stimulation frequency, there was a shortening of APD that was the same irrespective of gender and under these stimulated conditions (−) chromanol 293B now prolonged APD to a similar extent in fibres from both sexes and (iv) however, when looking across stimulation rates in the presence of isoproterenol, fibres from female and male animals behaved differently: in fibres from females, APD was prolonged at 0.2 Hz relative to APD at 1 Hz, while in fibres from males APD shortened in these conditions. Subsequent addition of (−) chromanol 293B at 0.2 Hz restored APD to that seen at 1 Hz for fibres from both sexes.
The IK1 current contributes to the ventricular action potential (Tamargo et al., 2004). At the concentration used in this study (10 μM), BaCl2 lengthened APD in a reverse frequency-dependent manner in ventricular papillary muscles isolated from mongrel dogs (Biliczki et al., 2002; Lengyel et al., 2004). Our data in beagle dog Purkinje fibres are consistent with these previous studies (Table 1). Moreover, IK1 block lengthened beagle dog Purkinje APD in fibres from female and male animals to a similar extent (Table 1). A recent study demonstrated that female rabbit ventricular myocytes have a significantly lower IK1 current density than cells from males which may contribute to the gender difference in the QT interval in rabbit hearts (Liu et al., 1998). Thus, it can be postulated that the absence of gender differences in IK1 block in beagle dog Purkinje fibres could be a consequence of a similar IK1 current density in both sexes, a hypothesis that remains to be confirmed. Recently, it has been shown that mongrel dog ventricular myocytes appear to have a large ‘repolarisation reserve'. However, when this ‘repolarisation reserve' is reduced by an IKr blocker (Biliczki et al., 2002), remodelling or genetic disorders, IK1 current inhibition with BaCl2 can result in excessive and potentially proarrhythmic prolongation of the ventricular APD. Therefore, the similar prolongation of APD evoked by BaCl2 in beagle dog Purkinje fibres from female and male animals (Table 1) might suggest that an IK1-related proarrhythmic prolongation of the ventricular APD would be the same in either sex. It would be valuable to evaluate whether there are gender differences in the ‘repolarisation reserve' in the beagle dog Purkinje preparation or in other cardiac preparations.
Although the IKs current is suggested to be an important contributor to the repolarisation of the cardiac action potential (Tamargo et al., 2004), its exact role in ventricular repolarisation remains under intense evaluation. Likewise the data reported by different authors in mongrel dog and rabbit heart preparations (see Introduction) (−) chromanol 293B did not change basal APD in beagle dog fibres from either sex (Table 1). Thus, a gender-related difference appears not to be a contributing factor to the lack of IKs blocker-induced changes in beagle dog APD under basal conditions (Table 1). Recently, studies have shown the importance of β-adrenoceptor stimulation in elucidating the effects of IKs blockers on APD in mongrel dog heart preparations (Han et al., 2001; Stengl et al., 2003; Volders et al., 2003). We observed that during β-adrenoceptor stimulation there was a clear effect of IKs blockade on APD in beagle dog Purkinje fibres (Figures 1, 2 and 3). Moreover, in the presence of isoproterenol, action potentials in fibres from male dogs get shorter when changing the stimulation rate from 1.0 to 0.2 Hz, while the opposite is seen in fibres from female dogs (Figures 2 and 4b). Most importantly, this alteration was reversed by (−) chromanol 293B (Figures 2 and 4b). The present investigation is the first to report gender-related differences in the contribution of isoproterenol-stimulated IKs to canine action potential repolarisation at low pacing rates. The mechanism of this difference remains to be elucidated. Such an important functional role of stimulated-IKs may provide a ‘brake' that would further decrease the incidence of proarrhythmic events in male animals. This assumption may be credible based on three observations: (i) IKr blockers prolong APD to a much greater extent at low rather than fast pacing rates (Nattel & Singh, 1999), (ii) IKr-prolonging effects are reduced in the presence of isoproterenol (Schreieck et al., 1997) and (iii) there is a gender difference in humans with respect to the autonomic modulation of ventricular repolarisation (Nakagawa et al., 2005). Thus, under conditions in which risk factors for Torsades de Pointes are combined (i.e. IKr block and low pacing rate), gender differences in the autonomic modulation of IKs may partially account for the greater susceptibility of female hearts to arrhythmogenesis.
Available evidence from studies involving human subjects and those involving experimental animals indicate a role for the gonadal steroids in the sex differences in cardiac repolarisation and susceptibility to arrhythmias (see review by James et al., 2005). The potential exists for the gonadal steroids to regulate the transcription of genes in the heart (i.e. expression of depolarising inward currents and repolarising outward currents of the cardiac action potential). Steroid hormones also induce rapid physiological responses through nongenomic pathways (i.e. effect of oestradiol-17β on APD, IKr and inward cardiac Ca2+ current). Additionally, the modulation by testosterone of the affinity of the hERG channel to blocking drugs may contribute to the sex differences in susceptibility to drug-induced Torsades de Pointes (Shuba et al., 2001). Although the underlying mechanisms to the sex differences are yet to be completely elucidated, our data from the beagle dog heart point to gender differences in the β-adrenoceptor modulation of IKs that could be a contributing factor to the reported decreased susceptibility of male hearts to arrhythmias (Figures 2 and 4b).
Our data are not in agreement with those reported in rabbit Purkinje fibres. Lu et al. (2005) showed that during β-adrenoceptor stimulation, (−) chromanol 293B induced APD prolongation in isolated rabbit papillary muscles and ventricular trabeculae, but not Purkinje fibres. The reasons why there are different effects of IKs blockade on APD in dog Purkinje preparations (isolated cells (Han et al., 2001) and fibres (this study)) and those in rabbit Purkinje fibres (Lu et al., 2005) are unknown. Although different preparations or selectivity of IKs blockers (chromanol 293B in the paper by Han et al. compared to (−) chromanol 293B in the paper by Lu et al. and this study) used in these studies may not explain these differences, species, rabbit versus dog, may play a role in these findings. As a result of these different results of IKs blockade on APD in Purkinje fibres, the choice of animal species and gender should be carefully selected to provide the mechanistic understanding needed for the preclinical cardiac evaluation of new drugs. Since the beagle dog is a commonly used preclinical species to test the effects of new drugs on cardiac repolarisation (Gralinski, 2003), the present study endorses the use of β-adrenoceptor-stimulated beagle Purkinje fibres as a suitable model to investigate the contribution of IKs to action potential repolarisation and detect unwanted IKs-blocking properties of new drugs.
Although the female and male animals used in this study were neither age nor weight matched, the weight range for females falls within that for males and the animals used were all sexually mature (see Methods). Despite the fact that our results are consistent with those reported in recent studies on the contribution of IK1 and IKs to canine action potential repolarisation, we cannot rule out factors other than gender that might contribute to the findings reported in this study. Moreover, clinical data suggest that the degree of drug-induced prolongation of the QT interval varies during the menstrual cycle (Rodriguez et al., 2001). Given that no quantitative measurement was performed to control for different states of oestrus, an extrapolation of the female canine data to premenopausal women cannot be made.
We chose concentrations of BaCl2 and (−) chromanol 293B that would, based on previous reports, be expected to selectively block IK1 and IKs, respectively (Bosch et al., 1998; Liu et al., 2001; Sun et al., 2001; Biliczki et al., 2002). However, it would strengthen our conclusions if we could demonstrate this using myocytes isolated from beagle dog Purkinje fibres.
In summary, in the present study we provide experimental evidence suggesting no gender differences in the contribution of IK1 to beagle dog action potential repolarisation at baseline. Our results also indicate that β-adrenoceptor stimulation is necessary for IKs to contribute significantly to beagle dog action potential repolarisation. Most importantly, the present investigation is the first to report that gender differences in the autonomic modulation of IKs may partially account for the greater susceptibility of female hearts to arrhythmogenesis.
Acknowledgments
We express our gratitude to Jackie A. Moors, Sue Egerton and Ann J. Woods for their expert technical assistance and thank Drs Karen L. Philp and Göran Duker for reviewing the manuscript and providing helpful suggestions.
Abbreviations
- APD
action potential duration
- APD50 and APD90
duration of the action potential at 50 and 90% repolarisation
- IK1
inward rectifier cardiac K+ current
- IKr
rapid delayed rectifier cardiac K+ current
- IKs
slow delayed rectifier cardiac K+ current
References
- ABI-GERGES N., PHILP K., POLLARD C., WAKEFIELD I., HAMMOND T.G., VALENTIN J.P. Sex differences in ventricular repolarization: from cardiac electrophysiology to Torsades de Pointes. Fundam. Clin. Pharmacol. 2004a;18:139–151. doi: 10.1111/j.1472-8206.2004.00230.x. [DOI] [PubMed] [Google Scholar]
- ABI-GERGES N., SMALL B.G., LAWRENCE C.L., HAMMOND T.G., VALENTIN J.P., POLLARD C.E. Evidence for gender differences in electrophysiological properties of canine Purkinje fibres. Br. J. Pharmacol. 2004b;142:1255–1264. doi: 10.1038/sj.bjp.0705880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANON Committee for Proprietary Medical Products (CPMP). Points to consider: The assessment of the potential of QT interval prolongation by non-cardiovascular medicinal products. The European Agency for the evaluation of medicinal products 1997. London, 17 December. Reference CPMP/ICH/986/96
- ANON ICH E14 Note for guidance on the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs 2005a. London, 25 May. Reference CHMP/ICH/2/04
- ANON ICH S7B Note for guidance on the nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals 2005b. London, 25 May. Reference CHMP/ICH/423/02 [PubMed]
- BILICZKI P., VIRAG L., IOST N., PAPP J.G., VARRO A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br. J. Pharmacol. 2002;137:361–368. doi: 10.1038/sj.bjp.0704881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSCH R.F., GASPO R., BUSCH A.E., LANG H.J., LI G.R., NATTEL S. Effects of the chromanol 293B, a selective blocker of the slow, component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovasc. Res. 1998;38:441–450. doi: 10.1016/s0008-6363(98)00021-2. [DOI] [PubMed] [Google Scholar]
- CHIANG C.E. Congenital and acquired long QT syndrome. Current concepts and management. Cardiol. Rev. 2004;12:222–234. doi: 10.1097/01.crd.0000123842.42287.cf. [DOI] [PubMed] [Google Scholar]
- GOGELEIN H., BRUGGEMANN A., GERLACH U., BRENDEL J., BUSCH A.E. Inhibition of IKs channels by HMR 1556. Naunyn. Schmiedebergs Arch. Pharmacol. 2000;362:480–488. doi: 10.1007/s002100000284. [DOI] [PubMed] [Google Scholar]
- GRALINSKI M.R. The dog's role in the preclinical assessment of QT interval prolongation. Toxicol. Pathol. 2003;31 (Suppl):11–16. doi: 10.1080/01926230390174887. [DOI] [PubMed] [Google Scholar]
- HAN W., WANG Z., NATTEL S. Slow delayed rectifier current and repolarization in canine cardiac Purkinje cells. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1075–H1080. doi: 10.1152/ajpheart.2001.280.3.H1075. [DOI] [PubMed] [Google Scholar]
- JAMES A.F., CHOISY S.C.M., HANCOX J.C.Recent advances in understanding sex differences in cardiac repolarization Prog. Biophys. Mol. Biol. 2005. in press (doi: 10.1016/j.pbiomolbio.2005.05.010) [DOI] [PubMed]
- KEATING M.T., SANGUINETTI M.C. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569–580. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- LENGYEL C., DEZSI L., BILICZKI P., HORVATH C., VIRAG L., IOST N., NEMETH M., TALOSI L., PAPP J.G., VARRO A. Effect of a neuroprotective drug, eliprodil on cardiac repolarisation: importance of the decreased repolarisation reserve in the development of proarrhythmic risk. Br. J. Pharmacol. 2004;143:152–158. doi: 10.1038/sj.bjp.0705901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENGYEL C., IOST N., VIRAG L., VARRO A., LATHROP D.A., PAPP J.G. Pharmacological block of the slow component of the outward delayed rectifier current (IKs) fails to lengthen rabbit ventricular muscle QT(c) and action potential duration. Br. J. Pharmacol. 2001;132:101–110. doi: 10.1038/sj.bjp.0703777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU G.X., DERST C., SCHLICHTHORL G., HEINEN S., SEEBOHM G., BRUGGEMANN A., KUMMER W., VEH R.W., DAUT J., PREISIG-MULLER R. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J. Physiol. 2001;532:115–126. doi: 10.1111/j.1469-7793.2001.0115g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU X.K., KATCHMAN A., DRICI M.D., EBERT S.N., DUCIC I., MORAD M., WOOSLEY R.L. Gender difference in the cycle length-dependent QT and potassium currents in rabbits. J. Pharmacol. Exp. Ther. 1998;285:672–679. [PubMed] [Google Scholar]
- LU H.R., VLAMINCKX E., VAN DE WATER A., GALLACHER D.J. Both β-adrenergic receptor stimulation and cardiac tissue type have important roles in elucidating the functional effects of IKs channel blockers in vitro. J. Pharmacol. Toxicol. Methods. 2005;51:81–90. doi: 10.1016/j.vascn.2004.10.004. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA M., OOIE T., OU B., ICHINOSE M., TAKAHASHI M., HARA M., YONEMOCHI H., SAIKAWA T. Gender differences in autonomic modulation of ventricular repolarization in humans. J. Cardiovasc. Electrophysiol. 2005;16:278–284. doi: 10.1046/j.1540-8167.2005.40455.x. [DOI] [PubMed] [Google Scholar]
- NATTEL S., QUANTZ M.A. Pharmacological response of quinidine-induced early afterdepolarizations in canine Purkinje fibres: insights into underlying ionic mechanisms. Cardiovasc. Res. 1988;22:808–817. doi: 10.1093/cvr/22.11.808. [DOI] [PubMed] [Google Scholar]
- NATTEL S., SINGH B.N. Evolution, mechanisms, and classification of antiarrhythmic drugs: focus on class III actions. Am. J. Cardiol. 1999;84:11R–19R. doi: 10.1016/s0002-9149(99)00697-9. [DOI] [PubMed] [Google Scholar]
- PLASTER N.M., TAWIL R., TRISTANI-FIROUZI M., CANUN S., BENDAHHOU S., TSUNODA A., DONALDSON M.R., IANNACCONE S.T., BRUNT E., BAROHN R., CLARK J., DEYMEER F., GEORGE A.L., JR, FISH F.A., HAHN A., NITU A., OZDEMIR C., SERDAROGLU P., SUBRAMONY S.H., WOLFE G., FU Y.H., PTACEK L.J. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- REDFERN W.S., CARLSSON L., DAVIS A.S., LYNCH W.G., MACKENZIE I., PALETHORPE S., SIEGL P.K., STRANG I., SULLIVAN A.T., WALLIS R., CAMM A.J., HAMMOND T.G. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ I., KILBORN M.J., LIU X.K., PEZZULLO J.C., WOOSLEY R.L. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;285:1322–1326. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- SCHREIECK J., WANG Y., GJINI V., KORTH M., ZRENNER B., SCHOMIG A., SCHMITT C. Differential effect of β-adrenergic stimulation on the frequency-dependent electrophysiologic actions of the new class III antiarrhythmics dofetilide, ambasilide, and chromanol 293B. J. Cardiovasc. Electrophysiol. 1997;8:1420–1430. doi: 10.1111/j.1540-8167.1997.tb01039.x. [DOI] [PubMed] [Google Scholar]
- SHIMIZU W., ANTZELEVITCH C. Cellular basis for the ECG features of the LQT1 form of the long-QT syndrome: effects of β-adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and torsade de pointes. Circulation. 1998;98:2314–2322. doi: 10.1161/01.cir.98.21.2314. [DOI] [PubMed] [Google Scholar]
- SHUBA Y.M., DEGTIAR V.E., OSIPENKO V.N., NAIDENOV V.G., WOOSLEY R.L. Testosterone-mediated modulation of HERG blockade by proarrhythmic agents. Biochem. Pharm. 2001;62:41–49. doi: 10.1016/s0006-2952(01)00611-6. [DOI] [PubMed] [Google Scholar]
- STENGL M., VOLDERS P.G., THOMSEN M.B., SPATJENS R.L., SIPIDO K.R., VOS M.A. Accumulation of IKs in canine ventricular myocytes. J. Physiol. 2003;551 (Part 3):777–786. doi: 10.1113/jphysiol.2003.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUS S.M.J.M, STURKENBOOM M.C.J.M, BLEUMINK G.S., DIELEMAN J.P, VAN DER LEI J., DE GRAEFF P.A., KINGMA J.H., STRICKER B.H.CH. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur. Heart J. 2005;26:2007–2012. doi: 10.1093/eurheartj/ehi312. [DOI] [PubMed] [Google Scholar]
- SUN Z.Q., THOMAS G.P., ANTZELEVITCH C. Chromanol 293B inhibits slowly activating delayed rectifier and transient outward currents in canine left ventricular myocytes. J. Cardiovasc. Electrophysiol. 2001;12:472–478. doi: 10.1046/j.1540-8167.2001.00472.x. [DOI] [PubMed] [Google Scholar]
- TAMARGO J., CABALLERO R., GOMEZ R., VALENZUELA C., DELPON E. Pharmacology of cardiac potassium channels. Cardiovasc. Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- VARRO A., BALATI B., IOST N., TAKACS J., VIRAG L., LATHROP D.A., CSABA L., TALOSI L., PAPP J.G. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J. Physiol. 2000;523 (Part 1):67–81. doi: 10.1111/j.1469-7793.2000.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLDERS P.G., STENGL M., VAN OPSTAL J.M., GERLACH U., SPATJENS R.L., BEEKMAN J.D., SIPIDO K.R., VOS M.A. Probing the contribution of IKs to canine ventricular repolarization: key role for β-adrenergic receptor stimulation. Circulation. 2003;107:2753–2760. doi: 10.1161/01.CIR.0000068344.54010.B3. [DOI] [PubMed] [Google Scholar]
- ZICHA S., MOSS I., ALLEN B., VARRO A., PAPP J., DUMAINE R., ANTZELEVICH C., NATTEL S. Molecular basis of species-specific expression of repolarizing K+ currents in the heart. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1641–H1649. doi: 10.1152/ajpheart.00346.2003. [DOI] [PubMed] [Google Scholar]