Abstract
The basic secretagogues, such as compound 48/80 (c48/80) and mastoparans, are widely used histamine-releasing agents and their mechanism of action is commonly attributed to a direct, receptor-bypassing property to activate the Gi/o class of G proteins.
We tested here whether c48/80 could directly stimulate [35S]guanosine-5′-[γ-thio]triphosphate ([35S]GTPγS) binding to rat brain sections in an attempt to visualize the entire signaling pool of Gi/o in its native neuroanatomical context.
Instead of direct Gi/o activation, c48/80 (100 μg ml−1) from various suppliers stimulated brain phospholipase D (PLD) activity, leading to the generation of endogenous phospholipids capable of activating brain white matter-enriched, Gi/o-coupled lysophosphatidic acid (LPA) receptors. This response was sensitive to 1-butanol and was potently reversed by the LPA1/LPA3 receptor-selective antagonist Ki16425 (IC50 59±13 nM, mean±s.e.m.), and showed age-dependent decline, closely reflecting known developmental regulation of the PLD–LPA1 receptor axis in the CNS.
In addition, c48/80 was found to modestly activate hippocampal 5-HT1A receptors in a pH-dependent and antagonist-sensitive manner.
Consistent with the lack of direct Gi/o-activating properties in brain sections, c48/80 showed no activity in classical membrane [35S]GTPγS binding assays. Instead, c48/80 from one particular manufacturer elicited non-specific effect in these assays, therefore challenging the previous interpretations regarding the compound's ability to activate G proteins directly.
We conclude that c48/80 is not a receptor-bypassing general G protein activator but rather activates PLD, leading to generation of endogenous LPA receptor-activating phospholipids. This property may also contribute to the compound's ability to release histamine from mast cells.
Keywords: Compound 48/80, phospholipase D, lysophosphatidic acid, Ki16425, [35S]GTPγS binding assay, 5-HT1A receptor
Introduction
Basic secretagogues comprise a set of cationic amphiphilic drugs, as well as endo- and exogenous peptides, which similarly share basic head group combined with a hydrophobic core of the molecule. These classic pharmacological agents, like the wasp venom mastoparan (MP) and the synthetic compound 48/80 (c48/80), are able to elicit mast cell degranulation and possibly alter enzymatic activity via pathways distinct from those stimulated by immunoglobulin E (IgE)-bound antigens (Ferry et al., 2002). Whereas the latter is mediated by cell-surface receptors, a receptor-bypassing mechanism has been proposed for the basic secretagogues. In original observations, Higashijima et al. (1988; 1990) found that MP could activate purified heterotrimeric G proteins probably by enhancing the dissociation of GDP from Gα subunits, thus accelerating the event considered as a rate-limiting step in conventional G protein activation by receptors coupled to heterotrimeric G proteins (G protein-coupled receptors (GPCRs)) (Ferguson et al., 1986). Similar results have been published for other secretagogues, for example, c48/80 and substance P (Mousli et al., 1990a, 1990b). Together, these observations have led to the conception that the basic secretagogues could act as receptor mimetics and their ability to induce mast cell degradation would therefore at least partly depend on this receptor-bypassing G protein activation.
Pertussis toxin (Ptx) is often used to prove the involvement of heterotrimeric G proteins in certain signaling processes. Ptx ADP-ribosylates the C-terminal cysteine residues in the α-subunits of Gi/o class G proteins, rendering them unable to interact with the GPCRs (Gilman, 1987). In strict contrast to the IgE-elicited mast cell activation, the actions of the basic secretagogues on both G proteins and mast cells seem to be Ptx sensitive (Saito et al., 1987; Tomita et al., 1991), indicating association with the Gi/o-type G proteins. Interestingly, Gi/o represents the most ubiquitous G protein type in the mammalian brain and is already accessible using various research methods, including traditional [35S]guanosine-5′-[γ-thio]triphosphate ([35S]GTPγS) membrane binding assay and more novel functional receptor autoradiography using [35S]GTPγS (Sovago et al., 2001; Laitinen, 2004). The former approach has been applied to study the activating effects of MP (Higashijima et al., 1988) and c48/80 (Mousli et al., 1990b; Tomita et al., 1991) on purified G protein subunits. As far as we are aware, no studies have so far addressed the capability of these agents to stimulate [35S]GTPγS binding to brain sections. As imaginable, receptor-bypassing approach to detect G protein activity in defined anatomical brain loci independent of their cognate receptors in their native anatomical context might be beneficial in building up our knowledge in the field of G protein-coupled signaling pathways in CNS. Against this background, we have tested the hypothesis whether the basic secretagogues c48/80 and MP and MP's more potent analog mastoparan-7 (MP-7) could enhance the specific G protein labeling by [35S]GTPγS in rat brain cryosections and membrane preparations. In contrast to expectations, neither c48/80 nor MP could elicit any specific [35S]GTPγS binding in brain sections directly. Instead, c48/80 stimulated phospholipase D (PLD, EC 3.1.4.4) activity, subsequently leading to activation of lysophosphatidic acid (LPA) receptors through the formation of bioactive lipids. In classical membrane [35S]GTPγS binding assays where both receptor-dependent and MP-stimulated G protein activity was evident, c48/80 failed to activate G proteins. Moreover, in modestly alkaline conditions, c48/80 stimulated [35S]GTPγS binding through the activation of the 5-HT1A receptors. These data indicate that c48/80 is not a direct G protein activator, but rather stimulates G protein activity indirectly mainly via PLD-dependent generation of LPA receptor-activating phospholipids.
Methods
Chemicals
[35S]GTPγS (specific activity 1250 Ci mmol−1) was purchased from NEN Life Science products Inc. (Boston, MA, U.S.A.). 1-Butanol and tert-butanol were from Merck (Darmstat, Germany). MP-7 was from Bachem (Torrance, CA, U.S.A.). c48/80 was obtained from MP Biomedicals (Irvine, CA, U.S.A.), Biomol (Plymouth Meeting, PA, U.S.A.) or Sigma-Aldrich (St Louis, MO, U.S.A.). Majority of the other chemicals (bovine serum albumin (BSA), carbachol, dithiothreitol (DTT), 8-cyclopentyl-1,3-dipropylxanthine, guanosine diphosphate, guanosine-5′-(γ-thio)trisphosphate, 3-(4-[4-([1-(2-chlorophenyl)ethoxy]carbonyl amino)-3-methyl-5-isoxazolyl] benzylsulfanyl) propanoic acid (Ki16425), LPA, MP-7, N-ethylmaleimide (NEM), phosphatidic acid (PA) and protease inhibitor cocktail; P-2714) were purchased from Sigma (St Louis, MO, U.S.A.). All other chemicals used were of the finest purity available.
Animals
Experiments were performed using either 4- or 9-week-old male Wistar rats purchased from the National Laboratory Animal Center in Kuopio, Finland. Permission and approval for the animal experiments was obtained from the local ethics committee. The animals were housed in groups of five to six individuals per cage under standard laboratory conditions (12–12 h light–dark cycle, food and water ad libitum, 60% of relative humidity). The rats were decapitated 8–9 h after lights on, and within the next 5 min whole brain was dissected out, dipped briefly in isopentane chilled on dry ice and frozen in dry ice.
[35S]GTPγS autoradiography
Coronal and sagittal cryosections (20 μm thick) were cut at −19°C (Leica cryostat), thaw-mounted onto Superfrost®Plus slides (Menzel-Gläser, Germany) and dried for 1–4 h at room temperature under constant stream of air and stored thereafter at −80°C.
The [35S]GTPγS binding assay was conducted in three steps as previously described (Laitinen & Jokinen, 1998; Laitinen, 1999; Laitinen et al., 2001). The steps (20, 60 and 90 min, respectively) were performed in a sequential order in Tris-HCl-based buffer (Tris-HCl 50 mM pH 7.4, EDTA 1 mM, NaCl 100 mM, MgCl2 5 mM) at 20°C. To achieve an optimal signal-to-noise ratio, 2 mM GDP and 1 μM 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) were included throughout steps 2 and 3. DPCPX represents a potent and specific adenosine A1 receptor antagonist, which is routinely used in our laboratory to eliminate basal labeling due to tonic adenosine A1 receptor activity (Laitinen, 1999). Chemicals under interest were brought into the assay during step 3, which also contained 0.1% BSA (fatty acid free), 1 mM DTT and 153 pM [35S]GTPγS. To determine non-specific binding (Nsb), some slides were incubated in the presence of 10 μM GTPγS. After 90 min incubation, the slides were rinsed in ice-cold washing buffer (2 × 5 min, 50 mM Tris-HCl and 5 mM MgCl2). Finally, the slides were dipped briefly in 0°C deionized water, air-dried and placed together with [14C] standards (Amersham, Little Chalfont, Bucks, U.K.) in contact with the radiosensitive film (BioMax MR™, Kodak Scientific Imagin Film) for 3–5 days. The films were then developed for 3–5 min at 4°C with Kodak D-19 developer.
[35S]GTPγS membrane binding assay
A previously described method (Lorenzen et al., 1993; Savinainen et al., 2001) was utilized in the preparation of membranes. Briefly, forebrains from two rats were weighed and homogenized in nine volumes of ice-cold 0.32 M sucrose using a glass Teflon homogenizer. The homogenate was centrifuged at low speed (1000 × g for 10 min at 4°C) and the supernatant was centrifuged at high speed (100,000 × g for 10 min at 4°C). High-speed centrifugation was repeated twice after resuspending the pellet in ice-cold deionized water. Finally, membranes were resuspended in Tris-HCl (50 mM, pH 7.4) supplemented with EDTA (1 mM) and stored thereafter at −80°C.
The [35S]GTPγS membrane binding assays were performed as recently described (Savinainen et al., 2001), with minor modifications. Briefly, the final incubation volume (400 μl) contained 5 μg membrane protein in incubation buffer (Tris-HCl 50 mM pH 7.4, EDTA 1 mM, MgCl2 5 mM, NaCl 100 mM, GDP 10 μM, DTT 1 mM, BSA 0.5% and [35S]GTPγS ∼0.15 nM) plus the chemicals of interest, as detailed in the Results section. Incubation buffer was also supplemented with 1 μM DPCPX to suppress basal [35S]GTPγS binding due to endogenously formed adenosine (Savinainen et al., 2003). Nsb was assessed in the presence of 10 μM GTPγS. Incubation buffers (in 15 ml conical tubes) were kept on ice and the membrane was added less than 5 min before initiating the incubation by adding drugs in 60 μl of deionized water. Incubations continued for specified time (7.5–90 min, as detailed in the Results section) and were stopped by the addition of 4 ml of ice-cold washing buffer (50 mM Tris-HCl pH 7.4, 5 mM MgCl2), followed by filtration through glass fiber filters (Whatman GF/B) and two washes with the buffer. The filters were transferred to scintillation vials along with HiSafe3 (Wallac, Turku, Finland) scintillation liquid. After shaking (15 min), the tubes were centrifuged at 1000 × g and counted the next day with Wallac LKB 1213 Rackbeta.
Data and image analyses
Autoradiography films were scanned as negatives using HP scanjet 7400c scanner. Optical density on autoradiograms was measured using ImageJ, a freely available java-based image analysis software developed at the U.S. National Institutes of Health (http://rsb.info.nih.gov/ij/). Optical densities were converted to nCi g−1 with the help of nonlinear transformation built by the grayscale values of [14C] standards. Data were collected from four or six different sections representing 4–6 (4-week-old) or two (9-week-old) individual animals and are presented as percent of basal binding+s.e.m. after subtraction of Nsb. Membrane binding data are presented as mean±s.e.m. of three independent experiments performed in duplicate. Nsb was subtracted before calculating the results. Statistical differences were determined using one-way ANOVA with Tukey's multiple comparison post hoc test with P<0.05 considered as statistically significant. All of the data analysis was conducted using GraphPad Prism 3.0 for Windows. In Figure 4b, the sigmoidal dose–response curve for Ki16425 was likewise fitted in GraphPad Prism 3.0 using nonlinear regression. IC50 values for Ki16425 are presented as value±s.e.m.
Figure 4.
c48/80- and LPA-stimulated G protein activity in brain cryostat sections is mediated by LPA receptors, likely LPA1. (a) [35S]GTPγS autoradiography was conducted using a three-step protocol with DPCPX (1 μM) present throughout steps 2 and 3, as detailed in the Methods section. c48/80 (100 μg ml−1; MP Biomedicals) or LPA (5 μM in 0.1% fatty acid-free BSA) was included in the [35S]GTPγS labeling step of developing (4-week-old) rat brain sections along with or without the LPA1/LPA3 receptor-selective antagonist Ki16425 (5 μM). Note that Ki16425 clearly abolishes [35S]GTPγS binding responses to c48/80 and LPA throughout the white matter tracts. Like 1-butanol, Ki16425 also suppresses basal G protein activity in the white matter regions, indicating tonic LPA receptor activity in the developing rat brain. Abbreviations: cca, corpus callosum. Scale bar=5 mm. (b) Quantitative autoradiography data on the corpus callosum, selected to represent the white matter regions. Autoradiography images were digitized and bound radioactivity values were obtained from the digitized images for statistical analysis. Ki16425 dose-dependently decreases the basal [35S]GTPγS binding as well as that evoked by c48/80 (100 μg ml−1; MP Biomedicals) or LPA (5 μM) with IC50 values of 35±9, 59±59 and 87±19 nM, respectively. Values are mean±s.e.m. (or IC50±s.e.m.) representing six sections from six developing (4-week-old) animals.
Results
c48/80-stimulated [35S]GTPγS binding responses are NEM sensitive and restricted to the white matter tracts in the developing rat brain
Commercially available powders of c48/80 represent a mixture of condensation products of N-methyl-p-methoxyphenethylamine with formaldehyde. Thus, the degree of polymerization and the relative abundance of each oligomer are expected to vary between the products from different manufacturers. Moreover, it is possible that only certain oligomers possess any biological activity and the other oligomers may thus be considered as impurity of the product. Therefore, we tested several batches of c48/80 obtained from distinct companies. In contrast to our expectations for a general and widespread stimulation of brain Gi/o protein activity, c48/80 (either from MP Biomedicals or Sigma-Aldrich) at a concentration of 100 μg ml−1, which was previously used in G protein studies by Mousli et al. (1990b), stimulated [35S]GTγS binding selectively in the white matter regions of 4-week-old rat brain. Noticeably, the c48/80-labeled areas, for example, the corpus callosum, the anterior commissure, the fimbria of hippocampus and the cerebellar white matter tracts, were strikingly similar, with the distribution of [35S]GTPγS binding response induced by LPA (50 μM) represented in Figure 1a and previously shown in distinct reports (Waeber & Chiu, 1999; Laitinen et al., 2001; Laitinen, 2004). The statistical analysis performed (n=4, four different animals) in two selected white matter areas (corpus callosum and fimbria of the hippocampus) confirmed significant differences in labeling between basal and c48/80- or LPA-stimulated conditions (Figure 1b). In the hippocampus and the sensory cortex, two regions that were selected to represent the gray matter, the [35S]GTPγS binding was not statistically significantly elevated in the presence of any of these substances. Instead, LPA induced heterogonous labeling in the striatal white matter, whereas c48/80 batches did not (Figure 1b). According to our observations, inactivation of Gi/o proteins with Ptx is not feasible in brain sections, but instead, pretreatment with a sulfhydryl reagent, NEM, dramatically compromises both basal and receptor-mediated [35S]GTPγS labeling to heterotrimeric G proteins (Waeber & Chiu, 1999; Laitinen, 2004). In the present study, 20 min pretreatment with NEM (1 mM) decreased overall [35S]GTPγS binding and largely eliminated all specific responses (Figure 1a). However, despite the NEM treatment, which resulted in a complete loss of responses evoked by LPA or c48/80 from MP Biomedicals, c48/80 from Sigma-Aldrich could still induce a significant amount of overall [35S]GTPγS labeling throughout the brain sections (Figure 1a and inset in Figure 1b). Furthermore, such an increase was observed even if 10 μM unlabeled GTPγS was included together with the Sigma's product (data not shown), thus revealing a non-specific action of the Sigma-Aldrich product.
Figure 1.
[35S]GTPγS autoradiography of rat brain sections reveals LPA-mimicking and NEM-sensitive G protein activation in response to stimulation with c48/80. (a) [35S]GTPγS autoradiography was conducted using a three-step protocol with DPCPX (1 μM) present throughout steps 2 and 3, as detailed in the Methods section. c48/80 (100 μg ml−1) from two suppliers (MP Biomedicals and Sigma-Aldrich) or LPA (50 μM in 0.1% fatty acid-free BSA) was present during the [35S]GTPγS labeling in step 3. Some sections were treated with the irreversible Gi/o protein inhibitor NEM (1 mM, included in step 1 of the protocol). In the control panel (left), the white matter anatomical loci where LPA typically activates G proteins are indicated. Note the highly restricted and LPA-mimicking distribution of c48/80-stimulated G protein activity throughout the white matter tracts, including the anterior commissure (aca), the corpus callosum (cca) and the fimbria of the hippocampus (fi). Other abbreviations: ctx, cerebral cortex; hip, hippocampal structures; str, striatum. Scale bar=5 mm. (b) Quantitative autoradiography data on selected brain regions and on whole brain for NEM-pretreated sections (inset). Autoradiography images were digitized and bound radioactivity values were obtained from the digitized images for two white matter regions (corpus callosum (cca) and the fimbria of the hippocampus (fi)), two gray matter regions (cerebral cortex (ctx) and hippocampus (hip)), an area containing both white and gray matter (striatum (str)) and for whole section area, as detailed in the Methods section. Values are mean+s.e.m. representing sections from four individual animals. Risk level: *P<0.05 compared to basal in each specified brain region and #P<0.05 compared to NEM-pretreated basal on whole section radioactivity (one-way ANOVA with Tukey's multiple comparison).
Another batch of c48/80 from Sigma-Aldrich likewise increased [35S]GTPγS binding in both white and gray matter brain regions, whereas c48/80 obtained from Biomol, like the one from MP Biomedicals, stimulated [35S]GTPγS binding specifically to the white matter tracts (data not shown). In contrast to c48/80, the other basic secretagogues MP (10–100 μM) and MP-7 (10–100 μM) failed to evoke detectable [35S]GTPγS binding response under the assay conditions employed (data not shown). MP are peptides and therefore might be sensitive to enzymatic degradation under the assay conditions of [35S]GTPγS autoradiography. However, neither pretreatment with a protease inhibitor cocktail nor further manipulation of the assay conditions (omission of BSA and/or the reducting agent DTT) allowed detection of MP-stimulated G protein activity. Such manipulations had no effect on c48/80-induced [35S]GTPγS binding responses (data not shown).
c48/80 activates PLD in brain sections leading to stimulation of [35S]GTPγS binding via LPA receptors
As the Sigma-Aldrich's c48/80 clearly caused non-specific binding responses, all further autoradiography studies were conducted using c48/80 from MP Biomedicals. Closely overlapping anatomical distribution of binding patterns evoked by c48/80 and LPA suggests possible direct agonism of c48/80 at LPA receptors or, alternatively, stimulation of enzymatic pathways in brain sections leading to accumulation of LPA receptor-activating phospholipids, for example, endogenous LPA. One straightforward biosynthetic pathway to produce endogenous LPA consists of sequential actions of PLD followed by phospholipase A1/A2 (PLA1/PLA2, EC 3.1.1.32 and 3.1.1.4, respectively) activity on membrane phospholipids, such as phosphatidylcholine (Aoki, 2004). In these reactions, PLD activity first catalyzes the conversion of phosphatidylcholine to PA, which further serves as a precursor for LPA. Nevertheless, in the presence of primary alcohols, like 1-butanol, PLD prefers to catalyze transphosphatidylation reaction between the membrane lipids and alcohol, leading to the formation of biologically inactive phosphatidyl-1-butanol at the expense of PA production (Billah, 1993; Yu et al., 1996). This unique characteristic of the enzyme is a commonly used approach to establish the participation of PLD in certain biochemical processes. Today, at least four distinct LPA-sensing receptors (LPA1–4) have been molecularly identified in mammalian tissues (Aoki, 2004). From these, the LPA1 (formerly, EDG-2) receptor exhibits temporal expression pattern in the myelin-forming cells during the development of the white matter tracts (Allard et al., 1998; Cervera et al., 2002). Against this background, we employed 9-week-old rat brain sections along with the ones from 4-week-old rats to further investigate the role of LPA receptors and 1-butanol-sensitive PLD in c48/80-evoked [35S]GTPγS binding responses.
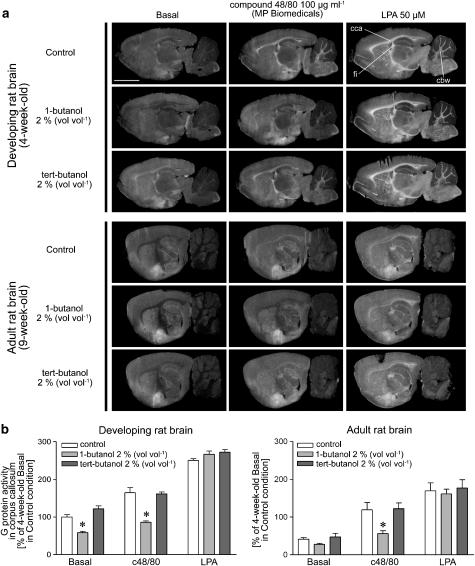
Effective 1-butanol concentrations for PLD transphosphatidylation in living cells usually settle around 0.5% (vol vol−1, 54 mM) (Chai et al., 2001; Chahdi et al., 2000), whereas the brain sections were found to require slightly higher concentrations. As demonstrated in Figure 2, 1-butanol 2% (vol vol−1, 216 mM) significantly inhibited c48/80-stimulated [35S]GTPγS binding responses in the white matter tracts (corpus callosum chosen to represent the white matter in quantification) in both developing and adult rat brain. 1-Butanol (2% vol vol−1) also reduced basal G protein activity in the white matter of developing but not in adult brain sections. As a negative control, inclusion of tert-butanol 2% (vol vol−1), which is not readily the substrate for transphosphatidylation (Billah, 1993), but exerts otherwise similar chemical properties as 1-butanol, did not affect basal or c48/80-stimulated [35S]GTPγS binding responses. Notably, LPA-evoked binding responses remained unaltered in the presence of both alcohols, indicating that LPA receptor function and subsequent G protein activation were not affected by this butanol concentration. Lower concentrations of 1-butanol (0.5 and 1% vol vol−1) were ineffective in brain sections (data not shown). Pretreatment of brain sections with 1-butanol (2% vol vol−1, present during step 2 of the protocol) was found insufficient to abolish basal or c48/80-evoked [35S]GTPγS binding to the white matter (data not shown), suggesting that the bioactive phospholipid was formed during the third incubation step.
Figure 2.
c48/80 stimulates G protein activity in the developing white matter tracts through 1-butanol-sensitive mechanisms likely involving PLD. (a) [35S]GTPγS autoradiography of developing (4-week-old) and adult (9-week-old) rat brain sections was conducted using a three-step protocol with DPCPX (1 μM) present throughout steps 2 and 3, as detailed in the Methods section. c48/80 (100 μg ml−1; MP Biomedicals) or LPA (50 μM in 0.1% fatty acid-free BSA) and the structural butanol isomers (2%, vol vol−1) were present during the [35S]GTPγS labeling step, as indicated. Note that the PLD inhibitor 1-butanol but not its inactive isomer tert-butanol selectively inhibits c48/80-stimulated G protein activity in the developing white matter tracts without affecting LPA-evoked responses. Note also age-dependent decline in basal and c48/80- or LPA-evoked [35S]GTPγS binding responses throughout the adult white matter tracts. Abbreviations: cca, corpus callosum; fi; the fimbria of the hippocampus; cbw, the cerebellar white matter (cbw). Scale bar=5 mm. (b) Quantitative autoradiography data on the corpus callosum of 4-week-old (left) and 9-week-old rats (right). Autoradiography images were digitized and bound radioactivity values were obtained from the digitized images, as detailed in the Methods section. Values are mean+s.e.m. representing four sections from four (developing) or two (adult) individual animals. Risk level: *P<0.05 compared to the respective control treatment in the corpus callosum of 4-week-old rat (one-way ANOVA with Tukey's multiple comparison).
In an attempt to clarify whether the PLD product was further processed by PLA2 activity to yield LPA, we tested the effect of known PLA2 inhibitors on c48/80-induced [35S]GTPγS binding. As shown in Figure 3, bromoenol lactone (calcium-independent PLA2), arachidonoyltrifluoromethyl ketone (cytosolic PLA2) and p-bromophenacyl bromide (secretatory PLA2) were each without significant effect, raising the possibility that the endogenous PA species, as a direct PLD product, or other routes of PA conversion could itself be responsible for the LPA mimicking signal.
Figure 3.
(a) Known phospholipase 2 (PLA2) inhibitors, bromoenol lactone (BEL, calcium-independent PLA2), arachidonoyltrifluoromethyl ketone (ATFMK, cytosolic PLA2) and p-bromophenacyl bromide (BPB, secretatory PLA2), have no effect on the c48/80-induced [35S]GTPγS binding to 4-week-old rat brain white matter areas. [35S]GTPγS autoradiography was conducted in three steps with DPCPX (1 μM) present throughout steps 2 and 3, as detailed in the Methods section. (b) Quantitative autoradiography data on the corpus callosum, selected to represent the white matter. The autoradiography images were digitized and bound radioactivity values were obtained from the digitized images for statistical analysis. Values are mean+s.e.m. representing four sections from four developing (4-week-old) animals. Scale bar=5 mm. c48/80 (100 μg ml−1; MP Biomedicals) was included in the labeling step of autoradiography along with the inhibitors (each at 50 μM) as indicated in the figure. No significant alterations were detected in c48/80-induced [35S]GTPγS binding when inhibitors were included. Risk level: P<0.05 compared to the respective control treatment in the corpus callosum.
The LPA-stimulated G protein activity in the CNS, as visualized by [35S]GTPγS autoradiography, is thought to reflect LPA1 receptor signaling (Waeber & Chiu, 1999; Laitinen et al., 2001; Laitinen, 2004). Importantly, the two major PLD isoforms, PLD1 and PLD2, which are responsible for free phospholipid production, likewise show enriched localization in the brain white matter areas (Colley et al., 1997; Saito et al., 2000). Especially, the PLD1-enriched brain regions, like corpus callosum, were strongly labeled with c48/80, whereas in the striatum, in which PLD expression is lower, no detectable signaling was observed albeit [35S]GTPγS binding to striatal white matter was induced by exogenous LPA (Figures 1 and 2). As evident from Figure 2, basal [35S]GTPγS binding was approximately 60% lower, and both c48/80- and LPA-stimulated binding responses were approximately 30% lower in the adult than in the developing rat brain. Such a decrease in LPA-evoked [35S]GTPγS binding responses between 4- and 9-week-old rat brain is in concordance with previous findings demonstrating the temporal decline in the expression of the LPA1 receptors in the white matter (Colley et al., 1997; Cervera et al., 2002). Thus, the equal reduction in c48/80- and LPA-induced [35S]GTPγS binding responses can be explained by the temporal decline in LPA1 receptor densities between 4- and 9-week-old rat brain. On the other hand, greater temporal decrease in basal G protein activity in the white matter may reflect the temporal alteration in the same, but in basal conditions only partially activated endogenous LPA1 receptor agonist-producing machinery. Indeed, the PLD, which in this point represents an obvious candidate as a mediator of c48/80-evoked [35S]GTPγS binding, shows diminishing expression during the development of the postnatal brain white matter (Saito et al., 2000).
The LPA receptor antagonist Ki16425 blocks both c48/80- and LPA-evoked [35S]GTPγS binding responses in rat brain sections
Ki16425 is a recently characterized compound possessing competitive antagonist properties toward the LPA receptor subtypes LPA1 and LPA3 and has been tested in various settings, including classical [35S]GTPγS membrane binding assays (Ohta et al., 2003). When applied to [35S]GTPγS autoradiography, Ki16425 dose-dependently diminished the [35S]GTPγS binding responses in the white matter (corpus callosum selected to represent the white matter tracts in quantification) elicited by c48/80 (100 μg ml−1) or LPA (5 μM). The IC50 values were 59±13 and 87±19 nM, respectively (Figure 4). Similar to 1-butanol (Figure 2), Ki16425 also suppressed the basal G protein activity in the white matter tracts (IC50 35±9 nM). Based on the selectivity of Ki16425 toward LPA1 or LPA3 receptors and the enriched localization of LPA1 receptor in the white matter, these results strongly suggest that both c48/80- and LPA-stimulated [35S]GTPγS binding responses in brain cryostat sections are mediated by LPA1 receptors.
Furthermore, the above results indicate that under the basal conditions of [35S]GTPγS autoradiography, tonic 1-butanol-sensitive PLD activity generates endogenous free phospholipids in sufficient amounts to activate G protein-coupled LPA receptors in the white matter tracts.
c48/80 exhibits 5-HT1A receptor-activating properties in alkaline pH conditions
A detailed mapping of c48/80-evoked binding responses revealed that in addition to the above-described LPA mimicking effect, the basic secretagogue also modestly stimulated the [35S]GTPγS binding to hippocampal structures that anatomically overlapped with the structures shown previously to be labeled by 5-HT1A receptor-selective agonist 8-hydroxy-2-(di-n-propilamino)-tetralin (8-OH-DPAT) (Sim et al., 1997). However, this effect was clearly detectable only when the third step of the [35S]GTPγS autoradiography protocol was conducted in slightly alkaline pH (8.40) conditions (Figure 5) and thereby may reflect the elevated proportion of non-ionized form of the basic compound at pH 8.40 as compared to the routinely used pH of 7.40. Although alkaline pH diminished basal [35S]GTPγS binding and eliminated both c48/80- and LPA-evoked responses in the white matter (Figure 5a, data not shown), it still allowed the detection of [35S]GTPγS binding responses elicited by 8-OH-DPAT and c48/80 in the hippocampus. Finally, both c48/80- and 8-OH-DPAT-induced [35S]GTPγS binding responses were reversed in the presence of the 5-HT1A receptor-selective antagonist 1-(2-methoxyphenyl)-4-(4-[2-phthalimido]butyl) piperazine hydrobromide (NAN-190) (Figure 5), further confirming the participation of 5-HT1A receptor in the c48/80-elicited [35S]GTPγS binding to the hippocampus.
Figure 5.
In alkaline conditions, c48/80 activates rat brain heterotrimeric G proteins through the 5-HT1A receptors. (a) [35S]GTPγS autoradiography was conducted using a three-step protocol with DPCPX (1 μM) present throughout steps 2 and 3, as detailed in the Methods section except that buffer pH was 8.40. At 100 μg ml−1, c48/80 stimulates [35S]GTPγS binding to the hippocampal structures (hip), identical to those labeled by the selective 5-HT1A receptor agonist 8-OH-DPAT (1 μM). Both c48/80- and 8-OH-DPAT-evoked responses are reversed by the selective 5-HT1A receptor antagonist NAN-190 (1 μM). Scale bar=5 mm. (b) Quantitative autoradiography data on the hippocampal region. Autoradiography images were digitized and bound radioactivity values were obtained from the digitized images, as detailed in the Methods section. Values are mean+s.e.m. representing sections from four individual animals. Risk level: *P<0.05 as compared to the basal and #P<0.05 as compared to the 8-OH-DPAT- or c48/80-induced response in the absence of NAN-190 (one-way ANOVA with Tukey's multiple comparison).
c48/80 has no apparent effect on the rate of specific [35S]GTPγS binding to rat forebrain membranes
As described above, c48/80 failed to activate the overall brain Gi/o protein pool in [35S]GTPγS autoradiographic studies, but rather indirectly stimulated [35S]GTPγS binding to the white matter through the stimulation of the PLD–LPA1 receptor axis. Moreover, the other direct G protein-activating agents tested, MP and MP-7, failed to stimulate [35S]GTPγS binding to brain sections. To rule out the possibility that the results with the basic secretagogues were merely characteristic to the [35S]GTPγS autoradiography approach, we tested the direct G protein-activating properties of these compounds also using the classic, filtration-based [35S]GTPγS membrane binding assay. Extent of Nsb was assessed for all compounds in the presence of 10 μM of non-labeled GTPγS. Consistent with the autoradiography studies, c48/80 (100 μg ml−1) from Sigma-Aldrich, but not from MP Biomedicals or Biomol (data not shown), elicited a significant amount of non-specific [35S]GTPγS labeling (Figure 6a). These data suggest that the non-specific effect seen with the Sigma's product could be due to an impurity, or more probably due to different proportions of distinct polymers and the increase in [35S]GTPγS binding might therefore result from non-specific interaction, for example, complex formation between the guanine nucleotides and some fraction of Sigma's c48/80. These findings along with the results obtained from the autoradiographic studies strongly suggest that G protein activation assays based on [35S]GTPγS binding techniques are unsuitable for studying the G protein-activating properties of Sigma's c48/80. Thus, again we used MP Biomedicals' product in further studies to clarify whether c48/80 could increase the rate of [35S]GTPγS binding to rat forebrain membranes. Furthermore, because the non-specific effect could be potentially present for the MP Biomedicals' product as well, we started all incubations by adding c48/80 or other drugs in order to minimize possible interactions with the guanine nucleotides during the preparative steps of the incubations. As shown in Figure 6b, c48/80 from MP Biomedicals failed to stimulate [35S]GTPγS binding at any of the time points tested, that is c48/80 did not affect the basal rate of [35S]GTPγS binding to membranes. In contrast, MP-7 (50 μM) enhanced the rate of [35S]GTPγS binding, resulting in approximately 40% increase in bound [35S]GTPγS during the 90 min incubation. The classic receptor-mediated G protein activation, produced by the muscarinic acetylcholine receptor agonist carbachol (100 μM), resulted in clear time-dependent [35S]GTPγS binding response up to 70% over basal (Figure 6b). For comparison, LPA (10 μM) stimulated [35S]GTPγS binding approximately 15% over basal during the 90 min incubation (data not shown).
Figure 6.
c48/80, unlike MP-7 or stimulation of G protein-coupled muscarinic acetylcholine receptors, fails to enhance the rate of [35S]GTPγS binding to rat forebrain membranes. Certain commercial batches of c48/80 were also found unsuitable for [35S]GTPγS-based G protein activation assays. (a) Classical [35S]GTPγS membrane binding assay was conducted, as detailed in the Methods section. Rat forebrain membranes were incubated for 90 min together with 100 μg ml−1 c48/80 from MP Biomedicals or Sigma-Aldrich in the presence of 0.15 nM [35S]GTPγS with or without 10 μM excess of non-labeled GTPγS. The data represent the percentage of bound radioactivity+s.e.m. from three independent experiments performed in duplicate. A batch of c48/80 from Sigma-Aldrich, but not from MP Biomedicals, causes a significant amount of [35S]GTPγS labeling, even when excess of non-labeled GTPγS is present, probably indicating, for example, chemical interaction between the guanine nucleotides and some portion of the c48/80 mixture. *P<0.05 compared to basal bound radioactivity at 90 min and #P<0.05 compared to Nsb in basal conditions. (b) Rat forebrain membranes were incubated in the presence of c48/80 (100 μg ml−1; MP Biomedicals), MP-7 (50 μM) or carbachol (100 μM) for the indicated times. Data are represented as percentage of bound radioactivity±s.e.m. from three independent experiments performed in duplicate. At all time points tested, MP-7 and carbachol, but not c48/80, significantly stimulated [35S]GTPγS labeling. Risk level: P<0.05 compared to basal condition in each time point.
Discussion
The results reported here provide the evidence that in rat brain sections, the putative G protein activator c48/80 activates Gi/o proteins indirectly through the stimulation of 1-butanol-sensitive PLD, which yields bioactive phospholipids or their precursors (LPA and/or PA) leading to LPA1 receptor-dependent G protein activation in rat brain white matter. In alkaline conditions, c48/80 was also found to indirectly stimulate [35S]GTPγS binding through the activation of the 5-HT1A receptors. In contrast, we found no evidence supporting the receptor-bypassing G protein-activating properties of c48/80. Instead, some batches (Sigma-Aldrich) of c48/80 exhibited characteristics that may lead to remarkable amounts of non-specific binding in [35S]GTPγS-based G protein activation assays. This non-specific effect might be due to different proportions of distinct polymers present in commercial batches and subsequent complex formation between the polymers and the radioligand. This severely restricts the feasibility of [35S]GTPγS binding methods in this particular case and may easily lead to erroneous conclusions concerning the actual pharmacological properties of c48/80. However, c48/80 from MP Biomedicals and Biomol were usable for the G protein activation assays, as these batches represented no significant amounts of non-specific binding.
In this study, we provided compelling evidence that c48/80 stimulates PLD activity in rat brain white matter. PLD has an emerging role in lipid-based cell signaling, as its primary product PA may itself act as a messenger and further serve as a precursor in the biosynthesis of several other signaling molecules (Aoki, 2004). Currently, two different isoforms of the enzyme are known, PLD1, which mainly localizes to the cytosol and its organelles, and PLD2, which appears to reside within the plasma membrane (Colley et al., 1997; Saito et al., 2000; Du et al., 2004). Both isoforms also exhibit a temporal expression pattern in the developing rat brain white matter areas (Saito et al., 2000). A link between the basic secretagogues and PLD has already been established, as Chahdi et al. (2000) demonstrated the ability of c48/80 to activate the enzyme in a 1-butanol-sensitive manner and probably through the G protein Gβγ subunits. Likewise, MP and its analogs have been shown to possess PLD-activating properties (Lee et al., 1998), especially toward the PLD2 isoform in cell membranes (Chahdi et al., 2003). Interestringly, LPA was recently identified as a histamine releaser from mast cells (Hashimoto et al., 2005), suggesting that the histamine-releasing action of c48/80 might be mediated through PLD activation and subsequent formation of endogenous species of PA and LPA.
LPA is also known to control several physiological events in the CNS, including astroglial cell proliferation and neuronal pathfinding, and is therefore needed for normal brain development (Anliker & Chun, 2004). The enzymatic pathway from PLD leading to PA or LPA formation and subsequent LPA receptor activation may therefore represent a relevant pathway for the production of free phospholipids controlling white matter development in juvenile animals. This concept is further supported by the highly temporal regulation of both LPA receptors and PLD enzymes in these brain regions of the growing animals (Allard et al., 1998; Weiner et al., 1998; Saito et al., 2000; Cervera et al., 2002; Anliker & Chun, 2004). Moreover, primary alcohols, like ethanol and 1-butanol, disrupt the astroglial cell proliferation and suppress PA formation probably through inhibition of the PLD pathway (Kotter & Klein, 1999). Consequently, it is possible that c48/80-stimulated and 1-butanol-sensitive PLD activity along with subsequent LPA receptor-mediated G protein activation, as demonstrated in this study, reflects that particular signaling pathway. However, freezing and thawing of the brain sections during the preparative steps of autoradiographic experiments effectively breaks the plasma membranes and related boundaries; thus, the observed results do not necessarily reflect a physiological event. For example, PLD1 has been associated with subcellular locations specialized for neurotransmitter release, such as growth cones in which the PLD1 product PA may physically alter membrane rigidity, promoting fusion of the synaptic vesicles (Humeau et al., 2001). Such PLD activity might reside within close proximity to the white matter-enriched LPA receptors, which in autoradiographic conditions could explain the c48/80-evoked [35S]GTPγS binding responses. Moreover, it is unlikely that lipophilic substances like PA or LPA could travel far from their site of biosynthesis in the brain sections, which further suggests that the bioactive molecule in c48/80-stimulated conditions is formed in close proximity of the LPA receptors.
After cleavage of PA from membrane phospholipids by PLD, the PLA1 or PLA2 activity has to release one of the acyl groups in order to yield an LPA molecule. Therefore, it might be possible that c48/80 induces its effects via increasing PLA activity, as Bronner et al. (1987) have proposed, thus leading to enhanced conversion of PA to LPA in brain sections. Nevertheless, in conditions where 1-butanol (2%) was included to rule out PLD participation, the synthetic dioleoyl-PA partly restored basal [35S]GTPγS binding to the white matter, which could not however be further stimulated with c48/80 (Supplementary figure), suggesting that PLAs are not the main targets for c48/80 effects. It should be noted that commercial dioleoyl-PA might contain residual LPA, making interpretation of the data even more difficult (Jalink et al., 1990). Along with the results obtained using inhibitors of calcium-independent cytosolic and secretatory PLA2, this notion suggests the existence of endogenous bioactive PA species that could act as LPA receptor agonists. Alternatively, LPA could be produced via additional, yet to be characterized biosynthetic pathways.
The abilty to activate PLD might be a common property among the cationic amphiphilic drugs like c48/80 and certain β-adrenoceptor-blocking agents (Bobeszko et al., 2002). For example, the classic β-blocker propranolol has been shown to activate PLD1 (Kiss, 1994; Chai et al., 2001). In line with these results, we noticed that millimolar concentrations of propranolol induce c48/80-mimicking, although weaker, yet fully 1-butanol-sensitive responses in the rat white matter tracts (V.A.B. Palomäki & J.T. Laitinen, unpublished observations).
Stimulation of [35S]GTPγS binding to hippocampal region by c48/80 probably reflects direct and selective agonism at the Gi/o-coupled 5-HT1A receptor by one or more polymers in the mixture. However, a clear and reproducible response was evident only at pH 8.40. Alkaline pH, which attenuated much of basal binding, did not alter [35S]GTPγS binding response elicited by the selective 5-HT1A receptor agonist 8-OH-DPAT, indicating that receptor–G protein coupling was unaffected. Instead, pH increase results in a higher proportion of non-ionized form of basic agents like c48/80, suggesting that 5-HT1A receptor might selectively favor the non-charged proportion of polymers present in c48/80.
In [35S]GTPγS autoradiography, a certain amount of heterogeneous [35S]GTPγS binding is usually observed even without adding stimulating agents, that is, under the basal conditions. The majority of this is due to brain sections' ability to generate adenosine during the incubations, which subsequently promotes widespread [35S]GTPγS binding through the Gi/o-coupled adenosine A1 receptors (Laitinen, 1999). This, however, can be effectively eliminated using A1 receptor-selective antagonist (DPCPX) or adenosine deaminase enzyme. The former approach was utilized in this study. The present results reveal another source of basal [35S]GTPγS signal in rat brain sections, namely tonic LPA receptor activity in the white matter areas, which appears to be due to endogenous PLD activity and subsequent PA and/or LPA formation. Noticeably, a prominent basal signal in the white matter is evident in several recent [35S]GTPγS autoradiography publications (Newman-Tancredi et al., 2001; Millan et al., 2003). As clearly demonstrated here, tonic LPA receptor-dependent basal G protein activity in the white matter can be efficiently eliminated utilizing 1-butanol (2% vol vol−1) or more subtly using submicromolar concentrations of the LPA receptor antagonist Ki16425. Such treatments are expected to increase the signal-to-noise ratio in [35S]GTPγS autoradiography and therefore further optimize the method. Elimination of tonic LPA receptor signaling should facilitate the detection of G protein activity mediated by other Gi/o-coupled receptors, especially in the white matter regions.
In conclusion, this report describes that the basic secretagogue c48/80 selectively stimulates PLD activity in the white matter of rat brain, leading to LPA receptor-dependent, likely LPA1-dependent, G protein activation in these regions. Moreover, in alkaline pH, c48/80 was found to selectively activate 5HT1A receptors in brain sections. In contrast, no evidence was found to support the proposed receptor-bypassing direct G protein-activating properties of c48/80 when studied using the [35S]GTPγS autoradiographic or membrane binding approach. Instead, we demonstrated that the Sigma-Aldrich batches of c48/80, which have been used in many previous studies, may non-specifically interact with the guanine nucleotides and therefore lead to erroneous conclusions regarding the actual G protein-activating properties of c48/80.
External data objects
Acknowledgments
The present results partly formed the experimental section of Ville Palomäki's graduate thesis for an M.Sc. degree in Pharmacy at the University of Kuopio. We thank Mrs Kati Mönkkönen for supervising the thesis and Dr Juha Savinainen for theoretical and practical advice. Mrs Tomoko Shimodaira participated in this study as an IFMSA exchange student and we acknowledge her contribution to the dose–response studies with Ki16425.
Abbreviations
- BSA
bovine serum albumin (essential fatty acid free)
- c48/80
compound 48/80
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- DTT
dithiothreitol
- GPCRs
G protein-coupled receptors
- GTPγS
guanosine-5′-(γ-thio)trisphosphate
- [35S]GTPγS
[35S]guanosine-5′-[γ-thio]triphosphate
- IgE
immunoglobulin E
- Ki16425
3-(4-[4-([1-(2-chlorophenyl)ethoxy]carbonyl amino)-3-methyl-5-isoxazolyl] benzylsulfanyl) propanoic acid
- LPA
lysophosphatidic acid
- MP
mastoparan
- MP-7
mastoparan-7
- NAN-190
1-(2-methoxyphenyl)-4-(4-[2-phthalimido]butyl) piperazine hydrobromide
- NEM
N-ethylmaleimide
- Nsb
non-specific binding
- 8-OH-DPAT
8-hydroxy-2-(di-n-propilamino)-tetralin
- PA
phosphatidic acid
- PLA
phospholipase A
- PLD
phospholipase D
- Ptx
pertussis toxin
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- ALLARD J., BARRON S., DIAZ J., LUBETZKI C., ZALC B., SCHWARTZ J.C., SOKOLOFF P. A rat G protein-coupled receptor selectively expressed in myelin-forming cells. Eur. J. Neurosci. 1998;10:1045–1053. doi: 10.1046/j.1460-9568.1998.00117.x. [DOI] [PubMed] [Google Scholar]
- ANLIKER B., CHUN J. Lysophospholipid G protein-coupled receptors. J. Biol. Chem. 2004;279:20555–20558. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- AOKI J. Mechanisms of lysophosphatidic acid production. Semin. Cell Dev. Biol. 2004;15:477–489. doi: 10.1016/j.semcdb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- BILLAH M.M. Phospholipase D and cell signaling. Curr. Opin. Immunol. 1993;5:114–123. doi: 10.1016/0952-7915(93)90090-f. [DOI] [PubMed] [Google Scholar]
- BOBESZKO M., CZAJKOWSKI R., WOJCIK M., SABALA P., LEI L., NALEPA I. Modulation by cationic amphiphilic drugs of serine base-exchange, phospholipase D and intracellular calcium homeostasis in glioma C6 cells. Pol. J. Pharmacol. 2002;54:483–493. [PubMed] [Google Scholar]
- BRONNER C., WIGGINS C., MONTE D., MARKI F., CAPRON A., LANDRY Y., FRANSON R.C. Compound 48/80 is a potent inhibitor of phospholipase C and a dual modulator of phospholipase A2 from human platelet. Biochim. Biophys. Acta. 1987;920:301–305. doi: 10.1016/0005-2760(87)90108-1. [DOI] [PubMed] [Google Scholar]
- CERVERA P., TIRARD M., BARRON S., ALLARD J., TROTTIER S., LACOMBE J., DAUMAS-DUPORT C., SOKOLOFF P. Immunohistological localization of the myelinating cell-specific receptor LP(A1) Glia. 2002;38:126–136. doi: 10.1002/glia.10054. [DOI] [PubMed] [Google Scholar]
- CHAHDI A., CHOI W.S., KIM Y.M., BEAVEN M.A. Mastoparan selectively activates phospholipase D2 in cell membranes. J. Biol. Chem. 2003;278:12039–12045. doi: 10.1074/jbc.M212084200. [DOI] [PubMed] [Google Scholar]
- CHAHDI A., FRAUNDORFER P.F., BEAVEN M.A. Compound 48/80 activates mast cell phospholipase D via heterotrimeric GTP-binding proteins. J. Pharmacol. Exp. Ther. 2000;292:122–130. [PubMed] [Google Scholar]
- CHAI M.Q., CHEN J.S., ZHAO S., SONG J.G. Propranolol increases phosphatidic acid level via activation of phospholipase D. Acta Pharmacol. Sin. 2001;22:777–784. [PubMed] [Google Scholar]
- COLLEY W.C., ALTSHULLER Y.M., SUE-LING C.K., COPELAND N.G., GILBERT D.J., JENKINS N.A., BRANCH K.D., TSIRKA S.E., BOLLAG R.J., FROHMAN M.A. Cloning and expression analysis of murine phospholipase D1. Biochem. J. 1997;326:745–753. doi: 10.1042/bj3260745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DU G., HUANG P., LIANG B.T., FROHMAN M.A. Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol. Biol. Cell. 2004;15:1024–1030. doi: 10.1091/mbc.E03-09-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON K.M., HIGASHIJIMA T., SMIGEL M.D., GILMAN A.G. The influence of bound GDP on the kinetics of guanine nucleotide binding to G proteins. J. Biol. Chem. 1986;261:7393–7399. [PubMed] [Google Scholar]
- FERRY X., BREHIN S., KAMEL R., LANDRY Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides. 2002;23:1507–1515. doi: 10.1016/s0196-9781(02)00090-6. [DOI] [PubMed] [Google Scholar]
- GILMAN A.G. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO T., OHATA H., MOMOSE K., HONDA K. Lysophosphatidic acid induces histamine release from mast cells and skin fragments. Pharmacology. 2005;75:13–20. doi: 10.1159/000085784. [DOI] [PubMed] [Google Scholar]
- HIGASHIJIMA T., BURNIER J., ROSS E.M. Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. Mechanism and structural determinants of activity. J. Biol. Chem. 1990;265:14176–14186. [PubMed] [Google Scholar]
- HIGASHIJIMA T., UZU S., NAKAJIMA T., ROSS E.M. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins) J. Biol. Chem. 1988;263:6491–6494. [PubMed] [Google Scholar]
- HUMEAU Y., VITALE N., CHASSEROT-GOLAZ S., DUPONT J.L., DU G., FROHMAN M.A., BADER M.F., BOULAIN B. A role for phospholipase D1 in neurotransmitter release. Proc. Natl. Acad. Sci. U.S.A. 2001;98:15300–15305. doi: 10.1073/pnas.261358698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JALINK K., VAN CORVEN E.J., MOOLENAAR W.H. Lysophosphatidic acid, but not phosphatidic acid, is a potent Ca2(+)-mobilizing stimulus for fibroblasts. Evidence for an extracellular site of action. J. Biol. Chem. 1990;265:12232–12239. [PubMed] [Google Scholar]
- KISS Z. Sphingosine-like stimulatory effects of propranolol on phospholipase D activity in NIH 3T3 fibroblasts. Biochem. Pharmacol. 1994;47:1581–1586. doi: 10.1016/0006-2952(94)90535-5. [DOI] [PubMed] [Google Scholar]
- KOTTER K., KLEIN J. Ethanol inhibits astroglial cell proliferation by disruption of phospholipase D-mediated signaling. J. Neurochem. 1999;73:2517–2523. doi: 10.1046/j.1471-4159.1999.0732517.x. [DOI] [PubMed] [Google Scholar]
- LAITINEN J.T. Selective detection of adenosine A1 receptor-dependent G protein activity in basal and stimulated conditions of rat brain [35S]guanosine 5′-(gamma-thio)triphosphate autoradiography. Neuroscience. 1999;90:1265–1279. doi: 10.1016/s0306-4522(98)00571-5. [DOI] [PubMed] [Google Scholar]
- LAITINEN J.T. [35S]GTPγS autoradiography: a powerful functional approach with expanding potential for neuropharmacological studies on receptors coupled to Gi family of G proteins. Curr. Neuropharmacol. 2004;2:191–206. [Google Scholar]
- LAITINEN J.T., JOKINEN M. Guanosine 5′-(gamma-[35S]thio)triphosphate autoradiography allows selective detection of histamine H3 receptor-dependent G protein activation in rat brain tissue sections. J. Neurochem. 1998;71:808–816. doi: 10.1046/j.1471-4159.1998.71020808.x. [DOI] [PubMed] [Google Scholar]
- LAITINEN J.T., URI A., RAIDARU G., MIETTINEN R. [(35)S]GTPgammaS autoradiography reveals a wide distribution of G(i/o)-linked ADP receptors in the nervous system: close similarities with the platelet P2Y(ADP) receptor. J. Neurochem. 2001;77:505–518. doi: 10.1046/j.1471-4159.2001.00265.x. [DOI] [PubMed] [Google Scholar]
- LEE S.Y., PARK N.G., CHOI M.U. Effects of mastoparan B and its analogs on the phospholipase D activity in L1210 cells. FEBS Lett. 1998;432:50–54. doi: 10.1016/s0014-5793(98)00831-x. [DOI] [PubMed] [Google Scholar]
- LORENZEN A., FUSS M., VOGT H., SCHWABE U. Measurement of guanine nucleotide-binding protein activation by A1 adenosine receptor agonists in bovine brain membranes: stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding. Mol. Pharmacol. 1993;44:115–123. [PubMed] [Google Scholar]
- MILLAN M.J., CUSSAC D., MILLIGAN G., CARR C., AUDINOT V., GOBERT A. The novel melatonin agonist agomelatine ( S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J. Pharmacol. Exp. Ther. 2003;306:954–964. doi: 10.1124/jpet.103.051797. [DOI] [PubMed] [Google Scholar]
- MOUSLI M., BRONNER C., BOCKAERT J., ROUOT B., LANDRY Y. Interaction of substance P, compound 48/80 and mastoparan with the alpha-subunit C-terminus of G protein. Immunol. Lett. 1990a;25:355–357. doi: 10.1016/0165-2478(90)90207-7. [DOI] [PubMed] [Google Scholar]
- MOUSLI M., BRONNER C., LANDRY Y., BOCKAERT J., ROUOT B. Direct activation of GTP-binding regulatory proteins (G proteins) by substance P and compound 48/80. FEBS Lett. 1990b;259:260–262. doi: 10.1016/0014-5793(90)80023-c. [DOI] [PubMed] [Google Scholar]
- NEWMAN-TANCREDI A., CUSSAC D., BROCCO M., RIVET J.M., CHAPUT C., TOUZARD M. Dopamine D2 receptor-mediated G protein activation in rat striatum: functional autoradiography and influence of unilateral 6-hydroxydopamine lesions of the substantia nigra. Brain Res. 2001;920:41–54. doi: 10.1016/s0006-8993(01)02927-4. [DOI] [PubMed] [Google Scholar]
- OHTA H., SATO K., MURATA N., DAMIRIN A., MALCHINKHUU E., KON J., KIMURA T., TOBO M., YAMAZAKI Y., WATANABE T., YAGI M., SATO M., SUZUKI R., MUROOKA H., SAKAI T., NISHITOBA T., IM D.S., NOCHI H., TAMOTO K., TOMURA H., OKAJIMA F. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol. Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- SAITO H., OKAJIMA F., MOLSKI T.F., SHA'AFI R.I., UI M., ISHIZAKA T. Effects of ADP-ribosylation of GTP-binding protein by pertussis toxin on immunoglobulin E-dependent and -independent histamine release from mast cells and basophils. J. Immunol. 1987;138:3927–3934. [PubMed] [Google Scholar]
- SAITO S., SAKAGAMI H., KONDO H. Localization of mRNAs for phospholipase D (PLD) type 1 and 2 in the brain of developing and mature rat. Brain Res. Dev. Brain Res. 2000;120:41–47. doi: 10.1016/s0165-3806(99)00189-3. [DOI] [PubMed] [Google Scholar]
- SAVINAINEN J.R., JÄRVINEN T., LAINE K., LAITINEN J.T. Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB(1) receptor-dependent G protein activation in rat cerebellar membranes. Br. J. Pharmacol. 2001;134:664–672. doi: 10.1038/sj.bjp.0704297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVINAINEN J.R., SAARIO S.M., NIEMI R., JÄRVINEN T., LAITINEN J.T. An optimized approach to study endocannabinoid signaling: evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors. Br. J. Pharmacol. 2003;140:1451–1459. doi: 10.1038/sj.bjp.0705577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIM L.J., XIAO R., CHILDERS S.R. In vitro autoradiographic localization of 5-HT1A receptor-activated G proteins in the rat brain. Brain Res. Bull. 1997;44:39–45. doi: 10.1016/s0361-9230(97)00061-0. [DOI] [PubMed] [Google Scholar]
- SOVAGO J., DUPUIS D.S., GULYAS B., HALL H. An overview on functional receptor autoradiography using [35S]GTPgammaS. Brain Res. Rev. 2001;38:149–164. doi: 10.1016/s0165-0173(01)00106-0. [DOI] [PubMed] [Google Scholar]
- TOMITA U., INANOBE A., KOBAYASHI I., TAKAHASHI K., UI M., KATADA T. Direct interactions of mastoparan and compound 48/80 with GTP-binding proteins. J. Biochem. (Tokyo) 1991;109:184–189. doi: 10.1093/oxfordjournals.jbchem.a123342. [DOI] [PubMed] [Google Scholar]
- WAEBER C., CHIU M.L. In vitro autoradiographic visualization of guanosine-5′-O-(3-[35S]thio)triphosphate binding stimulated by sphingosine 1-phosphate and lysophosphatidic acid. J. Neurochem. 1999;73:1212–1221. doi: 10.1046/j.1471-4159.1999.0731212.x. [DOI] [PubMed] [Google Scholar]
- WEINER J.A., HECHT J.H., CHUN J. Lysophosphatidic acid receptor gene vzg-1/lpA1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. J. Comp. Neurol. 1998;398:587–598. [PubMed] [Google Scholar]
- YU C.H., LIU S.Y., PANAGIA V. The transphosphatidylation activity of phospholipase D. Mol. Cell. Biochem. 1996;157:101–115. doi: 10.1007/BF00227886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.