Abstract
β2-Adrenoceptor agonists provide a potential therapy for muscle wasting and weakness, but their use may be limited by adverse effects on the heart, mediated in part, by β1-adrenoceptor activation.
Two β2-agonists, formoterol and salmeterol, are approved for treating asthma and have an extended duration of action and increased safety, associated with greater β2-adrenoceptor selectivity.
The pharmacological profiles of formoterol and salmeterol and their effects on skeletal and cardiac muscle mass were investigated in 12-week-old, male F344 rats. Formoterol and salmeterol were each administered via daily i.p. injection at one of seven doses (ranging from 1 to 2000 μg kg−1 day−1), for 4 weeks. Rats were anaesthetised and the EDL and soleus muscles and the heart were excised and weighed. Dose–response curves were constructed based on skeletal and cardiac muscle hypertrophy.
Formoterol was more potent than salmeterol, with a significantly lower ED50 in EDL muscles (1 and 130 μg kg−1 day−1, P <0.05), whereas salmeterol had greater intrinsic activity than formoterol in both EDL and soleus muscles (12% greater hypertrophy than formoterol). The drugs had similar potency and intrinsic activity in the heart, with a smaller leftward shift for formoterol than seen in skeletal muscle. A dose of 25 μg kg−1 day−1 of formoterol elicited greater EDL and soleus hypertrophy than salmeterol, but resulted in similar β-adrenoceptor downregulation.
These results show that doses as low as 1 μg kg−1 day−1 of formoterol can elicit significant muscle hypertrophy with minimal cardiac hypertrophy and provide important information regarding the potential therapeutic use of formoterol and salmeterol for muscle wasting.
Keywords: Adrenergic, heart, cardiac hypertrophy, receptors
Introduction
A severe loss of muscle mass and strength is associated with numerous conditions and disease states, including: dystrophy, cancer cachexia, chemotherapy, sepsis, acquired immune deficiency syndrome, burn injury, and sarcopenia (Barton & Morris, 2003; Jackman & Kandarian, 2004). The continual loss of muscle protein can lead to an increase in morbidity, and in extreme cases, an increased mortality rate. Thus, therapies that aim to alleviate the symptoms of muscle wasting are directed towards preserving existing muscle fibres, enhancing muscle fibre regeneration, and promoting muscle fibre growth. Agents that stimulate an increase in muscle size (hypertrophy), by either increasing protein synthesis or decreasing protein degradation, or both, have the potential to be applied clinically to combat muscle wasting conditions (Lynch, 2004).
Synthetic β2-adrenoceptor agonists (β2-agonists) were initially developed to facilitate bronchodilation to relieve asthma (Solis-Cohen, 1900). However, it became apparent that at doses higher than used therapeutically, β2-agonists were capable of eliciting significant skeletal muscle hypertrophy (Emery et al., 1984). Since then, β2-agonists such as clenbuterol and fenoterol have been examined in numerous animal models of muscle wasting (Maltin et al., 1987; Chen & Alway, 2000; Sneddon et al., 2000; Zeman et al., 2000; Beitzel et al., 2004; Ryall et al., 2004). Some agonists, such as albuterol, have been used in clinical trials for the treatment of neuromuscular disorders (Martineau et al., 1992; Kissel et al., 1998; 2001; Fowler et al., 2004). The first clinical trials using albuterol to treat young boys with facioscapulohumeral dystrophy, found that year-long administration at doses of 16 and 32 mg day−1 had only limited beneficial effects on strength, and was associated with some adverse cardiac-related events (Kissel et al., 1998). In a more recent study, Fowler et al. (2004) administered a lower dose of 8 mg day−1 of albuterol for 28 weeks to boys with Duchenne and Becker muscular dystrophy, and found modest increases in strength with no reported side effects. These results suggested that low doses of β2-agonists were well tolerated, but elicited only a modest improvement in skeletal muscle mass and strength.
In the past 15–20 years, much research on asthma has focused on extending the bronchodilating actions of β2-agonists, while maintaining or improving their safety profile (Löfdahl and Svedmyr, 1989; Ball et al., 1991; Guhan et al., 2000). To this end, two β2-agonists, formoterol fumarate and salmeterol xinafoate, have been recently approved in the U.S. for the treatment of asthma. These β2-agonists have an extended duration of action in relaxing smooth muscle compared with traditional asthma medications, such as albuterol and terbutaline (Löfdahl & Svedmyr, 1989; Roux et al., 1996; Waldeck, 1996). In addition to an extended duration of action, the safety profiles of formoterol and salmeterol, defined as the frequency of adverse events, have been improved significantly, because of an increased selectivity for the β2-adrenoceptor (Guhan et al., 2000; Pearlman et al., 2002). Since the skeletal muscle adrenoceptor population consists predominantly of β2-adrenoceptors, and cardiac muscle consists primarily of β1-adrenoceptors (Ryall et al., 2002; 2004; Gregorevic et al., 2005), a more selective β2-agonist is likely to lessen any adverse effects on cardiac performance. While cardiac muscle consists primarily of β1-adrenoceptors, there is a significant proportion of β2-adrenoceptors, generally in a ratio of 2 : 1 (Brodde, 1991; Ryall et al., 2002; Gregorevic et al., 2005). The cardiac β2-adrenoceptor is known to have dual coupling properties (Xiao et al., 1999; Kilts et al., 2000), whereby stimulation activates not only the traditional adrenoceptor–Gs–cAMP–PKA pathway, but also a Gi-mediated signal, believed to be involved in cell survival (Foerster et al., 2003; Pönicke et al., 2003; Bernstein et al., 2005). Formoterol is reported to have a β2 : β1 adrenoceptor selectivity ratio of between 200 and 400, while salmeterol has a selectivity ratio of >10,000 (Anderson, 1993; Johnson et al., 1993).
The exact mechanism for the extended duration of action of both formoterol and salmeterol is still unclear, although two hypotheses have been proposed. The first, and most popular explanation, is known as the ‘diffusion microkinetic hypothesis', and it relates to the greater lipophilic nature of formoterol and salmeterol (compared to other β-agonists, such as fenoterol and clenbuterol; Waldeck, 1996), a property conferred by a long carbon side-chain (Anderson, 1993). The second explanation relates to the presence of a secondary binding site, or ‘exo-site' (Johnson et al., 1993).
It has been reported previously that both formoterol and salmeterol are capable of producing significant muscle hypertrophy (Moore et al., 1994; Busquets et al., 2004) at high (milligram) doses, but the potency and maximal hypertrophic effect of these drugs on skeletal and cardiac muscle has yet to be determined fully. We hypothesised that due to their extended duration of action, a relatively low dose of formoterol or salmeterol administered to rats would elicit skeletal muscle hypertrophy, whereas their high β2- : β1-adrenoceptor selectivity ratio would minimise cardiac hypertrophy. In addition, we examined the β-adrenoceptor downregulation associated with 4 weeks of β-agonist administration to rats at a single therapeutic dose of 25 μg kg−1 day−1.
Methods
All experiments were approved by the Animal Experimentation Ethics Committee of The University of Melbourne, and were conducted in accordance with the guidelines for the care and use of experimental animals as outlined by the National Health and Medical Research Council of Australia. Rats were housed in a pathogen-free environment in standard cages with free access to drinking water and food. Rats were housed under an artificial light : dark cycle with light between 0600 and 1800 h.
β2-Agonist administration and tissue collection
Male 3-month-old Fischer 344 rats (F344, n=128 rats, body mass (BM) ∼270 g) obtained from the Animal Resource Centre (Canning Vale, Western Australia) were allocated into either control or one of two β2-agonist-treated groups. Treated rats (n=112, 8 rats dose−1) received 1,10, 25, 250, 500, 1000, or 2000 μg kg−1 of formoterol in saline, while salmeterol was administered in a 1 : 10 cremphor : saline vehicle due to its highly lipophilic nature. β2-Agonist treatment consisted of once daily intraperitoneal (i.p.) injections for a period of 4 weeks. Control rats received an identical volume of saline only (n=8), or a cremphor : saline mix (for salmeterol control, n=8). All animals were weighed daily throughout the study.
Following 4 weeks of treatment, rats were anaesthetised with sodium pentobarbitone (Nembutal, Rhone Merieux, Pinkenba, QLD, Australia: 60 mg kg−1, i.p.), with supplemental doses administered to maintain an adequate depth of anaesthesia, such that there was no response to tactile stimulation. The EDL (fast-twitch) and the soleus (slow-twitch) muscles were surgically excised from both hindlimbs, blotted on filter paper, trimmed of their tendons, and weighed on an analytical balance. The rats were killed by opening the thoracic cavity and immediate cardiac excision. The heart was blotted on filter paper, trimmed of large vessels, and weighed. All tissues were frozen immediately in thawing isopentane and then stored at −80°C for later biochemical analyses.
Muscle protein concentration
To determine the effect of daily injection of cremphor vehicle, skeletal muscle protein concentration was determined in saline control- and cremphor vehicle-treated animals using a Bradford protein assay (Bio-Rad, Hercules, CA, U.S.A.), with bovine serum albumin standards. Protein assays were completed in triplicate on 96-well microplates (Nalgene Nunc International, Rochester, NY, U.S.A.) and read on a Multiskan Spectrum microplate spectrophotometer, running Multiskan Spectrum software (V1.00, Thermo Electron Corporation, Milford, MA, U.S.A.).
Pharmacological characterisation of β-agonist-induced hypertrophy
Tissue mass (as a % of control) was plotted against the negative logarithm of dose (as mol kg−1 day−1, due to differences in molecular weights (MW) of formoterol fumarate, 841, and salmeterol xinafoate, 604). Nonlinear regression analysis was performed using GraphPad Prism v. 4.02 for Windows (GraphPad Software, San Diego, CA, U.S.A.) using the following sigmoidal dose–response (variable slope) relationship
where Ybot is the value at the bottom of the plateau, Ytop is the value at the top of the lateau, and X and Y are the dose–response variables.
β-Adrenoceptor radioligand-binding assay
A working dose of 25 μg kg−1 day−1 was chosen for an examination of agonist-induced β-adrenoceptor downregulation. This was the lowest dose at which both formoterol and salmeterol elicited skeletal muscle hypertrophy.
Both EDL and soleus muscle cell membrane samples were assayed for total β-adrenoceptor density using methods described in detail previously (Sillence et al., 1991; Beitzel et al., 2004; Ryall et al., 2004). Briefly, frozen EDL and soleus muscles were placed in 2 ml of ice-cold buffer A (mm: Tris (pH 7.0) 50, sucrose 250, EGTA 1; pH 7.4 at 4°C) and homogenised (Polytron PT 2100, Kinematica AG, Luzernerstrasse, Switzerland) separately for 30 s. Cell membrane fragments were prepared by centrifugation at 1,000, 10,000 and 100,000 × g at 4°C, with wash stages (in buffer A) between each centrifugation, to obtain the cell membrane fraction for analysis, as described previously (Sillence et al., 1991).
Single-point saturation assays were performed by incubating 400 μl cell membrane suspension with 50 μl [125I]iodocyanopindolol (135 pM; ICYP, the radioligand), and 50 μl of either buffer (to determine the total counts of ICYP bound to β2-adrenoceptors, in mM: 50 Tris (pH 7.7), 10 MgCl2, 150 NaCl; pH 7.4 at 37°C) or DL-propranolol (2 μM; a nonselective β-adrenoceptor antagonist that determines nonspecific binding of ICYP to the membrane) in polyethylene tubes (12 × 75 mm). Assays were initiated with the addition of cell membranes, and tubes were incubated for 90 min in a shaking water bath set at 37°C (130 cycles min−1). Separation of bound ligand from free ligand was achieved by filtering the contents of each tube through Whatman GF-C glass fibre filter papers (Whatman GF-C filter paper, Maidstone, U.K.) with 21 ml of ice-cold buffer, using a cell harvester (Brandel M-48R cell harvester, Biomedical Research and Development Labs, Gaithersburg, MD, U.S.A.). Radioactivity remaining on the filters was determined in a gamma counter (1470 Wizard-automatic gamma counter, Wallac OY, Turku, Finland) at a counting efficiency of 78%. Results were obtained as γ-radiation counts per minute for all tubes and then converted into concentration of β-adrenoceptor per milligram of protein (Sillence et al., 1991; Ryall et al., 2002; 2004; Beitzel et al., 2004). Previous experiments have shown that rat muscle contains a predominant population of β2-adrenoceptors, with β1-adrenoceptors usually undetectable by this technique (Sillence et al., 1991). Hence the β-adrenoceptors measured were designated β2-adrenoceptors.
β2-Adrenoceptor agonists
Formoterol fumarate dihydrate (2-hydroxy-5-[(1RS)-1-hydroxy-2-][[(1RS)-2-(p-methoxyphenyl)-1-methylethylamino]ethyl]formanilide fumarate dihydrate and salmeterol xinafoate (4-hydroxy-α[[[6-[4-phenylbutyl)oxy]hexyl]amino]methyl]1,3-benzenedimethanol) were kindly supplied by Astra-Zeneca (Molndal, Sweden).
Statistical analyses
Individual variables were compared between groups using separate one-way ANOVA with Fisher's least significant difference post hoc multiple comparison procedure used to determine significance between groups. Significance was set at P<0.05. All values are expressed as mean±s.e.m. unless specified otherwise.
Results
Body mass
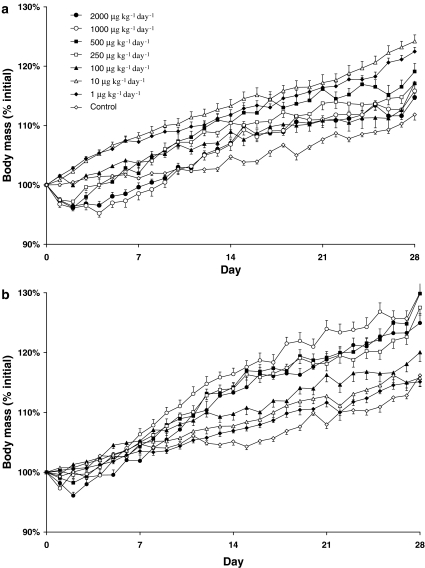
Grouped BM data, following administration of formoterol or salmeterol at different doses, are presented in Figure 1. Both control groups (saline and cremphor) exhibited a significant increase in BM during the 4-week experimental period (saline, 12±0.01%; cremphor, 16±0.01%, P<0.0001), indicating normal growth and development at this age. Interestingly, the cremphor control animals exhibited greater weight gain than saline control animals (P<0.05), and for this reason all further results will be compared relative to the respective controls.
Figure 1.
Increase in BM following 4 weeks of treatment with (a) formoterol, or (b) salmeterol. Note the initial drop of BM in the first 3–4 days associated with the high doses of both formoterol and salmeterol (initial BM: formoterol, 267±2 g; salmeterol, 268±2 g).
High doses of formoterol (2000, 1000, and 500 μg kg−1 day−1) administered for 4 weeks caused a significant decrease in BM from days 2 to 5, compared to saline-treated rats (P<0.05), after which time BM was equivalent to that of saline-treated rats. Following 28 days of treatment, only the highest dose of 2000 μg kg−1 day−1 failed to cause a significant increase in BM above that seen in saline control (response, in decreasing order of dose; 4, 7, 5, 6, 9, and 7%, respectively, P<0.05, Table 1).
Table 1.
Selected morphometric parameters following 4 weeks of daily formoterol administration (n=8 rats dose−1)
| Dose (μg kg−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control (vehicle) | 1 | 10 | 25 | 250 | 500 | 1000 | 2000 | |
| Final BM (g) | 298±4 | 320±6* | 325±5* | 315±5* | 313±3* | 318±3* | 311±4* | 307±3 |
| EDL mass (mg) | 104±1 | 117±2* | 133±2*† | 128±2* | 133±1*† | 141±1*† | 135±2* | 134±1* |
| EDL mass/BM | 0.35±0.01 | 0.42±0.01* | 0.41±0.01* | 0.40±0.01* | 0.43±0.01*† | 0.44±0.01* | 0.44±0.01* | 0.44±0.01* |
| Soleus mass (mg) | 94±1 | 105±2* | 106±1* | 107±2* | 109±2* | 118±2*† | 110±2* | 108±2* |
| Soleus mass/BM | 0.31±0.01 | 0.34±0.01* | 0.32±0.01 | 0.34±0.01* | 0.35±0.01* | 0.37±0.01*† | 0.35±0.01* | 0.35±0.01* |
| Heart mass (mg) | 661±12 | 693±14 | 751±10*† | 748±19* | 807±16*† | 830±17* | 802±9* | 821±9* |
| Heart mass/BM | 2.22±0.03 | 2.16±0.02 | 2.31±0.03*† | 2.36±0.02* | 2.58±0.05*† | 2.61±0.05* | 2.58±0.02* | 2.68±0.03*† |
BM: body mass, EDL: extensor digitorum longus
P<0.05 treated vs respective control
P<0.05 significantly different from previous (lower) dose. Results presented as mean±s.e.m.
Only the highest dose of salmeterol (2000 μg kg−1 day−1) was associated with an initial decrease in BM, which occurred from days 2 to 3, when compared to cremphor-treated rats (P<0.05). The higher doses of salmeterol (250, 500, 1000, and 2000 μg kg−1 day−1) all caused an increase in BM above that of cremphor-treated rats, following 4 weeks administration (25, 30, 30, and 28%, respectively, P<0.05, Table 2). while doses of 1, 10 and 25 μg kg−1 day−1 did not affect BM significantly. Cremphor vehicle-treated rats exhibited a lower protein concentration in both the EDL and soleus muscles (data not shown), likely indicating an increase in fluid retention.
Table 2.
Selected morphometric parameters following 4 weeks of daily salmeterol administration (n=8 rats dose−1)
| Dose (μg kg−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control (vehicle) | 1 | 10 | 25 | 250 | 500 | 1000 | 2000 | |
| Final BM (g) | 312±2 | 309±5 | 309±6 | 322±4 | 341±2* | 346±3* | 339±4* | 329±5* |
| EDL mass (mg) | 109±1 | 107±2 | 112±1† | 119±2*† | 140±2*† | 144±2* | 152±2*† | 152±2* |
| EDL/BM | 0.35±0.01 | 0.36±0.01 | 0.36±0.01 | 0.37±0.01* | 0.41±0.01*† | 0.41±0.01* | 0.45±0.01*† | 0.46±0.01* |
| Soleus mass (mg) | 97±1 | 101±2 | 102±2* | 106±1* | 117±2*† | 124±2*† | 123±2* | 124±2* |
| Soleus/BM | 0.31±0.01 | 0.33±0.01 | 0.33±0.01 | 0.33±0.01* | 0.34±0.01* | 0.36±0.01* | 0.36±0.01* | 0.38±0.01* |
| Heart mass (mg) | 689±6 | 677±10 | 699±19 | 732±12* | 798±13*† | 821±12*† | 863±19*† | 801±19* |
| Heart/BM | 2.21±0.03 | 2.19±0.02 | 2.26±0.05 | 2.28±0.03* | 2.35±0.03* | 2.37±0.02* | 2.55±0.05*† | 2.43±0.03* |
BM: body mass, EDL: extensor digitorum longus
P<0.05 treated vs respective control
P<0.05 significantly different from previous (lower) dose. Results presented as mean±s.e.m.
EDL, soleus, and heart mass
Formoterol treatment for 4 weeks of increased absolute EDL muscle mass above that of saline control rats at all doses investigated (P<0.05), with a maximal increase in mass observed at a dose of 500 μg kg−1 day−1 (36% greater than in saline control rats, P<0.05, Table 1). When corrected for changes in BM, formoterol elicited a maximal response in the EDL at doses of 250 μg kg−1 day−1 and above. Salmeterol treatment caused significant hypertrophy of the EDL muscle compared to cremphor control rats at doses of 25 μg kg−1 day−1 and above, with a maximal increase in absolute EDL muscle mass observed at doses above 1000 μg kg−1 day−1 (39% greater than cremphor control, P<0.05, Table 2). The EDL-to-BM ratio was increased at doses of 25 μg kg−1 day−1 and above, with a maximal response observed at doses of 1000 μg kg−1 day−1 and above (P<0.05).
Formoterol treatment increased absolute soleus muscle mass above that of saline control rats at all doses tested (P<0.05). As for the EDL muscle, a dose of 500 μg kg−1 day−1 of formoterol produced the greatest hypertrophy in the soleus muscle (26% above saline control). The soleus-to-BM ratio was increased at doses of 250 μg kg−1 day−1 and above, with a maximal increase observed at a dose of 500 μg kg−1 day−1. Salmeterol produced hypertrophy of the soleus muscle at doses of 10 μg kg−1 day−1 and above, and elicited a maximum response at doses above 500 μg kg−1 day−1 (28% above that of cremphor vehicle rats, P<0.05). When corrected for BM, salmeterol had a maximal effect at doses of 500 μg kg−1 day−1 and above.
In contrast to skeletal muscle, the lowest dose of formoterol treatment did not cause significant cardiac hypertrophy (Table 1). Doses of 10 μg kg−1 and above were required to elicit cardiac hypertrophy, with a maximal response observed at doses of 250 μg kg−1 day−1 and above (22, 26, 21, and 24% increases above that of control for doses of 250, 500, 1000, and 2000 μg kg−1 day−1, P<0.05). Formoterol induced the largest observed increase in the heart mass-to-BM ratio, which occurred at a dose of 2000 μg kg−1 day−1. Salmeterol administration for 4 weeks produced cardiac hypertrophy at doses of 25 μg kg−1 day−1 and above (P<0.05), with a maximal response occurring at doses of 1000, and 2000 μg kg−1 day−1 (25 and 16%, respectively, compared to that of cremphor vehicle, Table 2). The heart mass-to-BM ratio was increased in rats treated with doses of 25 μg kg−1 day−1 of salmeterol and above, with a maximal increase observed at a dose of 1000 μg kg−1 day−1.
Dose–response profiles of formoterol and salmeterol
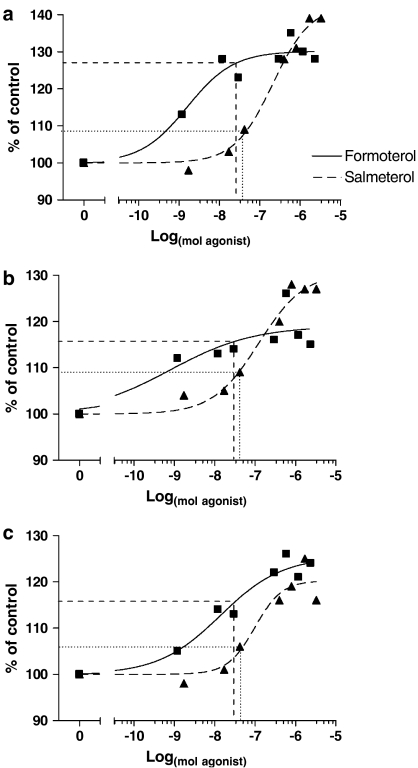
Dose–response curves were plotted for formoterol and salmeterol, based on EDL, soleus and cardiac hypertrophy relative to vehicle control (i.e. % of control, Figure 2). The relative potency (ED50) and intrinsic activity (Emax) values were calculated based on a nonlinear regression of β2-agonist dose and the percentage increase in mass above respective control (Table 3). From these profiles it was determined that formoterol and salmeterol exhibited significantly different curves for changes in EDL and soleus muscle, and heart mass (P<0.05). Emax was 12% greater in the EDL and the soleus muscles for salmeterol compared to formoterol administration (P<0.05). When ED50 of each drug was calculated, formoterol was more potent than salmeterol in the EDL muscle (P<0.05, Table 2), and exhibited a similar trend (not significant) in the soleus muscle.
Figure 2.
Dose–response graphs for (a) EDL, (b) soleus, and (c) heart following 4 weeks of treatment with either formoterol or salmeterol. Results are presented as percentage increase above control. Formoterol and salmeterol had different dose–response profiles (as determined by nonlinear regression) in both fast and slow-twitch skeletal muscle, as well as in cardiac muscle. Lines indicate response to a dose of 25 μg kg−1 day−1 of formoterol (dashed), or salmeterol (dotted). Note: The log-scale on the abscissa is expressed in mol due to different MW of formoterol fumarate (MW=841) and salmeterol xinafoate (MW=604).
Table 3.
Selected pharmacologic parameters following 4 weeks of daily formoterol or salmeterol administration (n=8 rats dose−1)
| Formoterol | Salmeterol | |
|---|---|---|
| EDL | ||
| Emax (% control) | 130.2±2.1 | 142.7±3.1* |
| Log ED50 (mM kg−1 day−1) | −5.8±0.3 | −3.7±0.9* |
| ED50 (μg kg−1 day−1) | (0.3⩽1⩽6) | (63⩽130⩽253)* |
| Soleus | ||
| Emax (% control) | 119.1±4.4 | 130.7±4.1* |
| Log ED50 (mM kg−1 day−1) | −6.1±0.9 | −3.9±0.3 |
| ED50 (μg kg−1 day−1) | (0.003⩽0.7⩽167) | (16⩽74⩽337) |
| Heart | ||
| Emax (% control) | 125.4±3.2 | 120.3±2.9 |
| Log ED50 (mM kg−1 day−1) | −4.9±0.3 | −4.0±0.3 |
| ED50 (μg kg−1 day−1) | (0.2⩽11⩽69) | (9⩽59⩽401) |
CI: confidence interval, EDL: extensor digitorum longus, ED50: drug concentration at half of the maximum effect, Emax: maximum hypertrophic effect.
P<0.05 formoterol vs salmeterol.
Note that ED50 values are presented as 95% CI.
In the heart, formoterol and salmeterol exhibited similar intrinsic activity and potency, with the formoterol dose–response curve shifted to the left slightly (P<0.05), so that at each dose measured the increase in cardiac mass was greater with formoterol treatment.
β-Adrenoceptor downregulation
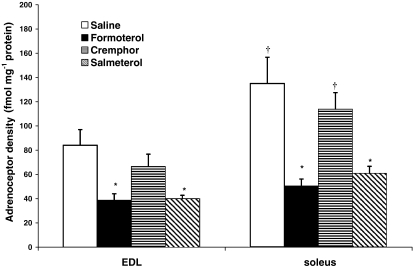
Radioligand assay was used to determine β-adrenoceptor density in EDL and soleus muscle cell membrane preparations following treatment with 25 μg kg−1 day−1 of formoterol or salmeterol, compared to their respective control levels (Figure 3).
Figure 3.
Adrenoceptor downregulation as a result of β2-agonist treatment. Both formoterol and salmeterol caused β-adrenoceptor downregulation at a dose of 25 μg kg−1 day−1 (*P<0.05 treated vs respective control; †P<0.05 EDL vs soleus).
From the saline control groups, it was determined that the soleus muscle contained 61% more β-adrenoceptors than the EDL (P<0.05), supporting previous results which have shown a greater β-adrenoceptor density in slow- than fast-twitch muscle. Compared to saline control, 4 weeks of formoterol treatment produced significant β-adrenoceptor downregulation in both the EDL and soleus muscles (54 and 63%, respectively). Salmeterol treatment also resulted in a significant downregulation of EDL and soleus β-adrenoceptor population (40 and 47%, compared to cremphor control, P<0.05).
Discussion
The most important finding of this study was that doses of less than 25 μg kg−1 day−1 of formoterol and salmeterol are capable of eliciting significant skeletal muscle hypertrophy with minimal or no cardiac hypertrophy, thus highlighting their significant clinical potential for muscle wasting conditions. While previous experiments had suggested that doses of more than 1000 μg kg−1 day−1, of β2-agonists such as clenbuterol were required to elicit skeletal muscle hypertrophy (Lynch et al., 2000; Sneddon et al., 2000; Ryall et al., 2002; 2004), we provide conclusive evidence that newer generation β2-agonists, formoterol, and to a lesser extent salmeterol, are capable of producing skeletal muscle hypertrophy at microgram doses.
Our pharmacologic characterisation of formoterol and salmeterol suggests that formoterol has a greater margin of selectivity between skeletal muscle and heart. When the formoterol ED50 values are compared between skeletal muscle and the heart, formoterol is 11- and 16-fold more selective for the EDL and soleus muscles, while salmeterol tended to be more selective for the heart, than skeletal muscle. These results provide important information for current clinical trials utilising low-dose β2-agonist therapy for muscle-wasting pathologies (Kissel et al., 2001; Fowler et al., 2004).
Although β2-adrenoceptor agonists have been proposed as possible treatments for skeletal muscle wasting (Maltin et al., 1987; Lynch et al., 2000; Sneddon et al., 2000; Zeman et al., 2000; Ryall et al., 2002; 2004), their clinical potential has been limited by the associated adverse effects, most notably cardiac hypertrophy (Duncan et al., 2000; Gregorevic et al., 2005). Chen & Alway (2000) proposed that a low dose of the β2-agonist clenbuterol may cause an increase in skeletal muscle mass and strength at the relatively low dose of 10 μg kg−1 day−1, but they observed only modest effects in rat slow-twitch skeletal muscle and no discernable effect in fast-twitch skeletal muscle. In contrast, our results show quite conclusively that formoterol elicits skeletal muscle hypertrophy in both fast- and slow-twitch skeletal muscles at a dose of only 1 μg kg−1 day−1, without a significant increase in cardiac mass.
Previous studies have shown that high (5 mg kg−1) doses of the β2-agonist clenbuterol induce apoptosis and necrosis in both the soleus muscle and the heart (Burniston et al., 2002; 2005). Our results suggest that doses as low as 1 μg kg−1 day−1 can be used to elicit skeletal muscle hypertrophy, thus minimizing any potential necrosis or apoptosis. Further work is required to determine whether the use of micromolar doses of these agents elicits apoptosis or necrosis.
β2-Agonist administration to animals, whether via drinking water, oral ingestion, or systemic administration (Benson et al., 1991; Moore et al., 1994; Smith et al., 2002), is associated with an initial drop of 5–10% of BM in the first 2–5 days of treatment. This response is believed to be due to stimulation of central β2-adrenoceptors in the hypothalamus, which causes a transitory suppression of appetite (Bendotti et al., 1986). A suppression of appetite, however short, may prove to be detrimental to a patient receiving treatment with a β2-agonist to reverse muscle wasting because they are already in a weakened state. Thus, our finding that the initial drop in BM only occurs at high doses (greater than 500 μg kg−1 day−1) of formoterol and salmeterol further supports the potential of a low-dose approach to β2-agonist therapy.
Many muscle-wasting conditions, such as that associated with the normal process of aging, require only small (10–15%) increases in muscle mass and strength to significantly improve the quality of life. We have shown previously that treating old rats with the β2-agonist fenoterol not only restored muscle mass and strength, but actually increased them to such an extent that muscles from treated old rats were larger and stronger than those from adult control rats (Ryall et al., 2004). This finding suggested that, in the case of age-related muscle-wasting, a maximal response was not required, and a dose of 1 μg kg−1 day−1 may be all that is required to increase muscle mass and strength in an elderly person that would allow them to complete the tasks of daily living.
The possible use of formoterol and salmeterol as a therapeutic intervention for muscle wasting is further supported by the fact that both drugs are currently in use for the prevention and treatment of asthma. Formoterol and salmeterol are administered via inhalation, at single doses of up to 120 and 500 μg, respectively (Palmqvist et al., 1999). Doses of 50 and 400 μg day−1 are recommended for maintenance therapy (Pearlman et al., 2002).
Previous studies using isolated smooth muscle preparations from both experimental animals and humans have found that salmeterol is less efficacious and less potent than formoterol in causing smooth muscle relaxation (Jeppsson et al., 1992; Lindén et al., 1993; Palmqvist et al., 1999). Our results for skeletal muscle potency are in agreement with previous studies on smooth muscle, with formoterol being more potent than salmeterol. However, our data on skeletal muscle mass suggest that, when administered in vivo, salmeterol has a higher intrinsic activity than formoterol. This result may be explained by the differences in lipophilicity of salmeterol and formoterol. Salmeterol, being highly lipophilic, is entirely localised to the lipid bilayer, whereas the less lipophilic formoterol is less strongly relocated to the lipid membrane and is more easily washed out (Ball et al., 1991; Anderson, 1993). Thus, salmeterol is likely to elicit a cAMP response for a longer duration. The importance of this would not necessarily be apparent in vitro, where smooth muscle contractile effects are recorded over several minutes or a few hours (Jeppsson et al., 1992). In vivo, however, our observations represent the effect of salmeterol over 24 h between successive injections. Thus, it is possible that a larger response might have been seen with formoterol if injections had been given with greater frequency.
The dose–response curves for salmeterol and formoterol provide evidence for the previously described, although yet to be defined, difference in response between fast- and slow-twitch skeletal muscle (Ryall et al., 2002; 2004; Beitzel et al., 2004). Both drugs elicited significantly greater hypertrophy in the EDL than soleus muscles, despite the fact that the soleus muscle has a greater density of β-adrenoceptors. At the dose examined, it was interesting to note that compared to the respective control, there seemed to be a greater absolute decrease in the density of adrenoceptors in the soleus than EDL muscle, for both formoterol and salmeterol. This observation could help explain the differences in response between fast- and slow-twitch skeletal muscles to β2-agonists, as it has been proposed that receptor downregulation is a significant factor in limiting the anabolic response to β-agonists (Rothwell et al., 1987; Sillence et al., 1991; Kim et al., 1992; Huang et al., 2000; Ryall et al., 2002). Further experiments are required to determine the exact mechanism for the different response between these fast and slow muscles.
In a previous study (Ryall et al., 2004), we demonstrated that another β2-agonist, fenoterol, induced β-adrenoceptor downregulation in the EDL, but not the soleus, muscles of 16-month-old F344 rats. This was in contrast to the current finding of downregulation in both the EDL and the soleus muscles of 12-week-old F344 rats. This could be attributed to a number of mechanisms about which we can only speculate, including a faster rate of adrenoceptor synthesis in soleus muscles of young rats, a hypothesis supported by the previous finding that fenoterol induced receptor downregulation in the soleus muscles of 16-week-old rats (Ryall et al., 2002). Nevertheless, despite this difference in sensitivity, it is clear from our data that this does not translate to a difference in responsiveness, as β-adrenoceptors in soleus muscles will downregulate if they are treated with a compound of sufficient intrinsic activity, and at a sufficient dose. Future experiments will need to look at the possible influence of advancing age on receptor downregulation.
The present study did not attempt to measure drug residues in the cell membrane fragment; thus, it is possible that there was some tissue contamination with residual formoterol or salmeterol. In theory, such contamination could have contributed to the apparent reduction in the number of binding sites for ICYP, which was attributed to receptor downregulation. As the degree of whole tissue contamination by residual drugs varies according to the lipophilicity and half-life of the compound in question (Sillence et al., 1993), it is not unreasonable to suggest that residual levels of salmeterol may have increased our measurement of β-adrenoceptor downregulation. However, due to the relatively high pK value for ICYP in rat skeletal muscle (pK=11.01; Sillence et al., 1993) and multiple wash stages in the membrane preparation procedure, we believe that residual contamination did not affect our measurements of β-adrenoceptor downregulation. In support of the effectiveness of the wash procedure is the finding that the β2-adrenoceptor downregulation caused by clenbuterol is counteracted by the β-adrenoceptor antagonist sotalol (Sillence et al., 1991), which would not have been apparent if residual clenbuterol had not been removed by our washing procedure. However, we cannot discount this possibility of drug residues altering our adrenoceptor measurements.
Finally, while our results show that the increase in EDL and soleus muscle mass is associated with a reduction in β-adrenoceptor density, it should be noted that we did not measure adrenoceptor density in the heart. Of particular importance to the current study was the examination of skeletal muscle hypertrophy compared with cardiac hypertrophy. We chose to look only at skeletal muscle adrenoceptors to determine whether we could elicit skeletal muscle hypertrophy without causing adrenoceptor downregulation. Thus, future studies should examine whether similar β-adrenoceptor downregulation occurs in the heart after treatment with either formoterol or salmeterol.
In conclusion, our findings demonstrate for the first time a novel pharmacological intervention for the treatment of muscle wasting, at doses that are currently used therapeutically for bronchodilation. The results provide important information regarding the optimal dose for formoterol and salmeterol for clinical application to muscle wasting conditions.
Acknowledgments
We thank Astra-Zeneca Inc., Molndal, Sweden for providing formoterol and salmeterol. This work was supported by research grants from the National Health and Medical Research Council (Australia), the Muscular Dystrophy Association (U.S.A.), and the Rebecca L. Cooper Medical Research Foundation. J.G.R. was supported by a Postgraduate Scholarship from the National Heart Foundation of Australia.
Abbreviations
- BM
body mass
- CI
confidence intervals
- ED50
drug dose at half of the maximum effect
- EDL
extensor digitorum longus
- Emax
maximum hypertrophic effect
- i.p.
intraperitoneal
- MW
molecular weight
References
- ANDERSON G.P. Formoterol: pharmacology, molecular basis of agonism, and mechanism of long duration of a highly potent and selective β2-adrenoceptor agonist bronchodilator. Life Sci. 1993;52:2145–2160. doi: 10.1016/0024-3205(93)90729-m. [DOI] [PubMed] [Google Scholar]
- BALL D.I., BRITTAIN R.T., COLEMAN R.A., DENYER L.H., JACK D., JOHNSON M., LUNTS L.H.C., NIALS A.T., SHELDRICK K.E., SKIDMORE I.F. Salmeterol, a novel, long-acting β2-adrenoceptor agonist: characterization of pharmacological activity invitro and invivo. Br. J. Pharmacol. 1991;104:665–671. doi: 10.1111/j.1476-5381.1991.tb12486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTON E., MORRIS C. Mechanisms and strategies to counter muscle atrophy. J. Gerontol. Series A. 2003;58:923–926. doi: 10.1093/gerona/58.10.m923. [DOI] [PubMed] [Google Scholar]
- BEITZEL F., GREGOREVIC P., RYALL J.G., PLANT D.R., SILLENCE M.N., LYNCH G.S. β2-Adrenoceptor agonist fenoterol enhances functional repair of regenerating rat skeletal muscle after injury. J. Appl. Physiol. 2004;96:1385–1392. doi: 10.1152/japplphysiol.01081.2003. [DOI] [PubMed] [Google Scholar]
- BENDOTTI C., VILLA M., SAMANIN R. Further evidence of the inhibitory role of perifornical hypothalamic beta-adrenergic receptors in the feeding behavior of hungry rats. Life Sci. 1986;38:259–266. doi: 10.1016/0024-3205(86)90311-5. [DOI] [PubMed] [Google Scholar]
- BENSON D.W., FOLEY-NELSON T., CHANCE W.T., ZHANG F.S., JAMES J.H., FISCHER J.E. Decreased myofibrillar protein breakdown following treatment with clenbuterol. J. Surg. Res. 1991;50:1–5. doi: 10.1016/0022-4804(91)90002-4. [DOI] [PubMed] [Google Scholar]
- BERNSTEIN D., FAJARDO G., ZHAO M., URASHIMA T., POWERS J., BERRY G., KOBILKA B.K. Differential cardioprotective/cardiotoxic effects mediated by β-adrenergic receptor subtypes. Am. J. Physiol. 2005;289:H2441–H2449. doi: 10.1152/ajpheart.00005.2005. [DOI] [PubMed] [Google Scholar]
- BRODDE O.E. β1- and β2-adrenoceptors in the human heart: properties, function, and alterations in chronic heart failure. Pharmacol. Rev. 1991;43:203–242. [PubMed] [Google Scholar]
- BURNISTON J.G., NG Y., CLARK W.A., COLYER J., TAN L.B., GOLDSPINK D.F. Myotoxic effects of clenbuterol in the rat heart and soleus muscle. J. Appl. Physiol. 2002;93:1824–1832. doi: 10.1152/japplphysiol.00139.2002. [DOI] [PubMed] [Google Scholar]
- BURNISTON J.G., TAN L.B., GOLDSPINK D.F. β2-Adrenergic receptor stimulation in vivo induces apoptosis in the rat heart and soleus muscle. J. Appl. Physiol. 2005;98:1379–1386. doi: 10.1152/japplphysiol.00642.2004. [DOI] [PubMed] [Google Scholar]
- BUSQUETS S., FIGUERAS M.T., FUSTER G., ALMENDRO V., MOORE-CARRASCO R., AMETLLER E., ARGILES J.M., LOPEZ-SORIANO F.J. Anticachetic effects of formoterol: a drug for potential treatment of muscle wasting. Cancer Res. 2004;64:6725–6735. doi: 10.1158/0008-5472.CAN-04-0425. [DOI] [PubMed] [Google Scholar]
- CHEN K.D., ALWAY S.E. A physiological level of clenbuterol does not prevent atrophy or loss of force in skeletal muscle of old rats. J. Appl. Physiol. 2000;89:606–612. doi: 10.1152/jappl.2000.89.2.606. [DOI] [PubMed] [Google Scholar]
- DUNCAN N.D., WILLIAMS D.A., LYNCH G.S. Deleterious effects of chronic clenbuterol treatment on endurance and sprint exercise performance in rats. Clin. Sci. 2000;98:329–347. [PubMed] [Google Scholar]
- EMERY P.W., ROTHWELL N.J., STOCK M.J., WINTER P.D. Chronic effects of β2-adrenergic agonists on body composition and protein synthesis in the rat. Biosci.Rep. 1984;4:83–91. doi: 10.1007/BF01120827. [DOI] [PubMed] [Google Scholar]
- FOERSTER K., GRONER F., MATTHES J., KOCH W.J., BIRNBAUMER L., HERZIG S. Cardioprotection specific for the G protein Gi2 in chronic adrenergic signaling through β2-adrenoceptors. Proc. Natl. Acad. Sci. U.S.A. 2003;100:14475–14480. doi: 10.1073/pnas.1936026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOWLER E.G., GRAVES M.C., WETZEL G.T., SPENCER M.J. Pilot trial of albuterol in Duchenne and Becker muscular dystrophy. Neurology. 2004;62:1006–1008. doi: 10.1212/01.wnl.0000118530.71646.0f. [DOI] [PubMed] [Google Scholar]
- GREGOREVIC P., RYALL J.G., PLANT D.R., SILLENCE M.N., LYNCH G.S. Chronic fenoterol administration impairs cardiac function in adult but not old rats. Am. J. Physiol. 2005;289:H344–H349. doi: 10.1152/ajpheart.01254.2004. [DOI] [PubMed] [Google Scholar]
- GUHAN A.R., COOPER S., OBORNE J., LEWIS S., BENNETT J., TATTERSFIELD A.E. Systemic effects of formoterol and salmeterol: a dose–response comparison in healthy subjects. Thorax. 2000;55:650–656. doi: 10.1136/thorax.55.8.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG H., GAZZOLA C., PEGG G.G., SILLENCE M.N. Differential effects of dexamethasone and clenbuterol on rat growth and on β2-adrenoceptors in lung and skeletal muscle. J. Animal Sci. 2000;78:604–608. doi: 10.2527/2000.783604x. [DOI] [PubMed] [Google Scholar]
- JACKMAN R.W., KANDARIAN S.C. The molecular basis of skeletal muscle atrophy. Am. J. Physiol. 2004;287:C834–C843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- JEPPSSON A.B., KÄLLSTRÖM B.L., WALDECK B. Studies on the interaction between formoterol and salmeterol in guinea-pig trachea invitro. Pharmacol. Toxicol. 1992;71:272–277. doi: 10.1111/j.1600-0773.1992.tb00982.x. [DOI] [PubMed] [Google Scholar]
- JOHNSON M., BUTCHERS P.R., COLEMAN R.A., NIALS A.T., STRONG P., SUMNER M.J., VARDEY C.J., WHELAN C.J. The pharmacology of salmeterol. Life Sci. 1993;52:2131–2143. doi: 10.1016/0024-3205(93)90728-l. [DOI] [PubMed] [Google Scholar]
- KILTS J.D., GERHARDT M.A., RICHARDSON M.D., SREERAM G., MACKENSEN G.B., GROCOTT H.P., WHITE W.D., DAVIS R.D., NEWMAN M.F., REVES J.G., SCHWINN D.A., KWATRA M.M. β2-Adrenergic and several other G protein-coupled receptors in human atrial membranes activate both Gs and Gi. Circ. Res. 2000;87:705–709. doi: 10.1161/01.res.87.8.705. [DOI] [PubMed] [Google Scholar]
- KIM Y.S., SAINZ R.D., SUMMERS R.J., MOLENAAR P. Cimaterol reduces beta-adrenergic receptor density in rat skeletal muscles. J. Animal. Sci. 1992;70:115–122. doi: 10.2527/1992.701115x. [DOI] [PubMed] [Google Scholar]
- KISSEL J.T., MCDERMOTT M.P., MENDELL J.R., KING W.M., PANDYA S., GRIGGS R.C., TAWIL R. Randomized, double-blind, placebo controlled trial of albuterol in facioscapulohumeral dystrophy. Neurology. 2001;57:1434–1440. doi: 10.1212/wnl.57.8.1434. [DOI] [PubMed] [Google Scholar]
- KISSEL J.T., MCDERMOTT M.P., NATARAJAN R., MENDELL J.R., PANDYA S., KING W.M., GRIGGS R.C., TAWIL R. Pilot trial of albuterol in facioscapulohumeral muscular dystrophy. Neurology. 1998;50:1402–1406. doi: 10.1212/wnl.50.5.1402. [DOI] [PubMed] [Google Scholar]
- LINDÉN A., BERGENDAL A., ULLMAN A., SKOOGH B.E., LÖFDAHL C.G. Salmeterol, formoterol, and salbutamol in the isolated guinea-pig trachea: differences in maximum relaxant effect and potency but not in functional antagonism. Thorax. 1993;48:547–553. doi: 10.1136/thx.48.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LÖFDAHL C.G., SVEDMYR N. Formoterol fumarate, a new β2-adrenoceptor agonist. Allergy. 1989;44:264–271. doi: 10.1111/j.1398-9995.1989.tb01068.x. [DOI] [PubMed] [Google Scholar]
- LYNCH G.S. Emerging drugs for sarcopenia: age-related muscle wasting. Expert Opin. Emerging Drugs. 2004;9:345–361. doi: 10.1517/14728214.9.2.345. [DOI] [PubMed] [Google Scholar]
- LYNCH G.S., HINKLE R.T., FAULKNER J.A. Power output of fast and slow skeletal muscles ofmdx (dystrophic) and control mice after clenbuterol treatment. Exp. Physiol. 2000;85:295–299. [PubMed] [Google Scholar]
- MALTIN C.A., HAY S.M., DELDAY M.L., SMITH F.G., LOBLEY G.E., REEDS P.J. Clenbuterol, a beta-agonist induces growth in innervated and denervated rat soleus muscle via apparently different mechanisms. Biosci. Rep. 1987;7:525–532. doi: 10.1007/BF01116510. [DOI] [PubMed] [Google Scholar]
- MARTINEAU L., HORAN M.A., ROTHWELL N.J., LITTLE R.A. Salbutamol, a β2-adrenoceptor agonist, increases skeletal muscle strength in young men. Clin. Sci. 1992;83:615–621. doi: 10.1042/cs0830615. [DOI] [PubMed] [Google Scholar]
- MOORE N.G., PEGG G.G., SILLENCE M.N. Anabolic effects of the β2-adrenoceptor agonist salmeterol are dependent on route of administration. Am. J. Physiol. 1994;267:E475–E484. doi: 10.1152/ajpendo.1994.267.3.E475. [DOI] [PubMed] [Google Scholar]
- PALMQVIST M., IBSEN T., MELLÉN A., LÖTVALL J. Comparison of the relative efficacy of formoterol and salmeterol in asthmatic patients. Am. J. Respir. Crit. Care Med. 1999;160:244–249. doi: 10.1164/ajrccm.160.1.9901063. [DOI] [PubMed] [Google Scholar]
- PEARLMAN D.S., KOTTAKIS J., TILL D., CIOPPA G.D. Formoterol delivered via a dry powder inhaler (Aerolizer): results from long-term clinical trials in children. Curr. Med. Res. Opin. 2002;18:445–455. doi: 10.1185/030079902125001254. [DOI] [PubMed] [Google Scholar]
- PÖNICKE K., HEINROTH-HOFFMANN I., BRODDE O.E. Role of β1- and β2-adrenoceptors in hypertrophic and apoptotic effects of noradrenaline and adrenaline in adult rat ventricular cardiomyocytes. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;367:592–599. doi: 10.1007/s00210-003-0754-z. [DOI] [PubMed] [Google Scholar]
- ROTHWELL N.J., STOCK M.J., SUDERA D.K. Changes in tissue blood flow and β-receptor density of skeletal muscle in rats treated with β2-adrenoceptor agonist clenbuterol. Br. J. Pharmacol. 1987;90:601–607. doi: 10.1111/j.1476-5381.1987.tb11211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUX F.J., GRANDORDY B., DOUGLAS J.S. Functional and binding characteristics of long-acting β2-agonists in lung and heart. Am. J. Respir. Crit. CareMed. 1996;153:1489–1495. doi: 10.1164/ajrccm.153.5.8630591. [DOI] [PubMed] [Google Scholar]
- RYALL J.G., GREGOREVIC P., PLANT D.R., SILLENCE M.N., LYNCH G.S. β2-Agonist fenoterol has greater effects on contractile function of rat skeletal muscles than clenbuterol. Am. J. Physiol. 2002;283:R1386–R1394. doi: 10.1152/ajpregu.00324.2002. [DOI] [PubMed] [Google Scholar]
- RYALL J.G., PLANT D.R., GREGOREVIC P., SILLENCE M.N., LYNCH G.S. β2-Agonist administration reverses muscle wasting and improves muscle function in aged rats. J. Physiol. 2004;555:175–188. doi: 10.1113/jphysiol.2003.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILLENCE M.N., MATTHEWS M.L., SPIERS W.G., PEGG G.G., LINDSAY D.B. Effects of clenbuterol, ICI118551 and sotalol on the growth of cardiac and skeletal muscle and on β2-adrenoceptor density in female rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;344:449–453. doi: 10.1007/BF00172585. [DOI] [PubMed] [Google Scholar]
- SILLENCE M.N., MOORE N.G., PEGG G.G., LINDSAY D.B. Ligand binding properties of putative β3-adrenoceptors compared in brown adipose tissue and in skeletal muscle membranes. Br. J. Pharmacol. 1993;109:1157–1163. doi: 10.1111/j.1476-5381.1993.tb13743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH W.N., DIRKS A., SUGIURA T., MULLER S., SCARPACE P., POWERS S.K. Alteration of contractile force and mass in the senescent diaphragm with β2-agonist treatment. J. Appl. Physiol. 2002;92:941–948. doi: 10.1152/japplphysiol.00576.2001. [DOI] [PubMed] [Google Scholar]
- SNEDDON A.A., DELDAY M.I., MALTIN C.A. Ame-lioration of denervation-induced atrophy by clenbuterol is associated with increased PKC-α activity. Am. J. Physiol. 2000;279:E188–E195. doi: 10.1152/ajpendo.2000.279.1.E188. [DOI] [PubMed] [Google Scholar]
- SOLIS-COHEN S. The use of adrenal substance in the treatment of asthma. J. Am. Med. Assoc. 1900;34:1164–1166. [Google Scholar]
- WALDECK B. Some pharmacodynamic aspects on long-acting β-adrenoceptor agonists. Gen. Pharmac. 1996;27:575–580. doi: 10.1016/0306-3623(95)02052-7. [DOI] [PubMed] [Google Scholar]
- XIAO R.P., AVDONIN P., ZHOU Y.Y., CHENG H., AKHTER S.A., ESCHENHAGEN T., LEFKOWITZ R.J., KOCH W.J., LAKATTA E.G. Coupling of β2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ. Res. 1999;84:43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]
- ZEMAN R.J., PENG H., DANON M.J., ETLINGER J.D. Clenbuterol reduces degeneration of exercised or aged dystrophic (mdx) muscle. Muscle Nerve. 2000;23:521–528. doi: 10.1002/(sici)1097-4598(200004)23:4<521::aid-mus10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]