Abstract
Although a second-generation histamine H1 blocker terfenadine induced torsades de pointes (TdP) arrhythmias in patients via the blockade of a rapid component of delayed rectifier K+ current (IKr), such action of terfenadine has not been detected in previous animal models.
We analysed the potential of the canine persistent atrioventricular block heart, a new in vivo proarrhythmia model, to detect a torsadogenic effect of terfenadine of an oral dose of 3 or 30 mg kg−1. The doses can provide therapeutic to supra-therapeutic plasma concentrations as an anti-histamine.
In 2 weeks of bradycardiac heart model, there were no significant changes in any of the electrocardiogram parameters after the administration of both doses of terfenadine.
In 4–6 weeks of bradycardiac heart model, the low dose of terfenadine hardly affected any of the electrocardiogram parameters except that it induced TdP in one out of six animals. The high dose significantly decreased the atrial rate and ventricular rate, prolonged the QT interval, and induced TdP in five out of six animals. Moreover, temporal variability of repolarization increased after the high-dose administration.
These results suggest that long-term bradycardia caused by atrioventricular block can remodel the canine heart to detect terfenadine-induced TdP.
Keywords: Terfenadine, QT interval prolongation, torsades de pointes, chronic atrioventricular block dog
Introduction
Drug-induced QT interval prolongation is often associated with the onset of torsades de pointes (TdP) resulting in a life-threatening ventricular arrhythmia (Belardinelli et al., 2003; Redfern et al., 2003). To avoid the occurrence of such dangerous events, new drug candidates are being carefully evaluated according to the guideline ICH S7B for safety pharmacology studies (The ICH Steering Committee, 2005). While most of the drugs that induced TdP have been shown to inhibit a rapid component of delayed rectifier K+ currents (IKr) (Belardinelli et al., 2003; Redfern et al., 2003), some drugs possessing IKr-blocking property in vitro, such as sildenafil and verapamil, did not induce QT interval prolongation or TdP in vivo (Zhang et al., 1999; Geelen et al., 2000; Shiina et al., 2000; Sugiyama et al., 2001). Therefore, it is important to develop a model that can predict proarrhythmic potential of drug candidates with high sensitivity, specificity and reproducibility.
α-Chloralose-anesthetized rabbits have been widely used as an in vivo proarrhythmia model for assessing the QT prolonging drugs, including class III antiarrhythmics, quinolones and prokinetics (Carlsson et al., 1990; 1997; Chiba et al., 2004). A second-generation histamine H1 blocker, terfenadine, has induced TdP clinically via the IKr blockade after its overdose or by coadministration with a cytochrome P-450 inhibitor (Monahan et al., 1990; Tarantino et al., 2005); however, the torsadogenic action of the drug has not been detected in the rabbit model (Lu et al., 2000; Batey and Coker, 2002). In addition, previous studies have indicated that terfenadine hardly affects the repolarization interval of the isolated heart tissues (Pinney et al., 1995; Gintant et al., 2001; Masumiya et al., 2004) due to its multichannel blocking property including K+, Na+ and L-type Ca2+ channels (Ming and Nordin, 1995; Liu et al., 1997; Tarantino et al., 2005).
Recently, chronic atrioventricular block dogs have been developed as a new in vivo proarrhythmic model for assessing the risks of the QT prolonging drugs, including class III antiarrhythmics, psychotropics, quinolones and prokinetics (Vos et al., 1998; Sugiyama et al., 2002a, 2002b; Thomsen et al., 2003; 2004; Chiba et al., 2004; Satoh et al., 2004). In this model, electrical, mechanical and structural adaptations are known to be induced after the onset of bradycardia (Verduyn et al., 2001; Sugiyama et al., 2002a). Furthermore, recent electrophysiological studies have demonstrated that the QT interval was prolonged on the 7–14th day, and that K+ channel was downregulated on the 3rd day after creation of atrioventricular block (Schoenmakers et al., 2003; Stengl et al., 2004). However, information is still limited regarding the time course of changes in its potential to detect the drug-induced TdP after the onset of the bradycardia (Sugiyama et al., 2002a), which was assessed in this study. We propose that persistent bradycardia of 2 weeks will remodel the canine heart moderately, whereas that of 4–6 weeks can do it severely (Sugiyama et al., 2002a).
Methods
All experiments were performed according to Guidelines for Animal Experiments, University of Yamanashi. A total of 10 beagle dogs of either sex weighing about 10 kg were used in this study.
Production of complete atrioventricular block
The catheter ablation technique of atrioventricular node was employed as previously described (Sugiyama et al., 2002a; Takahara et al., 2004). The dogs were anaesthetized with pentobarbital sodium (30 mg kg−1, i.v.). After intubation with a cuffed endotracheal tube, the dogs were artificially ventilated with a room air using a volume-limited ventilator (SN-408-3; Shinano, Tokyo, Japan). Tidal volume and respiratory rate were set at 20 ml kg−1 and 15 strokes min−1, respectively. To prevent blood clotting, heparin calcium (100 IU kg−1) was intravenously administered. The surface lead II electrocardiogram (ECG) was continuously monitored using a polygraph system (RM-6000; Nihon-Kohden, Tokyo, Japan). A quad-polar electrodes catheter with a large tip of 4 mm (D7-DL-252; Cordis-Webster, Baldwin Park, CA, U.S.A.) was inserted through the right femoral vein using the standard percutaneous technique under sterile condition and positioned around the tricuspid valve watching the bipolar electrograms from the distal electrodes pair. The optimal site for the atrioventricular node ablation, namely the compact atrioventricular node, was determined on the basis of the intracardiac electrogram, of which a very small His deflection was recorded and atrium/ventricular voltage ratio was >2. The site was usually found at 1–2 cm proximal from the position where the largest His bundle electrogram was recorded. The power source for atrioventricular node ablation was an electrosurgical generator (MS-1500; Mera, Tokyo, Japan) delivering continuous unmodulated radiofrequency energy at a frequency of 500 kHz. After proper positioning, the radiofrequency energy of 20 W was delivered for 10 s from the tip electrode to an indifferent patch electrode positioned on the animal's back, which was continued then for 30 s if junctional rhythm was induced. The end point of this procedure was the development of the complete atrioventricular block with an onset of stable idioventricular escaped rhythm.
Holter ECG recording
A Holter recording and analysis system (QR2100 and HS1000, Fukuda ME Kogyo, Tokyo, Japan) was used to record and analyse ECG over 24 h. The effects of terfenadine on the atrial rate, ventricular rate, QT interval and corrected QT interval (QTc) as well as the proarrhythmic effects were assessed without anaesthesia. These values were expressed as the mean of three consecutive complexes. QTc was calculated using the Van de Water's formula (Van de Water et al., 1989). TdP was defined as a polymorphic ventricular tachycardia, of which QRS complex twisted around the baseline, lasting ⩾6 consecutive beats (Satoh & Zipes, 1996).
Experimental protocol
Experiment 1: ECG was recorded without anaesthesia at 24 h before and 0.5, 24 h, 1, 2, 4 and 8 weeks after the atrioventricular node ablation (n=4).
Experiment 2: At 2 weeks after the induction of complete atrioventricular block (n=6), 3 or 30 mg kg−1 of terfenadine was orally administered as a powder using a gelatin capsule, which will provide therapeutic to supra-therapeutic plasma concentrations as an anti-histamine (Ferguson et al., 1985; Usui et al., 1998) 2 h after the start of ECG monitoring. Initially, the low-dose of terfenadine was orally administered, and then 2 days later, 10 times higher dose was orally administered. At 4–6 weeks after the induction of complete atrioventricular block, the same protocol was applied to the same animal group (n=6). Since the time at maximum plasma concentration (Tmax) and t1/2 of orally administered terfenadine are reported to be 1.3 and 15.1 h, respectively (Lalonde et al., 1996), plasma concentration of terfenadine at 2 weeks after the oral administration can be estimated to be 1/100,000 (0.01%) of its maximum plasma concentration (Cmax).
Beat-to beat analysis
ECG of 51 consecutive beats under stable idioventricular automaticity was recorded before and after the drug administration. Poincaré plots with QTn versus QTn+1 were prepared for each of two analysis time points. The mean orthogonal distance from the diagonal to the points of the Poincaré plot was determined as short-term variability (=∑∣QTn+1–QTn∣/[50 × √2]). On the other hand, the mean distance to the mean of the parameter parallel to the diagonal of the Poincaré plot was determined as long-term variability (=∑∣QTn+1+QTn−2QTmean∣/[50 × √2]). These nomenclatures are adopted from heart rate variability investigations using Holter monitoring in humans (Brennan et al., 2001), which have been applied to the QT interval of normal dogs and chronic atrioventricular block dogs (Thomsen et al., 2004; Schneider et al., 2005).
Drugs
The following drugs were purchased: terfenadine (Sigma, St Louis, MO, U.S.A.), pentobarbital sodium (Tokyo Kasei, Tokyo, Japan) and heparin calcium (Mitsui, Tokyo, Japan).
Statistics
Data are presented as the mean±s.e.m. The statistical comparisons within a parameter were evaluated by one-way, repeated-measures analysis of variance (ANOVA) followed by Contrasts for mean values comparison. A P-value <0.05 was considered statistically significant.
Results
Time course of change in the QT interval after the induction of complete atrioventricular block (experiment 1)
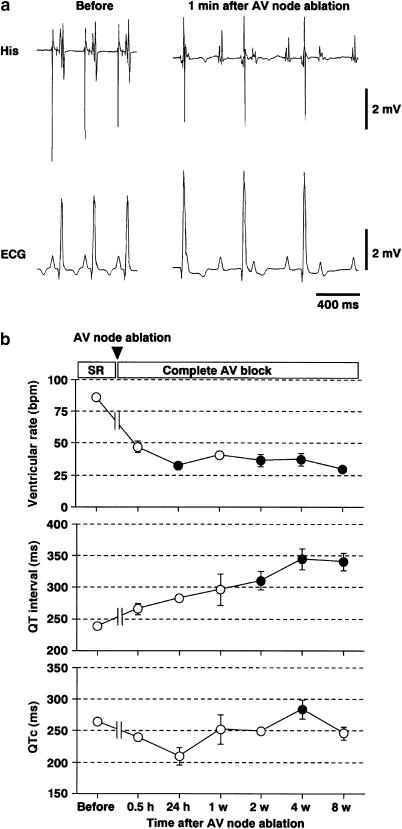
Figure 1a shows typical tracings of ECG from a dog before and 1 min after the atrioventricular node ablation, whereas Figure 1b summarized the time courses of the heart rate, QT interval and QTc after the induction of complete atrioventricular block (n=4). Before the surgery, the ventricular rate, QT interval and QTc were 86±2 beats min−1, 239±6 and 265±7 ms, respectively. At 24 h after the atrioventricular node ablation, these were 33±2 beats min−1, 289±8 and 209±14 ms, respectively. This bradycardia of <40 beats min−1 continued throughout the experiment. The QT interval was gradually prolonged after the induction of complete atrioventricular block, and significant changes were observed for 2–8 weeks. On the other hand, significant prolongation of the QTc was detected only at 4 weeks.
Figure 1.
Electrocardiogram of complete atrioventricular block dogs. (a) Typical tracings of His bundle electrogram (His) and lead II electrocardiogram (ECG) before and after the atrioventricular (AV) node ablation. (b) Time course of the heart rate, QT interval and corrected QT (QTc) after the AV node ablation (n=4). Data are presented as mean±s.e.m. SR: sinus rhythm. Closed symbols represent statistically significant differences from each value at 0.5 h after the AV node ablation by P<0.05.
Torsadogenic action of terfenadine (experiment 2)
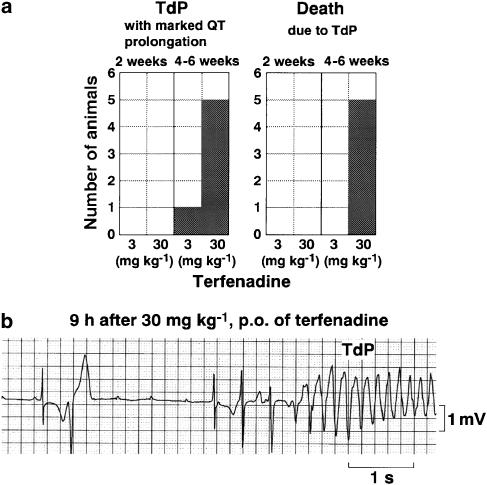
The number of animals showing TdP is summarized in Figure 2a, whereas a typical tracing of terfenadine-induced TdP is depicted in Figure 2b. In 2 weeks of bradycardiac heart model (2 weeks model), no TdP was detected after administration of both doses of terfenadine. In 4–6 weeks of bradycardiac heart model (4–6 weeks model), the number of episodes of TdP arrhythmias increased in a dose-dependent manner. At 80 min after the administration of the low dose, one episode of TdP was detected in one animal out of six, which lasted for 2.3 s and was spontaneously terminated. In this animal, the QT interval was 440 ms just before the onset of TdP, and the morphology of ECG during TdP was similar to that observed after the high dose. After the administration of the high dose, 2±1 episodes of short duration of TdP (2.7±0.2 s) were detected in five out of six animals, and the dogs lost consciousness after the latest TdP that degenerated into ventricular fibrillation, leading to death. The initial TdP was observed at 11.2±3.3 h after the drug administration, whereas the latest TdP leading to the animal's death was induced at 14.6±2.3 h. Onset of TdP followed the R on T phenomenon. The QT interval was 464±10 ms just before the onset of TdP.
Figure 2.
Terfenadine-induced TdP. (a) Summary of proarrhythmic effects of terfenadine in the 2 and 4–6 weeks of bradycardiac heart models. (b) A typical tracing of torsades de pointes (TdP) observed at 9 h after the oral administration of 30 mg kg−1 of terfenadine in a dog of the 4–6 weeks model.
Effects of terfenadine on the electrocardiogram parameters (experiment 2)
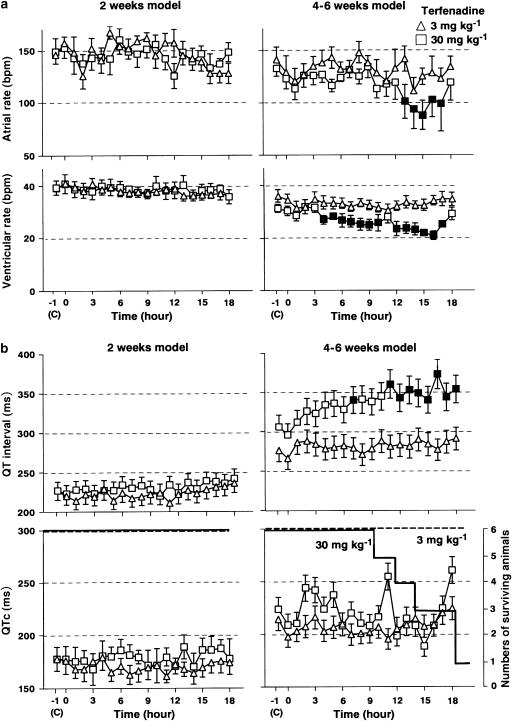
The time courses of the changes in the ECG parameters are summarized in Figure 3. In the 2 weeks model, the pre drug control values of the atrial rate, ventricular rate, QT interval and QTc were 146±8, 39±3 beats min−1, 227±6 and 183±8 ms in the low-dose group (n=6), and 149±13, 39±3 beats min−1, 227±8 and 178±11 ms in the high-dose group (n=6), respectively. In the 4–6 weeks model, those were 141±12, 36±2 beats min−1, 276±10 and 214±9 ms in the low-dose group (n=6), and 133±8, 31±2 beats min−1, 306±10 and 224±15 ms in the high-dose group (n=6), respectively. The pre drug control values of the QT interval and QTc in the 4–6 weeks model were significantly longer than those in the 2 weeks model. Meanwhile, no significant difference was detected in the pre drug control values of the atrial rate or ventricular rate between the 2 weeks and 4–6 weeks model. In the 2 weeks model, the administration of both doses of terfenadine did not affect any of these parameters. In the 4–6 weeks model, the administration of the low dose did not affect any of these parameters, whereas the high dose significantly decreased the atrial rate for 13–17 h and ventricular rate for 4–10 and 12–17 h, and increased the QT interval at 7 and for 11–18 h. Significant changes were not detected in QTc.
Figure 3.
Effects of terfenadine on the electrocardiogram. (a) Time courses of the effects of terfenadine on the atrial rate and ventricular rate in the 2 weeks and 4–6 weeks of bradycardiac heart models (n=6). (b) Time courses of the effects of terfenadine on the QT interval, corrected QT (QTc) and number of surviving animals (n=6). Data are presented as mean±s.e.m. Closed symbols represent statistically significant differences from each pre drug control (c) value by P<0.05.
Beat-to-beat analysis (experiment 2)
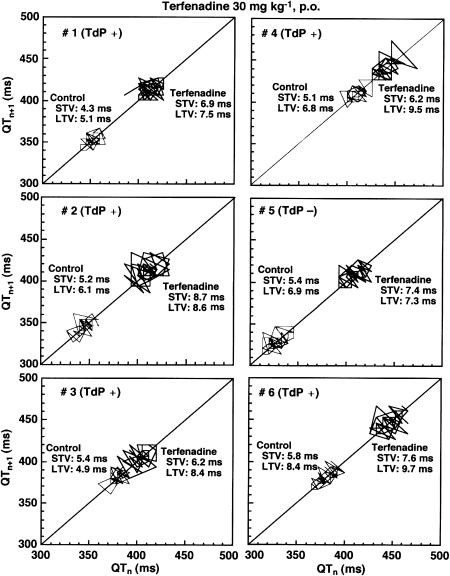
Beat-to-beat analysis was employed for dogs receiving 30 mg kg−1 of terfenadine to assess relationship between the progression of remodelling process and torsadogenic potential of the model. ECG of 51 consecutive beats under stable idioventricular rhythm was recorded from six dogs of the 2 weeks model before and 12.7±1.8 h after terfenadine administration and from six dogs of the 4–6 weeks model before and 14.0±1.1 h after drug administration. As shown in Figure 4, the QT interval, short-term variability and long-term variability of each dog increased. Table 1 summarizes the short-term variability and long-term variability in the 2 and 4–6 weeks models. Although terfenadine hardly affected the short-term variability or long-term variability in the 2 weeks model, the drug significantly increased these parameters in the 4–6 weeks model. It should be noted that #5 dog of the 4–6 weeks model, which did not complicate TdP, showed the smallest increment in the long-term variability (+0.4 ms, Figure 4).
Figure 4.
Poincaré plots of the QT interval assessed in the 4–6 weeks of bradycardiac heart model. A total of 51 beats were plotted for each of the two analysis time points; before and after 30 mg kg−1, p.o. of terfenadine administration. Torsades de pointes (TdP) was induced in five out of six dogs (#1–4 and 6). STV: short-term variability, LTV: long-term variability.
Table 1.
Effects of a torsadogenic dose of terfenadine (30 mg kg−1, p.o.) on the QT interval variability in the atrioventricular (AV) block heart
| Time after the onset of AV block | Short-term variability (ms) | Long-term variability (ms) | ||||
|---|---|---|---|---|---|---|
| Baseline | Terfenadine | Change | Baseline | Terfenadine | Change | |
| 2 weeks (n=6) | 5.4±0.4 | 5.1±0.2 | −0.3±0.4 | 6.2±0.4 | 6.7±0.7 | +0.5±0.8 |
| 4–6 weeks (n=6) | 5.2±0.2 | 7.2±0.4** | +2.0±0.4†† | 6.4±0.5 | 8.5±0.4** | +2.2±0.5 |
Data are presented as mean±s.e.m.
P<0.01, compared with the respective baseline values;
P<0.01, compared with change in variability of the 2 weeks model.
Discussion
In the present study, TdP was detected with high reproducibility after terfenadine administration in the 4–6 weeks model, whereas it was not induced in the 2 weeks model. This was in good accordance with our observation using a 5-HT4 agonist cisapride in the canine chronic atrioventricular block model (Sugiyama et al., 2002a). The K+ currents, including a slow component of delayed rectifier K+ currents (IKs) and IKr, generally compensate each other to secure the repolarization process; namely, the repolarization reserve (Roden, 1998), and the intact canine heart has been shown to possess wider safety margin for pharmacological IKr blockade than human (Biliczki et al., 2002; Satoh et al., 2005). Since the significant downregulation of the IKs and IKr has been demonstrated in the cardiomyocytes of dogs at least 3 days after the onset of complete atrioventricular node block (Volders et al., 1999; Schoenmakers et al., 2003; Stengl et al., 2004), similar electrophysiological changes may have occurred in our atrioventricular block model. Furthermore, as shown in Figure 1b, the QT interval at 4 weeks was longer than that at 2 weeks, which may suggest the development of remodelling process of the heart related to the decreased repolarization reserve, resulting in enhancement of the drug-induced QT interval prolongation and onset of TdP. Also, experimental conditions may alter sensitivity to detect the drug-induced TdP, since a class III antiarrhythmic drug dofetilide has been shown to induce such arrhythmias in anaesthetized mongrel dogs at both 2 and 5 weeks after the onset of atrioventricular block (Schoenmakers et al., 2003).
In our previous studies with the chronic atrioventricular block dogs, torsadogenic doses of cisapride, sulpiride or nifekalant hardly affected the atrial and/or idioventricular rhythm (Sugiyama et al., 2002a, 2002b; Satoh et al., 2004). Meanwhile as shown in the results, the supra-therapeutic dose of terfenadine prolonged the QT interval leading to induction of TdP along with marked reduction of the atrial and ventricular rate in the 4–6 weeks model. The inhibition of the ventricular automaticity can be explained by Na+ channel-blocking property and IKr-inhibitory action of terfenadine (Sugiyama et al., 1994; Ming and Nordin, 1995; Liu et al., 1997; Usui et al., 1998; Takahara et al., 2005). The fact that terfenadine decreased the idioventricular rate only in the 4–6 weeks model indicates that the longer-term bradycardia may have remodelled the Purkinje, resulting in the increased sensitivity of phase 4 depolarization. Furthermore, since slow ventricular rhythm has been demonstrated to enhance electrical vulnerability in the ventricular muscle (Sugiyama & Hashimoto, 2002; Sugiyama et al., 2002a), the enhanced bradycardiac effect of terfenadine can increase its torsadogenicity associated with IKr-inhibitory action, which may partly explain strong proarrhythmic effects of terfenadine in the 4–6 weeks model as well as the clinical case reports (Monahan et al., 1990).
In previous reports, terfenadine did not cause TdP in the α-chloralose-anaesthetized rabbit model (Lu et al., 2000; Batey and Coker, 2002). In a recent study using fluoroquinolone antibacterial drugs, sensitivity of the rabbit model for detecting TdP was significantly less than that of the canine chronic atrioventricular block model (Chiba et al., 2004). Since an α-adrenoceptor agonist, such as methoxiamine or phenylephrine, was administered to enhance the induction of TdP in the rabbit model (Carlsson et al., 1990; Lu et al., 2000; Batey and Coker, 2002), the multiple ion channel-blocking effects of terfenadine (Ming & Nordin, 1995; Liu et al., 1997) might have counteracted the effects of pharmacological cardiac α-adrenoceptor stimulation of the heart, resulting in the decrease of the sensitivity.
It was clearly demonstrated that the interventricular dispersion of repolarization plays a key role in the induction of acquired type of TdP using the chronic atrioventricular block dogs (Vos et al., 1998; Schoenmakers et al., 2003). In addition to spatial heterogeneity of cardiac repolarization, its temporal heterogeneity has been analysed in the in vivo canine models (Eckardt et al., 2002; Thomsen et al., 2004; Schneider et al., 2005), since class III antiarrhythmic or IKr blocking drugs indeed produce a repolarization instability (Hondeghem & Hoffmann, 2003). Furthermore, a recent study using chronic atrioventricular block dogs has shown the utility of short-term variability in predicting the drug-induced TdP (Thomsen et al., 2004). In this study, a torsadogenic dose of terfenadine significantly increased both short-term and long-term variability of repolarization in the 4–6 weeks model. However, there was no change in baseline values of the short-term variability of repolarization between the 2 and 4–6 weeks models, which may suggest that the bradycardiac effect of terfenadine may also have promoted the induction of TdP. More importantly, change in the long-term variability was the smallest in a dog that did not complicate TdP in the 4–6 weeks group. Therefore, analysis of long-term as well as short-term variability of repolarization may be reliable in predicting the proarrhythmic potential of a drug before the onset of TdP.
In conclusion, long-term bradycardia caused by atrioventricular block can remodel the heart severely to detect terfenadine-induced TdP. Therefore, the current canine model can be useful for detecting proarrhythmic potential of the drug candidates with unknown multifarious pharmacological actions in addition to IKr blockade.
Acknowledgments
This study was supported in part by Grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (#17590216), The Pharmacological Research Foundation, Tokyo, and the TV Yamanashi Science Development Fund.
Abbreviations
- ECG
electrocardiogram
- ICH
the international conference on harmonization of technical requirements for registration of pharmaceuticals for human use
- IKr
a rapid component of delayed rectifier K+ currents
- IKs
a slow component of delayed rectifier K+ currents
- TdP
torsades de pointes
- QTc
corrected QT interval
References
- BATEY A.J., COKER S.J. Proarrhythmic potential of halofantrine, terfenadine and clofilium in a modified in vivo model of torsade de pointes. Br. J. Pharmacol. 2002;135:1003–1012. doi: 10.1038/sj.bjp.0704550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELARDINELLI L., ANTZELEVITCH C., VOS M.A. Assessing predictors of drug-induced torsade de pointes. Trends. Pharmacol. Sci. 2003;24:619–625. doi: 10.1016/j.tips.2003.10.002. [DOI] [PubMed] [Google Scholar]
- BILICZKI P., VIRÁG L., IOST N., PAPP J.G., VARRÓ A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br. J. Pharmacol. 2002;137:361–368. doi: 10.1038/sj.bjp.0704881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRENNAN M., PALANISWAMI M., KAMEN P. Do existing measures of Poincaré plot geometry reflect nonlinear features of heart rate variability. IEEE Trans. Biomed. Eng. 2001;48:1342–1347. doi: 10.1109/10.959330. [DOI] [PubMed] [Google Scholar]
- CARLSSON L., ALMGREN O., DUKER G. QTU-prolongation and torsades de pointes induced by putative class III antiarrhythmic agents in the rabbit: etiology and interventions. J. Cardiovasc. Pharmacol. 1990;16:276–285. doi: 10.1097/00005344-199008000-00014. [DOI] [PubMed] [Google Scholar]
- CARLSSON L., AMOS G.J., ANDERSSON B., DREWS L., DUKER G., WADSTEDT G. Electrophysiological characterization of the prokinetic agents cisapride and mosapride in vivo and in vitro: implications for proarrhythmic potential. J. Pharmacol. Exp. Ther. 1997;282:220–227. [PubMed] [Google Scholar]
- CHIBA K., SUGIYAMA A., HAGIWARA T., TAKAHASHI S., TAKASUNA K., HASHIMOTO K. In vivo experimental approach for the risk assessment of fluoroquinolone antibacterial agents-induced long QT syndrome. Eur. J. Pharmacol. 2004;486:189–200. doi: 10.1016/j.ejphar.2003.12.014. [DOI] [PubMed] [Google Scholar]
- ECKARDT L., BREITHARDT G., HAVERKAMP W. Electrophysiologic characterization of the antipsychotic drug sertindole in a rabbit heart model of torsade de pointes: low torsadogenic potential despite QT prolongation. J. Pharmacol. Exp. Ther. 2002;300:64–71. doi: 10.1124/jpet.300.1.64. [DOI] [PubMed] [Google Scholar]
- FERGUSON J., MACDONALD K.J., KENICER K.J. Terfenadine and placebo compared in the treatment of chronic idiopathic urticaria: a randomised double-blind study. Br. J. Clin. Pharmacol. 1985;20:639–641. doi: 10.1111/j.1365-2125.1985.tb05122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEELEN P., DROLET B., RAIL J., BERUBE J., DALEAU P., ROUSSEAU G., CARDINAL R., O'HARA G.E., TURGEON J. Sildenafil (Viagra) prolongs cardiac repolarization by blocking the rapid component of the delayed rectifier potassium current. Circulation. 2000;102:275–277. doi: 10.1161/01.cir.102.3.275. [DOI] [PubMed] [Google Scholar]
- GINTANT G.A., LIMBERIS J.T., McDERMOTT J.S., WEGNER C.D., COX B.F. The canine Purkinje fiber: an in vitro model system for acquired long QT syndrome and drug-induced arrhythmogenesis. J. Cardiovasc. Pharmacol. 2001;37:607–618. doi: 10.1097/00005344-200105000-00012. [DOI] [PubMed] [Google Scholar]
- HONDEGHEM L.M., HOFFMANN P. Blinded test in isolated female rabbit heart reliably identifies action potential duration prolongation and proarrhythmic drugs: importance of triangulation, reverse use dependence, and instability. J. Cardiovasc. Pharmacol. 2003;41:14–24. doi: 10.1097/00005344-200301000-00003. [DOI] [PubMed] [Google Scholar]
- LALONDE R.L., LESSARD D., GAUDREAULT J. Population pharmacokinetics of terfenadine. Pharm. Res. 1996;13:832–838. doi: 10.1023/a:1016036624935. [DOI] [PubMed] [Google Scholar]
- LIU S., MELCHERT R.B., KENNEDY R.H. Inhibition of L-type Ca2+ channel current in rat ventricular myocytes by terfenadine. Circ. Res. 1997;81:202–210. doi: 10.1161/01.res.81.2.202. [DOI] [PubMed] [Google Scholar]
- LU H.R., REMEYSEN P., DE CLERCK F. Nonselective IKr-blockers do not induce torsades de pointes in the anesthetized rabbit during α1-adrenoceptor stimulation. J. Cardiovasc. Pharmacol. 2000;36:728–736. doi: 10.1097/00005344-200012000-00007. [DOI] [PubMed] [Google Scholar]
- MASUMIYA H., SAITO M., ITO M., MATSUDA T., NOGUCHI K., IIDA-TANAKA N., TANAKA H., SHIGENOBU K. Lack of action potential-prolonging effect of terfenadine on rabbit myocardial tissue preparations. Biol. Pharm. Bull. 2004;27:131–135. doi: 10.1248/bpb.27.131. [DOI] [PubMed] [Google Scholar]
- MING Z., NORDIN C. Terfenadine blocks time-dependent Ca2+, Na+, and K+ channels in guinea pig ventricular myocytes. J. Cardiovasc. Pharmacol. 1995;26:761–769. doi: 10.1097/00005344-199511000-00013. [DOI] [PubMed] [Google Scholar]
- MONAHAN B.P., FERGUSON C.L., KILLEAVY E.S., LLOYD B.K., TROY J., CANTILENA L.R., JR Torsades de pointes occurring in association with terfenadine use. JAMA. 1990;264:2788–2790. [PubMed] [Google Scholar]
- PINNEY S.P., KOLLER B.S., FRANZ M.R., WOOSLEY R.L. Terfenadine increases the QT interval in isolated guinea pig heart. J. Cardiovasc. Pharmacol. 1995;25:30–34. doi: 10.1097/00005344-199501000-00006. [DOI] [PubMed] [Google Scholar]
- REDFERN W.S., CARLSSON L., DAVIS A.S., LYNCH W.G., MACKENZIE I., PALETHORPE S., SIEGL P.K., STRANG I., SULLIVAN A.T., WALLIS R., CAMM A.J., HAMMOND T.G. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- RODEN D.M. Taking the ‘idio' out of ‘idiosyncratic': predicting torsades de pointes. Pacing. Clin. Electrophysiol. 1998;21:1029–1034. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- SATOH T., ZIPES D.P. Rapid rates during bradycardia prolong ventricular refractoriness and facilitate ventricular tachycardia induction with cesium in dogs. Circulation. 1996;94:217–227. doi: 10.1161/01.cir.94.2.217. [DOI] [PubMed] [Google Scholar]
- SATOH Y., SUGIYAMA A., TAKAHARA A., ANDO K., WANG K., HONSHO S., HASHIMOTO K. The antipsychotic and antiemetic drug prochlorperazine delays the ventricular repolarization of the in situ canine heart. J. Pharmacol. Sci. 2005;97:101–106. doi: 10.1254/jphs.fpj04038x. [DOI] [PubMed] [Google Scholar]
- SATOH Y., SUGIYAMA A., TAKAHARA A., CHIBA K., HASHIMOTO K. Electropharmacological and proarrhythmic effects of a class III antiarrhythmic drug nifekalant hydrochloride assessed using the in vivo canine models. J. Cardiovasc. Pharmacol. 2004;43:715–723. doi: 10.1097/00005344-200405000-00015. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER J., HAUSER R., ANDREAS J.O., LINZ K., JAHNEL U. Differential effects of human ether-a-go-go-related gene (HERG) blocking agents on QT duration variability in conscious dogs. Eur. J. Pharmacol. 2005;512:53–60. doi: 10.1016/j.ejphar.2005.01.042. [DOI] [PubMed] [Google Scholar]
- SCHOENMAKERS M., RAMAKERS C., VAN OPSTAL J.M., LEUNISSEN J.D., LONDONO C., VOS M.A. Asynchronous development of electrical remodeling and cardiac hypertrophy in the complete AV block dog. Cardiovasc. Res. 2003;59:351–359. doi: 10.1016/s0008-6363(03)00430-9. [DOI] [PubMed] [Google Scholar]
- SHIINA H., SUGIYAMA A., TAKAHARA A., SATOH Y., HASHIMOTO K. Comparison of the electropharmacological effects of verapamil and propranolol in the halothane-anesthetized in vivo canine model under monophasic action potential monitoring. Jpn. Circ. J. 2000;64:777–782. doi: 10.1253/jcj.64.777. [DOI] [PubMed] [Google Scholar]
- STENGL M., RAMAKERS C., NABAR A., DONKER D.W., VOS M.A., VOLDERS P.G. IKs downregulation in canine ventricular hypertrophy confers the loss of β-adrenergic-induced shortening of repolarization, favoring proarrhythmia. Circulation. 2004;110 (Suppl III):III–319. [Google Scholar]
- SUGIYAMA A., HASHIMOTO K. Effects of a typical IKr channel blocker sematilide on the relationship between ventricular repolarization, refractoriness and onset of torsades de pointes. Jpn. J. Pharmacol. 2002;88:414–421. doi: 10.1254/jjp.88.414. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA A., ISHIDA Y., SATOH Y., AOKI S., HORI M., AKIE Y., KOBAYASHI Y., HASHIMOTO K. Electrophysiological, anatomical and histological remodeling of the heart to AV block enhances susceptibility to arrhythmogenic effects of QT-prolonging drugs. Jpn. J. Pharmacol. 2002a;88:341–350. doi: 10.1254/jjp.88.341. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA A., MOTOMURA S., HASHIMOTO K. Utilization of an isolated, blood-perfused canine papillary muscle preparation as a model to assess efficacy and adversity of class I antiarrhythmic drugs. Jpn. J. Pharmacol. 1994;66:303–316. doi: 10.1254/jjp.66.303. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA A., SATOH Y., SHIINA H., TAKAHARA A., YONEYAMA M., HASHIMOTO K. Cardiac electrophysiologic and hemodynamic effects of sildenafil, a PDE5 inhibitor, in anesthetized dogs. J. Cardiovasc. Pharmacol. 2001;38:940–946. doi: 10.1097/00005344-200112000-00016. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA A., SATOH Y., SHIINA H., TAKEDA S., HASHIMOTO K. Torsadegenic action of the antipsychotic drug sulpiride assessed using in vivo canine models. J. Cardiovasc. Pharmacol. 2002b;40:235–245. doi: 10.1097/00005344-200208000-00009. [DOI] [PubMed] [Google Scholar]
- TAKAHARA A., SUGIYAMA A., HASHIMOTO K. Reduction of repolarization reserve by halothane anaesthesia sensitizes the guinea-pig heart for drug-induced QT interval prolongation. Br. J. Pharmacol. 2005;146:561–567. doi: 10.1038/sj.bjp.0706352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHARA A., SUGIYAMA A., SATOH Y., NAKAMURA Y., HASHIMOTO K. Cardiovascular effects of an L/N-type Ca2+ channel blocker cilnidipine assessed in the chronic atrioventricular conduction block dogs. J. Pharmacol. Sci. 2004;96:219–223. doi: 10.1254/jphs.scj04007x. [DOI] [PubMed] [Google Scholar]
- TARANTINO P., APPLETON N., LANSDELL K. Effect of trazodone on hERG channel current and QT-interval. Eur. J. Pharmacol. 2005;510:75–85. doi: 10.1016/j.ejphar.2005.01.009. [DOI] [PubMed] [Google Scholar]
- THE ICH STEERING COMMITTEE. The nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals (S7B). The international conference on harmonization of technical requirements for registration of pharmaceuticals for human use (ICH) 2005. The Guideline was recommended for adoption at Step 4 of the ICH process in May 2005 ()
- THOMSEN M.B., VERDUYN S.C., STENGL M., BEEKMAN J.D., DE PATER G., VAN OPSTAL J., VOLDERS P.G., VOS M.A. Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation. 2004;110:2453–2459. doi: 10.1161/01.CIR.0000145162.64183.C8. [DOI] [PubMed] [Google Scholar]
- THOMSEN M.B., VOLDERS P.G., STENGL M., SPATJENS R.L., BEEKMAN J.D., BISCHOFF U., KALL M.A., FREDERIKSEN K., MATZ J., VOS M.A. Electrophysiological safety of sertindole in dogs with normal and remodeled hearts. J. Pharmacol. Exp. Ther. 2003;307:776–784. doi: 10.1124/jpet.103.052753. [DOI] [PubMed] [Google Scholar]
- USUI T., SUGIYAMA A., ISHIDA Y., SATOH Y., SASAKI Y., HASHIMOTO K. Simultaneous assessment of the hemodynamic, cardiomechanical, and electrophysiological effects of terfenadine on the in vivo canine model. Heart Vessels. 1998;13:49–57. doi: 10.1007/BF01744586. [DOI] [PubMed] [Google Scholar]
- VAN DE WATER A., VERHEYEN J., XHONNEUX R., RENEMAN R.S. An improved method to correct the QT interval of the electrocardiogram for change in heart rate. J. Pharmacol. Methods. 1989;22:207–217. doi: 10.1016/0160-5402(89)90015-6. [DOI] [PubMed] [Google Scholar]
- VERDUYN S.C., RAMAKERS C., SNOEP G., LEUNISSEN J.D., WELLENS H.J., VOS M.A. Time course of structural adaptations in chronic AV block dogs: evidence for differential ventricular remodeling. Am. J. Physiol. 2001;280:H2882–H2890. doi: 10.1152/ajpheart.2001.280.6.H2882. [DOI] [PubMed] [Google Scholar]
- VOLDERS P.G., SIPIDO K.R., VOS M.A., SPATJENS R.L., LEUNISSEN J.D., CARMELIET E., WELLENS H.J. Downregulation of delayed rectifier K+ currents in dogs with chronic complete atrioventricular block and acquired torsades de pointes. Circulation. 1999;100:2455–2461. doi: 10.1161/01.cir.100.24.2455. [DOI] [PubMed] [Google Scholar]
- VOS M.A., DE GROOT S.H., VERDUYN S.C., VAN DER ZANDE J., LEUNISSEN H.D., CLEUTJENS J.P., VAN BILSEN M., DAEMEN M.J., SCHREUDER J.J., ALLESSIE M.A., WELLENS H.J. Enhanced susceptibility for acquired torsade de pointes arrhythmias in the dog with chronic, complete AV block is related to cardiac hypertrophy and electrical remodeling. Circulation. 1998;98:1125–1135. doi: 10.1161/01.cir.98.11.1125. [DOI] [PubMed] [Google Scholar]
- ZHANG S., ZHOU Z., GONG Q., MAKIELSKI J.C., JANUARY C.T. Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ. Res. 1999;84:989–998. doi: 10.1161/01.res.84.9.989. [DOI] [PubMed] [Google Scholar]