Abstract
Leukocyte locomotion over the lumen of inflamed endothelial cells is a critical step, following firm adhesion, in the inflammatory response. Once firmly adherent, the cell will spread and will either undergo diapedesis through individual vascular endothelial cells or will migrate to tight junctions before extravasating to the site of injury or infection. Little is known about the mechanisms of neutrophil spreading or locomotion, or how motility is affected by the physical environment. We performed a systematic study to investigate the effect of the type of adhesive ligand and shear stress on neutrophil motility by employing a parallel-plate flow chamber with reconstituted protein surfaces of E-selectin, E-selectin/PECAM-1, and E-selectin/ICAM-1. We find that the level and type of adhesive ligand and the shear rate are intertwined in affecting several metrics of migration, such as the migration velocity, random motility, index of migration, and the percentage of cells moving in the direction of flow. On surfaces with high levels of PECAM-1, there is a near doubling in random motility at a shear rate of 180 s−1 compared to the motility in the absence of flow. On surfaces with ICAM-1, neutrophil random motility exhibits a weaker response to shear rate, decreasing slightly when shear rate is increased from static conditions to 180 s−1, and is only slightly higher at 1000 s−1 than in the absence of flow. The random motility increases with increasing surface concentrations of E-selectin and PECAM-1 under static and flow conditions. Our findings illustrate that the endothelium may regulate neutrophil migration in postcapillary venules through the presentation of various adhesion ligands at sites of inflammation.
INTRODUCTION
Adhesion of leukocytes to inflamed vascular endothelium is a critical first step in the body's innate immune response and occurs through a well defined adhesion cascade consisting of rolling, firm adhesion, spreading, locomotion, and transmigration. Mechanisms responsible for the initial attachment and rolling of polymorphonuclear leukocytes (PMNs) are well characterized. The selectin family of adhesion molecules are responsible for initial tethering and rolling of PMNs to ligands present on inflamed endothelium (1–4). Additional proteins such as P-selectin glycoprotein-1 (PSGL-1) (5), I-domain of αLβ2 (6,7), and a yet to be determined ligand for E-selectin on PMNs allow for prolonged stable rolling interactions. During rolling, PMNs sense signals through selectin ligation (8) and the presentation of chemokines on the endothelial surface (9), which cause a rapid activation, up-regulation, and colocalization of β2 integrins to the tips of the microvilli, resulting in firm adhesion to members of the immunoglobulin superfamily such as intercellular adhesion molecule-1 (ICAM-1) (10,11). PMNs subsequently spread, undergo locomotion over the lumen wall (12), and extravasate through tight junctions (13) or take a transcellular route to the site of infection through much less well understood mechanisms (14).
The effects of the type of adhesion molecule and the level of shear stress on the initial attachment and rolling and subsequent firm adhesion of neutrophils have been well studied (1,11,15–17). It has been shown that the rolling flux of leukocytes in vivo decreases with increasing shear rate (18) and that cell deformability plays a key role in stabilizing rolling interactions at high shear rates, causing a plateau in rolling velocity as a function of shear rate (19). Selectins allow for initial attachment and rolling, whereas firm adhesion is a result of β2 integrins becoming activated and increasing their affinity to ICAM-1 (2,10,15). Although many of the mechanisms regarding adhesion ligand kinetics and shear with respect to capture and rolling have been elucidated, their effect on neutrophil motility has only recently been investigated.
Cinamon and co-workers (20) recently studied the combinatorial effect of hydrodynamic shear and chemoattractant signals on transendothelial migration (TEM). They found that PMNs were able to undergo TEM on HUVEC activated with high levels of TNF-α and expressing high levels of ICAM-1 and E-selectin in a β2-integrin-dependent manner, regardless of whether flow was imposed. However, flow became an important factor for TEM to occur on untreated or slightly activated endothelium, and TEM was substantially increased with the addition of platelet-activating factor (PAF) (20). Rainger et al. (21) found that increasing concentrations of platelet endothelial cell adhesion molecule-1 (PECAM-1 or CD31) increased the directional index of migration, which is the fraction of the trajectory that was in the direction of flow, at a shear rate of 50 s−1. They also found that increasing concentrations of fibronectin immobilized with P-selectin and BSA caused an increase in the directional index of migration. PECAM-1 has been found to be expressed over the entire luminal surface of the endothelium (22) and highly expressed on cell borders of endothelial cells and PMNs (23). It is unlikely that a soluble chemoattractant gradient can be set up under blood flow, so it has been postulated that PMNs can sense flow-generated stress through PECAM-1/PECAM-1 or αvβ3/PECAM-1 interactions that allow PMNs to find tight junctions (21,24). Furthermore, it has been seen by Carman and Springer (14) that an alternate and less common transmigration path exists directly through the endothelial cell, suggesting that haptotaxis is not required for TEM. Luu et al. (24) found that PMNs migrated at ∼6 μm/min and preferred the direction of flow while migrating on top of the endothelium as a result of PECAM-1/PECAM-1 interactions but migrated randomly at ∼14 μm/min while migrating under the endothelium where flow was not present.
Because PECAM-1 is found throughout the surface of the endothelial wall, and no obvious gradient of PECAM-1 is observed, it is possible that PECAM-1 is not responsible for the directed migration of PMNs to the endothelial tight junctions. Because there is an interplay between shear rates and adhesive ligands on rolling dynamics, it is possible that the motility of PMNs on endothelium is a more complex behavior where PECAM-1, ICAM-1, and other proteins are involved together with shear stress in aiding PMNs to the correct location for transmigration to occur, where this location can be either through tight junctions or through the endothelial cell itself. In this article we set out to study the interplay between the effects of shear rates on PMNs in the presence of various physiological adhesive ligands. To do this, we utilized a laminar-flow parallel-plate flow chamber with reconstituted protein surfaces composed of combinations of E-selectin, ICAM-1, and PECAM-1 because previous work has shown them to be major players during migration, and shear rates of 0, 180, and 1000 s−1 were employed to test physiological shear rates and compare to static conditions.
We found that the type of adhesive ligand and the shear rate were intertwined in affecting cell locomotion. We determined how various chemical surfaces with various shear rates affected migration velocity, random motility, directional index of migration, and the number of cells that move in the direction of shear. We found the trend for increasing random motility under static conditions to be E-selectin < E-selectin/PECAM-1 < E-selectin/ICAM-1. Once shear was imposed, the random motility on the E-selectin surface increased slightly as a function of shear. The random motility on the E-selectin/ICAM-1 surface decreased at 180 s−1 but nearly doubled at 1000 s−1 to become only slightly higher than that seen in the absence of shear. The random motility on an E-selectin/PECAM-1 surface, when a high level of PECAM-1 was present, nearly doubled when the shear rate was increased from 0 to 180 s−1 and increased slightly further at 1000 s−1. A weaker response was seen on a surface made solely of E-selectin, and reducing the amount of PECAM-1 on an E-selectin/PECAM-1 surface also led to a weak response. However, surfaces with a high level of PECAM-1 but a low level of E-selectin supported motility, suggesting that motility is driven strongly by the level of PECAM-1. We found that the directional index of migration increases with increasing shear rate for E-selectin and E-selectin/ICAM-1 surfaces; however, a leveling off is again seen for the E-selectin/PECAM-1 surfaces with high levels of PECAM-1 between 180 and 1000 s−1. Again, lower concentrations of PECAM-1 resulted in behavior resembling that on surfaces coated purely with E-selectin. Based on this evidence, we have proposed a force-based directed-motility model in which a combination of the adhesion ligands and the shear rate affects the probability that the cell will migrate upstream of the direction of flow. We found that different levels of shear rates were required to affect the percentage of cells that have a net movement in the direction of flow. At the highest shear rate tested (1000 s−1), the percentage of cells moving in the direction of flow (downstream) ranged from ∼75% for the E-selectin/PECAM-1 surface to ∼90% for the E-selectin surface. These results indicate a complex interplay of molecular mechanisms and shear rate that allows the endothelium to control motility.
MATERIALS AND METHODS
Proteins
Recombinant human ICAM-1, recombinant human E-selectin, and recombinant human PECAM-1 were purchased from R & D Systems (Minneapolis, MN). Anti-human PECAM-1 mAb that binds domain 1 of PECAM-1 was purchased from Ancell (Bayport, MN).
Substrate preparation
Eight-well, rectangular flexiPerm gaskets were placed on microscope slides cut from bacteriological polystyrene dishes. The surface enclosed by one of the eight wells was incubated overnight with 200 μl of the appropriate protein dilution in binding buffer (0.1 M NaHCO3, pH 9.2) at 4°C. Protein dilutions of 0.1 μg/ml E-selectin (low E-selectin), 1 μg/ml E-selectin (E-selectin), 1 μg/ml E-selectin + 4 μg/ml ICAM-1 (ICAM-1), 1 μg/ml E-selectin + 0.5 μg/ml PECAM-1 (low PECAM-1), 1 μg/ml E-selectin + 1 μg/ml PECAM-1 (moderate PECAM-1), and 1 μg/ml E-selectin + 4 μg/ml PECAM-1 (high PECAM-1) were used. After incubation, slides were washed and blocked with 0.5% Tween 20 (Sigma, St. Louis, MO) diluted in HBSS (without Ca and Mg) (Cambrex, Walkersville, MD) for at least 2 h at 4°C.
mAb blocking
The high-PECAM-1 surface was made and blocked as described above. Anti-human PECAM-1 was diluted in the 0.5% Tween 20 solution to 20 μg/ml, and 200 μl was added for 30 min immediately before the migration assay.
Surface site density
E-selectin, E-selecin/ICAM-1, and E-selectin/PECAM-1 substrates were prepared as described above. Substrates were first blocked with StartingBlock (Pierce, Rockford, IL) according to manufacturer's protocol, followed by 25% rat serum (Sigma) for 2 h. Rat serum blocking as well as all subsequent incubations and washes were performed at 4°C to prevent dissociation of proteins. Following blocking procedures, mouse anti-human E-selectin, ICAM-1, or PECAM-1 antibody (Ancell) was added at 2 μg/ml for 2 h. After three PBS washes, horseradish peroxidase (HRP)-conjugated rat anti-mouse secondary antibody (BD Pharmingen, San Diego, CA) was added at 1:1000 dilution for 2 h. Samples were then washed three more times with PBS before addition of Amplex Red ELISA substrate (Molecular Probes, Eugene, OR). Measurements were made after 30 min using a fluorescence plate reader with 485 nm excitation and 525 nm emission filters. Fluorescence signal was converted to ligand density using HRP concentration calibration and known substrate area and by assuming a 1:1:1 ratio of ligand/primary antibody/secondary antibody. We found surface site densities to be 80 E-selectin molecules/μm2 for the low-E-selectin surface, 291 ± 3 E-selectin molecules/μm2 for the E-selectin surface, 150 ± 21 E-selectin molecules and 192 ± 7 ICAM-1 molecules/μm2 for the ICAM-1 surface, 70 ± 14 E-selectin molecules and 1067 ± 28 PECAM-1 molecules/μm2 for the high-PECAM-1 surface, 101 ± 2 E-selectin molecules and 647 ± 135 PECAM-1 molecules/μm2 for the moderate-PECAM-1 surface, and 153 ± 14 E-selectin molecules and 241 ± 48 PECAM-1 molecules/μm2 for the low-PECAM-1 surface.
Neutrophil separation
Whole blood was taken from healthy adult donors into BD Vacutainers containing K3EDTA (Becton Dickinson, Franklin Lakes, NJ). Seven milliliters of whole blood was layered onto 4 ml of dextran density gradient (Robbins Scientific, Sunnyvale, CA) and centrifuged at 500× g for 60 min. The PMN layer was washed once with HBSS (without Ca and Mg). The PMNs were counted and placed in HBSS (without Ca and Mg) + 0.1% human serum (Golden West Biologicals, Temecula, CA) + 10 mM HEPES (BioWhittaker Walkersville, MD). An HBSS (without Ca and Mg) buffer containing 15 mM Ca2+ and 20 mM Mg2+ was added in a 1:10 dilution to the HBSS buffer and incubated at 37°C for 10 min before the flow assay.
Laminar flow assay for motility
A straight-channel, parallel-plate flow chamber was used for laminar flow assays. A straight channel template cut from 0.01-inch-thick Duralastic sheeting (Allied Biomedical, Goose Creek, SC) was placed over a protein-coated slide. The template and slide were then placed in the bottom well of the flow chamber. The flow chamber experiment was previously described (25,26): the assembled chamber was mounted on the stage of a Nikon Diaphot inverted microscope with phase-contrast optics (Nikon, Tokyo, Japan); 105 PMNs in 1 ml HBSS + 0.12 human serum + 10 mM HEPES + 1.5 mM Ca2+ + 2.0 mM Mg2+ were placed in a test tube and connected to the flow chamber using rubber tubing, and flow was initiated with an infusion/withdrawal syringe pump (Harvard Apparatus, South Natick, MA). Experiments were recorded using a Cohu black-and-white CCD camera (Cohu, San Diego, CA) and Sony SVO-9500MD S-VHS recorder (Sony Medical Systems, Montvale, NJ). For each experiment, wall shear stress (τw) was calculated as previously described (26). The parallel-plate flow chamber with reconstituted protein surfaces composed of E-selectin, E-selectin/ICAM-1, and E-selectin/PECAM-1 was utilized, and we activated PMNs with fMLF once a significant number of neutrophils were rolling on the surface by switching the buffer to HBSS + 1.5 mM Ca2+ + 2.0 mM Mg2+ + 2 nM fMLF. On activation with fMLF, the cells immediately became firmly adherent, spread, and were tracked as they migrated.
Data analysis
Still images taken every 10 s were analyzed using LabVIEW VI (National Instruments, Austin, TX) software. The x,y-positions of individual cells were found by calculating the cell's centroid and were tracked over the entire length of the experiment. This analysis was repeated for every neutrophil. The mean squared displacement (MSD) was calculated by time-averaging the square of the distance traversed in the x-direction plus the square of the distance traversed in the y-direction. The sliding time average was determined by calculating the MSD for every x-second interval and averaging the values to give the MSD at x seconds. The values for the migration regime were plotted versus time, and the data in the locomotion regime was fit to a Langevin-type equation, r2 = 4D (t − τ (1 − e−t/τ), where D is the random motility coefficient, r2 is the two-dimensional mean-squared displacement, t is time, and τ is the persistence time (27).
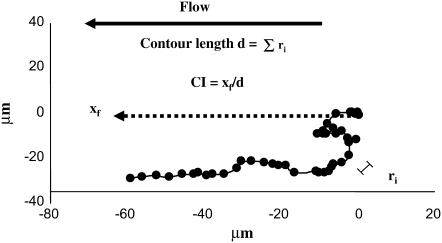
Several other definitions are used in the text to describe migration patterns, as shown in Fig. 1. In the assays, flow is directed right to left, xf is the distance that the cell traversed in the direction of flow, ri is the distance the cell traversed in a 10-s period, and d is the contour length of the cell trajectory defined as the sum of ri during migration (once the centroid has been displaced 5 μm). We define the chemotactic index (CI) of migration as xf/d, which is positive if the net translation is in the direction of flow. The migration velocity is calculated by dividing d by the time the cell was migrating. The percentage of cells with a positive index of migration is calculated by dividing the number of cells with a positive index of migration by the total number of cells.
FIGURE 1.
Parameter definitions. This illustrates a typical trajectory of a PMN during motility under shear flow. The initial coordinates of the cell are (0,0). Flow is directed right to left, xf is the distance that the cell traversed in the direction of flow, ri is the distance the cell traversed in a 10-s period, and d is the contour length of the cell trajectory defined as the sum of ri in the migration regime (once the centroid has displaced 5 μm). We define the index of migration, or CI, as xf/d, which is positive if the net translation is in the direction of flow.
Cells tracked for the six surfaces and three shear rates consist of low E-selectin (0 s−1, 4 d, 27 cells; 180 s−1, 4 d, 21 cells; 1000 s−1, 4 d, 25 cells), E-selectin (0 s−1, 4 d, 26 cells; 180 s−1, 4 d, 27 cells; 1000 s−1, 4 d, 24 cells), low PECAM-1 (0 s−1, 4 d, 36 cells; 180 s−1, 4 d, 28 cells; 1000 s−1, 4 d, 30 cells), moderate PECAM-1 (0 s−1, 4 d, 31 cells; 180 s−1, 4 d, 26 cells; 1000 s−1, 4 d, 28 cells), high PECAM-1 (0 s−1, 4 d, 28 cells; 180 s−1, 4 d, 25 cells; 1000 s−1, 5 d, 35 cells), mAb-blocked high PECAM-1 (180 s−1, 4 d, 25 cells), and ICAM-1 (0 s−1, 4 d, 24 cells; 180 s−1, 6 d, 38 cells; 1000 s−1, 4 d, 34 cells). The error bars indicate the standard error.
RESULTS
Migration velocity of neutrophils
PMNs were captured and rolled on the various surfaces until 2 nM fMLF was introduced into the inlet buffer. On fMLF injection, the cells immediately became firmly adherent and began to spread. Once the cell was fully spread, it began to migrate and was tracked during its entire trajectory. The period of spreading was approximately 1–2 min. To measure the migration velocity, the contour length of the trajectory of the PMNs in the migration regime, d, was measured and divided by the time that the cells were in the migration regime.
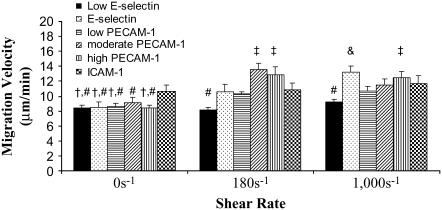
The migration velocities of neutrophils on low-E-selectin, E-selectin, low-PECAM-1, moderate-PECAM-1, high-PECAM-1, and ICAM-1 surfaces (see Materials and Methods) with shear rates of 0, 180, and 1000 s−1 were measured (Fig. 2). Under static conditions, the migration velocity was found to be highest on the ICAM-1 surface. The E-selectin and various PECAM-1 surfaces showed an increase in migration velocity at 180 s−1 from 0 s−1 but did not continue to increase when shear rate was increased to 1000 s−1. Migration on the low-E-selectin surface and the ICAM-1 surface showed little dependence on shear rate (Fig. 2). High-PECAM-1 surfaces blocked with 20 μg/ml anti-PECAM-1 resulted in a migration velocity of 7.4 ± 0.1 μm/min at 180 s−1, which is approximately equal to the migration velocity on the low-E-selectin surface.
FIGURE 2.
Migration velocity. (A) Migration velocities for surfaces coated in 0.1 μg/ml E-selectin (solid bars, low-E-selectin), 1 μg/ml E-selectin (black dots, E-selectin), 1 μg/ml E-selectin + 0.5 μg/ml PECAM-1 (horizontal black stripes, low-PECAM-1), 1 μg/ml E-selectin + 1 μg/ml PECAM-1 (diagonal black stripes, moderate-PECAM-1), 1 μg/ml E-selectin + 4 μg/ml PECAM-1 (vertical black stripes, high-PECAM-1), and 1 μg/ml E-selectin + 4 μg/ml ICAM-1, (checkered, ICAM-1) at 0 (first grouping), 180 (second grouping), and 1000 s−1 (third grouping). The velocities were found by dividing the contour length of the cell trajectory during the migration phase (d) by the time the cell is in its migration regime. The migration velocities are found from at least 4 d, and the error bars represent standard error. A two-way ANOVA and a least-squares means differences Tukey HSD test were performed using JMP 5.1 with α = 0.05 (# is statistically different from &, and † is statistically different from ‡).
Random motility
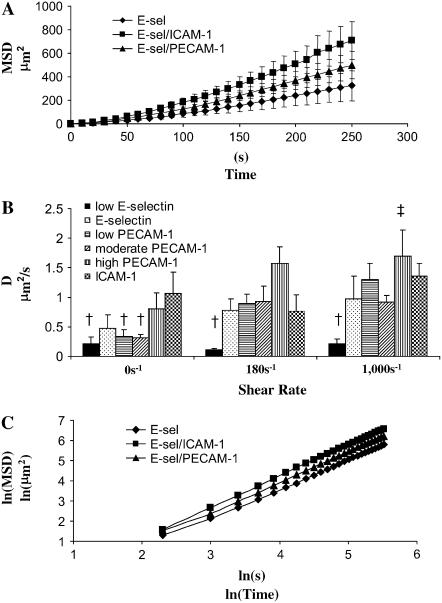
The MSD, r2, was calculated as described in Materials and Methods and plotted versus time; representative plots are shown in Fig. 3 A. Fig. 3 A contains data for PMNs migrating on E-selectin, ICAM-1, and high-PECAM-1 surfaces under static conditions.
FIGURE 3.
Random motility and persistence time. The two-dimensional mean squared displacement (MSD) was plotted versus time for the migration regime that does not include spreading or small translations up to 5 μm. The MSD was calculated using time averaging over the range of data. The average MSD for each run (includes at least five cells) was averaged with runs from other days (at least 4 days), and the standard error is reported. (A) Typical MSD curves. The two-dimensional MSD was plotted versus time, and the curves are shown for surfaces coated in 1 μg/ml E-selectin (diamond, E-selectin), 1 μg/ml E-selectin + 4 μg/ml ICAM-1 (square, ICAM-1), and 1 μg/ml E-selectin + 4 μg/ml PECAM-1 (triangle, high PECAM-1) under static conditions. (B) Measure of D. The curves from Fig. 4 A as well as similar curves for the 180 and 1000 s−1 shear rates were fit to determine D, the random motility coefficient. This was found for surfaces of 0.1 μg/ml E-selectin (solid bars, low-E-selectin), 1 μg/ml E-selectin (black dots, E-selectin), 1 μg/ml E-selectin + 0.5 μg/ml PECAM-1 (horizontal black stripes, low-PECAM-1), 1 μg/ml E-selectin + 1 μg/ml PECAM-1 (diagonal black stripes, moderate-PECAM-1), 1 μg/ml E-selectin + 4 μg/ml PECAM-1 (vertical black stripes, high-PECAM-1), and 1 μg/ml E-selectin + 4μg/ml ICAM-1 (checkered, ICAM-1) at 0 (first grouping), 180 (second grouping), and 1000 s−1 (third grouping). A two-way ANOVA and a least-squares means differences Tukey HSD test were performed using JMP 5.1 with α = 0.05 († is statistically different from ‡). (C) Measure of diffusiveness of cell motility. Plotting the curves from Fig. 4 A, 1 μg/ml E-selectin (diamond, E-selectin), 1 μg/ml E-selectin + 4 μg/ml ICAM-1 (square, ICAM-1), and 1 μg/ml E-selectin + 4 μg/ml PECAM-1 (triangle, high-PECAM-1) under static conditions, as the natural log of MSD versus natural log of time results in slopes of ∼1.42.
The random motility coefficients for neutrophils migrating on the low-E-selectin, E-selectin, low-PECAM-1, moderate-PECAM-1, high-PECAM-1, and ICAM-1 surfaces at shear rates of 0, 180, and 1000 s−1 were determined from a Langevin-type equation (see Materials and Methods) (Fig. 3 B). The random motility of neutrophil migration is dependent on the type of ligand and shear rate. The random motility under static conditions was found to follow the trend low E-selectin < E-selectin ≈ low PECAM-1 ≈ moderate PECAM-1 < high PECAM-1 < ICAM-1. The high motility on ICAM-1 and high-PECAM-1 surfaces is reasonable because migration has been found to be β2-integrin dependent (28), and PECAM-1/PECAM-1 interactions have been implicated in migration (24). Compared to static conditions, at 180 s−1, the random motility is not altered on an E-selectin surface, slightly decreases on the ICAM-1 surface, and is nearly double on the high-PECAM-1 surface. High-PECAM-1 surfaces blocked with 20 μg/ml anti-PECAM-1 resulted in a random motility coefficient of 0.23 ± 0.05 μm2/s at 180 s−1, which is in close agreement with the low-E-selectin surface. At 1000 s−1, the random motility for the ICAM-1 surface reaches the level achieved by the high-PECAM-1 surface at 180 s−1, whereas the random motility on the E-selectin surface only slightly increases as the shear rate goes from 0 to 180 to 1000 s−1. The random motility showed a much weaker response to shear stresses with lower concentrations of PECAM-1, similar to that seen with the low-E-selectin and E-selectin surface. The results on the low-E-selectin surface indicate that the increase in motility is a result of increasing amounts of PECAM-1 and not a decrease in the amount of E-selectin.
The persistence time, τ, found from fitting the MSD curves, was found to range from 30 to 100 s, which is on the same order as that found by others for PMNs under various conditions (29). Surprisingly, these values did not indicate any trend with respect to shear rate or adhesive chemistry, except that the low-E-selectin surface resulted in the lowest persistence time.
To characterize the randomness of neutrophil motility, the MSDs versus time were plotted for the 18 different conditions in which shear rate and surface chemistry were altered. These ln-ln plots of MSD versus time had an average slope of 1.42 ± 0.03, and the deviations did not indicate any specific trend. Three of these curves for random motility under static conditions for different surface chemistries are illustrated in Fig. 3 C. For reference, a slope of 1 would indicate that PMNs undergo random motion, whereas a slope of 3/2 would indicate that PMN motility is undergoing superdiffusive motion (30,31). Thus, a slope of ∼3/2, as shown in Fig. 3 C, indicates that neutrophil motility is superdiffusive, even under static conditions.
Directional index of migration and percentage of cells moving in the direction of flow
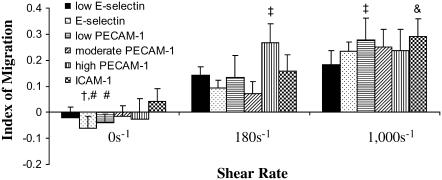
The directional index of migration is calculated by dividing the distance the cells traversed in the direction of flow by its contour length (xf/d). A schematic of the parameters measured can be seen in Fig. 1. The directional index of migration gives an indication of the amount of movement that is in the direction of flow versus the total trajectory.
The directional index of migration was found for PMNs migrating on six surface compositions (low E-selectin, E-selectin, low PECAM-1, moderate PECAM-1, high PECAM-1, and ICAM-1) under imposed shear rates of 0, 180, and 1000 s−1 (Fig. 4). Under static conditions, the cells display a zero index of migration, as expected. At nonzero shear rates, PMNs preferentially moved in the direction of flow in a shear rate– and ligand-dependent manner. For the low-E-selectin, E-selectin, low-PECAM-1, moderate-PECAM-1, and ICAM-1 surfaces, the index of migration increases with increasing shear rate. The index of migration increased more for the high-PECAM-1 surface for a shear rate of 180 s−1 than for the other surfaces but remained unchanged from 180 to 1000 s−1. At 180 s−1, the index of migration shows the trend of low E-selectin ≈ E-selectin ≈ low PECAM-1 ≈ moderate PECAM-1 ≈ ICAM-1 < high PECAM-1. High-PECAM-1 surfaces blocked with 20 μg/ml anti-PECAM-1 resulted in an index of migration of ∼0.14 ± 0.01 at 180 s−1, which is approximately equal to the index of migration of the low-E-selectin surface. All the surfaces resulted in a similar index of migration at 1000 s−1 with the low-E-selectin surface showing the smallest increase. This indicates that the vascular endothelium can regulate directionality of motion by presentation of adhesion proteins.
FIGURE 4.
Index of migration. (A) The index of migration (xf/d) was found for surfaces coated in 0.1 μg/ml E-selectin (solid bars, low-E-selectin), 1 μg/ml E-selectin (black dots, E-selectin), 1 μg/ml E-selectin + 0.5 μg/ml PECAM-1 (horizontal black stripes, low-PECAM-1), 1 μg/ml E-selectin + 1 μg/ml PECAM-1 (diagonal black stripes, moderate-PECAM-1), 1 μg/ml E-selectin + 4 μg/ml PECAM-1 (vertical black stripes, high-PECAM-1), and 1 μg/ml E-selectin + 4 μg/ml ICAM-1 (checkered, ICAM-1) at 0 (first grouping), 180 (second grouping), and 1000 s−1 (third grouping). The average index of migration is found from at least 4 d, and the error bars represent standard error. A two-way ANOVA and a least-squares means differences Tukey HSD test were performed using JMP 5.1 with α = 0.05 (# is statistically different from &, and † is statistically different from ‡).
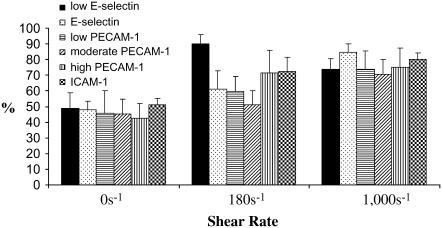
The percentage of cells that had a net movement in the direction of flow was measured for the low-E-selectin, E-selectin, low-PECAM-1, moderate-PECAM-1, high-PECAM-1, and ICAM-1 surfaces at shear rates of 0, 180, and 1000 s−1 (Fig. 5). Neutrophils preferentially migrate in the direction of shear in a ligand- and shear rate–dependent manner. At 0 s−1 there does not appear to be a preference to move in any direction. At 180 s−1, ICAM-1 and high-PECAM-1 surfaces appear to cause a slight increase in the percentage of cells moving in the direction of flow; however, the largest increase occurs on the low-E-selectin surface. Despite differences in the index of migration between high-PECAM-1 and ICAM-1 surfaces, there was no difference in the percentage of cells moving in the direction of shear of high-PECAM-1 versus ICAM-1 surfaces. High-PECAM-1 surfaces blocked with 20 μg/ml anti-PECAM-1 resulted in ∼76 ± 5% of neutrophils at 180 s−1 with a net movement in the direction of flow, which in this case remains approximately equal to that found for high-PECAM-1 surfaces. There are still a significant number of cells moving against the direction of shear at 1000 s−1 regardless of the surface composition, ranging from ∼10% to 30% of the cells.
FIGURE 5.
Percentage of cells with a net movement in the direction of flow. (A) The percentage of cells with a positive index of migration (xf) in the direction of shear was found for surfaces coated in 0.1 μg/ml E-selectin (solid bars, low-E-selectin), 1 μg/ml E-selectin (black dots, E-selectin), 1 μg/ml E-selectin + 0.5 μg/ml PECAM-1 (horizontal black stripes, low PECAM-1), 1 μg/ml E-selectin + 1 μg/ml PECAM-1 (diagonal black stripes, moderate-PECAM-1), 1 μg/ml E-selectin + 4 μg/ml PECAM-1 (vertical black stripes, high-PECAM-1), and 1 μg/ml E-selectin + 4 μg/ml ICAM-1 (checkered, ICAM-1) at 0 (first grouping), 180 (second grouping), and 1000 s−1 (third grouping). The average percentages are found from at least 4 d, and the error bars represent standard error. A two-way ANOVA and a least-squares means differences Tukey HSD test were performed using JMP 5.1 with α = 0.05 (no statistically significant differences were found).
DISCUSSION
There is evidence that chemoattractant signals, shear stress, and substrate chemistry are all parameters that are intertwined in determining neutrophil migration, as shown here and by others (23,32–35). The purpose of this study was to use a reconstituted protein system to determine the interplay between shear stress and substrate chemistry as they relate to neutrophil locomotion. E-selectin is known to recruit PMNs to sites of inflammation (2,36), whereas ICAM-1 has been shown to be largely responsible for subsequent firm adhesion (37). There is evidence that PECAM-1 interactions rapidly adjust the neutrophil cytoskeleton and cause integrin activation (38,39). Because all three molecules are present at sites of inflammation and have been shown to have key roles in neutrophil adhesion and motility, they were chosen for in-depth study in this paper. Dustin and Springer found ICAM-1 surface expression on activated human umbilical and saphenous vein endothelial cells to reach 5 × 106 sites/cell (40) or ∼6000 molecules/μm2 for an upper bound when 800 μm2 (41) is used as the cell surface area (42). Dustin and co-workers suggest that this number indicates up to a 40-fold increase over an unactivated endothelial cell, which would correspond to ∼150 molecules/μm2 (40). Hentzen and co-workers utilized flow cytometry to find a site density of 2.3 × 105 sites of ICAM-1 per unactivated endothelial cell, which translates to ∼290 molecules/μm2, and a site density of ∼1000 sites/μm2 for 4-h IL-1–activated endothelial cells (42,43). Our reported number of ∼192 molecules of ICAM-1/μm2 is on the same order as that reported by Dustin and Springer and Hentzen and co-workers for unstimulated endothelial cells. Furthermore, Langley and co-workers quantified the ratio of ICAM-1 to PECAM-1 for different organs in a murine model (44). They found that the ratio of ICAM-1 to PECAM-1 varies from ∼0.26 for heart tissue to ∼0.89 for lung tissue. In this study we looked at ICAM-1/PECAM-1 ratios of ∼0.18 to ∼0.8 if we compare the ICAM-1 surface to the three PECAM-1 surfaces, which appears to be within the physiological range. The authors are not aware of any quantitative measurements of E-selectin site densities, which may be because of the rapid up- and downregulation of E-selectin in vivo. The available data indicate that we are working on the low end of physiological expression for ICAM-1 and PECAM-1. A different method for adsorbing ICAM-1 to the surface would most likely need to be worked out in order to perform subsequent studies that look at the high end of physiological conditions. Three shear rates of 0, 180, and 1000 s−1 were tested in this study. Traditionally, most neutrophil migration studies were done in the absence of flow, but a wide range of shear rates are seen in vivo, which we attempted to mimic by using these three shear rates.
We measured the migration velocity, random motility coefficient, index of migration, and percentage of cells with a net positive index of migration for the six substrates tested at three shear rates. For the E-selectin, ICAM-1, and high PECAM-1 surfaces we found the following: 1) The migration velocity of PMNs increased on the E-selectin and high-PECAM-1 surfaces when flow was imposed, but the migration velocity on the ICAM-1 surface remained independent of shear rate. 2) Under static conditions, the random motility coefficient was found to follow the trend of E-selectin < high PECAM-1 < ICAM-1. The random motility coefficient more than doubled from the E-selectin to the ICAM-1 surface, which is in agreement with PMN migration being dominated by Mac-1 binding to ICAM-1 and subsequent cytoskeleton rearrangements (30). 3) When a shear rate of 180 s−1 was imposed, the random motility of the high-PECAM-1 surface showed a noted increase, whereas the random motility on an E-selectin surface remained nearly the same as that at 0 s−1; on an ICAM-1 surface, the random motility actually showed a slight decrease with shear rate. 4) At 1000 s−1, the random motility coefficient of ICAM-1 surface nearly doubled from that at 180 s−1, thus reaching the level achieved by the high-PECAM-1 surface at 180 s−1. Random motility further increased on the high-PECAM-1 surface when the shear rate was increased to 1000 s−1, whereas there was little increase in random motility on the E-selectin surface at 1000 s−1. 5) The index of migration was zero under static conditions for all three surfaces and increased with increasing shear rate for the E-selectin and ICAM-1 surfaces, whereas a maximum was reached for the high-PECAM-1 surface at a shear rate of 180 s−1. 6) An increase in the percentage of cells moving in the direction of flow was seen on the E-selectin surface at 1000 s−1, whereas a large increase was seen on the ICAM-1 and high-PECAM-1 surfaces at 180 s−1 shear rate. 7) Lower concentrations of PECAM-1 resulted in behavior that closely resembled that of the E-selectin surface, indicating that a high level of PECAM-1 would need to be presented along the endothelium to alter neutrophil migration. Furthermore, the low-E-selectin surface indicates that the increase in motility was caused by an increasing amount of PECAM-1 and not a decreasing amount of E-selectin. This conclusion was further verified by antibody blocking PECAM-1 on the high-PECAM-1 surface at 180 s−1, which resulted in a similar migration velocity, random motility coefficient, and index of migration as the low-E-selectin surface.
These data indicate that there is a complex relationship among adhesion ligand kinetics, shear rate, and possible signaling components activated through mechanical forces or the adhesion of specific proteins. It is clear that ICAM-1 and PECAM-1 become critical for locomotion under conditions of high shear stress, which may be a result of ICAM-1's strong interaction with activated β2 integrins presented on the PMN surface and by PECAM-1's possible signaling or less well understood adhesion dynamics. It is also apparent that there needs to be a combination of shear stress and the presence of high levels of PECAM-1 for the neutrophil to reach optimum levels of random motility. It seems counterintuitive that an increase in PECAM-1 on the surface, which should result in more bonds, would actually increase the random motility, and this could be further evidence that PECAM-1 causes a more rapid rearrangement of the cytoskeleton (38). Our data indicate that it is possible that a combination of a high level of PECAM-1/PECAM-1 interactions in combination with shear is required for signaling rapid cytoskeletal rearrangements. One may also expect the addition of ICAM-1 would reduce the random motility, but it is possible that rapid turnover of Mac-1 binding to ICAM-1 results in a more efficient random walk. It appears that ICAM-1 as well as PECAM-1 (21) is capable of acting as a flow-directing device depending on the shear rate imposed. From these results, it becomes unclear if there is a mechanotransduction element in flow-directed PMN motility or if it is simply a result of the opposite forces acting on the PMN (see discussion below). ICAM-1 itself results in the capture of a very low number of PMNs, whereas PECAM-1 alone on the surface results in no interactions of the PMNs from free stream (data not shown).
Plotting the MSD versus time and calculating the random motility coefficient give a measure of how well PMNs are able to randomly sample the surface. In the absence of a concentration gradient, which is likely the case as the cell is undergoing locomotion over the lumen wall, an increase in the random motility would increase the chances for PMNs to reach a tight junction to undergoTEM. Our data indicate that ICAM-1 and PECAM-1 are necessary on the endothelial surface to allow for an increased sampling of the surface. The MSD versus time was also plotted as a ln-ln plot. The slope was found to be 1.42 ± 0.03 for the 18 cases, which indicates a superdiffusive behavior (Fig. 3 C: 180 and 1000 s−1 shear rates not shown) (31). This is consistent with our knowledge of cytoskeleton machinery within PMNs (45,46). The motility is driven by actin polymerization in the leading lamellae and contractile actin/myosin assemblies in the uropod that drive locomotion within the cell. The fact that the slope does not approach 2, which would indicate a ballistic behavior, signifies that the locomotion is not being dominated by a drift component. However, our remaining data do suggest that the flow results in preferential movement in the direction of flow.
Rainger et al. (21) found that the index of migration of PMNs on surfaces coated with P-selectin and BSA increases with increasing concentrations of PECAM-1 or fibronectin at a shear rate of 50 s−1. They did not see a large increase in the index of migration until the coating had 2.5 or 5.0 μg/ml PECAM-1, resulting in indices of migration of ∼0.2 and 0.28, respectively, which are in close agreement with our results for the high-PECAM-1 surface at a shear rate of 180 s−1. Luu et al. (24) showed that PECAM-1/PECAM-1 interactions act as sensors for directed migration and migration velocity, using antibodies to block as PMNs migrated on and under human umbilical vein endothelial cells. Rainger et al. (35) found that PMNs' migration accelerates until it levels off on their various surface compositions. The migration velocity of neutrophils on ICAM-1 surfaces in Rainger et al. (35) is lower than the value we found on a similar surface, perhaps because they block the surfaces with BSA, which is also known to bind to Mac-1 (9).
We show that a comparable directional index of migration could be obtained at high shear rates on the ICAM-1 surface as with the high-PECAM-1 surface. We also show that the percentage of cells with a net positive index of migration was ligand and shear rate dependent and that an increase is seen with increasing shear for all surfaces. As shear rate increased from 0 to 180 s−1, an increase was noted on the ICAM-1 and high-PECAM-1 surfaces compared to the E-selectin surface alone. At 1000 s−1, the percentage of cells moving in the direction of flow on the E-selectin surface reaches nearly 90%, indicating that the probability of the transient bonds in the reverse direction being strong enough to counteract the force being imposed is very low. In contrast, the stronger adhesion on ICAM-1 and high-PECAM-1 surfaces leads to a decrease to 70–80% of the cells moving in the direction of flow.
Here, we have shown that it is the interplay between an adhesive molecule and shear stress that modulates directional migration. When ICAM-1 is presented on the substrate and a shear stress of 1000 s−1 is applied, the index of migration is similar to that on a PECAM-1 surface at a much lower shear rate. Thus, similar signaling events might be happening when PMNs are exposed to 1000 s−1 on ICAM-1 substrates as those at 180 s−1 on PECAM-1 substrates. It is possible that an alternative form of directed motility is taking place. This model is based only on physical forces acting on PMNs as explained next. A PMN might migrate along the substrate, stop, detach a little while lifting its lamellipod off the surface but keeping its uropod bound; the flow pushes the PMN, adhesion occurs, migration restarts, and so on, giving a “sense” of directionality to the migration. The shear stress necessary to observe this behavior occurs at different values for ICAM-1 and PECAM-1 because the binding force of the PECAM-1/PECAM-1 interaction is most likely different from the β2-integrin/ICAM-1 interaction. Further evidence for this hypothesis comes from observations of neutrophil migration using reflection interference contrast microscopy (H. Aranda-Espinoza, L. Smith, and D. Hammer, unpublished data), where the adhesion contact area of the PMN is often in the rear of the cell (uropod) as the lamellipod lifts up, perhaps to better sense its environment. The cells that had a net negative index of migration even under high shear rates may have spent a majority of their time migrating while a large portion of each was adhered to the surface. This would cause the entire cell to be closer to the surface, experience less force being imposed, and remain largely unaffected by external forces and capable of performing its random walk without bias.
In the future, similar assays to the ones described here will be performed utilizing reflection interference contrast microscopy and traction force microscopy. Reflection interference contrast microscopy will allow us to determine how these different adhesion ligands affect cell area and tight adhesion contacts during migration. Force traction measurements will allow us to determine the area of the cell that creates the greatest amount of force as well as the magnitude of the force that is created within the cell. In coupling these results, we hope to gain new insight on how adhesion ligands and shear affect neutrophil migration.
Acknowledgments
The authors thank Eric Johnston for writing the cell-tracking code, Stephanie Eucker for assistance with JMP 5.1, as well as John Crocker and Sally Zigmond for useful discussions.
We acknowledge support from National Institutes of Health grant HL18208.
References
- 1.Lawrence, M. B., and T. A. Springer. 1991. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 65:859–873. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence, M. B., and T. A. Springer. 1993. Neutrophils roll on E-selectin. J. Immunol. 151:6338–6346. [PubMed] [Google Scholar]
- 3.Buttrum, S. M., R. Hatton, and G. B. Nash. 1993. Selectin-mediated rolling of neutrophils on immobilized platelets. Blood. 82:1165–1174. [PubMed] [Google Scholar]
- 4.Lawrence, M. B., E. L. Berg, E. C. Butcher, and T. A. Springer. 1995. Rolling of lymphocytes and neutrophils on peripheral node addressin and subsequent arrest on ICAM-1 in shear flow. Eur. J. Immunol. 25:1025–1031. [DOI] [PubMed] [Google Scholar]
- 5.Mehta, P., K. D. Patel, T. M. Laue, H. P. Erickson, and R. P. McEver. 1997. Soluble monomeric P-selectin containing only the lectin and epidermal growth factor domains binds to P-selectin glycoprotein ligand-1 on leukocytes. Blood. 90:2381–2389. [PubMed] [Google Scholar]
- 6.Shimaoka, M., C. Lu, R. T. Palframan, U. H. von Andrian, A. McCormack, J. Takagi, and T. A. Springer. 2001. Reversibly locking a protein fold in an active conformation with a disulfide bond: integrin alphaL I domains with high affinity and antagonist activity in vivo. Proc. Natl. Acad. Sci. USA. 98:6009–6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salas, A., M. Shimaoka, A. N. Kogan, C. Harwood, U. H. von Andrian, and T. A. Springer. 2004. Rolling adhesion through an extended conformation of integrin alphaLbeta2 and relation to alpha I and beta I-like domain interaction. Immunity. 20:393–406. [DOI] [PubMed] [Google Scholar]
- 8.Simon, S. I., Y. Hu, D. Vestweber, and C. W. Smith. 2000. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J. Immunol. 164:4348–4358. [DOI] [PubMed] [Google Scholar]
- 9.Ley, K. 2003. Arrest chemokines. Microcirculation. 10:289–295. [DOI] [PubMed] [Google Scholar]
- 10.Takagi, J., and T. A. Springer. 2002. Integrin activation and structural rearrangement. Immunol. Rev. 186:141–163. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence, M. B., C. W. Smith, S. G. Eskin, and L. V. McIntire. 1990. Effect of venous shear stress on CD18-mediated neutrophil adhesion to cultured endothelium. Blood. 75:227–237. [PubMed] [Google Scholar]
- 12.Schenkel, A. R., Z. Mamdouh, and W. A. Muller. 2004. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat. Immunol. 5:393–400. [DOI] [PubMed] [Google Scholar]
- 13.Springer, T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 76:301–314. [DOI] [PubMed] [Google Scholar]
- 14.Carman, C. V., and T. A. Springer. 2004. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell. Biol. 167:377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbassi, O., T. K. Kishimoto, L. V. McIntire, D. C. Anderson, and C. W. Smith. 1993. E-selectin supports neutrophil rolling in vitro under conditions of flow. J. Clin. Invest. 92:2719–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiVietro, J. A., M. J. Smith, B. R. Smith, L. Petruzzelli, R. S. Larson, and M. B. Lawrence. 2001. Immobilized IL-8 triggers progressive activation of neutrophils rolling in vitro on P-selectin and intercellular adhesion molecule-1. J. Immunol. 167:4017–4025. [DOI] [PubMed] [Google Scholar]
- 17.Eniola, A. O., P. J. Willcox, and D. A. Hammer. 2003. Interplay between rolling and firm adhesion elucidated with a cell-free system engineered with two distinct receptor-ligand pairs. Biophys. J. 85:2720–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ley, K., and P. Gaehtgens. 1991. Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ. Res. 69:1034–1041. [DOI] [PubMed] [Google Scholar]
- 19.Lei, X., M. B. Lawrence, and C. Dong. 1999. Influence of cell deformation on leukocyte rolling adhesion in shear flow. J. Biomech. Eng. 121:636–643. [DOI] [PubMed] [Google Scholar]
- 20.Cinamon, G., V. Shinder, R. Shamri, and R. Alon. 2004. Chemoattractant signals and β2 integrin occupancy at apical endothelial contacts combine with shear stress signals to promote transendothelial neutrophil migration. J. Immunol. 173:7282–7291. [DOI] [PubMed] [Google Scholar]
- 21.Rainger, G. E., C. D. Buckley, D. L. Simmons, and G. B. Nash. 1999. Neutrophils sense flow-generated stress and direct their migration through alphaVbeta3-integrin. Am. J. Physiol. 276:H858–H864. [DOI] [PubMed] [Google Scholar]
- 22.Feng, D., J. A. Nagy, K. Pyne, H. F. Dvorak, and A. M. Dvorak. 2004. Ultrastructural localization of platelet endothelial cell adhesion molecule (PECAM-1, CD31) in vascular endothelium. J. Histochem. Cytochem. 52:87–101. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien, C. D., P. Lim, J. Sun, and S. M. Albelda. 2003. PECAM-1-dependent neutrophil transmigration is independent of monolayer PECAM-1 signaling or localization. Blood. 101:2816–2825. [DOI] [PubMed] [Google Scholar]
- 24.Luu, N. T., G. E. Rainger, C. D. Buckley, and G. B. Nash. 2003. CD31 regulates direction and rate of neutrophil migration over and under endothelial cells. J. Vasc. Res. 40:467–479. [DOI] [PubMed] [Google Scholar]
- 25.Rodgers, S. D., R. T. Camphausen, and D. A. Hammer. 2000. Sialyl Lewis(x)-mediated, PSGL-1-independent rolling adhesion on P-selectin. Biophys. J. 79:694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eniola, A. O., S. D. Rodgers, and D. A. Hammer. 2002. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes. Biomaterials. 23:2167–2177. [DOI] [PubMed] [Google Scholar]
- 27.Lauffenburger, D. A., and J. J. Linderman. 1993. Receptors: Models for Binding, Trafficking, and Signaling. Oxford University Press, New York.
- 28.Anderson, S. I., N. A. Hotchin, and G. B. Nash. 2000. Role of the cytoskeleton in rapid activation of CD11b/CD18 function and its subsequent downregulation in neutrophils. J. Cell. Sci. 113:2737–2745. [DOI] [PubMed] [Google Scholar]
- 29.Tranquillo, R. T., D. A. Lauffenburger, and S. H. Zigmond. 1988. A stochastic model for leukocyte random motility and chemotaxis based on receptor binding fluctuations. J. Cell Biol. 106:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheikh, S., W. B. Gratzer, J. C. Pinder, and G. B. Nash. 1997. Actin polymerisation regulates integrin-mediated adhesion as well as rigidity of neutrophils. Biochem. Biophys. Res. Commun. 238:910–915. [DOI] [PubMed] [Google Scholar]
- 31.Metzler, R., and J. Klafter. 2004. The restaurant at the end of the random walk: recent developments in the description of anomalous transport by fractional dynamics. J. Phys. A: Math. Gen. 37:R161–R208. [Google Scholar]
- 32.Tranquillo, R. T., S. H. Zigmond, and D. A. Lauffenburger. 1988. Measurement of the chemotaxis coefficient for human neutrophils in the under-agarose migration assay. Cell. Motil. Cytoskel. 11:1–15. [DOI] [PubMed] [Google Scholar]
- 33.Cassimeris, L., and S. H. Zigmond. 1990. Chemoattractant stimulation of polymorphonuclear leucocyte locomotion. Semin. Cell Biol. 1:125–134. [PubMed] [Google Scholar]
- 34.Sheikh, S., G. E. Rainger, Z. Gale, M. Rahman, and G. B. Nash. 2003. Exposure to fluid shear stress modulates the ability of endothelial cells to recruit neutrophils in response to tumor necrosis factor-alpha: a basis for local variations in vascular sensitivity to inflammation. Blood. 102:2828–2834. [DOI] [PubMed] [Google Scholar]
- 35.Rainger, G. E., C. Buckley, D. L. Simmons, and G. B. Nash. 1997. Cross-talk between cell adhesion molecules regulates the migration velocity of neutrophils. Curr. Biol. 7:316–325. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence, M. B., D. F. Bainton, and T. A. Springer. 1994. Neutrophil tethering to and rolling on E-selectin are separable by requirement for L-selectin. Immunity. 1:137–145. [DOI] [PubMed] [Google Scholar]
- 37.Neelamegham, S., A. D. Taylor, A. R. Burns, C. W. Smith, and S. I. Simon. 1998. Hydrodynamic shear shows distinct roles for LFA-1 and Mac-1 in neutrophil adhesion to intercellular adhesion molecule-1. Blood. 92:1626–1638. [PubMed] [Google Scholar]
- 38.Dimitrijevic, I., L. Axelsson, and T. Andersson. 2002. The adhesion receptor CD-31 can be primed to rapidly adjust the neutrophil cytoskeleton. Biochem. Biophys. Res. Commun. 292:1092–1097. [DOI] [PubMed] [Google Scholar]
- 39.Zhao, T., and P. J. Newman. 2001. Integrin activation by regulated dimerization and oligomerization of platelet endothelial cell adhesion molecule (PECAM)-1 from within the cell. J. Cell Biol. 152:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dustin, M. L., and T. A. Springer. 1988. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J. Cell Biol. 107:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbee, K. A., P. F. Davies, and R. Lal. 1994. Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. Circ. Res. 74:163–171. [DOI] [PubMed] [Google Scholar]
- 42.Lomakina, E. B., and R. E. Waugh. 2004. Micromechanical tests of adhesion dynamics between neutrophils and immobilized ICAM-1. Biophys. J. 86:1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hentzen, E. R., S. Neelamegham, G. S. Kansas, J. A. Benanti, L. V. McIntire, C. W. Smith, and S. I. Simon. 2000. Sequential binding of CD11a/CD18 and CD11b/CD18 defines neutrophil capture and stable adhesion to intercellular adhesion molecule-1. Blood. 95:911–920. [PubMed] [Google Scholar]
- 44.Langley, R. R., J. Russell, M. J. Eppihimer, S. J. Alexander, M. Gerritsen, R. D. Specian, and D. N. Granger. 1999. Quantification of murine endothelial cell adhesion molecules in solid tumors. Am. J. Physiol. 277:H1156–H1166. [DOI] [PubMed] [Google Scholar]
- 45.Wang, F., P. Herzmark, O. D. Weiner, S. Srinivasan, G. Servant, and H. R. Bourne. 2002. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat. Cell Biol. 4:513–518. [DOI] [PubMed] [Google Scholar]
- 46.Xu, J., F. Wang, A. Van Keymeulen, P. Herzmark, A. Straight, K. Kelly, Y. Takuwa, N. Sugimoto, T. Mitchison, and H. R. Bourne. 2003. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 114:201–214. [DOI] [PubMed] [Google Scholar]