Abstract
Outer membrane protein P5 of nontypeable (acapsulate) Haemophilus influenzae (NTHi P5) forms large pores in planar lipid bilayers between symmetric solutions that unpredictably display a nonzero reversal potential. Moreover, NTHi P5 has a high theoretical isoelectric point, calculated as 9.58, which is not in agreement with the experimental isoelectric point, determined as 6.3–6.8, or with its preference for cations, disproportionately strong at one side. These anomalous results intimate that NTHi P5 is associated with a polyanion. Chemical and immunological analyses revealed the presence of inorganic polyphosphate (polyP), and the amphiphilic, solvating polyester, poly-(R)-3-hydroxybutyrate, frequently associated with polyP. A sharp reduction in cation selectivity was observed after addition of Saccharomyces cerevisiae exopolyphosphatase X to the bilayer, providing functional evidence for the involvement of polyP in selectivity. The results suggest that NTHi P5 associates with polyP and poly-(R)-3-hydroxybutyrate to create large, cation-selective pores in the outer membrane of H. influenzae.
INTRODUCTION
Nontypeable (acapsulate) Haemophilus influenzae (NTHi) is a Gram-negative opportunistic pathogen, commonly associated with otitis media, chronic bronchitis, and other mucosal infections (1). Outer membrane protein P5 (NTHi P5) may play a role in NTHi pathogenesis by binding specifically to a variety of receptors on the host cell membrane, including respiratory mucin (2–5), bronchial epithelial cells (6), and the carcinoembryonic antigen family of cell adhesion molecules (7,8). The method of interaction of NTHI P5 with each of these receptors during colonization and infection is uncertain, but it is presumably mediated by surface-exposed domains.
The structure of NTHi P5 is not yet determined, but the sequence shows significant homology to that of outer membrane protein A of Escherichia coli (OmpA) (9). Circular dichroism studies indicate a β-strand content of 49–55% (10), as compared to the 40% β-strand content reported for E. coli OmpA by Sugawara et al. (11). Significant variation in the NTHi P5 gene has been noted during chronic infections (12); Webb and Cripps (10) found that the variable regions correspond to surface-exposed loops as predicted by alignment of NTHi P5 amino acid sequences with the topology model proposed by Vogel and Jähnig for the N-terminal segment of OmpA (13).
Our previous electrophysiological studies (14) have shown that recombinant NTHi P5, purified from inclusion bodies using the nonionic detergent, octylglucoside (OG-P5) forms pores in planar lipid bilayers that display a major conductance state of 1.1 ± 0.1 nS and high open probability (Po = 0.99) at 22°C. The large pores are well-defined and stable to changes in temperature or storage at low temperatures for extended periods. However, they are readily converted to narrow pores (58 ± 6 pS) when exposed to the ionic detergent, lithium dodecyl sulfate (LDS). The LDS-P5 narrow pores are stable at lower temperatures, but are converted to large pores by incubation at temperatures > 40°C, suggesting they are an intermediate or partially denatured form. During our recent studies of NTHi P5, we noted a nonzero reversal potential when the pores were in planar lipid bilayers between symmetric K+ solutions. Here we explore the molecular basis of this unexpected asymmetry in a large conductance pore.
MATERIALS AND METHODS
NTHi P5
Omp P5 from NTHi strain UC19, originally recovered from the sputum of a chronic bronchitis patient and cloned in plasmid pCU28 by Webb and Cripps (15) with an N-terminal extension of 10 residues that included six histidines. This clone was generously provided by Dr. Dianne C. Webb (University of Canberra, Australia).
Preparation of recombinant NTHi P5 protein
NTHi P5 was prepared as previously described (14). Briefly, plasmid pCU28, containing His-tagged P5 was transformed into B12 Gold competent cells and overexpressed by addition of 1 mM isopropyl-beta-D-thiogalactopyranoside. Inclusion bodies were collected and extracted with n-octyl-bD-glucopyranoside (10 mg/ml; OG-P5). The His-tag was inaccessible, consequently, OG-P5 was further purified by chromatography on Superdex S-200 (1.6 × 60 cm, HiPrep, Pharmacia) equilibrated with 0.4 M LiCl, 20 mM Tris-HCl, pH 7.5 containing 0.1% octylglucoside. Purified P5 was concentrated using Microcon tubes (10 Kd) to a final concentration of ∼22 μg/ml.
The partially denatured narrow pore form (LDS-P5) was prepared in the same manner, except that inclusion bodies were extracted with 2% LDS and chromatography eluent contained 0.1% LDS in place of 0.1% OG.
SDS-PAGE
Samples were diluted (1:1, v/v) with loading buffer (0.125 M Tris-Cl, 4% SDS, 20% glycerol, 10% dithiothreitol, 0.2% bromphenol blue) and heated for 3 min in a boiling water bath. Proteins were electrophoretically separated on 12% SDS-PAGE gels (Bio-Rad, Hercules, CA) using Tris-glycine sodium dodecyl sulfate (SDS) buffer (Bio-Rad) at constant voltage of 180 mV. Protein bands were visualized by staining with Coomassie brilliant Blue R-250.
Planar lipid bilayer measurements
Planar lipid bilayers were formed from a solution of synthetic diphytanoylphosphatidylcholine (DPhPC, Avanti Polar Lipids, Birmingham, AL) in n-decane (Aldrich) (17 mg/ml). The solution was used to paint a bilayer in an aperture of ∼150μm diameter in a Delrin cup (Warner Instruments, Hamden, CT) between symmetric aqueous bathing solutions at 22°C 1M KCl, 10 mM Tris, pH 7.1 or, for gradient studies, 150 mM KCl cis and 50 mM KCl trans, in 10 mM Tris, pH 7.3. The mean activity coefficients of electrolytes are 0.743 in 150 mM KCl and 0.816 in 50 mM KCl. Fluctuations of junction potential, estimated with the JPCALC of the pClamp software, were 0.5–0.8 mV. All salts were ultrapure (>99%) (Sigma-Aldrich, St. Louis, MO). Bilayer capacitances were in the range of 25–50 pF. After the bilayers were formed, 0.2–0.5 μl of the NTHi P5 in DPhPC vesicles (2 μg/ml) was added to the cis compartment with gentle stirring.
Recording and data analysis
Unitary currents were recorded with an integrating patch clamp amplifier (Axopatch 200A, Axon Instruments, Foster City, CA). The trans solution (voltage command side) was connected to the CV 201A head stage input, and the cis solution was held at virtual ground via a pair of matched Ag-AgCl electrodes. Currents through the voltage-clamped bilayers (background conductance) < 3 pS) were low-pass-filtered at 10 kHz (−3 dB cutoff, Besel type response) and recorded after digitization through an analog-to-digital converter (Digidata 1322A, Axon Instruments). Data were filtered at 100 Hz through an 8 pole Bessel filter (902LPF, Frequency Devices, Haverhill, MA) and digitized at 1 kHz using pClamp9 software (Axon Instruments). Single-channel conductance events were identified automatically and analyzed by using Clampfit9 software (Axon Instruments).
Determination of isoelectric point (pI)
The pI of NTHi P5 was determined by analytical gel isoelectric focusing (IEF) on 0.4 mm gels of 5% acrylamide/bisacrylamide (33.7:1), 5% 3–10 ampholytes, 20 mM C12M on gel support film using a Bio-Rad model 111 Mini IEF cell. Voltage steps were applied for 15 min at 100 V, 15 min at 200 V and 60 min at 450 V. IEF standards were from Bio-Rad. Gels were stained with Coomassie Brilliant Blue R-250, crocein scarlet (Bio-Rad IEF stain), and destained with 40% methanol, 10% acetic acid.
Extraction of inorganic polyphosphate (polyP) from NTHi P5
Proteinase K (200 μg/ml final conc.) was added to NTHi P5 (500 μg in 1.2 ml of 10 mM Hepes, pH 7.2, 0.05% OG) in a Teflon tube. After incubation at room temperature for 40 min, 5 μg polyP5 (Sigma-Aldrich) was added to serve as a carrier. Phenol/chloroform (1:1 w/v, equilibrated with 10 mM Hepes, pH 7.2) (1/2 vol) was added, the sample was gently mixed and then centrifuged at low speed to separate layers. The aqueous layer was transferred to a clean teflon tube. The remaining organic layer and interface was extracted 3× with 10 mM EDTA, pH 7.2 (0.25 ml per extraction). The aqueous layers were pooled and extracted 1× with 1/2 vol 1:1 phenol/chloroform and then 3× with chloroform (1 ml each extraction). Traces of chloroform remaining in the aqueous layer were removed with a stream of N2 gas.
Determination of polyP
PolyP was separated by electrophoresis on a 15% acrylamide gel (acrylamide:bis 19:1) with Tris/borate/EDTA (90/90/2.7 mM), pH 8.3 (16). Xylene cyanol (XC) and brom phenol blue (BPB) were used as markers; under these conditions, XC and BPB migrate to the same positions as polyP72 and polyP35 respectively. The gel was stained with 0.05% o-toluidine blue in 25% methanol, 5% glycerol; destaining was with 25% methanol, 5% glycerol.
Determination of poly-(R)-3-hydroxybutyrate (PHB)
The presence of PHB was detected by Western blot analysis of SDS-PAGE gels with anti-PHB IgG raised in rabbits to a synthetic 8-mer of R-3-hydroxybutyrate (courtesy of D. Seebach, ETH, Switzerland) by Metabolix. The identity of PHB was confirmed by a chemical assay as described by Huang and Reusch (18). Briefly, PHB was converted to crotonic acid by heating the dry sample in conc. sulfuric acid at 120°C for 40 min. The resulting crotonic acid was extracted, and chromatographed on an Aminex HPX-87H ion exclusion organic acid analysis column (Bio-Rad) using 0.014 N H2SO4 as eluent. The crotonic acid peak was identified by its elution time, ultrviolet absorption curve, and mass spectrum, and quantitated by comparison of the peak area with that of crotonic acid standards. PHB from Alcaligenes spp. (Sigma-Aldrich) subjected to the same procedure produces ∼50% of the theoretical yield of crotonic acid.
RESULTS
Asymmetry and cation selectivity of NTHi P5
NTHi P5, purified using the nonionic detergent OG (OG-P5) and inserted into DPhPC bilayers between symmetric solutions of 1M KCl, 10 mM Tris, pH 7.1 at 22°C, forms large pores (1.1 ± 0.1 nS) with high open probability (0.99) (14) that exhibit a nonzero reversal potential. A representative current record is shown in Fig. 1. This unexpected observation led us to examine its ion selectivity. For this study, OG-P5 was inserted into DPhPC bilayers between 3:1 gradient solutions of KCl (the channel was added to the cis compartment) and the reversal potential was determined. The gradient sides were then inverted and new values for the reversal potential were registered. A total of 21 such experiments were done at each side. OG-P5 exhibited cation selectivity on both sides, but to a different degree. On one side, it was slightly cation selective with Vrev = +9 mV (or −9 mV with reversed gradient); and on the other side, it was strongly cation selective with Vrev = +24 mV (or −24 mV) (Fig. 2), very to close to the Nernst theoretical potential for K+ of +26 mV (or −26 mV) (Fig. 2).
FIGURE 1.
(A) Representative current traces (from > 30 independent experiments) that demonstrate asymmetry of the pore formed by NTHi P5, purified with OG P5, and incorporated into planar bilayers of DiPhPC between symmetric solutions of 1 M KCl, 10 mM Tris, pH 7.3 at 22°C. (B) I/V relationship of OG-P5 in panel A.
FIGURE 2.
I/V plots for OG-P5 in planar bilayers of DiPhPC between 3:1 KCl gradients. The cis compartment (ground side) contained 150 mM KCl and the trans compartment (command side) contained 50 mM KCl. Channel inserted in orientation I (▾) exhibited slightly cationic selectivity with Vrev = +9 mV; orientation II (▵) provided strong cationic selectivity with Vrev = +24 mV. Data were summarized from a total of 19 experiments.
Determination of pI of NTHi P5
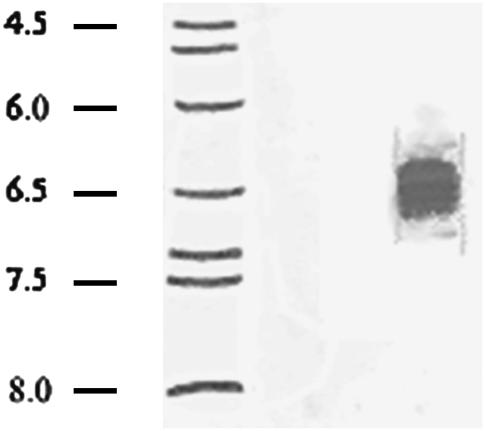
The preference of NTHi P5 for cations was inconsistent with the theoretical pI of the protein, calculated as 9.53 (ExPASy Compute pI/Mw tool). NTHi P5, used in this study, has been modified by addition of a hexa-Histidine tag at the N-terminal end (15), and this modification produces a protein with a slightly higher theoretical pI of 9.58. Therefore, the experimental pI of NTHi P5 was determined by analytical isoelectric focusing on acrylamide gels. The protein formed a wide band centering at pI 6.3 to pI 6.8 (Fig. 3). Notably, Reusch (19) had previously found a similar difference between theoretical and experimental pIs for the Streptomyces lividans potassium channel, KcsA. In this case, the difference was attributable to the presence of the polyanion, polyP, and polyP was associated with the amphiphilic, solvating, PHB. Consequently, NTHi P5 was examined for the presence of polyP and PHB.
FIGURE 3.
Analytical isoelectric focusing of NTHi P5. Focusing was carried out on 0.4 mm gels of 5% acrylamide/bisacrylamide (33.7:1), 20 mM C12M, 5% pH 3–10 ampholytes formed on gel support film. The gel was stained with Coomassie brilliant Blue R250, crocein scarlet, and destained with 40% methanol, 10% acetic acid. (Lane 1) pI standards; (lane 2) NTHi P5.
Determination of polyP and poly-R-3-hydroxybutyrate PHB
The presence of polyP in the protein was signaled by its metachromatic reaction to the cationic dye, o-toluidine blue. PolyP of > 5 residues causes a shift in the absorption maximum of o-toluidine blue toward shorter wavelengths, i.e., from 630 nm (blue) to 530 nm (violet-red) (20). Consequently, polyP stains a distinctive reddish-purple color on SDS-PAGE gels, whereas proteins and polynucleotides stain blue. PolyP was extracted from NTHi P5 (see Materials and Methods), separated by electrophoresis on a 15% polyacrylamide gel (acrylamide:bis; 19:1) and stained with o-toluidine blue (Fig. 4 A, lane 1). PolyP formed a wide band, probably due to hydrolysis during purification procedures, ranging from ∼70 to ∼40 residues. The identity of polyP was confirmed by observing the disappearance of this band when NTHi P5 was incubated with 1 μl Saccharomyces cerevisiae exopolyphosphatase X (scPPX1) (55,000 U/μl) (21) for 15 min before loading on the gel (lane 2).
FIGURE 4.
(A) Presence of polyP in NTHi P5. PolyP extracted from NTHi P5 (see Materials and Methods) and separated by electrophoresis on 15% polyacrylamide gels (acrylamide:bis; 19:1). Electrophoresis buffer was Tris/borate/EDTA (90:90:2.7 mM), pH 8.3. The gel was stained with 0.05% o-toluidine blue in 25% methanol, 5% glycerol; destaining was with 25% methanol, (Lane 1) polyP extracted from NTHi P5 (see Materials and Methods); (lane 2) extracted polyP after treatment with 1 μl S. cerevisiae PPX1 (55,000 units/μl) for 15 min before loading. 5% glycerol. XC comigrates with ∼ polyP72; BPB comigrates with ∼ polyP35 (16). (B) Presence of PHB in NTHi P5. (Lane 1) SDS-PAGE gel of NTHi P5; (lane 2) Western blot of a similar gel of NTHi P5 probed with anti PHB IgG. Second antibody was conjugated to alkaline phosphatase. Samples were heated for 3 min in boiling water before loading.
The presence of PHB in NTHi P5 was detected by Western blot analysis using anti-PHB IgG (Fig. 4 B, lane 2). Extraction with chloroform did not remove PHB from NTHi P5. The identity of PHB was confirmed and the amount determined by chemical assay in which PHB is converted to crotonic acid by heating in concentrated sulfuric acid (18). The crotonic assay is extracted and then separated and quantitated by HPLC (see Materials and Methods). The results of three trials indicate 56 ± 8 μg PHB/mg protein.
Effect of S. cerevisiae exopolyphosphatase PPX1 on selectivity and conductance of NTHi P5
Functional evidence for the presence of polyP in NTHi P5 and its involvement in NTHi P5 function was obtained by observing the effect of scPPX1on selectivity and conductance.
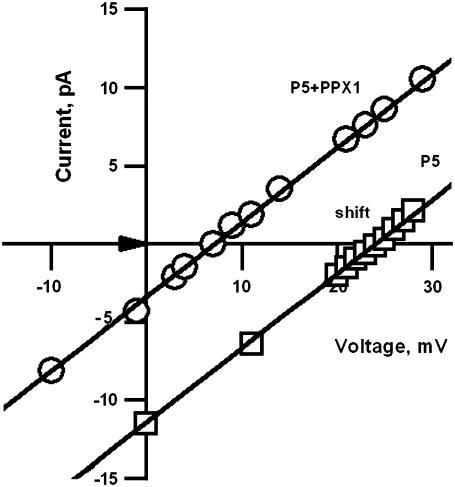
To determine the effect on selectivity, OG-P5 was inserted into DPhPC bilayers between 3:1 gradient solutions of KCl, pH 7.3 and the reversal potential was determined as +24 mV. The enzyme scPPX1 (1 μl) was added to the side of the pore exhibiting a high preference for cations (Vrev = +24 mV). After ∼5 min, the reversal potential dropped sharply to +15 mV and then to +7 mV (Fig. 5), approximately the value of the reversal potential at the opposite side (+9 mV). The effect was repeated in three experiments.
FIGURE 5.
Effect of S. cerevisiae PPX1 on cation selectivity of NTHi OG- P5. Partial I–V curves demonstrate the shift in the reversal potential of OG-P5 (solid line) after the addition of scPPX1 (dashed line). 1 μl scPPX1 (55,000 units/μl) was added to the compartment that exhibited high cationic selectivity with reversal potential of 24 mV. Experimental conditions are the same as in Fig. 2. Data were summarized from a total of three experiments.
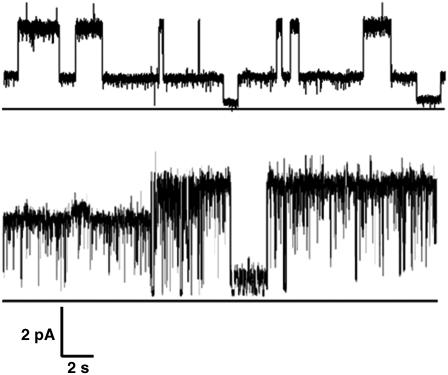
The conductance of OG-P5 is so large that a significant change on addition of scPPX1 could not be established. Hence, the effect of scPPX1 on conductance was examined for the narrow pore conformer obtained when inclusion bodies of NTHi P5 are extracted with LDS (LDS-P5), presumably by partial denaturation. In this case, LDS-P5 was incorporated in DPhPC bilayers between symmetric solutions of 1 M KCl, 10 mM Tris-Cl buffer, pH 7.1 at 22°C. Under these conditions, LDS-P5 forms low conductance pores with a major chord conductance of 58 ± 6 pS and minor chord conductance of 130 ± 15 pS (14) (top trace, Fig. 6). Approximately 5 min after addition of scPPX1 in the same concentration as above, there was a noticeable change in the gating mode of the channel, and ∼10 min after this, the chord conductance increased from 125 pS ± 15 pS to 165 pS ± 20 pS (bottom trace, Fig. 6). Three experiments were conducted and analyzed.
FIGURE 6.
Representative current records of LDS-P5 treated with scPPX1. (Top trace) LDS-P5 before addition of scPPX1. (Bottom trace) ∼10 min after the addition of 1 μl scPPX1 (55,000 units/μl) to the cis compartment. Experimental conditions and solvents are the same as in Fig. 1. Clamping potential was +30 mV. The closed state is delineated by a horizontal line under traces. The experiment was repeated three times with the same result.
DISCUSSION
Reconstituted NTHi P5 forms large pores (1.1 ± 0.1 nS) with high open probability (0.99) in planar lipid bilayers of DPhPC between symmetric solutions of 1M KCl, 10 mM Tris, pH 7.1 at 22°C (14), which unexpectedly display a nonzero reversal potential (Fig. 1). Here we find that this asymmetry reflects a selectivity for cations, slight at one side (Vrev = 9 mV) but close (Vrev = 24 mV) to the Nernst theoretical potential for K+ (Vrev = 26 mV) at the other side (Fig. 2).
Strong cation selectivity is unexpected at one side of a large open pore, and it is particularly surprising in a protein with a theoretical pI of 9.58. The large difference between this theoretical pI and the experimental pI (6.3–6.8) (Fig. 3) and the preference for cations suggested the presence of polyP, a polymorphic chain of negatively-charged tetrahedral phosphoryl residues. PolyP is a universal cell component (22–24) which has previously been found in other membrane transporters (19, 25–30). Its association with NTHi P5 was detected by the metachromatic reaction of the protein on SDS-PAGE gels to o-toluidine blue stain. PolyP, purified from NTHi P5, migrated on an acrylamide gel as a broad band (due to partial hydrolysis) of ∼40–70 units. The identity of polyP was confirmed by the degradation of this band when treated with S. cerevisiae exopolyphosphatase scPPX1 (Fig. 4 A). K(polyP) is water-soluble, hence the persistent binding of polyP to the protein throughout purification and electrophysiological studies in the presence of high concentrations of KCl indicates strong integration of polyP with NTHi P5.
In the proposed model of the 200 residue N-terminal of NTHi P5 by Webb and Cripps (10), the intracellular loops have a weak net negative charge whereas the extracellular loops have a strong net positive charge. This is irreconcilable with the observed pattern of cation selectivity in the planar bilayer, but may be explained by the presence of polyP. Assuming that polyP traverses the pore, the combined effects of the amino acid and polyP charges would make the intracellular side highly negative and the extracellular side weakly negative. Further assuming that the arrangement of polyP in the recombinant protein mirrors that in the native protein, this charge asymmetry may direct the movement of cations into the cell and/or inhibit their exit from the periplasm. However, it should be noted that this charge distribution is speculative since the structure of the large pore conformer of NTHi P5 is unknown.
The presence of polyP in NTHi P5 may account for both the low experimental pI and the strong attraction for cations. The broad range of the experimental pI (Fig. 3), centering at 6.3–6.8, may be attributed to variation in the amount of polyP associated with individual molecules as a result of differential loss of polyP during purification procedures. On addition of scPPX1, the protein band moves off the gel to high pI (above pI 8; not shown). The sharp decrease in cation selectivity on addition of polyphosphatase scPPX1 provides functional evidence of the integral role of polyP in cation selectivity (Fig. 6). Before treatment, the reversal potential at the strong selectivity side (24 mV) was close to the Nernst theoretical potential for K+ (26 mV); after addition of scPPX1, the reversal potential indicated only slight cation selectivity (7 mV). This remaining cation preference may be attributed to the negative charge of amino acid residues at this face or the presence of remnants of polyP that were inaccessible to the enzyme. PolyP occupies only a small area of the pore as evidence by an increase of only 40 pS in the narrow pore conductance after addition of scPPX1 (Fig. 6). This difference represents only ∼4% of the conductance of the large pore form.
PolyP in membrane proteins has frequently been found associated with the amphiphilic, solvating PHB, also a universal cell constituent (31,32). The presence of PHB in NTHi P5 was indicated by its positive reaction to anti-PHB IgG on Western blots (Fig. 4 B), and confirmed by a chemical assay (18) which indicated 56 ± 8 μg PHB/mg protein or ∼24 residues PHB per molecule. This value is likely understated due to loss by hydrolysis of the polyester during protein purification. It is probable that the interaction between PHB and NTHi P5 is covalent at one end, i.e., the CoA ester end of PHB may covalently bind to an amino acid in the pore, since the PHB was not removed from the protein by extraction with chloroform. Other interactions with the protein, such as hydrophobic bonding via the methyl groups and hydrogen or coordinate bonding via the ester carbonyl oxygens, would be noncovalent. Due to its amphiphilic nature, PHB may form a hydrophilic lining in the pore interior that eases the integration of the highly charged polyP and the transfer of cations.
In summary, our studies indicate that NTHi P5 associates with the polyanion, polyP, and the polyester, PHB, to form large pores in the outer membranes of H. influenzae that exhibit asymmetric cation-selectivity. This asymmetry of charge may help to direct movement of cations along the polyP backbone. Furthermore, the capacity of polyP to bind tightly to positive amino acid residues and that of PHB to bind to hydrophobic residues may be at least partially accountable for the attachment of NTHi P5 to receptors (2–8).
Acknowledgments
We thank Dr. Dianne C. Webb, University of Canberra, Australia, for providing a clone of Omp P5 from NTHi strain UC19 in plasmid pCU28, and Dr. Arthur Kornberg, Stanford University, for kindly and generously providing the enzyme S. cerevisiae exopolyphosphatase X.
We gratefully acknowledge support from Grant GM 05090 from the National Institutes of Health and Grant MCB- 0422938 from the National Science Foundation.
Eleonora Zakharian's present address is Dept. of Pharmacology and Physiology, University of Medicine and Dentistry of New Jersey, Newark, NJ 07103.
References
- 1.Hardy, G. G., S. M. Tudor, and J. W. St Geme 3rd. 2003. The pathogenesis of disease due to nontypeable Haemophilus influenzae. Methods Mol. Med. 71:1–28. [DOI] [PubMed] [Google Scholar]
- 2.Sirakova, T., P. E. Kolattukudy, D. Murwin, J. Billy, E. Leake, D. Lin, T. DeMaria, and I. Bakaletz. 1994. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect. Immun. 62:2002–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy, M. S., J. M. Bernstein, T. F. Murphy, and H. S. Faden. 1996. Binding between outer membrane proteins of nontypeable Haemophilus influenzae and human nasopharyngeal mucin. Infect. Immun. 64:1477–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy, M. S., T. F. Murphy, H. S. Faden, and J. M. Bernstein. 1997. Middle ear mucin glycoprotein: purification and interaction with nontypeable Haemophilus influenzae and Moraxella catarrhalis. Otolaryngol. Head Neck Surg. 116:175–180. [DOI] [PubMed] [Google Scholar]
- 5.Kubiet, M., R. Ramphal, A. Weber, and A. Smith. 2000. Pilus-mediated adherence of Haemophilus influenzae to human respiratory mucins. Infect. Immun. 68:3362–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swords, W. E., B. A. Buscher, K. Ver Steeg II, A. Preston, W. A. Nichols, J. N. Weiser, B. W. Gibson, and M. A. Apicella. 2000. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol. Microbiol. 37:13–27. [DOI] [PubMed] [Google Scholar]
- 7.Hill, D. J., M. A. Toleman, D. J. Evans, S. Villullas, L. Van Alphen, and M. Virji. 2001. The variable P5 proteins of typeable and non-typeable Haemophilus influenzae target human CEACAM1. Mol. Microbiol. 39:850–862. [DOI] [PubMed] [Google Scholar]
- 8.Avadhanula, V., C. A. Rodriguez, G. C. Ulett, L. O. Bakaletz, and E. E. Adderson, 2006. Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infect. Immun. 74: 830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munson, R. S. Jr., S. Grass, and R. West. 1993. Molecular cloning and sequence of the gene for outer membrane protein P5 of Haemophilus influenzae. Infect. Immun. 61:4017–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webb, D. C., and A. W. Cripps. 1998. Secondary structure and molecular analysis of interstrain variability in the P5 outer-membrane protein of nontypeable Haemophilus influenzae isolated from diverse anatomical sites. J. Med. Microbiol. 47:1059–1067. [DOI] [PubMed] [Google Scholar]
- 11.Sugawara, E., M. Steiert, S. Rouhani, and H. Nikaido. 1996. Secondary structure of the outer membrane proteins OmpA of Escherichia coli and OprF of Pseudomonas aeruginosa. J. Bacteriol. 178:6067–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duim, B., L. D. Bowler, P. P. Eijk, H. M. Jansen, J. Dankert, and L. van Alphen. 1997. Molecular variation in the major outer membrane protein P5 gene on nonencapsulated Haemophilus influenzae during chronic infection. Infect. Immun. 65:1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel, H., and F. Jähnig. 1986. Models for the structure of outer-membrane proteins of Escherichia coli derived from raman spectroscopy and prediction methods. J. Mol. Biol. 190:191–199. [DOI] [PubMed] [Google Scholar]
- 14.Zakharian, E., and R. N. Reusch (2006) Pore characteristics of nontypeable Haemophilus influenzae outer membrane protein A in planar lipid bilayers. Biophys. J. 91:3242–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webb, D. C., and A. W. Cripps. 1999. A method for the purification and refolding of a recombinant form of the nontypeable Haemophilus influenzae P5 outer membrane protein fused to polyhistidine. Protein Expr. Purif. 15:1–7. [DOI] [PubMed] [Google Scholar]
- 16.Clark, J. E., and H. W. Wood. 1987. Preparation of standards and determination of sizes of long-chain polyphosphates by gel electrophoresis. Anal. Biochem. 161:280–290. [DOI] [PubMed] [Google Scholar]
- 17.Reference deleted in proof.
- 18.Huang, R., and R. N. Reusch. 1996. Poly-(3-hydroxybutyrate) is associated with specific proteins in the cytoplasm and membranes of Escherichia coli. J. Biol. Chem. 271:22196–22202. [DOI] [PubMed] [Google Scholar]
- 19.Reusch, R. N. 1999. Streptomyces lividans potassium channel contains poly-(R)-3- hydroxybutyrate and inorganic polyphosphate. Biochem. 47:15666–15672. [DOI] [PubMed] [Google Scholar]
- 20.Griffin, J. B., N. M. Davidian, and R. Penniall. 1965. Studies of phosphorus metabolism by isolated nuclei. VII. Identification of polyphosphate as a product. J. Biol. Chem. 240:4427–4434. [PubMed] [Google Scholar]
- 21.Wurst, H., T. Shiba, and A. Kornberg. 1995. The gene for a major exopolyphosphatase of Saccharomyces cerevisiae. J. Bacteriol. 177:898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornberg, A., N. N. Rao, and D. Ault-Riché. 1999. Inorganic polyphosphate: a molecule of many functions. Annu. Rev. Biochem. 68:89–125. [DOI] [PubMed] [Google Scholar]
- 23.Kulaev, I. S., and V. Vagabov. T. 2005. The Biochemistry of Inorganic Polyphosphates, 2nd Ed. Wiley, New York.
- 24.Schröder, H. C., B. Lorenz, L. Kurz, and W. E. G. Müller. 1999. Inorganic polyphosphate in eukaryotes: enzymes, metabolism and function. Prog. Mol. Subcell. Biol. 23:45–81. [DOI] [PubMed] [Google Scholar]
- 25.Reusch, R. N., R. Huang, and L. L. Bramble. 1995. Poly-3-hydroxybutyrate/ polyphosphate complexes form voltage-activated Ca2+ channels in the plasma membranes of Escherichia coli. Biophys. J. 69:754–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reusch, R. N. 1999. Polyphosphate/poly-(R)-3-hydroxybutyrate) ion channels in cell membranes. Prog. Mol. Subcell. Biol. 23:151–182. [DOI] [PubMed] [Google Scholar]
- 27.Reusch, R. N., R. Huang, and D. Kosk-Kosicka. 1997. Novel components and enzymatic activities of the human erythrocyte plasma membrane calcium pump. FEBS Lett. 412:592–596. [DOI] [PubMed] [Google Scholar]
- 28.Reusch, R. N. 2000. Transmembrane ion transport by polyphosphate/poly-(R)-3- hydroxybutyrate complexes. Biochem. (Mosc). 65:280–295. [PubMed] [Google Scholar]
- 29.Zakharian, E., and R. N. Reusch. 2004. Functional evidence for a supramolecular structure for the Streptomyces lividans potassium channel KcsA. Biochem. Biophys. Res. Commun. 322:1059–1065. [DOI] [PubMed] [Google Scholar]
- 30.Pavlov, E., E. Zakharian, C. Bladen, C. T. Diao, C. Grimbly, R. N. Reusch, and R. French. 2005. A large, voltage-dependent channel, isolated from mitochondria by water-free chloroform extraction. Biophys. J. 88:2614–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reusch, R. N. 1992. Biological complexes of poly-beta-hydroxybutyrate. FEMS Microbiol. Rev. 9:119–129. [DOI] [PubMed] [Google Scholar]
- 32.Seebach, D., A. Brunner, H. M. Burger, J. Schneider, and R. N. Reusch. 1994. Isolation and 1H-NMR spectroscopic identification of poly(3-hydroxybutanoate) from prokaryotic and eukaryotic organisms. Eur. J. Biochem. 224:317–328. [DOI] [PubMed] [Google Scholar]