Summary
The regulation of host-mediated apoptosis by the E6 and E7 oncoproteins has garnered attention because it is believed to be an important strategy employed by high-risk (HR)-human papillomaviruses (HPVs) to evade immune surveillance. Additionally, the revelation that E5 can protect cells from tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-mediated apoptosis suggests that it may also play a role in undermining host defense mechanisms. Cellular transformation is an unintended consequence of persistent infection by HR-HPVs, and it is therefore likely that the primary function of E5, E6 and E7 is to regulate cell survival throughout the normal viral life cycle in order to ensure viral replication and promote the spread of progeny. The purpose of this article is to review the literature on the regulation of host-mediated apoptosis by E5, E6 and E7 that describe the mechanisms employed by HR-HPVs to persist in the host and create the conditions necessary for cellular transformation.
Introduction
Human papillomaviruses (HPVs) are small, nonenveloped, double-stranded DNA viruses whose natural cellular hosts are keratinocytes. Infection with HPV occurs below the surface of the epithelium in the basal layer, and the life cycle of the virus is closely connected with the differentiation program of the cells it infects. The most common phenotypical manifestations of HPV infection are warts and papillomas of the skin, and various genital hyperplastic epithelial lesions. Over 100 HPV genotypes have been identified and approximately 33% of these genotypes are associated with lesions of the genital tract. HPVs that infect the genital tract can be subdivided into two categories: low-risk and high-risk. Low-risk (LR)-HPVs such as types 6 and 11 generally cause benign warts which rarely progress to cancer. On the other hand, high-risk (HR)-HPVs such as types 16, 18, 31 and 45 are associated with the development of high-grade lesions (cervical intraepithelial neoplasia (CIN) 2/3) that can progress to cancer.
All HPVs have a common genomic organization and encode 8 proteins: E1, E2, E4, E5, E6, and E7 (early) and L1 and L2 (late). E1 and E2 are replication factors and are also involved in transcription control; E4 and E5 are believed to regulate late viral functions although their role is not clearly understood; E6 and E7 are oncoproteins; and L1 and L2 are structural proteins [48]. The E6 and E7 oncoproteins of the high-risk strains are the main contributors to malignant transformation [26, 56]. It is believed that a combination of persistent infection by HR strains along with the inability of the immune system to adequately clear the virus from infected cells are the main factors contributing to the integration of HPV genomes into the DNA of the host-a critical step in tumorigenesis. As a result of integration, the E2 open reading frame (ORF) is frequently disrupted, leading to a loss of E2 expression and its repressive action on E6 and E7, as well as a concomitant rise in the levels of these oncoproteins.
HR-HPVs, such as HPV 16 and HPV 18, play a pivotal role in the pathophysiology of cervical cancer [48, 51] which accounts for one-fifth of all cancerrelated deaths among women, and is the second most common cancer worldwide [62, 67]. Merck’s recently approved vaccine for HPV 16 and 18, and one under development by GlaxoSmithKline hold great promise for the eradication of this deadly disease [11]. While a considerable amount of research has been done on the role of HR-HPVs in the etiology of cervical cancer, many of the actions of these viruses that promote persistence in the host and cellular transformation have not been fully elucidated. A full understanding of the events that occur during the time HR-HPVs establish a successful infection may reveal targets for novel therapeutic approaches.
Immune defense mechanisms against HPV infection
HPVs are persistent viruses that can remain in their hosts for long periods of time before causing any ill effects. Generally, the host reacts to viral pathogens by generating both humoral and cell-mediated responses. Humoral responses are typically antibody-mediated and involve the secretion of antibodies such as immunoglobulin A (IgA) and immunoglobulin G (IgG) by B lymphocytes. Cell-mediated responses, on the other hand, are carried out by immune effector cells such as dendritic cells (DCs), natural killer (NK) cells, macrophages and T lymphocytes which secrete a number of cytokines including interferons (INF) and tumor necrosis factor (TNF), and up-regulate the expression of Fas ligand (FasL) and TNF-related apoptosis inducing ligand (TRAIL) on their cell surface.
In the case of HPV infection, the immune response is frequently weak or undetectable, and accompanied by little or no inflammation. Even when an immune response is elicited, it may not be able to clear the virus. Although HR-HPVs are ‘clever’ at evading immune detection, studies suggest that the immune system can be successful at controlling HPV infection [7]. Indeed, a number of reports indicate that systemic Th1 responses against HPV proteins are associated with health [93, 94]. These reports demonstrate that the immune system is successful at preventing many cases of HPV infection from progressing to malignancy and may explain instances of spontaneous regression of warts observed in some patients.
Langerhans cells (LC), a type of DC that monitors squamous mucosal surfaces, are an integral part of the immune system’s arsenal. These cells are likely the first immune cells to encounter HPV in low-grade squamous intraepithelial lesions (CIN 1). Work by several groups suggests that function of LCs may be impaired because of their low density and stunted dendritic processes within CIN 1 [1, 89]. However, using an organotypic culture model to approximate the microenvironment of CIN 1, Hurbert et al. demonstrated that LCs were fully capable of inducing apoptosis in HPV positive keratinocytes and that the interaction of LCs with HPV positive cells did not result in their demise [37]. Therefore, the ability of HPV to establish chronic infection is likely not the result of an inadequate host-immune response, but to the ability of the virus to evade host-immune surveillance mechanisms.
Host-mediated apoptosis
Apoptosis, a type of programmed cell death, plays an important role in organism development, cellular homeostasis and the pathophysiology of many diseases [70]. In the immune system, apoptosis is often triggered by cell surface receptors. Many of these receptors belong to the TNF receptor family including tumor necrosis factor receptor 1 (TNF R1), Fas, death receptor 4 (DR4), and death receptor 5 (DR5). These receptors bind to their cognate ligands, TNF, FasL, and TRAIL. The receptors and ligands that make up the TNF receptor and ligand families are also referred to as death receptors and death ligands, receptively, because their interaction triggers cell death [30]. Typically, cell death receptor-mediated apoptosis is induced by the binding of the death ligands (usually in the form of a homotrimer) to their cognate receptors, which then recruit adaptor molecules and initiator caspases such as FADD and procaspase-8 to their death domains (DD) to form the death inducing signaling complex (DISC). Initiator caspases are then activated by proximity induced cleavage at the DISC and in turn cleave and activate executioner caspases such as procaspase-3. Once activated, caspase-3 cleaves target substrates such as poly(ADP-ribose) polymerase (PARP) and Lamin B, leading to the demise of the cell. In Fas- and TRAIL-mediated apoptosis, this is commonly called the type I model and its hallmark is strong caspase-8 activation which results in robust DISC formation. In contrast, DISC formation in type II cells is weak and amplification of the death signal via the mitochondrial pathway is necessary for apoptosis. In this context, caspase-8 cleaves Bid, triggering mitochondrial depolarization and the release of cytochrome C. Following its release, cytochrome C forms a complex with (apoptosis protease activating factor 1) Apaf-1 and pro-caspase 9 called the apoptosome. The apoptosome, which is analogous in function to the DISC, mediates the activation of caspase-9 which in turn activates caspase-3. The intracellular events of apoptosis give way to the external characteristics of this form of cell death, which include chromatin condensation, phosphatidlyserine exposure, cytoplasmic shrinkage and membrane blebbing.
Not surprisingly, many viruses, including HPV, have developed numerous strategies to block host-mediated apoptosis. The ability of HPV to persist in the host for long periods of time without being eliminated attests to the sophistication of its evasion mechanisms. A growing body of evidence suggests that the oncoproteins of HR-HPVs (i.e., E6 and E7), as well as E5, can inhibit death receptor signaling at key points in the pathway. In so doing, HPV is able to regulate the survival of infected cells in order to facilitate its replication cycle and thus ensure the production and spread of progeny.
Modulation of apoptosis by E5
The role of E5 in protecting HPV from elimination by the host has not received much attention because of its weak transforming properties and the fact that the E5 open reading frame (ORF) is often deleted after the HPV genome has integrated into the DNA of the host [13, 53]. Furthermore, direct immunoblot analysis of E5 protein expression has been hindered by its low solubility and many studies have utilized tagged versions of the protein for easier analysis. HPV E5 is a hydrophobic protein and is expressed in cellular membrane structures such as the Golgi apparatus, endoplasmic reticulum and the nuclear membrane [12]. The exact function of E5 is not known but a number of its actions have been described. For example, HPV 16 E5 has been reported to interfere with the actin cytoskeleton and block endocytic trafficking [84]; modulate epidermal growth factor receptor signaling [14, 16, 65, 77]; induce c-jun expression via a ras- and PKC-dependent pathway [10]; trigger the up-regulation of diacylglycerol (DAG) and inositol phosphates in fibroblast cells [15]; and down-regulate the expression of surface MHC class I molecules [3].
With regard to its role in tumorigenesis, E5 has been shown to induce fibroblasts to form colonies in soft agar [65, 77], increase the efficiency of immortalization of keratinocytes by E6 and E7 [76], and impair cell-cell communication at gap junctions [59]. While these activities may not be necessary for maintaining the malignant status of infected cells, the findings of these studies and others suggest that E5 may play an important role in the initial phase of tumorigenesis. Indeed, prior to integration, when the HPV genome is episomal, the E5 mRNA is the most abundant viral transcript [74]. E5 has also been reported to inhibit the expression of the p21 tumor suppressor [88], which suggest that it may co-operate with E6 and E7 to transform keratinocytes. Interestingly, the E5 protein has been shown to protect HaCat keratinocytes from both Fas- and TRAIL-mediated apoptosis, albeit by different mechanisms [40]. In their study, Kabsch and Alonso showed that E5 inhibits Fas-induced apoptosis, in part, by decreasing the cell surface expression of the Fas receptor. While E5 did not down-regulate TRAIL receptor expression, it was found to inhibit TRAIL signaling by interfering with the formation of the TRAIL DISC and thereby inhibiting the cleavage of procaspases-8 and -3, as well as of PARP [40]. Therefore, it is possible that E5 interferes with the ability of the immune system to eliminate infected cells by impairing death receptor signaling. Together, the results of these studies provide strong evidence that the E5 contributes to the evasion of immune surveillance during the early stages of HPV infection.
Modulation of apoptosis by E6
The E6 oncoprotein has been widely studied because of its potent tumorigenic properties. E6 is a relatively small protein (150 amino acids) and is produced in two forms: a full-length version of ∼16 kDa and a smaller version of about half that size corresponding to the N-terminal half of the full-length protein [51]. One of the primary targets of E6 is the tumor suppressor p53 [71, 95]. In the early stages of HR-HPV infection, E7 induces a significant increase in cell proliferation as a result of its interaction with retinoblastoma (RB), which triggers the expression of p53 [48]. Under normal physiological conditions, this rise in p53 levels would lead to cell cycle arrest and/or apoptosis, depending on the intensity of the damage or stimulus. However, in this situation, E6 binds to p53 with the aid of E6-associated protein ligase (E6AP) and prevents p53 from inducing growth arrest and apoptosis by targeting it for degradation through the ubiquitin-proteasome pathway [38]. E6 also precludes the growth-suppressive activities of p53 by cytoplasmic sequestration and by transcriptional suppression of its target genes [51].
A number of proteins other than p53 are targeted by E6. These include proteins involved in the regulation of transcription and DNA replication such as p300/CBP [63, 98], IRF-3 [69], hMcm7 [43, 44], E6TP1 [28] and ADA3 [28, 97]; proteins involved in apoptosis and immune evasion such as Bak [79], c-Myc [34], TNF receptor 1 (TNF-R1) [23] and FADD [24]; proteins involved with epithelial organization and differentiation such as paxillin [87], E6BP/ERC-55 [9], zyxin [18], and fibulin-1 [20]; proteins involved in cell-cell adhesion, polarity, and proliferation control that contain a PDZ-binding motif such as hDLG [42, 45], hScrib [57], MAGI-1 [31, 81], MAGI-2, MAGI-3 [82], and MUPPI [46]; and proteins involved in DNA repair such as XRCCI and 6-0-methylguanine-DNA methyl transferase [73].
Two of these proteins, Bak and myc, were the first apoptosis-related targets of E6 to be identified. Thomas & Banks found that E6 inhibits Bak-mediated apoptosis by direct binding to Bak, an interaction that is conserved from HR-to LR-HPVs [79, 80]. In laryngeal cells, E6 was found to inhibit TNF-mediated apoptosis by reducing the expression of Bak without significantly affecting the expression of caspase-3 and -8 [19]. Like p53, both Bak and myc are ubiquitinated by E6AP and degraded in the ubiquitin-proteasome pathway [34, 79].
Over the past several years, our laboratory has discovered novel E6 binding partners that have helped to elucidate the role of E6 in immune evasion. We have shown that E6 can inhibit TNF-mediated apoptosis in mouse fibroblasts, human monocytes/histocytes and osteosarcoma cells by binding to the death domain of the TNF-R1 and preventing it from interacting with TRADD [23]. Paradoxically, stable transfection of E6 in human cells does not always result in protection from TNF-mediated apoptosis. We found that clones with a high level of E6 expression were more sensitive to apoptosis induced by TNF than clones with a low level of E6 expression [25]. This finding is consistent with literature reports which describe both the ‘sensitization’ [47, 90] and ‘protection’ properties of E6 [21]. With regard to Fas-mediated apoptosis, we have shown that E6 binds to FADD and mediates its degradation via the ubiquitin proteosome pathway [24]. Recently, we demonstrated that E6 can also protect human cells (HCT116) from TRAIL-induced apoptosis by accelerating the degradation of FADD and caspase-8 [29]. In these studies, the targeting of TNF-R1, FADD and caspase-8 by E6 resulted in the suppression of caspase activation and protection from apoptosis (Figure 1). However, our unpublished observations that E6 does not interact with TRADD or Fas suggests that the binding of E6 to TNF-R1, FADD and caspase-8 is specific.
Figure 1.
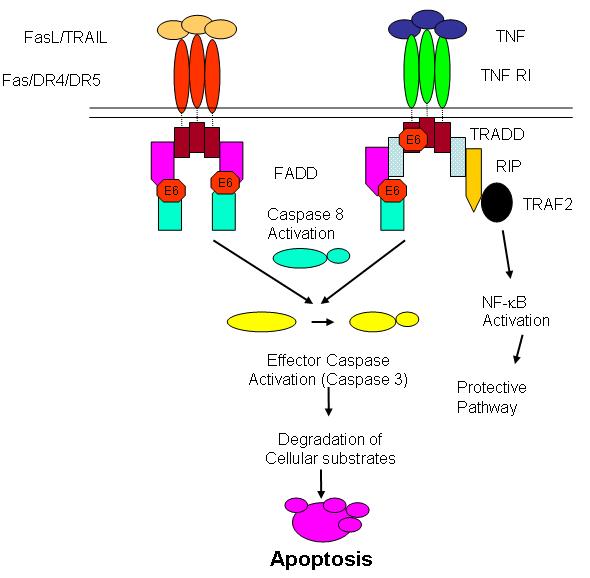
HPV 16 E6 exploits similarities in the apoptotic signaling pathways of TNF, FasL and TRAIL. E6 inhibits TNF- and Fas-mediated apoptosis by binding to TNF R1 and to FADD. E6 protects cells from TRAIL-mediated apoptosis by accelerating the degradation of FADD and caspase-8.
Interestingly, we also found that E6 does not interfere with the mitochondrial apoptotic pathway in our cellular models. We arrived at this conclusion based on the results of experiments in which the mitochondrial pathway in E6-expressing HCT116 and U2OS cells was activated by mitomycin C or ceramide. While both agents are known to trigger the mitochondrial apoptotic pathway, their ability to induce cell death in HCT116 and U2OS cells was not hindered in the presence of E6 [24, 25, 29]. However, others have found that the induction of apoptosis via Bak-and Bax-dependent pathways is impaired in the presence of E6 [79]. This implies that in some cases E6 may inhibit the mitochondrial pathway because it is well-known that both Bak and Bax can trigger cytochrome c release [33]. In the Thomas and Banks study cited above, p53-null mouse 10(1) cells and human Saos-2 cells lacking both p53 and Rb were transfected with Bak alone or Bak plus HPV 18 E6. Survival assays revealed that Bak-mediated apoptosis was markedly inhibited upon co-transfection of Bak with E6 [79]. Recently, Vogt et al. reported that the suppression of Bax activity by E6 was necessary for its (E6’s) antiapoptotic function [91]. The results of the study demonstrated that inhibition of E6 expression with RNAi in HPV-positive HeLa cells lead to the transactivation of the PUMA gene by p53, the activation of Bax, and its translocation to the mitochondria. These events were followed by the release of cytochrome c into the cytosol and the activation of caspase-3. The apparent discrepancy between our results and these two studies can be reconciled by noting that the ceramide- and mitomycin C signaling pathways may not require Bak or Bax for apoptosis induction via the mitochondria. Whether this is also true of death receptor-induced apoptosis may not be important because our results suggest that E6 inhibits death receptor-mediated apoptosis upstream of the mitochondrial apoptotic pathway.
The link between the association of E6 with certain cellular proteins and its oncogenic potential is not always obvious. A case in point is a group of proteins containing PDZ (postsynaptic density protein, discs large tumor suppressor, and the epithelial tight junction protein, Z0-1) domains. A number of these proteins are targeted by E6 and are necessary for both its transforming potential [92] and its ability to induce epithelial hyperplasia in mice [58]. A recent study by researchers at the University of Iowa provides convincing evidence that the PDZ-binding domain of E6 is important in mediating resistance to TNF-induced apoptosis [39]. According to the report, the expression of E6 in primary human airway epithelial cells (AECs) leads to an upregulation in the activity of p52-containing NF-κB complexes, as well as the transactivation of nuclear factor kappa B (NF-κB) responsive genes such as the inhibitor of apoptosis protein, cIAP-2. As a result of this cascade of events, AECs acquire partial resistance to TNF and experience protection from apoptosis. The authors also demonstrated that the protection provided by E6 could be abrogated by a mutant form of the protein that lacks the ability to bind the PDZ motif and to activate NF-κB. Intriguingly, this mutant protein retains the ability to bind and direct the degradation of p53. Taken together, the studies discussed above suggest that through the activities of E6, HR-HPVs can interfere with cellular apoptotic pathways by both p53-dependent and -independent mechanisms.
Modulation of apoptosis by E7
Like E6, the E7 oncoprotein of HR-HPVs is necessary for both cellular transformation and viral pathogenesis. E7’s primary target is retinoblastoma (Rb) and its related proteins including p107 and p130 [5, 22]. Under normal conditions, Rb forms a complex with histone deacetylase (HDAC) and binds to the E2F transcription factor in the G1 phase of the cell cycle. This prevents E2F from transactivating genes that are necessary for proliferation until the cell enters the S phase. However, when E7 is expressed in cells, it binds to Rb and HDAC and relieves their repression of E2F, resulting in the constitutive activation of E2F-responsive genes. The actions of E7 cause the cell to reenter the S phase where cellular replication factors that are necessary of viral replication are activated. While much is known about the interaction between E7 and Rb family members and the biological consequences of this association, a number of unanswered questions remain regarding the interaction of E7 and HDAC. One of these questions is why a mutation in the HDAC binding domain of the E7 oncoprotein of HPV 31 results in defective maintenance of viral episomes [49]. Several possibilities have been proposed [48]. One is that the binding of HDAC to E7 prevents it from deacetylating the E2F transcription factors, which causes these proteins to relocate outside the nucleus. Another more attractive possibility is that the interaction of these proteins uncovers an important, but as yet unrecognized, role of E7.
E7 binds to other proteins besides Rb and HDAC. They include proteins involved in cell cycle control such as cyclin-dependent kinase cdk2 and cycline A [86]; proteins regulating transcription such as the TATA box-binding protein and the AP1 transcription factors [2, 52, 64]; and proteins with other cellular functions such as TAF-110 and TBP [55], the S4 ATPase of the 26S proteosome [4], Mi2 [8], Interferon Regulatory Factor-1 [61], IGFBP-3 [50], M2-PK [54], Skip [68], and PP2A [66].
Besides its role in cell proliferation and viral replication, E7 also regulates apoptosis. However, its effect on cellular apoptotic pathways is pleiomorphic. For example, in some studies the actions of E7 appear to be anti-apoptotic. A case in point is a recent study by Yuan et al. which suggests that E7 can inhibit TNF-mediated apoptosis in keratinocytes by up-regulating the expression of the inhibitor of apoptosis protein, c-IAP2 [96]. In another study, it was reported that the expression of E7 in fibroblasts delayed Fas-mediated apoptosis and prevented TNF-mediated apoptosis by a mechanism involving the suppression of caspase-8 activation [83]. However, the majority of studies suggest that E7 serves in pro-apoptotic role. For instance, E7 has been shown to sensitize mouse lymphoma cells (JD3) to IFN-alpha-induced apoptosis [85]; co-expression of E7 and p21 induced apoptosis in U2OS osteosarcoma cells [41]; and overexpression of E7 in genital keratinocytes induced spontaneous cell death and sensitized the cells to TNF-mediated apoptosis [75]. The pleiotrophic effect of both E6 and E7 on apoptosis is indicative of their important role in immune evasion and underscores the complexity of HPV-host interactions.
Combined effects of E2, E6 and E7 on cell survival
Studies in which the activities of E6 and E7 have been examined independently (ie, by overexpression) have uncovered many of the molecular mechanisms employed by these proteins to modulate cell survival. However, in an attempt to mirror physiological conditions, other studies have examined the effect of E6/E7 on cell survival within the context of the whole HPV genome. One such study took advantage of the fact that E6 and E7 are expressed bicistronically [78] and investigated the consequences of siRNA-mediated transcriptional repression of the genes encoding these proteins. The authors found that inhibition of E6 and E7 with siRNA resulted in a significant reduction in DNA replication as measured by [3H]thymidine incorporation in HeLa cells [35]. Furthermore, they found that the reduction in cell proliferation was not due to apoptosis, but to senescence [35]. Thus, within the context of the whole HPV genome post-transcriptional silencing of E6 and E7 alone is sufficient to inhibit the growth of cervical carcinoma cells. It is noteworthy that similar observations have been achieved by exogenous expression of E2 in cervical carcinoma cells [32]. Not only does the overexpression of E2 result in senescence, but studies by the DiMaio, Gaston and Theirry laboratories have demonstrated that E2 can induce apoptotic cell death [6, 17, 60]. This finding and the fact that E2 can regulate the activities of E6 and E7 via transcriptional control or by direct interaction [27], suggests that HPV genome integration may result from a strong selective pressure on the virus to avoid E2-induced apoptosis while modulating the survival of infected cells through the activities of E6 and E7.
Conclusions
HPV, like many of its viral counterparts has developed sophisticated mechanisms to evade host defenses and establish a successful infection. The primary weapons at its disposal are the E5, E6 and E7 oncoproteins. Although the role of these proteins, particularly E6 and E7, in cellular transformation (an unintended consequence of the HPV life cycle) has been well-studied, less attention has been given to their role in HPV persistence in the host during the normal viral life cycle, and to their ability to circumvent host-mediated apoptosis. Recent reports suggest that an important effect of E5 might be to create conditions conducive to the integration of HPV DNA into the host genome, in part by fending off apoptotic stimuli from immune effector cells. As noted, E5 achieves this by modulating signal transduction triggered by apoptotic stimuli from these cells [40] and also by interfering with MHC class I protein expression [3]. Furthermore, E5 has transforming potential and can co-operate with E6 and E7 to immortalize cells [76]. Following intergration, the E5 ORF is usually deleted and the transcriptional repression of E6 and E7 by E2 is relieved, allowing these oncogenes and their protein products to take center stage.
The interaction of E6 and E7 with their primary cellular targets, p53 and Rb, respectively, plays an important role in the initiation of tumorigenesis, and these oncoproteins are also required for retaining the transformed status of infected cells [48].
Intriguingly, E6 and E7 can inhibit apoptosis induced by stimuli such as TNF as well as sensitize cells to TNF and other apoptotic stimuli [25, 83, 96]. The significance of these observations is still being investigated, but clues to their possible role in the viral life cycle are beginning to unfold. The fact that HPV encodes proteins that perform both functions (protection/sensitization) suggests that (1) during the viral life cycle both functions may be required, and/or that (2) E6 can compensate for E7 or vice versa if the expression of one or the other protein is lost (i.e., through deletion) or silenced (e.g., by antisense or siRNA molecules produced by the host) during the viral life cycle. Indeed, while the expression of both E6 and E7 are necessary to efficiently immortalize cells, they can also induce cell transformation on their own [36, 72]. This finding as well as the ability of both proteins to modulate cell survival suggests that there is some redundancy in their functions. If true, this would be highly advantageous to the virus as it tries to persist in the host and avoid elimination. Together, the studies presented here strongly suggest that targeting E5, E6 and E7 is a prudent strategy for therapies aimed at preventing the development of premalignant intraepithelial lesions and their progression to cancer.
References
- 1.al-Saleh W, Delvenne P, Arrese JE, Nikkels AF, Pierard GE, Boniver J. Inverse modulation of intraepithelial Langerhans’ cells and stromal macrophage/dendrocyte populations in human papillomavirus-associated squamous intraepithelial lesions of the cervix. Virchows Arch. 1995;427:41–8. doi: 10.1007/BF00203736. [DOI] [PubMed] [Google Scholar]
- 2.Antinore MJ, Birrer MJ, Patel D, Nader L, McCance DJ. The human papillomavirus type 16 E7 gene product interacts with and trans-activates the AP1 family of transcription factors. Embo J. 1996;15:1950–60. [PMC free article] [PubMed] [Google Scholar]
- 3.Ashrafi GH, Haghshenas MR, Marchetti B, O’Brien PM, Campo MS. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int J Cancer. 2005;113:276–83. doi: 10.1002/ijc.20558. [DOI] [PubMed] [Google Scholar]
- 4.Berezutskaya E, Bagchi S. The human papillomavirus E7 oncoprotein functionally interacts with the S4 subunit of the 26 S proteasome. J Biol Chem. 1997;272:30135–40. doi: 10.1074/jbc.272.48.30135. [DOI] [PubMed] [Google Scholar]
- 5.Berezutskaya E, Yu B, Morozov A, Raychaudhuri P, Bagchi S. Differential regulation of the pocket domains of the retinoblastoma family proteins by the HPV16 E7 oncoprotein. Cell Growth Differ. 1997;8:1277–86. [PubMed] [Google Scholar]
- 6.Blachon S, Bellanger S, Demeret C, Thierry F. Nucleo-cytoplasmic shuttling of high risk human Papillomavirus E2 proteins induces apoptosis. J Biol Chem. 2005;280:36088–98. doi: 10.1074/jbc.M505138200. [DOI] [PubMed] [Google Scholar]
- 7.Bontkes HJ, de Gruijl TD, Walboomers JM, Schiller JT, Dillner J, Helmerhorst TJ, Verheijen RH, Scheper RJ, Meijer CJ. Immune responses against human papillomavirus (HPV) type 16 virus-like particles in a cohort study of women with cervical intraepithelial neoplasia. II. Systemic but not local IgA responses correlate with clearance of HPV-16. J Gen Virol. 1999;80(Pt 2):409–17. doi: 10.1099/0022-1317-80-2-409. [DOI] [PubMed] [Google Scholar]
- 8.Brehm A, Nielsen SJ, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. Embo J. 1999;18:2449–58. doi: 10.1093/emboj/18.9.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JJ, Reid CE, Band V, Androphy EJ. Interaction of papillomavirus E6 oncoproteins with a putative calcium-binding protein. Science. 1995;269:529–31. doi: 10.1126/science.7624774. [DOI] [PubMed] [Google Scholar]
- 10.Chen SL, Huang CH, Tsai TC, Lu KY, Tsao YP. The regulation mechanism of c-jun and junB by human papillomavirus type 16 E5 oncoprotein. Arch Virol. 1996;141:791–800. doi: 10.1007/BF01718155. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J. Public health. High hopes and dilemmas for a cervical cancer vaccine. Science. 2005;308:618–21. doi: 10.1126/science.308.5722.618. [DOI] [PubMed] [Google Scholar]
- 12.Conrad M, Bubb VJ, Schlegel R. The human papillomavirus type 6 and 16 E5 proteins are membrane-associated proteins which associate with the 16-kilodalton pore-forming protein. J Virol. 1993;67:6170–8. doi: 10.1128/jvi.67.10.6170-6178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corden SA, Sant-Cassia LJ, Easton AJ, Morris AG. The integration of HPV-18 DNA in cervical carcinoma. Mol Pathol. 1999;52:275–82. doi: 10.1136/mp.52.5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crusius K, Auvinen E, Steuer B, Gaissert H, Alonso A. The human papillomavirus type 16 E5-protein modulates ligand-dependent activation of the EGF receptor family in the human epithelial cell line HaCaT. Exp Cell Res. 1998;241:76–83. doi: 10.1006/excr.1998.4024. [DOI] [PubMed] [Google Scholar]
- 15.Crusius K, Kaszkin M, Kinzel V, Alonso A. The human papillomavirus type 16 E5 protein modulates phospholipase C-gamma-1 activity and phosphatidyl inositol turnover in mouse fibroblasts. Oncogene. 1999;18:6714–8. doi: 10.1038/sj.onc.1203075. [DOI] [PubMed] [Google Scholar]
- 16.Crusius K, Rodriguez I, Alonso A. The human papillomavirus type 16 E5 protein modulates ERK1/2 and p38 MAP kinase activation by an EGFR-independent process in stressed human keratinocytes. Virus Genes. 2000;20:65–9. doi: 10.1023/a:1008112207824. [DOI] [PubMed] [Google Scholar]
- 17.DeFilippis RA, Goodwin EC, Wu L, DiMaio D. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J Virol. 2003;77:1551–63. doi: 10.1128/JVI.77.2.1551-1563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degenhardt YY, Silverstein SJ. Gps2, a protein partner for human papillomavirus E6 proteins. J Virol. 2001;75:151–60. doi: 10.1128/JVI.75.1.151-160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du J, Chen GG, Vlantis AC, Chan PK, Tsang RK, van Hasselt CA. Resistance to apoptosis of HPV 16-infected laryngeal cancer cells is associated with decreased Bak and increased Bcl-2 expression. Cancer Lett. 2004;205:81–8. doi: 10.1016/j.canlet.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 20.Du M, Fan X, Hong E, Chen JJ. Interaction of oncogenic papillomavirus E6 proteins with fibulin-1. Biochem Biophys Res Commun. 2002;296:962–9. doi: 10.1016/s0006-291x(02)02041-7. [DOI] [PubMed] [Google Scholar]
- 21.Duerksen-Hughes PJ, Yang J, Schwartz SB. HPV 16 E6 blocks TNF-mediated apoptosis in mouse fibroblast LM cells. Virology. 1999;264:55–65. doi: 10.1006/viro.1999.9977. [DOI] [PubMed] [Google Scholar]
- 22.Dyson N, Howley PM, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–7. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 23.Filippova M, Song H, Connolly JL, Dermody TS, Duerksen-Hughes PJ. The human papillomavirus 16 E6 protein binds to tumor necrosis factor (TNF) R1 and protects cells from TNF-induced apoptosis. J Biol Chem. 2002;277:21730–9. doi: 10.1074/jbc.M200113200. [DOI] [PubMed] [Google Scholar]
- 24.Filippova M, Parkhurst L, Duerksen-Hughes PJ. The human papillomavirus 16 E6 protein binds to Fas-associated death domain and protects cells from Fas-triggered apoptosis. J Biol Chem. 2004;279:25729–44. doi: 10.1074/jbc.M401172200. [DOI] [PubMed] [Google Scholar]
- 25.Filippova M, Brown-Bryan TA, Casiano CA, Duerksen-Hughes PJ. The human papillomavirus 16 E6 protein can either protect or further sensitize cells to TNF: effect of dose. Cell Death Differ. 2005 doi: 10.1038/sj.cdd.4401678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finzer P, Aguilar-Lemarroy A, Rosl F. The role of human papillomavirus oncoproteins E6 and E7 in apoptosis. Cancer Lett. 2002;188:15–24. doi: 10.1016/s0304-3835(02)00431-7. [DOI] [PubMed] [Google Scholar]
- 27.Gammoh N, Grm HS, Massimi P, Banks L. Regulation of human papillomavirus type 16 E7 activity through direct protein interaction with the E2 transcriptional activator. J Virol. 2006;80:1787–97. doi: 10.1128/JVI.80.4.1787-1797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Q, Srinivasan S, Boyer SN, Wazer DE, Band V. The E6 oncoproteins of high-risk papillomaviruses bind to a novel putative GAP protein, E6TP1, and target it for degradation. Mol Cell Biol. 1999;19:733–44. doi: 10.1128/mcb.19.1.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garnett TO, Filippova M, Duerksen-Hughes PJ. Accelerated degradation of FADD and procaspase 8 in cells expressing human papilloma virus 16 E6 impairs TRAIL-mediated apoptosis. Cell Death Differ. 2006 doi: 10.1038/sj.cdd.4401886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaur U, Aggarwal BB. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol. 2003;66:1403–8. doi: 10.1016/s0006-2952(03)00490-8. [DOI] [PubMed] [Google Scholar]
- 31.Glaunsinger BA, Lee SS, Thomas M, Banks L, Javier R. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene. 2000;19:5270–80. doi: 10.1038/sj.onc.1203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodwin EC, Yang E, Lee CJ, Lee HW, DiMaio D, Hwang ES. Rapid induction of senescence in human cervical carcinoma cells. Proc Natl Acad Sci U S A. 2000;97:10978–83. doi: 10.1073/pnas.97.20.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green DR. At the gates of death. Cancer Cell. 2006;9:328–30. doi: 10.1016/j.ccr.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Gross-Mesilaty S, Reinstein E, Bercovich B, Tobias KE, Schwartz AL, Kahana C, Ciechanover A. Basal and human papillomavirus E6 oncoprotein-induced degradation of Myc proteins by the ubiquitin pathway. Proc Natl Acad Sci U S A. 1998;95:8058–63. doi: 10.1073/pnas.95.14.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall AH, Alexander KA. RNA interference of human papillomavirus type 18 E6 and E7 induces senescence in HeLa cells. J Virol. 2003;77:6066–9. doi: 10.1128/JVI.77.10.6066-6069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herber R, Liem A, Pitot H, Lambert PF. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J Virol. 1996;70:1873–81. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hubert P, Giannini SL, Vanderplasschen A, Franzen-Detrooz E, Jacobs N, Boniver J, Delvenne P. Dendritic cells induce the death of human papillomavirus-transformed keratinocytes. Faseb J. 2001;15:2521–3. doi: 10.1096/fj.00-0872fje. [DOI] [PubMed] [Google Scholar]
- 38.Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. Embo J. 1991;10:4129–35. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.James MA, Lee JH, Klingelhutz AJ. Human Papillomavirus Type 16 E6 Activates NF-{kappa}B, Induces cIAP-2 Expression, and Protects against Apoptosis in a PDZ Binding Motif-Dependent Manner. J Virol. 2006;80:5301–7. doi: 10.1128/JVI.01942-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kabsch K, Alonso A. The human papillomavirus type 16 E5 protein impairs TRAIL- and FasL-mediated apoptosis in HaCaT cells by different mechanisms. J Virol. 2002;76:12162–72. doi: 10.1128/JVI.76.23.12162-12172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaznelson DW, Bruun S, Monrad A, Gjerlov S, Birk J, Ropke C, Norrild B. Simultaneous human papilloma virus type 16 E7 and cdk inhibitor p21 expression induces apoptosis and cathepsin B activation. Virology. 2004;320:301–12. doi: 10.1016/j.virol.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 42.Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci U S A. 1997;94:11612–6. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhne C, Banks L. E3-ubiquitin ligase/E6-AP links multicopy maintenance protein 7 to the ubiquitination pathway by a novel motif, the L2G box. J Biol Chem. 1998;273:34302–9. doi: 10.1074/jbc.273.51.34302. [DOI] [PubMed] [Google Scholar]
- 44.Kukimoto I, Aihara S, Yoshiike K, Kanda T. Human papillomavirus oncoprotein E6 binds to the C-terminal region of human minichromosome maintenance 7 protein. Biochem Biophys Res Commun. 1998;249:258–62. doi: 10.1006/bbrc.1998.9066. [DOI] [PubMed] [Google Scholar]
- 45.Lee SS, Weiss RS, Javier RT. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci U S A. 1997;94:6670–5. doi: 10.1073/pnas.94.13.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SS, Glaunsinger B, Mantovani F, Banks L, Javier RT. Multi-PDZ domain protein MUPP1 is a cellular target for both adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J Virol. 2000;74:9680–93. doi: 10.1128/jvi.74.20.9680-9693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Tergaonkar V, Krishna S, Androphy EJ. Human papillomavirus type 16 E6-enhanced susceptibility of L929 cells to tumor necrosis factor alpha correlates with increased accumulation of reactive oxygen species. J Biol Chem. 1999;274:24819–27. doi: 10.1074/jbc.274.35.24819. [DOI] [PubMed] [Google Scholar]
- 48.Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev. 2004;68:362–72. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Longworth MS, Laimins LA. The binding of histone deacetylases and the integrity of zinc finger-like motifs of the E7 protein are essential for the life cycle of human papillomavirus type 31. J Virol. 2004;78:3533–41. doi: 10.1128/JVI.78.7.3533-3541.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mannhardt B, Weinzimer SA, Wagner M, Fiedler M, Cohen P, Jansen-Durr P, Zwerschke W. Human papillomavirus type 16 E7 oncoprotein binds and inactivates growth-inhibitory insulin-like growth factor binding protein 3. Mol Cell Biol. 2000;20:6483–95. doi: 10.1128/mcb.20.17.6483-6495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mantovani F, Banks L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001;20:7874–7887. doi: 10.1038/sj.onc.1204869. [DOI] [PubMed] [Google Scholar]
- 52.Massimi P, Pim D, Storey A, Banks L. HPV-16 E7 and adenovirus E1a complex formation with TATA box binding protein is enhanced by casein kinase II phosphorylation. Oncogene. 1996;12:2325–30. [PubMed] [Google Scholar]
- 53.Massimi P, Banks L. Transformation assays for HPV oncoproteins. Methods Mol Med. 2005;119:381–95. doi: 10.1385/1-59259-982-6:381. [DOI] [PubMed] [Google Scholar]
- 54.Mazurek S, Zwerschke W, Jansen-Durr P, Eigenbrodt E. Metabolic cooperation between different oncogenes during cell transformation: interaction between activated ras and HPV-16 E7. Oncogene. 2001;20:6891–8. doi: 10.1038/sj.onc.1204792. [DOI] [PubMed] [Google Scholar]
- 55.Mazzarelli JM, Atkins GB, Geisberg JV, Ricciardi RP. The viral oncoproteins Ad5 E1A, HPV16 E7 and SV40 TAg bind a common region of the TBP-associated factor-110. Oncogene. 1995;11:1859–64. [PubMed] [Google Scholar]
- 56.Munger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Research. 2002;89:213–228. doi: 10.1016/s0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 57.Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol. 2000;20:8244–53. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen ML, Nguyen MM, Lee D, Griep AE, Lambert PF. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6’s induction of epithelial hyperplasia in vivo. J Virol. 2003;77:6957–64. doi: 10.1128/JVI.77.12.6957-6964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oelze I, Kartenbeck J, Crusius K, Alonso A. Human papillomavirus type 16 E5 protein affects cell-cell communication in an epithelial cell line. J Virol. 1995;69:4489–94. doi: 10.1128/jvi.69.7.4489-4494.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parish JL, Kowalczyk A, Chen HT, Roeder GE, Sessions R, Buckle M, Gaston K. E2 proteins from high- and low-risk human papillomavirus types differ in their ability to bind p53 and induce apoptotic cell death. J Virol. 2006;80:4580–90. doi: 10.1128/JVI.80.9.4580-4590.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park JS, Kim EJ, Kwon HJ, Hwang ES, Namkoong SE, Um SJ. Inactivation of interferon regulatory factor-1 tumor suppressor protein by HPV E7 oncoprotein. Implication for the E7-mediated immune evasion mechanism in cervical carcinogenesis. J Biol Chem. 2000;275:6764–9. doi: 10.1074/jbc.275.10.6764. [DOI] [PubMed] [Google Scholar]
- 62.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. International Journal of Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 63.Patel D, Huang SM, Baglia LA, McCance DJ. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. Embo J. 1999;18:5061–72. doi: 10.1093/emboj/18.18.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillips AC, Vousden KH. Analysis of the interaction between human papillomavirus type 16 E7 and the TATA-binding protein, TBP. J Gen Virol. 1997;78(Pt 4):905–9. doi: 10.1099/0022-1317-78-4-905. [DOI] [PubMed] [Google Scholar]
- 65.Pim D, Collins M, Banks L. Human papillomavirus type 16 E5 gene stimulates the transforming activity of the epidermal growth factor receptor. Oncogene. 1992;7:27–32. [PubMed] [Google Scholar]
- 66.Pim D, Massimi P, Dilworth SM, Banks L. Activation of the protein kinase B pathway by the HPV-16 E7 oncoprotein occurs through a mechanism involving interaction with PP2A. Oncogene. 2005;24:7830–8. doi: 10.1038/sj.onc.1208935. [DOI] [PubMed] [Google Scholar]
- 67.Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. International Journal of Cancer. 2002;97:72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- 68.Prathapam T, Kuhne C, Banks L. The HPV-16 E7 oncoprotein binds Skip and suppresses its transcriptional activity. Oncogene. 2001;20:7677–85. doi: 10.1038/sj.onc.1204960. [DOI] [PubMed] [Google Scholar]
- 69.Ronco LV, Karpova AY, Vidal M, Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12:2061–72. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rudin CM, Thompson CB. Apoptosis and disease: regulation and clinical relevance of programmed cell death. Annu Rev Med. 1997;48:267–81. doi: 10.1146/annurev.med.48.1.267. [DOI] [PubMed] [Google Scholar]
- 71.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–36. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 72.Song S, Pitot HC, Lambert PF. The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic animals. J Virol. 1999;73:5887–93. doi: 10.1128/jvi.73.7.5887-5893.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Srivenugopal KS, Ali-Osman F. The DNA repair protein, O(6)-methylguanine-DNA methyltransferase is a proteolytic target for the E6 human papillomavirus oncoprotein. Oncogene. 2002;21:5940–5. doi: 10.1038/sj.onc.1205762. [DOI] [PubMed] [Google Scholar]
- 74.Stoler MH, Rhodes CR, Whitbeck A, Wolinsky SM, Chow LT, Broker TR. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Hum Pathol. 1992;23:117–28. doi: 10.1016/0046-8177(92)90232-r. [DOI] [PubMed] [Google Scholar]
- 75.Stoppler H, Stoppler MC, Johnson E, Simbulan-Rosenthal CM, Smulson ME, Iyer S, Rosenthal DS, Schlegel R. The E7 protein of human papillomavirus type 16 sensitizes primary human keratinocytes to apoptosis. Oncogene. 1998;17:1207–14. doi: 10.1038/sj.onc.1202053. [DOI] [PubMed] [Google Scholar]
- 76.Stoppler MC, Straight SW, Tsao G, Schlegel R, McCance DJ. The E5 gene of HPV-16 enhances keratinocyte immortalization by full-length DNA. Virology. 1996;223:251–4. doi: 10.1006/viro.1996.0475. [DOI] [PubMed] [Google Scholar]
- 77.Straight SW, Hinkle PM, Jewers RJ, McCance DJ. The E5 oncoprotein of human papillomavirus type 16 transforms fibroblasts and effects the downregulation of the epidermal growth factor receptor in keratinocytes. J Virol. 1993;67:4521–32. doi: 10.1128/jvi.67.8.4521-4532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang S, Tao M, McCoy JP, Jr., Zheng ZM. The E7 oncoprotein is translated from spliced E6*I transcripts in high-risk human papillomavirus type 16-or type 18-positive cervical cancer cell lines via translation reinitiation. J Virol. 2006;80:4249–63. doi: 10.1128/JVI.80.9.4249-4263.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thomas M, Banks L. Inhibition of Bak-induced apoptosis by HPV-18 E6. Oncogene. 1998;17:2943–54. doi: 10.1038/sj.onc.1202223. [DOI] [PubMed] [Google Scholar]
- 80.Thomas M, Banks L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. J Gen Virol. 1999;80(Pt 6):1513–7. doi: 10.1099/0022-1317-80-6-1513. [DOI] [PubMed] [Google Scholar]
- 81.Thomas M, Glaunsinger B, Pim D, Javier R, Banks L. HPV E6 and MAGUK protein interactions: determination of the molecular basis for specific protein recognition and degradation. Oncogene. 2001;20:5431–9. doi: 10.1038/sj.onc.1204719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomas M, Laura R, Hepner K, Guccione E, Sawyers C, Lasky L, Banks L. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene. 2002;21:5088–96. doi: 10.1038/sj.onc.1205668. [DOI] [PubMed] [Google Scholar]
- 83.Thompson DA, Zacny V, Belinsky GS, Classon M, Jones DL, Schlegel R, Munger K. The HPV E7 oncoprotein inhibits tumor necrosis factor alpha-mediated apoptosis in normal human fibroblasts. Oncogene. 2001;20:3629–40. doi: 10.1038/sj.onc.1204483. [DOI] [PubMed] [Google Scholar]
- 84.Thomsen P, van Deurs B, Norrild B, Kayser L. The HPV16 E5 oncogene inhibits endocytic trafficking. Oncogene. 2000;19:6023–32. doi: 10.1038/sj.onc.1204010. [DOI] [PubMed] [Google Scholar]
- 85.Thyrell L, Sangfelt O, Zhivotovsky B, Pokrovskaja K, Wang Y, Einhorn S, Grander D. The HPV-16 E7 oncogene sensitizes malignant cells to IFN-alpha-induced apoptosis. J Interferon Cytokine Res. 2005;25:63–72. doi: 10.1089/jir.2005.25.63. [DOI] [PubMed] [Google Scholar]
- 86.Tommasino M, Adamczewski JP, Carlotti F, Barth CF, Manetti R, Contorni M, Cavalieri F, Hunt T, Crawford L. HPV16 E7 protein associates with the protein kinase p33CDK2 and cyclin A. Oncogene. 1993;8:195–202. [PubMed] [Google Scholar]
- 87.Tong X, Howley PM. The bovine papillomavirus E6 oncoprotein interacts with paxillin and disrupts the actin cytoskeleton. Proc Natl Acad Sci U S A. 1997;94:4412–7. doi: 10.1073/pnas.94.9.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsao YP, Li LY, Tsai TC, Chen SL. Human papillomavirus type 11 and 16 E5 represses p21(WafI/SdiI/CipI) gene expression in fibroblasts and keratinocytes. J Virol. 1996;70:7535–9. doi: 10.1128/jvi.70.11.7535-7539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Viac J, Chardonnet Y, Chignol MC, Schmitt D. Papilloma viruses, warts, carcinoma and Langerhans cells. In Vivo. 1993;7:207–12. [PubMed] [Google Scholar]
- 90.Vikhanskaya F, Falugi C, Valente P, Russo P. Human papillomavirus type 16 E6-enhanced susceptibility to apoptosis induced by TNF in A2780 human ovarian cancer cell line. Int J Cancer. 2002;97:732–9. doi: 10.1002/ijc.10114. [DOI] [PubMed] [Google Scholar]
- 91.Vogt M, Butz K, Dymalla S, Semzow J, Hoppe-Seyler F. Inhibition of Bax activity is crucial for the antiapoptotic function of the human papillomavirus E6 oncoprotein. Oncogene. 2006 doi: 10.1038/sj.onc.1209429. [DOI] [PubMed] [Google Scholar]
- 92.Watson RA, Thomas M, Banks L, Roberts S. Activity of the human papillomavirus E6 PDZ-binding motif correlates with an enhanced morphological transformation of immortalized human keratinocytes. J Cell Sci. 2003;116:4925–34. doi: 10.1242/jcs.00809. [DOI] [PubMed] [Google Scholar]
- 93.Welters MJ, de Jong A, van den Eeden SJ, van der Hulst JM, Kwappenberg KM, Hassane S, Franken KL, Drijfhout JW, Fleuren GJ, Kenter G, Melief CJ, Offringa R, van der Burg SH. Frequent display of human papillomavirus type 16 E6-specific memory t-Helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003;63:636–41. [PubMed] [Google Scholar]
- 94.Welters MJ, van der Logt P, van den Eeden SJ, Kwappenberg KM, Drijfhout JW, Fleuren GJ, Kenter GG, Melief CJ, van der Burg SH, Offringa R. Detection of human papillomavirus type 18 E6 and E7-specific CD4+ T-helper 1 immunity in relation to health versus disease. Int J Cancer. 2006;118:950–6. doi: 10.1002/ijc.21459. [DOI] [PubMed] [Google Scholar]
- 95.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–9. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 96.Yuan H, Fu F, Zhuo J, Wang W, Nishitani J, An DS, Chen IS, Liu X. Human papillomavirus type 16 E6 and E7 oncoproteins upregulate c-IAP2 gene expression and confer resistance to apoptosis. Oncogene. 2005;24:5069–78. doi: 10.1038/sj.onc.1208691. [DOI] [PubMed] [Google Scholar]
- 97.Zeng M, Kumar A, Meng G, Gao Q, Dimri G, Wazer D, Band H, Band V. Human papilloma virus 16 E6 oncoprotein inhibits retinoic X receptor-mediated transactivation by targeting human ADA3 coactivator. J Biol Chem. 2002;277:45611–8. doi: 10.1074/jbc.M208447200. [DOI] [PubMed] [Google Scholar]
- 98.Zimmermann H, Degenkolbe R, Bernard HU, O’Connor MJ. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J Virol. 1999;73:6209–19. doi: 10.1128/jvi.73.8.6209-6219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


