Abstract
Older adults have more difficulty than younger adults appropriately directing their behavior when the required response is in competition with a prepotent response. The authors varied the difficulty of inhibiting a prepotent eye movement response by varying the response cue (peripheral onset or central arrow). The response cue manipulation did not affect prosaccade accuracy and latency for either age group and did not affect younger adults' antisaccades. Older adults' antisaccades were slower in the peripheral cue condition than in the central arrow condition. These findings are taken as support for the inhibitory deficit hypothesis of aging (L. Hasher, R. T. Zacks, & C. P. May, 1999).
Keywords: antisaccade, aging, inhibition
Older adults have more difficulty than younger adults appropriately directing their behavior when the required response is in competition with a prepotent or highly practiced response. Looking away from an onset stimulus when the appropriate response is to look in the opposite direction is more difficult for older than for younger adults (Butler, Zacks, & Henderson, 1999; Olincy, Ross, Youngd, & Freedman, 1997). Furthermore, when a cue to stop the response to the target stimulus is presented, older adults have more difficulty withholding their response than do younger adults (Kramer, Humphrey, Larish, Logan, & Strayer, 1994; May & Hasher, 1998; Williams, Ponesse, Schachar, & Logan, 1999). Likewise, older adults have more difficulty withholding the word reading response to a color word when required to identify the color of the word (Davidson, Zacks, & Williams, 2003; West, 1999; but see Verhaeghen & De Meersman, 1998).
Each of these tasks requires that a prepotent or automatic response be prevented. For this reason, age-related declines in performance on these tasks have been taken as support for the inhibitory deficit hypothesis of aging (Hasher & Zacks, 1988; Hasher, Zacks, & May, 1999). This theory posits that age-related changes in cognitive abilities (including language comprehension and memory) are caused by impaired inhibitory processes that lead to three types of functional declines: (a) reduced ability to restrict access of irrelevant information to working memory, (b) reduced ability to delete information from working memory that has become irrelevant to the current task, and (c) reduced ability to restrain responding when a prepotent response is incorrect (Connelly, Hasher, & Zacks, 1991; Hartman & Hasher, 1994; Hasher & Zacks, 1988; Zacks, Hasher, Doren, Hamm, & Attig, 1987; Zacks, Radvansky, & Hasher, 1996). Older adults' reduced restraint ability would lead to slower and more error-prone performance when a conflicting response is also possible.
Although the inhibitory deficit account has received extensive support (for a review, see Hasher, Zacks, & May, 1999; but see Burke, 1997; McDowd, 1997), an alternative explanation of agerelated performance deficits in interference tasks has been proposed that does not require inhibition to play a role. The goal neglect theory proposes that age differences in performance on behavioral control tasks are due to a failure to maintain the current task goal in a state of activation that is sufficient to guide behavior (De Jong, Berendsen, & Cools, 1999; De Jong, 2001; West, 2001). According to this view, correct performance in conflict situations requires adequate activation, or availability, of the current task goals; inhibition of competing, but incorrect, responses is not a component of successful task performance. Goal activation is an effortful process that varies with the cognitive control abilities of the individual. During task performance, if goal activation is not sufficient when the stimulus is presented, correct performance may be slowed by the time needed to reactivate the current task goal. In some instances goal activation may be so low that the goal cannot be reactivated and a more practiced or prepotent response will be executed instead. Because of age-related increases in goal neglect, older adults have more difficulty than do younger adults in maintaining optimal levels of goal activation and therefore perform more slowly and make more errors in interference situations than do younger adults (De Jong, 2001; De Jong et al., 1999; West, 2001).
The Study
The current study varied the inhibitory difficulty of an antisaccade task, while keeping the demands of maintaining the task goals the same. In the typical antisaccade task, the viewer is asked to look in the opposite direction of a peripheral onset stimulus. The antisaccade procedure elicits two competing response programs: an incorrect, involuntary eye movement toward the peripheral stimulus and a correct, voluntary response in the opposite direction (Forbes & Klein, 1996; Hallet, 1978; Hallet & Adams, 1980; for a review, see Everling & Fischer, 1998).
Older adults have more difficulty performing antisaccades than do younger adults. Not only are older adults more likely to incorrectly look toward a peripheral onset than are younger adults (Bojko, Kramer, & Peterson, 2004; Butler et al., 1999; Olincy et al., 1997; Nieuwenhuis, Ridderinkhof, De Jong, Kok, & van der Molen, 2000; Klein, Fischer, Hartnegg, Heiss, & Roth, 2000), but when antisaccade latencies are compared with saccade latencies in a control condition, older adults often are slowed more than are younger adults (Fischer, Biscaldi, & Gezeck, 1997; Nieuwenhuis et al., 2000; but see Munoz, Broughton, Goldring, & Armstrong, 1998).
In the present study, we varied the inhibitory difficulty of the task by varying the cue used to signal the direction of the correct eye movement response. In the standard antisaccade condition, the inhibitory demands are high because of the use of a peripheral onset to signal the direction in which the participant should not look. Peripheral onsets automatically attract attention, and an eye movement is involuntarily programmed to that location (e.g., Theeuwes, Kramer, & Hahn, 1999). According to the inhibitory hypothesis, performing the correct task (looking in the opposite direction) requires the incorrect response program to be inhibited before its programming is completed. Inhibition must be applied in a trial-by-trial manner to the incorrect response. To reduce the inhibitory demands, we used a different type of direction cue: a centrally presented arrow. Arrow cues also automatically direct attention to the location they point to (Hommel, Pratt, Colzato, & Godijn, 2001; Tipples, 2002), but programming a saccade in response to an arrow cue requires voluntary saccade programming that is subserved by frontal brain regions (Pierrot-Deseilligny, Rivaud, Gaymard, Muri & Vermersch, 1995). Because voluntary saccade programming is more effortful, the incorrect response should be less likely to be programmed following the central arrow cue than the peripheral onset cue and therefore the inhibitory demands of the task should be reduced in the central cue conditions.
Performing antisaccades in response to central arrow cues should be faster and/or more accurate than performing antisaccades in response to a peripheral onset. In addition, if older adults have more difficulty suppressing their erroneous responses than do younger adults, the effect of the type of cue should be larger for them. This manipulation is not expected to influence the difficulty of maintaining the task goal, however, because the instructions to the participants and trial events will only differ by the cue used. If antisaccade accuracy and latency are only a function of the activation of the current task goal (to look in the opposite direction) and not related to whether inhibition is needed to suppress the programming of a competing response, older adults should be affected in the same way as younger adults by the different cue conditions.
Method
Participants
Thirty-three younger adults (age range = 18-23 years) were recruited from undergraduate psychology courses and were either compensated $7/hr for their participation or given partial course credit. One younger adult (from the central cue condition) was excluded because his or her eye movements could not be reliably tracked. Thirty-nine community-dwelling older adults (age range = 61-85 years) were recruited from the Lansing, Michigan area and compensated $10/hr for their participation. Seven older adults were excluded from the analyses: 2 because they were not performing the task correctly and 5 because their eye movements could not be reliably tracked. Younger and older adults reporting learning disabilities, psychoactive medications, or a history of brain trauma (including stroke) were excluded from the sample. All participants were able to discriminate letters that subtended 0.6° of visual angle.
Mean ages, vocabulary test scores, and years of education by age group are presented in Table 1. Education did not differ by age group, F(1, 59) = 2.87, MSE = 3.73, p = .10, or by cue condition (F < 1). Vocabulary scores were higher for older adults than for younger adults, F(1, 60) = 42.0, MSE = 14.83, p < .01, but did not differ by cue condition, F(1, 60) = 2.93, MSE = 14.83, p = .09. Older adults in the central cue condition were a little older, on average, than those in the peripheral cue condition. This difference, although not reliable, F(1, 30) = 3.01, MSE = 23.93, p = .09, would reduce differences between the cue conditions because the younger group was in the more difficult inhibitory condition. For younger adults, age did not vary by cue condition (F < 1).
Table 1.
Participant Characteristics by Cue Condition
| Peripheral |
Central |
|||
|---|---|---|---|---|
| Characteristic | M | SD | M | SD |
| Younger adults | ||||
| Age (years) | 20.9 | 1.3 | 21.1 | 1.2 |
| Education (years) | 14.8 | 1.0 | 15.1 | 2.2 |
| Vocabulary | 27.8 | 4.1 | 30.1 | 3.5 |
| Older adults | ||||
| Age (years) | 71.9 | 5.3 | 74.9 | 4.4 |
| Education (years) | 15.6 | 2.1 | 15.9 | 2.1 |
| Vocabulary | 34.7 | 4.2 | 35.7 | 3.6 |
Design
This experiment had a 2 (age group: younger or older) × 2 (eye movement task: prosaccade or antisaccade) × 2 (cue type: central or peripheral) design, with age group and cue type as between-subjects variables and eye movement task as a within-subjects variable.
Apparatus and Stimuli
Eye movements were recorded with an ISCAN RK-416 high-speed eyetracker (ISCAN, Burlington, MA) that uses an infrared video-based system to compute and horizontally track the center of the pupil in the right eye. Signals were generated by the eyetracker at a frequency of 120 Hz, allowing saccade latencies to be calculated with a temporal resolution of 8.33 ms. The spatial resolution of the apparatus is 0.2° of visual angle. Stimuli were displayed at a resolution of 800 × 600 pixels on a NEC Multisync XE15 monitor controlled by a Pentium PC-compatible computer.
A chin and forehead rest was used to stabilize the participant's head at a viewing distance of 49 cm. The fixation display contained a white cross in the center of a black screen flanked by white boxes, one to the left and one to the right. The fixation cross and boxes subtended visual angles of 0.8° and 1.2°, respectively, and the distance from fixation to the inside edge of the box was 10.5°. The central arrow cue subtended the same visual angle as the fixation stimulus (0.8°). The perceptual identification target was a circle (0.6°) with a gap (0.2°) in the top or bottom.
Procedure
First, the apparatus was explained to the participant, and the participant signed a consent form. Then, while sitting in the chin and forehead rest, the participant's acuity was tested with a photocopied miniature version of the Snellen vision chart taped to the computer monitor.
Following the acuity test, participants read instructions describing the prosaccade condition and then were shown the displays that would occur in each trial while the experimenter explained the task. The instructions emphasized that when the cue (onset or arrow) appeared, it was important to move the eyes as quickly and as accurately as possible. Participants were told to press the button that corresponded to the location of the gap, either top or bottom, and to guess if they were unsure. Prosaccades were always performed first to allow participants to become familiar with the displays and timing parameters (see Butler et al., 1999). The same instruction procedure was used prior to the antisaccade condition. Forty trials were presented in each block.
The sequence of events for each trial is illustrated in Figure 1. At the beginning of a block of trials, the fixation display (the fixation cross flanked by box place markers) was presented. After checking the calibration, the experimenter initiated a string of eight trials. The fixation display was presented for an additional 300 ms followed by the task instruction for 800 ms (“TOWARD” for prosaccade and “OPPOSITE” for antisaccade). The task instruction was replaced by the fixation screen for 1,800 ms. Then, the fixation cross disappeared, and 200 ms later the cue (onset or arrow) was presented for 400 ms. Following the cue, the target display was presented for 150 ms. In the prosaccade condition, the perceptual identification target (a circle with a gap) was presented in the location signaled by the cue; in the antisaccade condition, it appeared in the opposite box. Simultaneous with the presentation of the target, a full circle was presented at the nontarget location to prevent older adults from using the onset of the target to guide their response (Nieuwenhuis et al., 2000). After the target display, both boxes appeared empty until a button press response was made. Following the button press, the fixation cross flashed in the center of the screen for 500 ms at 50-ms intervals.
Figure 1.
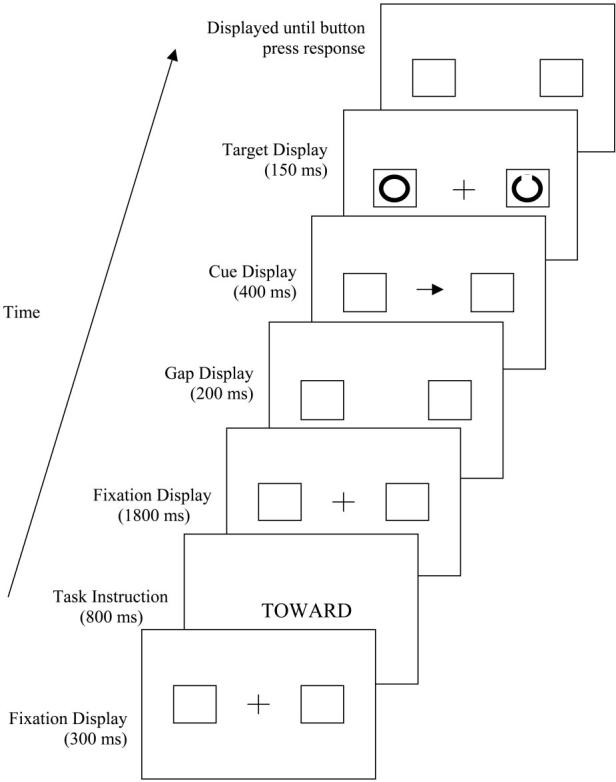
Illustration of a prosaccade trial in the central cue condition. On an antisaccade trial, the full circle would have appeared in the box the arrow pointed toward and the gap circle would have appeared in the opposite box. In the peripheral onset condition, the fixation cross was replaced with a double-headed arrow and the center of one of the peripheral boxes was turned white. In the experimental displays, stimuli were white presented on a black background.
In the central cue condition, the direction cue was a single-headed arrow presented at fixation. The direction cue in the peripheral onset condition was one of the box place markers turning white. In the prosaccade condition, participants were told to look toward the box the arrow pointed at or that turned white; in the antisaccade condition, participants were told to look at the box opposite from the box the arrow pointed toward or at the box opposite from the box that turned white. In addition, a double-headed arrow was presented at fixation simultaneous with the peripheral cue, hence equating the peripheral and central cue conditions on the need to disengage the oculomotor system from fixation before the eye movement response could be initiated (Kingstone & Klein, 1993).
Data Analysis
Any samples of less than 15 ms were removed from the eye tracking record as representing saccades. Fixations were coded as the time between saccades, and the position of a fixation was calculated by averaging the position of the samples during that continuous time period, weighted for the duration of each sample. The initial saccade was the first change in fixation position that was greater than 1° from the previous fixation and that occurred following the presentation of the cue. The latency of the initial saccade was the time from the presentation of the cue until the end of the fixation prior to the initial saccade. Correct prosaccades were initial saccades toward the location of the peripheral onset or direction of the arrow. Correct antisaccades were initial saccades in the opposite direction from the peripheral onset or direction of the arrow. Trials were excluded from all analyses if the initial fixation was not within 4° of the center of the screen. These initial fixation samples could have occurred because of either poor calibration or anticipatory eye movements. Two additional types of trials were excluded from the analyses: (a) trials that the experimenter had hand-coded as having poor calibration because of subject movements and (b) trials with saccade latencies less than 75 ms or greater than 1000 ms, indicating that the saccade was not in response to the stimulus.
Results
More data were excluded for the older (M = 20.8%, SD = 16.1) than the younger adults (M = 6.2%, SD = 10.1), F(1, 60) = 27.55, MSE = 0.02, p < .01, and more data were excluded in the peripheral (M = 17.0%, SD = 15.6) than in the central cue condition (M = 9.9%, SD = 14.2), F(1, 60) = 6.51, MSE = 0.02, p < .02. Age group and cue type did not interact (F < 1); there was no difference between the eye movement tasks in percent of trials excluded (F < 1); and age group, cue type, and eye movement task did not enter into any interactions (Fs < 1). Because the age difference in percentage of trials excluded was the same for the two cue conditions, it is unlikely that it accounts for the effect of cue condition reported below.
Saccade direction accuracy and mean correct saccade latencies from the prosaccade and antisaccade conditions were submitted to repeated measures analyses of variance with age group and cue type as between-subject variables and eye task as a within-subject variable. For all analyses, the rejection level for inferring statistical significance was set at .05.
Mean saccade direction accuracies and latencies are presented in Table 2. Prosaccades (M = 97.2%, SD = 4.6) were more accurate than antisaccades (M = 76.7%, SD = 17.1), F(1, 60) = 94.28, MSE = 0.01, p < .01, and younger adults (M = 90.8%, SD = 8.0) were more accurate than older adults (M = 83.1%, SD = 17.5), F(1, 60) = 13.93, MSE = 0.01, p < .01. Age group interacted with task such that older adults showed a greater difference between prosaccade (M = 95.7%, SD = 5.9) and antisaccade (M = 70.5%, SD = 17.6) direction accuracy than did younger adults (M = 98.8%, SD = 1.9, and M = 82.9%, SD = 14.2, respectively), F(1, 60) = 4.84, MSE = 0.01, p = .03. There was no effect of cue type, and it did not enter into any interactions (Fs < 1.1).
Table 2.
Mean Saccade Accuracies and Latencies by Cue Condition
| Younger adults |
Older adults |
|||||||
|---|---|---|---|---|---|---|---|---|
| Central |
Peripheral |
Central |
Peripheral |
|||||
| Response | M | SE | M | SE | M | SE | M | SE |
| Accuracy | ||||||||
| Prosaccade | 99.0 | 0.3 | 98.5 | 0.6 | 96.3 | 0.9 | 95.1 | 2.0 |
| Antisaccade | 85.7 | 3.6 | 80.1 | 3.7 | 69.4 | 4.6 | 71.7 | 4.6 |
| Latency | ||||||||
| Prosaccade | 299 | 12.5 | 281 | 14.0 | 372 | 19.1 | 363 | 19.1 |
| Antisaccade | 329 | 11.2 | 329 | 15.5 | 390 | 15.7 | 446 | 20.1 |
| Antisaccade errors | 265 | 7.6 | 230 | 10.5 | 343 | 21.5 | 268 | 13.2 |
Note. Mean antisaccade error latencies for young adults in the central arrow cue condition excluded 1 participant who did not make any antisaccade errors. Antisaccade errors incorrect saccades in the antisaccade condition.
Mean correct saccade latencies for each participant were based on 10 or more observations. Antisaccades (M = 373 ms, SD = 78) were slower than prosaccades (M = 329 ms, SD = 67), F(1, 60) = 53.64, MSE = 1187.19, p < .01, and older adults (M = 393 ms, SD = 67) were slower than younger adults (M = 309 ms, SD = 51), F(1, 60) = 38.79, MSE = 5728.15, p < .01. The main effect of cue type was not significant (F < 1), but cue did enter into an interaction with task, F(1, 60) = 11.55, MSE = 1187.19, p < .01, that was modulated by a marginally significant three-way interaction that included age group, F(1, 60) = 3.79, MSE = 1187.19, p = .056. However, t tests indicated that the cue type did not affect the latencies of younger adults' prosaccades nor antisaccades (ts = -0.96 and 0.02, respectively). Likewise, older adults' prosaccade latencies were not different in the peripheral and arrow cue conditions, t(30) =-0.45, p = .66. However, older adults in the peripheral cue condition were slower to initiate correct antisaccades than older adults in the arrow cue condition, t(30) = 2.25, p = .03.
To evaluate the impact of our cue manipulation, the saccade latencies of error saccades in the antisaccade condition and correct saccades in the prosaccade condition were compared by age group and cue type. Two analyses were conducted: one on data from the 31 younger and 32 older adults with at least one error saccade latency and the second on data from the 20 younger and 25 older adults with at least four error saccade latencies. The analysis reported here and means reported in Table 2 were based on the larger sample. The same pattern of effects was observed in the smaller sample except for the absence of the cue by task interaction. Cue type did affect saccade latencies. Consistent with the idea that saccades toward peripheral onsets are harder to suppress, saccades toward peripheral onsets were faster (M = 286 ms, SD = 63) than saccades consistent with the pointing direction of the arrow (M = 319 ms, SD = 66), F(1, 59) = 8.20, MSE = 4254.11, p < .01. Correct responses in the prosaccade condition were slower (M = 328 ms, SD = 68) than error saccades in the antisaccade condition (M = 277 ms,SD = 68), F(1, 59) = 43.77, MSE = 1887.36, p < .01. The effect of eye movement task was modulated by an interaction with the cue type, F(1, 59) = 7.86, MSE = 1887.36, p < .01, indicating that in the peripheral onset condition there was a greater difference between the latencies of saccades from the two tasks (73 ms) than in the central arrow condition (30 ms). Older adults' saccade latencies (M = 337 ms, SD = 71) were slower than younger adults' latencies (M = 268 ms, SD = 45), F(1, 59) = 34.99, MSE = 4254.11, p < .01, but age group did not enter into any interactions with cue type or task (Fs < 2.1). Following the eye movement(s), a response was made to the target (gap on top or bottom of circle). Because discrimination was contingent on the speed and accuracy of the eye movement(s), button press reaction times and accuracies mirrored them.
Discussion
In this experiment, the inhibitory difficulty of antisaccade performance was varied by means of different stimuli to cue looking direction; either a peripheral onset or a central arrow cue was used. Older adults were slower to move their eyes in the opposite direction from the peripheral onset than in the opposite direction from where an arrow was pointing. Younger adults' correct antisaccade latencies were unaffected by the cue type. Older adults were slower to initiate antisaccades when the inhibitory demands of the task were higher, but younger adults' antisaccade latencies were unaffected by the inhibitory difficulty of the task. This finding is taken as support for the inhibitory deficit hypothesis of aging (Hasher & Zacks, 1988; Hasher et al., 1999).
One might ask whether the age difference observed was the result of age differences in spatial orienting rather than eye movement control. Although early research on attentional control in older adults was inconclusive, recent studies have suggested that older adults have more difficulty removing attention from a location cued by an onset than from a location cued by a central arrow (Juola, Koshino, Warner, McMickell & Peterson, 2000; Lincourt, Folk, & Hoyer, 1997; for a review, see Kramer, Scialfa, Peterson, & Irwin, 2001). Antisaccades differ in potentially significant ways from these attention tasks, however. Antisaccade cues signal an immediate response, always occur in the wrong location (0% valid), but always signal the correct response (100% predictive). Attentional cues have their effect on responses that occur after a delay and under conditions of varying validity and predictivity. Although age differences in attentional processes may exist, their role in the results of our study is unclear.
Perhaps surprisingly, the effects of the cue were overall quite small. Saccade accuracy was unaffected by cue type, and there was no effect of cue type on the antisaccade latencies of the younger adults. Like peripheral onsets though, arrow cues automatically directed attention (Hommel et al., 2001; Tipples, 2002). Furthermore, unlike previous research (e.g., Logan & Irwin, 2000), we tried to equate the cueing conditions on the need for oculomotor disengagement (Kingstone & Klein, 1993). Specifically, because the arrow cue was a new stimulus presented at fixation (conditions known to slow voluntary saccade initiation; Saslow, 1967), we also presented a stimulus (a double-headed arrow) simultaneously with the presentation of the peripheral cue. Presentation of a central stimulus simultaneously with the peripheral cue slows saccade initiation toward the cue (Walker, Deubel, Schneider, & Findlay, 1997). According to our data, young adults' voluntary, antisaccade latencies and accuracies are the same when the cue conditions are equated for oculomotor disengagement. However, it should be noted our cue manipulation still did have an effect. Saccades toward peripheral onsets were initiated more quickly than saccades consistent with the direction of the arrow cue by both younger and older adults.
The effect of the cue manipulation was only observed in older adults' correct saccade latencies and not their accuracies. It is not clear why this is the case. Perhaps it reflects a speed-accuracy trade-off; rather than allowing the difficulty of inhibition to lead to even more saccade direction errors, older adults sacrificed some of their antisaccade programming time to devote more effort to stopping the incorrect responses.
The goal neglect view has trouble accounting for the slower performance of older adults in the onset than in the arrow condition. According to the goal neglect view, the speed of programming an incorrect saccade consistent with the cue direction should not influence correct (antisaccade) performance. In both cue conditions, the goal being maintained is the same: to look in the opposite direction of the location indicated by the cue. Because the task to be performed was the same in both cue conditions, goal neglect should have been equally likely in both.
Even though the goal structure was the same, the cue type influenced older adults' performance more than younger adults' performance. In a direct comparison of antisaccade performance in the two cue conditions, the antisaccade latencies of older adults were slower when the inhibitory demands of the task were higher (in the peripheral cue condition) than when the inhibitory demands were lessened (in the central cue condition), but there was no effect of cue condition on the antisaccade latencies of the young adults. Age differences in performing antisaccades are related to the difficulty of inhibition, a result that supports the inhibitory deficit hypothesis of aging (Hasher & Zacks, 1988; Hasher et al., 1999; Zacks & Hasher, 1997).
Footnotes
This article is based on Karin M. Butler's dissertation. This research was first presented at the Cognitive Aging Conference, April 2002. This research was supported by National Institute of Aging Grant AGO 4306 to Lynn Hasher and Rose T. Zacks and National Science Foundation Grant SBR 9617274 to John Henderson. We thank Tom Carr, John Henderson, and Eric Altmann for helpful discussion of this research, and we thank Katie Paquette, Kristina Pecora, Sarah Burnett, Kate Arrington, and Kiel Christianson for their help in data collection and participant recruiting.
References
- Bojko A, Kramer AF, Peterson MS. Age equivalence in switch costs for prosaccade and antisaccade tasks. Psychology and Aging. 2004;19:226–234. doi: 10.1037/0882-7974.19.1.226. [DOI] [PubMed] [Google Scholar]
- Burke D. Language, aging, and inhibitory deficits: Evaluation of a theory. Journal of Gerontology, Series B: Psychological Sciences and Social Sciences. 1997;52:P254–P264. doi: 10.1093/geronb/52b.6.p254. [DOI] [PubMed] [Google Scholar]
- Butler KM, Zacks RT, Henderson JM. Suppression of reflexive saccades in younger and older adults: Age comparisons on an antisaccade task. Memory &Cognition. 1999;27:584–589. doi: 10.3758/bf03211552. [DOI] [PubMed] [Google Scholar]
- Connelly SL, Hasher L, Zacks RT. Age and reading: The impact of distraction. Psychology and Aging. 1991;6:533–541. doi: 10.1037//0882-7974.6.4.533. [DOI] [PubMed] [Google Scholar]
- Davidson DJ, Zacks RT, Williams CC. Stroop interference, practice, and aging. Aging, Neuropsychology and Cognition. 2003;10:85–98. doi: 10.1076/anec.10.2.85.14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong R. Adult age differences in goal activation and goal maintenance. European Journal of Cognitive Psychology. 2001;13:71–89. [Google Scholar]
- De Jong R, Berendsen E, Cools R. Goal neglect and inhibitory limitations: Dissociable causes of interference effects in conflict situations. Acta Psychologica. 1999;101:379–394. doi: 10.1016/s0001-6918(99)00012-8. [DOI] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: A review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Fischer B, Biscaldi M, Gezeck S. On the development of voluntary and reflexive components in human saccade generation. Brain Research. 1997;754:285–297. doi: 10.1016/s0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- Forbes K, Klein RM. The magnitude of the fixation offset effect with endogenously and exogenously controlled saccades. Journal of Cognitive Neuroscience. 1996;8:344–352. doi: 10.1162/jocn.1996.8.4.344. [DOI] [PubMed] [Google Scholar]
- Hallet PE. Primary and secondary saccades to goals defined by instructions. Vision Research. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hallet PE, Adams BD. The predictability of saccadic latency in a novel voluntary oculomotor task. Vision Research. 1980;20:329–339. doi: 10.1016/0042-6989(80)90019-x. [DOI] [PubMed] [Google Scholar]
- Hartman M, Hasher L. Aging and suppression: Memory for previously relevant information. Psychology and Aging. 1994;6:587–594. doi: 10.1037//0882-7974.6.4.587. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. The Psychology of Learning and Motivation. 1988;22:193–225. [Google Scholar]
- Hasher L, Zacks RT, May CP. Inhibitory control, circadian arousal, and age. In: Gopher D, Koriat A, editors. Attention and performance: XVII. Cognitive regulation of performance: Interaction of theory and application. MIT Press; Cambridge, MA: 1999. pp. 653–675. [Google Scholar]
- Hommel B, Pratt J, Colzato L, Godijn R. Symbolic control of visual attention. Psychological Science. 2001;12:360–365. doi: 10.1111/1467-9280.00367. [DOI] [PubMed] [Google Scholar]
- Juola JF, Koshino H, Warner CB, McMickell M, Peterson MS. Automatic and voluntary control of attention in young and older adults. American Journal of Psychology. 2000;113:159–178. [PubMed] [Google Scholar]
- Kingstone A, Klein RM. Visual offsets facilitate saccadic latency: Does predisengagement of visuospatial attention mediate this gap effect? Journal of Experimental Psychology: Human Perception and Performance. 1993;19:1251–1265. doi: 10.1037//0096-1523.19.6.1251. [DOI] [PubMed] [Google Scholar]
- Klein C, Fischer B, Hartnegg K, Heiss WH, Roth M. Optomotor and neuropsychological performance in old age. Experimental Brain Research. 2000;135:141–154. doi: 10.1007/s002210000506. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Humphrey DG, Larish JF, Logan GD, Strayer DL. Aging and inhibition: Beyond a unitary view of inhibitory processing in attention. Psychology and Aging. 1994;9:491–512. [PubMed] [Google Scholar]
- Kramer AF, Scialfa CT, Peterson MS, Irwin DE. Attentional capture, attentional control, and aging. In: Folk CL, Gibson BS, editors. Attraction, distraction and action: Multiple perspectives on attentional capture. Elsevier Science; Amsterdam, the Netherlands: 2001. pp. 293–322. [Google Scholar]
- Lincourt AE, Folk CL, Hoyer WJ. Effects of aging on voluntary and involuntary shifts of attention. Aging, Neuropsychology, &Cognition. 1997;4:290–303. doi: 10.1080/13825589708256654. [DOI] [PubMed] [Google Scholar]
- Logan GD, Irwin DE. Don't look! Don't touch! Inhibitory control of eye and hand movements. Psychonomic Bulletin &Review. 2000;7:107–112. doi: 10.3758/bf03210728. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L. Synchrony effects in inhibitory control over thought and action. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:363–379. doi: 10.1037//0096-1523.24.2.363. [DOI] [PubMed] [Google Scholar]
- McDowd JM. Inhibition in attention and aging. Journal of Gerontology, Series B: Psychological Sciences and Social Sciences. 1997;52:P265–P273. doi: 10.1093/geronb/52b.6.p265. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof RR, De Jong R, Kok A, van der Molen MW. Inhibitory inefficiency and failures of intention activation: Age-related decline in the control of saccadic eye movements. Psychology and Aging. 2000;15:635–647. doi: 10.1037//0882-7974.15.4.635. [DOI] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Youngd DA, Freedman R. Age diminishes performance on an antisaccade eye movement task. Neurobiology of Aging. 1997;18:483–489. doi: 10.1016/s0197-4580(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Muri R, Vermersch A-I. Cortical control of saccades. Neurological Progress. 1995;37:557–567. doi: 10.1002/ana.410370504. [DOI] [PubMed] [Google Scholar]
- Saslow MG. Latency for saccadic eye movement. Journal of the Optical Society of America. 1967;57:1030–1033. doi: 10.1364/josa.57.001030. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Kramer AF, Hahn S. Influence of attentional capture on oculomotor control. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:1595–1608. doi: 10.1037//0096-1523.25.6.1595. [DOI] [PubMed] [Google Scholar]
- Tipples J. Eye gaze is not unique: Automatic orienting in response to uninformative arrows. Psychonomic Bulletin &Review. 2002;9:314–318. doi: 10.3758/bf03196287. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, De Meersman L. Aging and the Stroop effect: A meta-analysis. Psychology and Aging. 1998;13:120–126. doi: 10.1037//0882-7974.13.1.120. [DOI] [PubMed] [Google Scholar]
- Walker R, Deubel H, Schneider WX, Findlay JM. Effect of remote distractors on saccade programming: Evidence for an extended fixation zone. Journal of Neurophysiology. 1997;78:1108–1119. doi: 10.1152/jn.1997.78.2.1108. [DOI] [PubMed] [Google Scholar]
- West R. Age differences in lapses of intention in the Stroop task. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1999;54:P34–P43. doi: 10.1093/geronb/54b.1.p34. [DOI] [PubMed] [Google Scholar]
- West R. The transient nature of executive control processes in younger and older adults. European Journal of Cognitive Psychology. 2001;13:91–106. [Google Scholar]
- Williams BR, Ponesse JR, Schachar RJ, Logan GD. Development of inhibitory control across the life span. Developmental Psychology. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Hasher L. Cognitive gerontology and attentional inhibition: A reply to Burke and McDowd. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1997;52:P274–P283. doi: 10.1093/geronb/52b.6.p274. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Hasher L, Doren B, Hamm V, Attig MS. Encoding and memory of explicit and implicit information. Journal of Gerontology. 1987;42:418–422. doi: 10.1093/geronj/42.4.418. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Radvansky GA, Hasher L. Studies of directed forgetting in older adults. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22:143–156. doi: 10.1037//0278-7393.22.1.143. [DOI] [PubMed] [Google Scholar]


