Abstract
We studied the neuronal basis of the motivational response to two powerful but radically different rewards—cocaine and maternal nurturing of pups in the postpartum rat (dam) which is in a unique motivational state. We used a place preference method designed to offer a choice between cues associated with a natural reinforcer (pups) and those associated with a pharmacologic reinforcer (cocaine). Using c-Fos or cocaine- and amphetamine-regulated transcript (CART) immunocytochemistry, we identified the neuronal groups that are activated when the dams expressed a preference for either cues-associated with pups or cues-associated with cocaine. Dams that preferred the cocaine-associated cues had more c-Fos positive neurons in medial prefrontal cortex, nucleus accumbens, and basolateral nucleus of amygdala than pup-associated cue preferring dams or control. Except for the accumbens, there was activation of neurons in these same regions with the pup-associated cue preference. In the nucleus accumbens only CART-immunoreactive (not c-Fos) neurons were activated with pup-cue preference. Notably, the medial preoptic area was the single area where greater activation of neurons was seen with a preference for pup-associated versus cocaine-associated cues. These responses were identified in the absence of the stimuli (cocaine or pups) and are proposed to be, in part, activation of these neurons related to motivational processing. Neither the distribution of neurons responding to pup-associated cue preference nor the demonstration that CART-expressing neurons are responsive to reward-associated cue preference has been previously reported. We hypothesize that the expression of preference for cocaine versus pup-associated cues is made possible by the concerted activity of these regionally distributed networks of neurons that are in part specific to the preference response.
Keywords: conditioned place preference, maternal behavior, medial preoptic area, nucleus accumbens, basolateral amygdala, medial prefrontal cortex
Abbreviations: Acb, nucleus accumbens; ANOVA, analysis of variance; BlA, basal-lateral amygdala; CART, cocaine-and amphetamine-regulated transcript; Cg1, anterior cingulate cortex; HSD, Tukey-Kramer honest significant difference; IL, infralimbic cortex; IR, immunoreactive/immunoreactivity; mPFC, medial prefrontal cortex; MPOA, medial preoptic area; PFC, prefrontal cortex; PrL, prelimbic cortex
We studied the neuronal basis of the motivational response to two powerful but radically different rewards—cocaine and maternal nurturing of pups (Hauser and Gandelman, 1985; Koob et al., 1987; Wise and Bozarth, 1987; Fleming et al., 1989; Stewart and Badiani, 1993; Tzschentke, 1998; Lee et al., 1999b). The high incidence of cocaine abuse in women of reproductive age and the correlation of cocaine abuse with poor caregiving suggest that the choice between these two rewards underlies a social problem (Chasnoff, 1988, 1995; Kandel et al., 1985; Roland and Volpe, 1989).
Psychomotor stimulants, such as cocaine and amphetamine, are powerful reinforcers, readily inducing self-administration and place preference responses across diverse animal models (Morutto and Phillips, 1998; Stewart and Badiani, 1993; Tzschentke, 1998). Maternal behavior is crucial for all mammalian species, including humans, and is one of the most highly motivated, naturally occurring behaviors. The young are powerful reinforcers and rewarding stimuli to maternal mammals (Hauser and Gandelman, 1985; Fleming et al., 1989; Lee et al., 1999a; Fahrbach and Pfaff, 1982). The intensity of this phenomenon has been documented by a pup self-administration procedure, in which dams lever press to gain access to pups (Lee et al., 1999a), and place preference responses, where dams choose an environment they have been conditioned to associate with pups (Fleming et al., 1989, 1994; Lee et al., 1999a; Mattson et al., 2001).
Whereas experimental studies of motivation typically examine the salience of a single reward, animals in natural settings must choose among many reinforcers. Hence, we used a choice paradigm based on the place preference method (Aigner and Balster, 1978; Nader and Woolverton, 1991, 1992; Parker, 1992). It presents maternal rats with a choice between cues associated with a natural reinforcer (pups) and those associated with a pharmacologic reinforcer (cocaine). Previously, we found that most dams preferred pup-associated cues up to 8 days postpartum but after that preferred cocaine-associated cues (Mattson et al., 2001, 2003). In the present study, we determined which neurons were activated during those preference responses.
The immediate early genes and their related proteins, e.g. c-Fos, participate in the early stages of cellular, including neuronal, activities. Systemic cocaine administration induces c-Fos in neurons within specific brain regions (Graybiel et al., 1990; Young et al., 1991; Hope et al., 1992), as do cocaine-associated cues in the absence of cocaine (Brown et al., 1992; Crawford et al., 1995; Neisewander et al., 2000; Uslander et al., 2001). Immunoreactivity for c-Fos has been used to identify neurons that are activated in consummatory aspects of maternal behavior (Numan, 1994; Kalinichev et al., 2000) but not appetitive components of the behavior.
Cocaine- and amphetamine-regulated transcript (CART) peptide is involved in many processes, including feeding, development, and stress response (Kuhar and Dall Vechia, 1999; Kask et al., 2000). This marker is thought to indicate processes related to reward, because acute cocaine increases CART mRNA and peptide in structures involved in reward, such as nucleus accumbens (Douglass et al., 1995; Couceyro et al., 1997; Smith et al., 1997, 1999; Koylu et al., 1998; Hurd and Fagergren, 2000).
We used a well-established model of maternal behavior, the postpartum rat (Numan and Insel, 2003), because this animal is in a unique motivational state that may provide insight into the fundamental properties of the reward system. Using c-Fos and CART peptide immunocytochemistry, we identified the neuronal groups that became activated during preference for cues associated with pups or cocaine. We focused on the following brain structures: nucleus accumbens (Acb), prefrontal cortex (PFC), and basal-lateral amygdala (BlA) (Koob et al., 1987; Kalivas and Barnes, 1994; Robbins and Everitt, 1999; Spanagel and Weiss, 1999; Rosenkranz and Grace, 2001), which are thought to underlie motivation, and the medial preoptic area (MPOA), which may mediate pup reinforcement (Lee et al., 1999a,b). We hypothesize that specific neuronal subsets within these brain regions underlie the postpartum females’ different behavioral responses to pup- and cocaine-associated cues. We determined the number and neuroanatomical distribution of c-Fos-immunoreactive (IR) or CART-IR neurons activated by preference for stimulus-associated cues in dams that preferred pup-associated cues versus cocaine-associated cues, and in control dams.
EXPERIMENTAL PROCEDURES
Subjects
We used female Sprague–Dawley rats (90–120 days old) from the colony maintained in the Laboratory Animal Facility at Rutgers University (Newark, NJ, USA). This colony consists of animals bred from animals originally purchased from Charles River Laboratories (Wilmington, MA, USA); the colony is maintained as genetically consistent with their Sprague–Dawley strain by regular and frequent purchase of males and females to serve as breeders. The Laboratory Animal Facility is accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC). All animals were treated in strict accordance with guidelines established by Rutgers University and National Institutes of Health USPHS Manual Guide for the Care and Use of Laboratory Animals. Care was taken to minimize the number of animals used and their suffering. These female rats (n=17) were bred by facility staff in accordance with the protocols developed by Mayer and Rosenblatt (1980, 1998), which regulate the duration of pregnancy and the timing of parturition on gestational day 22. On postpartum day 1, all pups were briefly removed from their mothers, co-mingled, and then litters of seven, randomly selected, pups returned to dams.
Prior to the start of the place preference conditioning paradigm, the expression of maternal behavior was tested in all dams using our standard behavioral assay in the home cage, and normal locomotor activity was verified using an Accuscan locomotor system. All further details as described in Mattson et al. (2003).
We tested the conditioned place preference of our experimental animals on day 10 postpartum because we had already established (Mattson et al., 2003), that on day 10 postpartum about half the dams prefer pup-associated cues and half prefer cocaine-associated cues; otherwise these animals are behaviorally and endocrinologically identical. The animals were conditioned to associate different sets of neutral cues with either pups or cocaine on days 6–9, and then testing was done on day 10 postpartum, as described below.
Place preference paradigm
This method is used widely in research on behavioral and neural processes involved in reward and reinforcement (Tzschentke, 1998). After a conditioning period in which the rat learns to associate an unconditioned stimulus with a conditioned stimulus, the preference of the rat for the conditioned cues is tested in the absence of the unconditioned stimulus. The time the rat spends in the chambers with the stimulus-associated cues indicates the motivation or desire for the stimulus. The exact details of the method are as described in Mattson et al. (2003).
Conditioning with pups or cocaine
The unconditioned stimulus was either a 10 mg/kg s.c. injection of cocaine or three pups from the commingled group. Each unconditioned stimulus was presented in different chambers for 2 h, one stimulus per day in an alternating counterbalanced sequence for 4 days. The dams were deprived of pups for 1 h before conditioning on the two pup-cue conditioning days.
Controls exposed to chambers and cues but not unconditioned stimuli
The control group received no unconditioned stimulus-cue conditioning; they were exposed only to the cues that served as the conditioned stimuli for the experimental group (Bardo and Bevins, 2000; Mattson et al., 2003). Animals were exposed to each cue-decorated chamber for 2 h in an alternating sequence on postpartum days 6–9, matching the parameters for the treatment of the experimental group. This procedure gave both the control and experimental groups equal time in the conditioning apparatus, in a counterbalanced sequence of conditioning chambers and contextual cues, plus equal handling. The control group had home cage conditions identical to those of the experimental animals.
Drug administration
Cocaine hydrochloride in a highly purified (>90%) powder form was provided by the National Institute on Drug Abuse (Research Triangle Park, NC, USA). It was dissolved in sterile 0.9% buffered physiological saline. Dams received 10 mg/kg s.c. injections of cocaine in the dorsal caudal flank region at a concentration of 4.5 mg/mL. The two injections were administered in opposite flank regions.
Testing
The dams were tested for their chamber preference on postpartum day 10, the day after the final conditioning session. They were deprived of their pups for 1 h before testing. The time the dams spent in each chamber was recorded, as well as the behaviors exhibited in each chamber, as described in Mattson et al. (2001, 2003). For the preference test, no unconditioned stimuli were present; the dams were tested in a drug-free state and with no pups present. The center chamber served as a neutral control, as no cue-stimulus conditioning or exposure was done in this chamber.
After the 60 min testing period, the dams remained in the apparatus for an additional 60 min and their location was noted every 10 min. The dams did not leave their preferred cue-associated chamber during this additional time, by choice, and thus spent a minimum of 1.5 h in their preferred cue-associated chamber. This time is sufficient to induce maximal IR protein levels of the immediate early gene product, c-Fos, and (Graybiel et al., 1990; Hope et al., 1994) CART peptide (Douglass et al., 1995).
Preference criterion
This criterion was derived empirically by Mattson et al. (2001) to distinguish dams that prefer pup- or cocaine-associated cues or had no preference (no animals fell into this category). In brief, the criterion requires that dams had to spend at least 50% of their time in one chamber with the caveat that the time must also be 25% greater than the time spent in either of the other two chambers. This was a rigorous criterion set to ensure a clear behavioral distinction between the two groups.
Tissue preparation and immunocytochemical protocols
c-Fos immunocytochemical procedures were conducted exactly according to the protocols previously published in our laboratory (DonCarlos et al., 1991; Hnatczuk et al., 1994; Kalinichev et al., 2000). To control for methodologically induced variability, sections from each behavioral group were processed at the same time, such that each immunocytochemical batch contained tissue from controls as well as dams from each preference group. The antic-Fos rabbit polyclonal antibody was Ab-5; 1:2000 dilution from Oncogene Science, Uniondale, NY, USA.
CART-peptide immunocytochemical procedures were based on published work from the laboratory of Dr. M. Kuhar (Emory University), who provided a gift of the antibody (Dall Vechia et al., 2000; Koylu et al., 2000). The protocol used for CART immunocytochemistry was the same as that described for c-Fos, with minor variation. The anti-CART peptide rabbit polyclonal antibody was against the C4 region of the peptide sequence (Douglass et al., 1995; Koylu et al., 1997, 1998, 1999), and a final primary antibody dilution was 1:5000 (Koylu et al., 1997, 1998).
Analysis of IR
We analyzed neurons expressing either c-Fos-immunoreactivity or CART peptide-IR according to established protocols from our laboratory (DonCarlos et al., 1991; Hnatczuk et al., 1994; Kalinichev et al., 2000). A cell was determined to express c-Fos-IR or CART-IR through visual inspection using a Zeiss microscope (Carl Zeiss, Thornwood, NY, USA). c-Fos is localized in the nucleus, whereas CART is localized throughout the cytoplasm of the soma and processes of neurons, including in their terminals. The labeled neurons have nuclei (c-Fos) or cytoplasm (CART) that is black or dark purple because the Ni-DAB reaction product is substantially darker than the background staining. The labeled neurons were analyzed within the anatomic structures as defined by the atlas of Paxinos and Watson (1998).
First, a qualitative analysis of labeled neurons was done to obtain a relative ranking (no staining, low, medium, high) as to the amount of IR in selected neuroanatomical structures (the Acb core and shell subdivisions, dorsal striatum, piriform cortex, medial PFC [including the anterior cingulate cortex, prelimbic cortex, and infralimbic cortex], BlA, and MPOA). The qualitative analysis was conducted using anatomically matched sections from a subset of animals in each group.
Next, an initial quantitative analysis was done by an observer who visually counted the number of IR cells in the core and shell subdivisions of the Acb in a subset of sections. Cells were counted with the aid of a Zeiss Axioplan microscope (×20 objective lens, ×10 ocular lens) equipped with a grid placed in one ocular lens. This grid covered a 0.25 mm2 area, which provided a constant anatomic area within the Acb core and shell for analysis. The cell counts obtained using this technique were then compared with counts obtained using our automated analysis system (see below), and the automated counts were found to be within ±5% of the observer counts, validating the automated counting process.
Finally, the majority of the cell counting was carried out using a Zeiss Axioskop microscope attached to the MicroComputer Imaging Device, an image-analysis system (Imaging Research, Inc., St. Catherines, Ontario, Canada) for automated cell counting. The automated analysis system permitted expedient and efficient analysis. Neurons were counted unilaterally at no less than two different anatomic levels for each anatomic structure (the Acb core and shell subdivisions, dorsal striatum, piriform cortex, medial PFC [including the anterior cingulate cortex, prelimbic cortex, and infralimbic cortex], BlA, and MPOA, Fig. 1). All further details were carried out as in Kalinichev et al. (2000).
Fig. 1.
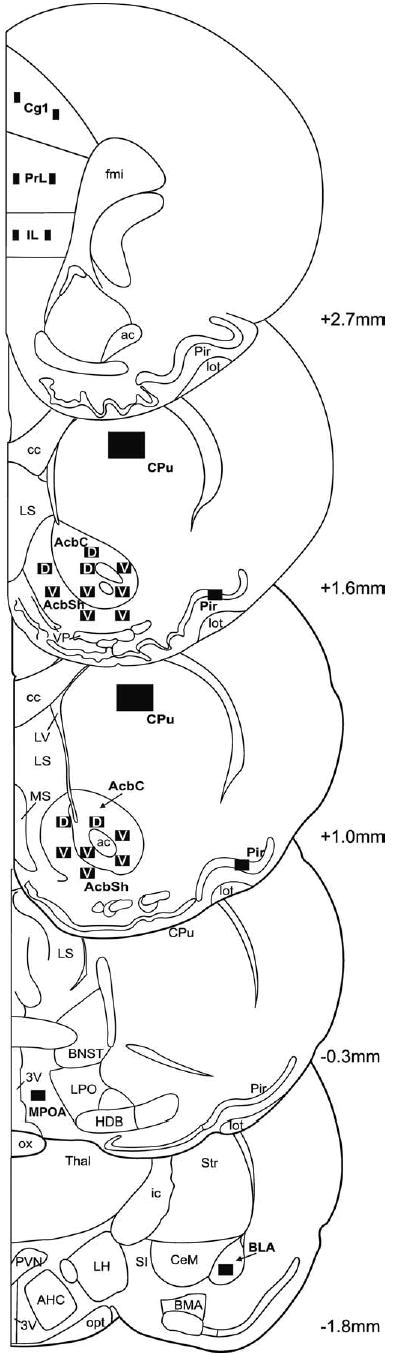
Schematic diagrams and bregma levels of the regions of analysis. Scanned areas are depicted by black rectangles. Subregions of Acb labeled with a “D” indicate the dorsal portion of the subdivisions; those labeled with a “V” indicate the ventral portion of the subdivisions. Size of sampling areas included in the Experimental Procedures.
Within the Acb, labeled neurons were counted at four rostral–caudal levels (bregma +1.6, +1.2, +1.0, +0.7); at each of these levels both the core and shell subdivisions were analyzed using the segregation described by Zaborszky et al. (1985). Within each subdivision at each rostral–caudal level, all labeled neurons within four or five areas of 0.14 mm2 were counted, as shown in Fig. 1. In the piriform cortex, 0.14 mm2 of layer 2 (Haberly and Price, 1978) and 0.5 mm2 of the dorsal striatum were analyzed in two of the same sections that were used for Acb analysis (bregma +1.6, +1.0). See Fig. 1 for further details.
In both the BlA (bregma −1.80, −2.12) and the MPOA (bregma −0.3, −0.8), two rostral–caudal levels were sampled, and an area of 0.14 mm2 was counted at each level (Fig. 1). Neurons of the medial prefrontal cortex (mPFC) were counted within three separate subdivisions in two layers (2 and 5) that are prominently efferent layers (Sesack et al., 1989) using an analysis area of 0.04 mm2 at two bregma levels (+3.2 and +2.7) (Fig. 1). The mPFC, consisting of the prelimbic (PrL), infralimbic (IL), and anterior cingulate (Cg1) cortices, was identified according to established neuroanatomical criteria (Krettek and Price, 1977). Localization of the cortical layers was set according to the parameters established by Gabbott et al. (1997).
At the start of each analysis session, the image-analysis system was set to use a particular microscope objective, light intensity, diaphragm width, and Köhler setting to optimize the visualization of signal, and then the system setting was further verified with a slide that served as a biological standard. For this purpose, a standard area within a slide that contained c-Fos-IR neurons was recounted to confirm that the same number of neurons with the same optical density per neuron was being measured each day. This process ensured that the analysis system was stable throughout the extended analytic process. Sections from both experimental and control groups were always counted during each analysis session. Sets of adjacent serial sections from each group were stained with Cresyl Violet to aid in determining the anatomic borders and cortical layers. The analysis was conducted by two experimenters who were unaware of the experimental condition or behavioral response of the animal. The inter-rater reliability between cell counts was well within 5%.
Neuronal distribution maps
The anatomical distribution of the IR neurons was mapped according to the outlines of the anatomical structures using a mid-range objective (×5) on a Zeiss microscope that was interfaced with the Neurolucida program (MicroBrightField, Inc., Colchester, VT, USA), and then a cell-by-cell representation of all Fos-IR or CART-IR neurons was plotted on this map. The Neurolucida program allows cell-by-cell plotting (×20) of all labeled neurons in their exact anatomic location with respect to each other and the anatomic structures within each section (all further details as in Kalinichev et al., 2000).
Photomicrographs
Photomicrographs were taken with a Zeiss Axiocam digital camera attached to a Zeiss microscope. The digital camera was interfaced with a personal computer, and the images were captured using Image Pro Plus and Adobe Photoshop software.
Statistical analysis
Bartlett’s test for homogeneity of variance was applied before performing factorial analysis of variance (ANOVA) followed by the Tukey-Kramer honest significant difference (HSD) post hoc test to examine the main effect and interactions of group and anatomical level for each neuroanatomical area analyzed. A repeated-measures ANOVA was performed on the pre-and post-conditioning place preference times. Statistical significance was set at P<0.05.
RESULTS
Place preference
During the preconditioning test before exposure to the unconditioned stimulus, dams spent, on average, equal amounts of time (33%) in each chamber of the apparatus (not statistically different [F (2, 14)=0.50, P>0.05; Fig. 2A]). Thus, before conditioning, the apparatus was neutral, and no dam had a preference for any chamber. After conditioning with both of the unconditioned stimuli (cocaine and pups) and the associated cues (conditioned stimuli), all the dams in the experimental group (n=12) developed a preference for cues associated with one of those stimuli. A repeated-measures ANOVA comparing time in the specific chambers before and after conditioning indicated that these times were statistically different, demonstrating a significant effect of conditioning (F (4, 42)=25.19, P< 0.001).
Fig. 2.
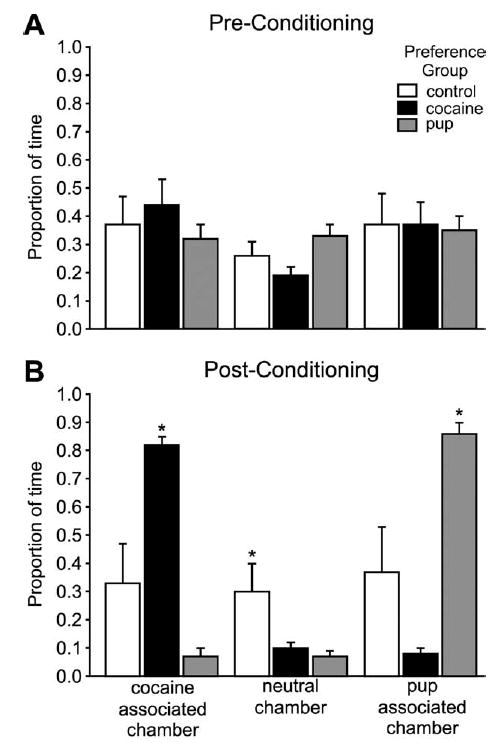
Behavioral data. (A) Mean (±S.E.M.) proportion of time spent by the dams in each stimulus-associated cues before the conditioning phase of the place preference procedure. (B) Mean (±S.E.M.) proportion of time spent by the dams in each stimulus-associated cues after conditioning, during the place preference test. Asterisks indicate a significant difference between groups in each stimulus-associated cues, P<0.05.
The dams that preferred the cocaine-associated chamber (n=6) spent significantly more time in the cocaine-associated chamber than in either of the other two chambers (F (2, 15)=288.25, P<0.001; Fig. 2B). Similarly, the dams that preferred the pup-associated chamber (n=6) spent significantly more time in that chamber (F (2, 15)=191.48, P<0.001; Fig. 2B). The control dams (n=5), which were never exposed to unconditioned stimuli as part of the conditioning process, spent equal time in all chambers in the post-conditioning test, and these times were not statistically different from the preconditioning times. No dam in the control group had any preference for a chamber, and therefore as expected, without exposure to unconditioned stimuli, there was no behavioral effect of exposure to the apparatus.
All dams actively explored all chambers throughout the testing period; no dam slept in the apparatus during testing. The number of entrances made into each chamber during the testing period was nearly equivalent for all dams.
Prior to conditioning, the maternal behavior of the dams was examined (not under the influence of cocaine) to ensure that all were behaviorally equivalent in their ability to express maternal behavior (consummatory aspect of maternal behavior). All dams expressed equal levels of maternal behavior, including pup retrieving, ano-genital licking, nursing, maternal aggression, and nest building (for full details of tests and data see Mattson et al., 2003). An additional subset of dams (brains not used for analysis) with known preferences for cues-associated with cocaine or pups established was tested for baseline (after saline) and cocaine-stimulated locomotor (10 mg/kg) activity. The dams’ baseline and drug-stimulated locomotor activity was equal regardless of preference group.
Quantitative analysis of c-Fos IR
In addition to the significant behavioral effect of conditioning in the experimental groups, the conditioning paradigm also resulted in increased numbers of c-Fos-IR neurons within specific, limited, neuroanatomical regions. As can be seen in the photomicrographs, c-Fos-IR nuclei were identified by the presence of black or dark purple reaction product within neuronal nuclei (see for example Fig. 4A–C, bottom).
Fig. 4.
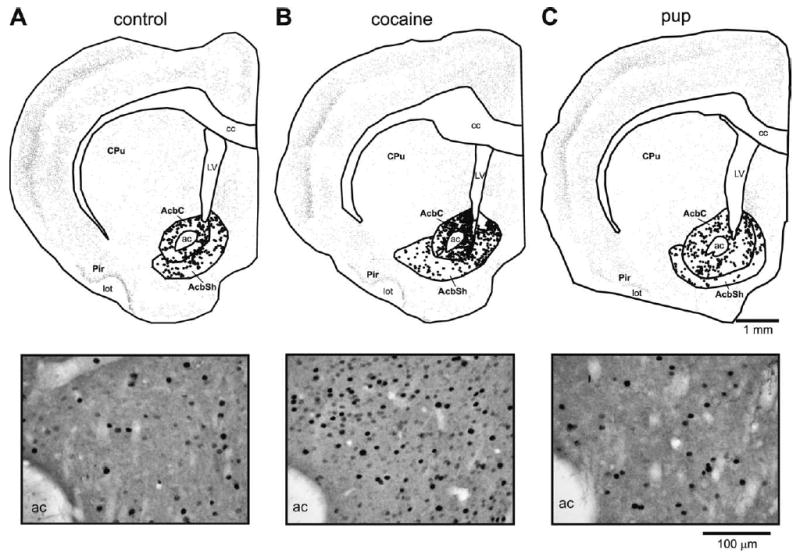
Top: Anatomically exact Neurolucida-generated illustrations of representative sections from each preference group in the Acb at bregma level + 1.6 mm; other details of figure as in Fig. 3. Bottom: Black and white photomicrographs from the same sections as the Neurolucida maps.
mPFC
Dams that preferred the cocaine-associated cues had the most c-Fos-IR neurons in the mPFC, whereas those that preferred the pup-associated cues had the second greatest number or were equal to the controls dams, which consistently had the fewest. The dams that preferred the cocaine-associated cues had statistically significantly more c-Fos-IR neurons in both layers 2 and 5 than the dams that preferred the pup-associated cues and the control group in the PrL (Fig. 3A, D), IL (Fig. 3B, E), and Cg1 (Fig. 3C, F) subregions. The dams that preferred the pup-associated cues had more c-Fos-IR neurons than the control group in layer 5 of the IL subregion (Fig. 3B, E; F (2, 12)=79.02, P<0.001; HSD (12)=6.03) and in layers 2 and 5 of Cg1 subregion (Fig. 3C, F; F (2, 12)=67.01, P<0.001; HSD=8.84; and F (2, 12)=79.11, P<0.001; HSD (12)=8.27, respectively).
Fig. 3.
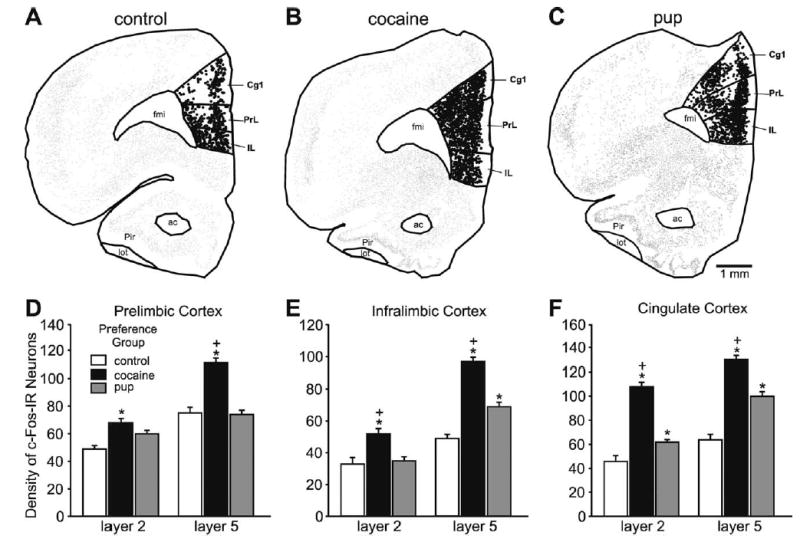
Top: Anatomically exact Neurolucida-generated illustrations of representative sections from the control group and each preference group in the mPFC at bregma level +3.2 mm. All c-Fos-IR neurons are represented in the illustrations. A single dot represents a c-Fos-IR neuron; larger dots represent neurons that were counted, here the c-Fos-IR neurons in the subregions of the mPFC, PrL, IL and Cg1, cortices. (A) Control group. (B) Cocaine-associated cues. (C) Pup-associated cues. Bottom: Histograms showing the mean (±S.E.M.) number of c-Fos-IR neurons in layers 2 and 5 of the three mPFC subregions from each preference group and control. Asterisks indicate a significant difference between that group and the control group, P<0.05. Plus signs indicate a significant difference between the experimental groups, P<0.05. Statistical details in the Results.
Acb
The dams that preferred the cocaine-associated cues had statistically significantly more c-Fos-IR neurons than dams that preferred the pup-associated cues or controls in both the shell or the core subdivisions (Figs. 4, 5A; F (2, 14)=19.49, P<0.001; HSD (14)=9.89; F (2, 14)=19.66, P<0.001; HSD (14)=10.30). These differences were most prominent in the dorsal and rostral regions of the both core and shell (Fig. 5B; F (2, 14)=33.43, P<0.001; HSD (14)=13.47; F (2, 14)=21.59, P<0.001; HSD (14)=15.15; Fig. 5C, D; F (2, 52)=43.79, P<0.001; HSD (52)=5.78). There were no differences in the number of c-Fos-IR neurons between the control group and dams that preferred the pup-associated cues (Figs. 4, 5A–D).
Fig. 5.
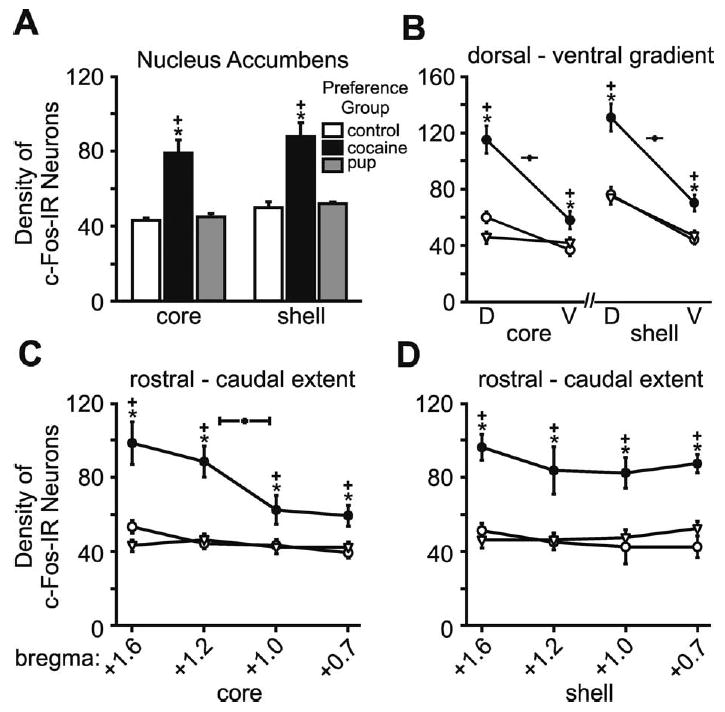
Number of c-Fos-IR neurons in the Acb core and shell subdivisions for each preference group and control. (A) Histograms show the mean (±S.E.M.) number of c-Fos-IR neurons within each subdivision. (B) Graphs show the mean (±S.E.M.) number of c-Fos-IR neurons along the dorsal-ventral gradient in the core (right) and shell (left). (C) Graphs show the mean (±S.E.M.) number of c-Fos-IR neurons along the rostral-caudal extent in the core, which were counted at four anatomic levels. (D) Graphs show the mean (±S.E.M.) number of c-Fos-IR neurons along the rostral-caudal extent in the shell, counted at the same four anatomic levels as the core. Asterisks indicate a significant difference from the control group, P<0.05. Plus signs indicate a significant difference from the other preference group, P<0.05. Statistical details in the Results.
BIA
Dams that preferred the cocaine-associated cues had more c-Fos-IR neurons than those that preferred the pup-associated cues, and both preference groups had more than the controls (Fig. 6A–C). These differences were all statistically significant (Fig. 6D; F (2, 14)=164.62, P<0.001; HSD (14)=5.46).
Fig. 6.
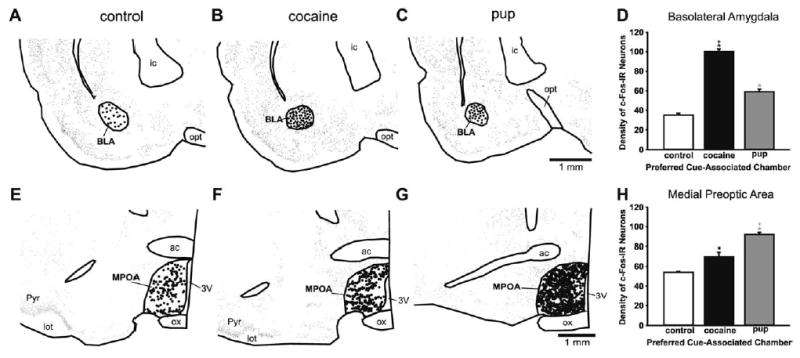
Top: Anatomically exact Neurolucida-generated illustrations of representative sections in the basolateral amygdala at bregma level −1.8 mm from the control group and each preference group; further illustration details as in Fig. 3. (A) Control group. (B) Cocaine-associated cues. (C) Pup-associated cues. (D) Histograms show the mean (±S.E.M.) number of c-Fos-IR neurons in the basolateral amygdala. Bottom: Anatomically exact Neurolucida-generated illustrations of representative cross-sections in the MPOA at bregma level −0.30 mm for each preference group, illustration details as in Fig. 3. (E) Control. (F) Cocaine-associated cues. (G) Pup-associated cues. (H) Number of c-Fos-IR neurons in the MPOA. Histograms from each preference group show the mean (±S.E.M.) number of c-Fos-IR neurons. All asterisks indicate a significant difference from the control group, P<0.05. Plus signs indicate a significant difference between the experimental groups, P<0.05. Statistical details in the Results.
MPOA
This was the single region in which dams that preferred the pup-associated cues had a significant increase in c-Fos-IR neurons over the dams that preferred the cocaine-associated cues (Fig. 6E–H). Both preference groups had more c-Fos-IR neurons than controls and all the differences among the three groups were statistically significant (F (2, 14)=25.54, P<0.001; HSD (14)=7.60).
Quantitative analysis of CART peptide IR
The Acb, core and shell subdivisions, piriform cortex, and the MPOA contained abundant CART-IR neuronal soma and fibers and were analyzed for the number of CART-IR neuronal cell bodies. The dorsal striatum, mPFC, and BlA had very few IR neuronal somas and processes and were not analyzed further. Cells that were CART-IR could be morphologically distinguished to be neurons and the CART-IR was found throughout the cytoplasm both in the soma and in extensive processes (Fig. 7). Both the anatomical distribution of CART-IR neurons and the cellular distribution of CART-IR are in accordance with reports in the literature (Koylu et al., 1998; Kuhar and Yoho, 1999; Smith et al., 1999; Kuhar et al., 2000).
Fig. 7.
Black and white photomicrograph illustrating the neuronal distribution of CART-IR. CART is localized in the cytoplasm of the soma and processes of neurons. The absence of staining in the nucleus (white in color) is not clearly visible due to the focal plane of the image.
Acb
There were more CART-IR neurons in the Acb shell than in the Acb core, while within the divisions these neurons were fairly homogeneously distributed (Fig. 8A–C, G; F (1, 28)=35.07, P<0.001; HSD (28)=2.81). Within the Acb core both preference groups had very substantially more CART-IR neurons than the controls, and the dams that preferred the cocaine-associated cues had modestly more than the dams that preferred the pup-associated cues (Fig. 8G, left). The differences among all three groups were all statistically significant (F (2, 14)=291.37, P=0.001; HSD=3.64). Within the Acb shell the dams both preference groups also had very substantially, and statistically significantly more, CART-IR neurons than the control group (Fig. 8G, right; F (2, 14)=236.98, P<0.001; HSD=3.55). The number of CART-IR neurons did not differ between the dams that preferred the cocaine-associated cues versus the pup-associated cues.
Fig. 8.
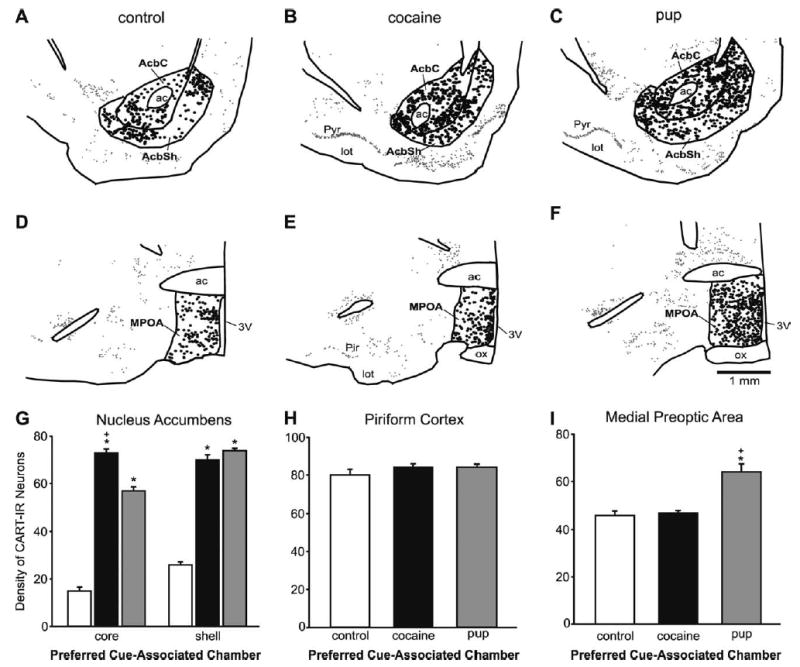
Top: Exact Neurolucida-generated anatomic illustrations of representative sections through the Acb at bregma level +1.6 mm from the control group and each preference group. All CART-IR neurons are represented in the illustrations. A single dot represents a CART-IR neuron; larger dots represent the CART-IR neurons that were counted, here in the Acb core and shell regions. The piriform cortex analysis was also conducted at this bregma level. (A) Control group. (B) Cocaine-associated cues. (C) Pup-associated cues. Middle: Exact Neurolucida-generated anatomic illustrations of representative sections in the MPOA at bregma level −0.30 mm from the control group and each preference group. Further illustration details as in Top of this figure. (D) Representative section from a control dam. (E) Representative section from a dam that preferred the cocaine-associated cues. (F) Representative section from a dam that preferred the pup-associated cues. (G) Number of CART-IR neurons in the core and shell of the Acb. Histograms from each preference group show the mean (±S.E.M.) number of CART-IR neurons. (H) Number of CART-IR neurons in the piriform cortex (control region). There were no significant differences among the groups. (I) Number of CART-IR neurons in the MPOA. Asterisks indicate a significant difference from the control group, P<0.05. Plus signs indicate a significant difference between the experimental groups, P<0.05.
MPOA
Dams that preferred the pup-associated cues had significantly more CART-IR neurons in the MPOA than both the dams that preferred the cocaine-associated cues and the control dams (Fig. 8D–F, I; F (2, 14)=18.04, P<0.001; HSD (14)=5.10). There was no significant difference between the dams that preferred the cocaine-associated cues and the control group.
Control brain regions: dorsal striatum and piriform cortex
Neurons that were c-Fos-IR were counted in the dorsal striatum and piriform cortex and CART-IR neurons were counted in the piriform cortex. There were no statistically significant differences among any of the preference or control groups in the number of c-Fos-IR neurons (Fig. 9A, B) or CART-IR (Fig. 8H) neurons in these regions.
Fig. 9.
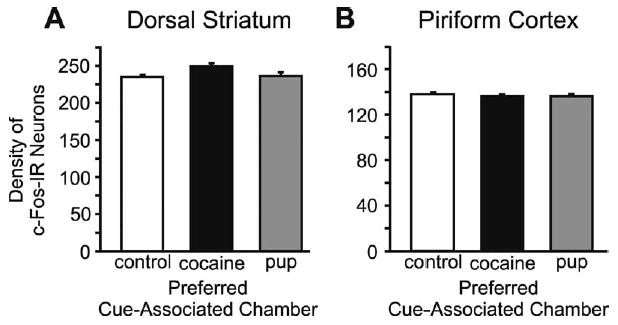
(A) Number of c-Fos-IR neurons in the dorsal striatum. (B) Number of c-Fos-IR neurons in the piriform cortex, these were control regions. Histograms from each preference group and controls show the mean (±S.E.M.) number of c-Fos-IR neurons. There were no significant differences among the groups in either structure.
DISCUSSION
We found populations of c-Fos- or CART-IR neurons that varied in size and distribution according to the animal’s preference for cocaine versus pup stimulus-cue associations. Our novel findings include activation of an orderly distributed pattern of neurons in preference states, the activation of neurons by pup-cue association preference, and the use of CART as a marker of any conditioned preference. Cues associated with the drug stimulus activated more neurons compared with the natural reward in all but one region, the MPOA. This is in accord with the idea that a drug stimulus is reinforcing because it powerfully subsumes the functions of a system evolved to respond to natural rewards (Wise, 1998). While increases in c-Fos-IR neurons are routinely interpreted as increased neuronal activity, the interpretation of increased CART-IR is not established in the literature. Since CART has the characteristics of a secreted peptide, however, increased CART-IR can be reasonably interpreted as a response to greater release, or neuronal preparation for this.
The activation of neurons we document is very specific to preference state, and cannot be attributed to activation of neurons supporting general locomotor or olfactory function since brain regions mediating motor and olfactory processes (dorsal striatum and piriform cortex) did not show increased cFos-IR or CART neurons with preference state. Furthermore, two exposures to cocaine during conditioning do not increase locomotor activity during the test for cue-stimulus association (Franklin and Druhan, 2000a,b), suggesting that our paradigm would not alter locomotor function. Other evidence that speaks to the regional specificity of locomotor versus motivational responses to these stimuli comes from studies showing that direct stimulation with cocaine or pups increased Fos expression in the dorsal striatum or piriform cortex, while cocaine-associated cues did not (Graybiel et al., 1990; Young et al., 1991; Brown et al., 1992; Fleming et al., 1994; Neisewander et al., 2000).
The increased numbers of IR neurons we report also cannot be due to a direct response to the unconditioned stimuli, because the rats’ only stimulus exposure was during conditioning; the unconditioned stimuli were not present during the test. Our randomized, counterbalanced procedure assured that equal numbers of subjects received alternate stimuli during the final conditioning session. Thus, the final exposure to cocaine as an unconditioned stimulus occurred 24 or 48 h before the test, when c-Fos has returned to basal levels (Hope et al., 1994). Responses to pups during home cage maternal behavior prior to testing also cannot be a confounding effect because the control and experimental dams had identical home cage exposure to pups before testing.
Most of the neurons activated by preference state are in brain regions known to be involved in motivational processes for reinforcing stimuli, including drugs (Bardo, 1998; Wise, 1998; Kalivas and Nakamura, 1999) and pups (Hansen et al., 1993; Lee et al., 1999b). Others reported similar activation of c-Fos-IR neurons with preference for cues associated with either pharmacologic or several natural reinforcers in place-preference paradigms (Brown et al., 1992; Crawford et al., 1995; Franklin and Druhan, 2000a; Schroeder et al., 2001). The neuronal populations that we uncovered as responsive to stimulus-associated cues, in the complete absence of the unconditioned stimulus, were found in many of the same brain regions (e.g. Acb, PFC, MPOA) where neurons responsive to the unconditioned stimuli themselves, suckling or cocaine have been found. In these regions, neurons active after cocaine administration (Graybiel et al., 1990) or in response to pups during the expression of maternal behavior (Numan and Insel, 2003) have been identified previously with c-Fos and by fMRI (Ferris et al., 2004) in a novel examination of response to suckling and cocaine exposure in the postpartum dam.
We have organized the discussion of the populations of activated neurons in a proposed functional hierarchy, ordered from the top down. The functional repertoire of the mPFC includes cognitive complexity, setting of emotional tone, and initiation of effortful behaviors to achieve rewards (Birrell and Brown, 2000; Brown and Bowman, 2002; Leonard, 1969; Groenewegen and Uylings, 2000; Peoples, 2002; Saper, 2000; Robbins, 2000; Sesack et al., 1989; Shidara and Richmond, 2002; Walton et al., 2002). These capacities make it an excellent candidate as a region that initiates the expression of preference. Our finding that all three of the mPFC had populations of preference-responsive neurons, adds strength to this hypothesis. Since layers 2 and 5 of these regions are the origin of corticocortical and corticofugal efferents, including to the Acb, BlA, and MPOA, the literature shows us an established efferent route for information flow from cortex to these downstream regions (Sesack et al., 1989; Groenewegen and Uylings, 2000; Saper, 2000). Furthermore, our finding that layer 5, more a source of corticofugal efferents, had more c-Fos-IR activated neurons than layer 2, suggests an emphasis on activation in the cortical outflow layer signaling to lower levels in a functional hierarchy.
Dams that preferred cocaine-associated cues had more c-Fos-IR neurons in both output layers of all three subdivisions of the mPFC, which was consistent with previous data (Franklin and Druhan, 2000a,b; Neisewander et al., 2000). Our finding that only pharmacologic reward-cue associations activated certain subregions suggests functional segregation within the mPFC, while our novel finding that preference for cues associated with pups increased the number of c-Fos-IR neurons in the IL and Cg1 suggesting participation in maternal motivation. This is also suggested by recent fMRI work showed higher responses in the Cg1 of human mothers after their infant cried (Lorberbaum et al., 1999) and the several prefrontal subregions with suckling of pups in the rat (Ferris et al., 2004). Our findings of such activation in the IL and PrL together with the finding that these areas are activated by preference for cues associated with other natural stimuli (Schroeder et al., 2001), also suggests that these mPFC subregions may mediate a more general natural reward response/recognition capacity.
Immediately downstream from the mPFC, the Acb neurons that are responsive to preference choices were found in both overlapping and partially separable subsets. Preference for cocaine-associated cues activated a population of Fos- and a population of CART-IR-expressing neurons in Acb core and shell, but only a population of the CART-IR neuron was activated with preference for pup-associated cues. The simplest conclusion is that different neurons respond to the different cue-associated preferences. However, the equal response of CART-IR neurons to both stimuli and the fact that both CART and FOS populations were responsive to the cocaine-associated cue preference complicate this conclusion. It is possible that the same neurons express both markers but that Fos is not involved with this particular natural reward-cue association. Alternatively, the neuronal populations responding to this particular set of drug and the natural reward-cue associations could be only partially separable. Identification of additional characteristics, e.g. connections or neurochemistry of these neurons, will be needed to determine which of these possibilities is correct.
Others have also reported higher numbers of c-Fos-expressing neurons in the Acb core and shell with preference for cocaine-associated cues compared with control (Neisewander et al., 2000). Neurons in separable populations within the Acb respond selectively in electrophysiological recording to drug and natural reinforcement (Carelli et al., 2000; Carelli and Wondolowski, 2003), and fMRI demonstrates response to cocaine in the Acb in rat (Ferris et al., 2004). Although dopaminergic stimulation and memory consolidation resulting from interaction with pups occur in the Acb, and fMRI detected a response to suckling in the Acb of rat, it is not necessary for home-cage maternal behavior or in operant responding to pups (Hansen et al., 1993; Lee et al., 1999a,b; Numan and Insel, 2003; Ferris et al., 2004).
The MPOA is the only region where the cues associated with the natural stimulus activated more c-Fos and CART-IR neurons than did the cues associated with the pharmacologic stimulus and control conditions. This region is also downstream from the mPFC (Saper, 2000). Cocaine-associated cues resulted in a smaller response among Fos-expressing neurons and no response from CART-IR neurons. Our most recent findings show that preference for cues associated with pups versus cues associated with pup-sized neutral objects also activates more c-Fos-IR neurons in the MPOA (unpublished findings). Thus, it appears that the area is fundamentally involved in pup-responsiveness, even without the contrast of a reward association such as to cocaine. Together these patterns suggest that a substantially separable population of neurons responds to this natural versus drug reward-cue associations. Similarly, Schroeder et al. (2001) found that in this region associations to a different natural reinforcer (chocolate) activated more neurons than did the drug reinforcer associations. The greater response to a natural reward-cue association over a drug-cue association may occur in other specific regions and raises the idea of regionally specialized responses that do not follow the established idea of more neurons activated by drug stimuli than natural stimuli.
The MPOA is critical to the expression of maternal behavior and is directly responsive to cocaine, as site-specific infusion of cocaine into the MPOA impairs the expression of maternal behavior (Vernotica et al., 1999). Our results extend our understanding of the role of the MPOA in the motivational aspects of maternal behavior by demonstrating that this region responded to a purely appetitive measure of motivational status. Our data accord well with findings that the MPOA is involved in operant responses for delivery of pups (Lee et al., 1999a). The MPOA may have a combined role in recognizing the pup as a highly salient reinforcer and thereby focusing the consummatory aspects of the behavior. There is a strong case that the MPOA has a remarkably analogous dual functional role for the appetitive and consummatory aspects of male sexual behavior (Balthazart et al., 1998; Hull et al., 1999). The fact that the MPOA mediates both appetitive and consummatory components of several behaviors suggests that it has a functional capacity that supersedes the particulars of the behaviors; how specific anatomical and neurochemical neuronal subsets within the MPOA carry out this dual capacity remains to be determined.
The activation of neurons in the basolateral amygdala is consistent with its involvement in conditioned reward processes and furthermore this region interacts with the mPFC over notable reciprocal connections to this region. Although both stimulus-cue associations resulted in a higher number of c-Fos-IR neurons in the BlA, the greater number activated by the drug-cue association suggests that distinct populations respond to the two stimulus-cue associations. Higher c-Fos-IR expression with the cocaine-paired environment accords with the findings of others (Brown et al., 1992; Neisewander et al., 2000). The BlA and other amygdaloid nuclei have a role in conditioned responses to psychostimulant (Hiroi and White, 1991; Brown and Fibiger, 1993; Parkinson et al., 2001; Fuchs et al., 2002) or pups as stimuli (Lee et al., 1999a).
We propose that preference for cues associated with the unconditioned stimulus is the product of a top-down hierarchical activation of neurons we have identified. As suggested by classical models of cortical efferent regulation, and the connections and functions of these structures, we propose that the PFC, after receiving cue-specific sensory information and memory information via projections from sensory and association areas of the cortex, initiates a descending cascade of efferent activity to the Acb, ventral tegmental area, BlA, and MPOA (Groenewegen et al., 1996; Groenewegen and Uylings, 2000; Saper, 2000; Eichenbaum and Cohen, 2001; Numan and Insel, 2003). The BlA and mPFC are involved in the detection and recognition of different rewards and the execution of unconditioned and conditioned responses (DeCoteau et al., 1997; Hitchcott et al., 1997; Meil and See, 1997). It has been proposed that the BlA regulates the response to the stimulus, whereas the mPFC integrates memory with the response.
The behavioral expression of the preference is then due to subsequent outflow of limbic and preoptic area structures via the ventral pallidum, and particular mesencephalic and brain stem structures. The flow of information across the structures that are likely to be important for the expression of preference probably depends on glutametergic efferents, most notably from the mPFC (Kalivas and Nakamura, 1999; Park et al., 2002; McFarland et al., 2003). We propose that the specificity of the preference choices in our paradigm may reside in the activation or inhibition of particular subsets of these glutametergic efferents to the accumbens, BlA, and MPOA, which might subsequently modulate the behavioral outcome of the cortical initiation. This may be a feed-forward process with an accumulating effect of the brain regions outside the cortex to modify the preference response, such that a cortical initiation may be dependent to a certain extent on the ‘set point’ or excitability of these regions. Integration of the hormonal and sensory state of the female with the corticofugal signals is likely critical for ordered responses to associations with natural stimuli that are dependent upon the hormonal status and maternal state of the female. The MPOA is very likely one site of such integration.
Acknowledgments
We thank Dr. Michael Kuhar for the gift of the CART primary antibody and Sharon Williams and Carolina Olazabal for technical assistance. We also thank Dr. Lazslo Zaborzsky for the use of his Neurolucida equipment and anatomic expertise and Derek Buhl for training on the Neurolucida equipment. This research was supported by RO1DA014025; PHS 1R25 GM608226.
References
- Aigner TG, Balster RL. Choice behavior in rhesus monkeys: cocaine versus food. Science. 1978;201:534–535. doi: 10.1126/science.96531. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Gerard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to out preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EE, Fibiger HC. Differential effects of excitotoxic lesions of the amygdala on cocaine-induced conditioned locomotion and conditioned place preference108. Psychopharmacology (Berl) 1993;113:123–130. doi: 10.1007/BF02244344. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J. Selective encoding of cocaine versus natural rewards by nucleus accumbens neurons is not related to chronic drug exposure. J Neurosci. 2003;23:11214–11223. doi: 10.1523/JNEUROSCI.23-35-11214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnoff IJ. Drugs, alcohol, pregnancy and parenting. Boston: Kluwer Academic Publishers; 1988. [Google Scholar]
- Chasnoff IJ. In: Cocaine: effects on pregnancy and the neonate. In: Drugs, alcohol, pregnancy, and parenting. Chasnoff IJ, editor. Dordrecht: Kluwer Academic Publishers; 1995. pp. 97–103. [Google Scholar]
- Couceyro PR, Koylu EO, Kuhar MJ. Further studies on the anatomical distribution of CART by in situ hybridization. J Chem Neuroanat. 1997;12:229–241. doi: 10.1016/s0891-0618(97)00212-3. [DOI] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. The effects of the kappa agonist U-50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacology (Berl) 1995;120:392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- Dall Vechia S, Lambert PD, Couceyro PC, Kuhar MJ, Smith Y. CART peptide immunoreactivity in the hypothalamus and pituitary in monkeys: analysis of ultrastructural features and synaptic connections in the paraventricular nucleus. J Comp Neurol. 2000;416:291–308. doi: 10.1002/(sici)1096-9861(20000117)416:3<291::aid-cne2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, Kesner RP, Williams JM. Short-term memory for food reward magnitude: the role of the prefrontal cortex. Behav Brain Res. 1997;88:239–249. doi: 10.1016/s0166-4328(97)00044-2. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Monroy E, Morrell JI. Distribution of estrogen receptor-immunoreactive cells in the forebrain of the female guinea pig. J Comp Neurol. 1991;305:591–612. doi: 10.1002/cne.903050406. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. Working memory and the prefrontal cortex. In: Eichenbaum H, Cohen NJ, editors. From conditioning to conscious recollection: Memory systems of the brain. New York: Oxford University Press; 2001. pp. 471–506. [Google Scholar]
- Fahrbach SE, Pfaff DW. Hormonal and neural mechanisms underlying maternal behavior in the rat. In: Pfaff DW, editor. The physiological mechanisms of motivation. New York: Springer-Verlag; 1982. pp. 253–285. [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2004;25:149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Cheung U, Myhal N, Kessler Z. Effects of maternal hormones on “timidity” and attraction to pup-related odors in female rats. Physiol Behav. 1989;46:449–453. doi: 10.1016/0031-9384(89)90019-x. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Suh EJ, Korsmit M, Rusak B. Activation of Fos-like immunoreactivity in the medial preoptic area and limbic structures by maternal and social interactions in rats. Behav Neurosci. 1994;108:724–734. doi: 10.1037//0735-7044.108.4.724. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci. 2000a;12:2097–2106. doi: 10.1046/j.1460-9568.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Involvement of the nucleus accumbens and medial prefrontal cortex in the expression of conditioned hyperactivity to a cocaine-associated environment in rats. Neuro-psychopharmacology. 2000b;23:633–644. doi: 10.1016/S0893-133X(00)00162-7. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Weber SM, Rice HJ, Neisewander JL. Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain Res. 2002;929:15–25. doi: 10.1016/s0006-8993(01)03366-2. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol. 1997;377:465–499. doi: 10.1002/(sici)1096-9861(19970127)377:4<465::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. J Comp Neurol. 1978;178:711–740. doi: 10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45:673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Hauser H, Gandelman R. Lever pressing for pups: evidence for hormonal influence upon maternal behavior of mice. Horm Behav. 1985;19:454–468. doi: 10.1016/0018-506x(85)90041-8. [DOI] [PubMed] [Google Scholar]
- Hiroi N, White NM. The lateral nucleus of the amygdala mediates expression of the amphetamine-produced conditioned place preference. J Neurosci. 1991;11:2107–2116. doi: 10.1523/JNEUROSCI.11-07-02107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcott PK, Bonardi CM, Phillips GD. Enhanced stimulusreward learning by intra-amygdala administration of a D3 dopamine receptor agonist. Psychopharmacology (Berl) 1997;133:240–248. doi: 10.1007/s002130050397. [DOI] [PubMed] [Google Scholar]
- Hnatczuk OC, Lisciotto CA, DonCarlos LL, Carter CS, Morrell JI. Estrogen receptor immunoreactivity in specific brain areas of the prairie vole (Microtus ochrogaster) is altered by sexual receptivity and genetic sex. J Neuroendocrinol. 1994;6:89–100. doi: 10.1111/j.1365-2826.1994.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci U S A. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, Moses J. Hormone-neurotransmitter interactions in the control of sexual behavior. Behav Brain Res. 1999;105:105–116. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Fagergren P. Human cocaine- and amphetamine-regulated transcript (CART) mRNA is highly expressed in limbic-and sensory-related brain regions. J Comp Neurol. 2000;425:583–598. doi: 10.1002/1096-9861(20001002)425:4<583::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Rosenblatt JS, Nakabeppu Y, Morrell JI. Induction of c-fos-like and fosB-like immunoreactivity reveals forebrain neuronal populations involved differentially in pup-mediated maternal behavior in juvenile and adult rats. J Comp Neurol. 2000;416:45–78. doi: 10.1002/(sici)1096-9861(20000103)416:1<45::aid-cne5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Barnes CD. Limbic motor circuits and neuropsychiatry. Boca Raton: CRC Press; 1994. [Google Scholar]
- Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999;9:223–227. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Murphy D, Karus D. Cocaine use in America: Epidemiologic and clinical perspectives. NIDA. 1985;(61):67–110. [Google Scholar]
- Kask A, Schioth HB, Mutulis F, Wikberg JE, Rago L. Anorexigenic cocaine- and amphetamine-regulated transcript peptide intensifies fear reactions in rats. Brain Res. 2000;857:283–285. doi: 10.1016/s0006-8993(99)02383-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Vaccarino FJ, Amaltric M, Bloom FE. Positive reinforcement properties of drugs: search for neural substrates. In: Engel J, Oreland L, editors. Brain reward systems and drug abuse. New York: Raven Press; 1987. pp. 35–50. [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, Kuhar MJ. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol. 1997;9:823–833. doi: 10.1046/j.1365-2826.1997.00651.x. [DOI] [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–132. [PubMed] [Google Scholar]
- Koylu EO, Smith Y, Couceyro PR, Kuhar MJ. CART peptides colocalize with tyrosine hydroxylase neurons in rat locus coeruleus. Synapse. 1999;31:309–311. doi: 10.1002/(SICI)1098-2396(19990315)31:4<309::AID-SYN10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Koylu EO, Weruaga E, Balkan B, Alonso JR, Kuhar MJ, Pogun S. Co-localization of cart peptide immunoreactivity and nitric oxide synthase activity in rat hypothalamus. Brain Res. 2000;868:352–357. doi: 10.1016/s0006-8993(00)02290-3. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Dall Vechia SE. CART peptides: novel addiction- and feeding-related neuropeptides. Trends Neurosci. 1999;22:316–320. doi: 10.1016/s0166-2236(98)01377-0. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Yoho LL. CART peptide analysis by Western blotting. Synapse. 1999;33:163–171. doi: 10.1002/(SICI)1098-2396(19990901)33:3<163::AID-SYN1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Adams LD, Hunter RG, Vechia SD, Smith Y. CART peptides. Regul Pept. 2000;89:1–6. doi: 10.1016/s0167-0115(00)00096-3. [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the MPOA and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 1999a;100:15–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- Lee A, Li M, Watchus J, Fleming AS. Neuroanatomical basis of maternal memory in postpartum rats: selective role for the nucleus accumbens. Behav Neurosci. 1999b;113:523–538. doi: 10.1037//0735-7044.113.3.523. [DOI] [PubMed] [Google Scholar]
- Leonard CM. The prefrontal cortex of the rat. I. Cortical projection of the mediodorsal nucleus II. Efferent connections. Brain Res. 1969;12:321–343. doi: 10.1016/0006-8993(69)90003-1. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Dubno JR, Horwitz AR, Nahas Z, Teneback CC, Bloomer CW, Bohning DE, Vincent D, Johnson MR, Emmanuel N, Brawman-Mintzer O, Book SW, Lydiard RB, Ballenger JC, George MS. Feasibility of using fMRI to study mothers responding to infant cries. Depress Anxiety. 1999;10:99–104. doi: 10.1002/(sici)1520-6394(1999)10:3<99::aid-da2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams SE, Rosenblatt JS, Morrell JI. Preferences for cocaine- or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharmacology (Berl) 2003;167:1–8. doi: 10.1007/s00213-002-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. Hormonal interaction with stimulus and situational factors in the initiation of maternal behavior in nonpregnant rats. J Comp Physiol Psychol. 1980;94:1040–1059. doi: 10.1037/h0077744. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. A method for regulating the duration of pregnancy and the time of parturition in Sprague–Dawley rats (Charles River CD strain) Dev Psychobiol. 1998;32:131–136. [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Morutto SL, Phillips GD. Interactions between sulpiride infusions within the perifornical region of the lateral hypothalamus and the nucleus accumbens on measures of locomotor activity and conditioned place preference. Behav Pharmacol. 1998;9:345–55. [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Cocaine vs. food choice in rhesus monkeys: effects of increasing the response cost for cocaine. NIDA Res Monogr. 1991;105:621. [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing response requirement on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 1992;108:295–300. doi: 10.1007/BF02245115. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M. Maternal behavior. In: Knobil E, Neil JD, editors. The physiology of reproduction. New York: Raven Press; 1994. pp. 223–283. [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer-Verlag; 2003. [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA. Place conditioning in a three or four choice apparatus: Role of stimulus novelty in drug-induced place conditioning. Behav Neurosci. 1992;106:294–306. doi: 10.1037//0735-7044.106.2.294. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Crofts HS, McGuigan M, Tomic DL, Everitt BJ, Roberts AC. The role of the primate amygdala in conditioned reinforcement. J Neurosci. 2001;21:7770–7780. doi: 10.1523/JNEUROSCI.21-19-07770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain atlas in stereotaxic coordinates. New York: Academic Press; 1998. [Google Scholar]
- Peoples LL. Neuroscience. Will, anterior cingulate cortex, and addiction. Science. 2002;296:1623–1624. doi: 10.1126/science.1072997. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Motivation and reward. In: Zigmond M, et al., editors. Fundamental neuroscience. New York: Academic Press; 1999. pp. 1245–1278. [Google Scholar]
- Robbins TW. From arousal to cognition: the integrative position of the prefrontal cortex. Prog Brain Res. 2000;126:469–483. doi: 10.1016/S0079-6123(00)26030-5. [DOI] [PubMed] [Google Scholar]
- Roland EH, Volpe JJ. Effect of maternal cocaine use on the fetus and newborn: review of the literature. Pediatr Neurosci. 1989;15:88–94. doi: 10.1159/000120449. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdale of rats. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper C. Hypothalamic connections with the cerebral cortex. Prog Brain Res. 2000;126:40–48. doi: 10.1016/S0079-6123(00)26005-6. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Binzak JM, Kelley AM. A common profile of prefrontal cortical activation following exposure to nicotine or chocolate-associated contextual cues. Neuroscience. 2001;105:535–545. doi: 10.1016/s0306-4522(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shidara M, Richmond BJ. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science. 2002;296:1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- Smith Y, Koylu EO, Couceyro P, Kuhar MJ. Ultrastructural localization of CART (cocaine- and amphetamine- regulated transcript) peptides in the nucleus accumbens of monkeys. Synapse. 1997;27:90–94. doi: 10.1002/(SICI)1098-2396(199709)27:1<90::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Smith Y, Kieval J, Couceyro PR, Kuhar MJ. CART peptideimmunoreactive neurones in the nucleus accumbens in monkeys: ultrastructural analysis, colocalization studies, and synaptic interactions with dopaminergic afferents. J Comp Neurol. 1999;407:491–511. doi: 10.1002/(sici)1096-9861(19990517)407:4<491::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Uslander J, Badiani A, Norton CS, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine and cocaine induce different patterns of c-fos mRNA expression in the striatum and subthalamic nucleus depending on environmental context. Eur J Neurosci. 2001;13:1977–1983. doi: 10.1046/j.0953-816x.2001.01574.x. [DOI] [PubMed] [Google Scholar]
- Vernotica EM, Rosenblatt JS, Morrell JI. Microinfusion of cocaine into the medial preoptic area or nucleus accumbens transiently impairs maternal behavior in the rat. Behav Neurosci. 1999;113:377–390. doi: 10.1037//0735-7044.113.2.377. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MF. The role of rat medial frontal cortex in effort-based decision making. J Neurosci. 2002;22:10996–11003. doi: 10.1523/JNEUROSCI.22-24-10996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci U S A. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Alheid GF, Beinfeld MC, Eiden LE, Heimer L, Palkovits M. Cholecystokinin innervation of the ventral striatum: a morphological and radioimmunological study. Neuroscience. 1985;14:427–453. doi: 10.1016/0306-4522(85)90302-1. [DOI] [PubMed] [Google Scholar]


