Abstract
We have systematically reviewed the presence, functional responses and regulation of α1-, α2- and β-adrenoceptors in the bladder, urethra and prostate, with special emphasis on human tissues and receptor subtypes.
α1-Adrenoceptors are only poorly expressed and play a limited functional role in the detrusor. α1-Adrenoceptors, particularly their α1A-subtype, show a more pronounced expression and promote contraction of the bladder neck, urethra and prostate to enhance bladder outlet resistance, particularly in elderly men with enlarged prostates. α1-Adrenoceptor agonists are important in the treatment of symptoms of benign prostatic hyperplasia, but their beneficial effects may involve receptors within and outside the prostate.
α2-Adrenoceptors, mainly their α2A-subtype, are expressed in bladder, urethra and prostate. They mediate pre-junctional inhibition of neurotransmitter release and also a weak contractile effect in the urethra of some species, but not humans. Their overall post-junctional function in the lower urinary tract remains largely unclear.
β-Adrenoceptors mediate relaxation of smooth muscle in the bladder, urethra and prostate. The available tools have limited the unequivocal identification of receptor subtypes at the protein and functional levels, but it appears that the β3- and β2-subtypes are important in the human bladder and urethra, respectively. β3-Adrenoceptor agonists are promising drug candidates for the treatment of the overactive bladder.
We propose that the overall function of adrenoceptors in the lower urinary tract is to promote urinary continence. Further elucidation of the functional roles of their subtypes will help a better understanding of voiding dysfunction and its treatment.
Keywords: Bladder, urethra, prostate, α1-adrenoceptor, α2-adrenoceptor, β-adrenoceptor
Introduction
The lower urinary tract is responsible for urine storage and voiding (see Andersson & Wein, 2004). During the storage phase of the micturition cycle, the bladder relaxes to accommodate increasing volumes of urine at acceptable pressure, and the bladder neck and urethra contract to provide resistance to prevent involuntary leakage. During the micturition phase, the bladder neck and urethral muscles relax to allow the detrusor to contract and expel urine without major resistance. While the prostate does not appear to play a major physiological role in continence, its enlargement in patients with benign prostatic hyperplasia (BPH) can increase bladder outlet resistance and thereby disturb physiological voiding.
Diseases of the lower urinary tract are frequent in the general population. They include the syndrome of the overactive bladder (OAB), which is defined as urgency, with or without incontinence, usually accompanied by frequency and nocturia (Abrams et al., 2002), and is present in about 16% of the population aged 40 years and over (Milsom et al., 2001). Another frequent condition is stress urinary incontinence, a condition largely affecting the female population. Its reported prevalence in the general female population ranges between 5 and 37% (Hampel et al., 2004). The reasons for this remarkable heterogeneity include differences between study populations and the use of varying definitions of the condition. More consistently, stress incontinence accounts for approximately 80% of incontinence in women (Hampel et al., 2004). While BPH is a very frequent condition in elderly males, its prevalence estimates depend on whether the histological diagnosis of BPH or the associated bothersome symptoms are assessed, the latter being reported in about 30% of men aged 50–80 in population-based studies (Berges et al., 2001).
The autonomic nervous system plays a key role in the regulation of lower urinary tract function (see Bannowsky & Juenemann, 2003; Michel et al., 2005c). Its sympathetic innervation occurs via the hypogastric nerve arising from the nucleus intermediolateralis of spinal cord segments Th12–L2. Noradrenaline released from these nerves can act on all three classes of adrenoceptors, that is, α1-, α2- and β-adrenoceptors. Three receptor subtypes have been cloned within each of these classes and are designated as α1A (in earlier papers, sometimes also referred to as α1A/D or α1C), α1B, α1D, α2A (its rodent analogue sometimes referred to as α2D), α2B, α2C, β1, β2 and β3 (see Bylund et al., 1994; Hieble et al., 1995). Multiple splice variants of the α1A-adrenoceptor have been reported, but they have a very similar ligand recognition profile and hence their pharmacological relevance remains unclear (Hirasawa et al., 1995; Chang et al., 1998; Daniels et al., 1999). Moreover, the α1A-adrenoceptor gene product can exhibit low affinity for prazosin and several other drugs upon expression in some cell types, and this phenotype is often referred to as ‘α1L' (Ford et al., 1997; Daniels et al., 1999). Similarly, the β1-adrenoceptor gene product can exhibit low affinity for propranolol and several other drugs upon expression in some cell types, and this phenotype is sometimes referred to as ‘β4' and sometimes as ‘atypical β-adrenoceptor' (Joseph et al., 2003; 2004). Moreover, it should be considered that single-nucleotide polymorphisms exist for most of the nine cloned human adrenoceptor subtypes, which could lead to altered tissue responses (see Leineweber et al., 2004; Lei et al., 2005). The present manuscript reviews the expression, functional responses and regulation of each of these adrenoceptor subtypes in the bladder, urethra and prostate, and discusses their therapeutic implications and potential value as drug targets.
Bladder
The bladder stores and expels urine. The force needed to expel it during the voiding phase of the micturition cycle is generated by the detrusor smooth muscle (sometimes with the help of increasing intra-abdominal pressure), which is anatomically largely found in the bladder dome. In contrast, the bladder neck is involved in generating resistance during the filling phase of the micturition cycle to help prevent involuntary urine leakage. Therefore, the bladder neck is functionally more closely related to the urethra than to the detrusor. The trigone and bladder base are anatomically located close to the bladder neck. Interestingly, the bladder neck appears to have a much denser sympathetic innervation than the detrusor, and the role of neuronally released noradrenaline in activating adrenoceptors expressed in the detrusor has not been well established.
α1-Adrenoceptors
mRNA expression
The presence of α1-adrenoceptor subtype mRNA in the urinary bladder has been assessed in rats, mice, monkeys and humans, with rats and humans apparently differing considerably. Using competitive RT–PCR, α1A-, α1B- and α1D-adrenoceptors were found to account for 95, 1 and 4%, respectively, of total α1-adrenoceptor mRNA in rat bladder (Scofield et al., 1995). Another study based upon RNase protection assays has reported a roughly similar abundance of all three subtypes in whole rat bladder, but did not provide quantitative information (Malloy et al., 1998), whereas a later, more quantitative report from those investigators based upon competitive RT–PCR has shown the presence of the three subtypes in a ratio of 70 : 5 : 25% (Hampel et al., 2002). Microarray analysis detected hybridization signals for the α1A-adrenoceptor, but not for any other α-adrenoceptor subtype (Lluel et al., 2003b). For each of the three subtypes, expression in the rat bladder base was shown to be markedly greater than in the detrusor (Yono et al., 2004). A predominant expression of the α1A-subtype was qualitatively confirmed using in situ hybridization studies, which found a strong expression of this subtype in the urothelium, a moderate expression in smooth muscle (quantitatively similar to that in prostate smooth muscle), but no presence in connective tissue; bladder dome and bladder base were similar in this regard (Walden et al., 1997). The same study also found a very similar situation in the rhesus monkey bladder (Walden et al., 1997). In contrast, that study detected a moderate expression of α1A-adrenoceptors in the human bladder dome smooth muscle (quantitatively similar to that in prostate smooth muscle), but not in bladder dome connective tissue or urothelium or in prostate epithelium (Walden et al., 1997). Using real-time PCR, other investigators confirmed a moderate expression of α1-adrenoceptor mRNA in the human bladder (corresponding to only 3% of β-adrenoceptor mRNA abundance), to which α1A-, α1B- and α1D-adrenoceptors contributed 33, 53 and 14%, respectively (Nomiya & Yamaguchi, 2003). RT–PCR studies reported a dominant abundance of α1A- and α1D-adrenoceptor mRNA with less, if any, α1B-adrenoceptor mRNA in the human bladder (Sigala et al., 2004). Other investigators, using RNase protection assays, detected α1A-, α1B- and α1D-adrenoceptors in a 34 : 0 : 66% ratio (Malloy et al., 1998). The predominance of α1D-adrenoceptor mRNA has been confirmed in a recent study from the same group using two independent sets of samples using quantitative real-time PCR confirmed by RNase protection assays (Schwinn, personal communication). Real-time PCR studies in mice reported α1A-, α1B- and α1D-adrenoceptors in a 42 : 8 : 50% ratio (Chen et al., 2005), that is, in a roughly similar ratio as in the human bladder. Taken together, the total quantity of α1-adrenoceptor mRNA expression in the detrusor appears low. While the α1A-adrenoceptor is the most abundant subtype in the rat bladder, the relative contributions of α1-adrenoceptor subtypes in the human bladder remain controversial.
Protein expression
The presence of α1-adrenoceptors in the detrusor of rats, rabbits, guinea-pigs, pigs, cats, monkeys and humans has been examined at the protein level using radioligand-binding studies in tissue homogenates and, in some cases, receptor autoradiography. Using [125I]BE 2254 (also known as [125I]HEAT) as the radioligand, a low density of α1-adrenoceptors (≈7 fmol mg−1 protein) was found in rat bladder, which was shown to represent a homogeneous population of α1A-adrenoceptors (Hampel et al., 2002). A low density of α1-adrenoceptors was confirmed in autoradiography studies using [3H]prazosin (Monneron et al., 2000). Saturation-binding experiments with the α1A-selective radioligand [3H]L-771,688 also detected a relatively low density of this subtype in the rat bladder as compared to several other tissues (Chang et al., 2000). In rabbit bladder, a slightly greater but still only moderate α1-adrenoceptor density (14–18 fmol mg−1 protein) was reported using [125I]BE 2254 (Tsujimoto et al., 1986) or [3H]prazosin (Latifpour et al., 1990). Autoradiography studies using [3H]prazosin also detected only few, if any, α1-adrenoceptors in the guinea-pig, cat and female pig bladder (Monneron et al., 2000). Using the same radioligand in membrane preparations, no quantifiable amounts of α1-adrenoceptors were detected in male or female porcine detrusor (Goepel et al., 1997). Autoradiography studies with [3H]prazosin found very little α1-adrenoceptor expression at the protein level in the urothelial or smooth muscle layers of the rhesus monkey detrusor (Walden et al., 1997). Studies in the human detrusor using [125I]BE 2254 as the radioligand reported a low α1-adrenoceptor density (≈6 fmol mg−1 protein); based upon competition studies with BMY 7378, 66% of these were described as α1D-adrenoceptors (Malloy et al., 1998). A low level of α1-adrenoceptor expression at the protein level in the detrusor was confirmed using [125I]BE 2254 by other investigators (Sigala et al., 2004). Using Western blots with subtype-selective antibodies, the latter study demonstrated the presence of all three subtypes at the protein level in the human detrusor, but did not provide subtype-specific quantification (Sigala et al., 2004). Another group, however, using [3H]prazosin as the radioligand, has not detected quantifiable numbers of α1-adrenoceptors in the human detrusor (Goepel et al., 1997). Thus, the overall density of α1-adrenoceptors in the detrusor of various species, including humans, is low.
The presence of α1-adrenoceptors has also been investigated in the trigone, bladder base and/or bladder neck of several species. In this regard, pigs appear to be the only species where α1-adrenoceptors have not been detected in the bladder neck (Goepel et al., 1997). In the rabbit bladder base, early studies had found α1-adrenoceptors of an unspecified subtype (Andersson et al., 1984; Larsson et al., 1986; Levin et al., 1988). Direct comparative studies in rats (Monneron et al., 2000) and rabbits (Latifpour et al., 1990) reported greater α1-adrenoceptor binding in the trigone and bladder base, respectively, than in the dome. Similar autoradiography studies with [3H]prazosin found greater α1-adrenoceptor expression in the monkey bladder base than detrusor; based upon competition by the highly α1A-selective SNAP 5272, the latter appeared to predominantly represent α1A-adrenoceptors (Figure 1) (Walden et al., 1997). α1-Adrenoceptors have also been found in the human bladder base (Levin et al., 1988). This was confirmed by other investigators, using not only radioligand binding but also Western blotting with subtype-selective antibodies, which detected all three subtypes (Sigala et al., 2004). Thus, in agreement with the mRNA measurements, radioligand binding and receptor autoradiography studies have detected only low densities of α1-adrenoceptors in the detrusor of several species, including humans; in this regard, detection by [125I]BE 2254 appears to be more sensitive than that by [3H]prazosin. A more consistent, and in some direct comparative studies greater, α1-adrenoceptor expression was seen in the trigone/bladder base/bladder neck region. While the α1A-adrenoceptor appears to be the most abundant subtype in healthy rats, the α1D-adrenoceptor appears to be the most abundant subtype in humans.
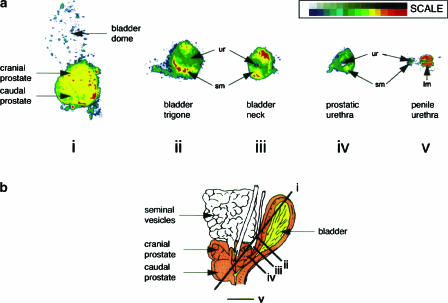
Figure 1.
Presence of α1A-adrenoceptor protein in the lower urinary tract of the monkey. Receptors were localized by autoradiography, using [3H]praozsin and defining non-specific binding in the presence of SNAP 5272. Receptor autoradiograms were scanned into computer as a 16 grey scale image. The 16 grey levels corresponding to specific α1A-adrenoceptor receptor binding were each assigned colour (see scale) to allow subtle differences in film exposure to be easily visible. Sections show bladder dome and prostate (i), bladder trigone (ii), bladder base (iii), prostatic urethra (iv) and penile urethra (v). Key: sm (smooth muscle); ur (urothelium); lm (longitudinal muscle). Schematic representation of monkey urinary tract together with the orientation of sectioning planes (i)–(v) shown in (b). Taken with permission from Walden et al. (1997).
In vitro function
In vitro studies on the functional role of α1-adrenoceptors in the urinary bladder have focused not only on direct contractile effects but also on the modulation of neurotransmitter release. The α1-adrenoceptor agonists phenylephrine and methoxamine enhanced the field stimulation-induced release of both noradrenaline and acetylcholine in the isolated rat bladder (Somogyi et al., 1995). Phenylephrine also increased the basal release of noradrenaline, but not of acetylcholine. While the phenylephrine effect on acetylcholine release was blocked by the α1-adrenoceptor antagonist terazosin, that on noradrenaline release was not, indicating that the latter may have been β-adrenoceptor-mediated. The increased acetylcholine release was accompanied by an enhancement of field stimulation-induced contraction, which was mainly seen at low-frequency nerve stimulation and at low extracellular Ca2+ concentrations. It was concluded that cholinergic nerve terminals in rat bladder express α1-adrenoceptors that facilitate acetylcholine release. Later studies from these investigators confirmed the initial observations and further demonstrated that the facilitation of acetylcholine release was largely, if not exclusively, mediated by α1A-adrenoceptors (Szell et al., 2000). An α1-adrenoceptor-mediated depolarization of parasympathetic nerves in the vesical ganglia has also been demonstrated in cats (Nakamura et al., 1984).
The possible direct contractile effects of α1-adrenoceptor stimulation have been investigated in rat, rabbit, guinea-pig and human bladder. In the rat detrusor, α1-adrenoceptor agonists such as phenylephrine produced only weak contractions, that is, in the range of 10–43% of those reached by muscarinic receptor stimulation or receptor-independently by KCl (Kolta et al., 1984; Somogyi et al., 1995; Lluel et al., 2000; 2003a, 2003b; Szell et al., 2000). The effect appears to be mediated by a subtype with low affinity for the α1D-selective BMY 7378, most likely the α1A-adrenoceptor (Lluel et al., 2003b). Interestingly, and in line with the data on α1-adrenoceptor expression at the protein level (see above), the phenylephrine-induced contraction was about three times as large in rat bladder neck as compared to the detrusor (Lluel et al., 2003a). One study demonstrates that the weak direct contractile effects of α1-adrenoceptor agonists in the rat detrusor occur via chloroethylclonidine-sensitive α1B- or α1D-adrenoceptors, that is, a different subtype than the one mediating enhanced acetylcholine release and hence indirect contractile effects (Szell et al., 2000). The direct contractile effects of α1-adrenoceptor agonists in the rabbit detrusor were also weak (Ueda et al., 1984; Tsujimoto et al., 1986; Latifpour et al., 1990). However, several direct comparative studies demonstrate greater α1-adrenoceptor-mediated contraction in rabbit trigone (Ueda et al., 1984) and bladder base (Latifpour et al., 1990). Interestingly, some data show that the α1-adrenoceptor subtype mediating the contraction of rabbit trigone, bladder base and/or bladder neck resembles the cloned α1A-adrenoceptor (Honda & Nakagawa, 1986; van der Graaf et al., 1997; Kava et al., 1998; Williams et al., 1999), but has relatively low affinity for prazosin (pA2 8.0–8.4), indicating that it may belong to the α1L-phenotype of the α1A-adrenoceptor (Lefevre-Borg et al., 1993; Deplanne & Galzin, 1996; van der Graaf et al., 1997; Kava et al., 1998; Williams et al., 1999) (a more detailed discussion of the α1L-phenotype is given in the prostate section). Based upon the high potency of the antagonist L-771,688 (also known as SNAP 6383) in inhibiting the contractile effects of the agonist A61603, the receptor mediating contraction of the monkey bladder neck was also classified as being α1A (Chang et al., 2000).
In analogy to rats and rabbits, studies in the human detrusor have found only very weak contraction (up to 5% of the maximum muscarinic response) by the α1-adrenoceptor agonist phenylephrine (Nomiya & Yamaguchi, 2003). A more robust contraction was observed in studies with the human bladder base and bladder neck (Caine et al., 1975). In the latter tissue, contraction was potently elicited by the α1A-selective agonist A-61603 and inhibited potently by the α1A-selective antagonist L-771,688 (Chang et al., 2000). In contrast to the rabbit bladder neck, however, responses in the human bladder base exhibited high potency for prazosin (pA2 8.9) (Kunisawa et al., 1985), indicating that the α1L-phenotype of the α1A-adrenoceptor was not involved.
In contrast to rats, rabbits and humans, α1-adrenoceptor stimulation in the isolate guinea-pig bladder did not enhance, but rather inhibited, the amplitude and frequency of phasic contractions (Gillespie, 2004), but the reasons for such species differences remain unclear. Thus, in most species, including humans, α1-adrenoceptor stimulation produces only weak detrusor contraction, whereas a stronger contraction is observed for the trigone, bladder base and/or bladder neck. The physiological relevance of this, however, remains unclear, since the bladder neck appears largely under the control of the parasympathetic (and perhaps nonadrenergic–noncholinergic) rather than the sympathetic nervous system (Deplanne et al., 1998).
In vivo function
The in vivo analysis of a role for α1-adrenoceptors in the regulation of bladder function is complicated by the fact that both central and peripheral receptors may be involved and may serve distinct functions. In anaesthetized rats, intra-thecal injections of prazosin inhibited bladder contraction evoked from the locus coeruleus (Yoshimura et al., 1988). Using continuous cystometry in conscious rats, doxazosin given intra-thecally was shown to reduce the size of the bladder pressure (Ishizuka et al., 1996b). Two studies have investigated the α1-adrenoceptor subtypes involved in the central stimulation of bladder contraction. Reductions in the height of isovolumetric contraction were reported for the α1A-adrenoceptor antagonist RS 100,329 given intra-thecally and for the moderately α1B-selective antagonist (+)-cyclazosin; however, the latter effect was not dose-related (Yoshiyama & De Groat, 2001). Further, both drugs increased the frequency of these contractions, while the α1D-adrenoceptor antagonist BMY 7378 had no effect. Naftopidil, which may have some selectivity for α1D-adrenoceptors, given intra-thecally, was reported to inhibit the appearance of regular isovolumetric bladder contractions and reduce their height (Sugaya et al., 2002). In addition, tamsulosin, which has high affinity for both α1A- and α1D-adrenoceptors, was also reported to inhibit the appearance of these contractions. These studies demonstrate that central, most likely spinal, α1-adrenoceptors are involved in stimulating bladder contractility, and that an α1A-adrenoceptor is the most likely candidate mediating such effects.
Studies with systemic administration of α1-adrenoceptor antagonists have yielded less consistent results. For example, i.v. α1-adrenoceptor antagonists inhibited the sympathetic control of the bladder by reducing hypogastric nerve activity (Danuser & Thor, 1995; Ramage & Wyllie, 1995) and somatic activity to the urethra (Danuser & Thor, 1995). However, spontaneous bladder contractions, presumably mediated by the parasympathetic nervous system, were unaffected (Ramage & Wyllie, 1995). Others compared the intra-thecal and intra-arterial effects of doxazosin, phentolamine, prazosin, tamsulosin and yohimbine upon cystometric parameters in anaesthetized rats (Jeong & Lee, 2000); based upon differences between drugs and modes of administration, these authors proposed that α1-adrenoceptors suppress the micturition effect via a peripheral mechanism, whereas α2-adrenoceptors do so via a central mechanism. Finally, antagonists selective for α1A-, α1B- and α1D-adrenoceptors, that is, RS-100,329, RS-51,385 and BMY 7378, given i.v. to anaesthetized rats, were found to have no effect on bladder contraction height induced by infusion of saline into the bladder, but the associated reflex urethral contractions were attenuated by blockade of α1A/D-adrenoceptors (Conley et al., 2001). Both RS-100,329 and BMY 7378 also decreased resting urethral pressure. The failure to see any changes in evoked bladder contraction may reflect a difference in the method being used to evoke it and/or the route of administration. However, the increase in frequency observed for intra-thecal α1A-adrenoceptor antagonists would be expected to be translated into a decrease in the volume threshold, but this was also not observed.
Thus, despite some ongoing controversies, the overall in vivo data suggest that at a spinal level α1-adrenoceptors are probably involved in mediating bladder contractions and decreasing the frequency of micturition. Therefore, systemically administered α1-adrenoceptor antagonists that penetrate into the central nervous system may predominantly inhibit bladder contractions. On the other hand, α1-adrenoceptors are also involved in the peripheral control of the sympathetic supply to the bladder and thus storage. In this respect, stimulation of the hypogastric nerve has also been shown to facilitate cholinergic transmission at the level of the pelvic ganglia via the action of α1-adrenoceptors (Keast et al., 1990) and thus also enhancing bladder contractions. Interestingly, it has been reported that in anaesthetized dogs the α-adrenoceptor agonist midodrine did not affect bladder capacity in young animals, but reduced it in old animals (Takahashi et al., 1996).
Treatment with a very high dose of the α1-adrenoceptor antagonist doxazosin (30 mg kg−1 orally) was reported to attenuate obstruction-induced bladder hypertrophy (Das et al., 2002). However, these findings are difficult to interpret since another study in nonobstructed rats found that doxazosin (2 or 4 mg kg−1 s.c. plus 4 mg kg−1 orally) increased the weight of the bladder base and, in at least some dose groups, upregulated α1A-adrenoceptor mRNA in the bladder base (Yono et al., 2004), and also because the doxazosin doses in both studies are very high as compared to a therapeutic dose of 4–8 mg per patient. Such high doses of doxazosin may have growth-inhibiting or apoptotic effects on the lower urinary tract, which are independent of α1-adrenoceptors (Walden et al., 2004).
Regulation of receptor expression and function
Some studies have investigated a possible regulation of the role of α1-adrenoceptors in bladder function by gender, ageing and bladder outlet obstruction. Expression of α1A-adrenoceptor mRNA was similar in the detrusor and bladder base of male and female rats (Walden et al., 1997), and the number of α1-adrenoceptor-binding sites was also similar in the detrusor and bladder base of male and female rabbits (Latifpour et al., 1990). A study in humans confirmed a lack of gender effect on α1-adrenoceptor binding in detrusor and bladder neck, but found a significantly greater α1-adrenoceptor density in female than in male trigone; this study also reported on the quantity of α1A-, α1B- and α1D-adrenoceptor mRNA in all three regions of both genders, but did not provide a statistical analysis of the observed differences (Sigala et al., 2004).
Ageing studies on α1-adrenoceptors in the bladder have been reported for rats, rabbits and dogs. A comparison of 7-, 17- and 29-month-old Fischer rats did not detect significant alterations in the maximum effects or potency of phenylephrine (Kolta et al., 1984). This was confirmed in studies on 10- and 30-month-old female (Lluel et al., 2000) and male Wistar rats (Lluel et al., 2003b), as well as in 6- and 24-month-old male Sprague–Dawley rats (Lluel et al., 2003a). However, the former two studies surprisingly reported a markedly increased noradrenaline-induced contraction in aged animals, which was not explained by a possible α2-adrenoceptor stimulation (Lluel et al., 2000; 2003b). Since the same studies did not detect differential expression of any α-adrenoceptor subtype in a microarray analysis, the phenylephrine findings appear somewhat more plausible than the noradrenaline findings. Ageing was also reported not to affect the number of α1-adrenoceptor-binding sites in detrusor and bladder base of 6 months vs 4.5–5-year-old male or female rabbits (Latifpour et al., 1990). On the other hand, studies in anaesthetized dogs found reductions of bladder capacity upon systemic administration of the α1-adrenoceptor agonists in 68-month-old, but not in 12-month-old, animals; the interpretation of these findings, however, is complicated by the fact that the old dogs had been parous, whereas the young ones were nonparous (Takahashi et al., 1996). Thus, the overall data suggest that neither gender nor ageing has a major effect on α1-adrenoceptor function in the bladder.
Due to the high prevalence of BPH, bladder outlet obstruction is frequent in elderly men. Since it has been speculated that the α1-adrenoceptor antagonist-induced symptom relief in BPH patients may involve effects on bladder function (see Michel, 2002; Roehrborn & Schwinn, 2004), potential alterations of α1-adrenoceptors have been investigated in the bladder of animal models of obstruction and in patients. Studies in a rat model of obstruction found an unchanged total α1-adrenoceptor mRNA and radioligand binding. However, this was accompanied by a reduction of α1A- and an increase of α1D-adrenoceptor mRNA; in competition-binding experiments, α1D-adrenoceptors had been undetectable in control rats, but represented approximately 40% of all α1-adrenoceptors in obstructed animals (Hampel et al., 2002). The contractile effects of phenylephrine were reported to remain unchanged in obstructed patients (Nomiya & Yamaguchi, 2003). Further studies are needed to define the role of α1-adrenoceptors in the detrusor in settings of bladder outlet obstruction.
Clinical implications
In conclusion, α1-adrenoceptors appear to play a small functional role if any in the detrusor of healthy animals and humans. Since some of the beneficial effects of α1-adrenoceptor antagonists in BPH patients cannot easily be explained solely based upon prostatic α1-adrenoceptors (see the section on prostate), it nevertheless appears plausible that those located in the detrusor may have therapeutic relevance. This hypothesis, however, remains to be tested. In a similar vein, it has been reported in a small group of patients with spinal cord injury that treatment with the α1-adrenoceptor antagonist terazosin increases bladder compliance and results in less incontinence and dysreflexia (Swierzewski et al., 1994), but it remains unclear whether this reflects a direct effect on the bladder or an indirect effect. On the other hand, α1-adrenoceptors may play a more prominent functional role in the bladder neck and hence the regulation of bladder outlet resistance. Antagonizing their function may contribute to the beneficial effects of α1-adrenoceptor antagonists in BPH patients (see the section on prostate), whereas their stimulation provides a potential target in the treatment of stress incontinence (see the section on urethra).
α2-Adrenoceptors
mRNA and protein expression
To the best of our knowledge, the presence of α2-adrenoceptor subtype mRNA in the bladder has not been reported. At the protein level, however, radioligand-binding studies have detected α2-adrenoceptors in the detrusor and bladder base/bladder neck of rabbits (Andersson et al., 1984; Levin et al., 1988; Latifpour et al., 1990), pigs (Goepel et al., 1997) and humans (Levin et al., 1988; Goepel et al., 1997). Their density in the rabbit bladder base was reported to be smaller (Levin et al., 1988), larger (Andersson et al., 1984) and similar (Latifpour et al., 1990) to that of α1-adrenoceptors within the same tissue. In the porcine and human bladder, their density (15–25 and 40 fmol mg−1 protein, respectively) clearly exceeded that of α1-adrenoceptors, but was somewhat smaller than that of β-adrenoceptors within the same study (Goepel et al., 1997). In competition-binding experiments in porcine and human bladder, a predominant, if not exclusive, population of α2A-adrenoceptors was found (Goepel et al., 1997).
In vitro function
Only few studies have assessed the functional role of α2-adrenoceptors in the bladder. The pre-junctional inhibition of neurotransmitter release from both post-ganglionic sympathetic and parasympathetic nerve terminals is the best-established function of α2-adrenoceptors in most tissues. Consistent with this concept, α2-adrenoceptor stimulation inhibited field stimulation-induced contraction of rat bladder in a tetrodotoxin, but not hexamethonium-sensitive, manner (Santicioli et al., 1983; Maggi et al., 1985). Moreover, α2-adrenoceptor stimulation also inhibits parasympathetic nerve activity in the bladder of rabbits (Tsurusaki et al., 1990) and cats (Nakamura et al., 1984; Keast et al., 1990) by an effect on the vesical parasympathetic ganglion. Despite the considerable abundance of α2-adrenoceptors in bladder homogenates, their post-junctional function has not been established as they do not mediate contractile effects in the rabbit detrusor (Ueda et al., 1984), whole guinea-pig bladder (Gillespie, 2004) or human bladder base (Kunisawa et al., 1985).
In vivo function
In vivo studies on α2-adrenoceptor function in the bladder are often difficult to interpret because central and peripheral α2-adrenoceptor stimulation may not have the same effects; they may even partly counteract each other and their relative roles may depend on the use of anaesthetized vs conscious animals. In anaesthetized rats α2-adrenoceptor stimulation reduced volume-induced bladder contraction (Maggi et al., 1985; Harada & Constantinou, 1993). Using somewhat different methods, an opposite conclusion was drawn from a more recent study (Jeong & Lee, 2000). However, all of these studies agree that the site of action of the α2-adrenoceptor agonists and antagonists is in the spinal cord rather than in the periphery. An initial report in conscious rats reported that intra-thecal and intra-arterial (close the bladder) administration of an α2-adrenoceptor agonist reduced micturition pressure, bladder capacity and micturition volume; while an α2-antagonist inhibited the effects of intra-thecal agonist, it mimicked those of the peripheral administration (Ishizuka et al., 1996a). In contrast, other studies found that α2-adrenoceptor agonists increase the frequency of bladder contractions (Durant et al., 1988; Kontani et al., 2000) and voiding (Harada & Constantinou, 1993), but the interpretation of this finding would be complicated by a diuretic effect of the agonist (Harada & Constantinou, 1993). Similar to the situation in anaesthetized rats, all of the above studies agree that α2-adrenoceptors in the spinal cord are likely to be the main source of modulation of bladder function.
Regulation of receptor expression and function
Studies in rabbit detrusor or bladder base reported a similar α2-adrenoceptor density in male and female animals as well as in young (6 months) and old (4.5–5 years) rabbits (Latifpour et al., 1990). A study in male and female pigs confirmed the lack of gender difference in detrusor and bladder neck, with α2A-adrenoceptors being the only detectable subtype in all groups (Goepel et al., 1997). Based upon all of the above data, α2-adrenoceptors are not considered a promising target for the treatment of voiding disorders.
β-Adrenoceptors
mRNA expression
The presence of β-adrenoceptors in the rat and human bladder at the mRNA level has been studied using Northern blots, PCR and in situ hybridization. Messenger RNA for all three β-adrenoceptor subtypes has been detected in rats (Seguchi et al., 1998; Fujimura et al., 1999; Matsubara et al., 2002). It has been claimed that the β3-adrenoceptor may be the most abundant subtype (Fujimura et al., 1999), but no specific quantitative data were reported. Studies in the human bladder have also detected mRNA for all three β-adrenoceptor subtypes (Fujimura et al., 1999; Igawa et al., 1999; Takeda et al., 1999; Li et al., 2003; Nomiya & Yamaguchi, 2003). Based upon quantitative PCR experiments, it appears that the β3-adrenoceptor accounts for more than 95% of all β-adrenoceptor mRNA in the human bladder (Nomiya & Yamaguchi, 2003). The presence of β3-adrenoceptor mRNA in the human detrusor has also been confirmed in in situ hybridization studies (Takeda et al., 1999).
Protein expression
The identification of β-adrenoceptors at the protein level is typically based upon binding studies with radioligands such as [125I]iodocyanopindolol, [125I]iodopindolol, [3H]CGP 12,177 or [3H]dihydroalprenolol. [125I]iodocyanopindolol and [3H]CGP 12,177 have much lower affinity for β3- than for β1- or β2-adrenoceptors (Hoffmann et al., 2004; Baker, 2005). Data from our lab confirm this and further demonstrate that [3H]dihydroalprenolol yields a similarly poor labelling of β3-adrenoceptors (Niclauss et al., unpublished observations), a finding that is entirely consistent with the low β3-adrenoceptor affinity of unlabelled alprenolol (Hoffmann et al., 2004). While high concentrations of [125I]iodocyanopindolol and [3H]CGP 12,177 have successfully been used to label β3-adrenoceptors in transfected cells, the use of similarly high concentrations in tissues yields very high nonspecific binding and will saturate β1- and β2-adrenoceptors. Both problems make the detection of β3-adrenoceptors in tissues expressing mixed β-adrenoceptor subtype populations virtually impossible. A potential alternative would be the use of a β3-adrenoceptor-selective radioligand such as [3H]SB 206,606. However, this ligand has only high nanomolar affinity for β3-adrenoceptors (Kd values of 200–500 nM) (Muzzin et al., 1994; Klaus et al., 1995). Therefore, [3H]SB 206,606 is also a poor choice for the labelling of β3-adrenoceptors in tissues. These technical limitations must be considered when interpreting existing radioligand-binding data in the bladder and other tissues.
Radioligand-binding studies on bladder β-adrenoceptors have been reported for rats, rabbits, pigs and humans. Saturation-binding studies with various radioligands have reported 6–42 fmol mg−1 protein in rats (Nishimoto et al., 1995; Ma et al., 2002), 60–92 fmol mg−1 protein in rabbits (Levin et al., 1988; Latifpour et al., 1990; Morita et al., 1998), 30–154 fmol mg−1 protein in pigs (Goepel et al., 1997; Yamanishi et al., 2002b, 2002c) and 22–60 fmol mg−1 protein in humans (Levin et al., 1988; Goepel et al., 1997; Morita et al., 2000; Li et al., 2003). Limited attempts have been made to identify the receptor subtypes in the bladder by radioligand binding. Based upon competition studies with the β2-selective antagonist ICI 118,551 and a β1-selective antagonist, sites in the rabbit (Latifpour et al., 1990) and human bladder (Goepel et al., 1997) were reported to largely belong to the β2-subtype. On the other hand, three studies in the porcine bladder detected few, if any, high-affinity sites for ICI 118,551, and the β1-selective antagonist CGP 20,712A recognized largely low-affinity sites in those studies (Goepel et al., 1997; Yamanishi et al., 2002b, 2002c). Two of the studies additionally report about 60% high-affinity sites for SR 59,230A (Yamanishi et al., 2002b, 2002c); the latter authors interpreted these findings as evidence in favour of the presence of a population of largely β3-adrenoceptors. However, three reasons argue against this interpretation: Firstly, ICI 118,551 may not be β2-selective in pigs (Goepel et al., 1996), which make the low affinity of this compound in the porcine bladder difficult to interpret. Secondly, while SR 59,230A can be used to functionally block β3-adrenoceptors, it is not selective for this subtype and, at least in humans, has even slightly lower affinity for β3- than for β1- and β2-adrenoceptors (Hoffmann et al., 2004). Thirdly, the radioligands used in all of the above studies are unlikely to label a major fraction of possibly present β3-adrenoceptors due to their low affinity for this subtype (at least in humans; see above). Therefore, we consider the presently available pig data to be inconclusive. This does not exclude the presence of β3-adrenoceptors at the protein level in any of these species, but the currently available radioligand-binding techniques are probably inadequate to detect their presence. Hence, the reported densities of β-adrenoceptors in the bladder may represent an underestimation if the additional presence of β3-adrenoceptors is taken into account.
In vitro function
Since activation of adenylyl cyclase is the prototypical signalling pathway of β-adrenoceptors, it is not surprising that an isoprenaline-stimulated, propranolol-sensitive elevation of cAMP content has also been reported in rat bladder (Derweesh et al., 2000; Ma et al., 2002; Uchida et al., 2005). However, various recent studies have questioned whether this can sufficiently explain β-adrenoceptor-mediated smooth muscle relaxation (Horinouchi et al., 2003; Peters & Michel, 2003; Tanaka et al., 2003). One study in rat bladder demonstrated that the concentration–response relationships for isoprenaline, clenbuterol and FR 165,101 for relaxation and cAMP elevations were largely superimposable in noncontracted muscle; however, no such relationship was observed during KCl-induced contraction (Uchida et al., 2005). Accordingly, the adenylyl cyclase inhibitor SQ 22,536, in a concentration where it fully suppressed cAMP formation, inhibited rat bladder relaxation by all three agonists in the absence of pre-contraction, but not in its presence (Uchida et al., 2005). Similarly, SQ 22,536 and the protein kinase A inhibitors H7, H89 and Rp-cAMPs, if anything, inhibited isoprenaline-induced relaxation of rat bladder only against passive tension, but not against KCl-induced tension in another study (Frazier et al., 2005a). These data demonstrate that, at least in rats, elevation of cAMP is relevant for the regulation of bladder smooth muscle tone against passive tension, but not in the presence of a depolarizing stimulus such as KCl. Interestingly, a combination of adenylyl and guanylyl cyclase inhibitors (SQ 22,536 and ODQ) caused the strongest inhibition of relaxation against passive tension, but was also inactive against KCl-induced tension (Frazier et al., 2005a).
A possible modulation of membrane potential, ion-channel activity and intracellular ion concentrations has been studied as an alternative means of β-adrenoceptor control of bladder function. In guinea-pig bladder smooth muscle bundles exhibiting spontaneous action potentials, isoprenaline was found to hyperpolarize the cells, prevent action potentials and reduce the associated Ca2+ transients; the elevation of membrane potential was blocked by protein kinase A inhibitors and by high extracellular K+ concentrations, but not by K+ channel inhibitors (Nakahira et al., 2001). In other studies, both isoprenaline and the receptor-independent adenylyl cyclase activator forskolin were shown to increase iberiotoxin-sensitive K+ currents in guinea-pig bladder smooth muscle cells, and such stimulation was sensitive to a peptidergic inhibitor of protein kinase A (Kobayashi et al., 2000). In a later study, these investigators also demonstrated propranolol-sensitive isoprenaline inhibition of Ba2+ current through L-type Ca2+ channels due to a shift of steady-state for inactivation by 11 mV; this effect was apparently mediated by protein kinase A, but did not involve protein kinase G (Kobayashi et al., 2003). Other investigators reported that isoprenaline caused marginal increases in Ca2+ currents after large conditioning depolarizations (but not in their absence) in the guinea-pig bladder, and that this effect was not mimicked by forskolin (Smith et al., 1999). On the other hand, a third group found that isoprenaline causes intracellular Ca2+ sparks and activates voltage-dependent Ca2+ channels in guinea-pig bladder, and proposed that this may underlie the activation of large-conductance, iberiotoxin-sensitive K+ channel (Petkov & Nelson, 2005). Differences in the electrophysiological procedures used by the two groups may have contributed to this apparent controversy. Activation of iberiotoxin-sensitive K+ channels can relax the urinary bladder (Malysz et al., 2004). Several studies have assessed the functional relevance of ion channel modulation by β-adrenoceptor stimulation. Studies using KCl-precontracted bladder strips from guinea-pigs (Kobayashi et al., 2000) or rats (Frazier et al., 2005a; Uchida et al., 2005) have consistently found that K+ channel blockers such as iberiotoxin or charybdotoxin inhibit isoprenaline-induced bladder relaxation. Interestingly, the latter two studies also report that relaxation against passive tension is not sensitive to those toxins.
Prostaglandins may play a role in bladder contraction by several agents such as protease-activated receptors or bradykinin (Nakahara et al., 2003; 2004; Chopra et al., 2005). Therefore, it is surprising that prostaglandins were also postulated to play a permissive role for β-adrenoceptor-mediated relaxation of the urinary bladder (Bolle et al., 1999).
The key function of β-adrenoceptors in the bladder is smooth muscle relaxation and an increase in bladder compliance during the filling phase of the micturition cycle. The interpretation of in vitro bladder relaxation experiments has to take into account that the results are sensitive to the experimental conditions. Thus, it has been found that the β-adrenoceptor agonist isoprenaline was approximately six times more potent when tested against passive tension than when tested against KCl-induced bladder tone in rats (Frazier et al., 2005a; Uchida et al., 2005). This is consistent with indirect comparisons in the published literature, where a pEC50 for isoprenaline of 8.3 (Yamazaki et al., 1998) vs 7.2 (Longhurst & Levendusky, 1999) and of 9.1 (Yamazaki et al., 1998) vs 7.3 (Oshita et al., 1997) were reported in rats and rabbits, respectively, for passive tension vs pre-contraction. In a comparison between KCl-induced and carbachol-induced tension in rat isolated detrusor, isoprenaline was significantly less potent and effective against the latter (Longhurst & Levendusky, 1999). Moreover, the choice of passive tension vs pre-contraction for relaxation experiments may also affect the underlying signal transduction of the β-adrenoceptor response (Frazier et al., 2005a; Uchida et al., 2005). A second methodological consideration relates to the use of muscarinic receptor agonists to induce bladder pre-contraction in combination with β-adrenoceptor agonists such as BRL 37,344 to induce relaxation. This drug has affinity for muscarinic acetylcholine receptors in the same concentration range where it acts as a β-adrenoceptor agonist (Kubota et al., 2002); hence, data using this combination may at least partly reflect direct muscarinic receptor antagonism rather than β-adrenoceptor agonism (see below).
A relaxation of bladder smooth muscle by β-adrenoceptor agonists has been demonstrated against passive tension (Igawa et al., 2001; Takeda et al., 2002a), endothelin receptor-mediated (Takeda et al., 2003), muscarinic receptor-mediated (Seguchi et al., 1998; Nomiya & Yamaguchi, 2003) and KCl-induced pre-contraction (Nishimoto et al., 1995; Yamanishi et al., 2003a) or against field stimulation-induced tone (Nishimoto et al., 1995; Hudman et al., 2001). Moreover, relaxation responses have been demonstrated in the detrusor of various species, including rats (Kolta et al., 1984; Nishimoto et al., 1995; Oshita et al., 1997; Seguchi et al., 1998; Yamazaki et al., 1998; Fujimura et al., 1999; Longhurst & Levendusky, 1999; Lluel et al., 2000; Morita et al., 2000; Woods et al., 2001; Matsubara et al., 2002; Inci et al., 2003; Malysz et al., 2004; Uchida et al., 2005; Frazier et al., 2005a), mouse (Matsui et al., 2003), rabbits (Oshita et al., 1997; Morita et al., 1998; 2000; Yamazaki et al., 1998; Bing et al., 2003), guinea-pigs (Li et al., 1992; Gopalakrishnan et al., 1999; Kobayashi et al., 2000; Malysz et al., 2004), ferrets (Takeda et al., 2000a), cats (Nergardh et al., 1977), dogs (Yamazaki et al., 1998), pigs (Yamanishi et al., 2002b, 2002c; 2003a), monkeys (Takeda et al., 2002a) and humans (Nergardh et al., 1977; Fujimura et al., 1999; Igawa et al., 1999; 2001; Takeda et al., 1999; Morita et al., 2000; Nomiya & Yamaguchi, 2003). In contrast, β-adrenoceptor stimulation did not consistently relax the basal tone of the human bladder neck (Caine et al., 1975).
Some studies have performed direct inter-species comparisons regarding the ability of β-adrenoceptor agonists to induce bladder relaxation. Such comparisons of, for example, rat vs dog (Takeda et al., 2003), rat vs rabbit (Oshita et al., 1997) or rat vs rabbit vs dog (Yamazaki et al., 1998) have consistently reported that the maximum effects of an agonist without subtype selectivity, such as isoprenaline, were similar in various species. However, within the same study, the rank order of isoprenaline potency consistently was rabbit>rat>dog, suggesting that rabbits may have the largest and dogs the smallest receptor reserve for this response, respectively. Similar inter-species comparisons with subtype-selective β-adrenoceptor agonists are more difficult to interpret, since the subtype being involved may differ between species.
Functional studies into the β-adrenoceptor subtypes mediating bladder relaxation have been hampered by several problems. Firstly, some drugs proposed to be β3-adrenoceptor-selective agonists may have effects independent of β-adrenoceptors. For example, it was reported that both BRL 37,344 and SR 58,611 can cause vasodilatation, which is insensitive to β-adrenoceptor antagonists (Brahmadevara et al., 2003). Moreover, BRL 37,344 was reported to be a direct muscarinic receptor antagonist (Kubota et al., 2002) and α1-adrenoceptor antagonist (Leblais et al., 2005). Secondly, no truly β3-adrenoceptor-selective antagonist has been described. Thus, SR 59,230, the most frequently used drug to antagonize β3-adrenoceptors, does not discriminate human β-adrenoceptor subtypes (Hoffmann et al., 2004) and, similarly to the chemically related bupranolol, may also be an α1-adrenoceptor antagonist (Leblais et al., 2005). When binding to β3-adrenoceptors, SR 59,230 may exhibit agonist rather than antagonist properties in some tissues (Horinouchi & Koike, 2001). Such limitations should be taken into account when interpreting the functional data presented below.
Studies in various species have used agonist and antagonist potency to identify the functional involvement of β-adrenoceptor subtypes in bladder relaxation. Since absolute agonist potency may differ between species even for nonsubtype-selective agonists (see above), the former approach has used either rank orders of potency of various agonists or the potency of highly subtype-selective agonists to classify the receptor subtype being involved. Most studies have been reported from rats. Based upon a high potency of β3-selective agonists such as CL 316,243 (Woods et al., 2001) and FK175 (Fujimura et al., 1999), it has been proposed that rat bladder relaxation predominantly occurs via this subtype. However, studies assessing the rank order of potency of multiple subtype-selective agonists have proposed a mixed involvement of β2- and β3-adrenoceptors in rat bladder relaxation in most cases. These were based upon rank orders such as isoprenaline=procaterol (β2-selective)>CL 316,243>dobutamine (β1-selective) (Takeda et al., 2003), CL 316,243⩾isoprenaline⩾procaterol (Takeda et al., 2000b), isoprenaline⩾CL 316,243⩾procaterol>dobutamine (Yamazaki et al., 1998), BRL 37,344⩾isoprenaline (Oshita et al., 1997), isoprenaline=GS-332 (β3-selective)⩾clenbuterol (β2-selective) (Morita et al., 2000) or isoprenaline>FR 165101 (β3-selective)⩾clenbuterol≫dobutamine (Uchida et al., 2005). One study, based upon a rank order of agonist potency of isoprenaline>BRL 37,344⩾T-0509 (β1-selective)>terbutaline (β2-selective)⩾SR 58,611 (β3-selective), has even proposed a mixed involvement of β1-, β2- and β3-adrenoceptors in rat bladder relaxation (Longhurst & Levendusky, 1999). Antagonist studies have reported that ICI 118,551 inhibits the effects of clenbuterol against low-, but not high-frequency field stimulation (Hudman et al., 2000). Relaxant effects of the β3-agonist FK175 were moderately inhibited by the nonselective bupranolol, but not by even high concentrations of the β1-selective CGP 20,712 or the β2-selective ICI 118,551 (Fujimura et al., 1999). Similarly, relaxation induced by BRL 37,344 was not inhibited by low propranolol concentrations, but by CGP 12,177 or SR 59,230 when added atop of propranolol; in the same study, relaxation by CGP 12,177 was not affected even by high propranolol concentrations (Longhurst & Levendusky, 1999). These data indicate that β2- and β3-selective agonists may indeed cause rat bladder relaxation via their cognate receptor subtypes. With regard to nonsubtype-selective agonists such as isoprenaline or noradrenaline, several studies report relatively poor antagonism by propranolol, metoprolol, butoxamine or ICI 118,551 (Oshita et al., 1997; Seguchi et al., 1998; Longhurst & Levendusky, 1999). However, SR 59,230, which should inhibit the cloned β3-adrenoceptor, also caused only poor isoprenaline antagonism (Longhurst & Levendusky, 1999). Taken together, these data argue against a strong involvement of β1- and β2-adrenoceptors, but also fail to provide clear evidence for a β3-adrenoceptor. Interestingly, the isoprenaline-induced cAMP response in rat bladder was fully sensitive to propranolol (Ma et al., 2002), which is in line with the proposal that β-adrenoceptor-mediated bladder relaxation occurs largely cAMP-independent (Frazier et al., 2005a; Uchida et al., 2005).
In vitro relaxation studies in rabbit bladder have reported agonist rank orders of potency of isoprenaline⩾adrenaline>noradrenaline⩾BRL 37,344 (Oshita et al., 1997), procaterol>isoprenaline>adrenaline⩾CGP 12,177>noradrenaline⩾dobutamine>CL 316,243 (Yamazaki et al., 1998) or clenbuterol≫GS-332 (Morita et al., 2000). Propranolol, bupranolol and ICI 118,551 antagonized the isoprenaline-induced relaxation with high potency, whereas CGP 20,712, in concentrations up to 100 nM, had no effect (Oshita et al., 1997; Yamazaki et al., 1998). Taken together, these data demonstrate that relaxation of the rabbit detrusor is predominantly mediated by a β2-adrenoceptor.
In the porcine detrusor, there was a rank order of potency of salbutamol (β2-agonist)>noradrenaline>BRL 37,344>CGP 12,177 (the latter two being partial agonists only); while the BRL 37,344 response was antagonized by SR 59,230, the corresponding Schild slope was significantly smaller than unity (Yamanishi et al., 2002a). The same investigators also reported a low potency of BRL 37,344 sensitive to SR 59,233 in the porcine bladder base (Yamanishi et al., 2002c). More recently, these authors also reported porcine bladder base relaxation by isoprenaline and salbutamol (Yamanishi et al., 2003a). CGP 20,712 did not inhibit the isoprenaline responses, whereas propranolol and ICI 118,551 caused inhibition, but with a Schild slope of less than unity; in contrast, ICI 118,551 inhibited the salbutamol responses with high potency and a Schild slope close to unity. Another group of investigators found an order of potency of isoprenaline=adrenaline⩾procaterol⩾BRL 37,344>CGP 12,177⩾salbutamol>CL 316,243⩾noradrenaline; in this regard, BRL 37,344, CL 316,243 and, surprisingly, noradrenaline were reported to be partial agonists and CGP 12,177 was found to be a weak partial agonist (Badawi et al., 2005). Taken together, these findings suggest that both β2-adrenoceptors and an additional subtype, possibly β3-adrenoceptors, mediate porcine bladder relaxation.
Data from several other animal species are too limited or controversial to allow definitive conclusions. In guinea-pigs, a predominant role of β1-adrenoceptors was proposed based upon relaxation by dobutamine, but not by BRL 37,344, salbutamol or clenbuterol, and antagonism of the isoprenaline, noradrenaline and adrenaline responses by atenolol (Yamamoto et al., 1998). Another study also proposed an involvement of β1-adrenoceptors based upon partial antagonism of the isoprenaline response by metoprolol, but reported an even greater role of β2-adrenoceptors based upon partial agonism by salbutamol and terbutaline and antagonism of the isoprenaline response by ICI 118,551 (Li et al., 1992). A more recent study based upon whole bladder contraction reported relaxation by noradrenaline and BRL 37,344, but not by formoterol (β2-selective) (Gillespie, 2004). Limited data from one study in cats have suggested a predominant involvement of β1-adrenoceptors (Nergardh et al., 1977). One study in ferrets has proposed a primary involvement of β3-adrenoceptors based upon an agonist rank order of potency of BRL 37,344>CGP 12,177⩾isoprenaline⩾CL 316,243>dobutamine⩾procaterol and upon antagonism of the isoprenaline response by SR 58,894, but not by CGP 20,712 or ICI 118,551 (Takeda et al., 2000a). One study in dogs reported an agonist rank order of potency of CL 316,243>isoprenaline⩾CGP 12,177>noradrenaline⩾dobutamine⩾procaterol⩾adrenaline, and that the isoprenaline-induced relaxation was inhibited with high potency by bupranolol, but not by CGP 20,712 or ICI 118,551 (Yamazaki et al., 1998); the same group later confirmed the rank order of CL 316,243>dobutamine≈procaterol (Takeda et al., 2003), suggesting predominantly an involvement of β3-adrenoceptors. A study in Cynomolgus monkeys found an agonist rank order of potency of isoprenaline>noradrenaline⩾CGP 12,177>BRL 37,344⩾adrenaline>dobutamine⩾salbutamol⩾procaterol, with the β1-selective xamoterol being a very weak partial agonist; the effects of isoprenaline were inhibited by bupranolol, but not by CGP 20,712 or ICI 118,551 (Takeda et al., 2002a), suggesting a predominant involvement of a β3-adrenoceptor.
Early reports on human bladder relaxation already proposed that this does not occur via a β1- or β2-adrenoceptor (Nergardh et al., 1977). Several more recent studies suggest that it indeed occurs via a β3-adrenoceptor. Igawa et al. (1998) originally reported relaxation of the human bladder (inhibited by bupranolol), whereas dobutamine, procaterol and CGP 12,177 caused much smaller if any relaxation. Thereafter, they reported an agonist order of potency of BRL 37,344⩾isoprenaline⩾noradrenaline⩾adrenaline⩾CGP 12,177⩾CL 316,243; in that study, isoprenaline responses were inhibited by SR 58,894, but only poorly by ICI 118,551 and not at all by CGP 20,712 (Figure 2) (Igawa et al., 1999). Another study from the same group reported an order of BRL 37,344⩾isoprenaline>CGP 12,177⩾CL 316,243, with all but isoprenaline being partial agonists (Igawa et al., 2001). Another study reported a rank order of potency of BRL 37,344>CGP 12,177>isoprenaline, with the former two being partial agonists only, and the β3-adrenoceptor agonist ZD 7114 being a very poor partial agonist; the isoprenaline responses were inhibited by SR 59,230, but not by butoxamine and atenolol (Takeda et al., 1999). In another study, isoprenaline and the β3-adrenoceptor selective agonist L 755,507, but not dobutamine or clenbuterol, relaxed carbachol-contracted human bladder strips (Nomiya & Yamaguchi, 2003). A very recent study reported a rank order of potency of isoprenaline>procaterol=CL 316,243=salbutamol, with the latter three compounds being considerably less effective than isoprenaline (Badawi et al., 2005). Finally, GS 332 was found to be more potent in the human bladder than clenbuterol in another study (Morita et al., 2000). In agreement with the predominant expression of β3-adrenoceptor mRNA in the human bladder (see above), these data demonstrate that this subtype is also most important for bladder relaxation in vitro. With the possible exception of ferrets and monkeys, the role of this subtype in other animal species is less prominent.
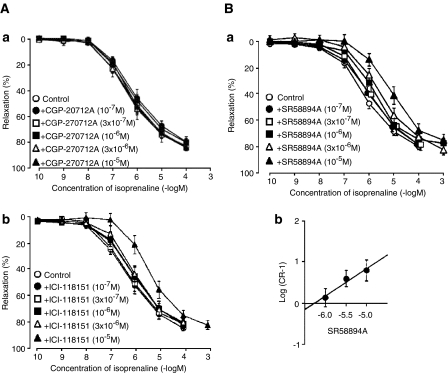
Figure 2.
Inhibition of isoprenaline-induced relaxation of human bladder detrusor by the β1-antagonist CGP 20,712, the β2-antagonist ICI 118,551 and the nonselective antagonist SR 58,894. Taken with permission from Igawa et al. (1999).
In vivo function
Functional in vivo effects on bladder function can be assessed in several ways. Noninvasive studies frequently look at micturition frequency, which is a key symptom of OAB (see Abrams et al., 2002). Invasive studies are based upon the insertion of a catheter coupled to a pressure transducer into the bladder and subsequent filling of the bladder endogenously or by installation of fluid. This allows various types of measurements, including the frequency of bladder contractions, maximum detrusor pressure, filling volume at first contraction or bladder compliance, all of which are typically also assessed in urodynamic studies in humans (see Abrams et al., 2002). Moreover, it should be considered that the effects of systemically administered drugs on bladder function are not necessarily mediated by drug targets located in the bladder (see the above section on bladder α1-adrenoceptors). Finally, the use of anaesthetized vs conscious animals may differentially affect the endogenous sympathetic tone.
Studies in rats (Lecci et al., 1998; Takeda et al., 2000b; 2003; Kaidoh et al., 2002; Tucci et al., 2002), ferrets (Takeda et al., 2000a) and monkeys (Takeda et al., 2002a) demonstrate that β-adrenoceptor agonists such as isoprenaline can reduce intra-vesical pressure, indicating that this is a consistent feature in biology. On the other hand, propranolol had little, if any, effects on bladder function on its own (Durant et al., 1988), indicating that either there is little endogenous β-adrenergic tone under the chosen experimental conditions and/or that the receptor mediating the bladder effects is propranolol-insensitive, that is, distinct from β1- and β2-adrenoceptors. Consistent with the latter possibility, neither i.v. terbutaline nor oral propranolol affected intra-vesical pressure in healthy women (Thind et al., 1993b), but both drugs caused small increases in bladder volume in another study in healthy women (Norlen et al., 1978).
A comparison of intra-peritoneal vs intra-thecal administration of isoprenaline in conscious, chronically instrumented rats with measurement of the contraction frequency of the urinary bladder demonstrated a peripheral site of action (Durant et al., 1988). This is consistent with the finding that β-adrenoceptor agonists reduce bladder pressure both in conscious (Kaidoh et al., 2002) and anaesthetized rats (Lecci et al., 1998; Takeda et al., 2000b; 2003; Tucci et al., 2002).
In a model of distension-induced bladder activity under isovolumetric conditions in urethane-anaesthetized rats, the reduction of intra-vesical pressure by i.v. isoprenaline decreased with increasing intra-vesical volumes (Lecci et al., 1998), possibly reflecting a physiological increase in endogenous β-adrenergic tone with increased bladder filling. In pentobarbital-anaesthetized rats, isoprenaline-induced reduction of bladder tone was attenuated by the cyclooxygenase inhibitor indomethacin, the Ca2+ flux blocker ruthenium red and the neurokinin A receptor antagonist MEN-10376, whereas the phosphodiesterase inhibitor papaverine did not affect them (Tucci et al., 2002). These data were interpreted to suggest that β-adrenoceptor-mediated bladder relaxations in vivo involve prostaglandins, neurokinin A and capsaicin-sensitive nerve fibres. Moreover, the lack of effect of papaverine is consistent with a cAMP-independent relaxation that has been demonstrated in vitro (Frazier et al., 2005a; Uchida et al., 2005).
In urethane-anaesthetized rats, isoprenaline, the β2-agonist procaterol and the β3-agonist CL 316,243 dose-dependently lowered intra-vesical pressure; CL 316,243 also increased bladder capacity and micturition intervals and reduced micturition pressure, whereas procaterol only increased bladder capacity and residual volume (Takeda et al., 2000b). Neither drug altered the total micturition volume, but their combination had somewhat greater effects on micturition interval, bladder capacity and residual volume than either drug alone. In conscious, unrestrained rats, i.v. procaterol did not affect voiding pressure relative to vehicle, and had little effect on bladder capacity, whereas CL 316,243 had no effect on bladder capacity but reduced voiding pressure (Kaidoh et al., 2002). Both procaterol and CL 316,243 reduced intra-vesical pressure in another study in urethane-anaesthetized rats; the procaterol effect was inhibited by ICI 118,551 and the CL 316,243 effect by the β3-adrenoceptor antagonist L 748,337, whereas neither antagonist affected the response to the other agonist (Takeda et al., 2003). In pentobarbital-anaesthetized ferrets, isoprenaline and CL 316,243 dose-dependently reduced bladder pressure, whereas dobutamine and procaterol had little effect (Takeda et al., 2000a). Taken together, these data suggest that both β2- and β3-adrenoceptors contribute to bladder relaxation in rats in vivo, whereas only β3-adrenoceptors are involved in ferrets. Both conclusions are consistent with the available in vitro data (see above).
In this context, it is interesting to note that the β3-adrenoceptor agonists (in contrast to nonsubtype-selective or β2-selective agonists) consistently had only small, if any, cardiovascular effects in the above studies (Takeda et al., 2000b; 2003; Kaidoh et al., 2002), indicating a possible safety advantage. On the other hand, two β3-adrenoceptor agonists, ZD 7114 and ZD 2079, were reported to induce cystitis and renal tubular necrosis upon chronic dosing in male and female rats (Waghe et al., 1999), but it remains unclear whether this is a specific effect of these two compounds or related to their mechanism of action; moreover, it is unclear whether this is limited to rats or can be extrapolated to other species.
Regulation of receptor expression and function
A possible gender effect on β-adrenoceptor-mediated regulation of bladder function has been studied in rabbits and rats. Radioligand-binding studies with [3H]dihydroalprenolol as the radioligand have found a significantly greater receptor number in young female as compared to young male rabbits in the bladder base, but no such differences were seen in the bladder base of older rabbits or in the bladder dome of either age group (Latifpour et al., 1990). Another study with the same radioligand confirmed a greater receptor number in the trigonal part (but not the detrusor) of young adult female as compared to male rabbits (Morita et al., 1998). Within the same study, this was confirmed functionally by a greater isoprenaline-induced relaxation in female than in male trigonal, but not detrusor muscle. Similarly, a study on ovariectomized Wistar rats reported a reduced potency for BRL 37,344 in relaxing bladder strips as compared to control- or oestrogen-treated ovariectomized rats; similar differences for isoprenaline did not reach statistical significance (Matsubara et al., 2002). On the other hand, relaxant responses for the weak partial agonist CGP 12,177 were reduced in female relative to male Wistar rats, but no such alterations was seen for the agonists BRL 37,344, isoprenaline and noradrenaline (Frazier et al., 2005b).
A regulation of β-adrenoceptor responsiveness with age has been demonstrated in several species. A binding study with [3H]dihydroalprenolol reported that the number of β-adrenoceptors increased in rabbit bladder dome and base with age (Latifpour et al., 1990). In contrast, a study using [125I]iodopindolol in 1- vs 3- vs 22-month-old male Fischer 344 rats reported an age-related decrease in receptor density (Nishimoto et al., 1995). A similar decrease in [3H]dihydroalprenolol-binding sites was also reported for human bladder (Li et al., 2003). Consistent with these findings, an age-related reduction of isoprenaline-stimulated cAMP formation has been found in a comparison of bladders from 3-, 6- and 24-month-old male Fischer 344 rats (Derweesh et al., 2000). The latter was accompanied by an increase in the expression of α-subunits of Gs, Go and Gi proteins, with the latter two increasing more than Gs and hence shifting the overall balance towards inhibition rather than stimulation of adenylyl cyclase. In line with these biochemical findings, it was reported from a comparison of 1- vs 3- vs 22-month-old male Fischer 344 rats that bladder relaxation by noradrenaline or isoprenaline against KCl-induced tone was attenuated, involving a reduction in agonist potency and maximum effects; isoprenaline effects against field stimulation-induced tone were similarly reduced (Nishimoto et al., 1995). Within that study, relaxation responses to forskolin, but not those to dibutyryl-cAMP, were also reduced with age, indicating that an alteration prior to cAMP formation rather than in cAMP responsiveness is involved. While these biochemical and functional studies in male Fischer 344 rats are consistent with a reduced β-adrenoceptor function with age, not all studies have confirmed that. Thus, one study in 7- vs 17- vs 29-month-old male Fischer 344 rats detected no alteration in the potency or efficacy of isoprenaline to relax isolated bladder strips (Kolta et al., 1984). Similarly, a study in 3- vs 23-month-old male Wistar rats reported similar concentration-dependent bladder strip relaxation by isoprenaline, noradrenaline, BRL 37,344 and CGP 12,177 in both age groups (Frazier et al., 2005b). A study in 10- vs 30-month-old female Wistar/Rij rats also found similar isoprenaline-induced bladder strip relaxation in both age groups (Lluel et al., 2000). Finally, a study comparing newborn, 1- and 4-month-old Sprague–Dawley rats also did not detect alterations of β-adrenoceptor-mediated bladder relaxation (Tugay et al., 2003), a finding probably more related to development than to ageing. In this context, it should be noted that studies on age-related differences of muscarinic receptor responsiveness in the bladder have found major strain differences, with Wistar rats most closely resembling the situation in humans (Schneider et al., 2005). A single and limited study in humans has reported that bladder relaxation responses to isoprenaline and BRL 37,344 and also receptor-independently to forskolin and dibutyryl-cAMP are lower in a group of subjects in their mid-60s than in those in their late 20s (Li et al., 2003), indicating that the observed difference may at least partly relate to an overall reduced ability to relax rather than a specific β-adrenoceptor desensitization.
Several studies have investigated the effects of β-adrenoceptor agonists in animal models of bladder dysfunction. Some of them compared such effects with those in healthy animals to test the possible alterations by disease, whereas other studies looked at the pathological condition only to determine whether β-adrenoceptor agonists might be effective therapeutics in such settings. Spontaneously hypertensive rats are a genetic animal model, which exhibits several features of OAB, including increased urinary frequency and reduced bladder capacity. A comparison of male spontaneously hypertensive with normotensive Wistar Kyoto rats detected a reduced bladder relaxation in response to noradrenaline and isoprenaline, but not to the partial agonist BRL 37,344 (Frazier et al., 2005b). OAB-like symptoms can also occur secondarily to bladder outlet obstruction. CL 316,243 dose-dependently inhibited spontaneous bladder contraction in obstructed rats, but a direct comparison with healthy rats (who have much less if any such spontaneous contractions) was not reported (Woods et al., 2001). When bladder hyper-reflexia was induced by intra-vesical installation of acetic acid, CL 316,243 also concentration-dependently reduced bladder contractions; comparison to the obstruction data from the same study indicates that the hyper-reflexic model may be more sensitive to this agonist (Woods et al., 2001). Bladder hyperactivity can also be induced by intra-vesical installation of prostaglandin E2. In this model, CL 316,243 dose-dependently increased micturition interval and micturition volume and decreased basal pressure, whereas threshold pressure and micturition pressure were not affected; on the other hand, procaterol reduced threshold pressure, but did not significantly affect the other parameters (Takeda et al., 2002b). Bladder hyper-reflexia can also be induced by cerebral infarction, which impairs some of the central nervous control of the bladder. While CL 316,243 had little effect on bladder capacity in control animals, it dose-dependently restored the reduced bladder capacity in cerebral infarction rats, and a similar restoration of bladder capacity was seen with procaterol; neither drug normalized voiding pressures within the tested dose range (Kaidoh et al., 2002).
Few studies have looked into alterations of β-adrenoceptor responsiveness in the bladder of patients. A limiting factor of all these studies is the problem of obtaining tissue from matched healthy controls. Apparently tumour-free tissue from cancer patients is most frequently used as control; while this appears the only feasible option, it remains unclear how representative such tissue is for healthy subjects. One study compared the relaxation of isolated bladder strips without pre-contraction by the β-adrenoceptor agonists isoprenaline, BRL 37,344, CL 316,243 and CGP 12,177 in patients with low bladder compliance, hyperreflexic bladders and controls; agonist potency was similar in all three groups for each agonist, and maximum effects were also similar across groups for the agonists, except for an increased effect of CGP 12,177 in low-compliance bladders (Igawa et al., 2001). Another study has reported on the relaxation of field stimulation-contracted bladder strips from patients with urodynamically confirmed urge incontinence with those from continent patients without a history of incontinence; clenbuterol caused only weak relaxation in control subjects at both 1 and 40 Hz stimulation, but significantly greater relaxation in strips from incontinent patients (Hudman et al., 2001). A comparison of bladder from males with and without bladder outlet obstruction detected statistically significant differences in the abundance of β1-, β2- or β3-adrenoceptor mRNA between groups; similarly, potency and maximum effects of isoprenaline and the β3-selective L 755,507 were similar in both groups (Nomiya & Yamaguchi, 2003). Taken together, the limited available animal and human data do not provide conclusive evidence for an alteration of β-adrenoceptor function in states of bladder dysfunction.
Clinical implications
The available data demonstrate that β-adrenoceptor agonists relax urinary bladder from various species including humans. In humans, this occurs largely, if not exclusively, via a β3-adrenoceptor. Several animal studies suggest that selective β3-agonists will have much fewer, if any, cardiovascular side effects as compared to agonists acting on other β-adrenoceptor subtypes (Takeda et al., 2000b; 2003; Kaidoh et al., 2002). Moreover, β-adrenoceptor agonists improved symptoms in various rat models of bladder dysfunction, and small pilot studies reported beneficial effects with terbutaline (Lindholm & Lose, 1986) and clenbuterol (Gruneberger, 1984). Against this background, several pharmaceutical companies are developing β3-adrenoceptor agonists for the treatment of OAB. Some of them have recently reported positive proof-of-concept studies with their selective agonists in OAB patients; while none of the underlying studies have published in the peer-reviewed literature, such findings would seem to indicate that bladder β3-adrenoceptors are a potentially important drug target.
Urethra
The urethra functions not only as a passive conduit for the urine being passed from the bladder but also actively contributes to bladder outlet resistance and hence the maintenance of continence during the filling/storage phase of the micturition cycle. In contrast to the other tissues covered in this manuscript, the urethra contains both smooth muscle, frequently referred to as the ‘internal urethral sphincter', and striated muscle, frequently referred to as the ‘external urethral sphincter'. Since sympathetic fibres primarily innervate the smooth muscle, much of the data reviewed below refer to the smooth muscle portion of the urethra only. However, it should be noted that striated urethral muscle also expresses adrenoceptors and that centrally located adrenoceptors may indirectly affect striated muscle function in the urethra by modulating the activity of the somatic pelvic nerves (see Michel et al., 2005c). Moreover, studies on the urethra have often been used as a substitute for the prostate in the evaluation of α1-adrenoceptor antagonist for the treatment of LUT symptoms suggestive of BPH.
α1-Adrenoceptors
mRNA and protein expression
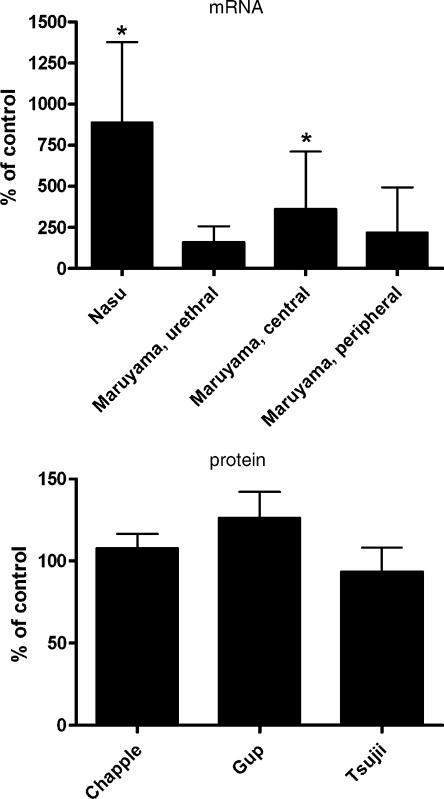
The presence of α1-adrenoceptors in the urethra has been assessed at the mRNA and protein level. Studies in rats have detected a rank order of abundance of α1A>α1B⩾α1D using real-time PCR (Yono et al., 2004). In the human proximal urethra, RNase protection assays detected the α1A-adrenoceptor as the most abundant subtype in male and female samples, whereas α1D-adrenoceptor mRNA was seen only in female samples and α1B-adrenoceptor mRNA in neither; this resulted in an α1A : α1B : α1D ratio of 100 : 0 : 0 in males and 90 : 0 : 10 in females (Nasu et al., 1998). In situ hybridization studies confirmed these findings and localized the α1A-signal to the urethral smooth muscle (Nasu et al., 1998). In situ hybridization studies in the penile urethra of the rhesus monkey have found no α1A-adrenoceptor signal in the urothelium or surrounding connective tissue, but in the smooth muscle and longitudinal striated muscle layers (Walden et al., 1997), with the functional significance of the latter remaining unclear.
Receptor autoradiography studies under conditions preferentially detecting α1A-adrenoceptors confirmed the presence of this subtype at the protein level in smooth and longitudinal muscle layers of the monkey urethra (Figure 1) (Walden et al., 1997). Radioligand-binding studies in tissue homogenates confirmed the presence of α1-adrenoceptors in the rabbit urethra; these studies demonstrated an abundance similar to that in bladder base and exceeding that in the detrusor (Andersson et al., 1984; Larsson et al., 1986; Latifpour et al., 1990; Testa et al., 1993). Within the rabbit urethra, proximal and distal parts were reported to express similar α1-adrenoceptor densities (Larsson et al., 1986). A study comparing urethral α1-adrenoceptors in multiple species in radioligand-binding experiments found a rank order of abundance of rat>human>dog>rabbit; based upon a lack of their inactivation by chloroethylclonidine, it was proposed that the α1-adrenoceptors in these species belong largely, if not exclusively, to the α1A-subtype (Testa et al., 1993). Thus, the urethra of various species including humans contains α1-adrenoceptors at the mRNA and protein levels, which appear to largely belong to the α1A-subtype.
In vitro and in vivo functions
Various studies have assessed the role of α1-adrenoceptors in the contractile tone of the urethra. Most of these were done in rabbits, but limited data have also been presented for rats, dogs and humans. In contrast to the bladder and prostate (see above and below), the adrenoceptor-mediated contraction of the rabbit urethra involves both α1- and α2-adrenoceptors (Ueda et al., 1984; Larsson et al., 1986). Field stimulation studies indicate that the proximal urethra appears largely under the control of the parasympathetic nervous system, whereas the medial and distal urethra are to a larger extent controlled by the sympathetic nervous system (Deplanne et al., 1998). The overall contribution of an α-adrenoceptor mechanism relative to muscarinic mechanism to nerve stimulation-induced urethral contraction appears limited (van der Werf & Creed, 2002). Nevertheless, it was found that the potency of an α1-adrenoceptor agonist did not differ between rabbit proximal and distal urethra (Larsson et al., 1986). A study involving multiple subtype-selective antagonists found that the contraction of the rabbit urethra is mediated by the α1A-adrenoceptor (Testa et al., 1993). This was confirmed by a high affinity of the moderately α1A-selective tamsulosin in other studies on rabbit urethra (Honda & Nakagawa, 1986). However, multiple studies have demonstrated that the α1A-adrenoceptor mediating the contraction of rabbit urethra has rather low affinity for prazosin (Lefevre-Borg et al., 1993; Testa et al., 1993; Deplanne & Galzin, 1996; van der Graaf et al., 1997), demonstrating that it exhibits the α1L-phenotype of the α1A-adrenoceptor (see also sections on bladder and prostate). Mechanistically, it was reported that α1A-adrenoceptor-mediated contraction of the rabbit urethra is moderately sensitive to inhibition by the Ca2+-entry blocker nifedipine (Testa et al., 1993). Apparently, α1-adrenoceptor-mediated urethral contraction has also been demonstrated in rats (Lluel et al., 2003a), dogs (Testa et al., 1993) and humans (Kunisawa et al., 1985). However, the latter was reported to differ from the situation in rabbits in two important ways: Firstly, contraction of the human urethra appeared to involve only α1- and no α2-adrenoceptors. Secondly, the α1-adrenoceptor-mediating contraction of the human urethra exhibited high affinity for prazosin, indicating that it does not represent the α1L-phenotype repeatedly found in rabbits.
Two studies in anaesthetized cats found that hypogastric nerve-induced urethral contraction is α1-adrenoceptor-mediated (Lefevre-Borg et al., 1993; Springer et al., 1994). The α1-adrenoceptor agonist midodrine also increased urethral pressure in anaesthetized rats (Takeda et al., 2003). Studies with several subtype-selective α1-adrenoceptor and 5-HT receptor antagonists demonstrate that α1A/D-adrenoceptors and 5-HT1A receptors are involved in urethral contraction via the micturition reflex; the anatomical location of these receptors has not been fully clarified (Conley et al., 2001). Studies in healthy women found that prazosin (1 mg kg−1) reduced the intra-urethral static pressure, but did not affect responses to coughing or squeezing (Thind et al., 1992; 1993a). Numerous other in vivo studies on the effects of α1-adrenoceptor agonist and antagonists on intra-urethral pressure (IUP) have been performed in animals, but the observed effects can largely be attributed to effects on prostatic rather than urethral α1-adrenoceptors (Akiyama et al., 1999), and hence will be discussed in the prostate section of this manuscript (see below).
Regulation of receptor expression and function
Few studies have investigated a physiological or pathophysiological regulation of urethral α1-adrenoceptors. They were reported not to be affected by gender at the mRNA level in humans (Nasu et al., 1998) or at the protein level in rabbits (Latifpour et al., 1990). In contrast, α1-adrenoceptor-mediated urethral contraction was reported to be markedly reduced in 24- vs 6-month-old rats (Lluel et al., 2003a). One study reported that chronic treatment with a high dose of the antagonist doxazosin upregulates α1A-adrenoceptor mRNA in rat urethra; however, this finding remains difficult to interpret, since several other dosing regimens within the same study did not reveal such upregulation (Yono et al., 2004).
Clinical implications
Based upon the contribution of the urethra to bladder outlet resistance and the role of α1-adrenoceptors in controlling urethral tone, α1-adrenoceptors are a potential target in the treatment of stress incontinence. Several α1-adrenoceptor agonists, particularly those with selectivity for the α1A-subtype, have been developed pre-clinically for this indication, including NS-49 (Obika et al., 1995), A-61603 (Knepper et al., 1995) and Ro 115–1240 (Blue et al., 2004). The latter compound has been successfully tested in a clinical proof-of-concept study in stress incontinence patients (Musselman et al., 2004), but its clinical development has been discontinued due to undisclosed reasons. Whether the beneficial effects of the combined serotonin/noradrenaline uptake inhibitor duloxetine in stress incontinence apart from its spinal cord-mediated enhancement of the somatic pudendal nerve activity also involve a direct effect on the urethral smooth muscle remains to be determined (see Michel et al., 2005c).
α2-Adrenoceptors
mRNA and protein expression
To the best of our knowledge, the presence of α2-adrenoceptor subtype mRNA in the urethra has not been reported. At the protein level, urethral α2-adrenoceptors have repeatedly been identified in radioligand-binding studies in rabbits (Andersson et al., 1984; Larsson et al., 1986; Latifpour et al., 1990). One of these studies found that the density of α2-adrenoceptors increases from the proximal to the distal urethra (Larsson et al., 1986). Based upon prazosin affinity, the urethral α2-adrenoceptors appear to belong largely, if not exclusively, to the α2A-subtype (Latifpour et al., 1990), which is consistent with those in bladder and prostate (see above and below). The density of rabbit urethral α2-adrenoceptors appears not to be regulated by gender or age (Latifpour et al., 1990).
In vitro and in vivo function
Functionally, in contrast to the studies in the bladder and prostate (see above and below), α2-adrenoceptors can elicit urethral contraction, which is quantitatively at least as strong as that mediated by α1-adrenoceptors, in rabbits (Andersson et al., 1984; Ueda et al., 1984; Larsson et al., 1986) and horses (Garcia-Sacristan et al., 1984). In line with the finding that α2-adrenoceptor expression increases in the distal urethra, an enhanced α2-adrenoceptor-mediated contraction was found in this part of the rabbit urethra (Larsson et al., 1986). In line with the in vitro data in rabbits, in vivo studies in dogs reported that i.v. clonidine can increase IUP via an α2-adrenoceptor, and that this effect accounted for about 50% of that of adrenaline; whether the difference between the two is due to an α1-adrenoceptor component in the adrenaline response or due to the partial agonism of clonidine remains to be determined (Shapiro et al., 1987). In contrast to rabbits and dogs, however, no α2-adrenoceptor-mediated contraction of the human urethra was found in vitro (Kunisawa et al., 1985). Therefore, little consideration has been given to the possibility of urethral α2-adrenoceptors being a potential therapeutic target in urological disease.
β-Adrenoceptors
mRNA and protein expression
To the best of our knowledge, the presence of β-adrenoceptor subtype mRNA in the urethra has not been reported. We are aware of only three studies that have quantified urethral β-adrenoceptors at the protein level using radioligand binding. One study reported a similar receptor density in the urethra of young and old male and female rabbits (Latifpour et al., 1990). Within the study, the β-adrenoceptor density in the urethra was somewhat lower than that in the bladder base and much lower than in the detrusor. In all four groups, the β2-selective antagonist ICI 118,551 had a much higher affinity in competition-binding studies than the β1-selective antagonist ICI 89,406, indicating that the urethral β-adrenoceptors in the rabbit urethra belong largely, if not exclusively, to the β2-subtype. A study in female pigs demonstrated binding sites with a low affinity for the β1-selective CGP 20,712A, whereas the β2-selective ICI 118,551 yielded biphasic fits with approximately 30% high-affinity sites; SR 59,230A, an antagonist that is often used to block β3-adrenoceptors but is not selective for this subtype and actually has slightly higher affinity for β1- and β2-adrenoceptors (Hoffmann et al., 2004), yielded monophasic competition curves (Yamanishi et al., 2002c). These data are difficult to interpret (see the above discussion of data on bladder base from same paper). The low affinity of CGP 20,712A appears to exclude the presence of β1-adrenoceptors. While it would be logical to assume that the low-affinity component of the ICI 118,551 competition curves represents β3-adrenoceptors, the study has used [3H]dihydroalprenolol as the radioligand, which is unlikely to detect β3-adrenoceptors (Hoffmann et al., 2004). Finally, one study has used [125I]iodocyanopindolol to detect β-adrenoceptors in the human external urethral sphincter, but no detailed subtype characterization was performed (Morita et al., 2000). Therefore, the identity of the β-adrenoceptor subtype in the urethra remains unclear.
In vitro function
Functional in vitro studies on urethral β-adrenoceptors have been performed in rat, dog, pig and horse. In rats and dogs, the maximum relaxant effects of several β-adrenoceptor agonists were only about half of those seen in bladder detrusor or trigone within the same study; the maximum relaxant effects were greater in the proximal than in the distal canine urethra (Takeda et al., 2003). Attempts to identify the β-adrenoceptor subtype mediating urethral relaxation have been based upon rank orders of potency of subtype-selective agonists and on their inhibition by subtype-selective antagonists. In the rat urethra, the β2-selective agonist procaterol was more potent than the β3-selective CL 316,243, and the β1-selective dobutamine was least potent (Takeda et al., 2003). Limited data in the equine urethra indicate involvement of a β2-adrenoceptor (Garcia-Sacristan et al., 1984). In the canine urethra, the agonist rank order of potency was procaterol>dobutamine=CL 316,243, which clearly differed from that observed in the detrusor within the same study, where CL 316,243 had been the most potent agonist (Takeda et al., 2003). In the porcine urethra, the β3-selective BRL 37,344 was more potent than the nonselective isoprenaline, with the β2-selective salbutamol being least potent (Yamanishi et al., 2003b). BRL 37,344 relaxed the urethra about 300 times more potently than the bladder base (Yamanishi et al., 2002c). The nonselective antagonist propranolol inhibited isoprenaline-induced relaxation with high potency (pA2 8.55), but had a Schild slope of less than unity (Yamanishi et al., 2003b). While even high concentrations of the β1-selective CGP 20,712A had no effect against isoprenaline, the β2-selective ICI 118,551 inhibited the effects of both salbutamol and isoprenaline with high potency and a Schild slope close to unity (Yamanishi et al., 2003b). SR 59,230A inhibited the effects of BRL 37,344 (Yamanishi et al., 2002c) and isoprenaline (Yamanishi et al., 2003b), with apparent pA2 values of 7.72 and 7.38, respectively, but in either case the Schild slope was less than unity, which makes interpretation of the data difficult. Taken together, these data demonstrate that the functional importance of β-adrenoceptors for smooth muscle tone in the urethra, particularly in its distal parts, is less than in the bladder. This is consistent with the lower β-adrenoceptor expression level in the urethra as compared to the bladder (Latifpour et al., 1990). This is also in line with studies demonstrating that the nerve stimulation-induced relaxation of the urethra is largely mediated by NO, whereas β-adrenergic mechanisms account for a minor component only (van der Werf & Creed, 2002). Relaxation of the rat, dog and pig urethra appears to involve a strong β2-adrenoceptor component; an additional involvement of a β3-adrenoceptor appears possible, particularly in the porcine urethra, whereas β1-adrenoceptors do not appear important in either species. Since the urethra also contains striated muscle (external urethral sphincter), it appears interesting to note that β-adrenoceptor stimulation has been reported to cause contraction of the striated urethral muscle (possibly via β2-adrenoceptors), which may contribute to the overall effects on urethral resistance (Morita et al., 2000).
In vivo function
A possible in vivo role of urethral β-adrenoceptors has been investigated in rats, cats and humans. In anaesthetized rats the β2-agonist procaterol reduced urethral pressure, while the β3-agonist CL 316,243 had less, if any, effects; the pressure reductions by procaterol were inhibited by the β2-antagonist ICI 118,551 (Takeda et al., 2003). Hypogastric nerve stimulation in cats caused propranolol-sensitive urethral relaxation, which was further enhanced in the presence of the noradrenaline uptake inhibitors nisoxetine and tomoxetine (Springer et al., 1994). In healthy women, systemic administration of the β2-adrenoceptor agonist terbutaline reduced resting urethral pressure, whereas the antagonist propranolol had no effect (Thind et al., 1993a, 1993b).
Clinical implications
In conclusion, the stimulation of β-adrenoceptors by exogenous agonists can induce urethral relaxation, an effect predominantly involving the β2-subtype. Endogenous catecholamines do not appear to have major effects on urethral β-adrenoceptors. Since even the effects of exogenous agonists on the urethra are small relative to those on the bladder, it appears unlikely that the use of β-adrenoceptor agonists, particularly those selective for the β3-subtype, for the treatment of OAB will have no major adverse effects on urethral tone.
Prostate
In contrast to bladder and urethra, which are largely muscular tissues, the prostate contains both stromal, that is, smooth muscle, and epithelial, that is, glandular, elements, which serve different functions. The ratio of smooth muscle and glandular elements differs markedly between species, and few species mimic the strong stromal component in the human prostate (Lepor et al., 1994). The histological state of human BPH is associated with a relative increase in the smooth muscle component of the prostate (Bartsch et al., 1979; Shapiro et al., 1992). The enlarged prostate is believed to contribute to increased bladder outlet resistance in two ways. Firstly, the enlargement itself can narrow the urethral lumen, and this is referred to as the static component of bladder outlet obstruction. Secondly, the contraction of bladder smooth muscle can additionally narrow the urethral lumen, and this is referred to as the dynamic component of bladder outlet obstruction. The high prevalence of BPH in elderly men has sparked a large number of studies into prostatic adrenoceptors, particularly α1-adrenoceptors.
α1-Adrenoceptors
mRNA expression
Prostatic α1-adrenoceptors have received considerable attention in the last decade due to the clinical success of α1-adrenoceptor antagonists in the treatment of symptoms of BPH (see Roehrborn & Schwinn, 2004). The presence of α1-adrenoceptor subtype mRNA has been assessed in the prostate of various species, including rats, rabbits, monkeys and humans. An early study on rat prostate based upon a competitive RT–PCR technique described the presence of α1A-, α1B- and α1D-adrenoceptor mRNA in a 99 : 1 : 0% ratio (Scofield et al., 1995). Other studies in rat prostate also using RT–PCR (Rokosh et al., 1994; Homma et al., 2000) or real-time PCR (Foster et al., 2004) did not confirm this strong dominance and rather described a roughly similar abundance of all three subtypes. On the other hand, an in situ hybridization study on rat prostate detected only the α1A-adrenoceptor, which was primarily localized in the prostatic smooth muscle cells (Walden et al., 1997). The same study reported similar findings for the monkey prostate (Walden et al., 1997). A study in rabbits has looked only at the α1D-subtype mRNA, and found this to be highly abundant in the prostate, that is, at a relative density only surpassed by the vas deferens and thoracic aorta in a panel of 16 tissues (Suzuki et al., 1997).
Owing to the obvious therapeutic implications, various studies have reported on α1-adrenoceptor subtype mRNA in the human prostate. While early studies based upon RT–PCR have detected only α1A-adrenoceptor mRNA in the human prostate (Hirasawa et al., 1993), later studies using Northern blots, RT–PCR or RNase protection assays have typically also detected α1D-adrenoceptor mRNA (Price et al., 1993; Faure et al., 1994; Weinberg et al., 1994; Tseng-Crank et al., 1995; Moriyama et al., 1996; Nasu et al., 1996). The overall data indicate that the approximate relative ratio of α1A-, α1B- and α1D-adrenoceptor mRNA in the human prostate is 70 : 0 : 30%. In line with this relative dominance of the α1A-adrenoceptor at the mRNA level, an in situ hybridization study has detected only this subtype (Walden et al., 1997). The α1-adrenoceptor subtype expression appears similar in the peri-urethral, base, apex and lateral lobe regions of the human prostate (Faure et al., 1994) or in its urethral, central and peripheral regions (Moriyama et al., 1998). At the cellular level, α1A-adrenoceptor mRNA expression was found both in stromal (smooth muscle) and epithelial (glandular) cells (Tseng-Crank et al., 1995). Messenger RNA for α1B- and α1D-adrenoceptors was found in primary cultures of human prostatic smooth muscle cells in some (Boesch et al., 1999), but not other, studies (Tseng-Crank et al., 1995). Of note, at least four splice variants exist of the α1A-adrenoceptor, and the human prostate expresses all of them, with variant 4 exhibiting the greatest abundance (Chang et al., 1998). The epithelial prostate cancer cell lines PC3 and Du145 have been reported to express mRNA for all three α1-adrenoceptor subtypes (Tseng-Crank et al., 1995). Taken together, at least in rats and humans considerable amounts of data demonstrate that the α1A-subtype is the most abundant α1-adrenoceptor at the mRNA level.
Protein expression
The presence of α1-adrenoceptors at the protein level has been investigated in radioligand-binding and receptor autoradiography studies in a variety of species, including rats, rabbits, pigs, dogs, monkeys and humans. While indirect comparisons between species are difficult, some studies have concomitantly investigated multiple species. Despite the major histological differences between species with regard to stromal content, it was reported that the density of α1-adrenoceptor-binding sites differed only modestly among them, with a rank order of abundance of human⩾rat⩾dog⩾rabbit (Testa et al., 1993). Studies in human tissues have investigated the intra-prostatic distribution of α1-adrenoceptors at the macroscopic and microscopic levels. One study reported that the α1-adrenoceptor density was greater in the adenoma as compared to the submucosal tissue of the prostatic urethra in enucleated hyperplastic prostate tissue (Kawabe et al., 1990). On the other hand, similar receptor densities were found in the central and peripheral zones of BPH tissue (Chapple et al., 1989). Another study divided the prostate into eight different regions and detected similar α1-adrenoceptor densities and similar phenylephrine-induced contractions in all of them (Lepor et al., 1993a). At the microscopic levels, receptor autoradiography studies have localized rat and human prostatic α1-adrenoceptors predominantly to the stroma rather than the glandular tissue, and within the stroma largely to smooth muscle rather than fibroblasts or connective tissue (Chapple et al., 1989; Killam et al., 1995). This was confirmed at higher resolution using fluorescent approaches (MacKenzie et al., 2000).
The relative presence of α1-adrenoceptor subtypes has been investigated in the prostate of various species. Early studies have used the ability of chloroethylclonidine to subtype-selectively alkylate α1-adrenoceptors so as to identify subtypes in the prostate. However, this approach has limited validity because chloroethylclonidine can potentially alkylate all subtypes of α1- and even α2-adrenoceptors if incubation concentration, time and temperatures are high, long and warm enough, respectively (Michel et al., 1993). One study using an incubation of prostatic membranes with 10 μM chloroethylclonidine for 30 min at 37°C has reported that >80% of receptors in the rat and human, >90% of all receptors in the rabbit and almost all receptors in the canine prostate are chloroethylclonidine-insensitive, and proposed them to belong to the α1A-subtype (Testa et al., 1993). On the other hand, a study using less stringent incubation conditions (10 μM chloroethylclonidine for 30 min at room temperature) reported only 77, 44 and 56% chloroethylclonidine-insensitive α1-adrenoceptor-binding sites in rat, canine and human prostate, respectively (Lepor et al., 1994). Expectedly, studies using a tritiated form of moderately α1A-selective tamsulosin as the radioligand have detected only chloroethylclonidine (10 μM for 10 min at 37°C) resistant sites in the rat prostate (Yazawa & Honda, 1993).
Later studies have rather used competition-binding experiments with subtype-selective drugs to characterize α1-adrenoceptors in the prostate. One study in rats detected low affinity of the α1D-selective BMY 7378 in rat prostate, indicating that this subtype is largely absent in rat prostate at the protein level; the same study detected monophasic competition curves of high affinity for the somewhat α1A-selective 5-methylurapidil, suggesting that this is the most abundant subtype at the protein level in rat prostate (Deng et al., 1996). Supporting this conclusion, studies with [3H]tamsulosin as the radioligand also found high affinity of the α1A-selective WB 4101 and 5-methylurapidil (Yazawa & Honda, 1993). Studies in rabbit prostate also reported at least 50% of α1-adrenoceptor-binding sites to have high affinity for WB 4101 and 5-methylurapidil (Hiraoka et al., 1995). One study in dog prostate detected mainly low-affinity sites for WB 4101 (Ohmura et al., 1993), but the poor selectivity of this drug makes the data difficult to interpret. Based upon competition by a single concentration of the highly α1A-selective antagonist SNAP 5272, autoradiographic studies reported that this subtype accounts for at least 80% of α1-adrenoceptor protein in the monkey prostate (Figure 1) (Walden et al., 1997). In an early study of the human prostate, the potency of a panel of six drugs (prazosin, phentolamine, 5-methylurapidil, urapidil, spiperone, WB 4101) to compete for [3H]prazosin binding correlated best with that for binding to rabbit liver (α1A-adrenoceptors), somewhat less with that for binding to chloroethylclonidine-treated rat hippocampus (α1D-adrenoceptors) and worst with that for binding to rat liver (α1B-adrenoceptors), suggesting that the receptors in the human prostate largely belong to the α1A-subtype (Testa et al., 1993). A similar approach was used by other investigators, who substituted the prototypical animal tissues with cell lines transfected with the cloned human receptors; in such experiments, the affinity of a panel of 12 drugs, some of which, such as the stereoisomers of niguldipine, have much greater subtype selectivity than those used in earlier studies, correlated very well with that at α1A-adrenoceptors, but not with that at either other subtype (Tseng-Crank et al., 1995). A study from our own laboratory has used narrowly spaced concentration increments of 5-methylurapidil, phentolamine, tamsulosin and WB 4101 in competition-binding studies with human prostate membranes (Michel et al., 1996). This has allowed detecting biphasic competition curves for all four agents despite their limited selectivity for α1A-adrenoceptors, and the percentage of high-affinity sites for these agents ranged between 61 and 79%, confirming that the α1A-adrenoceptor is the most abundant subtype in the human prostate (Figure 3). In conclusion, it appears that the α1A-subtype is the most abundant α1-adrenoceptor subtype in the rat and human prostate at the protein level, and limited data suggest a similar situation in rabbits and monkeys. The remaining receptors appear to belong largely to the α1B-subtype, with α1D-adrenoceptors apparently being absent at the protein level. While the large abundance of α1A-adrenoceptor protein is in agreement with the mRNA data, no such agreement between mRNA and protein data exists for α1B- and α1D-adrenoceptors. The reasons for the poor detection of α1D-adrenoceptor protein despite the presence of corresponding mRNA remain to be elucidated, but similar discrepancies for mRNA and protein expression of this subtype have been found in many other tissues of several species (Yang et al., 1997; 1998b).
Figure 3.
Competition of subtype-selective antagonists for [3H]prazosin binding to human prostate membranes. Data are means of 4–5 experiments (error bars deleted for clarity). Modified with permission from Michel et al. (1996).
In vitro function
Studies in guinea-pig prostate have demonstrated that α1-adrenoceptor stimulation enhances inositol phosphate formation, apparently via an α1A-like subtype (Haynes & Hill, 1997). Experiments with rat prostatic neuroendocrine cells demonstrated α1-adrenoceptor agonist-induced Ca2+ elevations, which were abolished by the phospholipase C inhibitor U 73,122 (Kim et al., 2003). Since α1-adrenoceptor stimulation can promote cellular growth in several cell types, it has been studied whether this is also the case in cultured prostatic smooth muscle cells. However, several studies with human tissue failed to detect growth modulation in these cells by α1-adrenoceptor agonists or antagonists (Boesch et al., 1999; Michel et al., 2000b). Whether this reflects of lack of such responses in prostatic stromal cells or rather a loss of α1-adrenoceptor expression under cell culture conditions (Ohmi et al., 1999) has remained unclear. The latter possibility is supported by some reports demonstrating modulation of prostatic cell growth upon treatment with α1-adrenoceptor agonists and antagonists in vivo (see below).
Most functional in vitro studies have focused on prostate contraction. This has been assessed by videoimaging of human prostatic stromal cell contractions in the presence of α1-adrenoceptor agonists and antagonists in some cases (Corvin et al., 1998), but the vast majority of studies has used classical organ bath approaches with prostatic strips from various species. α-Adrenoceptor-mediated contraction of the prostate of rabbits (Honda et al., 1985), dogs (Felsen et al., 1994; Delaflotte et al., 1996) and humans (Hieble et al., 1985; Lepor et al., 1988b; Chapple et al., 1989; Yu et al., 1994) appears to occur largely, if not exclusively, via α1- rather than α2-adrenoceptors; such findings have been obtained by comparing the effects of α1-selective agonists such as phenylephrine with those of α2-selective agonists such as UK 14,304, or by comparing the antagonism of noradrenaline by α1-selective antagonists such as prazosin with that of α2-selective antagonists such as rauwolscine. The predominant, if not exclusive, mediation of prostate contraction via α1- rather than α2-adrenoceptors is further supported by a potent antagonism of field stimulation-induced contraction by prazosin (Tsujii et al., 1992; Guh et al., 1995). At least within the rat (Steidle et al., 1989) and human prostate (Lepor et al., 1994), all regions appear to have similar contractile responsiveness to α1-adrenoceptor stimulation.
The identification of α1- relative to α2-adrenoceptors is classically based upon a high affinity of the antagonist prazosin for the former (see Bylund et al., 1994). However, it has repeatedly been reported that prazosin has lower potency than expected (but, nevertheless, higher than that for α2-adrenoceptors) for some α1-adrenoceptor responses. Based upon such observations, it has been proposed to classify α1-adrenoceptor responses with relatively low prazosin affinity as α1L (Muramatsu et al., 1990; Ohmura et al., 1992); in this scheme, all three cloned α1-adrenoceptor subtypes have high affinity for prazosin and are designated as α1H. Despite extensive searches and completion of the sequencing of the human genome, a corresponding gene for the proposed α1L-adrenoceptor has not been identified. Rather, it has been reported that transfection of cells that lack functional α1-adrenoceptors with the cDNA encoding the α1A-adrenoceptor can induce the presence of a receptor with relatively low prazosin affinity under some experimental conditions; on the other hand, prazosin low-affinity sites did not become detectable in cells transfected with α1B- or α1D-adrenoceptors (Ford et al., 1997; Daniels et al., 1999). Therefore, it is now generally assumed that the relatively low prazosin affinity observed in some settings represents a phenotype of the cloned α1A-adrenoceptor rather than a distinct receptor subtype. Nevertheless, the α1L phenomenon is important for the pharmacology of the prostate, since it affects not only prazosin but also other antagonists with a quinazoline structure, such as alfuzosin, doxazosin and terazosin, which are routinely used in BPH treatment; in contrast, antagonists from other chemical classes such as tamsulosin do not discriminate the classical α1A pharmacology and its α1L phenotype (Ford et al., 1997; Daniels et al., 1999). With regard to α1A/L-adrenoceptors in the prostate, most studies have been performed in rabbits and humans. Binding affinities of both prazosin and tamsulosin in human prostate membranes were consistent with their reported affinities at cloned α1A-adrenoceptors (Figure 4), and studies in isolated human prostatic cells using fluorescent ligands have reported even higher affinities, possibly reflecting technical differences between mixed tissue and isolated cell approaches (MacKenzie et al., 2000). On the other hand, the reported functional potencies of prazosin in the rabbit prostate were about 1.5 log units lower than its affinity in binding studies with cloned α1A-adrenoceptors or in human prostate membranes (Figure 4). These data demonstrate that the α1-adrenoceptor mediating contraction of the rabbit prostate has the characteristics of an α1L-adrenoceptor. Similarly low prazosin potencies were found in functional studies with the canine prostate (range pA2 7.90–8.55) (Ohmura et al., 1993; Buckner et al., 1996; Leonardi et al., 1997) or rat prostate (pKB 8.13–8.78) (Killam et al., 1995; Homma et al., 2000). On the other hand, tamsulosin has similar affinity for the classical α1A-adrenoceptor and its α1L phenotype in rabbit prostate (Figure 4), and this was confirmed in radioligand-binding studies with [3H]prazosin in bovine prostate, where tamsulosin had a similarly high affinity at prazosin high- and low-affinity sites (pKi 9.13 and 8.99, respectively) (Maruyama et al., 1998). Other radioligand-binding studies suggested that the canine prostate expresses a much larger abundance of low- than high-affinity binding sites for [3H]prazosin (Ohmura et al., 1993). Studies in human prostate have yielded a less clear picture. Thus, the reported functional potencies of prazosin were only slightly lower than to be expected based upon the affinity estimates from the radioligand-binding studies, and higher than the functional estimates in the rabbit prostate (Figure 4). Interestingly, functional affinity estimates (pA2 or pKB values) for other quinazolines in rabbit prostate (alfuzosin: median 7.22, range 7.19–7.25 (two studies), doxazosin: 7.01 (one study), terazosin: median 7.71, range 7.46–7.96 (two studies)) were also much lower than their pKi values in binding studies with human prostate (alfuzosin: median 8.18, range 7.46–8.29 (four studies), doxazosin: median 8.40, range 8.20–8.60 (two studies), terazosin: median 8.52, range 7.96–8.98 (five studies)) or cloned α1A-adrenoceptors (alfuzosin: median 8.20, range 7.99–8.42 (five studies), doxazosin: median 8.56, range 8.34–8.89 (five studies), terazosin: median 8.35, range 7.58–8.53 (seven studies)), whereas much smaller discrepancies were observed for human prostate (alfuzosin: not reported; doxazosin: median 8.32, range 8.20–8.43 (two studies), terazosin: median 7.60, range 7.38–8.48 (three studies)) (Hieble et al., 1985; Chapple et al., 1989; Morita & Kondo, 1992a; Yamada et al., 1992; Lefevre-Borg et al., 1993; Testa et al., 1993; 1996; Faure et al., 1994; Forray et al., 1994; Goetz et al., 1994; Teng et al., 1994; Yu et al., 1994; Hancock et al., 1995; Marshall et al., 1995; Tseng-Crank et al., 1995; Chueh et al., 1996; Ford et al., 1996; Hatano et al., 1996; Kenny et al., 1996; Michel et al., 1996; Leonardi et al., 1997; Martin et al., 1997; Noble et al., 1997; Chang et al., 2000). Therefore, the overall evidence suggests that the α1-adrenoceptors mediating contraction of the rabbit and canine prostate have relatively low affinity for prazosin and other quinazolines; in contrast, the receptor mediating contraction of the human prostate has only moderately lower affinity for prazosin and other quinazolines than would be expected based upon their affinity in binding studies, and hence cannot be unequivocally classified as α1L.
Figure 4.
Potency and affinity of prazosin and tamsulosin at cloned α1A-adrenoceptors and those in human and rabbit prostate. Data are from individual studies (filled circles) and their means (horizontal lines), and given as pKi (binding studies) or pKB/pA2 (functional studies), as taken from the following references: Hieble et al. (1985); Chapple et al. (1989); Morita & Kondo (1992a); Yamada et al. (1992); Lefevre-Borg et al. (1993); Testa et al. (1993; 1996); Faure et al. (1994); Forray et al. (1994); Goetz et al. (1994); Teng et al. (1994); Yu et al. (1994); Hancock et al. (1995); Marshall et al. (1995); Tseng-Crank et al. (1995); Chueh et al. (1996); Ford et al. (1996); Hatano et al. (1996); Kenny et al. (1996); Michel et al. (1996); Leonardi et al. (1997); Martin et al. (1997); Noble et al. (1997); Chang et al. (2000).
Numerous studies have investigated which of the cloned α1-adrenoceptor subtypes mediates prostate contraction in vitro. Similar to the radioligand-binding studies in this tissue, the early studies have relied largely on the ability of the subtype-selectively alkylating chloroethylclonidine to identify receptor subtypes. Later studies have used panels of drugs with largely moderate subtype selectivity and correlated their potencies in the prostate with their affinities at the cloned receptor subtypes. Highly subtype-selective drugs (mostly selective for α1A-adrenoceptors) were only used in the most recent studies and have enabled an unequivocal identification of the receptor subtype being involved.
A study comparing α1-adrenoceptor-mediated prostate contraction in rats with those in dogs and humans reported only negligible contraction in rats despite a greater density of receptors than in dogs, and hence did not allow for identification of receptor subtypes (Lepor et al., 1994). However, most other studies reported more substantial contractions of the rat prostate. Based upon a lack of effect of chloroethylclonidine (10 μM for 10 or 30 min at 37°C), it was proposed that rat prostate contraction in response to phenylephrine is mediated by an α1A-adrenoceptor (Yazawa & Honda, 1993). Since 30 nM of the α1D-selective BMY 7378 did not shift the concentration–response curve for rat prostate, it was proposed that this subtype may not be involved (Deng et al., 1996). More definitive conclusions could be based upon highly α1A-selective antagonists such as L-771,688 (also known as SNAP 6383) (Chang et al., 2000) or SNAP 7915 (Lagu et al., 2000), which had a subnanomolar KB value in rat prostate. Moreover, the α1A-selective agonist A-61603 was an effective contractile agent in rat prostate (Chang et al., 2000; Lagu et al., 2000). Therefore, it appears that contraction of rat prostate is mediated predominantly, if not exclusively, by an α1A-adrenoceptor. Based upon limited data, that is, insensitivity towards chloroethylclonidine and high affinity for 5-methylurapidil, a similar situation has been proposed in guinea-pig prostate (Haynes & Hill, 1997).
As stated above, contraction of the rabbit prostate occurs via an α1-adrenoceptor with relatively low prazosin affinity (Honda et al., 1985; Hiraoka et al., 1995; Delaflotte et al., 1996; Leonardi et al., 1997; Martin et al., 1997). Since studies with cloned receptors have detected such low-affinity states for prazosin only with the α1A- but not with the α1B- or α1D-adrenoceptor (Ford et al., 1997), these data already provided indirect evidence for a predominant involvement of the α1A-adrenoceptor. Several studies with the moderately α1A-selective antagonist tamsulosin support this conclusion (Honda et al., 1985; Honda & Nakagawa, 1986; Yamagishi et al., 1996; Martin et al., 1997; Taguchi et al., 1997). One study reported only low affinity for the moderately α1A-selective antagonists WB 4101 and 5-methylurapidil, but suggested that this might be due to their poor recognition of the α1L-phenotype (Hiraoka et al., 1995). However, somewhat greater potencies for both antagonists (but not for prazosin) were reported in another study, which also found a low potency of BMY 7378 (Delaflotte et al., 1996). Based upon correlation of potencies of a panel of five drugs with those at cloned subtypes, this study proposed involvement of an α1A-adrenoceptor. Based upon a similar approach with a panel of six drugs, the same conclusion was reached (Martin et al., 1997). Another report from the same laboratory, but with largely distinct authors, reported identical values for all six drugs (van der Graaf et al., 1997). More definitive conclusions can be based upon the high potency of highly α1A-selective antagonists such as silodosin (formerly known as KMD 3213) (Yamagishi et al., 1996) or B8805–033 (Eltze et al., 2001), which also had high potency. Taken together, these data demonstrate that contraction of the rabbit prostate occurs via an α1A-adrenoceptor with low affinity for prazosin.
As stated above, contraction of the canine prostate, similar to that of the rabbit, occurs via an α1-adrenoceptor with relatively low prazosin potency (Ohmura et al., 1993; Buckner et al., 1996; Leonardi et al., 1997). With regard to the cloned subtypes being involved, initial studies in the canine prostate reported that chloroethylclonidine (10 μM for 30 min at 37°C) reduced phenylephrine-induced contraction by 53% (Lepor et al., 1994). On the other hand, several moderately subtype-selective antagonists had Schild slopes around unity in the canine prostate, indicating the involvement of a single subtype only (Hancock et al., 1995). In later studies from the same investigators, the potency of a panel of 11 antagonists correlated better with that at the cloned α1A- than the other α1-adrenoceptor subtypes; moreover, in such studies the α1A-selective A-61603 was a full agonist for canine prostate contraction (Buckner et al., 1996). Other investigators reached a similar conclusion based upon a panel of 14 antagonists (Leonardi et al., 1997). Moreover, highly α1A-selective antagonists such as SNAP 7915 (Lagu et al., 2000) and L-771,688 (Chang et al., 2000) also had high potency in the canine prostate. Therefore, contraction of the canine prostate also involves predominantly, if not exclusively, an α1A-adrenoceptor.
Early studies into the α1-adrenoceptor subtype mediating contraction of the human prostate were primarily based upon chloroethylclonidine (10–100 μM for 30 min at 37°C) and have reported 30–80% inhibition of agonist- or field stimulation-induced contraction (Lepor et al., 1993b; 1994; Teng et al., 1994; Guh et al., 1995; Marshall et al., 1995). While these experiments did not allow definitive conclusions, later experimental approaches have consistently supported the suggestion that the contraction of the human prostate occurs predominantly, if not exclusively, via an α1A-adrenoceptor. This was based upon a high potency of moderately α1A-selective drugs such as WB 4101, 5-methylurapidil, SB 216,469 and tamsulosin (Marshall et al., 1995; Chess-Williams et al., 1996; Chueh et al., 1996; Noble et al., 1997), and upon the correlation of the potency of panels of antagonists with those at cloned receptor subtypes (Forray et al., 1994; Marshall et al., 1995; 1996; Ford et al., 1996; Kenny et al., 1996; Testa et al., 1996). Most recently, highly α1A-selective antagonists such as L 771,688 (Chang et al., 2000), SNAP 7915 (Lagu et al., 2000) or B 8805–033 (Eltze et al., 2001) have allowed definitive conclusions. They are in line with potent and effective agonism by α1A-selective drugs such as A 61603 (Chang et al., 2000; Lagu et al., 2000). Taken together, the overall evidence demonstrates that contraction of prostate of rats, guinea-pigs, rabbits, dogs and humans in response to exogenous agonists occurs largely, if not exclusively, via the α1A-subtype. Some studies in isolated human prostate have used field stimulation to release endogenous agonist (Yu et al., 1994; Guh et al., 1995; Chueh et al., 1996). Those studies unequivocally reported high potency for α1A-selective antagonists and hence demonstrate that human prostate contraction not only in response to exogenous but also to endogenous release agonist occurs via this subtype.
In contrast to the range of subtyping reports, only few studies have addressed the possible underlying mechanisms. Thus, contractions induced by exogenous noradrenaline (Teng et al., 1994) and by endogenous agonist released by field stimulation (Guh et al., 1995; 1996; Haynes & Hill, 1997) were sensitive to Ca2+ entry blockers such as nifedipine. One study in rats found that the protein kinase C inhibitors calphostin C and bisindolylmaleimide I did not affect noradrenaline-induced contraction (Ramasamy et al., 2002).
In vivo function
In vivo studies on prostate function have typically measured alterations of IUP. In the interpretation of such data, it has to be taken into account that this response is a composite measure of the contractile force developed by the urethra and the surrounding prostate (see also the section on urethra). However, the contribution of the prostate appears to dominate because phenylephrine-induced IUP elevations were about 80% smaller in prostate-ablated male, castrated male or female as compared to prostate-intact rats (Akiyama et al., 1999). The systemic administration of α1-adrenoceptor agonists such as noradrenaline, adrenaline or phenylephrine has been shown to increase IUP in anaesthetized rats (Guilmard et al., 1996; Martin et al., 1997; Akiyama et al., 1999), anaesthetized cats (Lefevre-Borg et al., 1993) and anaesthetized dogs (Breslin et al., 1993; Kenny et al., 1994; 1996; Testa et al., 1997; Witte et al., 1997; 2002; Pulito et al., 2000; Eltze et al., 2001) and conscious dogs (Brune et al., 2002). Similar IUP elevations have also been produced by the systemic administration of α1A-selective agonists such as A 61603 (Knepper et al., 1995). Agonist-induced IUP elevations were inhibited by various α1-adrenoceptor antagonists, including the clinically used alfuzosin (Lefevre-Borg et al., 1993; Kenny et al., 1994; Guilmard et al., 1996; Martin et al., 1997; Testa et al., 1997; Akiyama et al., 1999), doxazosin (Kenny et al., 1994; 1996; Martin et al., 1997; Witte et al., 2002), tamsulosin (Breslin et al., 1993; Kenny et al., 1994; 1996; Martin et al., 1997; Testa et al., 1997; Akiyama et al., 1999; Pulito et al., 2000; Brune et al., 2002; Witte et al., 2002) and terazosin (Breslin et al., 1993; Kenny et al., 1994; Martin et al., 1997; Testa et al., 1997; Witte et al., 1997; 2002; Akiyama et al., 1999; Brune et al., 2002). Similar inhibition was also obtained when IUP elevations had been induced by stimulation of the hypogastric nerves (Lefevre-Borg et al., 1993; Leonardi et al., 1997; Sato et al., 2001), indicating its physiological relevance.
Many studies have compared the effects of antagonists on IUP with those on blood pressure. Most such studies appear to have been designed with the primary aim of demonstrating that the drug manufactured by the sponsoring company is superior to the comparator drugs in this regard rather than with a genuine interest to better understand the adrenergic control of prostatic function. Nevertheless, it appears from the overall body of evidence that drugs with selectivity for α1A-adrenoceptors produce less blood pressure lowering for any given level of antagonism of the IUP response (Kenny et al., 1996; Testa et al., 1997; Akiyama et al., 1999; Pulito et al., 2000; Eltze et al., 2001). Together with the numerous in vitro studies on prostate contraction (see above), these data clearly demonstrate that prostate contraction occurs mainly, if not exclusively, via an α1A-adrenoceptor. In agreement with these animal data, it has been found that nonselective α1-adrenoceptor antagonists (Martorana et al., 1997; Witjes et al., 1997), the α1A/D-selective tamsulosin (Abrams et al., 1998) and the α1A-selective RO700004 (Blue et al., 2002) can lower bladder outlet resistance in patients in vivo. However, the overall effect of α1-adrenoceptor antagonists on bladder outlet resistance in patients is small and detected only inconsistently (see Kortmann et al., 2003).
Based upon the role of α1-adrenoceptors in the modulation of cell growth in many other tissues, this possibility has also been investigated in the prostate. Studies in rat have found induction of atypical prostatic hyperplasia upon chronic (26 days) treatment with the agonist phenylephrine; these effects were blocked by the α1A-antagonist RS 100,329, but not by BMY 3738, cyclazosin or yohimbine (Marinese et al., 2003). Treatment of BPH patients has been reported to reduce smooth muscle myosin heavy chain mRNA expression relative to control or androgen-ablated patients (Lin et al., 2001). Other studies have found that treatment with doxazosin and other quinazoline α1-adrenoceptor antagonists induces apoptosis in the human prostate (Kyprianou et al., 1998; Chon et al., 1999; Tahmatzopoulos & Kyprianou, 2004). However, the assessment of apoptosis was largely based upon the TUNEL assay, which is known to frequently yield false-positive results, and no alterations of overall prostate size were seen with doxazosin relative to placebo in a large multi-year study (McConnell et al., 2003). Therefore, it appears unlikely that therapeutic doses of α1-adrenoceptor antagonists indeed modulate prostate growth in an appreciable manner.
Regulation of receptor expression and function
An increase in α1-adrenoceptors with age was reported for rabbit prostate, but failed to reach statistical significance (Kondo et al., 1992). Patients with diabetes suffer from greater BPH symptoms than those without (Michel et al., 2000a). If this would be α-adrenoceptor-related, an enhanced responsiveness of the receptors would be expected in diabetes. However, a study in rats with streptozotocin-induced diabetes reported the opposite, that is, a reduced potency and efficacy of noradrenaline to contract the prostate in vitro, and insulin treatment in vivo restored the maximum effects but not the agonist potency; the protein kinase C inhibitor calphostin C (but not bisindolylmaleimide I) restored the contractile effects of noradrenaline in diabetic rats (Ramasamy et al., 2002). The authors interpreted these findings to indicate that an increased protein kinase C activity in prostates from diabetic rats may impair α1-adrenoceptor function; desensitization of α1-adrenoceptor function by protein kinase C activation is well documented (Yang et al., 1998a).
Most studies on the regulation of prostatic α1-adrenoceptor expression and function were performed in the context of BPH, which develops in an androgen-dependent manner. Accordingly, the effect of androgen removal on prostatic α1-adrenoceptors has been studied in castrated rats. Castration causes a major shrinkage of the prostate. This was accompanied by a markedly loss of α1-adrenoceptor-mediated elevations of IUP in vivo (Akiyama et al., 1999). In vitro studies found that strips from castrated rats developed a greater tension in response to α1-adrenoceptor stimulation, but relative to KCl-induced contraction no change was observed (Homma et al., 2000), indicating that the enhanced responses are related to an altered overall tissue responsiveness and change in the glandular/stromal ratio. However, the potency of phenylephrine decreased in castrated rats. On the other hand, receptor expression at the protein level remained unchanged when expressed relative to tissue weight and even increased when expressed per mg protein, although α1A-adrenoceptor mRNA expression was reduced (Homma et al., 2000). A similar receptor increase relative to protein content was also observed by others after castration (Takahashi et al., 2002).
In contrast to the rat studies, a seven-fold increase in total α1-adrenoceptor mRNA, largely due to an increase in α1A-adrenoceptor mRNA, was reported in the prostate from BPH patients (Nasu et al., 1996). A later study found numerically more α1A-adrenoceptor mRNA in the urethral, central and peripheral areas of the prostate from BPH as compared to control patients, but the differences were statistically significant only for the central area (Figure 5) (Moriyama et al., 1998). While an increased receptor density was also found at the protein level in some studies (Morita & Kondo, 1992b; Kondo et al., 1993), others reported a similar density of α1-adrenoceptors in the prostate from BPH and control patients (Figure 5) (Chapple et al., 1989; Tsujii et al., 1992), and another study found a similar density in prostates from men with symptomatic vs asymptomatic BPH (Gup et al., 1990). Accordingly, a fourth study reported a lack of correlation between the density of prostatic α1-adrenoceptors and the size of the adenoma within a group of BPH patients (Kawabe et al., 1990). A report on increased contraction of prostatic tissue from BPH patients in response to phenylephrine does not necessarily contradict these findings, because that study found similarly increased contraction in response to prostaglandin E2 and F2α and also receptor-independently in response to KCl (Kitada & Kumazawa, 1987), suggesting that the enhanced responses may not be related to alterations in adrenoceptor expression, but rather to the increased contribution of smooth muscle cells in the hyperplastic human prostate. Moreover, another study found similar contractile responses in patients with symptomatic and asymptomatic BPH (Gup et al., 1989).
Figure 5.
α1A-Adrenoceptor mRNA (upper panel) and total α1-adrenoceptor protein expression (lower panel) in prostates from BPH patients. Data are expressed as % of the corresponding control group and taken from Chapple et al. (1989); Gup et al. (1990); Tsujii et al. (1992); Nasu et al. (1996); Moriyama et al. (1998). *P<0.05 vs control in the respective study. Note that Moriyama et al. have studied the urethral, central and peripheral zones in parallel.
Since α-adrenoceptor antagonists are the most frequent form of rational medical BPH treatment, the effects of agonist and antagonist treatment on receptor expression have been studied. In cultured human prostatic smooth muscle cells, surprisingly only α1B- and α1D-, but no α1A-, adrenoceptor mRNA was detected; neither expression was altered by treatment with the agonist phenylephrine or the antagonist doxazosin (Boesch et al., 1999). In in vivo studies, an 8-week treatment with doxazosin also failed to alter the expression of rat prostatic α1-adrenoceptor subtype mRNA, and only a 12-week treatment with very high doxazosin doses (2 and 4 mg kg−1) caused detectable upregulation (Foster et al., 2004). Given the inverse agonism of doxazosin (Hein et al., 2001) and the known regulation of all three subtypes by agonists (Yang et al., 1999), these findings are difficult to understand.
Clinical implications
Based upon the above data, it is obvious that α1-adrenoceptor antagonists are an option for the treatment of BPH, and indeed they have become the most widely used form of medical treatment worldwide (see Roehrborn & Schwinn, 2004). Original concepts have assumed that their beneficial effects are largely mediated by the α1-adrenoceptors mediating prostate smooth muscle contraction, that is, α1A-adrenoceptors. In this line of thought, α1A-selective antagonists may be fully effective, but lack some of the side effects associated with blockade of the other subtypes, for example, in the vasculature (Rudner et al., 1999). While the excellent tolerability of the moderately α1A-selective tamsulosin is in line with this reasoning, several lines of evidence have challenged this view. Firstly, alfuzosin, which has similar affinity for all α1-adrenoceptor subtypes except for those in the α1L-state, has a similarly good tolerability profile as tamsulosin (see Michel et al., 2001). Secondly, mechanistic models in which symptom relief in BPH patients is related to prostatic smooth muscle relaxation imply that α1-adrenoceptor antagonists relieve BPH symptoms by lowering bladder outlet obstruction. While such effects have been found in some clinical studies, the overall evidence suggests that clinically used doses of α1-adrenoceptor antagonists have little effect on bladder outlet obstruction (see Kortmann et al., 2003). Thirdly, and perhaps most importantly, α1-adrenoceptor antagonists were also shown to improve typical BPH symptoms in animal models of bladder outlet obstructions under experimental conditions where a reduction of bladder outlet resistance was not possible (Broten et al., 1998; Gu et al., 2004). Taken together, these data suggest that mechanisms distinct from prostatic smooth muscle relaxation may contribute to the α1-adrenoceptor antagonist-induced symptom relief in BPH patients. Candidates for such alternative mechanisms include α1-adrenoceptors, possibly α1D-adrenoceptors, in the bladder or in parts of the spinal cord involved in micturition control. However, the anatomical and pharmacological identity of such sites remains to be determined.
Another conceptually important point is the finding that some α1-adrenoceptor antagonists, specifically alfuzosin and tamsulosin, have little effect on blood pressure at doses where they clearly relieve BPH symptoms (see Michel et al., 2001). Several not mutually exclusive theories have been proposed in this regard. One possibility is that all three α1-adrenoceptor subtypes contribute to vascular resistance (see Guimaraes & Moura, 2001). This implies that no α1-adrenoceptor antagonist could ever be fully free of vascular effects, but that subtype-selective antagonists may have fewer than others. Based upon the above, it appears that inhibition of α1A- and perhaps also α1D-adrenoceptors is sufficient for a full therapeutic response in BPH and hence a drug with little affinity for α1B-adrenoceptors may have less effect on the vasculature. In this context, it should be considered that the relative role of α1B-adrenoceptors increases in blood vessels of the elderly (Rudner et al., 1999). This might explain why tamsulosin has little vascular effects, but it cannot explain a relatively similar cardiovascular profile of alfuzosin that does not discriminate between α1-adrenoceptor subtypes (Michel & Insel, 1994). A second theory relates to a possible differential tissue distribution of some α1-adrenoceptor antagonists. Thus, it has been demonstrated that both alfuzosin and tamsulosin enrich in tissues of the lower urinary tract as compared to other tissues or the blood stream (Sato et al., 2001; Mottet et al., 2003; Romic et al., 2003). However, it remains unclear whether other drugs with greater blood pressure effects, for example, doxazosin or terazosin, lack such properties despite being chemically rather similar to alfuzosin. Thirdly, it has been proposed that other pharmacokinetic factors, that is, a late tmax and a small Cmax/Ctrough ratio, may contribute. Indeed, formulations fulfilling those criteria have been introduced for alfuzosin, doxazosin and tamsulosin, and in all three cases this has resulted in moderate improvements in tolerability (Kirby et al., 2001; Roehrborn et al., 2003; Chapple et al., 2005; Michel et al., 2005a, 2005b). While all of the above are plausible hypotheses, it must be emphasized that each remains to be proven and that they are not mutually exclusive.
α2-Adrenoceptors
mRNA and protein expression
Based upon PCR (Eason & Liggett, 1993) and RNase protection assays (Perälä et al., 1992), all three cloned α2-adrenoceptor subtypes are expressed at the mRNA level in the human prostate. Studies at the protein level have sometimes been performed using immunohistochemistry (Slater et al., 2000) and receptor autoradiography (James et al., 1989; Felsen et al., 1994), but in most cases have relied upon radioligand-binding experiments with tissue homogenates. In young and old rats, α2A-adrenoceptor immunoreactivity was mainly detected in the prostatic epithelium (Slater et al., 2000). Studies in canine prostates have reported a similar density of total α2-adrenoceptors in the proximal, midportion and distal region (Shapiro et al., 1987), and on a histological level they were largely found in the epithelium (Felsen et al., 1994). Autoradiographic studies in the human prostate have localized the α2-adrenoceptors mainly in association with blood vessels and, to a lesser extent, with the prostate epithelium (James et al., 1989). Competition radioligand-binding studies reported that prostatic α2-adrenoceptors belong predominantly, if not exclusively, to the α2A-subtype (Goepel et al., 1997). Several studies in the human prostate have compared the density of α2- and α1-adrenoceptor-binding sites. While some studies reported a similar abundance of both receptors at the protein level (Hedlund et al., 1985; Shapiro & Lepor, 1986; Lepor et al., 1988a, 1988b; Gup et al., 1990; Morita & Kondo, 1992a, 1992b), others reported significantly fewer α2- than α1-adrenoceptors (Chapple et al., 1989; James et al., 1989; Yamada et al., 1992); some studies also reported much fewer α2- than α1-adrenoceptors, but the difference did not reach statistical significance with small patient numbers (Kawabe et al., 1990; Hatano et al., 1996).
In vitro and in vivo functions
Similar to most other tissues, α2-adrenoceptor agonists and antagonists can inhibit or enhance, respectively, the pre-junctional noradrenaline release in the human prostate (Hedlund et al., 1985). Accordingly, the α2-adrenoceptor agonist clonidine inhibited the field stimulation-induced contraction of the human prostate (Guh et al., 1995). In rat prostate neuroendocrine cells α2-adrenoceptor agonists can inhibit high-voltage-operated Ca2+ channels in a pertussis toxin-sensitive manner (Kim et al., 2003), but the effect of this on the overall prostatic function remains unclear.
In line with the above studies demonstrating the presence of prostatic α2-adrenoceptors in the epithelium rather than the stroma, however, no post-junctional contractile function of prostatic α2-adrenoceptors has been identified, since they contribute little to α-adrenergic contraction in dogs (Somers et al., 1989; Felsen et al., 1994), horses (Garcia-Sacristan et al., 1984) or humans (Hedlund et al., 1985; Hieble et al., 1985; Lepor et al., 1988b; Chapple et al., 1989; Gup et al., 1989; Steidle et al., 1989; Hatano et al., 1996). In light of the α2-adrenoceptor-mediated in vitro contraction of the rabbit urethra (see above) but not the prostate, in vivo data with systemic administration of α2-adrenoceptor drugs on canine IUP are interpreted to represent effects upon the urethra rather than the prostate (Shapiro et al., 1987). One study has reported that, in a rat model of androgen/oestrogen-induced BPH, chronic administration of the α2-agonist atipamezole increased prostatic compliance, while an α1-agonist did not (Constantinou & Omata, 1996); unfortunately, no follow-up of this interesting study has been reported.
Regulation of receptor expression and function
While no major alterations in α2A-adrenoceptor immunoreactivity were found in histochemical studies comparing prostates from 12-week- and 18-month-old rats (Slater et al., 2000), another study comparing 6-month- and 4.5–5-year-old rabbits found a numerically higher overall α2-adrenoceptor density in the older animals, but the difference failed to reach statistical significance (Kondo et al., 1992). Four studies in patients with BPH have reported an increased α2-adrenoceptor density as compared to controls (Chapple et al., 1989; Gup et al., 1990; Morita & Kondo, 1992b; Kondo et al., 1993). While this finding seems remarkably consistent, its relevance remains unclear due to the lack of knowledge regarding physiological functions of this receptor in the prostate (apart from pre-junctional inhibition). For this very reason, α2-adrenoceptors are not considered to represent a therapeutic target at present.
β-Adrenoceptors
mRNA and protein expression
The presence of β-adrenoceptor subtype mRNA has been studied in a limited way only. One study using Northern blot analysis has demonstrated the presence of β2-adrenoceptor mRNA in rats (Collins et al., 1988), and another study using RNase protection assays without previous PCR amplification has reported the presence of β3-adrenoceptor mRNA in humans (Berkowitz et al., 1995).
The presence of prostatic β-adrenoceptor subtypes at the protein level has been studied by radioligand-binding and immunological techniques. Saturation-binding studies using [3H]dihydroalprenolol or [125I]iodocyanopindolol have reported 125–1000 fmol mg−1 protein in rats (Poyet et al., 1986b; Collins et al., 1988; Guthrie et al., 1990; Gousse et al., 1991; Fukumoto et al., 1993; Chen et al., 1995), 60 fmol mg−1 protein in pigs (Goepel et al., 1997) and 40–280 fmol mg−1 protein in humans (Tsujii et al., 1992; Goepel et al., 1997). Three of these studies have performed a characterization of the β-adrenoceptor subtypes being present using competition binding, and report a predominant contribution of the β2-subtype in rats, pigs and humans (Poyet et al., 1986b; Gousse et al., 1991; Goepel et al., 1997). However, all of these studies have employed radioligand concentrations that are unlikely to detect β3-adrenoceptors (Hoffmann et al., 2004). This did not allow detection of possibly present β3-adrenoceptors and may underestimate the total β-adrenoceptor density. Indeed, immunohistochemical studies with a selective antibody have detected the presence of β1- and β3-adrenoceptors in the rat and human prostate, respectively (Chamberlain et al., 1999; Slater et al., 2000).
In vitro and in vivo functions
The functional presence of β-adrenoceptors in the rat, guinea-pig and human prostate has been demonstrated by adenylyl cyclase stimulation studies, largely involving β2-adrenoceptors (Shima et al., 1980; Poyet et al., 1986a; Purvis et al., 1986; Solano et al., 1994; Carmena et al., 1995; 1997; Chen et al., 1995; Haynes & Hill, 1997; Juarranz et al., 1998). While β-adrenoceptor stimulation did not inhibit α1-adrenoceptor-mediated inositol phosphate formation (Haynes & Hill, 1997) and did not alter basal prostatic tone (Caine et al., 1975), it has been shown to inhibit α1-adrenoceptor-mediated, field stimulation-induced or receptor-independent prostate contraction in rats (Kalodimos & Ventura, 2001), guinea-pigs (Haynes & Hill, 1997), dogs (Normandin & Lodge, 1996), horses (Garcia-Sacristan et al., 1984) and humans (Tsujii et al., 1992; Drescher et al., 1994). Based upon subtype-selective agonists and antagonists, the relaxation responses against field stimulation and against α1-adrenoceptor agonist were reported to occur via different receptors in guinea-pigs, that is, via β1-adrenoceptors for field stimulation and via β2-adrenoceptors for the α1-response (Haynes & Hill, 1997). In rat prostate, the nonselective agonists isoprenaline and adrenaline, the β1-selective noradrenaline and RO363, and the β2-selective salbutamol relaxed field stimulation-induced contraction, whereas the β3-selective BRL 37,344 did not; on the other hand, the β2-antagonist ICI 118,551 antagonized responses to isoprenaline and salbutamol, while the β1-antagonist atenolol did not (Kalodimos & Ventura, 2001). Relaxation of field stimulation-induced contraction affected the nifedipine-sensitive component only, whereas relaxation of the α1-response inhibited the nifedipine-sensitive and -insensitive components of contraction (Haynes & Hill, 1997). Since the relaxations were mimicked by the adenylyl cyclase stimulator forskolin and by phosphodiesterase inhibitors in various species (Tsujii et al., 1992; Drescher et al., 1994; Chen et al., 1995; Normandin & Lodge, 1996; Kalodimos & Ventura, 2001), these appear to be cAMP-mediated. β-Adrenoceptor stimulation has also been reported to regulate gene transcription in the rat prostate (Guthrie et al., 1990). Some functional data also demonstrate the presence of β-adrenoceptors, most likely β2-adrenoceptors, in the LNCaP human prostate cancer cell line (Nagmani et al., 2003). To the best of our knowledge, in vivo studies on the β-adrenoceptor-mediated regulation of prostatic function have not been reported, apart from the above-mentioned studies on IUP, which may partly reflect prostatic effects.
Regulation of receptor expression and function
A possible regulation of the prostatic β-adrenoceptor expression and function by age has been studied in rats (Chen et al., 1995). The number of [3H]dihydroalprenolol-binding sites increased somewhat between 4 and 12 weeks of age and remained at a similar level up to an age of 52 weeks. In contrast, isoprenaline-stimulated adenylyl cyclase activity increased stronger between the ages of 2 and 12 weeks, and then continuously declined until the age of 104 weeks. Concomitantly, the ratio of Gs/Gi α-subunits, as assessed by toxin-catalyzed ADP ribosylation, also was lowest at 2 weeks, maximum at 12 weeks, and declined at 52 and 104 weeks. On the other hand, an immunohistochemical study has reported an increased β1-adrenoceptor density in 20- vs 3-month-old rats (Slater et al., 2000). Thus, a reduced prostatic β-adrenoceptor function in immature and old rats appears to reflect predominantly a change in G-protein function rather than one in receptor expression. Accordingly, a study in rabbits did not detect any alterations of β-adrenoceptor-binding sites with age (Kondo et al., 1992).
A study in tissue specimens from control patients and those with BPH has found a significant reduction of β-adrenoceptor, assessed as [3H]dihydroalprenolol-binding sites, by about 40% in the latter group (Tsujii et al., 1992). This was accompanied by a loss of isoprenaline-induced (but not forskolin-induced) prostate relaxation; concomitantly, propranolol enhanced noradrenaline-induced contraction in control, but not in BPH tissue. Together, these data suggest that a reduced receptor density is the cause of an impaired β-adrenoceptor-mediated relaxation in BPH.
Since the prostate is an androgen-dependent tissue, the modulation of prostatic β-adrenoceptor expression and function by androgens has been studied in rats. While castration reduced prostatic β-adrenoceptor density at the protein level and testosterone replacement restored it, castration had little effect on β2-adrenoceptor mRNA and testosterone replacement had only transient increasing effects (Collins et al., 1988). Upregulation of β2-adrenoceptor mRNA by androgen has also been observed in the intact rat prostate (Guthrie et al., 1990). The functional relevance of this is supported by the observation that castration reduced β-adrenoceptor-mediated adenylyl cyclase stimulation in the prostate, and this was also restored by androgen replacement; basal and forskolin-stimulated adenylyl cyclase activity exhibited a similar androgen-dependent pattern (Poyet et al., 1986a; Purvis et al., 1986). Thus, β-adrenoceptor expression and function in the prostate are androgen-dependent, and this may involve transcriptional and post-transcriptional effects at the receptor level, as well as receptor-independent effect.
Three studies have evaluated the effect of diabetes on prostatic β-adrenoceptors using the rat streptozotocin model. The initial report described a marked reduction in prostatic β-adrenoceptors in diabetes (shown to be a predominant β2-adrenoceptor population), which was fully restored upon insulin substitution (Gousse et al., 1991). A later study from the same group confirmed these findings, and reported that such regulation was not accompanied by alterations of the relative contribution of agonist high- and low-affinity sites (Fukumoto et al., 1993). Other investigators found that diabetes markedly reduced isoprenaline- or forskolin-stimulated adenylyl cyclase activity in rat prostate, but this was not corrected by insulin treatment; in that study, diabetes was also associated with a reduced expression of Gs, Gi1/2 and Gi3/o proteins, which also was not fully restored by insulin treatment (Carmena et al., 1997). Finally, it has also been reported that chronic alcohol intake increases isoprenaline-induced adenylyl cyclase activity as well as expression of Gs and Gi1/2 proteins in rat prostate (Juarranz et al., 1998).
Clinical implications
Taken together, these data demonstrate that all three β-adrenoceptor subtypes appear to be expressed in the prostate at the mRNA and protein levels, but precise quantitative information on their relative contribution is lacking. β-Adrenoceptor stimulation in the prostate causes smooth muscle relaxation, an effect involving both β1- and β2-adrenoceptors; while a single study in rats argues against an involvement of β3-adrenoceptors, the present data are too limited to allow definitive conclusions. Ageing, ambient androgen levels and diabetes can regulate prostatic β-adrenoceptor expression and function.
Conclusions
In conclusion, adrenoceptors in the lower urinary tract predominantly mediate continence-supporting functions, that is, a relaxation of smooth muscle in the detrusor via β-adrenoceptors and hence increased bladder compliance and a contraction of smooth muscle in the bladder neck, urethra and prostate via α1-adrenoceptors. In humans, the former appears to involve mainly β3-adrenoceptors, and the latter mainly α1A-adrenoceptors. α2-Adrenoceptors, possibly from the α2A-subtype, mediate pre-junctional inhibition of transmitter release, but the great abundance of this subtype makes other, as yet undefined functions likely. Most of the six other adrenoceptor subtypes are also expressed to varying extents in the lower urinary tract, but their function remains unclear. In pathophysiological settings, particularly during states of bladder outlet obstruction in elderly men with enlarged prostates, α1-adrenoceptors may contribute to the symptoms and hence antagonists of these receptors effectively relieve such symptoms. β3-Adrenoceptor agonists may be a useful form of treatment of bladder dysfunction in the context of OAB, but perhaps also in the context of BPH. The latter possibility makes the possible combination of α1-adrenoceptor antagonist with β3-adrenoceptor agonist treatment potentially interesting.
Acknowledgments
Work in our lab on the subject of this article has been funded in part by the Deutsche Forschungsgemeinschaft, Astellas and Boehringer Ingelheim. We thank Dr A.G. Ramage for his help with the section on central nervous α1-adrenoceptors.
Glossary
- BPH
benign prostatic hyperplasia
- OAB
overactive bladder
- RNase
ribonuclease
- RT–PCR
reverse transcriptase–polymerase chain reaction
References
- ABRAMS P., CARDOZO L., FALL M., GRIFFITHS D., ROSIER P., ULMSTEN U., VAN KERREBROECK P., VICTOR A., WEIN A. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourol. Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- ABRAMS P., TAMMELA T.L., HELLSTRÖM P., MARBERGER M., DJAVAN B.R., LUNGLMAYR G., PANOS P., LAVAL K.-U. European pressure-flow investigation of tamsulosin in men with lower urinary tract symptoms (LUTS) suggestive of benign prostatic obstruction (BPO) – ESPRIT study. J. Urol. 1998;159:256. [Google Scholar]
- AKIYAMA K., HORA M., TATEMICHI S., MASUDA N., NAKAMURA S., YAMAGISHI R., KITAZAWA M. KMD-3213, a uroselective and long-acting a1a-adrenoceptor antagonist, tested in a novel rat model. J. Pharmacol. Exp. Ther. 1999;291:81–91. [PubMed] [Google Scholar]
- ANDERSSON K.-E., WEIN A.J. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol. Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.-E., LARSSON B., SJOGREN C. Characterization of α-adrenoceptors in the female rabbit urethra. Br. J. Pharmacol. 1984;81:293–300. doi: 10.1111/j.1476-5381.1984.tb10078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BADAWI J.K., UECELEHAN H., HATZINGER M., MICHEL M.S., HAFERKAMP A., BROSS S. Relaxant effects of b-adrenergic agonists on porcine and human detrusor muscle. Acta Physiol. Scand. 2005;185:151–159. doi: 10.1111/j.1365-201X.2005.01474.x. [DOI] [PubMed] [Google Scholar]
- BAKER J.G. The selectivity of b-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br. J. Pharmacol. 2005;141:317–322. doi: 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANNOWSKY A., JUENEMANN K.-P. Innervation and function of the female urinary bladder and urethra. EAU Update Series. 2003;1:120–127. [Google Scholar]
- BARTSCH G., MÜLLER H.R., OBERHOLZER M., ROHR H.P. Light microscopic stereological analysis of the normal human prostate and of benign prostatic hyperplasia. J. Urol. 1979;122:487–491. doi: 10.1016/s0022-5347(17)56476-9. [DOI] [PubMed] [Google Scholar]
- BERGES R.R., PIENTKA L., HÖFNER K., SENGE T., JONAS U. Male lower urinary tract symptoms and related health care seeking in Germany. Eur. Urol. 2001;39:682–687. doi: 10.1159/000052527. [DOI] [PubMed] [Google Scholar]
- BERKOWITZ D.E., NARDONE N.A., SMILEY R.M., PRICE D.T., KREUTTER D.K., FREMEAU R.T., SCHWINN D.A. Distribution of β3-adrenoceptor mRNA in human tissues. Eur. J. Pharmacol. 1995;289:223–228. doi: 10.1016/0922-4106(95)90098-5. [DOI] [PubMed] [Google Scholar]
- BING W., CHANG S., HYPOLITE J.A., DISANTO M.E., ZDERIC S.A., ROLF L., WEIN A.J., CHACKO S. Obstruction-induced changes in urinary bladder smooth muscle contractility: a role for rho kinase. Am. J. Physiol. 2003;285:F990–F997. doi: 10.1152/ajprenal.00378.2002. [DOI] [PubMed] [Google Scholar]
- BLUE D., ZINNER N., GRINO P., GABLES C., CRAGER M., FORD A.P.D.W.2002RO700004, a selective α1A-adrenoceptor antagonist, does not improve lower urinary tract symptoms in men with benign prostatic hyperplasia J. Urol. 167(Suppl.)26511743334 [Google Scholar]
- BLUE D.R., DANIELS D.V., GEVER J.R., JETT M.F., O'YANG C., TANG H.M., WILL T.J., FORD A.P.D.W. Pharmacological characteristics of Ro 115–1240, a selective α1A/1L-adrenoceptor agonist: a potential therapy of stress urinary incontinence. BJU Int. 2004;93:162–170. doi: 10.1111/j.1464-410x.2004.04577.x. [DOI] [PubMed] [Google Scholar]
- BOESCH S.T., CORVIN S., ZHANG J., ROGATSCH H., BARTSCH G., KLOCKER H. Modulation of the differentiation status of cultured prostatic smooth muscle cells by an α1-adrenergic receptor antagonist. Prostate. 1999;39:226–233. doi: 10.1002/(sici)1097-0045(19990601)39:4<226::aid-pros2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- BOLLE P., FIDANZA S., TUCCI P. Response to isoprenaline of rabbit detrusor muscle following exposure to 5, 8, 11, 14 eicosatetraenoic acid. Role of prostanoids on beta-adrenergic-evoked response. J. Auton. Pharmacol. 1999;19:161–165. doi: 10.1046/j.1365-2680.1999.00130.x. [DOI] [PubMed] [Google Scholar]
- BRAHMADEVARA N., SHAW A.M., MACDONALD A. Evidence against β3-adrenoceptors or low affinity state of β2-adrenoceptors mediating relaxation in rat isolated aorta. Br. J. Pharmacol. 2003;138:99–106. doi: 10.1038/sj.bjp.0705017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRESLIN D., FIELDS D.W., CHOU T.-C., MARION D.N., KANE M., VAUGHAN E.D., JR, FELSEN D. Medical management of benign prostatic hyperplasia: a canine model comparing the in vivo efficacy of alpha-1 adrenergic antagonists in the prostate. J. Urol. 1993;149:395–399. doi: 10.1016/s0022-5347(17)36102-5. [DOI] [PubMed] [Google Scholar]
- BROTEN T., SCOTT A., SIEGL P.K.S., FORRAY C., LAGU B., NAGARATHNAM D., WONG W.C., MARZABADI M., MURALI DHAR T.G., GLUCHOWSKI C. Alpha-1 adrenoceptor blockade inhibits detrusor instability in rats with bladder outlet obstruction. FASEB J. 1998;12:A445. [Google Scholar]
- BRUNE M.E., KATWALA S.P., MILICIC I., WITTE D.G., KERWIN J.F., JR, MEYER M.D., HANCOCK A.A., WILLIAMS M. Effect of fiduxosin, an antagonist selective for α1A- and α1D-adrenoceptors, on intraurethral and arterial pressure responses in conscious dogs. J. Pharmacol. Exp. Ther. 2002;300:487–494. doi: 10.1124/jpet.300.2.487. [DOI] [PubMed] [Google Scholar]
- BUCKNER S.A., OHEIM K.W., MORSE P.A., KNEPPER S.M., HANCOCK A.A. α1-Adrenoceptor-induced contractility in rat aorta is mediated by the α1D subtype. Eur. J. Pharmacol. 1996;297:241–248. doi: 10.1016/0014-2999(95)00755-5. [DOI] [PubMed] [Google Scholar]
- BYLUND D.B., EIKENBERG D.C., HIEBLE J.P., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., MOLINOFF P.B., RUFFOLO R.R., JR, TRENDELENBURG U. IV. International Union of Pharmacology Nomenclature of Adrenoceptors. Pharmacol. Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- CAINE M., RAZ S., ZEIGLER M. Adrenergic and cholinergic receptors in the human prostate, prostatic capsule and bladder neck. Br. J. Urol. 1975;47:193–202. doi: 10.1111/j.1464-410x.1975.tb03947.x. [DOI] [PubMed] [Google Scholar]
- CARMENA M.J., CLEMENTE C., CARRERO I., SOLANO R.M., PRIETO J.C. G-proteins and β-adrenergic stimulation of adenylate cyclase activity in the diabetic rat prostate. Prostate. 1997;33:46–54. doi: 10.1002/(sici)1097-0045(19970915)33:1<46::aid-pros8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- CARMENA M.J., GARCIA-PARAMIO P., SOLANO R.M., PRIETO J.C. Protein kinase C regulation of the adenylyl cyclase system in rat prostatic epithelium. Prostate. 1995;27:204–211. doi: 10.1002/pros.2990270405. [DOI] [PubMed] [Google Scholar]
- CHAMBERLAIN P.D., JENNINGS K.H., PAUL F., CORDELL J., HOLMES S.D., PARK J., CHAMBERS J., SENNITT M.V., STOCK M.J., CAWTHORNE M.A., YOUNG P.W., MURPHY G.J. The tissue distribution of the human β3-adrenoceptor studied using a monoclonal antibody: direct evidence of the β3-adrenoceptor in human adipose tissue, atrium and skeletal muscle. Int. J. Obesity. 1999;23:1057–1065. doi: 10.1038/sj.ijo.0801039. [DOI] [PubMed] [Google Scholar]
- CHANG D.J., CHANG T.K., YAMANISHI S.S., SALAZAR F.H.R., KOSAKA A.H., KHARE R., BHAKTA S., JASPER J.R., SHIEH I.-S., LESNICK J.D., FORD A.P.D.W., DANIELS D.V., EGLEN R.M., CLARKE D.E., BACH C., CHAN H.W. Molecular cloning, genomic characterization and expression of novel human α1A-adrenoceptor isoforms. FEBS Lett. 1998;422:279–283. doi: 10.1016/s0014-5793(98)00024-6. [DOI] [PubMed] [Google Scholar]
- CHANG R.S.L., CHEN T.-B., O'MALLEY S.S., PETTIBONE D.J., DISALVO J., FRANCIS B., BOCK M.G., FREIDINGER R., NAGARATHNAM D., MIAO S.W., SHEN Q., LAGU B., DHAR T.G.M., TYAGARAJAN S., MARZABADI M.R., WONG W.C., GLUCHOWSKI C., FORRAY C. In vitro studies on L-771, 688 (SNAP 6383), a new potent and selective α1A-adrenoceptor antagonist. Eur. J. Pharmacol. 2000;409:301–312. doi: 10.1016/s0014-2999(00)00854-2. [DOI] [PubMed] [Google Scholar]
- CHAPPLE C.R., AL-SHUKRI S.H., GATTEGNO B., HOLMES S., MARTINEZ-SAGARRA J.M., SCARPA R.M., VAN VIERSSEN TRIP O.B., VIK V., VAN DER PUTTEN-SLOB I. Tamsulosin oral controlled absorption system (OCAS) in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH): efficacy and tolerability in a placebo and active comparator controlled phase 3a study. Eur. Urol. Suppl. 2005;4:33–44. [Google Scholar]
- CHAPPLE C.R., AUBRY M.L., JAMES S., GREENGRASS P.M., BURNSTOCK G., TURNER-WARWICK R.T., MILROY E.J.G., DAVEY M.J. Characterisation of human prostatic adrenoceptors using pharmacology receptor binding and localisation. Br. J. Urol. 1989;63:487–496. doi: 10.1111/j.1464-410x.1989.tb05942.x. [DOI] [PubMed] [Google Scholar]
- CHEN C., ISHIKAWA Y., AMANO I., EGUCHI T., ISHIDA H. Age-dependent changes in response of rat prostatic tissues to isoproterenol and forskolin: changes with sexual maturation in function of G proteins. Mech. Ageing Dev. 1995;81:1–13. doi: 10.1016/0047-6374(94)01577-9. [DOI] [PubMed] [Google Scholar]
- CHEN Q., TAKAHASHI S., ZHONG S., HOSODA C., ZHENG H.-Y., OGUSHI T., FUJIMURA T., OHTA N., TANOUE A., TSUJIMOTO G., KITAMURA T. Function of the lower urinary tract in mice lacking α1d-adrenoceptor. J. Urol. 2005;174:370–374. doi: 10.1097/01.ju.0000161210.17365.cc. [DOI] [PubMed] [Google Scholar]
- CHESS-WILLIAMS R., CHAPPLE C.R., VERFÜRTH F., NOBLE A.J., COULDWELL C.J., MICHEL M.C. The effects of SB 216469, an antagonist which discriminates between the α1A-adrenoceptor and the human prostatic α1-adrenoceptor. Br. J. Pharmacol. 1996;119:1093–1100. doi: 10.1111/j.1476-5381.1996.tb16009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHON J.K., BORKOWSKI A., PARTIN A.W., ISAACS J.T., JACOBS S.C., KYPRIANOU N. α1-Adrenoceptor antagonists terazosin and doxazosin induce prostate apoptosis without affecting cell proliferation in patients with benign prostatic hyperplasia. J. Urol. 1999;161:2002–2008. [PubMed] [Google Scholar]
- CHOPRA B., BARRICK S.R., MEYERS S., BECKEL J.M., ZEIDEL M.L., FORD A.P.D.W., DE GROAT W.C., BIRDER L.A. Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J. Physiol. (London) 2005;562:859–871. doi: 10.1113/jphysiol.2004.071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUEH S.-C., GUH J.-H., CHEN J., LAI M.-K., KO F.-N., TENG C.-M. Inhibition by tamsulosin of tension responses of human hyperplastic prostate to electrical field stimulation. Eur. J. Pharmacol. 1996;305:177–180. doi: 10.1016/0014-2999(96)00197-5. [DOI] [PubMed] [Google Scholar]
- COLLINS S., QUARMBY V.E., FRENCH F.S., LEFKOWITZ R.J., CARON M.G. Regulation of the β2-adrenergic receptor and its mRNA in the rat ventral prostate by testosterone. FEBS Lett. 1988;233:173–176. doi: 10.1016/0014-5793(88)81378-4. [DOI] [PubMed] [Google Scholar]
- CONLEY R.K., WILLIAMS T.J., FORD A.P.D.W., RAMAGE A.G. The role of α1-adrenoceptors and 5-HT1A receptors in the control of the micturition reflex in male anaesthetized rats. Br. J. Pharmacol. 2001;133:61–72. doi: 10.1038/sj.bjp.0704043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONSTANTINOU C.E., OMATA S. Analysis of the relative biomechanical effects of alpha 1 and alpha 2 antagonists in modifying the compliance of the prostate and micturition parameters of the hormonally manipulated male rat. Neurourol. Urodyn. 1996;15:85–101. doi: 10.1002/(SICI)1520-6777(1996)15:1<85::AID-NAU9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- CORVIN S., BÖSCH S.T., EDER I., THURNHER M., BARTSCH G., KLOCKER H. Videoimaging of prostatic stromal-cell contraction: an in vitro model for studying drug effects. Prostate. 1998;37:209–214. doi: 10.1002/(sici)1097-0045(19981201)37:4<209::aid-pros1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- DANIELS D.V., GEVER J.R., JASPER J.R., KAVA M.S., LESNICK J.D., MELOY T.D., STEPAN G., WILLIAMS T.J., CLARKE D.E., CHANG D.J., FORD A.P.D.W. Human cloned α1A-adrenoceptor isoforms display α1L-adrenoceptor pharmacology in functional studies. Eur. J. Pharmacol. 1999;370:337–343. doi: 10.1016/s0014-2999(99)00154-5. [DOI] [PubMed] [Google Scholar]
- DANUSER H., THOR K.B. Inhibition of central sympathetic and somatic outflow to the lower urinary tract of the cat by the α1-adrenergic receptor antagonist prazosin. J. Urol. 1995;153:1308–1312. [PubMed] [Google Scholar]
- DAS A.K., LEGGETT R.E., WHITBECK C., EAGEN G., LEVIN R.M. Effect of doxazosin on rat urinary bladder function after partial outlet obstruction. Neurourol. Urodyn. 2002;21:160–166. doi: 10.1002/nau.10045. [DOI] [PubMed] [Google Scholar]
- DELAFLOTTE S., AUGUET M., CHABRIER P.E. Pharmacological evidence that different α1 adrenoceptor subtypes mediate contraction in rabbit prostate and hypogastric artery. Acta Physiol. Scand. 1996;158:241–251. doi: 10.1046/j.1365-201X.1996.565310000.x. [DOI] [PubMed] [Google Scholar]
- DENG X.F., CHEMTOB S., VARMA D.R. Characterization of α1D-adrenoceptor subtype in rat myocardium, aorta and other tissues. Br. J. Pharmacol. 1996;119:269–276. doi: 10.1111/j.1476-5381.1996.tb15981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEPLANNE V., GALZIN A.M. Functional characterization of alpha-1-adrenoceptor subtypes in the prostatic urethra and trigone of male rabbit. J. Pharmacol. Exp. Ther. 1996;278:527–534. [PubMed] [Google Scholar]
- DEPLANNE V., PALEA S., ANGEL I. The adrenergic, cholinergic and NANC nerve-mediated contractions of the female rabbit bladder neck and proximal, medial and distal urethra. Br. J. Pharmacol. 1998;123:1517–1524. doi: 10.1038/sj.bjp.0701757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DERWEESH I.H., WHEELER M.A., WEISS R.M. Alterations in G-proteins and β-adrenergic responsive adenylyl cyclase in rat urinary bladder during ageing. J. Pharmacol. Exp. Ther. 2000;294:969–974. [PubMed] [Google Scholar]
- DRESCHER P., ECKERT R.E., MADSEN P.O. Smooth muscle contractility in prostatic hyperplasia: role of cyclic adenosine monophosphate. Prostate. 1994;25:76–80. doi: 10.1002/pros.2990250204. [DOI] [PubMed] [Google Scholar]
- DURANT P.A., LUCAS P.C., YAKSH T.L. Micturition in the unanaesthetized rat: spinal vs peripheral pharmacology of the adrenergic system. J. Pharmacol. Exp. Ther. 1988;245:426–435. [PubMed] [Google Scholar]
- EASON M.G., LIGGETT S.B. Human α2-adrenergic receptor subtype distribution: widespread and subtype-selective expression of α2C10, α2C4, and α2C2 mRNA in multiple tissues. Mol. Pharmacol. 1993;44:70–75. [PubMed] [Google Scholar]
- ELTZE M., BOER R., MICHEL M.C., HEIN P., TESTA R., ULRICH W.-R., KOLASSA N., SANDERS K.H. In vitro and in vivo uroselectivity of B8805–033, an antagonist with high affinity at prostatic α1A- vs α1B- and α1D-adrenoceptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:649–662. doi: 10.1007/s002100100413. [DOI] [PubMed] [Google Scholar]
- FAURE C., PIMOULE C., VALLANCIEN G., LANGER S.Z., GRAHAM D. Identification of α1-adrenoceptor subtypes present in the human prostate. Life Sci. 1994;54:1595–1605. doi: 10.1016/0024-3205(94)90031-0. [DOI] [PubMed] [Google Scholar]
- FELSEN D., ERNSBERGER P., SUTARIA P.M., NEJAT R.J., NGUYEN P., MAY M., BRESLIN D.S., MARION D.N., VAUGHAN E.D., JR Identification, localization and functional analysis of imidazoline and alpha adrenergic receptors in canine prostate. J. Pharmacol. Exp. Ther. 1994;268:1063–1071. [PubMed] [Google Scholar]
- FORD A.P.D.W., ARREDONDO N.F., BLUE D.R., BONHAUS D.W., JASPER J.R., KAVA M.S., LESNICK J., PFISTER J.R., SHIEH I.A., VIMONT R.L., WILLIAMS T.J., MCNEAL J.E., STAMEY T.A., CLARKE D.E. RS-17053 (N-[2-cyclopropylmethoxyphenoxy)ethyl]-5-chloro-a, a-dimethyl-1H-indole-3-ethanamine hydrochloride), a selective α1A-adrenoceptor antagonist, displays low affinity for functional α1A-adrenoceptors in human prostate: implications for adrenoceptor classification. Mol. Pharmacol. 1996;49:209–215. [PubMed] [Google Scholar]
- FORD A.P.D.W., DANIELS D.V., CHANG D.J., GEVER J.R., JASPER J.R., LESNICK J.D., CLARKE D.E. Pharmacological pleiotropism of the human recombinant α1A-adrenoceptor: implications for α1-adrenoceptor classification. Br. J. Pharmacol. 1997;121:1127–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORRAY C., BARD J.A., WETZEL J.M., CHIU G., SHAPIRO E., TANG R., LEPOR H., HARTIG P.R., WEINSHANK R.L., BRANCHEK T.A., GLUCHOWSKI C. The α1-adrenergic receptor that mediates smooth muscle contraction in human prostate has the pharmacological properties of the cloned human α1C subtype. Mol. Pharmacol. 1994;45:703–708. [PubMed] [Google Scholar]
- FOSTER H.E., JR, YONO M., SHIN D., TAKAHASHI W., POURESMAIL M., AFIATPOUR P., LATIFPOUR J. Effects of chronic administration of doxazosin on α1-adrenoceptors in the rat prostate. J. Urol. 2004;172:2465–2470. doi: 10.1097/01.ju.0000138475.89790.88. [DOI] [PubMed] [Google Scholar]
- FRAZIER E.P., MATHY M.-J., PETERS S.L.M., MICHEL M.C. Does cyclic AMP mediate rat urinary bladder relaxation by isoproterenol. J. Pharmacol. Exp. Ther. 2005a;313:260–267. doi: 10.1124/jpet.104.077768. [DOI] [PubMed] [Google Scholar]
- FRAZIER E.P., SCHNEIDER T., MICHEL M.C. β-Adrenergic urinary bladder relaxation in rats prone to overactive bladder. FASEB J. 2005b;19:A535. [Google Scholar]
- FUJIMURA T., TAMURA K., TSUTSUMI T., YAMAMOTO T., NAKAMURA K., KOIBUCHI Y., KOBAYASHI M., YAMAGUCHI O. Expression and possible functional role of the β3-adrenoceptor in human and rat detrusor muscle. J. Urol. 1999;161:680–685. [PubMed] [Google Scholar]
- FUKUMOTO Y., YOSHIDA M., DOKITA S., KAMAI T., WEISS R.M., LATIFPOUR J. The reversal effect of insulin on diabetes-induced alterations in beta adrenergic and muscarinic receptors in rat prostate. J. Urol. 1993;149:1602–1607. doi: 10.1016/s0022-5347(17)36459-5. [DOI] [PubMed] [Google Scholar]
- GARCIA-SACRISTAN A., CASANUEVA C.R., CASTILLA C., LABADIA A. Adrenergic receptors in the urethra and prostate of the horse. Res. Vet. Sci. 1984;36:57–60. [PubMed] [Google Scholar]
- GILLESPIE J.I. Noradrenaline inhibits autonomic activity in the isolated guinea pig bladder. BJU Int. 2004;93:401–409. doi: 10.1111/j.1464-410x.2003.04626.x. [DOI] [PubMed] [Google Scholar]
- GOEPEL M., ERDBRÜGGER W., WITTMANN A., MICHEL M.C. Atypical affinities of ICI 118, 551 at porcine β2-adrenoceptors. Br. J. Pharmacol. 1996;117 (Suppl.):198. [Google Scholar]
- GOEPEL M., WITTMANN A., RÜBBEN H., MICHEL M.C. Comparison of adrenoceptor subtype expression in porcine and human bladder and prostate. Urol. Res. 1997;25:199–206. doi: 10.1007/BF00941983. [DOI] [PubMed] [Google Scholar]
- GOETZ A.S., LUTZ M.W., RIMELE T.J., SAUSSY D.L., JR Charactarization of alpha-1 adrenoceptor subtypes in human and canine prostate membranes. J. Pharmacol. Exp. Ther. 1994;271:1228–1233. [PubMed] [Google Scholar]
- GOPALAKRISHNAN M., WHITEAKER K.L., MOLINARI E.J., DAVIS-TABER R., SCOTT V.E.S., SHIEH C.-C., BUCKNER S.A., MILICIC I., CAIN J.C., POSTL S., SULLIVAN J.P., BRIONI J.D. Characterization of the ATP-sensitive potassium channels (KATP) expressed in guinea pig bladder smooth muscle cells. J. Pharmacol. Exp. Ther. 1999;289:551–558. [PubMed] [Google Scholar]
- GOUSSE A., YOSHIDA M., WEISS R.M., LATIPOUR J. Beta adrenergic receptor alterations in diabetic rat prostate: effects of insulin and dietary myoinositol. Prostate. 1991;19:121–131. doi: 10.1002/pros.2990190205. [DOI] [PubMed] [Google Scholar]
- GRUNEBERGER A. Treatment of motor urge incontinence with clenbuterol and flavoxate hydrochloride. Br. J. Obstetr. Gynaecol. 1984;91:275–278. [PubMed] [Google Scholar]
- GU B., REITER J.P., SCHWINN D.A., SMITH M.P., KORSTANJE C., THOR K.B., DOLBER P.C. Effects of α1-adrenergic receptor subtype selective antagonists on lower urinary tract function in rats with bladder outlet obstruction. J. Urol. 2004;172:758–762. doi: 10.1097/01.ju.0000131609.96301.e6. [DOI] [PubMed] [Google Scholar]
- GUH J.-H., CHUEH S.-C., KO F.-N., TENG C.-M. Characterization of α1-adrenoceptor subtypes in tension response of human prostate to electrical field stimulation. Br. J. Pharmacol. 1995;115:142–146. doi: 10.1111/j.1476-5381.1995.tb16331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUH J.-H., KO F.-N., CHUEH S.-C., LAI M.-K., TENG C.-M. Ouabain-induced increases in resting tone of human hyperplastic prostate following repeated noradrenaline and electrical field stimulation. Br. J. Pharmacol. 1996;117:1716–1720. doi: 10.1111/j.1476-5381.1996.tb15344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUILMARD C., AUGUET M., CHABRIER P.E. Pharmacological characterization of alpha1-adrenoceptor subtype mediating regulation of arterial pressure and urethral perfusion pressure in the anaesthetized rat. J. Auton. Pharmacol. 1996;16:197–203. doi: 10.1111/j.1474-8673.1996.tb00423.x. [DOI] [PubMed] [Google Scholar]
- GUIMARAES S., MOURA D. Vascular adrenoceptors: an update. Pharmacol. Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- GUP D.I., SHAPIRO E., BAUMANN M., LEPOR H. Contractile properties of human prostate adenomas and the development of infravesical obstruction. Prostate. 1989;15:105–114. doi: 10.1002/pros.2990150204. [DOI] [PubMed] [Google Scholar]
- GUP D.I., SHAPIRO E., BAUMANN M., LEPOR H. Autonomic receptors in human prostate adenomas. J. Urol. 1990;143:179–185. doi: 10.1016/s0022-5347(17)39906-8. [DOI] [PubMed] [Google Scholar]
- GUTHRIE P.D., FREEMAN M.R., LIAO S.T., CHUNG L.W. Regulation of gene expression in rat prostate by androgen and β-adrenergic receptor pathways. Mol. Endocrinol. 1990;4:1343–1353. doi: 10.1210/mend-4-9-1343. [DOI] [PubMed] [Google Scholar]
- HAMPEL C., ARTIBANI W., ESPUNA PONS M., HAAB F., JACKSON S., ROMERO J., GAVART S., PAPANICOLAOU S. Understanding the burden of stress urinary incontinence in Europe: a qualitative review of the literature. Eur. Urol. 2004;46:15–27. doi: 10.1016/j.eururo.2004.02.003. [DOI] [PubMed] [Google Scholar]
- HAMPEL C., DOLBER P.C., SMITH M.P., SAVIC D.L., THÜROFF J.W., THOR K.B., SCHWINN D.A. Modulation of bladder α1-adrenergic receptor subtype expression by bladder outlet obstruction. J. Urol. 2002;167:1513–1521. [PubMed] [Google Scholar]
- HANCOCK A.A., BUCKNER S.A., IRELAND L.M., KNEPPER S.M., KERWIN J.F., JR Actions of terazosin and its enantiomers at subtypes of α1- and α2-adrenoceptors. J. Receptor Signal Transd. Res. 1995;15:863–885. doi: 10.3109/10799899509049862. [DOI] [PubMed] [Google Scholar]
- HARADA T., CONSTANTINOU C.E. The effect of alpha2 agonists and antagonists on the lower urinary tract of the rat. J. Urol. 1993;149:159–164. doi: 10.1016/s0022-5347(17)36030-5. [DOI] [PubMed] [Google Scholar]
- HATANO A., TANG R., WALDEN P.D., LEPOR H. The α-adrenoceptor antagonist properties of the enantiomers of doxazosin in the human prostate. Eur. J. Pharmacol. 1996;313:135–143. doi: 10.1016/0014-2999(96)00502-x. [DOI] [PubMed] [Google Scholar]
- HAYNES J.M., HILL S.J. β-Adrenoceptor-mediated inhibition of α1-adrenoceptor-mediated and field stimulation-induced contractile responses in the prostate of the guinea pig. Br. J. Pharmacol. 1997;122:1067–1074. doi: 10.1038/sj.bjp.0701494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDLUND H., ANDERSSON K.-E., LARSSON B. Alpha-adrenoceptors and muscarinic receptors in the isolated human prostate. J. Urol. 1985;134:1291–1298. doi: 10.1016/s0022-5347(17)47714-7. [DOI] [PubMed] [Google Scholar]
- HEIN P., GOEPEL M., COTECCHIA S., MICHEL M.C. A quantitative analysis of antagonism and inverse agonism at wild-type and constitutively active hamster α1B-adrenoceptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:34–39. doi: 10.1007/s002100000329. [DOI] [PubMed] [Google Scholar]
- HIEBLE J.P., BYLUND D.B., CLARKE D.E., EIKENBURG D.C., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., RUFFOLO R.R., JR International Union of Pharmacology X. Recommendation for nomenclature of α1-adrenoceptors: consensus update. Pharmacol. Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- HIEBLE J.P., CAINE M., ZALAZNIK E. In vitro characterization of the α-adrenoceptors in human prostate. Eur. J. Pharmacol. 1985;107:111–117. doi: 10.1016/0014-2999(85)90048-2. [DOI] [PubMed] [Google Scholar]
- HIRAOKA Y., OHMURA T., SAKAMOTO S., HAYASHI H., MURAMATSU I. Identification of α1-adrenoceptor subtypes in the rabbit prostate. J. Auton. Pharmacol. 1995;15:271–278. doi: 10.1111/j.1474-8673.1995.tb00310.x. [DOI] [PubMed] [Google Scholar]
- HIRASAWA A., HORIE K., TANAKA T., TAKAGAKI K., MURAI M., YANO J., TSUJIMOTO G. Cloning, functional expression and tissue distribution of human cDNA for the α1C-adrenergic receptor. Biochem. Biophys. Res. Commun. 1993;195:902–909. doi: 10.1006/bbrc.1993.2130. [DOI] [PubMed] [Google Scholar]
- HIRASAWA A., SHIBATA K., HORIE K., TAKEI Y., OBIKA K., TANAKA T., MURAMOTO N., TAKAGAKI K., YANO J., TSUJIMOTO G. Cloning, functional expression and tissue distribution of human α1C-adrenoceptor splice variants. FEBS Lett. 1995;363:256–260. doi: 10.1016/0014-5793(95)00330-c. [DOI] [PubMed] [Google Scholar]
- HOFFMANN C., LEITZ M.R., OBERDORF-MAASS S., LOHSE M.J., KLOTZ K.-N. Comparative pharmacology of human β-adrenergic receptor subtypes – characterization of stably transfected receptors in CHO cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 2004;369:151–159. doi: 10.1007/s00210-003-0860-y. [DOI] [PubMed] [Google Scholar]
- HOMMA Y., HAMADA K., NAKAYAMA Y., TSUJIMOTO G., KAWABE K. Effect of castration on contraction and α1-adrenoceptor expression in rat prostate. Br. J. Pharmacol. 2000;131:1454–1460. doi: 10.1038/sj.bjp.0703706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONDA K., NAKAGAWA C. Alpha-1 adrenoceptor antagonist effects of the optical isomers of YM-12617 in rabbit lower urinary tract and prostate. J. Pharmacol. Exp. Ther. 1986;239:512–516. [PubMed] [Google Scholar]
- HONDA K., MIYATA-OSAWA A., TAKENAKA T. α1-Adrenoceptor subtype mediating contraction of the smooth muscle in the lower urinary tract and prostate of rabbits. Naunyn-Schmiedeberg's Arch. Pharmacol. 1985;330:16–21. doi: 10.1007/BF00586704. [DOI] [PubMed] [Google Scholar]
- HORINOUCHI T., KOIKE K. Agonist activity of SR59230A at atypical β-adrenoceptors in guinea pig gastric fundus and duodenum. Eur. J. Pharmacol. 2001;416:165–168. doi: 10.1016/s0014-2999(01)00854-8. [DOI] [PubMed] [Google Scholar]
- HORINOUCHI T., TANAKA Y., KOIKE K. Evidence for the primary role for 4-aminopyridine-sensitive KV channels in β3-adrenoceptor-mediated, cyclic AMP-independent relaxations of guinea-pig gastrointestinal smooth muscle. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;367:193–203. doi: 10.1007/s00210-002-0658-3. [DOI] [PubMed] [Google Scholar]
- HUDMAN D., ELLIOTT R.A., NORMAN R.I. Inhibition of the contractile response of the rat detrusor muscle by the β2-adrenoceptor agonist clenbuterol. Eur. J. Pharmacol. 2000;392:79–85. doi: 10.1016/s0014-2999(00)00107-2. [DOI] [PubMed] [Google Scholar]
- HUDMAN D., ELLIOTT R.A., WHITAKKER P., TERRY T.R., SANDHU D.P., NORMAN R.I. Inhibition of the contractile responses of isolated human and rat bladders by clenbuterol. J. Urol. 2001;166:1969–1973. [PubMed] [Google Scholar]
- IGAWA Y., YAMAZAKI Y., TAKEDA H., AKAHANE M., AJISAWA Y., YONEYAMA T., NISHIZAWA O. Possible β3-adrenoceptor-mediated relaxation of the human detrusor. Acta Physiol. Scand. 1998;164:117–118. doi: 10.1046/j.1365-201X.1998.00406.x. [DOI] [PubMed] [Google Scholar]
- IGAWA Y., YAMAZAKI Y., TAKEDA H., HAYAKAWA K., AKAHANE M., AJISAWA Y., YONEYAMA T., NISHIZAWA O., ANDERSSON K.-E. Functional and molecular biological evidence for a possible β3-adrenoceptor in the human detrusor muscle. Br. J. Pharmacol. 1999;126:819–825. doi: 10.1038/sj.bjp.0702358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGAWA Y., YAMAZAKI Y., TAKEDA H., KAIDOH K., AKAHANE M., AJISAWA Y., YONEYAMA T., NISHIZAWA O., ANDERSSON K.-E. Relaxant effects of isoproterenol and selective β3-adrenoceptor agonists on normal, low compliant and hyperreflexic human bladders. J. Urol. 2001;165:240–244. doi: 10.1097/00005392-200101000-00071. [DOI] [PubMed] [Google Scholar]
- INCI K., ISMAILOGLU U.B., SAHIN A., SUNUR A., SAHIN-ERDEMLI I. The effect of inflammation on rat urinary bladder-dependent relaxation in coaxial bioassay system. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;367:547–552. doi: 10.1007/s00210-003-0710-y. [DOI] [PubMed] [Google Scholar]
- ISHIZUKA O., MATTIASSON A., ANDERSSON K.-E. Role of spinal and peripheral alpha2 adrenoceptors in micturition in normal conscious rats. J. Urol. 1996a;156:1853–1857. [PubMed] [Google Scholar]
- ISHIZUKA O., PERSSON K., MATTIASSON A., NAYLOR A.M., WYLLIE M.G., ANDERSSON K.-E. Micturition in conscious rats with and without bladder outlet obstruction: role of spinal α1-adrenoceptors. Br. J. Pharmacol. 1996b;117:962–966. doi: 10.1111/j.1476-5381.1996.tb15288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES S., CHAPPLE C.R., PHILLIPS M.I., GREENGRASS P.M., DAVEY M.J., TURNER-WARWICK R.T., MILROY E.J.G., BURNSTOCK G. Autoradiographic analysis of alpha-adrenoceptors and muscarinic cholinergic receptors in the hyperplastic human prostate. J. Urol. 1989;142:438–444. doi: 10.1016/s0022-5347(17)38780-3. [DOI] [PubMed] [Google Scholar]
- JEONG M.S., LEE J.G. The role of spinal and peripheral α1- and α2-adrenoceptors on bladder activity induced by bladder distension and anaesthetized rat. BJU Int. 2000;85:925–931. doi: 10.1046/j.1464-410x.2000.00572.x. [DOI] [PubMed] [Google Scholar]
- JOSEPH S.S., COLLEDGE W.H., KAUMANN A.J. Aspartate138 is required for the high-affinity ligand binding site but not for the low-affinity binding site of the β1-adrenoceptor. Naunyn-Schmiedeberg's Arch. Pharmacol. 2004;370:223–226. doi: 10.1007/s00210-004-0962-1. [DOI] [PubMed] [Google Scholar]
- JOSEPH S.S., LYNHAM J.A., MOLENAAR P., GRACE A.A., COLLEDGE W.H., KAUMANN A.J. Intrinsic sympathomimetic activity of (−)-pindolol mediated through a (−)-propranolol-resistant site of the β1-adrenoceptor in human atrium and recombinant receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;368:496–503. doi: 10.1007/s00210-003-0835-z. [DOI] [PubMed] [Google Scholar]
- JUARRANZ M.G., GUIJARRO L.G., BODEGA G., PRIETO J.C. G-Protein regulation of adenylate cyclase activity in rat prostatic membranes after chronic ethanol ingestion. Prostate. 1998;36:226–234. doi: 10.1002/(sici)1097-0045(19980901)36:4<226::aid-pros3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- KAIDOH K., IGAWA Y., TAKEDA H., YAMAZAKI Y., AKAHANE S., MIYATA H., AJISAWA Y., NISHIZAWA O., ANDERSSON K.-E. Effects of selective β2 and β3-adrenoceptor agonists in detrusor hyperreflexia in conscious cerebral infarcted rats. J. Urol. 2002;168:1247–1252. doi: 10.1016/S0022-5347(05)64634-4. [DOI] [PubMed] [Google Scholar]
- KALODIMOS P.J., VENTURA S. β2-Adrenoceptor-mediated inhibition of field stimulation induced contractile responses of the smooth muscle of the rat prostate gland. Eur. J. Pharmacol. 2001;431:81–89. doi: 10.1016/s0014-2999(01)01414-5. [DOI] [PubMed] [Google Scholar]
- KAVA M.S., BLUE D.R., JR, VIMONT R.L., CLARKE D.E., FORD A.P.D.W. α1L-Adrenoceptor mediation of smooth muscle contraction in rabbit bladder neck: a model for lower urinary tract tissues of man. Br. J. Pharmacol. 1998;123:1359–1366. doi: 10.1038/sj.bjp.0701748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABE K., MORIYAMA N., HAMADA K., ISHIMA T. Density and localization of alpha1-adrenoceptors in hypertrophied prostate. J. Urol. 1990;143:592–595. doi: 10.1016/s0022-5347(17)40036-x. [DOI] [PubMed] [Google Scholar]
- KEAST J.R., KAWATANI M., DE GROAT W.C. Sympathetic modulation of cholinergic transmission in cat vesical ganglia is mediated by α1- and α2-adrenoceptors. Am. J. Physiol. 1990;258:R44–R50. doi: 10.1152/ajpregu.1990.258.1.R44. [DOI] [PubMed] [Google Scholar]
- KENNY B.A., MILLER A.M., WILLIAMSON I.J.R., O'CONNELL J., CHALMERS D.H., NAYLOR A.M. Evaluation of the pharmacological selectivity profile of α1 adrenoceptor antagonists at prostatic α1 adrenoceptors: binding, functional and in vivo studies. Br. J. Pharmacol. 1996;118:871–878. doi: 10.1111/j.1476-5381.1996.tb15480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNY B.A., READ A.M., NAYLOR A.M., GREENGRASS P.M., CARTER A.J., WYLLIE M.G. Effect of alpha1 adrenoceptor antagonists on prostatic pressure and blood pressure in the anesthetized dog. Urology. 1994;44:52–57. doi: 10.1016/s0090-4295(94)80009-x. [DOI] [PubMed] [Google Scholar]
- KILLAM A.L., WATTS S.W., COHEN M.L. Role of α1-adrenoceptors and 5-HT2 receptors in serotonin-induced contraction of rat prostate: autoradiographical and functional studies. Eur. J. Pharmacol. 1995;273:7–14. doi: 10.1016/0014-2999(94)00613-c. [DOI] [PubMed] [Google Scholar]
- KIM J.H., SHIN S.Y., NAM J.H., HONG E.-K., CHUNG Y.-S., JEONG J.Y., KANG J., UHM D.-Y., KIM S.J. Adrenergic regulation of the intracellular [Ca2+] and voltage-operated Ca2+ channel currents in the rat prostate neuroendocrine cells. Prostate. 2003;57:99–110. doi: 10.1002/pros.10277. [DOI] [PubMed] [Google Scholar]
- KIRBY R.S., ANDERSEN M., GRATZKE P., DAHLSTRAND C., HOYE K. A combined analysis of double-blind trials of the efficacy and tolerability of doxazosin-gastrointestinal therapeutic system, doxazosin standard and placebo in patients with benign prostatic hyperplasia. BJU Int. 2001;87:192–200. doi: 10.1046/j.1464-410x.2001.02032.x. [DOI] [PubMed] [Google Scholar]
- KITADA S., KUMAZAWA J. Pharmacological characteristics of smooth muscle in benign prostatic hyperplasia and normal prostatic tissue. J. Urol. 1987;138:158–160. doi: 10.1016/s0022-5347(17)43034-5. [DOI] [PubMed] [Google Scholar]
- KLAUS S., MUZZIN P., REVELLI J.-P., CAWTHORNE M.A., GIACOBINO J.-P., RICQUIER D. Control of β3-adrenergic receptor gene expression in brown adipocytes in culture. Mol. Cell. Endocrinol. 1995;109:189–195. doi: 10.1016/0303-7207(95)03502-x. [DOI] [PubMed] [Google Scholar]
- KNEPPER S.M., BUCKNER S.A., BRUNE M.E., DEBERNARDIS J.F., MEYER M.D., HANCOCK A.A. A-61603, a potent α1-adrenergic receptor agonist, selective for the α1A receptor subtype. J. Pharmacol. Exp. Ther. 1995;274:97–103. [PubMed] [Google Scholar]
- KOBAYASHI H., ADACHI-AKAHANE S., NAGAO T. Involvement of BKCa channels in the relaxation of detrusor muscle viaβ-adrenoceptors. Eur. J. Pharmacol. 2000;404:231–238. doi: 10.1016/s0014-2999(00)00606-3. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI H., MIWA T., NAGAO T., ADACHI-AKAHANE S. Negative modulation of L-type Ca2+ channels viaβ-adrenoceptor stimulation in guinea-pig detrusor smooth muscle cells. Eur. J. Pharmacol. 2003;470:9–15. doi: 10.1016/s0014-2999(03)01762-x. [DOI] [PubMed] [Google Scholar]
- KOLTA M.G., WALLACE L.J., GERALD M.C. Age-related changes in sensitivity of rat urinary bladder to autonomic agents. Mech. Ageing Dev. 1984;27:183–188. doi: 10.1016/0047-6374(84)90043-5. [DOI] [PubMed] [Google Scholar]
- KONDO S., TASHIMA Y., MORITA T. Adenergic and cholinergic muscarinic receptors in the prostate of young and old rabbits. Urol. Int. 1992;49:201–205. doi: 10.1159/000282426. [DOI] [PubMed] [Google Scholar]
- KONDO S., TASHIMA Y., MORITA T. Quantitative analysis of adrenergic alpha-1 and alpha-2 receptors in human prostatic urethral tissue. Br. J. Urol. 1993;72:68–73. doi: 10.1111/j.1464-410x.1993.tb06461.x. [DOI] [PubMed] [Google Scholar]
- KONTANI H., TSUJI T., KIMURA S. Effects of adrenergic α2-receptor agonists on urinary bladder contraction in conscious rats. Jpn. J. Pharmacol. 2000;84:381–390. doi: 10.1254/jjp.84.381. [DOI] [PubMed] [Google Scholar]
- KORTMANN B.B.M., FLORATOS D.L., KIEMENEY L.A.L.M., WIJKSTRA H., DE LA ROSETTE J.J.M.C.H. Urodynamic effects of alpha-adrenoceptor blockers: a review of clinical trials. Urology. 2003;62:1–9. doi: 10.1016/s0090-4295(02)02113-1. [DOI] [PubMed] [Google Scholar]
- KUBOTA Y., NAKAHARA T., YUNOKI M., MITANI A., MARUKO T., SAKAMOTO K., ISHII K. Inhibitory mechanism of BRL37344 on muscarinic receptor-mediated contractions of the rat urinary bladder smooth muscle. Naunyn-Schmiedeberg's Arch. Pharmacol. 2002;366:198–203. doi: 10.1007/s00210-002-0602-6. [DOI] [PubMed] [Google Scholar]
- KUNISAWA Y., KAWABE K., NIIJIMA T., HONDA K., TAKENAKA T. A pharmacological study of alpha adrenergic receptor subtypes in smooth muscle of human urinary bladder base and prostatic urethra. J. Urol. 1985;134:396–398. doi: 10.1016/s0022-5347(17)47185-0. [DOI] [PubMed] [Google Scholar]
- KYPRIANOU N., LITVAK J.P., BORKOWSKI A., JACOBS S.C. Induction of prostate apoptosis by doxazosin in benign prostatic hyperplasia. J. Urol. 1998;159:1810–1815. doi: 10.1016/S0022-5347(01)63162-8. [DOI] [PubMed] [Google Scholar]
- LAGU B., TIAN D., JEON Y., LI C., WETZEL J.M., NAGARATHNAM D., SHEN Q., FORRAY C., CHANG R.S.L., BROTEN T.P., RANSOM R.W., CHAN T.-B., O'MALLEY S.S., SCHORN T.W., RODRIGUES A.D., KASSAHUN K., PETTIBONE D.J., FREIDINGER R., GLUCHOWSKI C. De novo design of a novel oxazolidinone analogue as a potent and selective α1A adrenergic receptor antagonist with high oral bioavailability. J. Med. Chem. 2000;43:2775–2778. doi: 10.1021/jm000085e. [DOI] [PubMed] [Google Scholar]
- LARSSON B., SJÖGREN C., ANDERSSON K.-E. Regional distribution of α-adrenoceptor subtypes in the female rabbit urethra. Acta Physiol. Scand. 1986;126:39–43. doi: 10.1111/j.1748-1716.1986.tb07786.x. [DOI] [PubMed] [Google Scholar]
- LATIFPOUR J., KONDO S., O'HOLLAREN B., MORITA T., WEISS R.M. Autonomic receptors in urinary tract: sex and age differences. J. Pharmacol. Exp. Ther. 1990;253:661–667. [PubMed] [Google Scholar]
- LEBLAIS V., POURAGEAUD F., IVORRA M.D., MARTHAN R., MULLER B. Comparison of the α-adrenoceptor-mediated effects of β3-adrenoceptor ligands in rat pulmonary artery. Naunyn-Schmiedeberg's Arch. Pharmacol. 2005;371:535–539. doi: 10.1007/s00210-005-1067-1. [DOI] [PubMed] [Google Scholar]
- LECCI A., GIULIANI S., TRAMONTANA M., SANTICIOLI P., CRISCUOLI M., DION S., MAGGI C.A. Bladder distension and activation of the efferent function of sensory fibres: similarities with the effect of capsaicin. Br. J. Pharmacol. 1998;124:259–266. doi: 10.1038/sj.bjp.0701820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEVRE-BORG F., O'CONNOR S.E., SCHOEMAKER H., HICKS P.E., LECHAIRE J., GAUTIER E., PIERRE F., PIMOULE C., MANOURY P., LANGER S.Z. Alfuzosin, a selective α1-adrenoceptor antagonist in the lower urinary tract. Br. J. Pharmacol. 1993;109:1282–1289. doi: 10.1111/j.1476-5381.1993.tb13762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEI B., MORRIS D.P., SMITH M.P., SVETKEY L.P., NEWMAN M.F., ROTTER J.I., BUCHANAN T.A., BECKSTROM-STERNBERG S.M., GREEN E.D., SCHWINN D.A. Novel human α1a-adrenoceptor single nucleotide polymorphisms alter receptor pharmacology and biological function. Naunyn-Schmiedeberg's Arch. Pharmacol. 2005;371:229–239. doi: 10.1007/s00210-005-1019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEINEWEBER K., BÜSCHER R., BRUCK H., BRODDE O.-E. Adrenoceptor polymorphisms. Naunyn-Schmiedeberg's Arch. Pharmacol. 2004;369:1–22. doi: 10.1007/s00210-003-0824-2. [DOI] [PubMed] [Google Scholar]
- LEONARDI A., HIEBLE J.P., GUARNERI L., NASELSKY D.P., POGGESI E., SIRONI G., SULPIZIO A.C., TESTA R. Pharmacological characterization of the uroselective alpha-1 antagonist Rec 15/2739 (SB 216469): role of the alpha-1L adrenoceptor in tissue selectivity, part I. J. Pharmacol. Exp. Ther. 1997;281:1272–1283. [PubMed] [Google Scholar]
- LEPOR H., BAUMANN M., SHAPIRO E. The alpha adrenergic binding properties of terazosin in the human prostate adenoma. J. Urol. 1988a;140:664–667. doi: 10.1016/s0022-5347(17)41751-4. [DOI] [PubMed] [Google Scholar]
- LEPOR H., GUP D.I., BAUMANN M., SHAPIRO E. Laboratory assessment of terazosin and alpha-1 blockade in prostatic hyperplasia. Urology. 1988b;32 (Suppl):21–26. [PubMed] [Google Scholar]
- LEPOR H., TANG R., SHAPIRO E. The alpha-adrenoceptor subtype mediating the tension of human prostatic smooth muscle. Prostate. 1993b;22:301–307. doi: 10.1002/pros.2990220404. [DOI] [PubMed] [Google Scholar]
- LEPOR H., TANG R., MERETYK S., SHAPIRO E. Binding and functional properties of alpha1 adrenoceptors in different regions of the human prostate. J. Urol. 1993a;150:253–256. doi: 10.1016/s0022-5347(17)35457-5. [DOI] [PubMed] [Google Scholar]
- LEPOR H., ZHANG W., KOBAYASHI S., TANG R., WANG B., SHAPIRO E. A comparison of the binding and functional properties of alpha-1 adrenoceptors and area density of smooth muscle in the human, canine and rat prostate. J. Pharmacol. Exp. Ther. 1994;270:722–727. [PubMed] [Google Scholar]
- LEVIN R.M., RUGGIERI M.R., WEIN A.J. Identification of receptor subtypes in the rabbit and human urinary bladder by selective radio-ligand binding. J. Urol. 1988;139:844–848. doi: 10.1016/s0022-5347(17)42659-0. [DOI] [PubMed] [Google Scholar]
- LI G., LI K., LI Z., WANG P. Age-dependent changes in β-adrenoceptor function in human detrusor and possible mechanisms. Chin. Med. J. 2003;116:1511–1514. [PubMed] [Google Scholar]
- LI J.H., YASAY G.D., KAU S.T. β-Adrenoceptor subtypes in the detrusor of guinea-pig urinary bladder. Pharmacology. 1992;44:13–18. doi: 10.1159/000138868. [DOI] [PubMed] [Google Scholar]
- LIN V.K., BENAIM E.A., MCCONNELL J.D. Alpha-blockade downregulates myosin heavy chain gene expression in human benign prostatic hyperplasia. Urology. 2001;57:170–175. doi: 10.1016/s0090-4295(00)00842-6. [DOI] [PubMed] [Google Scholar]
- LINDHOLM P., LOSE G. Terbutaline (Bricanyl) in the treatment of female urge incontinence. Urol. Int. 1986;41:158–160. doi: 10.1159/000281188. [DOI] [PubMed] [Google Scholar]
- LLUEL P., DEPLANNE V., HEUDES D., BRUNEVAL P., PALEA S. Age-related changes in urethrovesical coordination in male rats: relationship with bladder instability. Am. J. Physiol. 2003a;284:R1287–R1295. doi: 10.1152/ajpregu.00499.2001. [DOI] [PubMed] [Google Scholar]
- LLUEL P., PALEA S., BARRAS M., GRANDADAM F., HEUDES D., BRUNEVAL P., CORMAN B., MARTIN D.J. Functional and morphological modifications of the urinary bladder in aging female rats. Am. J. Physiol. 2000;278:R964–R972. doi: 10.1152/ajpregu.2000.278.4.R964. [DOI] [PubMed] [Google Scholar]
- LLUEL P., SALEA S., RBIERE P., BARRAS M., TEILLET L., CORMAN B. Increased adrenergic contractility and decreased mRNA expression of NOS III in aging rat urinary bladders. Fundam. Clin. Pharmacol. 2003b;17:633–641. doi: 10.1046/j.1472-8206.2003.00187.x. [DOI] [PubMed] [Google Scholar]
- LONGHURST P.A., LEVENDUSKY M. Pharmacological characterization of β-adrenoceptors mediating relaxation of the rat urinary bladder in vitro. Br. J. Pharmacol. 1999;127:1744–1750. doi: 10.1038/sj.bjp.0702709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA F.H., HIGASHIRA-HOSHI H., ITOH Y. Functional muscarinic M2 and M3 receptors and β-adrenoceptors in cultured rat bladder smooth muscle. Life Sci. 2002;70:1159–1172. doi: 10.1016/s0024-3205(01)01488-6. [DOI] [PubMed] [Google Scholar]
- MACKENZIE J.F., DALY C.J., PEDIANI J.D., MCGRATH J.C. Quantitative imaging in live human cells reveals intracellular α1-adrenoceptor ligand-binding sites. J. Pharmacol. Exp. Ther. 2000;294:434–443. [PubMed] [Google Scholar]
- MAGGI C.A., SANTICIOLI P., FURIO M., MELI A. Dual effects of clonidine on micturition reflex in urethane anesthetized rats. J. Pharmacol. Exp. Ther. 1985;235:528–536. [PubMed] [Google Scholar]
- MALLOY B.J., PRICE D.T., PRICE R.R., BIENSTOCK A.M., DOLE M.K., FUNK B.L., RUDNER X.L., RICHARDSON C.D., DONATUCCI C.F., SCHWINN D.A. α1-Adrenergic receptor subtypes in human detrusor. J. Urol. 1998;160:937–943. doi: 10.1016/S0022-5347(01)62836-2. [DOI] [PubMed] [Google Scholar]
- MALYSZ J., BUCKNER S.A., DAZA A.V., MILICIC I., PEREZ-MEDRANO A., GOPALAKRISHNAN M. Functional characterization of large conductance calcium-activated K+ channel openers in bladder and vascular smooth muscle. Naunyn-Schmiedeberg's Arch. Pharmacol. 2004;369:481–489. doi: 10.1007/s00210-004-0920-y. [DOI] [PubMed] [Google Scholar]
- MARINESE D., PATEL R., WALDEN P.D. Mechanistic investigation of the adrenergic induction of ventral prostate hyperplasia in mice. Prostate. 2003;54:230–237. doi: 10.1002/pros.10170. [DOI] [PubMed] [Google Scholar]
- MARSHALL I., BURT R.P., CHAPPLE C.R. Noradrenaline contractions of human prostate mediated by α1A- (α1c-) adrenoceptor subtype. Br. J. Pharmacol. 1995;115:781–786. doi: 10.1111/j.1476-5381.1995.tb15001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL I., BURT R.P., GREEN G.M., HUSSAIN M.B., CHAPPLE C.R. Different subtypes of α1A-adrenoceptor mediating contraction of rat epididymal vas deferens, rat hepatic portal vein and human prostate distinguished by the antagonist RS 17053. Br. J. Pharmacol. 1996;119:407–415. doi: 10.1111/j.1476-5381.1996.tb16001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN D.J., LLUEL P., GUILLOT E., COSTE A., JAMMES D., ANGEL I. Comparative alpha-1 adrenoceptor subtype selectivity and functional uroselectivity of alpha-1 adrenoceptor antagonists. J. Pharmacol. Exp. Ther. 1997;282:228–235. [PubMed] [Google Scholar]
- MARTORANA G., GIBERTI C., DI SILVERIO F., VON HELAND M., RIGATTI P., COLOMBO R., CASADEI G., PACIFICO P. Effects of short-term treatment with the α1-blocker alfuzosin on urodynamic pressure/flow parameters in patients with benign prostatic hyperplasia. Eur. Urol. 1997;32:47–53. [PubMed] [Google Scholar]
- MARUYAMA K., NAKAMURA T., YOSHIHARA T., FUKUTOMI J., SUGIYAMA K., HATTORIM K., OHNUKI T., WATANABE K., NAGATOMO T. Tamsulosin: assessment of affinity of 3H-prazosin bindings to two α1-adrenoceptor subtypes (α1H and α1L) in bovine prostate and rat heart and brain. Gen. Pharmacol. 1998;31:597–600. doi: 10.1016/s0306-3623(98)00048-2. [DOI] [PubMed] [Google Scholar]
- MATSUBARA S., OKADA H., SHIRAKAWA T., GOTOH A., KUNO T., KAMIDONO S. Estrogen levels influence beta-3-adrenoceptor-mediated relaxation of the female rat detrusor muscle. Urology. 2002;59:621–625. doi: 10.1016/s0090-4295(01)01583-7. [DOI] [PubMed] [Google Scholar]
- MATSUI M., GRIFFIN M.T., SHEHNAZ D., TAKETO M.M., EHLERT F.J. Increased relaxant action of forskolin and isoproterenol against muscarinic agonist-induced contractions in smooth muscle from M2 receptor knockout mice. J. Pharmacol. Exp. Ther. 2003;305:106–113. doi: 10.1124/jpet.102.044701. [DOI] [PubMed] [Google Scholar]
- MCCONNELL J.D., ROEHRBORN C.G., BAUTISTA O., ANDRIOLE G.L., DIXON C.M., KUSEK J.W., LEPOR H., MCVARY K.T., NYBERG L.M., CLARKE H.S., CRAWFORD E.D., DIOKNO A.C., FOLEY J.P., FOSTER H.E., JACOBS S.C., KAPLAN S.A., KREDER K.J., LIEBER M.M., LUCIA M.S., MILLER G.J., MENON M., MILAM D.F., RAMSDELL J.W., SCHENKMAN N.S., SLAWIN K.M., SMITH J.A. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. New Engl. J. Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C. Potential role of α1-adrenoceptor subtypes in the aetiology of LUTS. Eur. Urol. Suppl. 2002;1:5–13. [Google Scholar]
- MICHEL M.C., INSEL P.A. Comparison of cloned and pharmacologically defined rat tissue α1-adrenoceptor subtypes. Naunyn-Schmiedeberg's Arch. Pharmacol. 1994;350:136–142. doi: 10.1007/BF00241087. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., FLANNERY M.T., NARAYAN P. Worldwide experience with alfuzosin and tamsulosin. Urology. 2001;58:508–516. doi: 10.1016/s0090-4295(01)01335-8. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., GRÜBBEL B., TAGUCHI K., VERFÜRTH F., OTTO T., KRÖPFL D. Drugs for treatment of benign prostatic hyperplasia: affinity comparison at cloned α1-adrenoceptor subtypes and in human prostate. J. Auton. Pharmacol. 1996;16:21–28. doi: 10.1111/j.1474-8673.1996.tb00352.x. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., KERKER J., BRANCHEK T.A., FORRAY C. Selective irreversible binding of chloroethylclonidine at α1- and α2-adrenoceptor subtypes. Mol. Pharmacol. 1993;44:1165–1170. [PubMed] [Google Scholar]
- MICHEL M.C., KORSTANJE C., KRAUWINKEL W., SHEAR M., DAVIES J., QUARTEL A. Cardiovascular safety of the oral controlled absorption system (OCAS) formulation of tamsulosin compared to the modified release (MR) formulation. Eur. Urol. Suppl. 2005a;4:53–60. [Google Scholar]
- MICHEL M.C., KORSTANJE C., KRAUWINKEL W., SHEAR M., DAVIES J., QUARTEL A. Comparison of vascular α1-adrenoceptor antagonism of tamsulosin oral controlled absorption system (OCAS) and modified release (MR) formulations. Eur. Urol. Suppl. 2005b;4:45–52. [Google Scholar]
- MICHEL M.C., MEHLBURGER L., SCHUMACHER H., BRESSEL H.-U., GOEPEL M. Effect of diabetes on lower urinary tract symptoms in patients with benign prostatic hyperplasia. J. Urol. 2000a;163:1725–1729. [PubMed] [Google Scholar]
- MICHEL M.C., OELKE M., PETERS S.L.M. The neuro–urological connection. Eur. Urol. Suppl. 2005c;4:18–28. [Google Scholar]
- MICHEL M.C., SCHÄFERS R.F., GOEPEL M. α-Blockers and lower urinary tract function: more than smooth muscle relaxation. BJU Int. 2000b;86 (Suppl. 2):23–30. doi: 10.1046/j.1464-410x.2000.00094.x. [DOI] [PubMed] [Google Scholar]
- MILSOM I., ABRAMS P., CARDOZO L., ROBERTS R.G., THÜROFF J.W., WEIN A.J. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760–766. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- MONNERON M.-C., GILLBERG P.G., OHMAN B., ALBERTS P. In vitro α-adrenoceptor autoradiography of the urethra and urinary bladder of the female pig, cat, guinea-pig and rat. Scand. J. Urol. Nephrol. 2000;34:233–238. doi: 10.1080/003655900750041951. [DOI] [PubMed] [Google Scholar]
- MORITA T., KONDO S. Comparison of selective alpha-1 blockade for alpha-receptors in human hypertrophied prostatic adenomas. Japan. J. Urol. 1992a;3:334–337. doi: 10.5980/jpnjurol1989.83.334. [DOI] [PubMed] [Google Scholar]
- MORITA T., KONDO S. Quantitative analysis of alpha-adrenoceptors in human prostatic capsule, adenoma and urethra – a comparison between normal and hypertrophied prostate. Nippon Hinyokika Gakkai Zasshi. 1992b;83:328–333. doi: 10.5980/jpnjurol1989.83.328. [DOI] [PubMed] [Google Scholar]
- MORITA T., IIZUKA H., IWATA T., KONDO S. Function and distribution of β3-adrenoceptors in rat, rabbit and human urinary bladder and external urethral sphincter. J. Smooth Muscle Res. 2000;36:21–32. doi: 10.1540/jsmr.36.21. [DOI] [PubMed] [Google Scholar]
- MORITA T., MASUDA H., TOSAKA A., ISHIZAKA K., TSUHII T., KONDO S. Sex differences in function and distribution of β-adrenoceptors in rabbit urinary bladder. J. Urol. 1998;159:555–558. doi: 10.1016/s0022-5347(01)63982-x. [DOI] [PubMed] [Google Scholar]
- MORIYAMA N., KURIMOTO S., HORIE S., NASU K., TANAKA T., YANO K., HIRANO H., TSUJIMOTO G., KAWABE K. Detection of α1-adrenoceptor subtypes in human hypertrophied prostate by in situ hybridization. Histochem. J. 1996;28:283–288. doi: 10.1007/BF02409016. [DOI] [PubMed] [Google Scholar]
- MORIYAMA N., YAMAGUCHI T., TAKEUCHI T., SAKAMOTO E., UEKI T., TSUJIMOTO G., KAWABE K. Semiquantitative evaluation of α1A-adrenoceptor subtype mRNA in human hypertrophied and non-hypertrophied prostates: regional comparison. Life Sci. 1998;64:201–210. doi: 10.1016/s0024-3205(98)00552-9. [DOI] [PubMed] [Google Scholar]
- MOTTET N., BREESSOLE F., DELMAS V., ROBERT M., COSTA P. Prostatic tissue distribution of alfuzosin in patients with benign prostatic hyperplasia following repeated oral administration. Eur. Urol. 2003;44:101–105. doi: 10.1016/s0302-2838(03)00154-4. [DOI] [PubMed] [Google Scholar]
- MURAMATSU I., OHMURA T., KIGOSHI S., HASHIMOTO S., OSHITA M. Pharmacological subclassification of α1-adrenoceptors in vascular smooth muscle. Br. J. Pharmacol. 1990;99:197–201. doi: 10.1111/j.1476-5381.1990.tb14678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSSELMAN D.M., FORD A.P.D.W., GENNEVOIS D.J., HARBISON M.L., LAURENT A.L., MOKATRIN A.S., STOLTZ R.R., BLUE D.R. A randomized crossover study to evaluate Ro 115–1240, a selective α1A/L-adrenoceptor partial agonist in women with stress urinary incontinence. BJU Int. 2004;93:78–83. doi: 10.1111/j.1464-410x.2004.04560.x. [DOI] [PubMed] [Google Scholar]
- MUZZIN P., BOSS O., MATHIS N., REVELLI J.-P., GIACOBINO J.-P., WILLCOCKS K., BADMAN G.F., CANTELLO B.C.C., HINDLEY R.M., CAWTHORNE M.A. Characterization of a new, highly specific, β3-adrenergic receptor radioligand, [3H]SB 206606. Mol. Pharmacol. 1994;46:357–363. [PubMed] [Google Scholar]
- NAGMANI R., PASCO D.S., SALAS R.D., FELLER D.R. Evaluation of β-adrenergic receptor subtypes in the human prostate cancer cell line-LNCaP. Biochem. Pharmacol. 2003;65:1489–1494. doi: 10.1016/s0006-2952(03)00105-9. [DOI] [PubMed] [Google Scholar]
- NAKAHARA T., KUBOTA Y., MITANI A., MARUKO T., SAKAMOTO K., ISHII K. Protease-activated receptor-2-mediated contraction in the rat urinary bladder: the role of urinary bladder mucosa. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;367:211–213. doi: 10.1007/s00210-002-0687-y. [DOI] [PubMed] [Google Scholar]
- NAKAHARA T., KUBOTA Y., SAITO M., SAKAMOTO K., ISHII K. Protease-activated receptor-2-mediated contraction of urinary bladder is enhanced in cyclophosphamide-treated rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 2004;369:212–219. doi: 10.1007/s00210-003-0847-8. [DOI] [PubMed] [Google Scholar]
- NAKAHIRA Y., HASHITANI H., FUKUTA H., SASAKI S., KOHRI K., SUZUKI H. Effects of isoproterenol on spontaneous excitations in detrusor muscle cells of the guinea pig. J. Urol. 2001;166:335–340. [PubMed] [Google Scholar]
- NAKAMURA T., YOSHIMURA M., SHINNICK-GALLAGHER P., GALLAGHER J.P., AKASU T. α2 and α1-Adrenoceptors mediate opposite actions on parasympathetic neurons. Brain Res. 1984;323:349–353. doi: 10.1016/0006-8993(84)90312-3. [DOI] [PubMed] [Google Scholar]
- NASU K., MORIYAMA N., FUKASAWA R., TSUJIMOTO G., TANAKA T., YANO J., KAWABE K. Quantification and distribution of α1-adrenoceptor subtype mRNAs in human proximal urethra. Br. J. Pharmacol. 1998;123:1289–1293. doi: 10.1038/sj.bjp.0701731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASU K., MORIYAMA N., KAWABE K., TSUJIMOTO G., MURAI M., TANAKA T., YANO J. Quantification and distribution of α1-adrenoceptor subtype mRNAs in human prostate: comparison of benign hypertrophied tissue and non-hypertrophied tissue. Br. J. Pharmacol. 1996;119:797–803. doi: 10.1111/j.1476-5381.1996.tb15742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NERGARDH A., BOREUS L.O., NAGLO A.S. Characterization of the adrenergic beta-receptor in the urinary bladder of man and cat. Acta Pharmacol. Toxicol. 1977;40:14–21. doi: 10.1111/j.1600-0773.1977.tb02049.x. [DOI] [PubMed] [Google Scholar]
- NISHIMOTO T., LATIFPOUR J., WHEELER M.A., YOSHIDA M., WEISS R.M. Age-dependent alterations in β-adrenergic responsiveness of rat detrusor smooth muscle. J. Urol. 1995;153:1701–1705. [PubMed] [Google Scholar]
- NOBLE A.J., CHESS-WILLIAMS R., COULDWELL C., FURUKAWA K., UCHYIUMA T., KORSTANJE C., CHAPPLE C.R. The effects of tamsulosin, a high affinity antagonist at functional α1A- and α1D-adrenoceptor subtypes. Br. J. Pharmacol. 1997;120:231–238. doi: 10.1038/sj.bjp.0700907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOMIYA M., YAMAGUCHI O. A quantitative analysis of mRNA expression of α1 and β-adrenoceptor subtypes and their functional roles in human normal and obstructed bladders. J. Urol. 2003;170:649–653. doi: 10.1097/01.ju.0000067621.62736.7c. [DOI] [PubMed] [Google Scholar]
- NORLEN L., SUNDIN T., WAAGSTEIN F. Effect of β-adrenoceptor stimulation on the human bladder in vivo. Urol. Int. 1978;33:355–358. doi: 10.1159/000280222. [DOI] [PubMed] [Google Scholar]
- NORMANDIN D.E., LODGE N.J. Pharmacological characterization of the isolated canine prostate. J. Urol. 1996;155:1758–1761. [PubMed] [Google Scholar]
- OBIKA K., SHIBATA A., HORIE K., FOGLAR R., KIMURA K., TSUJIMOTO G. NS-49, a novel α1a-adrenoceptor-selective agonist characterization using recombinant human α1-adrenoceptors. Eur. J. Pharmacol. 1995;291:327–334. doi: 10.1016/0922-4106(95)90073-x. [DOI] [PubMed] [Google Scholar]
- OHMI K., SHINOURA H., NAKAYAMA Y., GODA N., TSUJIMOTO G. Characterization of α1-adrenoceptors expressed in a novel vascular smooth muscle cell line cloned from p53 knockout mice, P53LMAC01 (AC01) cells. Br. J. Pharmacol. 1999;127:756–762. doi: 10.1038/sj.bjp.0702588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHMURA T., OSHITA M., KIGOSHI S., MURAMATSU I. Identification of α1-adrenoceptor subtypes in the rat vas deferens: binding and functional studies. Br. J. Pharmacol. 1992;107:697–704. doi: 10.1111/j.1476-5381.1992.tb14509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHMURA T., SAKAMOTO S., HAYASHI H., KIGOSHI S., MURAMATSU I. Identification of α1-adrenoceptor subtypes in the dog prostate. Urol. Res. 1993;21:211–215. doi: 10.1007/BF00590038. [DOI] [PubMed] [Google Scholar]
- OSHITA M., HIRAOKA Y., WATANABE Y. Characterization of β-adrenoceptors in urinary bladder: comparison between rat and rabbit. Br. J. Pharmacol. 1997;122:1720–1724. doi: 10.1038/sj.bjp.0701562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERÄLÄ M., HIRVONEN H., KALIMO H., ALA-UOTILA S., REGAN J.W., AKERMAN K.E.O., SCHEININ M. Differential expression of two α2-adrenergic receptor subtype mRNAs in human tissues. Mol. Brain Res. 1992;16:57–63. doi: 10.1016/0169-328x(92)90193-f. [DOI] [PubMed] [Google Scholar]
- PETERS S.L.M., MICHEL M.C. cAMP-independent relaxation of smooth muscle cells via Gs-coupled receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;368:329–330. doi: 10.1007/s00210-003-0816-2. [DOI] [PubMed] [Google Scholar]
- PETKOV G.V., NELSON M.T. Differential regulation of Ca2+-activated K+ channels by β-adrenoceptors in guinea pig urinary bladder smooth muscle. Am. J. Physiol. 2005;288:C1255–C1263. doi: 10.1152/ajpcell.00381.2004. [DOI] [PubMed] [Google Scholar]
- POYET P., GAGNE B., LABRIE F. Characteristics of the β-adrenergic stimulation of adenylate cyclase activity in rat ventral prostate and its modulation by androgens. Prostate. 1986a;9:237–245. doi: 10.1002/pros.2990090304. [DOI] [PubMed] [Google Scholar]
- POYET P., GAGNE B., LAVOIE M., LABRIE F. Characteristics of the β-adrenergic receptor in the rat ventral prostate using [125I]cyanopindolol. Mol. Cell. Endocrinol. 1986b;48:59–67. doi: 10.1016/0303-7207(86)90166-8. [DOI] [PubMed] [Google Scholar]
- PRICE D.T., SCHWINN D.A., LOMASNEY J.W., ALLEN L.F., CARON M.G., LEFKOWITZ R.J. Identification, quantification, and localization of mRNA for three distinct alpha1 adrenergic receptor subtypes in human prostate. J. Urol. 1993;150:546–551. doi: 10.1016/s0022-5347(17)35544-1. [DOI] [PubMed] [Google Scholar]
- PULITO V.L., LI X., VARGA S.S., MULCAHY L.S., CLARK K.S., HALBERT S.A., REITZ A.B., MURRAY W.V., JOLLIFFE L.K. An investigation of the uroselective properties of four novel α1a-adrenergic receptor subtype-selective antagonists. J. Pharmacol. Exp. Ther. 2000;294:224–229. [PubMed] [Google Scholar]
- PURVIS K., RUI H., GORDELADZE J.O., ATTRAMADAL H. Hormonal activation of the adenylyl cyclases of the rat and human prostate gland. Prostate. 1986;8:11–24. doi: 10.1002/pros.2990080104. [DOI] [PubMed] [Google Scholar]
- RAMAGE A.G., WYLLIE M.G. A comparison of the effects of doxazosin and terazosin on the spontaneous sympathetic drive to the bladder and related organs in anaesthetized cats. Eur. J. Pharmacol. 1995;294:645–650. doi: 10.1016/0014-2999(95)00599-4. [DOI] [PubMed] [Google Scholar]
- RAMASAMY S., HODGSON W.C., VENTURA S. Protein kinase C and the sub-sensitivity and sub-reactivity of the diabetic rat prostate gland to noradrenaline. Eur. J. Pharmacol. 2002;434:151–161. doi: 10.1016/s0014-2999(01)01541-2. [DOI] [PubMed] [Google Scholar]
- ROEHRBORN C., VAN KERREBROECK P., NORDLING J. Safety and efficacy of alfuzosin 10 mg once-daily in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a pooled analysis of three double-blind, placebo-controlled studies. BJU Int. 2003;92:257–261. doi: 10.1046/j.1464-410x.2003.04309.x. [DOI] [PubMed] [Google Scholar]
- ROEHRBORN C.G., SCHWINN D.A. α1-Adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J. Urol. 2004;171:1029–1035. doi: 10.1097/01.ju.0000097026.43866.cc. [DOI] [PubMed] [Google Scholar]
- ROKOSH D.G., BAILEY B.A., STEWART A.F.R., KARNS L.R., LONG C.S., SIMPSON P.C. Distribution of α1C-adrenergic receptor mRNA in adult rat tissues by RNase protection assay and comparison with α1B and α1D. Biochem. Biophys. Res. Commun. 1994;200:1177–1184. doi: 10.1006/bbrc.1994.1575. [DOI] [PubMed] [Google Scholar]
- ROMIC I., KISS T., KISBENEDEK L., KONDAS J., TORZSOK F., MILAK M., AVIS M., KRAUWINKEL W., SWART P., KORSTANJE C.2003Tamsulosin drug ratio in prostate versus free fraction in plasma supports pharmacokinetic (PK) contribution to its uroselectivity J. Urol. 169(Suppl)28812478165 [Google Scholar]
- RUDNER X.L., BERKOWITZ B.A., BOOTH J.V., FUNK B.L., COZART K.L., D'AMICO E.B., EL-MOALEM H., PAGE S.O., RICHARDSON C.D., WINTERS B., MARUCCI L., SCHWINN D.A. Subtype specific regulation of human vascular α1-adrenergic receptors by vessel bed and age. Circulation. 1999;100:2336–2343. doi: 10.1161/01.cir.100.23.2336. [DOI] [PubMed] [Google Scholar]
- SANTICIOLI P., MAGGI C.A., MELI A. The effects of clonidine on electrically-induced contractions of rat detrusor strips in vitro. J. Auton. Pharmacol. 1983;3:161–166. doi: 10.1111/j.1474-8673.1983.tb00531.x. [DOI] [PubMed] [Google Scholar]
- SATO S., OHTAKE A., MATSUSHIMA H., SAITOH C., USUDA S., MIYATA K. Pharmacological effect of tamsulosin in relation to dog plasma and tissue concentrations: prostatic and urethral retention possibly contributes to uroselectivity of tamsulosin. J. Pharmacol. Exp. Ther. 2001;296:697–703. [PubMed] [Google Scholar]
- SCHNEIDER T., HEIN P., MICHEL-REHER M., MICHEL M.C. Effects of ageing on muscarinic receptor subtypes and function in rat urinary bladder. Naunyn-Schmiedeberg's Arch. Pharmacol. 2005;372:71–78. doi: 10.1007/s00210-005-1084-0. [DOI] [PubMed] [Google Scholar]
- SCOFIELD M.A., LIU F., ABEL P.W., JEFFRIES W.B. Quantification of steady state expression of mRNA for alpha-1 adrenergic receptor subtypes using reverse transcription and a competitive polymerase chain reaction. J. Pharmacol. Exp. Ther. 1995;275:1035–1042. [PubMed] [Google Scholar]
- SEGUCHI H., NISHIMURA J., ZHOU Y., NIIRO N., KUMAZAWA J., KANAIDE H. Expression of β3-adrenoceptors in rat detrusor muscle. J. Urol. 1998;159:2197–2201. doi: 10.1016/S0022-5347(01)63305-6. [DOI] [PubMed] [Google Scholar]
- SHAPIRO E., LEPOR H. α2 adrenergic receptors in hyperplastic human prostate: identification and characterization using [3H]rauwolscine. J. Urol. 1986;135:1038–1042. doi: 10.1016/s0022-5347(17)45971-4. [DOI] [PubMed] [Google Scholar]
- SHAPIRO E., BECICH M.J., HARTANTO V., LEPOR H. The relative proportion of stromal and epithelial hyperplasia is related to the development of symptomatic benign prostatic hyperplasia. J. Urol. 1992;147:1293–1297. doi: 10.1016/s0022-5347(17)37546-8. [DOI] [PubMed] [Google Scholar]
- SHAPIRO E., TSITLIJK J.E., LEPOR H. α2 adrenergic receptors in canine prostate: biochemical and functional correlations. J. Urol. 1987;137:565–570. doi: 10.1016/s0022-5347(17)44107-3. [DOI] [PubMed] [Google Scholar]
- SHIMA S., KAWASHIMA Y., HIRAI M., ASAKURA M. Effect of adrenergic stimulation on adenylate cyclase activity in rat prostate. Biochim. Biophys. Acta. 1980;628:255–262. doi: 10.1016/0304-4165(80)90374-8. [DOI] [PubMed] [Google Scholar]
- SIGALA S., PERONI A., MIRABELLA G., FORNARI S., PALAZZOLO F., PEZZOTTI G., SIMEONE C., COSCIANI CUNICO S., SPANO P.F. Alpha1 adrenoceptor subtypes in human urinary bladder: sex and regional comparison. Life Sci. 2004;76:417–427. doi: 10.1016/j.lfs.2004.09.008. [DOI] [PubMed] [Google Scholar]
- SLATER M., BARDEN J.A., MURPHY C.R. Tyrosine kinase A, autonomic and transmitter receptors, but not innervation, are upregulated in the aging rat prostate. Acta Histochem. 2000;102:427–438. doi: 10.1078/0065-1281-00565. [DOI] [PubMed] [Google Scholar]
- SMITH L.M., KAJIOKA S., BRADING A.F., NAKAYAMA S. Effects of phosphorylation-related drugs on slow Ca2+ tail current in guinea-pig detrusor cells. Eur. J. Pharmacol. 1999;370:187–193. doi: 10.1016/s0014-2999(99)00119-3. [DOI] [PubMed] [Google Scholar]
- SOLANO R.M., CARMENA M.J., GUIJARRO L.G., PRIETO J.C. Neuropeptide Y inhibits vasoactive intestinal peptide-stimulated adenylyl cyclase in rat ventral prostate. Neuropeptides. 1994;27:31–37. doi: 10.1016/0143-4179(94)90014-0. [DOI] [PubMed] [Google Scholar]
- SOMERS W.J., FELSEN D., CHOU T.C., MARION D.N., CHERNESKY C.E., VAUGHAN E.D., JR An in vivo evaluation of alpha adrenergic receptors in canine prostate. J. Urol. 1989;141:1230–1233. doi: 10.1016/s0022-5347(17)41227-4. [DOI] [PubMed] [Google Scholar]
- SOMOGYI G.T., TANOWITZ M., DE GROAT W.C. Prejunctional facilitatory α1-adrenoceptors in the rat urinary bladder. Br. J. Pharmacol. 1995;114:1710–1716. doi: 10.1111/j.1476-5381.1995.tb14961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPRINGER J.P., KROPP B.P., THOR K.B. Facilitatory and inhibitory effects of selective norepinephrine reuptake inhibitors on hypogastric nerve-evoked urethral contractions in the cat: a prominent role of urethral β-adrenergic receptors. J. Urol. 1994;152:515–519. doi: 10.1016/s0022-5347(17)32785-4. [DOI] [PubMed] [Google Scholar]
- STEIDLE C.P., COHEN M.L., HOOVER D.M., NEUBAUER B.L. Comparative contractile responses among ventral, dorsal, and lateral lobes of the rat prostate. Prostate. 1989;15:53–63. doi: 10.1002/pros.2990150106. [DOI] [PubMed] [Google Scholar]
- SUGAYA K., NISHIJIMA S., MIYAZATO M., ASHITOMI K., HATANO T., OGAWA Y. Effects of intrathecal injection of tamsulosin and naftopidil, alpha-1A and -1D adrenergic receptor antagonists, on bladder activity in rats. Neurosci. Lett. 2002;328:74–76. doi: 10.1016/s0304-3940(02)00459-7. [DOI] [PubMed] [Google Scholar]
- SUZUKI F., MIYAMOTO S., TAKITA M., OSHITA M., WATANABE Y., KAKIZUKA A., NARUMIYA S., TANIGUCHI T., MURAMATSU I. Cloning, functional expression and tissue distribution of rabbit α1d-adrenoceptor. Biochim. Biophys. Acta. 1997;1323:6–11. doi: 10.1016/s0005-2736(96)00229-5. [DOI] [PubMed] [Google Scholar]
- SWIERZEWSKI S.J.I., GORMLEY E.A., BELVILLE W.D., SWEETSER P.M., WAN J., MCGUIRE E.J. The effect of terazosin on bladder function in the spinal cord injured patient. J. Urol. 1994;151:951–954. doi: 10.1016/s0022-5347(17)35132-7. [DOI] [PubMed] [Google Scholar]
- SZELL E.A., YAMAMOTO T., DE GROAT W.C., SOMOGYI G.T. Smooth muscle and parasympathetic nerve terminals in the rat urinary bladder have different subtypes of α1 adrenoceptors. Br. J. Pharmacol. 2000;130:1685–1691. doi: 10.1038/sj.bjp.0703475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGUCHI K., SAITOH M., SATO S., ASANO M., MICHEL M.C. Effects of tamsulosin metabolites at alpha-1 adrenoceptor subtypes. J. Pharmacol. Exp. Ther. 1997;280:1–5. [PubMed] [Google Scholar]
- TAHMATZOPOULOS A., KYPRIANOU N. Apoptotic impact of α1-blockers on prostate cancer growth: a myth or an inviting reality. Prostate. 2004;59:91–100. doi: 10.1002/pros.10357. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI S., MORIYAMA N., YAMAZAKI R., KAWABE K. Urodynamic analysis of age-related changes of α1-adrenoceptor responsiveness in female beagle dogs. J. Urol. 1996;156:1485–1488. [PubMed] [Google Scholar]
- TAKAHASHI W., AFIATPOUR P., FOSTER H.E., JR, IKEDA K., WADA Y., WEIS R.M., LATIFPOUR J. The effect of castration on endothelins, their receptors and endothelin converting enzyme in rat prostate. Naunyn-Schmiedeberg's Arch. Pharmacol. 2002;366:166–176. doi: 10.1007/s00210-002-0575-5. [DOI] [PubMed] [Google Scholar]
- TAKEDA H., IGAWA Y., KOMATSU Y., YAMAZAKI Y., AKAHANE M., NISHIZAWA O., AJISAWA Y. Characterization of β-adrenoceptor subtypes in the ferret urinary bladder in vitro and in vivo. Eur. J. Pharmacol. 2000a;403:147–155. doi: 10.1016/s0014-2999(00)00586-0. [DOI] [PubMed] [Google Scholar]
- TAKEDA H., MATSUZAWA A., IGAWA Y., YAMAZAKI Y., KAIDOH K., AKAHANE S., KOJIMA M., MIYATA H., AKAHANE M., NISHIZAWA O. Functional characterization of β-adrenoceptor subtypes in the canine and rat lower urinary tract. J. Urol. 2003;170:654–658. doi: 10.1097/01.ju.0000074622.50255.a8. [DOI] [PubMed] [Google Scholar]
- TAKEDA H., YAMAZAKI Y., AKAHANE M., AKAHANE S., MIYATA H., IGAWA Y., NISHIZAWA O. Characterization of β-adrenoceptor subtype in bladder smooth muscle of cynomolgus monkey. Jpn. J. Pharmacol. 2002a;88:108–113. doi: 10.1254/jjp.88.108. [DOI] [PubMed] [Google Scholar]
- TAKEDA H., YAMAZAKI Y., AKAHANE M., IGAWA Y., AJISAWA Y., NISHIZAWA O. Role of β3-adrenoceptor in urine storage in the rat: comparison between the selective β3-adrenoceptor agonist, CL316, 243, and various smooth muscle relaxants. J. Pharmacol. Exp. Ther. 2000b;293:939–945. [PubMed] [Google Scholar]
- TAKEDA H., YAMAZAKI Y., IGAWA Y., KAIDOH K., AKAHANE S., MIYATA H., NISHIZAWA O., AKAHANE M., ANDERSSON K.-E. Effects of β3-adrenoceptor stimulation on prostaglandin E2-induced bladder hyperreactivity and on the cardiovascular system in conscious rats. Neurourol. Urodyn. 2002b;21:558–565. doi: 10.1002/nau.10034. [DOI] [PubMed] [Google Scholar]
- TAKEDA M., OBARA K., MIZUSAWA T., TOMITA Y., ARAI K., TSUTSUI T., HATANO A., TAKAHASHI K., NOMURA S. Evidence for β3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: analysis by molecular biological and pharmacologocial methods. J. Pharmacol. Exp. Ther. 1999;288:1367–1373. [PubMed] [Google Scholar]
- TANAKA Y., YAMASHITA Y., YAMAKI F., HORINOUCHI T., SHIGENOBU K., KOIKE K. Evidence for a significant role of a Gs-triggered mechanism unrelated to the activation of adenylyl cyclase in the cyclic AMP-independent relaxant response of guinea-pig tracheal smooth muscle. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;368:437–441. doi: 10.1007/s00210-003-0809-1. [DOI] [PubMed] [Google Scholar]
- TENG C.-M., GUH J.-H., KO F.-N. Functional identification of α1-adrenoceptor subtypes in human prostate: comparison with those in rat vas deferens and spleen. Eur. J. Pharmacol. 1994;265:61–66. doi: 10.1016/0014-2999(94)90223-2. [DOI] [PubMed] [Google Scholar]
- TESTA R., GUARNERI L., ANGELICO P., POGGESI E., TADDEI C., SIRONI G., COLOMBO D., SULPIZIO A.C., NASELSKY D.P., HIEBLE J.P., LEONARDI A. Pharmacological characterization of the uroselective alpha-1 antagonist Rec 15/2739 (SB 216469): role of the alpha-1L adrenoceptor in tissue selectivity, part II. J. Pharmacol. Exp. Ther. 1997;281:1284–1293. [PubMed] [Google Scholar]
- TESTA R., GUARNERI L., IBBA M., STRADA G., POGGESI E., TADDEI C., SIMONAZZI I., LEONARDI A. Characterization of α1-adrenoceptor subtypes in prostate and prostatic urethra of rat, rabbit, dog and man. Eur. J. Pharmacol. 1993;249:307–315. doi: 10.1016/0014-2999(93)90527-o. [DOI] [PubMed] [Google Scholar]
- TESTA R., GUARNERI L., TADDEI C., POGGESI E., ANGELICO P., SARTANI A., LEONARDI A., GOFRIT O.N., MERETYK S., CAINE M. Functional antagonistic activity of Rec 15/2739, a novel alpha-1 antagonist selective for the lower urinary tract, on noradrenaline-induced contraction of human prostate and mesenteric artery. J. Pharmacol. Exp. Ther. 1996;277:1237–1246. [PubMed] [Google Scholar]
- THIND P., LOSE G., COLSTRUP H., ANDERSSON K.-E. The effects of α-adrenoceptor stimulation and blockade on the static urethral sphincter function in healthy females. Scand. J. Urol. Nephrol. 1992;26:219–225. doi: 10.3109/00365599209180872. [DOI] [PubMed] [Google Scholar]
- THIND P., LOSE G., COLSTRUP H., ANDERSSON K.-E. The effect of pharmacological stimulation and blockade of autonomic receptors on the urethral pressure and power generation during coughing and squeezing of the pelvic floor in healthy females. Scand. J. Urol. Nephrol. 1993a;27:519–525. doi: 10.3109/00365599309182286. [DOI] [PubMed] [Google Scholar]
- THIND P., LOSE G., COLSTRUP H., ANDERSSON K.-E. The influence of β-adrenoceptor and muscarinic receptor agonists and antagonists on the static urethral closure function in healthy females. Scand. J. Urol. Nephrol. 1993b;27:31–38. doi: 10.3109/00365599309180411. [DOI] [PubMed] [Google Scholar]
- TSENG-CRANK J., KOST T., GOETZ A., HAZUM S., ROBERSON K.M., HAIZLIP J., GODINOT N., ROBERTSON C.N., SAUSSY D. The α1C-adrenoceptor in human prostate: cloning, functional expression, and localization to specific prostatic cell types. Br. J. Pharmacol. 1995;115:1475–1485. doi: 10.1111/j.1476-5381.1995.tb16640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUJII T., AZUMA H., YAMAGUCHI T., OSHIMA H. A possible role of decreased relaxation mediated by β-adrenoceptors in bladder outlet obstruction by benign prostatic hypertrophy. Br. J. Pharmacol. 1992;107:803–807. doi: 10.1111/j.1476-5381.1992.tb14527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUJIMOTO G., TIMMINS P.V., HOFFMAN B.B. Alpha adrenergic receptors in the rabbit bladder base smooth muscle: alpha-1 adrenergic receptors mediate contractile responses. J. Pharmacol. Exp. Ther. 1986;236:384–389. [PubMed] [Google Scholar]
- TSURUSAKI M., YOSHIDA M., AKASU T., NAGATSU I. α2-Adrenoceptors mediate the inhibition of cholinergic transmission in parasympathetic ganglia of the rabbit urinary bladder. Synapse. 1990;5:233–240. doi: 10.1002/syn.890050309. [DOI] [PubMed] [Google Scholar]
- TUCCI P., BARTOCCI C., BOLLE P. Cyclo-oxygenase- and capsaicin-sensitive afferent fibres affect beta-adrenoceptor-evoked response in the rat urinary bladder. Pharmacology. 2002;64:57–62. doi: 10.1159/000056151. [DOI] [PubMed] [Google Scholar]
- TUGAY M., YILDIZ F., UTKAN T., GACAR N., ULAK G., ERDEN F. Age-related smooth muscle reactivity changes in the rat bladder: an in vitro study. Pharmacol. Res. 2003;48:329–334. doi: 10.1016/s1043-6618(03)00105-1. [DOI] [PubMed] [Google Scholar]
- UCHIDA H., SHISHIDO K., NOMIYA M., YAMAGUCHI O. Involvement of cyclic AMP-dependent and -independent mechanisms in the relaxation of rat detrusor muscle viaβ-adrenoceptors. Eur. J. Pharmacol. 2005;518:195–202. doi: 10.1016/j.ejphar.2005.06.029. [DOI] [PubMed] [Google Scholar]
- UEDA S., SATAKE N., SHIBATA S. α1- and α2-adrenoceptors in the smooth muscle of isolated rabbity urinary bladder and urethra. Eur. J. Pharmacol. 1984;103:249–254. doi: 10.1016/0014-2999(84)90484-9. [DOI] [PubMed] [Google Scholar]
- VAN DER GRAAF P.H., DEPLANNE V., DUQUENNE C., ANGEL I. Analysis of α1-adrenoceptors in rabbit lower urinary tract and mesenteric artery. Eur. J. Pharmacol. 1997;327:25–32. doi: 10.1016/s0014-2999(97)89674-4. [DOI] [PubMed] [Google Scholar]
- VAN DER WERF B.A., CREED K.E. Mechanical properties and innervation of the smooth muscle layers of the urethra of greyhounds. BJU Int. 2002;90:588–595. doi: 10.1046/j.1464-410x.2002.02971.x. [DOI] [PubMed] [Google Scholar]
- WAGHE M., WESTWOOD R., NUNN G., KALINOWSKI A., ALDRIDGE A. Urinary tract toxicity in rats following administration of beta 3-adrenoceptor agonists. Toxicol. Pathol. 1999;27:165–170. doi: 10.1177/019262339902700203. [DOI] [PubMed] [Google Scholar]
- WALDEN P.D., DURKIN M.M., LEPOR H., WETZEL J.M., GLUCHOWSKI C., GUSTAFSON E.L. Localization of mRNA and receptor binding sites for the α1A-adrenoceptor subtype in the rat, monkey and human urinary bladder and prostate. J. Urol. 1997;157:1032–1038. [PubMed] [Google Scholar]
- WALDEN P.D., GLOBINA Y., NIEDER A. Induction of anoikis by doxazosin in prostate cancer cells is associated with activation of caspase-3 and a reduction of focal adhesion kinase. Urol. Res. 2004;32:261–265. doi: 10.1007/s00240-003-0365-7. [DOI] [PubMed] [Google Scholar]
- WEINBERG D.H., TRIVEDI P., TAN C.P., MITRA S., PERKINS-BARROW A., BORKOWSKI D., STRADER C.D., BAYNE M. Cloning, expression and characterization of human α adrenergic receptors α1A, α1B and α1C. Biochem. Biophys. Res. Commun. 1994;201:1296–1304. doi: 10.1006/bbrc.1994.1845. [DOI] [PubMed] [Google Scholar]
- WILLIAMS T.J., BLUE D.R., DANIELS D.V., DAVIS B., ELWORTHY T., GEBER J.R., KAVA M.S., MORGANS D., PADILLA F., TASSA S., VIMONT R.L., CHAPPLE C.R., CHESS-WILLIAMS R., EGLEN R.M., CLARKE D.E., FORD A.P.D.W. In vitro α1-adrenoceptor pharmacology of Ro 70.0004 and RS-100329, novel α1A-adrenoceptor selective antagonists. Br. J. Pharmacol. 1999;127:252–258. doi: 10.1038/sj.bjp.0702541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITJES W.P.J., ROSIER P.F.W.M., CARIS C.T.M., DEBRUYNE F.M.J., DE LA ROSETTE J.J.M.C.H. Urodynamic and clinical effects of terazosin therapy in symptomatic patients with and without bladder outflow obstruction: a stratified analysis. Urology. 1997;49:197–206. doi: 10.1016/S0090-4295(96)00490-6. [DOI] [PubMed] [Google Scholar]
- WITTE D.G., BRUNE M.E., KATWALA S.P., MILICIC I., KERWIN J.F., JR, HANCOCK A.A. Relationships between pharmacokinetics and blockade of agonist-induced prostatic intraurethral pressure and mean arterial pressure in the conscious dog after single and repeated daily oral administration of terazosin. J. Pharmacol. Exp. Ther. 1997;282:891–898. [PubMed] [Google Scholar]
- WITTE D.G., BRUNE M.E., KATWALA S.P., MILICIC I., STOLARIK D., HUI Y.-H., MARSH K.C., KERWIN J.F., JR, MEYER M.D., HANCOCK A.A. Modeling of relationships between pharmacokinetics and blockade of agonist-induced elevation of intraurethral pressure and mean arterial pressure in conscious dogs treated with α1-adrenoceptor antagonists. J. Pharmacol. Exp. Ther. 2002;300:495–504. doi: 10.1124/jpet.300.2.495. [DOI] [PubMed] [Google Scholar]
- WOODS M., CARSON N., NORTON N.W., SHELDON J.H., ARGENTIERI T.M. Efficacy of the β3-adrenergic receptor agonist CL-316243 on experimental bladder hyperreflexia and detrusor instability in the rat. J. Urol. 2001;166:1142–1147. [PubMed] [Google Scholar]
- YAMADA S., SUZUKI M., KATO Y., KIMURA R., MORI R., MATSUMOTO K., MARUYAMA M., KAWABE K. Binding characteristics of naftopidil and α1-adrenoceptor antagonists to prostatic α-adrenoceptors in benign prostatic hypertrophy. Life Sci. 1992;50:127–135. doi: 10.1016/0024-3205(92)90294-y. [DOI] [PubMed] [Google Scholar]
- YAMAGISHI R., AKIYAMA K., NAKAMURA S., HORA M., MASUDA N., MATSUZAWA A., MURATA S., UJIIE A., KURASHINA Y., IIZUKA K., KITAZAWA M. Effect of KMD-3213, an α1a-adrenoceptor-selective antagonist, on the contractions of rabbit prostate and rabbit and rat aorta. Eur. J. Pharmacol. 1996;315:73–79. doi: 10.1016/s0014-2999(96)00589-4. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO Y.L., MORI A., KOIKE K. Adrenoceptor in the detrusor of guinea pig bladder. J. Smooth Muscle Res. 1998;34:233–242. doi: 10.1540/jsmr.34.233. [DOI] [PubMed] [Google Scholar]
- YAMANISHI T., CHAPPLE C.R., YASUDA K., YOSHIDA K., CHESS-WILLIAMS R. The role of β3-adrenoceptors in mediating relaxation of porcine detrusor muscle. Br. J. Pharmacol. 2002b;135:129–134. doi: 10.1038/sj.bjp.0704470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMANISHI T., CHAPPLE C.R., YASUDA K., YOSHIDA K., CHESS-WILLIAMS R. The role of M2 muscarinic receptor subtypes mediating contraction of the circular and longitudinal smooth muscle of the pig proximal urethra. J. Urol. 2002a;168:308–314. [PubMed] [Google Scholar]
- YAMANISHI T., CHAPPLE C.R., YASUDA K., YOSHIDA K., CHESS-WILLIAMS R. Role of β-adrenoceptor subtypes in mediating relaxation of the pig bladder trigonal muscle in vitro. Neurourol. Urodyn. 2003a;22:338–342. doi: 10.1002/nau.10130. [DOI] [PubMed] [Google Scholar]
- YAMANISHI T., CHAPPLE C.R., YASUDA K., YOSHIDA K., CHESS-WILLIAMS R. Identification of β-adrenoceptor subtypes in lower urinary tract of the female pig. J. Urol. 2002c;168:2706–2710. doi: 10.1016/S0022-5347(05)64248-6. [DOI] [PubMed] [Google Scholar]
- YAMANISHI T., CHAPPLE C.R., YASUDA K., YOSHIDA K., CHESS-WILLIAMS R. The functional role of β-adrenoceptor subtypes in mediating relaxation of pig urethral smooth muscle. J. Urol. 2003b;170:2508–2511. doi: 10.1097/01.ju.0000085596.11247.78. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI Y., TAKEDA H., AKAHANE M., IGAWA Y., NISHIZAWA O., AJISAWA Y. Species differences in the distribution of β-adrenoceptor subtypes in bladder smooth muscle. Br. J. Pharmacol. 1998;124:593–599. doi: 10.1038/sj.bjp.0701870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG M., BÜSCHER R., TAGUCHI K., GRÜBBEL B., INSEL P.A., MICHEL M.C. Protein kinase C does not mediate phenylephrine-induced down-regulation of Madin–Darby canine kidney cell alpha-1B adrenoceptors. J. Pharmacol. Exp. Ther. 1998a;286:36–43. [PubMed] [Google Scholar]
- YANG M., REESE J., COTECCHIA S., MICHEL M.C. Murine alpha1-adrenoceptor subtypes. I. Radioligand binding studies. J. Pharmacol. Exp. Ther. 1998b;286:841–847. [PubMed] [Google Scholar]
- YANG M., RUAN J., VOLLER M., SCHALKEN J., MICHEL M.C. Differential regulation of human α1-adrenoceptor subtypes. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:439–446. doi: 10.1007/pl00005373. [DOI] [PubMed] [Google Scholar]
- YANG M., VERFÜRTH F., BÜSCHER R., MICHEL M.C. Is α1D-adrenoceptor protein detectable in rat tissues. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;355:438–446. doi: 10.1007/pl00004966. [DOI] [PubMed] [Google Scholar]
- YAZAWA H., HONDA K. α1-Adrenoceptor subtype in the rat prostate is preferentially the a1A type. Jpn. J. Pharmacol. 1993;62:297–304. doi: 10.1254/jjp.62.297. [DOI] [PubMed] [Google Scholar]
- YONO M., FOSTER H.E., JR, TAKAHASHI W., POURESMAIL M., LATIFPOUR J. Doxazosin-induced up-regulation of α1A-adrenoceptor mRNA in the rat lower urinary tract. Can. J. Physiol. Pharmacol. 2004;82:872–878. doi: 10.1139/y04-098. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA N., SASA M., OHNO Y., YOSHIDA O., TAKAORI S. Contraction of urinary bladder by central norepinephrine originating in the locus coeruleus. J. Urol. 1988;139:423–427. doi: 10.1016/s0022-5347(17)42448-7. [DOI] [PubMed] [Google Scholar]
- YOSHIYAMA M., DE GROAT W.C. Role of spinal α1-adrenoceptor subtypes in the bladder reflex in anesthetized rats. Am. J. Physiol. 2001;280:R1414–R1419. doi: 10.1152/ajpregu.2001.280.5.R1414. [DOI] [PubMed] [Google Scholar]
- YU S.-M., KO F.-N., CHUEH S.-C., CHEN J., CHEN S.-C., CHEN C.-C., TENG C.-M. Effects of dicentrine, a novel α1-adrenoceptor antagonist, on human hyperplastic prostates. Eur. J. Pharmacol. 1994;252:29–34. doi: 10.1016/0014-2999(94)90571-1. [DOI] [PubMed] [Google Scholar]