Abstract
Lower urinary tract symptoms (LUTS) are present in many common urological syndromes. However, their current suboptimal management by muscarinic and α1-adrenoceptor antagonists leaves a significant opportunity for the discovery and development of superior medicines. As potential targets for such therapeutics, purinoceptors have emerged over the last two decades from investigations that have established a prominent role for ATP in the regulation of urinary bladder function under normal and pathophysiological conditions. In particular, evidence suggests that ATP signaling via P2X1 receptors participates in the efferent control of detrusor smooth muscle excitability, and that this function may be heightened in disease and aging. ATP also appears to be involved in bladder sensation, via activation of P2X3 and P2X2/3 receptors on sensory afferent neurons, both within the bladder itself and possibly at central synapses. Such findings are based on results from classical pharmacological and localization studies in non-human and human tissues, knockout mice, and studies using recently identified pharmacological antagonists – some of which possess attributes that offer the potential for optimization into candidate drug molecules. Based on recent advances in this field, it is clearly possible that the development of selective antagonists for these receptors will occur that could lead to therapies offering better relief of sensory and motor symptoms for patients, while minimizing the systemic side effects that limit current medicines.
Keywords: ATP, urinary bladder, P2X1 receptors, P2X3 receptors, P2X receptor antagonist
Background
The collective term ‘lower urinary tract symptoms' (often as the acronym ‘LUTS') has become common in urological specialties in recent years (Abrams et al., 2002). LUTS include urinary urgency, frequency, nocturia, discomfort, urge incontinence and obstruction, and are variously manifested in syndromes such as overactive bladder (OAB) and benign prostatic hyperplasia (BPH). In patients with the more morbid conditions of chronic abacterial prostatitis/chronic pelvic pain syndrome (CAP/CPPS) and interstitial cystitis (IC), these symptoms present with a persistent or phasic burden of pain emanating from the pelvic region (Egan & Krieger, 1997; Kream & Carr, 1999; Nickel, 2003). The prevalence of LUTS is extremely high; it is estimated that in the seven major pharmaceutical markets (U.S.A., Japan, U.K., France, Germany, Italy, and Spain), in excess of 100 million men and women are afflicted with the bothersome LUTS of OAB and BPH (Caddle et al., 2003; Fernandes & Cocoros, 2005). A smaller but still significant number of patients, possibly up to 12 million, are estimated to fit the diagnosis of CAP/CPPS and IC (Jones & Nyberg, 1997; Krieger et al., 2003; Schaeffer, 2003; Clemens et al., 2005).
Current treatment options for patients with LUTS include the nonselective muscarinic antagonists (e.g. tolterodine, oxybutynin, trospium) that are used principally in patients with OAB and urinary urge incontinence (Chapple, 2000; Wein, 2001; Michel et al., 2005), and the selective α1-adrenoceptor antagonists (e.g. tamsulosin, doxazosin, alfuzosin) that are widely used in men with obstructive BPH (Speakman et al., 2004). Although these treatments offer some improvement for patients with LUTS, the improvement is often modest, and the benefits appear to be especially limited with respect to sensory symptoms such as urgency, nocturia, discomfort, and pain. Accordingly, a recent review of 32 randomized, placebo-controlled trials of muscarinic antagonists in OAB revealed that use of oxybutynin and tolterodine, on average, results in only one fewer leakage episode or micturition event per 48 h compared to placebo, with a minimal effect on sensory symptoms (Herbison et al., 2003). Muscarinic antagonists also produce many systemic side effects that significantly impair treatment tolerability, including dry mouth, constipation, blurred vision, dry eyes, drowsiness, and cognitive decline. As a consequence, persistence with long-term muscarinic antagonist therapy has been a constant challenge in patients with OAB (Haab & Castro-Diaz, 2005), and likely reflects the marginal efficacy and challenging tolerability profile of these medications. Incremental approaches aimed at improving the effectiveness of muscarinic antagonists include extended release and once daily formulations of oxybutynin and tolterodine, and development of the M3-selective muscarinic antagonists darifenacin and solifenacin (Cardozo et al., 2004; Chapple et al., 2004; 2005; Haab et al., 2004). However, the persistence of significant side effects with these newer medications, especially gastrointestinal, raises justifiable questions as to whether this mechanism can ever be ideally optimized to create more effective clinical relief. Moreover, despite many years of muscarinic antagonist use, little compelling evidence has surfaced that the pathophysiology of LUTS relates to dysregulation of cholinergic control of urinary storage and voiding.
Selective α1-adrenoceptor antagonists, tamsulosin in particular due to its lower cardiovascular side effects (Milani & Djavan, 2005; Muzzonigro, 2005), are the preferred first line of therapy for patients with BPH/LUTS (Speakman et al., 2004). However, their effect on irritative or storage symptoms is not especially impressive and continuing use seems most likely limited to men presenting with urinary obstruction.
Given the limitations of ‘gold-standard' treatments, it is clear that alternative, novel therapeutic approaches are needed that more effectively target LUTS (Fernandes & Cocoros, 2005). Novel interventions in several mechanistic areas have surfaced through preclinical studies, some of which have advanced into proof-of-concept clinical testing. These potential therapeutic targets include tachykinin receptors, various potassium, calcium, and sodium channels, β-adrenoceptors, TRPV channels, and purinoceptors, among others. Despite efforts in these areas, reports of success have been rare (Wein, 2001; Wein & Hanno, 2002; Lecci & Maggi, 2005). Other novel therapeutic interventions such as intradetrusor botulinum toxin (Botox) and intravesical treatment with neurotoxic agents such as capsaicin or resiniferatoxin (RTX) offer some symptom improvement in certain conditions; however, the improvement is varied and the route of administration unattractive (Fowler, 2000; Zermann et al., 2000; Reitz et al., 2004; Smith et al., 2004).
Purinergic regulation of lower urinary tract function
Purinergic transmission has increasingly been recognized as playing a role in lower urinary tract function, regulating both afferent and efferent signaling pathways controlling urine storage and elimination (Figures 1 and 2). The scope of purinergic signaling within the lower urinary tract is vast, and involves multiple receptors for ATP including the P2Y and P2X receptor families (Burnstock, 2000). Currently, eight metabotropic P2Y receptors (Abbracchio et al., 2003), and seven P2X (P2X1−7) receptor subunits for ionotropic ATP receptors are known (North, 2002). P2X receptors can form either homomultimeric or heteromultimeric receptors, and are believed to exist in their native conformation as trimers (Nicke et al., 1998). Several reviews have comprehensively covered the role of these diverse receptor subtypes in lower urinary tract function (Burnstock, 2000; Andersson & Wein, 2004). In this review, we provide a historical perspective on ATP signaling within the urinary bladder, with a particular focus on selected P2X channels where recent biological and chemical advances suggest that medicinal exploitation can be achieved. Specifically, we focus on P2X1 receptors that likely play a significant role in efferent regulation of detrusor smooth muscle excitability and contraction, and P2X3 and P2X2/3 receptors that mediate sensory functions, including afferent modulation of urinary storage and elimination.
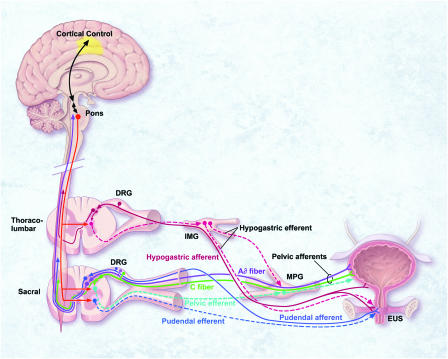
Figure 1.
Schematic diagram of the neural circuits controlling continence and micturition. The majority of Aδ- and C-afferents that innervate the urinary bladder and urethra are found in pelvic nerves, which also contain parasympathetic efferents originating from the sacral spinal cord. The remaining bladder afferents are carried by hypogastric nerves, which also contain sympathetic efferents originating from the thoracolumber spinal cord. Sacral somatic afferent and efferent innervation to the external urethral sphincter is via pudendal nerves. Under normal physiological conditions in adults, the micturition reflex is controlled predominantly by Aδ afferents communicating via the spinal cord to supraspinal centers in the pons and cortex. Under pathophysiological conditions or with aging, spinal reflex mechanisms mediated by C-fibre afferents may become dominant.
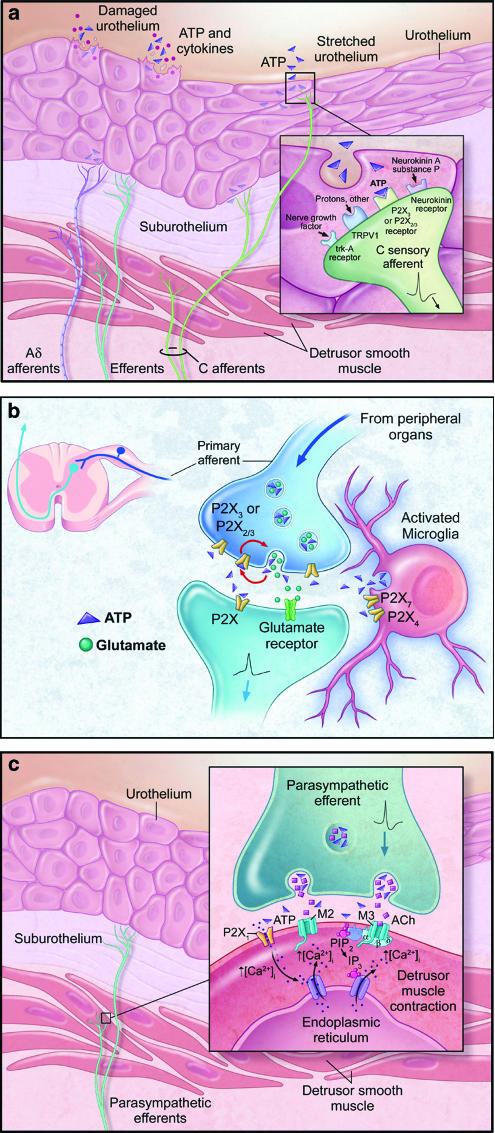
Figure 2.
Schematic diagrams showing the roles of ATP and P2X receptors in the micturition pathway. (a) Mechanical distension or damage to the urothelium causes release of ATP, and this release is augmented in disease states such as interstitial cystitis, benign prostate hyperplasia, or spinal cord injury. ATP acts on P2X3 and P2X2/3 receptors on the peripheral terminals of Aδ- and C-bladder afferents, where it may convey mechanosensory and nociceptive information to the spinal cord. (b) At the central terminals of primary sensory afferents within the dorsal horn of the spinal cord, ATP may be coreleased with glutamate. P2X receptors are expressed on both presynaptic and postsynaptic membranes. Presynaptic P2X3 and P2X2/3 receptors are thought to be important in facilitating glutamate release. In addition, P2X4 and P2X7 receptors present on microglia may mediate inflammatory responses, thus contributing to hyperexcitability at these synapses. (c) Excitation of parasympathetic efferents causes corelease of ATP with acetylcholine from the nerve terminal. These neurotransmitters act on P2X1 and muscarinic (M3) receptors, respectively, present on the postjunctional membrane to cause detrusor smooth muscle contraction.
Efferent control of urinary bladder function – role of P2X1 receptors
The cholinergic contribution to parasympathetically mediated detrusor smooth muscle contraction is well established. However, it is also recognized that in most mammalian species, part of the bladder contraction response evoked by transmural nerve stimulation is atropine-resistant and purinergic. The concept of purinergic signaling in the lower urinary tract emerged through the pioneering work of Burnstock (1972), who demonstrated that ATP was the neurotransmitter involved in atropine-resistant, nonadrenergic, noncholinergic (NANC) contractions in the guinea pig urinary bladder. Although broad acceptance of this idea awaited the cloning of receptors for ATP in the mid-1990s, a considerable amount of evidence was amassed in the intervening years to support the idea of purinergic signaling in the lower urinary tract (Burnstock, 2000). Some key early findings included the demonstration that NANC-mediated detrusor smooth muscle contractions could be mimicked by ATP (Burnstock, 1972; Burnstock et al., 1978; Dean & Downie, 1978) and the more stable ATP analog alpha, beta-methylene ATP (α,β-meATP) (Kasakov & Burnstock, 1983; Hoyle & Burnstock, 1985). NANC- and ATP-mediated detrusor smooth muscle contractions could also be suppressed by desensitization with α,β-meATP, or by various nonselective purinergic antagonists such as quinidine, reactive blue 2, suramin, and pyridoxal-phosphate-6-azophenyl-2′-4′-disulfonic acid (PPADS), without depressing responses to acetylcholine (Dean & Downie, 1978; Kasakov & Burnstock, 1983; Hoyle & Burnstock, 1985; Brading & Williams, 1990; Ziganshin et al., 1993; Tong et al., 1997; King et al., 1997). Ecto-ATPase inhibitors were shown to potentiate NANC and ATP responses in the guinea pig bladder (Hourani & Chown, 1989; Westfall et al., 1997), and release of ATP in response to transmural stimulation of NANC nerves was demonstrated (Burnstock et al., 1978; Tong et al., 1997). Electrophysiological recordings from isolated detrusor smooth muscle cells from guinea pig, rabbit, and pig also showed that ATP and α,β-meATP elicited dose-dependent, membrane depolarization and inward currents that showed rapid desensitization (Fujii, 1988; Inoue & Brading, 1990; 1991). Similar to the contractile response of detrusor smooth muscle strips, desensitization with α,β-meATP blocked ATP-induced currents in isolated myocytes. These findings are consistent with ATP being an excitatory neurotransmitter in the urinary bladder.
Although NANC-mediated detrusor smooth muscle contractions are clearly identifiable in some species, the purinergic contribution to nerve-mediated bladder contraction varies with species, age, and frequency of stimulation (Andersson & Wein, 2004). The purinergic component in bladder strips can vary from being dominant in cat, mouse, and rabbit, to moderate in guinea pig, rat, and dog, to less pronounced compared to cholinergic responses in pig and human (Sibley, 1984; Levin et al., 1990; Andersson, 1993; Wust et al., 2002). In the normal human bladder, atropine-resistant, nerve-mediated contractions have been observed by some investigators (Sjogren et al., 1982; Cowan & Daniel, 1983; Bayliss et al., 1999), but not by others (Sibley, 1984; Kinder & Mundy, 1985). Atropine-resistant responses may reflect only a small portion of the contraction of the human bladder under normal physiological conditions. However, many studies have shown that the purinergic component of human bladder contraction is significantly increased with age and under various pathological conditions of the lower urinary tract (see below).
Evidence of purinergic involvement in nerve-mediated detrusor contraction has also come from whole organ and in vivo studies measuring bladder pressure changes in response to stimulation. In vitro whole bladder studies in rabbit and cat demonstrated that ATP and transmural nerve stimulation, in the presence of atropine, produced transient rises in intravesical pressure (Levin & Wein, 1982; Levin et al., 1990; Chancellor et al., 1992). In a conscious rat cystometry model, arterial administration of ATP and α,β-meATP close to the bladder produced rapid, phasic contractions that were desensitized by α,β-meATP (Igawa et al., 1993). In a pithed rat model, spinal electrical stimulation (L6-S2) evoked an increase in intravesical pressure that was sensitive to PPADS, thus demonstrating the importance of peripheral purinergic neurotransmission in the bladder reflex (Hegde et al., 1998). In an anesthetized rat model, contractile responses of the bladder to pelvic nerve stimulation were further characterized as consisting of a phasic purinergic component predominating at low stimulation frequencies, followed by a tonic, cholinergic component at higher stimulation frequencies (Nunn & Newgreen, 1999).
The recent availability of P2X receptor antagonists with improved subtype selectivity, and P2X1 gene knockout mice, subsequently confirmed the involvement of P2X1 receptors in atropine-resistant detrusor contraction (Figures 2c and 3). In a distension-evoked micturition reflex model in anesthetized rats, intravenous administration of the P2X1, P2X3 receptor antagonist di-inosine pentaphosphate (IP5I), or a novel P2X1 receptor antagonist RO116-6446 (IC50 at recombinant rat P2X1 receptor of ∼3 μM), caused a significant attenuation of phasic isovolumetric bladder contractions without affecting the volume or pressure thresholds for evoking the micturition reflex (King et al., 2004). Moreover, in P2X1-deficient mice, P2X receptor-mediated inward currents were abolished in detrusor smooth muscle cells (Vial & Evans, 2000). Neurogenic bladder contractions in these mice were also reduced by ∼70% compared to P2X1 wild-type mice, while carbachol-mediated responses were unaffected. Supporting these findings, dense P2X1-like immunoreactivity is found in the detrusor smooth muscle (Lee et al., 2000; Elneil et al., 2001; Vial & Evans, 2001), and in close apposition to motor nerve varicosities in the rat detrusor (Hansen et al., 1998). Quantitative mRNA studies have also shown that P2X1 is the most abundant P2X receptor subtype in the adult human bladder (O'Reilly et al., 2001). Collectively, these data confirm that P2X1 receptors play a substantial role in parasympathetic neuronal control of urinary bladder function, although to varying extents across species from rodent to man.
Figure 3.
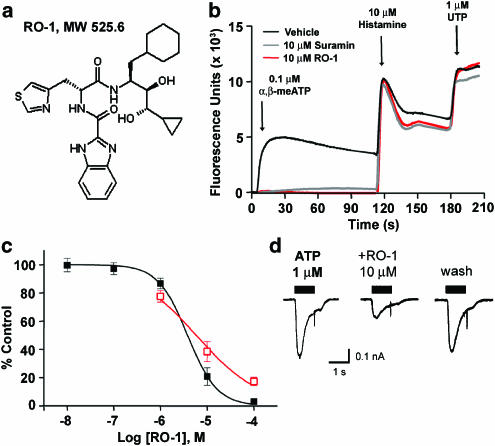
Structure and in vitro pharmacological properties of RO-1, a selective P2X1 antagonist. (a) Chemical structure of RO-1. (b) Cytosolic calcium flux evoked by 0.1 μM α,β-meATP (first peak), 10 μM histamine (second peak), and 1 μM UTP (third peak) in Fluo-3-loaded CHOK1 cells expressing recombinant human P2X1 receptors. In all, 10 μM suramin and 10 μM RO-1 blocked α,β-meATP-evoked cytosolic calcium flux, but did not inhibit calcium flux evoked by histamine or UTP acting on endogenous histamine and UTP-sensitive, suramin-insensitive P2Y receptors in CHOK1 cells. (c) Concentration-effect curves showing the inhibition of cytosolic calcium flux evoked by 0.1 μM α,β-meATP in Fluo-3-loaded CHOK1 cells expressing recombinant human P2X1 receptors (filled black squares; pIC50=5.5), or currents evoked by 1 μM ATP in patch-clamped, dissociated rat bladder smooth muscle cells (open red squares; pIC50=5.2). (d) Representative patch-clamp recordings from dissociated rat bladder smooth muscle cells showing inhibition of ATP-evoked currents by RO-1. The inhibition of ATP-evoked currents could be reversed following washout of RO-1 from the extracellular bath solution.
Sensory functions of ATP – role of P2X3 and P2X2/3 receptors
A sensory role for ATP can be traced to the early study of Holton (1959), who showed that ATP released from sensory nerves during antidromic stimulation caused vasodilation in the rabbit ear artery. It is likely that multiple purinergic pathways and receptors are involved in the sensory actions of ATP. However, a crucial role has been proposed for homomultimeric P2X3 and heteromultimeric P2X2/3 receptors in mediating the primary sensory effects of ATP (Burnstock, 2001; Jarvis, 2003). P2X3 and P2X2/3 receptors are predominantly localized on small-to-medium diameter C-fiber and Aδ sensory neurons within the dorsal root ganglia (DRG) and other sensory ganglia (Vulchanova et al., 1997; Bradbury et al., 1998; Dunn et al., 2001), and on peripheral nerve terminals in tissues including the urinary bladder (Cockayne et al., 2000) (Figure 2a). P2X3 and P2X2/3 receptors are also present on the central projections of primary sensory neurons within the dorsal horn of the spinal cord, where they may modulate glutamate release (Gu & MacDermott, 1997; Vulchanova et al., 1998; Nakatsuka & Gu, 2001; Nakatsuka et al., 2003) (Figure 2b).
Early reports suggested that ATP was involved in pain, including the demonstration that ATP applied to a blister base in healthy human volunteers was associated with heightened pain sensation (Collier et al., 1966; Bleehen et al., 1976; Bleehen & Keele, 1977). In addition, ATP applied to forearm skin by iontophoresis caused mild painful responses that were enhanced by sensitization with UV irradiation or intradermal capsaicin (Hamilton et al., 2000). Intracutaneous injection of ATP (Hilliges et al., 2002), or direct infusion of ATP into skeletal muscle (Mork et al., 2003), also caused pain in human volunteers. Recent studies in animals using more selective pharmacological and genetic tools have established a crucial role for P2X3 and P2X2/3 receptors in both peripheral and centrally mediated pain facilitation. Studies using the P2X1, P2X3 and P2X2/3 selective antagonist 2′,3′-O-(2,4,6-trinitrophenyl)-ATP (TNP-ATP) (Tsuda et al., 1999a, 1999b; Jarvis et al., 2001; Honore et al., 2002b; Ueno et al., 2003), and the P2X3, P2X2/3 selective antagonist A-317491 (Jarvis et al., 2002; McGaraughty et al., 2003; Wu et al., 2004), have shown that peripheral and spinal P2X3 and P2X2/3 receptors are involved in persistent, chronic neuropathic, and inflammatory pain. Mice deficient in P2X3, P2X2, or both receptor subunits (Cockayne et al., 2000; Souslova et al., 2000; Cockayne et al., 2005), as well as animals treated with P2X3-selective antisense (Barclay et al., 2002; Honore et al., 2002a; Inoue et al., 2003) or short interfering RNA (siRNA) (Dorn et al., 2004) revealed comparable findings. These data provide strong preclinical evidence that P2X3 and P2X2/3 receptors are important in pain circuitry in vivo, and suggest that antagonism of P2X3 and/or P2X2/3 receptors may have potential therapeutic utility in the management of chronic pain conditions.
An important role for ATP and P2X3-containing receptors in the mechanosensory regulation of urinary bladder function has also emerged (Burnstock, 2001). Sensory afferent innervation is essential for the normal control of urinary bladder function, coordinating compliance and excitation during the storage and elimination phases of the micturition reflex. The urinary bladder is innervated by the pelvic and hypogastric/lumbar splanchnic nerves with cell bodies in the lumbosacral and thoracolumbar DRG, respectively (Figure 1). Under normal physiological conditions, it is believed that the predominant sensory afferents involved in detecting bladder volume changes are the Aδ pelvic nerve afferents which convey information about the state of bladder fullness to spinal and supraspinal centers coordinating the micturition reflex (Habler et al., 1993; de Groat et al., 1999; Andersson & Wein, 2004). In contrast, the normally silent pelvic afferent C-fibers are thought to assume a prominent role under pathophysiological conditions, where they become hyperexcitable and convey information about noxious, inflammatory, or painful stimuli, and evoke reflex contractions mainly through a localized spinal reflex (Habler et al., 1990; de Groat et al., 1998; Yoshimura & de Groat, 1999). C-fiber afferents within the hypogastric/lumbar splanchnic nerve can also facilitate the effects of noxious chemical irritation within the urinary bladder (Mitsui et al., 2001). Thus, hyperexcitability of C-fibers in different functional pathways may contribute to the underlying pathophysiology of LUTS, including increased sensations of urgency and pain (Yoshimura et al., 2002).
Anatomically, the urinary bladder is innervated by sensory nerve fibers that project into the suburothelial lamina propria, urothelium, and detrusor smooth muscle (Figure 2a). P2X3 immunoreactivity has been found on many of these nerve fibers, and also on bladder epithelial cells (Cockayne et al., 2000; Lee et al., 2000; Elneil et al., 2001; Vlaskovska et al., 2001; Yiangou et al., 2001; Birder et al., 2004; Wang et al., 2005), thus a role may exist for P2X3 receptors in regulating sensory functions of these cells. Numerous studies have shown that ATP is released from the bladder urothelium in response to distension (Ferguson et al., 1997; Vlaskovska et al., 2001; Wang et al., 2005), and these findings can be mimicked in isolated urothelial cell cultures (Sun & Chai, 2002; Birder et al., 2003). Studies using an isolated bladder-pelvic nerve preparation in either rats (Namasivayam et al., 1999) or mice (Vlaskovska et al., 2001; Rong et al., 2002) have also shown that distension leads to increased afferent nerve activity that is mimicked by ATP and/or α,β-meATP. Intravesical infusion of ATP or α,β-meATP can directly stimulate bladder overactivity in conscious rats, in a manner that is concentration dependent and sensitive to TNP-ATP (Pandita & Andersson, 2002). Conversely, intravesical infusion of suramin or PPADS can inhibit nonvoiding bladder contractions in bladder outlet-obstructed rats, and increase bladder capacity in normal conscious rats (Cova et al., 1999; Velasco et al., 2003).
Studies in P2X3- and P2X2-deficient mice have been instrumental in demonstrating the importance of homomultimeric P2X3 and heteromultimeric P2X2/3 receptors in regulating bladder reflex excitability. Urinary bladder reflexes in response to filling are reduced in anesthetized P2X3, P2X2, and P2X2/P2X3 double mutant mice (Cockayne et al., 2000; 2005), despite normal levels of distension-evoked ATP release from the bladder urothelium (Vlaskovska et al., 2001). Bladder pelvic afferents from P2X-deficient mice display altered electrophysiological responses, as measured by an increased volume threshold for activation in response to bladder distension (Vlaskovska et al., 2001; Cockayne et al., 2005). Single unit activity recordings confirmed the reduced afferent mechanosensitivity in P2X-deficient mice; however, it remains to be determined whether these deficits reflect changes in the sensitivity of Aδ and/or C-fiber afferents. Supporting these findings, recent studies (Zhong et al., 2003; Dang et al., 2005a) have shown that labelled rat bladder sensory afferents projecting via the pelvic nerve express both P2X3 and P2X2/3 receptors, with a clear predominance of P2X2/3 heteromultimers. Accordingly, electrophysiological recordings from these afferents (lumbosacral DRG) showed that >80% responded to ATP and α,β-meATP with persistent, slowly desensitizing currents characters of the P2X2/3 receptor. Bladder afferents projecting via the hypogastric/lumbar splanchnic nerve (thoracolumbar DRG) also contain currents consistent with P2X3 and P2X2/3 receptors (Dang et al., 2005a); however, less is known about the importance of P2X receptors on these sympathetic sensory afferents.
A mechanosensory transduction pathway within the micturition reflex is therefore postulated wherein ATP released from the urothelium activates P2X3 and/or P2X2/3 receptors on submucosal primary afferents (Figure 2a). ATP and α,β-meATP have been shown to not only activate low- and high-threshold bladder afferents directly but also to sensitize their mechanosensory responses (Vlaskovska et al., 2001; Rong et al., 2002). Bladder inflammation can also sensitize and enhance P2X receptor function on pelvic and hypogastric/lumbar splanchnic afferents in the lumbosacral and thoracolumbar DRG (Dang et al., 2005b). Thus, P2X3 and P2X2/3 receptors may be important in sensing volume changes during normal bladder filling, and may participate in lowering the threshold for C-fiber activation under pathophysiological conditions.
Several studies have investigated the role of C-fibers in models of ATP-induced bladder overactivity. Selective deletion of nonpeptidergic C-fibers (i.e. P2X3-expressing C-fibers) via intrathecal administration of an IB4-conjugated saporin molecule reduced both ATP- and capsaicin-induced bladder overactivity in conscious rats (Nishiguchi et al., 2004). Two recent reports further demonstrated the importance of spinal endogenous ATP and P2X receptors in chemical irritation-induced (Masuda et al., 2005) or spinal cord injury-induced (Salas et al., 2005) bladder overactivity in rats. It has also been shown that in patients with neurogenic detrusor overactivity, who were successfully treated with RTX, the density of P2X3-immunoreactive nerve fibers in the bladder was significantly reduced compared with that observed in nonresponder patients (Brady et al., 2004). Collectively, these data suggest that P2X3-containing receptors are present on sensory fibers within the urinary bladder, and at central synapses in the spinal cord, where they may play a role in mediating bladder hyperexcitability, at least under certain experimental or pathophysiological conditions.
Altered ATP and P2X receptor function in pathophysiology
In several reports of efferent and afferent mechanisms controlling lower urinary tract function, evidence suggests that ATP and/or P2X receptor-mediated responses are heightened in pathological situations. Changes in purinergic responses associated with efferent control of the micturition reflex have been observed in various bladder disease states, as well as in aging, where conditions associated with LUTS are common. For example, an increased atropine-resistant component of ∼50% of the neurogenic bladder contraction response was observed in tissues from men with BPH and detrusor overactivity (Sjogren et al., 1982), and in women with IC (Palea et al., 1993) or idiopathic detrusor instability (O'Reilly et al., 2002). Similar, but variable, degrees of atropine-resistant detrusor contractions have been observed in other studies of patients with BPH and detrusor overactivity (Nergardh & Kinn, 1983; Sibley, 1984; Chapple & Smith, 1994). Among subsets of patients with unstable bladder, atropine resistant responses were present in those with idiopathic detrusor instability or detrusor instability secondary to obstruction, but not in patients with neurogenic detrusor instability (Bayliss et al., 1999).
Recent studies have explored the basis for the enhanced purinergic neurotransmission that emerges in certain unstable bladder conditions. Studies by Fry and co-workers have suggested that purinergic-mediated contractions are not due to altered sensitivities of the detrusor smooth muscle cells to ATP or cholinergic agonists (Wu, 1999), but instead may result from reduced extracellular hydrolysis of ATP (Fry et al., 2002; Harvey, 2002). They found that ATP is significantly more potent at generating detrusor contractions in diseased bladder biopsies compared to control stable bladders, and that this is likely due to the fact that ATPase activity in unstable bladders is ∼50% of that measured in stable bladder biopsies (Harvey, 2002). Studies of age-related changes in detrusor contractility have further demonstrated a significant positive correlation between age and the purinergic component of detrusor contraction (Lieu et al., 1997; Yoshida et al., 2001), and shown that aging is associated with increased release of ATP from isolated detrusor smooth muscle tissue (Yoshida et al., 2004). These studies also demonstrated that aging is negatively correlated with cholinergic neurotransmission and decreased release of ACh from isolated detrusor smooth muscle tissue.
Numerous human and non-human studies have also shown that ATP release from the bladder urothelium is augmented under certain pathophysiological conditions. Urothelial cells from cats with a naturally occurring cystitis (FIC) exhibit enhanced hypotonic-evoked release of ATP (Birder et al., 2003). ATP release from urothelial cells is also markedly increased in rats subjected to spinal cord injury, in which bladder hyperreflexia develops (Khera et al., 2004), and in rats following chemically induced bladder inflammation (Smith et al., 2005). Following spinal cord injury in rats, an increase in basal and bladder stimulation-evoked ATP release has also been observed within the lumbosacral spinal cord by microdialysis (Salas et al., 2005). In patients with IC (Sun et al., 2001; Sun & Chai, 2002), or LUTS secondary to BPH (Sun et al., 2002), stretch-activated ATP release from bladder urothelial cells is augmented compared to age-matched controls. P2X3 receptor expression also appears to be abnormally upregulated in response to stretch in bladder urothelial cells from IC patients (Sun & Chai, 2004). An increased density of P2X3 and TRPV1-expressing nerve fibers has also been found in the bladders of patients with neurogenic detrusor overactivity, and following treatment with RTX, responder patients showed diminished levels of both TRPV1 and P2X3 immunoreactivity (Brady et al., 2004).
The mechanism(s) by which urothelial ATP release is augmented under pathophysiological conditions is not entirely clear. However, intravesical treatment with Botox can inhibit the augmented urothelial release of ATP following spinal cord injury or chemically induced cystitis, suggesting that vesicular release may be altered (Khera et al., 2004; Smith et al., 2005). In mice lacking the TRPV1 receptor, distension- or hypotonic-evoked release of ATP from the bladder urothelium is significantly reduced, further raising the possibility that activation of TRPV1 receptors may be a mechanosensory stimulus involved in distension-evoked release of ATP during bladder filling (Birder et al., 2002).
P2X1, P2X3, and P2X2/3 receptors as therapeutic targets for lower urinary tract disorders
Given these emergent roles of ATP, modulation of P2X receptor activity has surfaced as a potential point of therapeutic intervention in diseases of the lower urinary tract. Among the P2X receptor class, antagonism of P2X1, P2X3, and P2X2/3 receptors appears to be the most biologically reasonable. However, exploiting the full therapeutic potential of the purinoceptor family will require more than sound biological rationale. Novel medicines must possess the right combination of potency and selectivity with suitable drug-like properties, such as oral bioavailability, metabolic stability, and optimal distribution characteristics. The ligands used to discover and delineate the purinoceptor family (e.g. suramin, PPADS, reactive blue 2) represent very poor starting points for drug discovery, and although significant advances have been made in recent years in developing ligands with increased potency and selectivity at some P2 receptors (Jacobson et al., 2004), little progress has been reported on drug-like ligands. Indeed, most of the ligands highlighted in purinoceptor medicinal chemistry reports violate more than one of the so-called Lipinski rules; a standard that provides a rough guide to drug likeness within pharmaceutical discovery (Lipinski et al., 2001).
In 2002, data were published for the first time on a selective P2X3/P2X2/3 small molecule antagonist, A-317491 (Jarvis et al., 2002). Activation of recombinant and native P2X3 and P2X2/3 receptors was inhibited by submicromolar concentrations of A-317491, and efficacy was demonstrated in several models of chronic inflammatory and neuropathic pain. A-317491 dose-dependently decreased nociceptive responses evoked by intraplantar injection of formalin or complete Freund's adjuvant with ED50 values of 50 and 30 μmol kg−1, s.c., respectively (McGaraughty et al., 2003). Reports on the use of this antagonist to study lower urinary tract function have been limited; however, one report suggested an inhibition of non-micturition bladder contractions and an increase in bladder capacity in a rat spinal cord injury model (Lu et al., 2002). While the discovery of this molecule has provided some advance over the large, nonselective, polyanionic antagonists described to this point (e.g. suramin, reactive blue 2), the poor pharmacokinetic properties of A-317491 (a tricarboxylic acid with poor oral bioavailability, high protein binding, and poor tissue distribution) may make it unattractive for medicinal development.
The paucity of available chemically attractive lead molecules directed efforts at Roche Pharmaceuticals, Palo Alto, towards novel lead discovery using high throughput screening (HTS) of the Roche compound library. Since calcium passes through open P2X channels, functional activation of these channels was quantitated using a fluorescence change evoked by cytosolic calcium flux in the presence of the calcium-sensitive dye, Fluo-3, and measured using a fluorometric imaging plate reader (FLIPR) (Jaime-Figueroa et al., 2005) (Figures 3b and 4b). Three FLIPR-based HTS campaigns targeting homomultimeric hP2X1, hP2X2, and rP2X3 receptors were conducted concurrently to identify compounds capable of inhibiting α,β-meATP (P2X1 and P2X3)- or ATP (P2X2)-evoked cytosolic calcium flux, without interfering with a secondary calcium flux produced by the calcium ionophore, ionomycin (see Figure 4b). In contrast to the P2X1 and P2X3 HTS screening campaigns, the HTS screen targeting P2X2 resulted in no chemically tractable small molecule leads. It therefore remains to be seen whether P2X2 is a feasible target for medicinal intervention.
Figure 4.
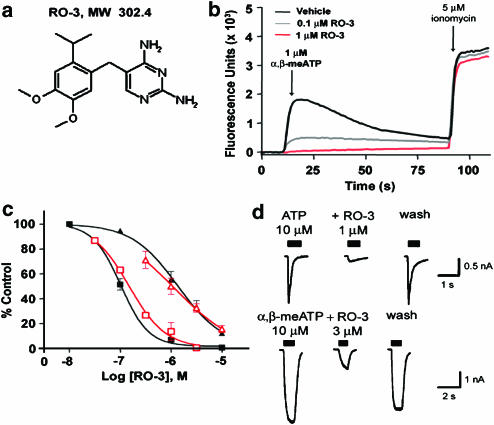
Structure and in vitro pharmacological properties of RO-3, a selective P2X3 and P2X2/3 antagonist. (a) Chemical structure of RO-3. (b) Cytosolic calcium flux evoked by 1 μM α,β-MeATP (first peak) and 5 μM ionomycin (second peak) in Fluo-3-loaded CHOK1 cells expressing recombinant rat P2X3 receptors. RO-3 at 0.1 and 1 μM blocked α,β-meATP-evoked cytosolic calcium flux, but did not inhibit calcium flux evoked by ionomycin. (c) Concentration-effect curves showing inhibition of cytosolic calcium flux evoked by 1 μM α,β-meATP in Fluo-3-loaded CHOK1 cells expressing recombinant rat P2X3 receptors (filled black squares; pIC50=7.0) or 5 μM α,β-meATP in Fluo-3-loaded 1321N1 astrocytoma cells expressing recombinant human P2X2/3 receptors (filled black triangles; pIC50=5.9). Also shown are concentration-effect curves for inhibition of currents evoked by 10 μM ATP or α,β-meATP in patch-clamped, dissociated rat thoracolumbar dorsal root ganglion (open red squares; pIC50=6.8) or nodose ganglion neurons (open red triangles; pIC50=5.9), respectively. (d) Representative patch-clamp recordings from dissociated rat thoracolumbar DRG (upper panel) or nodose ganglion (lower panel) neurons showing the inhibition of ATP- or α,β-meATP-evoked currents by RO-3. The inhibition of ATP- or α,β-meATP-evoked currents could be reversed by washout of RO-3 from the extracellular bath solution.
The P2X1 HTS screening campaign resulted in discovery of an antagonist from a series of dipeptide compounds, prepared initially as potential renin inhibitors (Jaime-Figueroa et al., 2005). Subsequent optimization efforts resulted in the discovery of RO-1 (Figure 3a), a novel, small molecule antagonist of moderate potency (pIC50=5.5, Figure 3c) with selectivity over homomultimeric P2X2 and P2X3, and heteromultimeric P2X2/3 receptors (IC50>100 μM at all three receptors). RO-1 (10 μM) nearly abolished calcium responses evoked by 1 μM ATP in patch-clamped, dissociated rat bladder smooth muscle cells (Figure 3d), and also greatly reduced rat tail artery ring contractions evoked by electrical field stimulation (1–10 μM RO-1, 1–64 Hz in the presence of 3 μM prazosin) (Gever et al., 2004). In tissue bath studies examining NANC responses, contractions evoked by β,γ-meATP in rat detrusor smooth muscle strips, or ATP in rat tail artery rings, were significantly reduced with as little as 0.1 μM RO-1 and almost completely abolished by 10 μM RO-1 (Gever et al., 2004). In a distension-evoked micturition reflex model in anesthetized rats, the magnitude of phasic isovolumetric bladder contractions was significantly attenuated by intravenous administration of 1 or 10 μmol kg−1 of RO116-6446 (RO-1) (King et al., 2004), a finding consistent with the decreased detrusor contraction observed in tissue bath studies. These results illustrate the feasibility of identifying P2X1 receptor antagonists that are neither nucleotides nor polyanionic compounds, and provide increased confidence that this target can be successfully exploited to identify therapeutically attractive molecules. However, despite apparent selectivity, optimization of potency was not successful with this class of compounds. It thus remains to be seen whether a more potent P2X1 receptor antagonist can be developed that will allow for a more effective modulation of efferent purinergic mechanisms. Moreover, in vivo studies examining the effects of selective P2X1 receptor antagonists on other smooth muscle preparations (especially vascular) that contain P2X1 receptors would be necessary to determine whether safe and tolerable antagonism of P2X1 receptors can be imparted to modify urinary function.
The P2X3 HTS screening campaign resulted in the discovery of two distinct chemical series. The first was a series of diaminopyrimidine containing molecules related in structure to the antibacterial drug trimethoprim. Subsequent optimization of this series resulted in a number of small molecule dual P2X3/P2X2/3 antagonists, exemplified by RO-3 (Figure 4a). RO-3 is a potent inhibitor of human homomultimeric P2X3 (pIC50=7.0) and heteromultimeric P2X2/3 (pIC50=5.9) receptors (Figure 4c). These potency estimates were confirmed using patch-clamp electrophysiology of rat thoracolumber dorsal root (P2X3 pIC50=6.8) and nodose (P2X2/3 pIC50=5.9) ganglion neurons (Figure 4c and d). RO-3 showed selectivity for P2X3 and P2X2/3 over all other functional homomultimeric P2X receptors (IC50 >10 μM at P2X1,2,4,5,7), despite its relatively small size (MW 302.4 Da). The presence of a substituted diaminopyrimidine moiety in the molecule, a substructure common to many well-known inhibitors of human protein kinases, prompted further selectivity testing against this protein family. When tested in an ATP-site competition-binding assay (Fabian et al., 2005) against a panel of 121 human protein kinases, no inhibition of >50% at 10 μM was observed. An extensive selectivity profile generated by Cerep (Paris, France) also showed little or no inhibition of radioligand binding, or function, at 74 receptors, transporters, and enzymes by 10 μM RO-3; the only exception being the melatonin (ML1) receptor with a pIC50=6.4. Based on this selectivity profile, the pharmacological effects of RO-3 can be reasonably attributed to P2X3 and P2X2/3 receptor antagonism.
As with the P2X1 receptor antagonist described above, the identification of this series of P2X3/P2X2/3 receptor antagonists represents a significant advance in the discovery of drug-like P2X antagonists, and the first reported non-nucleotide, nonpolyanionic low molecular weight compound. RO-3 has moderate to high metabolic stability in rat and human hepatocytes and liver microsomes, and is highly permeable, orally bioavailable (14%), and has a reasonable in vivo plasma half-life (t1/2=0.41 h) in rats. RO-3 is also widely distributed to tissues following administration, with low plasma protein binding (48.6%) and good CNS penetration (brain to plasma ratio=0.8). This diaminopyrimidine represents the first class of P2X3- or P2X2/3-specific probes to allow simultaneous assessment of the impact of blocking homomultimeric P2X3 and heteromultimer P2X2/3 receptors in peripheral and central tissues. Initial assessments have been undertaken to examine the properties of RO-3 in a variety of ex vivo whole organ preparations and in vivo rodent models. In a guinea pig ureter-afferent nerve preparation, and mouse bladder-pelvic nerve preparation, RO-3 dose-dependently reduced afferent nerve activity induced by distension or α,β-meATP. Early in vivo data indicate that RO-3 has activity in several rodent models of pain, as well as in cystometry models optimized to measure various parameters associated with sensory regulation of the micturition reflex.
Conclusion
The impact and significance of purinergic signaling in LUT function – and its potential relevance to disease – has become greatly substantiated over recent years, particularly with the development of gene knockout mice and the emergence of novel pharmacological probes. Efforts focused on P2X1 and P2X3/P2X2/3 antagonism represent hypothesis-driven approaches, and progress has been made in these areas. Gene knockout and pharmacological data for other P2 receptors (e.g. P2X4 and P2X7) may also offer hints at opportunities in visceral organ diseases associated with inflammation and pain; however, the role of these P2X receptors in LUT function has not yet been established.
It is also clear that the path from pharmacological probe to therapeutic probe, and then to novel differentiated medicines is a complex and challenging one. Thus, it may be some time before therapeutic potential is fully appreciated. A potential concern is that there are many receptors for ATP (P2X and P2Y), and there may be sufficient redundancy built into targeted biological systems such that blockade at any one receptor or ion channel will be too subtle to have clear clinical impact.
P2X3 and P2X2/3 regulation of sensory mechanisms in the lower urinary tract appears to be the most attractive purinergic opportunity currently surfacing, and advances have been made in the identification of chemical entities with properties suitable for medicinal optimization. To date, focus has been somewhat limited to P2X3 and P2X2/3 receptor involvement in afferent mechanisms within peripheral target tissues. However, recent reports (Masuda et al., 2005; Salas et al., 2005) suggest that attention has turned to the role of ATP and P2X3, P2X2/3 receptors at central synapses in the spinal cord where central sensitization may occur. Moving forward, a greater focus is warranted on the function of these receptors across visceral sensory pathways (e.g. hypogastric and pudendal afferents) (Figure 1) to gain a more comprehensive insight into integrated function of the bladder, urethra, and sphincters (Mitsui et al., 2001; Yoshimura et al., 2003; Dang et al., 2005a).
Lastly, it is well known that C-fibers are heterogeneous, and that the population with the highest level of P2X3 expression is the nonpeptidergic subpopulation that binds the isolectin IB4 and has its central projections within inner lamina II of the dorsal horn. A recent study showed that IB4-binding nociceptive neurons link pain signals in the periphery to predominantly limbic regions of the brain (i.e. amygdala, hypothalamus, and globus pallidus), via projection neurons of the deep dorsal horn (Braz et al., 2005). The interesting suggestion posed by these authors is that these neurons may contribute more to the affective component of the pain experience than to the sensory discriminative component. Whether activation of P2X3 and/or P2X2/3 receptors is important in this circuitry, and whether these associations provide a key to targeting the symptomatology of frequency, urgency, and pain in chronic disorders of the lower urinary tract remains to be determined.
Acknowledgments
We express sincere thanks to Professor Geoffrey Burnstock, Professor Stephen B. McMahon, Dr Philip M. Dunn, Dr Gillian E. Knight, and Dr Weifang Rong for their many contributions to the P2X work conducted at Roche Palo Alto. We also thank Silvia Patrick for information support.
Glossary
- ATP
adenosine-5′-triphosphate
- α,β-meATP
alpha, beta-methylene ATP
- Botox
botulinum toxin
- BPH
benign prostatic hyperplasia
- CAP/CPPS
chronic abacterial prostatitis/chronic pelvic pain syndrome
- DRG
dorsal root ganglia
- FLIPR
fluorometric imaging plate reader
- IC
interstitial cystitis
- LUTS
lower urinary tract symptoms
- NANC
nonadrenergic, noncholinergic
- OAB
overactive bladder
- PPADS
pyridoxal-phosphate-6-azophenyl-2′-4′-disulfonic acid
- RTX
resiniferatoxin
- TNP-ATP
2′,3′-O-(2,4,6-trinitrophenyl)-ATP
References
- ABBRACCHIO M.P., BOEYNAEMS J.M., BARNARD E.A., BOYER J.L., KENNEDY C., MIRAS-PORTUGAL M.T., KING B.F., GACHET C., JACOBSON K.A., WEISMAN G.A., BURNSTOCK G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol. Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ABRAMS P., CARDOZO L., FALL M., GRIFFITHS D., ROSIER P., ULMSTEN U., VAN KERREBROECK P., VICTOR A., WEIN A. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourol. Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol. Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- ANDERSSON K.E., WEIN A. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol. Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- BARCLAY J., PATEL S., DORN G., WOTHERSPOON G., MOFFATT S., EUNSON L., ABDEL'AL S., NATT F., HALL J., WINTER J., BEVAN S., WISHART W., FOX A., GANJU P. Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J. Neurosci. 2002;22:8139–8147. doi: 10.1523/JNEUROSCI.22-18-08139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYLISS M., WU C., NEWGREEN D., MUNDY A.R., FRY C.H. A quantitative study of atropine-resistant contractile responses in human detrusor smooth muscle, from stable, unstable and obstructed bladders. J. Urol. 1999;162:1833–1839. [PubMed] [Google Scholar]
- BIRDER L.A., BARRICK S.R., ROPPOLO J.R., KANAI A.J., DE GROAT W.C., KISS S., BUFFINGTON C.A. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am. J. Physiol. (Renal Physiol.) 2003;285:F423–F429. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- BIRDER L.A., NAKAMURA Y., KISS S., NEALEN M.L., BARRICK S., KANAI A.J., WANG E., RUIZ G., DE GROAT W.C., APODACA G., WATKINS S., CATERINA M.J. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat. Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- BIRDER L.A., RUAN H.Z., CHOPRA B., XIANG Z., BARRICK S., BUFFINGTON C.A., ROPPOLO J.R., FORD A.P.D.W., DE GROAT W.C., BURNSTOCK G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am. J. Physiol. (Renal Physiol.) 2004;287:F1084–F1091. doi: 10.1152/ajprenal.00118.2004. [DOI] [PubMed] [Google Scholar]
- BLEEHEN T., HOBBIGER F., KEELE C.A. Identification of algogenic substances in human erythrocytes. J. Physiol. 1976;262:131–149. doi: 10.1113/jphysiol.1976.sp011589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEEHEN T., KEELE C.A. Observations on the algogenic actions of adenosine compounds on the human skin blister base preparation. Pain. 1977;3:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- BRADBURY E.J., BURNSTOCK G., MCMAHON S.B. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol. Cell. Neurosci. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- BRADING A.F., WILLIAMS J.H. Contractile responses of smooth muscle strips from rat and guinea-pig urinary bladder to transmural stimulation: effects of atropine and alpha, beta-methylene ATP. Br. J. Pharmacol. 1990;99:493–498. doi: 10.1111/j.1476-5381.1990.tb12956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADY C.M., APOSTOLIDIS A., YIANGOU Y., BAECKER P.A., FORD A.P., FREEMAN A., JACQUES T.S., FOWLER C.J., ANAND P. P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur. Urol. 2004;46:247–253. doi: 10.1016/j.eururo.2003.12.017. [DOI] [PubMed] [Google Scholar]
- BRAZ J.M., NASSAR M.A., WOOD J.N., BASBAUM A.I. Parallel ‘pain' pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Purinergic nerves. Pharmacol. Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- BURNSTOCK G.2000Purinergic signalling in lower urinary tract Handbook of Experimental Pharmacologyed. Abbracchio, M.P. & Williams, M. pp. 423–515.Berlin: Springer Verlag [Google Scholar]
- BURNSTOCK G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol. Sci. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., COCKS T., CROWE R., KASAKOV L. Purinergic innervation of the guinea-pig urinary bladder. Br. J. Pharmacol. 1978;63:125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CADDLE M., BERG C., SZNEKE P.2003Benign prostatic hyperplasiaSeptember 2003, Decision Resources Mosaic Study #39. Waltham, Mass.: Decision Resources [Google Scholar]
- CARDOZO L., LISEC M., MILLARD R., VAN VIERSSEN TRIP O., KUZMIN I., DROGENDIJK T.E., HUANG M., RIDDER A.M. Randomized, double-blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J. Urol. 2004;172:1919–1924. doi: 10.1097/01.ju.0000140729.07840.16. [DOI] [PubMed] [Google Scholar]
- CHANCELLOR M.B., KAPLAN S.A., BLAIVAS J.G. The cholinergic and purinergic components of detrusor contractility in a whole rabbit bladder model. J. Urol. 1992;148:906–909. doi: 10.1016/s0022-5347(17)36775-7. [DOI] [PubMed] [Google Scholar]
- CHAPPLE C., STEERS W., NORTON P., MILLARD R., KRALIDIS G., GLAVIND K., ABRAMS P. A pooled analysis of three phase III studies to investigate the efficacy, tolerability and safety of darifenacin, a muscarinic M3 selective receptor antagonist, in the treatment of overactive bladder. BJU Int. 2005;95:993–1001. doi: 10.1111/j.1464-410X.2005.05454.x. [DOI] [PubMed] [Google Scholar]
- CHAPPLE C.R. Muscarinic receptor antagonists in the treatment of overactive bladder. Urology. 2000;55:33–46. doi: 10.1016/s0090-4295(99)00492-6. [DOI] [PubMed] [Google Scholar]
- CHAPPLE C.R., RECHBERGER T., AL SHUKRI S., MEFFAN P., EVERAERT K., HUANG M., RIDDER A. Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU Int. 2004;93:303–310. doi: 10.1111/j.1464-410x.2004.04606.x. [DOI] [PubMed] [Google Scholar]
- CHAPPLE C.R., SMITH D. The pathophysiological changes in the bladder obstructed by benign prostatic hyperplasia. Br. J. Urol. 1994;73:117–123. doi: 10.1111/j.1464-410x.1994.tb07477.x. [DOI] [PubMed] [Google Scholar]
- CLEMENS J.Q., MEEHAN R.T., ROSETTI M.C., GAO S.Y., CALHOUN E.A. Prevalence and incidence of interstitial cystitis in a managed care population. J. Urol. 2005;173:98–102. doi: 10.1097/01.ju.0000146114.53828.82. [DOI] [PubMed] [Google Scholar]
- COCKAYNE D.A., DUNN P.M., ZHONG Y., RONG W., HAMILTON S.G., KNIGHT G.E., RUAN H.Z., MA B., YIP P., NUNN P., MCMAHON S.B., BURNSTOCK G., FORD A.P.D.W. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J. Physiol. 2005;567:621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCKAYNE D.A., HAMILTON S.G., ZHU Q.-M., DUNN P.M., ZHONG Y., NOVAKOVIC S., MALMBERG A.B., CAIN G., BERSON A., KASSOTAKIS L., HEDLEY L., LACHNIT W.G., BURNSTOCK G., MCMAHON S.B., FORD A.P.D.W. Urinary bladder hyporeflexia and reduced pain-related behavior in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- COLLIER H.O., JAMES G.W., SCHNEIDER C. Antagonism by aspirin and fenamates of bronchoconstriction and nociception induced by adenosine-5′-triphosphate. Nature. 1966;212:411–412. doi: 10.1038/212411a0. [DOI] [PubMed] [Google Scholar]
- COVA R., GUARNERI L., ANGELICO P., VELASCO C., LEONARDI A., TESTA R. Effects of infravesical suramin and PPADS on cystometrographic parameters in conscious rats. Urodinamica. 1999;9:162–168. [Google Scholar]
- COWAN W.D., DANIEL E.E. Human female bladder and its noncholinergic contractile function. Can J. Physiol. Pharmacol. 1983;61:1236–1246. doi: 10.1139/y83-182. [DOI] [PubMed] [Google Scholar]
- DANG K., BIELEFELDT K., GEBHART G.F. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J. Neurosci. 2005a;25:3973–3984. doi: 10.1523/JNEUROSCI.5239-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANG K., BURNES L., KARDOS S., BIELEFELDT K., GEBHART G.F.2005bCyclophosphamide-induced bladder inflammation enhances P2X receptor function and sensitizes rat lumbosacral and thoracolumbar dorsal root ganglion neuronsProgram No. 170.2. 2005 Abstract Viewer/Itinerary Planner. 35th Annual Meeting of the Society for Neuroscience. Washington, DC; November 12–16, 2005. [Google Scholar]
- DEAN D.M., DOWNIE J.W. Contribution of adrenergic and ‘purinergic' neurotransmission to contraction in rabbit detrusor. J. Pharmacol. Exp. Ther. 1978;207:431–445. [PubMed] [Google Scholar]
- DE GROAT W.C., ARAKI I., VIZZARD M.A., YOSHIYAMA M., YOSHIMURA N., SUGAYA K., TAI C., ROPPOLO J.R. Developmental and injury induced plasticity in the micturition reflex pathway. Behav. Brain Res. 1998;92:127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- DE GROAT W.C., DOWNIE J.W., LEVIN R.M., LIN A.T.L., MORRISON J.F.B., NISHIZAWA O., STEERS W.D., THOR K.B.1999Basic neurophysiology and neuropharmacology Incontinence: 1st International Consultation on Incontinence, June 28–July 1, 1998, Monacoed. Abrams, P., Khoury, S. & Wein, A. pp. 105–154.St Helier, Jersey, UK: Health Publication [Google Scholar]
- DORN G., PATEL S., WOTHERSPOON G., HEMMINGS-MIESZCZAK M., BARCLAY J., NATT F.J.C., MARTIN P., BEVAN S., FOX A., GANJU P., WISHART W., HALL J. siRNA relieves chronic neuropathic pain. Nucleic Acids Res. 2004;32:e49. doi: 10.1093/nar/gnh044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNN P.M., ZHONG Y., BURNSTOCK G. P2X receptors in peripheral neurons. Prog. Neurobiol. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- EGAN K.J., KRIEGER J.L. Chronic abacterial prostatitis – a urological chronic pain syndrome. Pain. 1997;69:213–218. doi: 10.1016/S0304-3959(96)03203-4. [DOI] [PubMed] [Google Scholar]
- ELNEIL S., SKEPPER J.N., KIDD E.J., WILLIAMSON J.G., FERGUSON D.R. Distribution of P2X(1) and P2X(3) receptors in the rat and human urinary bladder. Pharmacology. 2001;63:120–128. doi: 10.1159/000056122. [DOI] [PubMed] [Google Scholar]
- FABIAN M.A., BIGGS W.H., TREIBER D.K., ATTERIDGE C.E., AZIMIOARA M.D., BENEDETTI M.G., CARTER T.A., CICERI P., EDEEN P.T., FLOYD M., FORD J.M., GALVIN M., GERLACH J.L., GROTZFELD R.M., HERRGARD S., INSKO D.E., INSKO M.A., LAI A.G., LELIAS J.M., MEHTA S.A., MILANOV Z.V., VELASCO A.M., WODICKA L.M., PATEL H.K., ZARRINKAR P.P., LOCKHART D.J. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat. Biotech. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- FERGUSON D.R., KENNEDY I., BURTON T.J. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes – a possible sensory mechanism. J. Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDES D., COCOROS N.2005Urge urinary incontinence August 2005, Decision Resources Mosaic Study #1. Waltham, Mass: Decision Resources [Google Scholar]
- FOWLER C.J. Intravesical treatment of overactive bladder. Urology. 2000;55:60–64. doi: 10.1016/s0090-4295(99)00498-7. [DOI] [PubMed] [Google Scholar]
- FRY C.H., SKENNERTON D., WOOD D., WU C. The cellular basis of contraction in human detrusor smooth muscle from patients with stable and unstable bladders. Urology. 2002;59:3–12. doi: 10.1016/s0090-4295(01)01632-6. [DOI] [PubMed] [Google Scholar]
- FUJII K. Evidence for adenosine triphosphate as an excitatory transmitter in guinea-pig, rabbit and pig urinary bladder. J. Physiol. 1988;404:39–52. doi: 10.1113/jphysiol.1988.sp017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEVER J.R., PADILLA F., KNIGHT G.F., DUNN P.M., TRAN A., MANDEL D.A., HEGDE S.S., JIAME-FIGUEROA S., GREENHOUSE R.J., LACHNIT W.G., BURNSTOCK G., FORD A.P.D.W.2004In vitro and in vivo characterization of RO0437626, a novel and selective P2X1 antagonistOn Purines 2004: 4th International Symposium on Nucleosides and Nucleotides. Chapel Hill, NC; June 6–9 2004, p. 86. [Google Scholar]
- GU J.G., MACDERMOTT A.B. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- HAAB F., CASTRO-DIAZ D. Persistence with antimuscarinic therapy in patients with overactive bladder. Int. J. Clin. Prac. 2005;59:931–937. doi: 10.1111/j.1368-5031.2005.00617.x. [DOI] [PubMed] [Google Scholar]
- HAAB F., STEWART L., DWYER P. Darifenacin, an M3 selective receptor antagonist, is an effective and well-tolerated once-daily treatment for overactive bladder. Eur. Urol. 2004;45:420–429. doi: 10.1016/j.eururo.2004.01.008. [DOI] [PubMed] [Google Scholar]
- HABLER H.J., JANIG W., KOLTZENBURG M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J. Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABLER H.J., JANIG W., KOLTZENBURG M. Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. J. Physiol. 1993;463:449–460. doi: 10.1113/jphysiol.1993.sp019604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON S.G., WARBURTON J., BHATTACHARJEE A., WARD J., MCMAHON S.B. ATP in human skin elicits a dose related pain response which is potentiated under conditions of hyperalgesia. Brain. 2000;123:1238–1246. doi: 10.1093/brain/123.6.1238. [DOI] [PubMed] [Google Scholar]
- HANSEN M.A., BALCAR V.J., BARDEN J.A., BENNETT M.R. The distribution of single P2x1-receptor clusters on smooth muscle cells in relation to nerve varicosities in the rat urinary bladder. J. Neurocytol. 1998;27:529–539. doi: 10.1023/a:1006908010642. [DOI] [PubMed] [Google Scholar]
- HARVEY R. The contractile potency of adenosine triphosphate and ecto-adenosine triphosphatase activity in guinea pig detrusor and detrusor from patients with a stable, unstable or obstructed bladder. J. Urol. 2002;168:1235–1239. doi: 10.1016/S0022-5347(05)64632-0. [DOI] [PubMed] [Google Scholar]
- HEGDE S.S., MANDEL D.A., WILFORD M.R., BRIAUD S., FORD A.P.D.W., EGLEN R.M. Evidence for purinergic neurotransmission in the urinary bladder of pithed rats. Eur. J. Pharmacol. 1998;349:75–82. doi: 10.1016/s0014-2999(98)00173-3. [DOI] [PubMed] [Google Scholar]
- HERBISON P., HAY-SMITH J., ELLIS G., MOORE K. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: systematic review. Br. Med. J. 2003;326:841–844. doi: 10.1136/bmj.326.7394.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLIGES M., WEIDNER C., SCHMELZ M., SCHMIDT R., ORSTAVIK K., TOREBJORK E., HANDWERKER H. ATP responses in human C nociceptors. Pain. 2002;98:59–68. doi: 10.1016/s0304-3959(01)00469-9. [DOI] [PubMed] [Google Scholar]
- HOLTON P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J. Physiol. 1959;145:494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONORE P., KAGE K., MIKUSA J., WATT A.T., JOHNSTON J.F., WYATT J.R., FALTYNEK C.R., JARVIS M.F., LYNCH K. Analgesic profile of intrathecal P2X3 antisense oligonucleotide treatment in chronic inflammatory and neuropathic pain states in rats. Pain. 2002a;99:11–19. doi: 10.1016/s0304-3959(02)00032-5. [DOI] [PubMed] [Google Scholar]
- HONORE P., MIKUSA J., BIANCHI B., MCDONALD H., CARTMELL J., FALTYNEK C., JARVIS M.F. TNP-ATP, a potent P2X3 receptor antagonist, blocks acetic acid-induced abdominal constriction in mice: comparison with reference analgesics. Pain. 2002b;96:99–105. doi: 10.1016/s0304-3959(01)00434-1. [DOI] [PubMed] [Google Scholar]
- HOURANI S.M., CHOWN J.A. The effects of some possible inhibitors of ectonucleotidases on the breakdown and pharmacological effects of ATP in the guinea-pig urinary bladder. Gen. Pharmacol. 1989;20:413–416. doi: 10.1016/0306-3623(89)90188-2. [DOI] [PubMed] [Google Scholar]
- HOYLE C.H.V., BURNSTOCK G. Atropine-resistant excitatory junction potentials in rabbit bladder are blocked by α,β-methylene ATP. Eur. J. Pharmacol. 1985;114:239–240. doi: 10.1016/0014-2999(85)90635-1. [DOI] [PubMed] [Google Scholar]
- IGAWA Y., MATTIASSON A., ANDERSSON K.-E. Functional importance of cholinergic and purinergic neurotransmission for micturition contraction in the normal, unanaesthetized rat. Br. J. Pharmacol. 1993;109:473–479. doi: 10.1111/j.1476-5381.1993.tb13593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE K., TSUDA M., KOIZUMI S. ATP induced three types of pain behaviors, including allodynia. Drug Dev. Res. 2003;59:56–63. [Google Scholar]
- INOUE R., BRADING A.F. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br. J. Pharmacol. 1990;100:619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE R., BRADING A.F. Human, pig and guinea-pig bladder smooth muscle cell generate similar inward currents in response to purinoceptor activation. Br. J. Pharmacol. 1991;103:1840–1841. doi: 10.1111/j.1476-5381.1991.tb12338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON K.A., COSTANZI S., OHNO M., JOSHI B.V., BESADA P., XU B., TCHILIBON S. Molecular recognition at purine and pyrimidine nucleotide (P2) receptors. Curr. Topics Med. Chem. 2004;4:805–819. doi: 10.2174/1568026043450961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAIME-FIGUEROA S., GREENHOUSE R., PADILLA F., DILLON M.P., GEVER J.R., FORD A.P.D.W. Discovery and synthesis of a novel and selective drug-like P2X1 antagonist. Bioorg. Med. Chem. Lett. 2005;15:3292–3295. doi: 10.1016/j.bmcl.2005.04.049. [DOI] [PubMed] [Google Scholar]
- JARVIS M.F., BURGARD E.C., MCGARAUGHTY S., HONORE P., LYNCH K., BRENNAN T.J., SUBIETA A., VAN BIESEN T., CARTMELL J., BIANCHI B., NIFORATOS W., KAGE K., YU H., MIKUSA J., WISMER C.T., ZHU C.Z., CHU K., LEE C.H., STEWART A.O., POLAKOWSKI J., COX B.F., KOWALUK E., WILLIAMS M., SULLIVAN J., FALTYNEK C. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc. Nat. Acad. Sci. U.S.A. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARVIS M.F., WISMER C.T., SCHWEITZER E., YU H., VAN BIESEN T., LYNCH K.J., BURGARD E.C., KOWALUK E.A. Modulation of BzATP and formalin induced nociception: attenuation by the P2X receptor antagonist, TNP-ATP and enhancement by the P2X3 allosteric modulator, cibacron blue. Br. J. Pharmacol. 2001;132:259–269. doi: 10.1038/sj.bjp.0703793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARVIS M.J. Contributions of P2X3 homomeric and heteromeric channels to acute and chronic pain. Exp. Opin. Ther. Targets. 2003;7:513–522. doi: 10.1517/14728222.7.4.513. [DOI] [PubMed] [Google Scholar]
- JONES C.A., NYBERG L.M. Epidemiology of interstitial cystitis. Urology. 1997;49:2–9. doi: 10.1016/s0090-4295(99)80327-6. [DOI] [PubMed] [Google Scholar]
- KASAKOV L., BURNSTOCK G. The use of the slowly degradable analog, α,β-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur. J. Pharmacol. 1983;86:291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- KHERA M., SOMOGYI G.T., KISS S., BOONE T.B., SMITH C.P. Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem. Int. 2004;45:987–993. doi: 10.1016/j.neuint.2004.06.001. [DOI] [PubMed] [Google Scholar]
- KINDER R.B., MUNDY A.R. Atropine blockade of nerve-mediated stimulation of the human detrusor. Br. J. Urol. 1985;57:418–421. doi: 10.1111/j.1464-410x.1985.tb06301.x. [DOI] [PubMed] [Google Scholar]
- KING B.F., KNOWLES I.D., BURNSTOCK G., RAMAGE A.G. Investigation of the effects of P2 purinoceptor ligands on the micturition reflex in female urethane-anaesthetized rats. Br. J. Pharmacol. 2004;142:519–530. doi: 10.1038/sj.bjp.0705790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING J.A., HUDDART H., STAFF W.G. Purinergic modulation of rat urinary bladder detrusor smooth muscle. Gen. Pharmacol. 1997;29:597–604. doi: 10.1016/s0306-3623(96)00573-3. [DOI] [PubMed] [Google Scholar]
- KREAM R.M., CARR D.B. Interstitial cystitis. A complex visceral pain syndrome Focus: Pain Forum. 1999;8:139–145. [Google Scholar]
- KRIEGER J.N., RILEY D.E., CHEAH P.Y., LIONG M.L., YUEN K.H. Epidemiology of prostatitis: new evidence for a world-wide problem. World J. Urol. 2003;21:70–74. doi: 10.1007/s00345-003-0329-0. [DOI] [PubMed] [Google Scholar]
- LECCI A., MAGGI C.A. Overactive urinary bladder: targeting sensory pathways. Drug Disc. Today: Ther. Strat. 2005;2:15–23. [Google Scholar]
- LEE H.Y., BRADINI M., BURNSTOCK G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J. Urol. 2000;163:2002–2007. [PubMed] [Google Scholar]
- LEVIN R.M., LONGHURST P.A., KATO K., MCGUIRE E.J., ELBADAWI A., WEIN A.J. Comparative physiology and pharmacology of the cat and rabbit urinary bladder. J. Urol. 1990;143:848–852. doi: 10.1016/s0022-5347(17)40115-7. [DOI] [PubMed] [Google Scholar]
- LEVIN R.M., WEIN A.J. Response of the in vitro whole bladder (rabbit) preparation to autonomic agonists. J. Urol. 1982;128:1087–1090. doi: 10.1016/s0022-5347(17)53350-9. [DOI] [PubMed] [Google Scholar]
- LIEU P.K., SA'ADU A., ORUGUN E.O., MALONE-LEE J.G. The influence of age on isometric and isotonic rat detrusor contractions. J. Gerontol.: Med. Sci. 1997;52:M94–M96. doi: 10.1093/gerona/52a.2.m94. [DOI] [PubMed] [Google Scholar]
- LIPINSKI C.A., LOMBARDO F., DOMINY B.W., FEENEY P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- LU S., FRASER M., CHANCELLOR M., SUN L., DE GROAT W.C. Evaluation of voiding dysfunction and purinergic mechanism in awake long-term spinal cord injured rats: comparison of metabolism cage and cystometrogram measurements. J. Urol. 2002;167:276. [Google Scholar]
- MASUDA H., CHANCELLOR M., KIHARA K., DE GROAT W., YOSHIMURA N.2005Evidence for the involvement of spinal endogenous ATP and P2X receptors in detrusor overactivity caused by acetic acid, acrolein or cyclophosphamide. 35th Annual Meeting of the International Continence Society, Montreal, Canada, August 28–September 2, 2005.
- MCGARAUGHTY S., WISMER C.T., ZHU C.Z., MIKUSA J., HONORE P., CHU K.L., LEE C.H., FALTYNEK C.R., JARVIS M.F. Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br. J. Pharmacol. 2003;140:1381–1388. doi: 10.1038/sj.bjp.0705574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHEL M.C., OELKE M., ZINNER N. Novel muscarinic antagonists to treat incontinence and/or overactive bladder. Drug Disc. Today: Ther. Strat. 2005;2:1–6. [Google Scholar]
- MILANI S., DJAVAN B. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: latest update on α1-adrenoceptor antagonists. BJU Int. 2005;95:29–36. doi: 10.1111/j.1464-410X.2005.05485.x. [DOI] [PubMed] [Google Scholar]
- MITSUI T., KAKIZAKI H., MATSUURA S., AMEDA K., YOSHIOKA M., KOYANAGI T. Afferent fibers of the hypogastric nerves are involved in the facilitating effects of chemical bladder irritation in rats. J. Neurophysiol. 2001;86:2276–2284. doi: 10.1152/jn.2001.86.5.2276. [DOI] [PubMed] [Google Scholar]
- MORK H., ASHINA M., BENDTSEN L., OLESEN J., JENSEN R. Experimental muscle pain and tenderness following infusion of endogenous substances in humans. Eur. J. Pain. 2003;7:145–153. doi: 10.1016/S1090-3801(02)00096-4. [DOI] [PubMed] [Google Scholar]
- MUZZONIGRO G. Tamsulosin in the treatment of LUTS/BPH: an Italian multicentre trial. Arch. Ital. Urol. Androl. 2005;77:13–17. [PubMed] [Google Scholar]
- NAKATSUKA T., GU J.G. ATP P2X receptor-mediated enhancement of glutamate release and evoked EPSCs in dorsal horn neurons of the rat spinal cord. J. Neurosci. 2001;21:6522–6531. doi: 10.1523/JNEUROSCI.21-17-06522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATSUKA T., TSUZUKI K., LING J.X., SONOBE H., GU J.G. Distinct roles of P2X receptors in modulating glutamate release at different primary sensory synapses in rat spinal cord. J. Neurophysiol. 2003;89:3243–3252. doi: 10.1152/jn.01172.2002. [DOI] [PubMed] [Google Scholar]
- NAMASIVAYAM S., EARDLEY I., MORRISON J.F.B. Purinergic sensory neurotransmission in the urinary bladder: an in vitro study in the rat. Br. J. Urol. Int. 1999;84:854–860. doi: 10.1046/j.1464-410x.1999.00310.x. [DOI] [PubMed] [Google Scholar]
- NERGARDH A., KINN A.C. Neurotransmission in activation of the contractile response in the human urinary bladder. Scand. J. Urol. Nephrol. 1983;17:153–157. doi: 10.3109/00365598309180160. [DOI] [PubMed] [Google Scholar]
- NICKE A., BAEUMERT H.G., RETTINGER J., EICHELE A., LAMBRECHT G., MUTSCHLER E., SCHMALZING G. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICKEL J.C. Recommendations for the evaluation of patients with prostatitis. World J. Urol. 2003;21:75–81. doi: 10.1007/s00345-003-0328-1. [DOI] [PubMed] [Google Scholar]
- NISHIGUCHI J., SASAKI K., SEKI S., CHANCELLOR M.B., ERICKSON K.A., DE GROAT W.C., KUMON H., YOSHIMURA N. Effects of isolectin B4-conjugated saporin, a targeting cytotoxin, on bladder overactivity induced by bladder irritation. Eur. J. Neurosci. 2004;20:474–482. doi: 10.1111/j.1460-9568.2004.03508.x. [DOI] [PubMed] [Google Scholar]
- NORTH R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- NUNN P.A., NEWGREEN D.T.1999An investigation into the bladder responses induced via pelvic nerve stimulation in the anesthetized rat Br. J. Pharmacol. 126227P10051140 [Google Scholar]
- O'REILLY B.A., KOSAKA A.H., CHANG T.K., FORD A.P.D.W., POPERT R., RYMER J.M., MCMAHON S.B. A quantitative analysis of purinoceptor expression in human fetal and adult bladders. J. Urol. 2001;165:1730–1734. [PubMed] [Google Scholar]
- O'REILLY B.A., KOSAKA A.H., KNIGHT G.F., CHANG T.K., FORD A.P.D.W., RYMER J.M., POPERT R., BURNSTOCK G., MCMAHON S.B. P2X receptors and their role in female idiopathic detrusor instability. J. Urol. 2002;167:157–164. [PubMed] [Google Scholar]
- PALEA S., ARTIBANI W., OSTARDO E., TRIST D.G., PIETRA C. Evidence for purinergic neurotransmission in human urinary bladder affected by interstitial cyctitis. J. Urol. 1993;150:2007–2012. doi: 10.1016/s0022-5347(17)35955-4. [DOI] [PubMed] [Google Scholar]
- PANDITA R.K., ANDERSSON K.-E. Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J. Urol. 2002;168:1230–1234. doi: 10.1016/S0022-5347(05)64631-9. [DOI] [PubMed] [Google Scholar]
- REITZ A., STOHRER M., KRAMER G., DEL POPOLO G., CHARTIER-KASTLER E., PANNEK J., BURGDORFER H., GOCKING K., MADERSBACHER H., SCHUMACHER S., RICHTER R., VON TOBEL J., CHURCH B. European experience of 200 cases treated with botulinum-A toxin injections into the detrusor muscle for urinary incontinence due to neurogenic detrusor overactivity. Eur. Urol. 2004;45:510–515. doi: 10.1016/j.eururo.2003.12.004. [DOI] [PubMed] [Google Scholar]
- RONG W., SPYER K.M., BURNSTOCK G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J. Physiol. 2002;541:591–600. doi: 10.1113/jphysiol.2001.013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALAS N.A., SMITH C.P., KISS S., BOONE T.B., SOMOGYI G.T. Intravesical cholinergic receptor activated spinal ATP release in normal and chronic spinal cord injured rats. J. Urol. 2005;173:45. [Google Scholar]
- SCHAEFFER A.J. Epidemiology and demographics of prostatitis. Andrologia. 2003;35:252–257. doi: 10.1046/j.1439-0272.2003.00584.x. [DOI] [PubMed] [Google Scholar]
- SIBLEY G.N. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J. Physiol. 1984;354:431–443. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SJOGREN C., ANDERSSON K.-E., HUSTED S., MATTIASSON A., MOLLER-MADSEN B. Atropine resistance of transmurally stimulated isolated human bladder muscle. J. Urol. 1982;128:1368–1371. doi: 10.1016/s0022-5347(17)53509-0. [DOI] [PubMed] [Google Scholar]
- SMITH C.P., RADZISZEWSKI P., BORKOWSKI A., SOMOGYI G.T., BOONE T.B., CHANCELLOR M.B. Botulinum toxin A has antinociceptive effects in treating interstitial cystitis. Urology. 2004;64:871–875. doi: 10.1016/j.urology.2004.06.073. [DOI] [PubMed] [Google Scholar]
- SMITH C.P., VEMULAKONDA V.M., KISS S., BOONE T.B., SOMOGYI G.T. Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: Effect of botulinum toxin A. Neurochem. Int. 2005;47:291–297. doi: 10.1016/j.neuint.2005.04.021. [DOI] [PubMed] [Google Scholar]
- SOUSLOVA V., CESARE P., DING Y., AKOPIAN A.N., STANFA L., SUZUKI R., CARPENTER K., DICKENSON A., BOYCE S., HILL R., NEBENIUS-OOSTHUIZEN D., SMITH A.J.H., KIDD E.J., WOOD J.N. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–1017. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- SPEAKMAN M.J., KIRBY R.S., JOYCE A., ABRAMS P., POCOCK R. Guideline for the primary care management of male lower urinary tract symptoms. BJU Int. 2004;93:985–990. doi: 10.1111/j.1464-410X.2004.04765.x. [DOI] [PubMed] [Google Scholar]
- SUN Y., CHAI T.C. Effects of dimethyl sulphoxide and heparin on stretch-activated ATP release by bladder urothelial cells from patients with interstitial cystitis. BJU Int. 2002;90:381–385. doi: 10.1046/j.1464-410x.2002.02912.x. [DOI] [PubMed] [Google Scholar]
- SUN Y., CHAI T.C. Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J. Urol. 2004;171:448–452. doi: 10.1097/01.ju.0000099660.46774.3c. [DOI] [PubMed] [Google Scholar]
- SUN Y., KEAY S., DEDEYNE P.G., CHAI T.C. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J. Urol. 2001;166:1951–1956. [PubMed] [Google Scholar]
- SUN Y., MALOSSI J., JACOBS S.C., CHAI T.C. Effect of doxazosin on stretch-activated adenosine triphosphate release in bladder urothelial cells from patients with benign prostatic hyperplasia. Urology. 2002;60:351–356. doi: 10.1016/s0090-4295(02)01710-7. [DOI] [PubMed] [Google Scholar]
- TONG Y.C., HUNG Y.C., SHINOZUKA K., KUNITOMO M., CHENG J.T. Evidence of adenosine 5′-triphosphate release from nerve and P2x-purinoceptor mediated contraction during electrical stimulation of rat urinary bladder smooth muscle. J. Urol. 1997;158:1973–1977. doi: 10.1016/s0022-5347(01)64196-x. [DOI] [PubMed] [Google Scholar]
- TSUDA M., UENO S., INOUE K. Evidence for the involvement of spinal endogenous ATP and P2X receptors in nociceptive responses caused by formalin and capsaicin in mice. Br. J. Pharmacol. 1999a;128:1497–1504. doi: 10.1038/sj.bjp.0702960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUDA M., UENO S., INOUE K. In vivo pathway of thermal hyperalgesia by intrathecal administration of α,β-methylene ATP in mouse spinal cord: involvement of the glutamate-NMDA receptor system. Br. J. Pharmacol. 1999b;127:449–456. doi: 10.1038/sj.bjp.0702582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UENO S., MORIYAMA T., HONDA K., KAMIYA H., SAKURADA T., KATSURAGI T. Involvement of P2X2 and P2X3 receptors in neuropathic pain in a mouse model of chronic constriction injury. Drug Dev. Res. 2003;59:104–111. [Google Scholar]
- VELASCO C., GUARNERI L., LEONARDI A., TESTA R. Effects of intravenous and infravesical administration of suramin, terazosin and BMY 7378 on bladder instability in conscious rats with bladder outlet obstruction. BJU Int. 2003;92:131–136. doi: 10.1046/j.1464-410x.2003.04281.x. [DOI] [PubMed] [Google Scholar]
- VIAL C., EVANS R.J. P2X receptor expression in mouse urinary bladder and the requirement of P2X1 receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br. J. Pharmacol. 2000;131:1489–1495. doi: 10.1038/sj.bjp.0703720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIAL C., EVANS R.J. Smooth muscle does not have a common P2X receptor phenotype: expression, ontogeny and function of P2X1 receptors in mouse ileum, bladder and reproductive systems. Auton. Neurosci. Basic Clin. 2001;92:56–64. doi: 10.1016/S1566-0702(01)00319-8. [DOI] [PubMed] [Google Scholar]
- VLASKOVSKA M., KASAKOV L., RONG W., BODIN P., BARDINI M., COCKAYNE D.A., FORD A.P.D.W., BURNSTOCK G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J. Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VULCHANOVA L., RIEDL M.S., SHUSTER S.J., BUELL G., SURPRENANT A., NORTH R.A., ELDE R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- VULCHANOVA L., RIEDL M.S., SHUSTER S.J., STONE L.S., HARGREAVES K.M., BUELL G., SURPRENANT A., NORTH R.A., ELDE R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur. J. Neurosci. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- WANG E.C.Y., LEE J.M., RUIZ W.G., BALESTREIRE E.M., VON BODUNGEN M., BARRICK S., COCKAYNE D.A., BIRDER L.A., APODACA G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J. Clin. Inves. 2005;115:2412–2422. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIN A.J. Pharmacological agents for the treatment of urinary incontinence due to overactive bladder. Expert Opin. Inves. Drugs. 2001;10:65–83. doi: 10.1517/13543784.10.1.65. [DOI] [PubMed] [Google Scholar]
- WEIN A.J., HANNO P.M. Targets for therapy of the painful bladder. Urology. 2002;59:68–73. doi: 10.1016/s0090-4295(01)01640-5. [DOI] [PubMed] [Google Scholar]
- WESTFALL T.D., KENNEDY C., SNEDDON P. The ecto-ATPase inhibitor ARL 67156 enhances parasympathetic neurotransmission in the guinea-pig urinary bladder. Eur. J. Pharmacol. 1997;329:169–173. [PubMed] [Google Scholar]
- WU C. A comparison of the mode of action of ATP and carbachol on isolated human detrusor smooth muscle. J. Urol. 1999;162:1840. [PubMed] [Google Scholar]
- WU G., WHITESIDE G.T., LEE G., NOLAN S., NIOSI M., PEARSON M.S., ILYIN V.I. A-317491, a selective P2X3/P2X2/3 receptor antagonist, reverses inflammatory mechanical hyperalgesia through action at peripheral receptors in rats. Eur. J. Pharmacol. 2004;504:45–53. doi: 10.1016/j.ejphar.2004.09.056. [DOI] [PubMed] [Google Scholar]
- WUST M., AVERBECK B., REIF S., BRATER M., RAVENS U. Different responses to drugs against overactive bladder in detrusor muscle of pig, guinea pig and mouse. Eur. J. Pharmacol. 2002;454:59–69. doi: 10.1016/s0014-2999(02)02478-0. [DOI] [PubMed] [Google Scholar]
- YIANGOU Y., FACER P., FORD A., BRADY C., WISEMAN O., FOWLER C.J., ANAND P. Capsaicin receptor VR1 and ATP-gated ion channel P2X3 in human urinary bladder. BJU Int. 2001;87:774–779. doi: 10.1046/j.1464-410x.2001.02190.x. [DOI] [PubMed] [Google Scholar]
- YOSHIDA M., HOMMA Y., INADOME A., YONO M., SESHITA H., MIYAMOTO Y., MURAKAMI S., KAWABE K., UEDA S. Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscle. Exp. Gerontol. 2001;36:99–109. doi: 10.1016/s0531-5565(00)00175-3. [DOI] [PubMed] [Google Scholar]
- YOSHIDA M., MIYAMAE K., IWASHITA H., OTANI M., INADOME A. Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology. 2004;63:17–23. doi: 10.1016/j.urology.2003.11.003. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA N., DE GROAT W.C. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J. Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIMURA N., SEKI S., CHANCELLOR M.B., DE GROAT W.C., UEDA T. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology. 2002;59:61–67. doi: 10.1016/s0090-4295(01)01639-9. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA N., SEKI S., ERICKSON K.A., ERICKSON V.L., CHANCELLOR M.B., DE GROAT W.C. Histological and electrical properties of rat dorsal root ganglion neurons innervating the lower urinary tract. J. Neurosci. 2003;23:4355–4361. doi: 10.1523/JNEUROSCI.23-10-04355.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZERMANN D.H., ISHIGOOKA M., SCHUBERT J., SCHMIDT R.A. Perisphincteric injection of botulinum toxin type A. Eur. Urol. 2000;38:393–399. doi: 10.1159/000020314. [DOI] [PubMed] [Google Scholar]
- ZHONG Y., BANNING A.S., COCKAYNE D.A., FORD A.P.D.W., BURNSTOCK G., MCMAHON S.B. Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience. 2003;120:667–675. doi: 10.1016/s0306-4522(03)00243-4. [DOI] [PubMed] [Google Scholar]
- ZIGANSHIN A.U., HOYLE C.H., BO X., LAMBRECHT G., MUTSCHLER E., BAUMERT H.G., BURNSTOCK G. PPADS selectively antagonizes P2X-purinoceptor-mediated responses in the rabbit urinary bladder. Br. J. Pharmacol. 1993;110:1491–1495. doi: 10.1111/j.1476-5381.1993.tb13990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]