Abstract
The intricate balance maintained between cell growth and proliferation factors and apoptosis-inducing factors is fundamental to the regulation of prostate growth. Disruptions in this homeostasis often trigger the loss of apoptosis and the over-expression of factors promoting cell survival and proliferation, inevitably leading to tumorigenesis and cancer. Deregulation of prostate growth during prostate cancer development and progression is characterized by apoptotic evasion, uncontrolled proliferation, and increased invasive potential. Thus, in advanced stages of disease progression, surviving prostate tumour cells acquire the ability to migrate and invade heterotopic tissues, with the bone and lymph nodes being the most common sites for human prostate cancer metastasis. The challenges in the implementation of effective therapeutic strategies for the treatment of advanced metastatic prostate cancer reflect the multidimensional nature and functional significance of antiapoptotic pathways in the emergence of therapeutic resistance of prostate tumours. In this chapter, we discuss the current understanding of the molecular mechanisms governing growth factor signalling pathways with often overlapping functions that contribute to loss of apoptosis control and activation of cell proliferation towards aggressive prostate tumorigenic growth and metastatic behaviour. While a full understanding of the prosurvival characteristics of these growth factor pathways is still evolving, the impact that growth factors such a epidermal growth factor and transforming growth factor-β can be recognized by the vigorous attempts at therapeutic targeting of their key signalling steps.
Keywords: Apoptosis, hypoxia, angiogenesis, anoikis, prostate cancer, IGF-1, FGF, VEGF, TGF-β, growth factor signalling
Introduction
The role of deregulation of apoptosis in human tumorigenesis is complex and linked to successive and interdependent genetic events that gradually lead to tumour formation. The intricate balance maintained between cell growth and proliferation factors and apoptosis-inducing factors is fundamental to the regulation of prostate growth. Disruptions in this homeostasis often trigger the loss of apoptosis and the overexpression of factors promoting cell survival and proliferation. The inevitable result is a dysfunctional signalling pathway leading to tumorigenesis and cancer. The deregulation of prostate growth in prostate cancer cells is distinguished by apoptotic evasion, uncontrolled proliferation, and loss of differentiation. In addition, cancer cells gain the ability to migrate and invade heterotopic tissues, with bone being the most common site of human prostate cancer metastasis (see Scher & Chung, 1994).
Prostate cancer is the most common malignancy and is the second leading cause of cancer death in males (see Society American Cancer, 2005). Nearly 230,000 cases of prostate cancer were reported in 2004, with approximately 30,000 of them resulting in patient death (see Lara et al., 2004). Most early tumours are androgen-dependent, and thus the most common therapeutic option is to deprive the tumour of androgens via surgical or medical castration (see Gnanapragasam et al., 2003). While chemotherapy and androgen ablation have proven to have significant effects on the early stages of prostate cancer, advanced prostate cancer is resilient to such treatments. Despite the early efficacy of androgen ablation, prostate tumours eventually relapse into a hormone-refractory (androgen-independent) disease, with devastating results on morbidity and mortality rates (see Isaacs, 1994; Lara et al., 2004). The transition to androgen independence is followed by metastasis, with greater than 80% of all tumours metastasizing to bone (see Scher & Chung, 1994).
Biochemically, prostate cancer progression associated the deregulation of specific growth factors with their respective signalling pathways (see Djakiew, 2000; Stangelberger et al., 2005). Growth factors can be placed into three categories: positive growth factors, which promote growth and proliferation; negative growth factors, which regulate and inhibit cell growth and proliferation, and often induce apoptosis, and angiogenic growth factors, which provide the growth factors necessary to build vascular and oxygen supplies necessary to tissue growth and survival. Insulin-like growth factor-1 (IGF-1) and its associated signalling pathway is one of the most significant positive growth-promoting signal transduction pathways, while the fibroblast growth factor (FGF) family of growth factors plays the role of both a positive growth factor and an angiogenic growth factor. Transforming growth factor-β (TGF-β) is a negative growth factor crucial to the regulation of cell differentiation and proliferation (see Zhu & Kyprianou, 2005). Progression of prostate cancer is dependent on angiogenesis, mediated primarily via the increased expression of vascular endothelial growth factor (VEGF). Molecular dissection of the deregulation of growth factor signalling pathways in prostate tumorigenesis may provide promising new therapeutic targets for prostate cancer.
Degradation of extracellular matrix (ECM)-surrounding tumours is a critical step in the invasion and metastasis of malignant epithelial cells. The degradation process is mainly mediated by zinc-dependent matrix metalloproteases (MMPs) produced by stromal cells. An increasing amount of evidence suggests that cancer cells can stimulate MMP production in a paracrine manner. The epithelial–stromal interactions play a prominent role in prostate cancer progression, thus tumour-derived factors such as EMMPRIN (MMP inducer), recently found to be highly expressed on the cell surface of highly aggressive human prostate cancer cells (see Rennebecke et al., 2005), may provide mechanistic and clinically relevant insights into the functional contribution of tumour cell surface proteins in prostate cancer development.
Post-translational modifications of cell surface proteins and their associated proteins also play important role in apoptotic signalling pathways. Focal adhesion kinase (FAK) and integrin-linked kinase are two integrin-associated proteins that can trigger downstream signalling pathways and result in anoikis (detachment-induced apoptosis) (see Attwell et al., 2003), Rho family GTPases (see Ryromaa et al., 2000), phosphatidylinositol 3K-Akt (PI3K-Akt) kinase (see McFall et al., 2001) and mitogen-activated protein kinases (MAPK) (see Slack-Davis et al., 2003) are reported to be targets of integrin-mediated signalling. Introduction of a constitutively active form of FAK into anchorage-dependent cells can render cells to become anchorage-independent (see Slack-Davis et al., 2003), while activation of PI3K-Akt can block anoikis in transformed and cancer cells, while inhibition of PI3K can induce anoikis (see McFall et al., 2001). It is clear that proper expression levels and post-translational modification states of cell surface and intracellular proteins that might be partners for the growth factor receptors and their signalling effectors, respectively, which are critical for prostate homeostasis, deregulation of which would contribute to prostate tumour progression and metastasis.
In this review, we will discuss the current understanding of the functional contribution of these growth factor signalling pathways in prostate tumorigenesis, as well as the mechanistic and therapeutic significance of their deregulation in prostate cancer progression and development of novel treatment approaches for advanced disease.
Cell growth: a balancing act
Insulin-like growth factor 1
IGF-1 exerts a highly mitogenic activity in cells (see Wu et al., 2001). In addition, IGF-1 is often used to enhance the early healing of bones, as it (in conjunction with TGF-β) induces bone regeneration (see Schmidmaier et al., 2004; Srouji et al., 2005). The IGF signalling axis consists of a complicated network of IGFs, IGF-binding proteins (IGFBPs), IGF tyrosine kinase receptors (IGF-Rs) and IGF-binding protein proteases (see Moschos & Mantzoros, 2002). Nearly all normal tissues produce low levels of IGF-1, but higher amounts are found in tissues during adolescence – a stage at which cells are growing and proliferating at faster rates (see Djavan et al., 2001). Effective binding of IGF-1 ligand to the IGF-1 receptor leads to the activation of signalling pathways that contribute to nearly 50% of cell growth and proliferation, according to IGF signalling models (see Baserga et al., 2003).
IGF-1, which is produced by prostatic stromal cells in response to androgen stimulation, works in a paracrine manner by stimulating the surrounding prostatic epithelial cells, resulting in increased proliferation (see Moschos & Mantzoros, 2002; Bogdanos et al., 2003; Garrison & Kyprianou, 2004). Proliferation of prostate cancer cells is stimulated by an activated IGF-1 signalling pathway (see Stattin et al., 2004). In normal cells, the IGF-1 pathway is inhibited by the IGF binding proteins. IGFBPs bind to IGF-1 with high affinity, effectively sequestering IGF-1 and preventing pathway activation through interaction with its receptor (see Grimberg & Cohen, 2000; Stewart & Weigel, 2005). Nearly 99% of free IGF is bound to IGFBPs in normal cells, with most being bound to IGFBP-3 (see Djavan et al., 2001; Moschos & Mantzoros, 2002).
The downstream targets of the IGF-1 signalling axis ultimately promote cell survival. The primary cell survival pathway activated in the IGF-1 axis is the PI3/Akt signalling pathway (see Dillin et al., 2002). Binding of the IGF-1 ligand to the IGF-1R results in the phosphorylation (and activation) of phosphoinositol-3 kinase (PI3). PI3 then further activates the Akt pathway, resulting in the phosphorylation (deactivation) of the proapoptotic Bad protein and effectively blocking apoptosis (see Moschos & Mantzoros, 2002). In addition to PI3/Akt pathway activation, IGF-1 also induces the activation of the MAPK pathway via the Ras protein. Moreover, a downstream target of the Ras/MAPK pathway is the proapoptotic protein Bad, which becomes deactivated upon phosphorylation, leading to cell survival and proliferation (see Moschos & Mantzoros, 2002).
A direct correlation between high plasma IGF-1 levels and prostate cancer progression has led to the implication of IGF-1 as an aetiologic factor of prostate cancer (see Stattin et al., 2004). As such, high serum levels of IGF-1 become promising predictors for prostate cancer and increased risk of malignancy (see Mantzoros et al., 1997; Wolk et al., 1998; Khosravi et al., 2001). IGF-1 is commonly overexpressed in the prostatic stroma, exerting its mitogenic action on prostatic epithelial cells in a paracrine manner (see Tennant et al., 1996). Targeting the Igf-1 gene in the prostatic stroma has emerged as a potentially attractive modality for treating prostate cancer.
One must also consider additional steps in the IGF-1 signalling pathway as molecular targets. For instance, downregulating the IGF-1R (which is constitutively expressed in prostatic epithelial cells) induces apoptosis in prostate cancer cells (see Reiss et al., 1998; Djavan et al., 2001; Baserga et al., 2003). Another possibility would be to upregulate IGFBP expression, which could lead to the binding of any excess IGF-1, inhibiting the IGF-1 signalling axis (see Nickerson et al., 1997). Indeed, the use of a new 5α-reductase inhibitor, epristeride, promises such a therapeutic approach. In preliminary studies, epristeride has been shown to lower IGF-1 protein and mRNA levels in both the stromal and epithelial BPH cells (see Wu et al., 2001). In addition, epristeride increases TGF-β expression, pointing to potential crosstalk between two growth factor signalling pathways.
Fibroblast growth factors
The FGF family contains 22 members and four different receptors (FGFRs) that bind the FGFs with very high affinity (see Ropiquet et al., 1999; Ornitz & Itoh, 2001). FGFs are highly conserved polypeptide growth factors that play a formidable role in development, angiogenesis, growth and proliferation, and when overexpressed, tumour formation (see Ornitz & Itoh, 2001; Smith et al., 2001). One of the more unique characteristics of FGFs is their high affinity for heparin sulphate proteoglycans, and heparin analogues, in the ECM (see Gospodarowicz & Cheng, 1986; Ornitz & Itoh, 2001). Each FGF has distinct FGF receptor and heparin-binding regions, and the ability to bind heparin in the ECM not only protects FGFs from degradation but also creates somewhat of an extracellular, growth factor repository (see Gospodarowicz & Cheng, 1986; Faham et al., 1998; Ornitz & Itoh, 2001).
Three specific FGFs play a significant role in the development of prostate cancer: FGF-2 (also known as basic FGF, or bFGF), FGF-7, and FGF-8. FGF-2 acts as a mitogen for prostatic stromal cells, and exerts its effect mainly in an autocrine manner (see Ropiquet et al., 1999; Garrison & Kyprianou, 2004). FGF-2 also maintains the ability to contribute to angiogenesis (see Mydlo et al., 1988). In contrast, FGF-7 exercises its effect in a paracrine manner, acting as a mitogen for prostatic epithelial cells (see Ittman & Mansukhani, 1997). The mechanism of action for FGF-8 has not been entirely elucidated, but FGF-8 is thought to play a role in carcinogenesis due to its overexpression in prostate cancer cells.
Recent evidence indicates that hypoxia induces FGF-2 and FGF-7 production, secretion, and, in some cases, the development of prostatic stromal and epithelial hyperplasia (see Berger et al., 2003). FGF is secreted by the stromal cells via a Na+/K+ ATPase pump (see Florkiewicz et al., 1998). Upon ligand release, FGF receptors, which contain both immunoglobin- and heparin-like binding domains, are able to bind to FGFs with extraordinarily high affinity, initiating the tyrosine kinase activity of the receptor (see Johnson et al., 1990). Once activated, the FGFRs target the downstream MAPK pathway, resulting in cell survival, proliferation, and angiogenesis (see Tsang & Dawid, 2004; Yamada et al., 2004).
A growing body of evidence documents both the direct and indirect contribution of FGF-2 and FGF-7 to prostate tumorigenesis. FGF-2 and FGF-7 levels are found in abnormally high levels (2–3-fold higher) in both benign and malignant prostate cells (see Cronauer et al., 1997; Ropiquet et al., 1999). In addition, the FGF-8 growth factor is overexpressed in approximately 60% of tumours with a Gleason grade of 7 and nearly all tumours (92%) with a Gleason grade of 8 or higher (see Gnanapragasam et al., 2003). High levels of all three of these FGFs in hyperplasic tissues are often indicative of unmediated proliferation, tumour metastasis, and extremely low survival rates (see Dorkin et al., 1999; Ropiquet et al., 1999). Targeting the FGF signalling axis is crucial to halting the powerful tumorigenic capabilities of the FGF family.
Anvirizel, a novel FGF-targeting drug, is an extract of the evergreen tree Nerium oleander and is currently undergoing clinical evaluations as a potential mode of treatment. The active components of Anvirizel appear to be the cardiac glycosides oleandrin and oleandrigenin (see Smith et al., 2001). Anvirizel exerts its mechanism of action by interfering with specific membrane Na+/K+ ATPase pumps, effectively inhibiting FGF-2 export (see Florkiewicz et al., 1998; Smith et al., 2001). The lack of extracellular FGF-2 caused by Anvirizel prevents the activation of the FGF-2 signalling pathway, thus inhibiting prostate cancer cell proliferation in vivo in both PC-3 and DU-145 prostate cancer cells (see Smith et al., 2001); a similar effect was observed in breast, lung, and melanoma cancer cells (see Smith et al., 2001; Manna et al., 2000; McConkey et al., 2000). As such, the FGF signalling axis is emerging as a clinically exciting target of molecular intervention and justifiably warrants further exploration and targeted therapeutic development.
Apoptosis players in the prostate
Transforming growth factor-β
In the normal prostate, TGF-β inhibits epithelial cell proliferation and stimulates apoptosis, thus acting in a tumour suppressor-like manner (see Bello-DeOcampo & Tindall, 2003). TGF-β signal transduction is initiated by binding of the TGF-β ligand to two distinct cell surface receptors (TβRI and TβRII), both of which have serine/threonine kinase domains (see Bello-DeOcampo & Tindall, 2003; Motyl & Gajewska, 2004; Feng & Derynck, 2005). Originally named for its ability to stimulate fibroblast growth, TGF-β has proven to be a critical regulator of prostate cell growth due to its ability to inhibit epithelial cell proliferation and induce apoptosis (see Massague et al., 1992; Zhu & Kyprianou, 2005).
TGF-β is released from prostatic stromal cells and exerts its impact in a paracrine manner, inhibiting prostatic epithelial cell growth and inducing apoptosis (see Wu et al., 2001; Bhowmick et al., 2004). TβRII is the primary receptor target for TGF-β, and upon binding, TβRII heterodimerizes with TβRI to initiate an intracellular signal transduction cascade (see Guo & Kyprianou, 1999). TGF-β exhibits pleiotropy, and as such, the TGF-β signalling axis stimulates a wide array of downstream targets – all of which have antiproliferative or apoptotic effects. Once the TβRI/TβRII heterodimer is formed, the serine/threonine kinase activity of the receptors is activated, effectively targeting the SMAD proteins as the primary intracellular effectors of TGF-β signalling. Phosphorylation of the SMAD proteins, namely SMAD-2 and SMAD-3, initiates the transduction of the TGF-β signal from the cell membrane to the nucleus (see Massague, 1998; Motyl & Gajewska, 2004). Upon nuclear translocation, the phosphorylated SMAD proteins trigger the activation of a series of transcription factors that dictate the proliferative and/or apoptotic outcomes of the cells (see Bello-DeOcampo & Tindall, 2003). The transcription of Bax, a proapoptotic factor that deactivates that antiapoptotic factor Bcl-2, is upregulated. In addition, the SMAD-activated transcription factors downregulate the transcription of the cell survival factor Bcl-2 (see Guo & Kyprianou, 1999). Further, the cell cycle is effectively halted by the increased expression of the cyclin-dependent kinase inhibitor p27Kip1 (see Guo & Kyprianou, 1999). Transcription activated by the TGF-β/SMAD signalling pathway leads to increased expression of IGFBP-3, the primary binding protein involved in sequestering the positive growth factor IGF-1 (see Nickerson et al., 1997; Motyl & Gajewska, 2004). Finally, the activated SMAD also has an effect in the cytosol, activating the apoptosis initiation factor caspase-1 (see Guo & Kyprianou, 1999).
Normal benign prostate cells express TGF-β in normal levels, while prostate cancer cells tend to overexpress TGF-β (see Perry et al., 1997; Lee et al., 1999; Zhu & Kyprianou, 2005). While the growth factor TGF-β might be overexpressed in the vast majority of prostate tumours, the important facet to examine is the direct correlation between prostate cancer progression and decreased TGF-β receptor expression (see Wikstrom et al., 1999). Receptor downregulation, mainly that of TβRII, and the upregulation of TGF-β is typically associated with the invasive, hormone-refractory forms of prostate cancer (see Guo et al., 1997; Shariat et al., 2004). Yet, the apoptotic potency of the TGF-β signalling pathway remains present, even in malignant cells. Studies have shown that overexpression of the TβRII receptor in prostate cancer cells generates an apoptotic response, comparable to that observed in normal prostate cells (see Hsing et al., 1996; Tu et al., 1996).
Mechanistically, TGF-β apoptotic signalling has been partnered with many key apoptotic regulators. The cell survival factor Bcl-2 can inhibit apoptosis typically induced by TGF-β in normal prostatic epithelial cells (see Bruckheimer & Kyprianou, 2002). Upregulation of prostate-specific antigen (PSA), often a hallmark of prostate cancer development, also inhibits the apoptotic ability of TGF-β (see Kang et al., 2001). Interestingly, androgens negatively regulate the expression of both TGF-β and its receptors, thus providing a molecular basis for the marked enhancement of TGF-β-induced prostate epithelial apoptosis following androgen ablation (see Wikstrom et al., 1999; Zatelli et al., 2000; Zhu & Kyprianou, 2005). There appears to be a considerably active crosstalk between the TGF-β signalling pathway and the androgen signalling axis, the degradation of which may functionally contribute to tumorigenesis (see Guo & Kyprianou, 1999; Gerdes et al., 2004; Zhu & Kyprianou, 2005).
Considering a dysfunctional TGF-β signalling pathway in prostate tumorigenesis proves attractive for new steps of therapeutic targeting. The loss of TβRII expression is quickly becoming a potential marker for prostate tumour progression. Being able to restore TβRII expression (or overexpressing it) in hormone-refractory prostate cancer cells could effectively reduce tumorigenicity and induce caspase-1-mediated apoptosis (see Guo & Kyprianou, 1999). The 5α-reductase inhibitor, epristeride, the same drug shown to inhibit IGF-1 mRNA expression, has been shown to increase TβRII expression, again asserting evidence of crosstalk between these two pathways (see Wu et al., 2001). Quinazoline-based α1-andrenoreceptor blockers, such as doxazosin and terazosin, have also been shown to induce the activation of the TGF-β signalling axis (see Partin et al., 2003). Clearly, TGF-β and its associated signalling pathway present a biochemically attractive avenue for tumour suppression.
Building a blood supply: the angiogenesis route
Vascular endothelial growth factor
Transformed cells would encounter many obstacles to tumour growth and progression, including hypoxia and nutrient deprivation, changes in cell–cell and cell–matrix interactions, inflammatory and growth inhibitory cytokines, and cell cycle checkpoints. Moreover, angiogenesis plays a vital role in tumour growth and progression (see Semenza, 2002a, 2002b; Nybert et al., 2005). A tumour cannot progress beyond 2–5 mm in diameter without procuring its own blood supply (see Kim et al., 1993; Lara et al., 2004; Gray et al., 2005). Among the factors that induce neovascularization, VEGF is perhaps the most widely studied (see Gray et al., 2005). VEGF serves as a mitogen for endothelial cells, stimulating cells to divide and promoting angiogenesis (see Ferrara & Henzel, 1989; Jackson et al., 2002). VEGF transduces its signal via the action of two types tyrosine kinase receptors located on endothelial cell membranes, VEGFR-I and VEGFR-II (see Ferrara et al., 2003). There is considerable evidence indicating that VEGF expression decreases significantly in response to androgen ablation (see Joseph et al., 1997; Sordello et al., 1998; Stewart et al., 2001; Lara et al., 2004).
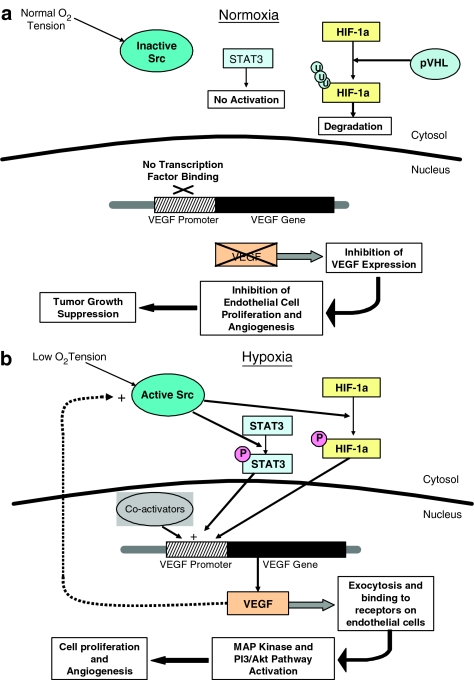
An intact VEGF signalling pathway is critical to tumorigenesis and the expression of VEGF is mediated heavily by the binding of signal transducer/activator of transcription-3 (STAT3) and hypoxia inducible factor 1-α (HIF-1α) to the promoter region of the VEGF gene (see Wei et al., 2003; Gray et al., 2005). As a tumour grows, the supply of oxygen that is able to reach neoplastic cells gradually decreases, leading to a condition aptly labelled hypoxia. The low oxygen tension present in hypoxic conditions stimulates the activation of Src, a tyrosine kinase that phosphorylates HIF-1α and STAT3 (see Semenza, 2002a, 2002b; Gray et al., 2005). Activated forms of HIF-1α and STAT3 both dimerize, and upon nuclear translocation, they activate a variety of hypoxic response elements – namely the expression of VEGF (Figure 1b) (see Fu et al., 2005). Once VEGF is released, it binds to VEGF receptors on adjacent endothelial cells and induces a series of cell survival and mitogenic pathways, primarily through the PI3/Akt pathway and the Ras-mediated MAP kinase pathway. VEGF may also exert its action by positively feeding back on the Src protein in the cytosol, maintaining the VEGF-promoting stimulus. Thus, Src, HIF-1α, and STAT3 act to regulate cell survival (see Semenza, 2003). In normal cells, VEGF is present in very low amounts (if at all) since activation of transcription factors STAT3 and HIF-1α is strictly regulated (see Fu et al., 2005). In normoxia (normal oxygen levels), the Src protein is inactive and, as such, cannot phosphorylate STAT3 or HIF-1α (Figure 1b). Inactive STAT3 does not dimerize or get transported to the nucleus, and any inactive HIF-1α is subsequently ubiquitinated and targeted for degradation by the von Hippel–Lindau protein (see Ivan et al., 2001; Jaakkola et al., 2001; Masson et al., 2001; Yu et al., 2001; Min et al., 2002; Fu et al., 2005). Inhibiting STAT3 and HIF-1α promoter site binding effectively reduces the transcription of VEGF, consequently preventing any neovascularization and thus preventing tumour progression (Figure 1a) (see Gray et al., 2005; Nybert et al., 2005).
Figure 1.
Angiogenesis outcomes: hypoxia and VEGF signalling. Involvement of angiogenesis in tumour formation. Under normal oxygen levels (a), the Src protein is inactive, consequently preventing the phosphorylation and activation of STAT3 and HIF-1α. These inactive transcription factors cannot bind to the VEGF promoter and therefore prevent the expression of the VEGF gene, effectively halting angiogenesis and suppressing tumour growth. Hypoxic conditions (b) activate the Src protein, which then phosphorylates STAT3 and HIF-1α. These active transcription factors bind to the VEGF promoter region, leading to the expression of VEGF, subsequent endothelial cell proliferation, and angiogenesis.
Angiogenic growth factors tend to be maintained in low levels in normal cells, keeping a steady balance between pro- and anti-angiogenesis, which often tilts in favour of angiogenic prevention (see Nybert et al., 2005). However, the disruption of the cellular ‘balance' and subsequent upregulation of VEGF, whether in response to improper signalling or hypoxia, is responsible for the development of aggressive, invasive metastases (see Ferrer et al., 1998; 1999; Nybert et al., 2005). VEGF expression is quite high in most prostate cancers and thus serves as a target for therapeutic treatments (see Lara et al., 2004). Rosiglitazone, a thiazolidinedione PPAR-γ ligand used in the treatment of diabetes, has been shown to inhibit VEGF production in tumour cells, successfully suppressing their growth (see Kubota et al., 1998; Panigrahy et al., 2002). Monoclonal antibodies specific against VEGF injected into DU-145 prostate tumour mouse xenografts have also been shown to prevent angiogenesis (see Borgstrom et al., 1998). The ability to downregulate VEGF expression is a critical step in arresting prostate tumour growth and progression, thus presenting several attractive possibilities for therapeutic intervention (see Jiang et al., 1997; Wiener et al., 1999; Gray et al., 2005).
Signalling crosstalk: growth factor pathways find common cell ground
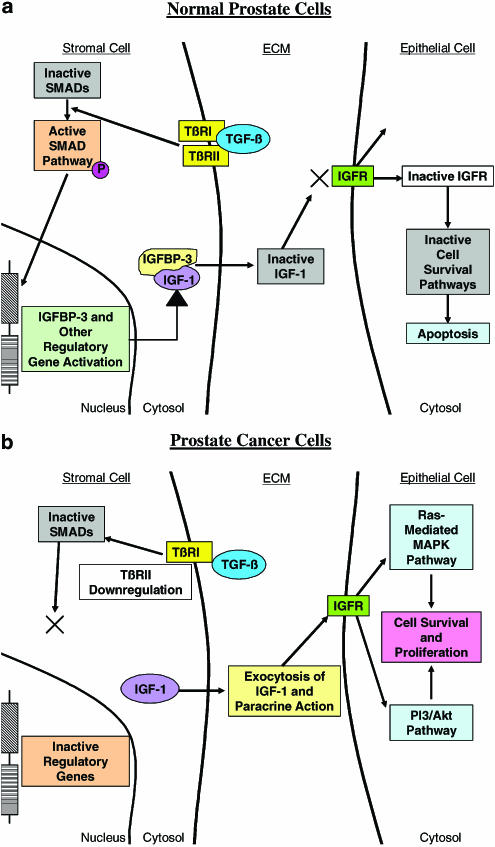
Examination of just a few of these growth factor pathways has revealed evidence of considerable crosstalk that exists between the stromal and epithelial cells of the prostate. Clearly, the growth factors expressed by stromal/fibroblast cells can exert a paracrine growth influence by binding to receptors on adjacent epithelial cells, or can exert an autocrine influence by binding to receptors on other stromal cells. Epithelial cells can thus be stimulated to release growth factors that can induce stromal cell growth, and thus the stage is set for a cyclic pathway of crosstalk between the stroma and epithelium of the prostate. One can appreciate from Figure 2 that crosstalk between stromal and epithelial cells is epitomized by the IGF-1 and TGF-β pathways. Direct pathway activation of TGF-β signalling in the normal prostate induces the expression of IGFBP-3, which prevents activation of the IGF-1 growth and survival pathway (Figure 2a). Conversely, dysfunctional TGF-β signalling can lead to increased activation of the IGF-1 growth factor pathway, eventually leading to tumorigenesis (Figure 2b). Another facet of the crosstalk involves the shared downstream effectors of the various growth factor signalling pathways. A classic example of such a communal intracellular target is the PI3/Akt signalling pathway. IGF-1-mediated receptor activation immediately targets the PI3/Akt pathway and subsequently deactivates the proapoptotic protein Bad; VEGF operates by the same signalling mechanism. Other signal transduction pathways, including the MAPK pathway, also serve as downstream for effectors for IGF-1, VEGF, and even for TGF-β.
Figure 2.
In normal prostate epithelial cells (a), TGF-β binds to the TβRI and TβRII receptors, activating the SMAD signalling pathway and inducing the transcription of IGFBP-3 and other regulatory genes. Some of these genes inactivate IGF-1 and (b) prevent it from binding to IGF receptors on adjacent cells. Loss of IGF signalling inactivates specific cell survival pathways, leading to apoptosis. (b) Downregulation/loss of TβRII, severely limits the anti-growth regulatory activity of TGF-β. Positive growth factors, like IGF, become active and bind to their receptors – initiating cell survival and proliferation pathways. TGF-β/IGF-1 crosstalk in normal and malignant prostate.
Pharmacological exploitation of the critical crosstalk events between the various growth factor signalling pathways provides promising therapeutic possibilities for prostate tumour targeting. Doxazosin and terazosin are quinazoline-based α1-adrenoceptor antagonists that are clinically effective in the relief of symptoms of BPH via their ability to selectively antagonize the α1-adrenoceptors and relax prostate smooth muscle tissue (see Kirby & Pool, 1997; Kyprianou, 2003). Recent experimental and clinical evidence, however, indicates that induction of prostate epithelial and smooth muscle cell apoptosis by doxazosin and terazosin is one of the molecular mechanisms contributing to the overall long-term clinical efficacy of these medications in improving lower urinary tract symptoms in BPH patients (see Kyprianou, 2003), as well as suppression of tumour growth of androgen-independent human prostate cancer xenografts (see Kyprianou & Benning, 2000; Benning & Kyprianou, 2002; Tahmatzopoulos & Kyprianou, 2004). More recent evidence established the ability of the quinazoline-based α1-adrenoceptor antagonist, doxazosin, but not the sulphonamide-based α1-adrenoceptor antagonist, tamsulosin, to trigger the phenomenon of anoikis, inhibit cell adhesion, and induce apoptosis of benign and malignant prostate epithelial cells and tumour-derived endothelial cells (see Keledjian et al., 2005; Garrison & Kyprianou, 2006). Both quinazoline-based α1-adrenoceptor antagonists (doxazosin and terazosin) can directly target VEGF-mediated angiogenesis and inhibit endothelial cell adhesion and migration (see Keledjian et al., 2005), via a death receptor-mediated apoptotic signalling (see Garrison & Kyprianou, 2006). Doxazosin also interferes with FGF-2 growth signalling and restimulates the TGF-β signalling pathway, which is absent in tumour cells (see Shaw et al., 2004; Tahmatzopoulos et al., 2004). Additional signalling mechanisms involving disruption of cell attachment to the ECM and subsequent induction of anoikis have been functionally implicated in a crosstalk with integrin signalling to be driving the cell death action of the quinazolines against the prostate. Indeed, cell detachment emerges as a critical event in apoptosis induction by doxazosin, which is a consequence of loss of cell attachment (anoikis induction), rather than the contributing executioner of apoptotic cell death (by the drug). Ongoing studies focus on dissecting the molecular mechanism underlying the antigrowth action of the quinazoline-based α1-adrenoceptor antagonists, doxazosin and terazosin, towards the development of a drug-discovery structure–function approach for the identification of the therapeutic efficacy of novel lead quinazoline-based compounds.
Summary
Future directions must involve the powerful molecular technology that is becoming increasingly available in combination with bioinformatics approaches. Mass spectrometry analysis can be directly applied to identify unknown growth actors and their signalling patterns and characterize post-translational modification and protein abundance (see Mann et al., 2001). Ongoing studies in this laboratory seek to specifically identify novel players of the TGF-β intracellular signalling that facilitates the intercellular protein circuitry in prostate cancer cells towards apoptosis induction. This is accomplished by introduction of the 2-DE MS/MS technique to compare the differential response of androgen-sensitive and TGF-β-resistant to androgen-sensitive and TGF-β1-responsive prostate cancer cells to TGF-β1 treatment. While such analysis represents only one example of the plethora of possibilities in the application of the proteomics approach towards the identification of novel signalling partners of a known growth factor, and their receptors, the value for such analysis in dissecting critical targets in key growth factor signalling pathways towards apoptosis induction in human prostate cancer cells holds considerable diagnostic, prognostic, and therapeutic promise for the detection and treatment of a disease so pervasive and debilitating that it threatens a large population of ageing North American males.
Deregulation of growth factors, such as IGF-1, FGFs, and VEGF, coupled with the loss of the TGF-β signalling pathway both play a role in prostate cancer development and progression. The growth factor signalling pathways regulating apoptosis and proliferation offer significant molecular targets for therapeutic targeting of hormone-refractory prostate cancer. Dysfunctional apoptotic programming leading to loss/suppression of apoptosis has been heavily implicated in prostate cancer progression; thus, reinstating apoptosis holds considerable promise as the molecular basis of effective therapeutic approaches for the treatment of hormone-refractory tumours.
Acknowledgments
These studies were supported by the following: Pfizer Educational Grant, MERCK Educational Grant, NIH 1NIDDK R01 Grant, DK 53525-06, and an UROdoc Research Grant. A. Ryan Reynolds was a recipient of a Markey Cancer Center Summer Research Fellowship at the University of Kentucky. We thank Dr Rangall G. Rowland, Chief, Division of Urology at the University of Kentucky Medical Center, for useful discussions.
Glossary
- BPH
benign prostatic hyperplasia
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- IGF-1
insulin-like growth factor-1
- MAPK
mitogen-activated protein kinase
- PSA
prostate-specific antigen
- TGF-β
transforming growth factor-β
- TβRII
TGF-β receptor II
- VEFG
vascular endothelial growth factor
References
- ATTWELL S., ROSKELLEY C., MILLS J., TROUSSARD A., WU C., DEDHAR S. The integrin-linked kinase (ILK) suppresses anoikis. Oncogene. 2003;19:3811–3815. doi: 10.1038/sj.onc.1203711. [DOI] [PubMed] [Google Scholar]
- BASERGA R., PERUZZI F., REISS K. The IGF-1 receptor in cancer biology. Int. J. Cancer. 2003;107:873–877. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- BENNING C.M., KYPRIANOU N. Quinazoline-derived α1-adrenoceptor antagonists induce prostate cancer cell apoptosis via an α1-adrenoceptor-indepedent action. Cancer Res. 2002;62:597–603. [PubMed] [Google Scholar]
- BELLO-DEOCAMPO D., TINDALL D.J. TGF-β/Smad signaling in prostate cancer. Curr. Drug Targets. 2003;4:197–207. doi: 10.2174/1389450033491118. [DOI] [PubMed] [Google Scholar]
- BERGER A.P., KOFLER K., BEKTIC J., ROGATSCH H., STEINER H., BARTSCH G., KLOCKER H. Increased growth factor prodcution in a human prostatic stromal cell culture model caused by hypoxia. Prostate. 2003;57:57–65. doi: 10.1002/pros.10279. [DOI] [PubMed] [Google Scholar]
- BHOWMICK N.A., CHYTIL A., PLIETH D., GORSKA A.E., DUMONT N., SHAPPELL S., WASHINGTON M.K., NEILSON E.G., MOSES H.L. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–850. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- BOGDANOS J., KARAMANOLAKIS D., TENTA R., TSINTAVIS A., MILATHIANAKIS C., MITSIADES C., KOUTSILIERIS M. Endocrine/paracrine/autocrine survival factor activity of bone microenvironment participates in the development of androgen ablation and chemotherapy refractoriness of prostate cancer metastasis in skeleton. Endocr. Relat. Cancer. 2003;10:279–289. doi: 10.1677/erc.0.0100279. [DOI] [PubMed] [Google Scholar]
- BORGSTROM P., BOURDON M.A., HILLAN K.J., SRIRAMARAO P., FERRARA N. Neutralizing anti-vascular endothelial growth factor antibody completely inhibits angiogenesis and growth of human prostate carcinoma micro tumours in vivo. Prostate. 1998;35:1–10. doi: 10.1002/(sici)1097-0045(19980401)35:1<1::aid-pros1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- BRUCKHEIMER E.M., KYPRIANOU N. Bcl-2 antagonizes the combined apoptotic effect of transforming growth factor-β dihydrotestosterone in prostate cancer cells. Prostate. 2002;53:133–142. doi: 10.1002/pros.10143. [DOI] [PubMed] [Google Scholar]
- CRONAUER M.V., HITTMAIR A., EDER I.E., HOBISCH A., CULIG Z., RAMONER R., ZHANG J., BARTSCH G., REISSIGL A., RADMAYR C., THURNHER M., KLOCKER H. Basic fibroblast growth factor levels in cancer cells and in sera of patients suffering from proliferative disorders of the prostate. Prostate. 1997;31:223–233. doi: 10.1002/(sici)1097-0045(19970601)31:4<223::aid-pros3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- DILLIN A., CRAWFORD D.K., KENYON C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- DJAKIEW D. Deregulated expression of growth factors and their receptors in the development of prostate cancer. Prostate. 2000;42:150–160. doi: 10.1002/(sici)1097-0045(20000201)42:2<150::aid-pros10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- DJAVAN B., WALDERT M., SEITZ C., MARBERGER M. Insulin-like growth factors and prostate cancer. World J. Urol. 2001;19:225–233. doi: 10.1007/s003450100220. [DOI] [PubMed] [Google Scholar]
- DORKIN T.J., ROBINSON M.C., MARSH C., BJARTELL A., NEAL D.E., LEUNG H.Y. FGF-8 over-expression in prostate cancer is associated with decreased patient survival and persists in androgen independent disease. Oncogene. 1999;18:2755–2761. doi: 10.1038/sj.onc.1202624. [DOI] [PubMed] [Google Scholar]
- FAHAM S., LINHARDT R.J., REES D.C. Diversity does make a difference: fibroblast growth factor-heparin interactions. Curr. Opin. Struct. Biol. 1998;8:578–586. doi: 10.1016/s0959-440x(98)80147-4. [DOI] [PubMed] [Google Scholar]
- FENG X.S., DERYNCK R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- FERRARA N., GERBER H.P., LECOUTER J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- FERRARA N., HENZEL W.J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophy. Res. Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- FERRER F.A., MILLER L.J., ANDRAWIS R.I., KURTZMAN S.H., ALBERTSEN P.C., LAUDONE V.P., KREUTZER D.L. Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology. 1998;51:161–167. doi: 10.1016/s0090-4295(97)00491-3. [DOI] [PubMed] [Google Scholar]
- FERRER F.A., MILLER L.J., LINDQUIST R., KOWALCZYK P., LAUDONE V.P., ALBERTSEN P.C., KREUTZER D.L. Expression of vascular endothelial growth factor receptors in human prostate cancer. Urology. 1999;54:567–572. doi: 10.1016/s0090-4295(99)00156-9. [DOI] [PubMed] [Google Scholar]
- FLORKIEWICZ R.Z., ANCHIN J., BAIRD A. The inhibition of fibroblast growth factor-2 export by cardenolides implies a novel function for the catalytic subunit of Na+/K+-ATPase. J. Biol. Chem. 1998;273:544–551. doi: 10.1074/jbc.273.1.544. [DOI] [PubMed] [Google Scholar]
- FU X.S., CHOI E., BUBLEY G.J., BALK S.P. Identification of hypoxia-inducible factor-1α (HIF-1α) polymorphism as a mutation in prostate cancer that prevents normoxia-induced degradation. Prostate. 2005;63:215–221. doi: 10.1002/pros.20190. [DOI] [PubMed] [Google Scholar]
- GARRISON J.B., KYPRIANOU N. Novel targeting of apoptosis pathways for prostate cancer therapy. Cur. Cancer Drug. Targets. 2004;4:85–95. doi: 10.2174/1568009043481623. [DOI] [PubMed] [Google Scholar]
- GARRISON J.B., KYPRIANOU N. Doxazosin induces apoptosis of benign and malignant prostate cells via a death-receptor mediated pathway. Cancer Res. 2006;66:1–9. doi: 10.1158/0008-5472.CAN-05-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERDES M.J., LARSEN M., DANG T.D., RESSLER S.J., TUXHORN J.A., ROWLEY D.R. Regulation of rate prostate stromal cell myodifferentiation by androgen and TGF-β1. Prostate. 2004;58:299–307. doi: 10.1002/pros.10327. [DOI] [PubMed] [Google Scholar]
- GNANAPRAGASAM V.J., ROBINSON M.C., MARSH C., ROBSON C.N., HAMDY F.C., LEUNG H.Y. FGF8 isoform b expression in human prostate cancer. Br. J. Cancer. 2003;88:1432–1438. doi: 10.1038/sj.bjc.6600875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSPODAROWICZ D., CHENG J. Heparin protects basic and acidic FGF from inactivation. J. Cell. Physiol. 1986;128:475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- GRAY M.J., ZHANG J., ELLIS L.M., SEMENZA G.L., EVANS D.B., WATOWICH S.S., GALLICK G.E. HIF-1α, STAT3, CBP/p300 and REF-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24:3110–3120. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- GRIMBERG A., COHEN P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J. Cell. Phys. 2000;183:1–9. doi: 10.1002/(SICI)1097-4652(200004)183:1<1::AID-JCP1>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUO Y., JACOBS S.C., KYPRIANOU N. Down-regulation of protein and mRNA expression for transforming growth factor-beta (TGF-beta1) type I and type II receptors in human prostate cancer. Int. J. Cancer. 1997;71:573–579. doi: 10.1002/(sici)1097-0215(19970516)71:4<573::aid-ijc11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- GUO Y., KYPRIANOU N. Restoration of transforming growth factor beta signaling pathway in human prostate cancer cells suppresses tumourigenicity via induction of caspase-1-mediated apoptosis. Cancer Res. 1999;59:1366–1371. [PubMed] [Google Scholar]
- HSING A.Y., KADOMATSU K., BONHAM M.J., DANIELPOUR D. Regulation of apoptosis induced by transforming growth factor-β1 in nontumourigenic and tumourigenic rat prostatic epithelial cell lines. Cancer Res. 1996;56:5146–5149. [PubMed] [Google Scholar]
- ISAACS J.T. Role of androgens in prostatic cancer. Vitam. Horm. 1994;49:433–502. doi: 10.1016/s0083-6729(08)61152-8. [DOI] [PubMed] [Google Scholar]
- ITTMAN M., MANSUKHANI A. Expression of fibroblast growth factors (FGFs) and FGF receptors in human prostate. J. Urol. 1997;157:351–356. [PubMed] [Google Scholar]
- IVAN M., KONDO K., YANG H., KIM W., VALIANDO J., OHH M., SALIC A., ASARA J.M., LANE W.S., KAELIN W.G., JR. HIF-α targeted for VHLmediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- JAAKKOLA P., MOLE D.R., TIAN Y.M., WILSON M.I., GIELBERT J., GASKELL S.J., KRIEGSHEIM A., HEBESTREIT H.F., MUKHERJI M., SCHOFIELD C.J., MAXWELL P.H., PUGH C.W., RATCLIFFE P.J. Targeting of HIF-α to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- JACKSON M.W., ROBERTS J.S., HECKFORD S.E., RICCIARDELLI C., STAHL J., CHOONG C., HORSFALL D.J., TILLEY W.D. A potential autocrine role for vascular endothelial growth factor in prostate cancer. Cancer Res. 2002;62:854–859. [PubMed] [Google Scholar]
- JIANG B.H., AGANI F., PASSANITI A., SEMENZA G.L. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumour progression. Cancer Res. 1997;57:5328–5335. [PubMed] [Google Scholar]
- JOHNSON D.E., LEE P.L., LU J., WILLIAMS L.T. Diverse forms of a receptor for acidic and basic fibroblast growth factors. Mol. Cell. Biol. 1990;10:4728–4736. doi: 10.1128/mcb.10.9.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSEPH I.B., NELSON J.B., DENMEADE S.R., ISAACS J.T. Androgens regulate vascular endothelial growth factor content in normal and malignant prostatic tissue. Clin. Cancer Res. 1997;3:2507–2511. [PubMed] [Google Scholar]
- KANG H.Y., LIN H.K., HU Y.C., YEH S., HUANG K.E., CHANG C. From transforming growth factor-β signaling to androgen action: identification of Smad3 as an androgen receptor co-regulator in prostate cancer cells. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3018–3023. doi: 10.1073/pnas.061305498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELEDJIAN K., GARRISON J.B., KYPRIANOU N. Doxazosin inhibits human vascular endothelial cell adhesion, migration, and invasion. J. Cell. Biochem. 2005;94:374–388. doi: 10.1002/jcb.20240. [DOI] [PubMed] [Google Scholar]
- KHOSRAVI J., DIAMANDI A., MISTRY J., SCORILAS A. Insulin-like growth factor I (IGF-I) and IGF-binding protein-3 in benign prostatic hyperplasia and prostate cancer. J. Clin. Endocrinol. Metab. 2001;86:694–699. doi: 10.1210/jcem.86.2.7211. [DOI] [PubMed] [Google Scholar]
- KIM K.J., LI B., WINER J., ARMANINI M., GILLETT N., PHILLIPS H.S., FERRARA N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- KIRBY R.S., POOL J.L. α1-Adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br. J. Urol. 1997);80:521–532. doi: 10.1046/j.1464-410x.1997.00247.x. [DOI] [PubMed] [Google Scholar]
- KUBOTA T., KOSHIZUKA K., WILLIAMSON E.A., ASOU H., SAID J.W., HOLDEN S., MIYOSHI I., KOEFFLER H.P. Ligand for peroxisome proliferator-activated receptor-γ (troglitazone) has potent antitumour effect against human prostate cancer both in vitro and in vivo. Cancer Res. 1998;58:3344–3352. [PubMed] [Google Scholar]
- KYPRIANOU N. Doxazosin and Terazosin suppress prostate growth by inducing apoptosis: Clinical significance. J. Urol. 2003;169:1520–1525. doi: 10.1097/01.ju.0000033280.29453.72. [DOI] [PubMed] [Google Scholar]
- KYPRIANOU N., BENNING C.M. Suppression of human prostate cancer cell growth by α-1-adrenoreceptor antagonists Doxazosin and Terazosin via induction of apoptosis. Cancer Res. 2000;60:4550–4555. [PubMed] [Google Scholar]
- LARA P.N., JR., TWARDOWSKI P., QUINN D.I. Angiogenesis-targeted therapies in prostate cancer. Clin. Prostate Cancer. 2004;3:165–173. doi: 10.3816/cgc.2004.n.027. [DOI] [PubMed] [Google Scholar]
- LEE C., SINTICH S.M., MATHEWS E.P., SHAH A.H., KUNDO S.D., PERRY K.T., CHO J.S., ILIO K.Y., CRONAUER M.V., JANULIS L., SENSIBAR J.A. Transforming growth factor-β in benign and malignant prostate. Prostate. 1999;39:285–290. doi: 10.1002/(sici)1097-0045(19990601)39:4<285::aid-pros9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- MCFALL A., ULKU A., LAMBERT Q.T., KUSA A., ROGERS-GRAHAM K., DER C.J. Oncogenic Ras blocks anoikis by activation of a novel effector pathway independent of phosphatidylinosotol 3-kinase. Mol. Cell. Biol. 2001);21:5488–5489. doi: 10.1128/MCB.21.16.5488-5499.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANN M., HENDRICKSON R.C., PANDLEY A. Analysis of proteins and proteomes by mass spectrometry. Ann. Rev. Biochem. 2001);70:437–473. doi: 10.1146/annurev.biochem.70.1.437. [DOI] [PubMed] [Google Scholar]
- MANNA S.K., SAH N.K., NEWMAN R.A., CISNEROS A., AGGARWAL B.B. Oleandrin suppresses activation of nuclear transcription factor-κB, activator protein-1, and c-Jun NH2-terminal kinase. Cancer Res. 2000;60:3838–3847. [PubMed] [Google Scholar]
- MANTZOROS C.S., TZONOU A., SIGNORELLO L.B. Insulin-like growth factor 1 in relation to prostate cancer and benign prostatic hyperplasia. Br. J. Cancer. 1997;76:1115–1118. doi: 10.1038/bjc.1997.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSAGUE J. TGF-β signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- MASSAGUE J., CHEIFETZ S., LAIHO M., RALPH D.A., WEIS F.M., ZENTELLA A. Transforming growth factor-β. Cancer Surv. 1992;12:81–103. [PubMed] [Google Scholar]
- MASSON N., WILLAM C., MAXWELL P.H., PUGH C.W., RATCLIFFE P.J. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCONKEY D.J., LIN Y., NUTT L.K., OZEL H.Z., NEWMAN R.A. Cardiac glycosides stimulate Ca2+ increases and apoptosis in androgen-independent, metastatic human prostate adenocarcinoma cells. Cancer Res. 2000;60:3807–3812. [PubMed] [Google Scholar]
- MIN J.H., YANG H., IVAN M., GERTLER F., KAELIN W.G., JR., PAVLETICH N.P. Structure of an HIF-1alpha-pVHL complex: hydroxyproline recognition in signaling. Science. 2002;296:1886–1889. doi: 10.1126/science.1073440. [DOI] [PubMed] [Google Scholar]
- MOSCHOS S.J., MANTZOROS C.S. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology. 2002;64:317–332. doi: 10.1159/000066230. [DOI] [PubMed] [Google Scholar]
- MOTYL T., GAJEWSKA M. IGF-binding proteins mediate TGF-β1-induced apoptosis in bovine mammary epithelial BME-UV1 cells. Comp. Biochem. Physiol. C (Toxicol. Pharmacol.) 2004;139:65–75. doi: 10.1016/j.cca.2004.09.006. [DOI] [PubMed] [Google Scholar]
- MYDLO J.H., MICHAELI J., HESTON W.D., FAIR W.R. Expression of basic fibroblast growth factor mRNA in benign prostatic hyperplasia and prostatic carcinoma. Prostate. 1988;13:241–247. doi: 10.1002/pros.2990130306. [DOI] [PubMed] [Google Scholar]
- NICKERSON T., HUYNH H., POLLAK M. Insulin-like growth factor binding protein-3 induces apoptosis in MCF-7 breast cancer cells. Biochem. Biophysics. Res. Commun. 1997;237:690–693. doi: 10.1006/bbrc.1997.7089. [DOI] [PubMed] [Google Scholar]
- NYBERT P., XIE L., KALLUIR R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967–3979. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- ORNITZ D.M., ITOH N. Fibroblast growth factors. Genome Biol. 2001;2:3005.1–3005.12. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANIGRAHY D., SINGER S., SHEN L.Q., BUTTERFIELD C.E., FREEDMAN D.A., CHEN E.J., MOSES M.A., KILROY S., DUENSING S., FLETCHER C., FLETCHER J.A., HLATKY L., HAHNFELDT P., FOLKMAN J., KAIPAINEN A. PPARγ ligands inhibit primary tumour growth and metastasis by inhibiting angiogenesis. J. Clin. Invest. 2002;110:923–932. doi: 10.1172/JCI15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARTIN J.V., ANGLIN I.E., KYPRIANOU N. Quinazoline-based alpha 1-adrenoreceptor antagonists induce prostate cancer cell apoptosis via TGF- β signaling and IκB induction. Br. J. Cancer. 2003;88:1615–1621. doi: 10.1038/sj.bjc.6600961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY K.T., ANTHONY C.T., STEINER M.S. Immunohistochemical localization of TGF-β1, TGF-β2, and TGF-β3 in normal and malignant human prostate. Prostate. 1997;33:133–140. doi: 10.1002/(sici)1097-0045(19971001)33:2<133::aid-pros7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- REISS K., D'AMBROSIO C., TU X., TU C., BASERGA R. Inhibition of tumour growth by a dominant negative mutant of the insulin-like growth factor I receptor with the by-stander effect. Clin. Cancer Res. 1998;4:2647–2655. [PubMed] [Google Scholar]
- RENNEBECK G., MARTELLI M, KYPRIANOU N. Anoikis and survival connections in the tumor microenvironment: is there a role in prostate cancer metastasis. Cancer Res. 2005;65:11230–11235. doi: 10.1158/0008-5472.CAN-05-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROPIQUET F., GIRI D., LAMB D.J., ITTMAN M. FGF7 and FGF2 are increased in benign prostatic hyperplasia and are associated with increased proliferation. J. Urol. 1999;162:595–599. [PubMed] [Google Scholar]
- RYROMAA M., LEHMAN K., DOWNWARD J. Matrix detachment induces caspase-dependent cytochrome C release from mitochondria: inhibition by PKB/Akt but not Raf signaling. Oncogene. 2000;19:4461–4468. doi: 10.1038/sj.onc.1203805. [DOI] [PubMed] [Google Scholar]
- SCHER H.I., CHUNG L.W. Bone metastases: improving the therapeutic index. Semin. Oncol. 1994;21:630–656. [PubMed] [Google Scholar]
- SCHMIDMAIER G., WILDEMANN B., OSTAPOWICZ D., KANDZIORA F., STANGE R., HAAS N.P., RASCHKE M. Long-term effects of local growth factor (IGF-1 and TGF-β1) treatment on fracture healing: A safety study for using growth factors. J. Orthop. Res. 2004;22:514–519. doi: 10.1016/j.orthres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- SEMENZA G. Signal transduction to hypoxia-inducible factor 1. Biochem. Pharmacol. 2002a;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- SEMENZA G.L. Involvement of hypoxia-inducible factor 1 in human cancer. Intern. Med. 2002b;41:79–83. doi: 10.2169/internalmedicine.41.79. [DOI] [PubMed] [Google Scholar]
- SEMENZA G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- SHARIAT S.F., KATTAN M.W., TRAXEL E., ANDREWS B., ZHU K., WHEELER T.M., SLAWIN K.M. Association of pre-and post-operative plasma levels of transforming growth factor-beta(1) and interleukin 6 and its soluble receptor with prostate cancer progression. Clin. Cancer Res. 2004;10:1992–1999. doi: 10.1158/1078-0432.ccr-0768-03. [DOI] [PubMed] [Google Scholar]
- SHAW Y.-J., YANG Y.-T., GARRISON J.B., KYPRIANOU N., CHEN C.-S. Pharmacological exploitation of the α1-adrenoreceptor antagonist Doxazosin to develop a novel class of antitumour agents that block intracellular protein kinase B/Akt activation. J. Med. Chem. 2004;47:4453–4462. doi: 10.1021/jm049752k. [DOI] [PubMed] [Google Scholar]
- SLACK-DAVIS J.K., EBLEN S.T., ZECEVIC M., BOEMER S.A., TARCAFAIVI A., DIAZ H.B., MARCHALL M.S., WEBER M.J., PARSON J.T., CATLING A.D. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J. Cell. Biol. 2003);162:281–291. doi: 10.1083/jcb.200212141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH J.A., MADDEN T., VIJJESWARAPU M., NEWMAN R.A. Inhibition of the export of fibroblast growth factor-2 (FGF-2) from prostate cancer cell lines PC3 and DU145 by Anvirzel and its cardiac glycoside component, oleandrin. Biochem. Pharmacol. 2001;62:469–472. doi: 10.1016/s0006-2952(01)00690-6. [DOI] [PubMed] [Google Scholar]
- Society American Cancer Cancer Facts and Figures. 2005.
- SORDELLO S., BERTRAND N., PLOUET J. Vascular endothelial growth factor is up-regulated in vitro and in vivo by androgens. Biochem. Biophysic. Res. Commun. 1998;251:287–290. doi: 10.1006/bbrc.1998.9328. [DOI] [PubMed] [Google Scholar]
- SROUJI S., RACHMIEL A., BLUMENFELD I., LIVNE E. Mandibular defect by TGF-β and IGF-1 released from a biodegradable osteoconductive hydrogel. J. Craniomaxillofac. Surg. 2005;33:79–84. doi: 10.1016/j.jcms.2004.09.003. [DOI] [PubMed] [Google Scholar]
- STANGELBERGER A., SCHALLY A.V., VARGA J.L., HAMMANN B.D., GROOT K., HALMOS G., CAI R.-Z., ZARANDI M. Antagonists of growth hormone releasing hormone (GHRH) and of bombesin/gastrin releasing peptide (BN/GRP) suppress the expression of VEGF, bFGF, and receptors of the EGF/HER family in PC-3 and DU-145 human androgen-independent prostate cancers. Prostate. 2005;64:303–315. doi: 10.1002/pros.20262. [DOI] [PubMed] [Google Scholar]
- STATTIN P., RINALDI S., BIESSY C., STENMAN U.-H., HALLMANS G., KAAKS R. High levels of circulating insulin-like growth factor-I increase prostate cancer risk: a prospective study in a population-based nonscreened cohort. J. Clin. Oncol. 2004;22:3104–3112. doi: 10.1200/JCO.2004.10.105. [DOI] [PubMed] [Google Scholar]
- STEWART L.V., WEIGEL N.L. Role of insulin-like growth factor binding proteins in Iα,25-dihydroxyvitamin D3-induced growth inhibition of human prostate cancer cells. Prostate. 2005;64:9–19. doi: 10.1002/pros.20212. [DOI] [PubMed] [Google Scholar]
- STEWART R.J., PANIGRAHY D., FLYNN E., FOLKMAN J. Vascular endothelial growth factor expression and tumour angiogenesis are regulated by androgens in hormone responsive human prostate carcinoma: evidence for androgen dependent destabilization of vascular endothelial growth factor transcripts. J. Urol. 2001;165:688–693. doi: 10.1097/00005392-200102000-00095. [DOI] [PubMed] [Google Scholar]
- TAHMATZOPOULOS A., KYPRIANOU N. Apoptotic impact of α1-blockers on prostate cancer: a myth or an inviting reality. Prostate. 2004;59:91–100. doi: 10.1002/pros.10357. [DOI] [PubMed] [Google Scholar]
- TAHMATZOPOULOS A., ROWLAND R.G., KYPRIANOU N. The role of α blockers in the management of prostate cancer. Expert. Opin. Pharmacother. 2004;5:1279–1285. doi: 10.1517/14656566.5.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TENNANT M.K., THRASHER J.B., TWOMEY P.A., DRIVDAHL R.H., BIRNBAUM R.S., PLYMATE S.R. Protein and messenger ribonucleic acid (mRNA for the type I insulin-like growth factor (IGF) receptor is decreased and IGF-II mRNA is increased in human prostate carcinoma compared to benign prostate epithelia. J. Clin. Endocrinol. Metab. 1996;81:3774–37782. doi: 10.1210/jcem.81.10.8855837. [DOI] [PubMed] [Google Scholar]
- TSANG M., DAWID I.B. Promotion and attenuation of FGF signaling through the Ras-MAP kinase pathway. Sci. STKE. 2004;228:317. doi: 10.1126/stke.2282004pe17. [DOI] [PubMed] [Google Scholar]
- TU H., JACOBS S.C., BORKOWSKI A., KYPRIANOU N. Significance of apoptosis in prostate cancer progression: relationship with cell proliferation, TGF-β1 and bcl-2 expression. Int. J. Cancer. 1996;69:357–363. doi: 10.1002/(SICI)1097-0215(19961021)69:5<357::AID-IJC1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- WEI D., LE X., ZHENG L., WANG L., FREY J.A., GAO A.C., PENG Z., HUANG S., XIONG H.Q., ABBRUZZESE J.L., XIE K. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- WIENER J.R., NAKANO K., KRUZELOCK R.P., BUCANA C.D., BAST R.C., JR., GALLICK G.E. Decreased Src tyrosine kinase activity inhibits malignant human ovarian cancer tumour growth in a nude mouse model. Clin. Cancer Res. 1999;5:2164–2170. [PubMed] [Google Scholar]
- WIKSTROM P., WESTIN P., STATTIN P., DAMBER J.E., BERGH A. Early castration-induced upregulation of transforming growth factor beta-1 and its receptors is associated with tumour cell apoptosis and a major decline in serum prostate-specific antigen in prostate cancer patients. Prostate. 1999;38:268–277. doi: 10.1002/(sici)1097-0045(19990301)38:4<268::aid-pros2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- WOLK A., MANTZOROS C.S., ANDERSSON S.O., BERGSTROM R., SIGNORELLO L.B., LAGIOU P., ADAMI H.O., TRICHOPOULOS D. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case–control study. J. Natl. Cancer Inst. 1998;90:911–915. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- WU S.-F., SUN H.-Z., QI X.-D., TU Z.-H. Effect of Epristeride on the expression of IGF-1 and TGF-β receptors in androgen-induced castrated rat prostate. Exp. Biol. Med. 2001;226:954–960. doi: 10.1177/153537020122601012. [DOI] [PubMed] [Google Scholar]
- YAMADA S., TAKETOMI T., YOSHIMURA A. Model analysis of difference between EGF pathway and FGF pathway. Biochem. Biophys. Res. Commun. 2004;314:1113–1120. doi: 10.1016/j.bbrc.2004.01.009. [DOI] [PubMed] [Google Scholar]
- YU F., WHITE S.B., ZHAO Q., LEE F.S. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZATELLI M.C., ROSSI R., DEGLI UBERTI E.C. Androgen influences transforming growth factor-β gene expression in human adrenocortical cells. J. Clin. Endocrinol. Metab. 2000;85:847–852. doi: 10.1210/jcem.85.2.6350. [DOI] [PubMed] [Google Scholar]
- ZHU B., KYPRIANOU N.2005Transforming growth factor beta and prostate cancer Cytokines and Cancered. Platanias, L. pp. 157–173.New York: Kluwer Academic Publishers; [DOI] [PubMed] [Google Scholar]